Abstract
For a whole century, citrate has been used as an in vitro anticoagulant via chelation of calcium. Later, also EDTA was introduced as an anticoagulant. An often overlooked fact is that zinc is bound to citrate and EDTA with affinities much greater than that for calcium, imposing problems in biomedical research. In vivo, proteins of the S100 family are released from leukocytes and known to bind calcium. Some of them, e.g., calprotectin, also chelate zinc. Thus, at an inflamed site, the ratio between Ca2+ and Zn2+ is changed. This mechanism is of importance for the modulation of the activation of a fascinating family of post-translationally acting calcium-dependent thiol enzymes, the transglutaminases, which are inhibited by zinc. This presentation illustrates the complexity of in vitro studies with zinc. Moreover, it exemplifies the role of Zn2+ in pathophysiological situations such as celiac disease and neurodegeneration.
Keywords: Autoimmunity, Celiac disease, Citrate, Neurodegeneration, Transglutaminase, Zinc
Highlights
-
•
Citrate, EDTA and DTT bind zinc as well as calcium.
-
•
At inflammation, calprotectin binds Zn2+, which leads to low concentrations of the ion.
-
•
Zn2+ inhibits the activation of transglutaminases and peptidylarginine deiminases.
Abbreviation list
- Ca
calcium
- CP
calprotectin
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- FXIII
factor XIII
- PAD
peptidylarginine deiminase
- TG
transglutaminase
- TG2
transglutaminase type 2
- Zn
zinc
1. Introduction
Citrate additive to blood tubes was introduced to bind calcium and thereby prevent blood coagulation [1]. The method represents a milestone in medicine history and is applied globally. However, an often overlooked effect shadows the success since citrate also binds zinc ions. After a century, this drawback still has consequences for biomedical research in enzymology. With this background, we want to highlight the complexity of in vitro studies with zinc but also illustrate how this trace metal physiologically modulates the activation of transglutaminases (TGs), a post-translationally acting family of thiol enzymes with multifaceted properties.
2. Zinc in the human body
After iron, zinc is the most abundant transition metal in man and is found in body fluids and in the nuclei, cytoplasm and membrane of all cells in the body. About 90% of the total 2–3 g are found in the skin, skeletal and muscles, while the highest concentrations have been reported in the prostate gland [2], retina [3], and the insulin-producing β-cells [4]. Zinc is bound to numerous proteins with varying affinities [5]. The serum concentration of zinc is normally 11–18 μmol/L [6].
The zinc homeostasis is controlled by metallothioneins and specific zinc transporters (ZIP and ZnT) [7]. The biological role of zinc can be divided into structural, catalytic and regulatory functions. The physiological significance of zinc is reflected by the symptoms of zinc deficiency. The first cases were described in the 1960s with hypogonadism and poor height growth due to one-sided low-zinc diet [8]. Simultaneous intake of other foods, especially salts of phytic acid which bind zinc in the intestine, can also reduce zinc absorption [9]. Other causes of zinc malabsorption may be damaged intestinal mucosa, e.g., in untreated celiac disease. Later, the importance of zinc for the immune system has been elucidated [10].
2.1. Biochemical aspects
The affinity of chelators such as citrate and EDTA is significantly greater for Zn2+ than for Ca2+ [11]. In practice, this means that the concentration of free Zn2+ never can be restored in blood that has been anticoagulated with these agents. In laboratory experiments, addition of EDTA to labile, calcium-dependent thiol enzymes is common to prevent accidental activation. Moreover, dithiothreitol (DTT) is used to protect against oxidation. Interestingly, DTT also binds Zn2+ with high affinity [12]. Remarkably, in vitro studies on zinc with these additives are still common.
The concentration of active Zn2+ (free or transiently protein-bound with low affinity) varies highly. The S100 family is a unique class of cell- and tissue-specific proteins which have been associated with calcium binding [13]. However, of the 25 human members of the S100, at least 10 also chelate zinc, with affinities that greatly exceed the binding for calcium [14]. The most well-known example is the heterodimer calprotectin (CP; S100A8/A9). CP is released from neutrophils and monocytes/macrophages [15]. Interestingly, Ca2+ increases the affinity between CP and Zn2+ [14]. Therefore, the concentration of active Zn2+ is reduced in inflammation. With respect to the binding of Zn2+, CP may physiologically be said to correspond to the in vitro properties of citrate, EDTA, and DTT.
About 300 enzymes are zinc-dependent [5]. In addition, zinc can inhibit the activation of at least two fascinating families of enzymes, namely the TGs and the citrullinating peptidylarginine deiminases (PADs). These are post-translationally acting, calcium-dependent thiol enzymes considered to have pathophysiological effects in major autoimmune diseases [16]. At a site of inflammation, an altered ratio of Ca2+/Zn2+ orchestrated by S100, can explain the modulation of enzyme activity.
2.2. Transglutaminases
The human TG family comprises eight thiol enzymes including coagulation factor XIII (FXIII) and a protein called band 4.2 [17]. The enzymes are present both intracellularly and extracellularly in the body. The eight enzymes have the same sequence of amino acids around the active center; the cysteine residue (GQCWV).
TGs catalyze the formation of proteolytically stable intermolecular ε-(γ-glutamyl)lysine pseudo-peptides between specific protein-bound lysine and glutamine residues [18,19]. The process is called transamidation and takes place in several steps. Ammonia is released upon formation of a thioester intermediate. In the absence of an amine group, water can serve as the second substrate, thereby hydrolyzing glutamine residues to glutamate (Fig. 1). In transamidation, the formation of the thioester is rate limiting [20]. In hydrolysis, on the other hand, the last step limits the rate [21].
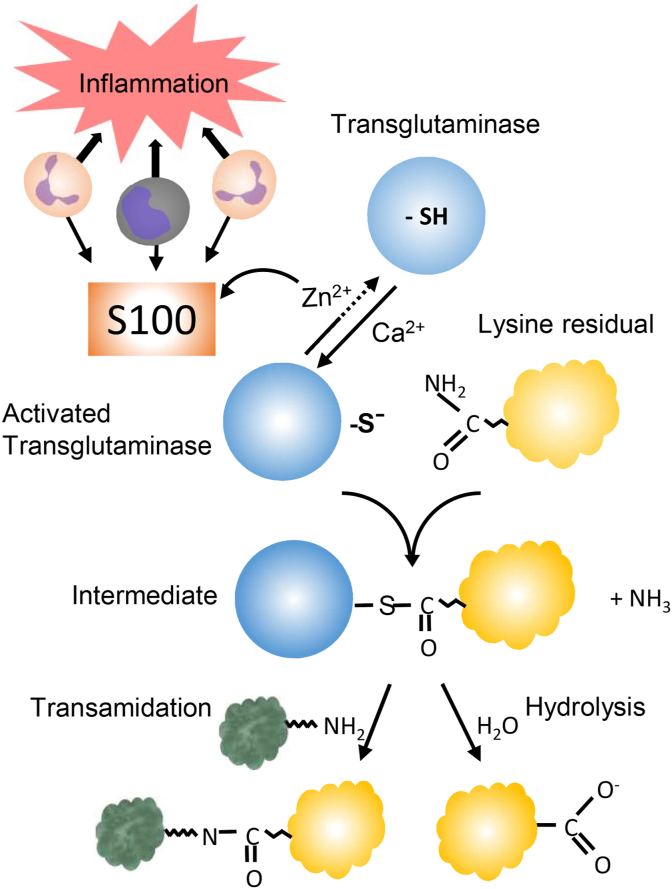
Fig. 1.
Zinc-modulated activation of transglutaminase. Leukocytes at a site of inflammation release S100-proteins which sequester Zn2+, thus facilitating the calcium-dependent activation of the enzyme. The intermediary thioester formed between the enzyme and a glutamine residue is then attacked by a primary amine, resulting in a transamidation. In the absence of an amine, water acts as the nucleophile, resulting in hydrolysis of the glutamine residue. In the transamidation, the formation of the thioester is rate limiting. In hydrolysis, the last step is rate limiting. Since the intermediate is essential for the reactions, both transamidation and hydrolysis (deamidation) are inhibited by zinc.
The discovery that Zn2+ inhibits TGs was made in the mid-1970s with a fluorescent technique that enables continuous measurement of transamidase activity [20]. Initially, the effect of metal ions was studied on thrombin-activated plasma FXIII. When Zn2+ did not activate the process, Ca2+ was added as a control, but still without effect. The finding initiated a detailed study that showed that zinc concentrations within the physiological range reversibly and competitively inhibited the calcium activation of thrombin-activated FXIII [22]. Moreover, Zn2+ prevented the Ca2+ -dependent alkylation of the catalytically essential cysteine sulfhydryl group of the enzyme with 14C-iodoacetamide [22,23]. Zinc also inhibited transamidation and deamidation, catalyzed by TG [22,24].
2.3. Factor XIII
FXIII is the only thiol enzyme among the classical coagulation factors. FXIII stabilizes the fibrin network during bleeding by catalyzing the formation of polymers between γ or α fibrin monomers [25]. The zymogen is a heterodimer, A2B2. The A-chain is formed in the bone marrow and the B-chain in the liver. Upon activation, thrombin cleaves a peptide in the A chain. Ca2+ has a dual function, partly when the A- and B-chains are separated and then when the A-chain changes in conformation, thereby exposing the active center, the cysteine residue [23]. Zn2+ inhibits the latter step competitively and reversibly [22].
Hereditary deficiency of FXIII is very rare and can lead to severe bleeding, defective wound healing and pregnancy complications [26]. Moreover, acquired deficiency has been reported during inflammatory conditions [27,28]. In vitro, citrullination of cellular FXIII (A2) gradually reduces the transamidating activity. Interestingly, citrullinated FXIII is an antigen to serum from rheumatoid arthritis (RA) patients [29], adding FXIII to the numerous lists of potential agents involved in the pathogenesis of RA.
2.4. Transglutaminase type 2 (TG2)
Upon Ca2+-induced activation of TG2 in vitro, the protein opens, exposing the cysteine group of the active center. Zn2+ inhibits this activation [22]. In vivo experiments on experimental animals show that TG2 is not active under normal physiological conditions despite the presence of Ca2+. However, the enzyme is activated after chemical or physical induction of tissue damage [30]. The reduction by thioredoxin and glutathione of an abrogating disulfide between two vicinal cysteine residues outside the active center may contribute to the activation of TG2 [31] (Fig. 2). Since Zn2+ is a stable ion, it does not participate directly in redox processes. Nevertheless, Zn2+ binds to thiols such as glutathione with high affinity [32].
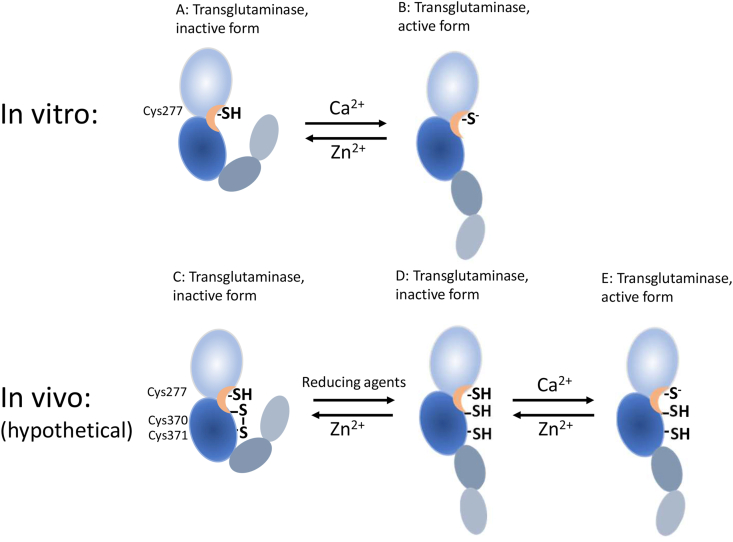
Fig. 2.
The proposed involvement of zinc in the activation of TG2. In vitro, Zn2+ competes with the binding of Ca2+ and the exposition of the active site cysteine thiol is blocked. In vivo a disulfide formed between two vicinal cysteine residues might inhibit the activation. When this disulfide is reduced, the enzyme is activated. Normal levels of Zn2+ prevents this reaction by binding to the thiol group of the reducing agent. When the concentration of active Zn2+ is reduced due to the release of S100-proteins such as calprotectin, the activation of TG2 can be completed.
TG2 are considered to be associated with a number of physiological functions, including apoptosis and receptor-mediated endocytosis, but has been particularly noted for its role in pathological contexts such as celiac disease and neurodegenerative diseases [17,19].
2.5. Transglutaminase type 2 and celiac disease
In celiac disease, antibodies are developed against both deamidated gluten peptides and TG2, the latter constituting the antigen to the histologically detected endomysial antibodies [33]. The affinity between TG2 and serum from subjects with celiac disease increases several folds when calcium is present [34,35], i.e., when the enzyme is activated. Zn2+ in low concentrations neutralizes this effect [34]. Celiac antibodies do not affect TG2 activity [34], which strengthens the hypothesis that the active center of the enzyme is masked to a thioester in the intermediate. These data have led to the hypothesis that the neoantigen consists of a Michaelis-Menten complex, in this case a thioester formed between the active thiol group of TG2 and a glutamine residue of partially deamidated gliadin (Fig. 3). This hypothesis has generated new ideas about the development of autoimmunity [34,36].
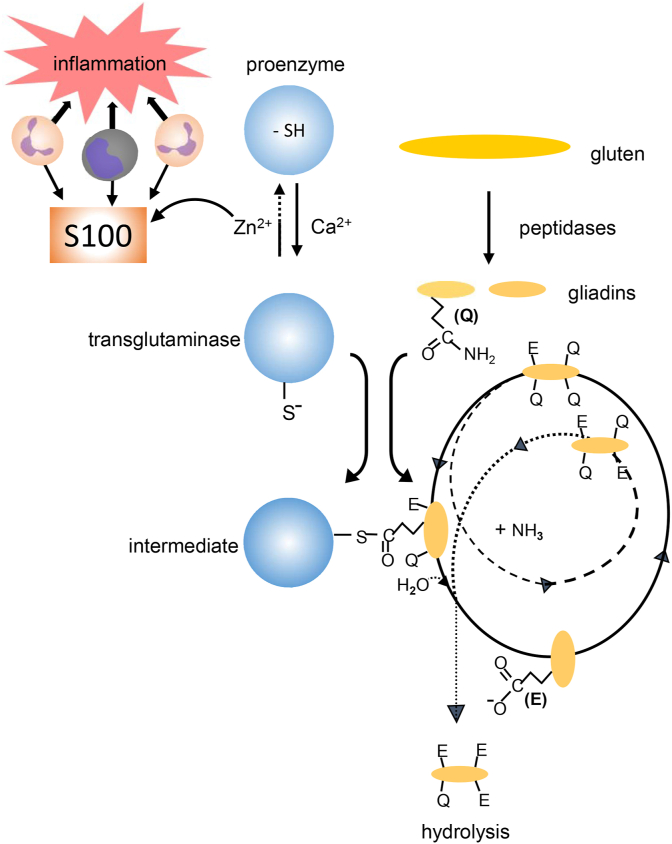
Fig. 3.
Postulated initiation of celiac disease. S100-proteins released from inflammatory cells chelate Zn2+, facilitating the calcium-dependent activation of transglutaminase type 2 (TG2). In a given order, specific glutamine residues (Q) in dietary gluten are deamidated (hydrolyzed) via TG2-catalysis, forming negatively charged glutamate residues (E). During the repeated process, a thioester intermediate is formed between the active site of the enzyme and a glutamine residue of a partly deamidated gliadin peptide. Since this complex is rather long-lived, it can function as an antigen resulting in the formation of antibodies against both deamidated gliadins and against TG2.
The pathogenesis of dermatitis herpetiformis (skin celiac disease) is considered to be similar to that of celiac disease. The major difference is that the isoenzyme is epidermal TG type 3 (TG3) [37].
The established treatment for celiac disease is a gluten-free diet. In vitro, zinc chloride and ascorbic palmitate, a dietary supplement that has some structural homology to α2-gliadin, have been shown to attenuate TG2 activity. This combination has been suggested to produce celiac-safe products [24].
2.6. Transglutaminase in neurodegenerative diseases
A common feature of several neurodegenerative diseases is the formation of stable, sparingly soluble protein aggregates. TG-catalyzed effects on proteins in the central nervous system (CNS) are therefore an exciting field of research with many perspectives on Alzheimer's, Parkinson's and Huntington's diseases and also on amyotrophic lateral sclerosis (ALS). Amyloid-β, hyperphosphorylated tau and α-synuclein are in vitro substrates for TGs [38]. A special case is Huntington's disease where the mutated gene leads to sequences of extra glutamine residues that may form substrates for TG [39]. Homeostasis for zinc is particularly complex in the brain [40], which may lead to a disturbed balance between Ca2+ and Zn2+, and explain a harmful activation of TG in inflamed parts of the CNS.
3. Conclusions
In vitro, citrate is routinely used to bind calcium ions and thus prevent blood clotting. An often overlooked aspect is that citrate, like EDTA and dithiothreitol (DTT), also chelate zinc. Therefore, laboratory studies with Zn2+ require major consideration of good laboratory practice.
S100 is a family of proteins known to chelate calcium. Of the 25 members, 10 of them including CP, also bind zinc. During inflammation, CP is released from neutrophils and monocytes/macrophages. With respect to the binding of Zn2+, CP has physiologically similar properties as citrate, EDTA, and DTT have in vitro. Therefore, the concentration of reactive Zn2+ is reduced at a site of inflammation.
Competitively, Zn2+ inhibits the calcium-dependent activation of TGs. Thus, the activation of TGs is facilitated during inflammation, supporting their physiological functions. Occasionally, this activation might induce pathophysiological reactions as illustrated by celiac disease and possibly also neurodegenerative illnesses.
The current presentation illustrates the complexity of in vitro studies with zinc, and how zinc binding additives may influence the catalytic processes of several thiol enzymes. Awareness of these processes are necessary in the evaluation of the laboratory findings concerning zinc-dependent processes.
Credit author statement
Conceptualization, P.S.; Data curation, P.S.; Formal analysis, P.S.; Funding acquisition, B.O.; Investigation; P.S., Methodology, P.S.; Project administration; B.O.; Resources; not applicable; Software, not applicable; Supervision, B.O.; Validation, P.S.; Visualization, B.R.; Roles/Writing - original draft, P.S.; Writing - review & Editing, B.R., B.O. All authors revised the manuscript critically and approved the final version for submission.
Funding
This study was supported by research grants from the Development Foundation of Region Skåne. The funders had no involvement in any aspect of the study and writing of the report.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Pål Stenberg, Email: pal.stenberg@med.lu.se.
Bodil Roth, Email: bodil.roth@med.lu.se.
Bodil Ohlsson, Email: bodil.ohlsson@med.lu.se.
References
- 1.Robertson O.H. A method of citrated blood transfusion. Br. Med. J. 1918;1:477–479. doi: 10.1136/bmj.1.2991.477. PMID: 20769016; PMCID: PMC2340403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costello L.C., Franklin R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016;611:100–112. doi: 10.1016/j.abb.2016.04.014. Epub 2016 Apr 27. PMID: 27132038; PMCID: PMC5083243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert R., Peto T., Lengyel I., Emri E. Zinc nutrition and inflammation in the aging retina. Mol. Nutr. Food Res. 2019;63 doi: 10.1002/mnfr.201801049. Epub 2019 Jun 27. PMID: 31148351. [DOI] [PubMed] [Google Scholar]
- 4.Davidson H.W., Wenzlau J.M., O'Brien R.M. Zinc transporter 8 (ZnT8) and β cell function. Trends Endocrinol. Metabol. 2014;25:415–424. doi: 10.1016/j.tem.2014.03.008. Epub 2014 Apr 18. PMID: 24751356; PMCID: PMC4112161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallee B.L., Falchuk K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. 10.1152/physrev.1993.73.1.79. PMID: 8419966. [DOI] [PubMed] [Google Scholar]
- 6.http://analysportalen-labmedicin.skane.se/
- 7.Kambe T., Tsuji T., Hashimoto A., Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015;95:749–784. doi: 10.1152/physrev.00035.2014. PMID: 26084690. [DOI] [PubMed] [Google Scholar]
- 8.Prasad A.S., Miale A., Jr., Farid Z., Sandstead H.H., Schulert A.R. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypogonadism. J. Lab. Clin. Med. 1963;61:537–549. PMID: 13985937. [PubMed] [Google Scholar]
- 9.Gibson R.S., Raboy V., King J.C. Implications of phytate in plant-based foods for iron and zinc bioavailability, setting dietary requirements, and formulating programs and policies. Nutr. Rev. 2018;76:793–804. doi: 10.1093/nutrit/nuy028. PMID: 30010865. [DOI] [PubMed] [Google Scholar]
- 10.Wessels I., Maywald M., Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9:1286. doi: 10.3390/nu9121286. PMID: 29186856; PMCID: PMC5748737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furia T.E. CRC Handbook of Food Additives. second ed. 1972. Stability constants (log K1) of various metal chelates. (Chapter 6) [Google Scholar]
- 12.Cornell N.W., Crivaro K.E. Stability constant for the zinc-dithiothreitol complex. Anal. Biochem. 1972;47:203–208. doi: 10.1016/0003-2697(72)90293-x. PMID: 5031113. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez L.L., Garrie K., Turner M.D. Role of S100 proteins in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867:118677. doi: 10.1016/j.bbamcr.2020.118677. Epub 2020 Feb 11. PMID: 32057918. [DOI] [PubMed] [Google Scholar]
- 14.Gilston B.A., Skaar E.P., Chazin W.J. Binding of transition metals to S100 proteins. Sci. China Life Sci. 2016;59:792–801. doi: 10.1007/s11427-016-5088-4. PMID: 27430886; PMCID: PMC5123432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ometto F., Friso L., Astorri D., Botsios C., Raffeiner B., Punzi L., Doria A. Calprotectin in rheumatic diseases. Exp. Biol. Med. 2017;242(8):859–873. doi: 10.1177/1535370216681551. Epub 2016 Jan 1. PMID: 27895095; PMCID: PMC5407536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenberg P., Roth B., Wollheim F.A. Peptidylarginine deiminases and the pathogenesis of rheumatoid arthritis: a reflection of the involvement of transglutaminase in coeliac disease. Eur. J. Intern. Med. 2009;20:749–755. doi: 10.1016/j.ejim.2009.08.007. Epub 2009 Sep 19. PMID: 19892302. [DOI] [PubMed] [Google Scholar]
- 17.Lorand L., Iismaa S.E. Transglutaminase diseases: from biochemistry to the bedside. Faseb. J. 2019;33:3–12. doi: 10.1096/fj.201801544R. Erratum in: FASEB J. 2019;33:4653. PMID: 30593123. [DOI] [PubMed] [Google Scholar]
- 18.Lorand L., Stenberg P. CRC Handbook of Biochemistry and Molecular Biology 3rd Edition Proteins Volume II. 1976. Endo-γ-glutamine:ε-lysine transferases. Enzymes which cross-link proteins. Editor G.D. Fasman. [Google Scholar]
- 19.Beninati S., Facchiano F., Piacentini M. Transglutaminases: future perspectives. Amino Acids. 2013;44:1–9. doi: 10.1007/s00726-012-1431-7. Epub 2012 Nov 30. PMID: 23283580. [DOI] [PubMed] [Google Scholar]
- 20.Stenberg P., Curtis C.G., Wing D., Tong Y.S., Credo R.B., Gray A., Lorand L. Tra nsamidase kinetics. Amide formation in the enzymic reactions of thiol esters with amines. Biochem. J. 1975;147:155–163. PMID: 239698; PMCID: PMC1165385. [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis C.G., Stenberg P., Brown K.L., Baron A., Chen K., Gray A. Kinetics of transamidating enzymes. Production of thiol in the reactions of thiol esters with fibrinoligase. Biochemistry. 1974;13:3257–3262. doi: 10.1021/bi00713a012. PMID: 4841063. [DOI] [PubMed] [Google Scholar]
- 22.Credo R.B., Stenberg P., Tong Y.S., Lorand L. Inhibition of fibrinoligase and transglutaminase by zinc ions. Fed. Proc. San Fransisco. 1976;7 [Google Scholar]
- 23.Curtis C.G., Brown K.L., Credo R.B., Domanik R.A., Gray A., Stenberg P., Lorand L. Calcium-dependent unmasking of active center cysteine during activation of fibrin stabilizing factor. Biochemistry. 1974;13:3774–3780. doi: 10.1021/bi00715a024. PMID: 4859234. [DOI] [PubMed] [Google Scholar]
- 24.Engstrom N., Saenz-Méndez P., Scheers J., Scheers N. Towards Celiac-safe foods: decreasing the affinity of transglutaminase 2 for gliadin by addition of ascorbyl palmitate and ZnCl2 as detoxifiers. Sci. Rep. 2017;7:77. doi: 10.1038/s41598-017-00174-z. PMID: 28250436; PMCID: PMC5427931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorand L. Factor XIII and the clotting of fibrinogen: from basic research to medicine. J. Thromb. Haemostasis. 2005;3:1337–1348. doi: 10.1111/j.1538-7836.2005.01213.x. PMID: 15978088. [DOI] [PubMed] [Google Scholar]
- 26.Karimi M., Peyvandi F., Naderi M., Shapiro A. Factor XIII deficiency diagnosis: challenges and tools. Int. J. Lab. Hematol. 2018;40:3–11. doi: 10.1111/ijlh.12756. PMID: 29027765. [DOI] [PubMed] [Google Scholar]
- 27.Yan M.T.S., Rydz N., Goodyear D., Sholzberg M. Acquired factor XIII deficiency: a review. Transfus. Apher. Sci. 2018;57:724–730. doi: 10.1016/j.transci.2018.10.013. PMID: 30446212. [DOI] [PubMed] [Google Scholar]
- 28.Ichinose A. Japanese Collaborative Research Group on AH13. Autoimmune acquired factor XIII deficiency due to anti-factor XIII/13 antibodies: a summary of 93 patients. Blood Rev. 2017;31:37–45. doi: 10.1016/j.blre.2016.08.002. Epub 2016 Aug 11. PMID: 27542511. [DOI] [PubMed] [Google Scholar]
- 29.Roth B., Wollheim F., Stenberg P. Cellular factor XIII and peptidylarginine deiminase-catalysed citrullination in rheumatoid arthritis. Ann. Rheum. Dis. 2010;69(Suppl 2) A9. http://dx.doi.org/10.113/ard.2010.129577i. [Google Scholar]
- 30.Siegel M., Strand P., Watts R.E., Choi K., Jabri B., Omary M.B., Khosla C. Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PloS One. 2008;3 doi: 10.1371/journal.pone.0001861. PMID: 18365016; PMCID: PMC2267210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palanski B.A., Khosla C. Cystamine and disulfiram inhibit human transglutaminase 2 via an oxidative mechanism. Biochemistry. 2018;57:3359–3363. doi: 10.1021/acs.biochem.8b00204. PMID: 29570977; PMCID: PMC6008213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oteiza P.I. Zinc and the modulation of redox homeostasis. Free Radic. Biol. Med. 2012;53:1748–1759. doi: 10.1016/j.freeradbiomed.2012.08.568. Epub 2012 Aug 25. PMID: 22960578; PMCID: PMC3506432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dieterich W., Ehnis T., Bauer M., Donner P., Volta U., Riecken E.O., Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat. Med. 1997;3:797–801. doi: 10.1038/nm0797-797. PMID: 9212111. [DOI] [PubMed] [Google Scholar]
- 34.Roth E.B., Sjöberg K., Stenberg P. Biochemical and immuno-pathological aspects of tissue transglutaminase in coeliac disease. Autoimmunity. 2003;36:221–226. doi: 10.1080/0891693031000118974. PMID: 14563015. [DOI] [PubMed] [Google Scholar]
- 35.Agardh D., Roth B., Lernmark A., Stenberg P. Calcium activation of tissue transglutaminase in radioligand binding and enzyme-linked autoantibody immunoassays in childhood celiac disease. Clin. Chim. Acta. 2005;358:95–103. doi: 10.1016/j.cccn.2005.02.027. PMID: 15946660. [DOI] [PubMed] [Google Scholar]
- 36.Stenberg P., Roth B. Zinc is the modulator of the calcium-dependent activation of post-translationally acting thiol-enzymes in autoimmune diseases. Med. Hypotheses. 2015;84:331–335. doi: 10.1016/j.mehy.2015.01.022. PMID: 25660831. [DOI] [PubMed] [Google Scholar]
- 37.Sárdy M., Kárpáti S., Merkl B., Paulsson M., Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J. Exp. Med. 2002;195:747–757. doi: 10.1084/jem.20011299. PMID: 11901200; PMCID: PMC2193738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeitner T.M., Pinto J.T., Krasnikov B.F., Horswill M., Cooper A. J. Transglutaminases and neurodegeneration. J. Neurochem. 2009;109(Suppl 1):160–166. doi: 10.1111/j.1471-4159.2009.05843.x. (Suppl 1) PMID: 19393023; PMCID: PMC2752967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Min B., Chung K.C. New insight into transglutaminase 2 and link to neurodegenerative diseases. BMB Rep. 2018;51:5–13. doi: 10.5483/bmbrep.2018.51.1.227. PMID: 29187283; PMCID: PMC5796628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sensi S.L., Paoletti P., Bush A.I., Sekler I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 2009;10:780–791. doi: 10.1038/nrn2734. PMID: 19826435. [DOI] [PubMed] [Google Scholar]