Abstract
The columbic efficiency, removal efficiency and voltage production of seven different combinations of carbon (acetic acid, albumin and sucrose) with nutrients (C:N, C:P, C:S, C:N:S, C:P:S, C:N:P and C: N:S:P) were investigated at three different ratios (20:1, 15:1 and 10:1). The effects of various pH values were also explored for these combinations of carbon, and sulfur compounds (pH 6–8). The highest columbic efficiency (75.8%), COD removal efficiency (86%) and voltage (667 mV) were recorded when the acetic acid was used in the MFC and the lowest columbic efficiency (12.8%), removal efficiency (37.6%) and voltage (145 mV) were observed in case of albumin. A marked increase in columbic efficiency, removal efficiency and voltage production were seen with the rise in the pH value from 6 to 8. The lowest columbic efficiency, removal efficiency and voltage production were seen at pH 6 and highest at pH 8. At each investigated pH, the highest removal efficiency, columbic efficiency, and voltage were found at substrate ratio of 20:1 while lower at 10:1. At all pH values, the carbon to nutrient ratios seemed to have followed a similar trend i.e., the COD removal efficiency, columbic efficiency and voltage generation was found in the order C:N > C:N:S > C:N:S:P > C:N:P > C:S > C:P:S > C:P. In all cases, nitrogen showed a higher removal as compared to phosphorous and sulfur.
Keywords: Bioenergy, Columbic efficiency, Electronic equivalents, Substrate ratios, Voltage generation
Highlights
-
•
The effect of substrate ratios on the voltage production in MFC was investigated.
-
•
Substrate ratios studied at three pH values (6~8).
-
•
The best performance was noted for acetic acid as a substrate.
-
•
The performance of MFC had linear relation with rising pH.
-
•
Nitrogen showed higher removal as compared to phosphorous and sulfur removal.
Bioenergy; Columbic efficiency; Electronic equivalents; Substrate ratios; Voltage generation.
1. Introduction
Industrial wastewater contains large amounts of organic matter, inorganic nutrients (like nitrogen, phosphorus, and sulfur) along with a wide array of other harmful pollutants that could adversely affect the environment. Hence, it is very important to treat such pollutant laden wastewater before releasing it into the natural water bodies. There are several ways to treat wastewater (chemical, physical, and biological processes), but sustainable wastewater treatment is the need of the day. Sustainable wastewater treatment not only aims at water reuse but also energy recovery and nutrient management (Goswami et al., 2019). Simultaneous removal of carbon, nitrogen and sulfur is possible using conventional wastewater treatment systems (Abeysiriwardana-Arachchige et al., 2020; Diaz-Elsayed et al., 2019; Castellanos et al., 2021). Although conventional wastewater treatment systems can remove/recover nutrients, it cannot produce electricity. Studies have shown that MFCs can use NO3- as a cathodic electron acceptor, allowing the simultaneous C removal at the anode and N at the cathode (Ge et al., 2020; Vijay et al., 2020; Deng et al., 2018; Kelly and He, 2014). Biological sulfur removal can also be accomplished in a MFC (Li et al., 2021; Cai et al., 2017; Luo et al., 2020). Phosphorous was demonstrated to be recovered in a MFC (Huang et al., 2017; Geng et al., 2018; Liu et al., 2018). Simultaneous anaerobic sulfide and nitrate removal was coupled with electricity generation as suggested by Cai et al. (2013).
Removal of individual or a combination of two nutrients have been studied, however, no prior study has focused on the simultaneous removal of carbon, nitrogen, phosphorous and Sulfur in a MFC using an abiotic cathode. In the current experiment, nitrogen, phosphorous and sulfur were individually used as well as in combinations (N, P, S, N:P, N:S, P: N and N:P:S) as electron acceptors in the cathodic chamber. Oxygen is the most commonly used electron acceptor in the cathodic compartment of aerobic MFCs; however, high aeration costs make it a less feasible option (Strik et al., 2011). Oxygen from air can be directly used by an aerobic cathode but they require catalysts which are usually expensive (TerHeijne et al., 2008). Hence, various nutrients can be a good alternative to oxygen as final electron acceptors.
Although above studies have focused on the removal of single nutrient or a combination of two nutrients, but none of the studies have been done on the simultaneous removal of carbon, nitrogen, sulfur, and phosphorous removal in MFC. Nutrient removal has been studied in the anodic chamber containing microbial communities in most of the previous studies; however, a simultaneous removal of nutrients has not been studied in the cathodic chamber of MFC containing abiotic cathode. In the current experiment nitrogen, phosphorous and sulfur were treated not only individually but in their mutual combinations (N, P, S, N:P, N:S, P: N and N:P:S) also as electron acceptors in the abiotic cathodic chamber. The purpose of this study was to find the most suitable ratio of carbon and nitrogen, phosphorus, and sulfur at a suitable pH where a maximum carbon and nutrient removal could be realized along electricity production.
2. Materials and methods
2.1. MFC construction
Three dual chamber of MFCs made of perplex glass were constructed as shown in Figure 1. Both anode and cathode of each MFC had a working volume of 500 mL each. Cation exchange membrane (CEM, CMI-7000, Membrane International, Inc. USA) was used to separate the two compartments. Titanium wire (17 cm2) was used as both anode and cathode. The working volume of the reactor was 500 ml. The MFC was provided with two ports on top while two ports were provided on lateral walls to drain off the influents or effluent. Sludge (150 ml) from a functional anaerobic MFC was added to the anodic chamber as a source of exoelectrogens. The MFC could be plugged to make it anaerobic. The whole apparatus was placed in dark to avoid photoinhibition and growth of photosynthetic microbial species.
Figure 1.
Illustration of various components of microbial fuel cell used in the study (H = positively charged hydrogen atoms, PEM = Proton exchange membrane, e = electrons flowing in the circuit).
Substrates used in the experiment included acetic acid, sucrose, and albumin. They were chosen after screening different sources of carbon. Synthetic solutions of nitrates, sulphates and phosphates were made based on their molar concentrations. Each substrate solution (300 ml) was added to the anode along with the trace element solution made according to (Mahmood et al., 2007). Acetic acid, sucrose and albumin were used in the experiment (carbon sources) were added to the anode while nutrient solutions of nitrates, phosphates and sulphates were used in cathode. The substrates and nutrients were added to the MFCs in different ratios i.e., 10:1, 15:1 and 20:1. These ratios were administered based on the molar concentrations and corresponding COD concentrations. For example, COD (carbon source) was maintained in range of 250–500 mg/L while the COD of nutrients like N, S and P was in range of 50–100 mg/L. Another important factor pH was also tested (three points tested were pH 6, 7 and 8). The external resistance was maintained at 1000 Ω. Nitrogen gas was purged through the MFC was for 15 min and then it was sealed maintain anaerobic conditions.
2.2. MFC operation
The synthetic solutions of each substrates (acetic acid, sucrose, and albumin) were used as anolytes in the anodic chamber where anaerobic conditions were maintained. Buffer solution (1M KMnO4) along with nutrient solution were added to the cathodic chamber to maintain the pH at desired level. Hydraulic retention time (HRT) was 24 h after which the effluent obtained along influent were analyzed for water quality parameters. The work of Pasupuleti et al., (2016) has demonstrated that higher HRT would be useful to enhance treatment and higher energy output. The MFC was operated in batch mode at 35 °C (closed circuit). Each experiment was carried out thrice and the internal resistance was kept at 1000 Ω.
2.3. Analytical procedure
The COD, pH, sulphate, nitrate, and phosphate concentration of both influent and effluent were analyzed according to the standard methods for wastewater analysis (APHA, 2005). Vola closed reflux colorimetric method using digester (HACH - LTG 082.99.40001) measured COD (APHA, 2005). The nitrates, phosphates and sulphates were measured by UV-VIS Spectrophotometer (IRMeCO UV-Vis, U2020).
Columbic efficiency was found using formula:
| Columbic efficiency = Cp/Cti x100 |
Where: Cp is total Columbs, Cti is theoretical amount of columbs. To find numbers of columbs the following formula was used Cmax = F f SCOD V (Cmax is the columbic efficiency). F is Faradays constant (96,485 C/mol of electrons), f is 1 mol of electrons generated per 8 g of COD. V is volume in liters.
3. Results and discussion
3.1. Removal efficiency, columbic efficiency and voltage production
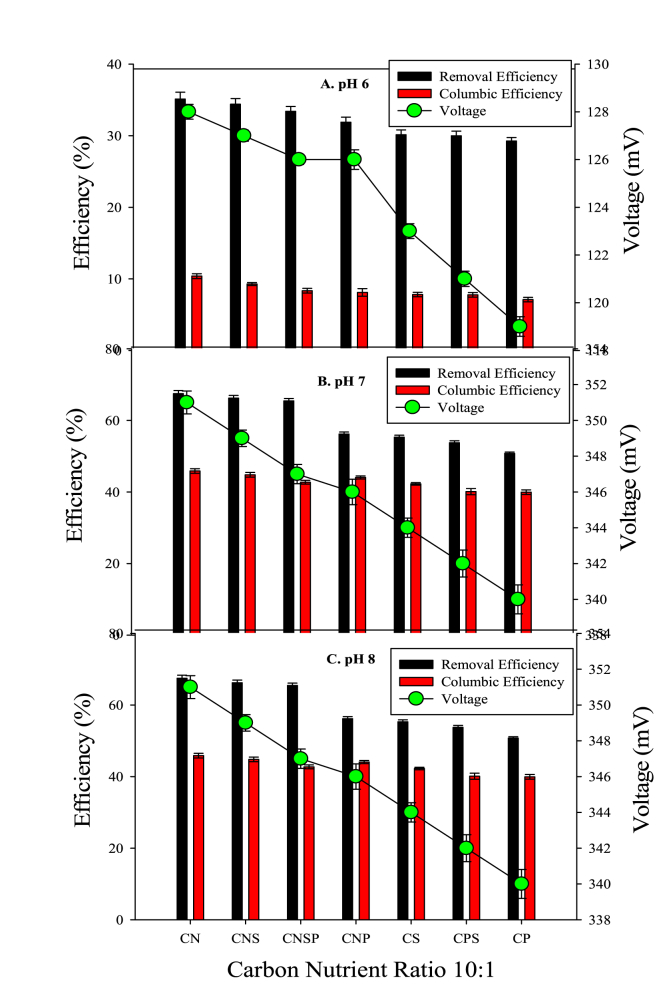
The CE, RE and voltage production of seven combinations of carbon with nutrients (C:N, C:P, C:S, C: N:S, C:P:S, C:N:P and C:N:S:P) were studied at different ratios (20:1, 15:1 and 10:1). The best performance of MFC was evident for 20:1 and at pH 8; hence, the following result section will describe results for pH 8 at 20:1 ratio.
For the carbon nutrient ratio of 10:1 and varying the pH; it was seen that at pH 6 (Figure 2), C:P showed the least promising results when COD RE, CE and voltage was measured that is 29.2%, 8.4% and 127 mV, respectively. C: N exhibited the highest COD removal (35.1%), CE (10.3%) and voltage 128 mV. At pH 7 C:P showed the lowest voltage value i.e., 221 mV. The lowest RE and CE i.e. 41.9% and 20.7%, respectively, were also shown by C:P. The highest COD RE (50.9%), CE (25.9%) and voltage (229 mV) was exhibited by C: N (Figure 2B). When the pH was kept at 8, the C:P showed a lowest COD RE (55.3%) and CE (42.2%). The voltage output was also the lowest (348 mV). C: N showed highest voltage value i.e., 351 mV along with the highest RE and CE i.e., 67.4% and 45.8% (Figure 2C).
Figure 2.
Effect of pH on removal efficiency, columbic efficiency and voltage using acetic acid at 10:1. A: The performance of MFC using acetic acid at pH 6; B: The performance of MFC using acetic acid at pH 7; C: The performance of MFC using acetic acid at pH 8.
For the C:N ratio of 20:1 at pH 6; the CE, RE and voltage were 18.2%, 42.7% and 187 mV, respectively (Figure 3). On the other hand, C:P showed the lowest CE (15.3%) with RE (36%) and voltage output (171 mV) (Figure 3G). At pH 7, the CE was in the range of 44.7%–54%, RE ranged from 66.8%-76.7% and voltage was in the range of 509 mV–530 mV (Figure 3H). Increasing the pH to 8 improved the CE, RE and voltage production (Figure 3I). C:N showed the highest CE (75.8%), RE (86%) and voltage (667 mV) while C:P showed the lowest CE (70%), RE (83%) and voltage (642 mV). The results were found in the order C: N > C: N:S > C: N:S:P > C:N:P > C:S > C:P:S > C:P.
Figure 3.
Effect of pH on removal efficiency, columbic efficiency and voltage using acetic acid at 20:1. G: The performance of MFC using acetic acid at pH 6; H: The performance of MFC using acetic acid at pH 7; I: The performance of MFC using acetic acid at pH 8.
The comparison of current and power density has been presented in Tables 1A, 1B, 1C.
Table 1A.
The relationship of current (I) and power density (PD) for acetic acid at various ratios and pH values.
| Acetic acid | ||||||
|---|---|---|---|---|---|---|
| Ratio | pH 6 |
pH 7 |
pH 8 |
|||
| I (mA) | P.D (mW/m2) | I (mA) | P.D (mW/m2) | I (mA) | P.D (mW/m2) | |
|
10:01 | ||||||
| CN | 0.128 | 9.60 × 10−2 | 0.224 | 2.95 × 10−1 | 0.351 | 7.25 × 10−1 |
| CNS | 0.127 | 9.48 × 10−2 | 0.221 | 2.87 × 10−1 | 0.349 | 7.16 × 10−1 |
| CNSP | 0.126 | 9.33 × 10−2 | 0.218 | 2.8 × 10-1 | 0.347 | 7.08 × 10−1 |
| CNP | 0.126 | 9.33 × 10−2 | 0.215 | 2.72 × 10−1 | 0.346 | 7.04 × 10−1 |
| CS | 0.123 | 8.89 × 10−2 | 0.213 | 2.67 × 10−1 | 0.344 | 6.96 × 10−1 |
| CPS | 0.121 | 8.61 × 10−2 | 0.209 | 2.57 × 10−1 | 0.342 | 6.88 × 10−1 |
| CP |
0.119 |
8.33 × 10−2 |
0.202 |
2.40 × 10−1 |
0.34 |
6.80 × 10−1 |
|
15:01 | ||||||
| CN | 0.193 | 2.19 × 10−1 | 0.38 | 8.49 × 10−1 | 0.527 | 1.63 |
| CNS | 0.19 | 2.12 × 10−1 | 0.377 | 8.36 × 10−1 | 0.527 | 1.63 |
| CNSP | 0.188 | 2.07 × 10−1 | 0.367 | 7.92 × 10−1 | 0.526 | 1.63 |
| CNP | 0.186 | 2.03 × 10−1 | 0.365 | 7.84 × 10−1 | 0.524 | 1.62 |
| CS | 0.185 | 2.01 × 10−1 | 0.364 | 7.79 × 10−1 | 0.52 | 1.59 |
| CPS | 0.183 | 1.96 × 10−1 | 0.362 | 7.71 × 10−1 | 0.518 | 1.58 |
| CP |
0.182 |
1.94 × 10−1 |
0.359 |
7.58 × 10−1 |
0.516 |
1.57 |
|
20:01 | ||||||
| CN | 0.187 | 2.05 × 10−1 | 0.529 | 1.65 | 0.667 | 2.62 |
| CNS | 0.185 | 2.01 × 10−1 | 0.526 | 1.63 | 0.657 | 2.54 |
| CNSP | 0.184 | 1.99 × 10−1 | 0.525 | 1.62 | 0.653 | 2.51 |
| CNP | 0.182 | 1.94 × 10−1 | 0.52 | 1.59 | 0.651 | 2.49 |
| CS | 0.18 | 1.90 × 10−1 | 0.51 | 1.53 | 0.65 | 2.49 |
| CPS | 0.175 | 1.80 × 10−1 | 0.506 | 1.51 | 0.645 | 2.45 |
| CP | 0.171 | 1.72 × 10−1 | 0.495 | 1.44 | 0.642 | 2.42 |
Table 1B.
The relationship of current (I) and power density (PD) for sucrose at various ratios and pH values.
| Sucrose | ||||||
|---|---|---|---|---|---|---|
| Ratio | pH6 |
pH7 |
pH8 |
|||
| I (mA) | P.D (mW/m2) | I (mA) | P.D (mW/m2) | I (mA) | P.D (mW/m2) | |
|
10:01 | ||||||
| CN | 0.099 | 5.76 × 10−2 | 0.201 | 2.38 × 10−1 | 0.341 | 6.84 × 10−1 |
| CNS | 0.091 | 4.87 × 10−2 | 0.187 | 2.06 × 10−1 | 0.341 | 6.84 × 10−1 |
| CNSP | 0.088 | 4.55 × 10−2 | 0.181 | 1.93 × 10−1 | 0.335 | 6.60 × 10−1 |
| CNP | 0.086 | 4.35 × 10−2 | 0.175 | 1.80 × 10−1 | 0.32 | 6.02 × 10−1 |
| CS | 0.084 | 4.15 × 10−2 | 0.161 | 1.52 × 10−1 | 0.312 | 5.73 × 10−1 |
| CPS | 0.08 | 3.76 × 10−2 | 0.156 | 1.43 × 10−1 | 0.225 | 2.98 × 10−1 |
| CP |
0.076 |
3.39 × 10−2 |
0.15 |
1.32 × 10−1 |
0.221 |
2.87 × 10−1 |
|
15:01 | ||||||
| CN | 0.135 | 1.07 × 10−1 | 0.33 | 6.41 × 10−1 | 0.576 | 1.95 |
| CNS | 0.134 | 1.05 × 10−1 | 0.324 | 6.18 × 10−1 | 0.571 | 1.92 |
| CNSP | 0.132 | 1.02 × 10−1 | 0.316 | 5.87 × 10−1 | 0.563 | 1.86 |
| CNP | 0.129 | 9.78 × 10−2 | 0.252 | 3.74 × 10−1 | 0.559 | 1.84 |
| CS | 0.12 | 8.47 × 10−2 | 0.224 | 2.95 × 10−1 | 0.51 | 1.53 |
| CPS | 0.119 | 8.33 × 10−2 | 0.214 | 2.69 × 10−1 | 0.487 | 1.40 |
| CP |
0.116 |
7.91 × 10−2 |
0.21 |
2.59 × 10−1 |
0.469 |
1.29 |
|
20:01 | ||||||
| CN | 0.194 | 2.21 × 10−1 | 0.565 | 1.88 | 0.624 | 2.29 |
| CNS | 0.186 | 2.03 × 10−1 | 0.564 | 1.87 | 0.62 | 2.26 |
| CNSP | 0.184 | 1.99 × 10−1 | 0.56 | 1.84 | 0.618 | 2.25 |
| CNP | 0.172 | 1.74 × 10−1 | 0.555 | 1.81 | 0.614 | 2.22 |
| CS | 0.169 | 1.68 × 10−1 | 0.542 | 1.73 | 0.61 | 2.19 |
| CPS | 0.163 | 1.56 × 10−1 | 0.535 | 1.68 | 0.607 | 2.17 |
| CP | 0.143 | 1.20 × 10−1 | 0.532 | 1.66 | 0.589 | 2.04 |
Table 1C.
The relationship of current (I) and power density (PD) for albumin at various ratios and pH values.
| Albumin | ||||||
|---|---|---|---|---|---|---|
| Ratio | pH6 |
pH7 |
pH8 |
|||
| I (mA) | P.D (mW/m2) | I (mA) | P.D (mW/m2) | I (mA) | P.D (mW/m2) | |
|
10:01 | ||||||
| CN | 0.082 | 3.95 × 10−2 | 0.147 | 1.27 × 10−1 | 0.172 | 1.74 × 10−1 |
| CNS | 0.078 | 3.57 | 0.141 | 1.17 × 10−1 | 0.172 | 1.74 × 10−1 |
| CNSP | 0.07 | 2.88 × 10−2 | 0.136 | 1.09 × 10−1 | 0.17 | 1.70 × 10−1 |
| CNP | 0.064 | 2.40 × 10−2 | 0.134 | 1.06 × 10−1 | 0.165 | 1.60 × 10−1 |
| CS | 0.06 | 2.11 × 10−2 | 0.131 | 1.01 × 10−1 | 0.159 | 1.48 × 10−1 |
| CPS | 0.055 | 1.77 × 10−2 | 0.127 | 9.49 × 10−2 | 0.151 | 1.34 × 10−1 |
| CP |
0.033 |
6.40E-06 |
0.121 |
8.61 × 10−2 |
0.145 |
1.23 × 10−1 |
|
15:01 | ||||||
| CN | 0.136 | 1.08 × 10−1 | 0.203 | 2.42 × 10−1 | 0.206 | 2.49 × 10−1 |
| CNS | 0.134 | 1.05 × 10−1 | 0.185 | 2.01 × 10−1 | 0.205 | 2.47 × 10−1 |
| CNSP | 0.133 | 1.04 × 10−1 | 0.188 | 2.08 × 10−1 | 0.204 | 2.44 × 10−1 |
| CNP | 0.132 | 1.02 × 10−1 | 0.176 | 1.82 × 10−1 | 0.203 | 2.42 × 10−1 |
| CS | 0.131 | 1.00 × 10−1 | 0.141 | 1.17 × 10−1 | 0.199 | 2.37 × 10−1 |
| CPS | 0.129 | 9.78 × 10−2 | 0.129 | 9.79 × 10−2 | 0.197 | 2.28 × 10−1 |
| CP |
0.125 |
9.19 × 10−2 |
0.122 |
8.76 × 10−1 |
0.192 |
2.16 × 10−1 |
|
20:01 | ||||||
| CN | 0.178 | 1.86 × 10−1 | 3.25 | 6.21 × 10−1 | 0.479 | 1.34 |
| CNS | 0.167 | 1.64 × 10−1 | 0.32 | 6.02 × 10−1 | 0.471 | 1.30 |
| CNSP | 0.16 | 1.50 × 10−1 | 0.299 | 5.26 × 10−1 | 0.469 | 1.29 |
| CNP | 0.156 | 1.43 × 10−1 | 0.282 | 4.68 × 10−1 | 0.467 | 1.28 |
| CS | 0.151 | 1.34 × 10−1 | 0.28 | 4.61 × 10−1 | 0.464 | 1.26 |
| CPS | 0.153 | 1.37 × 10−1 | 0.279 | 4.58 × 10−1 | 0.462 | 1.25 |
| CP | 0.143 | 1.20 × 10−1 | 0.273 | 4.38 × 10−1 | 0.46 | 1.24 |
3.1.1. Sucrose removal
Using sucrose as carbon source, at carbon to nutrient ratio of 20:1 and pH 6, the C:P yielded the lowest COD RE (38%), CE (14.5%) and voltage production (151 mV) and the results were shown in Figure 4.
Figure 4.
Effect of pH on Removal Efficiency, Columbic Efficiency and Voltage using Sucrose at 20:1. G: The performance of MFC using sucrose at pH 6; H: The performance of MFC using sucrose at pH 7; I: The performance of MFC using sucrose at pH 8.
Whereas the highest RE, CE, and voltage production of 42.7%,18.3% and 194 mV respectively was achieved with C: N (Figure 4G). When the pH was increased from pH 6 to pH 7, an increase in the CE, RE and amount of voltage was seen. Similarly, C:P again yielded the lowest COD RE, CE, and voltage production of 66.2%, 43 % and 532 mV, respectively, at pH 7 (Figure 4H). The highest COD RE of 78.4%, CE of 61.1% and voltage production of 565 mV was recorded for C: N at pH 7. An increase in pH from pH 7 to pH 8 resulted in the increase of the three parameters being studied namely COD RE, CE, and voltage production. In the case of pH 8, the lowest COD RE, CE, and voltage production of 69%, 58% and 589 mV respectively were recorded for C:P, whereas the highest of 86%, 73.9% and 624 mV respectively was observed for C: N (Figure 4I). At all the three ratios and pH COD RE was found in the following order C: N > C: N:S > C: N:S:P > C: N:P > C:S > C:P:S > C:P. CE and voltage production followed the same order.
3.1.2. Albumin
Keeping carbon nutrient ratio constant at 10:1 and pH was varied then at pH 6 (Figure 5A) C:P showed the least COD RE, CE and voltage that is 16.2%, 2.3 % and 33 mV, respectively. C: N exhibited the highest COD removal (22.2%), CE (6.7%) and voltage 83 mV. At pH 7, C:P again yielded the lowest results for COD RE, CE, and voltage with values of 27%, 7.7% and 129 mV, respectively. At pH 7, C: N exhibited the highest COD RE, CE, and voltage that is 35.8%, 12.8% and 147 mV respectively (Figure 5B). Increasing the pH to 8 further increased the CE, RE and voltage production (Figure 5C). C: N showed the highest CE (27 %), RE (50.6%) and voltage (174 mV) while C:P showed the lowest CE (12.8%), RE (37.6%) and voltage (145 mV).
Figure 5.
Effect of pH on Removal Efficiency, Columbic Efficiency and Voltage using Albumin at 10:1. A: The performance of MFC using albumin at pH 6; B: The performance of MFC using albumin at pH 7; C: The performance of MFC using albumin at pH 8.
At carbon nutrient ratio 15:1, C:P yielded the lowest COD RE of 14.5%, CE of 6.9% and voltage production of 124 mV at pH 6. Whereas the highest RE, CE, and voltage production of 30.8%, 9.1% and 137 mV respectively were achieved with C: N (Figure 6D). When the pH was increased from pH 6 to pH 7 an increase in the CE, RE and amount of voltage was seen. When the pH was increased to 7 the CE ranged from 11.1% - 14.7%, RE ranged from 32.7 % - 44.4 % and voltage was in the range of 129 mV–180 mV (Figure 6E). When the pH was kept at 8 C:P showed the lowest COD RE (40.7 %) and CE (16.5 %). The voltage output was also the lowest for C:P (192 mV). C: N showed highest voltage value i.e., 210 mV along with the highest RE and CE i.e., 58% and 39.6% (Figure 6F).
Figure 6.
Effect of pH on Removal Efficiency, Columbic Efficiency and Voltage using Albumin at 15:1. D: The performance of MFC using albumin at pH 6; E: The performance of MFC using albumin at pH 7; F: The performance of MFC using albumin at pH 8.
In the next series of experiments carbon nutrient ratio was kept constant at 20:1 and pH was varied (Figure 7). When the pH was kept at pH 6 the highest CE (15.3%), RE (40%) and voltage (160 mV) was found for C: N. On the other hand, C:P showed the lowest CE (10%), RE (32%) and voltage output (143 mV) (Figure 7G). Similarly, at pH 7, C:P again yielded the lowest COD RE, CE, and voltage production of 44.9%, 17.6% and 273 mV respectively. The highest COD RE of 55.5 %, CE of 17.6 % and voltage production of 325 mV was recorded for C: N at pH 7 (Figure 7H). When the pH was kept at 8 C:P showed the lowest COD RE (60.4%) and CE (35%). The voltage output was also the lowest for C:P (460 mV). C:N showed highest voltage value i.e. 472 mV along with the highest RE and CE i.e. 66.1% and 42.5% (Figure 7I). For all the three ratios CE, COD RE and voltage generation was in the order C: N > C: N:S > C: N:S:P > C: N:P > C:S > C:P:S > C:P. The highest was exhibited by C: N and the lowest by C:P.
Figure 7.
Effect of pH on Removal Efficiency, Columbic Efficiency and Voltage using Albumin at 20:1. G: The performance of MFC using albumin at pH 6; H: The performance of MFC using albumin at pH 7; I: The performance of MFC using albumin at pH 8.
The type of cathodic electron acceptor seems to exert a great influence on the functioning of MFC. Voltage generation is greatly influenced by the cathode electron acceptor (Gurung and Oh, 2012). How much voltage can be generated depends on the redox potential of the nutrient being used as electron acceptor. Nitrate has lower redox potential as compared to sulphate and phosphate hence, more voltage output was seen in the cases where nitrate was used a final electron acceptor. Hence, electron acceptors with lower redox potential help in greater voltage output (Cai et al., 2016). Electron acceptors are the accelerants that help in speeding up the forward reaction of biodegradation (Pandit et al., 2011). Higher rate of biodegradation translates into higher removal and columbic efficiencies.
A marked increase in CE, RE and voltage production were seen when the pH was increased from 6 to 8. The lowest CE, RE and voltage production were seen at pH 6 and highest at pH 8. At lower pH 6 the difference between RE and CE was significant. The pH of MFC can greatly affect MFC performance. The voltage output as well as RE of COD is greatly influenced by pH (Puig et al., 2010). Studies have found that MFC shows lowest current density at pH 6 and that an increase in pH results in an increase in the voltage production (He et al., 2008). The pH value of 8 seems to favor the anaerobic communities in MFC. When the pH is increased the conditions become favorable for the growth and production of electrogenic bacteria (Mohamed et al., 2020). This is the reason why a significant difference could be seen at various applied pH values.
3.2. Substrate transformation in MFC
3.2.1. Nitrogen removal
The lowest nitrogen RE was recorded using albumin as the substrate at pH 6 and ration of 10:1, whereas the highest nitrogen RE was observed using acetic acid as the substrate at a pH of 8 and 20:1. When acetic acid was used as the substrate highest nitrogen RE (85%) was achieved at pH 8 and 20:1 and the lowest RE was observed at pH 6 and 10:1 When sucrose was used as a substrate highest nitrogen RE (72.8%) was seen at pH 8 and 20:1 and the lowest RE (29.6%) was seen at pH 6 and 10:1. Using albumin resulted in the lowest of the three substrates with a highest and lowest RE of 60.7% and 14.3% respectively. The highest RE using albumin was observed at pH 8 and a ratio of 20:1 and the lowest RE at pH 6 with a ratio of 10:1 (Figure 8A).
Figure 8.
Nitrogen, phosphorus and sulfur removal at various pH and ratios. A: Nitrogen removal at various pH and ratios; B: Phosphorus removal at various pH and ratios; C: Sulfur removal at various pH and ratios.
3.2.2. Phosphorus removal
The highest phosphorus removal was achieved using acetic acid at a pH of 8 with a ratio of 20:1, whereas the lowest phosphorus removal was observed using albumin as a substrate with a ratio of 10:1 at pH 6. When acetic acid was used as substrate, the highest removal of the substrate was seen at pH 6 and ratio 20:1, whereas the lowest removal percentage of phosphorus using acetic acid was observed at pH 6 with a ratio of 10:1. In the case of sucrose, the highest RE of phosphorus (47.4%) was also achieved at a pH of 8 and 20:1 and the lowest RE (18.8%) was observed at pH 6 with and 10:1. Using albumin as a substrate resulted in the lowest RE of all substrates. The lowest and highest RE of 5% and 25% respectively were recorded while using pH of 6 and ratio 10:1 and pH 8 and ratio 20:1 respectively (Figure 8B).
3.2.3. Sulfur removal using MFC
The highest sulfur removal (74%) was achieved using acetic acid at pH 8 and a ratio of 20:1 and the lowest sulfur removal (11.2%) was observed using albumin as a substrate at pH 6 with a ratio of 10:1. While using acetic acid as the substrate the lowest sulfur RE (32%) was achieved at pH 6 and ratio 10:1. The highest removal in the case of acetic acid was achieved at pH 8 and a ratio of 20:1. The lowest RE (28%) using sucrose was observed at 10:1 and pH6 and the highest (70%) RE was seen at pH 8 and 20:1. In the case of sucrose, the lowest RE was achieved using pH 6 and 10:1 whereas the highest RE with sucrose was achieved at pH 8 and 20:1. Using albumin the lowest and highest sulfur removal achieved was 11.2% and 63.4% respectively. The lowest RE using albumin was observed at pH 6 and 10:1 and the highest RE was achieved using pH 8 and a ratio of 20:1 (Figure 8C).
The redox potentials of nitrogen and oxygen are very close to each other and hence it can be used as an electron acceptor in the cathodic cell (Han et al., 2020). Virdis et al. (2011) found that in a MFC treating synthetic wastewater containing acetate nitrate removal of 94.1% could be achieved. When cathodic nitrate accepts electrons, it turns into nitrogen gas. A coupled MFC system comprising of an oxic-biocathode MFC (O-MFC) and an anoxic-biocathode MFC (A-MFC) was implemented for simultaneous removal of C and N from wastewater (Xie et al., 2011). The MFC system obtained a maximum COD, NH4+-N and total nitrogen (TN) removal rate of 98.8%, 97.4% and 97.3%, respectively, at an A-MFC external resistance of 5 Ω.
Sulphates in the cathodic chambers can act as electron acceptors. When Sulfide and acetate (C source) was treated using MFC (Rabaey et al., 2006). Ninety eight percent of the sulfide and 46% of the acetate was removed. MFC removed SO4−2 via sulfide. This demonstrates that effluents can be polished by a MFC for both residual C and S compounds. Izadi and Rahimnejad (2014) used a dual chamber MFC to investigate the removal of S. The initial concentration of sulfide in the anode compartment was 0.4 g/L and it was completely removed after 3 days of MFC operation. The maximum generated voltage, power and current density were 988.915 mV, 346.746 mW.m-2, 1285.64 mA m−2.
Amount of phosphorus removal was different for each substate and it was in the order acetic acid > sucrose > albumin. Varying nutrient ratio and pH influenced the removal percentage of phosphorous. For each substrate, most phosphorous removal was recorded at 20:1 and pH 8. At pH 6 and 10:1 the least phosphorous removal was seen. Phosphate can act an electron acceptor but requires a large amount of energy to accept electrons because of its high redox potential. An increase in pH can help in the precipitation of phosphates and hence result it its removal (Tao et al., 2015).
Not too many studies have been done on phosphorous removal using MFC. However, there are studies showing that phosphorous removal is possible using MFC. Air-cathode single chamber MFCs were operated with swine wastewater and 70%–82% of the phosphorous was removed from the influent (Ichihashi and Hirooka, 2012). Using MFC (Zang et al., 2012) were able to remove C and P from urine. The removal efficiencies for PO43- and COD were found out to be 42.6% and 62.4% respectively. The power density of 0.9 W m3 was obtained.
3.2.4. Simultaneous Nitrogen, phosphorous and sulfur removal
Simultaneous removal of nutrients namely nitrogen, phosphorous and sulfur was studied using acetic acid as carbon source in the MFC and was presented in Figure 9. At pH 6 the lowest nitrogen (39%), sulfur (35%) and phosphorous (25%) RE was seen when the carbon nutrient ratio was kept 10:1. The RE increased when the carbon nutrient ratio 15:1 was used and the highest was noted when 20:1 carbon nutrient ratio was used in the MFC i.e., 48% nitrogen removal, 41% sulfur removal and 36% phosphorous removal was noted. When the pH was increased to 7, an increase was seen in the removal efficiencies was noted. At carbon nutrient ratio of 10:1 and pH 7 the lowest nutrient removal efficiencies were noted. When the carbon nutrient ratio of 15:1 was used, an increase in the removal efficiencies was noted. The highest nitrogen (61%), sulfur (56%), and phosphorous (47%) removal efficiencies were noted when the carbon nutrient ratio 20:1 was used in the experiment. Increasing the pH to pH 8 showed the best results (higher nutrient removal efficiencies as compared to pH 6 and pH 7). At pH 8 the highest RE was noted when the carbon nutrient ratio was kept 20:1 i.e., nitrogen RE was 81%, sulfur RE was 77% and phosphorous RE was 64% (Figure 9).
Figure 9.
Simultaneous Nitrogen, Phosphorous and Sulfur removal at various pH and ratios.
When sucrose was used as a carbon source in the MFC and simultaneous removal of nitrogen, phosphorous and sulfur was studied it was observed that the lowest nitrogen (32%), sulfur (30%) and phosphorous (21%) RE was seen when the carbon nutrient ratio was kept 10:1. The RE became higher when the carbon nutrient ratio 15:1 was used. The highest nitrogen removal (42%), sulfur removal (39%) and phosphorous removal (27%) was noted when 20:1 carbon nutrient ratio was used in the MFC (Figure 9).
At carbon nutrient ratio of 10:1 and pH 7 the lowest nutrient removal efficiencies were noted. When the carbon nutrient ratio of 15:1 was used, an increase in the removal efficiencies was noted. The highest nitrogen (60%), sulfur (51%), and phosphorous (42%) removal efficiencies were noted when the carbon nutrient ratio 20:1 was used in the experiment. At pH 8 the highest RE was noted when the carbon nutrient ratio was kept 20:1 i.e., nitrogen RE was 72%, sulfur RE was 67% and phosphorous RE was 59% (Figure 9).
In the last set of experiments albumin was used as a substrate and simultaneous removal of the nutrients was studied. The lowest nitrogen (14%), sulfur (12%) and phosphorous (10%) RE was seen when the carbon nutrient ratio was kept 10:1. The RE became higher when the carbon nutrient ratio 15:1 was used. The highest nitrogen removal (22%), sulfur removal (20%) and phosphorous removal (19%) was noted when 20:1 carbon nutrient ratio was used in the MFC. When the pH was increased to 7, an increase was seen in the removal efficiencies was seen. At carbon nutrient ratio of 10:1 and pH 7 the lowest nutrient removal efficiencies were noted. When the carbon nutrient ratio of 15:1 was used, an increase in the removal efficiencies was noted. The highest nitrogen (36%), sulfur (32%), and phosphorous (29%) removal efficiencies were noted when the carbon nutrient ratio 20:1 was used in the experiment. At pH 8, the lowest nitrogen (40%), sulfur (35%) and phosphorous (34%) RE was seen when the carbon nutrient ratio was kept 10:1. The RE increased when the carbon nutrient ratio 15:1 was used and the highest was noted when 20:1 carbon nutrient ratio was used in the MFC i.e., 56% nitrogen removal, 49% sulfur removal and 42% phosphorous removal was noted.
The results show that all the three substrates showed best results at pH 8 and carbon nutrient ratio of 20:1. Among the three substrates acetic acid showed the highest nutrient removal. The second highest nutrient RE was shown by sucrose. The least nutrient RE was shown by albumin. The nutrient removal followed a similar trend for all substrates i.e., nitrogen showed the highest RE followed by sulfur. The least was shown by phosphorous. Experiments carried out using nutrients in combination (C: N:S, C:P:S, C:P: N, C:P:N:S) showed that more than one nutrient can be removed simultaneously using an MFC. The results were found in the order C: N > C: N:S > C: N:S:P > C: N:P > C:S > C:P:S > C:P. The best simultaneous removal of nutrients was achieved when acetic acid was used in the system and pH 8 was maintained while keeping the carbon nutrient ratio at 20:1. Simultaneous anaerobic sulfide and nitrate removal in the anodic chamber coupled with electricity generation has been extensively studied (Cai et al., 2013, 2014, 2020). However, simultaneous removal of nitrogen and sulfur, phosphorous and sulfur, phosphorus and nitrogen and the combination of the three nutrients in the cathodic chamber of MFC has not been researched extensively till now. This study showed that the simultaneous removal of these combinations of nutrients can be achieved in the cathodic chamber of MFC. Out of the three nutrients, nitrogen showed the highest removal followed by sulfur. The least removal was shown by phosphorous. The reason behind this is their redox potentials which are in the order phosphate > sulphate > nitrate. Nitrate has the lowest redox potential out of the three i.e. (0.74 V). Low redox potential means that it most easily accepts the electrons coming from the anodic chamber (Sun et al., 2013) When nitrate accepts electrons, it gets reduced and turns into nitrogen gas. Sulphate can also accept electrons and turn in to elemental sulfur. Phosphorus removal was the lowest because it is not a very good electron acceptor because of endergonic reduction potential i.e., it requires a large amount of energy.
The best nutrient removal was obtained at pH 8. At pH 8 electricity producing thrive resulting in more generation of electrons in the anode that could be accepted by the electron acceptors in the cathodic chamber. Law et al. (2011) investigated how pH range effects removal of N. They found that the nitrogen removal increased as the pH was increased. The maximum removal was found at pH 8.0. Similarly (Guštin &Marinšek, 2011), found that ammonia stripping bench plant removed 92.8% of ammonium and 88.3% of total nitrogen from the anaerobic digestion effluent at high pH. It is because a high pH changes ammonia/ammonium ratio in favor of ammonia. Swine wastewater has been studied in a single chamber MFC for struvite precipitation (Ichihashi and Hirooka, 2012). It was found that 70–82% of phosphorus was removed, and struvite precipitation only occurred on the cathode surface when electrolyte pH was around 8 (Zhai et al., 2012). studied the removal of sulfur in MFC. They found that highest sulfur recovery efficiency (78.6 ± 8.3%) and CE (58.6 ± 1.6%) occurred at a pH 8.
MFC showed the highest nutrient removal when acetic acid was used as a carbon source. The results were found in the order Acetic Acid > Sucrose > Albumin. Carbon source greatly influences microorganism metabolism (Mitra and Mishra, 2018). Acetic acid is a simple compound and can be degraded easily by microbes resulting in the release of electrons to the anode. These electrons then move to the cathode and reduce the nutrients there acting as electron acceptors hence resulting in nutrient removal. Sucrose is more complex than acetic acid and its degradation releases fewer electrons as compared to acetic acid. Albumin is the most complex among the three and its degradation offers the least number of electrons. Number of electrons released greatly effects how much nutrient removal takes place. Hence, simpler compounds like acetic acid are more easily used up by exoelectrogens as compared to macromolecules (Yang et al., 2019). Studies have shown that acetic acid is a better electron donor for exoelectrogens as compared to longer chain compounds (Freguia et al., 2010). It has been reported that MFCs are best option to recover nutrients like ammonium and phosphate from wastewater (Ye et al., 2019).
In case of each substrate, the nutrient removal was found in the order 20:1 > 15:1 > 10:1. Most nutrient removal was found when the ratio was 20:1. It might be due to a higher amount of organic matter at 20:1 that can be degraded by microorganisms. More organic matter degradation means higher number of electrons available to reduce nutrient ions acting as final electron acceptors. Electrons and protons released by redox reactions in the anode move to the cathode resulting in bio-potential which helps in voltage generation (Mohan et al., 2009). The increase in loading rate results in an increase power generation (Goud et al., 2011). The energy generated by MFC is different with different electron donors (Sun et al., 2009).
4. Conclusion
The MFC exhibited highest columbic efficiency, COD removal efficiency, nutrient removal efficiency and voltage when acetic acid was used in the MFC and lowest was observed for albumin. Increasing the pH from pH 6 to pH 8 resulted in an increased in the parameters being studied. At each pH, the highest columbic efficiency, COD removal efficiency, nutrient removal efficiency and voltage was observed at 20:1 and lowest was seen at 10:1. In all experiments best results were seen when C: N was used in the system and least promising ones were observed for C:P. In all experiment's nitrogen showed the highest removal efficiency and phosphorous exhibited the least removal efficiency.
Declarations
Author contribution statement
Madiha Tariq: Performed the experiments; Analyzed and interpreted the data.
Jin Wang: Contributed reagents, materials, analysis tools or data.
Adeel Jalal Malik & Mohammed Salim Akhter: Analyzed and interpreted the data; Wrote the paper.
Qaisar Mahmood: Conceived and designed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The data that has been used is confidential.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Jin Wang, Email: wangjin100@sjtu.edu.cn.
Qaisar Mahmood, Email: drqaisar@cuiatd.edu.pk.
References
- Abeysiriwardana-Arachchige I.S.A., Munasinghe-Arachchige S.P., Delanka-Pedige H.M.K., Nirmalakhandan N. Removal and recovery of nutrients from municipal sewage: algal vs. conventional approaches. Water Res. 2020;175:115709. doi: 10.1016/j.watres.2020.115709. [DOI] [PubMed] [Google Scholar]
- APHA . Standard methods for the examination of water and wastewater. 21st Ed. American Public Health Association/American Water Works Association/Water Environment Federation; Washington DC: 2005. [Google Scholar]
- Cai J., Qaisar M., Sun Y. Effect of external resistance on substrate removal and electricity generation in microbial fuel cell treating sulfide and nitrate simultaneously. Environ. Sci. Pollut. Control Ser. 2020;27(1):238–249. doi: 10.1007/s11356-019-06960-8. [DOI] [PubMed] [Google Scholar]
- Cai J., Zheng P., Mahmood Q. Effect of cathode electron acceptors on simultaneous anaerobic sulfide and nitrate removal in microbial fuel cell. Water Sci. Technol. 2016;73(4):947–954. doi: 10.2166/wst.2015.570. [DOI] [PubMed] [Google Scholar]
- Cai J., Zheng P., Qaisar M., Xing Y. Effect of operating modes on simultaneous anaerobic sulfide and nitrate removal in microbial fuel cell. J. Ind. Microbiol. Biotechnol. 2014;41(5):795–802. doi: 10.1007/s10295-014-1425-4. [DOI] [PubMed] [Google Scholar]
- Cai J., Zheng P., Qaisar M., Zhang J. Elemental sulfur recovery of biological sulfide removal process from wastewater: a review. Crit. Rev. Environ. Sci. Technol. 2017;47(21):2079–2099. [Google Scholar]
- Cai J., Zheng P., Zhang J., Xie Z., Li W., Sun P. Simultaneous anaerobic sulfide and nitrate removal coupled with electricity generation in microbial fuel cell. Bioresour. Technol. 2013;129:224–228. doi: 10.1016/j.biortech.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Castellanos R.M., Dias J.M.R., Bassin I.D., Dezotti M., Bassin J.P. Effect of sludge age on aerobic granular sludge: addressing nutrient removal performance and biomass stability. Process Saf. Environ. Protect. 2021;149:212–222. [Google Scholar]
- Deng L., Ngo H.H., Guo W., Wang J., Zhang H. Evaluation of a new sponge addition-microbial fuel cell system for removing nutrient from low C/N ratio wastewater. Chem. Eng. J. 2018;338:166–175. [Google Scholar]
- Diaz-Elsayed N., Rezaei N., Guo T., Mohebbi S., Zhang Q. Wastewater-based resource recovery technologies across scale: a review. Resour. Conserv. Recycl. 2019;145:94–112. [Google Scholar]
- Freguia S., Teh E.H., Boon N., Leung K.M., Keller J., Rabaey K. Microbial fuel cells operating on mixed fatty acids. Bioresour. Technol. 2010;101(4):1233–1238. doi: 10.1016/j.biortech.2009.09.054. [DOI] [PubMed] [Google Scholar]
- Ge X., Cao X., Song X., Wang Y., Si Z., Zhao Y., Tesfahunegn A.A. Bioenergy generation and simultaneous nitrate and phosphorus removal in a pyrite-based constructed wetland-microbial fuel cell. Bioresour. Technol. 2020;296:122350. doi: 10.1016/j.biortech.2019.122350. [DOI] [PubMed] [Google Scholar]
- Geng Y.K., Wang Y., Pan X.R., Sheng G.P. Electricity generation and in situ phosphate recovery from enhanced biological phosphorus removal sludge by electrodialysis membrane bioreactor. Bioresour. Technol. 2018;247:471–476. doi: 10.1016/j.biortech.2017.09.118. [DOI] [PubMed] [Google Scholar]
- Goswami L., Kumar R.V., Pakshirajan K., Pugazhenthi G. A novel integrated biodegradation—microfiltration system for sustainable wastewater treatment and energy recovery. J. Hazard Mater. 2019;365:707–715. doi: 10.1016/j.jhazmat.2018.11.029. [DOI] [PubMed] [Google Scholar]
- Goud R.K., Babu P.S., Mohan S.V. Canteen based composite food waste as potential anodic fuel for bioelectricity generation in single chambered microbial fuel cell (MFC): bio-electrochemical evaluation under increasing substrate loading condition. Int. J. Hydrogen Energy. 2011;36(10):6210–6218. [Google Scholar]
- Gurung A., Oh S.E. Effects of electron donors and acceptors in generating bioelectrical energy using microbial fuel cells. Korean J. Environ. Agric. 2012;31(1):24–29. [Google Scholar]
- Guštin S., Marinšek-Logar R. Effect of ph, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Saf. Environ. Protect. 2011;89(1):61–66. [Google Scholar]
- Han X., Qu Y., Wu J., Li D., Ren N., Feng Y. Nitric oxide reduction by microbial fuel cell with carbon-based gas diffusion cathode for power generation and gas purification. J. Hazard Mater. 2020;399:122878. doi: 10.1016/j.jhazmat.2020.122878. [DOI] [PubMed] [Google Scholar]
- He Z., Huang Y., Manohar A.K., Mansfeld F. Effect of electrolyte pH on the rate of the anodic and cathodic reactions in an air-cathode microbial fuel cell. Bioelectrochemistry. 2008;74(1):78–82. doi: 10.1016/j.bioelechem.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Huang Y., Huang Y., Liao Q., Fu Q., Xia A., Zhu X. Improving phosphorus removal efficiency and Chlorella vulgaris growth in high-phosphate MFC wastewater by frequent addition of small amounts of nitrate. Int. J. Hydrogen Energy. 2017;42(45):27749–27758. [Google Scholar]
- Ichihashi O., Hirooka K. Removal and recovery of phosphorus as struvite from swine wastewater using microbial fuel cell. Bioresour. Technol. 2012;114:303–307. doi: 10.1016/j.biortech.2012.02.124. [DOI] [PubMed] [Google Scholar]
- Izadi P., Rahimnejad M. Simultaneous electricity generation and sulfide removal via a dual chamber microbial fuel cell. Biofuel Res. J. 2014;1(1):34–38. [Google Scholar]
- Kelly P.T., He Z. Nutrients removal and recovery in bioelectrochemical systems: a review. Bioresour. Technol. 2014;153:351–360. doi: 10.1016/j.biortech.2013.12.046. [DOI] [PubMed] [Google Scholar]
- Law Y., Lant P., Yuan Z. The effect of ph on N 2 O production under aerobic conditions in a partial nitritation system. Water Res. 2011;45(18):5934–5944. doi: 10.1016/j.watres.2011.08.055. [DOI] [PubMed] [Google Scholar]
- Li L.I., Qin D.A.I., Sai Z.H.A.N.G., Hao L.I.U. Degradation efficiency and mechanism of sulfur-containing azo dye wastewater by microbial fuel cell under different pH conditions. Chin. J. Environ. Eng. 2021;15(1):115–125. [Google Scholar]
- Liu T., Chen X., Wang X., Zheng S., Yang L. Highly effective wastewater phosphorus removal by phosphorus accumulating organism combined with magnetic sorbent MFC@ La (OH) 3. Chem. Eng. J. 2018;335:443–449. [Google Scholar]
- Luo H., Bai J., He J., Liu G., Lu Y., Zhang R., Zeng C. Sulfate reduction and elemental sulfur recovery using photoelectric microbial electrolysis cell. Sci. Total Environ. 2020;728:138685. doi: 10.1016/j.scitotenv.2020.138685. [DOI] [PubMed] [Google Scholar]
- Mahmood Q., Zheng P., Cai J., Wu D., Hu B., Li J. Anoxic sulfide biooxidation using nitrite as electron acceptor. J. Hazard Mater. 2007;147:249–256. doi: 10.1016/j.jhazmat.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Mitra M., Mishra S. Effect of glucose on growth and fatty acid composition of an euryhaline eustigmatophyte Nannochloropsis oceanica under mixotrophic culture condition. Bioresour. Technol. Rep. 2018;3:147–153. [Google Scholar]
- Mohamed S.N., Hiraman P.A., Muthukumar K., Jayabalan T. Bioelectricity production from kitchen wastewater using microbial fuel cell with photosynthetic algal cathode. Bioresour. Technol. 2020;295:122226. doi: 10.1016/j.biortech.2019.122226. [DOI] [PubMed] [Google Scholar]
- Mohan S.V., Raghavulu S.V., Peri D., Sarma P.N. Integrated function of microbial fuel cell (MFC) as bio-electrochemical treatment system associated with bioelectricity generation under higher substrate load. Biosens. Bioelectron. 2009;24(7):2021–2027. doi: 10.1016/j.bios.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Pasupuleti S.B., Srikanth S., Dominguez-Benetton X., Mohan S.V., Pant D. Dual gas diffusion cathode design for microbial fuel cell (MFC): optimizing the suitable mode of operation in terms of bioelectrochemical and bioelectro-kinetic evaluation. J. Chem. Technol. Biotechnol. 2016;91(3):624–639. [Google Scholar]
- Pandit S., Sengupta A., Kale S., Das D. Performance of electron acceptors in catholyte of a two-chambered microbial fuel cell using anion exchange membrane. Bioresour. Technol. 2011;102(3):2736–2744. doi: 10.1016/j.biortech.2010.11.038. [DOI] [PubMed] [Google Scholar]
- Puig S., Serra M., Coma M., Cabré M., Balaguer M.D., Colprim J. Effect of pH on nutrient dynamics and electricity production using microbial fuel cells. Bioresour. Technol. 2010;101(24):9594–9599. doi: 10.1016/j.biortech.2010.07.082. [DOI] [PubMed] [Google Scholar]
- Rabaey K., Van de Sompel K., Maignien L., Boon N., Aelterman P., Clauwaert P., Verstraete W. Microbial fuel cells for sulfide removal. Environ. Sci. Technol. 2006;40(17):5218–5224. doi: 10.1021/es060382u. [DOI] [PubMed] [Google Scholar]
- Strik D.P., Timmers R.A., Helder M., Steinbusch K.J., Hamelers H.V., Buisman C.J. Microbial solar cells: applying photosynthetic and electrochemically active organisms. Trends Biotechnol. 2011;29(1):41–49. doi: 10.1016/j.tibtech.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Sun J., Li W., Li Y., Hu Y., Zhang Y. Redox mediator enhanced simultaneous decolorization of azo dye and bioelectricity generation in air-cathode microbial fuel cell. Bioresour. Technol. 2013;142:407–414. doi: 10.1016/j.biortech.2013.05.039. [DOI] [PubMed] [Google Scholar]
- Sun M., Mu Z.X., Chen Y.P., Sheng G.P., Liu X.W., Chen Y.Z., Ma F. Microbe-assisted sulfide oxidation in the anode of a microbial fuel cell. Environ. Sci. Technol. 2009;43(9):3372–3377. doi: 10.1021/es802809m. [DOI] [PubMed] [Google Scholar]
- Tao Q., Zhou S., Luo J., Yuan J. Nutrient removal and electricity production from wastewater using microbial fuel cell technique. Desalination. 2015;365:92–98. [Google Scholar]
- TerHeijne A., Hamelers H.V.M., Saakes M., Buisman C.J.N. Performance of non-porous graphite and titanium-based anodes in microbial fuel cells. Electrochim. Acta. 2008;53:5697–5703. [Google Scholar]
- Vijay A., Khandelwal A., Chhabra M., Vincent T. Microbial fuel cell for simultaneous removal of uranium (VI) and nitrate. Chem. Eng. J. 2020;388:124157. [Google Scholar]
- Virdis B., Read S.T., Rabaey K., Rozendal R.A., Yuan Z., Keller J. Biofilm stratification during simultaneous nitrification and denitrification (SND) at a biocathode. Bioresour. Technol. 2011;102(1):334–341. doi: 10.1016/j.biortech.2010.06.155. [DOI] [PubMed] [Google Scholar]
- Xie S., Liang P., Chen Y., Xia X., Huang X. Simultaneous carbon and nitrogen removal using anoxic/anoxic-biocathode microbial fuel cells coupled system. Bioresour. Technol. 2011;102(1):348–354. doi: 10.1016/j.biortech.2010.07.046. [DOI] [PubMed] [Google Scholar]
- Yang G., Wang J., Zhang H., Jia H., Zhang Y., Cui Z., Gao F. Maximizing energy recovery from homeostasis in microbial fuel cell by synergistic conversion of short-chain volatile fatty acid. Bioresour. Technol. Rep. 2019;7:100200. [Google Scholar]
- Ye Y., Ngo H.H., Guo W., Liu Y., Chang S.W., Nguyen D.D., Zhang X. Feasibility study on a double chamber microbial fuel cell for nutrient recovery from municipal wastewater. Chem. Eng. J. 2019;358:236–242. [Google Scholar]
- Zang G.L., Sheng G.P., Li W.W., Tong Z.H., Zeng R.J., Shi C., Yu H.Q. Nutrient removal and energy production in a urine treatment process using magnesium ammonium phosphate precipitation and a microbial fuel cell technique. Phys. Chem. Chem. Phys. 2012;14(6):1978–1984. doi: 10.1039/c2cp23402e. [DOI] [PubMed] [Google Scholar]
- Zhai L.F., Song W., Tong Z.H., Sun M. A fuel-cell-assisted iron redox process for simultaneous sulfur recovery and electricity production from synthetic sulfide wastewater. J. Hazard Mater. 2012;243:350–356. doi: 10.1016/j.jhazmat.2012.10.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.