Graphical abstract
Keywords: Colorectal cancer, Drimia calcarata, Phytochemicals, Cytotoxicity, Anticancer and apoptosis, p53, STAT5B
Highlights
-
•
For the first time, phytochemical analysis and antioxidant activity of D. calcarata water and methanol extracts were elucidated.
-
•
The D. calcarata water extract demonstrated the highest total phenolic, tannin and flavonoid contents and the best antioxidant activity.
-
•
This work also deciphered the anticancer activities and molecular mechanism of D. calcarata extracts against HT-29 and Caco-2 cells.
-
•
The Caco-2 cells were significantly more sensitive to D. calcarata ME as compare to HT-29 cells, which were more sensitive to D. calcarata WE.
-
•
Methanol extract upregulated p53 expression in both cell lines and selectively induced the expression of STAT5B mRNA.
Abstract
Colorectal cancer is the fourth leading cause of oncological-related deaths and the third most diagnosed malignancy, worldwide. The emergence of chemoresistance is a fundamental drawback of colorectal cancer therapies and there is an urgent need for novel plant-derived therapeutics. In this regard, other compounds are needed to improve the efficacy of treatment against colorectal cancer. Medicinal plants have been effectively used by traditional doctors for decades to treat various ailments with little to no side effects. Drimia calcarata (D. calcarata) is one of the plants used by Pedi people in South Africa to treat a plethora of ailments. However, the anticancer therapeutic use of D. calcarata is less understood. Thus, this study was aimed at evaluating the potential anticancer activities of D. calcarata extracts against human colorectal cancer cells. The phytochemical analysis and antioxidant activity were analysed using LC–MS, DPPH, and FRAP. The inhibitory effects and IC50 values of D. calcarata extracts were determined using the MTT assay. Induction of cellular apoptosis was assessed using fluorescence microscopy, the Muse® Cell Analyser, and gene expression analysis by Polymerase Chain Reaction (PCR). Water extract (WE) demonstrated high phenolic, tannin, and flavonoid contents than the methanol extract (ME). LC–MS data demonstrated strong differences between the ME and WE. Moreover, WE showed the best antioxidant activity than ME. The MTT data showed that both ME and WE had no significant activity against human embryonic kidney Hek 293 cell line that served as non-cancer control cells. Caco-2 cells demonstrated high sensitivity to the ME and demonstrated resistance toward the WE, while HT-29 cells exhibited sensitivity to both D. calcarata extracts. The expression of apoptosis regulatory genes assessed by PCR revealed an upregulation of p53 by ME, accompanied by downregulation of Bcl-2 and high expression of Bax after treatment with curcumin. The Bax gene was undetected in HT-29 cells. The methanol extract induced mitochondrial-mediated apoptosis in colorectal Caco-2 and HT-29 cells and WE induced the extrinsic apoptotic pathway in HT-29 cells. ME downregulated STAT1, 3, and 5B in HT-29 cells. The D. calcarata bulb extracts, therefore, contain potential anticancer agents that can be further targeted for cancer therapeutics.
1. Introduction
Colorectal cancer is a standout amongst other comprehended neoplasms from a genetic perspective [1]. Colorectal cancer results from an anomalous development of epithelial cells inside the colon and rectum of the large intestines. It is a major public health problem; the fourth leading cause of oncological-related deaths and the third most diagnosed malignancy, worldwide [2,3]. The risk factors that cause colorectal cancer include age, overweight, physical inactivity, unhealthy diets, alcohol consumption, and smoking [4,5]. Additional risk factors include the family history of colorectal cancer, heredity conditions like polyposis and hereditary nonpolyposis colorectal cancer, and individual history of inflammatory bowel disease, polyps, and other cancers [6,7]. Early detection of colorectal cancer early is critical, bearing in mind the negative result on survival conferred by spreading cancer [8]. Metastatic disease has previously been viewed as fatal, causing massive effects and interfering with the physiological condition [9]. However, no predominant mutations have been related to metastatic colorectal malignant growth [10]. The regular strategies utilised for colorectal cancer treatment include surgery, radiotherapy, and chemotherapy [11]. If diagnosed early via colonoscopy, surgery is often successfully carried out to remove the tumour. Thus, colorectal malignant growth can be effectively treated when identified early; however, at a later stage when the ailment has spread to different tissues, it becomes an arduous task to treat this disease [12]. Late diagnosis can result in a poor prognosis, but advanced colorectal cancer can be controlled by chemotherapy and radiotherapy [13]. For metastatic colorectal cancer patients, around 75 % continue to live beyond 1 year, 35 % beyond 3 years, and less than 20 % beyond 5 years after diagnosis. The prime treatment for unresectable metastatic colorectal cancer is a systemic therapy. Clinical trials done in the previous five years have proved that modifying treatment to the molecular and pathologic features of the tumour enhances overall survival [14], even though the clinical outcomes and treatment responses of each patient with colorectal cancer differ greatly [15], the use of standardized treatment possibilities is limited in standard practice [16,17]. As a component of first- and second-line combination therapies, oxaliplatin is used to treat metastatic colorectal cancer. The response rate has significantly improved by more than 50 % and led to a significant increase in median survival times [18,19]. Nevertheless, most colorectal cancer patients ultimately develop drug resistance, and the five-year survival rate for progressive colorectal cancer patients is less than 10 % [20]. Thus, it is important to irradiate the mechanism of chemoresistance due to the fact this understanding may also strengthen new techniques to overcome drug resistance in colorectal cancer patients.
The emergence of chemoresistance is a fundamental drawback of colorectal cancer therapies and there is an urgent need for novel plant-derived therapeutics. In this regard, other compounds are needed to increase the efficacy of treatment against colorectal cancer. Natural compounds have received increasing attention as potential adjuvant therapy against cancer to improve tumour response to treatment. However, the efficacy of classic chemotherapeutic agents is reduced by the insurgence of chemoresistance. In this context, the search for alternative therapeutic approaches has received great attention. Above all, some bioactive compounds, including gambogic acid isolated from Garcinia hanburyi, hinder cell growth, promote apoptosis, and overcome drug resistance in colorectal cancer cells [21]. In particular, emerging data demonstrate that some natural compounds including S-adenosylL-methionine, have the potential to overcome drug resistance in colorectal cancer cells devoid of p53 [22].
Medicinal plants have been the foundation of traditional remedies for decades and continue to grant new prescriptions to humanity [23], with little or no side effects [24]. They are rich in bioactive compounds, which has led to their exploration for potential anticancer drug leads. There is a huge number of medicinal plants that are currently used, worldwide, and Drimia calcarata (D. calcarata) is one of the plants used by Pedi people in South Africa to treat a plethora of ailments [25]; however, its anticancer activities remain poorly understood. Drimia species contain a colossal number of natural compounds, which include cardiac glycosides, saponins, terpenes, alkaloids, and flavonoids with beneficial biological properties that include; antifungal, antiviral, antibacterial, anti-inflammatory, antioxidant, and insecticidal [[26], [27], [28]]. It has been shown that natural compounds from medicinal plants have anticancer activities, and regulate signal transduction pathways, modulating proteins that control cell cycle progression and inducing of apoptotic pathways [29]. Previously, riparianin was isolated from D. calcarata dichloromethane extract and implicated to have anticancer activities against breast (MCF-7), renal (TK10) and melanoma (UACC62) cancer cells [30]. However, the implicated anticancer activities of riparianin against different cancers were only based on the MTT assay, thus the underlying molecular mechanisms remain unclear. Previously, Caco-2 and HT-29 colorectal cancer cells have been subjected to various plant extracts. Glycyrrhiza glabra induced apoptosis of HT-29 cells through down-regulation of HSP90 gene expression [31]. The Garcinia mangostana extract exhibited effective cytotoxicity through the induction of intrinsic cell death, inhibition of cell movement, cell invasion, and clonogenicity. Furthermore, extract promoted the MAPK/ERK, c-Myc/Max, and p53-mediated pathways [32]. Moreover, Origanum vulgare ethanol extract leads to growth arrest and apoptosis induction of colorectal Caco-2 cells in a dose- and time-dependent manner [33].
Many chemotherapeutic drugs result in DNA damage that is picked up by p53; the cell would then be able to attempt to patch up the damage or initiate cell death. However, when the tumour suppressor gene p53 is defective, it becomes more challenging for chemotherapy to be effective [34]. The p53 mutant tumours showed a better clinical outcome compared to wild-type p53 tumours. Jackson et al. [35] demonstrated that the activity of wild-type p53 obstructs chemotherapy effect and showed the necessity to re-evaluate the philosophy for p53 in cancer therapy. STAT5 and p53 are co-localised in proximal promoters on the chromatin at 463 genomic positions and p53 relies on STAT5 activation to bind to the chromatin whereas STAT5 does not rely on p53 [36,37]. In addition, STAT1 interrelated with p53 and increased apoptosis by DNA damage [38]. Furthermore, oncostatin induced apoptotic sensitization via p53 and STAT5 collaboration in osteoblasts [39]. Signal transducers and activators of transcription (STAT) is a group of transcription factors (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6) having a significant role in various physiological processes, such as cell differentiation, cell survival, or cell development [40,41]. STAT1 plays a role as a tumour suppressor gene, while substantial evidence applies that STAT3 and STAT5 have appeared to play a role in tumour growth and proliferation [42,43]. Regulation of STATs and STAT-linked signalling pathways has been detected in diseased states, such as chronic inflammatory bowel diseases and malignant transformation [44]. STATs might signify a novel molecular target for therapeutic interventions [45,46]. Although there is enough evidence from phytochemical data supporting the anticancer activity of Drimia plants, however, the anticancer therapeutic use of D. calcarata and the underlying molecular mechanisms remain unclear. For this reason, the purpose of this study was to assess the potential anticancer activities of D. calcarata extracts against human colorectal cancer cells.
2. Methods and materials
2.1. Plant collection and verification
The Drimia calcarata plantwas collected at Phalakwane village, Ga-Mphahlele in Limpopo province, South Africa. The plant materials were submitted to the University of Limpopo Larry Leach Herbarium for identification and verification (voucher specimen number UNIN 121629).
2.2. Preparation of the plant extracts
The D. calcarata bulbs were cleansed, air-dried, and crushed into a fine powder. The extraction was carried out using methanol and water (1:10 w/v) as previously reported [47]. The samples were filtered into pre-weighed beaker glass and air-dried. The dried plant extracts were reconstituted in acetone for phytochemical analysis and dimethylsulphoxide (DMSO) [Sarchem, RSA] for all cell culture-based assays.
2.3. Phytochemical screening
The extracts were screened for the presence of tannins, phenols, quinones, anthraquinones, steroids, terpenoids, flavonoids, chaconnes, anthocyanins, xanthones, flavones, flavonols, alkaloids, carbohydrates, cardic glycosides, saponins, proteins, coumarins, phlobatannins and leucoanthocyanins turns following the protocol previously reported [47,48].
2.4. Total phenolic content (TPC) determination
The TPC amount in acetone dissolved extract was measured using Folin-Ciocalteau method, following a protocol previously reported [49]. Briefly, 100 μL of the extract and 900 μL of the distilled water were mixed in a 15 mL centrifuge tube. To this concoction, Folin-Ciocalteau (FC) reagent (100 μL) was added and left samples at 25 °C for 1 h and 30 min. Following incubation, 7% sodium carbonate (Na2CO3) [1000 μL] was added to each sample and adjusted with distilled water to 2500 μL. Gallic acid was utilised as a standard compound and analysed similarly to the experimental samples. The samples were left for 1 h and 30 min at 25 °C and the absorbances were measured at 550 nm using a microtitre plate reader (Promega, USA).
2.5. Total tannin content (TTC) determination
Folin-Ciocalteau method was chosen to measure the tannin content as previously described [50]. A hundred microliters (100 μL) of the acetone dissolved extract of different concentrations were added to 15 mL centrifuge tubes with 2500 μL of distilled water. This was followed by the addition of 2 M FC reagent (100 μL) and 35 % Na2CO3 (500 μL) solution to the mixtures. The final volume was adjusted using distilled water to 5000 μL and samples were left for 30 min at 25 °C. Tannic acid was used to prepare standard solutions similar to the experimental samples. Absorbances were measured at 725 nm using a microtitre plate reader (Promega, USA).
2.6. Total flavonoid content (TFC) determination
To measure TFC, the previously described method [51] was utilised. Briefly, 100 μL of each concentration, 10 % aluminium chloride (100 μL), 1 M potassium acetate (100 μL), and distilled water (2800 μL) were mixed and incubated for 30 min at 25 °C. Quercetin was employed as a standard compound. The absorbance readings were taken using a microtitre plate reader (Promega, USA) at 415 nm.
2.7. Quantitative 2, 2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assay
Free radical scavenging activities of the D. calcarata methanol extract (ME) and water extract (WE) were quantified and compared using the DPPH assay, following a protocol previously described [52], with some modification. Briefly, 500 μL of different bulb extract concentrations were prepared and 1000 μL of 60 μg/mL DPPH (Sigma-Aldrich, USA) solution was added. Ascorbic acid served as positive control. The samples were left at 25 °C for 30 min in the dark. After the incubation time had elapsed, the absorbance readings of the samples were taken at 517 nm using a microtitre plate reader (Promega, USA) and the formula (1) was used to calculate the percentage inhibition.
| (1) |
2.8. Ferric Ion reducing antioxidant power
The ferric ion reducing antioxidant power (FRAP) of ME and WE was determined using following the method previously described [50]. Different concentrations of the bulb extracts were prepared using acetone. Five hundred microlitres (500 μL) of different concentrations were mixed with 500 μL of 0.2 M sodium phosphate buffer (pH 6.6), followed by the addition of 500 μL of 1 % potassium ferricyanide and vortexed. The mixtures were left for 20 min at 50 °C. After incubation, 400 μL of 10 % trichloroacetic acid was added and centrifuged for 30 min at 3000 rpm. From the resulting supernatant, 1000 μL was added to a new 15 mL centrifuge tube. To each tube, a volume of 5000 μL distilled water was added, followed by the addition of 1000 μL of 0.1 % ferric chloride. The positive control used for this assay was ascorbic acid. The absorbance readings at 700 nm were taken utilizing the Promega microtiter plate reader (Promega, USA). The formula (2), below, was used to calculate the percentage reducing power.
| (2) |
2.9. Liquid chromatography-mass spectrometry (LC–MS) analysis
Four different stock cocktails (ranging from 1 mg/mL) for water extract (WE) and methanol extract (ME) were prepared quantitatively to enable differentiation of isomers and compounds with comparable elemental formulas. Fifty percent methanol in water containing 1% formic acid was used to prepare the cocktails. The LC–MS analysis was done as previously described [53]. Calibration, calculation, and the rest of the settings were done using polyalanine [54].
Cell culture and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) Cytotoxicity Assay
Cell culture and MTT assay were performed following a method as previously reported [55]. Dulbecco’s Modified Eagle Medium (Hyclone, USA) containing 10 % fetal bovine serum (Hyclone, USA) and 1% antibiotic mixture of Penicillin and Streptomycin (Biowest, USA) was used to culture cells in an incubator containing 5% CO2 at 37 °C. MTT assay was employed to assess the activities of ME and WE. Briefly, 1 × 103 cells/well were cultured overnight. Following incubation, treatment was done using different concentrations (15.63−1000 μg/mL) of D. calcarata extracts, solvent controls (0.25 % DMSO and 0.25 % H2O), and positive control (50 μM curcumin) for 24 h. Following treatment, the cells in each well plate were subjected to a 5 mg/mL MTT reagent (10 μL) [ThermoFischer Scientific, USA] and incubated in the CO2 incubator for 3 h. After incubation, the formed crystals were dissolved by adding 100 μL DMSO and placed in the dark for an hour at 25 °C. Thereafter, measured the absorbance readings using a microtitre plate reader (Promega, USA) at 560 nm. The cell viability was calculated using the formula (3).
| (3) |
2.10. Morphological examination by fluorescence microscopy
Cancer cells (2 × 103 cells/well) were cultured plates overnight. Followed by treatment with IC50 values of ME and WE, for 24 h. The experiment included the following controls: solvent controls (0.25 % DMSO and 0.25 % H2O), positive control (50 μM curcumin), and the untreated control. Following treatment, the cells were fixed in 3.7 % paraformaldehyde for 10 min at 25 °C. The cells were then stained using 1 μg/mL acridine orange/ethidium bromide (AO/EB) [ThermoFischer Scientific, USA] and placed in the dark for 15 min at 25 °C. The cells were then washed with 1 X PBS and morphological features were observed under the Eclipse Ti-U fluorescence microscope while images were captured using a DSRI-1 camera (Nikon Instruments Inc., USA).
2.11. Apoptosis assay
To determine if ME and WE induce apoptosis, Annexin V and Dead Cell Kit (Merck-Millipore, Germany) was employed as per the manufacturer’s protocol. Briefly, 2 × 103 cells/well were seeded. Following overnight incubation, 24 h treatment with IC50 values and the controls was carried. Following treatment, 1X trypsin was used to detach the cells, which was deactivated with a complete medium and centrifuged. Following centrifugation, 100 μL DMEM containing 1% FBS was used to re-suspend the pellets. The Annexin V and Dead Cell reagent (Merck-Millipore, Germany) was added, and samples were left in the dark for 20 min at 25 °C. The Muse® Cell Analyser (Merck-Millipore, Germany) was used to analyse the samples.
2.12. Caspase 3/7 activation assay
To further confirm whether ME and WE induced caspase-dependent apoptosis, the Muse® caspase3/7 activation Kit (Merck-Millipore, Germany) was employed. Briefly, the cells (2 × 103 cells/well) were cultured in plates. Following incubation, 24 h treatment with the IC50 s and the controls was carried out. The cells were detached from the plate using 1X trypsin, which was deactivated in complete medium, followed by collection at 300 x g for 5 min using centrifugation. The samples were re-suspended in 50 μL of 1X Assay Buffer and 5 μL of Muse® Caspase-3/7 Reagent (Merck-Millipore, Germany). The samples were thoroughly mixed by gently pipetting up and down for 5 s. The tube caps were loosened and incubated samples for 30 min in the 37 °C chamber. After incubation, the Muse® Caspase 7-AAD (Merck-Millipore, Germany) [150 μL] was added to each tube and placed in the dark at 25 °C for 5 min. The Muse® Cell Analyser (Merck-Millipore, Germany) was used to analyse the samples.
2.13. Polymerase chain reaction (PCR) components
ZR® RNA MiniPrep Kit (Zymo Research, USA) was used for total RNA was extracted, and manufacturer’s instructions were followed. The complementary deoxyribonucleic acid (cDNA) was synthesised using Promega AMV II Reverse Transcription System (USA). The EmeraldAmp® GT PCR Kit (Takara Bio, USA) was employed for the amplification of apoptosis-related genes and STAT genes. Amplification using different primer sets shown in Table 1 was done using T100™ Thermal Cycler (BioRad, USA).. The PCR products were mixed with the novel juice (Cat no. LD001-1000) [Celtic Molecular Diagnostic, South Africa]. Samples were visualised using 2 % agarose gels which were viewed using D-DiGit Gel Scanner (Analytical technology, South Africa). The band densities from three independent experiments were measure using Image J software.
Table 1.
The primer sequences of apoptosis-related genes and STAT genes.
| Gene | Forward Primers | Reverse Primer |
|---|---|---|
| p53 | 5′- GTTGCCCAGGCTGGAGTGGAG -3′ | 5′- GGCTGAGACAGGTGGATCGC -3′ |
| Bcl-2 | 5′- GCACCGGGCATCTTCTCCTC-3′ | 5′- CCGAGATGTCCAGCCAGCTG -3′ |
| Bax | 5′-GGGTGGTTGGGTGAGACTC-3′ | 5′-AGACACGTAAGGAAACGCATTA-3′ |
| STAT1 | 5′-GCCCCGATGGTCTCATTCCG-3′ | 5′-GTCCTTCAACAGGGCACGCT-3′ |
| STAT3 | 5′-TGCCTGCGGCATCCTTCTGC-3′ | 5′-ACAGGCGTGAGCCACCATGC-3′ |
| STAT5B | 5′-GGATGGGTGCATCGGGGAAG-3′ | 5′-TCTCAGAGGCAGGTGCTGGT-3′ |
| GAPDH | AGCTGAACGGGAAGCTCACT | TGCTGTAGCCAAATTCGTTG |
2.14. Statistical significance
GraphPad Prism Version 6.0 was employed for graphical data analysis presented as mean ± standard error of the mean (SEM). The one-way ANOVA Tukey Kramer Multiple Comparison Test was used to verify the statistical significance. The asterisk (*) (**) and (***) indicated p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001, respectively.
3. Results and discussion
3.1. Drimia calcarata (D. calcarata) bulb extracts contain different phenolic compounds
Traditional medicine continues to play a very important role within primary health care across the globe. Drimia plants are known to contain a colossal number of natural compounds, which include cardiac glycosides, saponins, terpenes, alkaloids, and flavonoids with significant pharmacological properties [26,27,56,57]. Qualitative phytochemical screening was done to check the presence of different phytochemical groups, which include tannins, phenols, quinones, anthraquinones, steroids, terpenoids, flavonoids, chalcones, anthocyanins, xanthones, flavones, flavonols, alkaloids, carbohydrates, cardic glycosides, saponins, proteins, coumarins, phlobatannins, and leucoanthocyanins turns in the methanol extract (ME) and water extract (WE). As shown in Table 2, both D. calcarata extracts contain tannins, phenols, steroids, terpenoids, and cardic glycosides. The flavonols, xanthones, flavones, and anthraquinones were only present in WE. This suggests that WE contained several classes of flavonoids. This group of compounds is well-known for its potential antioxidant activity, antitumor, antimicrobial, and anti-inflammatory health benefits [[58], [59], [60]]. Farnesiferol C, a coumarin isolated from Ferula asafoetida displayed an anticancer effect on the breast cancer MFC-7 cells [61].
Table 2.
The presence/absence of various secondary metabolites in the methanol extract (ME) and water extract (WE) of D. calcarata.
| Phytochemicals | ME | WE |
|---|---|---|
| Tannins | + | ++ |
| Phenols | + | ++ |
| Quinones | + | + |
| Anthraquinones | – | + |
| Steroids | + | ++ |
| Terpenoids | + | ++ |
| Flavonoids | ++ | + |
| Chalcones | – | – |
| Anthocyanins | – | – |
| Xanthones and flavones | – | + |
| Flavonols | – | + |
| Alkaloids | – | – |
| Carbohydrates | + | – |
| Cardic glycosides | + | ++ |
| Saponins | + | ++ |
| Proteins | ++ | – |
| Coumarins | ++ | + |
| Phlobatannins | – | – |
| Leucoanthocyanins turns | + | ++ |
Key: (++) Strong intensity reaction.
(+) Medium intensity reaction.
(-) Not detected.
Carbohydrates and proteins were only detected in ME. Polysaccharides are secondary metabolites that exist in abundance in plants [62]. Plant polysaccharides displayed wide biological activities, which include anticancer [63,64], antioxidant [65], antimicrobial [66], antidiabetic [67] and wound healing [68] benefits. Various tumour-promoting signals contain serine, threonine, or tyrosine phosphorylation that operates as the main switch for target proteins. In non-cancerous cells, phosphoregulation is coordinated by protein kinases and phosphatases. Numerous malignancies are classified through deviant activation of protein kinases whose action is frequently vital, and sometimes enough, to result in cancer [69]. Chalcones, anthocyanins, phlobatannins, and alkaloids were absent in all the extracts.
3.2. Total phenolic content (TPC), total tannin content (TTC) and total flavonoid content (TFC)
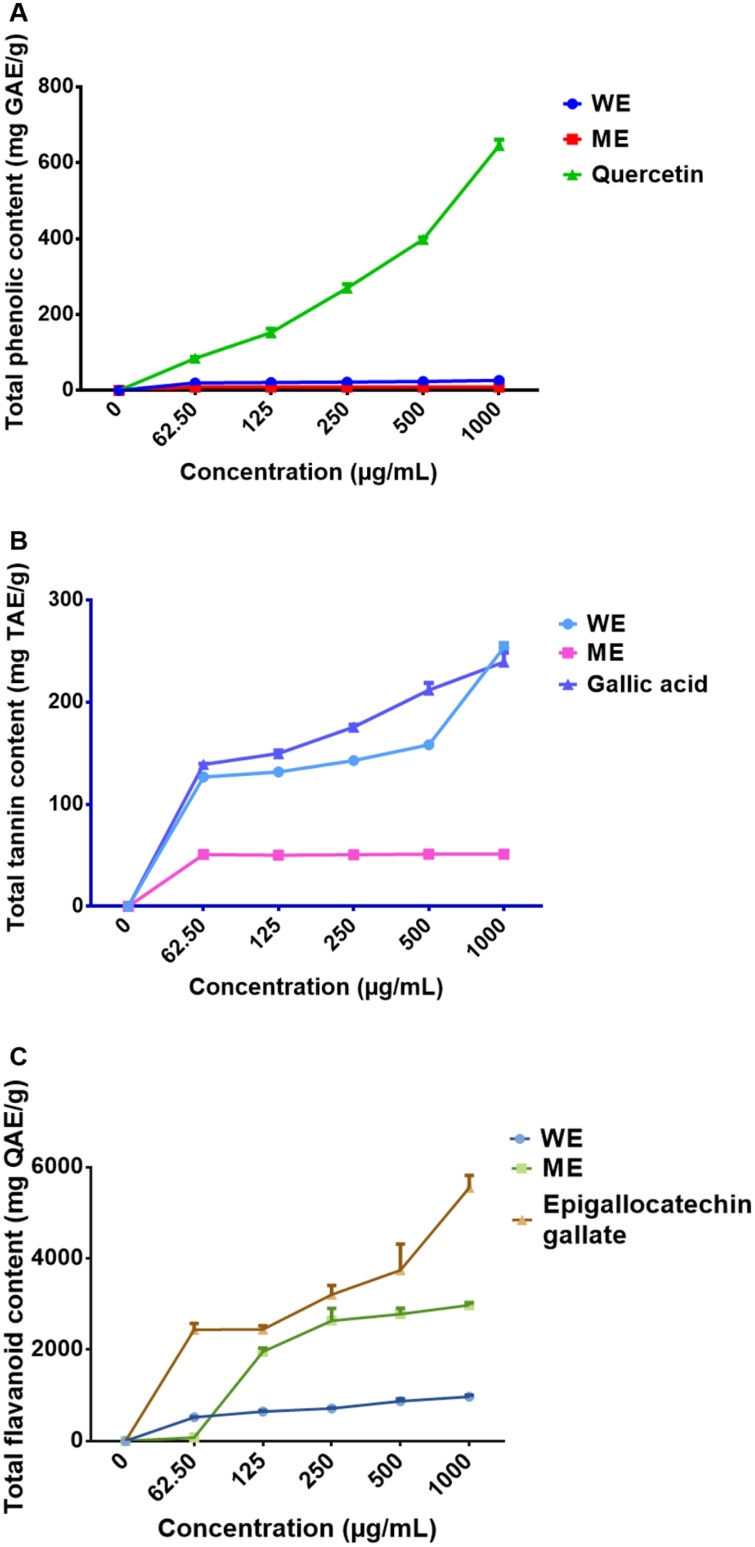
The concentration of TPC in ME and WE of D. calcarata was evaluated. As shown in Fig. 1 and Table A1 (Supplementary data), the linear equation of gallic acid was found to be y = 1.6185x +0.0171. The TPC in extracts was expressed as gallic acid equivalents and WE was found to contain a significantly high TPC of 26.654 ± 0.153 (p < 0.001), compared to ME, which contained a TPC of (9.383 ± 0.029). Quercetin was used as the positive control. The TTC of each extract was determined as tannic acid equivalent using the linear regression from the standard curves. The linear equation of tannic acid was found to be y = 0.2061x +0.0027. The WE contained a significantly higher (p < 0.001) TTC of 253.580 ± 4.356 compared to ME which displayed TTC of 51.306 ± 0.957 (p < 0.001). The results of total tannin content in the D. calcarata extracts are displayed in Fig. 1B and Table A2 (Supplementary data). The TFC was expressed in terms of quercetin equivalents. As shown in Fig. 1C and Table A3, WE showed significantly (p < 0.001) higher TFC 971.33 ± 33.198 at 1000 μg/mL than the ME 866.67 ± 20.667. Epigallocatechin (5547.3 ± 271.64) was used as a positive control. Recently, Yadav et al. [70] reported higher TPC in D. indica diploid (D11) and D. indica triploid (D12) methanol bulb extract than dichloromethane and water extract. TTC was higher in the bulb methanol extract of D. razii than other Drimia species while TFC was found to be higher in methanol bulb extract than DCM and water extracts of D. raogibikei.
Fig. 1.
The total phenolic (A), total tannin (B) and total flavonoid (C) contents of the water extract (WE) and methanol extract (ME) represented as gallic acid, tannic acid and quercetin equivalents (GAE, TAE and QAE in mg/mL), respectively. The differences were found to be statistically significant, (*** P < 0.001) compare to the blank control. Quercetin, gallic acid and epigallocatechin gallate were used as positive controls, respectively.
3.3. Quantitative 2, 2-Diphenyl-1-picrylhydrazyl (DPPH) and free radical antioxidant power (FRAP)
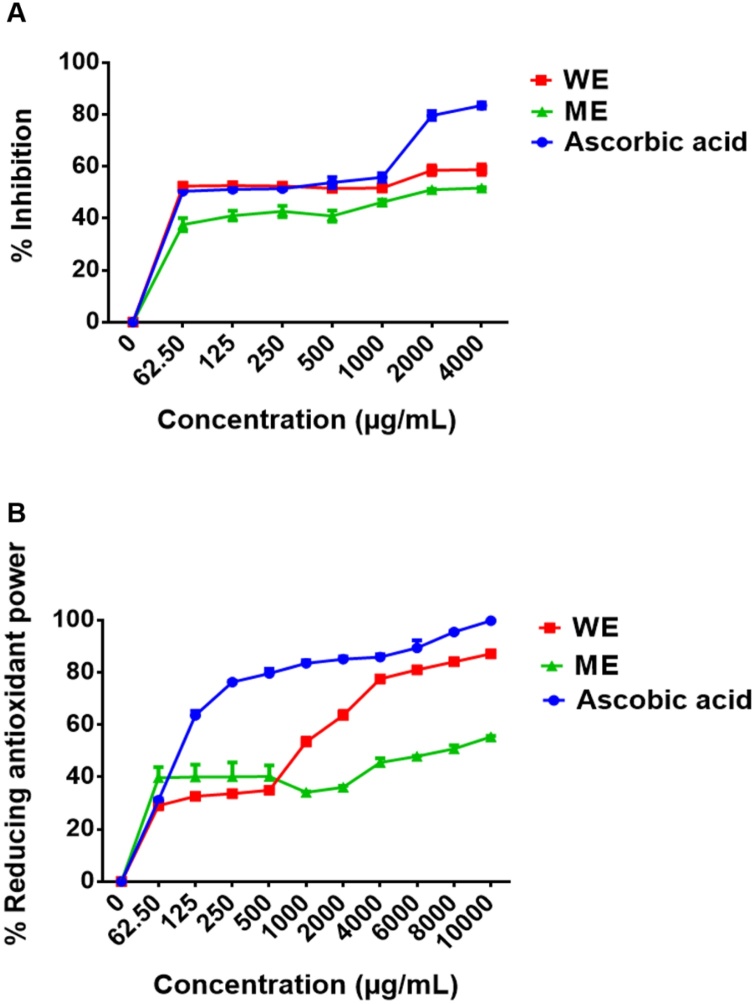
Studies previously reported the association between antioxidant activity, TPC, and TFC in selected medicinal plants [71,72]. As shown in Fig. 2, there were significant differences in total antioxidant capacity between the D. calcarata methanol extract (ME) and water extract (WE). The antioxidant activity of D. calcarata extracts was evaluated using both DPPH and FRAP assays. D. calcarata water extract (WE) showed the best antioxidant activity in both assays. It exhibited the lowest half-maximum effective concentration (EC50) values of 62.50 μg/mL and 1000 μg/mL for the DPPH scavenging activity and the ferric reducing antioxidant power, respectively. The antioxidant activity of WE may be attributed to the Quercetin derivative, Dihydrophaseic acid hexoside, and Limonin-17-beta-D-glucoside (1-). These compounds were previously reported to have antioxidant activity [[73], [74], [75]]. The EC50 value for WE was the same as that of ascorbic acid (positive control) in the DPPH assay and much higher than that of ascorbic acid in the FRAP assay. The ME showed less DPPH scavenging activity and ferric reducing antioxidant power with the EC50 values of 2000 μg/mL and 10 000 μg/mL, respectively. The antioxidant activity of ME can be attributed to compounds such as salvianolic acid A, Isorhamnetin derivative, Taxifolin 4′-glucoside, Petunidin 3,5-Di-O-Beta-d-Glucoside, Hydroxyoleuropein, Oleuropein, and Limonin-17-beta-d-glucoside (1-) [73,74,[76], [77], [78]]. Previously, DPPH inhibition was significantly higher in dichloromethane and methanol extract than water extract [68]. The DPPH inhibition was significantly higher in dichloromethanebulb extract of D. coromandeliana followed by dichloromethane bulb extract of D. raogibikei [70]. Previously, Rajput et al. [26] reported the highest DPPH scavenging activity in bulb extracts of D. coromandeliana. The smaller the EC50 value, the stronger the antioxidant activity [56].
Fig. 2.
DPPH scavenging activity (A) and reducing antioxidant power activity (B) of D. calcarata water extract (WE) and methanol extract (ME). The differences were found to be statistically significant (*** P < 0.001) compare to the blank control. Ascorbic acid was used as positive control.
3.4. Chemical composition of D. calcarata WE and ME by liquid chromatography mass spectrometry (LC–MS)
A high-resolution LC–MS-based approach was used to study the phytochemical variations in D. calcarata bulb extracts. As shown in Table 3, two compounds were only present in the water extract (Ficuspirolide and 6β-Acetoxyscillarenin3-Oβ-d-glucoside(1→4)-α-l-rhamnoside) with 4 compounds only present in the methanol extract (Isorhamnetin derivative, Taxifolin 4′-glucoside, Scillirubroside/scilliphaeosidin-glucoside and 6β-Acetoxyscillarenin3-Oβ-d-glucoside(1→4)-α-l-rhamnoside). These were previously found in other Drimia plants. Additionally, for the first time, 9 compounds were identified in the D. calcarata water extract, namely; Pantothenate, Quercetin derivative, Dihydrophaseic acid hexoside, Vanillic acid hexoside, 2-hydroxyethyl 4-acetyl-4-methyl-5-oxohexanoate, Psoralene, Baccatin III, Cyanidin 3-O-(6ꞌꞌ acetyl) glucoside, and Limonin-17-beta-d-glucoside(1-) and only one compound was unidentified (retention time: 10.8, molecular weight: 351.1276), while 10 new compounds were identified for the first time in the methanol D. calcarata, and these are Salvianolic acid A, Protocatechuic acid glucoside, Rhamnosyl-di-hexosyl quercetin sulphate, Dihydrophaseic acid hexoside, Eriodictyol 7-O-glucoside, Petunidin 3,5-Di-O-Beta-d-Glucoside, 2-hydroxyethyl 4-acetyl-4-methyl-5-oxohexanoate, Hydroxyoleuropein, Oleuropein and Limonin-17-beta-d-glucoside and only one unidentified compound (retention time:16.07, molecular weight: 685.1863). Dihydrophaseic acid hexoside, 2-hydroxyethyl 4-acetyl-4-methyl-5-oxohexanoate, Limonin-17-beta-d-glucoside (1-) and 6β-Acetoxyscillarenin3-Oβ-d-glucoside (1→4)-α-l-rhamnoside were found in both ME and WE. The data showed strong phytochemical differences between the bulb solvent extractions. Previously, Moodley et al. [30] attributed the activity of D. calcarata to a single compound, riparianin (Formula: C33H42O13; Molecular weight = 646.7 g/mol), which was identified in the dichloromethane extract. The compound was not found in the ME and WE; this might be due to a difference in the polarity of the solvents. The obtained chromatograms are shown in Fig. A1 (ZM_ Unilim_190917_27) for WE and Fig. S1 (ZM_Unilim_190917_26) for ME. Cytotoxicity analysis of Drimia calcarata extracts using the MTT assay
Table 3.
Identification of phenolic compounds from D. calcarata water extract and methanol extract by LC–MS.
| Water Extract | ||||||
|---|---|---|---|---|---|---|
| Compound | Retention Time (Min) | M-H | M-H Formula | Biological activities | Reference | Compound CID |
| Pantothenate | 5.81 | 218.1039 | C9H16NO5 | Biosynthesis of co-enzyme A | [74] | 6613 |
| Ficuspirolide | 6.46 | 240.0503 | C13H20O4 | Unknown | [56,61] | 100987513 |
| Quercetin derivative | 6.66 | 299.0767 | C15H10O7 | Antioxidant, anticancer |
[76,77] | 5280343 |
| Dihydrophaseic acid hexoside | 8.62 | 443.1891 | C21H31O10 | Antioxidant, anticancer |
[78] | – |
| Vanillic acid 4-Beta-d-Glucoside | 10.32 | 329.0876 | C14H18O9 | Anticancer | [79,80] | 14132336 |
| Unidentified | 10.98 | 351.1276 | Unknown | Unknown | – | |
| 2-hydroxyethyl 4-acetyl-4-methyl-5-oxohexanoate | 13.38 13.96 |
229.1070 229.1070 |
C11H17O5 | Unknown | – | 57557709 |
| Psoralene | 16.01 | 187.0979 | C11H6O3 | Anticancer | [81] | 6199 |
| Baccatin III | 17.85 | 603.2449 | C31H38O11 | Anticancer | [82] | 65366 |
| Cyanidin 3-O-(6ꞌꞌ acetyl) glucoside | 18.74 | 491.2128 | C23H23O12 | Anticancer | [83,84] | 15714477 |
| Limonin-17-beta-d-glucoside | 20.90 | 649.2471 | C32H42O14 | Anticancer, antioxidant and hepatoprotection | [83,85,86,87] | 24820753 |
| 6β-Acetoxyscillarenin3-Oβ-d-glucoside(1→4)-α-l-rhamnoside | 21.91 21.94 22.01 22.87 |
753.3328 753.3335 753.3345 753.3326 |
C38H54O15 | Unknown | [56] | – |
| Methanol Extract | ||||||
|---|---|---|---|---|---|---|
| Peak number | Retention Time (Min) | M-H | M-H Formula | Biological activities | Reference | |
| Salvianolic acid A | 4.96 | 492.1812 | C26H22O10 | Antioxidant and cardioprotective capacity | [88,89] | 5281793 |
| Protocatechuic acid glucoside | 5.47 | 315.0711 | C13H16O9 | Antidiabetic | [90,91] | 11972438 |
| Isorhamnetin derivative | 6.45 | 373.0757 | C16H12O7 | Anticancer and antioxidant | [92,93] | 5281654 |
| Rhamnosyl-di-hexosyl quercetin sulphate | 7.65 | 915.2181 | C33H38O28S | Unknown | [94] | 129849106 |
| Dihydrophaseic acid 4-O-beta-d-glucoside | 8.62 | 443.1893 | C21H31O10 | Antioxidant, anticancer |
[79] | 11988281 |
| Eriodictyol 7-O-glucoside | 10.26 | 449.1064 | C21H21O11 | Cell survival | [95,96] | 13254473 |
| Taxifolin 4′-glucoside | 12.19 12.25 |
465.1044 465.1044 |
C21H22O12 | Antioxidant, anticancer | [78,79] | 71587141 |
| Petunidin 3,5-Di-O-Beta-d-Glucoside | 13.38 | 641.1724 | C28H33O17 | Antioxidant | [97,98] | 75184857 |
| 2-hydroxyethyl 4-acetyl-4-methyl-5-oxohexanoate | 13.94 14.00 |
229.1070 229.1070 |
C11H17O5 | Unknown | – | 57557709 |
| Unidentified | 16.07 | 685.1863 | Unknown | Unknown | ||
| Hydroxyoleuropein | 17.49 | 557,1857 | C25H32O14 | Antioxidant | [99,100] | 6440747 |
| Oleuropein | 18.26 18.31 |
539.1779 539.1798 |
C25H32O13 | Antioxidant, anticancer, antimicrobial, cardioprotective capacity and anti-inflammatory | [101,102,103,104] | 5281544 |
| Scillirubroside/scilliphaeosidin-glucoside | 19.38 | 607.2750 | C28H31O15 | Unknown | [93] | – |
| Limonin-17-beta-d-glucoside | 20.87 | 649.2489 | C32H41O14 | Anticancer, antioxidant and hepatoprotection | [83,85,86,87] | 46878414 |
| 6β-Acetoxyscillarenin3-Oβ-d-glucoside(1→4)-α-l-rhamnoside | 21.87 21.99 |
753.3318 753.3318 |
C38H54O15 | Unknown | [56] | – |
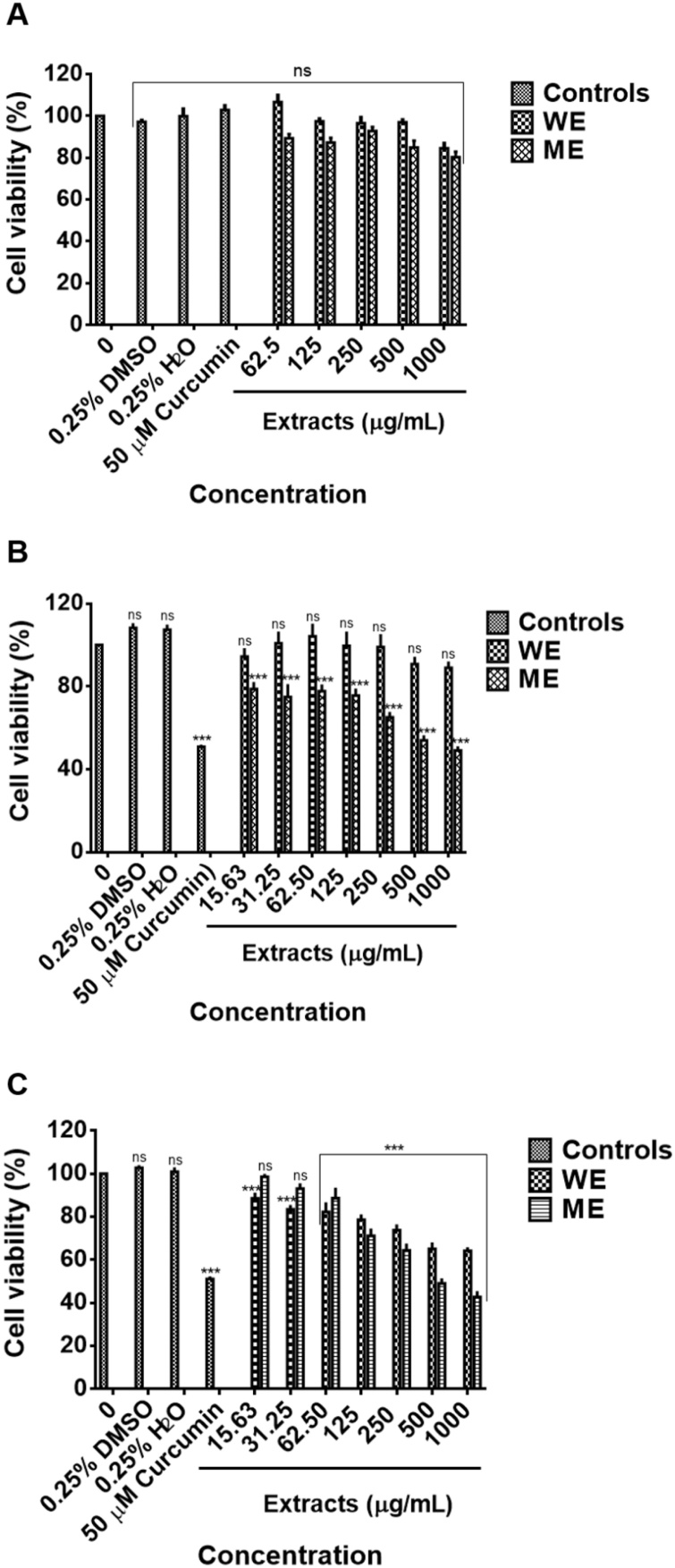
To determine the safety and potential use of plant extracts as the source of therapeutics, it is pivotal to investigate their cytotoxic and non-cytotoxic concentrations [105]. Due to an increased number of cancer-related deaths and shortage of effective chemotherapy, research studies are required to come up with novel alternatives for treatment and prevention, and herbal medicine plays a crucial role in the discovery of such new drugs [106,107]. The potential cytotoxic effects of D. calcarata ME and WE were evaluated for the first time. A shown in Fig. 3A, both WE and ME had no significant cytotoxic effect on the human embryonic kidney HEK-293 cells. The results suggest the safety of the D. calcarata extracts. As shown in Fig. 3B & C, the colorectal cancer cells Caco-2 and HT-29 had varying sensitivities towards methanol extract. The culture conditions can massively impact the integrity of the Caco-2 monolayer, as well as the permeability of drugs [108]. The lack of efficacy against the Caco-2 cells may be attributed to lack of solubility, thus, as suggested by Lechanteur et al. [108] suggested that the behaviour of these cells may affect the efficacy of the drugs. Thus, in future, it would fruitful to reprogramme these cells to improve drug uptake. Previously, Caco-2 cells were epigenetically reprogrammed and altered into CD4+ cells with nano-sized complexes of amphiphilic poly-(N-vinylpyrrolidone) [PVP] with miRNA-152 and piRNA-30074 [109]. The WE extract demonstrated a cytotoxic effect against the HT-29 cells and had no significant effect on the Caco-2 cells. An IC50 of 450 μg/mL for ME was used for the treatment of Caco-2 cells and 500 μg/mL for both ME and WE for the treatment of HT-29 cells. For the WE extract, an IC50 could not be deduced due to lack of activity, thus 500 μg/mL WE was chosen to further confirm the inactivity against Caco-2 cells. Curcumin (50 μM) was used as a positive control, 0.25 % DMSO, and 0.25 % H2O as solvent controls. Previously, D. maritima exhibited anticancer activity against malignant human neuroblastoma (SH-SY5Y) cell line, non-small cell lung cancer (NSCLC) [A549] cell line, human cervical cell lines, (SiHa and HeLa) [4,16,110]. Furthermore, altissimin, a flavonoid C-apioglucoside fromDrimia altissima demonstrated solid in vitro anticancer activity against cervical HeLa cells [111]. MTT assay has been used as a drug sensitivity test (DST), in vitro; however, this method has some drawbacks/limitations [112]. For instance, this assay cannot differentiate between apoptotic and necrotic cells and therefore does not reveal the mechanism of action [113,114]. Detection of apoptosis in treated cancer cells is more valuable than assessing only cancer cell viability. Additionally, riparianin isolated from D. calcarata was implicated in anticancer activities against breast (MCF-7), renal (TK10), and melanoma (UACC62) cancer cells based on MTT assay only, but the anticancer mechanism of the dichloromethane extract was not explored. The chemical composition of D. calcarata water extract included; Quercetin derivative, Dihydrophaseic acid hexoside, Vanillic acid 4-Beta-d-Glucoside, Psoralene, Baccatin III, Cyanidin 3-O-(6ꞌꞌ acetyl) glucoside and Limonin-17-beta-d-glucoside and methanol extract included; Isorhamnetin derivative, Dihydrophaseic acid 4-O-beta-d-glucoside, Taxifolin 4′-glucoside, Oleuropein and Limonin-17-beta-d-glucoside, and have been demonstrated to have anticancer activities in previous reports as indicated in Table 3. The results suggest that some of these phytochemicals may stimulate the anticancer activity of D. calcarata. Since the anticancer activity of the extracts should be mainly attributed to the identified secondary metabolites, these findings warrant further study at the molecular level to certainly launch the anticancer value of these phytochemicals.
Fig. 3.
Cytotoxic effect of ME and WE against Human embryonic kidney HEK-293 cells (A), colorectal Caco-2 cancer cells (B) and colorectal HT-29 cancer cells (C). Non cancer cells were treated with D. calcarata extracts at concentrations ranging from 62.5 to 1000 μg/mL and cancer cells from 15. 63 to 1000 μg/mL. The difference was found to be statistically nonsignificant (ns) after treatment of the noncancerous HEK-293 cell line with WE and ME. Both ME and WE significantly (*** P < 0.001) reduced the viability of the HT-29 cells. WE had no effect on Caco-2 cells.
3.5. Apoptosis analysis
Apoptosis was primarily defined by its morphological features, which include chromatin condensation, nuclear fragmentation, membrane blebbing, and cell shrinkage [115]. To elucidate whether the inhibition of cell proliferation by ME and WE was linked with cellular apoptosis induction, the effects of both extracts were first investigated through fluorescence microscopy using acridine orange/ethidium bromide (AO/EB) dual staining. Secondly, by in vitro assays Muse® Annexin and Dead Cell assay, Muse® Caspase 3/7 Activation assay and lastly, Polymerase Chain Reaction (PCR).
3.6. AO/EB dual staining
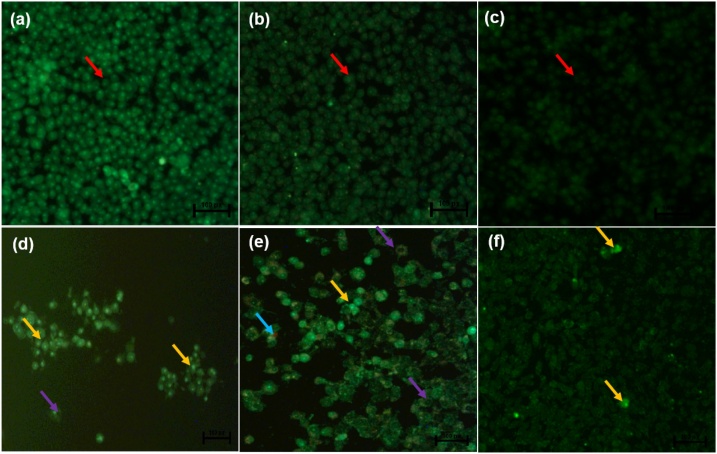
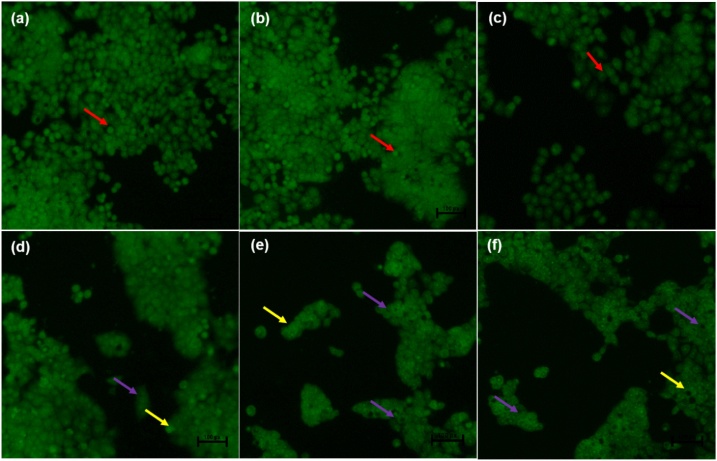
Dual acridine orange/ethidium bromide (AO/EB) fluorescent staining, visualised under a fluorescent microscope, has been employed to categorise apoptosis-related changes of cell membranes during the process of cell death. Moreover, this technique can also accurately differentiate cells in dissimilar stages of apoptosis [116]. The untreated control Caco-2 cells (Fig. 4a), 0.25 % DMSO treated cells and 0.25 % H2O-treated cells (Fig. 4b) displayed intact nuclear (red arrows), while curcumin- and ME-treated Caco-2 cells (Fig. 4d & e) showed cell shrinkage (purple arrows), cells going through early apoptosis which fluoresced a green/yellow colour (yellow arrows) and cells going through the late stage of apoptosis which showed uneven orange fluorescence at their periphery (blue arrows) but WE treated Caco-2 cells (Fig. 4f) displayed no apoptotic cells. Treatment of the HT-29 cells with curcumin and both extracts caused cell shrinkage (purple arrows) and cells going through early apoptosis, which fluoresced bright green (yellow arrows) [Fig. 5j–l] and the number of viable cells decreased tremendously.
Fig. 4.
Nuclear morphology of Caco-2 cells after acridine orange/ethidium bromide (AO/EB) staining: The figure shows untreated Caco-2 cells - (a), 0.25 % DMSO-treated Caco-2 cells (b), 0.25 % H2O-treated Caco-2 cells (c), 50 μM Curcumin-treated Caco-2 cells (d), -450 μg/mL ME-treated Caco-2 cells (e), -500 μg/mL WE-treated Caco-2 cells (f). The images were taken at 20X magnification using the Eclipse Ti-U fluorescence microscope (Nickon Instruments Inc. USA) and images were captured at 20X magnification. The red arrows indicate intact nuclei, while purple arrows display loss of membrane integrity. The yellow arrows show cells undergoing early apoptosis while the blue arrows show cells at late apoptosis. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Fig. 5.
Nuclear morphology of HT-29 cells after acridine orange/ethidium bromide (AO/EB) staining: The figure shows untreated HT29 cells (a), 0.25 % DMSO-treated HT29 cells (b), 0.25 % H2O-treated HT29 cells (c), -50 μM Curcumin-treated HT29 cells (d), 450 μg/mL ME-treated HT29 cells (e), -500 μg/mL WE-treated HT29 cells (f). The cells were taken at 20X magnification utilizing an Eclipse Ti-U fluorescence microscope (Nickon Instruments Inc. USA). The red arrows indicate intact nuclei, while purple arrows display loss of membrane integrity. The yellow arrows show cells undergoing early apoptosis while the blue arrows show cells at late apoptosis. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Previously, Liu et al. [117] AO/EB was used for apoptosis detection in Osteosarcoma Cells. Cells undergoing early apoptosis were noticeable by crescent-shaped or granular yellow-green acridine orange nuclear staining, while those undergoing late-stage of apoptosis were patent with orange nuclear ethidium bromide staining. Additionally, treatment of HT-29 cells with vanillin resulted in reduced cancer cell number, and also features of apoptosis-like cell blebbing were observed [118].
3.7. Annexin V and caspase 3/7 activation
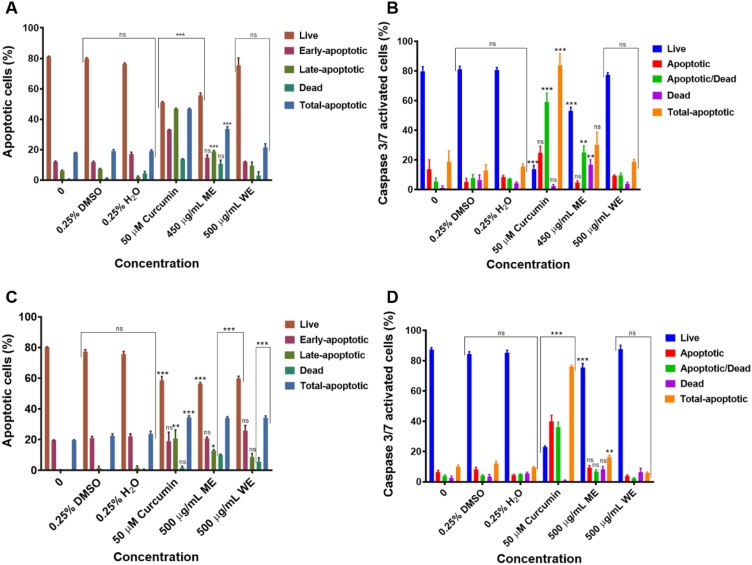
To further confirm the apoptotic activity of the D. calcarata, the Muse® Annexin V and Dead Cell assay and Muse® Caspase 3/7 assay were carried out. Treatment of Caco-2 cells with 450 μg/mL of the ME and 50 μM curcumin significantly (p < 0.001) induced late apoptosis, while the WE did not affect Caco-2 cells (Figs. 6A and A2 [Supplementary data]). ME and curcumin significantly (p < 0.01 and p < 0.001) induced the activation of the effector caspases, including 3 and 7 in Caco-2 cells (Figs. 6B and A4 [Supplementary data]). Interestingly, both D. calcarata extracts induced early apoptosis in HT-29 cells (Figs. 6C and A3 [Supplementary data]). ME and curcumin were also significantly induced caspases -3 and -7 activation in HT-29 cells (p < 0.01; p < 0.001). The WE did not affect the activation of caspases -3 and -7 in both cell lines (Figs. 6D and A5 [Supplementary data]). Previously, D. maritima induced cell death in human breast cancer cells via the intrinsic apoptotic pathway [119]. Baccatin III promoted apoptosis in JR4-Jurkat cells with possible participation of anti-apoptotic Bcl-2 and mitochondrial membrane damage [120]. Furthermore, limonin glucoside induced apoptosis through a reduction in the transcription ratio of Bcl-2/Bax and initiating the release of cytochrome c from mitochondria to cytosol, which confirm the activation of the intrinsic apoptotic pathway in SW480 colon cancer cells [121]. Oleuropein has been demonstrated to promote breast tumour cell death through a p53-dependent pathway, which is regulated by Bax and Bcl-2 genes [122]. These compounds have been found present in the D. calcarata extracts. Therefore, phytochemicals present in D. calcarata extracts may have therapeutic potential in colorectal cancer by inducing apoptosis.
Fig. 6.
Apoptosis evaluation and Caspase3/7 activation in Caco-2 (A&B) and HT-29 (C&D) cells after 24 h treatment. In A, B, C and D, solvent controls had no significant (ns) effects on both Caco-2 and HT-29 cells. The positive control agent significantly (p < 0.001) induced apoptosis of both colorectal cancer cells.
3.8. Expression of apoptotic related genes
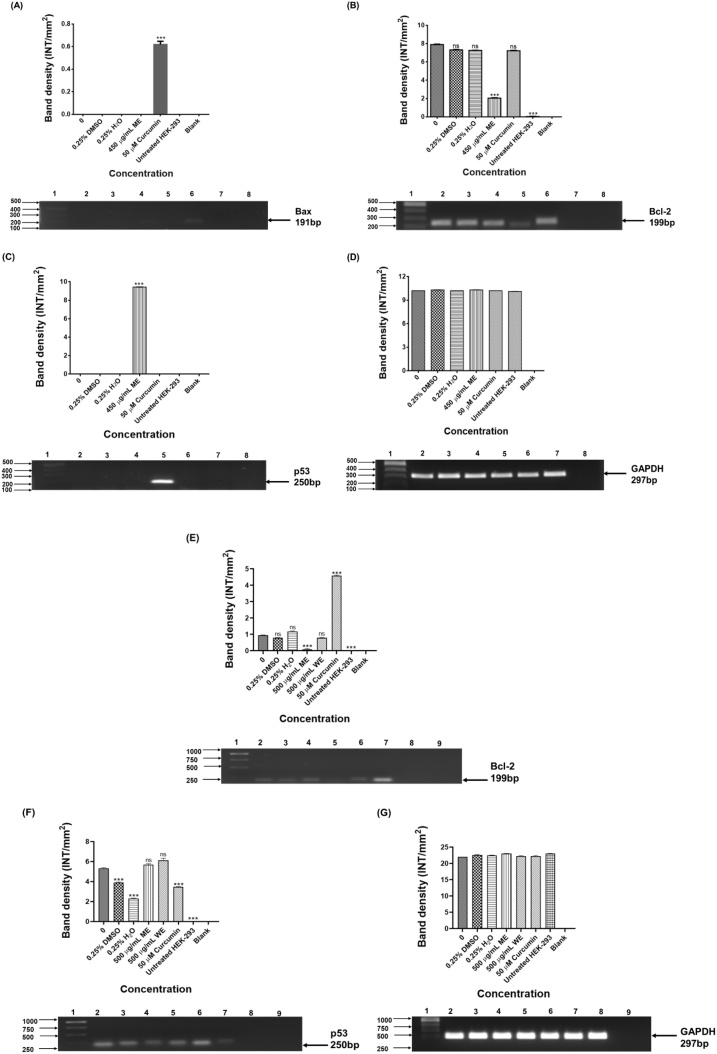
Bcl-2 and Bax are members of the Bcl-2 family, which are named from B-cell lymphoma-2 [123], which regulate apoptosis by acting as anti- or pro-apoptotic regulators that are involved in a wide variety of cellular activities. Bax is an oncogene regarded as having a proapoptotic effect. It has also been suggested that the ratio of Bax to Bcl‐2 proteins is the dominant factor that determines whether expression of these oncogenes results in pro‐ or antiapoptotic effects [124]. In this study, Bax was undetected in both untreated colorectal cancer cells and D. calcarata WE and ME treated Caco-2 and HT-29. Bax upregulation was only detected after the treatment of Caco-2 cells with 50 μM curcumin (Fig. 7A). Bcl-2 is mostly reported as an antiapoptotic regulator, thus promoting tumorigenesis by stimulating anti-apoptotic activities [[110], [111], [112]]. As shown in Fig. 7, Bcl-2 was highly expressed in untreated Caco-2 cells (Fig. 7B) than untreated HT-29 cells (Fig. 7E). ME significantly (p < 0.001) downregulated the expression of Bcl-2 in both cell lines, while WE and curcumin-treated HT-29 showed no significant effect on the expression of Bcl-2. Decreased expression of Bcl‐2 in both colorectal cancer cells can be credited with the apoptosis prompted by ME since overexpression of Bcl-2 supports tumour cell growth and resistance to apoptosis.
Fig. 7.
PCR analysis of apoptotic genes in Caco-2 (A-Bax, B-Bcl-2, C-p53 &D-GAPDH) and HT-29 (E-Bcl-2, F-p53 & G-GAPDH) cells. Caco-2 cells: Lane 1: 100 bp Marker, Lane 2: untreated, Lane 3: 0.25 % DMSO, Lane 4: 0.25 % H2O, Lane 5: 450 μg/mL ME, Lane 6: 50 μM curcumin, Lane 7: HEK-293 cells and Lane 8: blank. HT-29: Lane 1: 1000 bp DNA ladder, Lane 2: untreated, Lane 3: 0.25 % DMSO, Lane 4: 0.25 % H2O, Lane 5: 62.5 μg/mL ME, Lane 6: 250 μg/mL WE, Lane 7: 50 μM curcumin, Lane 8: HEK-293 cells and Lane 9: blank. GAPDH was used as a loading control.
The p53 was not detected in the untreated Caco-2 cells, but the treatment of Caco-2 cells with ME, lead to its upregulation (Fig. 7C). Interestingly, both ME and WE upregulated the expression of p53 in HT-29 (Fig. 7F). The results suggest that the D. calcarata extracts increase cancer cell apoptosis through a p53-dependent pathway. In agreement, He et al. [125], previously concluded that the general health of patients with colorectal cancer improved following curcumin treatment through the mechanism of increased p53 molecule expression in tumour cells and as a result, speeding up tumour cell apoptosis. Curcumin-treated Caco-2 cells showed no expression of p53, while curcumin-treated HT-29 cells resulted in the significant (p ≤ 0.001) downregulation of p53 expression. In agreement, curcumin resulted in the downregulation of p53 in HT-29 [125]. This might be due to the fact that HT-29 colorectal cancer cells are p53 mutated cells, regardless of the p53 status, curcumin is considered to be active against colorectal cancer cells [126]. Previously, Gamet-Payrastre et al. [127] also reported the expression of p53, with no detection of Bcl-2 and elevated expression level of the proapoptotic protein Bax in HT-29. A previous report suggested that p53 induces the mitochondrial cell death pathway [128]. Moreover, in other diseases, curcumin protected rat liver from streptozotocin-induced diabetic pathophysiology by counteracting reactive oxygen species and preventing the activation of p53 and MAPKs mediated stress response pathways [129]. Downregulation of Bcl-2 unblocks the release of cytochrome c from the mitochondria for initiation of apoptosis, thus ME induced mitochondrial-mediated apoptosis in colorectal Caco-2 and HT-29 cells. This was supported by the upregulation of p53 in both cell lines by ME. Previously, human breast cancer cells went through intrinsic apoptosis after treatment with D. maritima [118]. GAPDH was used as the loading control (Fig. 7D and G).
3.9. Expression of signal transducers and activators of transcription (STAT) genes
Colorectal cancer study has defined many biomarkers for diagnostic, prognostic, and predictive functions that either alone or as part of a panel would help advance patient's clinical management. At present, it is obvious that colorectal cancer is a polygenic disease occurring due to epigenetic as well as genetic manifestations in a numeral of genes with an unparalleled role for the maintenance of standard cellular physiological conditions [130]. Such genes may be tumour suppressor genes, oncogenes, mismatch repair genes, and cell cycle regulating genes in colon mucosal cells [131]. Consequently, research studies aim to describe new markers for prognosis and prediction of the biological behaviour of a particular therapeutic procedure [132]. The wild-type colorectal cancers may be less sensitive to medicinal plants extracts than p53-mutant colorectal cancers. Additionally, STAT5B can also be targeted for combinatorial therapy.
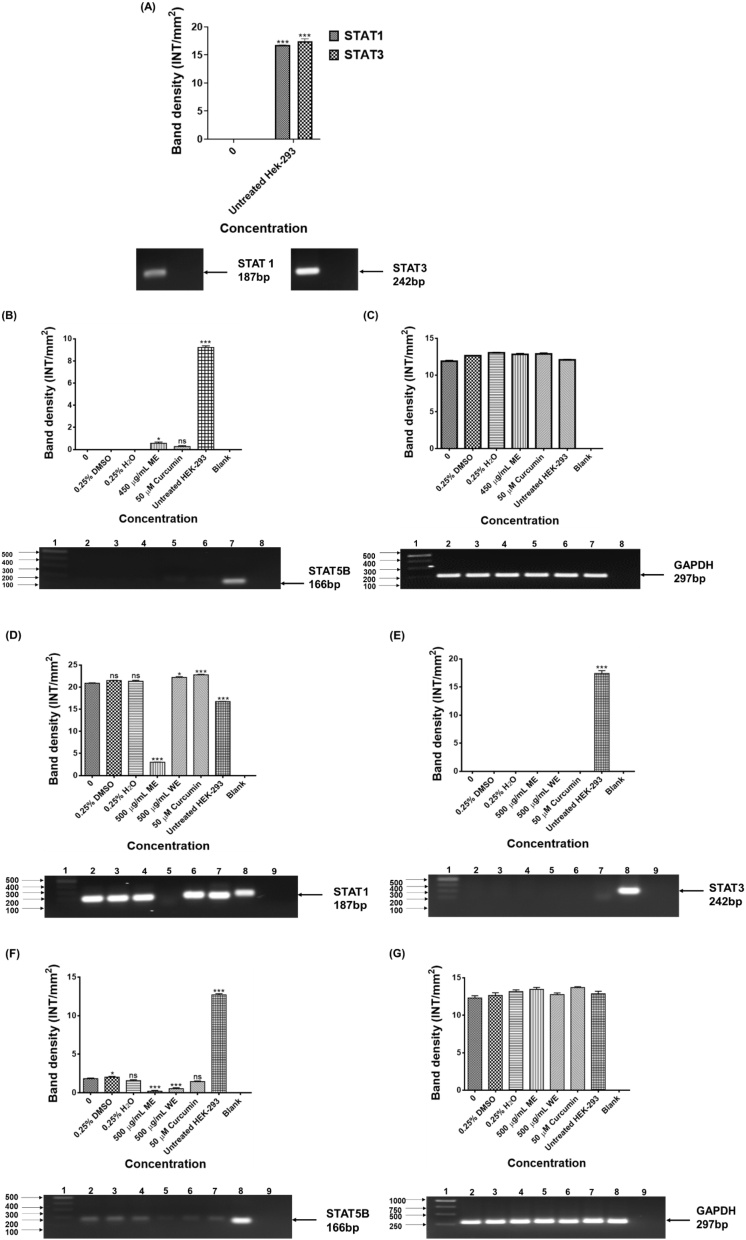
Transcription factors are adaptor molecules that recognise regulatory sequences in the DNA and aim at the cluster of proteins that regulate gene expression. A few transcription factors are overactive in many human cancer cells, making them significant targets for the development of therapeutic drugs [133]. As shown in Fig. 8A, the expression of STAT1 and STAT3 were not observed in the untreated and treated Caco-2 cells, the expression was only detected in the untreated HEK-293 cells. No expression of STA5B was observed in the untreated Caco-2, DMSO and H2O treated Caco-2 cells. Treatment of Caco-2 with ME and curcumin resulted in upregulation of STAT5B, which is highly expressed in HEK-293 cells (Fig. 8B). As shown in Fig. 8D, ME downregulated the expression of STAT1 in HT-29 cells. No expression of STAT3 in the HT-29 cells before and after treatment (Fig. 8E). Treatment with ME, WE, and curcumin downregulated the expression of STAT5B (Fig. 8F). The expression of STAT genes in colorectal cancer is unclear. STAT1, STAT3, and STAT5 activation are typically associated with increased tumour proliferation, metastasis, survival, and invasion [[134], [135], [136]]. During the progression of cancer, additional signalling pathways turn out to be activated in cells, but most congregate into major downstream networks. As part of an extended network, STAT5 signal transducers can interact in crosstalk with other pathways to enable co-operation or antagonistic actions of various growth factors. Research report demonstrated that comparable to normal developmental programs, oncogenic functions of STAT5 rely on molecular crosstalk with PI3K/AKT signalling for the initiation and in some instances the progression, of breast cancer. The multitude by which STATs can interact with individual mediators of the PI3K/AKT signalling cascade may provide diverse opportunities for targeting signalling nodes within molecular networks that are crucial for the survival of cancer cells [137]. Moreover, the AKT and STAT5 pathways appear most critical and may result in drug-resistant chronic myeloid leukemia and systemic mastocytosis. Thus, targeting STAT5 and AKT could be a stimulating approach in human malignancies. There is an unmet need to come up with novel drugs which will target STAT-regulated genes in cancer. GAPDH was used as the loading control (Fig. 8C and G).
Fig. 8.
PCR analysis of STATS genes in HEK-293 (A-STAT1& STAT3), Caco-2 (C-STAT5B &D-GAPDH) and HT-29 (D-STAT1, E-STAT3, F-STAT5B & G-GAPDH) cells. STAT1, STAT3, STAT5B and GAPDH in colorectal cancer cells after 24 h treatment D. calcarata extracts. Caco-2 cells: Lane 1: 100 bp Marker, Lane 2: untreated, Lane 3: 0.25 % DMSO, Lane 4: 0.25 % H2O, Lane 5: 450 μg/mL ME, Lane 6: 50 μM curcumin, Lane 7: HEK-293 cells and Lane 8: blank. HT-29: Lane 1: 1000 bp DNA ladder, Lane 2: untreated, Lane 3: 0.25 % DMSO, Lane 4: 0.25 % H2O, Lane 5: 62.5 μg/mL ME, Lane 6: 250 μg/mL WE, Lane 7: 50 μM curcumin, Lane 8: HEK-293 cells and Lane 9: blank. GAPDH was used as a loading control.
4. Conclusion
The D. calcarata methanol extract (ME) demonstrated cytotoxicity against colorectal cancer Caco-2 and HT-29 cells, meanwhile, water extract (WE) showed selective cytotoxicity towards the colorectal cancer cells. The expression of apoptosis-related regulatory genes assessed by PCR revealed an up-regulation of p53 while Bcl-2 was downregulated. Upregulation of Bax after treatment with curcumin was observed. The Bax gene was undetected in HT-29 cells. The mode of cell death induced by ME was suggestive of an intrinsic apoptotic pathway and p53-dependent mechanism in both colorectal cancer cells. ME downregulated the expression of STAT1, 3, and 5B in HT-29 cells. Our findings indicate that phytochemicals present in Drimia calcarata have a potential for natural therapeutic product development for the treatment of colorectal cancer. However, further investigations are now needed to establish the precise mechanism of action for the compounds with unknown biological activities and to elucidate their therapeutic effectiveness.
Author’s contribution
K. Laka—Methodology, Formal analysis, Investigation, Writing—original draft, review & editing, K.B.F Mapheto - Resources, Z. Mbita- Resources, Writing—review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This work was financially supported by the National Research Foundation (NRF) of South Africa (UID: 113858). The University of Limpopo Research and Development Office for financial contribution.
Handling Editor: Dr. Aristidis Tsatsakis
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2021.06.015.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Radtke F., Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307(5717):1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 2.Ciasca G., Papi M., Minelli E., Palmieri V., De Spirito M. Changes in cellular mechanical properties during onset or progression of colorectal cancer. World J. Gastroenterol. 2016;22(32):7203. doi: 10.3748/wjg.v22.i32.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 4.Islami F., Goding Sauer A., Miller K.D., Siegel R.L., Fedewa S.A., Jacobs E.J., McCullough M.L., Patel A.V., Ma J., Soerjomataram I., Flanders W.D. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018;68(1):31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 5.Keum N., Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019;16(12):713–732. doi: 10.1038/s41575-019-0189-8. [DOI] [PubMed] [Google Scholar]
- 6.Jasperson K.W., Tuohy T.M., Neklason D.W., Burt R.W. Hereditary and familial colon cancer. Gastroenterology. 2010;138(6):2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amersi F., Agustin M., Ko C.Y. Colorectal cancer: epidemiology, risk factors, and health services. Clin. Colon Rectal Surg. 2005;18(3):133. doi: 10.1055/s-2005-916274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Geest L.G., Koopman M., Verhoef C., Elferink M.A., de Wilt J.H. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin. Exp. Metastasis. 2015;32(5):457–465. doi: 10.1007/s10585-015-9719-0. [DOI] [PubMed] [Google Scholar]
- 9.Riihimäki M., Hemminki A., Fallah M., Thomsen H., Sundquist K., Sundquist J., Hemminki K. Metastatic sites and survival in lung cancer. Lung cancer. 2014;86(1):78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Mlecnik B., Bindea G., Kirilovsky A., Angell H.K., Obenauf A.C., Tosolini M., Church S.E., Maby P., Vasaturo A., Angelova M., Fredriksen T. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci. Transl. Med. 2016;8(327) doi: 10.1126/scitranslmed.aad6352. 327ra26-327ra26. [DOI] [PubMed] [Google Scholar]
- 11.Mishra J., Drummond J., Quazi S.H., Karanki S.S., Shaw J.J., Chen B., Kumar N. Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Crit. Rev. Oncol. Hematol. 2013;86(3):232–250. doi: 10.1016/j.critrevonc.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen C.H., Gögenur M., Madsen M.T., Gögenur I. The effect of time from diagnosis to surgery on oncological outcomes in patients undergoing surgery for colon cancer: a systematic review. Eur. J. Surg. Oncol. 2018;44(10):1479–1485. doi: 10.1016/j.ejso.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Wessel B.E., Coldwell D. Colon cancer metastasis to the sternum: palliative treatment with radiofrequency ablation and cement injection. Radiol. Case Rep. 2016;11(4):357–360. doi: 10.1016/j.radcr.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biller L.H., Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325(7):669–685. doi: 10.1001/jama.2021.0106. [DOI] [PubMed] [Google Scholar]
- 15.Linnekamp J.F., Wang X., Medema J.P., Vermeulen L. Colorectal cancer heterogeneity and targeted therapy: a case for molecular disease subtypes. Cancer Res. 2015;75(2):245–249. doi: 10.1158/0008-5472.CAN-14-2240. [DOI] [PubMed] [Google Scholar]
- 16.Dienstmann R., Salazar R., Tabernero J. Personalizing colon cancer adjuvant therapy: selecting optimal treatments for individual patients. J. Clin. Oncol. 2015;33(16):1787–1796. doi: 10.1200/JCO.2014.60.0213. [DOI] [PubMed] [Google Scholar]
- 17.Dienstmann R., Vermeulen L., Guinney J., Kopetz S., Tejpar S., Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer. 2017;17(2):79–92. doi: 10.1038/nrc.2016.126. [DOI] [PubMed] [Google Scholar]
- 18.Cremolini C., Schirripa M., Antoniotti C., Moretto R., Salvatore L., Masi G., Falcone A., Loupakis F. First-line chemotherapy for mCRC—a review and evidence-based algorithm. Nat. Rev. Clin. Oncol. 2015;12(10):607–619. doi: 10.1038/nrclinonc.2015.129. [DOI] [PubMed] [Google Scholar]
- 19.Gargett M., Haddad C., Kneebone A., Booth J.T., Hardcastle N. Clinical impact of removing respiratory motion during liver SABR. Radiat. Oncol. 2019;14(1):1–9. doi: 10.1186/s13014-019-1300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciombor K.K., Wu C., Goldberg R.M. Recent therapeutic advances in the treatment of colorectal cancer. Annu. Rev. Med. 2015;66:83–95. doi: 10.1146/annurev-med-051513-102539. [DOI] [PubMed] [Google Scholar]
- 21.Wen C., Huang L., Chen J., Lin M., Li W., Lu B., Rutnam Z.J., Iwamoto A., Wang Z., Yang X., Liu H. Gambogic acid inhibits growth, induces apoptosis, and overcomes drug resistance in human colorectal cancer cells. Int. J. Oncol. 2015;47(5):1663–1671. doi: 10.3892/ijo.2015.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosca L., Pagano M., Pecoraro A., Borzacchiello L., Mele L., Cacciapuoti G., Porcelli M., Russo G., Russo A. S-adenosyl-l-Methionine overcomes uL3-mediated drug resistance in p53 deleted colon cancer cells. Int. J. Mol. Sci. 2021;22(1):103. doi: 10.3390/ijms22010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren Y., Kinghorn A.D. Natural product triterpenoids and their semi-synthetic derivatives with potential anticancer activity. Planta Med. 2019 doi: 10.1055/a-0832-2383. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaya H.O. Biodiversity, traditional medicine and public health care in Eastern and Southern Africa. Pula: Botswana J. Afr. Stud. 2017;3(1):16–30. [Google Scholar]
- 25.Semenya S.S., Potgieter M.J., Erasmus L.J.C. Indigenous plant species used by Bapedi healers to treat sexually transmitted infections: their distribution, harvesting, conservation and threats. South Afr. J. Bot. 2013;87:66–75. [Google Scholar]
- 26.Rajput B., Golave A., Yadav S., Jadhav J.P. Total phenolic concentrations and antioxidant activities in Drimia sp. J. Herbs Spices Med. Plants. 2018;24(1):28–36. [Google Scholar]
- 27.Baskaran P., Singh S., Van Staden J. In vitro propagation, proscillaridin A production and antibacterial activity in Drimia robusta. Plant Cell Tissue Organ Culture (PCTOC) 2013;114(2):259–267. [Google Scholar]
- 28.Fouché G., Cragg G.M., Pillay P., Kolesnikova N., Maharaj V.J., Senabe J. In vitro anticancer screening of South African plants. J. Ethnopharmacol. 2008;119(3):455–461. doi: 10.1016/j.jep.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Ulrich S., Wolter F., Stein J.M. Molecular mechanisms of the chemopreventive effects of resveratrol and its analogs in carcinogenesis. Mol. Nutr. Food Res. 2005;49(5):452–461. doi: 10.1002/mnfr.200400081. [DOI] [PubMed] [Google Scholar]
- 30.Moodley N., Crouch N.R., Mulholland D.A. Bufadienolides from Drimia macrocentra and Urginea riparia (Hyacinthaceae: urgineoideae) Phytochemistry. 2007;68(19):2415–2419. doi: 10.1016/j.phytochem.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 31.Nourazarian S.M., Nourazarian A., Majidinia M., Roshaniasl E. Effect of root extracts of medicinal herb Glycyrrhiza glabra on HSP90 gene expression and apoptosis in the HT-29 colon cancer cell line. Asian Pacific J. Cancer Prev. 2016;16(18):8563–8566. doi: 10.7314/apjcp.2015.16.18.8563. [DOI] [PubMed] [Google Scholar]
- 32.Aisha A.F., Abu-Salah K.M., Ismail Z., Majid A.M.S.A. In vitro and in vivo anti-colon cancer effects of Garcinia mangostana xanthones extract. BMC Complement. Altern. Med. 2012;12(1):104. doi: 10.1186/1472-6882-12-104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Savini I., Arnone R., Catani M.V., Avigliano L. Origanum vulgare induces apoptosis in human colon cancer caco2 cells. Nutr. Cancer. 2009;61(3):381–389. doi: 10.1080/01635580802582769. [DOI] [PubMed] [Google Scholar]
- 34.Breen L., Heenan M., Amberger-Murphy V., Clynes M. Investigation of the role of p53 in chemotherapy resistance of lung cancer cell lines. Anticancer Res. 2007;27(3A):1361–1364. [PubMed] [Google Scholar]
- 35.Jackson J.G., Pant V., Li Q., Chang L.L., Quintás-Cardama A., Garza D., Tavana O., Yang P., Manshouri T., Li Y., El-Naggar A.K. p53-mediated senescence impairs the apoptotic response to chemotherapy and clinical outcome in breast cancer. Cancer Cell. 2012;21(6):793–806. doi: 10.1016/j.ccr.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girardot M., Pecquet C., Chachoua I., Van Hees J., Guibert S., Ferrant A., Knoops L., Baxter E.J., Beer P.A., Giraudier S., Moriggl R. Persistent STAT5 activation in myeloid neoplasms recruits p53 into gene regulation. Oncogene. 2015;34(10):1323–1332. doi: 10.1038/onc.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fritsche M., Mundt M., Merkle C., Jähne R., Groner B. p53 suppresses cytokine induced, Stat5 mediated activation of transcription. Mol. Cell. Endocrinol. 1998;143(1-2):143–154. doi: 10.1016/S0303-7207(98)00140-3. [DOI] [PubMed] [Google Scholar]
- 38.Townsend P.A., Scarabelli T.M., Davidson S.M., Knight R.A., Latchman D.S., Stephanou A. STAT-1 interacts with p53 to enhance DNA damage-induced apoptosis. J. Biol. Chem. 2004;279(7):5811–5820. doi: 10.1074/jbc.M302637200. [DOI] [PubMed] [Google Scholar]
- 39.Chipoy C., Brounais B., Trichet V., Battaglia S., Berreur M., Oliver L., Juin P., Redini F., Heymann D., Blanchard F. Sensitization of osteosarcoma cells to apoptosis by oncostatin M depends on STAT5 and p53. Oncogene. 2007;26(46):6653–6664. doi: 10.1038/sj.onc.1210492. [DOI] [PubMed] [Google Scholar]
- 40.Liang Q.C., Xiong H., Zhao Z.W., Jia D., Li W.X., Qin H.Z., Deng J.P., Gao L., Zhang H., Gao G.D. Inhibition of transcription factor STAT5b suppresses proliferation, induces G1 cell cycle arrest and reduces tumor cell invasion in human glioblastoma multiforme cells. Cancer Lett. 2009;273(1):164–171. doi: 10.1016/j.canlet.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Bromberg J., Darnell J.E. The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19(21):2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- 42.Ma X.T., YU L.W., WANG S. Molecular mechanism of Stat5b signaling pathway in regulating the expression of Bcl-2 family members and promoting survival of human colon cancer cells. Chin. J. Exper. Surg. 2005;10:4. [Google Scholar]
- 43.Eloff J.N. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 1998;60(1):1–8. doi: 10.1016/s0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 44.Mudter J., Weigmann B., Bartsch B., Kiesslich R., Strand D., Galle P.R., Lehr H.A., Schmidt J., Neurath M.F. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am. J. Gastroenterol. 2005;100(1):64–72. doi: 10.1111/j.1572-0241.2005.40615.x. [DOI] [PubMed] [Google Scholar]
- 45.Chiarle R., Simmons W.J., Cai H., Dhall G., Zamo A., Raz R., Karras J.G., Levy D.E., Inghirami G. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat. Med. 2005;11(6):623–629. doi: 10.1038/nm1249. [DOI] [PubMed] [Google Scholar]
- 46.Nam S., Buettner R., Turkson J., Kim D., Cheng J.Q., Muehlbeyer S., Hippe F., Vatter S., Merz K.H., Eisenbrand G., Jove R. Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2005;102(17):5998–6003. doi: 10.1073/pnas.0409467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maria R., Shirley M., Xavier C., Jaime S., David V., Rosa S., Jodie D. Preliminary phytochemical screening, total phenolic content and antibacterial activity of thirteen native species from Guayas province Ecuador. J. King Saud Univ.-Sci. 2018;30(4):500–505. [Google Scholar]
- 48.Elledge S.J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274(5293):1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 49.Matotoka M.M., Masoko P. Phytochemical analysis, antioxidant, antibacterial and combinational effects of medicinal plants used by Bapedi traditional healers to prepare herbal mixtures. J. Med. Plants Res. 2018;12(29):563–574. [Google Scholar]
- 50.Kudumela R.G., Masoko P. In vitro assessment of selected medicinal plants used by the Bapedi Community in South Africa for treatment of bacterial infections. J. Evid. Integr. Med. 2018:23. doi: 10.1177/2515690X18762736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beseni B.K., Matsebatlela T.M., Bagla V.P., Njanje I., Poopedi K., Mbazima V., Mampuru L., Mokgotho M.P. Potential Antiglycation and Hypoglycaemic Effects of Toona ciliata M. Roem. and Schkuhria pinnata Lam. Thell. Crude Extracts in Differentiated C2C12 Cells. Evid. Based Complement. Altern. Med. 2019 doi: 10.1155/2019/5406862. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brand-Williams W., Cuvelier M.E., Berset C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995;28(1):25–30. [Google Scholar]
- 53.Stander M.A., Van Wyk B.E., Taylor M.J., Long H.S. Analysis of phenolic compounds in rooibos tea (Aspalathus linearis) with a comparison of flavonoid-based compounds in natural populations of plants from different regions. J. Agric. Food Chem. 2017;65(47):10270–10281. doi: 10.1021/acs.jafc.7b03942. [DOI] [PubMed] [Google Scholar]
- 54.Rautenbach M., Vlok N.M., Eyéghé-Bickong H.A., van der Merwe M.J., Stander M.A. An electrospray ionization mass spectrometry study on the “In Vacuo” hetero-oligomers formed by the antimicrobial peptides, surfactin and gramicidin S. J. Am. Soc. Mass Spectrom. 2017;28(8):1623–1637. doi: 10.1007/s13361-017-1685-0. [DOI] [PubMed] [Google Scholar]
- 55.Laka K., Makgoo L., Mbita Z. Survivin splice variants in arsenic trioxide (As2O3)-Induced deactivation of PI3K and MAPK cell signalling pathways in MCF-7 cells. Genes. 2019;10(1):41. doi: 10.3390/genes10010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kakouri E., Kanakis C., Trigas P., Tarantilis P.A. Characterization of the chemical composition of Drimia numidica plant parts using high-resolution mass spectrometry: study of their total phenolic content and antioxidant activity. Anal. Bioanal. Chem. 2019;411(14):3135–3150. doi: 10.1007/s00216-019-01781-7. [DOI] [PubMed] [Google Scholar]
- 57.Patil J.R., Jayaprakasha G.K., Murthy K.C., Chetti M.B., Patil B.S. Characterization of Citrus aurantifolia bioactive compounds and their inhibition of human pancreatic cancer cells through apoptosis. Microchem. J. 2010;94(2):108–117. [Google Scholar]
- 58.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016:5. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mamadalieva N.Z., Herrmann F., El‐Readi M.Z., Tahrani A., Hamoud R., Egamberdieva D.R., Azimova S.S., Wink M. Flavonoids in Scutellaria immaculata and S. ramosissima (Lamiaceae) and their biological activity. J. Pharm. Pharmacol. 2011;63(10):1346–1357. doi: 10.1111/j.2042-7158.2011.01336.x. [DOI] [PubMed] [Google Scholar]
- 60.Manivannan V., Johnson M. Total phenolic, tannin, triterpenoid, flavonoid and sterol contents, anti-diabetic, anti-inflammatory and cytotoxic activities of Tectaria paradoxa (Fee.) Sledge. Toxicol. Rep. 2020;7:1465–1468. doi: 10.1016/j.toxrep.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hasanzadeh D., Mahdavi M., Dehghan G., Charoudeh H.N. Farnesiferol C induces cell cycle arrest and apoptosis mediated by oxidative stress in MCF-7 cell line. Toxicol. Rep. 2017;4:420–426. doi: 10.1016/j.toxrep.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding X., Hou Y., Hou W. Structure feature and antitumor activity of a novel polysaccharide isolated from Lactarius deliciosus Gray. Carbohydr. Polym. 2012;89(2):397–402. doi: 10.1016/j.carbpol.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 63.Jia X., Zhang C., Qiu J., Wang L., Bao J., Wang K., Zhang Y., Chen M., Wan J., Su H., Han J. Purification, structural characterization and anticancer activity of the novel polysaccharides from Rhynchosia minima root. Carbohydr. Polym. 2015;132:67–71. doi: 10.1016/j.carbpol.2015.05.059. [DOI] [PubMed] [Google Scholar]
- 64.Suresh V., Senthilkumar N., Thangam R., Rajkumar M., Anbazhagan C., Rengasamy R., Gunasekaran P., Kannan S., Palani P. Separation, purification and preliminary characterization of sulfated polysaccharides from Sargassum plagiophyllum and its in vitro anticancer and antioxidant activity. Process. Biochem. 2013;48(2):364–373. [Google Scholar]
- 65.Yang W., Wang Y., Li X., Yu P. Purification and structural characterization of Chinese yam polysaccharide and its activities. Carbohydr. Polym. 2015;117:1021–1027. doi: 10.1016/j.carbpol.2014.09.082. [DOI] [PubMed] [Google Scholar]
- 66.Krichen F., Karoud W., Sila A., Abdelmalek B.E., Ghorbel R., Ellouz-Chaabouni S., Bougatef A. Extraction, characterization and antimicrobial activity of sulfated polysaccharides from fish skins. Int. J. Biol. Macromol. 2015;75:283–289. doi: 10.1016/j.ijbiomac.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 67.Ferreira S.B., Sodero A.C., Cardoso M.F., Lima E.S., Kaiser C.R., Silva F.P., Jr, Ferreira V.F. Synthesis, biological activity, and molecular modeling studies of 1 h-1, 2, 3-triazole derivatives of carbohydrates as α-glucosidases inhibitors. J. Med. Chem. 2010;53(6):2364–2375. doi: 10.1021/jm901265h. [DOI] [PubMed] [Google Scholar]
- 68.Lazareva E.B., Spiridonova T.G., Chernega E.N., Plesskaia L.G., Grunenkova I.V., Smirnov S.V., Men’shikov D.D. Topical pectins for the treatment of burn wounds. Antibiot. Chemother. 2002;47(9):9. [PubMed] [Google Scholar]
- 69.Bononi A., Agnoletto C., De Marchi E., Marchi S., Patergnani S., Bonora M., Giorgi C., Missiroli S., Poletti F., Rimessi A., Pinton P. Protein kinases and phosphatases in the control of cell fate. Enzyme Res. 2011 doi: 10.4061/2011/329098. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yadav P.B., Lekhak U.M., Ghane S.G., Lekhak M.M. Phytochemicals, antioxidants, estimation of cardiac glycoside (Scillaren A) and detection of major metabolites using LC-MS from Drimia species. South Afr. J. Bot. 2020 [Google Scholar]
- 71.Cai Y., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74(17):2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun J., Chu Y.F., Wu X., Liu R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002;50(25):7449–7454. doi: 10.1021/jf0207530. [DOI] [PubMed] [Google Scholar]
- 73.Mirahmadi S.F., Norouzi R. Chemical composition, phenolic content, free radical scavenging and antifungal activities of Achillea biebersteinii. Food Biosci. 2017;18:53–59. [Google Scholar]
- 74.Rodríguez-Pérez C., Quirantes-Piné R., Fernández-Gutiérrez A., Segura-Carretero A. Comparative characterization of phenolic and other polar compounds in Spanish melon cultivars by using high-performance liquid chromatography coupled to electrospray ionization quadrupole-time of flight mass spectrometry. Food Res. Int. 2013;54(2):1519–1527. [Google Scholar]
- 75.Kuo Y.H., Li Y.C. Three new compounds, ficusone, ficuspirolide, and ficusolide from the heartwood of Ficus microcarpa. Chem. Pharm. Bull. 1999;47(3):299–301. doi: 10.1248/cpb.48.1862. [DOI] [PubMed] [Google Scholar]
- 76.Danihelová M., Veverka M., Šturdík E., Jantová S. Antioxidant action and cytotoxicity on HeLa and NIH-3T3 cells of new quercetin derivatives. Interdiscip. Toxicol. 2013;6(4):209–216. doi: 10.2478/intox-2013-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rhimi W., Camarda A., Saidi M., Boulila A., Otranto D., Cafarchia C. Chemical characterization and acaricidal activity of Drimia maritima (L) bulbs and Dittrichia viscosa leaves against Dermanyssus gallinae. Vet. Parasitol. 2019;268:61–66. doi: 10.1016/j.vetpar.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 78.De Rosso M., Tonidandel L., Larcher R., Nicolini G., Dalla Vedova A., De Marchi F., Gardiman M., Giust M., Flamini R. Identification of new flavonols in hybrid grapes by combined liquid chromatography–mass spectrometry approaches. Food Chem. 2014;163:244–251. doi: 10.1016/j.foodchem.2014.04.110. [DOI] [PubMed] [Google Scholar]
- 79.Navarro M., Arnaez E., Moreira I., Quesada S., Azofeifa G., Wilhelm K., Vargas F., Chen P. Polyphenolic characterization, antioxidant, and cytotoxic activities of Mangifera indica cultivars from Costa rica. Foods. 2019;8(9):384. doi: 10.3390/foods8090384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gong J., Zhou S., Yang S. Vanillic acid suppresses HIF-1α expression via inhibition of mTOR/p70S6K/4E-BP1 and Raf/MEK/ERK pathways in human colon cancer HCT116 cells. Int. J. Mol. Sci. 2019;20(3):465. doi: 10.3390/ijms20030465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu S.B., Pang F., Wen Y., Zhang H.F., Zhao Z., Hu J.F. Antiproliferative and apoptotic activities of linear furocoumarins from Notopterygium incisum on cancer cell lines. Planta Med. 2010;76(01):82–85. doi: 10.1055/s-0029-1185971. [DOI] [PubMed] [Google Scholar]
- 82.Lee Y.H., Lee Y.R., Park C.S., Im S.A., Song S., Hong J.T., Whang B.Y., Kim K., Lee C.K. Baccatin III, a precursor for the semisynthesis of paclitaxel, inhibits the accumulation and suppressive activity of myeloid-derived suppressor cells in tumor-bearing mice. Int. Immunopharmacol. 2014;21(2):487–493. doi: 10.1016/j.intimp.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 83.Brito A., Areche C., Sepúlveda B., Kennelly E.J., Simirgiotis M.J. Anthocyanin characterization, total phenolic quantification and antioxidant features of some Chilean edible berry extracts. Molecules. 2014;19(8):10936–10955. doi: 10.3390/molecules190810936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sorrenti V., Vanella L., Acquaviva R., Cardile V., Giofrè S., Di Giacomo C. Cyanidin induces apoptosis and differentiation in prostate cancer cells. Int. J. Oncol. 2015;47(4):1303–1310. doi: 10.3892/ijo.2015.3130. [DOI] [PubMed] [Google Scholar]
- 85.Kelley D.S., Adkins Y.C., Zunino S.J., Woodhouse L.R., Bonnel E.L., Breksa A.P., III, Manners G.D., Mackey B.E. Citrus limonin glucoside supplementation decreased biomarkers of liver disease and inflammation in overweight human adults. J. Funct. Foods. 2015;12:271–281. [Google Scholar]
- 86.Jayaprakasha G.K., Jadegoud Y., Nagana Gowda G.A., Patil B.S. Bioactive compounds from sour orange inhibit colon cancer cell proliferation and induce cell cycle arrest. J. Agric. Food Chem. 2010;58(1):180–186. doi: 10.1021/jf9027816. [DOI] [PubMed] [Google Scholar]
- 87.Mandadi K.K., Jayaprakasha G.K., Bhat N.G., Patil B.S. Red Mexican grapefruit: a novel source for bioactive limonoids and their antioxidant activity. Zeitschrift für Naturforschung C. 2007;62(3-4):179–188. doi: 10.1515/znc-2007-3-405. [DOI] [PubMed] [Google Scholar]
- 88.Wang S.B., Tian S., Yang F., Yang H.G., Yang X.Y., Du G.H. Cardioprotective effect of salvianolic acid A on isoproterenol-induced myocardial infarction in rats. Eur. J. Pharmacol. 2009;615(1-3):125–132. doi: 10.1016/j.ejphar.2009.04.061. [DOI] [PubMed] [Google Scholar]
- 89.Muhammad H., Omar M.H., Isa M.L., Thani N.S.I.A., Rasid E.N.I., Awang N. Male reproductive toxicity studies of Orthosiphon stamineus aqueous extract in Sprague Dawley rats. J. Med. Plants. 2018;6(5):07–14. [Google Scholar]
- 90.Abu-Reidah I.M., Arráez-Román D., Segura-Carretero A., Fernández-Gutiérrez A. Profiling of phenolic and other polar constituents from hydro-methanolic extract of watermelon (Citrullus lanatus) by means of accurate-mass spectrometry (HPLC–ESI–QTOF–MS) Food Res. Int. 2013;51(1):354–362. doi: 10.1016/j.foodres.2016.10.018. [DOI] [Google Scholar]
- 91.Scazzocchio B., Varì R., Filesi C., D’Archivio M., Santangelo C., Giovannini C., Iacovelli A., Silecchia G., Volti G.L., Galvano F., Masella R. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARγ activity in human omental adipocytes. Diabetes. 2011;60(9):2234–2244. doi: 10.2337/db10-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu Q., Kroon P.A., Shao H., Needs P.W., Yang X. Differential effects of quercetin and two of its derivatives, isorhamnetin and isorhamnetin-3-glucuronide, in inhibiting the proliferation of human breast-cancer MCF-7 cells. J. Agric. Food Chem. 2018;66(27):7181–7189. doi: 10.1021/acs.jafc.8b02420. [DOI] [PubMed] [Google Scholar]
- 93.Knittel D.N., Stintzing F.C., Kammerer D.R. Simultaneous determination of bufadienolides and phenolic compounds in sea squill (Drimia maritima (L.) Stearn) by HPLC-DAD-MS n as a means to differentiate individual plant parts and developmental stages. Anal. Bioanal. Chem. 2014;406(24):6035–6050. doi: 10.1007/s00216-014-8008-0. [DOI] [PubMed] [Google Scholar]
- 94.Deve A.S., Kumaresan K., Rapheal V.S. Extraction process optimization of polyphenols from Indian Citrus sinensis–as novel antiglycative agents in the management of diabetes mellitus. J. Diabetes Metab. Disord. 2014;13(1):11. doi: 10.1186/2251-6581-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu Q., Zhang D.D., Wang L., Lou H., Ren D. Eriodictyol-7-O-glucoside, a novel Nrf2 activator, confers protection against cisplatin-induced toxicity. Food Chem. Toxicol. 2012;50(6):1927–1932. doi: 10.1016/j.fct.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 96.Areias F.M., Valentao P., Andrade P.B., Ferreres F., Seabra R.M. Phenolic fingerprint of peppermint leaves. Food Chem. 2001;73(3):307–311. [Google Scholar]
- 97.Li J., Hu L., Zhou T., Gong X., Jiang R., Li H., Kuang G., Wan J., Li H. Taxifolin inhibits breast cancer cells proliferation, migration and invasion by promoting mesenchymal to epithelial transition via β-catenin signaling. Life Sci. 2019;232:116617. doi: 10.1016/j.lfs.2019.116617. [DOI] [PubMed] [Google Scholar]
- 98.Wang R., Zhu X., Wang Q., Li X., Wang E., Zhao Q., Wang Q., Cao H. The anti-tumour effect of taxifolin on lung cancer via suppressing stemness and epithelial-mesenchymal transition in vitro and oncogenesis in nude mice. Ann. Transl. Med. 2020;8(9) doi: 10.21037/atm-20-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rajan V.K., Ragi C., Muraleedharan K. A computational exploration into the structure, antioxidant capacity, toxicity and drug-like activity of the anthocyanidin “Petunidin”. Heliyon. 2019;5(7):02115. doi: 10.1016/j.heliyon.2019.e02115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alcalde-Eon C., Saavedra G., de Pascual-Teresa S., Rivas-Gonzalo J.C. Liquid chromatography–mass spectrometry identification of anthocyanins of isla oca (Oxalis tuberosa, Mol.) tubers. J. Chromatogr. A. 2004;1054(1-2):211–215. doi: 10.1016/j.chroma.2004.08.074. [DOI] [PubMed] [Google Scholar]
- 101.Shirzad H., Niknam V., Taheri M., Ebrahimzadeh H. Ultrasound-assisted extraction process of phenolic antioxidants from Olive leaves: a nutraceutical study using RSM and LC–ESI–DAD–MS. J. Food Sci. Technol. 2017;54(8):2361–2371. doi: 10.1007/s13197-017-2676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pérez-Bonilla M., Salido S., van Beek T.A., de Waard P., Linares-Palomino P.J., Sánchez A., Altarejos J. Isolation of antioxidative secoiridoids from olive wood (Olea europaea L.) guided by on-line HPLC–DAD–radical scavenging detection. Food Chem. 2011;124(1):36–41. [Google Scholar]
- 103.Longo E., Morozova K., Scampicchio M. Effect of light irradiation on the antioxidant stability of oleuropein. Food Chem. 2017;237:91–97. doi: 10.1016/j.foodchem.2017.05.099. [DOI] [PubMed] [Google Scholar]
- 104.Hamdi H.K., Castellon R. Oleuropein, a non-toxic olive iridoid, is an anti-tumour agent and cytoskeleton disruptor. Biochem. Biophys. Res. Commun. 2005;334(3):769–778. doi: 10.1007/s13659-014-0003-9. [DOI] [PubMed] [Google Scholar]
- 105.Visioli F., Galli C., Galli G., Caruso D. Biological activities and metabolic fate of olive oil phenols. Eur. J. Lipid Sci. Technol. 2002;104(9‐10):677–684. [Google Scholar]
- 106.Bisignano G., Tomaino A., Cascio R.L., Crisafi G., Uccella N., Saija A. On the in‐vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 1999;51(8):971–974. doi: 10.1211/0022357991773258. [DOI] [PubMed] [Google Scholar]
- 107.Ernst E., Pittler M.H. Risks associated with herbal medicinal products. Wiener Med. Wochenschr. 2002;152(7‐8):183–189. doi: 10.1046/j.1563-258x.2002.01112.x. [DOI] [PubMed] [Google Scholar]
- 108.Lechanteur A., Almeida A., Sarmento B. Elucidation of the impact of cell culture conditions of Caco-2 cell monolayer on barrier integrity and intestinal permeability. Eur. J. Pharm. Biopharm. 2017 doi: 10.1016/j.ejpb.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 109.Klimenko O.V., Shtilman M. Reprogramming of CaCo2 colorectal cancer cells after using the complex of poly-(N-vinylpyrrolidone) with small non-coding RNAs. Toxicol. Rep. 2019;6:186–192. doi: 10.1016/j.toxrep.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.George S., Bhalerao S.V., Lidstone E.A., Ahmad I.S., Abbasi A., Cunningham B.T., Watkin K.L. Cytotoxicity screening of Bangladeshi medicinal plant extracts on pancreatic cancer cells. BMC Complement. Altern. Med. 2010;10(1):52. doi: 10.1186/1472-6882-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lau S.T., Lin Z.X., Liao Y., Zhao M., Cheng C.H., Leung P.S. Brucein D induces apoptosis in pancreatic adenocarcinoma cell line PANC-1 through the activation of p38-mitogen activated protein kinase. Cancer Lett. 2009;281(1):42–52. doi: 10.1016/j.canlet.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 112.Merghoub N., Benbacer L., Amzazi S., Morjani H., El-Mzibri M. Cytotoxic effect of some Moroccan medicinal plant extracts on human cervical cell lines. J. Med. Plants Res. 2009;3(12):1045–1050. [Google Scholar]
- 113.Nyambe M.N., Beukes D.R., Van De Venter M., Swanepoel B., Hlangothi B.G. Isolation and characterisation of altissimin: a novel cytotoxic flavonoid C-apioglucoside from Drimia altissima (Asparagaceae) Nat. Prod. Res. 2019:1–9. doi: 10.1080/14786419.2019.1596097. [DOI] [PubMed] [Google Scholar]
- 114.Sargent J.M. Chemosensitivity Testing in Oncology. Springer; Berlin, Heidelberg: 2003. The use of the MTT assay to study drug resistance in fresh tumour samples; pp. 13–25. [DOI] [PubMed] [Google Scholar]
- 115.Phillips R.M., Bibby M.C., Double J.A. A critical appraisal of the predictive value of in vitro chemosensitivity assays. JNCI: J. Natl. Cancer Inst. 1990;82(18):1457–1468. doi: 10.1093/jnci/82.18.1457. [DOI] [PubMed] [Google Scholar]
- 116.Carmichael J., Mitchell J.B., DeGraff W.G., Gamson J., Gazdar A.F., Johnson B.E., Glatstein E., Minna J.D. Chemosensitivity testing of human lung cancer cell lines using the MTT assay. Br. J. Cancer. 1988;57(6):540–547. doi: 10.1038/bjc.1988.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu K., Liu P.C., Liu R., Wu X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Monit. Basic Res. 2015;21:15. doi: 10.12659/MSMBR.893327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lowe S.W., Lin A.W. Apoptosis in cancer. Carcinogenesis. 2000;21(3):485–495. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 119.Ho K., Yazan L.S., Ismail N., Ismail M. Apoptosis and cell cycle arrest of human colorectal cancer cell line HT-29 induced by vanillin. Cancer Epidemiol. 2009;33(2):155–160. doi: 10.1016/j.canep.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 120.Hamzeloo-Moghadam M., Aghaei M., Abdolmohammadi M.H., Khalaj A., Fallahian F. Cytotoxic effect of Drimia maritima bulb extract and induction of mitochondrial apoptotic signaling in human breast cancer cells, MCF-7 and MDA-MB-468. Onco. Ther. 2018;11:7669–7677. doi: 10.2147/OTT.S182786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chakravarthi B.V., Sujay R., Kuriakose G.C., Karande A.A., Jayabaskaran C. Inhibition of cancer cell proliferation and apoptosis-inducing activity of fungal taxol and its precursor baccatin III purified from endophytic Fusarium solani. Cancer Cell Int. 2013;13(1):105. doi: 10.1186/1475-2867-13-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chidambara Murthy K.N., Jayaprakasha G.K., Kumar V., Rathore K.S., Patil B.S. Citrus limonin and its glucoside inhibit colon adenocarcinoma cell proliferation through apoptosis. J. Agric. Food Chem. 2011;59(6):2314–2323. doi: 10.1021/jf104498p. [DOI] [PubMed] [Google Scholar]
- 123.Hassan Z.K., Elamin M.H., Omer S.A., Daghestani M.H., Al-Olayan E.S., Elobeid M.A., Virk P. Oleuropein induces apoptosis via the p53 pathway in breast cancer cells. Asian Pac. J. Cancer Prev. 2013;14:6739–6742. doi: 10.7314/APJCP.2013.14.11.6739. [DOI] [PubMed] [Google Scholar]
- 124.Pegoraro L., Palumbo A., Erikson J., Falda M., Giovanazzo B., Emanuel B.S., Rovera G., Nowell P.C., Croce C.M. A 14; 18 and an 8; 14 chromosome translocation in a cell line derived from an acute B-cell leukemia. Proc. Natl. Acad. Sci. U. S. A. 1984;81(22):7166–7170. doi: 10.1073/pnas.81.22.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.He Z.Y., Shi C.B., Wen H., Li F.L., Wang B.L., Wang J. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest. 2011;29(3):208–213. doi: 10.3109/07357907.2010.550592. [DOI] [PubMed] [Google Scholar]
- 126.Van Erk M.J., Teuling E., Staal Y.C., Huybers S., Van Bladeren P.J., Aarts J.M., Van Ommen B. Time-and dose-dependent effects of curcumin on gene expression in human colon cancer cells. J. Carcinog. 2004;3:8. doi: 10.1186/1477-3163-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gamet-Payrastre L., Li P., Lumeau S., Cassar G., Dupont M.A., Chevolleau S., Gasc N., Tulliez J., Tercé F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60(5):1426–1433. [PubMed] [Google Scholar]
- 128.Goel A., Boland C.R. Epigenetics of colorectal cancer. Gastroenterology. 2012;143(6):1442–1460. doi: 10.1053/j.gastro.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Migliore L., Migheli F., Spisni R., Coppedè F. Genetics, cytogenetics, and epigenetics of colorectal cancer. J. Biomed. Biotechnol. 2011 doi: 10.1155/2011/792362. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gonzalez-Pons M., Cruz-Correa M. Colorectal cancer biomarkers: where are we now? Comput. Biomed. Res. 2015 doi: 10.1155/2015/149014. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Das J., Ghosh J., Manna P., Sil P.C. Taurine protects rat testes against doxorubicin-induced oxidative stress as well as p53, Fas and caspase 12-mediated apoptosis. Amino Acids. 2012;42(5):1839–1855. doi: 10.1007/s00726-011-0904-4. [DOI] [PubMed] [Google Scholar]
- 132.Ghosh S., Bhattacharyya S., Rashid K., Sil P.C. Curcumin protects rat liver from streptozotocin-induced diabetic pathophysiology by counteracting reactive oxygen species and inhibiting the activation of p53 and MAPKs mediated stress response pathways. Toxicol. Rep. 2015;2:365–376. doi: 10.1016/j.toxrep.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Robinson R.L., Sharma A., Bai S., Heneidi S., Lee T.J., Kodeboyina S.K., Patel N., Sharma S. Comparative STAT3-regulated gene expression profile in renal cell carcinoma subtypes. Front. Oncol. 2019;9:72. doi: 10.3389/fonc.2019.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heppler L.N., Frank D.A. Targeting oncogenic transcription factors: therapeutic implications of endogenous STAT inhibitors. Trends Cancer. 2017;3(12):816–827. doi: 10.1016/j.trecan.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Adamkova L., Souckova K., Kovarik J. Transcription protein STAT1: biology and relation to cancer. Folia Biol. 2007;53(1):1. [PubMed] [Google Scholar]
- 136.Rädler P.D., Wehde B.L., Wagner K.U. Crosstalk between STAT5 activation and PI3K/AKT functions in normal and transformed mammary epithelial cells. Mol. Cell. Endocrinol. 2017;451:31–39. doi: 10.1016/j.mce.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bibi S., Arslanhan M.D., Langenfeld F., Jeanningros S., Cerny-Reiterer S., Hadzijusufovic E., Tchertanov L., Moriggl R., Valent P., Arock M. Co-operating STAT5 and AKT signalling pathways in chronic myeloid leukemia and mastocytosis: possible new targets of therapy. Haematological. 2014;99(3):417. doi: 10.3324/haematol.2013.098442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.