Abstract
Coronavirus disease 2019 (COVID-19) is a pandemic viral disease affecting also obstetric patients and uncertainties exist about the prognostic role of inflammatory biomarkers and hemocytometry values in patients with this infection. To clarify that, we have assessed the values of several inflammatory biomarkers and hemocytometry variables in a cohort of obstetric patients hospitalized with COVID-19 and we have correlated the values at admission with the need of oxygen supplementation during the hospitalization. Overall, among 62 (27.3%) pregnant women and 165 (72.7%) postpartum women, 21 (9.2%) patients received oxygen supplementation and 2 (0.9%) required admission to intensive care unit but none died. During hospitalization leukocytes (p < 0.001), neutrophils (p < 0.001), neutrophils to lymphocytes ratio (p < 0.001) and C reactive protein (p < 0.001) decreased significantly, whereas lymphocytes (p < 0.001), platelets (p < 0.001) and ferritin (p = 0.001) increased. Lymphocyte values at admission were correlated with oxygen need, with a 26% higher risk of oxygen supplementation for each 1000 cells decreases. Overall, in obstetric patients hospitalized with COVID-19, C reactive protein is the inflammatory biomarker that better mirrors the course of the disease whereas D-dimer or ferritin are not reliable predictors of poor outcome. Care to the need of oxygen supplementation should be reserved to patients with reduced lymphocyte values at admission.
Subject terms: Predictive markers, Viral infection
Introduction
The pandemic of coronavirus disease 2019 (COVID-2019), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is affecting many women during pregnancy and in the postpartum worldwide. Even if the earliest data from China indicated that pregnant women infected by SARS-CoV-2 did not experience a worse outcome than non-pregnant women of the same age1, according to newest reports, pregnant women are more likely to deliver preterm, to have an increased risk of maternal death and of being admitted to the intensive care unit (ICU)2,3. In addition to its impact on the cardio-pulmonary physiology, pregnancy is characterized by several shifts in the woman immunologic profile. Particularly, in preparation of delivery, a pro-inflammatory state occurs with immune cells migrating into the myometrium and high levels of pro-inflammatory cytokines found both in the cervical tissue and in the peripheral blood4. Unfortunately, a peculiar characteristic of COVID-19 is the release of a large amount of inflammatory cytokines, a condition that in severe cases resembles the macrophages activation syndrome5. Some inflammatory biomarkers have been considered as tools to monitor the evolution of COVID-19, namely C reactive protein (CRP), lactate dehydrogenase (LDH), ferritin and D-dimer6–8. Also alterations of the leukocytes count, such as lymphocytopenia or an elevated neutrophils to lymphocytes ratio (NLR), seems correlated with disease severity9,10.
Unfortunately, the role of the above-mentioned markers has not been studied during pregnancy and in the postpartum in women infected by SARS-CoV-2. We do not know their evolution during the disease and whether their values can be adopted at admission to guide clinical choices. In this retrospective cohort of obstetric patients admitted with COVID-19 at our tertiary referral centre, we assessed the baseline values and the trend of inflammatory biomarkers and hemocytometry variables and their association with the severity of COVID-19.
Results
Demographic and clinical characteristics
We enrolled 227 women. At discharge, 62 patients (27.3%) were still pregnant, whereas 165 (72.7%) delivered during the hospitalization. The median gestational age at admission was 34 weeks and 3 days. Overall, no death occurred in our cohort, 21 (9.2%) patients required oxygen supplementation, and only 2 (0.9%) were admitted to the ICU. In both cases the reason was an acute pulmonary embolism leading to respiratory insufficiency in patients not receiving venous thromboembolism prophylaxis with enoxaparin. Pregnant women had higher mean body mass index (p < 0.05) and lower gestational age (p < 0.05) compared with those who delivered and reached the virologic cure in a longer period (p < 0.05) and reported more frequently fever, dyspnoea, cough and asthenia as symptoms (p < 0.05). Since March 30, 2020, all patient (200, 87.7%) started at admission prophylactic enoxaparin at the dosage of 100 IU/Kg daily. Immunomodulatory therapy with hydroxychloroquine 200 mg every 12 h was started until May 2020 in those with lung involvement at chest X-ray (20, 8.8%). Table 1 shows the clinical and demographic characteristics of the patients enrolled.
Table 1.
Demographic and clinical characteristics of obstetric patients with COVID-19.
| All (n = 227) | Postpartum women (n = 165) | Pregnant women (n = 62) | p | |
|---|---|---|---|---|
| Age (years) | 31.9 (6.1) | 32 (6.3) | 31 (5.8) | |
| Ethnicity | ||||
| Caucasic | 125 (54.8%) | 100 (60.6%) | 25 (40.3%) | < 0.05 |
| Black | 4 (1.8%) | 1 (0.6%) | 3 (4.8%) | < 0.05 |
| Hispanic | 50 (21.9%) | 31 (18.8%) | 19 (30.6%) | < 0.05 |
| Arab | 48 (21.1%) | 33 (20%) | 15 (24.2%) | ns |
| BMI | 24.6 (5.4) | 23.8 (5.7) | 28.1 (5) | < 0.05 |
| Gestational age at admission (weeks + days) | 34 + 3 | 37 + 2 | 27.4 + 5 | < 0.05 |
| Time since symptoms (days) | 4.7 (3.7) | 4.6 (3.84) | 5.4 (2) | ns |
| Hospitalization length (days) | 10.5 (8.9) | 10.9 (9.8) | 9.1(6.2) | ns |
| Time for clinical cure (days) | 6.4 (5.2) | 5.1 (3.7) | 8.3 (6.5) | < 0.05 |
| Time for virologic cure (days) | 22 (33.8) | 22.5 (42) | 22 (9.8) | ns |
| Symptoms | 102 (54.8%) | 50 (30.3%) | 52 (83.8%) | < 0.05 |
| Fever | 60 (26.3%) | 36 (21.8%) | 24 (38.7%) | < 0.05 |
| Dyspnoea | 28 (12.3%) | 9 (5.4%) | 19 (30.6%) | < 0.05 |
| Cough | 64 (28.1%) | 25 (15.1%) | 39 (62.9%) | < 0.05 |
| Coryza | 35 (15.4%) | 22 (13.3%) | 13 (21%) | ns |
| Asthenia | 22 (9.6%) | 5 (3%) | 17 (27.4%) | < 0.05 |
| Anosmia/ageusia | 13 (5.7%) | 7 (4.2%) | 6 (9.7%) | < 0.05 |
| Nausea/diarrhoea | 7 (3.1%) | 4 (2.42%) | 3 (4.8%) | ns |
| Pharyngitis | 22 (9.6%) | 13 (7.9%) | 9 (14.5%) | ns |
| Chest X-ray/Chest computed tomography | 88 (38.6%) | 42 (25.4%) | 46 (74.2%) | |
| Reduced transparency/Ground glass | 35 (15.4%) | 12 (7.3%) | 23 (37.1%) | < 0.05 |
| Interstitial thickening/Crazy paving | 45 (19.7%) | 19 (11.5%) | 26 (42%) | < 0.05 |
| Consolidation | 25 (11%) | 11 (6.6%) | 14 (22.6%) | ns |
| Therapy | ||||
| Lopinavir/Ritonavir | 1 (0.4%) | 0 | 1 (2.1%) | ns |
| Hydroxychloroquine | 20 (8.8%) | 13 (7.9%) | 7 (11.3%) | ns |
| LMWH | 200 (87.7%) | 151 (95.5%) | 49 (79%) | < 0.05 |
| Remdesivir | 1 (0.4%) | 0 | 1 (2.1%) | ns |
| Oxygen supplementation | 21 (9.2%) | 8 (4.8%) | 13 (21%) | < 0.05 |
Among pregnant women are included those patients who did not deliver during hospitalization, among postpartum women those who did. All data are expressed as mean with standard deviation. (BMI body mass index, LMWH low-molecular-weight heparin, CPAP continuous positive airways pressure, ICU intensive care unit).
Inflammatory biomarkers and white blood cells trend during hospitalization
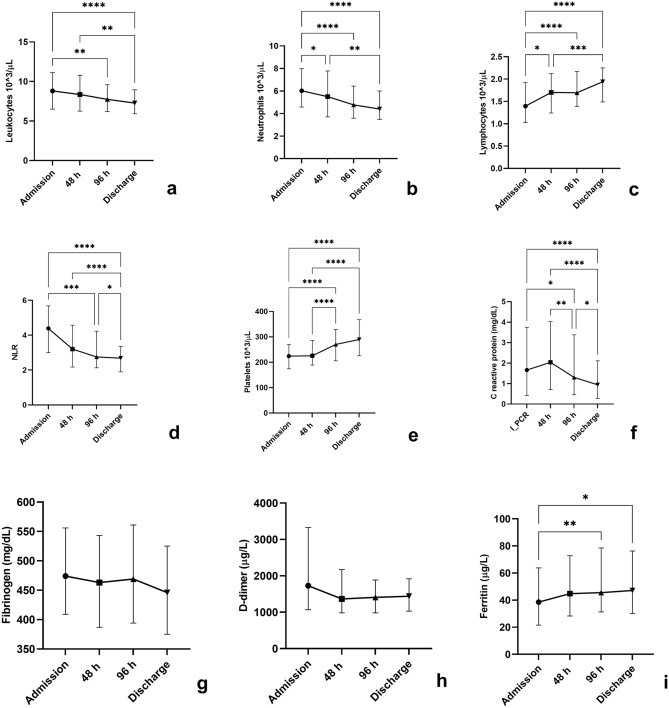
At admission, the median lymphocytes value was 1,395 cells/µL. The median values of NLR, C reactive protein, D-dimer, procalcitonin and fibrinogen were all above the upper reference limits (4.4, 1.66 mg/dL, 1,727 µg/L, 0.06 µg/L and 474 mg/dL, respectively). During the observation time leukocytes (p < 0.001), neutrophils (p < 0.001), NLR (p < 0.001) and CRP (p < 0.001) values decreased whereas lymphocytes (p < 0.001), platelets (p < 0.001) and ferritin (p = 0.001) increased. Table 2 summarizes inflammatory biomarkers and white blood cells trend during hospitalization. The results of multiple comparisons tests performed to assess differences among the same variable at different timepoints are shown in Fig. 1.
Table 2.
Values (median and interquartile range) of inflammatory biomarkers and hemocytometry variables at different study timepoints.
| Admission | 48 h | 96 h | Discharge | p | |
|---|---|---|---|---|---|
| Leukocytes (cells/mL) | 8810 (6510–11,120) | 8360 (6255–10,790) | 7740 (6190–9595) | 7270 (5920–8940) | < 0.001 |
| Neutrophils (cells/mL) | 6020 (4580–7990) | 5500 (3700–7780) | 4770 (3590–6440) | 4400 (3480–6000) | < 0.001 |
| Lymphocytes (cells/mL) | 1395 (1028–1923) | 1700 (1240–2125) | 1695 (1388–2170) | 1940 (1488–2250) | < 0.001 |
| Platelets (106 cells/mL) | 224 (174–270) | 225 (189–286) | 270 (205–328) | 289 (225–367) | < 0.001 |
| NLR | 4.4 (3–5.7) | 3.2 (2.2–4.6) | 2.7 (2.1–4.2) | 2.7 (1.9–3.3) | < 0.001 |
| Ferritin (µg/L) | 38.5 (21.5–64) | 44.8 (28.2–72.7) | 45.5 (31.2–78.5)) | 47.1 (30–76.2) | < 0.001 |
| C reactive protein (mg/dL) | 1.66 (0.41–3.75) | 2 (0.7–4) | 1.3 (0.4–3.4) | 0.9 (0.3–2.1) | < 0.001 |
| D-dimer (µg/L) | 1727 (1069–3328) | 1364 (980–2,171) | 1408 (982–1886) | 1439 (1029–1917) | 0.0248 |
| Procalcitonin (µg/L) | 0.06 (0.045–0.1) | 0.06 (0.04–0.08) | 0.05 (0.03–0.07) | 0.04 (0.03–0.06) | < 0.001 |
| Fibrinogen (mg/dL) | 474 (409–556) | 463 (387–543) | 469 (394–561) | 446 (375–525) | ns |
| LDH (U/L) | 186 (155–236) | 189 (141–245) | 203 (154–264) | 166 (138–214) | ns |
| AST (U/L) | 59 (41–107) | 55 (45–82) | 53 (42–72) | 45 (36–54) | 0.042 |
| ALT (U/L) | 17 (13–24) | 19 (13–29) | 24 (15–40) | 24 (15–40) | < 0.001 |
NLR neutrophils to lymphocytes ratio, LDH lactate dehydrogenase, AST aspartate aminotransferase, ALT alanine aminotransferase.
Figure 1.
Leukocytes (a), neutrophils (b), lymphocytes (c), neutrophils to lymphocytes (NLR) ratio (d), platelets (e), C reactive protein (f), fibrinogen (g), D-dimer (h) and ferritin (i) values assessed at admission, at 48 and 96 h from admission and at discharge. Data are shown as mean with standard deviation. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
Predictors of oxygen need
The independent effect of different variables at baseline on the likelihood of receiving oxygen supplementation was interrogated by a univariate logistic regression analysis and the results are reported in Table 3. The model proved to be significant only for lymphocytes and explained 94% (Nagelkerke R2) of the variance. Reduced lymphocytes value was associated with an increased likelihood of oxygen supplementation. A multiple logistic regression model was built, including lymphocytes, CRP and D-dimer. Lymphocytes value maintained its association with oxygen supplementation in the multivariate model.
Table 3.
Simple logistic regression and multiple logistic regression models for oxygen supplementation at baseline. In the multivariate logistic regression were included lymphocytes, CRP and D-dimer.
| Simple logistic regression | Multiple logistic regression | |||
|---|---|---|---|---|
| Odds ratio (95%CI) | p | Odds ratio (95%CI) | p | |
| Leukocytes | 0.94 (0.82–1.05) | ns | ||
| Neutrophils | 1.021 (0.88–1.15) | ns | ||
| Lymphocytes | 0.26 (0.10–0.7) | 0.005 | 4.5 (1.34–15.1) | 0.02 |
| NLR | 1.108 (0.977–1.246) | ns | ||
| CRP | 1.008 (0.958–1.036) | ns | 1 (0.5–1.02) | ns |
| PCT | 1.90 (0.024–19.20) | ns | ||
| Fibrinogen | 1.001 (0.996–1.005) | ns | ||
| d-Dimer | 1 (0.99–1) | ns | 1 (1–1) | ns |
| Haemoglobin | 0.77 (0.54–1.01) | ns | ||
| Ferritin | 1.003 (0.99–1.01) | ns | ||
NLR neutrophils to lymphocytes ratio, CRP C reactive protein, PCT procalcitonin, CI confidence interval.
Discussion
Our findings show how, among the variables assessed in our study, CRP was the inflammatory biomarker which varied more significantly during the course of COVID-19 in obstetric patients, supporting its employment as a tool to monitor the evolution of the disease. Among hemocytometry parameters, leukocytes, neutrophils, lymphocytes, platelets, and NLR are those with values which changed significantly. Considering their widespread availability in any hospitalized patient, they also should be considered in assessing disease progression. Interestingly, low lymphocytes value at admission was associated with a higher likelihood of receiving oxygen supplementation during hospitalization, suggesting that additional care should be reserved to patients presenting with reduced values of these blood cells.
Our findings are in agreement with the results of previous studies highlighting how COVID-19 patients are characterized by a relevant systemic inflammation, which acts as a driver of morbidity and mortality. Particularly, lymphocytopenia9, a high NLR11 and high values of CRP12 had all been linked with disease severity or mortality. Our work confirms that these variables show abnormal values also in obstetric patients and mirrors the course of the disease.
Intriguingly, D-dimer values, despite being well above the upper reference limit, were not associated with oxygen need and did not vary significantly during the hospitalization. A mounting amount of evidence is supporting the role of vascular endothelialitis and thrombosis in the pathogenesis of COVID-1913,14, and D-dimer values above 1000 µg/L have been associated with a poor prognosis15. In our cohort D-dimer values were above this limit in all the timepoints. It is possible that the administration of antithrombotic prophylaxis with enoxaparin in almost 90% of the patients have prevented some additional thromboembolic events. Moreover, it should be recognized that pregnancy is characterized by a physiologic increase of D-dimer values, especially in the third trimester16,17. However, in the specific setting of pregnant women, published data on the prognostic role of D-dimer are so far conflicting and further study are needed18,19.
To our surprise ferritin values were well above those observed in normal pregnancies20, which is characterized by a physiologic anaemic state and where high levels of ferritin, especially in the third trimester, are associated with negative outcomes (e.g. preterm delivery and gestational diabetes)21. At the same time, our values were well below those observed in several cohorts of non-pregnant COVID-19 patients22. For example, Chen and colleagues observed a median ferritin of 337 µg/L among their patients with moderate COVID-198. Therefore, it is possible to speculate that the inflammatory status caused by SARS-CoV-2 leads to an increase of serum ferritin also in obstetric patients, but this increase is partially concealed by the low levels of this molecule physiologically encountered in this population.
When compared to similar cohorts of obstetric patients with COVID-19, we have a striking similarity in terms of displayed symptoms. Fever, cough and dyspnoea were the three most frequently reported symptoms also in the large British UKOSS (UK Obstetric Surveillance System) cohort and in two Italian cohorts23–25. Interestingly, we have found that the severity of COVID-19 in pregnant women or in the postpartum was inferior to that previously reported in the literature. Indeed, we did not observe any death and admission to the ICU was required in only two patients. Di Toro and colleagues in their systematic review of the literature reported an admission rate to the ICU of 8%, with 3 stillbirths and 5 maternal deaths26.
Several differences emerged between the two groups of obstetric patients composing our cohort, those who did not deliver during hospitalization (pregnant women) and those who did (postpartum women). The first group displayed a more severe clinical pattern, with a longer time to clinical cure, a higher prevalence of symptoms, more radiographic findings, and a higher need of oxygen. Our study was not designed to investigate the causes of this difference, but it could be speculated that the presence of the foetus, compressing the other abdominal viscera and reducing the space for lung expansion, could have exacerbated symptoms such as dyspnoea and justify the higher need of oxygen in this group. This physical condition hardly explains the higher frequency of fever reported among pregnant women, which could be related to the different hormonal asset specific of this group. It is a well-known fact that during gestation pregnancy-associated hormones alter the immune response and disease pathogenesis, with other respiratory viral infection such as influenza occurring with enhanced severity in this population27. Unfortunately, quantification of hormones was not performed in our cohort, but these findings suggest that efforts should be devoted in understanding the impact of sexual hormones on COVID-19 severity.
We recognize that our work has some limitations. First, is a single centre study, and therefore the conclusions can apply only to the specific population afferent to the hospital in which the study was performed. Considering that our hospital was the referring centre for obstetric patients with COVID-19 of a large portion of Lombardy region, the most populous Italian region, and the large variation in ethnicities taken in care, we can suggest that this element did not impact the results. Second, it is a retrospective study, and some analysis (i.d. surveillance nasal swab) were performed for clinical/epidemiologic reasons and not to ascertain biologic differences.
Based on the results of our study, a minimum set of analyses composed of hemocytometry plus CRP should be performed in obstetric patients with COVID-19 at admission and during hospitalization, to assess disease severity and follow the evolution of the disease. Specific care to oxygen need should be reserved to those patients presenting with low values of leukocytes and lymphocytes at admission.
Methods
Study population
We enrolled a cohort of pregnant women consecutively admitted at the COVID-19 Maternity Hub at the Foundation IRCCS Ca' Granda Ospedale Maggiore Policlinico in Milan, Italy with a diagnosis of COVID-19 in the period from March 10, 2020 to April 24, 2020. Clinical and demographic data were extracted from the electronic medical records.
The diagnosis of COVID-19 was established by the detection of SARS-CoV-2 in a nasopharyngeal swab. The nasopharyngeal swab was performed either in all pregnant women admitted for obstetric indication or in women with at least one of the following signs or symptoms: fever, cough, dyspnoea, asthenia, myalgia, coryza, sore throat, headache, ageusia or dysgeusia, anosmia or parosmia, ocular symptoms, diarrhoea, nausea, and vomit. All SARS-CoV-2 positive cases were admitted in a dedicated COVID-19 ward. Inflammatory biomarkers and hemocytometry variables were assessed at admission, at 48 h and 96 h after admission and at discharge. The execution of chest X-ray or chest computed tomography (CT) was reserved to patients with respiratory symptoms, according to clinical judgment. The patients enrolled were subdivided in two groups: women who delivered during hospitalization or women who did not.
Clinical cure was defined as the absence of fever, a respiratory rate < 22 breaths per minutes and peripheral haemoglobin saturation values > 95% for at least three days. All patients repeated two nasopharyngeal swabs at an interval of 24 h, 14 days after discharge or 14 days after clinical cure if still hospitalized for other reasons until October 2020. If both the swabs resulted negative the patient was considered cured (virologic cure) of the infection and not requiring anymore isolation measures anymore. Since October 2020 only one nasopharyngeal swab to assess virologic cure was performed, 10 days after the first positive nasopharyngeal swab or 10 days after symptoms onset.
Pro-inflammatory biomarkers assessment and white blood cell profile
Procalcitonin (PCT) and ferritin were measured with electrochemiluminescent immunoassays (ECLIA) on a Roche Cobas e801 instrument (Roche Diagnostics, Monza, Italy). C-reactive protein (CRP) was measured with an immunoturbidimetric method and lactate dehydrogenase (LDH), alanine and aspartate aminotransferase (ALT, AST) with the International Federation of Clinical Chemistry (IFCC) optimized methods on a Roche Cobas c702 instrument (Roche Diagnostics, Monza, Italy). Fibrinogen and D-dimer were measured with ACL Top (Werfen, Milano, Italy). Hemocytometry analysis was performed with Sysmex XN 9000 (Dasit, Cornaredo, Italy).
SARS-CoV-2 detection
Two different methods were used for viral detection. The first one consisted in Seegene Inc reagents (Seoul, Korea), RNA extraction with STARMag Universal Universal Cartridge kit on Nimbus instrument (Hamilton, Agrate Brianza, Italy) and amplification with Allplex 2019-nCoV assay, while the second employed a GeneFinder COVID-19 Plus RealAmp Kit (OSANG Healthcare, Anyangcheondong-ro, Dongan-gu, Anyang-si, Gyeonggi-do, Korea) on ELITech InGenius instrument (Torino, Italy). Both assays identify the virus by multiplex rRT-PCR targeting three viral genes (E, RdRP and N).
Statistical analysis and ethics
Descriptive statistics were obtained for all the variables reported for this study. Groups were compared with unpaired t test or Mann–Whitney test and Fisher's exact test. The trend of different variables during hospitalization was assessed with Friedmann test and Dunn's multiple comparisons test. Univariate logistic regression models were employed to assess correlation between variables at baseline and oxygen supplementation. Multivariate logistic regression models were then built including significant variables at univariate analysis and variables with a biologic correlate. A p value < 0.05 was deemed statistically significant. All the analysis was performed with SPSS Statistics 23 (IBM Corp, USA). The study was approved by the Comitato Etico Milano Area 2 (#339_2020) and conducted according to the Declaration of Helsinki. An informed consent was obtained from all the patients enrolled.
Acknowledgements
The authors are thankful to Valeria Castelli for the help in data collection.
Author contributions
A.L., E.F., A.B. and A.G. designed the study. S.D. and L.L.P. collected the data. A.L. performed the analysis and wrote the first draft of the manuscript. A.L. and A.C. wrote the second draft of the manuscript. All the authors revised the final version of the manuscript.
Data availability
All data will be available on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/28/2021
The original online version of this Article was revised: In the original version of this Article the author name Letizia Li Piani was incorrectly indexed. The original Article has been corrected.
References
- 1.Chen H, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allotey J, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ. 2020;370:3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zambrano LD, et al. Update: Characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 Infection by pregnancy status—United States, January 22–October 3, 2020. Morb. Mortal. Wkly. Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bränn E, Edvinsson Å, Rostedt Punga A, Sundström-Poromaa I, Skalkidou A. Inflammatory and anti-inflammatory markers in plasma: From late pregnancy to early postpartum. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-018-38304-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulert GS, Grom AA. Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Annu. Rev. Med. 2015;66:145–159. doi: 10.1146/annurev-med-061813-012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G, et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020 doi: 10.1172/jci137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lombardi A, et al. Early phases of COVID-19 are characterized by a reduction in lymphocyte populations and the presence of atypical monocytes. Front. Immunol. 2020;11:1–9. doi: 10.3389/fimmu.2020.560330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J. Transl. Med. 2020;18:1–12. doi: 10.1186/s12967-019-02189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Z, et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect. Dis. 2020;95:332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinelli I, et al. Pulmonary embolism in a young pregnant woman with Covid-19. Thromb. Res. 2020 doi: 10.1016/j.jns.2019.116544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GutiérrezGarcía I, et al. D-dimer during pregnancy: Establishing trimester-specific reference intervals. Scand. J. Clin. Lab. Invest. 2018;78:439–442. doi: 10.1080/00365513.2018.1488177. [DOI] [PubMed] [Google Scholar]
- 17.Murphy N, et al. Gestation-specific D-dimer reference ranges: A cross-sectional study. BJOG Int. J. Obstet. Gynaecol. 2015;122:395–400. doi: 10.1111/1471-0528.12855. [DOI] [PubMed] [Google Scholar]
- 18.van der Pol LM, et al. Pregnancy-adapted YEARS algorithm for diagnosis of suspected pulmonary embolism. N. Engl. J. Med. 2019;380:1139–1149. doi: 10.1056/NEJMoa1813865. [DOI] [PubMed] [Google Scholar]
- 19.Goodacre S, et al. The DiPEP study: An observational study of the diagnostic accuracy of clinical assessment, D-dimer and chest X-ray for suspected pulmonary embolism in pregnancy and postpartum. BJOG An Int. J. Obstet. Gynaecol. 2019;126:383–392. doi: 10.1111/1471-0528.15286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daru J, Allotey J, Peña-Rosas JP, Khan KS. Serum ferritin thresholds for the diagnosis of iron deficiency in pregnancy: A systematic review. Transfus. Med. 2017;27:167–174. doi: 10.1111/tme.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scholl TO. Iron status during pregnancy: Setting the stage for mother and infant. Am. J. Clin. Nutr. 2005;81:1218–1222. doi: 10.1093/ajcn/81.5.1218. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Hu Y, Yu J, Ma T. Retrospective analysis of laboratory testing in 54 patients with severe- or critical-type 2019 novel coronavirus pneumonia. Lab. Investig. 2020 doi: 10.1038/s41374-020-0431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savasi VM, et al. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID-19) Obstet. Gynecol. 2020;1:1–7. doi: 10.1097/AOG.0000000000003979. [DOI] [PubMed] [Google Scholar]
- 24.Ferrazzi E, et al. Vaginal delivery in SARS-CoV-2 infected pregnant women in Northern Italy: A retrospective analysis. BJOG. 2020;24:1–6. doi: 10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knight M, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: National population based cohort study. BMJ. 2020;369:1–10. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Toro F, et al. Impact of COVID-19 on maternal and neonatal outcomes: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021;27:36–46. doi: 10.1016/j.cmi.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 2012;62:263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will be available on request.