Abstract
Elevated plasma vitamin B12 has been associated with solid cancers, based on a single B12 measurement. We evaluated the incidence of solid cancers following B12 measurement in patients with persistent elevated B12, compared to patients without elevated B12 and to patients with non-persistent elevated B12. The study population included patients with at least two plasma B12 measurements without already known elevated-B12-related causes. Patients with elevated plasma B12 (≥ 1000 ng/L) at first measurement (n = 344) were matched for age and sex with patients having 2 normal B12 measurements (< 1000 ng/L) (NN group, n = 344). The patients with elevated plasma B12 at first measurement were split into 2 groups, according to the presence (EE group, n = 144) or the absence (EN group, n = 200) of persistent elevated plasma B12 at second measurement. We compared the cancer-free survival during 60 months between the groups after adjustment for the other elevated-B12-related causes in a survival competing risk model. Compared to the NN group, a persistent elevated plasma B12 ≥ 1000 ng/mL was strongly associated with the occurrence of solid cancer (HR 5.90 [95% CI 2.79–12.45], p < 0.001), contrary to non-persistent plasma B12 elevation (p = 0.29). These results could help to select patients in whom the screening for solid cancers would be of interest.
Subject terms: Biomarkers, Cancer, Metabolic disorders
Introduction
The plasma vitamin B12 (B12) measurement is mainly conducted to detect vitamin B12 deficiency, but the incidental finding of elevated B12 is not uncommon1,2. An elevated B12 is generally defined as a level higher than the upper limit of the normal range, around 1000 ± 100 ng/L (738 ± 73.8 pmol/L)2–4. Elevated B12 has been associated with various diseases1,5,6: liver diseases7–10, myeloid blood malignancies10–13, chronic renal failure3,14, autoimmune or inflammatory diseases3, Gaucher disease15. An association between elevated B12 and solid cancers has been demonstrated by two population-based cohort studies16,17 and persists after adjustment for the other elevated-B12-related causes1.
No consensus exists concerning a diagnostic strategy in case of incidental finding of elevated B125,18,19. Some causes can be explored with simple investigations (blood count, renal function evaluation, liver cytolysis and cholestasis parameters, and liver echography) but solid cancer exploration often requires imaging or endoscopic examinations. The risk of solid cancer seems insufficient to systematically perform these invasive and expansive investigations in case of incidental discovery of elevated B12. Patients in whom active screening should be discussed need to be better targeted to allow early diagnosis and limit unnecessary investigations.
In studies evaluating the association between solid cancer and elevated B12, a single measurement was sufficient to define an elevated B12 level1,16,17. In our own daily practice, we observed spontaneous normalizations of elevated B12 level after the resolution of acute disorders (severe infections, acute inflammatory state due to immune or inflammatory diseases). We therefore hypothesized that acute conditions could have temporarily raised the B12 level. On the contrary, elevated B12 levels encountered in some cancers could be correlated with the tumor mass or the granulocytic immune response5,6,20. Consequently, if elevated B12 levels are caused by some cancers, elevated B12 should persist as long as the cancer persists.
The objective of this study was to evaluate the proportion of incident solid cancers in patients with persistent elevated B12 level, compared to patients without elevated B12 and to patients with non-persistent elevated B12.
Patients and methods
Ethics and statement for study checklist
This study was approved by the bioethical committee of Angers University Hospital (n°2019/105) and has been conducted in accordance with the Declaration of Helsinki. Patients gave informed consent. We applied the strengthening the reporting of observational studies in epidemiology (STROBE) statement to observational studies.
Study population
This study included patients aged 18 years and over, admitted to Angers University Hospital between January 2007 and May 2015. Patients were required to have undergone two B12 measurements at 2 different times (T1 and T2) at 1 to 48 months intervals. Assays were initially performed to search for B12 deficiencies. Assays realized in intensive care and maternity units were excluded because of the metabolic changes observed in these patients21–23.
Patients with identified elevated-B12-related causes including a solid cancer before/at T1 were excluded. The elevated-B12-related causes were: acute liver disease (acute elevation of transaminases to more than 2 times normal) or chronic liver disease (dysmorphic ultrasound appearance, persistent biological signs of hepatocellular insufficiency, and/or histology suggestive of cirrhosis), severe chronic renal failure (MDRD clearance ≤ 30 mL/min/1.73 m2), autoimmune or inflammatory disease, Gaucher disease, myeloid blood malignancy, active solid cancer, and cancer treated over the last 5 years1,2,6. Patients with pernicious anemia or those supplemented with vitamin B12 were also excluded. In the case of solid cancer diagnosed between T1 and T2, patients were excluded if the T2 assay was performed at more than 1 month after cancer treatment initiation.
Plasma vitamin B12 assay
The B12 measurements were centralized in the biochemistry laboratory of Angers University Hospital. The tests were carried out on an immunoanalytical system ADVIA Centaur (SIEMENS HEALTHCARE DIAGNOSTICS Inc. Tarrytown, NY, USA) with ADVIA Centaur VB12 reagents.
An elevated B12 level was defined as ≥ 1000 ng/L1,4,24–26.
In patients with three or more B12 measurements and at least one B12 ≥ 1000 ng/L, T1 was selected as the first test with B12 ≥ 1000 ng/L. In the absence of B12 ≥ 1000 ng/L, T1 was randomly selected between the first and the penultimate B12 measurement. T2 was the test immediately following T1 with at least 1 month between T1 and T2. As the study aimed at comparing persistent and non-persistent elevated B12, patients who had only the last measurement ≥ 1000 ng/L were excluded.
Groups’ constitution
Patients with B12 ≥ 1000 ng/L at T1 were selected and matched with a control group. Control patients were randomly selected among those with B12 < 1000 ng/L at T1 and T2, with a ratio 1:1, matching for sex, age and the number of B12 measurements (2, 3, ≥ 4) during the study period. These control patients defined the NN (normal/normal) group. Then, the patients with B12 ≥ 1000 ng/L at T1 were split into 2 groups, those having B12 ≥ 1000 ng/L at T2 (EE group) and those having B12 < 1000 ng/L at T2 (EN group). Thus, 3 groups of patients were defined according to B12 status: EE (elevated/elevated), EN (elevated/normal) and NN. As explained above, patients in the EE or EN groups could have had previous normal B12 measurements before the first elevated one (T1).
Collected data
All patient records have been fully reviewed.
The following general data were collected: sex, age, B12 levels, dates of B12 measurement, and death date. We collected the incident elevated-B12-related causes, including solid cancers, which appeared within 60 months following T1, and their date of occurrence.
Solid cancers were defined as non-hematological malignancies. The site of primary cancer (according to the International Classification of Diseases for Oncology classification), presence of metastasis and site of metastasis were collected.
Statistical methods
The quantitative data were presented in medians and quartiles and compared using a Student t test or an ANOVA. The categorical data were presented as absolute values and as percentages and were compared using Chi-squared test.
Time-to-event curves for incident solid cancer were presented as Kaplan–Meier curves and were compared with a log-rank test. Follow-up was limited at 60 months. Loss of follow-up was censored. The influence of covariate (including age, sex, and all the elevated-B12-related causes) on the occurrence of solid cancer was evaluated with a survival competing risk model (package cmprsk from R) with the death as the competing risk. The proportional hazard assumption was checked with 2 different methods: graphically by plotting the log(minuslog) curves and by studying the interaction with time. The alpha risk was 5%. The hazard ratios (HR) were presented with a confidence interval of 95%. The analyses were carried out using GRAPHPAD Prism v6.01 (GRAPHPAD SOFTWARE, La Jolla, CA, USA) and R software (version 3.5.1, R-project.org, Vienna, Austria).
Results
Description of the population
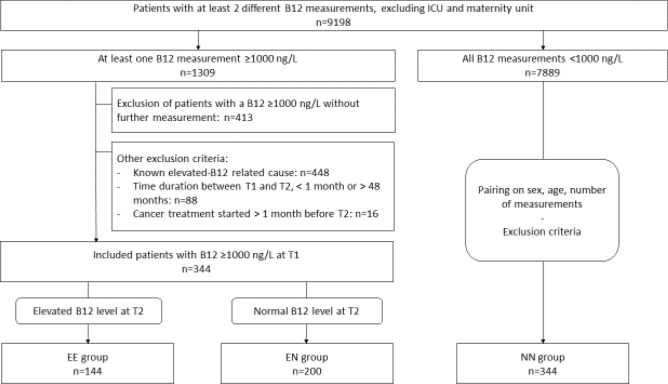
Between January 2007 and May 2015, 9,198 patients underwent at least 2 measurements of B12 in our center, excluding the intensive care and maternity units. Among these patients, plasma B12 ≥ 1000 ng/L at T1 were found in 344 patients without any known elevated-B12-related causes. Among these 344, 144 (41.9%) patients had a B12 ≥ 1000 ng/L at T2 (EE group) and 200 (58.1%) patients had a B12 < 1000 ng/L at T2 (EN group). The 344 patients in the NN group were randomly selected from the 7,889 patients with plasma B12 < 1000 ng/L at T1 and T2. The flowchart is detailed in Fig. 1.
Figure 1.
Flowchart.
The study population included 688 patients with a median age of 79 [64–86] years. There were 256/688 men (37.2%), and the median time from T1 to the last hospitalization or consultation was 3.2 [1.5–5.2] years.
The age of the patients was similar in the 3 groups (Table 1). The proportion of men was higher in the EE group, but with no significant difference (p = 0.06). B12 at T1 was higher in the EE group than in the EN group (p < 0.001). The death rate was higher in the EE group than in the other groups (p < 0.001).
Table 1.
Comparison of the 3 groups.
| EE group (n = 144) | EN group (n = 200) | NN group (n = 344) | p-value | |
|---|---|---|---|---|
| Age (at T1) | 78 [68–87] | 80 [60–86] | 79 [64–86] | 0.74 |
| Sex (men) | 64 (44.4%) | 64 (32.0%) | 128 (37.2%) | 0.06 |
| Number of realized measurements | ||||
| 2 measurements | 86 (59.8%) | 109 (54.5%) | 195 (56.7%) | 0.85 |
| 3 measurements | 29 (20.1%) | 49 (24.5%) | 74 (21.5%) | |
| ≥ 4 measurements | 29 (20.1%) | 42 (21.0%) | 75 (21.8%) | |
| Interval T1-T2 (days) | 252 [81–504] | 272 [92–523] | 250 [119–496] | 0.82 |
| Plasma vitamin B12 at T1 (ng/L) | 1521 [1206–2000] | 1177 [1063–1414] | 364 [287–509] | < 0.001 |
| Plasma vitamin B12 at T2 (ng/L) | 1544 [1226–2000] | 613 [427–804] | 370 [281–495] | < 0.001 |
| Time between T1 and last admission (years) | 2.7 [0.7–4.7] | 3.1 [1.5–5.3] | 3.6 [1.8–5.3] | 0.01 |
| Death | 47 (32.6%) | 32 (16.0%) | 44 (12.8%) | < 0.001 |
| Diseases diagnosed during the follow-up period | ||||
| At least 1 elevated-B12-related cause | 75 (52.1%) | 34 (17.0%) | 38 (11.0%) | < 0.001 |
| Chronic liver disease | 32 (22.2%) | 11 (5.5%) | 5 (1.5%) | < 0.001 |
| Severe renal failurea | 0 (0%) | 2 (1.0%) | 0 (0%) | 0.09 |
| Autoimmune/inflammatory disease | 3 (2.1%) | 1 (0.5%) | 4 (1.2%) | 0.43 |
| Myeloid blood malignancy | 25 (17.4%) | 6 (3.0%) | 13 (3.8%) | < 0.001 |
| Solid cancer | 30 (20.8%) | 12 (6.0%) | 14 (4.1%) | < 0.001 |
| Without metastasis | 14 (9.7%) | 7 (3.5%) | 9 (2.6%) | 0.002 |
| With metastasis | 16 (11.1%) | 5 (2.5%) | 5 (1.5%) | < 0.001 |
| Lymphoid blood malignancy | 2 (1.4%) | 2 (1.0%) | 3 (0.9%) | 0.87 |
The 3 groups were compared by ANOVA or Chi-squared test, as appropriate.
aCreatinine clearance with MDRD formula ≤ 30 mL/min/1.73 m2.
Incident causes of elevated B12
At least one cause of elevated B12 was diagnosed in the follow-up of 75/144 (52.1%) patients in the EE group, 34/200 (17.0%) in the EN group, and 38/448 (11.0%) in the NN group (p < 0.001, Table 1).
Among the causes of elevated B12, incident myeloid blood malignancies and chronic liver diseases were more frequent in the EE group (p < 0.001 and p < 0.001, respectively). The frequency of myeloid blood malignancy did not differ between the EN and NN groups, and chronic liver disease was more common in the EN group than in the NN group.
Incident solid cancer was diagnosed in 30/144 (20.8%) patients in the EE group, 12/200 (6.0%) in the EN group and 13/344 (3.8%) in the NN group (p < 0.001). Compared with the reference NN group, the EE group was associated with the occurrence of solid cancers with or without metastases (p < 0.001 and p < 0.001, respectively), contrary to the EN group (p = 0.56 and p = 0.38, respectively). Solid cancers and metastases sites are presented in Table S1, supplemental material.
Solid cancer-free survival adjusted for age, sex, and other elevated-B12-related causes
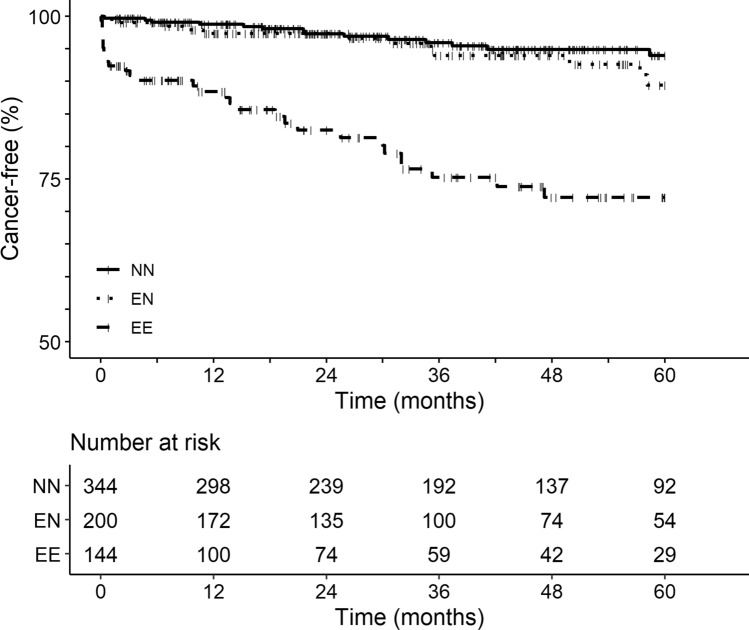
The cancer-free survival significantly differed between the 3 groups (p < 0.001, Fig. 2). The difference was linked to a more frequent occurrence of incident solid cancer in the EE group (p < 0.001 compared with EN and NN groups), as the EN and NN groups did not differ (p = 0.28).
Figure 2.
Cancer-free survival in the 3 groups. Events only referred to solid cancers and not to blood malignancies. Loss of follow-up was censored. The influence of covariate (including age, sex, and all the elevated-B12-related causes) on the occurrence of solid cancer was evaluated with a survival competing risk model (package cmprsk from R) with the death as the competing risk.
Compared with the NN group as a reference, only the EE group was significantly associated with incident cancer with HR 5.90 [95% CI 2.79–12.45] (p < 0.001), contrary to the EN group (HR 1.52 [95% CI 0.70–3.30], p = 0.29) (Table 2).
Table 2.
Strength of association between incident solid cancers within 60 months and the 3 groups in an adjusted competing risk survival model with the death as a competing risk.
| Hazard ratio (95%CI) | p-value | |
|---|---|---|
| Group | ||
| NN | Reference | – |
| EN | 1.52 (0.70–3.30) | 0.29 |
| EE | 5.90 (2.79–12.45) | < 0.001 |
| Agea | 1.01 (0.23–1.93) | 0.98 |
| Sex (men) | 1.27 (0.71–2.26) | 0.42 |
| Myeloid blood malignancies | 0.28 (0.07–1.16) | 0.08 |
| Lymphoid blood malignancies | 2.69 (0.53–13.53) | 0.23 |
| Chronic liver diseases | 1.39 (0.57–3.40) | 0.46 |
| Severe chronic kidney failure | 0.41 (0.05–3.53) | 0.41 |
| Autoimmune/inflammatory diseases | 1.14 (0.17–7.56) | 0.89 |
aAge as dichotomous variable with the median age as a threshold (< or ≥ 79 years).
Discussion
In the case of incidental finding of elevated B12, the relevance of an active search for solid cancer necessitating invasive and expensive examinations remains debated5,18,19. Indeed, few studies analyzed the incidence of solid cancers following the incidental finding of elevated B12 and no studies linked elevated B12 to solid cancer after adjusting for other causes of elevated B12. Our study aimed at evaluating the incidence of solid cancers in patients with persistent elevated B12, in comparison with patients without elevated B12 and those with non-persistent elevated B12.
The association between elevated B12 and solid cancers was demonstrated by two population-based studies: a B12 > 800 pmol/L (1084 ng/L) was associated with a diagnosis of cancer in the following year with a Standardized Incidence Ratio of 6.3 [95% CI 5.7–6.9] in a Danish cohort16, and a B12 between 800 and 1000 pmol/L (1084–1355 ng/L) was associated with an Incidence Rate Ratio of 2.9 [95% CI 2.4–3.5] in a British cohort17. However, to our knowledge, no study has evaluated this association according to the persistence of this B12 elevation. In our study, an increased risk of cancer was associated with patients having a persistent elevated B12 (group EE) but not with those showing transient elevated B12 (group EN). For the first time, we demonstrate that the risk of incident solid cancer in the case of transiently elevated B12 is similar to that of patients without elevated B12, whereas this risk is strongly increased in the case of persistent elevated B12.
The incidence of solid cancers in the EE group argues for checking the persistence of B12 elevation to justify investigations in case of incidental finding of B12 elevation. The occurrence of solid cancers in 20.8% of patients in the EE group could justify careful clinical surveillance and raises the question of carrying out complementary investigations. In our study, a significant number of solid cancers were diagnosed more than one year after T1 (51.5% in the EE group). This raises the question of the potential interest in repeating investigations 1 year later.
This is interesting for also exploring the other causes related to elevated B12. Indeed, the frequency of incident diagnoses of chronic liver diseases (32/144, 22.2%) and myeloid blood malignancies (25/144, 17.4%) in the EE group also confirmed the interest in screening these pathologies in case of persistent elevated B125,18,19.
Some authors suggested that the development of solid cancers could be secondary to the elevated B1227,28. Indeed, vitamin B12 is the cofactor of methionine synthase, which is implicated in methylation reaction and in the synthesis of purine bases29,30, and these functions are crucial in tumor-initiating cells and cell proliferation30,31. On the contrary, we think that certain cancers would be, directly or indirectly, responsible for B12 elevation. Moreover, Arendt et al. showed that the SIR of cancers was higher within the year following B12 measurement than in subsequent years16. This supports the presence of undiagnosed subclinical cancer rather than a hypothetical role of the elevated B12 in the development of cancer.
In this study, we limited the analyses to cancers occurring within 60 months following B12 measurement, because it appeared to be difficult to establish the causal link between a subclinical solid cancer and an elevated plasma B12 beyond a period of 5 years32–35.
The mechanism of elevated B12 in case of solid cancer is poorly understood5,6,20. The prognostic nature of elevated B12 in solid cancers suggested the question of a possible link with the tumor mass or the capacity for proliferation20. The first hypothesis consists in the secretion of a tumor mediator increasing the bioavailability of vitamin B12, promoting the synthesis of nucleic acids by cancer cells. The second hypothesis is that of the release of haptocorrins by the granulocytic cells involved in the anti-tumor response.
Our study has some limitations. The incidence of elevated-B12-related causes could be underestimated due to the retrospective and non-interventional nature of the study. However, this would identically impact the 3 groups. We cannot exclude that the elevated B12 level may have prompted some physicians to look for underlying cancer in patients of the EE and EN groups, but this limitation is imbalanced by the prolonged follow-up (3.2 [1.5–5.2] years in the whole population, with a longer follow-up in the NN group), which could have enabled detection of cancers that have not been actively searched initially. We are not able to exclude loss of information due to the fact that follow-up was carried out in several centers. Nevertheless, the bulk of the study data came from the university hospital, which is the main healthcare facility in the region, so the risk of information loss appears to be reduced and is not biased between the groups. We set the period of at least 1 month between the 2 measurements to allow normalization of B12 in case of acute transient elevation. However, the study methodology did not allow for determination of the most suitable time to perform this control. The higher proportion of men in the EE group could have biased the risk of incident cancer, since men are more exposed to environmental risk factors36. To avoid this, we included the sex of patients as a variable of adjustment in the survival model and, finally, sex did not significantly influence the occurrence of cancer at 60 months. We also observed a higher mortality in the EE group, which could possibly bias the incidence of solid cancers. We anticipated this bias using a survival competing risk model with the death as the competing risk. Patients in our study were hospitalized, so the results need to be confirmed in an outpatient population. Our results are support looking for the cause of an incidental finding of elevated B12, however we cannot propose B12 measurement as a screening marker for solid cancer at this stage.
Conclusion
The persistence of elevated B12 was associated with a high incidence of solid cancer at 60 months, in contrast to a transient B12 elevation. Solid cancers represent one of the main diagnoses found in patients with unexplained and persistent elevated B12. An unexplained elevated B12 level should be confirmed later by a second measurement, which could help to identify patients in whom the screening for solid cancers would be of interest.
Supplementary Information
Author contributions
V.L. and G.U. contributed to conception and design, to acquisition, analysis and interpretation of data, drafted the manuscript and critically revised the manuscript for important intellectual content. F.C. contributed to analysis and interpretation of data and critically revised the manuscript for important intellectual content. C.L., A.G., O.C., A.P. and C.L. contributed to interpretation of data and critically revised the manuscript for important intellectual content.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-92945-y.
References
- 1.Urbanski G, et al. Strength of the association of elevated vitamin B12 and solid cancers: An adjusted case-control study. J. Clin. Med. 2020;9:474. doi: 10.3390/jcm9020474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andres E, Serraj K, Zhu J, Vermorken AJM. The pathophysiology of elevated vitamin B12 in clinical practice. QJM. 2013;106:505–515. doi: 10.1093/qjmed/hct051. [DOI] [PubMed] [Google Scholar]
- 3.Chiche L, et al. Implications cliniques de la découverte d’une hypervitaminémie B12 en médecine interne. Rev. Med. Interne. 2008;29:187–194. doi: 10.1016/j.revmed.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Cappello S, et al. Elevated plasma vitamin B12 concentrations are independent predictors of in-hospital mortality in adult patients at nutritional risk. Nutrients. 2016;9:1. doi: 10.3390/nu9010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serraj K, Mecili M, Housni I, Andrès E. Hypervitaminémie B12: physiopathologie et intérêt en pratique clinique. La Presse Méd. 2011;40:1120–1127. doi: 10.1016/j.lpm.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Arendt JFB, Nexo E. Cobalamin related parameters and disease patterns in patients with increased serum cobalamin levels. PLoS ONE. 2012;7:e45979. doi: 10.1371/journal.pone.0045979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonsen K, et al. Vitamin B 12 and its binding proteins in hepatocellular carcinoma and chronic liver diseases. Scand. J. Gastroenterol. 2014;49:1096–1102. doi: 10.3109/00365521.2014.921325. [DOI] [PubMed] [Google Scholar]
- 8.Baker H, Leevy CB, DeAngelis B, Frank O, Baker ER. Cobalamin (vitamin B12) and holotranscobalamin changes in plasma and liver tissue in alcoholics with liver disease. J. Am. Coll. Nutr. 1998;17:235–238. doi: 10.1080/07315724.1998.10718752. [DOI] [PubMed] [Google Scholar]
- 9.Lambert D, et al. Alcoholic cirrhosis and cobalamin metabolism. Digestion. 1997;58:64–71. doi: 10.1159/000201425. [DOI] [PubMed] [Google Scholar]
- 10.Shipton MJ, Thachil J. Vitamin B12 deficiency: A 21st century perspective. Clin. Med. 2015;15:145–150. doi: 10.7861/clinmedicine.15-2-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gimsing P. Cobalamin metabolism in chronic myelogenous leukemia. Dan. Med. Bull. 1998;45:459–479. [PubMed] [Google Scholar]
- 12.Gimsing P. Cobalamin forms and analogues in plasma and myeloid cells during chronic myelogenous leukaemia related to clinical condition. Br. J. Haematol. 2008;89:812–819. doi: 10.1111/j.1365-2141.1995.tb08419.x. [DOI] [PubMed] [Google Scholar]
- 13.Vlasveld LT, Bos GMJ, Ermens AAM, Bakker JA, Lindemans J. Hyperhomocysteinemia and functional cobalamin deficiency due to granulocytosis-induced alterations in the cobalamin-binding protein. Haematologica. 2006;91:394–396. [PubMed] [Google Scholar]
- 14.McMahon GM, et al. The association between vitamin B12, albuminuria and reduced kidney function: An observational cohort study. BMC Nephrol. 2015;16:235. doi: 10.1186/1471-2369-16-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannibal L, et al. Hampered vitamin B12 metabolism in gaucher disease? J. Inborn Errors Metab. Screen. 2017;5:232640981769235. doi: 10.1177/2326409817692359. [DOI] [Google Scholar]
- 16.Arendt JFB, Pedersen L, Nexo E, Sørensen HT. Elevated plasma vitamin B12 levels as a marker for cancer: A population-based cohort study. J. Natl. Cancer Inst. 2013;105:1799–1805. doi: 10.1093/jnci/djt315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arendt JFH, Sørensen HT, Horsfall LJ, Petersen I. Elevated vitamin B12 levels and cancer risk in UK primary care: A THIN database cohort study. Cancer Epidemiol. Biomark. Prev. 2019;28:814–821. doi: 10.1158/1055-9965.EPI-17-1136. [DOI] [PubMed] [Google Scholar]
- 18.Arendt JFB, Nexo E. Unexpected high plasma cobalamin/Proposal for a diagnostic strategy. Clin. Chem. Lab. Med. 2013;51:545. doi: 10.1515/cclm-2012-0545. [DOI] [PubMed] [Google Scholar]
- 19.Ermens AAM, Vlasveld LT, Lindemans J. Significance of elevated cobalamin (vitamin B12) levels in blood. Clin. Biochem. 2003;36:585–590. doi: 10.1016/j.clinbiochem.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Arendt JFH, Farkas DK, Pedersen L, Nexo E, Sørensen HT. Elevated plasma vitamin B12 levels and cancer prognosis: A population-based cohort study. Cancer Epidemiol. 2016;40:158–165. doi: 10.1016/j.canep.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Sviri S, et al. Increased vitamin B12 levels are associated with mortality in critically ill medical patients. Clin. Nutr. 2012;31:53–59. doi: 10.1016/j.clnu.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Greibe E, et al. Uptake of cobalamin and markers of cobalamin status: a longitudinal study of healthy pregnant women. Clin. Chem. Lab. Med. 2011;49:11. doi: 10.1515/CCLM.2011.682. [DOI] [PubMed] [Google Scholar]
- 23.Milman N, Byg K-E, Bergholt T, Eriksen L, Hvas A-M. Cobalamin status during normal pregnancy and postpartum: A longitudinal study comprising 406 Danish women. Eur. J. Haematol. 2006;76:521–525. doi: 10.1111/j.0902-4441.2006.t01-1-EJH2550.x. [DOI] [PubMed] [Google Scholar]
- 24.Arshad M, et al. Assessment of the serum levels of hemoglobin, ferritin, and vitamin B12 in a sample of Iranian population with morbid obesity. Jo. Minimally Invasive Surg. Sci. 2016;5:2. [Google Scholar]
- 25.Berg RL, Shaw GR. Laboratory evaluation for vitamin B12 deficiency: The case for cascade testing. Clin. Med. Res. 2013;11:7–15. doi: 10.3121/cmr.2012.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frenkel EP, White JD, Reisch JS, Sheehan RG. Comparison of two methods for radioassay of vitamin B12 in serum. Clin. Chem. 1973;19:1357–1360. doi: 10.1093/clinchem/19.12.1357. [DOI] [PubMed] [Google Scholar]
- 27.Zhao L, Wei Y, Song A, Li Y. Association study between genome-wide significant variants of vitamin B12 metabolism and gastric cancer in a han Chinese population: Unexpected Role of Vitamin B12 Metabolism Genes. IUBMB Life. 2016;68:303–310. doi: 10.1002/iub.1485. [DOI] [PubMed] [Google Scholar]
- 28.Fanidi A, et al. Is high vitamin B12 status a cause of lung cancer? Is high vitamin B12 status a cause of lung cancer? Int. J. Cancer. 2019 doi: 10.1002/ijc.32033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green R, et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primers. 2017;3:1. doi: 10.1038/nrdp.2017.40. [DOI] [PubMed] [Google Scholar]
- 30.Newman AC, Maddocks ODK. One-carbon metabolism in cancer. Br. J. Cancer. 2017;116:1499–1504. doi: 10.1038/bjc.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, et al. Methionine is a metabolic dependency of tumor-initiating cells. Nat. Med. 2019;25:825–837. doi: 10.1038/s41591-019-0423-5. [DOI] [PubMed] [Google Scholar]
- 32.Seijo LM, et al. Biomarkers in lung cancer screening: achievements, promises, and challenges. J. Thorac. Oncol. 2019;14:343–357. doi: 10.1016/j.jtho.2018.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tørring ML, Frydenberg M, Hansen RP, Olesen F, Vedsted P. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: A cohort study in primary care. Eur. J. Cancer. 2013;49:2187–2198. doi: 10.1016/j.ejca.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 34.Brousselle A, et al. Explaining time elapsed prior to cancer diagnosis: patients’ perspectives. BMC Health Serv. Res. 2017;17:1. doi: 10.1186/s12913-017-2390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen RP, Vedsted P, Sokolowski I, Søndergaard J, Olesen F. Time intervals from first symptom to treatment of cancer: a cohort study of 2,212 newly diagnosed cancer patients. BMC Health Serv. Res. 2011;11:1–10. doi: 10.1186/1472-6963-11-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whiteman DC, Wilson LF. The fractions of cancer attributable to modifiable factors: A global review. Cancer Epidemiol. 2016;44:203–221. doi: 10.1016/j.canep.2016.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.