Dear Editor,
Although the incidence and risk factors of venous thromboembolism [VTE] is well characterized in multiple myeloma [MM], little is known regarding the characterization of arterial thromboembolism [ATE] in the context of modern anti-myeloma therapy. Since MM is a cancer of older adults with several shared risk factors for cancer and cardiovascular disease, ATE remains an area of major concern. Notably, population-based studies from Sweden have demonstrated an increased risk of ATE in MM compared to matched controls, with an incidence of 3.8% in the first year [1]. Another study by the HOVON Group reported a high ATE incidence of 5.6% among newly diagnosed transplant-eligible MM patients receiving doxorubicin-based regimens [2]. However, data from older literature captured ATE incidence prior to the introduction of proteasome inhibitors and immunomodulatory drugs (IMiDs). The objective of our retrospective cohort study was to investigate the incidence, risk factors, and nature of ATE events in newly diagnosed MM in the context of modern anti-myeloma therapy.
We included all consecutive MM patients treated at the Cleveland Clinic from 1/1/2008 to 12/31/2018. We identified potential baseline disease-related, patient-related and treatment-related risk factors a priori and extracted from medical records. The primary endpoint was to estimate ATE incidence in the first year after treatment initiation. Secondary endpoints were to identify risk factors for ATE and association of ATE with overall survival [OS].
We calculated the cumulative incidence of ATE by 12 months. Death without ATE was a competing risk for ATE. Fine and Gray regression was used to identify univariate risk factors for ATE and results were reported as hazard ratio [HR] with 95% confidence intervals [CI]. We tested the following pre-treatment variables: year of treatment, age, sex, race, immunoglobulin subtype, International Staging System [ISS] stage, bone marrow plasma cell percentage, M-protein, involved/uninvolved free light chain ratio, karyotype, high-risk FISH, lactate dehydrogenase, creatinine, calcium, hemoglobin, prior ATE, prior VTE, body mass index, diabetes mellitus, chronic kidney disease, hypertension, hyperlipidemia, total leukocyte count, platelet count, liver disease, acute infection (within 90 days before treatment), erythropoietin use, clotting disorders, autoimmune disease, hyperviscosity, dexamethasone dose, doxorubicin use, multi-agent chemotherapy, smoking status, IMiD use, and concurrent anti-platelet/anti-thrombotic therapy. If HR could not be calculated, Gray test was used instead to assess association with ATE; this occurs for variables with categories that have 0% or 100% ATE events. Survival based on occurrence of ATE was assessed by landmark analyses at 6 and 12 months. For this analysis, Kaplan–Meier survival estimates at 5 years were calculated for those with or without ATE by the landmark along with log-rank test p value.
A total of 1029 consecutive patients met inclusion criteria, of whom 934 patients with available data on initial anti-myeloma therapy were analyzed. The baseline clinical and demographic characteristics are summarized in Table 1. The median age at treatment initiation was 63 years [range, 22–94]. Approximately one-fifth of patients were Black and more than half were male. The cohort was roughly equally divided into ISS stages I, II, and III disease, with one-fourth of patients having high-risk FISH cytogenetics at diagnosis. ATE before MM diagnosis was present in 12% of patients. Approximately one-fifth were actively smoking or had quit within 10 years prior to MM diagnosis. A 3-drug induction regimen with VRD (bortezomib-lenalidomide-dexamethasone) or VCD (bortezomib-cyclophosphamide-dexamethasone) was initiated in 52% of patients. Less than 6% had received high-dose dexamethasone [>16o mg per cycle]. The following anti-platelet/anti-thrombotic agents were administered: none [33%], low-dose aspirin [55%], prophylactic low molecular weight heparin [LMWH] (4%), and warfarin or therapeutic LMWH [7%].
Table 1.
Baseline clinical and demographic characteristics.
| Variable [Number of Patients with Available Data] | Patients [934] | |
|---|---|---|
| N | % | |
| Male sex [934] | 516 | 55.2 |
| Race [925]: | ||
| White | 738 | 79.8 |
| Black | 175 | 18.9 |
| Other | 12 | 1.3 |
| Age at treatment initiation[934] | Median: 63 years [22–94] | NA |
| Multiple Myeloma Subtype [934]: | ||
| IgG | 477 | 51.1 |
| IgA | 209 | 22.4 |
| IgM | 11 | 1.2 |
| Others | 237 | 25.4 |
| Albumin [g/dl] [817] | Median: 3.7 [1.1–5.2] | NA |
| Β-2 microglobulin [mcg/ml] [762] | Median: 3.8 [0.2–78.0] | |
| ISS stage [808] | ||
| I | 267 | 33.0 |
| II | 260 | 32.2 |
| III | 281 | 34.8 |
| Percentage BMPCs [866] | 50 [1–100] | NA |
| Serum M-protein [g/dl] [822] | Median: 1.95 [0–10.49] | NA |
| Involved/Uninvolved sFLC ratio [743] | 80 [0.5–64,775] | NA |
| Abnormal Metaphase Cytogenetics [781] | 146 | 18.7 |
| High-Risk FISH Cytogeneticsa [604] | 143 | 23.7 |
| LDH > ULN [643] | 178 | 27.7 |
| History of ATE [934] | 114 | 12.2 |
| History of VTE [931] | 60 | 6.4 |
| BMI [799] | ||
| <25 | 208 | 26 |
| 25–29.9 | 291 | 36.4 |
| 30–34.9 | 195 | 24.4 |
| 35–39.9 | 66 | 8.3 |
| ≥40 | 39 | 4.9 |
| History of Cardiac Diseaseb [933] | 164 | 17.6 |
| History of DM [934] | 157 | 16.8 |
| History of CKD [931] | 106 | 11.4 |
| History of HTN [932] | 464 | 49.8 |
| History of HLD [923] | 288 | 31.2 |
| Acute Infection at Diagnosisc [932] | 37 | 4.0 |
| History of Autoimmune Disease [925] | 60 | 6.5 |
| Smoking History [922]d | ||
| Never | 523 | 56.7 |
| Former | 222 | 24.1 |
| Current | 177 | 19.2 |
| Initial Treatment Regimen [934]: | ||
| VRD | 378 | 40.5 |
| VD | 204 | 21.8 |
| RD | 179 | 19.2 |
| VCD | 104 | 11.1 |
| Others | 69 | 7.4 |
| Dexamethasone Dose per Cycle [900]: | ||
| <120 mg | 168 | 18.7 |
| 120–60 mg | 681 | 75.7 |
| >160 mg | 51 | 5.7 |
| Initial Thromboprophylaxis Regimen [863] | ||
| None | 288 | 33.4 |
| ASA | 477 | 55.3 |
| Prophylactic LMWH | 35 | 4.1 |
| Warfarin/Therapeutic LMWH | 63 | 7.3 |
ISS International Staging System, BMPC Bone Marrow Plasma Cells, NA Not Applicable, sFLC Serum Free Light Chain, FISH Fluorescence in situ Hybridization, LDH Lactate Dehydrogenase, ULN Upper Limit of Normal, ATE Arterial Thromboembolism, VTE Venous Thromboembolism, BMI Body Mass Index, DM Diabetes Mellitus, CKD Chronic Kidney Disease, HTN Hypertension, HLD Hyperlipidemia, VRD Bortezomib-Lenalidomide-Dexamethasone, VD Bortezomib-Dexamethasone, RD Lenalidomide-Dexamethasone, VCD Bortezomib-Cyclophosphamide-Dexamethasone, ASA Aspirin, LMWH Low Molecular Weight Heparin.
aHigh‐risk FISH abnormality was defined by the presence of deletion(17p), t(4;14), t(14;16), and/or t(14;20).
bCardiac disease was defined as congestive heart failure, coronary artery disease [including acute myocardial infarction], and/or arrhythmia.
cDefined as acute infection within 90 days prior to treatment initiation.
dCurrent smokers were defined as patients who were smoking at diagnosis or had quit smoking less than 10 years before diagnosis. Former smokers were defined as patients who had quit smoking more than 10 years before diagnosis.
A total of 25 ATE events were observed within a year of treatment initiation. The cumulative incidence of ATE at 6 and 12 months was 2.0% [95% CI, 1.2–3.0] and 2.7% [95% CI, 1.8–4.0] respectively. The Kaplan–Meier curve for cumulative incidence of ATE, along with that of VTE for comparison, is shown in Fig. 1. The median time to ATE from treatment initiation was 4.8 months [range, 0.2–11.5 mo.]. The nature of ATEs are as follows: acute ischemic stroke [n = 10; 40%], acute myocardial infarction [n = 10; 40%], transient ischemic attack [n = 2; 8%], peripheral artery embolism [n = 2; 8%], and superior mesenteric artery thrombosis [n = 1; 4%]. Of 15 patients for whom response status at the time of ATE was available, 4 had very good partial response [VGPR], 7 had PR, and 4 had stable disease.
Fig. 1. Incidence of arterial and venous thromboembolism in the first year after treatment initiation.
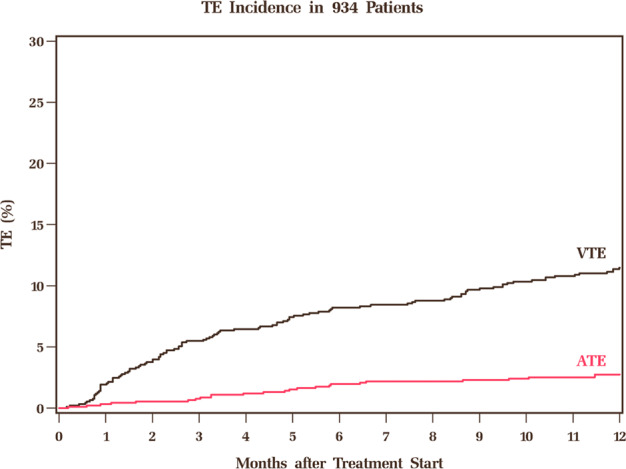
ATE Arterial Thromboembolism, VTE Venous Thromboembolism.
On univariate analysis, the following factors were significantly predictive of ATE: ISS stage III disease [vs I/II; HR = 2.62; 95% CI, 1.06–6.51; p = 0.038], prior ATE [HR = 6.86; 95% CI, 3.14–15.0; p < 0.001], diabetes mellitus [HR = 2.89; 95% CI, 1.28–6.52; p = 0.011], chronic kidney disease [HR = 3.58; 95% CI, 1.48–8.69; p = 0.005], hyperlipidemia [HR = 2.66; 95% CI, 1.19–5.92; p = 0.017], acute infection [HR = 4.92; 95% CI, 1.71–14.2; p = 0.003], and current smoker [vs former/never smoker; HR = 2.37; 95% CI, 1.05–5.35; p = 0.037]. Of note, use of IMiDs in induction therapy was not associated with an increase in ATE risk [HR = 0.54; 95% CI, 0.24–1.20; p = 0.13]. We did not find any significant association between the use of thromboprophylactic agents and incidence of ATE.
Subsequently, we evaluated the association of ATE with OS. Of 25 patients with ATE events, nine were alive with a median follow-up after ATE diagnosis of 23.9 months [range, 7.9-71.8 months]. The 5-year OS among patients with or without ATE at 6-month landmark was 39% and 60% respectively [p = 0.004] and that at 12-month landmark was 45% and 63% respectively [p < 0.001]. The Kaplan–Meier curves for survival using landmark analysis at 6 and 12 months is shown in the figure in Supplementary Appendix A.
Our study demonstrates that the incidence of ATE in the first year after myeloma diagnosis is 2.7% in the current era, which is substantially lower than prior estimates with cytotoxic therapies. Notably, the high ATE incidence (5.6%) seen in the study by the HOVON group was in the context of doxorubicin-based regimens [2], which could be due to the pro-coagulant effect of doxorubicin on platelets [3]. Our data is in line with the recently published Myeloma XI trial, which showed an ATE incidence of 1.3% and 2.4% in transplant-eligible and transplant-ineligible pathways respectively [4]. Furthermore, data from our study confirms the association of ATE with worsened OS in the current era, consistent with the Swedish myeloma database and Myeloma XI trial [4,5,]. However, we acknowledge, that there may not be a causal relationship between ATE and survival, since ATE is associated with several co-morbidities and a high tumor burden, which may be the drivers of mortality in these patients. The only tumor-specific risk factor that we could identify was ISS stage III, which implies a high tumor burden. Although IMiD use is a well-established risk factor for VTE in MM [6], the association with ATE is less well defined. Due to a low event rate, it remains difficult to observe meaningful difference in ATE incidence between treatment arms in randomized clinical trials [7]. With a large sample size, our study shows that IMiD use may not associated with increased ATE incidence in the first year of treatment. However, prolonged IMiD maintenance therapy beyond first year may be associated with a small increase in ATE risk, as shown in the Myeloma XI trial [4].
Our study has limitations. First, our database did not have incidence of clonal hematopoiesis of indeterminate potential [CHIP], which is a non-modifiable risk-factor for atherosclerotic cardiovascular disease [8]. However, a recent study on 629 transplanted myeloma patients did not demonstrate CHIP as a predictor of ATE in this population at a median follow-up of ~10 years [9]. Second, despite a large sample size, the total number of ATE events was low, hence, multivariable analysis of risk factors could not be performed. Unfortunately, confounding can be present in retrospective analyses; hence, we need studies with more events to identify independent risk factors for ATE. Third, we did not have a matched control group of patients without myeloma. However, based on data from the SEER-Medicare linked database, the 6-month cumulative incidence of ATE in patients with cancer and matched controls is 4.7% and 2.2% respectively, with the highest risk in patients with lung cancer (8.3%) [10]. Hence, the incidence of ATE in patients with MM treated in the modern era was comparable to Medicare enrollees without a cancer diagnosis.
In summary, we show a small but significant risk of ATE in the first year after myeloma diagnosis in the current era, with several modifiable risk factors, including diabetes, hyperlipidemia, and smoking. A history of ATE was present in 12% of patients prior to myeloma diagnosis, which was the strongest risk factor for subsequent ATE. Patients with myeloma should undergo a thorough risk assessment for ATE at diagnosis. There is a paucity of data on primary prophylaxis for ATE in patients with cancer. A phase III RCT comparing LMWH (nadroparin) to placebo for prevention of VTE and ATE in solid tumor patients demonstrated a decrease in ATE risk (stroke and peripheral thrombosis) by 50% (0.4% in nadroparin arm and 0.8% in placebo arm) [11]. However, the primary endpoint included a composite of VTE and ATE and lacked power to detect a difference in ATE specifically. Similarly, a recent update from the CASSINI trial demonstrated a numerically lower incidence of ATE with rivaroxaban compared to placebo (1.0% vs 1.7%), however, was not statistically significant likely due to low number of events [12]. Future studies should assess the role of primary and secondary prophylaxis for ATE, especially in high-risk patients.
Supplementary information
Author contributions
RC designed the research and wrote the paper. AAK designed the research and approved the final draft of the manuscript. LR performed statistical analysis, provided critical input in study design and approved the final draft of the manuscript. JV, AVMG, BMF, JK, CJS, and FA performed patient management, provided input in the paper, and approved the final draft of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41408-021-00513-4.
References
- 1.Kristinsson SY, Pfeiffer RM, Björkholm M, Goldin LR, Schulman S, Blimark C, et al. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: a population-based study. Blood. 2010;115:4991–8. doi: 10.1182/blood-2009-11-252072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libourel EJ, Sonneveld P, van der Holt B, de Maat MP, Leebeek FW. High incidence of arterial thrombosis in young patients treated for multiple myeloma: results of a prospective cohort study. Blood. 2010;116:22–26. doi: 10.1182/blood-2009-12-257519. [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Lim KM, Noh JY, Kim K, Kang S, Chang YK, et al. Doxorubicin-induced platelet procoagulant activities: an important clue for chemotherapy-associated thrombosis. Toxicol Sci. 2011;124:215–24. doi: 10.1093/toxsci/kfr222. [DOI] [PubMed] [Google Scholar]
- 4.Bradbury CA, Craig Z, Cook G, Pawlyn C, Cairns DA, Hockaday A, et al. Thrombosis in patients with myeloma treated in the Myeloma IX and Myeloma XI phase 3 randomized controlled trials. Blood. 2020;136:1091–104. doi: 10.1182/blood.2020005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristinsson SY, Pfeiffer RM, Björkholm M, Schulman S, Landgren O. Thrombosis is associated with inferior survival in multiple myeloma. Haematologica. 2012;97:1603–7. doi: 10.3324/haematol.2012.064444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palumbo A, Rajkumar SV, Dimopoulos MA, Richardson PG, San Miguel J, Barlogie B, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414–23. doi: 10.1038/sj.leu.2405062. [DOI] [PubMed] [Google Scholar]
- 7.Maharaj S, Chang S, Seegobin K, Serrano-Santiago I, Zuberi L. Increased risk of arterial thromboembolic events with combination lenalidomide/dexamethasone therapy for multiple myeloma. Expert Rev Anticancer Ther. 2017;17:585–91. doi: 10.1080/14737140.2017.1330153. [DOI] [PubMed] [Google Scholar]
- 8.Jaiswal S et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. 2017;377:111-21. [DOI] [PMC free article] [PubMed]
- 9.Mouhieddine TH, Sperling AS, Redd R, Park J, Leventhal M, Gibson CJ, et al. Clonal hematopoiesis is associated with adverse outcomes in multiple myeloma patients undergoing transplant. Nat Commun. 2020;11:2996. doi: 10.1038/s41467-020-16805-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind M, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–38. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agnelli G, Gussoni G, Bianchini C, Verso M, Mandalà M, Cavanna L, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10:943–9. doi: 10.1016/S1470-2045(09)70232-3. [DOI] [PubMed] [Google Scholar]
- 12.Khorana AA, McNamara MG, Kakkar AK, Streiff MB, Riess H, Vijapurkar U, et al. Assessing full benefit of rivaroxaban prophylaxis in high-risk ambulatory patients with cancer: thromboembolic events in the randomized CASSINI Trial. TH Open. 2020;4:e107–e112. doi: 10.1055/s-0040-1712143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


