Abstract
This case illustrates a false positive F18 FDG PET CT in the left axilla of a woman being treated for metastatic breast cancer after COVID-19 vaccination. Follow-up ultrasound of the axilla indicated no metastasis, indicating that the lymphadenopathy was likely due to an immune response following vaccination. This case report, in conjunction with prior studies of other vaccines with similar findings suggest that providers should be aware of potential false positive imaging following COVID-19 vaccination. In light of these findings, clinicians and imaging providers should record the date and side of the vaccination and inform patient of potential false positive results to reduce patient anxiety and unnecessary tests as COVID-19 vaccines become widely available.
Keywords: Case report, COVID-19, Vaccination, False-positive, Breast cancer
Background
There are over 150,000 women currently living with metastatic breast cancer in the United States. (Mariotto et al., 2017) Given recent and continuing advances in treatment and in our understanding of disease biology, this number is growing as women are living longer on treatment and enjoying better quality of life.Current clinical guidelines suggest that these women should be routinely monitored with systemic imaging while undergoing therapy, in order to understand their ongoing response to treatment and minimize the risk of uncontrolled cancer progression. (Cardoso et al., 2020)
Outside of the context of clinical trials, there are not specific imaging recommendations for monitoring these patients, so providers work with their patients to determine imaging type and frequency. (Cardoso et al., 2020)F 18 Fluorodeoxyglucose Positron Emission Tomographywith Computed Tomography (FDG PET/CT) has shown high accuracy in diagnosing progressive disease in metastatic breast cancer, and therefore is commonly used by providers to monitor progression in these patients. (JH et al., 2016) It is common practice for patients with metastatic breast cancer receiving treatment to undergo FDG-PET/ CT every three to four months to track their disease progression or regression. While this practice can facilitate early findings of progression, it also poses a risk of false positive results. FDG-PET/ CT identifies rapidly proliferating cells, which is a hallmark of cancer but can also have other causes, such as inflammation, infection, or acute immune responses. The risk of a false positive leads to uncertainty and fear in these patients, which is important for patients and providers to take into account. This is particularly important in the context of the current COVID-19 pandemic, when now at long last COVID-19 vaccines are finally available, and are recommended for metastatic breast cancer patients. (Coronavirus Disease 2019)Here we present a case of a patient on active treatment for metastatic breast cancer who had a false positive FDG-PET/ CT following administration of the COVID-19 vaccine.
Case presentation
A 48 year-old woman was diagnosed with a clinical stage 1 cancer of the left breast in late 2011.Her biopsy showed invasive mammary carcinoma with mixed terminal ductal and lobular features, ER 98%, PR 99%, Her2 2+/negative, Ki67 12%. She underwent a bilateral mastectomy and left sentinel lymph node removal. The right breast was negative for malignancy; the left breast revealed invasive lobular carcinoma, measuring 1.2 × 1.1 × 1.0 cm, and with associated lobular carcinoma in situ (LCIS). There was no evidence of lymphovascular invasion. Margins and nodes were negative.Oncotype testing was performed with a score of 14, and she was determined not to require chemotherapy.She was started on Tamoxifen.
Six years later, the patient presented to the emergency department with severe back pain impairing ambulation. An MRI showed abnormal signal and heterogeneous enhancement with involvement of pedicles greater on right and left of T9. Loss of height and mild paraspinal soft tissue changes were also noted. A biopsy of the T9 region revealed atypical cells, and CT CAP was performed which revealed the lesion at T9 and in addition, two small liver lesions thought to be possible hemangiomas vs metastases.Given this uncertainty, a PET CT was performed which revealed multiple lytic and hypermetabolic foci in the axial skeleton, one liver lesion that was felt to be almost certainly a metastasis given SUV of 6.1, and another liver lesion that was felt to be equivocal. Biopsy of the liver lesion revealed metastasis from known breast primary.
With the diagnosis of metastatic breast cancer confirmed, the patient was initiated on treatment with letrozole, palbociclib and denosumab. She was subsequently monitored with FDG PET CT given that CT scans had previously been equivocal, and FDG PET CTs for 32 months after initiation of therapy revealed excellent clinical response. Scans continued to be clear until a routine monitoring PET CT in January of 2021 revealed an interval increase in size, number and metabolic activity of left axillary and subpectoral nodes suggestive of recurrent disease (see Fig. 1., Fig. 2. ). However, when a left axillary ultrasound was preformed 11 days later in anticipation of biopsy, the lymph nodes appeared normal (Fig. 3 ).
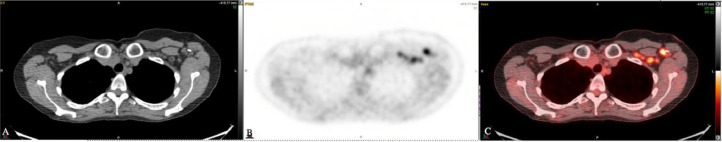
Fig. 1.
Axial FDG PET/CT images of the thorax A. Computed tomographic images demonstrate the presence of at least 10 lymph nodes in the left axillary and retro pectoral area, the side where the cancer was initially diagnosed. The largest lymph node measures 1.0 × 0.9 cm and has a thickened cortex. All these lymph nodes are new compared to prior study and enlarged compared to the contralateral side. B. PET images demonstrate increase metabolic activity of these lymph nodes with a Max SUV of 4.1 for the lymph node adjacent to a surgical clip. C. Fused FDG PET/CT images demonstrate the described findings.
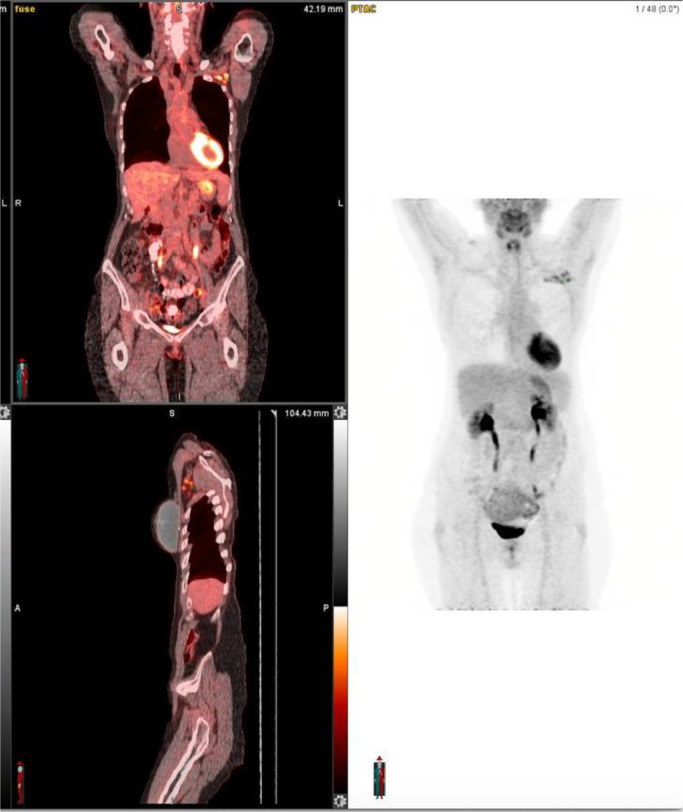
Fig. 2.
FA.DG PET/CT images. A and B: Coronal and Sagittal planes of the whole body FDG PET/CT images. C. MIP (Maximum intensity projection) FDG PET/CT image. These images demonstrate physiologic distribution of radiotracer with the only abnormal findings being the left axillary and retro pectoral metabolically active lymph nodes (arrows) . Note, there was no liver or bone uptake to suggest active metastatic disease.
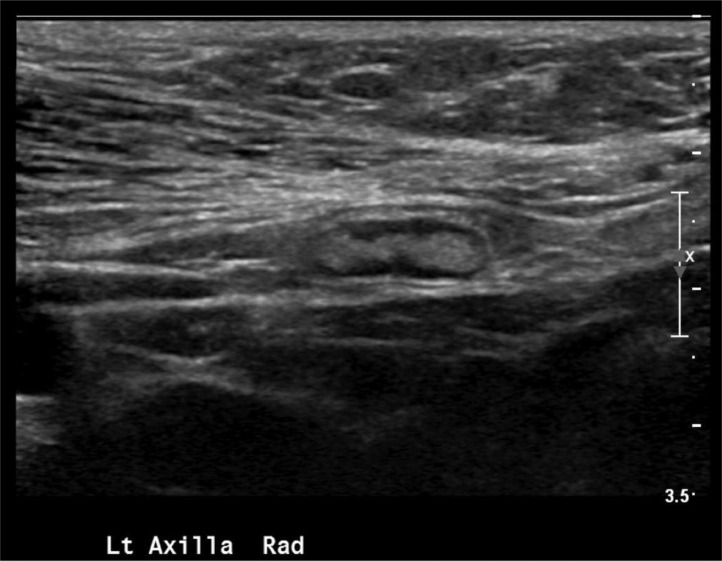
Fig. 3.
Ultrasound images of the left axilla demonstrate a sagittal view of a normal appearing lymph node, with no cortical thickening or abnormal shape to suggest the presence of malignancy. Other lymph nodes demonstrated normal appearance.
Upon further investigation, the patient had received her first dose of the Moderna COVID-19 vaccine in her left arm one week prior to the January PET CT, which is very likely to have caused this false positive PET CT finding.
Discussion
Research has yet to elucidate the full impact of the COVID-19 vaccines on FDG PET/CT findings due to their novelty. However, findings from other vaccine studies and this case study indicate that the immune response stimulated by vaccination may lead to false-positive findings.
A collection of studies conducted after the H1N1 influenza pandemic and following vaccination efforts indicated that vaccine-induced false-positive FDG PET/CT were likely to occur.(Burger et al., 2011;Panagiotidis et al., 2010) One study examining post-vaccination FDG PET/CT in patients with malignant tumors found that nearly 20% of the patients who had been vaccinated in the prior 14 days had positive lymph node findings, all of which resolved, indicating that they were vaccine- and not malignancy-related.(Burger et al., 2011)
Coates et al. (2017) reported comparable findings from Cervarix and Gardasil vaccination. All participants showed ipsilateral node uptake of FDG in the axillary lymph node one week after vaccination. Some participants even showed contralateral lymph activity up to one month following vaccination, likely due to the immunogenicity of the vaccines.(Coates et al., 2017) The nature of the vaccines and the age of the participants studied may have impacted the differing severities of lymph activation seen in the H1N1 and HPV vaccines.
Recently emerging evidence suggests that both the Moderna and Pfizer-BioNTech COVID-19 vaccines may also cause transient axially lymphadenopathy. (8, 9) Lymphadenopathy assessed by physical examination was reported as a solicited adverse event in 11.6% of patients after receiving the first dose and 16.0% of patients after receiving the second dose of the Moderna vaccine with a mean duration of one to two days. (8) In the Pfizer-BioNTech COVID-19 vaccine trials, 64 patients in the vaccine group reported lymphadenopathy compared with only 6 patients in the placebo group. (9)Patients who reported lymphadenopathy noticed it within 2–4 days post-vaccination and it lasted, on average, 10 days. Rates and duration of lymphadenopathy in both trials were reported based on clinical assessment using physical examination, so rates and duration of subclinical adenopathy appreciable on mammography and PET/CT are likely greater. Another recent study identified five case studies of axillary lymphadenopathy occurring post COVID-19 vaccination in oncology patients that mimicked metastases on FDG PET/CT. (10)
In light of these findings, new guidelines have been released by the Society of Breast Imaging (SBI), which include scheduling screening exams such as mammograms prior to the first dose of the COVID-19 vaccine or four to six weeks following the second dose, if it does not unduly delay care. (Grimm et al., 2021) Additionally, SBI recommends obtaining information about vaccine status, timing of vaccine, and site of vaccination at the time of breast imaging and informing patients about the potential for a false positive exam due to vaccination prior to conducting imaging studies in order to reduce patient anxiety. (Grimm et al., 2021)These findings indicate the need for further study of lymph node activation following COVID-19 vaccination to inform imaging guidelines as vaccine distribution becomes widespread.
As more people with malignancies who undergo routine imaging are vaccinated against COVID-19, more clarity about the impact of the vaccine on FDG PET/CT will be gained. B and T lymphocyte proliferation with vaccination do indicate the potential for false-positive imaging.(Sahin et al., 2020) Therefore, it may be useful to get a clear vaccination history and wait at least a month after vaccination to perform imaging unless symptoms of cancer progression are evident, to decrease the risk of false positive scans and to reduce patient anxiety. If an FDG PET/CT scan is indicated, it is recommended to inject the radiotracer in the opposite side of the breast involved, which in case of trace infiltration can produce uptake in the lymph nodes. Patients should ask providers to administer the vaccine on the opposite side of the breast cancer.
Conclusion
FDG PET/CTs are the most sensitive tool we currently have for monitoring metastatic breast cancer. It has always been important for providers to take into account all factors that can impact a patient's presentation aside from cancer, and in the current era, COVID-19 vaccination timing is an additional element that should be considered when scheduling imaging and discussing risks and benefits with patients. Clinicians and imaging providers should be aware of these findings and should record the date and side of the vaccination to avoid patient anxiety and unnecessary tests.
Author contribution statement
Rebecca Schapiro, MPH: Conceptualization, Writing – original draft preparation
Valeria Moncayo, MD: Data curation, Writing – reviewing and editing
Jane Meisel, MD: Conceptualization, Supervision, Writing – reviewing and editing
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Informed consent was received from the patient to present these findings.
Patient consent statement
The authors received written, informed consent from the patient to publish this case.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mariotto A.B., Etzioni R., Hurlbert M., Penberthy L., Mayer M. Estimation of the Number of Women Living with Metastatic Breast Cancer in the United States. Cancer Epidemiol. Prev. Biomark. 2017;26(6):809–815. doi: 10.1158/1055-9965.EPI-16-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso F., Paluch-Shimon S., Senkus E., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann. Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JH O., Lodge M.A., Wahl R.L. Practical PERCIST: a Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology. 2016;280(2):576–584. doi: 10.1148/radiol.2016142043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronavirus Disease 2019(COVID-19) Resources for the Cancer Care Community. Accessed February 27, 2021. https://www.nccn.org/covid-19/
- Burger I.A., Husmann L., Hany T.F., Schmid D.T., Schaefer N.G. Incidence and Intensity of F-18 FDG Uptake After Vaccination With H1N1 Vaccine. Clin. Nucl. Med. 2011;36(10):848–853. doi: 10.1097/RLU.0b013e3182177322. [DOI] [PubMed] [Google Scholar]
- Panagiotidis E., Exarhos D., Housianakou I., Bournazos A., Datseris I. FDG uptake in axillary lymph nodes after vaccination against pandemic (H1N1) Eur. Radiol. 2010;20(5):1251–1253. doi: 10.1007/s00330-010-1719-5. [DOI] [PubMed] [Google Scholar]
- Coates E.E., Costner P.J., Nason M.C., et al. Lymph Node Activation by PET/CT Following Vaccination With Licensed Vaccines for Human Papillomaviruses. Clin. Nucl. Med. 2017;42(5):329–334. doi: 10.1097/RLU.0000000000001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LocalReactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: moderna COVID-19 Vaccine | CDC. Published February 19, 2021. Accessed February 27, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html.
- 9.Reactions and Adverse Events of the Pfizer-BioNTech COVID-19 Vaccine | CDC. Published February 19, 2021. Accessed February 27, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
- 10.ÖzütemizC., KrystosekL.A., ChurchA.L., et al.Lymphadenopathy in COVID-19 Vaccine Recipients: diagnostic Dilemma in Oncology Patients. Radiology. Published online February 24, 2021:210275. doi:10.1148/radiol.2021210275 [DOI] [PMC free article] [PubMed]
- GrimmL., DestounisS., DoganB., et al.SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination.; 2021:3.
- Sahin U., Muik A., Derhovanessian E., et al. COVID-19 vaccine BNT162b1 elicits human antibody and T H 1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]