Abstract
Using a fixel-based analysis (FBA), we assessed the fiber-specific white matter (WM) alterations in nonmedicated patients with early-stage Parkinson’s disease (PD) with tremor-dominant (TD; n = 53; mean age, 61.7 ± 8.7 years) and postural instability and gait disorder (PIGD; n = 27; mean age, 57.8 ± 8.1 years) motor subtypes and age- and sex-matched healthy controls (HC; n = 43; mean age, 61.6 ± 9.2 years) from Parkinson’s Progression Markers Initiative dataset. FBA revealed significantly increased macrostructural fiber cross section and a combination of fiber density and cross section metrics within the corticospinal tract in patients with TD-PD compared with HC. Nonetheless, no significant changes in FBA-derived metrics were found in patients with PIGD-PD compared with HC or patients with TD-PD. Our results may provide evidence of WM neural compensation mechanisms in patients with TD-PD marked by increases in fiber bundle size and the ability to relay information between brain regions.
Subject terms: Diagnostic markers, Parkinson's disease
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder, mainly characterized by cardinal motor symptoms, including rigidity, resting tremor, and bradykinesia, as manifestations of selective loss of dopaminergic neurons in the substantia nigra pars compacta and the widespread aggregation of α-synuclein in the form of Lewy neurites and Lewy bodies1. Based on predominance of specific motor signs at the time of diagnosis, two main subtypes of Parkinson’s disease (PD) are described: tremor dominant (TD) and postural instability and gait disorder (PIGD)2. Discrete patterns of clinical progression have been suggested in TD-PD and PIGD-PD. PIGD-PD is associated with a faster rate of motor decline and weaker response to levodopa treatment3, while TD-PD is known to have a better prognosis and slower disease progression2. Different clinical presentations and progression of the two PD motor subtypes suggest distinct underlying neural mechanisms. Prior postmortem studies have demonstrated that patients with PIGD-PD had more cell loss in dopaminergic and nondopaminergic neural circuits than the patients with TD-PD4. However, while detailed knowledge of the white matter (WM) changes in TD-PD and PIGD-PD, particularly in the early stage, is essential to guide future disease-modifying strategies successfully, to date, the WM structural alterations in TD-PD and PIGD-PD have not been fully elucidated.
Diffusion tensor imaging (DTI) is currently the most widely used approach to estimate changes in WM microstructural integrity in PD5. PD has generally been associated with reduced fractional anisotropy (FA)6. FA corresponds to the degree of directionality of water diffusion and reflects the white matter integrity7. However, a recent study in patients with early-stage TD-PD reported greater FA in WM motor pathways compared with healthy controls (HC) and patients with PIGD-PD, which was interpreted as a result of greater organization of the WM to compensate for PD pathology8. However, the interpretation might not be strictly accurate, given the limitations of the tensor model. The inability to resolve multiple fiber orientations in regions of crossing or kissing fibers, which includes up to 90% of the WM voxels in the brain, may contribute to unreliable estimation of DTI measures in these regions9. A selective loss of specific fiber directions in crossing-fiber regions may also result in increased FA10. Furthermore, FA is influenced by numerous tissue characteristics, including axonal diameter, fiber density, tissue geometry, and degree of myelination;11 thus, interpretation of change in such metric must be done with care.
Advances in diffusion modeling techniques allow quantification of WM fiber properties in the presence of complex fiber geometry9,12. Fixel-based analysis (FBA) is a framework that facilitates measurement of specific fiber-bundle populations within a voxel or so-called “fixel”13,14. In brief, FBA utilizes the fiber orientation distributions (FODs) estimated using constrained spherical deconvolution (CSD) techniques, which enables the measurement of microstructural differences in fiber density (FD), macrostructural differences in fiber bundle cross section (FC), or differences arising from a combination of FD and FC (fiber density and cross section [FDC])14. In the developing brain, an increase in fixel metrics might result from increasing axonal diameter or axon count15,16, whereas a decrease in those metrics could occur due to axonal loss or atrophy, such as in PD17–19.
It remains unknown whether the WM microstructural changes detected in early-stage PD, particularly in TD-PD on DTI (indexed by increases in FA)8,20,21, reflect selective neurodegenerative or compensatory mechanisms. Moreover, understanding the PD pathogenesis at the initial stages is crucial for treating these patients more efficiently. In the current cross-sectional study, we applied FBA to evaluate fiber-specific WM alterations in nonmedicated patients with early-stage TD-PD and PIGD-PD using Parkinson’s Progression Markers Initiative (PPMI) dataset. Unlike DTI, we hypothesized that FBA may help in differentiating between selective neural loss and neural compensation, marked by a decrease or increase in fixel metrics, respectively. Further, we also hypothesized that WM neural compensation would be present in patients with TD-PD.
Results
Demographics and clinical characteristics
We included 123 participants from the PPMI dataset divided into three groups: 43 HC (25 men; mean age, 61.6 ± 9.2 years), 53 patients with TD-PD (32 men; mean age, 61.7 ± 8.7 years), and 27 patients with PIGD-PD (15 men; mean age, 57.8 ± 8.1 years). As shown in Table 1, the groups were not significantly different in terms of age, sex, and years of education. All subjects were without cognitive decline (Montreal Cognitive Assessment [MoCA] score ≥ 26); however, the MoCA scores were significantly higher in patients with PIGD-PD than in those with TD-PD (P = 0.013), while no significant differences were found between HC and either patients with TD-PD or PIGD-PD.
Table 1.
Demographic characteristics of the participants.
| HC | TD-PD | PIGD-PD | P-value | ||||
|---|---|---|---|---|---|---|---|
| HC vs. TD-PD vs. PIGD-PD | HC vs. TD-PD | HC vs. PIGD-PD | TD-PD vs. PIGD-PD | ||||
| Number of subjects | 43 | 53 | 27 | ||||
| Sex (male/female) | 25/18 | 32/21 | 15/12 | 0.92a | N/A | N/A | N/A |
| Age (years) | 61.6 ± 9.2 | 61.7 ± 8.7 | 57.8 ± 8.1 | 0.13b | N/A | N/A | N/A |
| Years of education | 15.2 ± 2.8 | 15.1 ± 2.9 | 15.0 ± 3.2 | 0.99d | N/A | N/A | N/A |
| MoCA | 28.4 ± 1.1 | 28.0 ± 1.3 | 28.7 ± 1.3 | 0.030d | 0.11e | 0.18e | 0.013e |
| Mean SBR | 2.3 ± 0.4 | 1.3 ± 0.3 | 1.3 ± 0.4 | <0.0001b | <0.0001c | <0.0001c | 0.995c |
| Dominant side (Rt/Lt) | N/A | 28/25 | 11/16 | ||||
| Disease duration (months) | N/A | 7.3 ± 8.5 | 5.8 ± 6.3 | N/A | N/A | N/A | 0.77d |
| Hoehn and Yahr stage | N/A | 1.5 ± 0.5 | 1.6 ± 0.5 | N/A | N/A | N/A | 0.39d |
| MDS-UPDRS-III | 0.6 ± 1.5 | 21.0 ± 7.9 | 21.7 ± 9.4 | <0.0001d | <0.0001e | <0.0001e | 0.88e |
| Mean progression of motor signs | N/A | 6.1 ± 5.7 | 6.2 ± 4.1 | N/A | N/A | N/A | 0.57e |
HC healthy control, Lt left, MoCA Montreal Cognitive Assessment, N/A not applicable, PIGD-PD patients with postural instability and gait disorder Parkinson’s disease, Rt right, TD-PD patients with tremor-dominant Parkinson’s disease, MDS-UPDRS-III Movement Disorder Society-Unified Parkinson’s Disease Rating Scale Part III.
Statistical analyses were performed using achi-square test, bone-way analysis of variance (ANOVA) test, cTukey HSD test, dKruskal–Wallis test, and eMann–Whitney U test.
As expected, the mean striatal binding ratio (SBR) of patients with TD-PD and PIGD-PD was significantly lower (P < 0.0001) than in HC, while there was no difference between patients with TD-PD and PIGD-PD. Although not statistically significant, patients with TD-PD had slightly longer disease duration, but lower Hoehn and Yahr stage, Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III total score, and mean progression of motor signs compared with patients with PIGD-PD.
Whole-brain FBA
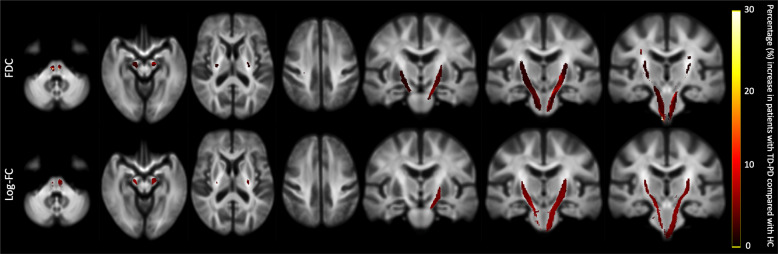
Figure 1 shows the whole brain FBA results for the log-FC and FDC. Compared with HC, patients with TD-PD showed significantly (family-wise error [FWE]-corrected P-value < 0.05) higher log-FC and FDC in the bilateral corticospinal tracts (CSTs). No significant differences were observed between other groups (HC vs. PIGD-PD; TD-PD vs. PIGD-PD). There were no WM tracts indicating decreased FD, log-FC, and FDC in patients with PD as opposed to the HC.
Fig. 1. Fiber tract-specific significant FDC and log-FC increases in patients with TD-PD compared to HC.
Streamline segments were cropped from the template tractogram to include only streamline points that corresponded with significant fixels (family-wise error-corrected P < 0.05). Streamlines were colored by percentage effect increase of log-transformed FC and FDC in the TD-PD group compared with the HC group. FC fiber cross section, FDC fiber density and cross section, HC healthy controls, TD-PD patients with tremor-dominant Parkinson’s disease.
Whole-brain voxel-based analysis (VBA)
No significant changes in FA and mean diffusivity (MD) were demonstrated between any of the tested groups: HC vs. TD-PD, HC vs. PIGD-PD, and TD-PD vs. PIGD-PD.
Tract-of-interest (TOI) analysis
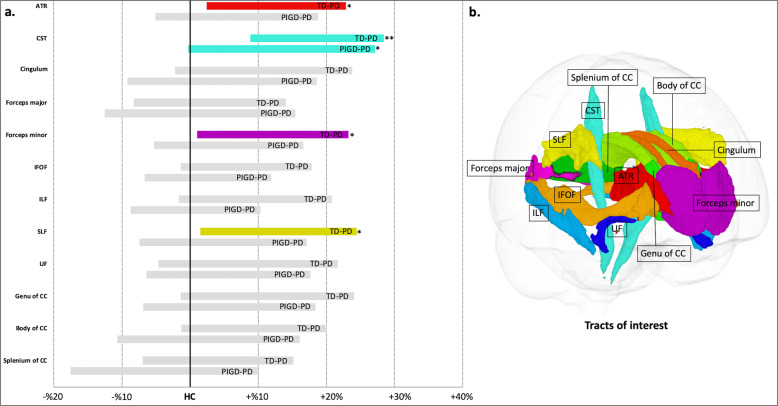
Significantly higher mean FDC value was demonstrated in patients with TD-PD compared with HC in the CST (P = 0.000036), with large-effect size (Cohen’s d = −1.06; Fig. 2 and Table 2). Compared with the HC, there was a trend toward a higher mean FDC value, with medium-effect sizes in the anterior thalamic radiation (ATR; P = 0.012, Cohen’s d = −0.61), forceps minor (P = 0.024, Cohen’s d = −0.55), and superior longitudinal fasciculus (SLF; P = 0.012, Cohen’s d = −0.65) in patients with TD-PD and in the CST in patients with PIGD-PD (P = 0.017, Cohen’s d = −0.63). There were no significant differences between TD-PD and PIGD-PD. There were no significant correlations between FDC value in patients with TD-PD or PIGD-PD and the disease duration, mean SBR, MDS-UPDRS part III total score, mean progression of motor signs, or MoCA scores.
Fig. 2. White matter tract of interest analysis.
a Increases (displayed in color; *P = 0.0045−0.05, **P < 0.0045) in a combined fiber density and cross section (FDC) metric in patients with TD-PD and PIGD-PD visualized as percentage difference from the HC. b Tracts of interest. ATR anterior thalamic radiation, CC corpus callosum, CST corticospinal tract, IFOF inferior fronto-occipital fasciculus, ILF inferior longitudinal fasciculus, PIGD-PD patients with postural instability and gait disorder Parkinson’s disease, SLF superior longitudinal fasciculus, TD-PD patients with tremor-dominant Parkinson’s disease, UF uncinate fasciculus.
Table 2.
Fiber density and cross section values of white matter tracts.
| HC | TD-PD | PIGD-PD | P-value | Cohen’s d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | HC vs. TD-PD vs. PIGD-PD | HC vs. TD-PDa | HC vs. PIGD-PDa | TD-PD vs. PIGD-PDa | HC vs. TD-PD | HC vs. PIGD-PD | TD-PD vs. PIGD-PD | |
| ATR | 0.49 | 0.05 | 0.52 | 0.05 | 0.50 | 0.06 | 0.014 | 0.012 | 0.26 | 1.00 | −0.61 | −0.30 | 0.28 |
| CST | 0.74 | 0.06 | 0.81 | 0.07 | 0.79 | 0.10 | 0.000049 | 0.000036 | 0.017 | 0.97 | −1.07 | −0.65 | 0.25 |
| Cingulum | 0.61 | 0.06 | 0.65 | 0.08 | 0.63 | 0.08 | 0.089 | 0.084 | 0.89 | 1.00 | −0.48 | −0.21 | 0.24 |
| Forceps major | 0.69 | 0.08 | 0.70 | 0.08 | 0.70 | 0.10 | 0.84 | 1.00 | 1.00 | 1.00 | −0.12 | −0.06 | 0.06 |
| Forceps minor | 0.46 | 0.05 | 0.48 | 0.05 | 0.47 | 0.05 | 0.028 | 0.024 | 0.91 | 0.66 | −0.55 | −0.25 | 0.31 |
| IFOF | 0.58 | 0.06 | 0.61 | 0.06 | 0.59 | 0.05 | 0.31 | 0.096 | 1.00 | 0.77 | −0.44 | −0.14 | 0.30 |
| ILF | 0.54 | 0.05 | 0.57 | 0.06 | 0.54 | 0.05 | 0.051 | 0.072 | 1.00 | 0.26 | −0.46 | −0.04 | 0.42 |
| SLF | 0.64 | 0.06 | 0.68 | 0.07 | 0.66 | 0.08 | 0.013 | 0.012 | 1.00 | 0.31 | −0.63 | −0.23 | 0.36 |
| UF | 0.60 | 0.06 | 0.63 | 0.08 | 0.62 | 0.07 | 0.18 | 0.24 | 0.59 | 1.00 | −0.37 | −0.26 | 0.12 |
| Genu of CC | 0.65 | 0.09 | 0.69 | 0.08 | 0.67 | 0.08 | 0.10 | 0.10 | 0.99 | 1.00 | −0.44 | −0.22 | 0.23 |
| Body of CC | 0.74 | 0.08 | 0.78 | 0.08 | 0.75 | 0.10 | 0.13 | 0.14 | 1.00 | 0.70 | −0.43 | −0.11 | 0.30 |
| Splenium of CC | 0.79 | 0.11 | 0.81 | 0.09 | 0.78 | 0.11 | 0.52 | 1.00 | 1.00 | 0.84 | −0.16 | 0.14 | 0.33 |
ATR anterior thalamic radiation, CST corticospinal tract, HC healthy controls, IFOF inferior fronto-occipital fasciculus, ILF inferior longitudinal fasciculus, PIGD-PD patients with postural instability and gait disorder Parkinson’s disease, SD standard deviation, SLF superior longitudinal fasciculus, TD-PD patients with tremor-dominant Parkinson’s disease.
aAnalysis of covariance with Bonferroni-adjusted post hoc tests.
Discussion
In the current study, FBA was used to measure fiber-specific WM changes in nonmedicated patients with early-stage TD and PIGD motor subtypes of PD. Increased WM integrity was demonstrated in specific fiber bundles of patients with TD-PD compared with HC. These findings may shed light on our understanding of WM changes related to motor subtypes of PD and can be helpful in the development of disease-modifying therapies.
Our findings of changes in the CST and other WM areas, including ATR, forceps minor, and SLF, are consistent with previous work using tensor-derived metrics that showed higher FA in patients with TD-PD compared with HC8. Higher log FC and FDC in patients with TD-PD indicate WM macrostructural changes marked by an increase in fiber-bundle size with a higher ability to relay information between the brain regions, respectively14. A study evaluating the microstructure of cross-sectioned axons localized to WM tracts within the dorsal striatum of early-stage α-synuclein transgenic mouse brain reported an increased number of axons as a result of axon outgrowth and arborization22. Incorporating the histopathological findings, the significant changes in log-FC and FDC in the CST of patients with TD-PD are likely due to the increased number of axons that subsequently enlarged the fiber-bundle size (Fig. 3). Nevertheless, further research, including postmortem histology studies, is required to establish the relationship between physiological tissue changes and fixel metrics.
Fig. 3. The interpretation of fixel-based metrics changes.
a Representative images show the enlargement of fiber orientation distributions’ amplitude, which reflects an increase in intra-axonal volume12, in the corticospinal tract (CST) in a patient with tremor-dominant Parkinson’s disease (TD-PD) compared with healthy control. b The schematic represents a fiber-bundle cross section (blue circles represent axons, and the grid represents imaging voxels). The increase in intra-axonal volume might indicate a fiber-bundle enlargement due to increases in axon number, as indexed by greater fiber cross section (FC) and a combined measure of fiber density and cross section (FDC).
The observed changes in fixel metrics in the fiber-specific WM tracts provide valuable evidence for neural compensation mechanisms in nonmedicated patients with early-stage TD-PD. A previous study has highlighted the role of dopamine- and non-dopamine-mediated compensatory mechanisms both within and outside the basal ganglia of patients with PD23, where one manifestation of the neural compensation might be structural remodeling of neural circuitry24. As a consequence, PD has a long premotor or prodromal period during which compensatory mechanisms take place to delay the clinical onset of disabling manifestations, and in the symptomatic period, the neural compensation also has a role in reducing the severity of motor symptoms24,25. In line with previous studies8,26,27, despite longer disease duration, patients with TD-PD had relatively lower Hoehn and Yahr stages, MDS-UPDRS part III total score, and mean progression of motor signs, as well as higher mean SBR compared with patients with PIGD-PD. Here our results might suggest that the WM neural compensation mechanisms predominantly occurring in the CST could be a key to milder disease severity in TD-PD. The CST is the main WM pathway that conducts motor impulses from the primary motor cortex to the spinal cord, which makes it essential to motor performance in PD20. Previous studies showed that the growth in the CST might occur to accommodate the loss of excitability in corticostriatal circuit in the early stage of PD19,28. Furthermore, the changes in the CST might be related to the fact that pathological changes in PD progress in an inverse pattern of brain development29, considering that most rapid maturation is present in the CST and slowest in the genus of corpus callosum in early childhood15. It is also consistent with several previous studies in PD that showed higher FD in the CST with decreased FD in the corpus callosum19 and higher FA in the CST with reduced FA within the substantia nigra, corpus callosum, and cingulate gyrus6.
A combination of basal ganglia pathology (i.e., pallidal dopamine depletion) and compensation in the cerebello–thalamo–cortical circuit was suggested to lead to tremor in PD, explaining the existence of tremor and a more benign disease course in the same patients30. Despite the fact that TD-PD has relatively benign nigrostriatal degeneration compared with PIGD-PD, there is also evidence that resting tremor might emerge as a collateral effect of cerebral mechanisms that compensate for pathophysiological changes producing akinesia31. In 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine primate PD models, tremor usually appears several days after akinesia and rigidity; thus, it was assumed that the appearance of tremor coincides with the time when compensatory mechanisms are presumably being activated2. Furthermore, a study with positron emission tomography found higher metabolic activity in M1 that was closely linked to tremor characteristics32. The M1, which plays a key role in generating neural impulses that regulate movements, is the target of output from basal ganglia and cerebellum and the site from where a part of the corticospinal descending pathway originates33. Thus, it is possible that the compensatory changes in the primary M1 may render this region more susceptible to pathological influences (tremor triggers) from the basal ganglia30.
It is also worth noting that the changes in fiber-bundle size are more prominent than changes in density. In contrast, Li et al.19 applied the FBA and found higher FD (without significant changes in log FC and FDC) in the CST of treated patients with PD with early (Hoehn and Yahr 1–1.5) and middle (Hoehn and Yahr 2–2.5) stages with unknown disease duration compared with HC. The disagreement between our study and the study from Li et al.19 may partly originate from the different sample characteristics and acquisition schemes. Importantly, unlike this study, they did not categorize the PD patients based on the motor subtypes. Moreover, it is known that dopamine replacement therapy is also responsible for neuroplasticity in the WM34. These findings might also suggest that WM changes in fiber density and bundle size occur via separate trajectories, which might depend on the timing of the scans. Indeed, the changes of axons might first be detected as a change in FD, and over time further manifest as a difference in log FC14. In a longitudinal study of patients with PD with longer disease duration (6.3 ± 5.0 years at baseline) compared with our study, reductions in FD were evident between the first follow-up assessment and baseline, whereas log FC was found to decrease only between the second and first follow-up assessment17. Those observations suggest that the first manifestation of WM degeneration in PD might be related to axonal degeneration, subsequently followed by fiber-bundle atrophy17. In the same manner, we speculate that neural compensation in PD first increases the FD and causes further enlargement of the fiber bundle.
In contrast to previous studies using PPMI data8,21, we found no significant inter-group differences in tensor metrics (FA and MD). This may be due to sample differences (i.e., different inclusion criteria) or differences in the analysis methods used between our and previous studies. The evaluation of DTI metrics is indeed influenced by the analysis techniques35. Previous studies utilized a tract-based spatial statistics (TBSS) analysis, the current most commonly used method for VBA of WM diffusion-weighted imaging data36. This method applied the skeleton projection step, which intends to reduce the effects of local misregistration. TBSS is generally considered as a whole-brain voxel-based method; however, in fact, only a very small percentage of the WM is investigated since the skeletonization and projection step are based on regions with high FA and therefore, the majority of WM voxels with crossing fibers and low FA are excluded from the analysis. Thus, the projection step is often thought to be less biologically plausible and to have reduced detection accuracy than a whole-brain VBA37,38.
Our findings should be interpreted in the context of some limitations. We performed our analysis with single nonzero b-value “single-shell” data only, with limited diffusion-weighting (b = 1000 s/mm2) rather than multi-shell with higher b-value data that would have better angular resolution and an improved ability to account for partial volume effects39. To mitigate this, we utilized the single-shell three-tissue-constrained spherical deconvolution (SS3T-CSD) method, which is capable of isolating the diffusion signal attributable to WM by modeling and removing the contribution from gray matter (GM) and cerebrospinal fluid (CSF)40. Further, reverse-phase encoded images were not available for PPMI diffusion-weighted images, and as such, we could not perform correction for magnetic susceptibility-related image distortion that might introduce greater variance in the fixel metrics41. It is also important to note, however, that the direct link between fixel metrics and cellular properties has not been robustly validated. Future studies should examine the underlying biological properties measured by fixel metrics. This study only included a small sample size, with a different number of subjects in each group (HC = 43, TD-PD = 53, and PIGD-PD = 27). The proportion of patients with PIGD manifestations in the PPMI cohort is indeed small42. These limitations might have limited the power of the statistical analyses and led to false-positive or -negative findings, especially in the PIGD-PD group. A PIGD motor subtype has a higher risk of cognitive decline; however, in this study, MoCA scores were significantly lower in TD-PD than PIGD-PD. We suspected that this resulted from decreased statistical power given that previous PPMI studies evaluating baseline data of patients with TD-PD and PIGD-PD with larger sample sizes showed no significant difference in the MoCA score between the groups27,42. Further, a previous PPMI-based DTI study with a smaller sample size (TD-PD, N = 52; PIGD-PD, N = 13) than our study demonstrated no significant difference in the MoCA score between patients with TD-PD and PIGD-PD8. Regardless, all subjects in the current study were without cognitive decline. Intriguingly, in TOI analysis, a nonsignificantly higher mean FDC value was demonstrated in the CST of PIGD-PD group compared with HC. Also, FDC was not significantly different between TD and PIGD groups. We suspect that this was the result of decreased statistical power due to the small sample size. It is also possible that neural compensation occurs in PIGD-PD, but in earlier or shorter times than TD-PD. However, it is important to bear in mind that these results are based solely on cross-sectional data, and so such speculations have to be taken with caution. The limitations of motor clinical scales, which limit the precision of measurement of motor symptoms and impact in early PD43, might have prevented detecting differences in clinical motor scores between the TD-PD and PIGD-PD. In Hoehn and Yahr staging, stage II does not necessarily have a more severe motor disability than stage I44, while the MDS-UPDRS part III comprises more tremor-related items than posture or gait-related items45. Studies evaluating baseline and follow-up (up to 4 years) clinical scores of PD motor subtypes using PPMI data have indeed showed that the differences of Hoehn and Yahr stage or MDS-UPDRS part III total score between TD-PD and PGID-PD were greater with increasing disease duration8,27. We also speculated that the narrow range of clinical scores and nonlinear WM changes in the early stage of PD were responsible for the lack of correlation between FDC and clinical measures. Finally, in some patients, tremor severity tends to decrease instead of worsening during disease progression. In this context, the failure of compensatory mechanisms in later stages of the disease leads to gradual disappearance of tremor30. Taken together, future longitudinal studies starting from the prodromal to late stages with larger sample sizes are necessary to fully depict the clinical trajectory of fixel-based measure changes in particular fiber pathways and the correlation between fixel-based measures and motor-severity scales in patients with TD-PD and PIGD-PD. Although longitudinal data are also available in the PPMI cohort, it is difficult to directly compare them with the baseline data because most of the patients received treatment by the time of follow-up.
In summary, we implemented FBA and found that patients with TD-PD exhibited growth within the CST. The increase in log FC and FDC in the CST might suggest WM neural compensation mechanisms in early-stage TD-PD marked by enlarged fiber bundle size along with a higher ability to relay information between the brain regions, respectively. Due to this study’s exploratory nature, future studies with histopathological verification are necessary to validate our findings. Further, considering the limitations of this study, the results must be interpreted with caution.
Methods
Study participants
The demographic, clinical, and diffusion-weighted imaging data of participants used in this study were downloaded through a standard application process from the PPMI website (http://www.ppmi-info.org/)46. The PPMI study was approved by the Institutional Review Board of all participating sites, and written informed consent was obtained from all subjects.
In the current study, we analyzed the baseline data from 80 patients with PD and 43 age- and gender-matched HC. The participants were included in this study if they met the following criteria: (1) they were without cognitive decline, with MoCA score ≥ 2647; (2) they had complete data on education years, mean SBR, diffusion-weighted images, and three-dimensional T1-weighted images. Patients with PD were included if they met the following criteria: (1) age at PD onset > 40 years; (2) complete clinical data, including disease duration, Hoehn and Yahr stage, and MDS-UDRS part III total score; (3) at early-stage of PD (Hoehn and Yahr stage ≤ 2 and disease duration < 5 years)48, considering that only PD patients with Hoehn and Yahr < 3 had been included in the baseline PPMI cohort46; (4) they were not on any PD medications at baseline; (5) confirmed striatal dopamine deficits; (6) the diagnosis of idiopathic PD was maintained after more than two years of follow-up. In the PPMI cohort, the motor subtypes of patients with PD were categorized into TD, intermediate, or PIGD49 based on the formula published by Stebbins et al.45. Here, patients with PD were included only if they had a predominant type of motor signs (TD-PD, n = 53; PIGD-PD, n = 27) at the disease onset. The mean progression of motor signs over time was then calculated as the total MDS-UPDRS part III score divided by disease duration in months26.
Diffusion-weighted imaging acquisition
The diffusion-weighted imaging data used in the current study were acquired using a standardized protocol across 11 different sites on 3 Tesla TIM Trio Siemens scanners equipped with a 12-channel head coil. The PPMI diffusion-weighted imaging sequence parameters were obtained using single-shot echo-planar imaging sequence with the following parameters: number of diffusion-encoding directions = 64, b-value = 1000 s/mm2, number of nondiffusion (b0) image = 1, repetition time = 900 ms, echo time = 88 ms, matrix size = 116 × 116, slices = 72, flip angle = 90°, voxel resolution = 1.98 × 1.98 mm2, and slice thickness = 2.0 mm. More information on the diffusion-weighted imaging acquisition is available online at http://www.ppmi-info.org/.
Whole-brain FBA
The FBA was implemented using MRtrix3 (http://mrtrix.org) following the recommended pipeline14. Preprocessing of diffusion-weighted images included denoising50, removal of Gibbs ringing artifacts, eddy-current and motion-induced distortion correction51, bias field correction52, and upsampling spatial resolution to an isotropic voxel size of 1.3 mm12. Following the preprocessing steps, FODs for each subject were calculated using SS3T-CSD approach using a group-averaged response function of each tissue type (WM, GM, and CSF)53,54. This step was performed using MRtrix3Tissue (http://3tissue.github.io/), a fork of MRtrix355. In brief, SS3T-CSD allows the estimation of fixed anisotropic single-fiber WM response function and fixed isotropic GM and CSF response functions across the brain using single-shell + b = 0 s/mm2 data40,53. The remaining processing steps included overall image-intensity normalization on subjects’ FOD images to make FOD amplitudes comparable across participants using the median b = 0 intensity, generation of a study-specific FOD template using FOD images from all subjects with linear and nonlinear registration56, and finally, each subject’s FOD image was subsequently registered to the template12,56.
Following the processing steps, three fixel metrics derived from the FBA were calculated as follows:
FD, a microscopic estimate of the axonal density or packing. In the context of spherical deconvolution57 and the apparent fiber-density12 frameworks, the amplitude of the FOD along a particular direction is proportional to the magnitude of the diffusion-weighted signal in the perpendicular (radial) plane, hence, to the intra-axonal volume along the corresponding orientation12. FD was calculated as the volume of intra-axonal compartment per unit volume of tissue14.
FC, a morphological measure of the macroscopic change in cross-sectional size of fiber bundle. FC is calculated as the extent of distortion in bundle cross section that is required to warp a participant’s FOD into the template image14. Prior to statistical analysis, FC was log-transformed (log FC) to ensure zero-centered and normally distributed data. Log FC > 1 or < 1 in the subject compared with the template frame of reference implied a larger or smaller fiber cross section, respectively14.
FDC, a combined measure of FD and FC, calculated as FD multiplied by FC for each fixel. FDC reflects the changes at both microscopic and macroscopic levels, thereby providing sensitivity to any differences related to the capacity of the WM to transmit information14,16.
Whole-brain VBA
FA and MD were calculated using MRtrix3 commands on the same preprocessed images used for FBA. FA and MD maps were subsequently generated in each subject’s space and then transformed to the population template space, by using the same warp generated during the FOD registration step for FBA.
TOI analysis
We further performed TOI analysis in WM tracts associated with early-stage PD8,58, using Johns Hopkins University’s ICBM-DTI-81 WM tractography and labels atlases59,60 to guide categorization. The average FDC was computed across each defined tract per participant—namely, ATR, inferior fronto-occipital fasciculus, SLF, inferior longitudinal fasciculus, cingulum, CST, forceps minor, forceps major, genus, body, and splenium of corpus callosum. FDC was chosen for TOI analysis because it is a combined metric of both microstructural and macrostructural changes representing the overall ability to relay information; hence, it is likely to be the most sensitive of the three fixel metrics14.
Volume-based morphometry
Intracranial volume (ICV) of all subjects was obtained using FreeSurfer version 6.0.0 (http://surfer.nmr.mgh.harvard.edu/fswiki) with the recon-all pipeline61. We used log-transformed ICV (log-ICV) as a nuisance covariate in whole-brain FBA statistical analysis.
Statistical analysis
Demographic and clinical assessments
All statistical analyses, except for whole-brain FBA and VBA, were performed using IBM SPSS Statistics version 25.0 (IBM Corporation, Armonk, NY, USA). Kolmogorov–Smirnov test was used to assess the normality of the data. The demographic and clinical data were analyzed using the unpaired t tests or Mann–Whitney U for two groups; one-way analysis of variance (ANOVA; post hoc analysis: Tukey test) or Kruskal–Wallis test were used for comparison of three groups, for normally or non-normally distributed data, respectively, and for continuous variables, while the chi-square test was used for categorical variables. Statistical significance was set at P < 0.05.
Whole-brain FBA
A general linear model (GLM) framework was utilized to compare FD, log FC, and FDC between (i) HC and TD-PD, (ii) HC and PIGD-PD, and (iii) TD-PD and PIGD-PD, with age, sex, and years of education included as nuisance covariates. To avoid false-positive results, additionally, log-ICV was used as a nuisance covariate for log FC and FDC (but not FD) to remove global effects of brain scaling resulting from the registration to a template14,62. Connectivity-based fixel enhancement for statistical inference13 with 2 million streamlines from the template tractogram and default smoothing parameters (smoothing = 10-mm full-width at half-maximum, C = 0.5, E = 2, and H = 3)13 was used. FWE-corrected P-values were then assigned to each fixel using non-parametric permutation testing over 5000 permutations.
Whole-brain VBA
Voxel-wise statistical analysis was performed in template space with threshold-free cluster enhancement using default parameters (dh = 0.1, E = 0.5, H = 2)63 across the whole brain for the same comparison as in the FBA. An FWE-corrected P < 0.05 was considered statistically significant.
TOI analysis
Mean FDC value was compared across groups using a one-way analysis of covariance (ANCOVA) followed by pairwise post hoc comparisons (Bonferroni test: HC vs. TD-PD, HC vs. PIGD-PD, and TD-PD vs. PIGD-PD) with age, sex, and years of education as covariates. The effect size was calculated using Cohen’s d to evaluate the statistical power of the relationship determined during intergroup comparisons. Effect sizes of 0.2, 0.5, and 0.8 were classified as small, medium, and large, respectively64. Correlational analyses of mean tract FDC with the disease duration or mean SBR or MDS-UPDRS part III total score or mean progression of motor signs or MoCA scores of the TD-PD and PIGD-PD were then performed using partial correlation test with age, sex, and years of education included as nuisance covariates. The Bonferroni correction was then used for multiple comparisons of 11 WM tracts with a significance level of P < 0.05/11 = 0.0045.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The funding sources of PPMI are available on www.ppmi-info.org/fundingpartners. CA is an overseas researcher under Postdoctoral Fellowship of Japan Society for the Promotion of Science (JSPS). This research was also supported by JSPS KAKENHI, Grant/Award Numbers: 18H02772, 19K17244, and JP16H06280; Brain/MINDS Beyond program from AMED, Grant/Award Numbers: JP19dm0307024 and JP19dm0307101; Grant-in-Aid for Special Research in Subsidies for ordinary expenses of private schools from The Promotion and Mutual Aid Corporation for Private Schools of Japan; and Juntendo Research Branding Project.
Author contributions
C.A., K.K., Y.S., W.U., T.O., T.H., N.H. and S.A. contributed to the conception and design of the study. C.A., K.K., Y.S., W.U., S.F., A.H., T.A. and A.W. contributed to the data collection, acquisition, and analysis of data. C.A., K.K., T.H., T.O., N.H. and S.A. contributed to drafting the paper and preparing the figures. All authors have reviewed and approved the contents of the paper.
Data availability
Data used in the preparation of this paper were obtained from the PPMI database (www.ppmi-info.org/data). PPMI data are freely accessible to researchers from the following link: http://www.ppmi-info.org/access-data-specimens/download-data. Access to data collected by PPMI data can be requested by completing the online application (including signing the Data Use Agreement and providing some detailed information). The datasets analyzed during the current study are available from the corresponding author on reasonable request and with permission of PPMI.
Code availability
All neuroimaging analyses performed in this paper were performed with freely available neuroimaging tools, namely MRtrix3 (http://mrtrix.org/) and FreeSurfer (http://surfer.nmr.mgh.harvard.edu/fswiki). Statistical analyses were performed using IBM SPSS Statistics version 25.0 (IBM Corporation, Armonk, NY, USA).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christina Andica, Email: christina@juntendo.ac.jp.
Koji Kamagata, Email: kkamagat@juntendo.ac.jp.
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-021-00197-4.
References
- 1.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 2.Zaidel A, Arkadir D, Israel Z, Bergman H. Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr. Opin. Neurol. 2009;22:387–393. doi: 10.1097/WCO.0b013e32832d9d67. [DOI] [PubMed] [Google Scholar]
- 3.Burn DJ, et al. Motor subtype and cognitive decline in Parkinson’s disease, Parkinson’s disease with dementia, and dementia with Lewy bodies. J. Neurol. Neurosurg. Psychiatry. 2006;77:585–589. doi: 10.1136/jnnp.2005.081711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1991;50:743–755. doi: 10.1097/00005072-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Andica C, et al. MR Biomarkers of degenerative brain disorders derived from diffusion imaging. J. Magn. Reson Imaging. 2020;52:1620–1636. doi: 10.1002/jmri.27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atkinson-Clement C, Pinto S, Eusebio A, Coulon O. Diffusion tensor imaging in Parkinson’s disease: review and meta-analysis. Neuroimage Clin. 2017;16:98–110. doi: 10.1016/j.nicl.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen MC, et al. Differential white matter regional alterations in motor subtypes of early drug-naive Parkinson’s disease patients. Neurorehabil. Neural Repair. 2018;32:129–141. doi: 10.1177/1545968317753075. [DOI] [PubMed] [Google Scholar]
- 9.Jeurissen B, Leemans A, Tournier JD, Jones DK, Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum. Brain Mapp. 2013;34:2747–2766. doi: 10.1002/hbm.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douaud G, et al. DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer’s disease. Neuroimage. 2011;55:880–890. doi: 10.1016/j.neuroimage.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- 12.Raffelt D, et al. Apparent Fibre Density: a novel measure for the analysis of diffusion-weighted magnetic resonance images. Neuroimage. 2012;59:3976–3994. doi: 10.1016/j.neuroimage.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 13.Raffelt DA, et al. Connectivity-based fixel enhancement: whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. Neuroimage. 2015;117:40–55. doi: 10.1016/j.neuroimage.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raffelt DA, et al. Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage. 2017;144:58–73. doi: 10.1016/j.neuroimage.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimond D, et al. Early childhood development of white matter fiber density and morphology. Neuroimage. 2020;210:116552. doi: 10.1016/j.neuroimage.2020.116552. [DOI] [PubMed] [Google Scholar]
- 16.Genc S, et al. Development of white matter fibre density and morphology over childhood: a longitudinal fixel-based analysis. Neuroimage. 2018;183:666–676. doi: 10.1016/j.neuroimage.2018.08.043. [DOI] [PubMed] [Google Scholar]
- 17.Rau YA, et al. A longitudinal fixel-based analysis of white matter alterations in patients with Parkinson’s disease. Neuroimage Clin. 2019;24:102098. doi: 10.1016/j.nicl.2019.102098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarkali A, et al. Fiber-specific white matter reductions in Parkinson hallucinations and visual dysfunction. Neurology. 2020;94:e1525–e1538. doi: 10.1212/WNL.0000000000009014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, et al. Fixel-based analysis reveals fiber-specific alterations during the progression of Parkinson’s disease. Neuroimage Clin. 2020;27:102355. doi: 10.1016/j.nicl.2020.102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mole JP, et al. Increased fractional anisotropy in the motor tracts of Parkinson’s disease suggests compensatory neuroplasticity or selective neurodegeneration. Eur. Radio. 2016;26:3327–3335. doi: 10.1007/s00330-015-4178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen MC, et al. White matter microstructural characteristics in newly diagnosed Parkinson’s disease: an unbiased whole-brain study. Sci. Rep. 2016;6:35601. doi: 10.1038/srep35601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schechter M, et al. A role for alpha-Synuclein in axon growth and its implications in corticostriatal glutamatergic plasticity in Parkinson’s disease. Mol. Neurodegener. 2020;15:24. doi: 10.1186/s13024-020-00370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bezard E, Gross CE, Brotchie JM. Presymptomatic compensation in Parkinson’s disease is not dopamine-mediated. Trends Neurosci. 2003;26:215–221. doi: 10.1016/S0166-2236(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 24.Brotchie J, Fitzer-Attas C. Mechanisms compensating for dopamine loss in early Parkinson disease. Neurology. 2009;72:S32–S38. doi: 10.1212/WNL.0b013e318198e0e9. [DOI] [PubMed] [Google Scholar]
- 25.Blesa J, et al. Compensatory mechanisms in Parkinson’s disease: circuits adaptations and role in disease modification. Exp. Neurol. 2017;298:148–161. doi: 10.1016/j.expneurol.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Baumann CR, Held U, Valko PO, Wienecke M, Waldvogel D. Body side and predominant motor features at the onset of Parkinson’s disease are linked to motor and nonmotor progression. Mov. Disord. 2014;29:207–213. doi: 10.1002/mds.25650. [DOI] [PubMed] [Google Scholar]
- 27.Lee JW, Song YS, Kim H, Ku BD, Lee WW. Alteration of tremor dominant and postural instability gait difficulty subtypes during the progression of Parkinson’s disease: Analysis of the PPMI cohort. Front Neurol. 2019;10:471. doi: 10.3389/fneur.2019.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arkadir D, Bergman H, Fahn S. Redundant dopaminergic activity may enable compensatory axonal sprouting in Parkinson disease. Neurology. 2014;82:1093–1098. doi: 10.1212/WNL.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 29.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 30.Helmich RC, Hallett M, Deuschl G, Toni I, Bloem BR. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain. 2012;135:3206–3226. doi: 10.1093/brain/aws023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivlin-Etzion M, Elias S, Heimer G, Bergman H. Computational physiology of the basal ganglia in Parkinson’s disease. Prog. Brain Res. 2010;183:259–273. doi: 10.1016/S0079-6123(10)83013-4. [DOI] [PubMed] [Google Scholar]
- 32.Burciu RG, Vaillancourt DE. Imaging of motor cortex physiology in Parkinson’s disease. Mov. Disord. 2018;33:1688–1699. doi: 10.1002/mds.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J. Neurosci. 1999;19:1446–1463. doi: 10.1523/JNEUROSCI.19-04-01446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhuang X, Mazzoni P, Kang UJ. The role of neuroplasticity in dopaminergic therapy for Parkinson disease. Nat. Rev. Neurol. 2013;9:248–256. doi: 10.1038/nrneurol.2013.57. [DOI] [PubMed] [Google Scholar]
- 35.Urger E, et al. Influence of analysis technique on measurement of diffusion tensor imaging parameters. AJR Am. J. Roentgenol. 2013;200:W510–W517. doi: 10.2214/AJR.12.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith SM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 37.Bach M, et al. Methodological considerations on tract-based spatial statistics (TBSS) Neuroimage. 2014;100:358–369. doi: 10.1016/j.neuroimage.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz CG, et al. Improved DTI registration allows voxel-based analysis that outperforms tract-based spatial statistics. Neuroimage. 2014;94:65–78. doi: 10.1016/j.neuroimage.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tournier JD, Calamante F, Connelly A. Determination of the appropriate b value and number of gradient directions for high-angular-resolution diffusion-weighted imaging. NMR Biomed. 2013;26:1775–1786. doi: 10.1002/nbm.3017. [DOI] [PubMed] [Google Scholar]
- 40.Dhollander T, Mito M, Raffelt D, Connelly A. Improved white matter response function estimation for 3-tissue constrained spherical deconvolution. 27th Int. Soc. Magn. Reson. Med. 2019;27:555. [Google Scholar]
- 41.Mito R, et al. Fibre-specific white matter reductions in Alzheimer’s disease and mild cognitive impairment. Brain. 2018;141:888–902. doi: 10.1093/brain/awx355. [DOI] [PubMed] [Google Scholar]
- 42.Aleksovski D, Miljkovic D, Bravi D, Antonini A. Disease progression in Parkinson subtypes: the PPMI dataset. Neurol. Sci. 2018;39:1971–1976. doi: 10.1007/s10072-018-3522-z. [DOI] [PubMed] [Google Scholar]
- 43.Regnault A, et al. Does the MDS-UPDRS provide the precision to assess progression in early Parkinson’s disease? Learnings from the Parkinson’s progression marker initiative cohort. J. Neurol. 2019;266:1927–1936. doi: 10.1007/s00415-019-09348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goetz CG, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov. Disord. 2004;19:1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 45.Stebbins GT, et al. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov. Disord. 2013;28:668–670. doi: 10.1002/mds.25383. [DOI] [PubMed] [Google Scholar]
- 46.Marek K, et al. The Parkinson’s progression markers initiative (PPMI) - establishing a PD biomarker cohort. Ann. Clin. Transl. Neurol. 2018;5:1460–1477. doi: 10.1002/acn3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalrymple-Alford JC, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75:1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 48.Bergamino M, Keeling EG, Mishra VR, Stokes AM, Walsh RR. Assessing white matter pathology in early-stage Parkinson disease using diffusion MRI: a systematic review. Front. Neurol. 2020;11:314. doi: 10.3389/fneur.2020.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jankovic J, et al. Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40:1529–1534. doi: 10.1212/WNL.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 50.Veraart J, Fieremans E, Novikov DS. Diffusion MRI noise mapping using random matrix theory. Magn. Reson Med. 2016;76:1582–1593. doi: 10.1002/mrm.26059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andersson JL, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tustison NJ, et al. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dhollander T, Connelly A. A novel iterative approach to reap the benefits of multi-tissue CSD from just single-shell (+ b = 0) diffusion MRI data. 24th Int. Soc. Magn. Reson. Med. 2016;24:3010. [Google Scholar]
- 54.Dhollander, T., Raffelt, D. & Connelly, A. Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. in ISMRM Workshop on Breaking the Barriers of Diffusion MRI (ISMRM, 2016).
- 55.Tournier JD, et al. MRtrix3: a fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202:116137. doi: 10.1016/j.neuroimage.2019.116137. [DOI] [PubMed] [Google Scholar]
- 56.Raffelt D, et al. Symmetric diffeomorphic registration of fibre orientation distributions. Neuroimage. 2011;56:1171–1180. doi: 10.1016/j.neuroimage.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 57.Tournier JD, Calamante F, Gadian DG, Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage. 2004;23:1176–1185. doi: 10.1016/j.neuroimage.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 58.Chen NK, et al. Alteration of diffusion-tensor magnetic resonance imaging measures in brain regions involved in early stages of Parkinson’s disease. Brain Connect. 2018;8:343–349. doi: 10.1089/brain.2017.0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hua K, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wakana S, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 62.Smith, R. E., Dhollander, T. & Connelly, A. On the regression of intracranial volume in Fixel-Based Analysis in 27th International Society of Magnetic Resonace in Medicine (International Society of Magnetic Resonace in Medicine, 2019).
- 63.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 64.Cohen J. A power primer. Psychol. Bull. 1992;112:155–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in the preparation of this paper were obtained from the PPMI database (www.ppmi-info.org/data). PPMI data are freely accessible to researchers from the following link: http://www.ppmi-info.org/access-data-specimens/download-data. Access to data collected by PPMI data can be requested by completing the online application (including signing the Data Use Agreement and providing some detailed information). The datasets analyzed during the current study are available from the corresponding author on reasonable request and with permission of PPMI.
All neuroimaging analyses performed in this paper were performed with freely available neuroimaging tools, namely MRtrix3 (http://mrtrix.org/) and FreeSurfer (http://surfer.nmr.mgh.harvard.edu/fswiki). Statistical analyses were performed using IBM SPSS Statistics version 25.0 (IBM Corporation, Armonk, NY, USA).