Abstract
Aim
A transition toward high serum folate concentrations has been noticed following the mandatory folate fortification. To explore this further, we studied the relationship between folate and health outcomes in population with chronic kidney disease (CKD).
Methods
We retrospectively explored the relationships between serum folate and risk of all-cause death in this population. We analyzed data of 2142 subjects with CKD who participated in the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Vital status was followed through December 31, 2006.
Results
Cox regression was used to estimate hazard ratios (HRs) of mortality for individuals with serum folate in rest quintiles compared with individuals with the fourth quintile. After an average follow-up of 57.4 months with 157 deaths recorded, a reversed J-shaped association was revealed after conducting multivariable adjustment. The mortality rate in population with lower and higher folate levels were 8.29% and 12.67%, respectively, and the corresponding adjusted HRs were 2.41 (95% confidence interval, CI=1.32–4.40) and 2.10 (1.20–3.70). Kaplan–Meier curve showed survival benefits for the fourth quintile of serum folate as compared to the first and fifth quintile.
Conclusion
Serum folate concentrations may influence all-cause mortality in a non-linear pattern in the CKD population. It is reasonable to recommend periodic surveillance in the CKD population to maintain the serum folate concentration in an appropriate level.
Keywords: serum folate, mortality, chronic kidney disease; CKD, National Health and Nutrition Examination Survey; NHANES, cohort study
Introduction
Whether folate supplement could bring survival benefit to population with chronic kidney disease (CKD) is so far controversial. Previous clinical trials indicated the presence of potential dosage-response relationship as to the treatment efficacy of folate. A trial enrolling 252 participants reported in 2010 indicated harmful effects with 2.5mg/d extra supplement based on folate fortification, with accelerated renal impairments and increased fatality.1 Another study stated folate supplement by 0.8mg/d could delay renal function impairment and bring survival benefits to a hypertensive CKD subgroup from China, without implementation of folate fortification.2 Folate consumption varied considerably among every single person after the initiation of folic acid fortification from 1998 in the US. Supraphysiologic concentrations of serum folate have been found in 38% of the elderly in the US.3 Since there was no consensus as to the recommended folate intake dosage, it is necessary to find and monitor the optimal level of serum folate in the CKD population, especially subjects from the US.
In this context, we sought to explore the relationship between serum folate concentration and all-cause mortality based on a large nationally representative US sample in the setting of a retrospective cohort study. It is speculated that there maybe a non-linear association between serum folate concentration and the risk of death. Lower or higher levels of serum folate were risk factors for death.
Materials and Methods
Study Participants
The study data were retrieved from the National Health and Nutrition Examination Survey (NHANES) 1999–2006. NHANES was conducted by the National Center for Health Statistics (NCHS) and used a stratified, multistage framework to achieve a nationally representative sample of the US population. The data combined various components including household interviews, physical examinations, and laboratory tests.
In this study, CKD was defined according to the estimated glomerular filtration rate (eGFR) and urinary albumin-creatinine ratios (ACRs). eGFR was calculated using the isotope dilution mass spectrometry 4-variable Modification of Diet in Renal Disease Study equation (MDRD).4 ACRs were acquired from urine samples and categorized as less than 30, 30 to 300, or greater than 300mg/g. CKD stages were defined as follows: stage 1, eGFR greater than 90 mL/min/1.73 m2 and ACR of 30 mg/g or greater; stage 2, eGFR of 60 to 89 mL/min/1.73 m2 and ACR of 30 mg/g or greater; stage 3, eGFR of 30 to 59 mL/min/1.73 m2; stage 4, eGFR of 15 to 29 mL/min/1.73 m2; and stage 5, eGFR less than 15 mL/min/1.73 m2.5
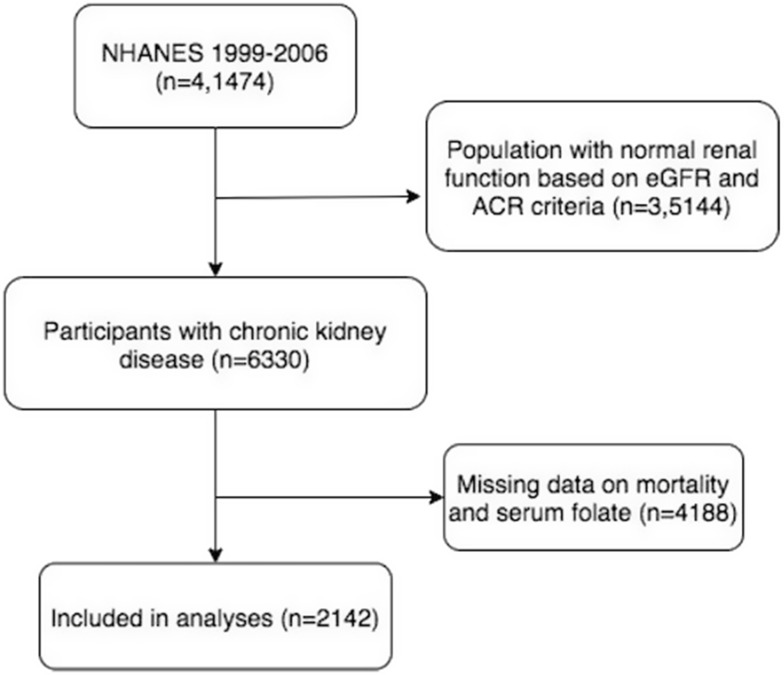
There were total 41,474 participants included in NHANES during 1999–2006, among which were 6330 CKD subjects as defined. After exclusion of 4188 cases due to missing information on mortality and serum folate concentration, 2142 subjects were enrolled in the final retrospective analysis (Figure 1). Due to the differentiated background between 2142 participants and the other 4188 excluded subjects, any conclusion drawn from this study may not be well fitted to CKD populations without information on serum folate and mortality (Supplement Material 1). The NHANES protocol was reviewed and approved by the NCHS’s Institutional Review Board (IRB), and the current study was exempt from ethics review by Georgia Southern University IRB committee according to Ethic Review Board Approval protocol #98-12 and protocol #2005-06.
Figure 1.
Flowchart of participation selection.
Data Collection
The Bio-Rad radioassay was used to detect serum folate concentrations between 1999 and 2006. Mortality outcome was determined by NHANES linked National Death Index (NDI) public-access files through December 31, 2006. A total of 12 factors (including social security number, sex, and date of birth) were used to link NHANES participants with the NDI to assess vital status. In this analysis, we only focused on all-cause death due to insufficient effective samples. Follow-up duration was obtained from the date of examination to the date of death or the end of 2006.
Covariates were included as potential confounders in the final models if they changed the estimates of serum folate on mortality by more than 10% or were significantly associated with mortality in the CKD population. The following covariates were selected a priori on the basis of established associations and/or plausible biological relations and tested: basic demographics including age at baseline, gender, race, body mass index (BMI); laboratory tests including serum glucose, phosphorus, total iron binding capacity (TIBC), serum vitamin B12, uric acid, albumin and inferred data eGFR; comorbidities and lifestyle information including hypertension and physical activity, folate consumption as dietary folate equivalents and food folate. Currently, the use of dietary folate equivalents is recommended for planning and evaluating the adequacy of people’s folate intake. According to the US Food and Drug Administration regulations, folic acid has been added to enriched cereal grains and thus affects hundreds of food products. The dietary folate equivalents provided by fortified foods equal the micrograms of food folate plus 1.7 times the micrograms of added folic acid.
Statistical Analysis
Previous studies have demonstrated that, when analyzing follow-up data, the results of weighted analyses were generally consistent with the conclusion drawn from un-weighted estimation, which have smaller difference.6 Accordingly, we perform the un-weighted estimates in this cohort study.
Baseline characteristics of participants are expressed as mean (standard deviation) (Gaussian distribution) or median (range) (Skewed distribution) for continuous variables, and as percentages for categorical variables. We used χ2 (categorical variables), One-Way ANOVA test (normal distribution), or Kruskal-Wallis H-test (skewed distribution) to test for differences among different serum folate (quintile). We used univariate and multivariate Cox proportional-hazards regression model to test the link between serum folate and all-cause mortality with three distinct models. Model 1 is the non-adjusted model with no covariates adjusted. Model 2 is the minimally-adjusted model with only sociodemographic variables adjusted. Model 3 is the fully-adjusted model with covariates presented above adjusted. Account for non-linear correlation between serum folate and all-cause mortality, we also used Cox proportional hazards regression model with cubic spline functions and the smooth curve fitting (penalized spline method) to address nonlinearity. Besides, two-piecewise Cox proportional-hazards regression model was also used to explain the nonlinearity further.
To text the robustness of our results, we performed a sensitivity analysis. We converted serum folate into a categorical variable according to the quintile, and calculated the P for trend in order to verify the results of serum folate as the continuous variable, and to examine the possibility of nonlinearity.
The proportional hazards assumption was checked by visual inspection of plots of log[-log(S)] against time, where S was the estimated survival function. Study participants who survived the entire follow-up period were censored on December 31, 2006.
All the analyses were performed with the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc, Boston, MA). p values less than 0.05 (two-sided) were considered statistically significant.
Results
The median serum folate concentration was 13 (1.7–184.5) ng/mL. The average follow-up of 57.4 months with a maximum of 71 was obtained from the 2142 subjects. A total of 157 (7.3%) deaths were documented. The main characteristics of the cohort at baseline are summarized in Table 1. Overall, participants with high serum folate were older and more likely to be female, of lower BMI, to have lower eGFR. Besides, participants with high serum folate were more likely to have higher levels of serum vitamin B12, consuming more folate as dietary folate equivalents and food folate.
Table 1.
Baseline Characteristic of the Study Participants with Chronic Kidney Disease (n=2142)
| Characteristics | Quintile Categories of Serum Folate, ng/mL | p-values | ||||
|---|---|---|---|---|---|---|
| Q1 (<8.7) | Q2 (8.7–11.4) | Q3 (11.5–14.6) | Q4 (14.7–19.1) | Q5 (≥19.2) | ||
| N | 422 | 431 | 428 | 427 | 434 | |
| Age (yr) | 45.42 (18.32) | 44.22 (18.03) | 48.54 (18.90) | 51.03 (19.48) | 59.73 (20.38) | <0.001 |
| BMI (Kg/m2) | 29.07 (6.59) | 28.86 (7.05) | 27.96 (5.93) | 27.40 (5.40) | 27.17 (5.45) | <0.001 |
| eGFR (mL/min/1.73 m2) | 46.87 (9.80) | 47.20 (9.73) | 44.94 (10.66) | 44.42 (10.66) | 42.51 (10.13) | <0.001 |
| Urinary albumin (ug/mL) | 9.00 (0.40–4870.00) | 8.40 (0.40–9910.00) | 8.40 (0.20–9820.00) | 7.60 (0.30–3580.00) | 8.40 (0.20–7570.00) | 0.064 |
| Urinary creatinine (g/L) | 1.28 (0.12–4.59) | 1.24 (0.09–5.34) | 1.15 (0.09–7.74) | 1.05 (0.07–5.48) | 0.86 (0.06–3.98) | <0.001 |
| Time to death or censorship from time of examination (months) | 56.12 (11.84) | 58.28 (9.40) | 57.76 (9.67) | 58.60 (8.79) | 56.24 (12.21) | <0.001 |
| Folate as dietary folate equivalents (mcg) | 410.00 (0.00–2781.00) | 423.00 (0.00–5715.00) | 454.00 (66.00–2388.00) | 477.00 (66.00–6635.00) | 494.00 (38.00–3126.00) | <0.001 |
| Food folate (mcg) | 157.50 (0.00–1557.00) | 178.00 (0.00–1209.00) | 184.00 (23.00–702.00) | 188.00 (16.00–1242.00) | 189.50 (12.00–1227.00) | <0.001 |
| Hemoglobin A1c (%) | 5.61 (1.01) | 5.65 (1.21) | 5.61 (1.11) | 5.58 (1.05) | 5.60 (1.06) | 0.895 |
| Serum Vitamin B12 (pg/mL) | 412.00 (100.00–9442.00) | 423.00 (94.00–52710.00) | 454.00 (80.00–16114.00) | 475.00 (94.00–2710.00) | 575.50 (100.00–3076.00) | <0.001 |
| Albumin (g/dL) | 4.13 (0.35) | 4.18 (0.33) | 4.24 (0.29) | 4.18 (0.33) | 4.14 (0.38) | <0.001 |
| Serum glucose (mg/dL) | 98.76 (37.64) | 98.10 (38.70) | 96.60 (33.37) | 95.75 (31.93) | 98.46 (34.23) | 0.680 |
| Phosphorus (mg/dL) | 3.73 (0.55) | 3.69 (0.53) | 3.72 (0.52) | 3.76 (0.57) | 3.82 (0.57) | 0.006 |
| Uric acid (mg/dL) | 5.17 (1.48) | 5.19 (1.54) | 5.20 (1.40) | 5.14 (1.47) | 5.33 (1.62) | 0.383 |
| TIBC, Frozen serum (ug/dL) | 371.44 (72.17) | 367.11 (63.66) | 374.23 (64.47) | 376.02 (65.96) | 368.16 (75.14) | 0.254 |
| Mortality | <0.001 | |||||
| Alive | 387 (91.71%) | 408 (94.66%) | 401 (93.69%) | 410 (96.02%) | 379 (87.33%) | |
| Death | 35 (8.29%) | 23 (5.34%) | 27 (6.31%) | 17 (3.98%) | 55 (12.67%) | |
| Sex | 0.004 | |||||
| Female | 250 (59.24%) | 259 (60.09%) | 260 (60.75%) | 282 (66.04%) | 303 (69.82%) | |
| Male | 172 (40.76%) | 172 (39.91%) | 168 (39.25%) | 145 (33.96%) | 131 (30.18%) | |
| Hypertension | <0.001 | |||||
| No | 285 (67.54%) | 320 (74.25%) | 302 (70.56%) | 308 (72.13%) | 232 (53.46%) | |
| Yes | 129 (30.57%) | 109 (25.29%) | 124 (28.97%) | 119 (27.87%) | 201 (46.31%) | |
| No records | 8 (1.90%) | 2 (0.46%) | 2 (0.47%) | 0 (0.00%) | 1 (0.23%) | |
| Physical activity | 0.005 | |||||
| Sit during the day | 138 (32.70%) | 123 (28.54%) | 118 (27.57%) | 92 (21.55%) | 110 (25.35%) | |
| Stand/Walk a lot | 111 (26.30%) | 117 (27.15%) | 107 (25.00%) | 87 (20.37%) | 113 (26.04%) | |
| Light load/Climb stairs often | 54 (12.80%) | 66 (15.31%) | 60 (14.02%) | 76 (17.80%) | 70 (16.13%) | |
| Heavy work/load | 97 (22.99%) | 103 (23.90%) | 112 (26.17%) | 144 (33.72%) | 119 (27.42%) | |
| No records | 22 (5.21%) | 22 (5.10%) | 31 (7.24%) | 28 (6.56%) | 22 (5.07%) | |
| Race | <0.001 | |||||
| Black | 114 (27.01%) | 108 (25.06%) | 73 (17.06%) | 61 (14.29%) | 40 (9.22%) | |
| Mexican American | 106 (25.12%) | 104 (24.13%) | 101 (23.60%) | 97 (22.72%) | 48 (11.06%) | |
| Other Hispanic | 12 (2.84%) | 23 (5.34%) | 22 (5.14%) | 21 (4.92%) | 14 (3.23%) | |
| Other ethnicity | 190 (45.02%) | 196 (45.48%) | 232 (54.21%) | 248 (58.08%) | 332 (76.50%) | |
| Chronic kidney disease | 0.002 | |||||
| Stage2 | 10 (2.37%) | 14 (3.25%) | 3 (0.70%) | 3 (0.70%) | 1 (0.23%) | |
| Stage3 | 386 (91.47%) | 397 (92.11%) | 392 (91.59%) | 389 (91.10%) | 398 (91.71%) | |
| Stage4 | 23 (5.45%) | 18 (4.18%) | 26 (6.07%) | 25 (5.85%) | 25 (5.76%) | |
| Stage5 | 3 (0.71%) | 2 (0.46%) | 7 (1.64%) | 10 (2.34%) | 10 (2.30%) | |
Notes: Values are presented as mean (standard deviation) or median (minimum-maximal) for continuous variables, and as number (percentage) for categorical variables.
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; TIBC, total iron binding capacity.
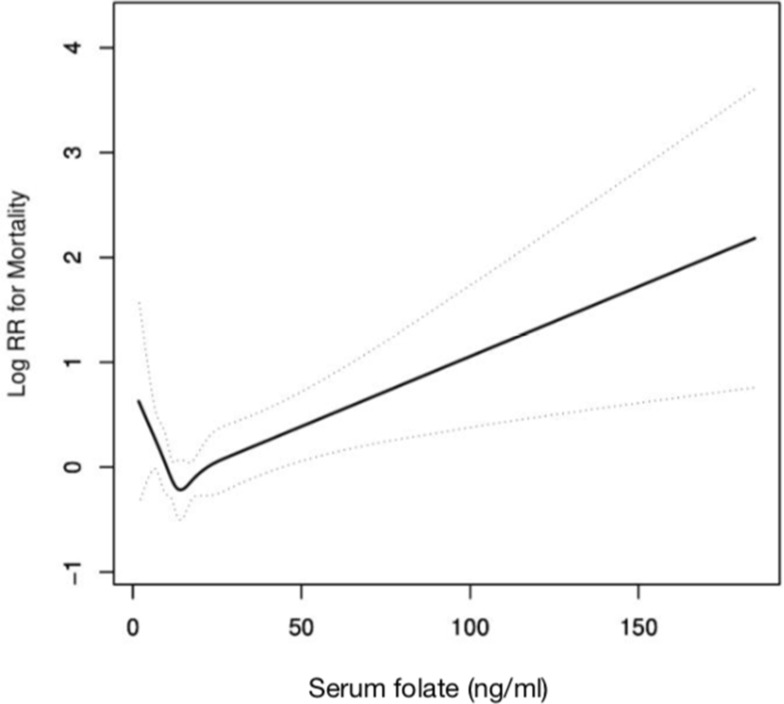
The death rate in 5 quintile categories were 8.29%, 5.34%, 6.31%, 3.98% and 12.67%, respectively. In the whole cohort, the lowest folate quintile was related to increased all-cause mortality (HR: 2.18, 95% CI: 1.22–3.89), without considering the effects of other confounders (Table 2). The association stayed stable in the fully adjusted model. In addition, the highest folate quintile was also linked to much greater rates of mortality as compared to the fourth quintile, further control for potential confounders did not diminish the effect size for the association. Thus, the correlation patterns between serum folate levels and mortality may be J-shaped. In this regard, we tested linearity between folate levels and mortality to explore whether there are nonlinear dose-response relationship, and the findings confirmed the existence of such relationship. The results of the nonlinear association between folate status, as a continuous variable, and mortality in the multivariate proportional hazards models are displayed in Figure 2. Further analysis indicated that there is an inflection point. As shown in Figure 2 and Table 3, the risk of all-cause mortality sharply dropped as folate concentration increased, reaching a relatively low level approximately in 14.8ng/mL that gradually leveled off displaying a reversed J-shaped pattern, with p for nonlinear=0.009.
Table 2.
Multivariate Analysis of Serum Folate Levels Associated with All-Cause Mortality
| Exposure | Model 1 HR (95% CI), p | Model 2 HR (95% CI), p | Model 3 HR (95% CI), p | Model 4 HR (95% CI), p |
|---|---|---|---|---|
| Serum folate (ng/mL) | 1.02 (1.02, 1.03) <0.0001 | 1.01 (1.00, 1.02) 0.0067 | 1.01 (1.00, 1.02) 0.0182 | 1.01 (1.00, 1.02) 0.0195 |
| Serum folate quintile | ||||
| Q1 (<8.7) | 2.18 (1.22, 3.89) 0.0083 | 2.81 (1.57, 5.03) 0.0005 | 2.41 (1.32, 4.40) 0.0043 | 2.47 (1.35, 4.53) 0.0034 |
| Q2 (8.7–11.4) | 1.35 (0.72, 2.52) 0.3497 | 1.93 (1.03, 3.63) 0.0413 | 1.69 (0.88, 3.24) 0.1128 | 1.69 (0.88, 3.25) 0.1123 |
| Q3 (11.5–14.6) | 1.61 (0.88, 2.95) 0.1247 | 1.82 (0.99, 3.33) 0.0539 | 1.92 (1.03, 3.58) 0.0395 | 1.93 (1.04, 3.60) 0.0380 |
| Q4 (14.7–19.1) | Reference | Reference | Reference | Reference |
| Q5 (≥19.2) | 3.32 (1.93, 5.73) <0.0001 | 2.09 (1.20, 3.62) 0.0089 | 2.10 (1.20, 3.70) 0.0097 | 2.09 (1.19, 3.67) 0.0107 |
| p for trend | 0.0001 | 0.1302 | 0.0713 | 0.0808 |
Notes: Model 1 adjusted for none. Model 2 adjusted for age, sex and race. Model 3 model 2 further adjusted for hypertension, physical activity, body mass index, eGFR, serum vitamin B12, albumin, serum glucose, phosphorus, uric acid, total iron binding capacity. Model 4 model 3 further adjusted for folate as dietary folate equivalents, food folate.
Figure 2.
Adjusted restricted cubic spline plots for all-cause mortality and serum folate concentrations. The potential confounders adjusted were as follows: age, sex, race, hypertension, physical activity, body mass index, eGFR, serum vitamin B12, albumin, serum glucose, phosphorus, uric acid, total iron binding capacity, folate as dietary folate equivalents, food folate.
Table 3.
Threshold Effect Analysis of Serum Folate on Mortality Using Piece-Wise Linear Regression Model
| Model | HR (95% CI), p |
|---|---|
| Linear analysis model | 1.01 (1.00, 1.02) 0.0195 |
| Two-piece-wise regression model | |
| Inflection point of serum folate (ng/mL) | 14.8 |
| < Inflection point | 0.94 (0.89, 0.99) 0.0236 |
| > Inflection point | 1.01 (1.01, 1.02) 0.0004 |
| Likelihood ratio test | 0.009 |
Notes: To assess threshold effect and estimate the risk of death associated with folate concentration, we dichotomized serum folate using the cutoff of 14.8ng/mL. The adjusted variances included in the Cox model were as following: age, sex, race, hypertension, physical activity, body mass index, eGFR, serum vitamin B12, albumin, serum glucose, phosphorus, uric acid, total iron binding capacity, folate as dietary folate equivalents, food folate.
Abbreviations: ACRs, urinary albumin-creatinine ratios; BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; IRB, Institutional Review Board; MDRD, Modification of Diet in Renal Disease Study equation; NCHS, National Center for Health Statistics; NDI, National Death Index; NHANES, National Health and Nutrition Examination Survey; TIBC, total iron binding capacity.
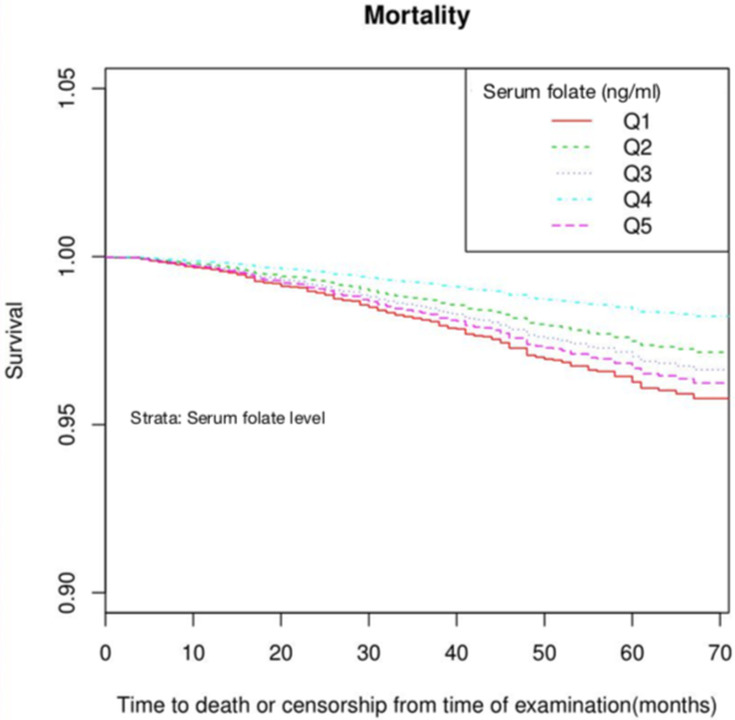
To specifically describe the tendency of hazard of death across the levels of serum folate overtime, the adjusted survival functions stratified by the level of serum folate were presented in Figure 3. For all-cause mortality, the fourth quintile (Q4) of folate level had the highest survivability compared with the other quintile, especially with the lowest quintile (Q1) (p<0.05 for Log rank test). The survival curve for the highest quintile (Q5) went nearly similar with the Q1 group.
Figure 3.
Survival probability by the level of serum folate. Quintile categories of serum folate: Q1<8.7ng/mL, Q2 8.7–11.4ng/mL, Q3 11.5–14.6ng/mL, Q4 14.7–19.1ng/mL, Q5 ≥19.2ng/mL. The potential confounders adjusted were as the same as Figure 2.
Discussion
The aim of the present study was to investigate the relationship between serum folate concentration and all-cause mortality. As the results indicated, the relationship between serum folate and risk of death was of non-linear pattern. The reversed J-shaped pattern meant that lower or higher levels of serum folate would result in increased mortality rate. The identification of this relationship in the CKD population was in consistent with our hypothesis.
Previous studies had revealed that folate has rewarding effects on endothelial function by decreasing plasma homocysteine,7,8 improving insulin sensitivity,9 reducing the risk of incident stroke.10 On the contrary, negative results, and opposite effects were also reported with different folate consumption levels.11,12 These reports indicated that there maybe a inconsistent dose-response relationship between folate and its physiological effect. Although the suitable reference range of serum folate has been well investigated in the general population, the optimal level for the CKD population remains understudied. Serum folate concentrations were typically low or low-normal in CKD population, especially in individuals not receiving folate supplements.13 First, a large scale of CKD population had high comorbidity burden and employed various medications, some of which may interfere with the bioavailability of nutrients and vitamins including folic acid.14 Furthermore, in CKD stage 5 population requiring regular dialysis, loss of small moleculars including serum folate was inevitable There were evidences supporting the benefit of folate supplement to CKD population.15 However, since excreting dysfunction of CKD, accumulation following continuous folate supplement may also cause aberrant elevation of serum folate level. The dose-response relationship of folate in the CKD population is thus far unclear. To address this issue was of crucial clinical value, especially for the CKD population from the US, where food fortification is in practice. To the best of our knowledge, this study is one of the largest cohorts analyzing the relationships between folate levels and all-cause mortality among the CKD population.
In this study, after a follow-up of average 57.4 months in a group of adults with CKD from a nationally representative sample of the US population, results indicate that serum folate levels are associated with all-cause mortality in a nonlinear pattern. As presented, persons with lower levels of folate are at increased risks of mortality. Elevated mortality risks are also noticed in persons with higher folate levels. These associations are obtained after adjusting for potential confounders.
Our results are roughly in agreement with a series of reports based on other population.16,17 Although the underlying mechanisms by which lower folate level contributes to mortality remain unclear, some feasible explanations could be considered. Reports stated that insufficient folate would interfere with the DNA methylation,18 yet others stated that folate deficiency could irritate a raised homocysteine level,19 which was recognized as a risk factor of death.20
Another concern was the possible adverse health effect of excess folate. We have noticed the increased all-cause mortality among those with high folate levels with regard to those with lower values as references in the CKD population. The association is biologically plausible. Suppressed immunity has been observed in women taking excess folic acid from supplements or fortification.21 Recent investigations had observed increased risk of abdominal aortic calcification or heart disease attacks among population with higher folate level, which indicates the possible adverse effects of excess folate.22,23 The mechanisms of the harmful effects have been suggested by former reports. One theory is that excess folate may induce obvious increase in the level of non-bound folate, which may in turn speed up folate’s degradation rate.24 It is also supposed that high folate concentrations could result in low extent of thymidylate and 5-methylenetetrahydrofolate, thus breaking DNA integrity and affecting protein composition.25
Of importance are the strengths of the present study. Compared with previous studies, the NHANES data set excludes the elderly institutionalized in places like nursing homes and hospitals; thus, allowing for the present study to expand the inferences from clinical setting to the community-dwelling population. Furthermore, the surveys of folate exposure, mortality and covariates are generally reliable because they were under the instruction of NCHS of the US. There are some limitations to our study as well. First, the data of serum folate were merely based on a single detection at baseline and it may not accurately account for the long-term folate level. Second, we are unable to state the influences of folate levels on more specific-caused mortality, like cardiovascular disease and diabetic mortality, due to insufficient effective sample size.
In summary, our findings suggest that serum folate concentrations may influence all-cause mortality in a non-linear pattern in the CKD population, indicating a reference range of 14.7–19.1ng/mL with the best survival outcome. Persons with lower or excess folate status are both at greater risk of mortality. The present study calls for more robust assessment on toxicologic implications associated with folate and clearly define its relationship with specific health conditions.
Conclusion
The existing evidences could not reach a consensus as regarding the benefit of folate supplement in the CKD populations. According to the result of this study, it’s reasonable to recommend periodic surveillance in the CKD population to maintain the serum folate concentration in an appropriate level.
Acknowledgments
This work was supported by the Science and Technology Bureau of Shantou City [grant number 181115234017142]. Li-Jun Yan and Fei-Ran Zhang are co-first authors and have contributed equally to this work.
Disclosure
The authors report no conflicts of interest and funding source had no role in study design, conduct, or reporting.
References
- 1.House AA, Eliasziw M, Cattran DC, et al. Effect of B-vitamin therapy on progression of diabetic nephropathy. JAMA. 2010;303(16):1603–1609. doi: 10.1001/jama.2010.490 [DOI] [PubMed] [Google Scholar]
- 2.Xu X, Qin X, Li Y, et al. Efficacy of folic acid therapy on the progression of chronic kidney disease the renal substudy of the China Stroke Primary Prevention Trial. JAMA Intern Med. 2016;176(10):1443–1450. doi: 10.1001/jamainternmed.2016.4687 [DOI] [PubMed] [Google Scholar]
- 3.Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr. 2005;82(2):442–450. doi: 10.1093/ajcn/82.2.442 [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004 [DOI] [PubMed] [Google Scholar]
- 5.Whaley-Connell AT, Sowers JR, Stevens LA, et al. CKD in the United States: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2008;51(4):S13–20. doi: 10.1053/j.ajkd.2007.12.016 [DOI] [PubMed] [Google Scholar]
- 6.Ingram DD, Makuc DM. Statistical issues in analyzing the NHANES I Epidemiologic Followup Study. Series 2: data evaluation and methods research. Vital Health Stat. 1994;121:1–30. [PubMed] [Google Scholar]
- 7.MacKenzie KE, Wiltshire EJ, Gent R, Hirte C, Piotto L, Couper JJ. Folate and vitamin B6 rapidly normalize endothelial dysfunction in children with type 1 diabetes mellitus. Pediatrics. 2006;118(1):242–253. doi: 10.1542/peds.2005-2143 [DOI] [PubMed] [Google Scholar]
- 8.Mierzecki A, Kloda K, Bukowska H, Chelstowski K, Makarewicz-Wujec M, Kozlowska-Wojciechowska M. Association between low-dose folic acid supplementation and blood lipids concentrations in male and female subjects with atherosclerosis risk factors. Med Sci Monit. 2013;19(1):733–739. doi: 10.12659/MSM.889087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gargari BP, Aghamohammadi V, Aliasgharzadeh A. Effect of folic acid supplementation on biochemical indices in overweight and obese men with type 2 diabetes. Diabetes Res Clin Pract. 2011;94(1):33–38. doi: 10.1016/j.diabres.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 10.Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313(13):1325–1335. doi: 10.1001/jama.2015.2274 [DOI] [PubMed] [Google Scholar]
- 11.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297(21):2351–2359. doi: 10.1001/jama.297.21.2351 [DOI] [PubMed] [Google Scholar]
- 12.Ebbing M, Bonaa KH, Nygard O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302(19):2119–2126. doi: 10.1001/jama.2009.1622 [DOI] [PubMed] [Google Scholar]
- 13.Dierkes J, Domröse U, Westphal S, et al. Cardiac troponin T predicts mortality in patients with end-stage renal disease. Circulation. 2000;102(16):1964–1969. doi: 10.1161/01.CIR.102.16.1964 [DOI] [PubMed] [Google Scholar]
- 14.Clase CM, Ki V, Holden RM. Water-soluble vitamins in people with low glomerular filtration rate or on dialysis: a review. Semin Dial. 2013;26(5):546–567. doi: 10.1111/sdi.12099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soohoo M, Ahmadi SF, Qader H, et al. Association of serum vitamin B12 and folate with mortality in incident hemodialysis patients. Nephrol Dial Transplant. 2017;32(6):1024–1032. doi: 10.1093/ndt/gfw090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee TY, Chiang EP, Shih YT, Lane HY, Lin JT, Wu CY. Lower serum folate is associated with development and invasiveness of gastric cancer. World J Gastroenterol. 2014;20(32):11313–11320. doi: 10.3748/wjg.v20.i32.11313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nkemjika S, Ifebi E, Cowan LT, et al. Association between serum folate and cardiovascular deaths among adults with hypertension. Eur J Clin Nutr. 2020;74(6):970–978. doi: 10.1038/s41430-019-0533-7 [DOI] [PubMed] [Google Scholar]
- 18.Wasson GR, McGlynn AP, McNulty H, et al. Global DNA and p53 region-specific hypomethylation in human colonic cells is induced by folate depletion and reversed by folate supplementation. J Nutr. 2006;136(11):2748–2753. doi: 10.1093/jn/136.11.2748 [DOI] [PubMed] [Google Scholar]
- 19.Waly MI, Ali A, Al-Nassri A, Al-Mukhaini M, Valliatte J, Al-Farsi Y. Low nourishment of B-vitamins is associated with hyperhomocysteinemia and oxidative stress in newly diagnosed cardiac patients. Exp Biol Med (Maywood). 2016;241(1):46–51. doi: 10.1177/1535370215596860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balsam A, El Kossi MM, Lord R, El Nahas AM. Cardiovascular disease on hemodialysis: predictors of atherosclerosis and survival. Hemodial Int. 2009;13(3):278–285. doi: 10.1111/j.1542-4758.2008.00337.x [DOI] [PubMed] [Google Scholar]
- 21.Cho E, Zhang X, Townsend MK, et al. Unmetabolized folic acid in prediagnostic plasma and the risk of colorectal cancer. J Natl Cancer Inst. 2015;107(12):djv260. doi: 10.1093/jnci/djv260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou L, Wen X, Peng Y, Guo M, Zhao L. Red blood cell folate and severe abdominal aortic calcification: results from the NHANES 2013–2014. Nutr Metab Cardiovasc Dis. 2021;31(1):186–192. doi: 10.1016/j.numecd.2020.08.020 [DOI] [PubMed] [Google Scholar]
- 23.Peng Y, Dong B, Wang Z. Serum folate concentrations and all-cause, cardiovascular disease and cancer mortality: a cohort study based on 1999–2010 National Health and Nutrition Examination Survey (NHANES). Int J Cardiol. 2016;219:136–142. doi: 10.1016/j.ijcard.2016.06.024 [DOI] [PubMed] [Google Scholar]
- 24.Oleinik NV, Krupenko NI, Reuland SN, Krupenko SA. Leucovorin-induced resistance against FDH growth suppressor effects occurs through DHFR up-regulation. Biochem Pharm. 2006;72(2):256–266. doi: 10.1016/j.bcp.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 25.Sauer J, Mason JB, Choi SW. Too much folate: a risk factor for cancer and cardiovascular disease? Curr Opin Clin Nutr Metab Care. 2009;12(1):30–36. doi: 10.1097/MCO.0b013e32831cec62 [DOI] [PMC free article] [PubMed] [Google Scholar]