Abstract
Purpose
External beam accelerated partial breast irradiation (APBI) is subject to treatment uncertainties that must be accounted for through planning target volume (PTV) margin. We hypothesize that magnetic resonance–guided radiation therapy with reduced PTV margins enabled by real-time cine magnetic resonance image (MRI) target monitoring results in better normal tissue sparing compared with computed tomography (CT)-guided radiation therapy with commonly used clinical PTV margins. In this study, we compare the plan quality of ViewRay MRIdian Linac forward planned intensity modulated radiation therapy and TrueBeam volumetric modulated arc therapy for a novel 3-fraction APBI schedule.
Methods and Materials
Targets and organs at risk (OARs) were segmented for 10 patients with breast cancer according to NSABP B39/RTOG 0413 protocol. A 3 mm margin was used to generate MR PTV3mm and CT PTV3mm plans, and a 10 mm margin was used for CT PTV10mm. An APBI schedule delivering 24.6 Gy to the clinical target volume and 23.4 Gy to the PTV in 3 fractions was used. OAR dose constraints were scaled down from existing 5-fraction APBI protocols. Target and OAR dose-volume metrics for the following data sets were analyzed using Wilcoxon matched-pairs signed-rank test: (1) MR PTV3mm versus CT PTV3mm plans and (2) MR PTV3mm versus CT PTV10mm.
Results
Average PTVs were 84.3 ± 51.9 cm3 and 82.6 ± 55 cm3 (P = .5) for MR PTV3mm and CT PTV3mm plans, respectively. PTV V23.4Gy, dose homogeneity index, conformity index (CI), and R50 were similar. There was no meaningful difference in OAR metrics, despite MR PTV3mm being larger than the CT PTV3mm in 70% of the patients. Average PTVs for MR PTV3mm and CT PTV10mm plans were 84.3 ± 51.9 cm3 and 131.7 ± 74.4 cm3, respectively (P = .002). PTV V23.4Gy was 99% ± 0.9% versus 97.6% ± 1.4% (P = .03) for MR PTV3mm and CT PTV10mm, respectively. Dose homogeneity index, CI, and R50 were similar. MR PTV3mm plans had better ipsilateral breast (V12.3Gy, 34.8% ± 12.7% vs 44.4% ± 10.9%, P = .002) and chest wall sparing (V24Gy, 8.5 ± 5.5 cm3 vs 21.8 ± 14.9 cm3, P = .004).
Conclusions
MR- and CT-based planning systems produced comparable plans when a 3 mm PTV margin was used for both plans. As expected, MR PTV3mm plans produced better ipsilateral breast and chest wall sparing compared with CT PTV10mm. The clinical relevance of these differences in dosimetric parameters is not known.
Introduction
Accelerated partial breast irradiation (APBI) refers to a broad array of radiation modalities that aim to treat the lumpectomy cavity with a margin to account for microscopic disease, thereby delivering radiation to a limited area of the body over a shorter period than conventional whole breast irradiation. This concept has garnered significant interest in early stage breast cancer and ductal carcinoma in situ. The GEC-ESTRO phase 3 APBI study1 and the Budapest trial2 provide compelling level I evidence for interstitial brachytherapy-mediated APBI. In the realm of external beam radiation therapy partial breast irradiation, IMPORT-LOW trial has demonstrated noninferiority of PBI (40 Gy in 15 fractions) compared with whole breast irradiation (40 Gy in 15 fractions).3 On the other hand, accelerated schedules like RAPID4 and NSABP B39/RTOG 04135 investigating 38.5 Gy in 10 fractions have noticed either worse cosmesis4 or marginally higher recurrence rates with APBI.5
From the large UK randomized studies, it appears that the α/β ratio for breast cancer cells is close to that of normal tissue late effects, suggesting that standard fractionation has limited benefits in breast tissue.6 Stereotactic body radiation therapy (SBRT) and hypofractionated radiation therapy offer a unique approach for treating localized tumors with ablative doses of radiation while sparing the surrounding organs at risk. Long-term results from a phase 3 study by University of Florence7 and a recent phase 1 dose-escalation study by Rahimi et al8 support the use of a 5-fraction APBI regime. More recently, Khan et al published favorable early cosmetic results after brachytherapy based 3-fraction APBI.9 Given encouraging results with 5-fraction external beam APBI regimes, we devised a 3-fraction APBI regimen that is noninvasive and could be delivered conveniently in 1 week.
Advances in image guidance for target localization and treatment delivery can help reduce PTV margins and in turn the volume of tissue receiving high-dose radiation. This is very relevant in breast APBI as single-institution studies have shown that higher volume of normal breast receiving prescription dose and 50% prescription dose correlate with worse cosmetic outcomes.10 Recent advances in radiation therapy techniques and delivery, including high-quality cone beam computed tomography (CT) scans for set-up verification, breathing management, and real-time imaging with or without gating has led to a reduction in PTV margins.11 In magnetic resonance–guided radiation therapy (MRgRT), a cine sagittal magnetic resonance image (MRI) is acquired before and during radiation delivery at 4 frames per second. For each cine image, the MRgRT system deforms the acquired lumpectomy contour and compares it to a predefined gating boundary/contour that is derived from the planning image. The system automatically sends a “beam off” signal if the lumpectomy contour is outside the gating boundary. This is an additional sophisticated tool to ensure accurate treatment delivery.12 In a study by Acharya et al, 30 patients were treated on a prospective study evaluating magnetic resonance (MR)-guided APBI. The clinical target volume (CTV) was defined as the surgical cavity with a 1 cm expansion with no additional margin for PTV. An MRI that was acquired before each treatment was used for localization. When intrafractional motion of the lumpectomy cavity was evaluated, the mean margin required for 90% of the cavity to be treated 90% of the time was only 0.7 mm (5th-95th percentile, 0-2.7 mm).13 In this study, without active breathing management, despite using no PTV margins, the mean difference in dose planned versus delivered was only 1%, further supporting reduced PTV margins with MRgRT.
We hypothesize that MRgRT APBI plans with reduced PTV margins (3 mm) might result in better normal tissue sparing compared with CT-guided RT with conventional PTV margins. In this study, we test this hypothesis by comparing the plan quality of ViewRay MRIdian Linac to that of TrueBeam STx (Varian Medical System) guided volumetric modulated arc therapy (VMAT).
Methods and Materials
After institutional review board approval, a total of 10 patients who previously received a lumpectomy boost on a Viewray Tri-Co60 MR system for breast cancer were selected for this dosimetric study. Patients whose lumpectomy cavity was clearly visible on both CT and MRI planning scans were included. Ratio of lumpectomy cavity to whole breast >30% was not used as an exclusion factor.
Image acquisition
MRI
ViewRay MRIdian Linac (ViewRay, Inc, Oakwood Village, OH), combines a 0.345 T field strength split-bore magnet with a 28 cm gap that contains 6 MV flattening filter free (linear accelerator (Linac).14 A TRUFI imaging sequence that is predominantly T2 weighted was used for image acquisition as this enabled better visualization of the lumpectomy cavity.13 Patients were scanned in supine position from chin to diaphragm using thoracic receiver surface coils to acquire 3 mm volumetric images.
CT
Patients were scanned supine on a breast board (Civco Medical Solutions) with both arms elevated above the head using arm support. Dummy surface coils were used to produce comparable breast deformation and attenuation to that of ViewRay MR simulation. A planning CT scan with 3 mm slice thickness was acquired using Siemens SOMATOM Definition Edge.
Contouring
A radiation oncology fellow (M.H.B.) contoured targets and organs at risk, and these were verified by a breast radiation oncologist. The surgical cavity, including surgical clips and any postsurgical changes, was contoured as one structure and labeled as the lumpectomy cavity. A 1-cm margin around the lumpectomy cavity was used to create the CTV after excluding chest wall and a 5 mm skin strip. Three different PTVs were generated.
At our institution, we use 3 mm MRgRT PTV margin for the following reasons: MRI geometric distortion, discrepancy between MRI isocenter to radiation therapy isocenter, multileaf collimator (MLC) position error, and uncertainties with voxel size and tracking.
-
1.
MR PTV3mm: A 3 mm margin was used to generate MR PTV3mm. Two different PTV margins were used for the CT plans: a 3 mm margin to compare the treatment planning system (TPS) of MR-Linac versus CT, and a 10 mm margin to compare previously studied PTV margins (MR-Linac 3 mm with real-time image guidance vs CT 10 mm margin without real-time image guidance, as per NSABP B39 and University of Florence).
-
2.
CT PTV3mm: A 3 mm PTV margin
-
3.
CT PTV10mm: A 10 mm PTV margin
Organs at risk including ipsilateral and contralateral breast, skin, chest wall, ipsilateral and contralateral lung, and heart were contoured as per the NSABP B39/RTOG 0413 protocol.
Rationale for 3-fraction APBI
A 3-fraction APBI schedule allows for patients in remote areas who travel to a distant treatment facility to complete their treatment in 5 days, without having to stay over the weekend. Given the relative safety of the 3-fraction APBI protocol demonstrated in a phase 1 study,15 we planned to investigate a novel MRI guided 3-fraction APBI protocol in a prospective study. As part of this study, we examined the feasibility of using MRIdian for APBI by performing current dosimetric study and comparing it with the standard CT platform.
Dose-volume constraints from Ontario Clinical Oncology Group protocol (NCT 02637024) and Rahimi et al phase 1 APBI study8 were used. All these doses were scaled appropriately using the biologically effective dose (BED) model: BED= D (1+[d/α/β]. Using an α/β ratio of 3.8 Gy (NSABP B39/RTOG 0413 protocol),16 30 Gy in 5 fractions, which was proven to be effective in the 5-fraction APBI protocol,17 would be equivalent to 24.6 Gy in 3 fractions (8.2 Gy per fraction).16,18 α/β values of 3.0 Gy were used for most organs at risk, apart from brachial plexus (α/β = 2.0) and heart (α/β = 1.5).19
Planning objectives
For dosimetric comparison, identical treatment planning objectives were used for both MR and CT plans. Dose constraints to target and organs at risk are shown in Table 1. Prescription dose was 24.6 Gy to CTV and 23.4 Gy to the PTV in 3 fractions.
Table 1.
Treatment planning objectives for 3-fraction APBI
| Structure | Dose objectives |
|---|---|
| Target evaluation | Interfraction interval should be ≥40 h |
| Whole breast (ipsilateral) | V12.3Gy <50% |
| PTV_EVAL | V23.4Gy ≥95% |
| PTV | D2cm3 <107% (26.3 Gy), Dmax115% (28.3 Gy) <0.5 cm3 |
| CTV | V24.6Gy ≥95% |
| PTV and CTV conformality index | 0.95-1.2 |
| OAR evaluation | |
| Contralateral breast | Point dose <0.9 Gy |
| Ipsilateral lung | V7.7Gy <10%, V2.7Gy <20% |
| Contralateral lung | V1.4Gy <15% |
| Heart (right-sided lesions) | Mean dose <0.9 Gy, V4.0Gy ≤0% |
| Heart (left sided lesion, excluding lower inner quadrant) | V2.6Gy<5%, Mean dose<1.8 Gy |
| Heart (left sided lesion, lower inner quadrant) | V3.9Gy <5%, Mean dose <1.8 Gy |
| Ipsilateral brachial plexus | V24Gy <3.0 cm3, Point Dmax <25.6 Gy |
| Chest wall | V24Gy <30 cm3, Point Dmax <26 Gy |
| Skin | D0.5cm3 ≤100% (24.6 Gy) |
Abbreviations: APBI = accelerated partial breast irradiation; CTV = clinical target volume; D2cm3 = dose to 2 cm3 of the target; Dmax = maximum dose to the target; OAR = organs at risk; PTV = planning target volume; Vx% = dose to x% of the target volume.
Plan generation
CT-Linac
VMAT plans were generated with Pinnacle (Philips) TPS using 2 arcs spanning 50 degrees to 180 degrees for right-side breast and 310 degrees to 180 degrees for left-side breast plans, with gantry angle spacing of 2 degrees. Truebeam STX system has 6 MV flattening filter free beams and MLCs with a capability to achieve 5 mm spatial resolution at the isocenter. Each plan was optimized with the progressive resolution optimizer. Dose distributions were calculated with the anisotropic analytic algorithm using a dose grid of 0.25 cm. TrueBeam STX is equipped with a 6 degree of freedom couch, which allows for more variable beam arrangement.
MR-Linac
ViewRay MRIdian Linac combines a 0.345 T field strength split-bore magnet MRI with a 28 cm gap that contains a 6 MV flattening filter free Linac. The MRIdian system uses step and shoot IMRT technique to deliver dose that is calculated using the Monte Carlo algorithm, incorporating the electron return effect.
As part of plan generation, the simulation CT scan was rigidly registered with the MRI to obtain electron density information for MR planning. Targets and organs at risk that were segmented on the MR simulation images were transferred to the MRIdian TPS. Seven to 15 beams were placed 15° to 30° apart to generate a step-and-shoot IMRT plan. During planning, the isocenter was placed near the PTV. Beams from the edge of the couch were restricted. A 0.2 cm grid size was use for dose calculation, and magnetic field was taken into consideration for optimization and final dose calculations.
Dosimetric analysis
CT-Linac and MR-Linac plans were compared using plan metrics such as near minimum dose (D98%, the dose to 98% of PTV), near maximum dose (D2%), median dose (D50%), conformity index (CI), and homogeneity index (HI) for PTV.
CI and HI were calculated as20
CI = Volume of the prescription isodose (27 Gy) / Volume of PTV
HI = (D2% − D98%)/ D50%
To evaluate the effect of intermediate dose on the normal tissue, R50% was calculated as
PTV R50% = Volume of tissue encompassed by 50% of the prescription dose (11.7 Gy)/Volume of prescription isodose (23.4 Gy)
Dosimetric data for organs at risk were also compared between the 2 plans. Two comparisons were done: (1) MR PTV3mm vs. CT PTV10mm and 2) MR PTV3mm versus CT PTV3mm. The first comparison was done to examine the effect of PTV margins used for treatment delivery on these 2 platforms. The second comparison was done to test the robustness of MRIdian Linac TPS. Uniform dose-volume constraints and treatment planning objectives criteria were used for all these different plans. Clinically relevant dose-volume metrics were analyzed using the Wilcoxon matched-pairs signed-rank test.
Results
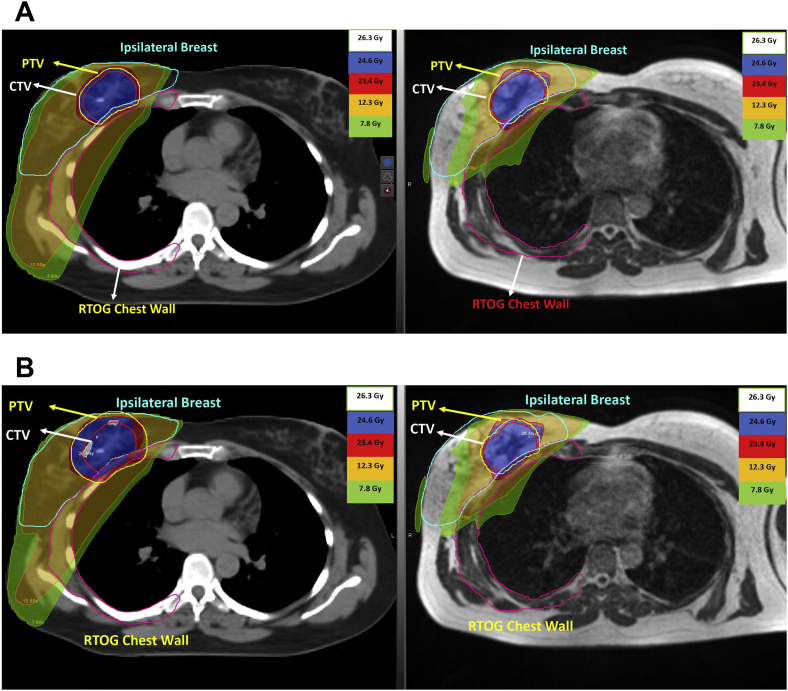
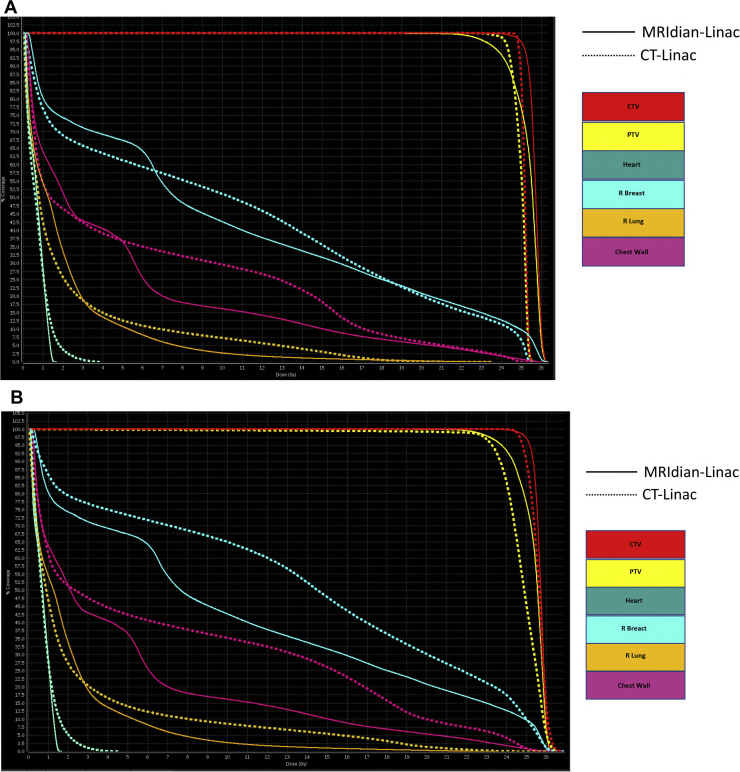
Out of the 10 patients selected for this dosimetric study, 7 patients had radiation therapy to left breast. An example of MR PTV3mm, CT PTV3mm and CT PTV10mm plans is shown in Figures 1 and 2.
Figure 1.
(A) CT-Linac PTV3mm and MRIdian-Linac PTV3mm contours and isodose lines. (B) CT-Linac PTV10mm and MRIdian-Linac PTV3mm contours and isodose lines.
Figure 2.
(A) Dose-volume histograms comparing CT-Linac PTV3mm and MRIdian-Linac PTV3mm plans. (B) Dose-volume histograms comparing CT-Linac PTV10mm and MRIdian-Linac PTV3mm plans.
MR PTV3mm and CT PTV3mm
PTV metrics
Dosimetric parameters of MR PTV3mm and CT PTV3mm plans are shown in Table 2. The average PTV was similar between both plans (84.3 ± 51.9 cm3 MR PTV3mm vs. 82.6 ± 55 cm3 CT PTV3mm, P = .5). Average ratio of PTV to ipsilateral breast volume was 13.0 (5.1) for MR PTV3mm and 11.4 (5.0) for CT PTV3mm plans (P = .03). Average volume receiving 26.3 Gy (107%) was less than 1 cm3 for both plans. Though there was a statistically significant difference in average D2% and average D50% between the 2 plans, corresponding absolute dosimetric differences were minor. Average CI was 1.0 for both plans and, average dose homogeneity index was similar. There was no statistically significant difference in the spread of intermediate doses (R50) between MR PTV3mm and CT PTV3mm plans.
Table 2.
Comparison of dosimetry between MRIdian-Linac PTV3mm and CT-Linac PTV3mm plans
| Variables | MRIdian-Linac PTV3mm Average (SD) | CT-Linac PTV3mm Average (SD) | P-value |
|---|---|---|---|
| Whole breast metrics | |||
| Ipsilateral breast volume (cm3) | 671.8 (360.0) | 751.0 (414.7) | .04∗ |
| V12.3 <50% | 34.8% (12.7) | 35.9% (11.4) | .5 |
| V12.3 (cm3) | 219.4 (117) | 251.3 (133.8) | .1 |
| PTV metrics | |||
| Volume (cm3) | 84.3 (51.9) | 82.6 (55) | .5 |
| Ratio of PTV/ipsilateral breast volume | 13.0 (5.1) | 11.4 (5.0) | .03∗ |
| V23.4 ≥95% | 99% (0.9) | 99.5% (0.5) | .2 |
| D2cc<26.3Gy | 26.2 Gy (0.2) | 25.6 Gy (0.3) | .002∗ |
| V26.3Gy (cm3) | 1.9 (3.5) | 0.07 (0.1) | .01∗ |
| D2% (Gy) | 26.2 (0.2) | 25.7 (0.3) | .004∗ |
| D50% (Gy) | 25.5 (0.2) | 25.1 (0.1) | .002∗ |
| D98% (Gy) | 23.8 (0.4) | 24 (0.2) | .5 |
| Max point dose (Gy) | 26.8 (0.3) | 26.2 (0.6) | .03∗ |
| DHI ratio | 0 | 0 | >.99 |
| CI ratio | 1.0 | 1.0 | >.99 |
| R50 ratio | 4.6 (1.3) | 5.9 (1.7) | .09 |
| Skin metrics | |||
| Max point dose (Gy) | 23.46 (2.09) | 22.89 (1.47) | .38 |
| Contralateral breast | |||
| Point dose (Gy) | 0.62 (0.2) | 0.66 (0.12) | .4 |
| Ipsilateral lung | |||
| V7.7 <10% | 3.7% (2.6) | 4.5% (3.4) | .6 |
| V2.7 <20% | 16.75% (6.4) | 13.95% (6.0) | .1 |
| Mean dose (Gy) | 1.7 (0.54) | 1.5 (0.55) | .4 |
| Contralateral lung | |||
| V1.4 <15% | 1.94% (3.64) | 2.04% (4.35) | .7 |
| Mean dose (Gy) | 0.34 (0.16) | 0.29 (0.12) | .4 |
| Total lung | |||
| Mean dose (Gy) | 0.97 (0.33) | 0.88 (0.33) | .5 |
| Heart | |||
| Mean dose (Gy) | 0.44 (0.2) | 0.38 (0.23) | .6 |
| V4.0 (%) | 0.06% (0.2) | 0.44% (1.3) | .6 |
| V2.6 (%) | 0.5% (0.9) | 0.8% (1.3) | .6 |
| RTOG chest wall | |||
| V24 (cm3) | 8.5 (5.5) | 8.2 (6.0) | .8 |
| Max point dose <26 Gy | 24.1 Gy (6.2) | 23.6 Gy (6.2) | .009∗ |
Abbreviations: CI = conformality index; D2cm3 = dose to 2 cm3 of the target; DHI = dose homogeneity index; Dmax = maximum dose; Dx = dose to x% of the target; PTV = planning target volume; SD = standard deviation; Vx = dose to x% of the target volume,
Statistically significant.
Organs at Risk
Average volume of ipsilateral breast receiving 23.4 Gy (V23.4) was higher for the MR PTV3mm plans (17.2% vs 14.3%, P = .004). This was seen in 8 out of 10 patients. Average maximum point dose to the chest wall was higher in the MR PTV3mm plans when compared to CT PTV3mm plans (24.2 ± 6.2 cm3 MR PTV3mm vs 23.6 ± 6.2 cm3 CT PTV3mm, P = .009). This was observed in 7 patients. There were no statistically significant differences in the ipsilateral/contralateral lung, heart, or skin metrics (Table 2). Overall, both MR PTV3mm and CT PTV3mm plans achieved the intended dose constraints with minimal variation, despite MR PTV3mm plans not having the benefit of dynamic IMRT planning. On average, the beam on time was 3 minutes and 1 minute, for MR PTV3mm and CT PTV3mm plans, respectively.
MR PTV3mm and CT PTV10mm
PTV metrics
The average values and standard deviation (SD) of MR PTV3mm and CT PTV10mm dosimetric parameters are shown in Table 3. Because MR PTV3mm and CT PTV10mm were generated using different margins, the average PTV of CT PTV10mm was larger than the MR PTV3mm by approximately 1.5 times (131.7 vs 84.3 cm3, P ≤ .002). On average, 99% (± 0.9%) of MR PTV3mm and 97.6% (± 1.4%) of CT PTV10mm received 23.4 Gy (P = .03). The average ratio of PTV to ipsilateral breast volume was 13.0 (5.1) for MR PTV3mm and 18.6 (6.4) for CT PTV10mm plans (P = .002). Most of the PTV metrics were better for MR PTV3mm plans compared with CT PTV10mm plans. The average PTV receiving 26.3 Gy was less than 1 cm3 for both plans. The CI was 0.99 for MR PTV3mm and 0.95 for CT PTV10mm plans (P = .02). The average dose homogeneity index was 0.1 and 0.08 for MR PTV3mm and CT PTV10mm plans, respectively (P = .02). This was reflected in the slightly higher average D2% for the MR PTV3mm plans (26.2 Gy vs 25.6 Gy, P = .006). The spread of intermediate dose (R50) was similar for both plans.
Table 3.
Comparison of dose metrics between MRIdian-Linac PTV3mm and CT-Linac PTV10mm plans
| Variables | MRIdian-Linac PTV3mm Average (SD) | CT-Linac PTV3mm Average (SD) | P-value |
|---|---|---|---|
| Whole breast metrics | |||
| Ipsilateral breast volume (cm3) | 671.8 (360.0) | 751.0 (414.7) | .04∗ |
| V12.3 <50% | 34.8% (12.7) | 44.4% (10.9) | .002∗ |
| V12.3 (cm3) | 219.4 (117) | 312.1 (147) | .002∗ |
| PTV metrics | |||
| Volume (cm3) | 84.3 (51.9) | 131.7 (74.4) | .002∗ |
| Ratio of PTV/ipsilateral breast volume | 13.0 (5.1) | 18.6 (6.4) | .002∗ |
| V23.4 ≥95% | 99% (0.9) | 97.6% (1.4) | .03∗ |
| D2cc <26.3 Gy | 26.1 Gy (0.19) | 25.7 Gy (0.33) | .01∗ |
| V26.3 Gy (cm3) | 0.9 (1.9) | 0.23 (0.67) | .06 |
| D2% (Gy) | 26.2 (0.17) | 25.6 (0.32) | .006∗ |
| D50% (Gy) | 25.5 (0.2) | 24.8 (0.17) | .002∗ |
| D98% (Gy) | 23.8 (0.4) | 23.5 (0.1) | .08 |
| Max point dose (Gy) | 26.8 (0.26) | 26.2 (0.36) | .01∗ |
| DHI ratio | 0.1 (0) | 0.08 (0.01) | .02∗ |
| CI ratio | 0.99 (0.03) | 0.95 (0.01) | .02∗ |
| R50 ratio | 4.6 (1.3) | 5.1 (1.4) | .5 |
| Skin metrics | |||
| Max point dose (Gy) | 23.46 (2.09) | 22.74 (1.58) | .43 |
| Contralateral breast | |||
| Point dose (Gy) | 0.6 (0.17) | 0.7 (0.2) | .2 |
| Ipsilateral lung | |||
| V7.7<10% | 3.7% (2.5) | 6.9% (3.2) | .02∗ |
| V2.7<20% | 16.7% (6.4) | 17.8% (5.3) | .99 |
| Mean dose (Gy) | 1.64 (0.55) | 2.0 (0.5) | .06 |
| Contralateral lung | |||
| V1.4 <15% | 1.9% (3.6) | 4.1% (6.1) | .64 |
| Mean dose (Gy) | 0.32 (0.16) | 0.4 (0.22) | .54 |
| Total lung | |||
| Mean dose (Gy) | 0.96 (0.36) | 1.2 (0.36) | .06 |
| Heart | |||
| Mean dose (Gy) | 0.4 (0.2) | 0.5 (0.2) | .6 |
| V4.0 (%) | 0.07% (0.2) | 0.34% (0.6) | .1 |
| V2.6 (%) | 0.5% (0.9) | 1.7% (2.0) | .1 |
| RTOG chest wall | |||
| V24 (cm3) | 8.5 (5.5) | 21.8 (14.9) | .004∗ |
| Max point dose <26 Gy | 24.1 Gy (6.2) | 23.8 Gy (5.8) | .2 |
Abbreviations: CI = conformality index; D2cm3 = dose to 2 cm3 of the target; DHI = dose homogeneity index; Dmax = maximum dose; Dx = dose to x% of the target; PTV = planning target volume; SD = standard deviation; Vx = dose to x% of the target volume.
Statistically significant.
Organs at risk
The average volume of ipsilateral breast receiving 12.3 Gy (50% of the CTV dose) was 1.4 times higher for CT PTV10mm compared with that of MR PTV3mm plans (312.1 ± 147 cm3 vs 219.4 ± 117 cm3, P = .002). V12.3Gy was lower in MR PTV3mm plans for 8 patients compared with CT PTV10mm plans, and it was similar between the 2 plans for 2 patients. Average volume of the ipsilateral lung receiving 7.7 Gy was lower for the MR PTV3mm plans (3.7% vs 6.9%, P = .02; Table 3). Eight out of the 10 patients had lower lung V7.7Gy and 1 patient had similar lung V7.7Gy for MR PTV3mm plans compared with CT PTV10mm plans. There was no statistically significant difference in the average mean heart dose or heart V2.6Gy or V4.0Gy between plans. Maximum skin dose was also similar between MR PTV3mm and CT PTV10mm plans. Average volume of the ipsilateral chest wall receiving 24 Gy (V24) was significantly higher for CT PTV10mm plans (21.8 ± 14.9 cm3 vs 8.5 ± 5.5 cm3, P = .004). Nine patients had lower chest wall V24Gy and 1 patient had similar chest V24Gy with MR PTV3mm plans compared with CT PTV10mm plans.
Discussion
Modern high-precision technology for radiation therapy planning and delivery has made it possible to investigate stereotactic radiosurgery and SBRT for tumors in various body sites, such as brain, lung, prostate, liver, kidneys, and bone. More recently, there has been increasing evidence that adjuvant APBI delivered via IMRT in 3 to 5 fractions is feasible and safe.9,21 We designed a prospective 3-fraction MR-guided external beam APBI protocol and performed a lead-in dosimetric study to test the robustness of MRIdian-Linac TPS.
In this dosimetric study, we have shown that MRIdian TPS generated 3-fraction breast APBI plans that are comparable to TrueBeam STX plans when a 3mm PTV margin is used, despite MR PTV3mm plans not having the benefit of dynamic IMRT planning. Volume of ipsilateral breast receiving 23.4 Gy and maximum point dose to the ipsilateral chest wall were the only 2 metrics that were different in the plan comparison. Although these metrics were slightly higher for the MR PTV3mm plans, the absolute difference was minimal (3% for ipsilateral breast V23.4Gy and 0.5Gy for chest wall maximum point dose). Individual data sets (MR sim and CT sim) were used to delineate targets for MRIdian TPS and Pinnacle, respectively, resulting in MR PTV3mm volumes being larger than CT PTV3mm volumes. This could account for the minimal differences in dosimetric values noted here (Table 2).
On the other hand, MR PTV3mm plans resulted in better ipsilateral breast and chest wall sparing compared with CT PTV10mm plans, mainly due the difference in PTV. Although the ipsilateral lung V7.7Gy was lower for MR PTV3mm plans, the absolute difference was minimal. Treatment of patients as per the NSABP B39/RTOG 0413 and University of Florence studies uses 10 mm PTV margins for CT-based external beam APBI planning. Radiation oncologists may elect to use smaller PTV margins for CT-guided treatment off-study, as per their institutional motion management guidelines. Advocates for MR-guided radiation therapy have started using 0 to 3 mm PTV margins for treatment plans delivered using real-time cine-MR gating, given the ability to detect and correct for intrafraction motion.13,22
As we have shown in Table 3, volume of ipsilateral breast receiving V95% and V50% are higher with the CT PTV10mm plans compared with the MR PTV3mm plans. In 2 external beam APBI studies that evaluated toxicity and cosmesis after 38.5 Gy in 10 fractions, treated twice daily, ipsilateral breast volumetric indices including V50% and V100% were associated with a high risk of subcutaneous fibrosis and fair/poor cosmesis.10,23 Regarding volumetric predictors for chest wall toxicity, pooled data from lung SBRT studies (48-60 Gy in 3-5 fractions) have shown that V30Gy <30 cm3 and V30Gy <70 cm3 result in rates of grade ≥2 chest wall pain of 17.3% and 27.8%; respectively.24 In our present study, we have used a conservative constraint of V24Gy <30 cm3 to limit chest wall toxicity. We have also shown that MR PTV3mm plans result in lower chest wall V24Gy compared with CT PTV10mm plans (8.5 vs 21.8 cm3, P = .004), which could potentially reduce the number of patients experiencing significant chest wall toxicity.
This is the first dosimetric study comparing CT-Linac with MRIdian-Linac for extremely hypofractionated APBI. One other study comparing MR-Linac with CT-Linac was done for pancreatic cancer, evaluating 40 Gy in 5 fractions. This study used 5 mm PTV and 3 mm planning risk volume margins for both plans and showed that dosimetrically similar plans were produced with MRIdian-Linac and CT-Linac,25 attesting to the feasibility of using of MRgRT in challenging anatomic sites such as the upper abdomen.
Our study has various limitations, the first and foremost being that the 3-fraction external beam APBI schedule used in this study has not been investigated in any other clinical studies. Recently, Khan et al published encouraging cosmesis results at a median follow-up of 12 months after 3-fraction APBI delivered using brachytherapy.9 In this phase 2 multi-institution study, 22.5 Gy in 3 fractions of 7.5 Gy was prescribed to the lumpectomy cavity plus 1.0 to 1.5 cm. Taking into account the significant dose heterogeneity inherent to brachytherapy planning, 7.5 Gy brachytherapy dose per fraction is comparable to our current external beam schedule delivering 8.2 Gy per fraction (109% of 7.5 Gy).
Another important limitation of our study is the generous 10 mm PTV margin that was used for CT plans. Investigators have used reduced PTV margins (5 mm) for CT-based APBI in combination with deep inspiratory breath hold compared with conventional 10 mm PTV margins.26 An important consideration in deep inspiration breath hold–based treatment delivery is the variability in organ position both during and between treatments that is accounted for by the 5 mm margin.27
Another area that needs further investigation is the accuracy of 0.35T MRI in delineating the breast surgical cavity. Studies comparing 3T diagnostic MRI with diagnostic CT have shown that the seroma cavity volumes were smaller on MRI scans, most likely due to clearer cavity definition on T2-weighted MRI sequences.28,29 Such studies have not been performed comparing 0.35T MRI with CT and or 3T MRI scans.
More importantly, although MR PTV3mm plans resulted in better ipsilateral breast and chest wall sparing compared with CT PTV10mm plans, the clinical relevance of these dosimetric differences is unknown. Granular cardiac dosimetric data based on important substructures such as the left anterior descending artery and left ventricle have not been evaluated in this study and will be investigated further in our ongoing prospective protocol. Total treatment time on MRIdian-Linac is 10 to 12 minutes due to the step and shoot treatment delivery system, lower gantry and MLC speed, and fixed collimator angles. Another practical issue to consider is elevating the breast coils using a special bracket during MR and CT planning image acquisition; this would help reduce interfraction variability that is introduced by variable breast deformation if the coils are placed directly on the breast tissue. In many cases, however, the ability of real-time tracking and online adaptation may outweigh any planning and delivery difficulties encountered with MRgRT.
In the current era of health care financial toxicity, studies investigating the cost-effectiveness of MR-guided radiation therapy and CT-based delivery platforms for delivering extreme hypofractionation schedules are needed.
Future directions
The concept of preoperative single-fraction radiation therapy for favorable early stage breast cancer is both convenient and attractive based on the hypothesis that smaller treatment volumes in the preoperative setting compared with adjuvant setting could potentially result in better cosmetic outcomes. This treatment paradigm also provides an opportunity to test biomarkers for radiation response. Two single-institution studies that used 15 Gy to 21 Gy single-fraction radiation therapy have reported favorable early cosmetic outcomes.30,31 MRI-guided radiation therapy could potentially contribute to this evolving paradigm by reducing PTV margins and thereby treatment volumes (clinical trials.gov: NCT03863301).
Conclusions
This study has shown that dosimetrically comparable 3-fraction APBI plans are produced with MRIdian-Linac (step and shoot IMRT) and CT-Linac (VMAT) when similar PTV margins (3 mm) were used. Step and shoot MR PTV3mm plans had only minimally increased dose to the ipsilateral breast and chest wall. As expected, MRI PTV3mm plans resulted in better ipsilateral breast and chest wall sparing compared with CT PTV10mm plans, mainly due to the difference in PTV margins. The clinical relevance of these dosimetric differences is not known.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: none.
Data Sharing Statement: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
This article was submitted to 2019 American Society for Radiation Oncology (ASTRO) Annual Meeting.
References
- 1.Strnad V., Ott O.J., Hildebrandt G. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: A randomised, phase 3, non-inferiority trial. Lancet. 2016;387:229–238. doi: 10.1016/S0140-6736(15)00471-7. [DOI] [PubMed] [Google Scholar]
- 2.Polgar C., Ott O.J., Hildebrandt G. Late side-effects and cosmetic results of accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: 5-year results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:259–268. doi: 10.1016/S1470-2045(17)30011-6. [DOI] [PubMed] [Google Scholar]
- 3.Coles C.E., Griffin C.L., Kirby A.M. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet. 2017;390:1048–1060. doi: 10.1016/S0140-6736(17)31145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivotto I.A., Whelan T.J., Parpia S. Interim cosmetic and toxicity results from RAPID: A randomized trial of accelerated partial breast irradiation using three-dimensional conformal external beam radiation therapy. J Clin Oncol. 2013;31:4038–4045. doi: 10.1200/JCO.2013.50.5511. [DOI] [PubMed] [Google Scholar]
- 5.Vicini F.A., Cecchini R.S., White J.R. San Antonio Breast Cancer Symposium; San Antonio, Texas: 2018. Primary results of NSABP B-39/RTOG 0413 (NRG Oncology): A randomized phase III study of conventional whole breast irradiation (WBI) versus partial breast irradiation (PBI) for women with stage 0, I, or II breast cancer. [Google Scholar]
- 6.Yarnold J., Ashton A., Bliss J. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: Long-term results of a randomised trial. Radiother Oncol. 2005;75:9–17. doi: 10.1016/j.radonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Meattini I., Marrazzo L., Saieva C. Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast cancer: Long-term results of the randomized phase III APBI-IMRT-florence trial. J Clin Oncol. 2020;38:4175–4183. doi: 10.1200/JCO.20.00650. [DOI] [PubMed] [Google Scholar]
- 8.Rahimi A., Thomas K.M., Spangler A. Preliminary results of a phase 1 dose-escalation trial for early-stage breast cancer using 5-fraction stereotactic body radiation therapy for partial-breast irradiation. Int J Radiat Oncol Biol Phys. 2017;98:196–205. doi: 10.1016/j.ijrobp.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Khan A.J., Chen P.Y., Yashar C. Three-fraction accelerated partial breast irradiation (APBI) delivered with brachytherapy applicators is feasible and safe: First results from the TRIUMPH-T trial. Int J Radiat Oncol Biol Phys. 2019;104:67–74. doi: 10.1016/j.ijrobp.2018.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jagsi R., Ben-David M.A., Moran J.M. Unacceptable cosmesis in a protocol investigating intensity-modulated radiotherapy with active breathing control for accelerated partial-breast irradiation. Int J Radiat Oncol Biol Phys. 2010;76:71–78. doi: 10.1016/j.ijrobp.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duma M.N., Baumann R., Budach W. Heart-sparing radiotherapy techniques in breast cancer patients: A recommendation of the breast cancer expert panel of the German Society Of Radiation Oncology (DEGRO) Strahlenther Onkol. 2019;195:861–871. doi: 10.1007/s00066-019-01495-w. [DOI] [PubMed] [Google Scholar]
- 12.Green OL, Rankine LJ, Cai B, et al. First clinical implementation of real-time, real anatomy tracking and radiation beam control [e-pub ahead of print]. Med Phys. https://doi.org/10.1002/mp.13002. Accessed February 2, 2021. [DOI] [PubMed]
- 13.Acharya S., Fischer-Valuck B.W., Mazur T.R. Magnetic resonance image guided radiation therapy for external beam accelerated partial-breast irradiation: Evaluation of delivered dose and intrafractional cavity motion. Int J Radiat Oncol Biol Phys. 2016;96:785–792. doi: 10.1016/j.ijrobp.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Menard C., van der Heide U.A. Introduction: Magnetic resonance imaging comes of age in radiation oncology. Semin Radiat Oncol. 2014;24:149–150. doi: 10.1016/j.semradonc.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Bondiau P.Y., Bahadoran P., Lallement M. Robotic stereotactic radioablation concomitant with neo-adjuvant chemotherapy for breast tumors. Int J Radiat Oncol Biol Phys. 2009;75:1041–1047. doi: 10.1016/j.ijrobp.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 16.Yarnold J., Bentzen S.M., Coles C., Haviland J. Hypofractionated whole-breast radiotherapy for women with early breast cancer: Myths and realities. Int J Radiat Oncol Biol Phys. 2011;79:1–9. doi: 10.1016/j.ijrobp.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Meattini I., Saieva C., Miccinesi G. Accelerated partial breast irradiation using intensity modulated radiotherapy versus whole breast irradiation: Health-related quality of life final analysis from the Florence phase 3 trial. Eur J Cancer. 2017;76:17–26. doi: 10.1016/j.ejca.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Nahum A.E. The radiobiology of hypofractionation. Clin Oncol (R Coll Radiol) 2015;27:260–269. doi: 10.1016/j.clon.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Appelt A.L., Vogelius I.R., Bentzen S.M. Modern hypofractionation schedules for tangential whole breast irradiation decrease the fraction size-corrected dose to the heart. Clin Oncol (R Coll Radiol) 2013;25:147–152. doi: 10.1016/j.clon.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Gregoire V., Mackie T.R. State of the art on dose prescription, reporting and recording in intensity-modulated radiation therapy (ICRU report No. 83) Cancer Radiother. 2011;15:555–559. doi: 10.1016/j.canrad.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Rahimi A., Timmerman R. New techniques for irradiating early stage breast cancer: Stereotactic partial breast irradiation. Semin Radiat Oncol. 2017;27:279–288. doi: 10.1016/j.semradonc.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Zou W., Dong L., Kevin Teo B.K. Current state of image guidance in radiation oncology: Implications for PTV margin expansion and adaptive therapy. Semin Radiat Oncol. 2018;28:238–247. doi: 10.1016/j.semradonc.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Leonard K.L., Hepel J.T., Hiatt J.R., Dipetrillo T.A., Price L.L., Wazer D.E. The effect of dose-volume parameters and interfraction interval on cosmetic outcome and toxicity after 3-dimensional conformal accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2013;85:623–629. doi: 10.1016/j.ijrobp.2012.06.052. [DOI] [PubMed] [Google Scholar]
- 24.Ma J.T., Liu Y., Sun L. Chest wall toxicity after stereotactic body radiation therapy: A pooled-analysis of 57 studies. Int J Radiat Oncol Biol Phys. 2019;103:843–850. doi: 10.1016/j.ijrobp.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 25.Ramey S.J., Padgett K.R., Lamichhane N. Dosimetric analysis of stereotactic body radiation therapy for pancreatic cancer using MR-guided Tri-(60)Co unit, MR-guided LINAC, and conventional LINAC-based plans. Pract Radiat Oncol. 2018;8:e312–e321. doi: 10.1016/j.prro.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Moran J.M., Ben-David M.A., Marsh R.B. Accelerated partial breast irradiation: What is dosimetric effect of advanced technology approaches? Int J Radiat Oncol Biol Phys. 2009;75:294–301. doi: 10.1016/j.ijrobp.2009.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Betgen A., Alderliesten T., Sonke J.J., van Vliet-Vroegindeweij C., Bartelink H., Remeijer P. Assessment of set-up variability during deep inspiration breath hold radiotherapy for breast cancer patients by 3D-surface imaging. Radiother Oncol. 2013;106:225–230. doi: 10.1016/j.radonc.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Pogson E.M., Delaney G.P., Ahern V. Comparison of magnetic resonance imaging and computed tomography for breast target volume delineation in prone and supine positions. Int J Radiat Oncol Biol Phys. 2016;96:905–912. doi: 10.1016/j.ijrobp.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Huang W., Currey A., Chen X. A comparison of lumpectomy cavity delineations between use of magnetic resonance imaging and computed tomography acquired with patient in prone position for radiation therapy planning of breast cancer. Int J Radiat Oncol Biol Phys. 2016;94:832–840. doi: 10.1016/j.ijrobp.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Guidolin K., Yaremko B., Lynn K. Stereotactic image-guided neoadjuvant ablative single-dose radiation, then lumpectomy, for early breast cancer: The SIGNAL prospective single-arm trial of single-dose radiation therapy. Curr Oncol. 2019;26:e334–e340. doi: 10.3747/co.26.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horton J.K., Blitzblau R.C., Yoo S. Preoperative single-fraction partial breast radiation therapy: A novel phase 1, dose-escalation protocol with radiation response biomarkers. Int J Radiat Oncol Biol Phys. 2015;92:846–855. doi: 10.1016/j.ijrobp.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]