Abstract
Purpose
This study aims to develop a local control risk stratification using recursive partitioning analysis (RPA) for patients receiving stereotactic body radiation therapy (SBRT) for metastatic cancer.
Methods and Materials
A single institutional database of 397 SBRT treatments to the liver, spine, and lymph nodes was constructed. All treatments required imaging follow-up to assess for local control. Cox proportional hazards analysis was implemented before the decision tree analysis. The data were split into training (70%), validation (10%), and testing (20%) sets for RPA to optimize the training set.
Results
In the study, 361 treatments were included in the local control analysis. Two-year local control was 71%. A decision tree analysis was used and the resulting model demonstrated 93.10% fidelity for the validation set and 87.67% for the test set. RPA class 3 was composed of patients with non-small cell lung cancer (NSCLC) primary tumors and treatment targets other than the cervical, thoracic, and lumbar spines. RPA class 2 included patients with primary cancers other than NSCLC or breast and treatments targets of the sacral spine or liver. RPA class 1 consisted of all other patients (including lymph node targets and patients with primary breast cancer). Classes 3, 2, and 1 demonstrated 3-year local controls rates of 29%, 50%, and 83%, respectively. On subgroup analysis using the Kaplan-Meier method, treatments for lymph nodes and primary ovarian disease demonstrated improved local control relative to other treatment targets (P < .005) and primary disease sites (P < .005), respectively.
Conclusions
A local control risk stratification model for SBRT to sites of metastatic disease was developed. Treatment target and primary tumor were identified as critical factors determining local control. NSCLC primary lesions have increased local failure for targets other than the cervical, thoracic, or lumbar spines, and improved local control was identified for lymph node sites and breast or ovarian primary tumors.
Introduction
Stereotactic body radiation therapy (SBRT), or stereotactic ablative radiation therapy (SABR), consists of the delivery of high doses of radiation therapy in relatively few fractions, and it has grown increasingly common as a treatment option.1, 2, 3, 4, 5 Its utility for the treatment of metastatic disease also has been an important area of interest, culminating in the SABR-COMET trial demonstrating improvement in overall survival with SABR in oligometastatic cancer.6
Metastatic cancer is increasingly common, especially as oncologic survival continues to improve.7 The underlying molecular mechanisms involved in the metastatic spread of disease are an active area of research, and hypotheses involving the acquisition of additional genetic alterations or a macrophage origin of metastasis have helped advance the field.8,9 In addition to the number of metastatic lesions, the location of metastatic disease has been found have prognostic value.10,11 The spine is the most common site of bone metastasis, and symptoms can be quite severe, ranging from severe pain to impairment of ambulation.12,13 The liver is also an important site of metastatic disease, especially in the setting of colorectal cancer.14, 15, 16 The spread of cancer to lymph nodes is complex, as it can represent regional or distant spread, depending on the lymph nodes involved.17,18 This variability has made developing stringent guidelines regarding their treatment quite difficult.
SBRT plays a role in the treatment paradigm of disease progression to these targets. SBRT for liver metastases has proven to be safe and effective, but the presence of other treatment options (eg, surgery and radiofrequency ablation) has somewhat limited its use.19, 20, 21, 22, 23 Even so, a recent multi-institutional study demonstrated encouraging local control rates, especially with smaller tumor volumes and higher biologically effective dose (BED).24 For spinal metastases, SBRT offers high rates of local control, and it even serves as an effective option in retreatment cases.25 It can be used in conjunction with surgical resection, and by sparing the neural tissues, toxicity can be minimized.26,27 Although there is relatively little literature regarding the utility of SBRT for lymph nodes, analyses often report local control >80%.28,29 Treatments aim to achieve durable disease remission via iterative utility of local therapy.29
The use of recursive partitioning analysis has shown utility in stratifying oligometastatic patients treated with ablative radiation therapy to predict overall survival, and it offers a useful tool to distinguish between different risk groups in large cohorts.30 The methodology has been applied to SBRT for spinal metastases, whole brain radiation, and SBRT for non-small cell lung cancer (NSCLC).31, 32, 33 The use of these machine learning techniques provides opportunities to distill feature importance and deduce correlations between potential predictive factors.
This study aims to develop a local control risk stratification using RPA for patients receiving SBRT for cancer metastatic to the liver, spine, or lymph nodes. A large single-institutional cohort is presented, and the effect of the site of primary disease is considered. We present a hypothesis-generating set of RPA classes to help identify patients who may benefit from SBRT for metastatic disease.
Methods and Materials
Treatment cohort
SBRT treatments at a single institution from 2007 to 2018 targeting the liver, spine, or lymph nodes were considered for inclusion in the study. Patients without clinical follow-up were excluded from the cohort, as were patients with other treatment targets (eg, solid lung lesions or bone metastases to areas other than the spine). Patients without imaging follow-up were excluded from the analysis of local control. Key descriptive factors were obtained via retrospective chart review from previous databases developed with institutional review board exemptions. This project was also exempt by the institutional review board.
Treatment planning and delivery
All cases involved the use of a full-body vacuum bag system for patient immobilization. This provided stability and consistency for the simulation CT scan. For liver treatments, an internal target volume (ITV) was constructed from the 4-dimensional CT scan to account for tumor motion, and doses were prescribed to the ITV. Treatments were generally delivered with 3-dimensional forward planned noncoplanar static field apertures and noncoplanar static arcs. For spine treatments, dose was prescribed to the planning target volume (PTV), which included the gross tumor volume (GTV) plus 3 to 5 mm of margin. Coplanar gantry angles were used to help preserve the superior and inferior dosimetric borders. Generally, multiple coplanar static gantry delivery was used, but noncoplanar static gantry was occasionally used for treatments involving particularly large volumes or extensive disease. The risk volume was designated as the spinal canal, and the delivery of 2 Gy less than the prescribed dose per fraction to the target 1 cc or 2 cc volume was used as the institutional organ at risk constraint. For hilar lymph nodes, dose was prescribed to the ITV, which was determined using serial CT scans throughout the breath cycle to account for tumor motion. Noncoplanar gantry angles were generally used. Finally, other lymph nodes involved dose delivery to the PTV (3-5 mm of margin expanding the GTV), and multiple noncoplanar static gantry delivery was used.
SBRT was delivered with a 6MV photon beam through a linear accelerator. A 2.5 mm to 4 mm width multileaf collimator was used. On-board cone beam CT was used before the initiation of therapy, and it was generally repeated 2 to 4 times during treatment to assess for motion. A robotic couch with 6 degrees of freedom was used to assist with patient alignment and target localization to the planning CT. Treatments were most commonly delivered once weekly in an effort to minimize toxicity.
Outcomes and follow-up
The primary endpoint of this study was local control. Overall survival was also noted. Local control was determined via CT and MRI scans obtained generally every 2 to 3 months after the completion of SBRT. Sometimes, PET/CT scans were also used for this purpose. Local failure was defined as tumor growth in the treated volume after treatment. Clinical follow-up was variable, including radiation oncologists, medical oncologists, pulmonologists, neurosurgeons, and others. All such clinical encounters were considered for the overall survival, which was computed from the completion of SBRT to the last date of interaction with a health care provider.
Statistical methods
Initial statistical methods used 2-tailed t tests for univariate analysis, Cox proportional hazards analysis, and the Kaplan-Meier method. A threshold of P < .05 was used to designate statistical significance in these analyses. Univariate analysis was generally conducted to consider binary results, and the results were correlated with findings on hazards analysis and Kaplan-Meier whenever possible. BED was determined as BED = total dose × (1 + dose per fraction/alpha/beta ratio), and an alpha/beta ratio of 10 Gy was used. For the hazards analysis, primary disease sites other than breast, NSCLC, colorectal, and ovarian were eliminated because there were too few such cases, and their inclusion resulted in very wide confidence intervals. Dose, fractions, and GTV were also eliminated owing to high correlations with BED and PTV. The sacral spine location was separated from C, T, or L spine because the sacral spine location independently met the threshold for feature importance (conducted before the decision tree analysis). Finally, the Kaplan-Meier method was incorporated to consider continuous variables. Chiefly, it was used to demonstrate differences in local control between different subgroups of the cohort using a univariable log-rank test.
Recursive partitioning analysis
Recursive partitioning analysis was conducted via decision tree analysis to develop a model to predict local failure in this cohort. The code was written using a set of Python scripts, and separate classes were designed to analyze the data. A range of open source statistical packages were used, including pandas, matplotlib, lifelines, numpy, and sklearn. The sklearn package provided the ExtraTreesClassifier, through which the feature importance analysis was undertaken. The decision tree analysis itself was carried out via DecisionTreeClassifier.
A feature importance analysis was performed to assess the relative importance values of each potential predictive factor for local control. A threshold value of 0.02 was used, and variables that failed to reach this threshold were eliminated from further analysis. Next, a correlation matrix and heatmap were produced to assess for correlations between the remaining variables. A threshold value of 0.50 was used, such that 2 variables with a correlation greater than 0.50 were not both retained for further analysis. In such cases, the variable with the higher importance value was kept, and the other variable was discarded.
The data were then split into training (70%), validation (10%), and testing sets (20%). This split allowed for optimization of the training set while maintaining sufficient samples in the testing and validation sets to minimize variance. The model was developed used the training set, and the separate validation and testing sets allowed for determination of the predictive power of the model. A decision tree analysis was iteratively performed through a range of split, leaf, and maximum depth criteria, as well as considering a range of features and selection criteria. The highest fidelity model generated through this method was retained and considered for further analysis.
Results
Treatment cohort
In the study, 293 patients with 397 treatments comprised the initial cohort. This data set consisted of a range of primary disease sites, including breast (21%), NSCLC (23%), colorectal (9%), and ovarian (17%). The median age at the start of treatment was 67.84 years, and the majority of treatments involved female patients. In addition, 84% of treatments involved prior chemotherapy, and 36% had prior radiation therapy at the treatment location. There were 159 treatments to the liver, 145 to the spine, and 93 to lymph nodes. Furthermore, 35 of the spine treatments involved the sacral spine. Lymph node treatments included 42 treatments of the hilar lymph nodes, and 51 treatments of ovarian primary disease metastatic to a range of primarily abdominal and perigastric lymph nodes. Treatments involved a median 24 Gy in 3 fractions, corresponding to a BED of 43.2 Gy. The most common liver dose-fractionation schemes were 30 Gy in 3 fractions, 36 Gy in 3 fractions, and 24 Gy in 3 fractions. Twenty-four Gy in 3 fractions was most frequently delivered for treatments to the spine. The median dose-fractionation for the hilar lymph nodes was 28 Gy in 4 fractions. Other lymph nodes were most commonly treated with 20 to 24 Gy in 3 to 4 fractions or 30 to 40 Gy in 3 to 5 fractions. The median GTV and PTV were 15.71 mL and 33.52 mL, respectively (Table 1).
Table 1.
The patient population involved in the study is described
| No. | Rate, % | |
|---|---|---|
| Primary disease site | ||
| Breast | 83 | 21 |
| NSCLC | 93 | 23 |
| Colorectal | 37 | 9 |
| Renal | 14 | 4 |
| Melanoma | 7 | 2 |
| Prostate | 15 | 4 |
| Multiple myeloma | 5 | 1 |
| Ovary | 69 | 17 |
| Other gyn | 12 | 3 |
| Head and neck | 10 | 3 |
| Other∗ | 52 | 13 |
| Median age (y) | 67.84 (31.47-92.31) | |
| Treatments for males | 134 | 34 |
| Treatments for females | 263 | 66 |
| Prior chemotherapy | 334 | 84 |
| Prior radiation at treatment location | 143 | 36 |
| Treatment site: liver | 159 | 40 |
| Cervical, thoracic, or lumbar spine | 110 | 28 |
| Sacral spine | 35 | 9 |
| Lymph node | 93 | 23 |
| Median dose (Gy) | 24 (6-50) | |
| Median fractions | 3 (1-6) | |
| Median BED (Gy) | 43.2 (9.6-112.5) | |
| Median GTV (cc) | 15.71 (0.3-368.8) | |
| Median PTV (cc) | 33.52 (0.4-586.09) |
Abbreviations: BED = biologically effective dose; GTV = gross tumor volume; NSCLC = non-small cell lung cancer; PTV = planning target volume.
The “other” primary tumors included: gastric, leiomyosarcoma, urothelial, pancreatic, and others.
Outcomes
The overall survival of the cohort is demonstrated in Figure 1a. The 2-year survival was 37%. After lesions without imaging follow-up were eliminated from subsequent analysis, the local control of the cohort was demonstrated in Fig 1b. In addition, 361 of the initial 397 were considered in this analysis. Two-year local control was 71% via Kaplan-Meier analysis, and the median time to failure was 10.81 months. A median 12.94 months of clinical follow-up was obtained with this patient group.
Figure 1.
The (a) overall survival and (b) local control for the entire patient cohort are shown. Treatments for lymph node targets (c) had improved local control relative to other targets (P <.005), and treatments with ovarian primary disease (d) had improved local control relative to other primary disease sites (P <.005). Abbreviation: NSCLC = non-small cell lung cancer.
Initial statistical analyses
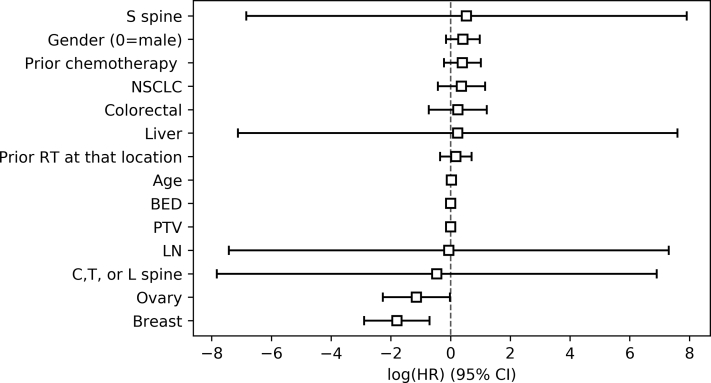
Initial statistical analyses involved using univariate analysis, Cox proportional hazards analysis, and the Kaplan-Meier method to determine predictive factors for local control. Univariate analysis demonstrated a number of factors that met statistical significance, including breast primary (P = 7.76 × 10−8), NSCLC primary (P = 3.85 × 10−5), and age (1.52 × 10−5). These results are tabulated in Table 2. Cox proportional hazards analysis was used to verify the relationships proposed via univariate analysis (Fig 2). Key factors involved in this analysis were primary tumor site, age, sex, prior treatment, treatment target, BED, and PTV. Again, breast primary (hazard ratio [HR] = 0.17 [0.06-0.49]) was predictive of local control, and ovarian primary was also protective (HR = 0.32 [0.10-0.98]). Increased age demonstrated a small increase in risk of local failure (HR = 1.03 [1.00-1.05]). On the other hand, prior chemotherapy, NSCLC primary, and treatment target failed to do so. In fact, the treatment target variables had very wide confidence intervals. The analysis was reattempted after eliminating those factors, but only minor changes in the results were obtained.
Table 2.
The predictors for local control are tabulated, along with the results of the univariate and Cox proportional hazards analyses
| Univariate | HR | Kaplan-Meier | |
|---|---|---|---|
| Primary tumor site | |||
| Breast | P = 7.76∗10−8 | HR = 0.27 (0.10-0.73), P <.005 | P = .63 |
| NSCLC | P = 3.85∗10−5 | HR = 1.44 (0.65-3.17), P = .37 | P = .38 |
| Colorectal | P = .78 | HR = 1.27 (0.48-3.36), P = .62 | ∗ |
| Renal | P = .52 | ∗ | ∗ |
| Melanoma | P = .71 | ∗ | ∗ |
| Prostate | P = .85 | ∗ | ∗ |
| Multiple myeloma | P = .45 | ∗ | ∗ |
| Ovary | P = .12 | HR = 0.32 (0.10-0.98), P = .05 | P <.005 |
| Other gyn | P = .6 | ∗ | ∗ |
| Head and neck | P = .0045 | ∗ | ∗ |
| Age | P = 1.52∗10−5 | HR = 1.03 (1.00-1.05), P = .03 | P = .81† |
| Sex | P = .66 | HR = 1.51 (0.86-2.66), P = .15 | ∗ |
| Prior chemotherapy | P = .021 | HR = 1.49 (0.80-2.75), P = .21 | P = .49 |
| Prior radiation therapy | P = .24 | HR = 1.20 (0.71-2.02), P = .51 | ∗ |
| Liver | P = .042 | HR = 1.27 (0.00-1992.56), P = .95 | ∗ |
| C, T, or L spine | P = 3.76∗10−4 | HR = 0.63 (0.00-996.11), P = .9 | ∗ |
| S spine | P = .066 | HR = 1.70 (0.00-2717.54), P = .89 | ∗ |
| Lymph node | P = 7.52∗10−4 | HR = 0.94 (0.00-1489.07), P = .99 | P <.005 |
| Dose | P = .3 | ∗ | ∗ |
| Fractions | P = 6.49∗10−4 | ∗ | ∗ |
| BED | P = .67 | HR = 1.00 (0.99-1.02), P = .72 | ∗ |
| GTV | P = .54 | ∗ | ∗ |
| PTV | P = .59 | HR = 1.00 (1.00-1.00), P = .9 | ∗ |
Abbreviations: BED = biologically effective dose; GTV = gross tumor volume; HR = hazard ratio; NSCLC = non-small cell lung cancer; PTV = planning target volume.
Indicates that the feature was dropped from the analysis.
Age >75 was used a threshold for this analysis.
Figure 2.
The Cox proportional hazards analysis for factors predictive of local failure is shown. Breast and ovarian primary disease were predictive of local control (hazard ratio [HR] = 0.17 and HR = 0.32, respectively), and increased age demonstrated increased risk of local failure (HR = 1.03). Abbreviations: CI = confidence interval; NSCLC = non-small cell lung cancer; PTV = planning target volume; LN = lymph node target.
Finally, Kaplan-Meier analysis was implemented to consider the factors achieving statistical significance via the other approaches. Regarding age, a threshold of age >75 met statistical significance on univariate analysis (P = .0001), but this threshold failed to demonstrate significance using the Kaplan-Meier method (P = .81). Similarly, prior chemotherapy (P = .49), breast primary (P = .63), and NSCLC primary (P = .38) all failed to demonstrate prognostic value for local control. Lymph node targets did show improved local control relative to other targets (P < .005). Additionally, ovarian primary tumors had improved local control relative to other primary tumors (P < .005; Fig 1c and d).
Decision tree analysis
First, a feature importance analysis resulted in the elimination of primary melanoma, prostate, multiple myeloma, ovary, other gynecology cancers, head and neck, renal, and also a liver target for the SBRT (Fig E1). Sacral spine cases met the importance threshold independently of the cervical, thoracic, and lumbar cases, so sacral cases were separated from the cervical, thoracic, and lumbar treatments.
Next a correlation matrix and heatmap were generated, which showed strong correlations between dose and fractions with BED and GTV with PTV. Factors with a correlation greater than 0.50 were eliminated, resulting in dose, fractions, and GTV being cut from the subsequent analysis. BED had a stronger initial feature importance than dose and fractions, which is why it was chosen instead of dose and fractions. The reasoning for keeping PTV was similar.
A decision tree analysis was iteratively performed through a range of split, leaf, and maximum depth criteria, as well as considering a range of features and selection criteria. The highest fidelity model demonstrated 93.10% fidelity for the validation set and 87.67% accuracy when subsequently applied to the test set (Fig E2).
RPA classes
From the decision tree analysis, 3 RPA classes were determined. RPA class 3 was composed of patients with NSCLC primary tumors and treatment targets other than the cervical, thoracic, and lumbar spine. RPA class 2 included patients with primary cancers other than NSCLC or breast and treatment targets of the sacral spine or liver. RPA class 1 consisted of all other patients. This included 3 groups from the decision-tree analysis: all cervical, thoracic, and lumbar spine treatments; lymph node treatments without primary NSCLC or breast tumors; and breast primary cancers with SBRT targets other than the cervical, thoracic, or lumbar spines.
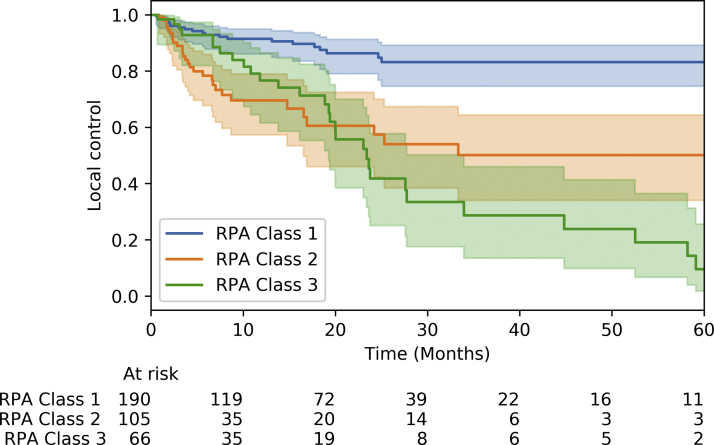
The RPA classes are demonstrated in Figure 3. RPA class 1 consisted of 190 treatments, with 21 local failures. RPA class 2 had 105 treatments, with 29 local failures. Finally, RPA class 3 included 66 treatments, with 31 local failures. Three-year local control rates for the classes computed via the Kaplan-Meier method were 83%, 50%, and 29%, respectively. Univariate analysis demonstrated statistically significance differences between each of the RPA classes, with respect to local control. Kaplan-Meier analysis demonstrated statistically significant differences between class 1 and class 2 (P = 1.0 × 10−4) and between class 1 and class 3 (P = 5.2 × 10−7). These results are included in Table 3.
Figure 3.
The 3 recursive partitioning analysis (RPA) classes are shown, with 3-year local control of 83%, 50%, and 29%, respectively.
Table 3.
Key outcomes results are tabulated. Bolded values indicate statistical significance
| No. | Rate | ||
|---|---|---|---|
| Treatments for local control | 361 | ||
| 2-y local control | 71% | ||
| 2-y overall survival | 37% | ||
| Median time to failure (mo) | 10.81 | ||
| Median follow-up (mo) | 12.94 | ||
| RPA outcomes | Class 1 | Class 2 | Class 3 |
| No. | 190 | 105 | 66 |
| Local failure | 21 | 29 | 31 |
| Crude local failure rate (%) | 11% | 28% | 47% |
| 3-y local control (%) | 83% | 50% | 29% |
| Median time to local failure (mo) | 15.38 | 5.5 | 11.25 |
| Univariate; Kaplan-Meier | Reference | 1.0 × 10−4; <0.005 | 5.2 × 10−7; <0.005 |
| Univariate; Kaplan-Meier | 1.0 × 10−4; <0.005 | Reference | 0.012; 0.52 |
Abbreviation: RPA = recursive partitioning analysis.
Discussion
The key result of this analysis is the development of 3 RPA classes for local control, a novel result compared with other RPA studies considering overall survival. These classes point toward the importance of primary disease site and treatment target in the local control achieved with SBRT. Critically, primary NSCLC disease resulted in poor local control, aside from when it involved treatment of the cervical, thoracic, or lumbar spines. Treatments targeting lymph nodes had high rates of efficacy, particularly when the primary disease site was not NSCLC. Furthermore, treatments to the liver and sacral spine were moderately efficacious, compared with treatments of the lymph nodes or C, T, or L spines. This fact was only amended if the primary tumor was NSCLC (poor local control) or breast (improved local control).
These results complement and build upon the foundation laid by the development of other prognostic models featuring large SBRT cohorts. Hong et al considered a large, multi-institutional cohort of 361 patients to consider factors predictive of overall survival. Their results also highlighted the importance of the primary disease site, as breast, kidney, and prostate cancer patients demonstrated improved overall survival.30
Notably, key dosimetric and volume factors (dose, BED, GTV, and PTV) failed to demonstrate statistical significance for local control in this large cohort. This result is somewhat surprising, as smaller target volume has been shown to be predictive of local control in SBRT targeting the lung, spine, and liver.34, 35, 36 Although it is likely that smaller tumors do have improved local control, the results here indicate that patients with larger tumors may still be considered as potential candidates for SBRT. Similarly, higher dose SBRT has generally been correlated with improved local control and even survival.30,37 The impressive results of this large single-institutional experience pose a hypothesis-generating question regarding the utility of low-dose SBRT in patients for whom high-dose therapy may be contraindicated.
Three key primary disease sites were identified. Treatments for ovarian cancer demonstrated improved local control relative to other treatment sites. Generally, outcomes in ovarian cancer are poor, and recurrence rates are high.38 Even so, SBRT has proven to be efficacious in this setting, with some local control rates reported as high as 92.9% at 1 year.39 Furthermore, toxicity is minimal, with Lazzari et al reporting no grade ≥3 toxicity.40 These results point toward the potential for the increased use of SBRT in ovarian cancer, but further study is needed. Similarly, patients with breast cancer may be predisposed to significant benefit with local therapy.30 On the other hand, NSCLC patients demonstrated poor local control, aside from treatment to the C, T, or L spines. Management of stage IV NSCLC is a complex medical scenario that requires multidisciplinary care and decision-making.41,42 Although SBRT can still play a role as local therapy for metastatic disease, careful consideration should be made for the addition of other treatment modalities, such as chemotherapy and immunotherapy. Furthermore, the initiation of palliative care early in the course of therapy for stage IV NSCLC may be of benefit.41
The fact that treatments for lymph node disease demonstrated improved local control was a surprising result, as there is relatively little data in the literature about SBRT use for this indication. Lymph node disease consists of a heterogenous group, as radiation therapy treatments can include regional lymph nodes or distant metastatic spread. For this reason, rigorous analysis of these outcomes is difficult. Even so, results have been encouraging. Yeung et al reported 1-year local control of 94%, and Loi et al noted only 15% local failure.28,29 Our results indicate that lymph node disease could be considered as an indication for treatment with SBRT in carefully selected patients with oligometastatic disease.
The cervical, thoracic and lumbar spine treatments demonstrated impressive rates of local control, and they comprised a large group within RPA class 1. This result that patients with spine metastases had improved local control is intriguing, and it correlates with the findings of Chen et al that patients with breast cancer with only bone metastasis had improved survival, relative to other sites of metastasis.10 Furthermore, C, T, and L spine treatments even had impressive local control for NSCLC primary tumors.
The fact the sacral spine treatments demonstrated independent feature importance relative to the C, T, and L spine treatments is a relatively novel observation. Zeng et al considered SBRT treatments of the cervical and sacral spines and noted decreased local control in the sacral spine at 2 years (78.7% vs 92.7%). They postulated that high rates of epidural disease and technical challenges specific to SBRT for the sacrum as possible explanations for this difference.43 Additional study is recommended to help clarify this distinction.
The primary limitation of this study involves inherent limitations of the data itself. The significant degree of overlap between patients with primary ovarian cancer and lymph node treatment targets in this data set puts into question which of the 2 factors is the most significant predictor of local control. Because primary ovarian cancer failed to meet the threshold for significance for inclusion in the decision tree analysis, it is most likely that the lymph node target was the more significant predictor, but additional study is needed. The data were obtained through retrospective review, which carries unavoidable biases. Furthermore, the dose-fractionation schemes used for the various treatment sites were variable, which may have obfuscated any predictive effects of dose or BED. Given the impressive local control rates with low-dose SBRT, especially for primary breast and ovarian disease, usage of alternative radiation therapy techniques aside from SBRT should also be strongly considered. Additionally, although single institutional study can be advantageous from the perspective of decreased variability between institutional treatment guidelines, it can limit the generalizability of a large cohort study. Finally, there is significant variability in the range of systemic treatments available for the different primary tumors. As systemic treatment options continue to evolve, different tumor histologies may gain a larger benefit from SBRT.
Conclusions
A local control risk stratification model for SBRT to sites of metastatic disease was developed. Treatment target and primary tumor were identified as critical factors determining local control. NSCLC primary lesions have increased local failure for targets other than the cervical, thoracic, or lumbar spines, and improved local control was identified for lymph node sites and breast or ovarian primary tumors.
Footnotes
Sources of support: There are no sources of funding to report.
Disclosures: The wife of Dr Kowalchuk is a senior technical product manager at GE Health care. No other authors have any conflicts or interests, competing interests, or disclosures.
Research data are available from the corresponding author upon reasonable request.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2020.10.025.
Supplementary Materials
Fig E1.
Fig E2.
References
- 1.Prezzano K.M., Ma S.J., Hermann G.M., Rivers C.I., Gomez-Suescun J.A., Singh A.K. Stereotactic body radiation therapy for non-small cell lung cancer: A review. World J Clin Oncol. 2019;10:14. doi: 10.5306/wjco.v10.i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haidar Y.M., Rahn D.A., III, Nath S. Comparison of outcomes following stereotactic body radiotherapy for non-small cell lung cancer in patients with and without pathological confirmation. Ther Adv Respir Dis. 2014;8:3–12. doi: 10.1177/1753465813512545. [DOI] [PubMed] [Google Scholar]
- 3.Grills I.S., Hope A.J., Guckenberger M. A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small cell lung cancer using daily online cone-beam computed tomography image-guided radiotherapy. J Thorac Oncol. 2012;7:1382–1393. doi: 10.1097/JTO.0b013e318260e00d. [DOI] [PubMed] [Google Scholar]
- 4.Jeppesen S.S., Schytte T., Jensen H.R., Brink C., Hansen O. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: An updated retrospective study on local failure and survival rates. Acta Oncologica. 2013;52:1552–1558. doi: 10.3109/0284186X.2013.813635. [DOI] [PubMed] [Google Scholar]
- 5.Timmerman R., Paulus R., Galvin J. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palma D.A., Olson R., Harrow S. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet. 2019;393:2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 7.Phillips J.L., Currow D.C. Cancer as a chronic disease. Collegian. 2010;17:47–50. doi: 10.1016/j.colegn.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Valastyan S., Weinberg R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seyfried T.N., Huysentruyt L.C. On the origin of cancer metastasis. Critical reviews in oncogenesis. Crit Rev Oncog. 2013;18:43. doi: 10.1615/critrevoncog.v18.i1-2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen M.T., Sun H.F., Zhao Y., Fu W.Y., Yang L.P., Gao S.P., Li L.D., Jiang H.L., Jin W. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: A SEER population-based analysis. Sci Rep. 2017;7:1–8. doi: 10.1038/s41598-017-10166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patanaphan V.I., Salazar O.M., Risco R.A. Breast cancer: Metastatic patterns and their prognosis. South Med. 1988;81:1109–1112. [PubMed] [Google Scholar]
- 12.Maccauro G., Spinelli M.S., Mauro S., Perisano C., Graci C., Rosa M.A. Physiopathology of spine metastasis. Int J Surg Oncol. 2011;2011 doi: 10.1155/2011/107969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delank K.S., Wendtner C., Eich H.T., Eysel P. The treatment of spinal metastases. Dtsch Arztebl Int. 2011;108:71. doi: 10.3238/arztebl.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbruzzese J.L., Abbruzzese M.C., Lenzi R., Hess K.R., Raber M.N. Analysis of a diagnostic strategy for patients with suspected tumors of unknown origin. J Clin Oncol. 1995;13:2094–2103. doi: 10.1200/JCO.1995.13.8.2094. [DOI] [PubMed] [Google Scholar]
- 15.Hugen N., Van de Velde C.J., De Wilt J.H., Nagtegaal I.D. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25:651–657. doi: 10.1093/annonc/mdt591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manfredi S., Lepage C., Hatem C., Coatmeur O., Faivre J., Bouvier A.M. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naxerova K., Reiter J.G., Brachtel E. Origins of lymphatic and distant metastases in human colorectal cancer. Science. 2017;357:55–60. doi: 10.1126/science.aai8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunner W., Widmann B., Marti L., Tarantino I., Schmied B.M., Warschkow R. Predictors for regional lymph node metastasis in T1 rectal cancer: A population-based SEER analysis. Surg Endosc. 2016;30:4405–4415. doi: 10.1007/s00464-016-4759-3. [DOI] [PubMed] [Google Scholar]
- 19.Schefter T.E., Kavanagh B.D., Timmerman R.D., Cardenes H.R., Baron A., Gaspar L.E. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. 2005;62:1371–1378. doi: 10.1016/j.ijrobp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Kavanagh B.D., Schefter T.E., Cardenes H.R. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol. 2006;4:848–855. doi: 10.1080/02841860600904870. [DOI] [PubMed] [Google Scholar]
- 21.Minami Y., Kudo M. Radiofrequency ablation of liver metastases from colorectal cancer: A literature review. Gut Liver. 2013;7:1. doi: 10.5009/gnl.2013.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garden O.J., Rees M., Poston G.J., Mirza D., Saunders M., Ledermann J., Primrose J.N., Parks R.W. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55(Suppl 3):iii1–iii8. doi: 10.1136/gut.2006.098053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simmonds P.C., Primrose J.N., Colquitt J.L., Garden O.J., Poston G.J., Rees M. Surgical resection of hepatic metastases from colorectal cancer: A systematic review of published studies. Br J Cancer. 2006;94:982–999. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahadevan A., Blanck O., Lanciano R. Stereotactic body radiotherapy (SBRT) for liver metastasis–clinical outcomes from the international multi-institutional RSSearch Patient Registry. Radiat Oncol. 2018;13:26. doi: 10.1186/s13014-018-0969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang U.K., Cho W.I., Kim M.S., Cho C.K., Lee D.H., Rhee C.H. Local tumor control after retreatment of spinal metastasis using stereotactic body radiotherapy: Comparison with initial treatment group. Acta Oncologica. 2012;51:589–595. doi: 10.3109/0284186X.2012.666637. [DOI] [PubMed] [Google Scholar]
- 26.Al-Omair A., Masucci L., Masson-Cote L. Surgical resection of epidural disease improves local control following postoperative spine stereotactic body radiotherapy. Neuro Oncol. 2013;15:1413–1419. doi: 10.1093/neuonc/not101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tseng C.L., Eppinga W., Charest-Morin R. Spine stereotactic body radiotherapy: Indications, outcomes, and points of caution. Global Spine. 2017;7:179–197. doi: 10.1177/2192568217694016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeung R., Hamm J., Liu M., Schellenberg D. Institutional analysis of stereotactic body radiotherapy (SBRT) for oligometastatic lymph node metastases. Radiat Oncol. 2017;12:105. doi: 10.1186/s13014-017-0820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loi M., Frelinghuysen M., Klass N.D. Locoregional control and survival after lymph node SBRT in oligometastatic disease. Clin Exp Metastasis. 2018;35:625–633. doi: 10.1007/s10585-018-9922-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong J.C., Ayala-Peacock D.N., Lee J. Classification for long-term survival in oligometastatic patients treated with ablative radiotherapy: A multi-institutional pooled analysis. PloS One. 2018;13 doi: 10.1371/journal.pone.0195149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao S.T., Koyfman S.A., Woody N. Recursive partitioning analysis index is predictive for overall survival in patients undergoing spine stereotactic body radiation therapy for spinal metastases. Int J Radiat Oncol Biol Phys. 2012;82:1738–1743. doi: 10.1016/j.ijrobp.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 32.Chidel M.A., Suh J.H., Reddy C.A., Chao S.T., Lundbeck M.F., Barnett G.H. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:993–999. doi: 10.1016/s0360-3016(00)00527-7. [DOI] [PubMed] [Google Scholar]
- 33.Matsuo Y., Shibuya K., Nagata Y. Prognostic factors in stereotactic body radiotherapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79:1104–1111. doi: 10.1016/j.ijrobp.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Parker S.M., Siochi R.A., Wen S., Mattes M.D. Impact of tumor size on local control and pneumonitis after stereotactic body radiation therapy for lung tumors. Pract Radiat Oncol. 2019;9:e90–e97. doi: 10.1016/j.prro.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta N., Zavitsanos P.J., Moldovan K. Local failure and vertebral body fracture risk using multifraction stereotactic body radiation therapy for spine metastases. Adv Radiat Oncol. 2018;3:245–251. doi: 10.1016/j.adro.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andratschke N.H., Nieder C., Heppt F., Molls M., Zimmermann F. Stereotactic radiation therapy for liver metastases: Factors affecting local control and survival. Radiat Oncol. 2015;10:69. doi: 10.1186/s13014-015-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kok E.N., Jansen E.P., Heeres B.C. High versus low dose stereotactic body radiation therapy for hepatic metastases. Clin Transl Radiat Oncol. 2020;20:45–50. doi: 10.1016/j.ctro.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ushijima K. Treatment for recurrent ovarian cancer—At first relapse. J Oncol. 2010;2010 doi: 10.1155/2010/497429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iftode C., D'Agostino G.R., Tozzi A. Stereotactic body radiation therapy in oligometastatic ovarian cancer: A promising therapeutic approach. Int J Gynecol Cancer. 2018;28:1507–1513. doi: 10.1097/IGC.0000000000001324. [DOI] [PubMed] [Google Scholar]
- 40.Lazzari R., Ronchi S., Gandini S. Stereotactic body radiation therapy for oligometastatic ovarian cancer: A step toward a drug holiday. Int J Radiat Oncol Biol Phys. 2018;101:650–660. doi: 10.1016/j.ijrobp.2018.03.058. [DOI] [PubMed] [Google Scholar]
- 41.Socinski M.A., Evans T., Gettinger S., Hensing T.A., Sequist L.V., Ireland B., Stinchcombe T.E. Treatment of stage IV non-small cell lung cancer: Diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e341S–e368S. doi: 10.1378/chest.12-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zappa C., Mousa S.A. Non-small cell lung cancer: Current treatment and future advances. Transl Lung Cancer Res. 2016;5:288. doi: 10.21037/tlcr.2016.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng K.L., Myrehaug S., Soliman H. Stereotactic body radiotherapy for spinal metastases at the extreme ends of the spine: Imaging-based outcomes for cervical and sacral metastases. Neurosurgery. 2019;85:605–612. doi: 10.1093/neuros/nyy393. [DOI] [PubMed] [Google Scholar]