Abstract
Purpose
Stereotactic body radiation therapy (SBRT) has demonstrated clinical benefits for patients with metastatic and/or unresectable cancer. Technical considerations of treatment delivery and nearby organs at risk can limit the use of SBRT in large tumors or those in unfavorable locations. Spatially fractionated radiation therapy (SFRT) may address this limitation because this technique can deliver high-dose radiation to discrete subvolume vertices inside a tumor target while restricting the remainder of the target to a safer lower dose. Indeed, SFRT, such as GRID, has been used to treat large tumors with reported dramatic tumor response and minimal side effects. Lattice is a modern approach to SFRT delivered with arc-based therapy, which may allow for safe, high-quality SBRT for large and/or deep tumors.
Methods and Materials
Herein, we report the results of a dosimetry and quality assurance feasibility study of Lattice SBRT in 11 patients with 12 tumor targets, each ≥10 cm in an axial dimension. Prior computed tomography simulation scans were used to generate volumetric modulated arc therapy Lattice SBRT plans that were then delivered on clinically available Linacs. Quality assurance testing included external portal imaging device and ion chamber analyses.
Results
All generated plans met the standard SBRT dose constraints, such as those from the American Association of Physicists in Medicine Task Group 101. Additionally, we provide a step-by-step approach to generate and deliver Lattice SBRT plans using commercially available treatment technology.
Conclusions
Lattice SBRT is currently being tested in a prospective trial for patients with metastatic cancer who need palliation of large tumors (NCT04553471, NCT04133415).
Introduction
Metastatic or unresectable cancer is responsible for significant morbidity and mortality in patients with solid tumors.1 Stereotactic body radiation therapy (SBRT) is emerging as a high-value treatment option for these patients, offering improved symptom palliation across cancer types and extended survival in oligometastatic populations. Unfortunately, SBRT can be difficult to deliver safely for large tumors. Large tumors may be near surrounding organs at risk (OARs), which can make planning difficult.2,3 Prior studies have shown that SBRT may be associated with unacceptable toxicity for tumors >5 cm.4,5
Spatially fractionated radiation therapy (SFRT) is a radiation therapy technique that theoretically allows for safe dose escalation for large tumors. Specialized beam collimation creates high-dose peaks organized throughout a target volume with intervening low-dose valleys.6 SFRT planned with a 2-dimensional technique, such as GRID radiation therapy, has been evaluated for large soft-tissue sarcomas, and is associated with excellent local control and low toxicity in prior case series.7,8 The GRID technique attempts to achieve a differential high-dose peak surrounded by lower-dose valleys, using either a precast block or multileaf collimators (MLCs).9 Although GRID is more widely accessible, newer SFRT techniques (eg, Lattice) offer improved dose distribution and OAR sparing compared with GRID. Lattice creates small, high-dose islands within a sea of lower doses covering the entire tumor volume. Compared with GRID, Lattice may be more beneficial for large or deep-seated tumors surrounded by OARs.10
To safely deliver SBRT to large and/or deep-seated tumors, we created a Lattice SBRT technique that delivers 2000 cGy in 5 fractions to the entire tumor target with a simultaneous integrated boost of 6670 cGy to vertices arranged geometrically inside the tumor. Herein, we describe the Lattice SBRT planning process, resulting dosimetric parameters of 12 pilot Lattice SBRT plans, and their quality assurance (QA) results.
Methods and Materials
This study was approved by our institutional review board. We retrospectively identified 12 large tumors >10 cm in 11 patients previously treated at our institution. Patient and tumor characteristics were extracted from the electronic medical record. A step-by-step guide to the Lattice SBRT contouring and treatment planning process is available in Supplementary Material E1. The Lattice SBRT prescription was created on the assumption that the tumor planning target volume (PTV) should receive at least a standard 5-fraction palliative dose of 2000 cGy. Spatially fractionated techniques have traditionally created a peak–valley dose gradient of approximately 100% to 30%6; therefore, a simultaneous integrated boost of 6670 cGy was selected as a Lattice boost dose prescription.
To generate the desired high-gradient dose distribution, our Lattice SBRT technique uses a geometric arrangement of spherical vertices, each with a diameter of 1.5 cm, 6-cm center-to-center spacing, and separation of 3.0 cm between each successive axial plane of spheres. A representative schematic is shown in Figure 1. The selection of these Lattice SBRT planning technique dimensions was based on previously published approaches, which used boost target vertices of 1 to 2 cm in diameter, spaced 2 to 3 cm apart throughout the gross tumor volume (GTV).11,12 These vertices are defined within a physician-contoured GTV (GTV_2000), which included all visually identifiable gross disease. The GTV_2000 was expanded by 0.5 to 1.0 cm to create the PTV_2000, which was to receive 2000 cGy in 5 fractions. To generate the geometric lattice, the axial plane with the largest cross dimension within the GTV_2000 was first selected. Then, a 3 × 3 × 3 cm grid guide was overlaid on the GTV_2000, and the high-dose target vertices were placed at the grid intersections using a 1.5 cm diameter 3-dimensional brush to create the PTV_6670. The PTV_6670 high-dose target vertices were alternated with 1.5 cm diameter avoidance vertices (ie, PTV_Avoid) such that the center of a PTV_6670 vertex was 3 cm apart from a PTV_Avoid vertex. This process was repeated every 3 cm in the superior–inferior direction with vertices offset by 3.0 cm with respect to axial slices above and below. All PTV_6670 vertices extending outside of a 5 mm contraction of the GTV_2000 were completely removed to minimize spill of the 30 Gy isodose volume outside the PTV_2000. Additionally, any PTV_6670 vertices located within 1.5 cm of an OAR were completely removed to limit the dose to normal tissue given any uncertainties at the time of treatment. Finally, a PTV_Control structure was made by expanding PTV_6670 by 8 mm and subtracting this from PTV_2000, and was used to assess dose falloff between adjacent PTV_6670 vertices.
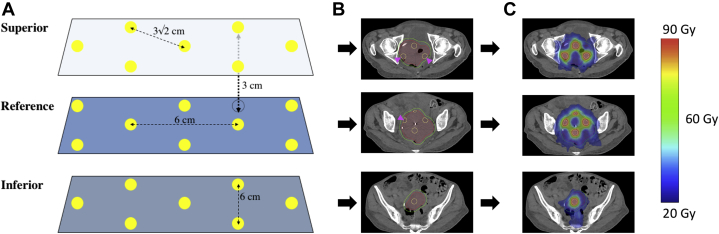
Figure 1.
(A) Geometric representation of sphere placement. Yellow dots represent the 1.5 cm diameter planning target volume (PTV)_6670 target vertices, and dotted line vertices represent the transposed target vertices from adjacent planes. Axial planes where vertices are placed are separated by 3 cm in plane. Within a plan, vertices are separated by 6 cm center to center (4.5 cm edge to edge) in orthogonal axes, and 3√2 cm along the diagonal. (B) Axial computed tomography slices of a target with the yellow outlined target vertices (PTV_6670) in each plane, red gross tumor volume_2000, and green PTV_2000. Magenta arrows denote cropped vertices in PTV_6670 that extend outside of the gross tumor volume_2000. (C) Dose distribution after volumetric modulated arc therapy planning for the target with blue representing 20 Gy and red 66.7 Gy. (A color version of this figure is available at https://doi.org/10.1016/j.adro.2020.100639.)
The Lattice plan generation attempted to achieve a goal of ≥95% prescription dose coverage to at least 95% of both the PTV_2000 and PTV_6670. To achieve the high-dose gradients, each of the interspaced PTV_Avoid vertices required a minimum dose between 19 and 20 Gy. High maximum point doses were allowed within the PTV_6670 vertices, with a maximum dose limited to 80 Gy. OAR constraints consistent with 5-fraction SBRT published in American Association of Physicists in Medicine (AAPM) Task Group 101 were used in planning directives.13
Volumetric modulated arc therapy (VMAT) was used to achieve the internal high-dose gradients, target coverages, and OAR objectives. VMAT plans are hypothesized to offer superior target coverage, reduce high-dose spill, and better spare OARs compared with 3-dimensional conformal radiation therapy (CRT) for Lattice radiation therapy.14,15 After an initial attempt to generate both 3-dimensional CRT and VMAT comparison plans for Lattice SBRT, we did not further pursue 3-dimensional CRT plans because, regardless of the number of beam angles, we would not achieve OAR dose constraints while attaining our desired dose gradient. All plans were delivered on a Varian Truebeam using standard Millennium 120 MLC (5 mm/10 mm MLC widths). Once the Lattice SBRT contouring and treatment planning process was finalized, planning was completed for all 12 tumors using a Varian Eclipse treatment planning system, version 15.6 (Varian Medical Systems, Palo Alto, CA) using patient computed tomography simulation scans.
After treatment planning, physician and physicist review, and plan approval, plan integrity and deliverability was evaluated per the standard clinical SBRT QA protocol. This included 2-dimensional external portal imaging device (EPID) portal dosimetry to measure fluence throughout each angle of the beam’s arc, 1-dimensional ion chamber (IC) absolute dose measurements within the PTV_6670 and PTV_Avoid (low-dose) vertices, and machine parameter delivery verification using an in-house log-file program called Dyna QA.16 For EPID portal dosimetry, 2-dimensional fluence maps were measured for each arc, and evaluated against the calculated fluence using 3%/3 mm (95% pass rate) and 2%/2 mm (90% pass rate) criteria across all pixels. The 2-dimensional planar measurements provided evidence of the accuracy of the high-dose gradients within the GTV_2000. The 1-dimensional IC measurements were completed using a small-field Exradin A16 IC (Standard Imaging, Middleton, WI) placed in an in-house designed solid water phantom at locations corresponding to the PTV_6670 and PTV_Avoid vertices, with 1 measurement in each structure captured per patient. A Dyna QA report was generated from the treatment machine log files and analyzed with in-house software (eg, MLC movement, gantry rotation, monitor units delivered).16
Results
The 11 patients had tumors of various histologies that were located in a range of anatomic locations (Table 1). Tumors had a median volume of 687.5 cc (range, 350–4440 cc) and a median greatest axial dimension of 12.75 cm (range, 10–18.5 cm). VMAT plans were created using flattening filter-free beams, and multiple full or partial noncoplanar arcs with couch kicks up to 15° based on available clearance of the treatment couch. Plans used 6MV or 10MV energies with 10MV plans overall resulting in less monitor units. A collimator rotation of 15° to 90° and jaw tracking were used for all plans. Treatment delivery times were acquired during the IC QA process, and ranged from 9 to 16 minutes (mean: 12.3 minutes), inclusive of couch kicks but exclusive of patient setup, imaging, or alignment.
Table 1.
Patient tumor characteristics for Lattice stereotactic body radiation therapy dosimetric and quality assurance analyses, ranked from smallest to largest volume
| Target | Histology | Site | Gross tumor volume, cm3 | Largest axial dimension, cm |
|---|---|---|---|---|
| 1 | Cervical squamous cell carcinoma | Central pelvis | 350 | 10 |
| 2 | Leiomyosarcoma | Right abdomen | 430 | 10.5 |
| 3 | Cholangiocarcinoma | Right abdomen | 450 | 10 |
| 4 | Melanoma | Mediastinum | 490 | 13 |
| 5∗ | Undifferentiated sarcoma | Left lung | 450 | 12.5 |
| 6∗ | Undifferentiated sarcoma | Right neck | 615 | 12 |
| 7 | Leiomyosarcoma | Left lung | 745 | 12.5 |
| 8 | Liposarcoma | Central pelvis | 1805 | 13 |
| 9 | Sarcomatoid carcinoma | Right abdomen | 940 | 13.5 |
| 10 | Liposarcoma | Right pelvis | 1035 | 14.5 |
| 11 | Melanoma | Left abdomen/pelvis | 1600 | 15.5 |
| 12 | Malignant peripheral nerve sheath tumor | Left abdomen/pelvis | 4475 | 18.5 |
Targets 5 and 6 are separate tumor targets from the same patient.
All plans, except 1, met the dose constraints for OARs (Table E1). The skin dose constraint could not be achieved in this patient who had a very large sarcoma metastasis to the neck where the tumor extended close to the skin. All targets achieved >95% coverage for the PTV_2000 and PTV_6670 (Table 2). Target 12, the largest target, achieved adequate dose coverage and OAR sparing; however, the Dmean of the PTV_Avoid was the highest of this series, indicating that achieving desired dose falloff in exceptionally large targets may be difficult. An additional metric, termed the Lattice composite, was defined as PTV_6670 divided by GTV_2000 to represent the volume of tumor target filled by high-dose vertices. All patients had a Lattice composite of approximately 2% to 4%.
Table 2.
Target dose coverage, Lattice composite (), number of noncoplanar arcs, MUs per Gy for each treatment plan, selectivity (), and gradient index of the PTV_6670 ()
| Target | Site | GTV, cm3 | V95%Rx |
PTV_Avoid Dmean, Gy | Lattice composite, % | Arcs, n | MU per cGy | Selectivity | Gradient index score | |
|---|---|---|---|---|---|---|---|---|---|---|
| PTV_6670 | PTV_2000 | |||||||||
| 1 | Central pelvis | 350 | 99.9 | 100 | 21.8 | 3.1 | 4 | 2.18 | 84.3 | 16.7 |
| 2 | Right abdomen | 430 | 99.8 | 100 | 26.7 | 2.3 | 4 | 3.63 | 67.7 | 14.5 |
| 3 | Right abdomen | 450 | 100 | 100 | 21.0 | 2.6 | 4 | 3.03 | 60.7 | 22.2 |
| 4 | Mediastinum | 490 | 100 | 100 | 21.3 | 1.9 | 5 | 3.29 | 59.2 | 19.1 |
| 5 | Left lung | 450 | 99.5 | 99.7 | 20.8 | 4.3 | 4 | 4.7 | 65.7 | 14.5 |
| 6 | Right neck | 615 | 89.6 | 96.5 | 22.2 | 3.1 | 3 | 3.31 | 60.0 | 14.4 |
| 7 | Left lung | 745 | 95.3 | 100 | 21.6 | 2.2 | 4 | 2.88 | 74.1 | 23.9 |
| 8 | Central pelvis | 1805 | 99.1 | 99.9 | 25.8 | 2.2 | 4 | 4.87 | 86.2 | 24.4 |
| 9 | Right abdomen | 940 | 99.8 | 100 | 22.4 | 2.3 | 4 | 5.48 | 67.0 | 11.2 |
| 10 | Right pelvis | 1035 | 97.2 | 99.4 | 22.2 | 2.3 | 4 | 3.76 | 79.0 | 26.2 |
| 11 | Left abdomen/pelvis | 1600 | 97.4 | 100 | 22.4 | 2.1 | 4 | 3.11 | 76.4 | 23.6 |
| 12 | Left abdomen/pelvis | 4475 | 100 | 99.9 | 29.1 | 1.9 | 8 | 4.76 | 75.4 | 31.9 |
Abbreviations: GTV = gross tumor volume; MU = monitor unit; PTV = planning target volume; Rx = prescription.
All 2-dimensional EPID, IC, and Dyna QA results achieved the thresholds specified (Table E2). The IC measurements, taken within the PTV_6670 structures, were within 3% of the expected dose predicted by the treatment planning system. A larger deviation of agreement was observed for the low-dose IC measurements taken in the PTV_Avoid structure, with no measurement exceeding 5% deviation.
Discussion
This study demonstrates that the proposed Lattice SBRT technique adheres to established SBRT safety guidelines, and can be planned and delivered on standard commercially available equipment. Lattice SBRT plans were successfully created for tumors ranging from 350 to 4440 cc, and the plans met tumor coverage objectives and OAR dose constraints for 5-fraction SBRT. The planning procedure was critical to consistently achieve the high-dose gradients characteristic of Lattice planning. The 1.5 cm diameter of PTV_6670 and PTV_Avoid vertices was selected for 3 reasons. First, the diameter represented a volume that approached the smallest limit that could be accurately, consistently, and safely delivered across multiple off-axis locations within the larger GTV_2000 tumor using a standard MLC-based delivery. Second, the diameter provided ample margin to minimize the impact of dose smearing caused by daily setup variations; thus, maintaining the desired 100% to 30% dose falloff. Third, the 1.5 cm spacing between the PTV_6670 and PTV_Avoid vertices represented the minimum achievable distance needed to create the desired dose falloff (Fig E1). Furthermore, the overall Lattice structure was critical in defining desired fluence paths to reach internal PTV_6670 vertices without increasing the dose to the PTV_Avoid vertices (Fig E2). The described planning method is not the exclusive means to create Lattice SBRT plans, but a consistent procedure that can be applied successfully across a broad range of tumor volumes, sites, and clinics.
All plans passed QA testing per a SBRT QA protocol with EPID portal dosimetry at 3%/3 mm and 2%/2 mm, IC absolute dose measurements at high- and low-dose points, and Dyna QA to confirm mechanical deliverability of plans. The increased variability in the low-dose IC measurements is attributed to the higher inaccuracy of the inter- and intra-MLC modeling and the associated length of time that low-dose regions are covered under the MLCs during the treatment process. Of note, the reported values are relative to the expected local dose produced in the treatment planning system, resulting in a deviation of approximately 5%, which is equivalent to 1 Gy in the low-dose measurements. This approximate 5% local deviation in the low-dose region corresponds to approximately 1% to 2% of the global prescription, and is within the 2% tolerance for IC measurements as specified in AAPM Task Group 218.17 Both the IC- and EPID-based measurements followed the program standards and QA recommendations provided in AAPM Task Group 218. EPID-based measurements are inherently perpendicular to the delivery direction; thus, the full arc-by-arc fluence distribution is evaluated against the corresponding distribution produced in the planning system.
This study has limitations inherent to its retrospective nature. For example, all patients were clinical treated per a palliative regime. As such, optimal patient positioning and immobilization for SBRT treatments was not always present. For example, in 2 lung cases, the patient’s arms were positioned down and directly in the beam path for all arcs. To maintain standard lung SBRT positioning, the arms were subsequently overridden to air. Additionally, many patients did not have immobilization common for SBRT treatments, including abdominal compression and body-length immobilization bags. However, these limitations would impact the delivery of the Lattice plans and not the planning procedure described in this study. Standard SBRT positioning, immobilization, and on-treatment imaging practices should be followed for any patients treated using the Lattice procedure described in this study.
We recently completed an evaluation of the safety of this regimen in a Phase a clinical trial for patients with large, unresectable tumors (NCT04133415), and are now evaluating its efficacy for patients with specific histologies (NCT04553471). Future work will evaluate delivering Lattice SBRT using intensity modulated proton therapy and magnetic resonance-guided adaptive radiation therapy platforms.
Conclusions
Lattice SBRT is an approach to deliver dose-escalated radiation to large tumors in a way that may surmount the limitations of conventional fractionation. Our approach uses VMAT to deliver high-dose islands within a sea of lower doses. Our approach is clinically and technically feasible, and being prospectively evaluated in 2 ongoing trials for patients with large, unresectable tumors.
Acknowledgments
The authors thank Jessica Hilliard, Jennifer Harris, and Tammy Price-Warfield for their contributions to the dosimetry and treatment planning process reported in this manuscript.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: Dr Knutson reports nonfinancial support from Varian Medical Systems outside of the submitted work. Dr Mutic reports grants and other support from Varian Medical Systems, Radialogica, and TreatSafely during the conduct of the study, as well as grants and other support from Varian Medical Systems outside of the submitted work. Dr Robinson reports grants and personal fees from Varian Medical Systems, personal fees from Astra Zeneca, Siemens Health care, and EMD Serono, as well as grants from Elekta and Merck outside of the submitted work. In addition, Dr Robinson has a patent (62/598,162) issued, as well as holds a leadership role and owns interest in Radialogica. Dr Spraker reports grants from Varian Medical Systems and the American College of Radiology, as well as personal fees from RadOncQuestions LLC outside of the submitted work. All other authors have no disclosures to declare.
Data sharing statement: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2020.100639.
Supplementary Materials
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Hartsell W.F., Scott C.B., Bruner D.W. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 3.Shiue K., Cerra-Franco A., Shapiro R. Histology, tumor volume, and radiation dose predict outcomes in NSCLC patients after stereotactic ablative radiotherapy. J Thorac Oncol. 2018;13:1549–1559. doi: 10.1016/j.jtho.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allibhai Z., Taremi M., Bezjak A. The impact of tumor size on outcomes after stereotactic body radiation therapy for medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;87:1064–1070. doi: 10.1016/j.ijrobp.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Videtic G.M.M., Donington J., Giuliani M. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol. 2017;7:295–301. doi: 10.1016/j.prro.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Billena C., Khan A.J. A current review of spatial fractionation: Back to the future? Int J Radiat Oncol Biol Phys. 2019;104:177–187. doi: 10.1016/j.ijrobp.2019.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohiuddin M., Miller T., Ronjon P., Malik U. Spatially fractionated grid radiation (SFGRT): A novel approach in the management of recurrent and unresectable soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2009;75:S526. [Google Scholar]
- 8.Mohiuddin M., Memon M., Nobah A. Locally advanced high-grade extremity soft tissue sarcoma: Response with novel approach to neoadjuvant chemoradiation using induction spatially fractionated GRID radiotherapy (SFGRT) J Clin Oncol. 2014;32 10575. [Google Scholar]
- 9.Peñagarícano J.A., Griffin R., Corry P., Moros E., Yan Y., Ratanatharathorn V. Spatially fractionated (GRID) therapy for large and bulky tumors. J Ark Med Soc. 2009;105:263–265. [PubMed] [Google Scholar]
- 10.Wu X., Ahmed M.M., Wright J., Gupta S., Pollack A. On modern technical approaches of three-dimensional high-dose Lattice radiotherapy (LRT) Cureus. 2010;2:e9. [Google Scholar]
- 11.Amendola B.E., Perez N.C., Wu X., Blanco Suarez J.M., Lu J.J., Amendola M. Improved outcome of treating locally advanced lung cancer with the use of Lattice radiotherapy (LRT): A case report. Clin Transl Radiat Oncol. 2018;9:68–71. doi: 10.1016/j.ctro.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amendola B.E., Perez N., Amendola M.A. Lattice radiotherapy with RapidArc for treatment of gynecological tumors: Dosimetric and early clinical evaluations. Cureus. 2010;2:e15. [Google Scholar]
- 13.Benedict S.H., Yenice K.M., Followill D. Stereotactic body radiation therapy: The report of AAPM Task Group 101: Stereotactic body radiation therapy: The report of TG101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 14.Gholami S., Severgnini M., Nedaie H.A., Longo F., Meigooni A.S. PO-0947: VMAT-based grid for spatially fractionated radiation therapy. Radiother Oncol. 2016;119:S460. [Google Scholar]
- 15.Sheikh K., Hrinivich W.T., Bell L.A. Comparison of treatment planning approaches for spatially fractionated irradiation of deep tumors. J Appl Clin Med Phys. 2019;20:125–133. doi: 10.1002/acm2.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rangaraj D., Zhu M., Yang D. Catching errors with patient-specific pretreatment machine log file analysis. Pract Radiat Oncol. 2013;3:80–90. doi: 10.1016/j.prro.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Miften M., Olch A., Mihailidis D. Tolerance limits and methodologies for IMRT measurement-based verification QA: Recommendations of AAPM Task Group No. 218. Med Phys. 2018;45:e53–e83. doi: 10.1002/mp.12810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.