Abstract
Background
The simultaneous occurrence of acute myocardial infarction, pulmonary embolism, and acute cerebral stroke is a rare concomitant finding that requires thorough aetiological investigation. Multiple reports note delayed COVID-19 arterial and venous thromboembolic complications. However, to the best of our knowledge, this is the first report of such a simultaneous finding after COVID-19.
Case summary
A 60-year-old male patient, with a history of Type II diabetes and no risk factors for thromboembolism, experienced simultaneous acute myocardial infarction, bilateral pulmonary embolism, and acute ischaemic stroke. The occurrence of these multi-systemic thromboembolic events made us rule out differential diagnoses of thrombophilia, systemic lupus erythematosus, antiphospholipid syndrome, vasculitis, cancer, disseminated intravascular coagulation, and paradoxical embolism through a patent foramen ovale. On laboratory analysis, the patient was positive for IgG SARS-COV2 antibodies, but negative for IgM antibodies and had two negative nasal polymerase chain reaction swab tests. After thorough aetiological investigation, the most probable diagnosis was thought to be delayed complications of COVID-19 infection.
Discussion
Multiple mechanisms, such as endothelial dysfunction, complement activation, and virus-induced antiphospholipid syndrome, may explain the hypercoagulable state related to COVID-19. To the best of our knowledge, this is the first case of concomitant multi-systemic thrombosis development, recognized as a delayed complication of COVID-19 infection. This highlights a need among cardiologists for an increased awareness of such late-onset complications. It also emphasizes the importance of identifying the optimal duration and dose of prophylactic anticoagulation as well as the characteristics of the population that would benefit from it after COVID-19.
Keywords: COVID-19, Venous thromboembolism, Arterial thrombosis, Case report
Learning points
The simultaneous occurrence of acute myocardial infarction, pulmonary embolism, and ischaemic stroke is a rare finding that poses the challenge of a thorough aetiological investigation.
Delayed arterial and venous thrombosis can occur in patients with COVID-19, particularly those with concomitant risk factors. In some cases, these symptoms can reveal the infection.
Studies are needed to determine the optimal duration and dose of prophylactic anticoagulation as well as the characteristics of the population that would benefit from it after SARS-CoV-2 infection.
Introduction
COVID-19 infection has been associated with multiple cardiac manifestations, and there are previous publications reporting the occurrence of arterial and venous thromboembolic events.1,2 However, the late-onset occurrence of simultaneous acute myocardial infarction, pulmonary embolism, and acute cerebral stroke in a patient affected by COVID-19 has never been reported before and requires extensive diagnostic investigation.
Timeline
| Timeline | Course of events |
|---|---|
| 3 days prior to admission | Typical angina chest pain associated with a dyspnoea Class II of the New York Heart Association |
| 20 h prior to admission | Left hemiplegia and confusion |
| Day 0 |
No chest pain on admission, stable haemodynamical status, polypnoea, SaO2 = 93% on room air, left hemi pyramidal syndrome Cerebral computed tomography (CT) scan: mid-cerebral artery ischaemic stroke Electrocardiogram (ECG): sinus rhythm, second-degree Mobitz 1 atrioventricular block, inferior STEMI High troponin levels, inflammatory syndrome Trans-thoracic echocardiogram: inferior and inferolateral wall motion abnormalities, no intracavitary thrombus, normal left ventricular ejection fraction Contrast chest CT scan: no aortic dissection, bilateral pulmonary embolism, bilateral lung condensation, and ground-glass opacities (COVID-19 Reporting and Data System 3) Supra-aortic vessel ultrasound: no plaques or stenosis |
| Day 1 |
24 h Holter ECG: sinus rhythm, no rhythm nor conduction abnormalities Result of reverse transcription-polymerase chain reaction (RT-PCR) COVID 19 nasal swab negative, positive SARS-COV2 IgG antibodies, negative IgM antibodies |
| Day 2 | Second RT-PCR COVID-19 swab negative |
| Day 3 |
Immunological tests for autoimmune diseases: negative Thrombophilic tests: negative Tumoral markers: negative Antiphospholipid antibodies: negative Doppler of temporal arteries: normal |
| Day 5 | Thoraco-Abdomino-Pelvic CT scan: not remarkable for any tumour, regression of the lung lesions |
| Day 13 | Transoesophageal echocardiography: no patent foramen oval, no left appendage thrombus |
| Day 14 |
Coronary angiography: tight stenosis spreads from the proximal right coronary artery (RCA) to the middle RCA Neurological re-assessment → started on optimal doses of enoxaparin |
| Day 18 | Discharge from hospital |
Case presentation
A 60-year-old North-African male patient, who was a non-smoker with a history of Type II diabetes mellitus presented to the emergency department 20 h after the onset of left hemiplegia. For 3 days prior, the patient suffered from typical anginal chest pain and New York Heart Association Class II heart failure symptoms. He reported no other symptoms that suggested recent infection or respiratory distress.
On admission, the patient was haemodynamically stable with normal vital signs, including blood pressure 142/93 mmHg, heart rate 77 b.p.m., and temperature 36.5°C, but tachypnoeic with a respiratory rate of 23 breaths per minute. Oxygen saturation on room air was 93%, but lung auscultation did not reveal any crackles, wheezing, or rhonchi. The cardiovascular examination was normal. On neurological examination, the patient did not follow orders correctly, with a Glasgow coma scale score of 14. He presented with left hemiplegia, hypotonia, and left sensory deficit. Capillary glycaemia was 3.2 g/dL, with glycosuria on the urine test strip, but no ketonuria was present.
Initial laboratory tests revealed elevated high-sensitivity troponin levels at 661 pg/mL [normal values (NV) 0.00–13.00 pg/mL] and inflammatory syndrome with an elevated white blood cell count, with a neutrophil predominance and normal platelet count. C-reactive protein was positive at 119 mg/L (NV 0.00–5.00 mg/L). The patient had dyslipidaemia. All other laboratory exams were normal.
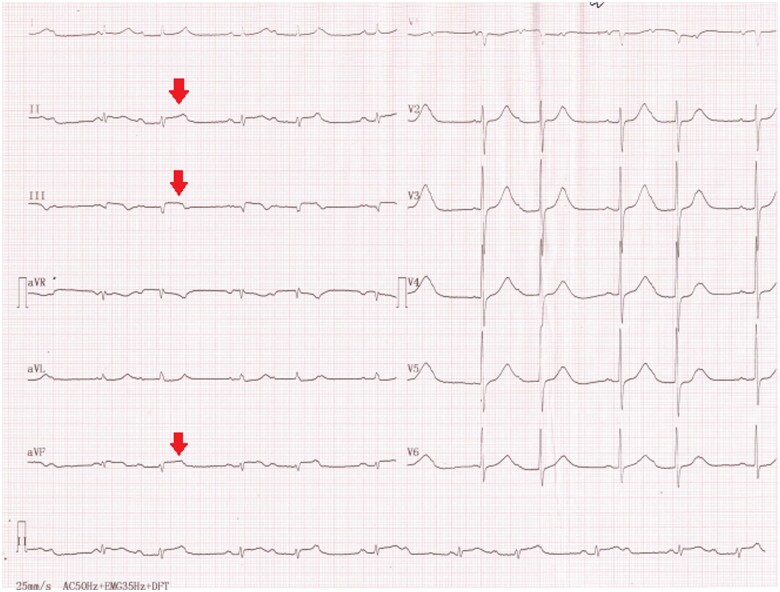
Electrocardiographic findings were suggestive of inferior STEMI with ST segment elevation in the II, III, and aVF leads and a concomitant second-degree Mobitz 1 atrioventricular block (Figure 1).
Figure 1.
Electrocardiographic findings on admission: inferior ST segment elevation and second-degree Mobitz 1 atrioventricular block.
Transthoracic echocardiography revealed normal dimensions of the left ventricle with hypokinesia of the basal and mid-inferior and inferolateral segments, and preserved ejection fraction. No valvular disease was detected and no other abnormalities from the right cavities, pericardium, or thoracic aorta were present. No intracavitary thrombus was found.
Afterwards, the patient was transferred to the imaging department for non-contrast computed tomography (CT) scan, which revealed an acute ischaemic stroke of the superficial and profound territory of the right middle cerebral artery.
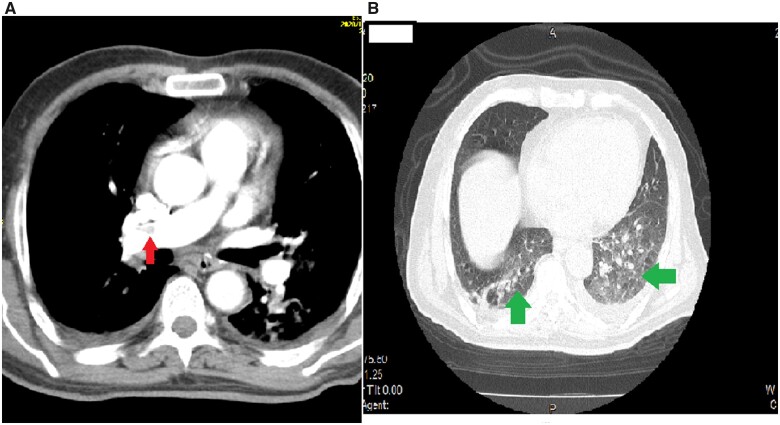
A chest CT angiogram (CTA) was performed to rule out aortic dissection that may have extended to the coronary arteries and the supra-aortic vessels; moreover, the CTA could perhaps elucidate the underlying cause of the stroke and acute myocardial infarction (AMI). Aortic dissection was ruled out but the presence of a thrombus in the proximal part of the right pulmonary artery and distal part of the left pulmonary artery was revealed. Ground-glass opacities could be observed in both lungs graded with the COVID-19 Reporting and Data System at Grade III (Figure 2).
Figure 2.
(A) Contrast chest computed tomography scan showing the right proximal pulmonary embolism (red arrow). (B) Pulmonary ground-glass opacities and condensation of both lungs graded COVID-19 Reporting and Data System 3 (green arrows).
D-Dimer levels were then measured and found to be high at 3194 µg/L (NV 0–500 µg/L). Fibrinogen levels were also high at 8.1 g/L (NV 1.5–2.5 g/L). The ferritin level was normal. A COVID-19 reverse transcription-polymerase chain reaction (PCR) nasal swab test was performed twice and was negative both times but the SARS-COV2 serology test was positive for IgG antibodies and negative for IgM.
The neurological team evaluated the impairment caused by the stroke by using the National Institutes of Health Stroke Scale (NIHSS) and assigned a score of 17. Despite the need for optimal doses of low molecular weight heparin, due to the high risk of intracranial haemorrhage, the patient was initially treated with aspirin 75 mg daily, low-dose enoxaparin (4000 UI daily), insulin, atorvastatin 80 mg daily, and lansoprazole 30 mg daily. The uptitration of enoxaparin was scheduled for Day 15 after the stroke according to the neurologist’s guidelines.
With regard to myocardial infarction, the patient was admitted 72 h after the onset of typical anginal chest pain, which is considered a long delay. However, his chest pain symptoms were no longer present at admission. Therefore, because the delay exceeded 48 h, primary percutaneous coronary intervention (PCI) was not performed, and according to the guidelines,3 a non-invasive test for the presence of residual myocardial viability was scheduled.
Further investigation was performed to identify the cause of this simultaneous development of acute myocardial infarction, bilateral pulmonary embolism, and cerebral ischaemic stroke.
To establish the diagnosis of a delayed COVID 19 complication, we thoroughly ruled out all other possible causes of this multi-systemic thrombotic state, as shown in Table 1.
Table 1.
Differential diagnosis of concomitant acute myocardial infarction, pulmonary embolism, and acute cerebral stroke
| Differential diagnosis |
|---|
| Myocardial infarction with intra-cardiac thrombi |
| Myocardial infarction with paroxysmal atrial fibrillation |
| Paradoxical embolism through a patent foramen ovale |
| Vasculitis |
| Systemic lupus erymathosus |
| Disseminated intravascular coagulation |
| Antiphospholipid syndrome |
| Thrombophilia |
| Paraneoplastic syndrome |
| COVID-19 infection |
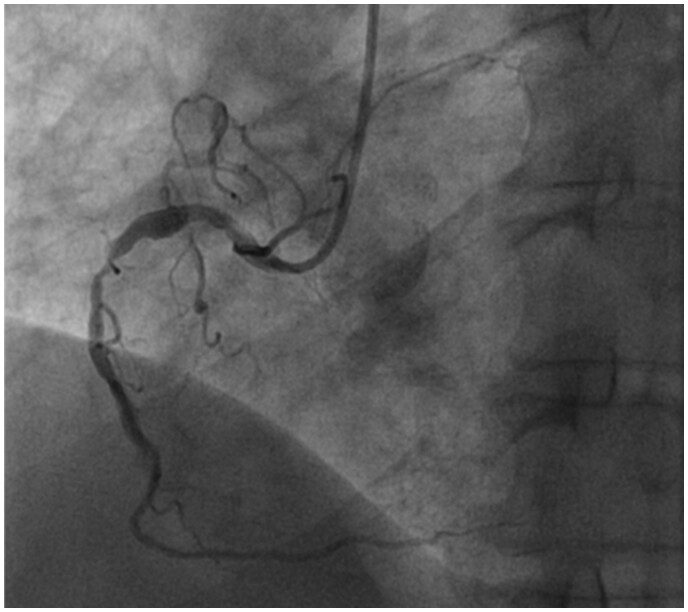
Coronary angiography performed on Day 14 (Supplementary material online, Videos 1, 2 and 3) revealed extensive multiple significant stenoses of the right coronary artery (Thrombolysis in Myocardial Infarction 3 flow) and an atheromatous left system without significant lesions (Figure 3).
Figure 3.
Coronary angiography performed at Day 14 showing extensive multiple significant stenoses of the right coronary artery.
A supra-aortic vessel ultrasound showed bilateral atheromatosis of both common carotid arteries but no significant stenosis.
A 24-h Holter electrocardiogram was performed to search for occult paroxysmal atrial fibrillation. No rhythm or conduction disturbances were detected.
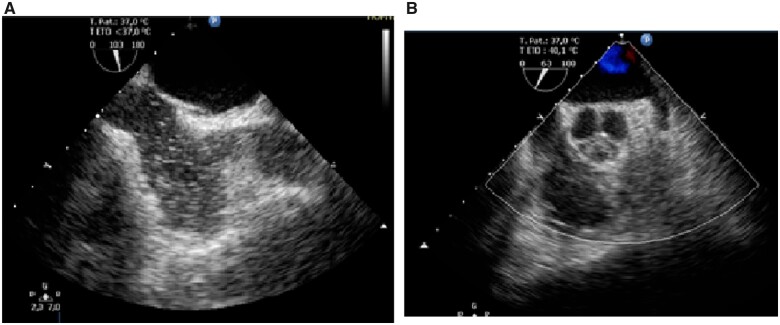
A transoesophageal echocardiography (TOE) examination with bubble contrast infusion and the Valsalva manoeuvre did not confirm a patent foramen ovale. No thrombi or other sources of emboli were detected according to the TOE (Figure 4).
Figure 4.
(A) Contrast transoesophageal echocardiography, bicaval view, no patent foramen oval, negative contrast bubble test. (B) Transoesophageal echocardiography, no left appendage thrombus.
Further investigation included Doppler examination of the lower limbs that was normal for deep venous thrombosis.
Immunological tests for autoimmune diseases, 24 h urine proteins, hepatitis B and C, human immunodeficiency virus, and syphilis serologies were negative. Antiphospholipid antibodies and thrombophilia tests were also negative. Colour Doppler ultrasound of the temporal arteries was normal.
The clinical presentation of the patient was not indicative of intravascular disseminated coagulation.
A thoraco-abdominopelvic CT scan was performed to search for a neoplasia, but no tumours or adenopathies were found. The plasmatic protein electrophoresis was normal.
The patient remained stable for the rest of his hospitalization, his neurological issues improved slightly, and he had better verbal responses, but maintained the same motor impairments. A follow-up CT scan showed an improvement of the lung lesions. The patient was discharged on Day 18, on apixaban 5 mg bid for 3 months, aspirin 75 mg daily, atorvastatin 80 mg daily, lansoprazole 30 mg per day, and insulin therapy. Delayed complications of COVID-19 infection were considered the most probable diagnosis.
Discussion
The article presents the case of a rare simultaneous manifestation of acute myocardial infarction, acute cerebral ischaemic stroke, and bilateral pulmonary embolism that developed in a single patient. Previously published studies have described such cases4 and the causes that provoked this multiple-organ thrombotic state are summarized in Table 1. Delayed complications of COVID-19 viral infection were found to be the most probable cause after a thorough aetiological investigation (Table 1), detection of IgG antibodies for SARS-COV2, and negative results for IgM antibodies and the nasal PCR COVID-19 tests.
Multiple mechanisms have been suggested to explain the hypercoagulable state in COVID-19 infection. Endothelial dysfunction,5 activation of the complement system,6 and an antiphospholipid syndrome induced by the virus7 are some of the mechanisms responsible for multiple thromboses. In some cases, COVID-19 infection is revealed by the presence of arterial or venous thrombosis.1 Thrombotic complications were also described despite the use of prophylactic antiplatelet or anticoagulant therapy.8
Our patient had many risk factors for cardiovascular disease. Due to diabetes, dyslipidaemia, his age, and the presence of carotid atherosclerotic plaques, he was considered to be at high risk for coronary artery disease. The inflammatory state due to SARS-COV2 infection could have further provoked instability of his condition. His hypercoagulable state could also explain the multifocal thrombosis.
As the patient was admitted 72 h after the onset of typical anginal chest pain, a delay that exceeded 48 h, primary PCI was not performed. According to the guidelines,3 a non-invasive test for the presence of residual myocardial viability was scheduled. In addition, as he was admitted with severe acute cerebral ischaemic stroke, the decision was to delay coronary angiography after the acute phase of cerebrovascular accident (CVA) because of his high risk of haemorrhagic transformation. After coronary angiography was performed on Day 14, a decision was made to administer conservative treatment. Unfortunately, no intravascular coronary imaging was performed to confirm a plaque rupture event due to its technical unavailability in our centre at that time.
Concerning the management of the patient, the large acute ischaemic CVA did not allow the introduction of anticoagulation, as the risk of haemorrhagic transformation was very high. Stroke guidelines advise delaying anticoagulation 2 weeks post-ischaemic stroke in patients with atrial fibrillation and severe stroke, but given conflicting advice regarding anticoagulation for coexisting pulmonary embolism. UK stroke guidelines suggest anticoagulation for proximal DVT or PE, while American Heart Association guidelines do not recommend initial anticoagulation in patients with moderate to severe stroke.9,10 As the patient’s stroke was severe, the delayed onset of optimal doses of heparin was decided after multidisciplinary discussion.
In this case, thromboembolic prophylaxis after COVID-19 was not suggested as the patient was asymptomatic for COVID-19 and had never previously undergone COVID-19 testing. This raises the cardiologic community’s concern that a vast number of undiagnosed asymptomatic patients with previous COVID-19 infection may be at risk of thromboembolic sequelae.
Conclusion
To the best of our knowledge, this is the first case of multi-systemic thromboembolic events revealing COVID-19 infection. The simultaneous development of acute myocardial infarction, bilateral pulmonary embolism, and acute ischaemic stroke is a rare finding. In our case, these findings were considered late-onset manifestations of COVID-19 infection. This emphasizes the importance of performing an extensive workup to reach the correct diagnosis and the significance of the duration of preventive anticoagulation during and after COVID-19 infection.
Lead author biography

Mariame Chakir studied human medicine at the Faculty of Medicine and Pharmacy of Marrakech, Morocco. She graduated in 2018. She is currently a third year medical resident at the cardiology department of the University Hospital Mohammed the VIth of Marrakech. She pursued a 2-year inter-universitary program in non-invasive cardiovascular imaging as well as a 1-year program in clinical research methods at Bordeaux University, France. She holds at present a Junior Editor position at the European Heart Journal-Case Reports.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: M.C. is a junior editor at the European Heart Journal-Case Reports.
All other authors declared no conflict of interest.
Funding: None declared.
Supplementary Material
Acknowledgements
Participation of Dr Joumana El Masrioui, Dr Sara Jourani, and Dr Ikram Hazzazi in the data collection is of notice and to be acknowledged.
Participation of the Department of Radiology of Mohammed the VIth University Hospital of Marrakech is gratefully acknowledged.
References
- 1. Schweblin C, Hachulla A, Roffi M, Glauser F.. Delayed manifestation of COVID-19 presenting as lower extremity multilevel arterial thrombosis: a case report. Eur Heart J Case Rep 2020;4:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roberts L, Whyte M, Georgiou L, Giron G, Czuprynska J, Rea C. et al. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood 2020;136:1347–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ibanez B, James S, Agewall S, Antunes M, Bucciarelli-Ducci C, Bueno H. et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2017;39:119–177. [DOI] [PubMed] [Google Scholar]
- 4. Barros-Gomes S, El Sabbagh A, Eleid MF, Mankrad SV.. Concomitant acute stroke, pulmonary and myocardial infarction due to in-transient thrombus across a patent foramen ovale. Echo Res Pract 2018;5:I9–I10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Libby P, Lüscher T.. COVID-19 is, in the end, an endothelial disease. Eur Heart J 2020;41:3038–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conway EM, Pryzdial ELG.. Is the COVID-19 thrombotic catastrophe complement-connected? J Thromb Haemost 2020;18:2812–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W. et al. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N Engl J Med 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kashi M, Jacquin A, Dakhil B, Zaimi R, Mahé E, Tella E. et al. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res 2020;192:75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stroke guidelines [Internet]. RCP London; 2021. https://www.rcplondon.ac.uk/guidelines-policy/stroke-guidelines. Mar 02, 2021.
- 10. Jauch E, Saver J, Adams H, Bruno A, Connors J, Demaerschalk B. et al. ; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.