Abstract
Population genomic analyses of high-altitude humans and other vertebrates have identified numerous candidate genes for hypoxia adaptation, and the physiological pathways implicated by such analyses suggest testable hypotheses about underlying mechanisms. Studies of highland natives that integrate genomic data with experimental measures of physiological performance capacities and subordinate traits are revealing associations between genotypes (e.g., hypoxia-inducible factor gene variants) and hypoxia-responsive phenotypes. The subsequent search for causal mechanisms is complicated by the fact that observed genotypic associations with hypoxia-induced phenotypes may reflect second-order consequences of selection-mediated changes in other (unmeasured) traits that are coupled with the focal trait via feedback regulation. Manipulative experiments to decipher circuits of feedback control and patterns of phenotypic integration can help identify causal relationships that underlie observed genotype–phenotype associations. Such experiments are critical for correct inferences about phenotypic targets of selection and mechanisms of adaptation.
Keywords: adaptation, altitude, hypoxia, genotype–phenotype association, hypoxia-inducible factor, EPAS1
Introduction
Genome-wide scans of DNA polymorphism in high-altitude human populations have revealed numerous loci that exhibit signatures of positive selection, many of which represent plausible candidate genes for hypoxia adaptation (Bigham et al. 2009, 2010; Aggarwal et al. 2010; Beall et al. 2010; Simonson et al. 2010; Yi et al. 2010; Peng et al. 2011; Xu et al. 2011; Alkorta-Aranburu et al. 2012; Scheinfeldt et al. 2012; Huerta-Sanchez et al. 2013; Xiang et al. 2013; Xing et al. 2013; Crawford et al. 2017; Hu et al. 2017; Yang et al. 2017; Arciero et al. 2018; Jeong et al. 2018). In Tibetan highlanders, such genome scans have consistently implicated central components of the hypoxia-inducible factor (HIF) signaling pathway, which orchestrates the transcriptional response to hypoxia (Beall et al. 2010; Simonson et al. 2010; Yi et al. 2010; Peng et al. 2011, 2017; Xu et al. 2011; Xiang et al. 2013; Yang et al. 2017; Jeong et al. 2018). Members of the HIF family of transcription factors exert O2-dependent control over the tissue-specific expression of myriad target genes and regulate diverse facets of the physiological response to hypoxia, including respiration, blood flow, vascular remodeling, and intermediary metabolism (Kaelin and Ratcliffe 2008; Lendahl et al. 2009; Majmundar et al. 2010; Greer et al. 2012; Semenza 2012, 2014; Samanta et al. 2017). Some HIF pathway genes such as EPAS1 (endothelial PAS domain containing protein 1), which encodes the O2-sensitive α subunit of the HIF-2 transcription factor, exhibit statistical evidence for positive selection in multiple high-altitude populations and species (Witt and Huerta-Sanchez 2019; Storz and Cheviron 2021). At face value, such patterns would seem to suggest that similar adaptive solutions have evolved repeatedly in response to the shared physiological challenge of environmental hypoxia. However, the extent to which shared signatures of positive selection reflect similarities in causal paths and phenotypic outcomes remains an open question. Comparative studies of systemic physiology in high-altitude humans and other vertebrates have revealed a far greater diversity of adaptive mechanisms than might be suggested by cross-referencing lists of candidate genes and gene ontology categories (Monge and Leon-Velarde 1991; Hochachka 1998; Beall 2006, 2007; Storz, Scott, et al. 2010; Gilbert-Kawai et al. 2014; Petousi and Robbins 2014; Ivy and Scott 2015; McClelland and Scott 2019; Storz et al. 2019; Storz and Scott 2019; O’Brien et al. 2020).
In studies of environmental adaptation, documenting an association between genotype and phenotype represents a necessary first step that can guide the design of follow-up experiments to test hypotheses about causal mechanisms. To identify and characterize mechanisms of adaptation to high-altitude hypoxia, focal phenotypes ideally represent fitness-related measures of whole-organism performance that reflect integrated physiological capacities (Bennett 1991; Storz et al. 2015, 2019; McClelland and Scott 2019; Storz and Scott 2019). Integrating such measures of systemic physiology with analyses of subordinate traits (respiratory, cardiovascular, and metabolic) can provide mechanistic insights into the chain of causation linking genotype and selected phenotype. For example, common-garden experiments involving high- and low-altitude deer mice (Peromyscus maniculatus) revealed that highland natives have evolved enhanced aerobic performance capacities in hypoxia owing to derived changes in numerous subordinate traits that alter the flux capacity of the O2-transport system, the oxidative capacity of tissue mitochondria, and the relationship between O2 consumption and ATP synthesis (Cheviron et al. 2012; Cheviron, Connaty et al. 2014; Lui et al. 2015; Scott et al. 2015, 2018; Ivy and Scott 2017; 2018; Lau et al. 2017; Mahalingam et al. 2017, 2020; Tate et al. 2017, 2020; Dawson et al. 2018; Nikel et al. 2018; Storz et al. 2019; Ivy et al. 2020). In addition to the examination of physiological performance capacities, the challenges of mammalian pregnancy at high altitude suggest that direct measurements of female reproductive success may capture significant variation in the fertility component of fitness (Moore 2001; Browne et al. 2015; Niermeyer et al. 2015; Grant et al. 2020).
Below I review and synthesize results from recent studies that illustrate how genomic data can be integrated with experimental physiology to yield insights into mechanisms of high-altitude adaptation. The review of recent work is organized according to the following progression, which does not necessarily follow the exact chronological sequence in which the various studies were performed: 1) analysis of genome-wide polymorphism data yields the discovery of candidate genes for hypoxia adaptation based on statistical signatures of positive selection; 2) guided by prior knowledge about pathways affected by allelic variation in a given candidate gene, experimental measurements of relevant physiological traits reveal altitude-related differences in mean phenotype; 3) experiments involving subjects with known genotypes reveal an association between specific allelic variants and phenotype; and 4) manipulative experiments using reverse genetics yield insights into causal mechanisms that underlie evolved differences in phenotype between high- and low-altitude natives. To date, several studies have followed this progression (with varying levels of completeness) through 2 or 3 of these steps. Such studies demonstrate how indirect, retrospective inferences about selection based on population genomic analyses can be integrated with mechanistic experiments to yield discoveries about the functional biology of adaptation. In genomic studies that involve relatively sparse or superficial measurements of physiological traits, a key interpretative challenge is that observed genotypic associations with hypoxia-responsive phenotypes may not reflect direct, causal relationships. Instead, such associations may reflect indirect effects of selection-mediated change in a separate, unmeasured trait (e.g., an upstream step in the same pathway) that is coupled with the measured trait via feedback regulation.
Fitness-Related Variation in Aerobic Performance Capacity in Hypoxia
In endotherms living in cold, hypoxic conditions at high altitude, ecologically important measures of whole-organism performance, such as capacities for sustained exercise and thermogenesis, are directly related to aerobic metabolism. An animal’s rate of aerobic metabolism can be measured as the rate of O2 consumption (VO2, typically measured in units of ml·min−1·kg−1) because O2 is required for ATP synthesis via oxidative phosphorylation in the mitochondria. The maximal rate of aerobic metabolism, which is measured by the maximal rate of O2 consumption (VO2max), is an index of whole-organism aerobic performance that reflects the integrated functioning of the cardiopulmonary/cardiovascular systems and muscle metabolism (Weibel et al. 1991; Taylor et al. 1996). This integrated functioning determines the flux capacity of the O2 transport pathway, which consists of serially integrated physiological processes representing diffusive and convective transfer steps (ventilation, pulmonary O2 diffusion, circulatory O2 delivery, and tissue O2 diffusion) that culminate in mitochondrial O2 utilization. VO2max can be elicited by challenging an animal’s exercise capacity (e.g., via forced treadmill running) or by challenging thermoregulatory capacity via acute cold exposure. When exposed to extreme cold, most eutherian mammals increase metabolic heat production via shivering or non-shivering thermogenesis. Thus, progressive reduction of ambient temperature will eventually elicit an animal’s maximal rate of metabolic heat production when it reaches the upper limit of O2 consumption.
At high altitude, the reduced partial pressure of O2 (PO2) of inspired air imposes constraints on aerobic metabolism and therefore impairs capacities for sustained exercise and thermogenesis (West 2006; Brutsaert 2008; Gonzalez and Kuwahira 2018; McClelland and Scott 2019). For endothermic vertebrates living at high altitude, the combination of increased thermoregulatory demand (due to low temperature) and reduced capacity for aerobic thermogenesis (due to low PO2) suggests that variation in cold-induced VO2max may have especially important fitness consequences. In small endotherms like rodents, the capacity for sustained thermogenesis in hypoxia may be critical for survival during daily or seasonal periods of extreme cold. In such conditions, individuals with higher capacities for aerobic thermogenesis can maintain their body core temperature at lower ambient air temperatures. Such individuals are less likely to succumb to hypothermia and are able to expand the active period of the torpor cycle, thereby increasing opportunities for foraging, mating, and other activities that may contribute to lifetime reproductive success (Hayes and O’Connor 1999; Sears et al. 2006). Measurements of field metabolic rates of high-altitude deer mice revealed that these animals are often operating close to their aerobic performance limits (Hayes 1989a, 1989b) and survivorship studies confirm that thermogenic capacity is subject to strong directional selection under natural conditions (Hayes and O’Connor 1999).
In summary, hypoxic VO2max provides an ecologically relevant and physiologically integrative measure of whole-organism aerobic performance. Quantification of the decrement in VO2max with increasing hypoxia can therefore provide the basis for an operational measure of high-altitude adaptation (Brutsaert 2008, 2016; McClelland and Scott 2019; Storz et al. 2019).
Association of EGLN1 Variants with Aerobic Capacity in Hypoxia
Quechua have lived for millennia in high-altitude regions of the Andes in Peru and Bolivia (Rademaker et al. 2014), and multiple lines of evidence indicate that they have higher limits of work performance in hypoxia relative to their nonindigenous and mestizo compatriots (Hochachka et al. 1991; Frisancho et al. 1995; Brutsaert et al. 2003; Brutsaert 2008, 2016). In fact, the impressive physical work capacities of Andean natives at high altitude were chronicled by Spanish conquistadors nearly 500 years ago (Monge 1948). Andean natives maintain a higher mean VO2max in hypoxia and suffer a smaller decrement in VO2max with increasing hypoxia relative to nonnative residents at the same altitude (Frisancho et al. 1995; Brutsaert et al. 2003; Brutsaert 2008, 2016). The genetic basis of this enhanced performance in hypoxia has yet to be elucidated. However, results of genome scans in native Andeans suggest that variation in HIF genes such as EGLN1 (egl-9 family hypoxia inducible factor) may have contributed to adaptation (Bigham et al. 2009, 2010; Foll et al. 2014; Crawford et al. 2017) and it is possible that some fraction of the phenotypic response to past selection is captured by general performance measures such as VO2max. Accordingly, Brutsaert et al. (2019) used a sample of 523 subjects (Quechua highlanders and non-Hispanic lowlanders) to test for an association between noncoding EGLN1 SNP variants and VO2max in hypoxia. EGLN1 encodes a prolyl hydroxylase (PHD2) that induces degradation of HIF in an O2-dependent manner. The choice of EGLN1 as a focal gene for the association study was also motivated by experimental evidence that loss-of-function mutations in EGLN1 are associated with a misregulation of O2 homeostasis, which affects erythropoiesis in humans (Percy et al. 2006; Lee and Percy 2011) and erthropoietic and ventilatory responses to environmental hypoxia in mice (Arsenault et al. 2013; Bishop et al. 2013).
The five EGLN1 SNPs were significantly associated with increased VO2max in hypoxia (equivalent to an altitude of 4,300 m) after controlling for population stratification (fig. 1A). Moreover, SNP alleles associated with high VO2max are present at highest frequency in Peruvian Quechua compared with 25 diverse lowland populations from the 1000 Genomes Project (fig. 1B and C). Genotypic effect sizes were large, as comparisons between test subjects that were 5-site homozygotes for the high VO2max alleles versus those who were alternative homozygotes for the low alleles revealed a statistically significant 13% difference in mean VO2max (33.97 vs. 30.42 ml·min−1·kg−1, respectively). The strong association between EGLN1 variants and VO2max in Quechua provides physiological context for interpreting the population genomic evidence for positive selection. The enrichment of derived, high VO2max EGLN1 alleles in the Quechua population is seemingly consistent with the hypothesis that past selection favored an increased aerobic capacity under conditions of chronic hypoxia or that it favored change in a related, unmeasured phenotype that resulted in increased aerobic capacity as an indirect, carryover effect. Given that the examined SNPs associated with high VO2max are intronic or are located outside the gene boundaries of EGLN1, and given that no coding polymorphisms in the gene were significantly associated with VO2max, the phenotypic effect of the causal variants must be mediated by changes in gene regulation. The identity of the causal variants, the molecular mechanism by which they exert their effect, and the many intermediary links that connect changes in EGLN1 regulation with changes in VO2max remain to be investigated.
Fig. 1.
In high-altitude Quechua, noncoding SNPs in EGLN1 are associated with aerobic exercise capacity (VO2max) in hypoxia. (A) Marginal mean values of VO2max for three alternative EGLN1 SNP genotypes (error bars = SEM) in a sample of Peruvian Quechua highlanders and non-Hispanic lowlanders. (B) Genotype frequencies for EGLN1 rs1769793 in Peruvian Quechua and non-Hispanic lowlanders from Syracuse, NY. The “TT” genotype is associated with high VO2max in hypoxia. (C) Allele frequencies of the T allele from the 1000 Genomes Project. Arrows denote frequencies for the Peruvian Quechua (PQU) and non-Hispanic Syracuse (SYR) population samples. Quechua have the highest recorded allele frequency of T worldwide. Data from Brutsaert et al. (2019) with permission.
HIF Genes, Hemoglobin Concentration, and Aerobic Capacity in Hypoxia
Genotypic associations with aerobic exercise capacity have not yet been documented in Tibetan highlanders, but a recent study demonstrated that VO2max of Tibetan males at 4,200 m is negatively correlated with hemoglobin (Hb) concentration (Simonson et al. 2015), a phenotype that is associated with allelic variation in HIF genes such as EPAS1 and EGLN1 (Beall et al. 2010; Simonson et al. 2010; Yi et al. 2010; Xiang et al. 2013; Peng et al. 2017; Tashi et al. 2017; Yang et al. 2017). In humans and other lowland mammals that ascend to high altitude, Hb concentration increases in a matter of days due to a reduction in blood plasma volume. Subsequently, over the span of >1 week, the elevated Hb concentration is sustained by renal synthesis and release of erythropoietin, a hormone that increases red blood cell production by stimulating the proliferation and differentiation of erythroid precursor cells in the bone marrow (Yoon et al. 2011; Siebenmann et al. 2017). During acclimatization to high altitude, the typical increase in Hb concentration can offset the reduction in arterial O2 saturation caused by the reduction in the PO2 of inspired air, thereby minimizing the reduction in arterial O2 content. Thus, if all else is equal, a higher Hb concentration should translate into an increase in convective O2 delivery to working muscles and an enhanced aerobic exercise capacity. This is the rationale for blood doping in cycling and other endurance sports. However, at high altitude, the hypoxia-induced increase in Hb concentration does not restore VO2max to sea level values (Calbet et al. 2002, 2003; Gonzalez and Kuwahira 2018) and it does not necessarily produce net improvements in circulatory O2 transport due to antagonistic interactions with interdependent steps in the O2-transport pathway.
High-altitude human populations in different parts of the world appear to have evolved different physiological responses to chronic hypoxia and therefore possess different average Hb concentrations at similar altitudes (Beall 2007; Gassmann et al. 2019). Consistent with the acclimatization response of lowland sojourners to high altitude, native Andeans living permanently at altitudes >4,300 m typically exhibit highly elevated blood Hb concentrations (Leon-Velarde et al. 2000). By contrast, Tibetans living at similar altitudes tend to have Hb concentrations that are only slightly higher than values expected for people living at sea level (Beall and Reichsman 1984; Beall and Goldstein 1987; Beall et al. 1998; Wu et al. 2005). At face value, the Tibetan pattern seems counterintuitive, due to the expectation that an increase in arterial O2 content should contribute to an enhancement of tissue O2 delivery and, hence, improved aerobic performance in hypoxia. However, in spite of their non-elevated Hb concentration, available evidence suggests that Tibetan highlanders have generally superior exercise capacities in hypoxia compared with acclimatized Han Chinese residents living at the same altitude (Sun et al. 1990; Ge et al. 1994; Niu et al. 1995; Chen et al. 1997).
The low (non-elevated) Hb concentration in Tibetans is strongly associated with derived, noncoding variants in and near the EPAS1 gene that show extreme frequency differences relative to Han Chinese and other lowland reference populations (Beall et al. 2010; Simonson et al. 2010; Yi et al. 2010; Peng et al. 2017; Yang et al. 2017). Although no causal relationship has been established, the fact that non-elevated Hb concentration is associated with derived allelic variants that appear to have increased in frequency due to positive selection suggests that the Tibetan phenotype may be adaptive. This inference is consistent with evidence that excessively elevated Hb concentration is physiologically counterproductive at high altitude (Villafuerte et al. 2004; Storz, Scott et al. 2010; Storz and Scott 2019) and suggests the hypothesis that a blunting of the normal hypoxia-induced increase in Hb concentration evolved as an adaptive mechanism of genetic compensation. One explanation for why the hypoxia-induced increase in Hb concentration may be nonadaptive is that the corresponding increase in blood viscosity compromises cardiac output and microcirculatory blood flow, thereby reducing tissue O2 delivery in spite of the increased arterial O2 content. An alternative explanation, predicted by results of theoretical models (Wagner 1996), is that an increase in Hb concentration limits the diffusive equilibration of O2 between alveolar gas and capillary blood in the lungs (thereby reducing the PO2 of arterial blood) and between the microcirculatory vessels of muscle and the mitochondria (thereby reducing overall tissue O2 extraction). The fact that hypoxia-tolerant highland mammal and bird species typically exhibit Hb concentrations that are within the range of sea-level values for closely related lowland species is also consistent with the idea that hypoxia-induced polycythemia is nonadaptive (Storz, Scott et al. 2010; Storz and Scott 2019).
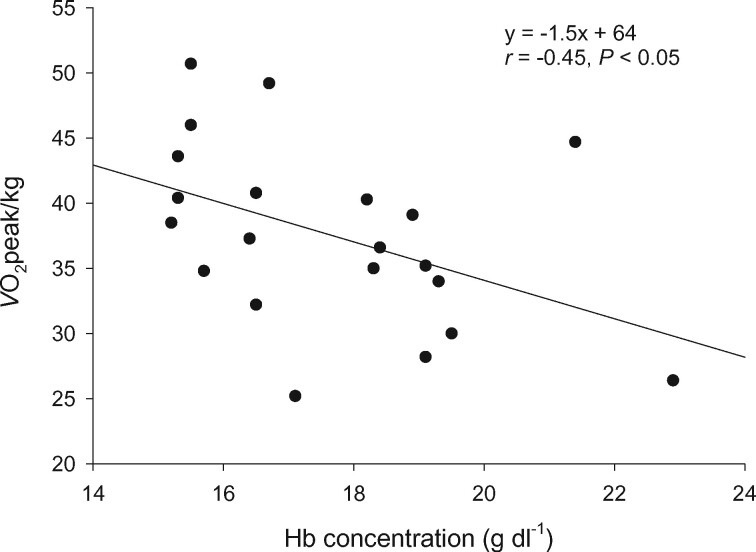
To assess the physiological consequences of variation in Hb concentration at high altitude, Simonson et al. (2015) investigated the determinants of aerobic exercise capacity by measuring VO2max and numerous subordinate traits in male Tibetans at 4,200 m. The authors examined each major step in the pathway for O2 transport from atmospheric air to the tissue mitochondria: ventilation, pulmonary diffusion capacity (diffusive conductance of O2 from the alveoli of the lungs to the pulmonary capillaries), cardiac output, and tissue diffusion capacity (diffusive conductance of O2 from tissue capillaries to the mitochondria of metabolizing cells). These linked steps represent conductances that are each expressed as the ratio between O2 flow and the O2 partial pressure difference across the conductance. Hb concentrations in the test subjects ranged from 15.2 to 22.9 g/dl, consistent with previous studies of Tibetan males at comparable altitudes, and exhibited a significant negative correlation with VO2max in hypoxia (fig. 2). This study also demonstrated that Hb concentration was negatively associated with cardiac output and O2 diffusion capacity of skeletal muscle—the two traits that explained most of the observed variance in VO2max (Simonson et al. 2015).
Fig. 2.
Relationship between Hb concentration and VO2max in 21 male Tibetan subjects at 4,200 m. From Simonson et al. (2015) with permission.
The Challenge of Identifying Phenotypic Targets of Selection
Given the strong evidence for positive selection on EPAS1 and the association between derived EPAS1 variants and non-elevated Hb concentration in Tibetans, it has often been implicitly assumed that Hb concentration represents the direct phenotypic target of selection. An alternative hypothesis is that the non-elevated Hb concentration represents a second-order consequence of changes in other traits regulated by HIF-2 that help sustain tissue oxygenation at low inspired PO2. Hb concentration is regulated by erythropoiesis and water balance via a feedback loop based on renal tissue PO2 (Donnelly 2003). Thus, attenuation of the hypoxia-induced increase in Hb concentration could be an indirect consequence of changes in any number of steps in the O2-transport pathway (ventilation, pulmonary O2 diffusion capacity, cardiac output, etc.) that help improve tissue oxygenation, thereby dampening the hypoxic signal that stimulates erythropoiesis and/or plasma volume contraction. Changes in numerous possible respiratory or cardiovascular traits could be mediated by selection on variation in EPAS1 (Storz, Scott et al. 2010; Petousi et al. 2014; Petousi and Robbins 2014; Simonson et al. 2015; Storz and Cheviron 2016; Storz and Scott 2019).
The non-elevated Hb concentration observed in Tibetan highlanders has traditionally been attributed to a blunted erythropoietic response to chronic hypoxia. However, recent work has demonstrated that Tibetans at high altitude actually have a higher total circulating Hb mass compared with acclimatized lowlanders—indicating that hypoxia-induced erythropoiesis is not attenuated—and they also have a considerably higher plasma volume than Andeans and lowlanders tested at similar altitudes (fig. 3A and B) (Stembridge et al. 2019). Consequently, Tibetans maintain blood volumes that are just as high as those of Andeans, but at a much lower Hb concentration (fig. 3C). Tibetans living at high altitude therefore benefit from an increased circulating Hb mass, which augments blood O2 transport capacity, and the expanded plasma volume prevents a corresponding increase in Hb concentration, which avoids viscosity-related impairments of cardiac function and microcirculatory blood flow. Accordingly, total Hb mass (but not Hb concentration) was positively correlated with VO2max in Tibetan subjects tested at 5,050 m (fig. 3D). The findings of Stembridge et al. (2019) highlight the importance of considering the functional integration of different components of higher-level performance phenotypes rather than focusing on individual components in isolation.
Fig. 3.
Variation in hematological traits among lowland natives at sea level and acclimatized lowlanders, native Tibetans (Sherpa), and Andeans at high altitude. (A) Andeans exhibit an elevated Hb concentration at high altitude relative to acclimatized lowlanders (LL HA) and Tibetans at high altitude. (B) Tibetans exhibit a significantly elevated plasma volume compared with acclimatized lowlanders (LL HA) and Andeans at high altitude. (C) Due to plasma volume expansion, Tibetans maintain blood volumes that are just as high as those of Andeans, but at a much lower Hb concentration. Consequently, Tibetan highlanders benefit from an augmented blood O2 transport capacity while avoiding viscosity-related impairments of cardiac function and microcirculatory blood flow. (D) Circulating Hb mass is positively correlated with VO2max in Tibetans tested at 5,050 m. LL SL, lowland natives tested at sea level; LL HA, lowland natives tested at high-altitude (5,050 m). Modified from Stembridge et al. (2019) with permission.
EPAS1 Genotype–Phenotype Associations in High-Altitude Deer Mice
Population genomic studies of high-altitude humans and other vertebrates have repeatedly identified EPAS1 as a candidate gene for hypoxia adaptation (Bigham and Lee 2014; Petousi and Robbins 2014; Simonson 2015; Witt and Huerta-Sanchez 2019; Storz and Cheviron 2021), but functional testing is lacking in all but a few cases and the phenotypic target of selection remains a mystery. An integrated genomic and physiological study of North American deer mice revealed striking evidence for altitude-related selection on EPAS1 polymorphism but different genotype–phenotype associations than those documented in Tibetan humans (Schweizer et al. 2019). Whereas nucleotide variation is restricted to noncoding sites in EPAS1 of Tibetan humans (Peng et al. 2011; Hu et al. 2017), a coding polymorphism exhibits the largest altitudinal difference in allele frequency across the EPAS1 gene of deer mice. This amino acid polymorphism exhibits a steep altitudinal cline in allele frequencies (fig. 4A) and genome-wide analyses of nucleotide variation provided strong evidence that the locus-specific pattern of differentiation reflects a history of altitude-related selection.
Fig. 4.
In North American deer mice (Peromyscus maniculatus), coding polymorphism in EPAS1 exhibits a striking pattern of altitudinal variation and contributes to variation in heart rate in hypoxia. (A) The derived amino acid variant exhibits a steep altitudinal cline in frequency from the Great Plains to the crest of the Front Range of the Southern Rocky Mountains. LN (Lincoln, NE; 430 m) and ME (summit of Mt. Evans; 4350 m) denote opposite ends of the altitudinal transect. (B) When exposed to severe hypoxia (12 kPa O2, the PO2 at the native altitude of the tested mice), high-altitude mice that were homozygous for the highland EPAS1 variant exhibited significantly higher resting heart rates than mice homozygous for the wild-type allele. From Schweizer et al. (2019) with permission.
Using segregating amino acid variation in an alpine population of deer mice living at 4,350 m in the Southern Rockies, Schweizer et al. (2019) tested for associations between EPAS1 genotype and numerous respiratory, cardiovascular, and metabolic phenotypes. In contrast to the case with Tibetan humans, the highland EPAS1 variant exhibited no association with Hb concentration (Schweizer et al. 2019). The only measured trait that exhibited a significant association with the derived, highland EPAS1 variant was an increased resting heart rate under hypoxia (fig. 4B). All else being equal, an increase in heart rate increases cardiac output and should therefore increase circulatory O2 delivery. During acclimatization to hypoxia, high-altitude deer mice increase cardiac output at VO2max, a plastic response that makes a significant contribution to aerobic capacity (Tate et al. 2020). Like the case with Hb concentration in Tibetan humans, it is not clear whether the increased heart rate in highland deer mice reflects a direct response to past selection, or whether it represents a secondary consequence of selectively mediated changes in other aspects of O2 sensing or O2 transport that are regulated by EPAS1.
The highland EPAS1 variant of deer mice was also associated with a downregulation of genes involved in catecholamine biosynthesis in the adrenal gland (Schweizer et al. 2019), a pathway that modulates heart rate and the vasoconstrictive response to hypoxia. Consistent with the observed association in vivo, subsequent experiments revealed that the highland EPAS1 variant is a loss-of-function mutation that reduces transcriptional activity because it impairs binding of HIF-2α to the transcriptional coactivator CREB-binding protein (Song et al. 2021).
Female Reproductive Success and the Challenge of Mammalian Pregnancy at High Altitude
Hypoxia-related problems during pregnancy impinge on the most critical period for reproduction and should therefore have an accentuated impact on fitness. Hypoxia contributes to hypertensive disorders (e.g., preeclampsia) and restricted fetal growth (intrauterine growth restriction, IUGR), which greatly increases the risk of stillbirth and infant mortality (Moore 2001; Browne et al. 2015; Niermeyer et al. 2015). Altitude-associated IUGR is typically caused by a slowing of fetal growth during the third trimester of pregnancy, not from a shortening of gestation (Moore 2001). The decline in birthweight with increasing altitude of residence appears to be a universal pattern in all human populations that have been studied, but the birthweight decline in infants born to women of Andean and Tibetan ancestry is roughly half that of infants born to European or Han Chinese women living at similar altitudes (Zamudio et al. 1993; Moore et al. 2001; Julian et al. 2007, 2009; Soria et al. 2013). The reduced susceptibility to IUGR among Andean natives clearly has a genetic basis, as infant birthweights at high-altitude are positively correlated with the fraction of indigenous ancestry of the parents (Julian et al. 2007; Bennett et al. 2008; Soria et al. 2013). These findings are consistent with historical chronicles of life in high Andean settlements during the Spanish conquest of South America, as it was well-documented that mestizo infants born to a Spanish father and indigenous mother had higher rates of survival at high altitude than criollo infants born to Spanish parents (Gonzales 2007). Altitude-associated IUGR also shows a trend in domesticated mammals, as highland breeds or species suffer less fetal growth restriction compared with lowland natives living at the same altitude (Parraguez et al. 2005).
Insights into the genetic and physiological mechanisms that underlie protection from hypertensive disorders of pregnancy and IUGR in high-altitude humans and other mammals could have great medical/veterinary benefits and could greatly enhance our understanding of hypoxia adaptation.
Genotypic Associations with Components of Female Reproductive Success
Motivated by results of population genomic analyses and a long history of physiological research on Tibetan natives (Beall et al. 2004; Beall 2007, 2014; Gilbert-Kawai et al. 2014), Jeong et al. (2018) used a sample of 1,000 ethnically Tibetan women to test for genome-wide associations with a suite of physiological and reproductive traits. The female study subjects were residents of villages located at altitudes of ∼3,000–4,000 m in the high Himalayan valleys of Nepal. The physiological phenotypes included a set of noninvasive hematological and cardiological measurements and the reproductive phenotypes were based on complete histories of pregnancy outcomes and offspring survival for women at post-reproductive ages. These records represent a rich source of data on lifetime reproductive success in a traditional society where—until recently—women had minimal access to modern medical care and contraceptive birth control (Cho et al. 2017). In addition to testing for genotype–phenotype associations within the set of Tibetan subjects, the authors used the genome-wide polymorphism data to test for evidence of polygenic responses to past selection on the measured phenotypes.
Analysis of genome-wide polymorphism data in the sample of Tibetan women revealed that derived variants at eight SNPs in an intron of EPAS1 exhibited a strong, negative association with Hb concentration. Data on the reproductive histories of the Tibetan women revealed that lower Hb concentration (measured at post-reproductive ages) is associated with a higher proportion of live births among pregnancies and with lower proportions and lower absolute numbers of still births and miscarriages (Cho et al. 2017; Jeong et al. 2018). This result is consistent with evidence from high-altitude populations that elevated Hb concentration and the associated increase in blood viscosity contribute to IUGR (Gonzales et al. 2009), an effect that may stem from a reduction in uterine artery blood flow and, hence, a reduced O2 delivery to the uteroplacental circulation (Zamudio et al. 1995; Moore et al. 2001; Wilson et al. 2007; Julian et al. 2008, 2009; Browne et al. 2015).
Consistent with earlier surveys of genome-wide variation based on different samples of Tibetans (Beall et al. 2010; Simonson et al. 2010; Yi et al. 2010; Peng et al. 2011; Xu et al. 2011; Hu et al. 2017; Yang et al. 2017), Jeong et al. (2018) found that EPAS1 and EGLN1 exhibited the strongest signals of positive selection. However, no SNPs in or near either gene exhibited statistically detectable associations with the measured reproductive phenotypes after correcting for multiple tests. This negative result may reflect a lack of statistical power, so it does not necessarily rule out the possibility that the surveyed variants have contributed to a past response to selection on the measured traits. Compared with a reference panel of control SNPs, the EPAS1 variants associated with low Hb concentration were present at a significantly higher frequency in Tibetans than in lowland reference populations. In Tibetans, this putative signature of polygenic adaptation was driven entirely by a single EPAS1 SNP where the derived allele associated with low Hb concentration was present at frequencies of 0.75 and 0.01 in Tibetans and Han Chinese, respectively. Based on the estimated genotypic effect sizes for the EPAS1 SNP alleles and the mean allele frequency difference between Tibetan and Han Chinese populations, Jeong et al. (Jeong et al. 2018) calculated that the EPAS1 variants explain 52% of the 1.1 g/dl difference in Hb concentration between Tibetan and Han Chinese women in the same age range. However, significant fractions of within- and between-population variation in Hb concentration remain unexplained and—outside of EPAS1—no other SNP alleles associated with low Hb concentration in Tibetans exhibited significant frequency differences with lowland populations. On the basis of these results, the authors concluded that Hb concentration may not represent the direct target of selection.
Association of PRKAA1 and EDNRA Variants with Infant Birth Weight at High Altitude
Using a panel of candidate genes for hypoxia adaptation that were implicated in genome scans of DNA polymorphism in native Andeans, Bigham et al. (2014) tested for genetic associations with altitude-related IUGR and other intermediate phenotypes in a cohort of Bolivian women with native Andean or mixed European ancestry. The authors detected significant associations between maternal genotypes at noncoding SNPs near two genes involved in O2 sensing and vascular control, the α-1 catalytic subunit of adenosine monophosphate-activated protein kinase (PRKAA1, also known as AMPK α1) and endothelin receptor type A (EDNRA). PRKAA1 also exhibited a significant association with a key subordinate trait, uterine artery diameter, an important determinant of uteroplacental blood flow that contributes to protection from altitude-associated IUGR (Zamudio et al. 1995; Julian et al. 2008, 2009; Browne et al. 2015). Finally, the derived PRKAA1 SNP allele associated with heavier birth weight and larger uterine artery diameter was present at significantly higher frequency in Andeans than in Europeans (0.88 vs. 0.73, respectively), consistent with well-documented differences in altitude-associated IUGR and uteroplacental blood flow in women from these two groups (Wilson et al. 2007; Julian et al. 2009). Interestingly, neither EPAS1 nor EGLN1 exhibited significant associations with infant birth weight or uterine artery diameter in the examined cohort of Andean and European women.
Although causal mutations and mechanistic effects have yet to be identified, the associations between specific maternal genotypes and infant birth weight suggest hypotheses about the physiological mechanisms by which high-altitude natives have evolved protection from hypoxia-induced IUGR. The studies of Jeong et al. (2018) and Bigham et al. (2014) focused on associations between maternal genotypes and pregnancy outcomes at high altitude. In future studies of high-altitude humans and other mammals it will also be of interest to investigate the influence of the fetal genotype and parent-of-origin effects (Grant et al. 2020).
Genetic Experiments Provide Insights into Mechanism and Process in Hypoxia Adaptation
Modifications of the HIF Pathway
Evidence for positive selection on HIF genes in high-altitude natives has motivated several follow-up studies to examine allelic differences in transcriptional regulation (Peng et al. 2017; Schweizer et al. 2019; Xin et al. 2020), molecular function in relation to HIF signaling (Lorenzo et al. 2014; Song et al. 2014, 2021; Liu et al. 2019) and higher-level physiological phenotypes involved in the response to hypoxia (Petousi et al. 2014; Brutsaert et al. 2019; Schweizer et al. 2019; Song et al. 2020). The product of the EGLN1 gene (PHD2) binds p23, a chaperone of the HSP90 pathway that promotes folding of client proteins, including HIF-α. The PHD2: p23 interaction recruits PHD2 to the HSP90 pathway, thereby facilitating O2-dependent hydroxylation of HIF-α (Arsenault et al. 2016), a posttranslational modification that targets HIF-α for degradation. The putatively adaptive Tibetan PHD2 allele is distinguished from the wildtype (lowland) allele by two amino acid mutations that flank the N-terminal domain responsible for binding p23 and other co-chaperones of the HSP90 pathway (Xiang et al. 2013; Lorenzo et al. 2014; Song et al. 2014, 2020). In vitro experiments revealed that the two mutations impair PHD2: p23 binding (Song et al. 2014), which compromises PHD2-induced hydroxylation of HIF-α. In hypoxia, the reduced rate of hydroxylation promotes the stabilization of HIF-α subunits, thereby facilitating dimerization with HIF-β in the nucleus and the subsequent transcriptional activation of target genes by the HIF-α/β heterodimer. The next question is how this Tibetan-specific modification of HIF signaling affects systemic physiology.
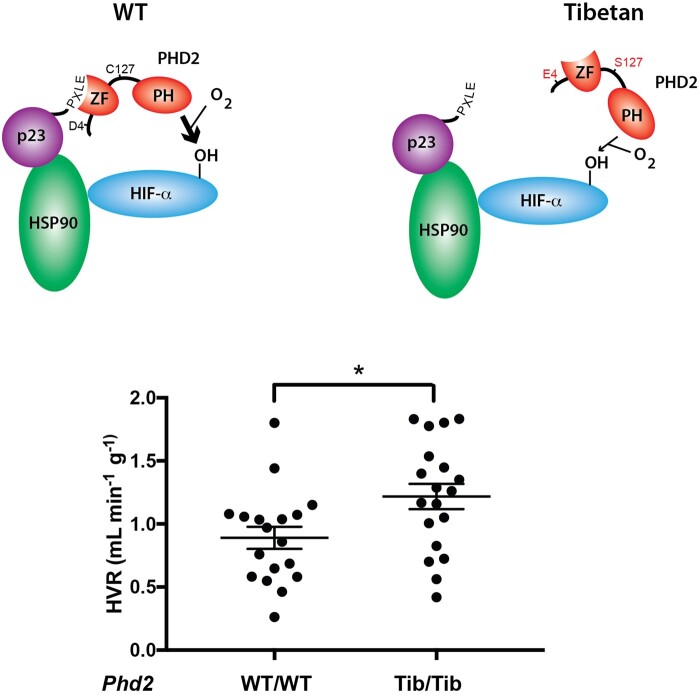
In lowland humans, the typical acclimatization response to acute hypoxia involves an increase in ventilation, a response that gradually diminishes with continued exposure (Ivy and Scott 2015; Pamenter and Powell 2016). At high altitude, the breathing pattern of Tibetans is similar to that of newly acclimatized lowlanders (and distinct from that of Andean highlanders) in that they maintain high resting ventilation and an enhanced ventilatory sensitivity to hypoxia at constant arterial CO2 concentration (Zhuang et al. 1993; Beall et al. 1997; Brutsaert 2007; Slessarev et al. 2010; Gilbert-Kawai et al. 2014). Experiments on knock-in mice revealed that the double-mutant Tibetan EGLN1 (PHD2) allele contributed to an augmentation of the hypoxic ventilatory response, recapitulating the Tibetan-specific respiratory phenotype (Song et al. 2020) (fig. 5). These experimental findings suggest that the Tibetan EGLN1 allele may play a role in mediating genetic assimilation of the ancestral acclimatization response to hypoxia. There is much left to discover regarding interactions between the products of EGLN1 (PHD2), EPAS1 (HIF-2α), and other components of the HIF pathway, and the manner in which evolved modifications appear to selectively activate and inhibit different outputs of the pathway in response to hypoxia.
Fig. 5.
In vivo experiments on knock-in mice reveal that the Tibetan EGLN1 (PHD2) allele is associated with an enhanced ventilatory response to hypoxia. Relative to mice that carry the wild-type human PHD2 allele, mice that are homozygous for the Tibetan-specific allele exhibit a greater hypoxic ventilatory response (HVR) when exposed to acute hypoxia (12% O2/3% CO2). The schematic diagram shows that Tibetan-specific mutations at PHD2 sites 4 and 17 impair the binding interaction between PHD2 and p23, a co-chaperone of the HSP90 pathway. Relative to wild-type PHD2, the zinc finger (ZF) binding domain of Tibetan PHD2 binds less readily to the “PXLE” motif of p23. Consequently, Tibetan PHD2 hydroxylates HIF-α less efficiently than the wild-type PHD2 (as indicated by difference in thickness of the arrow connecting PHD2 to HIF-α). Modified from Song et al. (2020) with permission.
Modifications of Hb Function, Blood-O2 Transport, and Aerobic Capacity in Hypoxia
In addition to population genomic surveys that nominate candidate genes for hypoxia adaptation based on signatures of positive selection, other studies have targeted candidate genes for experimental testing based on known physiological functions. Under conditions of severe hypoxia, theoretical and empirical results suggest that an increased Hb-O2 affinity may be adaptive if it helps safeguard arterial O2 saturation, and if it is accompanied by an increased tissue O2 diffusion capacity so that the augmentation of arterial O2 content translates into a corresponding increase in tissue O2 extraction (Storz 2016, 2019). Consistent with these predictions, the general trend—far more pronounced in birds than in mammals—is that highland taxa have convergently evolved increased Hb–O2 affinities relative to their lowland counterparts (Natarajan, Projecto-Garcia et al. 2015; Natarajan et al. 2016; Storz 2016, 2019; Zhu et al. 2018). Protein-engineering experiments have identified and characterized the specific amino acid replacements responsible for observed changes in Hb function, and have provided detailed insights into molecular mechanisms of biochemical adaptation (Natarajan et al. 2013, 2016, 2018; Projecto-Garcia et al. 2013; Galen et al. 2015; Natarajan, Projecto-Garcia, et al. 2015; Tufts et al. 2015; Kumar et al. 2017; Zhu et al. 2018; Signore et al. 2019; Signore and Storz 2020). In some cases, in vitro experiments that quantified the phenotypic effects of specific mutations have been integrated with population genetic analyses to test for corroborative evidence that the variants in question increased in frequency under the influence of positive selection (Storz and Kelly 2008; Storz et al. 2009, 2012; Storz, Runck et al. 2010; Cheviron, Natarajan et al. 2014; Galen et al. 2015; Natarajan, Projecto-Garcia, et al. 2015).
High-altitude deer mice have evolved a derived increase in Hb-O2 affinity relative to lowland conspecifics due to multiple amino acid replacements in duplicated genes that encode the α- and β-chain subunits of the α2β2 Hb heterotetramer (Storz et al. 2009; Storz, Runck et al. 2010; Natarajan et al. 2013; Natarajan, Hoffmann, et al. 2015; Jensen et al. 2016). Genetic crosses revealed that the evolved increase in Hb-O2 affinity contributes to an adaptive enhancement of aerobic capacity at high altitude (Chappell and Snyder 1984; Chappell et al. 1988). To examine the physiological mechanisms by which increases in Hb-O2 affinity affect whole-animal performance capacity in hypoxia, Wearing et al. (2020) created F2 interpopulation hybrids between highland and lowland deer mice to randomize associations between allelic α- and β-globin variants on an admixed genetic background. They then examined effects of alternative Hb variants on thermogenic VO2max and subordinate cardiorespiratory and hematological traits in hypoxia. In vivo measurements revealed that the genetically based increase in Hb-O2 affinity augments arterial O2 saturation in hypoxia (Tate et al. 2017, 2020). However, experimental results and mathematical modeling indicate that the increased arterial O2 saturation only translates into an enhancement of hypoxic VO2max when accompanied by a corresponding increase in tissue O2 diffusion capacity (Wearing et al. 2020). It is therefore notable that, in conjunction with the evolved increase in Hb-O2 affinity, deer mice native to high altitude have also evolved a skeletal muscle phenotype characterized by enhanced capacities for tissue O2 diffusion and O2 utilization owing to derived increases in capillary surface density, volume density of total and subsarcolemmal mitochondria, density of oxidative fiber types, and mitochondrial oxidative capacity (Lui et al. 2015; Scott et al. 2015, 2018; Mahalingam et al. 2017, 2020; Tate et al. 2017, 2020; Nikel et al. 2018). These discoveries regarding the determinants of hypoxic VO2max in deer mice highlight the importance of accounting for the functional integration of focal phenotypes and illustrate how the adaptive value of changes in one trait may be contingent on antecedent changes in other traits.
Coordinated Evolution of Interdependent Traits
Physiological responses to hypoxia involve coordinated changes in serially integrated traits that exert control over different steps in transport pathways for O2 and metabolic substrates (Gonzalez et al. 1993, 1994, 1998; Wagner 1996; Scott and Milsom 2006; Ivy and Scott 2015; McClelland et al. 2017; Tate et al. 2017, 2020; Gonzalez and Kuwahira 2018; McClelland and Scott 2019; Storz and Scott 2019). Consequently, patterns of developmental, functional, and genetic interdependence among such traits may exert a strong influence on the evolution of higher-level performance capacities such as VO2max.
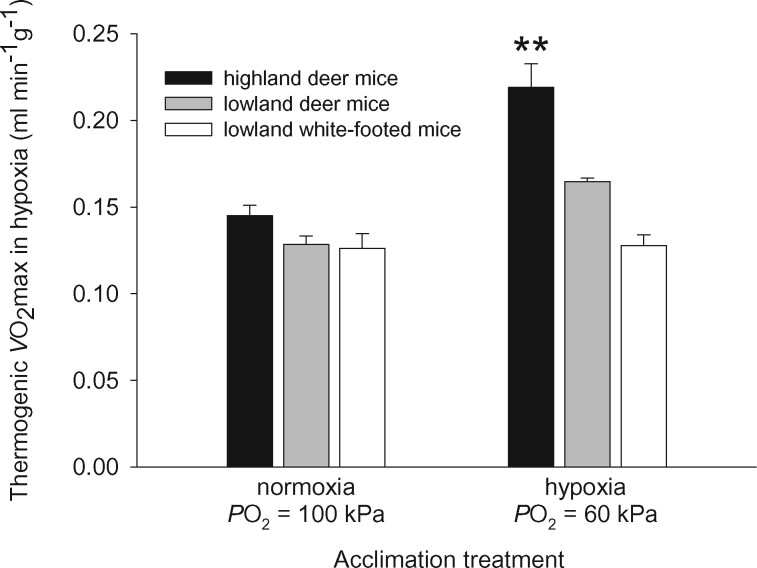
In hypoxia, high-altitude deer mice have significantly higher aerobic performance capacities than lowland conspecifics, both in terms of exercise-induced and cold-induced VO2max (Cheviron et al. 2012, 2013; Cheviron, Connaty et al. 2014; Lui et al. 2015; Lau et al. 2017; Tate et al. 2017, 2020). This augmented performance in hypoxia is partly attributable to an interaction between subordinate traits that govern different steps in the O2-transport pathway (Tate et al. 2017, 2020). For example, after 6–8 weeks of acclimation to hypoxia (barometric pressure = 60 kPa, PO2 = 12.5 kPa) at 25 °C, highland mice increase thermogenic VO2max 1.7-fold, a far more pronounced increase than that observed in lowlanders (fig. 6). The higher thermogenic VO2max in hypoxia-acclimated highland mice is largely explained by an increase in O2 transport to tissues involved in shivering and nonshivering thermogenesis (skeletal muscle and brown adipose tissue, respectively). This enhancement of O2-transport capacity in highland mice is attributable to the interaction between a hypoxia-induced increase in cardiac output in conjunction with genetically based increases in arterial O2 saturation and tissue O2 extraction (Tate et al. 2017, 2020). Evolved changes in these latter two traits in highland mice stem from increases in Hb-O2 affinity (Storz et al. 2009; Storz, Runck et al. 2010; Natarajan et al. 2013; Natarajan, Hoffmann et al. 2015; Jensen et al. 2016) and the capillary density and oxidative capacity of skeletal muscle (Lui et al. 2015; Scott et al. 2015, 2018; Mahalingam et al. 2017, 2020; Tate et al. 2017, 2020; Nikel et al. 2018). This example highlights how changes in whole-animal performance capacities may stem from interactions between both plastic and evolved changes in subordinate traits, an important consideration for the design and interpretation of association studies. In physiological studies of deer mice, transcriptomic analyses of O2-consuming tissues such as cardiac and skeletal muscle have shed light on mechanisms of plasticity in key phenotypes and have identified changes in regulatory networks that mediate both acclimatization and genetic adaptation to hypoxia (Cheviron et al. 2012; Cheviron, Connaty et al. 2014; Scott et al. 2015; Velotta et al. 2016, 2020; Schweizer et al. 2019).
Fig. 6.
Following acclimation, high-altitude deer mice (Peromyscus maniculatus) exhibit a higher thermogenic capacity in hypoxia relative to lowland conspecifics and the exclusively lowland white-footed mice (P. leucopus). Thermogenic capacity is measured as cold-induced VO2max in hypoxia. Data are means ± SEM. **Significant pairwise difference between highland deer mice and both lowland taxa within the same acclimation treatment. ***Data from Tate et al. (2020) with permission.
In the context of high-altitude adaptation, another important question about phenotypic integration concerns the extent to which hypoxia-induced responses in subordinate traits are synergistic or antagonistic with respect to higher-level performance capacities. Studies of hypoxic pulmonary hypertension and other altitude-related maladies in lowland natives indicate that some components of the ancestral acclimatization response to hypoxia are maladaptive. Differences in hypoxia acclimatization between highland and lowland natives suggest that the process of high-altitude adaptation may often involve directional selection on genetically based trait variation that mitigates the deleterious effects of environmentally induced changes. This form of genetic compensation is expected to produce counter-gradient patterns of altitudinal variation in hypoxia-responsive traits such that adaptive phenotypic differentiation between highland and lowland natives is cryptic under field conditions and is only revealed by experimental treatments that control for plasticity (Storz et al. 2010; Storz and Scott 2019, 2021; Storz and Cheviron 2021).
Future Outlook
Population genomic surveys are useful for generating hypotheses about the genetic basis of high-altitude adaptation, but such data need to be combined with experimental testing to provide insight into functional mechanisms and phenotypic targets of selection. Manipulative physiological experiments are required to determine whether observed genotype–phenotype associations reflect causal effects or indirect consequences of changes in other traits that control interdependent steps in the same pathway. Considerations of phenotypic integration illustrate how observed genotype–phenotype associations can mislead inferences about adaptive mechanisms when the measured phenotype (e.g., Hb concentration) represents a single, labile component of a higher level performance trait (e.g., VO2max). In Tibetan highlanders, for example, the integrated regulation of erythropoiesis and plasma volume maximizes the benefits of increased Hb mass (augmented arterial O2 content) while mitigating associated costs (increased blood viscosity or diffusion limitation). Since reaction norms of hypoxia-responsive traits like Hb concentration do not reveal the underlying pattern of integration with other traits, the causal mechanism underlying the enhancement of circulatory O2 transport in hypoxia only came to light once multiple components of trait variation were jointly examined in the context of systemic physiology (Stembridge et al. 2019). Future progress in our understanding of high-altitude adaptation will require integration of genomic data with mechanistic approaches in experimental physiology to dissect the functional, developmental, and genetic interdependence of subordinate traits that contribute to fitness-related performance capacities in hypoxia.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL087216) and the National Science Foundation (OIA-1736249). I thank C.M. Beall, T.D. Brutsaert, F.S. Lee, G.B. McClelland, L.G. Moore, C. Natarajan, G.R. Scott, and M. Stembridge for helpful discussion, and three anonymous reviewers for comments and suggestions.
References
- Aggarwal S, Negi S, Jha P, Singh PK, Stobdan T, Pasha MAQ, Ghosh S, Agrawal A, Prasher B, Mukerji M, et al. 2010. EGLN1 involvement in high-altitude adaptation revealed through genetic analysis of extreme constitution types defined in Ayurveda. Proc Natl Acad Sci U S A. 107(44):18961–18966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkorta-Aranburu G, Beall CM, Witonsky DB, Gebremedhin A, Pritchard JK, Di Rienzo A.. 2012. The genetic architecture of adaptations to high altitude in Ethiopia. PLoS Genet. 8(12):e1003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciero E, Kraaijenbrink TAsanHaber M, Mezzavilla M, Ayub Q, Wei W, Zhaxi PC, Yang HM, Jian W, et al. 2018. Demographic history and genetic adaptation in the Himalayan region inferred from genome-wide SNP genotypes of 49 populations. Mol Biol Evol. 35:1916–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault PR, Pei F, Lee R, Kerestes H, Percy MJ, Keith B, Simon MC, Lappin TRJ, Khurana TS, Lee FS.. 2013. A knock-in mouse model of human PHD2 gene-associated erythrocytosis establishes a haploinsufficiency mechanism. J Biol Chem. 288(47):33571–33584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault PR, Song DS, Chung YJ, Khurana TS, Lee FS.. 2016. The zinc finger of Prolyl Hydroxylase Domain Protein 2 is essential for efficient hydroxylation of hypoxia-inducible factor alpha. Mol Cell Biol. 36(18):2328–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM. 2006. Andean, Tibetan, and Ethiopian patterns of adaptation to high-altitude hypoxia. Integr Comp Biol. 46(1):18–24. [DOI] [PubMed] [Google Scholar]
- Beall CM. 2007. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci USA. 104(Suppl 1):8655–8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM. 2014. Adaptation to high altitude: phenotypes and genotypes. Annu Rev Anthropol. 43(1):251–272. [Google Scholar]
- Beall CM, Brittenham GM, Strohl KP, Blangero J, Williams-Blangero S, Goldstein MC, Decker MJ, Vargas E, Villena M, Soria R, et al. 1998. Hemoglobin concentration of high-altitude Tibetans and Bolivian Aymara. Am J Phys Anthropol. 106(3):385–400. [DOI] [PubMed] [Google Scholar]
- Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Li JC, Liang Y, McCormack M, et al. 2010. Natural selection on EPAS1 (HIF2α) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci U S A. 107(25):11459–11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM, Goldstein MC.. 1987. Hemoglobin concentration of pastoral nomads permanently resident at 4,850–5,450 meters in Tibet. Am J Phys Anthropol. 73(4):433–438. [DOI] [PubMed] [Google Scholar]
- Beall CM, Reichsman AB.. 1984. Hemoglobin levels in a Himalayan high-altitude population. Am J Phys Anthropol. 63(3):301–306. [DOI] [PubMed] [Google Scholar]
- Beall CM, Song KJ, Elston RC, Goldstein MC.. 2004. Higher offspring survival among Tibetan women with high oxygen saturation genotypes residing at 4,000 m. Proc Natl Acad Sci U S A. 101(39):14300–14304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM, Strohl KP, Blangero J, Williams-Blangero S, Almasy LA, Decker MJ, Worthman CM, Goldstein MC, Vargas E, Villena M, et al. 1997. Ventilation and hypoxic ventilatory response of Tibetan and Aymara high altitude natives. Am J Phys Anthropol. 104(4):427–447. [DOI] [PubMed] [Google Scholar]
- Bennett A, Sain SR, Vargas E, Moore LG.. 2008. Evidence that parent-of-origin affects birth-weight reductions at high altitude. Am J Hum Biol. 20(5):592–597. [DOI] [PubMed] [Google Scholar]
- Bennett AF. 1991. The evolution of activity capacity. J Exp Biol. 160:1–23. [DOI] [PubMed] [Google Scholar]
- Bigham A, Bauchet M, Pinto D, Mao XY, Akey JM, Mei R, Scherer SW, Julian CG, Wilson MJ, Herraez DL, et al. 2010. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet. 6(9):e1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham AW, Julian CG, Wilson MJ, Vargas E, Browne VA, Shriver MD, Moore LG.. 2014. Maternal PRKAA1 and EDNRA genotypes are associated with birth weight, and PRKAA1 with uterine artery diameter and metabolic homeostasis at high altitude. Physiol Genomics 46(18):687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham AW, Lee FS.. 2014. Human high-altitude adaptation: forward genetics meets the HIF pathway. Genes Dev. 28(20):2189–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham AW, Mao X, Mei R, Brutsaert T, Wilson MJ, Julian CG, Parra EJ, Akey JM, Moore LG, Shriver MD.. 2009. Identifying positive selection candidate loci for high-altitude adaptation in Andean populations. Hum Genomics 4(2):79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop T, Talbot NP, Turner PJ, Nicholls LG, Pascual A, Hodson EJ, Douglas G, Fielding JW, Smith TG, Demetriades M, et al. 2013. Carotid body hyperplasia and enhanced ventilatory responses to hypoxia in mice with heterozygous deficiency of PHD2. J Physiol. 591(14):3565–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne VA, Julian CG, Toledo-Jaldin L, Cioffi-Ragan D, Vargas E, Moore LG.. 2015. Uterine artery blood flow, fetal hypoxia and fetal growth. Phil Trans R Soc B 370(1663):20140068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert T. 2016. Why are high altitude natives so strong at high altitude? Nature vs. nurture: genetic factors vs. growth and development. Adv Exp Med Biol. 903:101–112. [DOI] [PubMed] [Google Scholar]
- Brutsaert TD. 2007. Population genetic aspects and phenotypic plasticity of ventilatory responses in high altitude natives. Respir Physiol Neurobiol. 158(2–3):151–160. [DOI] [PubMed] [Google Scholar]
- Brutsaert TD. 2008. Do high-altitude natives have enhanced exercise performance at altitude? Appl Physiol Nutr Metab. 33(3):582–592. [DOI] [PubMed] [Google Scholar]
- Brutsaert TD, Kiyamu M, Revollendo GE, Isherwood JL, Lee FS, Rivera-Ch M, Leon-Velarde F, Ghosh S, Bigham AW.. 2019. Association of EGLN1 gene with high aerobic capacity of Peruvian Quechua at high altitude. Proc Natl Acad Sci U S A. 116(48):24006–24011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert TD, Parra EJ, Shriver MD, Gamboa A, Palacios JA, Rivera M, Rodriguez I, Leon-Velarde F.. 2003. Spanish genetic admixture is associated with larger VO2max decrement from sea level to 4,338 m in Peruvian Quechua. J Appl Physiol. 95(2):519–528. [DOI] [PubMed] [Google Scholar]
- Calbet JAL, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B.. 2003. Why is VO2max after altitude acclimatization still reduced despite normalization of arterial O2 content? Am J Physiol Regul Integr Comp Physiol. 284(2):R304–R316. [DOI] [PubMed] [Google Scholar]
- Calbet JAL, Radegran G, Boushel R, Sondergaard H, Saltin B, Wagner PD.. 2002. Effect of blood haemoglobin concentration on VO2max and cardiovascular function in lowlanders acclimatised to 5260 m. J Physiol. 545(2):715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell MA, Hayes JP, Snyder LRG.. 1988. Hemoglobin polymorphisms in deer mice (Peromyscus maniculatus), physiology of β-globin variants and α-globin recombinants. Evolution 42(4):681–688. [DOI] [PubMed] [Google Scholar]
- Chappell MA, Snyder LRG.. 1984. Biochemical and physiological correlates of deer mouse α-chain hemoglobin polymorphisms. Proc Natl Acad Sci U S A. 81(17):5484–5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QH, Ge RL, Wang XZ, Chen HX, Wu TY, Kobayashi T, Yoshimura K.. 1997. Exercise performance of Tibetan and Han adolescents at altitudes of 3,417 and 4,300 m. J Appl Physiol. 83(2):661–667. [DOI] [PubMed] [Google Scholar]
- Cheviron ZA, Bachman GC, Connaty AD, McClelland GB, Storz JF.. 2012. Regulatory changes contribute to the adaptive enhancement of thermogenic capacity in high-altitude deer mice. Proc Natl Acad Sci U S A. 109(22):8635–8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron ZA, Bachman GC, Storz JF.. 2013. Contributions of phenotypic plasticity to differences in thermogenic performance between highland and lowland deer mice. J Exp Biol. 216(Pt 7):1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron ZA, Connaty AD, McClelland GB, Storz JF.. 2014. Functional genomics of adaptation to hypoxic cold-stress in high-altitude deer mice: transcriptomic plasticity and thermogenic performance. Evolution 68(1):48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron ZA, Natarajan C, Projecto-Garcia J, Eddy DK, Jones J, Carling MD, Witt CC, Moriyama H, Weber RE, Fago A, et al. 2014. Integrating evolutionary and functional tests of adaptive hypotheses: a case study of altitudinal differentiation in hemoglobin function in an Andean sparrow, Zonotrichia capensis. Mol Biol Evol. 31(11):2948–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JI, Basnyat B, Jeong C, Di Rienzo A, Childs G, Craig SR, Sun JY, Beall CM.. 2017. Ethnically Tibetan women in Nepal with low hemoglobin concentration have better reproductive outcomes. Evol Med Public Health. 2017(1):82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JE, Amaru R, Song J, Julian CG, Racimo F, Cheng JY, Guo X, Yao J, Ambale-Venkatesh B, Lima JA, et al. 2017. Natural selection on genes related to cardiovascular health in high-altitude adapted Andeans. Am J Hum Genet. 101(5):752–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson NJ, Lyons SA, Henry DA, Scott GR.. 2018. Effects of chronic hypoxia on diaphragm function in deer mice native to high altitude. Acta Physiol. 223(1):e13030. [DOI] [PubMed] [Google Scholar]
- Donnelly S. 2003. Why is erythropoietin made in the kidney? The kidney functions as a ‘critmeter’ to regulate the hematocrit. Adv Exp Med Biol.543:73–87. [DOI] [PubMed] [Google Scholar]
- Foll M, Gaggiotti OE, Daub JT, Vatsiou A, Excoffier L.. 2014. Widespread signals of convergent adaptation to high altitude in Asia and America. Am J Hum Genet. 95(4):394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisancho AR, Frisancho HG, Milotich M, Brutsaert T, Albalak R, Spielvogel H, Villena M, Vargas E, Soria R.. 1995. Developmental, genetic, and environmental components of aerobic capacity at high altitude. Am J Phys Anthropol. 96(4):431–442. [DOI] [PubMed] [Google Scholar]
- Galen SC, Natarajan C, Moriyama H, Weber RE, Fago A, Benham PM, Chavez AN, Cheviron ZA, Storz JF, Witt CC.. 2015. Contribution of a mutational hotspot to adaptive changes in hemoglobin function in high-altitude Andean house wrens. Proc Natl Acad Sci U S A. 112(45):13958–13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann M, Mairbaurl H, Livshits L, Seide S, Hackbusch M, Malczyk M, Kraut S, Gassmann NN, Weissmann N, Muckenthaler MU.. 2019. The increase in hemoglobin concentration with altitude varies among human populations. Ann N Y Acad Sci. 1450(1):204–220. [DOI] [PubMed] [Google Scholar]
- Ge RL, Chen QH, Wang LH, Gen D, Yang P, Kubo K, Fujimoto K, Matsuzawa Y, Yoshimura K, Takeoka M, et al. 1994. Higher exercise performance and lower VO2max in Tibetan than Han residents at 4,700 m altitude. J Appl Physiol. 77(2):684–691. [DOI] [PubMed] [Google Scholar]
- Gilbert-Kawai ET, Milledge JS, Grocott MPW, Martin DS.. 2014. King of the mountains: Tibetan and Sherpa physiological adaptations for life at high altitude. Physiology 29(6):388–402. [DOI] [PubMed] [Google Scholar]
- Gonzales GE. 2007. Peruvian contributions to the study on human reproduction at high altitude: from the chronicles of the Spanish conquest to the present. Respir Physiol Neurobiol. 158(2-3):172–179. [DOI] [PubMed] [Google Scholar]
- Gonzales GF, Steenland K, Tapia V.. 2009. Maternal hemoglobin level and fetal outcome at low and high altitudes. Am J Physiol Regul Integr Comp Physiol. 297(5):R1477–R1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez NC, Clancy RL, Moue Y, Richalet JP.. 1998. Increasing maximal heart rate increases maximal O2 uptake in rats acclimatized to simulated altitude. J Appl Physiol (1985)). 84(1):164–168. [DOI] [PubMed] [Google Scholar]
- Gonzalez NC, Clancy RL, Wagner PD.. 1993. Determinants of maximal oxygen-uptake in rats acclimated to simulated altitude. J Appl Physiol. 75(4):1608–1614. [DOI] [PubMed] [Google Scholar]
- Gonzalez NC, Erwig LP, Painter CF, Clancy RL, Wagner PD.. 1994. Effect of hematocrit on systemic O2 transport in hypoxic and normoxic exercise in rats. J Appl Physiol. 77(3):1341–1348. [DOI] [PubMed] [Google Scholar]
- Gonzalez NC, Kuwahira I.. 2018. Systemic oxygen transport with rest, exercise, and hypoxia: a comparison of humans, rats, and mice. Compr Physiol. 8(4):1537–1573. [DOI] [PubMed] [Google Scholar]
- Grant I, Soria R, Julian CG, Vargas E, Moore LG, Aiken CE, Giussani DA.. 2020. Parental ancestry and risk of early pregnancy loss at high altitude. FASEB J. 34(10):13741–13749. [DOI] [PubMed] [Google Scholar]
- Greer SN, Metcalf JL, Wang Y, Ohh M.. 2012. The updated biology of hypoxia-inducible factor. Embo J. 31(11):2448–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP. 1989a. Altitudinal and seasonal effects on aerobic metabolism of deer mice. J Comp Physiol B 159(4):453–459. [DOI] [PubMed] [Google Scholar]
- Hayes JP. 1989b. Field and maximal metabolic rates of deer mice (Peromyscus maniculatus) at low and high altitudes. Physiol Zool. 62(3):732–744. [Google Scholar]
- Hayes JP, O’Connor CS.. 1999. Natural selection on thermogenic capacity of high-altitude deer mice. Evolution. 53(4):1280–1287. [DOI] [PubMed] [Google Scholar]
- Hochachka PW. 1998. Mechanism and evolution of hypoxia-tolerance in humans. J Exp Biol. 201(Pt 8):1243–1254. [DOI] [PubMed] [Google Scholar]
- Hochachka PW, Stanley C, Matheson GO, McKenzie DC, Allen PS, Parkhouse WS.. 1991. Metabolic and work efficiencies during exercise in Andean natives. J Appl Physiol. 70(4):1720–1730. [DOI] [PubMed] [Google Scholar]
- Hu H, Petousi N, Glusman G, Yu Y, Bohlender R, Tashi T, Downie JM, Roach JC, Cole AM, Lorenzo FR, et al. 2017. Evolutionary history of Tibetans inferred from whole-genome sequencing. PLoS Genet. 13(4):e1006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Sanchez E, DeGiorgio M, Pagani L, Tarekegn A, Ekong R, Antao T, Cardona A, Montgomery HE, Cavalleri GL, Robbins PA, et al. 2013. Genetic signatures reveal high-altitude adaptation in a set of Ethiopian populations. Mol Biol Evol. 30(8):1877–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy CM, Greaves MA, Sangster ED, Robertson CE, Natarajan C, Storz JF, McClelland GB, Scott GR.. 2020. Ontogenesis of evolved changes in respiratory physiology in deer mice native to high altitude. J Exp Biol. 223(5):jeb219360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy CM, Scott GR.. 2015. Control of breathing and the circulation in high-altitude mammals and birds. Comp Biochem Physiol A Mol Integr Physiol. 186:66–74. [DOI] [PubMed] [Google Scholar]
- Ivy CM, Scott GR.. 2017. Control of breathing and ventilatory acclimatization to hypoxia in deer mice native to high altitudes. Acta Physiol. 221(4):266–282. [DOI] [PubMed] [Google Scholar]
- Ivy CM, Scott GR.. 2018. Evolved changes in breathing and CO2 sensitivity in deer native to high altitudes. Am J Physiol Regul Integr Comp Physiol. 315(5):R1027–R1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B, Storz JF, Fago A.. 2016. Bohr effect and temperature sensitivity of hemoglobins from highland and lowland deer mice. Comp Biochem Physiol A Mol Integr Physiol. 195:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong C, Witonsky DB, Basnyat B, Neupane M, Beall CM, Childs G, Craig SR, Novembre J, Di Rienzo A.. 2018. Detecting past and ongoing natural selection among ethnically Tibetan women at high altitude in Nepal. PLoS Genet. 14(9):e1007650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian CG, Galan HL, Wilson MJ, DeSilva W, Cioffi-Ragan D, Schwartz J, Moore LG.. 2008. Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am J Physiol Regul Integr Comp Physiol. 295(3):R906–R915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S, Moore LG.. 2007. High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch Dis Child Fetal Neonatal Ed. 92:372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian CG, Wilson MJ, Lopez M, Yamashiro H, Tellez W, Rodriguez A, Bigham AW, Shriver MD, Rodriguez C, Vargas E, et al. 2009. Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am J Physiol Regul Integr Comp Physiol. 296(5):R1564–R1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG, Ratcliffe PJ.. 2008. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 30(4):393–402. [DOI] [PubMed] [Google Scholar]
- Kumar A, Natarajan C, Moriyama H, Witt CC, Weber RE, Fago A, Storz JF.. 2017. Stability-mediated epistasis restricts accessible mutational pathways in the functional evolution of avian hemoglobin. Mol Biol Evol. 34(5):1240–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau D, Connaty A, Mahalingam S, Wall N, Cheviron ZA, Storz JF, Scott GR, McClelland GB.. 2017. Acclimation to hypoxia increases carbohydrate use during exercise in high-altitude deer mice. Am J Physiol Regul Integr Comp Physiol. 312(3):R400–R411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Percy MJ.. 2011. The HIF pathway and erythrocytosis. Annu Rev Pathol. 6:165–192. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Lee KL, Yang H, Poellinger L.. 2009. Generating specificity and diversity in the transcriptional response to hypoxia. Nat Rev Genet. 10(12):821–832. [DOI] [PubMed] [Google Scholar]
- Leon-Velarde F, Gamboa A, Chuquiza J, Esteba W, Rivera-Chira M, Monge C.. 2000. Hematological parameters in high altitude residents living at 4355, 4660, and 5500 meters above sea level. High Alt Med Biol. 1(2):97–104. [DOI] [PubMed] [Google Scholar]
- Liu XX, Zhang YL, Li YF, Pan JF, Wang DD, Chen WH, Zheng ZQ, He XH, Zhao QJ, Pu YB, et al. 2019. EPAS1 gain-of-function mutation contributes to high-altitude adaptation in Tibetan horses. Mol Biol Evol. 36(11):2591–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo FR, Huff C, Myllymaki M, Olenchock B, Swierczek S, Tashi T, Gordeuk V, Wuren T, Ri-Li G, McClain DA, et al. 2014. A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet. 46(9):951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui MA, Mahalingam S, Patel P, Connaty AD, Ivy CM, Cheviron ZA, Storz JF, McClelland GB, Scott GR.. 2015. High-altitude ancestry and hypoxia acclimation have distinct effects on exercise capacity and muscle phenotype in deer mice. Am J Physiol Regul Integr Comp Physiol. 308(9):R779–R791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam S, Cheviron ZA, Storz JF, McClelland GB, Scott GR.. 2020. Chronic cold exposure induces mitochondrial plasticity in deer mice native to high altitudes. J Physiol. 598(23):5411–5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam S, McClelland GB, Scott GR.. 2017. Evolved changes in the intracellular distribution and physiology of muscle mitochondria in high-altitude native deer mice. J Physiol. 595(14):4785–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WHJ, Simon MC.. 2010. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 40(2):294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland GB, Lyons SA, Robertson CE.. 2017. Fuel use in mammals: conserved patterns and evolved strategies for aerobic locomotion and thermogenesis. Integr Comp Biol. 57(2):231–239. [DOI] [PubMed] [Google Scholar]
- McClelland GB, Scott GR.. 2019. Evolved mechanisms of aerobic performance and hypoxia resistance in high-altitude natives. Annu Rev Physiol. 81:561–583. [DOI] [PubMed] [Google Scholar]
- Monge C. 1948. Acclimatization in the Andes. Baltimore (MD: ): The Johns Hopkins University Press. [Google Scholar]
- Monge C, Leon-Velarde F.. 1991. Physiological adaptation to high-altitude - oxygen-transport in mammals and birds. Physiol Rev. 71(4):1135–1172. [DOI] [PubMed] [Google Scholar]
- Moore LG. 2001. Human genetic adaptation to high altitude. High Alt Med Biol. 2(2):257–279. [DOI] [PubMed] [Google Scholar]
- Moore LG, Young D, McCullough RE, Droma T, Zamudio S.. 2001. Tibetan protection from intrauterine growth restriction (IUGR) and reproductive loss at high altitude. Am J Hum Biol. 13(5):635–644. [DOI] [PubMed] [Google Scholar]
- Natarajan C, Hoffmann FG, Lanier HC, Wolf CJ, Cheviron ZA, Spangler ML, Weber RE, Fago A, Storz JF.. 2015. Intraspecific polymorphism, interspecific divergence, and the origins of function-altering mutations in deer mouse hemoglobin. Mol Biol Evol. 32(4):978–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Hoffmann FG, Weber RE, Fago A, Witt CC, Storz JF.. 2016. Predictable convergence in hemoglobin function has unpredictable molecular underpinnings. Science. 354(6310):336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Inoguchi N, Weber RE, Fago A, Moriyama H, Storz JF.. 2013. Epistasis among adaptive mutations in deer mouse hemoglobin. Science 340(6138):1324–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Jendroszek A, Kumar A, Weber RE, Tame JRH, Fago A, Storz JF.. 2018. Molecular basis of hemoglobin adaptation in the high-flying bar-headed goose. PLoS Genet. 14(4):e1007331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Projecto-Garcia J, Moriyama H, Weber RE, Munoz-Fuentes V, Green AJ, Kopuchian C, Tubaro PL, Alza L, Bulgarella M, et al. 2015. Convergent evolution of hemoglobin function in high-altitude Andean waterfowl involves limited parallelism at the molecular sequence level. PLoS Genet. 11(12):e1005681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niermeyer S, Andrade MP, Vargas E, Moore LG.. 2015. Neonatal oxygenation, pulmonary hypertension, and evolutionary adaptation to high altitude. Pulm Circ. 5(1):48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikel KE, Shanishchara NK, Ivy CM, Dawson NJ, Scott GR.. 2018. Effects of hypoxia at different life stages on locomotory muscle phenotype in deer mice native to high altitudes. Comp Biochem Physiol B Biochem Mol Biol. 224:98–104. [DOI] [PubMed] [Google Scholar]
- Niu WZ, Wu YA, Li B, Chen NG, Song SZ.. 1995. Effects of long-term acclimatization in lowlanders migrating to high altitude: comparison with high-altitude residents. Eur J Appl Physiol Occup Physiol. 71(6):543–548. [DOI] [PubMed] [Google Scholar]
- O’Brien KA, Simonson TS, Murray AJ.. 2020. Metabolic adaptation to high altitude. Curr Opin Endocr Metab Res. 11:33–41. [Google Scholar]
- Pamenter ME, Powell FL.. 2016. Time domains of the hypoxic ventilatory response and their molecular basis. Compr Physiol. 6(3):1345–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parraguez VH, Atlagich M, Diaz R, Bruzzone ME, Behn C, Raggi LA.. 2005. Effect of hypobaric hypoxia on lamb intrauterine growth: comparison between high- and low-altitude native ewes. Reprod Fertil Dev. 17(5):497–505. [DOI] [PubMed] [Google Scholar]
- Peng Y, Cui CY, He YXOuzhuluobuZhang H, Yang DY, Zhang QBianbazhuomaYang LX, He YB, et al. 2017. Down-regulation of EPAS1 transcription and genetic adaptation of Tibetans to high-altitude hypoxia. Mol Biol Evol. 34:818–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Yang Z, Zhang H, Cui C, Qi X, Luo X, Tao X, Wu TOuzhuluobuBasang, et al. 2011. Genetic variations in Tibetan populations and high-altitude adaptation at the Himalayas. Mol Biol Evol. 28:1075–1081. [DOI] [PubMed] [Google Scholar]
- Percy MJ, Zhao Q, Flores A, Harrison C, Lappin TRJ, Maxwell PH, McMullin MF, Lee FS.. 2006. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci U S A. 103(3):654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petousi N, Croft QPP, Cavalleri GL, Cheng H-Y, Formenti F, Ishida K, Lunn D, McCormack M, Shianna KV, Talbot NP, et al. 2014. Tibetans living at sea level have a hyporesponsive hypoxia-inducible factor system and blunted physiological responses to hypoxia. J Appl Physiol. 116(7):893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petousi N, Robbins PA.. 2014. Human adaptation to the hypoxia of high altitude: the Tibetan paradigm from the pregenomic to the postgenomic era. J Appl Physiol. 116(7):875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Projecto-Garcia J, Natarajan C, Moriyama H, Weber RE, Fago A, Cheviron ZA, Dudley R, McGuire JA, Witt CC, Storz JF.. 2013. Repeated elevational transitions in hemoglobin function during the evolution of Andean hummingbirds. Proc Natl Acad Sci U S A. 110(51):20669–20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademaker K, Hodgins G, Moore K, Zarrillo S, Miller C, Bromley GRM, Leach P, Reid DA, Alvarez WY, Sandweiss DH.. 2014. Paleoindian settlement of the high-altitude Peruvian Andes. Science 346(6208):466–469. [DOI] [PubMed] [Google Scholar]
- Samanta D, Prabhakar NR, Semenza GL.. 2017. Systems biology of oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 9:0.1002/wsbm.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinfeldt LB, Soi S, Thompson S, Ranciaro A, Woldemeskel D, Beggs W, Lambert C, Jarvis JP, Abate D, Belay G, et al. 2012. Genetic adaptation to high altitude in the Ethiopian highlands. Genome Biol. 13(1):R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer RM, Velotta JP, Ivy CM, Jones MR, Muir SM, Bradburd GS, Storz JF, Scott GR, Cheviron ZA.. 2019. Physiological and genomic evidence that selection on the transcription factor Epas1 has altered cardiovascular function in high-altitude deer mice. PLoS Genet. 15(11):e1008420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GR, Elogio TS, Lui MA, Storz JF, Cheviron ZA.. 2015. Adaptive modifications of muscle phenotype in high-altitude deer mice are associated with evolved changes in gene regulation. Mol Biol Evol. 32(8):1962–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GR, Guo KH, Dawson NJ.. 2018. The mitochondrial basis for adaptive variation in aerobic performance in high-altitude deer mice. Integr Comp Biol. 58(3):506–518. [DOI] [PubMed] [Google Scholar]
- Scott GR, Milsom WK.. 2006. Flying high: a theoretical analysis of the factors limiting exercise performance in birds at altitude. Respir Physiol Neurobiol. 154(1–2):284–301. [DOI] [PubMed] [Google Scholar]
- Sears MW, Hayes JP, O'Connor CS, Geluso K, Sedinger JS.. 2006. Individual variation in thermogenic capacity affects above-ground activity of high-altitude deer mice. Funct Ecol. 20(1):97–104. [Google Scholar]
- Semenza GL. 2012. Hypoxia-inducible factors in physiology and medicine. Cell 148(3):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. 2014. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 9:47–71. [DOI] [PubMed] [Google Scholar]
- Siebenmann C, Robach P, Lundby C.. 2017. Regulation of blood volume in lowlanders exposed to high altitude. J Appl Physiol (1985)). 123(4):957–966. [DOI] [PubMed] [Google Scholar]
- Signore AV, Storz JF.. 2020. Biochemical pedomorphosis and genetic assimilation in the hypoxia adaptation of Tibetan antelope. Sci Adv. 6(25):eabb5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signore AV, Yang YZ, Yang QY, Qin G, Moriyama H, Ge RL, Storz JF.. 2019. Adaptive changes in hemoglobin function in high-altitude Tibetan canids were derived via gene conversion and introgression. Mol Biol Evol. 36(10):2227–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson TS. 2015. Altitude adaptation: a glimpse through various lenses. High Alt Med Biol. 16(2):125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson TS, Wei G, Wagner HE, Wuren T, Qin G, Yan M, Wagner PD, Ge RL.. 2015. Low haemoglobin concentration in Tibetan males is associated with greater high-altitude exercise capacity. J Physiol. 593(14):3207–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson TS, Yang Y, Huff CD, Yun H, Qin G, Witherspoon DJ, Bai Z, Lorenzo FR, Xing J, Jorde LB, et al. 2010. Genetic evidence for high-altitude adaptation in Tibet. Science 329(5987):72–75. [DOI] [PubMed] [Google Scholar]
- Slessarev M, Prisman E, Ito S, Watson RR, Jensen D, Preiss D, Greene R, Norboo T, Stobdan T, Diskit D, et al. 2010. Differences in the control of breathing between Himalayan and sea-level residents. J. Physiol. 588(9):1591–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, , Bigham AW, , Lee FS. Forthcoming 2021. High-altitude deer mouse Hypoxia inducible factor-2α shows defective interaction with CREB-binding protein. J Biol Chem. 100461. doi: 10.1016/j.jbc.2021.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Li LS, Arsenault PR, Tan Q, Bigham AW, Heaton-Johnson KJ, Master SR, Lee FS.. 2014. Defective Tibetan PHD2 binding to p23 links high altitude adaption to altered oxygen sensing. J Biol Chem. 289(21):14656–14665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Navalsky BE, Guan W, Ingersoll X, Wang T, Loro E, Eeles L, Matchett KB, Percy MJ, Walsby-Tickle J, et al. 2020. Tibetan PHD2, an allele with loss-of-function properties. Proc Natl Acad Sci U S A. 117(22):12230–122238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria R, Julian CG, Vargas E, Moore LG, Giussani DA.. 2013. Graduated effects of high-altitude hypoxia and highland ancestry on birth size. Pediatr Res. 74(6):633–638. [DOI] [PubMed] [Google Scholar]
- Stembridge M, Williams AM, Gasho C, Dawkins TG, Drane A, Villafuerte FC, Levine BD, Shave R, Ainslie PN.. 2019. The overlooked significance of plasma volume for successful adaptation to high altitude in Sherpa and Andean natives. Proc Natl Acad Sci U S A. 116(33):16177–16179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF. 2016. Hemoglobin-oxygen affinity in high-altitude vertebrates: is there evidence for an adaptive trend? J Exp Biol. 219(Pt 20):3190–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF. 2019. Hemoglobin: insights into protein structure, function, and evolution. Oxford: Oxford University Press. [Google Scholar]
- Storz JF, Bridgham JT, Kelly SA, Garland T. Jr.. 2015. Genetic approaches in comparative and evolutionary physiology. Am J Physiol Regul Integr Comp Physiol. 309(3):R197–R214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Cheviron ZA.. 2016. Functional genomic insights into regulatory mechanisms of high-altitude adaptation. Adv Exp Med Biol. 903:113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Cheviron ZA.. 2021. Physiological genomics of adaptation to high-altitude hypoxia. Annu Rev Anim Biosci. 9:149–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Cheviron ZA, McClelland GB, Scott GR.. 2019. Evolution of physiological performance capacities and environmental adaptation: insights from high-elevation deer mice (Peromyscus maniculatus). J Mammal. 100(3):910–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Kelly JK.. 2008. Effects of spatially varying selection on nucleotide diversity and linkage disequilibrium: insights from deer mouse globin genes. Genetics 180(1):367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Natarajan C, Cheviron ZA, Hoffmann FG, Kelly JK.. 2012. Altitudinal variation at duplicated β-globin genes in deer mice: effects of selection, recombination, and gene conversion. Genetics 190(1):203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Runck AM, Moriyama H, Weber RE, Fago A.. 2010. Genetic differences in hemoglobin function between highland and lowland deer mice. J Exp Biol. 213(Pt 15):2565–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Runck AM, Sabatino SJ, Kelly JK, Ferrand N, Moriyama H, Weber RE, Fago A.. 2009. Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc Natl Acad Sci U S A. 106(34):14450–14455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Scott GR.. 2019. Life ascending: mechanism and process in physiological adaptation to high-altitude hypoxia. Annu Rev Ecol Evol Syst. 50:503–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Scott GR.. 2021. Phenotypic plasticity, genetic assimilation, and genetic compensation in hypoxia adaptation of high-altitude vertebrates. Comp Biochem Physiol Part A Mol Integre Physiol. 253:110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Scott GR, Cheviron ZA.. 2010. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J Exp Biol. 213(Pt 24):4125–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SF, Droma TS, Zhang JG, Tao JX, Huang SY, McCullough RG, McCullough RE, Reeves CS, Reeves JT, Moore LG.. 1990. Greater maximal O2 uptakes and vital capacities in Tibetan than Han residents of Lhasa. Respir Physiol. 79(2):151–161. [DOI] [PubMed] [Google Scholar]
- Tashi T, Reading NS, Wuren T, Zhang X, Moore LG, Hu H, Tang F, Shestakova A, Lorenzo F, Burjanivova T, et al. 2017. Gain-of-function EGLN1 prolyl hydroxylase (PHD2 D4E: c 127S) in combination with EPAS1 (HIF-2α) polymorphism lowers hemoglobin concentration in Tibetan highlanders. J Mol Med. 95(6):665–670. [DOI] [PubMed] [Google Scholar]
- Tate KB, Ivy CM, Velotta JP, Storz JF, McClelland GB, Cheviron ZA, Scott GR.. 2017. Circulatory mechanisms underlying adaptive increases in thermogenic capacity in high-altitude deer mice. J Exp Biol. 220(Pt 20):3616–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate KB, Wearing OH, Ivy CM, Cheviron ZA, Storz JF, McClelland GB, Scott GR.. 2020. Coordinated changes across the O2 transport pathway underlie adaptive increases in thermogenic capacity in high-altitude deer mice. Proc Biol Sci. 287(1927):20192750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CR, Weibel ER, Weber JM, Vock R, Hoppeler H, Roberts TJ, Brichon G.. 1996. Design of the oxygen and substrate pathways.1. Model and strategy to test symmorphosis in a network structure. J Exp Biol. 199:1643–1649. [DOI] [PubMed] [Google Scholar]
- Tufts DM, Natarajan C, Revsbech IG, Projecto-Garcia J, Hoffman FG, Weber RE, Fago A, Moriyama H, Storz JF.. 2015. Epistasis constrains mutational pathways of hemoglobin adaptation in high-altitude pikas. Mol Biol Evol. 32(2):287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velotta JP, Jones J, Wolf CJ, Cheviron ZA.. 2016. Transcriptomic plasticity in brown adipose tissue contributes to an enhanced capacity for nonshivering thermogenesis in deer mice. Mol Ecol. 25(12):2870–2886. [DOI] [PubMed] [Google Scholar]
- Velotta JP, Robertson CE, Schweizer RM, McClelland GB, Cheviron ZA.. 2020. Adaptive shifts in gene regulation underlie a developmental delay in thermogenesis in high-altitude deer mice. Mol Biol Evol. 37(8):2309–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte FC, Cardenas R, Monge-C C.. 2004. Optimal hemoglobin concentration and high altitude: a theoretical approach for Andean men at rest. J Appl Physiol. 96(5):1581–1588. [DOI] [PubMed] [Google Scholar]
- Wagner PD. 1996. A theoretical analysis of factors determining VO2MAX at sea level and altitude. Respir Physiol. 106(3):329–343. [DOI] [PubMed] [Google Scholar]
- Wearing OH, Ivy CM, Gutierrez-Pinto N, Velotta JP, Campbell-Staton SC, Natarajan C, Cheviron ZA, Storz JF, Scott GR.. 2020. The adaptive benefit of increases in hemoglobin-O2 affinity is contingent on tissue O2 diffusing capacity in high-altitude deer mice. bioRxiv. doi.org/10.1101/2020.10.29.357665. [DOI] [PMC free article] [PubMed]
- Weibel ER, Taylor CR, Hoppeler H.. 1991. The concept of symmorphosis: a testable hypothesis of structure-function relationship. Proc Natl Acad Sci U S A. 88(22):10357–10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JB. 2006. Human responses to extreme altitudes. Integr Comp Biol. 46(1):25–34. [DOI] [PubMed] [Google Scholar]
- Wilson MJ, Lopez M, Vargas M, Julian C, Tellez W, Rodriguez A, Bigham A, Armaza JF, Niermeyer S, Shriver M, et al. 2007. Greater uterine artery blood flow during pregnancy in multigenerational (Andean) than shorter-term (European) high-altitude residents. Am J Physiol Regul Integr Comp Physiol. 293(3):R1313–R1324. [DOI] [PubMed] [Google Scholar]
- Witt KE, Huerta-Sanchez E.. 2019. Convergent evolution in human and domesticate adaptation to high-altitude environments. Philos Trans R Soc Lond B Biol Sci. 374(1777):20180235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TY, Wang XQ, Wei CY, Cheng HW, Wang XZ, Li Y, Ge D, Zhao HN, Young P, Li GL, et al. 2005. Hemoglobin levels in Qinghai-Tibet: different effects of gender for Tibetans vs. Han. J Appl Physiol. 98:598–604. [DOI] [PubMed] [Google Scholar]
- Xiang K, Ouzhuluobu Peng Y, Yang Z, Zhang X, Cui C, Zhang H, Li M, Zhang YBianba, et al. 2013. Identification of a Tibetan-specific mutation in the hypoxic gene EGLN1 and its contribution to high-altitude adaptation. Mol Biol Evol. 30:1889–1898. [DOI] [PubMed] [Google Scholar]
- Xin JX, Zhang H, He YX, Duren Z, Bai CJ, Chen L, Luo X, Yan DS, Zhang CY, Zhu X, et al. 2020. Chromatin accessibility landscape and regulatory network of high-altitude hypoxia adaptation. Nat Commun. 11:4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Wuren T, Simonson TS, Watkins WS, Witherspoon DJ, Wu W, Qin G, Huff CD, Jorde LB, Ge R-L.. 2013. Genomic analysis of natural selection and phenotypic variation in high-altitude Mongolians. PLoS Genet. 9(7):e1003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Li S, Yang Y, Tan J, Lou H, Jin W, Yang L, Pan X, Wang J, Shen Y, et al. 2011. A genome-wide search for signals of high-altitude adaptation in Tibetans. Mol Biol Evol. 28(2):1003–1011. [DOI] [PubMed] [Google Scholar]
- Yang J, Jin Z-B, Chen J, Huang X-F, Li X-M, Liang Y-B, Mao J-Y, Chen X, Zheng Z, Bakshi A, et al. 2017. Genetic signatures of high-altitude adaptation in Tibetans. Proc Natl Acad Sci U S A. 114(16):4189–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZXP, Pool JE, Xu X, Jiang H, Vinckenbosch N, Korneliussen TS, et al. 2010. Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329:75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon D, Ponka P, Prchal JT.. 2011. Hypoxia. 5. Hypoxia and hematopoiesis. Am J Physiol Cell Physiol Cell Physiol. 300(6):C1215–C1222. [DOI] [PubMed] [Google Scholar]
- Zamudio S, Droma T, Norkyel KY, Acharya G, Zamudio JA, Niermeyer SN, Moore LG.. 1993. Protection from intrauterine growth retardation in Tibetans at high altitude. Am J Phys Anthropol. 91(2):215–224. [DOI] [PubMed] [Google Scholar]
- Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C, Moore LG.. 1995. Effect of altitude on uterine artery blood flow during normal pregnancy. J Appl Physiol. 79(1):7–14. [DOI] [PubMed] [Google Scholar]
- Zhu X, Guan Y, Signore AV, Natarajan C, DuBay SG, Cheng Y, Han NJ, Song G, Qu Y, Moriyama H, et al. 2018. Divergent and parallel routes of biochemical adaptation in high-altitude passerine birds from the Qinghai-Tibet Plateau. Proc Natl Acad Sci U S A. 115(8):1865–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Droma T, Sun S, Janes C, McCullough RE, McCullough RG, Cymerman A, Huang SY, Reeves JT, Moore LG.. 1993. Hypoxic ventilatory responsiveness in Tibetan compared with Han residents of 3,658 m. J Appl Physiol. 74(1):303–311. [DOI] [PubMed] [Google Scholar]