Abstract
Background
Heart transplant recipients represent a particularly vulnerable patient population to the novel coronavirus disease 2019 (COVID-19) due to chronic immunosuppression and high rates of comorbidities. Currently, data are limited and evidence to guide management of heart transplant recipients with COVID-19 is sparse. In this case report, we provide a summary of the current literature as well as an in-depth analysis of our clinical decision-making.
Case summary
A 67-year-old female who underwent cardiac transplantation 1 year prior was found to have acute hypoxic respiratory failure due to COVID-19. Her immunosuppressant medications were modulated with discontinuation of mycophenolate and titration of tacrolimus troughs with a goal of 6–10 ng/dL. She was administered supportive treatment including convalescent plasma, remdesivir, and dexamethasone, in addition to antibiotic treatment that resulted in resolution of her symptoms within a matter of days despite her precarious disposition.
Discussion
This case demonstrates that it can be safe and efficacious to modulate immunosuppressant medications in cardiac transplant recipients in accordance with recommendations made by the International Society of Heart and Lung Transplantation. This case additionally demonstrates that aspects of the current literature regarding the management of COVID-19 can be safely extrapolated to cardiac transplant recipients. Providing supportive care with dexamethasone, remdesivir, and convalescent plasma as indicated can be beneficial in cardiac transplant recipients; although, the current literature regarding convalescent plasma and remdesivir is conflicting.
Keywords: COVID-19, Cardiac transplantation, Immunosuppressant medications, Case report
Learning points
Modifying immunosuppressant agents in line with recommendations made by International Society of Heart and Lung Transplantation can be safe and efficacious.
Due to the lack of data regarding management of coronavirus disease 2019 infection in cardiac transplant patients, cautious extrapolation of the current literature may be made.
Providing supportive care with dexamethasone, remdesivir, antibiotic therapy, and convalescent plasma as indicated can be beneficial in cardiac transplant recipients; although, the current literature regarding convalescent plasma is conflicting.
Introduction
Heart transplant recipients represent a particularly vulnerable patient population to coronavirus disease 2019 (COVID-19) because of chronic immunosuppression and high rates of comorbidities.1,2 Due to impaired immune defences from both underlying disease and treatment, immunocompromised patients with viral respiratory infections are at increased risk of more severe infection and increased rates of bacterial and fungal superinfection compared with their immunocompetent counterparts.3 Data regarding mortality in solid organ transplant patients and concomitant COVID-19 infection have been found to be similar to the general population; however, data are conflicting.4–6 Here, we provide a case report and discussion of a patient who previously underwent a cardiac transplantation and was found to be in acute hypoxic respiratory failure due to COVID-19.
Timeline
| 6 days prior to admission | Patient began experiencing symptoms of coronavirus disease 2019 (COVID-19) infection |
| 4 days prior to admission | Patient found to be COVID-19 positive |
| Hospital Day 1 | Patient having worsening symptoms of COVID-19 infection, requiring oxygen and hospitalization. Patient started on Intravascular fluids as well as supportive management for COVID-19 including convalescent plasma |
| Hospital Day 2 | Patient started on remdesivir. Her immunosuppressant regimen was adjusted in line with the International Society of Heart and Lung Transplantation |
| Hospital Day 6 | Patient clinically improving significantly not requiring oxygen |
| Hospital Day 7 | Patient was safely discharged home with instructions to continue isolation and follow-up with her primary and transplant physician to discuss the possibility of restarting her home immunosuppressant regimen |
Case presentation
A 67-year-old female with a prior history of cardiac transplant 1 year prior, on an immunosuppressant regimen, presented to University Medical Center (UMC) with worsening shortness of breath. Her symptoms of fever, headaches, and myalgias had started 6 days prior. Two days later, she was seen in clinic and diagnosed with COVID-19. At that time, she was paucisymptomatic and was sent home with over the counter medications. Her immunosuppressive regimen included mycophenolate 250 mg twice daily and tacrolimus 9 mg daily. On the day of admission to UMC, her symptoms of cough and dyspnoea worsened acutely. Her dyspnoea had become so severe that she was unable to walk around her home or take care of her activities of daily living.
In the emergency room, she was found to be dyspnoeic at rest requiring 4–5 L per minute nasal cannula to maintain saturations >90%. Her physical exam was unremarkable other than ‘mildly distant heart sounds, and globally diminished breath sounds, no rales, or wheezing noted’.
Labs were drawn that demonstrated leukopenia (2.7 k/mm3), lymphocytopenia (absolute lymphocyte count 0.72 k/mm3), and elevated creatinine (1.67 mg/dL). Furthermore, she was found to have elevated inflammatory markers (Table 1). Since the D-dimer was mildly elevated (0.62 mg/L), she was started on a prophylactic dose of enoxaparin 40 mg twice daily. Repeat testing confirmed COVID-19 infection. Troponins were negative. Brain natriuretic peptide (BNP) was elevated 136 pg/mL. Blood and respiratory cultures were drawn that demonstrated no growth throughout her admission.
Table 1.
Laboratory tests throughout admission
| Laboratory findings | Reference | Day 1 | Day 3 | Day 5 | Day 6 |
|---|---|---|---|---|---|
| Ferritin (ng/mL) | 7.0–271 | 207 | 225 | 161 | 139 |
| D-dimer (mg/L) | <0.49 | 0.41 | 0.62 (H) | 0.52 (H) | 0.61 (H) |
| C-reactive protein (mg/L) | <3 | 68.4(H) | 20.2(H) | — | 3.1 (H) |
| Lactate dehydrogenase (U/L) | 125–243 | 426 (H) | 437(H) | — | 320 (H) |
| Procalcitonin (ng/mL) | <0.04 | 0.06 (H) | — | — | <0.04 |
| Troponin I (ng/mL) | 0.02–0.04 | <0.006 | — | — | — |
| Creatinine (mg/dL) | 0.55–1.30 | 1.67 (H) | 1.25 | 1.18 | 1.17 |
| B-type natriuretic peptide (pg/mL) | <99 | 136 (H) | — | — | — |
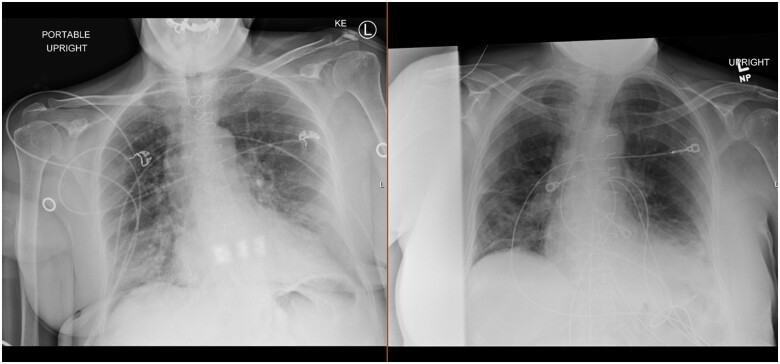
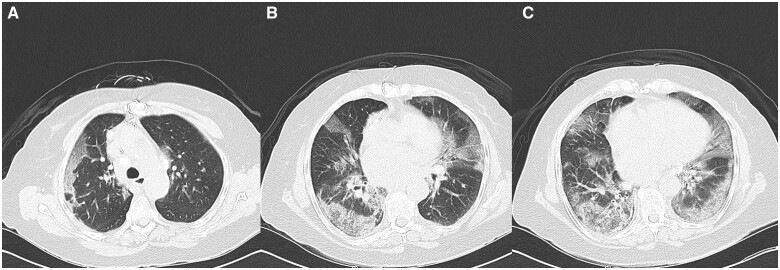
Chest X-ray demonstrated new increased density in the right lower lung concerning for infiltrate as well as central vascular prominence (Figure 1). A chest computed tomography (CT) was ordered to evaluate her developing COVID-19 pneumonia (Figure 2). Chest CT demonstrated diffuse peripheral and lower lung predominant ground-glass opacities, with some underlying septal thickening. Transthoracic echocardiogram demonstrated a normal left ventricular ejection fraction 55–60% and normal right ventricular systolic function.
Figure 1.
Left is from hospital admission Day 1 and right is from hospital admission Day 6 on completing course of treatment.
Figure 2.
(A) Computed tomography scan mid-thorax; (B) computed tomography scan of mid-thorax; (C) computed tomography scan base of thorax.
She was admitted to the coronary care unit because of her history of heart transplant and concern for progression of her acute hypoxic respiratory failure given her COVID-19 status. The case was discussed with infectious disease who initially held remdesivir given her elevated creatinine. She was instead given convalescent plasma, dexamethasone, and started on empiric antibiotics for possible community-acquired pneumonia. Clinically she appeared dehydrated; maintenance fluids were thus administered and resulted in improvement of her renal function back to baseline. The very next day, she was started on a course of remdesivir for 5 days.
Mycophenolate mofetil was stopped in line with recommendations from the International Society of Heart and Lung Transplantation (ISHLT). Initial tacrolimus trough level was found to be supratherapeutic (12.5 ng/mL), so it was initially held as well. Throughout the course of her admission, her renal function improved and her tacrolimus levels were adjusted to goal target of 6–10 ng/mL. Her leukopenia resolved; however, she continued to have a persistent lymphocytopenia consistent with her COVID-19 infection. Inflammatory markers trended downwards (Table 1).
She completed her courses of remdesivir and empiric antibiotics, and was weaned from oxygen completely. Repeat chest X-ray demonstrated significant improvement (Figure 1). She was discharged home with instructions to complete 20 days of isolation per recommendations by infectious disease. She was additionally instructed to follow-up with her primary care and transplant physicians to discuss restarting her home regimen of mycophenolate mofetil. She was discharged with dexamethasone 6 mg daily and tacrolimus extended release 8 mg daily.
Discussion
Herein, we report a case of an immunosuppressed heart transplant recipient who was found to be in acute hypoxic respiratory failure due to a COVID-19 infection. Due to her worsening clinical picture, she was started on a number of supportive measures in addition to titration of her immunosuppressant medications to help enhance her recovery. The case highlights a possible treatment strategy for patients with COVID-19 in the setting of an immunocompromised transplant recipient.
It is not uncommon to adjust immunosuppressant medications in the setting of viral infections.7 According to the guidance by the ISHLT, at this time there is no evidence to guide decisions regarding the use of COVID-19 strategies specifically in patients with thoracic transplant.8 A specific treatment for COVID-19 remains unavailable; therefore, the initial clinical management is based on supportive care including antibiotic therapy, oxygen supply, pausing of mycophenolate as described in ‘Guidance for cardiothoracic transplant and ventricular assist device centers regarding the SARS CoV-2 pandemic by the ISHLT’, and transition from sirolimus to tacrolimus; though specific data are lacking at this time.9,10
Results of a systematic review of SARS-CoV-1 highlights two in vitro studies that showed a potential benefit to tacrolimus by demonstrating that cell lines treated with tacrolimus inhibited viral replication of SARS-CoV-1 and resulted in a reduction of viral titres to undetectable levels.11 Tacrolimus has been found to not only inhibit lymphocyte activation, but to also effectively down-regulate the infiltration of inflammatory cells, which may be beneficial in the treatment of COVID-19.12,13 Mycophenolate, on the other hand, has been associated with more viral load and even fatal disease in in vivo studies.14 Additionally, mycophenolate has a number of drug–drug interactions that may complicate recovery.
Due to the lack of evidence to guide decision-making regarding the use of SARS-CoV-2 treatment strategies in patients with thoracic transplant, the ISHLT recommends careful extrapolation of current published data to help guide treatment in heart transplant recipients with concomitant SARS-CoV-2 infection. In accordance, we carefully extrapolated recommendations from the COVID-19 Guidelines Recommendation Panel of the National Institute of Health, providing our patient with a number of supportive measures including dexamethasone and remdesivir. Although we administered remdesivir, the World Health Organization has recently issued a conditional recommendation against its use in hospitalized patients, as there is currently no evidence that remdesivir improves survival and other outcomes in these patients. At the time of treatment, the COVID-19 Guidelines Panel was recommending for the use of convalescent plasma. Although we administered convalescent plasma, the COVID-19 guidelines panel recently updated their guidelines regarding convalescent plasma recommending against its use. Additionally, we adjusted her immunosuppressive medications in accordance with ISHLT’s recommendations allowing her to mount an inflammatory response without triggering significant inflammatory storm or acute rejection. Additionally, our patient’s D-dimer was elevated throughout her admission; however, she was not started on therapeutic anticoagulation because it was felt that the risks outweighed the benefit given her mild elevation in D-dimer. Instead, she was started on prophylactic enoxaparin 40 mg twice daily. Because of the prognostic role troponin and BNP play in COVID-19, these laboratories were drawn, however, were found to be unremarkable and only mildly elevated.15 These measures led to her clinical improvement and ultimately a positive outcome despite her precarious disposition.
In conclusion, more literature is required to determine if modulating immunosuppressant medications, as aligned with ISHLT’s recommendations, in heart transplant recipients experiencing acute hypoxic respiratory failure due to COVID-19 is safe and efficacious.
Lead author biography

Ariyon Schreiber performed his undergraduate education at the University of California, San Diego in Biochemistry and Cell Biology. He attended medical school in Michigan at Oakland University-William Beaumont School of Medicine. Currently, he is training in internal medicine at the University of Nevada, Las Vegas. His current research interests include COVID-19 and cardiovascular disease.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
The authors thank Dr. Yassin Naga, Dr. Christopher Nguyen, Dr. Brooke Lentz, Dr. Matthew Klinka, Dr. Jimmy Diep, and Jacky Schreiber.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
References
- 1. Latif F, Farr MA, Clerkin KJ, Habal MV, Takeda K, Naka Y. et al. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol 2020;5:e202159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singhvi A, Barghash M, Lala A, Mitter SS, Parikh A, Oliveros E. et al. Challenges in heart transplantation during COVID-19: a single-center experience. J Heart Lung Transplant 2020;39:894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manuel O, Estabrook M; American Society of Transplantation Infectious Diseases Community of Practice. RNA respiratory viral infections in solid organ transplant recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019;33:e13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rinaldi M, Bartoletti M, Bussini L, Pancaldi L, Pascale R, Comai G. et al. COVID-19 in solid organ transplant recipients: no difference in survival compared to general population. Transpl Infect Dis 2021;23:e13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miarons M, Larrosa-García M, García-García S, Los-Arcos I, Moreso F, Berastegui C. et al. ; on behalf of the Vall d’Hebron COVID-19 Working Group. COVID-19 in solid organ transplantation: a matched retrospective cohort study and evaluation of immunosuppression management. Transplantation 2021;105:138–150. [DOI] [PubMed] [Google Scholar]
- 6. Kates OS, Haydel BM, Florman SS, Rana MM, Chaudhry ZS, Ramesh MS. et al. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis 2020;ciaa1097. doi:10.1093/cid/ciaa1097.32766815 [Google Scholar]
- 7. Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L. et al. ; The Transplantation Society International CMV Consensus Group. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2018;102:900–931. [DOI] [PubMed] [Google Scholar]
- 8. Lee H, Mantell BS, Richmond ME, Law SP, Zuckerman WA, Addonizio LJ. et al. Varying presentations of COVID-19 in young heart transplant recipients: a case series. Pediatr Transplant 2020;24:e13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mathies D, Rauschning D, Wagner U, Mueller F, Maibaum M, Binnemann C. et al. A case of SARS-CoV-2 pneumonia with successful antiviral therapy in a 77-year-old man with a heart transplant. Am J Transplant 2020;20:1925–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aslam S, Grossi P, Teuteberg J, Adler E, Farrero M, Frigerio M. et al. Guidance for Cardiothoracic Transplant and Ventricular Assist Device Centers Regarding the SARS CoV-2 Pandemic. International Society of Heart & Lung Transplantation. 2020. [Google Scholar]
- 11. Russell B, Moss C, George G, Santaolalla A, Cope A, Papa S. et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience 2020;14:1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kobashigawa JA, Miller LW, Russell SD, Ewald GA, Zucker MJ, Goldberg LR. et al. ; the Study Investigators. Tacrolimus with mycophenolate mofetil (MMF) or sirolimus vs. cyclosporine with MMF in cardiac transplant patients: 1-year report. Am J Transplant 2006;6:1377–1386. [DOI] [PubMed] [Google Scholar]
- 13. Pereira R, Medeiros YS, Fröde TS.. Antiinflammatory effects of tacrolimus in a mouse model of pleurisy. Transpl Immunol 2006;16:105–111. [DOI] [PubMed] [Google Scholar]
- 14. Allison AC, Eugui EM.. Preferential suppression of lymphocyte proliferation by mycophenolic acid and predicted long-term effects of mycophenolate mofetil in transplantation. Transplant Proc 1994;26:3205–3210. [PubMed] [Google Scholar]
- 15. Arcari L, Luciani M, Cacciotti L, Musumeci MB, Spuntarelli V, Pistella E. et al. Incidence and determinants of high-sensitivity troponin and natriuretic peptides elevation at admission in hospitalized COVID-19 pneumonia patients. Intern Emerg Med 2020;15:1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.