Abstract
Populations of Escherichia coli selected in constant and fluctuating environments containing lactose often adapt by substituting mutations in the lacI repressor that cause constitutive expression of the lac operon. These mutations occur at a high rate and provide a significant benefit. Despite this, eight of 24 populations evolved for 8,000 generations in environments containing lactose contained no detectable repressor mutations. We report here on the basis of this observation. We find that, given relevant mutation rates, repressor mutations are expected to have fixed in all evolved populations if they had maintained the same fitness effect they confer when introduced to the ancestor. In fact, reconstruction experiments demonstrate that repressor mutations have become neutral or deleterious in those populations in which they were not detectable. Populations not fixing repressor mutations nevertheless reached the same fitness as those that did fix them, indicating that they followed an alternative evolutionary path that made redundant the potential benefit of the repressor mutation, but involved unique mutations of equivalent benefit. We identify a mutation occurring in the promoter region of the uspB gene as a candidate for influencing the selective choice between these paths. Our results detail an example of historical contingency leading to divergent evolutionary outcomes.
Keywords: adaptation; epistasis; gene expression; lac operon, experimental evolution
Introduction
“I am [speaking] of the central principle of all history—contingency. A historical explanation [rests] on an unpredictable sequence of antecedent states, where any major change in any step of the sequence would have altered the final result. This final result is therefore dependent, or contingent, upon everything that came before—the unerasable and determining signature of history.”
—Stephen J. Gould (1989) Wonderful Life, p. 283.
“Life is just one damned thing after another!”
—Lilian Bell (1909) The Concentrations of Bee, p. 241. The expression was used widely at that time.
Gould famously argued that the outcomes of biological evolution are often causally dependent on the precise series of changes that have occurred in the evolving lineages (Gould 1989; Blount et al. 2008, 2012; Zee et al. 2014). One possible basis for such historical contingency are epistatic interactions between mutations in determining fitness. Long-term evolution can be thought of as consisting of “one damned substitution after another,” to paraphrase the popular expression from 1909. If the effects of different mutations on fitness are completely independent of each other (i.e., if there is no epistasis for fitness), then later substitutions will not be causally dependent on earlier ones, and there will be no meaningful contingency. If, however, a mutation confers a benefit only in the presence of certain earlier mutations (i.e., if there is epistasis for fitness), then evolution will be historically contingent (Blount et al. 2018). In that case, the presence of a particular mutation determines the range of subsequent mutations that are likely to be favored. Epistasis for fitness means that even closely related populations might respond differently and unpredictably to similar selection pressures, depending on the substitutions they undergo, exactly as Gould envisaged (Blount et al. 2008; Wang et al. 2013; Harms and Thornton 2014; Spor et al. 2014; Zee et al. 2014; Shah et al. 2015; Phillips et al. 2016; Peng et al. 2018).
Historical contingency is difficult to examine because it requires disentangling the roles of chance and natural selection in determining evolutionary outcomes (Blount et al. 2018). One approach to this is to construct actual and alternative evolutionary pathways and determine the dependence of key phenotypes on the order in which mutations occur. This approach has been followed in several studies examining evolution of single genes, typically finding that epistasis creates a strong dependence of evolutionary outcome on the order in which mutations occur (Weinreich et al. 2006; Ogbunugafor and Hartl 2016; Starr et al. 2017). At the level of whole organisms, this approach is not generally feasible if large numbers of mutations have occurred. Instead, it is possible to use controlled laboratory experimental evolution to examine the evolutionary outcomes of replicated populations and determine statistically a signature of historical contingency (Blount et al. 2008, 2012; Zee et al. 2014).
Several experimental evolution studies have found that adaptive potential differs between different genotypes, often, at least in part, explained by differences in their initial fitness (Travisano et al. 1995; Barrick et al. 2010; Bedhomme et al. 2013; Kryazhimskiy et al. 2014; Perfeito et al. 2014; Wünsche et al. 2017; Johnson et al. 2019). The strong influence of fitness on mutation effect is supported by work that has directly measured the effect of adding specific mutations to diverse genetic backgrounds (Pearson et al. 2012; Wang et al. 2013; Kryazhimskiy et al. 2014; Wang et al. 2016). Nevertheless, specific genetic differences between founding strains can be influential (Blount et al. 2008, 2012; Zee et al. 2014). Indeed, historical contingency can affect replicate populations started from a common ancestor and evolved in a common environment (Collins and Bell 2004; Cooper and Lenski 2010; Wiser et al. 2013). In this case, contingency builds on chance differences in the origination and fixation of mutations. One example is the evolution of citrate utilization in an Escherichia coli long-term evolution experiment. In that work, one of 12 replicate populations evolved the ability to use citrate (Blount et al. 2008). Subsequent genetic analysis revealed that evolution of citrate utilization is rare because it depends on a series of prior mutational events (Quandt et al. 2014; Leon et al. 2018). Other examples of historical contingency have been inferred by finding genetically or phenotypically distinct evolutionary outcomes either dependent on early occurring mutations (Yedid and Bell 2002; Meyer et al. 2012) or from analysis of the total suite of changes occurring in evolved populations (Tenaillon et al. 2012).
We examine the basis of divergent evolution in lacI, the repressor of the lac operon genes that confer the ability to utilize lactose. Mutations in lacI fixed or rose to high frequency in 16 of 24 populations evolved for 8,000 generations in a long-term experiment that evolved populations of E. coli in environments containing combinations of glucose and lactose (Cooper and Lenski 2010; Satterwhite and Cooper 2015) (fig. 1A and supplementary fig. S1, Supplementary Material online). Absence of repressor mutations in the remaining eight populations was surprising because lacI mutations confer a large fitness benefit in the ancestor (fig. 1B) and occur at a high rate, due to the presence of a mutational hotspot (Farabaugh et al. 1978). Evolutionary simulations incorporating the measured lacI− mutation rate and fitness effect indicate that this divergence cannot be attributed to chance differences in mutation timing and success. When we added a lacI− mutation to strains isolated from populations that did not fix it, we found that it no longer confers any benefit, and, in fact, is often deleterious, indicating that it interacts negatively with previous substitutions. These results demonstrate that divergent adaptation in the lac operon is common and is due to effects contingent on previous adaptations.
Fig. 1.

lacI effect and distribution in 8,000 generation evolved populations. (A) Rows represent each of four evolution environments (Lac [lactose], G/L [alternating daily between glucose and lactose], G_L [alternating every 2,000 generations between glucose and lactose, starting with glucose], L_G [alternating every 2,000 generations between glucose and lactose, starting with lactose]) and columns the six replicate populations evolved in each environment. Details are in Materials and Methods. Dark blue cells indicate fixation of the lacI− mutation, light blue cells indicate a mixed population with lacI− still segregating, and white cells indicate that lacI− was undetectable. Details of screening are in Materials and Methods. (B) Fitness effect of the lacI− mutation added to the ancestor and assessed in three evolution environments relative to the original ancestor (following method described in Materials and Methods). Symbols show mean and lines 95% CIs of at least three replicate competitions in each treatment.
Results
Mutations in lacI Occur in about Half of Evolved Populations
We previously found that loss-of-function mutations in the lac operon repressor, lacI, were common in nine of 12 populations of E. coli evolved for 2,000 generations in environments either containing lactose (Lac) only or alternating daily between lactose and glucose (G/L) (Quan et al. 2012). Here, we find that after extension for a further 6,000 generations of evolution, lacI− mutations had fixed or become common in two more of these populations and in five of 12 derived populations that alternated between glucose and lactose every 2,000 generations (denoted G_L and L_G where the first letter indicates the sugar initially present during selection; fig. 1A). The absence of detectable lacI− mutations in eight of the 24 populations was surprising because a mutational hotspot in lacI causes inactivating frameshift mutations to occur at a high rate and those mutations provide a benefit in lactose-containing environments that outweigh an associated cost in glucose (Farabaugh et al. 1978; fig. 1). Herafter, we denote evolved populations that did and did not fix a lacI− mutation EvlacI− and EvlacI+, respectively.
A possible explanation for the failure of lacI− mutations to fix after 8,000 generations in multiple populations is that an earlier occurring substitution(s) reduced either the rate at which lacI− mutations occur or the benefit they confer when they do arise. We refer to this possibility as the contingency hypothesis. An alternative, noncontingent explanation is possible, however. We examine these hypotheses below.
Simulations Predict More lacI− Substitutions than Observed
An alternative to the contingency hypothesis is that lacI− mutations, although beneficial, have simply not occurred yet, or have occurred but have not yet had the time to reach high frequency. To evaluate this possibility, we simulated the evolution of populations with lacI− mutations available alongside a background pool of other beneficial and deleterious mutations (fig. 2). We used an individual-based model, incorporating estimates of mutation rates and effects, and allowing for competition between mutations (see Materials and Methods for details). Our results show that, as populations become larger, the frequency of substitution (fs) for a lacI− mutation rises quickly. With an effective population size of 105 individuals, lacI− fixed in most simulated populations (fs = 91.5% combining Lac, G_L and L_G selection regimes). The observed frequency in experimental populations was only fs = 12/24 = 50% (95% CI: 31.4–68.6%) despite the fact that they were much larger (Ne = 3.3 × 107) (Cooper and Lenski 2010). This difference is statistically significant (binomial test: P < 0.001). Thus, stochastic variation in mutation timing and competition cannot explain the high number of experimental populations retaining the ancestral lacI allele.
Fig. 2.
Simulated frequency of lacI− substitution (fs). Simulations were modeled in Lac and extrapolated to apply to G_L and L_G 2,000-generation fluctuating environments as detailed in Materials and Methods. Symbols indicate the proportion of replicate simulations (n = 100 except at Ie = 5 × 104 [n = 50] and Ne = 105 [n = 20]) in which lacI− fixed. Error bars are 95% CI. Means and errors were calculated using 1,000 boot-strapped samples. In the Lac treatment, populations were simulated for 8,000 generations. For G_L and L_G 2,000 generation fluctuating environments, the same simulated populations were sampled at 2,000 generations and frequency of lacI− substitution (fs) was calculated as detailed in Materials and Methods. The dashed line indicates the overall frequency of lacI− fixation at 8,000 generations among 24 experimentally evolved populations. Note that the two treatments are plotted with a small offset to avoid overplotting.
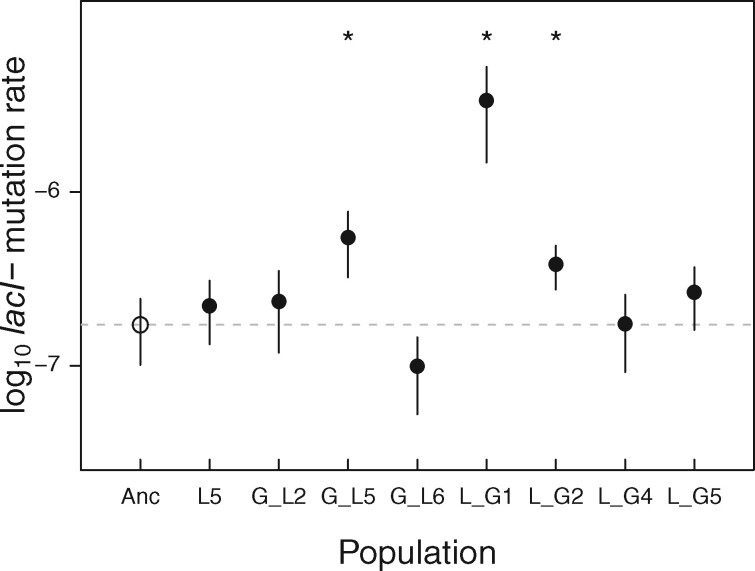
Evolved Clones Have Similar lacI− Mutation Rates to Ancestor
A second hypothesis for lacI− mutations failing to fix in some populations is that their mutation rate was lower in those populations. Indeed, mutation at the lacI hotspot involves a strand slippage mechanism that might be affected by DNA topology, which is a common target of selection in an experiment closely related to this one (Blount et al. 2008, 2012; Zee et al. 2014). To the extent that a potential change in lacI− mutation rate depends on previously substituted mutations, this possibility represents an example of contingency. We estimate the ancestral lacI− mutation rate at 1.72 ± 0.7 × 10−7 per cell per generation (95% CI), which is consistent with a previous estimate (Schaaper et al. 1986). If EvlacI+ populations evolved lower lacI− mutation rates, expected fixation times would be increased. In fact, five of eight clones isolated from EvlacI+ populations had lacI− mutation rates indistinguishable from the ancestor (fig. 3). The remaining clones had an elevated lacI− mutation rate. In the case of the L_G1 population, this elevation was substantial, presumably due to the presence of a 27-bp deletion in the mutS gene, which is likely to result in a general increase in the genomic mutation rate (supplementary table S1, Supplementary Material online).
Fig. 3.
Mutation rate to lacI− for 8,000 generation lacI+ev populations. Closed symbols indicate evolved populations and the open circle (and dashed line) indicates the mutation rate in the ancestor. Mean per locus mutation rates are calculated from n ≥ 10 replicates, and errors are 95% CIs. Asterisks indicate populations with a lacI mutation rate significantly different than the ancestor (t-test P < 0.05).
In summary, changes in mutation rate to lacI− do not explain why it has not fixed in so many evolved populations. We note, however, that this analysis does not exclude the possibility that populations might have evolved in a way causing beneficial mutations at loci other than lacI to occur at higher rates and that these mutations might outcompete mutations occurring at lacI (Gerrish and Lenski 1998). We consider this possibility unlikely, however, because it would delay but not prevent lacI− mutation substitutions. Moreover, results presented below support an alternative mechanism affecting lacI− fixation.
Fitness Effect of lacI− Is Lower in EvlacI+ Populations
To directly test for a change in the selective benefit of lacI− mutations as posited by the contingency hypothesis, we performed allelic replacement experiments to measure the fitness effect of lacI− mutations in clones isolated from all evolved populations. If the lacI− mutation was prevented from fixing due to epistasis, we expected it to be less beneficial, or even deleterious, in EvlacI+ populations.
We found that the lacI− mutation was beneficial in the 12 populations in which it fixed (fig. 4). Among clones isolated from populations selected in Lac, G_L, and L_G environments, the grand mean fitness effect of the lacI− mutation measured in the lactose environment is 9.66% (±2.29% [95% CI]), which is not significantly different from its effect in the ancestor (two-tailed t-test, P = 0.53). Among clones isolated from the G/L environment, the lacI− mutation conferred a significantly greater benefit than it did in the ancestor measured in that environment (8.50% vs. 4.05%, two-tailed t-test: P = 0.02). By contrast, the lacI− mutation conferred no benefit or was deleterious when introduced into clones isolated from the eight EvlacI+ populations, imposing a mean cost of 7% (±4.81% [95% CI]) when measured in lactose (compared with its effect in the ancestor, two-tailed t-test: P < 0.001) (fig. 4). These results reveal the presence of strong negative epistatic interactions between the lacI− mutation and one or more of the mutations that accumulated in the EvlacI+ populations, in agreement with the contingency hypothesis.
Fig. 4.
Fitness of lacI− in ancestor and 8,000 generation evolved populations. Solid symbols indicate the fitness measurements for the ancestor and for clones from populations evolved in lactose, G_L and L_G environments, which were measured in lactose. Hollow symbols indicate the ancestor and clones isolated from populations evolved in the G/L environment, which were measured in the G/L environment. Symbols indicate mean fitness estimates (n ≥ 3). Errors shown for ancestor are 95% CIs.
EvlacI+ Populations Have an Alternative Mechanism of Reducing Lag Time
To examine the basis of the different fitness effects of the lacI− mutation, we compared its effect on growth dynamics in the ancestor, and in EvlacI− and EvlacI+ clones. In the ancestor, the most striking effect of the lacI− mutation was a decrease in the time taken for the population to resume growth following transfer to fresh medium (i.e., its lag time) from 5.00 to 3.87 h (t-test: P < 0.001; fig. 5A and supplementary fig. S2, Supplementary Material online). This effect was also seen among EvlacI− clones (EvlacI− clones: lag = 2.96 h; lacI+ revertants: 5.60 h; paired t-test: P < 0.001; fig. 5B and supplementary fig. S2, Supplementary Material online). By contrast, the lacI− mutation increased lag time when added to EvlacI+ clones (EvlacI+ clones: lag = 2.96 h; lacI− derivatives: 3.11 h; paired t-test: P = 0.03; fig. 5C and supplementary fig. S2, Supplementary Material online). We found good agreement between direct competitions between lacI+/− strain pairs and virtual competitions based on growth curve parameters, indicating that growth parameters are meaningful measures of fitness components (supplementary fig. S2, Supplementary Material online).
Fig. 5.
Effect of lacI− mutation on growth dynamics of the ancestor and evolved clones. Red and blue lines indicate strains(s) with functional and nonfunctional lacI alleles, respectively. The green line in (D) indicates the ancestral lacI+ strain, as plotted in panel (A), and is included for reference. Shaded regions indicate SD (n ≥ 3). All growth curves performed in the lactose environment. (A) Ancestor with and without the lacI− mutation. The mutation significantly decreases lag time in the lactose assay environment. (B) Clones from EvlacI− populations. The lacI− mutation also decreases lag time in these populations. (C) Clones from EvlacI+ populations. The lacI− mutation confers a small cost when added to these clones. (D) Original evolved clones from EvlacI− and EvlacI+ populations. The shortened lag time dependent on the lacI− mutation in EvlacI− populations has been achieved through a different mutational mechanism in EvlacI+ populations. Lines indicate the mean of replicate growth curves for a single strain (A) or a grand mean of growth curves estimated for the multiple clones in the noted evolutionary group (B–D).
The absence of lacI− mutation benefit may be because EvlacI+ clones had substituted an alternative means of shortening lag time such that the effect of the lacI− mutation has become redundant. To test this possibility, we compared the lag times of EvlacI+ and EvlacI− clones. We found that lag times were indistinguishable, consistent with EvlacI+ populations having evolved an alternative means of shortening lag time (two-tailed t-test P = 0.96; fig. 5D).
EvlacI+ Populations Have Increased Sensitivity to a lac Operon Inducer
As a first step in examining the basis of the reduction of lag time among EvlacI+ populations, we measured changes in the expression of lac genes in environments with increasing concentrations of IPTG, a synthetic inducer. We found that clones from all EvlacI+ populations were more sensitive in responding to IPTG, reaching half maximum expression of LacZ, a gene product controlled by the LacI repressor, at a lower concentration of IPTG than the ancestor (supplementary fig. S4, Supplementary Material online). Qualitatively similar results were seen when we used an alternative inducer, TMG, and compared expression of a Plac-gfp reporter at 4 and 6 h following induction (supplementary fig. S5, Supplementary Material online). These findings are consistent with a higher sensitivity to IPTG and TMG inducers in the EvlacI+ strains.
An increase in inducer sensitivity could cause reduced lag times in EvlacI+ populations by allowing them to more quickly express lac genes following transfer to a fresh lactose supplemented environment. One mechanism through which this could occur is if basal lac expression was higher in EvlacI+ populations, which increases the probability that a cell will turn on lac operon expression and resume growth (Chu and Barnes 2016). However, in both the IPTG and TMG induction data experiments, basal lac expression appeared unchanged in EvlacI+ populations relative to the ancestor. We also considered the possibility of some change in the nature of lactose uptake. None of the evolved strains had mutations in the LacY permease protein so, to test the possibility that some other inducer import mechanism had evolved, we determined the reliance of lac induction on the LacY permease. To do this, we introduced a lacY loss-of-function mutation into the ancestor and the Lac5 EvlacI+ strain. (This strain evolved a strong antagonistic interaction with the lacI− mutation, see below.) We found that increased sensitivity of lac induction, in response to both lactose and TMG, was completely dependent on LacY (supplementary fig. S6, Supplementary Material online). We also found that TMG, but not lactose, was able to weakly induce lac genes independent of LacY, but that ability was not different between the ancestor and the Lac5 strain.
Reduced Benefit of lacI Mutations Is Caused by Mutations in uspB
To examine the nature of the mutations that interact with lacI− to reduce its benefit, we estimated the fitness effect of a lacI− mutation introduced into clones isolated at intervals along the evolutionary path of the eight EvlacI+ populations (fig. 6A). We reasoned that steep declines in lacI mutation effect would indicate the substitution of a negatively interacting mutation in the population. Among the tested populations, the steepest change was seen in the Lac5 population. Focusing on the beginning part of the evolution of this population, the effect of the lacI− mutation declined from conferring an 8.3% benefit in the ancestor to a mean cost of 0.9% among four independent clones isolated from this population at 500 generations of selection (fig. 6B). To determine the genetic basis of this change, the 500 generation evolved clones were sequenced and mutational changes relative to their ancestor identified (table 1). All four clones shared an IS150 insertion occurring 24-bp upstream of the uspB gene. The same mutation is present in clones isolated from this population after 4,000 and 8,000 generations of evolution.
Fig. 6.

Fitness effect of the lacI− mutation introduced into EvlacI+ clones. (A) Symbols indicate the effect of adding the lacI− mutation into clones from each EvlacI+ population isolated at indicated time points. Lines connect clones isolated from the same population at different points in time. The red rectangle indicates the population and 500 generation time point that clones were isolated from to analyze in more detail (shown in B). We were unable to transfer the lacI− mutation into a clone isolated at 2,000 generations from one EvlacI− population. The mutation effect trajectory of this population connects the ancestor and the 4,000 generation time point directly. (B) The effect of the lacI− mutation in the ancestor (black symbol) and clones from the 500 generation time point of the Lac5 population (red symbols). Clones 3 and 4 had the same genotype so are grouped here (table 1). Mean and 95% CI of replicate fitness estimates are shown (n = 3).
Table 1.
Mutations in 500 Generation Clones of the Lac5 Population.
| Clone |
||||
|---|---|---|---|---|
| Gene | 1 | 2 | 3 | 4 |
| uspB a ←::IS150→uspA | –24/–367b bp | –24/–367 bp | –24/–367 bp | –24/–367 bp |
| rbs | Δ5,943 bp | Δ7,590 bp | Δ7,590 bp | |
Also known as yhiO.
Numbers indicate position of the insertion relative to the start of the flanking genes.
To determine the interaction between lacI− and uspB mutations, we focused on a Lac5 8,000 generation evolved clone. The lacI− mutation confers a cost in this clone measured in the lactose environment (Lac5 lacI− vs. Lac5: relative fitness = 0.89 ± 0.09 [95% CI], P = 0.03). When we reverted the evolved uspB allele, the lacI− mutation conferred a 26% benefit, comparable with its effect in EvlacI− clones and greater than its effect in the ancestor (Lac5 uspBAnclacI− vs. Lac5 uspBAnc: relative fitness = 1.26 ± 0.09 [95% CI], P = 0.006). This result identifies the uspB mutation as being one cause of the historical contingency we see, acting to reduce the benefit conferred by lacI−. We note, however, that the situation is complex and might depend on higher order interactions. Insertion mutations upstream or in uspB are present in all of seven sequenced clones isolated from EvlacI+ populations, but also in clones isolated from four EvlacI− population (G_L3, G/L5, L_G6, and Lac1) (supplementary tables S1 and S2, Supplementary Material online). This pattern indicates that clones without the lacI− mutation are significantly more likely to have the uspB mutation, consistent with a negative genetic interaction (one-tailed Fisher’s exact test: P = 0.007). Nevertheless, that uspB and lacI− are sometimes found together suggests that other mutations might influence the relationship between them.
Divergent lac Regulation Strategies Do Not Promote Further Genetic Divergence
To test if the historical contingency influencing the effect of the lacI mutation extends to promoting continued divergence of EvlacI+ and EvlacI− populations along different evolutionary paths, we used Dice’s coefficient of similarity (s) to compare the sets of mutations found among each population group (supplementary tables S1 and S2, Supplementary Material online). This metric is bounded between 0, indicating completely dissimilar mutation sets, and 1, indicating identical mutation sets. We found no signal of higher mutation parallelism within compared with between populations that substitute different lacI strategies. On average, EvlacI+ clones were more similar to EvlacI− clones than to each other (within EvlacI+, Dice’s s = 0.045; between EvlacI+/−, s = 0.063; within EvlacI−, s = 0.073) (supplementary fig. S7A, Supplementary Material online). Moreover, no similarity estimate was significantly different to a null expectation based on randomly assigning sequenced clones to the EvlacI+ and EvlacI− group labels. A limitation to this analysis is that it groups populations that were selected in different environments. Although it would be ideal to consider the effect of lacI− on subsequent mutation accumulation separately for each selection environment, only one of four environments contains multiple populations of each lacI type, limiting our ability to distinguish repeated from random effects. We note, however, that, with one exception, clones from the different selection environments did not detectably differ in the sets of mutations they accumulated (supplementary fig. S7B, Supplementary Material online).
Discussion
We found that the absence of potentially beneficial lacI mutations in EvlacI+ populations was due to epistasis and is, therefore, a case of historical contingency. In other words, populations followed at least two distinct evolutionary paths contingent on the presence of some earlier mutational change. This early mutation caused the fitness effect of subsequent lacI− mutations to be neutral or deleterious in all EvlacI+ populations, instead of beneficial as in the ancestor and EvlacI− populations. We found no evidence to support alternative hypotheses of insufficient time or changes in mutation rate explaining the absence of lacI− mutations.
In the context of replicate evolved populations started from a common ancestor, as examined here, historical contingency stems from negative genetic interactions between early mutations and potentially beneficial mutations. At the physiological level, these interactions reflect that otherwise beneficial mutations are either redundant to, or interact antagonistically with, earlier substituting mutations. Redundancy could occur if there were two alternative mutational targets that cause the same phenotypic change. For example, mutations affecting either repressor proteins or operator binding sites (Quan et al. 2012). Antagonism could reflect, for example, alternative adaptive strategies that share some metabolite such that the evolution of one strategy changes the cellular physiological environment in a way that means the second is no longer beneficial (reviewed in De Visser et al. [2011]). Whereas redundancy allows for convergence in adaptive traits, even as populations might diverge genetically, antagonism between adaptive mechanisms predicts eventual phenotypic divergence as new mutations come to depend on the different cell environments caused by earlier ones (Szamecz et al. 2014).
We find that the shortened lag time conferred by lacI− mutations in the EvlacI− populations is matched in EvlacI+ populations, consistent with EvlacI+ populations substituting some alternative mutation(s) that makes lacI− redundant. This possibility is also supported by our findings that EvlacI+ and EvlacI− populations had indistinguishable growth curves, that EvlacI+ populations increased in their sensitivity to lac operon induction, and that the overall set of substituted mutations did not differ between the population groups. Indeed, it seems that “evolutionary routes are many, but the destinations are limited.” (Blount et al. 2008, 2012; Zee et al. 2014). A purely redundant mechanism of interaction does not, however, easily explain our finding that adding lacI− decreases fitness in five of eight EvlacI+ strains more than can be explained by only the cost of constitutive lac expression. These high costs indicate some direct antagonism between lacI− and alternative adaptations. To the extent that this antagonism reflects a distinct mechanistic basis of adaptation, albeit with similar immediate phenotypic effects, it might eventually lead to diverging selective opportunities and, therefore, evolutionary paths.
The mechanism by which the lacI− mutation shortens lag time likely depends on the lack of functional repressor causing cells to produce higher levels of lac gene products through stationary phase so that they can more quickly import and catabolize lactose in fresh medium (Quan et al. 2012; Chu and Barnes 2016). The most obvious candidate for a mutation interacting with lacI−, therefore, would be one causing the same higher lac activity. We did not find any candidate mutations in the canonical lac regulon of EvlacI+ clones, and, indeed, we did not detect any increase in basal lac expression among these clones. However, we did identify an IS150 insertion mutation occurring upstream of uspB as interacting with lacI in at least one population. The insertion occurs just upstream of uspB between the promoter and the start of the gene. It therefore seems likely to reduce or eliminate that gene’s expression, though IS150 has an outward-facing promoter and could also upregulate uspB (Schwartz et al. 1988). In either case, a link between UspB and lactose metabolism is unclear. UspB is an integral membrane protein that is up-regulated in stressful conditions, including starvation, and might play a role in mediating changes in membrane composition either directly or as a result of disrupted protein–protein interactions (Farewell et al. 1998; Liu et al. 2019). In principle, such changes could affect stationary phase membrane composition to facilitate lactose uptake although lactose induction of lac expression still depended on the LacY permease so any change in uptake must have been minor (supplementary fig. S6, Supplementary Material online). We did not rule out the possibility that uspB interacts indirectly with lactose metabolism, though the benefit of lacI− was lost in strains containing only uspB and one other mutation, a deletion of the rbs operon. The rbs deletion mutation occurred in almost all of our evolved populations, regardless of their lacI status, so it seems likely the interaction is, in fact, direct. Finally, we note that although the uspB mutation was found in all EvlacI+ clones, it was also found in one EvlacI− population, indicating that the interaction between uspB and lacI mutations might be influenced by other mutations.
Both EvlacI+ and EvlacI− populations are found in three of the four lactose-containing environments we consider, but at different frequencies. The ratio of lacI− absence to fixation (7:3) is substantially higher among the 2,000 generation fluctuating G_L and L_G selected populations than among those selected in the daily alternating G/L environment (0:5) (Fisher’s exact test: P = 0.026), even though populations spent the same total time in each environmental component. The lacI− mutation was also less likely to fix among G_L and L_G populations than among Lac populations, although this difference was not significant (Fisher’s exact test: P = 0.119). One explanation for these differences is that mutations selected in the glucose portion of the selection regime can interact negatively with lacI−, decreasing its benefit in lactose. Such mutations would not be expected to reach high frequency in the G/L daily fluctuating environments where they would compete directly with lacI− mutations for fixation.
How the history of a population affects its future evolution remains a major question in evolutionary biology (reviewed in Blount et al. [2018]). Does natural selection generally converge on high-fitness genotypes, such that evolutionary outcomes, even if not paths, are deterministic, or do antagonistic interactions cause chance differences in mutation occurrence and success to be built on, causing evolutionary outcomes to be contingent, and, therefore, unpredictable? Contingency leading to divergent fitness outcomes has been described in several controlled laboratory evolution experiments, even when comparing populations started from a common ancestor, and therefore having relatively few differences among which contingency can arise (Collins and Bell 2004; Blount et al. 2008; Beaumont et al. 2009; Cooper and Lenski 2010; Wiser et al. 2013). Divergence is commonly seen at the genetic level in these kinds of experiments, though the basis of differences is not usually examined. For example, whether distinct mutational pathways are likely to be equivalent or to affect available subsequent adaptive paths, and therefore likely to lead to eventual fitness differences. One example of the difficulty in evaluating this possibility comes from an experiment demonstrating a reproducibly different adaptive potential of genotypes differing crucially in mutations causing different residues in a gene affecting DNA supercoiling (Woods et al. 2011).
In summary, we describe an example of historical contingency affecting selection of lac regulation. Whereas approximately half of a set of replicate populations selected in lactose-containing environments quickly substituted a mutation in lacI that provided a benefit by decreasing lag time, remaining populations substituted other mutations that interacted negatively with lacI causing them to follow alternative evolutionary paths. At the point in our evolution experiment that was considered here, these paths are marked by distinct genetic, but not fitness, outcomes.
Materials and Methods
Bacterial Strains and Growth Conditions
Bacterial strains were isolated from populations evolved for 8,000 generations as part of a long-term evolution experiment. This experiment evolved replicate populations in seven different environmental treatments, four of which are considered here: lactose only (Lac), a combination of glucose and lactose fluctuating daily (G/L), or the same combination fluctuating every 2,000 generations with one treatment starting with glucose (G_L) and another with lactose (L_G) (Satterwhite and Cooper 2015) (supplementary fig. S1, Supplementary Material online). Evolution environments comprised these sugars added to base Davis–Mignoli (DM) medium at a concentration of 175 μg/ml (glucose) or 210 μg/ml (lactose). These concentrations support approximately equal densities of stationary phase bacteria (∼3.5 × 108 cfu/ml) (Cooper and Lenski 2010). Populations were propagated in 1 ml of medium in 96 × 2 ml well blocks for 8,000 generations, using a daily 1:100 serial transfer. Each population was initially homogeneous, such that de novo mutation was the only source of genetic variation. Samples were frozen at −80 °C, with glycerol as a cryoprotectant, every 500 generations. Six replicate populations were evolved in each environment, with three started from REL606 and three from REL607, a spontaneous Ara+ derivative (Lenski et al. 1991). The different ara markers allow strains to be differentiated by plating on tetrazolium-arabinose plates, on which ara+ strains form white colonies and ara− red colonies. The Ara marker does not affect fitness measurements in any of the assay environments (Cooper and Lenski 2010). Lysogeny broth (LB) was used for nonselective culturing, whereas the respective evolution environment of each clone was used for fitness assays and growth measurements.
Identification of lacI Mutations
To determine fixation of lacI mutations within evolved populations, we plated 8,000-generation population samples (1,000–3,000 cells) on TGX indicator medium (Blount et al. 2008, 2012; Schenk et al. 2012; Zee et al. 2014). This medium consists of 10 g/l tryptone, 2.5 g/l sodium chloride, 5 g/l glucose, and 30 mg/ml of the LacZ substrate 5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside (X-gal). Colony color on this medium indicates the level of LacZ activity in the absence of any recognized inducer, and thus distinguishes between ancestral repressed (white colonies) and mutant derepressed (blue colonies) lac expressing strains. Four populations had a mix of blue and white colonies and were not considered here. Strains were isolated from populations having >95% blue colonies and sequenced to confirm that derepression was due to a mutation in the lacI repressor. Evolved strains from populations that fixed a lacI mutation are denoted as EvlacI− and those from populations that maintained the ancestral allele are EvlacI+.
Strain Construction
To determine the fitness effect of lacI− mutations in evolved strains, we reverted them using the suicide plasmid pDS132::lacI+, as described previously (Satterwhite and Cooper 2015). Potential engineered revertants were screened for expected lacI+ phenotypes first on TGX medium. White colonies were streaked onto minimal medium supplemented with P-Gal. Clones that reverted to lacI+ cannot grow on this medium (Nghiem et al. 1988). Clones isolated from populations that retained the ancestral lacI allele were converted to lacI− by isolating spontaneous mutants selected on minimal medium supplemented with P-Gal as the only potential carbon source and with X-gal as a means to increase visual contrast of small colonies, making them easier to detect. In all cases, mutant isolation was confirmed by sequencing of the lacI gene. This also allowed us to confirm that selected lacI− strains had the same 4-base insertion/deletion mutations that dominated the mutations that fixed in the evolution experiment (Quan et al. 2012).
We used a combined CRISPR-recombineering approach to replace the evolved uspB allele (uspB::IS150) with the ancestral allele (Jiang et al. 2015). A uspB targeting sgRNA pTarget plasmid (pTarget-uspB) was constructed by using the NEBuilder HiFi DNA Assembly kit to assemble DNA fragments containing a donor region of the ancestral uspB locus, the pTarget vector backbone including a uspB N20 sequence, and two linking oligonucleotides (details in supplementary table S1, Supplementary Material online). The uspB donor DNA encoded by the resulting plasmid does not alter the N20 target sequence in the edited strain. To prevent persistent editing after DNA repair, we modified the procedure used by Jiang et al. (2015) by simultaneously treating cells with arabinose and lactose for 1.5 h following transformation of pCas containing cells with pTarget-uspB. This combination activates the red recombination system and cuts the pTarget plasmid, causing it to be lost from cells. Transformants were plated on kanamycin plates to select pCas carrying cells and then grown at 42 °C to select for loss of that plasmid. The candidate edited cells were screened and confirmed by colony PCR.
Mutation Rate Estimates
The rate of mutation to constitutive lac expression was estimated using a fluctuation test. For each clone, the freezer stock was inoculated into LB, grown overnight at 37 °C, and then diluted 1:1,000 into ten fresh 1 ml LB cultures. After overnight growth, a 100-μl sample from each replicate population was plated onto DM agar supplemented with X-gal and P-gal. A diluted sample was also plated onto LB agar plates to estimate total cell density. Plates were incubated at 37 °C for 48 h prior to counting.
To assess the plating efficiency of lacI− mutants on the X-gal + P-Gal selection plates, we compared the number of colonies growing when a small number of lacI− cells were plated by themselves on to permissive LB medium and when they were plated onto P-gal medium with an excess of a REL606 strain that had a lac operon deletion that made it unable to mutate to grow on P-Gal. The plating efficiency of lacI− cells was calculated as peff = mc/me, where mc is the number of lacI− mutants able to form visible colonies on P-Gal in combination with the deletion strain, and me is the number of mutants that form colonies when plated alone onto LB. Colony counts in the fluctuation test were multiplied by 1/peff to obtain corrected mutation counts. Following this correction, mutation rate analysis was carried out using the bz-rates estimator (http://www.lcqb.upmc.fr/bzrates) (Gillet-Markowska et al. 2015).
Individual-Based Simulations
The frequency of substitution (fs) for a lacI− mutation is a function of mutation rate, population size, and mutation dynamics. These dynamics, such as clonal interference and hitchhiking, can significantly affect fixation times. To estimate the expected fixation time for lacI− mutants in the long-term evolution experiment, we conducted individual-based simulations using a Wright–Fisher regime with a genome-wide mutation rate of U = 7 × 10−4, the mean genomic rate as calculated from relevant estimates (Wielgoss et al. 2011; Lee et al. 2012; Long et al. 2016), and a lacI+ to lacI− mutation rate of 1.72 × 10−7 (measured in the ancestor, see below). Competing background mutations were divided into three deleterious and three beneficial classes, each comprising a different proportion of occurring mutations (supplementary table S2, Supplementary Material online). Each background mutation occurs at a new site and acts on fitness independently of other mutations in a simulated individual. Mutations to lacI− conferred a fitness increase as measured in this work in the ancestor (8.31% in Lac, 4.05% in G/L; fig. 1B). The effective population size prevailing in the evolution experiment (N = 3.3 × 107) (Cooper and Lenski 2010) was too large for us to replicate simulations in a reasonable time frame. We therefore simulated population sizes up to 105. Fixation times are expected to be decreased at larger population sizes, therefore results at N = 105 provide a conservative estimate for the probability of a lacI− mutation fixing in the experimental populations. Populations were allowed to evolve for 8,000 generations or until the lacI− mutation fixed (frequency above 95%). For each population size, we simulated 100 replicate populations and recorded the proportion of these replicates that fixed the lacI− mutation. We then bootstrapped the simulation results 1,000 times to estimate the 95% CI for fs. For the 2,000-generation fluctuating environments, we simulated only the two 2,000 generations of evolution in lactose, and calculated fs as fs = f2k + f2k(1 − f2k), where f2k is the frequency of lacI− fixation during the first period of 2,000-generations of lactose selection. Assuming that lacI− mutations that did not fix during lactose selection periods were lost during any subsequent period of glucose selection, where they were costly, the second term gives the number of those populations expected to fix lacI− in the second period of lactose selection.
We did not simulate evolution in the G/L environment, as this requires estimation of the correlated effects of background mutations across glucose and lactose environments, which are ignored in the more slowly fluctuating 2,000 generation environments.
Fitness Assays
The relative fitness of a given strain was assayed relative to a reference with the opposite Lac (i.e., lacI+ vs. lacI−) or Ara (i.e., ara+ vs. ara−) marker. Fitness assays were carried out in identical conditions to a tested strain’s evolution environment. Prior to each assay, competitors were independently preconditioned to the competition environment for 1 day, except for the G/L environment, which was preconditioned over 2 days. For the G/L environment, cultures were diluted 100-fold from glucose to lactose for day two (for both preconditioning and competition steps). Following preconditioning, competitors were mixed at a 1:1 volume ratio and transferred with a 100-fold dilution into the competition environment. A sample was immediately plated on indicator agar (TGX or TA) in order to determine the starting frequency of the two competitors. At the end of the competition, samples were again plated on indicator agar. Absolute fitness of competitor, a, was calculated as wa = ln(100d×(Na(f)/Na(i)), where d is the number of competition days, and N is the number of colony-forming units at the initial (i) and final (f) time points. The relative fitness of competitor a relative to competitor b is then wa/b = wa/wb, and the selective advantage of a over b is s = wa/b−1.
Growth Rate Estimates and Virtual Competitions
Each clone was inoculated from a freezer stock into 1 ml of LB media and grown overnight. The following day, 1 μl of each culture was transferred into 1 ml of DM supplemented with 210 μg/ml lactose and incubated at 37 °C for 24 h. On day three, 2 μl of the preconditioned culture was transferred into 198 μl of the same media in a 96-well polystyrene plate. This plate was incubated in a VersaMax microplate reader (Molecular Dynamics, CA) and grown at 37 °C until cells reached stationary phase. The culture’s optical density at 450 nm (OD450) was measured every 5 min during growth. Growth curves were analyzed using an extension of the logistic model (Baranyi and Roberts 1994; Ram et al. 2019) as implemented at: https://multi-choice-comparison.shinyapps.io/growth_curves/. To check that estimated growth parameters were meaningful estimates of fitness components, we implemented a double-strain Baranyi–Roberts model to perform virtual competitions as mediated only through growth parameters separately estimated for each competitor (Ram et al. 2019). Virtual competition results were compared with direct competition fitness estimates. Briefly, virtual competitions model a common nutrient pool that is independently used for growth by two competitors depending on their measured growth parameters. The change in the density of each competitor was used to estimate a virtual relative fitness.
Genome Sequencing
Genomic DNA was isolated and purified using the Wizard Genomic DNA Purification Kit (Promega) following the protocol for Gram-negative bacteria at one-third volume. Double-stranded DNA was quantified using SYBR Green I Nucleic Acid Stain (Invitrogen) in a SpectraMax M5 Fluorescence Microplate Reader (Molecular Devices). Libraries were created following the Nextera XT DNA Library Prep Kit protocol, at one-quarter volume, with Nextera XT Index Kit v2 adapters (Illumina). Libraries were individually quantified using the Qubit dsDNA High Sensitivity Assay with a Qubit 2.0 Flourometer (ThermoFisher) and fragment size determined using the Agilent 2100 BioAnalyzer with a High Sensitivity DNA Analysis Kits (Agilent). Libraries were pooled and sequenced on an Illumina NextSeq, producing 150 bp, paired-end reads. The Breseq computational pipeline was used to align reads to the reference sequence and identify mutations (Deatherage and Barrick 2014).
Statistics
R3.5.3 used for plotting and mutation similarity analysis (R Core Team 2017). The mclustcomp function in package mclustcomp was used to estimate Dice’s coefficient of similarity comparing mutations fixed in clones isolated from different evolved populations. Simulations were performed using a custom Python script, which is available on request.
Supplementary Material
Supplementary data are available at Molecular Biology and Evolution online.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation (Grant No. DEB-1253650 to T.F.C. and Grant No. DEB-1354952 to R.B.R.A.) and the Royal Society of New Zealand Marsden fund (Grant No. 19-MAU-082 to T.F.C.).
Data Availability
T.F.C. will make the strains constructed in this study available to qualified recipients following completion of an institutional material transfer agreement. The results of competition experiments, summary input data, and analysis scripts that pertain to the experiments and analyses reported in this article have been deposited at https://doi.org/10.5061/dryad.0rxwdbs05.
References
- Baranyi J, Roberts TA.. 1994. A dynamic approach to predicting bacterial growth in food. Int J Food Microbiol. 23(3–4):277–294. [DOI] [PubMed] [Google Scholar]
- Barrick JE, Kauth MR, Strelioff CC, Lenski RE.. 2010. Escherichia coli rpoB mutants have increased evolvability in proportion to their fitness defects. Mol Biol Evol. 27(6):1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont HJE, Gallie J, Kost C, Ferguson GC, Rainey PB.. 2009. Experimental evolution of bet hedging. Nature 462(7269):90–93. [DOI] [PubMed] [Google Scholar]
- Bedhomme S, Lafforgue G, Elena SF.. 2013. Genotypic but not phenotypic historical contingency revealed by viral experimental evolution. BMC Evol Biol. 13:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount ZD, Barrick JE, Davidson CJ, Lenski RE.. 2012. Genomic analysis of a key innovation in an experimental Escherichia coli population. Nature 489(7417):513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount ZD, Borland CZ, Lenski RE.. 2008. Historical contingency and the evolution of a key innovation in an experimental population of Escherichia coli. Proc Natl Acad Sci U S A. 105(23):7899–7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount ZD, Lenski RE, Losos JB.. 2018. Contingency and determinism in evolution: replaying life’s tape. Science 362(6415):eaam5979–eaam6012. [DOI] [PubMed] [Google Scholar]
- Chu D, Barnes DJ.. 2016. The lag-phase during diauxic growth is a trade-off between fast adaptation and high growth rate. Sci Rep. 6:25191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S, Bell G.. 2004. Phenotypic consequences of 1,000 generations of selection at elevated CO2 in a green alga. Nature 431(7008):566–569. [DOI] [PubMed] [Google Scholar]
- Cooper TF, Lenski RE.. 2010. Experimental evolution with E. coli in diverse resource environments. I. Fluctuating environments promote divergence of replicate populations. BMC Evol Biol. 10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Visser JAGM, Cooper TF, Elena SF.. 2011. The causes of epistasis. Proc Biol Sci. 278(1725):3617–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatherage DE, Barrick JE.. 2014. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods Mol Biol. 1151:165–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh PJ, Schmeissner U, Hofer M, Miller JH.. 1978. Genetic studies of the lac repressor. VII. On the molecular nature of spontaneous hotspots in the lacI gene of Escherichia coli. J Mol Biol. 126(4):847–857. [DOI] [PubMed] [Google Scholar]
- Farewell A, Kvint K, Nyström T.. 1998. uspB, a new σS-regulated gene in Escherichia coli which is required for stationary-phase resistance to ethanol. J Bacteriol. 180(23):6140–6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrish PJ, Lenski RE.. 1998. The fate of competing beneficial mutations in an asexual population. Genetica 102–103:127–144. [PubMed] [Google Scholar]
- Gillet-Markowska A, Louvel G, Fischer G.. 2015. bz-rates: a web tool to estimate mutation rates from fluctuation analysis. G3 (Bethesda) 5:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MJ, Thornton JW.. 2014. Historical contingency and its biophysical basis in glucocorticoid receptor evolution. Nature 512(7513):203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Chen B, Duan C, Sun B, Yang J, Yang S.. 2015. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 System. Appl Environ Microbiol. 81(7):2506–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MS, Martsul A, Kryazhimskiy S, Desai MM.. 2019. Higher-fitness yeast genotypes are less robust to deleterious mutations. Science 366(6464):490–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryazhimskiy S, Rice DP, Jerison ER, Desai MM.. 2014. Global epistasis makes adaptation predictable despite sequence-level stochasticity. Science 344(6191):1519–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Popodi E, Tang H, Foster PL.. 2012. Rate and molecular spectrum of spontaneous mutations in the bacterium Escherichia coli as determined by whole-genome sequencing. Proc Nat Acad Sci U S A. 109(41):E2274–E2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenski RE, Rose MR, Simpson SC, Tadler SC.. 1991. Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. Am Nat. 138(6):1315–1341. [Google Scholar]
- Leon D, D’Alton S, Quandt EM, Barrick JE.. 2018. Innovation in an E. coli evolution experiment is contingent on maintaining adaptive potential until competition subsides. PLoS Genet. 14(4):e1007348–e1007422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhao L, Zhang Q, Huo N, Shi X, Li L, Jia L, Lu Y, Peng Y, Song Y.. 2019. Proteomics-based mechanistic investigation of Escherichia coli inactivation by pulsed electric field. Front Microbiol. 10:2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H, Miller SF, Strauss C, Zhao C, Cheng L, Ye Z, Griffin K, Te R, Lee H, Chen C-C, et al. 2016. Antibiotic treatment enhances the genome-wide mutation rate of target cells. Proc Natl Acad Sci U S A. 113(18):E2498–E2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JR, Dobias DT, Weitz JS, Barrick JE, Quick RT, Lenski RE.. 2012. Repeatability and contingency in the evolution of a key innovation in phage Lambda. Science 335(6067):428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem Y, Cabrera M, Cupples CG, Miller JH.. 1988. The mutY gene: a mutator locus in Escherichia coli that generates G.C→T. Proc Natl Acad Sci U S A. 85(8):2709–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbunugafor CB, Hartl D.. 2016. A pivot mutation impedes reverse evolution across an adaptive landscape for drug resistance in Plasmodium vivax. Malar J. 15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson VM, Miller CR, Rokyta DR.. 2012. The consistency of beneficial fitness effects of mutations across diverse genetic backgrounds. PLoS One 7(8):e43864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F, Widmann S, Wünsche A, Duan K, Donovan KA, Dobson RCJ, Lenski RE, Cooper TF.. 2018. Effects of beneficial mutations in pykF gene vary over time and across replicate populations in a long-term experiment with bacteria. Mol Biol Evol. 35(1):202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfeito L, Sousa A, Bataillon T, Gordo I.. 2014. Rates of fitness decline and rebound suggest pervasive epistasis. Evolution 68(1):150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KN, Castillo G, Wünsche A, Cooper TF.. 2016. Adaptation of Escherichia coli to glucose promotes evolvability in lactose. Evolution 70(2):465–470. [DOI] [PubMed] [Google Scholar]
- Quan S, Ray JCJ, Kwota Z, Duong T, Balázsi G, Cooper TF, Monds RD.. 2012. Adaptive evolution of the lactose utilization network in experimentally evolved populations of Escherichia coli. PLoS Genet. 8(1):e1002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt EM, Deatherage DE, Ellington AD, Georgiou G, Barrick JE.. 2014. Recursive genomewide recombination and sequencing reveals a key refinement step in the evolution of a metabolic innovation in Escherichia coli. Proc Natl Acad Sci U S A. 111(6):2217–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2017. R: a language and environment for statistical computing. Vienna (Austria: ). Available at: https://www.R-project.org/. [Google Scholar]
- Ram Y, Dellus-Gur E, Bibi M, Karkare K, Obolski U, Feldman MW, Cooper TF, Berman J, Hadany L.. 2019. Predicting microbial growth in a mixed culture from growth curve data. Proc Natl Acad Sci U S A. 116(29):14698–14707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterwhite RS, Cooper TF.. 2015. Constraints on adaptation of Escherichia coli to mixed-resource environments increase over time. Evolution 69(8):2067–2078. [DOI] [PubMed] [Google Scholar]
- Schaaper RM, Danforth BN, Glickman BW.. 1986. Mechanisms of spontaneous mutagenesis: an analysis of the spectrum of spontaneous mutation in the Escherichia coli lacI gene. J Mol Biol. 189(2):273–284. [DOI] [PubMed] [Google Scholar]
- Schenk MF, Szendro IG, Krug J, De Visser JAGM.. 2012. Quantifying the adaptive potential of an antibiotic resistance enzyme. PLoS Genet. 8(6):e1002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E, Herberger C, Rak B.. 1988. Second-element turn-on of gene expression in an IS1 insertion mutant. Mol Gen Genet. 211(2):282–289. [DOI] [PubMed] [Google Scholar]
- Shah P, McCandlish DM, Plotkin JB.. 2015. Contingency and entrenchment in protein evolution under purifying selection. Proc Natl Acad Sci U S A. 112(25):E3226–E3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spor A, Kvitek DJ, Nidelet T, Martin J, Legrand J, Dillmann C, Bourgais A, de Vienne D, Sherlock G, Sicard D.. 2014. Phenotypic and genotypic convergences are influenced by historical contingency and environment in yeast. Evolution 68(3):772–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr TN, Picton LK, Thornton JW.. 2017. Alternative evolutionary histories in the sequence space of an ancient protein. Nature 549(7672):409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szamecz B, Boross G, Kalapis D, Kovács K, Fekete G, Farkas Z, Lázár V, Hrtyan M, Kemmeren P, Groot Koerkamp MJA, et al. 2014. The genomic landscape of compensatory evolution. PLoS Biol. 12(8):e1001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenaillon O, Rodríguez-Verdugo A, Gaut RL, McDonald P, Bennett AF, Long AD, Gaut BS.. 2012. The molecular diversity of adaptive convergence. Science 335(6067):457–461. [DOI] [PubMed] [Google Scholar]
- Travisano M, Mongold J, Bennett A, Lenski R.. 1995. Experimental tests of the roles of adaptation, chance, and history in evolution. Science 267(5194):87–87. [DOI] [PubMed] [Google Scholar]
- Wang Y, Diaz Arenas C, Stoebel DM, Cooper TF.. 2013. Genetic background affects epistatic interactions between two beneficial mutations. Biol Lett. 9(1):20120328–20120328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Diaz Arenas C, Stoebel DM, Flynn K, Knapp E, Dillon MM, Wünsche A, Hatcher PJ, Moore FB-G, Cooper VS, et al. 2016. Benefit of transferred mutations is better predicted by the fitness of recipients than by their ecological or genetic relatedness. Proc Natl Acad Sci U S A. 113(18):5047–5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich DM, Delaney NF, Depristo MA, Hartl DL.. 2006. Darwinian evolution can follow only very few mutational paths to fitter proteins. Science 312(5770):111–114. [DOI] [PubMed] [Google Scholar]
- Wielgoss S, Barrick JE, Tenaillon O, Cruveiller S, Chane-Woon-Ming B, Médigue C, Lenski RE, Schneider D.. 2011. Mutation rate inferred from synonymous substitutions in a long-term evolution experiment with Escherichia coli. G3 (Bethesda) 1(3):183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiser MJ, Ribeck N, Lenski RE.. 2013. Long-term dynamics of adaptation in asexual populations. Science 342(6164):1364–1367. [DOI] [PubMed] [Google Scholar]
- Woods RJ, Barrick JE, Cooper TF, Shrestha U, Kauth MR, Lenski RE.. 2011. Second-order selection for evolvability in a large Escherichia coli population. Science 331(6023):1433–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wünsche A, Dinh DM, Satterwhite RS, Arenas CD, Stoebel DM, Cooper TF.. 2017. Diminishing-returns epistasis decreases adaptability along an evolutionary trajectory. Nat Ecol Evol. 1(4):61. [DOI] [PubMed] [Google Scholar]
- Yedid G, Bell G.. 2002. Macroevolution simulated with autonomously replicating computer programs. Nature 420(6917):810–812. [DOI] [PubMed] [Google Scholar]
- Zee PC, Mendes-Soares H, Yu Y-TN, Kraemer SA, Keller H, Ossowski S, Schneeberger K, Velicer GJ.. 2014. A shift from magnitude to sign epistasis during adaptive evolution of a bacterial social trait. Evolution 68(9):2701–2708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
T.F.C. will make the strains constructed in this study available to qualified recipients following completion of an institutional material transfer agreement. The results of competition experiments, summary input data, and analysis scripts that pertain to the experiments and analyses reported in this article have been deposited at https://doi.org/10.5061/dryad.0rxwdbs05.