Abstract
All physiological events in living organisms originated as specific chemical/biochemical signals on the cell surface and transmitted into the cytoplasm. This signal is translated within milliseconds–hours to a specific and unique order required to maintain optimum performance and homeostasis of living organisms. Examples of daily biological functions include neuronal communication and neurotransmission in the process of learning and memory, secretion (hormones, sweat, and saliva), muscle contraction, cellular growth, differentiation and migration during wound healing, and immunity to fight infections. Among the different transducers for such life-dependent signals is the large family of G protein-coupled receptors (GPCRs). GPCRs constitute roughly 800 genes, corresponding to 2% of the human genome. While GPCRs control a plethora of pathophysiological disorders, only approximately one-third of GPCR families have been deorphanized and characterized. Recent drug data show that around 40% of the recommended drugs available in the market target mainly GPCRs. In this review, we presented how such system signals, either through G protein or via other players, independent of G protein, function within the biological system. We also discussed drugs in the market or clinical trials targeting mainly GPCRs in various diseases, including cancer.
Keywords: Arrestin, Drugs, G proteins, GPCR, GRKs, Heterodimerization, Signaling
Abbreviations: CCR, Chemokine Receptor; cAMP, cyclic AMP; AC, Adenylyl Cyclase; COX, Cyclooxygenase; DAG, Diacylglycerol; ERK, Extracellular signal-Regulated Kinase; GRKs, G protein-coupled Receptor Kinases; GIP, Gastric Inhibitory Peptide; GLP1R, Glucagon-Like Peptide-1 Receptor; IP3, Inositol 1,4,5-triphosphate; PKA, Protein Kinase A; PIP2, Phosphatidylinositol-4,5-bisphosphate; MAPK, Mitogen-Activated Protein Kinase; NMDA, N-Methyl D-Aspartate; Nbs, Nanobodies; PAR-1, Protease Activated Receptor 1
1. Introduction
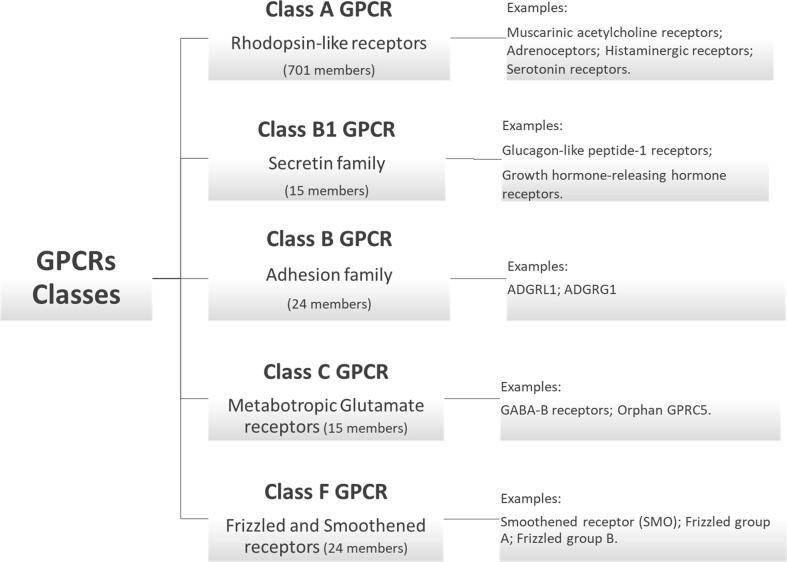
G protein-coupled receptors (GPCRs) transduce several extracellular signals to intracellular signaling by connecting with G proteins and arrestins. To understand GPCR signal transduction at the molecular level, GPCR structural organization and their transducer complex are essential in drug discovery (Hilger et al., 2018). GPCRs function as communication inboxes like peptides, proteins, lipids, sugars, and light energy. The received messages inform cells about the presence or absence of light-sustaining molecules or nutrients in the immediate surroundings, or they deliver messages sent by other cells. Over 1000 human GPCRs have been discovered, and each works differently regarding the signals they receive (Sommer et al., 2020). The new members’ list is growing daily with advances in new techniques, such as cryogenic electron microscopy and crystallography. The function of new GPCRs and the ligand they activate remain partially comprehended. Research is ongoing yearly to find novel ligands of orphan GPCR (GPCR without ligand is known as orphan GPCR). The GPCR family has four main subfamilies: Class A GPCRs (Rhodopsin-like receptors), Class B GPCRs (Secretin family), Class C GPCRs (Metabotropic Glutamate receptors), and Class F GPCRs (Frizzled and Smoothened receptors) (Fig. 1).
Fig. 1.
Flowchart showing the classification of GPCRs superfamily. Approximately 826 GPCRs members can be classified into five classes according to seven-transmembrane domain sequence homology (Fredriksson et al., 2003, Langenhan, 2020).
Class A GPCRs (Rhodopsin-like receptor): Class A is the biggest and most distinct class of the GPCR subfamily in humans and impacts every aspect of human life (Isberg et al., 2016, Kolakowski, 1994). This class is represented by the name of the rhodopsin-like receptor, where they are further subdivided into 19 subgroups based on a phylogenetic investigation (Joost and Methner, 2002). There are over 388 olfactory and 286 non-olfactory receptors affecting vital information in the human body. Among the characteristics of rhodopsin-like GPCRs is the presence of molecular switches in the seven-transmembrane helical bundles where the management of numerous functionally significant signature sequence patterns is present. Interestingly, the signature sequence plays a pivotal role in regulating the GPCR activation mechanism and signaling cascade, acting as a shuttle switch between active and inactive receptor conformation.
Class B GPCRs (Secretin family): Class B GPCRs are acknowledged as the secretin family comprising over 15 receptors for binding peptide hormones. The secretin family constitutes essential drug targets in several human afflictions, namely diabetes (type 2), cardiovascular disease, cancer, headache, neurodegeneration, and psychiatric disorders (Alexander et al., 2013, Pal and Xu, 2012). One of the most important members of class B GPCR is the glucagon-like peptide-1 receptor (GLP1R). GLP1R is mainly expressed in pancreatic β-cells, where it pairs between Gαq and Gαi proteins, regulating the cellular concentration of calcium and inhibiting intracellular cyclic AMP (cAMP respectively). Additionally, it activates adenylyl cyclase (AC) and elevates intracellular cAMP via stimulator G (Gαs) protein, major signaling pathways activating the synthesis and release of insulin and subsequently lowering glucose concentration (Nadkarni and Holz, 2014, Buteau, 2008). Therapies based on GLP1 and GLP1R agonists, such as dipeptidyl peptidase 4 inhibitors, affect glucose metabolism through numerous mechanisms. Glucose homeostasis entirely depends on the complex interaction of several hormones, such as insulin, amylin, gastrointestinal peptides, and glucagon. Aberrant regulation of these hormones may induce clinical manifestations and, finally, diabetes.
Class C GPCRs (Metabotropic Glutamate receptors): Class C GPCRs comprise mainly three domains: (i) the venus flytrap domain or module, possessing a large extracellular part responsible for ligand interaction and activation of receptors, (ii) cysteine-rich domain, and (iii) transmembrane domain. The cysteine-rich domain mainly transmits signals to the transmembrane domain from the extracellular domain (Kniazeff et al., 2011, Muto et al., 2007).
Class F GPCRs (Frizzled and Smoothened receptors): The frizzled receptor family belongs to the wingless and int-1 (Wnt) family as a signaling molecule (Wang et al., 2016). The genes of the frizzled receptors were first isolated from drosophila in 1989, followed by a discovery in mammals (Vinson et al., 1989). In mammals, 10 receptor subtypes have been identified and named frizzled 1–10 (Koike et al., 1999, Wang et al., 1996). Three signaling pathways have been characterized by frizzled receptors. The first pathway discovered in drosophila due to mutation in this receptor may induce cytoskeleton and structural dysfunction and is called planar cell polarity (Gubb and Garcia-Bellido, 1982). The second pathway considered classical wnt signaling, the frizzled-wnt interaction, may induce the inhibition of β-catenin into the nucleus to regulate transcription factors (Nusse, 2012). The third pathway for frizzled signaling is based on G protein signaling and is called the wnt-calcium pathway (Wang and Malbon, 2003).
2. G proteins
As the name indicates, when an external signaling particle binds to a GPCR, GPCRs communicate with G proteins that are commonly found in the cytosol, thereby triggering the conformational rearrangement within the GPCR. The ensuing conformational change triggers the interaction between the GPCR and the adjacent G protein. G proteins are specific proteins that bind to guanosine triphosphate (GTP) and guanosine diphosphate (GDP) nucleotides. Few G proteins, such as Ras signaling protein, are small proteins having a single subunit, while most G proteins associated with GPCRs are heterotrimeric. G proteins possess three polypeptides structured into two distinct, well-designed units: the α-subunit and the βγ-dimer. From an evolutionary perspective, the GTPase superfamily is conserved throughout evolution. Post-translationally, Gα and Gβγ-subunits are lipidated and predominantly confined at the surface of the inner leaflet of the plasma membrane. As mentioned above, α-subunit can inherently interact with the guanine nucleotide (GDP during the inactive state and GTP when activated). The domain exhibits GTPase activity enabling GTP hydrolysis and, subsequently, G protein deactivation (Cabrera-Vera et al., 2003). In the normal state, G protein exists as a heterotrimeric complex with GDP binding to the α-subunit. As the ligand binds to the receptor, the ensuing events induce a conformational change in the receptor, or binding may arise to stabilize the existing active conformation. The resulting processes cause the association of Gαβγ heterodimer. Heterotrimeric G proteins are also classified according to their Gα-subunit into four main groups (Fig. 1), Gαs, Gαi/o, Gαq/11, and Gα12/13. GPCRs often bind selectively to specific Gα protein family members, although more promiscuous coupling is also seen sometimes (Hermans, 2003). Further details about G proteins will be delivered under the signaling section.
3. G protein receptor kinases (GRKs)
There are seven GRKs categorized into three subfamilies depending upon sequence and structural similarity. The first subfamily is rhodopsin kinase, whose members are GRK1 and GRK7; the second subfamily is βARK whose members are GRK2 and GRK3, and the last subfamily is GRK-4-like subfamily (GRK4, GRK5, and GRK6) (Pitcher et al., 1998). The GRKs belong to the AGC protein kinase of serine/threonine kinases with the acronyms PKA, PKG, and PKC. Due to the presence of sequence alignments of kinase domains, the members are placed into this subgroup, as the catalytic domain of GRKs is intensely conserved, centrally positioned in the tri-domain structure (Pearce et al., 2010). Rhodopsin kinase is a member of GRK1, found mainly in mammalian retinal rod cells. Rhodopsin kinase phosphorylates light-activated rhodopsin, thereby binding to the arrestin to terminate the light-activated signaling mechanism.
Both GRK2 and GRK3 are closely associated and can phosphorylate at sites that stimulate arrestin-mediated receptor desensitization, internalization, and signal trafficking. Initially, GRK2 was identified as a protein kinase that phosphorylated the adrenergic (β-2) receptor. The GRK2 is mainly expressed in the heart, where it was proposed to treat heart failure (Lieu and Koch, 2019). Approaches are underway to find a molecule that can bind G protein βγ-subunit complex by inhibiting GRK2 activation often referred to as “βARKct” (Thal et al., 2012). To find GRK inhibitors, studies have demonstrated that paroxetine, FDA-approved anti-depressant and amlexanox, and anti-inflammatory immunomodulator, inhibit GRK2 and GRK5, respectively (Homan and Tesmer, 2014, Thal et al., 2012).
4. Arrestin
The first signaling mechanism of arrestin was deciphered in 1999, and receptor-bound arrestin was shown to exhibit Src-dependent activation of pro-proliferative mitogen-activated protein (MAP) kinase and extracellular signal-regulated kinase1/2 (ERK1/2) (Luttrell et al., 1999). Among the four β-arrestin isoforms identified, two are non-visual, arrestin-2 (β-arrestin 1) and arrestin-3 (β-arrestin 2), which are ubiquitously expressed and critical in GPCR desensitization (Pierce et al., 2002). In 2000, arrestin-3 was reported to scaffold ASK1-MKK4/7-JNK cascade receptor-dependently (McDonald et al., 2000). Recent findings have indicated that arrestin-mediated signaling responding to GPCR activation cannot be detected in “zero functional G cells” (total absence of G protein activity) due to genetic knockout of members of the Gs, Gq, and G12/13 families and inactivation of Gi family members by pertussis toxin (Grundmann et al., 2018, Alvarez-Curto et al., 2016). Also, arrestin-2/3 knockout cells, ERK1/2 phosphorylation reacting to numerous GPCR activation, which is regularly considered a characteristic of arrestin-mediated signaling, is like that in parental cells with a full complement of non-visual arrestins.
Initially, it was suggested that arrestin activation involves receptor interaction with two separate sites on arrestin: a “phosphorylation sensor” and an “activation sensor” with arrestin. When both sites are engaged and occupied, activation conformational change occurs in arrestin (Gurevich and Gurevich, 2006). Further studies ascertained that the receptor interacts with arrestin at two separate interfaces. The cytoplasmic tail of the receptor binds within positively charged residue in the N domain, while the receptor core (transmembrane helices and loops) binds between the N and C domains.
A plethora of studies confirmed that receptor core binding is not compulsory for activating arrestin and the Rp tail to encourage arrestin conformational modification and signaling (Cahill et al., 2017, Thomsen et al., 2016, Shukla et al., 2013). Recently, Latorraca et al. (2018) performed extensive all-atom molecular dynamics simulations of arrestins to establish whether the receptor core is significant in the activation mechanism. It was found that diverse receptor binding modes stimulate arrestin, postulate a structural establishment for designing functionally selective (biased) GPCR-targeted ligands with preferred properties on arrestin signaling. Numerous crystal studies of arrestin confirmed that upon activation, there is a twist of the C domain of around 20 degrees regarding the N domain (Zhou et al., 2017, Cahill et al., 2017, Shukla et al., 2013).
In addition to the important role of β-arrestin in regulating rapid receptor desensitization, internalization, and ubiquitination, it is clear that β-arrestin binds to GPCRs with differing affinity, and this induced the proposal of two classes of GPCR (classes A and B) defined by their β-arrestin1/2 selectivity/affinity and longevity of interaction (Oakley et al., 2000). Class A GPCRs, which include the β2-adrenoceptor, μ-opioid receptor, and D1 dopamine receptor, has a higher affinity for β-arrestin-2 compared to β-arrestin1, and the receptor-arrestin complex dissociates quite rapidly either at the cell surface or shortly after internalization, and this may account for the rapid recycling characteristic of this subgroup (Moore et al., 2007, Oakley et al., 2000). Class B GPCRs, which include the angiotensin II (AT1A) receptor, neurotensin 1 receptor, V2 vasopressin receptor, and neurokinin-1 receptor, have a similar affinity for both arrestins 2 and 3 and form prolonged associations with arrestin, allowing the receptor-arrestin complex to be observed within endosomes (Moore et al., 2007, Oakley et al., 2000). Thus, it has been demonstrated that the sustained binding of β-arrestin to V2 vasopressin receptors results in slow recycling, with the receptor taking approximately 4 h to recycle through endosomes and back to the plasma membrane (Oakley et al., 1999).
5. GPCR signaling
5.1. Classical GPCR signaling
It is presumed that GPCRs exist in two different conformations, either active or inactive “Binary switch” foundations for the classical protracted ternary complex model of GPCR-driven signaling (Samama et al., 1993). GPCR naming was presented after their communication with heterotrimeric guanine nucleotide-binding proteins (abbreviated as G proteins). The heterotrimeric G proteins function as transducers of the signal produced by the complex ligand-receptor, thereby activating or inhibiting particular signaling pathways via immediate communication with intracellular effector proteins. The temporary ternary complex, the agonist-receptor-G protein, assists in activating G protein via exchanging GDP for GTP on the α-subunit. Therefore, the receptor-ligand complex functions as a guanine-nucleotide exchange factor for the G protein heterotrimer. Exchanging GTP for GDP induces α-GTP discharge from βγ-dimer and following communication of the Gα and/or Gβγ subunits with effectors (Cabrera-Vera et al., 2003). Cessation of effector activation or inhibition is conveyed through GTP hydrolysis by the GTPase property of the α-subunit. After hydrolysis, the Gα-GDP quickly re-associates with the Gβγ complex to recreate the inactive G protein heterotrimer. The inherent GTPase property of the Gα-subunit can be expedited by accessory proteins called GTPase-activating proteins and are members of the regulator of the G protein signaling protein family (Pierce et al., 2002).
G proteins are classified according to the Gα-subunit into four main groups: Gαs, Gαi/o, Gαq/11, and Gα12/13. GPCRs frequently interact with particular Gα protein members, although more immoral binding has also been reported (Hermans, 2003).
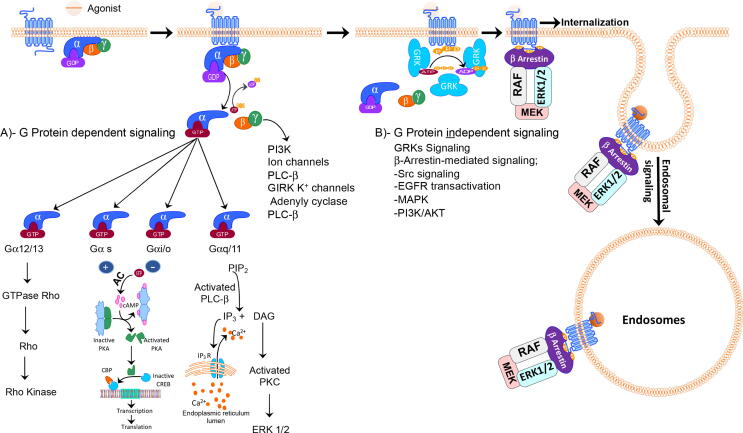
From ATP, the second messenger adenosine (cAMP) is synthesized once the activation of Gαs results in subsequent AC activation (Fig. 2). The rise in intracellular cAMP concentration activates cAMP-dependent PKA, which activates several physiological processes by phosphorylating particular cellular proteins. For instance, the PKA-dependent phosphorylation of myosin light-chain kinase instigates the relaxation of smooth muscle. cAMP production can also be triggered by Gβγ-subunits, which elevate AC activity by some isozymes (Clapham and Neer, 1997). Coupling to Gαi/o prevents AC activity, thereby decreasing the cAMP intracellular concentration. Gβγ-subunit of Gαi/o protein activation may also control some ion channels, comprising G protein-regulated, inwardly rectifying K+ channels, which are normally facilitated by the Gβγ-subunits of Gαi/o (Pierce et al., 2002). Gαq/11 protein subfamily members excite phosphoinositide turnover by activating phospholipase Cβ (PLCβ) and the hydrolysis of phosphatidylinositol-4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). In the endoplasmic reticulum, IP3 binds to IP3 receptors and induces Ca2+ release into the cytoplasm, whereas DAG activates the conventional and novel isoenzymes of PKC (Cordeaux and Hill, 2002). GPCR activation of Gα12/13 proteins may enlist guanine-nucleotide exchange factors for monomeric GTPases, for example, p115RhoGEF assisting GTP-for-GDP exchange (Kozasa et al., 1998). Similarly, GPCR also controls monomeric GTPases, such as Rho, to regulate some cellular events, including cell migration, proliferation, and angiogenesis (Heasman and Ridley, 2008) (Fig. 1). After activating and dissolving the heterotrimeric G protein, Gβγ controls the activity of the gamut of effectors involving AC, PLC, and various ion channels (Clapham and Neer, 1997). The capability of GPCRs to stimulate MAP kinase pathways and ERK was established to follow both G protein-dependent and β-arrestin-dependent pathways (Marinissen and Gutkind, 2001, Defea et al., 2000) (Fig. 2).
Fig. 2.
Schematic diagram of GPCRs signaling steps, A)- G protein-dependent signaling; Following binding of agonist to the extracellular domain of receptor results in conformation change of receptor including cytosolic c-Terminus leading to recruit specific heterotrimeric G protein. Exchange of GDP by GTP of Gα subunit (Gαq, Gαs, Gαi, and Gα12/13) results in dissociation of heterotrimeric G proteins and downstream signaling of Gα from βγ. B)- G protein independent signaling; Following termination of first wave signals by PKC, PKA, or GRKs that lead to β-Arrestins recruitment to c-terminus of the receptor. β-Arrestins will prevent further G proteins coupling, facilitate internalization of receptor and act as a scaffold for MAP kinase signaling as a second signal wave.
5.2. Non-classical GPCR signaling
GPCR activation and signaling are regulated by a process called receptor desensitization, which is characterized by the loss of receptor response due to prolonged or overstimulation (Lefkowitz, 1998). GPCR desensitization is described as the physical uncoupling of G proteins from their couple receptors, subsequently resulting in a loss in receptors’ capability to activate and initiate further intracellular signaling (Penela et al., 2003). The receptor desensitization process initiated by GPCR phosphorylation that can be provoked by second messenger kinases, such as PKA and PKC, which can phosphorylate and affect the responsiveness of both agonists occupied and unoccupied receptors, thereby producing heterologous desensitization (Willets et al., 2003, Hausdorff et al., 1990). Receptor phosphorylation can also be mediated by GRKs, seven isoforms of serine/threonine kinases belonging to the AGC kinase family (Homan and Tesmer, 2014), phosphorylating specifically agonist-occupied active GPCRs, mediating the homologous receptor desensitization process (Kelly et al., 2008, Willets et al., 2003) (Fig. 1). Receptor activation provokes GRK recruitment and consequently phosphorylates the receptor’s serine/threonine moieties in the third intracellular loop or C-terminal tail (Lefkowitz, 1993, Benovic et al., 1991), inducing the formation of phosphorylation barcoding, a specific phosphorylation configuration of the receptor (Penela et al., 2010). Receptor phosphorylation subsequently enhances the affinity for β-arrestin protein binding (Scheerer and Sommer, 2017). This physical association between GPCR and β-arrestin protein “phosphorylated GPCR-β-arrestin complexes” further inhibits GPCR and G-protein interaction. Accordingly, the termination of the G protein-dependent signaling cascade commences (Gurevich and Gurevich, 2019). Receptor-arrestin complexes are subjected to clathrin-mediated endocytosis. Depending on the interaction potency, the receptors undergo recycling, internalization into endosomes, or degradation (Penela et al., 2010, Kelly et al., 2008). The GPCRs can be classified according to the strength of the GPCR-β-arrestin interaction into class A GPCR, weak interaction receptors such as β2 adrenergic receptors. Thus, the formation of a transient receptor-β-arrestin complex undergoes recycling into the cell membrane. Also, class B GPCR characterized by strong receptor-β-arrestin interaction, such as vasopressin V2 receptor, induces sustained internalization into endosomes (Luttrell and Lefkowitz, 2002). Importantly, Thomsen et al. (2016) described the strong interaction between receptors and β-arrestin as supercomplexes or mega complexes mediating sustained/prolonged G protein signaling intracellularly.
Earlier evidence revealed that both GRKs and β-arrestins have multiple functions beyond GPCR regulations (Lefkowitz, 1998). They can interact with various intracellular substrates and contribute to signaling transduction G protein-independently, termed non-classical or non-canonical GPCR signaling (Shenoy and Lefkowitz, 2011, Penela et al., 2010).
GRKs are multi-domain proteins containing a central serine/threonine protein kinase catalytic domain, an N-terminal domain including a regulator of the G protein signaling homology domain, and various C-terminal domains (Homan and Tesmer, 2014, Penela et al., 2010). As multiple domain proteins, they can interact with diverse proteins or act as scaffolds for molecules implicated in cellular signaling. For instance, previous studies have shown that GRK2 interacts with clathrin, phosphoinositide 3-kinase, MEK, and caveolin-1(Jimenez-Sainz et al., 2006, Schutzer et al., 2005, Naga Prasad et al., 2002, Shiina et al., 2001). Moreover, GRK2 can be implicated in cellular signaling phosphorylation-dependently, such as R-Smad phosphorylation (Ho et al., 2005). GRK5 can also phosphorylate or interact with several proteins, such as p53, IκBα, PDGF, class II histone deacetylase 5, and activating-nuclear factor of activated T cells (Hullmann et al., 2014, Islam et al., 2013, Chen et al., 2010, Martini et al., 2008, Wu et al., 2006). Additionally, β-arrestins act as ligand-regulated adaptor scaffolds facilitating intracellular signaling via recruiting various proteins to activate GPCRs (Luttrell and Miller, 2013). Several studies have shown that β-arrestins act as scaffolds for ERK signaling (Ren et al., 2005, Luttrell et al., 2001). β-arrestin 1 interacts directly with MEK1 (Meng et al., 2009) (Fig. 2). Some GPCRs including parathyroid hormone receptor (PTHR) and thyroid-stimulating hormone receptor (TSHR) can generate cAMP after receptor internalization. These receptors exhibit the endosomal-mediated generation of cAMP intracellularly (Vilardaga et al., 2014). Furthermore, β-arrestin 2 is implicated in V2 receptor-mediated ERK1/2 activation (Ren et al., 2005). Kim et al. (2005) also reported that GRK5 and 6 mediated AT1 receptor phosphorylation enables β-arrestin 2-mediated ERK1/2 activation (Kim et al., 2005), indicating that β-arrestins play a major role in ERK signaling via β-arrestin-dependent mechanisms. Therefore, multiple phosphorylation-dependent and/or independent GRK interactions, or β-arrestins scaffolding, indicate that GRKs and β-arrestins might be implicated in regulating several cellular signaling cascades G-protein-independently.
5.3. G protein preassembly
In 2007, the concept of a pre-associated complex between PAR1 (protease-activated receptor 1) and Gαi1 protein was established after being transiently expressed in COS-7 cells (Ayoub et al., 2010, Ayoub et al., 2007). In 2011, Qin et al. proposed a mechanism by which some GPCRs assemble with G proteins before ligand-induced activation (Ayoub et al., 2012, Qin et al., 2011). The proposed mechanism of preassembly describes how M3 muscarinic acetylcholine receptors (M3R) form inactive-state complexes with Gq heterotrimers in intact cells. Substantial evidence for the existence of a preassembled complex has been ascertained by various techniques, including FRET (fluorescence resonance energy transfer) and BRET (bioluminescence resonance energy transfer) in live cells (Gales et al., 2006). The Preassembly of G proteins and GPCRs sometimes suggests rapid G protein activation and subsequent downstream signaling pathways. Any disruption in preassembly may inhibit downstream signaling. The preassembly theory postulates that the specific molecular organization of GPCRs and G proteins may allow a faster process of G protein activation due to a lack of time for the receptor and G protein for collision.
5.4. GPCR allosterism
The allostery concept in GPCR was first proposed by Monod, Wyman, Jacob, and Changeux and formalized the classical model called the Monod-Wyman-Changeux (MWC) (Monod et al., 1965, Monod et al., 1963). In the last two decades, the idea of targeting allosteric sites in GPCR has induced recent drug discovery for pharmaceutical firms (Conn et al., 2009).
The mechanistic understanding of allosterism at GPCR remains incompletely deciphered due to the non-availability of high-resolution structural information on GPCRs and their interacting cohorts (ligands and proteins) and complexes, thereby explaining the promiscuous nature of GPCR. Allosterism is a powerful mechanism of drug action in GPCR. Most drug discoveries are based on targeting the endogenous agonist-binding orthosteric site, ignoring spatially distinct and conformationally linked allosteric sites (Christopoulos et al., 2014). GPCRs are allosteric proteins because of the conformational transition mediating signal transduction, which includes a reciprocal cooperative interaction between the orthosteric-ligand binding site and intracellular transducer positioned around 40 Å distance (Lane et al., 2017). A classical hallmark of allosteric interaction is the ability of one substance to alter the affinity of other molecules through cooperative effects by either changing the association rate or dissociation rate, sometimes both.
The beauty of allosterism is that the mechanism is indistinguishable from the change in the isomerization constant of the unoccupied receptor. This factor explains the spontaneous activation of unoccupied receptors. Ligand binding to allosteric sites exhibits several advantages over orthosteric ligands or drugs. While allosteric ligands that enhance orthosteric agonist activity are called positive allosteric modulators (PAMs), those that inhibit orthosteric agonists are called negative allosteric modulators (NAMs). Also, those exerting no net effect on orthosteric ligands at equilibrium are called neutral allosteric ligands (NALs) (Christopoulos et al., 2014).
GPCR allosteric sites are generally found on the extracellular surface. However, few sites have been found on the intracellular side of the receptors (CCR4 and CXCR2). Domain swapping studies between CCR4 and CCR5 revealed that signal transduction inhibition by prazinyl sulfonamide compounds are presented through the C-terminal domain of CCR4 (Andrews et al., 2008). Recent pepducin discovery is produced by attaching a lipidated group (acyl chain) to a peptide corresponding to a portion of one of the loops of intracellular GPCR (Tressel et al., 2011). The pepducin binding with PAR-1 proposed that the receptor C-terminal tail is a possible interaction site. Thus, it was confirmed that pepducin is among the allosteric modulators of the GPCR function.
5.5. Therapeutic targeting of GPCRs
As of 2020, over 1500 drugs were approved by the FDA, where 460 drugs (36%) target mainly GPCRs. Presently, class A GPCR is targeted by most of the drugs (94%), followed by class B (4%), class C (2%), and class F (2%). Rask et al. reported that around 19% of the human genomes are targeted by drugs, which directly or indirectly affect GPCR (Rask-Andersen et al., 2011). Due to the diverse druggable properties of GPCR, the data showed most of the clinical trials were conducted by the US FDA and other countries for the clinical development of drugs. GPCRs are connected to numerous signaling cascade pathways, exhibiting their effect in minute concentrations (Table 1).
Table 1.
Application of different types of drugs on GPCRs.
| S. No | Targets/Name of receptor | Disease-associated | Mechanism of action | Name of drug | Manufacturer | Status |
|---|---|---|---|---|---|---|
| Anti-diabetic Drugs | ||||||
| 1. | AMY 1-3 | Type 1& 2 diabetes | Peptide agonist | Pramlintide | BMS/Amylin | Market |
| 2. | GLP-1 & GIP | Type 2 diabetes | Competitively inhibit DPP-4 | Sitagliptin (Januvia) | Merck & Co | Market |
| 3. | GLP-1 | Type-2 diabetes | Peptide agonist | Albiglutide | GSK | Market |
| 4. | GLP-2 | Type 2 diabetes | Peptide agonist | Teduglutide | NPS Allelix | Market |
| 5. | Prostanoid receptor | Ischemic stroke, pain, inflammation | Irreversibly acetylate Ser530 of COX-1, inhibits the generation of thromboxane A2 | Aspirin | Bayer Bitterfeld GmbH Germany | Market |
| 6. | Protease activated receptor-1 (PAR-1) | Myocardial infarction, coronary revascularization | Inhibits thrombin related platelet aggregation | Vorapaxar (Zontivity) | Merck and Co. | Market |
| Anti-platelet & Cardiac Drugs | ||||||
| 7. | P2Y12 | Acute coronary syndrome, peripheral artery disease | Selectively inhibits the binding of ADP to P2Y12 | Clopidogrel (Plavix) | Bristol-Myers-Squibb-Sanofi | Market |
| 8. | P2Y12 | Stent thrombosis & myocardial infarction | Blocks ADP-induced platelet activation and aggregation | Cangrelor (Kangreal) | Parsippanny | Market |
| 9. | A2A Adenosine receptor | Coronary vasodilator | Elevated coronary flow reserve via selective A2A receptor activation | Regadenoson (Lexiscan) | Astellas Pharma | Market |
| 10. | α-2 Adrenergic receptors | Myocardial ischemia, clinical anesthesia | Neuronal hyperpolarization | Dexmedetomidine (Precedex) | Pfizer | Market |
| 11. | Beta adrenergic receptor | Myocardial infarction, hypertension | Function as beta 1 receptor antagonists | Acebutolol (Sectral) | Sanofi Aventis | Sanofi Aventis |
| Miscellaneous drugs acting on different types of receptors | ||||||
| 12. | CTR | Osteoporosis, hypercalcemia, Paget’s hemiplegia | Acts primarily on bone | Calcitonin- Salmon | Novartis | Market |
| 13. | CGRP | Migraine | CGRP receptor mab | Galcanezumab (LY2951742) | Eli Lilly | Phase III |
| 14. | GHRH | HIV-associated lipodystrophy | Peptide agonist | Tesamorelin | Theratechnologies Inc | Market |
| 15. | PTH1 | Osteoporosis | Peptide agonist | Teriparatide | Eli Lilly | Market |
| 16. | CXCR4 | Cancer | Mobilizes hematopoietic stem cells | Plerixafor (Mozobil) | AnorMED/Genzyme | Market |
| 17. | α-1 Adrenergic receptors | Epistaxis, Drooping eyelids | Vasoconstrictor and targets subset of adrenoreceptors in Muller’s muscle of the eyelid | Oxymetazoline (Afrin, Otrivin) | Merck | Market |
| 18. | β-3 Adrenergic receptor | Overactive bladder | Relaxes detrusor smooth muscle | Mirabegron (Myrbetriq) | Astellas Pharma Inc | Market |
| 19. | AT1 receptor antagonists | High blood pressure, congestive heart problem | Selectively block the binding of angiotensin II to AT1 | Candesartan (Atacand) | AstraZeneca, Takeda Pharmaceuticals | Market |
| 20. | Bradykinin B2 receptor antagonist | Hereditary angioedema | Elevates vessel permeability dilates blood vessel and contracts smooth muscle cells | Icatibant (Firazr) | Shire Plc | Market |
| 21. | Calcium sensing receptor | Chronic kidney disease | Allosteric activator of the parathyroid gland | Etelcalcetide (Parsabiv) | Amgen | Market |
| 22. | MT1 and MT2 receptor | Insomnia and sleep disorders | Inhibits neuronal firing | Ramelteon (Rozerem) | Zydus Cadila | Market |
| 23. | NTS2 receptor | Conjunctivitis | Prevents histamine binding and activity | Levocabastine (Livostin) | Janssen Pharmaceutica | Market |
| 24. | Mu-opioid receptor | Primarily used for local anesthesia in surgery | Inhibits the release of numerous neurotransmitters | Alfentanil (Alfenta) | Janssen Pharmaceutica | Market |
| 25. | Prostaglandin E1 receptor | Stomach ulcer, postpartum bleeding | Inhibits basic & nocturnal gastric acid secretion | Misoprostol (Cytotec) | Pfizer | Market |
| 26. | Prostaglandin F receptor | Ocular hypertension, open-angle glaucoma | Increases outflow of aqueous humor | Latanoprost (Xalatan) | Pfizer | Market |
| Neurological drugs | ||||||
| 27. | GPR143 receptor | Parkinson disease | DOPA decarboxylase converts levodopa to dopamine | Levodopa | Sandoz | Market |
| 28. | Muscarinic acetylcholine receptor | Alzheimer’s disease | Donepezil reversibly inactivates the cholinesterase | Donepezil (Aricept) | Pfizer | Market |
| 29. | Serotonin 1A (5-HT1A) receptor | Schizophrenia, Bipolar disorder | Aripiprazole is a partial HT1A agonist | Aripiprazole (Abilify) | Bristol-Myers Squibb | Market |
| 30. | Kainate & NMDA receptor | Amyotrophic lateral sclerosis | Directly inhibit kainite and NMDA receptor | Riluzole (Rilutek) | Sanofi Aventis | Market |
| 31. | 5-HT3 receptor | Epilepsy | Repress the release of glutamate & aspartate in the CNS | Lamotrigine (Lamictal) | GlaxoSmithKline | Market |
Looking for examples of antiplatelet prescriptions/drugs, numerous antiplatelet prescribed by FDA approved drugs recommended by medical practitioners are aspirin, vorapaxar, and clopidogrel, which prevent blood coagulation having a history or risk of developing coronary artery diseases, myocardial infarction, stable angina, and acute ischemic stroke (Ahmed, 2019). Aspirin is the most commonly prescribed medicine worldwide due to its various features, such as anti-inflammatory, anti-thrombotic, anti-pyretic, and antiplatelet (Ittaman et al., 2014). The exact mechanism of action of aspirin was discovered in 1971; however, aspirin usage dates back to the prehistoric times of Mesopotamian, Greek, Galen, and Chinese civilization. The action mechanism involves irreversible cyclooxygenase (COX) inhibition and associated suppression of prostaglandin derivative synthesis, which signals prostaglandin receptors. Subsequent studies ascertained that anti-thrombotic aspirin effects were due to COX acetylation in platelets (Miner and Hoffhines, 2007). However, the dose-dependent effect of aspirin has been reported in numerous clinical trials in the last few decades. Also, vorapaxar, an antagonist of protease-activated receptor 1 (PAR-1), can reduce the metastasis and proliferation of cancerous cell lines. A recent study has shown that KPC-derived tumor cells with PAR-1 knockout considerably reduced tumor growth in the tail vein lung metastatic experiment, ensuing tumor cell injection into C57/BL6 mice. The study results ascertained that pancreatic ductal adenocarcinoma is determined by activating the coagulation system through a tumor cell-derived transcription factor. This establishes the modifications of tumor-immune cell cross-talk mainly by stimulating CD8+ T cell activity through PAR1/thrombin dependent signaling pathway (Yang et al., 2019). Clopidogrel is among the mainstay drugs of coronary heart disease as its active metabolite covalently interacts with the ADP binding site of P2Y12 receptor positioned on platelets, thereby thwarting the activation of the glycoprotein complex necessary for activating platelet (Savi et al., 2006).
Furthermore, diabetes is a chronic disease categorized by inadequate insulin production (type 1) or insulin responsiveness (type 2), causing increased glucose concentrations in the bloodstream. In the United States, around 9% of the population has diabetes mellitus, and anti-diabetes treatments are prescribed (Bullard et al., 2018). Glucagon-like peptide 1 (GLP-1) is a gastrointestinal peptide hormone secreted from the three main tissues of the human body: enteroendocrine L cells (distal intestine), alpha cells (pancreas), and the central nervous system. The hormone exhibits its effect by interacting with and activating members of class B GPCR, i.e. GLP-1 receptor (GLP-1R). GLP-1interaction with its receptor (GLP-1R) activates heterotrimeric Gαs protein, which consequently stimulates AC activity. The ensuing event induces cAMP formation, and the increased cAMP concentration, in turn, activates PKA and the cAMP-regulated guanine nucleotide exchange factor (Ozaki et al., 2000). In the next sections, we will elaborate more on “main players” mediating and regulating GPCR signaling, including G proteins, GRKs, and β-arrestins.
5.6. GPCR genetics
Understanding GPCR genetics is fundamental in GPCR pharmacology because variants in the gene sequence of the receptor could be combined with alterations in receptor function, thereby affecting drug response (Thompson et al., 2014). Additionally, a mutation in the GPCR gene might participate in creating pathological phenotype GPCR sequences inducing pathological condition development (Stoy and Gurevich, 2015). GPCR mutations can be classified based on their influence on receptor function and signaling capability into either mutation that amplifies signaling transduction, in this case, termed gain of function, or attenuates receptor-related signaling, called loss of function (Stoy and Gurevich, 2015, Thompson et al., 2014). Moreover, GPCR mutations influence receptors’ function via various mechanisms, such as changing receptor basal activity, ligand binding ability, receptor cell membrane expression, and receptor/G-proteins interactions (Stoy and Gurevich, 2015). For example, it has been reported that mutation in the 5-Alphahydroxytryptamine receptor 1B (5-HTR1B) gene alters receptor function via modification of the ligand-binding ability of the receptor (Kiel et al., 2000). Also, a mutation in the α2A-adrenergic receptor gene (ADRA2 A) increased agonist-dependent G protein coupling, inducing a gain of receptor function (Small et al., 2000). Similarly, β1-adrenergic receptor (ADRB1) gene polymorphisms induce gain in receptor function via enhancing both basal and agonist dependent effect, i.e., enhancing Gs-mediated adenylyl cyclase activation (Akhter et al., 2006, Mason et al., 1999). Conversely, missense variations in the calcium-sensing receptor (CaR) gene attenuate receptor transduction signaling capability and, therefore, loss of receptor function (Bai et al., 1997, Janicic et al., 1995). Another example of receptor function attenuation is a mutation in the gonadotropin-releasing hormone receptor (GNRHR) in the GPR54 gene, inducing loss of receptor function (de Roux et al., 2003). Also, the mutation in the endothelin receptor B (EDNRB) gene attenuates Gq coupling of the receptor, thereby affecting subsequent signaling (Imamura et al., 2000, Puffenberger et al., 1994).
Importantly, the consequence of GPCR gene variants is not limited to receptor activity or signaling transduction; gene variants can be a predisposition factor for pathological disease developments (GAO, 2021). GPCRs are implicated in various diseases, such as obesity, type II diabetes, cancer, cardiovascular, immunological, and neurodegenerative diseases (Heng et al., 2013). Mutation-induced GPCR dysfunctions have been linked to several pathological conditions. For example, a mutation in follicle-stimulating hormone receptor (FSHR) induces various consequences affecting reproductive system function (Simoni et al., 2002, Gromoll et al., 1996). Such mutations in FSHR induce familial spontaneous ovarian hyperstimulation syndrome (Tao, 2008). Additionally, a mutation in the thyroid-stimulating hormone receptor (TSHR) triggers familial gestational hyperthyroidism (Rodien et al., 1998). Another example is the melanocortin-4 receptor (MC4R), which is mostly expressed in neuronal cells and is critical in controlling eating behaviors and energy homeostatic regulation. Mutations in MC4R have been associated with obesity (Santini et al., 2009, Hinney et al., 2003). The mutation-induced disease has been reported with arginine vasopressin type 2 receptor (V2R), Gs-coupled GPCR. V2R is mainly expressed in the collecting duct cells of the kidney, mediating the enhancement of water permeability via PKA activation and consequently incorporating water channel aquaporin-2 (Juul et al., 2014). Loss of function mutations in V2R gene has been associated with nephrogenic diabetes insipidus characterized by an incapability to concentrate urine (Bichet, 2009).
Overall, mutated GPCRs are combined with alterations in receptor functions and subsequently various disease phenotypes. Identifying such receptor variants helps determine the molecular mechanisms participating in pathological conditions development. Additionally, understanding such translation from receptor variant genes into disease phenotype development will assist in establishing a way to prevent or treat GPCR mutations associated with pathological disorders.
6. GPCRs in drug discovery
6.1. GPCR nanobodies
In 2012, the Nobel Prize was awarded to Robert Lefkowitz and Brian Kobilka for their efforts and discoveries in the GPCR field, including the crystal structure of several GPCRs (Lefkowitz, 2013, Van Noorden, 2012). Such discoveries open very promising opportunities for drug discovery and GPCRs research. Three discoveries also discussed the bright future of the GPCR research field.
First, in addition to small chemical molecules, GPCRs can be targeted by monoclonal antibodies (mAbs), and several mAbs have been generated and approved as therapeutic targets, such as mogamulizumab (POTELIGEO®), in treating leukemia (Hutchings, 2020, Beck and Reichert, 2012). The advantage of using mAbs in treating various diseases is notable because they have a long half-life. However, producing mAbs is costly, and the route of administration (which cannot be taken orally) may be disadvantageous for such a venue (Mujic-Delic et al., 2014). Interestingly, immunizing camel or llama with GPCRs induces the production of antibodies with only heavy chains and is considered to be 10 times smaller than classical antibodies and named nanobodies (Nbs). The first GPCRs-Nbs generated are against chemokine receptors (CXCRs) (Maussang et al., 2013, Jahnichen et al., 2010). These generated Nbs harvested after immunization comprise a library of different Nbs with different pharmacological properties, such as agonists, antagonists, or inverse agonists (Jahnichen et al., 2010). In addition to the small size and selective single amino acid target of Nbs, several advantages have been observed for GPCRs-Nbs, including stability either thermal (up to 70 °C) or acidic environment (can be administered orally) (Harmsen et al., 2006, Dumoulin et al., 2002). Furthermore, Nbs exhibit a very short half-life (about 2 h), making them proper as diagnostic tools like imaging and can be PEGylated or incorporated into serum albumin to extend the duration of action up to several weeks (Mujic-Delic et al., 2014). Therefore, generating Nbs against various GPCRs in the future will hopefully increase selectivity toward therapeutic targets and reduce undesirable signals and effects. In Table 2, we prepared a list of therapeutic nanobodies under clinical trials, and a few have been approved by FDA for therapeutic application for treating patients.
Table 2.
Application of nanobodies against various diseases under investigation for therapeutic use.
| S. No | Name of Nanobody | Mechanism of Action | Name of Disease | Format | Manufacturer | Clinical trial (Phase) | References |
|---|---|---|---|---|---|---|---|
| 1. | DN 10, DN 13 | Interacts with metabotropic glutamate receptors | Impairment in CNS | NA | NA | NA | Scholler P et al., 2017 |
| 2. | ALX0141 | Anti-RANKL (key mediator of bone resorption) | Osteoporosis | Trivalent, bispecific | Albynx | I | |
| 3. | Vobarilizumab | IL-6 receptor antagonists | Systemic lupus erythematosus, Rheumatoid arthritis | Bivalent, bispecific | Albynx, Abbvie | II | |
| 4. | Caplacizumab | Blocks platelet aggregation and anti-von Willebrand factor | Thrombosis, acquired thrombotic thrombocytopenic purpurea | Bivalent, monospecific | Ablynx (Sanofi) | Approved | |
| 5. | Ozoralizumab | Effectively neutralizes TNF-α and interacts with HSA | Rheumatoid arthritis | Trivalent, bispecific | Ablynx, Taisho | II | |
| 6. | Lulizumab pegol | Modulators of CD28 | Systemic lupus erythematosus | Monomeric pegylated | Bristol-Myers Squibb | II | Shi R et al., 2016 |
| 7. | M6495 | Inhibitor of ADAMTS-5 | Osteoarthritis | Bifunctional | Merck | Ib | |
| 8. | TAS266 | Interacts death receptor-5 | Advanced solid tumors | Tetramer | Novartis pharmaceuticals | I | Papadopoulos et al |
| 9. | ARP1 | Rice-based expression system to allow ARP1 in children | Rotavirus diarrhea | Monomeric | CMC Vellore India | III completed | |
| 10. | 2Rs15d | Targets HER2 | Breast cancer | Monomeric radionuclide | Camel-IDS | I |
NA: Not applicable, HAS: Human serum albumin, ADAMTS-5: A Disintegrin And Metalloprotease with Thrombospondin-motifs-5.
6.2. GPCR-biased signaling
When GPCRs are exposed to a neutral agonist, such as morphine on μ-opioid receptor, an occupied receptor can generate several signal waves (non-biased agonist). In GPCR signaling, the ability of a molecule to selectively activate one pathway without affecting another pathway is called biased agonism. Biased signaling occurs at different signaling proteins, including G proteins, GRKs, β-arrestins, and even at levels of the allosteric binding site (Ilter et al., 2019). Since GPCR activation-induced two distinct signal waves, G protein-dependent signaling followed by β-arrestin-dependent signaling opens a new promising therapeutic future in the world of GPCRs. This is true since discovering such molecules dramatically lowers the adverse effects by turning off unwanted signals (Ilter et al., 2019). For example, the analgesic effect of morphine (neutral agonist) through the activation of μ-receptors is accompanied by several side effects, including constipation, respiratory depression, tolerance, nausea, and sedation. A recent study has shown that the analgesic effect is associated with the G-protein signaling pathway, while other unwanted effects are associated with β-arrestin-mediated signaling (Rankovic et al., 2016). Linking these effects to a specific signaling pathway induced the discovery of “TRV 130” (in phase II clinical trials) with a selective analgesic effect via G protein-dependent signals without β-arrestin signaling-mediated unfavorable effects (Dewire et al., 2013, Viscusi et al., 2016). Recently, the second molecule named PZM21 was discovered as a biased analgesic promising drug (Manglik et al., 2016). Furthermore, since most antipsychotic medications act as D2 antagonists, they cause many undesirable effects, including extrapyramidal unwanted effects. An FDA-approved drug since 2002, aripiprazole, is an excellent example of biased agonism since it preserves antipsychotic effects without undesirable effects via activating only β-arrestin-D2 signaling pathway but not the G protein-D2 signaling pathway (Rankovic et al., 2016). Considering the heart failure model, carvedilol induces β2-adrenergic receptors-mediated β-arrestin signaling wave against Gαs-dependent signaling having a cardioprotective effect advantage (Wisler et al., 2014). Furthermore, stimulation of the angiotensin II type 1 receptor (ATR1) induces Gαs-dependent signaling, which is considered to have a deleterious effect on the heart and kidney, and β-arrestin-mediated signaling having a cardiac and renal protective effect. Recently, a molecule, TRV120027, can selectively activate ATR1-β-arrestin-mediated signaling, thereby inducing a cardiorenal protective effect (Lymperopoulos, 2018, Boerrigter et al., 2012). Therefore, the biased agonism concept is a very promising field in GPCR drug discovery.
6.3. GPCR splice variants
Among the critical facts about GPCRs is the splice variant existence for most discovered receptors. Receptor genetic variations mainly occur at the orthosteric binding site, which induces differences in efficacy and potency (Hauser et al., 2018). Three types of variations were observed: missense variation, loss of function variation, and copy number variation. A previous study has shown that, among 108 GPCRs, each individual out of 2504 human samples has 68 missense variations in the coding region of 33% GPCRs. The same study also demonstrated how different splice variants of μ-opioid receptors exhibit significant pharmacological differences, including efficacy and potency. Another example is the significantly different antihypertensive responses to angiotensin receptor blockers, angiotensin converting enzyme (ACE) inhibitors, and β-blockers due to splice variants of angiotensin receptors, ACE, and β-adrenergic receptors, respectively (Johnson, 2008, Liggett et al., 2006, Mialet Perez et al., 2003). At the level of G protein coupling to receptors, a study of cholecystokinin A receptor using one drug that induced a couple to all the main four Gα isoforms Gαq, Gαi/o, Gαs, and Gα12/13 reveals the existence of two polymorphisms. One of the polymorphisms has increased efficacy to Gαq and Gαi/o but not Gαs and lacks efficacy to Gα12/13, while other polymorphisms lack efficacy and potency in all isoforms, except Gαs potency (Hauser et al., 2018). Therefore, considering genetic variations during diagnosis and drug discovery will dramatically avoid undesirable effects and tailor safe and personalized medicine.
6.4. Heterodimerization in GPCRs
In 1993, the term “heterodimerization” was unprecedentedly introduced to explain a specific type of direct interaction between various types of GPCRs (Zoli et al., 1993). The heterodimerization concept was later ascertained in studies where two non-functional GPCR monomers (GABAB1 and GABAB2) connect in a signaling heterodimer (Marshall et al., 1999). Considering their functions, GPCR heterodimers are classified into two categories: obligatory and non-obligatory GPCR heterodimers. Obligatory GPCR heterodimers need heterodimerization of GPCR protomers to work as functional receptors, and a few examples are GABA and taste receptors. Also, non-obligatory GPCR heterodimers comprise functional protomers and control the pharmacological and biochemical events of the promoters. GPCR functions depend not only on protomer and homodimer but also on heterodimers. Heterodimerization of GPCRs modulates molecular events of the GPCR protomer, and the ensuing process may cause ligand binding affinity, signal transduction, and internalization.
Clinically, heterodimerization of GPCRs is critical, as it was observed in the unusual crosstalk between different classes of drugs, exemplified by beta-blockers, where it may block signaling by both β-adrenergic and angiotensin receptors. This may be due to the heterodimerization of βARs and AT1 angiotensin receptors (Barki-Harrington et al., 2003). Likewise, the interaction between δ-OPR (opioid receptor) and μ-OPR has been proposed to explain the well-known results of δ-OPR on μ-OPR-mediated analgesia (Gomes et al., 2004). In a clinical setting, synergism and antagonism between drugs are crucial, as heterodimerization between receptors embodies a particular mechanism and perhaps underlies potential drug–drug interaction at the molecular level. The clinical significance of heterodimerization has also been observed in chemokine receptors. Chemokines are a family of cytokines responsible for activating leukocytes via GPCR stimulation. Chemokine receptors (CXCR4 and CCR5) serve as coreceptors for the entry of HIV into cells (Cairns and D'Souza, 1998). The polymorphism (V64I) in the CCR2 receptor is associated with a significantly reduced rate of AIDS progression (Table 2) (Smith et al., 1997). Mellado et al. (1999) revealed that the V64I polymorphism augments heteromerization between CCR2/CCR5 and CCR2/CXCR4. The above data suggest that heteromerization of CCRs is a fundamental factor aiding HIV in using these receptors to enter into the cells. Clinical implications of GPCR heteromerization of various diseases, such as asthma, cardiac failure, preeclampsia, AIDS, and Parkinson's disease, are presented in Table 3.
Table 3.
Some of the pathological manifestations due to heterodimerization of GPCRs.
| S. No | Disease | Receptors involved | Molecular mechanism | References |
|---|---|---|---|---|
| 1. | Asthma | EP1/B2AR |
|
McGraw DW et al., 2016 |
| 2. | Cardiac failure | AT1R/β-AR |
|
Barki-Harrington L et al., 2003; Barki-Harrington L et al., 2003 |
| 3. | Preeclampsia | AT1R/B2R |
|
Quitterer U et al., 2004; AbdAlla S et al., 2005 |
| 4. | AIDS | CCR2/CCR5/CXCR4 |
|
Mellado M et al., 1999; Lee B et al., 1998 |
| 5. | Parkinson disease | A2aR/D2R |
|
Fuxe K et al., 2005 and 2007 |
EP1: prostaglandin E1 receptor.
7. Conclusion
GPCRs are a family of cell surface receptors that respond to a plethora of external signals. Owing to their diverse functions, GPCRs and their associated signaling are considered vital to life. The interaction of signaling molecules with GPCRs marks G protein activation, thereby activating the production of various second messenger molecules. Through these molecular events, GPCRs assist in regulating various biological functions, starting from sensation to growth to hormone responses. Considering the multifaceted role of GPCR, it is imperative to comprehend the molecular events and the discovery of new drugs. GPCRs display their position as possible drug targets in a gamut of diseases, including cancer. Despite recent advances in drug discovery, it is essential to determine new avenues, as GPCRs are the most ubiquitous receptors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed, A.A.M.A., 2019. Antiplatelet drug management. In: Patient Blood Management in Cardiac Surgery. Springer, 51–60.
- Akhter S.A., D'Souza K.M., Petrashevskaya N.N., Mialet-Perez J., Liggett S.B. Myocardial beta1-adrenergic receptor polymorphisms affect functional recovery after ischemic injury. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1427–H1432. doi: 10.1152/ajpheart.00908.2005. [DOI] [PubMed] [Google Scholar]
- Alexander S.P., Benson H.E., Faccenda E., Pawson A.J., Sharman J.L., Spedding M., Peters J.A., Harmar A.J., Collaborators C. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br. J. Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Curto E., Inoue A., Jenkins L., Raihan S.Z., Prihandoko R., Tobin A.B., Milligan G. Targeted elimination of G proteins and arrestins defines their specific contributions to both intensity and duration of G Protein-coupled Receptor Signaling. J. Biol. Chem. 2016;291:27147–27159. doi: 10.1074/jbc.M116.754887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews G., Jones C., Wreggett K.A. An intracellular allosteric site for a specific class of antagonists of the CC chemokine G protein-coupled receptors CCR4 and CCR5. Mol. Pharmacol. 2008;73:855–867. doi: 10.1124/mol.107.039321. [DOI] [PubMed] [Google Scholar]
- Ayoub M.A., Al-Senaidy A., Pin J.P. Receptor-G protein interaction studied by bioluminescence resonance energy transfer: lessons from protease-activated receptor 1. Front. Endocrinol. (Lausanne) 2012;3:82. doi: 10.3389/fendo.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub M.A., Maurel D., Binet V., Fink M., Prezeau L., Ansanay H., Pin J.P. Real-time analysis of agonist-induced activation of protease-activated receptor 1/Galphai1 protein complex measured by bioluminescence resonance energy transfer in living cells. Mol. Pharmacol. 2007;71:1329–1340. doi: 10.1124/mol.106.030304. [DOI] [PubMed] [Google Scholar]
- Ayoub M.A., Trinquet E., Pfleger K.D., Pin J.P. Differential association modes of the thrombin receptor PAR1 with Galphai1, Galpha12, and beta-arrestin 1. FASEB J. 2010;24:3522–3535. doi: 10.1096/fj.10-154997. [DOI] [PubMed] [Google Scholar]
- Bai M., Janicic N., Trivedi S., Quinn S.J., Cole D.E., Brown E.M., Hendy G.N. Markedly reduced activity of mutant calcium-sensing receptor with an inserted Alu element from a kindred with familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. J. Clin. Invest. 1997;99:1917–1925. doi: 10.1172/JCI119359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barki-Harrington L., Luttrell L.M., Rockman H.A. Dual inhibition of beta-adrenergic and angiotensin II receptors by a single antagonist: a functional role for receptor-receptor interaction in vivo. Circulation. 2003;108:1611–1618. doi: 10.1161/01.CIR.0000092166.30360.78. [DOI] [PubMed] [Google Scholar]
- Beck A., Reichert J.M. Marketing approval of mogamulizumab: a triumph for glyco-engineering. MAbs. 2012;4:419–425. doi: 10.4161/mabs.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benovic J.L., Onorato J.J., Arriza J.L., Stone W.C., Lohse M., Jenkins N.A., Gilbert D.J., Copeland N.G., Caron M.G., Lefkowitz R.J. Cloning, expression, and chromosomal localization of beta-adrenergic receptor kinase 2. A new member of the receptor kinase family. J. Biol. Chem. 1991;266:14939–14946. [PubMed] [Google Scholar]
- Bichet D.G. V2R mutations and nephrogenic diabetes insipidus. Prog. Mol. Biol. Transl. Sci. 2009;89:15–29. doi: 10.1016/S1877-1173(09)89002-9. [DOI] [PubMed] [Google Scholar]
- Boerrigter G., Soergel D.G., Violin J.D., Lark M.W., Burnett J.C., Jr. TRV120027, a novel beta-arrestin biased ligand at the angiotensin II type I receptor, unloads the heart and maintains renal function when added to furosemide in experimental heart failure. Circ. Heart Fail. 2012;5:627–634. doi: 10.1161/CIRCHEARTFAILURE.112.969220. [DOI] [PubMed] [Google Scholar]
- Bullard K.M., Cowie C.C., Lessem S.E., Saydah S.H., Menke A., Geiss L.S., Orchard T.J., Rolka D.B., Imperatore G. Prevalence of Diagnosed Diabetes in Adults by Diabetes Type - United States, 2016. MMWR Morb. Mortal. Wkly Rep. 2018;67:359–361. doi: 10.15585/mmwr.mm6712a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buteau J. GLP-1 receptor signaling: effects on pancreatic beta-cell proliferation and survival. Dibetes Metab. 2008 doi: 10.1016/S1262-3636(08)73398-6. [DOI] [PubMed] [Google Scholar]
- Cabrera-Vera T.M., Vanhauwe J., Thomas T.O., Medkova M., Preininger A., Mazzoni M.R., Hamm H.E. Insights into G protein structure, function, and regulation. Endocr. Rev. 2003;24:765–781. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- Cahill T.J., 3rd, Thomsen A.R., Tarrasch J.T., Plouffe B., Nguyen A.H., Yang F., Huang L.Y., Kahsai A.W., Bassoni D.L., Gavino B.J., Lamerdin J.E., Triest S., Shukla A.K., Berger B., Little J.T., Antar A., Blanc A., Qu C.X., Chen X., Kawakami K., Inoue A., Aoki J., Steyaert J., Sun J.P., Bouvier M., Skiniotis G., Lefkowitz R.J. Distinct conformations of GPCR-beta-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc. Natl. Acad. Sci. U S A. 2017;114:2562–2567. doi: 10.1073/pnas.1701529114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J.S., D'Souza M.P. Chemokines and HIV-1 second receptors: the therapeutic connection. Nat. Med. 1998;4:563–568. doi: 10.1038/nm0598-563. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhu H., Yuan M., Fu J., Zhou Y., Ma L. G-protein-coupled receptor kinase 5 phosphorylates p53 and inhibits DNA damage-induced apoptosis. J. Biol. Chem. 2010;285:12823–12830. doi: 10.1074/jbc.M109.094243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos A., Changeux J.P., Catterall W.A., Fabbro D., Burris T.P., Cidlowski J.A., Olsen R.W., Peters J.A., Neubig R.R., Pin J.P., Sexton P.M., Kenakin T.P., Ehlert F.J., Spedding M., Langmead C.J. International union of basic and clinical pharmacology. XC. multisite pharmacology: recommendations for the nomenclature of receptor allosterism and allosteric ligands. Pharmacol. Rev. 2014;66:918–947. doi: 10.1124/pr.114.008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D.E., Neer E.J. G protein beta gamma subunits. Annu. Rev. Pharmacol. Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- Conn P.J., Christopoulos A., Lindsley C.W. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeaux Y., Hill S.J. Mechanisms of cross-talk between G-protein-coupled receptors. Neurosignals. 2002;11:45–57. doi: 10.1159/000057321. [DOI] [PubMed] [Google Scholar]
- de Roux N., Genin E., Carel J.C., Matsuda F., Chaussain J.L., Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defea K.A., Zalevsky J., Thoma M.S., Dery O., Mullins R.D., Bunnett N.W. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J. Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewire S.M., Yamashita D.S., Rominger D.H., Liu G., Cowan C.L., Graczyk T.M., Chen X.T., Pitis P.M., Gotchev D., Yuan C., Koblish M., Lark M.W., Violin J.D. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J. Pharmacol. Exp. Ther. 2013;344:708–717. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- Dumoulin M., Conrath K., van Meirhaeghe A., Meersman F., Heremans K., Frenken L.G., Muyldermans S., Wyns L., Matagne A. Single-domain antibody fragments with high conformational stability. Protein Sci. 2002;11:500–515. doi: 10.1110/ps.34602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson R., Lagerström M.C., Lundin L.-G., Schiöth H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003;63:1256. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Gales C., van Durm J.J., Schaak S., Pontier S., Percherancier Y., Audet M., Paris H., Bouvier M. Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat. Struct. Mol. Biol. 2006;13:778–786. doi: 10.1038/nsmb1134. [DOI] [PubMed] [Google Scholar]
- Gao W.J.R. Pharmacogenomics of GPCR genes in type 2 diabetes and obesity. Curr. Opin. Endocrine Metabolic Res. 2021;16:128–135. [Google Scholar]
- Gomes I., Gupta A., Filipovska J., Szeto H.H., Pintar J.E., Devi L.A. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc. Natl. Acad. Sci. U S A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromoll J., Simoni M., Nordhoff V., Behre H.M., de Geyter C., Nieschlag E. Functional and clinical consequences of mutations in the FSH receptor. Mol. Cell. Endocrinol. 1996;125:177–182. doi: 10.1016/s0303-7207(96)03949-4. [DOI] [PubMed] [Google Scholar]
- Grundmann M., Merten N., Malfacini D., Inoue A., Preis P., Simon K., Ruttiger N., Ziegler N., Benkel T., Schmitt N.K., Ishida S., Muller I., Reher R., Kawakami K., Inoue A., Rick U., Kuhl T., Imhof D., Aoki J., Konig G.M., Hoffmann C., Gomeza J., Wess J., Kostenis E. Lack of beta-arrestin signaling in the absence of active G proteins. Nat. Commun. 2018;9:341. doi: 10.1038/s41467-017-02661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubb D., Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J. Embryol. Exp. Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- Gurevich V.V., Gurevich E.V. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol. Ther. 2006;110:465–502. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich V.V., Gurevich E.V. GPCR signaling regulation: the role of GRKs and arrestins. Front. Pharmacol. 2019;10:125. doi: 10.3389/fphar.2019.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen M.M., van Solt C.B., Van Zijderveld Van Bemmel A.M., Niewold T.A., Van Zijderveld F.G. Selection and optimization of proteolytically stable llama single-domain antibody fragments for oral immunotherapy. Appl. Microbiol. Biotechnol. 2006;72:544–551. doi: 10.1007/s00253-005-0300-7. [DOI] [PubMed] [Google Scholar]
- Hausdorff W.P., Caron M.G., Lefkowitz R.J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- Hauser A.S., Chavali S., Masuho I., Jahn L.J., Martemyanov K.A., Gloriam D.E., Babu M.M. Pharmacogenomics of GPCR Drug Targets. Cell. 2018;172(41–54) doi: 10.1016/j.cell.2017.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman S.J., Ridley A.J. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Heng B.C., Aubel D., Fussenegger M. An overview of the diverse roles of G-protein coupled receptors (GPCRs) in the pathophysiology of various human diseases. Biotechnol. Adv. 2013;31:1676–1694. doi: 10.1016/j.biotechadv.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Hermans E. Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol. Ther. 2003;99:25–44. doi: 10.1016/s0163-7258(03)00051-2. [DOI] [PubMed] [Google Scholar]
- Hilger D., Masureel M., Kobilka B.K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 2018;25:4–12. doi: 10.1038/s41594-017-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinney A., Hohmann S., Geller F., Vogel C., Hess C., Wermter A.K., Brokamp B., Goldschmidt H., Siegfried W., Remschmidt H., Schafer H., Gudermann T., Hebebrand J. Melanocortin-4 receptor gene: case-control study and transmission disequilibrium test confirm that functionally relevant mutations are compatible with a major gene effect for extreme obesity. J. Clin. Endocrinol. Metab. 2003;88:4258–4267. doi: 10.1210/jc.2003-030233. [DOI] [PubMed] [Google Scholar]
- Ho J., Cocolakis E., Dumas V.M., Posner B.I., Laporte S.A., Lebrun J.J. The G protein-coupled receptor kinase-2 is a TGFbeta-inducible antagonist of TGFbeta signal transduction. EMBO J. 2005;24:3247–3258. doi: 10.1038/sj.emboj.7600794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan K.T., Tesmer J.J. Structural insights into G protein-coupled receptor kinase function. Curr. Opin. Cell Biol. 2014;27:25–31. doi: 10.1016/j.ceb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullmann J.E., Grisanti L.A., Makarewich C.A., Gao E., Gold J.I., Chuprun J.K., Tilley D.G., Houser S.R., Koch W.J. GRK5-mediated exacerbation of pathological cardiac hypertrophy involves facilitation of nuclear NFAT activity. Circ. Res. 2014;115:976–985. doi: 10.1161/CIRCRESAHA.116.304475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings C.J. A review of antibody-based therapeutics targeting G protein-coupled receptors: an update. Expert Opin. Biol. Ther. 2020;20:925–935. doi: 10.1080/14712598.2020.1745770. [DOI] [PubMed] [Google Scholar]
- Ilter M., Mansoor S., Sensoy O. Utilization of biased G protein-coupled receptorsignaling towards development of safer andpersonalized therapeutics. Molecules. 2019;24 doi: 10.3390/molecules24112052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura F., Arimoto I., Fujiyoshi Y., Doi T. W276 mutation in the endothelin receptor subtype B impairs Gq coupling but not Gi or Go coupling. Biochemistry. 2000;39:686–692. doi: 10.1021/bi991981z. [DOI] [PubMed] [Google Scholar]
- Isberg V., Mordalski S., Munk C., Rataj K., Harpsoe K., Hauser A.S., Vroling B., Bojarski A.J., Vriend G., Gloriam D.E. GPCRdb: an information system for G protein-coupled receptors. Nucleic Acids Res. 2016;44:D356–D364. doi: 10.1093/nar/gkv1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam K.N., Bae J.W., Gao E., Koch W.J. Regulation of nuclear factor kappaB (NF-kappaB) in the nucleus of cardiomyocytes by G protein-coupled receptor kinase 5 (GRK5) J. Biol. Chem. 2013;288:35683–35689. doi: 10.1074/jbc.M113.529347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittaman S.V., Vanwormer J.J., Rezkalla S.H. The role of aspirin in the prevention of cardiovascular disease. Clin. Med. Res. 2014;12:147–154. doi: 10.3121/cmr.2013.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnichen S., Blanchetot C., Maussang D., Gonzalez-Pajuelo M., Chow K.Y., Bosch L., de Vrieze S., Serruys B., Ulrichts H., Vandevelde W., Saunders M., de Haard H.J., Schols D., Leurs R., Vanlandschoot P., Verrips T., Smit M.J. CXCR4 nanobodies (VHH-based single variable domains) potently inhibit chemotaxis and HIV-1 replication and mobilize stem cells. Proc. Natl. Acad. Sci. U S A. 2010;107:20565–20570. doi: 10.1073/pnas.1012865107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicic N., Pausova Z., Cole D.E., Hendy G.N. Insertion of an Alu sequence in the Ca(2+)-sensing receptor gene in familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Am. J. Hum. Genet. 1995;56:880–886. [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Sainz M.C., Murga C., Kavelaars A., Jurado-Pueyo M., Krakstad B.F., Heijnen C.J., Mayor F., Jr., Aragay A.M. G protein-coupled receptor kinase 2 negatively regulates chemokine signaling at a level downstream from G protein subunits. Mol. Biol. Cell. 2006;17:25–31. doi: 10.1091/mbc.E05-05-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.A. Ethnic differences in cardiovascular drug response: potential contribution of pharmacogenetics. Circulation. 2008;118:1383–1393. doi: 10.1161/CIRCULATIONAHA.107.704023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joost P., Methner A. Phylogenetic analysis of 277 human G-protein-coupled receptors as a tool for the prediction of orphan receptor ligands. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-11-research0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul K.V., Bichet D.G., Nielsen S., Norgaard J.P. The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. Am. J. Physiol. Renal Physiol. 2014;306:F931–F940. doi: 10.1152/ajprenal.00604.2013. [DOI] [PubMed] [Google Scholar]
- Kelly E., Bailey C.P., Henderson G. Agonist-selective mechanisms of GPCR desensitization. Br. J. Pharmacol. 2008;153(Suppl 1):S379–S388. doi: 10.1038/sj.bjp.0707604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel S., Bruss M., Bonisch H., Gothert M. Pharmacological properties of the naturally occurring Phe-124-Cys variant of the human 5-HT1B receptor: changes in ligand binding, G-protein coupling and second messenger formation. Pharmacogenetics. 2000;10:655–666. doi: 10.1097/00008571-200010000-00008. [DOI] [PubMed] [Google Scholar]
- Kim J., Ahn S., Ren X.R., Whalen E.J., Reiter E., Wei H., Lefkowitz R.J. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc. Natl. Acad. Sci. U S A. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeff J.P.L., Rondard P., Pin J.P. Dimers and beyond: The functional puzzles of class C GPCRs. Pharmacol. Ther. 2011;130:9–25. doi: 10.1016/j.pharmthera.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Koike J., Takagi A., Miwa T., Hirai M., Terada M., Katoh M. Molecular cloning of Frizzled-10, a novel member of the Frizzled gene family. Biochem. Biophys. Res. Commun. 1999;262:39–43. doi: 10.1006/bbrc.1999.1161. [DOI] [PubMed] [Google Scholar]
- Kolakowski L.F., Jr. GCRDb: a G-protein-coupled receptor database. Recept Channels. 1994;2:1–7. [PubMed] [Google Scholar]
- Kozasa T., Jiang X., Hart M.J., Sternweis P.M., Singer W.D., Gilman A.G., Bollag G., Sternweis P.C. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- Lane J.R., May L.T., Parton R.G., Sexton P.M., Christopoulos A. A kinetic view of GPCR allostery and biased agonism. Nat. Chem. Biol. 2017;13:929–937. doi: 10.1038/nchembio.2431. [DOI] [PubMed] [Google Scholar]
- Langenhan T. Adhesion G protein–coupled receptors—Candidate metabotropic mechanosensors and novel drug targets. Basic Clin. Pharmacol. Toxicol. 2020;126:5–16. doi: 10.1111/bcpt.13223. [DOI] [PubMed] [Google Scholar]
- Latorraca, N.R., wang, J.K, Bauer, B, Townshend, S., olivieri, J.E, Xu, H.E., sommer, M.E., Dror, R.O., et al., 2018. Molecular mechanism of GPCR-mediated arrestin activation. Nature 557 (7705), 1476–4687 (Electronic). 10.1038/s41586-018-0077-3. In this issue. [DOI] [PMC free article] [PubMed]
- Lefkowitz R.J. G protein-coupled receptor kinases. Cell. 1993;74:409–412. doi: 10.1016/0092-8674(93)80042-d. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R.J. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J. Biol. Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R.J. A brief history of G-protein coupled receptors (Nobel Lecture) Angew. Chem. Int. Ed. Engl. 2013;52:6366–6378. doi: 10.1002/anie.201301924. [DOI] [PubMed] [Google Scholar]
- Lieu M., Koch W.J. GRK2 and GRK5 as therapeutic targets and their role in maladaptive and pathological cardiac hypertrophy. Expert Opin Ther Targets. 2019;23:201–214. doi: 10.1080/14728222.2019.1575363. [DOI] [PubMed] [Google Scholar]
- Liggett S.B., Mialet-Perez J., Thaneemit-Chen S., Weber S.A., Greene S.M., Hodne D., Nelson B., Morrison J., Domanski M.J., Wagoner L.E., Abraham W.T., Anderson J.L., Carlquist J.F., Krause-Steinrauf H.J., Lazzeroni L.C., Port J.D., Lavori P.W., Bristow M.R. A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc. Natl. Acad. Sci. U S A. 2006;103:11288–11293. doi: 10.1073/pnas.0509937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell L.M., Ferguson S.S., Daaka Y., Miller W.E., Maudsley S., Della Rocca G.J., Lin F., Kawakatsu H., Owada K., Luttrell D.K., Caron M.G., Lefkowitz R.J. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Luttrell L.M., Lefkowitz R.J. The role of β-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- Luttrell L.M., Miller W.E. Arrestins as regulators of kinases and phosphatases. Prog. Mol. Biol. Transl. Sci. 2013;118:115–147. doi: 10.1016/B978-0-12-394440-5.00005-X. [DOI] [PubMed] [Google Scholar]
- Luttrell L.M., Roudabush F.L., Choy E.W., Miller W.E., Field M.E., Pierce K.L., Lefkowitz R.J. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc. Natl. Acad. Sci. U S A. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymperopoulos A. Arrestins in the cardiovascular system: an update. Prog. Mol. Biol. Transl. Sci. 2018;159:27–57. doi: 10.1016/bs.pmbts.2018.07.003. [DOI] [PubMed] [Google Scholar]
- Manglik A., Lin H., Aryal D.K., McCorvy J.D., Dengler D., Corder G., Levit A., Kling R.C., Bernat V., Hubner H., Huang X.P., Sassano M.F., Giguere P.M., Lober S., Da D., Scherrer G., Kobilka B.K., Gmeiner P., Roth B.L., Shoichet B.K. Structure-based discovery of opioid analgesics with reduced side effects. Nature. 2016;537:185–190. doi: 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinissen M.J., Gutkind J.S. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol. Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- Marshall F.H., Jones K.A., Kaupmann K., Bettler B. GABAB receptors - the first 7TM heterodimers. Trends Pharmacol. Sci. 1999;20:396–399. doi: 10.1016/s0165-6147(99)01383-8. [DOI] [PubMed] [Google Scholar]
- Martini J.S., Raake P., Vinge L.E., Degeorge B.R., Jr., Chuprun J.K., Harris D.M., Gao E., Eckhart A.D., Pitcher J.A., Koch W.J. Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc. Natl. Acad. Sci. U S A. 2008;105:12457–12462. doi: 10.1073/pnas.0803153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D.A., Moore J.D., Green S.A., Liggett S.B. A gain-of-function polymorphism in a G-protein coupling domain of the human beta1-adrenergic receptor. J. Biol. Chem. 1999;274:12670–12674. doi: 10.1074/jbc.274.18.12670. [DOI] [PubMed] [Google Scholar]
- Maussang D., Mujic-Delic A., Descamps F.J., Stortelers C., Vanlandschoot P., Stigter-Van Walsum M., Vischer H.F., Van Roy M., Vosjan M., Gonzalez-Pajuelo M., Van Dongen G.A., Merchiers P., Van Rompaey P., Smit M.J. Llama-derived single variable domains (nanobodies) directed against chemokine receptor CXCR7 reduce head and neck cancer cell growth in vivo. J. Biol. Chem. 2013;288:29562–29572. doi: 10.1074/jbc.M113.498436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald P.H., Chow C.W., Miller W.E., Laporte S.A., Field M.E., Lin F.T., Davis R.J., Lefkowitz R.J. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- mellado M, Rodriguez-Frade J., Vila-coro A.J., de ana A.M., martinez A.C. Chemokine control of HIV-1 infection. nature. 1999;400(0028-0836 (Print)) doi: 10.1038/23382. In press. [DOI] [PubMed] [Google Scholar]
- Meng D., Lynch M.J., Huston E., Beyermann M., Eichhorst J., Adams D.R., Klussmann E., Houslay M.D., Baillie G.S. MEK1 binds directly to betaarrestin1, influencing both its phosphorylation by ERK and the timing of its isoprenaline-stimulated internalization. J. Biol. Chem. 2009;284:11425–11435. doi: 10.1074/jbc.M806395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mialet Perez J., Rathz D.A., Petrashevskaya N.N., Hahn H.S., Wagoner L.E., Schwartz A., Dorn G.W., Liggett S.B. Beta 1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nat. Med. 2003;9:1300–1305. doi: 10.1038/nm930. [DOI] [PubMed] [Google Scholar]
- Miner J., Hoffhines A. The discovery of aspirin's antithrombotic effects. Tex. Heart Inst. J. 2007;34:179–186. [PMC free article] [PubMed] [Google Scholar]
- Monod J., Changeux J.P., Jacob F. Allosteric proteins and cellular control systems. J. Mol. Biol. 1963;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- Monod J., Wyman J., Changeux J.P. On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Moore C.A., Milano S.K., Benovic J.L. Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- Mujic-Delic A., de Wit R.H., Verkaar F., Smit M.J. GPCR-targeting nanobodies: attractive research tools, diagnostics, and therapeutics. Trends Pharmacol. Sci. 2014;35:247–255. doi: 10.1016/j.tips.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Muto T.T.D., Morikawa K., Jingami H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc. Natl. Acad. Sci. U S A. 2007;104:3759–3854. doi: 10.1073/pnas.0611577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni P.C.O., Holz G.G. Regulation of glucose homeostasis by GLP-1. Prog. Mol. Biol. Transl. Sci. 2014;121:23–65. doi: 10.1016/B978-0-12-800101-1.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naga Prasad S.V., Laporte S.A., Chamberlain D., Caron M.G., Barak L., Rockman H.A. Phosphoinositide 3-kinase regulates beta2-adrenergic receptor endocytosis by AP-2 recruitment to the receptor/beta-arrestin complex. J. Cell Biol. 2002;158:563–575. doi: 10.1083/jcb.200202113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley R.H., Laporte S.A., Holt J.A., Barak L.S., Caron M.G. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J. Biol. Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- Oakley R.H., Laporte S.A., Holt J.A., Caron M.G., Barak L.S. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J. Biol. Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- Ozaki N., Shibasaki T., Kashima Y., Miki T., Takahashi K., Ueno H., Sunaga Y., Yano H., Matsuura Y., Iwanaga T., Takai Y., Seino S. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat. Cell Biol. 2000;2:805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- Pal K.M.K., Xu H.E. Structure and mechanism for recognition of peptide hormones by Class B G-protein-coupled receptors. Acta Pharmacol. Sin. 2012;3:300–311. doi: 10.1038/aps.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce L.R., Komander D., Alessi D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- Penela P., Murga C., Ribas C., Lafarga V., Mayor F., Jr. The complex G protein-coupled receptor kinase 2 (GRK2) interactome unveils new physiopathological targets. Br. J. Pharmacol. 2010;160:821–832. doi: 10.1111/j.1476-5381.2010.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penela P., Ribas C., Mayor F., Jr. Mechanisms of regulation of the expression and function of G protein-coupled receptor kinases. Cell. Signal. 2003;15:973–981. doi: 10.1016/s0898-6568(03)00099-8. [DOI] [PubMed] [Google Scholar]
- Pierce K.L., Premont R.T., Lefkowitz R.J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Pitcher J.A., Freedman N.J., Lefkowitz R.J. G protein-coupled receptor kinases. Annu. Rev. Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Puffenberger E.G., Hosoda K., Washington S.S., Nakao K., Dewit D., Yanagisawa M., Chakravart A. A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung's disease. Cell. 1994;79:1257–1266. doi: 10.1016/0092-8674(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Qin K., Dong C., Wu G., Lambert N.A. Inactive-state preassembly of G(q)-coupled receptors and G(q) heterotrimers. Nat. Chem. Biol. 2011;7:740–747. doi: 10.1038/nchembio.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankovic Z., Brust T.F., Bohn L.M. Biased agonism: An emerging paradigm in GPCR drug discovery. Bioorg. Med. Chem. Lett. 2016;26:241–250. doi: 10.1016/j.bmcl.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask-Andersen M., Almen M.S., Schioth H.B. Trends in the exploitation of novel drug targets. Nat Rev Drug Discov. 2011;10:579–590. doi: 10.1038/nrd3478. [DOI] [PubMed] [Google Scholar]
- Ren X.R., Reiter E., Ahn S., Kim J., Chen W., Lefkowitz R.J. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc. Natl. Acad. Sci. U S A. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodien P., Bremont C., Sanson M.L., Parma J., van Sande J., Costagliola S., Luton J.P., Vassart G., Duprez L. Familial gestational hyperthyroidism caused by a mutant thyrotropin receptor hypersensitive to human chorionic gonadotropin. N. Engl. J. Med. 1998;339:1823–1826. doi: 10.1056/NEJM199812173392505. [DOI] [PubMed] [Google Scholar]
- Samama P., Cotecchia S., Costa T., Lefkowitz R.J. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J. Biol. Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- Santini F., Maffei M., Pelosini C., Salvetti G., Scartabelli G., Pinchera A. Melanocortin-4 receptor mutations in obesity. Adv. Clin. Chem. 2009;48:95–109. [PubMed] [Google Scholar]
- Savi P., Zachayus J.L., Delesque-Touchard N., Labouret C., Herve C., Uzabiaga M.F., Pereillo J.M., Culouscou J.M., Bono F., Ferrara P., Herbert J.M. The active metabolite of Clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc. Natl. Acad. Sci. U S A. 2006;103:11069–11074. doi: 10.1073/pnas.0510446103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerer P., Sommer M.E. Structural mechanism of arrestin activation. Curr. Opin. Struct. Biol. 2017;45:160–169. doi: 10.1016/j.sbi.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Schutzer W.E., Reed J.F., Mader S.L. Decline in caveolin-1 expression and scaffolding of G protein receptor kinase-2 with age in Fischer 344 aortic vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H2457–H2464. doi: 10.1152/ajpheart.01090.2004. [DOI] [PubMed] [Google Scholar]
- Shenoy S.K., Lefkowitz R.J. beta-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol. Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina T., Arai K., Tanabe S., Yoshida N., Haga T., Nagao T., Kurose H. Clathrin box in G protein-coupled receptor kinase 2. J. Biol. Chem. 2001;276:33019–33026. doi: 10.1074/jbc.M100140200. [DOI] [PubMed] [Google Scholar]
- Shukla A.K., Manglik A., Kruse A.C., Xiao K., Reis R.I., Tseng W.C., Staus D.P., Hilger D., Uysal S., Huang L.Y., Paduch M., Tripathi-Shukla P., Koide A., Koide S., Weis W.I., Kossiakoff A.A., Kobilka B.K., Lefkowitz R.J. Structure of active beta-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature. 2013;497:137–141. doi: 10.1038/nature12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni M., Nieschlag E., Gromoll J. Isoforms and single nucleotide polymorphisms of the FSH receptor gene: implications for human reproduction. Hum Reprod Update. 2002;8:413–421. doi: 10.1093/humupd/8.5.413. [DOI] [PubMed] [Google Scholar]
- Small K.M., Forbes S.L., Brown K.M., Liggett S.B. An asn to lys polymorphism in the third intracellular loop of the human alpha 2A-adrenergic receptor imparts enhanced agonist-promoted Gi coupling. J. Biol. Chem. 2000;275:38518–38523. doi: 10.1074/jbc.M004550200. [DOI] [PubMed] [Google Scholar]
- Smith M.W., Dean M., Carrington M., Winkler C., Huttley G.A., Lomb D.A., Goedert J.J., O'Brien T.R., Jacobson L.P., Kaslow R., Buchbinder S., Vittinghoff E., Vlahov D., Hoots K., Hilgartner M.W., O'Brien S.J. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC), ALIVE Study. Science. 1997;277:959–965. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]
- Sommer M.E., Selent J., Carlsson J., de Graaf C., Gloriam D.E., Keseru G.M., Kosloff M., Mordalski S., Rizk A., Rosenkilde M.M., Sotelo E., Tiemann J.K.S., Tobin A., Vardjan N., Waldhoer M., Kolb P. The European Research Network on Signal Transduction (ERNEST): Toward a Multidimensional Holistic Understanding of G Protein-Coupled Receptor Signaling. ACS Pharmacol. Transl. Sci. 2020;3:361–370. doi: 10.1021/acsptsci.0c00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoy H., Gurevich V.V. How genetic errors in GPCRs affect their function: Possible therapeutic strategies. Genes Dis. 2015;2:108–132. doi: 10.1016/j.gendis.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y.X. Constitutive activation of G protein-coupled receptors and diseases: insights into mechanisms of activation and therapeutics. Pharmacol. Ther. 2008;120:129–148. doi: 10.1016/j.pharmthera.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal D.M., Homan K.T., Chen J., Wu E.K., Hinkle P.M., Huang Z.M., Chuprun J.K., Song J., Gao E., Cheung J.Y., Sklar L.A., Koch W.J., Tesmer J.J. Paroxetine is a direct inhibitor of g protein-coupled receptor kinase 2 and increases myocardial contractility. ACS Chem. Biol. 2012;7:1830–1839. doi: 10.1021/cb3003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M.D., Hendy G.N., Percy M.E., Bichet D.G., Cole D.E. G protein-coupled receptor mutations and human genetic disease. Methods Mol. Biol. 2014;1175:153–187. doi: 10.1007/978-1-4939-0956-8_8. [DOI] [PubMed] [Google Scholar]
- Thomsen A.R., Plouffe B., Cahill T.J., 3rd, Shukla A.K., Tarrasch J.T., Dosey A.M., Kahsai A.W., Strachan R.T., Pani B., Mahoney J.P., Huang L., Breton B., Heydenreich F.M., Sunahara R.K., Skiniotis G., Bouvier M., Lefkowitz R.J. GPCR-G protein-beta-arrestin super-complex mediates sustained G protein signaling. Cell. 2016;166:907–919. doi: 10.1016/j.cell.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tressel S.L., Koukos G., Tchernychev B., Jacques S.L., Covic L., Kuliopulos A. Pharmacology, biodistribution, and efficacy of GPCR-based pepducins in disease models. Methods Mol. Biol. 2011;683:259–275. doi: 10.1007/978-1-60761-919-2_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Noorden R. Nobel work boosts drug development. Nature. 2012;490:320. doi: 10.1038/490320a. [DOI] [PubMed] [Google Scholar]
- Vilardaga J.-P., Jean-Alphonse F.G., Gardella T.J. Endosomal generation of cAMP in GPCR signaling. Nat. Chem. Biol. 2014;10:700–706. doi: 10.1038/nchembio.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C.R., Conover S., Adler P.N. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338:263–264. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- Viscusi E.R., Webster L., Kuss M., Daniels S., Bolognese J.A., Zuckerman S., Soergel D.G., Subach R.A., Cook E., Skobieranda F. A randomized, phase 2 study investigating TRV130, a biased ligand of the mu-opioid receptor, for the intravenous treatment of acute pain. Pain. 2016;157:264–272. doi: 10.1097/j.pain.0000000000000363. [DOI] [PubMed] [Google Scholar]
- Wang H.Y., Malbon C.C. Wnt signaling, Ca2+, and cyclic GMP: visualizing Frizzled functions. Science. 2003;300:1529–1530. doi: 10.1126/science.1085259. [DOI] [PubMed] [Google Scholar]
- Wang Y., Chang H., Rattner A., Nathans J. Frizzled Receptors in Development and Disease. Curr. Top. Dev. Biol. 2016;117:113–139. doi: 10.1016/bs.ctdb.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Macke J.P., Abella B.S., Andreasson K., Worley P., Gilbert D.J., Copeland N.G., Jenkins N.A., Nathans J. A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J. Biol. Chem. 1996;271:4468–4476. doi: 10.1074/jbc.271.8.4468. [DOI] [PubMed] [Google Scholar]
- Willets J.M., Challiss R.A., Nahorski S.R. Non-visual GRKs: are we seeing the whole picture? Trends Pharmacol. Sci. 2003;24:626–633. doi: 10.1016/j.tips.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Wisler J.W., Xiao K., Thomsen A.R., Lefkowitz R.J. Recent developments in biased agonism. Curr. Opin. Cell Biol. 2014;27:18–24. doi: 10.1016/j.ceb.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.H., Goswami R., Cai X., Exum S.T., Huang X., Zhang L., Brian L., Premont R.T., Peppel K., Freedman N.J. Regulation of the platelet-derived growth factor receptor-beta by G protein-coupled receptor kinase-5 in vascular smooth muscle cells involves the phosphatase Shp2. J. Biol. Chem. 2006;281:37758–37772. doi: 10.1074/jbc.M605756200. [DOI] [PubMed] [Google Scholar]
- Yang Y., Stang A., Schweickert P.G., Lanman N.A., Paul E.N., Monia B.P., Revenko A.S., Palumbo J.S., Mullins E.S., Elzey B.D., Janssen E.M., Konieczny S.F., Flick M.J. Thrombin Signaling Promotes Pancreatic Adenocarcinoma through PAR-1-Dependent Immune Evasion. Cancer Res. 2019;79:3417–3430. doi: 10.1158/0008-5472.CAN-18-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.E., He Y., de Waal P.W., Gao X., Kang Y., van Eps N., Yin Y., Pal K., Goswami D., White T.A., Barty A., Latorraca N.R., Chapman H.N., Hubbell W.L., Dror R.O., Stevens R.C., Cherezov V., Gurevich V.V., Griffin P.R., Ernst O.P., Melcher K., Xu H.E. Identification of Phosphorylation Codes for Arrestin Recruitment by G Protein-Coupled Receptors. Cell. 2017;170(457–469) doi: 10.1016/j.cell.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M., Agnati L.F., Hedlund P.B., Li X.M., Ferre S., Fuxe K. Receptor-receptor interactions as an integrative mechanism in nerve cells. Mol. Neurobiol. 1993;7:293–334. doi: 10.1007/BF02769180. [DOI] [PubMed] [Google Scholar]