Abstract
Traditional medicines implicate consumption of plant crude extracts, which may consist of extensive phytochemical diversity. Overall, the most biologically active extract of Peganum harmala (seeds) exhibited significant cytotoxic activity on Artemia salina with LC50 value of 61.547 µg/mL, while P. harmala (roots) [LC50 = 124.229 µg/mL] and M. azedarach (fruits) [LC50 = 147.813 µg/mL] showed moderate cytotoxic potential. P. harmala (seeds) extract also showed the maximum antitumor potential with 52.278 µg/mL LC50. Branches of P. harmala and Morus alba were not active in both bioassays. These outcomes were further reinforced by the levels of phenolics and flavonoids checked against gallic acid and quercetin equivalents, respectively, by standard curves. Current study aims to isolate, structurally characterize and analyze the bioactive compound from plant extracts by using chromatographic and spectrophotometric techniques. Bioactivity guided isolation of extracts led to the isolation of PH-HM-16 from ethyl acetate fraction P. harmala seeds. Chemical structure of PH-HM-16 was elucidated by ESI-MS, 1H NMR, 13C NMR, HSQC and IR spectrum. The results demonstrated significant positive anticancer activities against six human cancer cell lines assessed through MTT cancer cell growth inhibition assay. PH-HM-16 was most effective against prostate cancer cell lines [IC50 = 17.63 µg/mL] followed by breast cancer cell line MCF7 [IC50 value of 41.81 µg/mL]. IC50 value of PH-HM-16 against human myeloid leukemia cell line HL-60 and human colorectal tumor cells HCT-116 was observed as 68.77 µg/mL and 71.54 µg/mL respectively. The IC 50 value of PH-HM-16 compound was not significant against human gastric cancer SGC-7901 (111.89 µg/mL) and human lung adenocarcinoma epithelial cell line A549 (176.04 µg/mL). Isolated bioactive metabolite PH-HM-16 possesses significant antitumor potential so this could be the first step to develop an effective anticancer agent. Hence, this compound represents a promising potential to be chemically standardized or developed into pharmaceuticals for the chemoprevention and/or the treatment of certain types of cancer, especially as adjuvant phytotherapeutics in conventional chemotherapy.
Keywords: Melia azedarach, Anticancer, Phytochemicals, Phenolics, Flavonoids
1. Introduction
Medicinal plants are an unlimited bioresources of therapeutic agents in conventional and traditional systems of medicine, food supplements, nutraceuticals, lead compounds or intermediates for synthetic drugs (Ncube et al., 2008). Traditional herbal remedies utilized for diverse bioactivities may work as a basis to recognize novel compounds that can be candidates for the development of new anticancer drugs. In particular, it may be assumed that the cytotoxic action of plant extracts points to diverse healing applications. Plants with cytotoxic activity may be not merely active against tumors, but also as abortifacients, contraceptives and against infectious maladies caused by protozoans. In this sense, cytotoxic properties are often indicative of anticancer potential (Mariani et al., 2014, Monteleone et al., 2012, Leonti et al., 2015). Ethno-historic records indicate that natural medicinal products are powerful reservoirs of effective phytotherapeutics with rarer side effects (Umadevi et al., 2013, Srinivas and Afolayan, 2007, Efferth et al., 2008).
Melia azedarach L. is prescribed in Chinese traditional medicine both orally and topically as antiparasitic and antifungal agent. This plant is a blood purifier and febrifuge whereas bark has vermifuge action (Phua et al., 2008). Crushed leaves in water are effective in skin infections (Iqbal et al., 2011); leaf extract is a blood purifier and painkiller whereas dry foliage is used to cure jaundice, allergy, constipation and leprosy (Mushtaq et al., 2012). Flowers and leaves are diuretic, emmenagogue and used to relieve headache and cold inflammation. Seeds are active in the treatment of rheumatism; seed powder is recommended for piles while seed oil is laxative, brain tonic and used in the treatment of earache and liver ailments (Ahmad et al., 2006, Iqbal et al., 2011). Roots are astringent, effective against vomiting, belching, heart disease, headache, fever, leukoderma, blood impurities, ulcer and uterine pains. Fruits are very useful as carminative and their powder are applicable for piles (Sultana et al., 2006).
Peganum harmala L. generally known as harmala is an extensively used medicinal plant of family Zygophyllaceae. Seeds are identified to be carminative, emetic, anthelmintic, galactagogue, aphrodisiac, diuretic, antithrombotic, emmenagogue, phlegmatic, purgative and strengthening the vision (Aghili, 2009, Tonkaboni and Momenin, 2007). They are used for psychosis, epilepsy, loss of memory, kidney stones, chronic headache, dropsy, rheumatism, colic, jaundice and sciatica (Aghili, 2009, Iqbal et al., 2011). Mixture of P. harmala and flax seeds along with honey has been used for the management of dyspnea. Decoction of seeds is efficient for treatment of laryngitis, sexual impotency, lung and liver diseases, as diuretic, demulcent and numbness (Ullah et al., 2013). Seed aqueous extract is beneficial for blood purification, while poultice from seeds is beneficial for numbness, paralysis, joint pain, back pain and coxalgia. The incense of seeds is efficient for toothache and repelling mosquitos (Hemmateenejad et al., 2006, Aghili, 2009, Tonkaboni and Momenin, 2007). Seed powder and extract, raw seeds and smoke of plant are active for the cure of abdominal/stomach and digestive problems, asthma, boils and pimples while alkaloids reveals antibacterial and antifungal potential (Shaheen et al., 2014, Sharma et al., 2011).
Morus genus (Moraceae) comprises a group of trees native from Asia, traditionally known as mulberries, and with a number of medicinal properties (Oliveira et al., 2015). Morus alba is plant widely used for ailments such as cough, asthma, bronchitis, insomnia, edema, wound healing, influenza, diabetes, nosebleeds and eye infections. It is also valuable for treatment of tinnitus, dizziness, urinary incontinence and constipation in elderly patients (Mhaskar et al., 2000). Plant is valuable in indigenous systems of medication for cooling, as purgative, laxative, diuretic, brain tonic, antibacterial, anthelmintic, in liver disorders, burning sensation and in vitiated condition of vata and pitta (Bae and Suh, 2007, Katsube et al., 2006, Machii et al., 2002). Extract of root and bark is effective against abdominal worms and considered as significant remedy to cure inflammation, cough, hepatitis, diabetes and cancer, while bark is well known for lowering blood pressure. Leaf extract is also widely utilized for wound healing, and sore throat as antiseptic and disinfectant (Gautam et al., 2007, Shinwari et al., 2003, Deshpande et al., 2008), as well as considered useful as anthelmintic, antibacterial, aphrodisiac, antirheumatic, diuretic, expectorant, antihypertensive, laxative and sedative agent (Dat et al., 2010, Husain et al., 2008, Khan et al., 2012a, Khan et al., 2012b, Qureshi et al., 2011).
Since ever, information on traditional herbal remedies has assisted in discovery of novel therapeutic leads with relevant bioactivity and less toxicity for cancer treatment, whereas basic research has emphasized their chemical modification and pharmacological profile (Bugs, 2004, Walsh and Amyes, 2004). This article aims to investigate the cytotoxicity and antitumor properties of different parts of important traditional plants for their development as possible source of phytotherapeutics as well as anticancer effect of isolated component against six selected human cancer cell lines.
2. Material and methodology
2.1. Plant selection
Three highly active species widely utilized as herbal remedies by local communities in salt range (Kallar Kahar, Pakistan) were selected for analysis (Table 1).
Table 1.
Plant selection based on ethnomedicinal importance.
| Plant Species | Family | Local Name | Part Used | Traditional uses | References |
|---|---|---|---|---|---|
| Morus alba | Moraceae | Toot, shahtoot | Leaves, branches | Pimples and warts, edema, eczema, swellings, antistress, inflamed eyes, astringent |
Khan et al., 2012 Zafar et al., 2013, Ahmad, 2007, Niyomkam et al., 2010, Leporatti and Ghedira, 2009, Devi et al., 2013, Dat et al., 2010 |
| Melia azedarach | Meliaceae | Dhraik | Leaves, fruits |
Piles, skin diseases, blood purifier, anti-allergic, insecticidal, chicken pox, smallpox, tumors, inflammatory diseases, pimples |
Iqbal et al., 2011, Naz et al., 2014, Faraga et al., 2011, Azam et al., 2013, Shabir et al., 2015, Akihisa et al., 2013 |
| Peganum harmala | Zygophyllaceae | Harmal | Roots, branches, leaves, seeds |
Blood purifier, antiseptic, healing ulcers, skin diseases, pimples and lumps on skin, anti-inflammatory, insecticidal |
Iqbal et al., 2011, Naz et al., 2014 |
2.2. Plant extracts
Melia azedarach (leaves and fruits), Peganum harmala (roots, branches, leaves and seeds) and Morus alba (leaves and branches) were shade dried. The plant samples were extracted twice using methanol (95%) at 25 °C temperature for 7 days by using filter paper Whatman No. 1. Filtered samples were concentrated by rotary evaporation in reduced pressure at 40 °C and then stored at 4 °C for further in vitro investigations.
2.3. Cytotoxicity assays
2.3.1. Preparation of the bioassays
Bioassays were performed in multiwell plates (0.45 μm) and sterilized seawater. Extracts were tested at several concentrations achieved by shifting the corresponding stock volume to multiple wells for evaporation. Seawater (5 mL) was added to individual wells in aqueous solution and allowed to evaporate. All assays were conducted in a temperature-controlled room at 28 °C, under a constant light regimen.
2.3.2. BSLT (Brine Shrimp Lethality Test)
Dried cysts (1 g/L) were incubated in a hatcher with strong aeration at 28–30 °C. After hatching (about 12 h), the phototropic nauplii were picked from lighted section by a glass capillary tube and concentrated in a small vial. Every test consisted in exposing sets of ten Artemia (12 h aged) to several concentrations of extracts. Toxicity was evaluated after 12 h (generally nauplii in instar I/II) and 24 h (nauplii in instar II/III). Mortality percentage was calculated by noting dead and alive nauplii. The death of larvae observed in the bioassay is due to bioactives and not to starvation as by comparing dead larvae in treatment to control. Besides, hatched nauplii can stay alive up to 48 h even without food as they nourish on yolk sac. Nevertheless, if control mortality is noticed, mortality was estimated as:
Mortality (%) = Survival in control (%) - Survival in treatment (%).
2.3.3. Toxicity testing criteria
Clarkson’s criterion for sample toxicity valuation categorizes in subsequent sequence: extracts with LC50 beyond 1000 µg/mL are considered as non-toxic; samples with LC50 in range of 500 to 1000 µg/mL are categorized as low toxic; extracts with 100–500 µg/mL LC50 are moderately toxic; whereas LC50 < 100 µg/mL was exhibited by highly toxic samples (Clarkson et al., 2004).
2.4. Inhibition of crown-gall tumorigenesis
Agrobacterium tumefaciens (At 10 strain) was grown at 28 °C for 48 h on yeast extract media. Segments of Solanum tuberosum L. were disinfected through immersing in mercuric chloride (10%) for half an hour. Cylinders (10 mm diameter) were made from the disinfected segments by sterilized cork borer; disks (0.5 cm diameter) were cut aseptically and placed in a 24 well culture plate comprising water agar (15%). Suspension of A. tumefaciens in phosphate buffered saline was standardized (1 × 109 CFU) through 0.97 ± 0.01 nm absorbance. Samples were prepared in Dimethyl sulfoxide (DMSO) in 1000 µg/mL and successively diluted to 500, 250, 125, 62.5, 31.25 and 15.625 μg/mL. Potassium dichromate, Vincristine and Etoposide were used as positive standards. Others controls included: DMSO with phosphate buffered saline, DMSO with bacterium and DMSO without bacterium. The test samples contained 100 µL water, 400 µL of the standardized bacterium suspension and 400 µL of drug or control solution. Each disk in the 24 well culture plate was overlaid with test solution (50 µL) and incubated at 25 °C. On day 21, disks were stained by Lugol's reagent [I2KI (5%)]. Number of tumors was observed on stained disks by dissecting microscope. Samples were analyzed in total of twelve replicates.
2.5. Total phenolic content
The Folin Ciocalteu assay was used to estimate the total phenolic content (Swain and Hillis, 1959). Sample (150 μL) and deionized distilled water (2400 μL) were mixed well with Folin–Ciocalteu reagent (150 μL; 0.25 N) using vortex and placed in a vial. Deionized distilled water was used as blank. After 10 min (time for reaction), Na2CO3 solution (300 µL; 1 N) was added and incubated in the darkness (25 °C; 90 min) and absorbance was read at 750 nm. Data were expressed as gallic acid equivalents (mg GAE/g dry mass) by gallic acid standard curve.
2.6. Total flavonoid content
The total flavonoid content was measured according to Park et al. (2008). The plant extract (50 mg) was dissolved in aqueous methanol (80%; 10 mL) and filtered by Whatman filter paper. Extract (0.3 mL), 30% methanol (3.4 mL), 0.5 M NaNO2 (0.15 mL) and AlCl3·6H2O (0.15 mL; 0.3 M) were added and left for 5 min; NaOH (1 M ; 1 mL) was poured into mixture. Absorbance of solution was measured against blank (reagent) on 506 nm. Standard curve was determined by using quercetin (standard mixture) and total flavonoids were expressed as quercetin equivalents (mg QE/g dry sample).
2.7. Isolation and structure elucidation
Column chromatography by following the procedure of Hammad et al. (2015) with minor modification was used for purification of bioactive component. The extract was adsorbed on a silica gel column by using various mixtures of solvent system starting with non-polar and changed by slightly increasing polarity. A glass column will uniformly packed with slurry of silica gel in the mobile phase solvent system. The mobile phases were applied on the top of the column and continuous elution were collected. Ethyl acetate fraction was chromatographed over silica gel column packed in chloroform. Total of 30 elutions were collected using chloroform:methanol (1.0–0.1) as mobile phase with increasing volumes of methanol solvent. All the collected eluents were evaporated to dryness by removing mixture of solvents under reduced pressure through rotary evaporator at 40 °C to get dried concentrated eluents. Different components eluted through the column were collected in glass vials (20 mL) for further (Thin layer chromatography (TLC) analysis. Thus, the most bioactive material was purified using this silica gel column successively eluted with a stepwise gradient with increasing volume of solvent till total elution were collected. Aliquots of 15–20 μL of the isolated fractions collected by column chromatography were applied as separate spots on a TLC plate about 1.5 cm from the edge (spotting line). After sample application, the plate were placed vertically into a solvent vapor saturated TLC chamber (12 × 15 × 10 cm). After the mobile phase moved about 80% from the spotting line, the plate was removed from the developing chamber and air dried in a fume hood to visualize spots within 1–2 min on the plate. The migrated spots on TLC plates representing various fractions/compounds, visualized with UV lamp at UV-254 nm. All 30 elutions were subjected to thin layer chromatography with chloroform:methanol as solvent system with varying concentrations (Fig. 9: Supplementary data). On the basis of their TLC profile, the same elutions were pooled together to form Group 1 to Group 5. Elution showing similar pattern were pooled together and used for further analysis (Houghton et al., 1996). Since Group 2 showed significant activity compared to other groups, it was again subjected to silica gel column chromatography and 19 eluents were collected in vials (Fig. 9: Supplementary data). Solvents system used for this was n-hexane and methanol with gradual increase in methanol concentration (1.0–0.1). These eluents were again subjected to pre coated silica gel TLC plates and the same eluents were combined to form Group 2a (elution 1–6), Group 2b (elution 7–11) and Group 2c (elution 12–19). HPLC analysis was carried out with photodiode array detector and LC column (150 × 4.6 mm). Two mobile phases A consisted of n-hexane and B ethyl acetate at flow rate of 0.5 mL/min were used. A gradient starting at 95% A changing to 10% was used for 10 min. After eluting, the column was equilibrated under initial conditions for 5 min. Total of 63 elutions were collected which were rotary evaporated and subjected to TLC. On the basis of TLC fingerprint profiling, similar groups were pooled together to get Group 2b.A, Group 2b.B, Group 2b.C and Group 2b.D. The isolated components were further characterized with the help of available spectroscopic techniques including Infrared spectroscopy (IR), Mass spectrometry (MS) and Nuclear Magnetic Resonance spectroscopy (NMR). Mass spectra of isolated components was obtained using mass spectrophotometer for molecular mass determination and ESI-MS spectral analysis was recorded in the positive ionization mode on mass spectrophotometer. IR Spectroscopy was measure the vibrations of atoms on the IR spectrophotometer (BUCK SCIENTIFIC) and based on this it was help to determine the functional groups. Nuclear Magnetic Resonance Spectroscopy (NMR) was performed with appropriate solvent to elucidate the structures of the isolated components (Vollhardt et al., 2007). Proton Nuclear Magnetic Resonance (1H NMR) Carbon Nuclear Magnetic Resonance (13C NMR) and Heteronuclear Single Quantum Coherence (HSQC) experiments were obtained on spectrometer (Bruker, 300 MHz, 500 MHz).
2.8. MTT cytotoxic assay
The growth of human cancer cell lines was evaluated using the colorimetric (3-[4,5-dimethylthiazol-2-yl]diphenyl tetrazolium bromide) MTT assay (Hayoto et al., 2006). Cell lines were incubated in 96-microwell plates and the metabolically active cells after 72 h of culture were determined by the intensity of the purple color (formazan product) quantitatively measured by spectrophotometry at 590 nm wavelength (Fort et al., 2018). The absorbance measured directly correlates to percentage of viable cells.
The percentage of cell viability and inhibition were calculated through following formula:
| % Cell viability = (Absorbance of treated cells / Absorbance of cells with vehicle solvent) × 100 |
| % Inhibition = 100 - % Cell viability |
3. Results and discussion
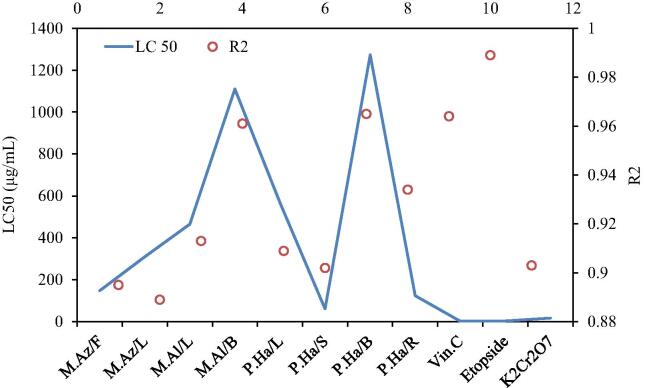
The cytotoxicity of all extracts was dose-dependent, even though toxicity was dependent on the plant parts studied too (Manar et al., 2008). Median lethality values of extracts ranged from 61.547 to 1272.543 µg/mL for P. harmala seeds (S) and P. harmala branches (B), respectively. In addition, P. harmala (S) caused 100% mortality of the brine shrimps within 24 h at 1000 µg/mL (Fig. 1). The outcomes of the brine shrimp lethality bioassay showed the P. harmala (S) extract to be extremely toxic to brine shrimp at 1000 µg/mL after 12 and 24 h exposure time. It was toxic at 500 µg/mL and 250 µg/mL after 12 and 24 h of exposure while slight toxicity was observed by the same extract at 62.5 µg/mL to 0.978 µg/mL as per Clarkson’s criteria. Slight toxicity was observed after 6 h of time exposure at the highest concentration tested, but no effect on lethality was recorded on lower concentrations after 6 h, and these outcomes are in accordance with earlier reports (Mackeen et al., 2000). The P. harmala (S) extract was highly toxic to shrimp larvae as per Clarkson’s toxicity criterion with median lethality value LC50 61.547 µg/mL obtained from logarithmic regression analysis (Fig. 2). Earlier reports showed the cytotoxic potential of plants as chief source of bioactives (Tawaha, 2006, Ali et al., 2011).
Fig. 1.
Brine Shrimp Lethality Test against logarithmic concentration of the plant extracts after 12 h and 24 h of exposure. Where M.Az/F:12 = Lethality of Melia azedarach (fruit) after 12 h; M.Az/L:12 = Lethality of Melia azedarach (Leaves) after 12 h; M.Al/L:12 = Lethality of Morus alba (Leaves) after 12 h; M.Al/B:12 = Lethality of Morus alba(Branches) after 12 h; P.Ha/L:12 = Lethality of Peganum harmala (Leaves) after 12 h; P.Ha/S:12 = Lethality of Peganum harmala (Seeds) after 12 h; P.Ha/B:12 = Lethality of Peganum harmala (Branches) after 12 h; P.Ha/R:12 = Lethality of Peganum harmala (Roots) after 12 h; Vin.C:12 = Lethality of Vincristine sulphate after 12 h; Etopside:12 = Lethality of Etopside after 12 h; K2Cr2O7:12 = Lethality of K2Cr2O7 (Potassium dichromate) after 12 h; M.Az/F:24 = Lethality of Melia azedarach (fruit) after 24 h; M.Az/L:24 = Lethality of Melia azedarach (Leaves) after 24 h; M.Al/L:24 = Lethality of Morus alba (Leaves) after 24 h; M.Al/B:24 = Lethality of Morus alba (Branches) after 24 h; P.Ha/L:24 = Lethality of Peganum harmala (Leaves) after 24 h; P.Ha/S:24 = Lethality of Peganum harmala (Seeds) after 24 h; P.Ha/B:24 = Lethality of Peganum harmala (Branches) after 24 h; P.Ha/R:24 = Lethality of Peganum harmala (Roots) after 24 h; Vin.C:24 = Lethality of Vincristine sulphate after 24 h; Etopside:24 = Lethality of Etopside after 24 h; K2Cr2O7:24 = Lethality of K2Cr2O7 (Potassium dichromate) after 24 h.
Fig. 2.
Cytotoxicity LC50 and R2 values determined through logarthmic regression analysis. Where M.Az/F = Melia azedarach (fruit); M.Az/L = Melia azedarach (Leaves); M.Al/L = Morus alba (Leaves); M.Al/B = Morus alba (Branches); P.Ha/L = Peganum harmala (Leaves); P.Ha/S = Peganum harmala (Seeds); P.Ha/B = Peganum harmala (Branches); P.Ha/R = Peganum harmala (Roots); Vin.C = Vincristine sulphate; Etopside = Etopside; K2Cr2O7 = K2Cr2O7 (Potassium dichromate).
P. harmala roots (R) exhibited moderate toxicity to nauplii with 124 µg/mL LC50. This extract showed to be toxic to nauplii on 1000–250 µg/mL concentration after 12 and 24 h exposure time and slight toxicity was observed against brine shrimps on six different concentrations ranged from 125 µg/mL to 0.96 µg/mL and after 12–24 h (Fig. 1). Finally, 0.978 µg/mL concentration of extract was not much effective against brine shrimp larvae (Fig. 1). Results help to predict the presence of compounds with potential anticancer activity (Moshi et al., 2010).
P. harmala leaf (L) extract exhibited toxicity in concentrations higher than 500 µg/mL. Cytotoxicity was moderate at concentration range between 250 and 15.625 µg/mL. P. harmala (L) extract remained ineffective at concentrations lower than 7.81 µg/mL after 12 h. Slight toxicity was observed 24 h after exposure to concentration range between 7.81 and 1.96 µg/mL and 0.978 µg/mL concentration was nontoxic after 12–24 h (Fig. 1). The LC50 value of P. harmala (L) extract after 24 h of exposure was 567.082 µg/mL falling in low toxicity class according to Clarkson’s standard (Table 2). This suggests that the toxic properties of P. harmala are due to the seeds and roots and strengthen the speculation for capability to yield anticancer agents. (Tanko et al., 2007). The P. harmala branch (B) extract was slightly toxic for the brine shrimp after 12 h of exposure at seven different concentrations ranging between 15.625 and 1000 µg/mL and nontoxic at lower concentrations. After 24 h of exposure, the P. harmala (B) extract was moderately lethal on nine concentrations tested at 3.91–1000 µg/mL (Fig. 1). However, the extract showed a toxic effect at 1.96 µg/mL and 0.978 µg/mL after 24 h. P. harmala (B) extract was found to be nontoxic with LC50 higher than 1000 µg/mL and was the least active with the highest LC50 1272.543 µg/mL among all assessed samples (Table 2). In few cases, thorough analysis of the cytotoxicity of plant parts allowed to highlight the cytotoxic compounds involved in the observed effects (Yvette et al., 2016).
Table 2.
Regression analysis of cytotoxic and antitumor activities.
| Sample | Part used |
Cytotoxicity |
Antitumor Activity |
Toxicity class |
|---|---|---|---|---|
| Regression Equation | Regression Equation | |||
| Melia azedarach | Fruits | y = 9.131ln(x) + 4.382 | y = 15.65ln(x) − 27.17 | Moderately toxic |
| Melia azedarach | Leaves | y = 8.042ln(x) + 3.858 | y = 10.25ln(x) − 9.303 | Moderately toxic |
| Morus alba | Leaves | y = 7.675ln(x) + 2.850 | y = 13.62ln(x) − 22.09 | Moderately toxic |
| Morus alba | Branches | y = 6.494ln(x) + 4.461 | y = 7.112ln(x) − 7.583 | Non toxic |
| Peganum harmala | Leaves | y = 7.530ln(x) + 2.256 | y = 12.05ln(x) − 22.85 | Low toxic |
| Peganum harmala | Seeds | y = 11.82ln(x) + 1.304 | y = 15.89ln(x) − 12.87 | Highly toxic |
| Peganum harmala | Branches | y = 6.231ln(x) + 5.456 | y = 8.385ln(x) − 20.75 | Non toxic |
| Peganum harmala | Roots | y = 10.35ln(x) + 0.091 | y = 14.24ln(x) − 21.37 | Moderately toxic |
| Vincristine C | – | y = 9.406ln(x) + 42.89 | y = 3.130ln(x) + 78.89 | Highly toxic |
| Etoposide | – | y = 9.669ln(x) + 37.62 | y = 3.087ln(x) + 78.77 | Highly toxic |
| K2Cr2O7 | – | y = 10.81ln(x) + 19.14 | – | Highly toxic |
M. alba (L) extract was toxic to brine shrimps at 500 µg/mL −1000 µg/mL concentrations, but slight toxicity was observed at 250 µg/mL to 1.96 µg/mL, and 0.978 µg/mL concentration was not effective to show any significant lethality on brine shrimps, after both 12 and 24 h exposure (Fig. 1). Overall, M. alba (L) extract was moderately lethal to shrimps with 310.327 µg/mL LC50 in the present study. 1000 µg/mL of M. alba (B) extract was toxic to brine shrimp nauplii after both 12 and 24 h exposure. Lethality values declined from 43 to 10 % with decreases in concentration from 500 µg/mL to 1.96 µg/mL. However, 0.978 µg/mL concentration of M. alba (B) extract revealed no significant lethality of nauplii after 12 and 24 h of exposure (Fig. 2). Based on calculated LC50 value of 1110.397 µg/mL after 24 h of exposure, M. alba (B) extract was categorized as nontoxic to nauplii (Fig. 2). Other plant species, however, showed no significant difference in percentage mortalities among different concentrations within the same species, signifying no brine shrimp lethality compared to that of control (Haque et al., 2009).
M. azedarach fruit (F) extract was toxic at concentrations of 250 µg/mL, 500 µg/mL and 1000 µg/mL. M. azedarach leaf (L) extract was toxic at 500 µg/mL and 1000 µg/mL. Slight toxicity against larvae was observed by M. azedarach (F) extract at concentration range 125–1.96 µg/mL after 12 h. The concentration 0.978 µg/mL was not lethal at that time. The 24 h exposure revealed slight toxicity on all tested concentrations in the range 0978–125 µg/mL. A similar trend of lethality was observed for M. azedarach (L) extract after 12 and 24 h. The 0.978 µg/mL dose of M. azedarach (L) extract was not much lethal for brine shrimp nauplii after 12 h, but slight toxicity was reported after 24 h (Fig. 1). M. azedarach (F) and M. azedarach (L) extracts exhibited toxicity to shrimp larvae with 147.814 µg/mL and 310.327 µg/mL median lethality LC50 values estimated through logarithmic regression analysis (Table 2). These results extend the experimental findings on cytotoxicity of the fruit extract of M. azedarach (Khan et al., 2014). Potassium dichromate, Etoposide and Vincristine sulphate served as the positive control for BSLT and found to be extremely toxic as per Clarkson’s lethality criterion (Carballo et al., 2002). The LC50 values for the positive controls were 17.37, 3.598 and 2.13 µg/mL respectively, after 24 h of exposure (Fig. 2).
In the present study, all samples confirmed high to moderate cytotoxic activity on brine shrimps, ranging from 3.45 to 7.37 probit values on log 10 (conc) − 0.0097 to 3.0000 (Fig. 3). P. harmala (S) showed the highest activity on shrimps with 7.37 probit value, followed by P. harmala (R) with probit number 6.04 at log 10 (conc) 3.0, while the branches of same species had 5.1 probit (Fig. 3). However, the probit value of P. harmala (L) was about in the same range (5.39). P. harmala (S) was found to be significant with probit value range 3.72–7.37 on log10 (c) − 0.0097 to 3.0, while P. harmala (R) had less variations with 3.59–6.04 probit values (Fig. 3). Conversely, P. harmala (L) and P. harmala (B) were not much active with probit value ranges 3.45–5.39 and 3.52–5.1, respectively. M. azedarach (F) also revealed significant results with 5.92 probit value of mortality percentage, different from that of M. azedarach (L) with 5.52 at log 10 (conc) 3.0. No significant difference was found between M. alba (L) and M. alba (B) at log10 (c) − 0.0097, but both samples showed a clear difference in cytotoxic activity on the shrimp assay at log10 (c) 3.0 with probit values from 5.39 to 5.15 (Fig. 3).
Fig. 3.
Probit analysis of cytotoxicity determination of eight plant extracts and three standards.Where M.Az/F = Melia azedarach (fruit); M.Az/L = Melia azedarach (Leaves); M.Al/L = Morus alba (Leaves); M.Al/B = Morus alba (Branches); P.Ha/L = Peganum harmala (Leaves); P.Ha/S = Peganum harmala (Seeds); P.Ha/B = Peganum harmala (Branches); P.Ha/R = Peganum harmala (Roots); Vin.C = Vincristine sulphate; Etopside = Etopside; K2Cr2O7 = K2Cr2O7 (Potassium dichromate).
Maximum mortality for general toxicity via BSLT was observed with 1000 µg/mL concentration whereas 0.978 and 1.96 µg/mL were the least active concentrations. This explains that degree of toxicity was proportional to concentrations of samples (Tanko et al., 2007). Furthermore, the brine shrimp lethality of plant bioactives correlates reasonably fine with their cytotoxic potential, thus providing novel therapeutic candidates for numerous maladies. Logarto et al. (2001) verified that there was a strong correlation (P < 0.05) in LC50 of BSLT and LD50 of acute oral toxicity in mice. This substantiates that brine shrimp lethality is valuable for the examination of plant extracts, to estimate their toxicity level. From a pharmacological perspective, a good association has been found with the BSLT used to discover antitumoral compounds from plant extracts (Billah et al., 2013). In our experimental conditions, trend of median lethal concentration, LC50 after 24 h, of the methanolic extracts was:
P. harmala (B) > M. alba (B) > P. harmala (L) > M. alba (L) > M. azedarch (L) > M. azedarach (F) > P. harmala (R) > P. harmala (S).
Although some species were more active after 24 h than after 12 h, in few cases there was no remarkable variation with time in the potential of the extracts (Ullah et al., 2013). After 24 h of exposure, bioactivity of the extracts amplified significantly because sensibility of brine shrimps increased. At this phase, nauplii possess II instar and show their high sensitivity to the treatment. The demise of the nauplii may be due to the influence of starvation or plant extract (Ali et al., 2013). To confirm the lethality of the plant extracts, control having only nauplii was also tested (Nondo et al., 2011). In any situation, hatched nauplii can stay alive up to 2 days without nutrition as yolk sac is sufficient to nourish. Normal yeast is supplemented as food of nauplii on the second day (Mbwambo et al., 2007). The effects of the plant species are consistent with the phytochemical data of these plants as a source of active cytotoxic and antitumor compounds (Bastos et al., 2009). So, our findings indicated BSLT as convenient bioassay to screen natural products in drug discovery process (Cyrus et al., 2008). The LC50 values of the plant extracts (at 24 h) were achieved through a plot of proportion of the shrimps killed alongside the concentrations of extracts and best-fit line was acquired from the statistics by means of regression analysis to calculate LC50 value (Roy et al., 2011).
In the present study, the potato disc bioassay was used as anti-tumor prescreen against plant crude extracts. Crown gall is a plant disease characterized by cell hyperplasia and hypertrophy caused by the gram negative bacterium Agrobacterium tumefaciens. It was shown that A. tumefaciens At 10 strain was very active in tumor production with 37.33 ± 1.05 tumor count per disc (Table 3). The bacterium has a large tumor-inducing plasmid that possesses a T-DNA that alters normal cells into self-directed tumor cells (Ahmad et al., 2016). Significant activity in the PDA was recorded in six plant extracts while two extracts were not effective (Fig. 4). The use of an antitumor prescreen assay, by means of plant systems, possesses several benefits as an option to animal systems for exploration of novel anticancer leads (Ayaz et al., 2016).
Table 3.
Inhibition of crown gall tumors by the plant extracts.
| Test material | Dose (μg/mL) | Tumor count per disc* | Inhibition of tumor [%] | IC50 (μg/mL) |
|---|---|---|---|---|
| Negative control | – | 37.33 ± 1.05 | ||
| Vincristine C | 1000 | 0.00 ± 0.00 | 100.00 | 9.8 |
| 500 | 0.00 ± 0.00 | 100.00 | ||
| 250 | 1.79 ± 0.59 | 95.20 | ||
| 125 | 2.13 ± 0.93 | 94.29 | ||
| 62.5 | 3.41 ± 0.72 | 90.87 | ||
| 31.25 | 3.93 ± 0.49 | 89.47 | ||
| 15.625 | 4.40 ± 1.21 | 88.21 | ||
| Etoposide | 1000 | 0.00 ± 0.00 | 100.00 | 9.00 |
| 500 | 0.00 ± 0.00 | 100.00 | ||
| 250 | 1.93 ± 0.21 | 94.83 | ||
| 125 | 2.85 ± 0.84 | 92.37 | ||
| 62.5 | 3.39 ± 0.30 | 90.92 | ||
| 31.25 | 4.08 ± 0.30 | 89.07 | ||
| 15.625 | 4.25 ± 1.17 | 88.62 | ||
| M.Az/F | 1000 | 4.63 ± 1.15 | 87.60 | 138.517 |
| 500 | 9.83 ± 0.92 | 73.67 | ||
| 250 | 17.89 ± 2.06 | 52.08 | ||
| 125 | 21.95 ± 2.71 | 41.20 | ||
| 62.5 | 24.78 ± 3.61 | 33.62 | ||
| 31.25 | 26.93 ± 0.94 | 27.86 | ||
| 15.625 | 28.75 ± 1.79 | 22.98 | ||
| M.Az/L | 1000 | 12.84 ± 1.40 | 65.60 | 325.596 |
| 500 | 17.04 ± 1.39 | 54.35 | ||
| 250 | 20.48 ± 2.05 | 45.14 | ||
| 125 | 23.61 ± 2.78 | 36.75 | ||
| 62.5 | 26.37 ± 3.31 | 29.36 | ||
| 31.25 | 26.91 ± 2.28 | 27.91 | ||
| 15.625 | 29.05 ± 4.01 | 22.18 | ||
| M.Al/L | 1000 | 10.84 ± 0.63 | 70.96 | 198.93 |
| 500 | 13.01 ± 1.83 | 65.15 | ||
| 250 | 17.21 ± 1.50 | 53.90 | ||
| 125 | 20.06 ± 0.27 | 46.26 | ||
| 62.5 | 27.67 ± 0.75 | 25.88 | ||
| 31.25 | 28.32 ± 1.05 | 24.14 | ||
| 15.625 | 30.05 ± 1.61 | 19.50 | ||
| M.Al/B | 1000 | 21.11 ± 2.10 | 43.45 | 3283.28 |
| 500 | 23.65 ± 2.91 | 36.65 | ||
| 250 | 25.98 ± 1.54 | 30.40 | ||
| 125 | 27.76 ± 2.27 | 25.64 | ||
| 62.5 | 29.65 ± 1.79 | 20.57 | ||
| 31.25 | 31.23 ± 2.26 | 16.34 | ||
| 15.625 | 32.01 ± 1.92 | 14.25 | ||
| P.Ha/L | 1000 | 13.98 ± 0.98 | 62.55 | 422.269 |
| 500 | 17.79 ± 1.57 | 52.34 | ||
| 250 | 19.96 ± 1.43 | 46.53 | ||
| 125 | 26.21 ± 1.49 | 29.79 | ||
| 62.5 | 29.14 ± 2.97 | 21.94 | ||
| 31.25 | 30.05 ± 1.77 | 19.50 | ||
| 15.625 | 31.85 ± 1.03 | 14.68 | ||
| P.Ha/S | 1000 | 1.87 ± 0.57 | 94.99 | 52.278 |
| 500 | 4.72 ± 0.86 | 87.36 | ||
| 250 | 10.04 ± 0.45 | 73.10 | ||
| 125 | 13.45 ± 1.60 | 63.97 | ||
| 62.5 | 15.3 ± 1.22 | 59.01 | ||
| 31.25 | 21.93 ± 1.81 | 41.25 | ||
| 15.625 | 27.04 ± 0.30 | 27.56 | ||
| P.Ha/B | 1000 | 24.29 ± 1.25 | 34.93 | 4617.86 |
| 500 | 25.23 ± 2.06 | 32.41 | ||
| 250 | 26.72 ± 1.73 | 28.42 | ||
| 125 | 30.06 ± 3.06 | 19.47 | ||
| 62.5 | 32.62 ± 4.29 | 12.62 | ||
| 31.25 | 34.31 ± 4.20 | 8.09 | ||
| 15.625 | 36.52 ± 2.84 | 2.17 | ||
| P.Ha/R | 1000 | 8.13 ± 0.58 | 78.22 | 150.196 |
| 500 | 13.44 ± 1.59 | 64.00 | ||
| 250 | 15.39 ± 0.14 | 58.77 | ||
| 125 | 17.66 ± 1.39 | 52.69 | ||
| 62.5 | 25.83 ± 0.32 | 30.81 | ||
| 31.25 | 26.97 ± 1.16 | 27.75 | ||
| 15.625 | 30.03 ± 0.90 | 19.56 |
(*mean of twelve replicates).
Where M.Az/F = Melia azedarach (fruit); M.Az/L = Melia azedarach (Leaves); M.Al/L = Morus alba (Leaves); M.Al/B = Morus alba (Branches); P.Ha/L = Peganum harmala (Leaves); P.Ha/S = Peganum harmala (Seeds); P.Ha/B = Peganum harmala (Branches); P.Ha/R = Peganum harmala (Roots).
Fig. 4.
Percentage Inhibition of Tumor at seven different concentartions.
M. azedarach (F) extract exhibited 87.60 and 73.67% inhibition of At 10 induced development of crown gall tumors on Solanum at concentrations of 1000 and 500 μg/disc, respectively. Activity decreased with decrease in concentration and the lowest activity (22.98%) was observed with M. azedarach (F) at 15.625 µg/mL. M. azedarach (F) extract caused 50% inhibition (IC50) of crown-gall tumors at concentration of 138.517 µg/mL (Fig. 5). M. azedarach (L) inhibited the formation of crown-gall tumors by 54.35, 45.14, 36.75, 29.36, 27.91 and 22.18% at concentrations of 1000–15.625 µg/mL (Fig. 4). The IC50 of crown-gall formation observed with M. azedarach (L) was found to be 325.596 μg/mL (Table 3). A relationship exists between concentration of samples and ability to restrain crown gall tumor development on potato discs (Khan et al., 2016).
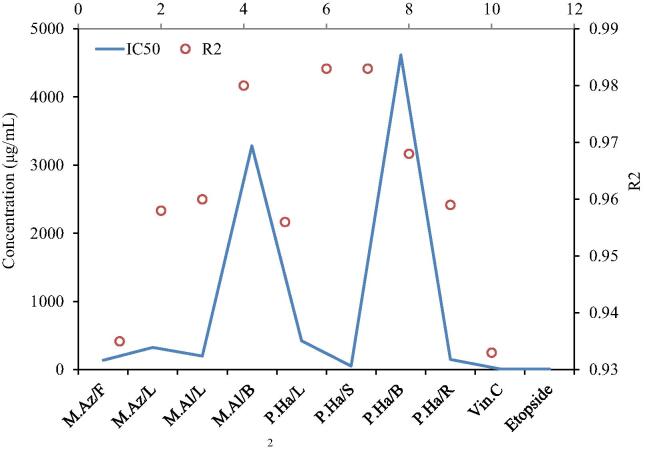
Fig. 5.
Antitumor activiy LC50 and R2 value determined through logarthmic regression analysis.Where M.Az/F = Melia azedarach (fruit); M.Az/L = Melia azedarach (Leaves); M.Al/L = Morus alba (Leaves); M.Al/B = Morus alba (Branches); P.Ha/L = Peganum harmala (Leaves); P.Ha/S = Peganum harmala (Seeds); P.Ha/B = Peganum harmala (Branches); P.Ha/R = Peganum harmala (Roots); Vin.C = Vincristine sulphate; Etopside = Etopside; K2Cr2O7 = K2Cr2O7 (Potassium dichromate).
M. alba (L) extract showed 70.96% inhibition at a dose of 1000 µg/mL. The inhibition potential declined from 65.15% to 46.26% with decrease in concentration from 500 to 125 μg/mL (Fig. 4). Tumor inhibition percentage was found to be<30% at concentration lower than 62.5 μg/mL and the lowest inhibition percentage was found to be 19.50% at 15.625 µg/mL of M. alba (L) extract. M. alba (B) was found to be a poor inhibitor of At induced crown gall tumors and exhibited 43.45% inhibition at 1000 μg/mL of extract concentration. The inhibition percentage extensively decreased with a reduction of 30% at concentrations < 250 μg/mL and 14.25% was the lowest activity obtained. IC50 values determined through logarithmic regression analysis of M. alba (L) and M. alba (B) extracts were 198.93 μg/mL and 3283.28 μg/mL, respectively, which suggested that different parts of the same plant showed huge difference in the inhibition potential of tumors on potato discs (Table 2).
Results showed that the extract of P. harmala (S) inhibited tumor growth in a concentration dependent trend (Table 3). Significant tumor inhibition was observed at five different concentrations from 1000 to 62.5 μg/mL but not at 31.25 and 15.625 μg/mL. P. harmala (S) showed very promising results with 94.99% inhibition of crown gall tumors at 1000 μg/mL extract (Fig. 4). Minimum (27.56%) tumor inhibition was recorded by 15.625 μg/mL of the same extract (Table 3). The extract of P. harmala (L) demonstrated 62.55% inhibition of the tumors at 1000 μg/mL whereas 500 μg/mL extract was able to cause 52.34% inhibition of crown gall tumors. Inhibitory effects of 250 μg/mL P. harmala (L) were 46.53% and the lowest activity (14.68%) was shown by the lowest concentration (15.625 μg/mL) tested among all seven concentrations tested (Fig. 4). P. harmala (R) showed significant inhibitory effect on the crown-gall tumor development as evident by the maximum inhibition of 78.22% at 1000 μg/mL. On the other hand, P. harmala (B) was found to be poorly active because of the maximum inhibition of 34.93% at the same concentration. The inhibitory activity of 64% and 32.41% at 500 μg/mL was observed with the crude extracts of P. harmala (R) and P. harmala (B), respectively. Percentage inhibition > 50% was exhibited by P. harmala (R) on concentrations > 125 μg/mL, but lower concentrations of the extract were not much effective against tumors. Nevertheless, 125 μg/mL of the P. harmala (B) extract was not sufficient to cause 20% inhibition of crown gall tumors. P. harmala (B) was considered almost inactive at 15.625 μg/mL concentration with the least value of percentage inhibition (2.17%) achieved in current study.
The differences in the IC50 values of four different parts of the P. harmala indicated the evident difference in potential of inhibition on crown gall tumor. The maximum IC50 value (4617.86 µg/mL) was shown by P. harmala (B) while the lowest IC50 (52.278 µg/mL) was reported by the P. harmala (S) (Fig. 5). The IC50 of P. harmala (R) (150.196 µg/mL) was lower than the P. harmala (S) one, but higher than that of P. harmala (L) (IC50 = 422.269 µg/mL). Vincristine and Etoposide (positive controls) caused 100% cancer inhibition at 1000–500 µg/mL concentrations. No significant difference in bioactivity between Etoposide and Vincristine was observed in current study, though a slight variation in inhibition along different concentrations was observed. The minimum activity of Vincristine was registered as 88.21% inhibition at 15.625 µg/mL concentration. IC50 values determined through logarithmic regression for Etoposide and Vincristine were 9 and 9.8 µg/mL, respectively (Fig. 5).
Animals and plants share few common mechanisms of tumorigenesis including the intracellular integration of exogenous genetic material; therefore, it might be predicted that some anticancer compounds prevent tumor formation and development in different organisms (Sahreen et al., 2015).
This work has emphasized the significance of brine shrimp bioassay to assess the biological activity of plants frequently used by several societies and ethnic groups for their therapeutic indications. Several reports have tried to associate the toxicity of phytochemicals from medicinal plants to Artemia salina with bioactivities like antiproliferative (Salawu et al., 2017), antiangiogenic (Ayaz et al., 2016), anticarcinogenic (Kumar et al., 2014) and antitumor (Wang et al., 2017) potentials, among others. Researchers have systematically performed this bioassay in initial evaluation of extracts recognized as antitumor agents (Arcanjo et al., 2012). This implies a positive correlation of brine shrimp lethality with antitumor activity in the development of new anticancer lead drugs from plants.
Flavonoid and phenolic contents of natural products are well known for many biological properties including antioxidant, anticarcinogenic, antineovascularization and antiproliferative activities and, consequently, they are considered as chemopreventive agents (Baba and Malik, 2015). Flavonoids can scavenge reactive oxygen species (ROS) in oxidative stress, condition that might promote carcinogenesis and/or angiogenesis. Likewise, phenolics are well known for their multitarget biological activity (Balasundram et al., 2006). Numerous preclinical studies suggested a protective role of phenolics in the prevention of colon, breast, ovary, prostate, lung and endometrium cancers (Galati and Brien, 2004, Baghel et al., 2012). Total phenolic content of samples was estimated through linear regression equation of calibration curve and expressed as gallic acid equivalent (mg GAE/g dry mass) (Fig. 8: Supplementary data). Results showed that the extracts of M. alba (L) and (B) were rich in phenolics with 543 ± 0.04 and 287 ± 0.01 mg GAE/g, respectively. P. harmala (S) extract also contained high levels of phenolics (271 ± 0.02 mg GAE/g). Results were in agreement with the previous study by Hassan et al. (2014) on numerous plant extracts screened for antiangiogenic potential and phenolic content. It was found that extracts of M. azedarach (L), P. harmala (B) and P. harmala (R) were poor of phenolics. On the other hand, P. harmala (L) and (F) extracts of M. azedarach exhibited moderate phenolic levels (113 ± 0.06 mg GAE/g and 107 ± 0.03 mg GAE/g, respectively). Phenolic compounds behave as metal chelating agents in the oxidative systems, thereby neutralizing free radical species. A number of evidence (Yeldandi et al., 2000, Galati and Brien, 2004) have proposed that reactive oxygen species (ROS) are crucial signaling molecules that activate cell growth and proliferation, and ultimately promote tumorigenesis and angiogenesis (Wang et al., 2017).
Total flavonoid content of samples was expressed in quercetin equivalent (QE) and calculated through regression analysis (Fig. 8: Supplementary data). Numerous studies reported the capability of flavonoids to inhibit cancer cell growth in vitro (Moghaddam et al., 2012, Delmulle et al., 2006, Chidambara et al., 2012). Flavonoids possess valuable antioxidant and antitumor potentials, and act as antiangiogenic agents through key mechanisms of carcinogenesis. Metabolic activation of (pro)carcinogens is hindered by flavonoids in early phases of carcinogenesis, while in progression stages, angiogenesis and metastases are inhibited due to induction of apoptosis (Clere et al., 2011). P. harmala (S) extract exhibited the highest levels of flavonoids, i.e., 449 ± 0.01 mg QE/g. Extracts of M. alba (L) and (B) showed high levels of flavonoids too, 291 ± 0.00 mg QE/g and 164 ± 0.03 mg QE/g, respectively. P. harmala (R) and (B) extracts exhibited low total flavonoid contents (28 ± 0.01 mg QE/g and 32 ± 0.06 mg QE/g, respectively) (Fig. 8: Supplementary data). P. harmala (L) contained moderate levels of total flavonoids with 73 ± 0.02 mg QE/g. Finally, M. azedarach (F) and (L) exhibited very low contents of total flavonoids, 12 ± 0.02 mg QE/g and 23 ± 0.00 mg QE/g, respectively (Fig. 8: Supplementary data). The cytotoxic and antitumor activities of plant extracts could be, at least in part, attributed to their high flavonoid content (Dai et al., 2013). Many studies have reported the occurrence of phenolic compounds and complex prenylated flavones in plant extracts, and thus their cytotoxic and antitumor activities could be ascribed to additive and/synergistic effects of bioactive phytochemicals (Elsayed et al., 2017). Correlation between phenolic/flavonoid content and cytotoxic activity is documented as polyphenols impair formation and development of angiogenesis (Pawlak et al., 2017). Consequently, polyphenols exert a pivotal role in counteracting angiogenesis, in addition to their capability to inhibit oxidation of free radicals as natural cytoprotective agents (Elkashak et al., 2017). In recent times, a huge number of studies have been focused on phenolics and flavonoids for the reduction of ROS generation in biological systems (Do et al., 2014). Indeed, oxidative stress generated by ROS plays a very critical role in the pathogenesis of chronic, degenerative diseases including cancer (Abozed et al., 2014).
3.1. Isolation of PH-HM-16
The five groups of elution were tested for their bioactivities after elutions and Group 2 was found to have significant cytotoxic potential with LC50 value of 25.84 µg/mL and was considerably effective to inhibit crown gall tumorgenesis with IC50 value of 28.36 µg/mL. All the three subgroups of group 2 were again tested for their bioactivities and Group 2b was found to be the most potent group with respect to its cytotoxic potential (LC50 value = 16.52 µg/mL) and antitumor activity (IC50 = 20.67 µg/mL) compared with Group 2a and Group 2c (Fig. 9: Supplementary data). The LC50 value for cytotoxic potential of Group 2a was 30.46 µg/mL and for Group 2c it was 28.14 µg/mL. While antitumor potential of Group 2a (IC50 = 38.10 µg/mL) and Group 2c (IC50 = 33.34 µg/mL) was also less than Group 2b, the latter was selected on the basis of high bioactivity to further check for the type of chemical constituents present therein (Fig. 9: Supplementary data). HPLC analysis leads to total of 63 elutions were collected and acquired Group 2b.A, Group 2b.B, Group 2b.C and Group 2b.D (Fig. 9: Supplementary data). All the groups were again checked for their bioactivities and Group 2b.A (elutions 4–13) was found to have significant cytotoxic (LC50 = 12.80 µg/mL) and antitumor (IC50 = 14.16) potentials. Finally, from Group 2b.A, pure colorless solid needles of the compound PH-HM-16 were isolated by recrystallization by using methanol:acetone mixture from the eluents obtained. Summary of the PH-HM-16 isolated is given in the Fig. 9: Supplementary data.
3.2. Identification and structure elucidation of isolated compound PH-HM-16
3.2.1. Physical characteristics of PH-HM-16.
PH-HM-16 was colorless, odorless, in form of needles with melting point range of 210 °C-214 °C. PH-HM-16 compound was soluble in acetone.
3.2.2. Spectroscopic analysis
Mass spectra of PH-HM-16 was recorded in the positive ionization mode on mass spectrophotometer. The molecular formula was assigned as C11H12N2O ([M + H]+, m/z 189.07683; calculated 189.23) by HRESIMS (Fig. 6a). ESI-MS spectral analysis of PH-HM-16 revealed dynamic energy of MS2 from protonated PH-HM-16 [M + H] + at 189.07683 m/z afforded the fragment ion at m/z 171.12705 by the loss of one water molecules (18 Da) (Fig. 6f). Consequently, a carbon–carbon double bond among C-1 and C-2 was formed through elimination reaction (Fig. 6f). The product ion at 154.67308 m/z was formed by elimination of ammonia (17 Da, NH3) (Fig. 6a).
Fig. 6.
a) Mass spectrum, b) Infrared spectrum, c) Proton Nuclear Magnetic Resonance spectrum, d) Carbon Nuclear Magnetic Resonance e) Heteronuclear Single Quantum Coherence f) Proposed structure and 3D conformer of PH-HM-16 isolated compound.
FTIR (Fourier Transform Infrared spectroscopy) spectrum of the isolated PH-HM-16 was determined by using KBr disk method on the IR spectrophotometer (BUCK SCIENTIFIC). The stretching vibration of hydroxyl group (3421 cm − 1) and olefinic groups (1508 cm − 1) was identified from the IR spectrum (Fig. 6b). The stretching for the hydroxyl group in the compounds appears in the range of 3287 cm−1 to 3487 cm−1 (Barud et al., 2013). The C-N in the structure of active principle was confirmed by FTIR which showed the characteristic absorbance peak spectrum at 1085 cm−1 (Fig. 6b) as this is the characteristic band range for the C-N group in the organic compounds (Oliveira et al., 2016).
1H NMR. Proton nuclear magnetic resonance spectra of PH-HM-16 in DMSO confirmed aromatic hydrogens in the structure by resonances at multiplet peaks δH 7.399–7.314 (m, H-7, H-8, H-9, H-10) indicated the presence of olefinic bond (Fig. 6c). Chemical shift for the olefinic bond was also in this range in the previous reports which validate the current results (Timmers et al., 2012). One triplet proton signal appeared at δH 5.1 (1H, t, H-1) was attributed to the hydroxymethine proton (Table 4). A range of isolated compounds clearly established that δH of the hydroxymethine proton was appeared in this range of chemical shift so this strengthen the interpretation of current spectrum (Montenegro et al., 2019). Proton NMR showed one proton signal at δH 4.78 (s, br, H5a, H5b) and three multiplet peaks centered at 2.15 (m, H-2a), 2.56 (m, H-2b) due to methylene and 3.50 (m) is assigned to the H-3a and H-3b protons (Fig. 6c).
Table 4.
NMR (1H and 13C) chemical shifts value of PH-HM-16.
| Position |
13C δC (ppm) |
1H δH (ppm) |
HSQC |
|---|---|---|---|
| 1 | 72.947 | 5.10 (t) | CH |
| 2 | 30.508 | 2.15 (m) 2.56 (m) |
CH2 |
| 3 | 48.263 | 3.50 (m) 3.50 (m) |
CH2 |
| 5 | 49.952 | 4.78 brs 4.78 brs |
CH2 |
| 6 | 120.896 | – | C |
| 7 | 124.313 | 7.39 (m) | CH |
| 8 | 126.799 | 7.33 (m) | CH |
| 9 | 127.766 | 7.31 (d) | CH |
| 10 | 129.423 | 7.37 (m) | CH |
| 11 | 143.715 | – | C |
| 12 | – | – | |
| 13 | 164.524 | – | C |
13C NMR spectroscopic experiment (DMSO) showed eleven carbon atoms with eight methylene and three methine resonances (Fig. 6f). It also showed the presence of a carbon (C-1) bearing a hydroxyl group at δC 72.947 which was attributed to confirmation of hydroxyl group in the isolated compound (Fig. 6d). 13C NMR peaks at the range of 70–79 could fairly be due to the carbon atom bearing hydroxyl group in the compound so this coincides with current spectrum (Sanches, 2005). The peaks observed in the downfield of spectra in the range δC 120–140 ppm can be assigned to greatly deshielded carbon atoms of the compound with C = C double bond (Edilu et al., 2015). Therefore, the presence of double bonds was supported by the appearance of three methine carbon signal at δC 120.896 (C-6), δ 143.715 (C-11) and resonance at 164.524 due to C-13 in the structure (Table 4). The position of benzene ring is confirmed by the appearance of 13C NMR spectrum at C-7 (124.313 ppm), C-8 (δ 126.799), C-9 (127.766 ppm) and C-10 (δC 129.423) indicating sp2 hybridized aromatic carbon in the compound (Fig. 6d). The rests of the carbons were appeared in aliphatic region at δC 30.508 (C-2), δ 48.263 because of C-3 in compound and δC at 49.952 ppm (C-5) chemical shifts with distinguishable peaks coincide with HSQC correlation thus identified as PH-HM-16 (Fig.0.11).
HSQC. The heteronuclear single quantum correlation or heteronuclear single quantum coherence experiment revealed the number of protons in the compound attached to specific carbon atoms (Fig. 6f). HSQC of the isolated compound PH-HM-16 is given in the Fig. 6e. It revealed that C-13 with resonance at δC 164.524 ppm and C-6 with chemical shift δC 120.896 ppm are not attached to any of the proton (Fig. 6f). Similarly, resonance at δC = 143.715 ppm in the spectrum due to C-11 was not linked with any of hydrogen in the HSQC spectra (Fig. 6e).
Hence this confirms that C-6, C-11 and C-13 might be the quaternary carbon in the structure of PH-HM-16 (Fig. 6f). Carbon-2 (δC = 30.508 ppm) is methylene carbon bonded to H-2a (m, δH = 2.15) and H-2b (m, δH = 2.56). Likewise, C-3 (δC = 48.263 ppm) is also attached with H-3a and H-3b protons (m, δH = 3.50 ppm) while the rest of carbon atoms in the molecule of PH-HM-16 are CH revealed in HSQC correlation (Fig. 6e).
4. Anticancer potential of PH-HM-16
Isolated compound PH-HM-16 was evaluated for its cytotoxic potential against six human cancer cell lines namely breast cancer (MCF7), human myeloid leukemia (HL-60), prostate cancer (PC-3), human gastric cancer (SGC-7901), human colorectal cancer (HCT-116) and human lung adenocarcinoma (A549) cell lines using 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assays. The cytotoxicity of isolated compound PH-HM-16 was examined at concentration ranging from 31.25 µg/mL to 500 µg/mL. The absorbance of all the samples was highly dependent on the concentration of compound used (Fridlender et al., 2015). Dose-related curves of percentage of cell viability (%) at each concentration was calculated by absorbance of each sample to negative control (Mathi et al., 2015). Results revealed that compound PH-HM-16 was significantly active against prostate cancer (PC-3) and breast cancer (MCF7) cell lines. The minimum absorbance of 0.116 nm and 0.123 nm was measured by the MCF7 and PC-3 cells, respectively, at 500 µg/mL of compound PH-HM-16, i.e., 14.181% and 15.037% cell viability, respectively (Fig. 10: Supplementary data). The value of absorbance increased from 0.186 nm to 0.371 nm in prostate cancer cell line and 0.247 to 0.423 nm in breast cancer cell line with decrease in the concentration of sample from 250 to 31.25 µg/mL and, thus strong correlation was found as expected (Fig. 10: Supplementary data). Similar trend in percentage of cell viability was observed in other cell lines (Fig. 7A). This is in strong correlation with toxicological profiles for such compounds and also with their in vivo toxicity (Fort et al., 2018). Significant cell growth inhibition was observed against prostate (PC-3) and breast (MCF7) cancer cell lines after treatment with PH-HM-16, with maximum inhibition of 84.96% and 85.81% at the dose of 500 µg/mL, and lowest inhibition of 54.65% and 48.29% at 31.25 µg/mL concentration, respectively (Fig. 7B). PH-HM-16 was most effective against PC3 cell line with IC50 value of 17.63 µg/mL followed by MCF7 with IC50 value of 41.81 µg/mL. This strongly agrees with a very recent report which clearly indicated significant in vitro anticancer activity by increasing concentration (Nguyen et al., 2020). Isolated compound PH-HM-16 showed moderate activity on human myeloid leukemia (HL-60) cell line. The minimum cell viability observed in this cell line was 23.59% with 0.193 nm absorbance at the dose of 500 µg/mL of PH-HM-16 (Fig. 10: Supplementary data). Gradual decrease in the dose level of the compound to 31.25 µg/mL increase the value of cell viability to 71.39% with absorbance of 0.584 nm (Fig. 7A). Percentage inhibition of human myeloid leukemia cells observed was in the range 28.61% to 76.40% at the concentration of 31.25 µg/mL to 500 µg/mL, with IC50 value of 68.77 µg/mL indicating a moderate efficacy of the compound (Table 5). Hence, anticancer activity of compound was highly concentration dependent (Solowey et al., 2014). Compound PH-HM-16 was weakly effective on human colorectal tumor cells (HCT-116), with maximum percentage inhibition 75.42% and absorbance value of 0.201 nm at the maximum dose of compound used (500 µg/mL), and the lowest inhibition observed was 37.41% with absorbance value of 0.512 nm at the lowest dose of the compound (31.25 µg/mL) used in the experiment (Fig. 7A). IC50 value against HCT-116 calls was observed to be 71.54 µg/mL (Table 5). HCT-116 cell line was also considered as sensitive in previous studies reported for anticancer activity of compounds isolated from Drechslera rostrata and Eurotium tonophilum (Alasmary et al., 2018). PH-HM-16 did not show significant inhibitory activity against human gastric cancer (SGC-7901) and human lung adenocarcinoma epithelial (A549) cell lines. Compound PH-HM-16 exhibited the lowest cell growth inhibition (15.16%) at the absorbance value of 0.694 nm at 31.25 µg/mL concentration of the compound (Fig. 10: Supplementary data). On the other hand, compound PH-HM-16 at dose of 31.25 µg/mL inhibited the SGC-7901 tumor cell growth by 36.92% (Fig. 7B). These results coincide with previous report about the anticancer potential of sesquiterpenoids isolated from the Solanum lyratum and also tested against stomach gastric cancer cell lines (Chen et al., 2017). The IC50 value of PH-HM-16 compound was not significant against SGC-7901 (111.89 µg/mL) and A549 (176.04 µg/mL) cancer cell lines (Table 5). These result implies that compound PH-HM-16 has significant cytotoxic activity against some cancer cell lines. The bioassays for the bioactive compound isolated clearly indicates that it might hold a promising perspective in the future for the development of anticancer agents.
Fig. 7.
A) Percentage cell viability and B) Percentage Inhibition of PH-HM-16 against six human cancer cell lines. Where HL-60 = Human myeloid leukemia cell line; PC-3 = Prostate cancer cell line; SGC-7901 = Human gastric cancer cell line; MCF-7 = Breast cancer cell line; HCT-116 = Human colorectal cancer cell line; Lung A549 = Human lung adenocarcinoma cell line.
Table 5.
Regression analysis and IC50 values of anticancer activity of PH-HM-16.
| Cell lines | IC 50 | Regression Equation | R2 |
|---|---|---|---|
| HL-60 | 68.77 | y = 15.838ln(x) − 17.008 | 0.7548 |
| PC-3 | 17.63 | y = 10.512ln(x) + 19.834 | 0.9898 |
| SGC-7901 | 111.89 | y = 12.593ln(x) − 9.4079 | 0.9698 |
| MCF-7 | 41.81 | y = 13.104ln(x) + 1.081 | 0.9646 |
| HCT116 | 71.54 | y = 13.845ln(x) − 9.1215 | 0.97 |
| Lung A549 | 176.04 | y = 18.783ln(x) − 47.122 | 0.9734 |
Where HL-60 = Human myeloid leukemia cell line; PC-3 = Prostate cancer cell line; SGC-7901 = Human gastric cancer cell line; MCF-7 = Breast cancer cell line; HCT-116 = Human colorectal cancer cell line; Lung A549 = Human lung adenocarcinoma cell line.
5. Conclusions
In conclusion, among the eight plant extracts tested, P. harmala (seeds + roots), M. azedarach (fruits) and M. alba (leaves) were the most biologically active extracts with significant antitumor and cytotoxic effects which might be due to presence of phytochemicals, including flavonoids and phenolics. Bioactivity guided isolation of extracts led us to the isolation of PH-HM-16 from fraction P. harmala seeds. The results demonstrated significant anticancer activity against six human cancer cell lines assessed through MTT cell growth inhibition assay. PH-HM-16 was most effective against prostate cancer (PC3) cell lines with IC50 value of 17.63 µg/mL followed by breast cancer (MCF7) cell line with IC50 value of 41.81 µg/mL. Hence, this compound shows a promising potential to be chemically standardized or developed into the food supplements or pharmaceuticals or as adjuvant agents for the chemoprevention and/or the treatment of certain types of cancer in association with conventional anticancer therapies. Therefore, our results could be promising in order to develop adjuvant phytotherapeutics for cancer treatment.
Funding
Funding Agency: Prince Sultan University; Saudi Arabia.
Grant No: N/A
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to acknowledge the support of Prince Sultan University for paying the Article Processing Charges (APC) of this publication.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2021.04.016.
Contributor Information
Yamin Bibi, Email: dryaminbibi@uaar.edu.pk.
Abdul Qayyum, Email: aqayyum@uoh.edu.pk.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abozed S.S., Elkalyoubi M., Abdelrashid A., Salama M.F. Total phenolic contents and antioxidant activities of various solvent extracts from whole wheat and bran. Ann. Agri. Sci. 2014;59(1):63–67. [Google Scholar]
- Aghili M.H. Tehran University of Medical Sciences; Tehran: 2009. Makhzan-al-Advia (Persian) p. 328. [Google Scholar]
- Ahmad M., Khan M.A., Manzoor S., Zafer M., Sultana S. Check list of Medicinal Flora of Tehsil Isakhel in District Mianwali. Pakistan. Ethnobot. Leaflets. 2006;10:41–48. [Google Scholar]
- Ahmad S., Ullah F., Ayaz M., Zeb A., Ullah F., Sadiq A. Antitumor and anti-angiogenic potentials of isolated crude saponins and various fractions of Rumex hastatus D. Don. Biol. Res. 2016;12(49):18–20. doi: 10.1186/s40659-016-0079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S.S. Medicinal plants from Lahore-Islamabad motorway (M-2) Pak. J. Bot. 2007;9(2):355–375. [Google Scholar]
- Akihisa T., Pan X., Nakamura Y., Kikuchi T., Takahashi N., Matsumoto M., Ogihara E., Fukatsu M., Koike K., Tokuda H. Limonoids from the fruits of Melia azedarach and their cytotoxic activities. Phytochemistry. 2013;89:59–70. doi: 10.1016/j.phytochem.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Alasmary F.A.S., Awaad A.S., Kamal M., Alqasoumid S., Zain M.E. Antitumor activity of extract and isolated compounds from Drechslera rostrata and Eurotium tonophilum. Saudi Pharm. J. 2018;26(2):279–285. doi: 10.1016/j.jsps.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali K.H., Elbeshbishy H.A., Elbadry A.A., Alkhalaf M. Cytotoxic activity of methanolic extract of Mentha longifolia and Ocimum basilicum against human breast cancer. Pak. J. Biol. Sci. 2013;16(23):1744–1750. doi: 10.3923/pjbs.2013.1744.1750. [DOI] [PubMed] [Google Scholar]
- Ali N., Shah S.W.A., Shah I., Ahmed G., Ghias M., Khan I. Cytotoxic and anthelmintic potential of crude saponins isolated from Achillea Wilhelmsii C. Koch and Teucrium stocksianum Boiss. BMC Complement. Altern. Med. 2011;11:106–112. doi: 10.1186/1472-6882-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcanjo D.D.R., Albuquerque A.C.M., Melo N.B., Santana L.C.L.R., Medeiros M.G.F., Cito A.M.G.L. Bioactivity evaluation against Artemia salina Leach of medicinal plants used in Brazilian Northeastern folk medicine. Braz. J. Biol. 2012;72(3):1–6. doi: 10.1590/s1519-69842012000300013. [DOI] [PubMed] [Google Scholar]
- Ayaz M., Junaid M., Ullah F., Sadiq A., Subhan F., Khan M.A., Ahmad W., Ali G., Imran M., Ahmad S. Molecularly characterized solvent extracts and saponins from Polygonum hydropiper L. show high anti-angiogenic, anti-tumor, brine shrimp, and fibroblast NIH/3T3 cell line cytotoxicity. Front. Pharmac. 2016;7:74–76. doi: 10.3389/fphar.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam M.M., Rashid A.N.M.M., Towfique N.M., Sen M.K., Nasrin S. Pharmacological potentials of Melia azedarach L. – a review. Am. J. Biosci. 2013;1(2):44–49. [Google Scholar]
- Baba S.A., Malik S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Uni. Sci. 2015;9(4):449–454. [Google Scholar]
- Bae S.H., Suh H.J. Antioxidant activities of five different mulberry cultivars in Korea. LWT Food Sci. Technol. 2007;40(6):955–962. [Google Scholar]
- Baghel S.S., Shrivastava N., Baghel P.A., Rajput S. A review of quercetin: antioxidant and anticancer properties. World J. Pharm. Pharmaceut. Sci. 2012;1(1):146–160. [Google Scholar]
- Balasundram N., Sundram K., Samman S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99(1):191–203. [Google Scholar]
- Barud H.S., Junior A.M.A., Saska S., Mestieri L.M., Campos J.A.D.B., Freitas R.M., Ferreira N.U., Nascimento A.P., Miguel F.G., Leitevaz M.M.O.L., Barizon E.A., Oliveira F.M., Gaspar A.M.M., Ribeiro S.J.L., Berretta A.A. Antimicrobial Brazilian propolis (EPP-AF) containing biocellulose membranes as promising biomaterial for skin wound healing. Evid. Based Complement. Alternat. Med. 2013:1–10. doi: 10.1155/2013/703024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos M.L., Lima M.R., Conserva L.M., Andrade V.S., Rocha E.M., Lemos R.P. Studies on the antimicrobial activity and brine shrimp toxicity of Zeyheria tuberculosa (Vell.) Bur. (Bignoniaceae) extracts and their main constituents. Ann. Clin. Microbiol. Antimicrob. 2009;18:8–16. doi: 10.1186/1476-0711-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M.M., Islam R., Khatun H., Parvin S., Islam E., Islam S.A., Mia A.A. Antibacterial, antidiarrhoeal, and cytotoxic activities of methanol extract and its fractions of Caesalpinia bonducella (L.) Roxb leaves. BMC Comp. Altern Med. 2013;12:13–101. doi: 10.1186/1472-6882-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugs B. Infectious Diseases Society of America, VA; Alexandria: 2004. No Drugs. [Google Scholar]
- Carballo J.L., Hernandez Z.L., Perez P., Garcia G.M.D. A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotech. 2002;2:17–21. doi: 10.1186/1472-6750-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Wu J., Zhang X.X., Wang Q., Yan S.H., Wang H.D., Liu S.L., Zou X. Anticancer activity of sesquiterpenoids extracted from Solanum lyratum via the induction of mitochondria-mediated apoptosis. Oncol. Lett. 2017;13(1):370–376. doi: 10.3892/ol.2016.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidambara M.K.N., Kim J., Vikram A., Patil B.S. Differential inhibition of human colon cancer cells by structurally similar flavonoids of citrus. Food Chem. 2012;132(1):27–34. doi: 10.1016/j.foodchem.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Clarkson C., Maharaj V.J., Crouch N.R., Grace O.M., Pillay P., Matsabisa M.G., Bhagwandin N., Smith P.J., Folb P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalized in South Africa. J. Ethnopharm. 2004;92:177–191. doi: 10.1016/j.jep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Clere N., Faure S., Martinez M.C., Andriantsitohaina R. Anticancer properties of flavonoids: roles in various stages of carcinogenesis. Cardiovasc. Hematol. Agents Med. Chem. 2011;9(2):62–77. doi: 10.2174/187152511796196498. [DOI] [PubMed] [Google Scholar]
- Cyrus W.G., Daniel G.W., Nanyingi M.O., Njonge F.K., Mbaria J.M. Antibacterial and cytotoxic activity of Kenyan medicinal plants. Mem. Inst. Oswaldo. Cruz. 2008;103(7):650–652. doi: 10.1590/s0074-02762008000700004. [DOI] [PubMed] [Google Scholar]
- Dai Z.J., Lu W.F., Gao J., Kang H.F., Ma Y.G., Zhang S.Q., Diao Y., Lin S., Xi J.W., Wu W.Y. Anti-angiogenic effect of the total flavonoids in Scutellaria barbata D. Don. BMC Complement. Altern. Med. 2013;13(1):1–10. doi: 10.1186/1472-6882-13-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat N.T., Binh P.T.X., Quynh L.T.P., Minh C.V., Huong H.T., Lee J.J. Cytotoxic prenylated flavonoids from Morus alba. Fitoterapia. 2010;81:1224–1227. doi: 10.1016/j.fitote.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Delmulle L., Bellahcene A., Dhooge W., Comhaire F., Roelens F., Huvaere K., Heyerick A., Castronovo V., Keukeleire D. Anti-proliferative properties of prenylated flavonoids from hops (Humulus lupulus L.) in human prostate cancer cell lines. Phytomedicine. 2006;13(9–10):732–734. doi: 10.1016/j.phymed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Deshpande, R.S., Smitha, B., Naveen, H., Neelakanta, N.T., 2008. Cultivation of medicinal crops and aromatic crops as a means of diversification in agriculture (consolidated report). P. 205.
- Devi B., Sharma N., Kumar D., Jeet K. Morus alba Linn: a phytopharmacological review. Int. J. Pharm. Pharmaceut. Sci. 2013;5(2):14–18. [Google Scholar]
- Do Q.D., Angkawijaya A.E., Nguyen P.L.T., Huynh L.H., Soetaredjo F.E., Ismadji S., Ju Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatic. J. Food Drug Analy. 2014;22(3):296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edilu A., Adane L., Woyessa D. In vitro antibacterial activities of compounds isolated from roots of Caylusea abyssinica. Ann. Clin. Microbiol. Antimicrob. 2015;14(15):1–8. doi: 10.1186/s12941-015-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferth T., Kahl S., Paulus K., Adams M., Rauh R., Boechzelt H., Hao X., Kaina B., Bauer R. Phytochemistry and pharmacogenomics of natural products derived from traditional Chinese medicine and Chinese material medica with activity against tumor cells. Mol. Cancer Ther. 2008;7:152–216. doi: 10.1158/1535-7163.MCT-07-0073. [DOI] [PubMed] [Google Scholar]
- Elkashak W.A., Osman S.M., Gaara A.H., Eltoumy S.A., Mohamed T.K., Brouard I. Phenolic metabolites, biological activities, and isolated compounds of Terminalia muelleri extract. Pharm. Biol. 2017;55(1):2277–2284. doi: 10.1080/13880209.2017.1406531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed H.E., Ebrahim H.Y., Mohyeldin M.M., Siddique A.B., Kamal A.M., Haggag E.G., El Sayed K.A. Rutin as a novel c-Met inhibitory lead for the control of triple negative breast malignancies. Nutr. Cancer. 2017;69(8):1256–1271. doi: 10.1080/01635581.2017.1367936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraga M., Ahmed M.H.M., Yousef H., Rahman A.A.H.A. Repellent and insecticidal activities of Melia azedarach L. against cotton leafworm, Spodoptera littoralis (Boisd.) Z. Naturforsch C. 2011;66(3–4):129–135. doi: 10.1515/znc-2011-3-406. [DOI] [PubMed] [Google Scholar]
- Fort R.S., Barnech J.M.T., Dourron J., Colazzo M., Crespo F.J.A., Duhagon M., Alvarez G. Isolation and structural characterization of bioactive molecules on prostate cancer from mayan traditional medicinal plants. Pharmaceuticals. 2018;11(7):1–16. doi: 10.3390/ph11030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender M., Kapulnik Y., Koltai H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015;6:1–9. doi: 10.3389/fpls.2015.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galati G., Brien P.J. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004;37(3):287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Gautam R., Saklani A., Jachak S.M. Indian medicinal plants as a source of antimycobacterial agents. J. Ethnopharmac. 2007;110:200–234. doi: 10.1016/j.jep.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Hammad E.A., Zeaiter A., Saliba N., Farra M., Talhouk S. Bioactivity of fractionated indigenous medicinal plant extracts of Phlomis damascena Born. and Ranunculus myosuroides against the cotton whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae) J. Entomol. Nematol. 2015;7(5):46–53. [Google Scholar]
- Hayota H., Debeirb O., Hamb P.V., Dammea M.V., Kissa R., Decaestecker C. Characterization of the activities of actin-affecting drugs on tumor cell migration. Toxicol. Appl. Pharmacol. 2006;21:30–40. doi: 10.1016/j.taap.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Haque M., Ullah M.O., Nahar K. In vitro antibacterial and cytotoxic activities of different parts of plant Swietenia mahagony. Pak. J. Biol. Sci. 2009;12(7):599–602. doi: 10.3923/pjbs.2009.599.602. [DOI] [PubMed] [Google Scholar]
- Hassan L.E.A., Ahamed M.B.K., Majid A.S.A., Baharetha H.M., Muslim N.S., Nassar Z.D., Majid M.S.A. Correlation of antiangiogenic, antioxidant and cytotoxic activities of some Sudanese medicinal plants with phenolic and flavonoid contents. BMC Comp. Altern. Med. 2014;14:406–410. doi: 10.1186/1472-6882-14-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmateenejad B., Abbaspour A., Maghami H., Miri R., Panjehshahin M.R. Partial least squares-based multivariate spectral calibration method for simultaneous determination of beta-carboline derivatives in Peganum harmala seed extracts. Anal. Chim. Acta. 2006;575(2):290–299. doi: 10.1016/j.aca.2006.05.093. [DOI] [PubMed] [Google Scholar]
- Husain S.Z., Malik R.N., Javaid M., Bibi S. Ethonobotanical properties and uses of medicinal plants of Morgah Biodiversity Park. Rawalpindi. Pak. J. Bot. 2008;40(5):1897–1911. [Google Scholar]
- Iqbal H., Sher Z., Khan Z.U. Medicinal plants from salt range Pind Dadan Khan, District Jhelum, Punjab. Pakistan. J. Med. Plants Res. 2011;5(11):2157–2168. [Google Scholar]
- Katsube T., Imawaka N., Kawano Y. Antioxidant flavonol glycosides in mulberry (Morus alba L.) leaves isolated based on LDL antioxidant activity. J. Food Chem. 2006;97:25–31. [Google Scholar]
- Khan I., Yasinzai M.M., Mehmood Z. Comparative study of green fruit extract of Melia azedarach Linn. with its ripe fruit extract for antileishmanial, larvicidal, antioxidant and cytotoxic activity. Am. J. Phytomed. Clin. Ther. 2014;2(2):442–454. [Google Scholar]
- Khan M., Rehman N., Khan A., Gilani A.H. Pharmacological basis for the medicinal use of Morus alba in gut and airways disorders. Bangladesh J. Pharmacol. 2012;7:289–298. [Google Scholar]
- Khan M.A., Khan M.A., Hussain M. Medicinal plants used in folk recipes by the inhabitants of Himalayan Region Poonch valley Azad Kashmir (Pakistan) J. Basic. Appl. Sci. 2012;8:35–45. [Google Scholar]
- Khan R.A., Khan M.R., Shah N.A., Sahreen S., Elahi S.N. Antitumor characterization of various fractions of Launaea procumbens. Toxicol. Ind. Health. 2016;32(1):188–191. doi: 10.1177/0748233713500823. [DOI] [PubMed] [Google Scholar]
- Kumar P.S., Febriyanti R.M., Sofyan F.F., Luftimas D.E., Abdulah R. Anticancer potential of Syzygium aromaticum L. in MCF-7 human breast cancer cell lines. Pharmacog. Res. 2014;6(4):350–354. doi: 10.4103/0974-8490.138291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonti, M., Dal, C.M., Weckerle, C.S., Laura, C., 2015. Reverse ethnopharmacology and drug discovery. In: Proceedings of the 15th International Congress of the International Society for Ethnopharmacology. Petra, Jordan.
- Leporatti M.L., Ghedira K. Comparative analysis of medicinal plants used in traditional medicine in Italy and Tunisia. J. Ethnobio. Ethnomed. 2009;5(31):1–8. doi: 10.1186/1746-4269-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logarto P.A., Silva Y.R., Guerra S.I., Iglesias B.L. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomed. 2001;8:395–400. doi: 10.1078/0944-7113-00044. [DOI] [PubMed] [Google Scholar]
- Machii H., Koyama A., Yamanouchi H. Mulberry for animal production. Mulberry breeding, cultivation and utilization in Japan. FAO Anim. Prod. Health Paper. 2002 [Google Scholar]
- Mackeen M.M., Ali A.M., Lajis N.H., Kawazu K., Hassan Z., Amran M., Habsah M., Mooi L.Y., Mohamed S.M. Antimicrobial, antioxidant, antitumour-promoting and cytotoxic activities of different plant part extracts of Garcinia atroviridis Griff. Ex T. Anders. J. Ethnopharmacol. 2000;72:395–402. doi: 10.1016/s0378-8741(00)00245-2. [DOI] [PubMed] [Google Scholar]
- Manar M.M., Hiyasat A.S., Darmani H. Toxicity testing of restorative dental materials using brine shrimp larvae (Artemia salina) J. Appl. Oral Sci. 2008;16(4):297–301. doi: 10.1590/S1678-77572008000400013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani F., Sena P., Roncucci L. Inflammatory pathways in the early steps of colorectal cancer development. World J. Gastroenterol. 2014;20:9716–9731. doi: 10.3748/wjg.v20.i29.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathi P., Das S., Nikhil K., Roy P., Yerra S., Ravada S.R., Bokka V.R., Botlagunta M. Isolation and characterization of the anticancer compound Piceatannol from Sophora interrupta Bedd. Int. J. Prev. Med. 2015;6:101–103. doi: 10.4103/2008-7802.167181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbwambo Z.H., Moshi M.J., Masimba P.J., Kapingu M.C., Nondo R.S. Antimicrobial activity and brine shrimp toxicity of extracts of Terminalia brownii roots and stem. BMC Complement. Altern. Med. 2007;30:7–9. doi: 10.1186/1472-6882-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhaskar K.S., Latter E.B., Caius J.S., Ram V.A. Indian Medicinal Plants. Sri Satguru Pub. 2000;3:3185–3189. [Google Scholar]
- Moghaddam G., Ebrahimi S.A., Rahbar R.N., Foroumadi A. Antiproliferative activity of flavonoids: influence of the sequential methoxylation state of the flavonoid structure. Phytother. Res. 2012;26(7):1023–1028. doi: 10.1002/ptr.3678. [DOI] [PubMed] [Google Scholar]
- Monteleone G., Pallone F., Stolfi C. The dual role of inflammation in colon carcinogenesis. Int. J. Mol. Sci. 2012;13:11071–11084. doi: 10.3390/ijms130911071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro I., Sanchez E., Werner E., Godoy P., Olguin Y., Caro N., Ehrenfeld N., Madrid A. Isolation and identification of compounds from the resinous exudate of Escallonia illinita Presl. and their anti-oomycete activity. BMC Chem. 2019;13:1–7. doi: 10.1186/s13065-019-0516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshi M.J., Innocent E., Magadula J.J., Otieno D.F., Weisheit A., Mbabazi P.K., Nondo R.S.O. Brine shrimp of some plants used as traditional medicine in Kagera Region, Northw West Tanzania, Tanzania. J. Health Res. 2010;12(1):63–67. doi: 10.4314/thrb.v12i1.56287. [DOI] [PubMed] [Google Scholar]
- Mushtaq A., Muhammad Z., Ajab K.M., Shazia S., Mujtaba S.G., Jan G. Ethnomedicinal Investigation of Phytomedicines among local communities of arid areas, Pakistan. Indian J. Tradit. Knowl. 2012;11(3):436–446. [Google Scholar]
- Naz I., Ahmad M., Hassan T.U. Ethnobotanical investigation of medicinal flora used by indigenous people in district Attock, Pakistan. J. Advanced Bot. Zoo. 2014;1(4):1–7. [Google Scholar]
- Ncube N.S., Afolayan A.J., Okoh A.I. Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. African J. Biotech. 2008;7(12):1797–1806. [Google Scholar]
- Nguyen N.H., Hoaita Q.T., Pham Q.T., Luong T.N.H., Phung V.T., Duong T.H., Giauvo V. Anticancer activity of novel plant extracts and compounds from Adenosma bracteosum (Bonati) in human lung and liver cancer cells. Molecules. 2020;25(2912):1–16. doi: 10.3390/molecules25122912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyomkam P., Kaewbumrung S., Kaewnpparat S., Panichayupakaranant P. Antibacterial activity of Thai herbal extracts on acne involved microorganism. Pharmaceut. Bio. 2010;48(4):1–7. doi: 10.3109/13880200903150443. [DOI] [PubMed] [Google Scholar]
- Nondo R.S., Mbwambo Z.H., Kidukuli A.W., Innocent E.M., Mihale M.J., Erasto P., Moshi M.J. Larvicidal, antimicrobial and brine shrimp activities of extracts from Cissampelos mucronata and Tephrosia villosa from coast region, Tanzania. BMC Comp. Altern. Med. 2011;23:11–33. doi: 10.1186/1472-6882-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira A.M., Mesquita M.S., Silva G.C., Lima E.O., Medeiros P.L., Paiva P.M.G., Souza I.A., Napoleao T.H. Evaluation of toxicity and antimicrobial activity of an ethanolic extract from leaves of Morus alba L. (Moraceae) Evid. Based Comp. Altern. Med. 2015;7–9 doi: 10.1155/2015/513978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira R.N., Mancini M.C., Oliveira F.C.S., Passos T.M., Quility B., Thire R.M.S., Mcguiness G.B. FTIR analysis and quantification of phenolsand flavonoids of five commercially available plants extracts used in wound healing. Revista Materia. 2016;21(3):767–779. [Google Scholar]
- Park Y.S., Jung S.T., Kang S.G., Heo B.K., Arancibia A.P., Toledo F., Drzewiecki J., Namiesnik J., Gorinstein S. Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem. 2008;107:640–648. [Google Scholar]
- Pawlak A., Miguel D.E., Kutkowska J., Obminska M.B., Rapak A., Lostao L.M. Flavopiridol strongly sensitizes canine lymphoma cells to TRAIL-induced Apoptosis. Anticancer Res. 2017;37(12):6655–6665. doi: 10.21873/anticanres.12123. [DOI] [PubMed] [Google Scholar]
- Phua D.H., Tsai W.J., Ger J., Deng J.F., Yang C.C. Human Melia azedarach poisoning. Clin. Toxicol. 2008;46(10):1067–1070. doi: 10.1080/15563650802310929. [DOI] [PubMed] [Google Scholar]
- Qureshi R., Maqsood M., Arshad M., Chaudhry A.K. Ethnomedicinal uses of plants by the people of Kadhi areas of Khushab, Punjab, Pakistan. Pak. J. Bot. 2011;43(1):121–133. [Google Scholar]
- Roy A., Biswas S.K., Chowdhury A., Shill M.C., Raihan S.Z., Muhit M.A. Phytochemical screening, cytotoxicity and antibacterial activities of two Bangladeshi medicinal plants. Pak. J. Biol. Sci. 2011;14(19):905–908. doi: 10.3923/pjbs.2011.905.908. [DOI] [PubMed] [Google Scholar]
- Sahreen S., Khan M.R., Khan R.A., Hadda T.B. Evaluation of phytochemical content, antimicrobial, cytotoxic and antitumor activities of extract from Rumex hastatus D. Don roots. BMC Complement. Altern. Med. 2015;3(15):211–213. doi: 10.1186/s12906-015-0736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salawu K.M., Ajaiyeoba E.O., Ogbole O.O., Adeniji J.A., Faleye T.C., Agunu A. Antioxidant, brine shrimp lethality, and antiproliferative properties of gel and leaf extracts of Aloe schweinfurthii and Aloe vera. J. Herbs. Spices & Med. Plants. 2017;4:263–271. [Google Scholar]
- Sanches N.R. An evaluation of antibacterial activities of Psidium guava (L) Braz. Arch. Biol. Tecnol. 2005;48:429–436. [Google Scholar]
- Shabir H., Khan M.S., Rehman H.U., Massod Z., Yousaf T., Majid A., Iqbal R. Ethnomedicinal uses of Xeric flora in Tehsil Banda Daud Shah collected from District Karak, KPK, Pakistan. World J. Zoo. 2015;10(2):59–69. [Google Scholar]
- Shaheen H., Qureshi R., Akram A., Gulfraz M. Inventory of medicinal flora from Thal Desert, Punjab, Pakistan. Afr. J. Tradit. Complement. Altern. Med. 2014;11(3):282–290. doi: 10.4314/ajtcam.v11i3.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Sharma M.S., Mishra A., Sharma S., Kumar B., Bhandari A. A review on Thar plants used in liver diseases. Int. J. Res. Pharm. Chem. 2011;1(2):1–13. [Google Scholar]
- Shinwari Z.K., Khan A.A., Nakaika T. Press, Al-Aziz Commun; Peshawar: 2003. Medicinal and other useful plants of District Swat Pakistan; p. 78. [Google Scholar]
- Solowey E., Lichtenstein M., Sallon S., Paavilainen H., Solowey E., Galski H.L. Evaluating medicinal plants for anticancer activity. Scientific W. J. 2014:1–12. doi: 10.1155/2014/721402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas K., Afolayan A.J. Anticancer drug design based on plant-derived natural products. Current Sci. 2007;92:906–908. [Google Scholar]
- Sultana S., Khan M.A., Ahmad M., Zafar M. Indigenous knowledge of folk herbal medicines by the women of district Chakwal, Pakistan. Ethnobot. Leaflets. 2006;10:243–253. [Google Scholar]
- Swain T., Hillis W.E. Phenolic constituents of Prunus domestica. I. The quantitative analysis of phenolic constituents. J. Sci. Food Agri. 1959;10:63–68. [Google Scholar]
- Tanko Y., Yaro A.H., Isa A.I., Yerima M., Salehe M.I.A., Mohamedi A. Toxicological and hypoglycaemic studies on the leaves of Cissampelos mucronata (Menispermaceae) on blood glucose levels of streptozotocin induced diabetic wistar rats. J. Med. Plant Res. 2007;1(5):113–116. [Google Scholar]
- Tawaha K.A. Cytotoxicity evaluation of Jordanian wild plants using brine shrimp lethality test. J. Appl. Sci. 2006;8(1):12–17. [Google Scholar]
- Timmers M.A., Dias D.A., Urban S. Application of HPLC-NMR in the identification of plocamenone and isoplocamenone from the marine red alga Plocamium angustum. Mar. Drugs. 2012;10(9):2089–2102. doi: 10.3390/md10092089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkaboni M.M., Momenin T. Shahid Beheshti University Med. Sci. Pub; Tehran: 2007. Persian; p. 150. [Google Scholar]
- Ullah M., Khan M.U., Mahmood A., Malik R.N., Hussain M., Wazir S.M., Daud M., Shinwari Z.K. An ethnobotanical survey of indigenous medicinal plants in Wana district South Waziristan Agency. Pakistan. J. Ethnopharmac. 2013;150:918–924. doi: 10.1016/j.jep.2013.09.032. [DOI] [PubMed] [Google Scholar]
- Umadevi M., Kumar K.P.S., Bhowmik D., Duraivel S. Traditionally used anticancer herbs in India. J. Med. Plants Stud. 2013;1(3):56–74. [Google Scholar]
- Vollhardt K., Peter C., Schore N. W.H. Freeman; New York: 2007. Organic chemistry structure and function. [Google Scholar]
- Walsh F.M., Amyes S.G. Microbiology and drug resistance mechanisms of fully resistant pathogens. Curr. Opin. Microbiol. 2004;7(5):439–444. doi: 10.1016/j.mib.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Wang F.F., Liu F., Shi C., Ma W., Wang K.J., Li N. Cytotoxic activities of fractions of the willow bracket medicinal mushroom, Phellinus igniarius (Agaricomycetes), and the induction of cell cycle arrest and apoptosis in MGC-803 cells. Int. J. Med. Mushrooms. 2017;19(6):561–570. doi: 10.1615/IntJMedMushrooms.v19.i6.70. [DOI] [PubMed] [Google Scholar]
- Yeldandi A.V., Rao M.S., Reddy J.K. Hydrogen peroxide generation in peroxisome proliferator-induced oncogenesis. Mutat. Res. 2000;448(2):159–177. doi: 10.1016/s0027-5107(99)00234-1. [DOI] [PubMed] [Google Scholar]
- Yvette M.K., Kelsey R.G., Constantine G., Karchesy J.J. Biological screening of selected Pacific Northwest forest plants using the brine shrimp (Artemia salina) toxicity bioassay. Springerplus. 2016;5:510–511. doi: 10.1186/s40064-016-2145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar M.S., Muhammad F., Javed I., Akhtar M., Khaliq T., Aslam B., Waheed A., Yasmin R., Zafar H. White mulberry (Morus alba): A brief phytochemical and pharmacological evaluations account. Int. J. Agric. Biol. 2013;15:612–620. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.