Abstract
Introduction
Sickle-cell disease (SCD) is one of the most common hematologic inherited disorders in Saudi Arabia. Vaso-occlusive pain crisis in SCD is a major cause for emergency visits and patients’ pain may be undertreated. This study presents a narrative literature review of current agents used to manage acute pain crisis in SCD patients presenting to the emergency department in hospitals of Saudi Arabia.
Method
We conducted a narrative review on relevant published articles about sickle cell disease pain crisis management in Saudi Arabia and included seven relevant studies based on our inclusion criteria.
Results
Using our search strategy, we included 7 studies Out of 4052. Studies included were conducted in different locations in the country. Four studies were in the Eastern region while only one in Western and One in Central regions. Those studies included around 2441 patients, in total. Morphine was used in 5 studies out of the 7 included. Pethidine was used in 4. One study used Isoxsuprine and another study used tinzaparin.
Conclusion
We found that continuous administration of IV morphine accompanied by oral analgesics including NSAIDs and acetaminophen is the most commonly used practice for treating SCD patients presenting with a vaso-occlusive pain crisis. Possible effectiveness of tinzaparin, isoxsuprine, and pethidine as therapeutic options may be considered. However, there was no recommendation for a certain agent to be prescribed. We recommend conducting further clinical randomized-controlled trials.
1. Introduction
1.1. Rationale
Sickle-cell disease (SCD) is the most common hematologic inherited disorder and has been identified by the World Health Organization (WHO) as a major public health problem (Alaa Al-Anazi et al., 2017). It is caused by an inherited hemoglobin S gene which associated with a substitution of amino acid valine for glutamic acid in position number six of the β chain, which is responsible for the production of a defective form of hemoglobin. (Alaa Al-Anazi et al., 2017, Mohieldin Elsayid et al., 2015, Alabdulaali, 2007) In the United States, about 72 000 people are affected by SCD and 2 million are carriers. While the prevalence in Africa estimated that 200,000 infants were born with this disease. The overall prevalence of the disease in Saudi Arabia ranges from 2% to 27%. (Alaa Al-Anazi et al., 2017, Jastaniah, 2011, Abd et al., 2019) There is a wide distribution pattern of the hemoglobin S(HbS) gene in different regions varying from 0 − 1% in the northern and central regions, and around 25% in some areas of the eastern region, to approximately 7% in the western, 12% in the southern region. (Mousa et al., 2010) The distribution of SCD cases in the Eastern region was (145 cases/10,000 population), and in the southern region was (24 cases/10,000 population) which is much higher than the western region (12 cases/10,000 population), and the central region (6 cases/10,000). There are nearly 4.2% of the population in Saudi Arabia who have the disease. (Alotaibi, 2019) Management of SCD has several modalities, which may include treatment with disease-modifying agents such as hydroxyurea, or by blood transfusions. (Rodgers, 2014, Estcourt et al., 2016) Several complications could occur in SCD patients including stroke, acute chest syndrome, pulmonary hypertension, end-organ damage and other multi-system complications (Vichinsky, 2014).
The leading cause of emergency department visits and the most common manifestation of sickle cell disease is vaso-occlusive crisis (VOC). Vaso-occlusive crises occur due to the obstruction of blood vessels with the characteristic “sickle” shape of the red blood cells in SCD patients causing ischemia to the supplied organ and resulting in pain. The frequency and intensity of painful crises is variable. Some patients have 6 or more episodes annually and others may have much less frequent episodes or may have none (Complications and Treatments of Sickle Cell Disease, 2019, Strouse, 2016).
1.2. Objective
In this study, we aim to have a literature review on current agents used in the management of sickle cell disease cases with acute pain crisis presenting to the emergency department in hospitals of Saudi Arabia.
1.3. Research question
What is the most commonly used agent for the management of acute pain episodes in sickle cell anemia patients in emergency departments in hospitals of Saudi Arabia?
2. Methods:
2.1. Study design
We initially searched the literature and it showed heterogeneity among the study designs. Therefore, we conducted a narrative review instead of a meta-analysis. Our inclusion criteria are shown below.
2.2. Participants, interventions, comparators
We included Saudi sickle cell disease patients who presented with vaso-occlusive painful crisis. Several pain management intervention modalities were performed which differed among the relevant articles. The studies were compared to the recommendations of the consensus opinion which is discussed within this research.
2.3. Systematic review protocol
A narrative review was performed using PubMed, Google Scholar, Cochrane Library, ScienceDirect, SageHub, Springer, KAU deepknowledge, and Saudi Medical Journal, searching for relevant articles published about sickle cell disease population in Saudi Arabia. Out of 4052 studies, only 7 studies could be included.
2.4. Search strategy
The exact keywords used in the search within the search engines were: “sickle cell disease acute pain management in Saudi Arabia”, and “sickle cell anemia pain in Saudi Arabia” using [Therapy] and [Broad] filters.
2.5. Data sources and data extraction
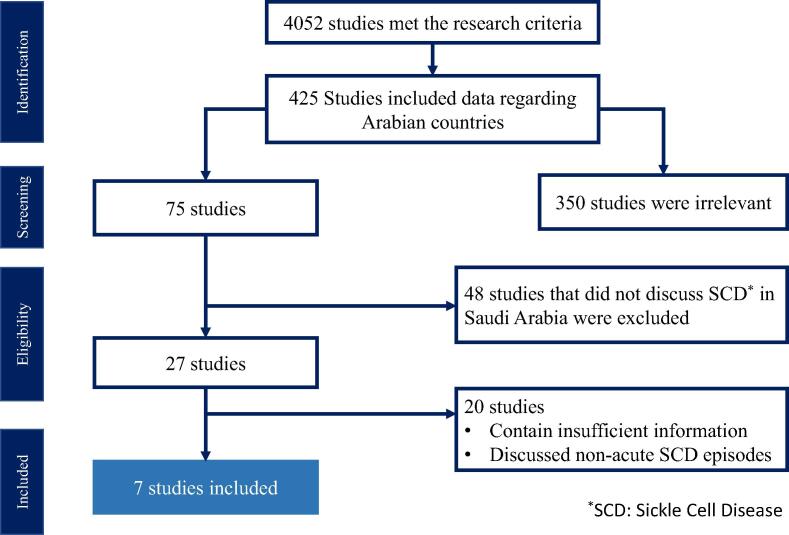
First, we screened the articles based on their titles and abstracts to determine relevance. We restricted our search to studies conducted in Saudi Arabia, written in English, regarding sickle cell disease and the management of vaso-occlusive pain. The total number of articles is shown in Fig. 1. Several studies were excluded from the study due to either they were irrelevant or had insufficient information
Fig. 1.
Flowchart of studies included.
2.6. Data analysis
A narrative synthesis of the studies was used.
3. Results
3.1. Study selection and characteristics
Using our search strategy, we found 4052 studies, only 7 studies met our inclusion criteria. All included studies were performed in Saudi Arabia. There were 4 studies conducted in the eastern region of Saudi Arabia, one in the western region, one in the central region, and one that included both Saudi Arabia and the Gulf region. There were 2 observational studies, 2 retrospective studies, one randomized clinical trial, one randomized comparative study, and one consensus opinion. Treatment modalities covered by these articles included numerous agents including opioid and non-opioid analgesics and other treatment methods. See Table 1 and Table 2.
Table 1.
Study-based characteristics of 7 articles.
| Study characteristics | Studies (n = 7) |
|---|---|
| Sample size | |
| 1–100 | 3 |
| 100–500 | 1 |
| greater than500 | 2 |
| Location | |
| Saudi Arabia and Gulf | 1 |
| Central region of Saudi Arabia | 1 |
| Eastern region of Saudi Arabia | 4 |
| Western region of Saudi Arabia | 1 |
| Type of study | |
| Observational study | 2 |
| Retrospective study | 2 |
| Randomized clinical trial | 1 |
| Randomized comparative study | 1 |
| Consensus opinion | 1 |
| Modality of treatment | |
| Morphine | 5 |
| Pethidine | 4 |
| Isoxsuprine | 1 |
| Tinzaparin | 1 |
Table 2.
Study characteristics and treatment modalities for emergency department management of acute vaso-occlusive pain.
| Author | Year | Sample | Location | Study design | Treatment | Outcome |
|---|---|---|---|---|---|---|
| E.Udezue, et al. | 2007 | 849 | Aramco Al-Hasa Health Center, KSA | Observational study | IV Morphine 5–7.5 mg q4h regularly for the first 24 h, then changed to PRN 5 mg morphine q6h | Regular intravenous narcotic analgesia for the initial 24 h supplemented by oral analgesia managed crisis effectively |
| E.Udezue, et al. | 2005 | 1154 | Aramco Al-Hasa Health Center, KSA | Observational study | IV Morphine 5–15 mg Q4h for the first 24 h combined with 1 g paracetamol PO | Morphine “regularly” was more effective than “on-demand” in VOC management |
| Mousa et al. | 2010 | Not specified | KSA and Gulf region | Consensus opinion |
Adults: Morphine 0.1 mg/Kg IV or (SC) q 20 min, Maintenance dose: morphine 0.05–0.1 mg/Kg SC or IV or PO, q 2–4 h, or as PCA. Children: morphine (0.1–0.15 mg/kg/dose, repeat every hour) Maintenance dose: additional 0.05 mg/kg morphine every 1–2 h |
__ |
| Alaa Al-Anazi, et al. | 2017 | 99 | King Abdulaziz Medical City, Riyadh, KSA | Retrospective chart review study | IV opioids (morphine, hydromorphone, fentanyl) or oral opioids (morphine, hydromorphone, oxycodone, Tylenol 3) in regular administration, versus patient controlled analgesia (In opioid equianalgesic dosing) | Intermittent IV morphine was more effective than PCA |
| Ali H. Al-Jam’a et al. | 1999 | 43 | Qatif Central Hospital, Dhahran Health Center, Dhahran and Dammam Central Hospital, in Dammam, KSA | Double-blind randomized comparative study | IM Isoxsuprine 5–10 mg or meperidine (pethidine) 50–100 mg | The study confirms potential effectiveness of isoxsuprine as a choice for treatment of VOC |
| Hashim M. Taha et al. | 2011 | 43 | King Abdulaziz Hospital, Al-Ahsa, KSA | Retrospective cohort study | Various agents used: Morphine, diclofenac, paracetamol, ibuprofen, tramadol, pethidine (No stated dosing) | 1. Significant number of pateints got IM analgesics2. Delay in initial administration of analgesics |
| Qari MH et al. | 2007 | 253 | King Abdulaziz University Hospital, King Fahd General Hospital, and King Abdulaziz Oncology Center, Jeddah, KSA | Prospective, randomized double-blind clinical trial | Tinzaparin 175 IU/kg SC OD for seven days + (supportive analgesia with morphine 1 mg/hr) | Tinzaparin displayed efficacy and safety in the management |
| Total | 2441 | |||||
KSA: Kingdom of Saudi Arabia. SCD: Sickle Cell Disease. VOC: Vaso-occlusive crisis. PCA: Patient-controlled analgesia. PRN: As needed. Q4h: every four hours. IM: Intramuscular. IV: Intravenous. PO: Orally
3.2. Synthesized findings
In this narrative review of 7 articles on the different treatment modalities in SCD patients who presented with a vaso-occlusive pain crisis to the emergency department, we found a range of treatment options used for management.
E.Udezue, et al. (Udezue and Herrera, 2007) performed an observational study by following a protocol that consists of 5–7.5 mg intravenous (IV) Morphine q4h regularly for the first 24 h of admission, then changed to 5 mg morphine q6h as needed, and accompanied with oral analgesia using paracetamol or non-steroidal anti-inflammatory drugs (NSAIDs). Results have shown adequate management of 80% of the SCD cases within 72 h of admission. The number of patients discharged was compared to the previous protocol used in the same hospital, 71% of the patients (following the old protocol) were discharged, versus 83% of patients discharged (following the new protocol) demonstrating a significantly higher percentage of patients discharged after 72 h of admission (P < 0.05). They have also found that males required higher narcotic analgesic dose than females with the same body weight, and had more persistent and prolonged pain crises, which they further investigated in another study.
E.Udezue, et al. (Udezue and Girshab, 2005) performed an observational study by comparing various acute pain management methods in SCD adult patients who present with VOC to the emergency department, in two different time periods (1995–1997) and (2000–2002). The first time period (1995–1997) included various treatment regimens which was using one of the following: 75 mg diclofenac administered intramuscularly (IM) every 8 h; 60–30 mg ketorolac given intravenously (IV) every 6 h, 50–100 mg pethidine given intravenously (IV) every 4 h as needed, IV morphine 5–15 mg every 4 h as needed. Whereas the standard treatment in the second time period (2000–2002) was 5–15 mg morphine administered intravenously every 4 h for the first 24 h, combined with oral paracetamol 1 g, or NSAID. Opiate analogs such as tramadol and (paracetamol with codeine), were beneficial options in those allergic to NSAIDs. Regular IV narcotic analgesia displayed superior effectiveness than intermittent or “as needed” analgesia which leads to a higher patient discharge rate. The definitive indicator used to assess enough pain relief was the number of patients discharged home or transferred to the hospital. Patients who had pain scores of 3–5 out of 5 were admitted to the hospital. They were discharged out when they maintained a decrease in pain score of ≥ 2 for at least four hours. Patients were assessed for adverse effects and no respiratory depression nor major side effects were seen.
Mousa et al. (Mousa et al., 2010) collected guidelines that were recommended by a committee of international clinical experts and local practitioners from various major hospitals located in Saudi Arabia and the Gulf region. These guidelines present a management algorithm of pain crises in SCD adults and children. If an adult patient has severe pain crisis and did not respond to oral analgesia, start intravenous (IV) or subcutaneous (SC) morphine 0.1 mg/Kg every 20 min. If the pain became under control, continue with the maintenance dose of morphine 0.05–0.1 mg/Kg SC or IV or orally (PO) every 2–4 h, and patient-controlled analgesia (PCA) may be used as an alternative. While if the pain crisis persists, start diamorphine 0.01 mg/Kg IV or SC, or use hydromorphine. As for children under 11 years of age, If the child presented with mild to moderate pain use paracetamol (15 mg/Kg per dose) plus codeine (1 mg/kg per dose given orally every 4 h) with or without ibuprofen (5–10 mg/kg per dose PO every 6–8 h), switch to oral analgesics such as paracetamol or ibuprofen when improved. If the child came with moderate to severe pain, start morphine (0.1–0.15 mg/kg/dose, repeated every hour). Extra 0.05 mg/kg of morphine administered every 1–2 h used as a maintenance dose until improvement occurs. Other adjuvant therapies in both adults and children included: hydroxyurea, tinzaparin, NSAIDs (ibuprofen, diclofenac, ketorolac), paracetamol, antiemetics, anxiolytics, and fentanyl patches (for adults only).
Alaa Al-Anazi et al. (Alaa Al-Anazi et al., 2017) retrospectively compared two groups of SCD patients for pain intensity and pain relief using either PCA or intermittent opioid therapy to manage their VOC episodes. The study included a total of 99 patients who are 14 years of age and above. The comparison took place within the first 72 h of admission. The first group (74 patients) were given intermittent IV morphine and the second group (25 patients) received PCA. The numerical pain score was measured (0–10) in all patients on admission (mean was 5.43 ± 1.73) and after treatment. Results demonstrate a significant reduction in pain score in the group that received intermittent IV morphine when compared to the PCA group (P < 0.0004), the mean pain score was found to be 3 in the first group (intermittent IV morphine) and 5 in the PCA group. The overall amount of morphine received within the first 72 h of admission was significantly higher in the group who received PCA (777 ± 175 mg) when compared to the first group (149 ± 74 mg) (P < 0.000003), concluding that intermittent administration of IV morphine was more effective in VOC pain management within the first 72 h of admission. All patients were investigated for the prevalence of any cardiovascular or respiratory adverse events and results have shown no signs of hypotension nor respiratory depression.
Ali H. Al-Jam’a et al. (Al-Jam'a et al., 1999) performed a double-blind randomized comparative study, they divided the patients to receive either isoxsuprine 5–10 mg or meperidine (pethidine) 50–100 mg IM. Overall, they observed no significant statistical difference among the two groups regarding their age, gender, weight, or height. Additionally, there was no significant difference in the degree of mobilization from the bed, duration of crisis, length of hospital days, side effect, and pulse or BP at multiple times of evaluation. Furthermore, there was no difference concerning the subjective symptoms of palpitation or somnolence, and no one reported nausea or vomiting. The pain was controlled better with conventional treatment (meperidine) only at 30 and 60 min. The degree of hemolysis did not show improvement with Isoxsuprine, as there were no significant differences in the total of hemoglobin level, total bilirubin, or reticulocyte count in both groups. The use of extra analgesics showed no statistically significant difference. Six patients within the isoxsuprine group and eight within the meperidine group needed extra analgesia (P = 0.6, chi-squared test). Five patients in each group required extra analgesia in the first two hours (P = 0.58, Fisher’s exact test). In conclusion, isoxsuprine shows similar effectiveness in treating sickle cell vaso-occlusive pain crises when compared to meperidine (pethidine).
Hashim M. Taha et al. (Taha and Rehmani, 2011) performed a retrospective cohort study to determine the time it takes for management & administration of analgesia to children and adolescents with SCD who present with painful crises in the emergency department (ED) from July 2006 to July 2007. The study included 43 patients who made 270 visits in total. Ages ranged between 5 and 18 years old, and the mean age was 12.1 years old, excluding those below 5 and above 18 years of age. Results have shown that male patients (176 [65.2%]) were presented more to the ED when compared to females (94 [34.8%]). Canadian Triage Acuity System was used to categorize the patients in the ED, which is classified into five levels. Level 3 (92 [34.1%]) and level 4 (158 [58.5%]) were the most triage type of visits. Although the international guidelines (95% confidence interval CI: 24.0–64.4) recommended 30 min as a standard time to administer the initial analgesics to SCD patients, the administration of the initial analgesics was delayed (42.2 ± 20.4) minutes, which is 40% higher than the standard time. Results have also found that morphine was the drug of choice for managing sever VOC pain, and other analgesics used were paracetamol (for mild to moderate pain), and codeine as an oral opioid for pediatrics, in addition to ibuprofen or other NSAIDs. Furthermore, almost fifth of the patients received analgesia through the IM route, which is not recommended due to the erratic absorption and the associated pain with injection. Most visits (237 [87.7%]) were permitted to leave the ED after an average length of stay of (183.9 ± 129.3) minutes, which is within the accepted duration of four hours.
Qari MH et al. (Qari et al., 2007) performed a randomized double-blind clinical trial by randomizing patients to receive either tinzaparin (175 IU/kg SC once daily for seven days) or placebo. They included SCD patients presenting to the ER who are 12 years of age and older and had pain that is severe enough to require narcotic analgesia. Data confirm that patients treated with tinzaparin showed a significant decreased in the number of days with the severest pain score (mean = 1.28 ± 0.20) vs placebo (1.74 ± 0.15), reduction in days of crisis (2.57 ± 0.45) vs placebo (4.35 ± 0.78), and reduction in duration of hospitalization (7.08 ± 1.8) vs placebo (12.06 ± 2.2) (p < 0.05). At entry, each group recorded similar pain scores. Pain resolved quickly or diminished from the first to the fourth day of treatment in the tinzaparin group. Two minor bleeding events were mentioned with tinzaparin treatment and were cured by discontinuation of tinzaparin therapy.
The following table summarizes the commonly encountered adverse effects in each study and the analgesics used. See Table 3.
Table 3.
Commonly encountered adverse effects for each study and analgesics used.
| Commonly encountered adverse effects | Analgesics used | Author |
|---|---|---|
| - Nausea and vomiting are common adverse effects of morphine. *The study did not specify number of incidence, but they mention that they used promethazine to decrease morphine induced nausea and vomiting. | IV Morphine Q4hr regularly for the first 24 h then changed to PRN Morphine Q6hr. | E. Udezue, et al. − 2007 |
| - No major side effects are reported, although few patients declined morphine occasionally because of drowsiness, sometimes worsened by promethazine.- Five cases of acute chest syndrome occurred in the first group and 10 in the second*Patients who were affected were transferred to the ICU and all survived. | IV Morphine regularly every four hours for the first 24 h, combined with PO Paracetamol or NSAIDs . | E. Udezue, et al. − 2005 |
| None specified | Morphine | Mousa et al. |
| - They defined adverse drug reaction as hypotension (systolic BP < 90 mmHg) and/or respiratory depression (respiratory rate < 12 breaths/min). - Over the 72 h of admission no signs of hypotension or respiratory depression had shown in both groups. | 1) Parenteral opioids: Morphine, Hydromorphone, and Fentanyl.2) Oral opioids: Morphine, Hydromorphone, Tylenol #3 (codeine), and Percocet (oxycodone). | Alaa Al-Anazi, et al. |
| - Side effects in the study were defined as any of the following: palpitation, somnolence, nausea, vomiting, tachycardia and hypotension. - There were no reported significant side effects in both groups. | 1) IM Isoxsuprine2) IM Meperidine | Ali H. Al-Jam'a et al. |
| None specified | Morphine, Voltaren (diclofenac), and Paracetamol. | Hashim M. Taha et al. |
| -Tinzaparin treatment was associated with two minor bleeding events that were reported and treated by cessation of tinzaparin. | Tinzaparin | Qari MH et al. |
IV: Intravenous, Q: Every, PRN: as needed, ICU: Intensive Care Unit, NSAID: non-steroidal anti-inflammatory drug, PO: Orally, BP: Blood pressure, IM: Intramuscular.
4. Discussion
4.1. Summary of key findings
We noticed that SCD patients tend to be undertreated for their vaso-occlusive pain crisis. Our first key finding was that among the seven reviewed articles morphine was the most used agent for treating VOC in sickle cell disease adult and pediatric population in Saudi Arabia, which was supported by five articles. (Alaa Al-Anazi et al., 2017, Udezue and Herrera, 2007, Udezue and Girshab, 2005 Mar, Mousa et al., 2010 Aug, Taha and Rehmani, 2011) Several dosing regimens of morphine were used, which were continuous IV infusion given regularly or as needed or using PCA with several routes of administration (PO, IV, SC). (Alaa Al-Anazi et al., 2017, Udezue and Girshab, 2005 Mar, Mousa et al., 2010 Aug) Also, dosing regimen was different by either using a fixed amount (IV 5–7.5 mg q4h, then switched to PRN 5 mg morphine q6h), (Udezue and Herrera, 2007) (IV morphine 5–15 mg Q4h) (Udezue and Girshab, 2005), or weight-based dosing in adults (IV or SC Morphine 0.1 mg/Kg q 20 min), then switched to (0.05–0.1 mg/Kg IV or PO or SC as a maintenance dose, q 2–4 h, or PCA). As for children (morphine 0.1–0.15 mg/kg/dose, repeated every hour, then switched to 0.05 mg/kg morphine q 1–2 h as a maintenance dose).
The second key finding is that Pethidine (Meperidine) was the second most used opioid in SCD painful crisis management in the emergency department. (Udezue and Herrera, 2007, Udezue and Girshab, 2005 Mar, Al-Jam'a et al., 1999 Mar, Taha and Rehmani, 2011) Pethidine is a short-acting and weak opioid analgesic, (Udezue and Girshab, 2005 Mar, Yale et al., 2000 Mar 1, Rees et al., 2003 Mar) but was associated with more addiction and side effects and had poor bioavailability. (Udezue and Herrera, 2007) Besides, norpethidine is a metabolite of pethidine and excreted renally which can cause seizures in patients with impaired renal function. (Udezue and Herrera, 2007, Rees et al., 2003 Mar) For these reasons the use of pethidine was discouraged. (Mousa et al., 2010) The intramuscular (IM) route is generally discouraged because of unstable absorption and the pain it causes. In addition, repeated pethidine injections through IM route can lead to muscular fibrosis causing decreased absorption from the injection site, which is why larger doses are needed and thus causing additional muscle fibrosis and worse, increasing the probability of drug addiction or dependence (Okpala and Tawil, 2002).
Male patients presented more to the emergency department than females, (Taha and Rehmani, 2011, Udezue and Girshab, 2004 May) and required up to 40% more opioid doses. In addition, the number of older males was significantly lower, suggesting either higher mortality in males or that older males are not experiencing pain as bad and therefore not presenting to the hospital. Furthermore, testosterone level in males may be the cause of more painful crises especially during the surge of testosterone in puberty,[20] and the decreased bioavailability of nitric oxide in the vascular endothelium of males with SCD may be a potential cause. (Gladwin et al., 2003) Interestingly, the suggested explanation for females being managed adequately on lower opioid doses was due to their familiarity of menstrual and birth pains, and due to a pharmacokinetic difference in opioid metabolism which was slower in females (Udezue and Girshab, 2005 Mar, Schwartz, 1999).
Our final key finding was that five articles followed similar protocols to the recommended consensus opinion. [14] However, we can still see hospitals use non-opioid therapeutic agents such as NSAIDs, Isosuxprine, and tinzaparin therapy in the management of acute SCD pain crisis.
The management of acute pain episodes is mainly supportive and involves bed rest, hydration, oxygen, and analgesia. (Gladwin et al., 2003 Jan 21, Delicou and Maragkos, 2013 Aug, Steinberg, 1999) The use of analgesia during the VOC may follow the three-step ladder recommended by the WHO for the management of cancer-related pain. However, it may be deemed inadequate (Rees et al., 2003).
There are numerous guidelines in the USA on management of VOC in SCD. The current recommendations in the US for treating VOC are in the report written by expert panel members Barbara P. Yawn et al. which included recommendations for the management of SCD from multiple aspects, including different age groups, acute and chronic complications, and health maintenance.
Adults and children in severe VOC pain should be started on parenteral opioids administered as around-the-clock either by patient-controlled analgesia (PCA), frequently scheduled doses, or as requested administration (strong recommendation, high quality of evidence). While those in mild to moderate VOC pain episodes are to be treated with nonsteroidal anti-inflammatory drugs (NSAIDs) in the absence of contraindications, (moderate strength recommendation, low quality of evidence). The use of incentive spirometry is strongly recommended to reduce the risk of acute chest syndrome (ACS) during hospitalization for VOC. Blood transfusion should not be done in children and adults with a VOC unless there are other indications for transfusion (moderate strength recommendation, low quality of evidence) . In addition, there is a strong recommendation for those who have more than 3 vaso-occlusive crises per year to be started on hydroxyurea therapy (Yawn et al., 2014).
There are other treatment options and routs of administration that can be used in the treatment of SCD during vaso-occlusive crises, however none are approved yet. Here we present a brief summary for each modality and its related recommendations. Pain management should include parenteral opioids for severe pain according to 2014 NIH recommendations, directed by an individualized or an institutional SCD-specific protocol. Furthermore, multiple protocols only use the oral (PO) or intranasal (IN) routes as a bridge to IV medications while others have IV medications as standard therapy (Paquin et al., 2020).
Medications through the IN route are administered easily, with a rapid onset of action, bypassing gastrointestinal and hepatic first-pass metabolism, avoiding the brain-blood barrier and specifically targeting the central nervous system, and are administered earlier than IV medications in different clinical scenarios including in SCD (Dale et al., 2002, Fein et al., 2017).
4.1.1. Intranasal fentanyl
Fentanyl is a selective opioid mu receptor agonist and can be given intranasally. (Paquin et al., 2020) Intranasal fentanyl (INF) works safely and effectively with minimal side effects in children with other painful conditions.
A randomized, double-blind, placebo-controlled trial, in the pediatric emergency department (PED), found that children who received INF 2 μg/kg (maximum 100 μg) for initial treatment of a VOC had a greater decrease in median pain score 20 min after the administration when compared to those who received placebo. Additionally, there were no significant adverse events due to INF. (Fein et al., 2017, Wolfe and Braude, 2010)
A single-center retrospective study examined whether the use of a new pain management pathway using intranasal (IN) fentanyl 2 mcg/kg/dose (maximum 100 mcg/dose) on patients with SCD seen in the ED for VOC leads to improved care, by decreasing the time needed for administration of the first opiate dose. The result of the study showed that the time to first opiate dose was 94.5 min in pre and 52.3 min in post protocol implementation, the number of patients treated with a non-intravenous opiate has been increased by 43%, and there was a 49% decrease in the number of IV line insertions in patients who were discharged from the ED (Paquin et al., 2020).
4.1.2. Transbuccal fentanyl
Fentanyl is an opioid agonist that selectively binds and activates mu-opioid receptors in the central nervous system resulting in hyperpolarization of the cell and inhibition of nerve activity. The transbuccal formulation of fentanyl is readily absorbed -upon contact with the buccal mucosa- into the systemic circulation resulting in effective and rapid analgesia (Arthur and Holder, 2012). One crossover clinical study evaluated the effect of fentanyl buccal tablet (FBT) as breakthrough analgesia in the early stage of pain management of adults with SCD during their severe vaso-occlusive crices. The first group were treated with ketorolac (0.86 mg/kg/day) and tramadol (7.2 mg/kg/day), and the second group received the same treatment with the addition of fentanyl buccal tablet (100 mcg given once, may be repeated with maximum daily dose of 400 mcg/day).
Results have shown a significant reduction in visual pain score (VAS) at 6 h when treating with fentanyl buccal tablet when compared with ketorolac and tramadol treatment. Transbuccal fentanyl can be a promising agent, however further investigation on a larger cohort is needed (De Franceschi et al., 2016).
4.1.3. Ketamine
Ketamine, an Nmethyl-D-aspartate (NMDA) receptor reversible antagonist, has a role in the management of acute pain crisis. The mechanism of ketamine in pain reduction is due to its ability - at subanesthetic doses - to play a role in counteracting hyperalgesia and to act as a protectant from opioid tolerance. Its use in VOC is currently under investigation.
In a systematic review and meta-analysis on the published literature on ketamine use during in VOC, ketamine showed potentially similar efficacy with other opioids in reducing the pain during VOC in SCD patients but with a higher rate of reported adverse events (Alghamdi and Al-Shahrani, 2020).
One randomized clinical trial showed that low-dose ketamine can be a potential analgesic as an adjunct to morphine for the treatment of moderate to severe acute pain. The study included 60 patients, however, only two of which were SCD cases. The study concluded that dosing of 0.3 mg/kg could be more effective than 0.15 mg/kg but can have minor adverse events (Beaudoin et al., 2014).
There is a research protocol for a randomized controlled trial undergoing in Saudi Arabia. It is done in the ED of a tertiary academic hospital in the eastern province (Alshahrani et al., 2019). The study aimed to evaluate whether the addition of ketamine to morphine can achieve better pain control and thus decreasing the number of repeated doses of opiates. They hypothesized that the early administration of ketamine would lead to a quicker improvement in pain score and lower the opioid requirements. It is a randomized, concealed, blinded, pragmatic parallel group, controlled trial enrolling adult patients with SCD and acute vaso-occlusive crisis pain. Patients are randomized to a treating arm receiving low-dose ketamine 0.3 mg/kg and a control group receiving standard dose of morphine 0.1 mg/kg, both arms receiving the treatment in normal saline and standard intravenous hydration. The primary efficacy aim is to validate whether the early use of ketamine for the management of acute sickle cell pain crisis will achieve a more effective reduction in pain severity scores. The secondary efficacy aim is to decrease the ED length of stay, the cumulative use of opioid during ED stays, the rate of hospital admission, development of any known side effects of the drugs used.
There is also a current RCT undergoing in the United states with a similar protocol. The researchers aim to examine the efficacy of 0.3 mg/kg dose of ketamine vs. placebo. (https://www.centerwatch.com/clinical-trials/listings/247682/sickle-cell-crisis-low-dose-ketamine-acute/?&radius=50&query=acute-pain&rnk=2, xxxx) There was a protocol for an RCT for the assessment of ketamine use in VOC sponsored by the University of south Florida, yet it was withdrawn in 2018 and was never proceeded with. (https://clinicaltrials.gov/ct2/show/NCT03502421, xxxx)
Consensus guidelines on the use of ketamine as an IV infusion included several indications and had specific recommendations for VOC. They conclude that ketamine may be considered for opioid dependent or opioid-tolerant SCD patients during their acute pain crises. However, evidence was limited to case series and case reports only. (Schwenk et al., 2018) In one case report and a literature review by Uprety et al, they found that 83.3% of 18 patients with SCD crises had significant improvement in pain with a reduction in opioid use with subanesthetic doses of ketamine. The case reported a patient who received a 7-day course of ketamine infusion with effective pain control. Exact doses were not provided in the study. (Uprety et al., 2014) In a case series by palm, et al. the researchers discussed the cases of 5 patients who were opioid tolerant SCD patients with VOC who received ketamine infusion dosed at 1 µg/kg/min and titrated up to 5 µg/kg/min, with durations varying from 5 to 9 days. Results have shown that ketamine can decrease pain in patients who require high-dose opioid analgesics during VOC with no serious complications (Palm et al., 2018).
4.2. Intranasal ketamine
There is a research protocol for a multicentered, RCT to assess the use of intranasal ketamine for pediatric SCD patients. Patients in the treatment group will receive intranasal ketamine dosed at 1 mg/kg at beginning of therapy, while the placebo group will receive the same volume of intranasal normal saline (Young et al., 2017).
4.2.1. Lidocaine
The only studies that tackled the use of lidocaine in VOC were retrospective in design and all concluded that lidocaine can have an effective role in the management of VOC with the need of further RCTs and prospective studies to be conducted.
One retrospective study evaluated the use of lidocaine and ketamine intravenous infusions as adjunct to opioids in SCD patients during their VOC. This study included 4 adolescent patients who acted as self-controls. The interventions results were compared to the management they received in their previous VOC admission.
Opioid consumption and length of stay during the active therapy admission was compared to a prior standard therapy admission. The study included patients were adolescents aged 13–17 years. They received ketamine and/or lidocaine infusions during seven active therapy admissions in VOC pain episodes. Results have shown a reduction in the opioid consumption seen in 3 patients out of 4, while one patient had a significantly increased opioid consumption when compared to his previous admission. Lidocaine was used in 2 patients and showed a reduction in opioids use with no adverse effects (Puri et al., 2019).
Another retrospective study evaluated the efficacy and safety of intravenous lidocaine as an adjunct to opioids in adults with sickle cell disease (SCD). The study was done on 11 SCD patients who received IV lidocaine initially dosed at 0.5–2.7 mg/kg/h (mean = 1 mg/kg/h), with a maximum mean dose at 1.5 mg/kg/h (range: 0.5–2.8 mg/kg/h). Fifteen intravenous lidocaine infusions were performed on those 11 patients. Eight of which reported to achieve at least 20% reduction in pain score and were considered clinically successful . A 32.2% reduction in morphine dose equivalent was seen in the eight patients when comparing the mean difference in morphine dose equivalent at 24 h before lidocaine infusion with the 24 h after.
During the first 24 h of the lidocaine infusion, other adjunct pain medications were discontinued in four patients. These agents included acetaminophen, ketorolac, ibuprofen, and gabapentin. However, the percent reduction was not consistent in all patient admissions, while one patient had 73.4% decrease in morphine dose equivalent, another had a 22.2% increase in requirement. Adverse effects associated with lidocaine use were dizziness and disorientation seen only in 2 patients (Nguyen et al., 2015).
4.2.2. Inhaled nitric oxide
Nitric oxide has a vasodilatory action. In SCD, there are alterations in the cofactor and substrate availability for endothelial nitric oxide synthase, leading to vasculopathy and emphasizing the potential role of reactive species like oxygen and nitrogen in SCD pathogenesis (Choudhuri et al., xxxx).
A narrative review by Aboursheid et al. discussed three clinical trials that evaluated the use of inhaled nitric oxide in VOC management. The authors concluded that the current clinical trials do not show enough evidence to determine the efficacy or the harm of using inhaled nitric oxide to treat SCD patients who present with VOC, and using the GRADE system (Grading of Recommendations, Assessment, Development and Evaluations) they identified the evidence as low quality (Aboursheid et al., 2019).
4.2.3. Inhaled methoxyflurane
Methoxyflurane, a previously used inhaled anesthetic, and a volatile hydrocarbon can be used at low doses and provide rapid analgesia within minutes for adults with pain associated with trauma. Inhaled methoxyflurane is available in an easily disposable self-administered inhaler. The dosing regimen used is mainly based on a low dose at 3 mL that can be administered twice per day with a maximum of 15 mL of total doses per week.
There are several ongoing RCTs that are assessing its efficacy and safety, and are all supporting its beneficial role in emergency analgesia. The main reported adverse effects were dizziness, somnolence, and headache.
No studies specifically addressed the use of inhaled methoxyflurane during VOC in SCD patients, however it may be considered a promising agent and it should be further studied in this patient population (Fabbri et al., 2020).
4.2.4. Nebulized morphine
One RCT compared the efficacy of nebulized morphine to the traditional IV morphine for treating severe post-traumatic pain. Doses used were 10 mg or 20 mg repeated every 10 min with a maximum of 3 nebulizations. Results have shown that the use of 10 mg nebulized morphine showed similar effectiveness and less adverse effects when compared to the intravenous route. Those who received the 20 mg dose had a significantly larger decrease in pain score. However, this study did not include patients with SCD (Grissa et al., 2015).
In a case report that assessed the use of nebulized morphine in two SCD patients who had acute chest pain showed a significant decrease in pain score within minutes. The dose used was 20 mg morphine sulphate in 3 or 5 mL of normal saline solution and was given every 6 h for a total of 10 days in the first patient, and 11 days in the second one. In addition, both patients were previously given intravenous opioids, morphine PCA for the first patient, IV hydromorphone and oral extended release morphine in the second patient. The authors concluded that nebulized morphine can be an effective modality of treatment that specifically targets acute chest pain in SCD while preventing the progression to acute chest syndrome (Ballas et al., 2004).
4.3. Limitations
We faced some limitations in our study. A limited number of studies contained relevant data. The included articles were very heterogeneous in their design and a few studies included small sample size, but they met the criteria for patients with SCD who present a vaso-occlusive crisis in the emergency department in Saudi Arabia. Inability to conduct a time weighted sum pain intensity differences (SPID) due to insufficient data. The included articles did not specify the rates and needs for rescue analgesia. Very little number of updated research in the area of VOC management and the response to opioids in Saudi Arabia. None of the seven included articles specified a clear recommendation on what agent to use in the management. Finally, the approach to VOC management in Saudi Arabia is somewhat inadequate regarding the non-opioid analgesic modalities that are used in Europe, USA and Australia which include the intranasal fentanyl, intranasal ketamine, intravenous ketamine, intravenous lidocaine, nitrous oxide and dexmedetomidine.
5. Conclusion
There are many agents used in SCD to manage acute pain crises which include opioids, NSAIDs, paracetamol, with the possible effectiveness of tinzaparin, isoxsuprine, and pethidine as therapeutic options. Based on our findings, no robust recommendation for a certain agent. We expect to see more recommendations based on randomized control trials rather than opinions.
Funding
None.
Competing interests
None declared.
Ethical approval
Not required.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to express our special thanks to Rayan Hejazi for the valuable support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alaa Al-Anazi LA-S, Maha Al-Ammari , Tariq Al-Debasi , Abdulmalik M. Alkatheri , Shmeylan Al-Harbi , Aiman A. Obaidat , et al. Assessment of patient-controlled analgesia versus intermittent opioid therapy to manage sickle-cell disease vaso-occlusive crisis in adult patients. Saudi J Anaesth2017 Oct-Dec. [DOI] [PMC free article] [PubMed]
- Mohieldin Elsayid MJA-S, Yasser Abdullah Alkulaibi , Abdullah Alanazi , and Shoeb Qureshi. Frequency distribution of sickle cell anemia, sickle cell trait and sickle/beta-thalassemia among anemic patients in Saudi Arabia. 2015 Aug. [DOI] [PMC free article] [PubMed]
- Alabdulaali M.K. Sickle cell disease patients in eastern province of Saudi Arabia suffer less severe acute chest syndrome than patients with African haplotypes. Ann Thorac Med. 2007 doi: 10.4103/1817-1737.36550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastaniah D.W. Epidemiology of sickle cell disease in Saudi Arabia. Ann Saudi Med. 2011 doi: 10.4103/0256-4947.81540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd Abeer A., Elmoneim Z.M.A.H., Mahmoud Badr Z., Bukhari Abdullah A., Almulla Abdulmalik A., Sonbol Abdullah M., Makhdoum Anas M. Causes of hospitalization in sickle cell diseased children in western region of Saudi Arabia. A single center study. Saudi Med J. 2019 doi: 10.15537/smj.2019.4.24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alotaibi Maha Mohammed. Sickle cell disease in Saudi Arabia: A challenge or not. Journal of epidemiology and global health. 2019;7(2):99–101. doi: 10.1016/j.jegh.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers GP. Hydroxyurea and other disease-modifying therapies in sickle cell disease. UpToDate. Schrier SL, Mahoney DH and Tirnauer JS (Eds). 2014.
- Estcourt LJ, Fortin PM, Hopewell S, Trivella M. Red blood cell transfusion to treat or prevent complications in sickle cell disease: an overview of Cochrane reviews. The Cochrane database of systematic reviews. 2016 Feb 8;2016(2). [DOI] [PMC free article] [PubMed]
- Vichinsky E.P. UpToDate; Waltham, MA: 2014. Overview of the clinical manifestations of sickle cell disease. [Google Scholar]
- Complications and Treatments of Sickle Cell Disease | CDC [Internet]. Centers for Disease Control and Prevention. 2019 [cited 9 December 2019]. Available from: https://www.cdc.gov/ncbddd/sicklecell/treatments.html#Pain
- Strouse J. Sickle cell disease. InHandbook of clinical neurology 2016 Jan 1 (Vol. 138, pp. 311-324). Elsevier. [DOI] [PubMed]
- Udezue E., Herrera E. Pain management in adult acute sickle cell pain crisis: a view point. West African journal of medicine. 2007;26(3):179–182. doi: 10.4314/wajm.v26i3.28305. [DOI] [PubMed] [Google Scholar]
- Udezue E., Girshab A.M. Observations on the management of acute pain crisis in adult sickle cell disease in eastern Saudi Arabia. Annals of Saudi medicine. 2005 Mar;25(2):115–119. doi: 10.5144/0256-4947.2005.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa S.A., Al Momen A., Al Sayegh F., Al Jaouni S., Nasrullah Z., Al Saeed H. Management of painful vaso-occlusive crisis of sickle-cell anemia: consensus opinion. Clinical and Applied Thrombosis/Hemostasis. 2010 Aug;16(4):365–376. doi: 10.1177/1076029609352661. [DOI] [PubMed] [Google Scholar]
- Al-Jam'a A.H., Al-Dabbous I.A., Rafiq M.S., Al-Khatti A., Al-Salem A.H., Al-Baharna A. Isoxsuprine in the treatment of sickle cell painful crises: a double-blind comparative study with narcotic analgesic. Annals of Saudi medicine. 1999 Mar;19(2):97–100. doi: 10.5144/0256-4947.1999.97. [DOI] [PubMed] [Google Scholar]
- Taha H.M., Rehmani R.S. Pain management of children and adolescents with sickle cell disease presenting to the emergency department. Saudi Med J. 2011;32(2):152–155. [PubMed] [Google Scholar]
- Qari M.H., Aljaouni S.K., Alardawi M.S., Fatani H., Alsayes F.M., Zografos P. Reduction of painful vaso-occlusive crisis of sickle cell anaemia by tinzaparin in a double-blind randomized trial. Thrombosis and haemostasis. 2007;98(08):392–396. [PubMed] [Google Scholar]
- Yale S.H., Nagib N., Guthrie T. Approach to Vaso-occlussive Crisis in Adults with Sickle Cell Disease. American family physician. 2000 Mar 1;61(5):1349–1356. [PubMed] [Google Scholar]
- Rees D.C., Olujohungbe A.D., Parker N.E., Stephens A.D., Telfer P., Wright J. Guidelines for the management of the acute painful crisis in sickle cell disease. British journal of haematology. 2003;120(5):744–752. doi: 10.1046/j.1365-2141.2003.04193.x. [DOI] [PubMed] [Google Scholar]
- Okpala I., Tawil A. Management of pain in sickle-cell disease. Journal of the Royal Society of Medicine. 2002;95(9):456–458. doi: 10.1258/jrsm.95.9.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udezue E., Girshab A.M. Differences between males and females in adult sickle cell pain crisis in eastern Saudi Arabia. Annals of Saudi medicine. 2004 May;24(3):179–182. doi: 10.5144/0256-4947.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin M.T., Schechter A.N., Ognibene F.P., Coles W.A., Reiter C.D., Schenke W.H. Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation. 2003 Jan 21;107(2):271–278. doi: 10.1161/01.cir.0000044943.12533.a8. [DOI] [PubMed] [Google Scholar]
- Schwartz J.B. Gender differences in response to drugs: pain medications. The journal of gender-specific medicine: JGSM: the official journal of the Partnership for Women's Health at Columbia. 1999;2(5):28–30. [PubMed] [Google Scholar]
- Delicou S., Maragkos K. Pain Management in Patients with Sickle Cell Disease-A Review. Hematology. 2013 Aug;1(1):30–36. [Google Scholar]
- Steinberg MH. Management of sickle cell disease. New England Journal of Medicine. 1999 Apr 1;340(13):1021-.. [DOI] [PubMed]
- Yawn B.P., Buchanan G.R., Afenyi-Annan A.N., Ballas S.K., Hassell K.L., James A.H., Jordan L., Lanzkron S.M., Lottenberg R., Savage W.J., Tanabe P.J. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. Jama. 2014 Sep 10;312(10):1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- Paquin H., Trottier E.D., Pastore Y., Robitaille N., Dore Bergeron M.-J., Bailey B. Evaluation of a clinical protocol using intranasal fentanyl for treatment of vaso-occlusive crisis in sickle cell patients in the emergency department. Paediatrics & Child Health. 2020;25(5):293–299. doi: 10.1093/pch/pxz022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale O., Hjortkjaer R., Kharasch E. Nasal administration of opioids for pain management in adults. Acta anaesthesiologica scandinavica. 2002;46(7):759–770. doi: 10.1034/j.1399-6576.2002.460702.x. [DOI] [PubMed] [Google Scholar]
- Fein D.M., Avner J.R., Scharbach K., Manwani D., Khine H. Intranasal fentanyl for initial treatment of vaso-occlusive crisis in sickle cell disease. Pediatric blood & cancer. 2017;64(6):e26332. doi: 10.1002/pbc.26332. [DOI] [PubMed] [Google Scholar]
- Wolfe T.R., Braude D.A. Intranasal medication delivery for children: a brief review and update. Pediatrics. 2010;126(3):532–537. doi: 10.1542/peds.2010-0616. [DOI] [PubMed] [Google Scholar]
- Arthur A.O., Holder P. A review of transbuccal fentanyl use in the emergency department. Pain research and treatment. 2012;2012 doi: 10.1155/2012/768796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Franceschi L., Mura P., Schweiger V., Vencato E., Quaglia F.M., Delmonte L., Evangelista M., Polati E., Olivieri O., Finco G. Fentanyl buccal tablet: a new breakthrough pain medication in early Management of Severe Vaso-Occlusive Crisis in sickle cell disease. Pain Practice. 2016 Jul;16(6):680–687. doi: 10.1111/papr.12313. [DOI] [PubMed] [Google Scholar]
- Alghamdi M., Al-Shahrani M. 38. Ketamine for painful sickle cell vaso-occlusive crises: a systematic review and meta-analysis. European Journal of Emergency Medicine. 2020 Aug 1;27(1):e14–e15. doi: 10.1097/01.mej.0000697876.99952.13. [DOI] [PubMed] [Google Scholar]
- Beaudoin F.L., Lin C., Guan W., Merchant R.C. Low-dose ketamine improves pain relief in patients receiving intravenous opioids for acute pain in the emergency department: results of a randomized, double-blind, clinical trial. Academic Emergency Medicine. 2014 Nov;21(11):1193–1202. doi: 10.1111/acem.12510. [DOI] [PubMed] [Google Scholar]
- Alshahrani M.S., Asonto L.P., El Tahan M.M., Al Sulaibikh A.H., Al Faraj S.Z., Al Mulhim A.A., Al Abbad M.F., Al Nahhash S.A., Aldarweesh M.N., Mahmoud A.M., Almaghraby N. Study protocol for a randomized, blinded, controlled trial of ketamine for acute painful crisis of sickle cell disease. Trials. 2019 Dec;20(1):1–7. doi: 10.1186/s13063-019-3394-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.centerwatch.com/clinical-trials/listings/247682/sickle-cell-crisis-low-dose-ketamine-acute/?&radius=50&query=acute-pain&rnk=2
- https://clinicaltrials.gov/ct2/show/NCT03502421
- Schwenk E.S., Viscusi E.R., Buvanendran A., Hurley R.W., Wasan A.D., Narouze S., Bhatia A., Davis F.N., Hooten W.M., Cohen S.P. Consensus guidelines on the use of intravenous ketamine infusions for acute pain management from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Regional Anesthesia & Pain Medicine. 2018 Jul 1;43(5):456–466. doi: 10.1097/AAP.0000000000000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uprety D., Baber A., Foy M. Ketamine infusion for sickle cell pain crisis refractory to opioids: a case report and review of literature. Annals of hematology. 2014 May 1;93(5):769–771. doi: 10.1007/s00277-013-1954-3. [DOI] [PubMed] [Google Scholar]
- Palm N., Floroff C., Hassig T.B., Boylan A., Kanter J. Low-dose ketamine infusion for adjunct management during vaso-occlusive episodes in adults with sickle cell disease: a case series. Journal of pain & palliative care pharmacotherapy. 2018 Jan 2;32(1):20–26. doi: 10.1080/15360288.2018.1468383. [DOI] [PubMed] [Google Scholar]
- Young J.R., Sawe H.R., Mfinanga J.A., Nshom E., Helm E., Moore C.G., Runyon M.S., Reynolds S.L. Subdissociative intranasal ketamine plus standard pain therapy versus standard pain therapy in the treatment of paediatric sickle cell disease vaso-occlusive crises in resource-limited settings: study protocol for a randomised controlled trial. BMJ open. 2017;7(7):e017190. doi: 10.1136/bmjopen-2017-017190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri L., Morgan K.J., Anghelescu D.L. Ketamine and lidocaine infusions decrease opioid consumption during vaso-occlusive crisis in adolescents with sickle cell disease. Current opinion in supportive and palliative care. 2019 Dec 1;13(4):402–407. doi: 10.1097/SPC.0000000000000437. [DOI] [PubMed] [Google Scholar]
- Nguyen N.L., Kome A.M., Lowe D.K., Coyne P., Hawks K.G. Intravenous lidocaine as an adjuvant for pain associated with sickle cell disease. Journal of pain & palliative care pharmacotherapy. 2015 Oct 2;29(4):359–364. doi: 10.3109/15360288.2015.1082009. [DOI] [PubMed] [Google Scholar]
- Choudhuri S, Ray B, Mohanty D. Medical Reports and Case Studies.
- Aboursheid T., Albaroudi O., Alahdab F. Inhaled nitric oxide for treating pain crises in people with sickle cell disease. Cochrane Database of Systematic Reviews. 2019;10 doi: 10.1002/14651858.CD011808.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri A., Ruggiano G., Collado S.G., Ricard-Hibon A., Restelli U., Sbrana G., Marinangeli F., Farina A., Coffey F. Role of inhaled methoxyflurane in the management of acute trauma pain. Journal of pain research. 2020;13:1547. doi: 10.2147/JPR.S252222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissa M.H., Boubaker H., Zorgati A., Beltaïef K., Zhani W., Msolli M.A., Bzeouich N., Bouida W., Boukef R., Nouira S. Efficacy and safety of nebulized morphine given at 2 different doses compared to IV titrated morphine in trauma pain. The American journal of emergency medicine. 2015;33(11):1557–1561. doi: 10.1016/j.ajem.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Ballas S.K., Viscusi E.R., Epstein K.R. Management of acute chest wall sickle cell pain with nebulized morphine. American journal of hematology. 2004;76(2):190–191. doi: 10.1002/ajh.20064. [DOI] [PubMed] [Google Scholar]