Abstract
Background
It is unclear whether the suggested link between COVID-19 during pregnancy and preeclampsia is an independent association or if these are caused by common risk factors.
Objective
This study aimed to quantify any independent association between COVID-19 during pregnancy and preeclampsia and to determine the effect of these variables on maternal and neonatal morbidity and mortality.
Study Design
This was a large, longitudinal, prospective, unmatched diagnosed and not-diagnosed observational study assessing the effect of COVID-19 during pregnancy on mothers and neonates. Two consecutive not-diagnosed women were concomitantly enrolled immediately after each diagnosed woman was identified, at any stage during pregnancy or delivery, and at the same level of care to minimize bias. Women and neonates were followed until hospital discharge using the standardized INTERGROWTH-21st protocols and electronic data management system. A total of 43 institutions in 18 countries contributed to the study sample. The independent association between the 2 entities was quantified with the risk factors known to be associated with preeclampsia analyzed in each group. The outcomes were compared among women with COVID-19 alone, preeclampsia alone, both conditions, and those without either of the 2 conditions.
Results
We enrolled 2184 pregnant women; of these, 725 (33.2%) were enrolled in the COVID-19 diagnosed and 1459 (66.8%) in the COVID-19 not-diagnosed groups. Of these women, 123 had preeclampsia of which 59 of 725 (8.1%) were in the COVID-19 diagnosed group and 64 of 1459 (4.4%) were in the not-diagnosed group (risk ratio, 1.86; 95% confidence interval, 1.32–2.61). After adjustment for sociodemographic factors and conditions associated with both COVID-19 and preeclampsia, the risk ratio for preeclampsia remained significant among all women (risk ratio, 1.77; 95% confidence interval, 1.25–2.52) and nulliparous women specifically (risk ratio, 1.89; 95% confidence interval, 1.17–3.05). There was a trend but no statistical significance among parous women (risk ratio, 1.64; 95% confidence interval, 0.99–2.73). The risk ratio for preterm birth for all women diagnosed with COVID-19 and preeclampsia was 4.05 (95% confidence interval, 2.99–5.49) and 6.26 (95% confidence interval, 4.35–9.00) for nulliparous women. Compared with women with neither condition diagnosed, the composite adverse perinatal outcome showed a stepwise increase in the risk ratio for COVID-19 without preeclampsia, preeclampsia without COVID-19, and COVID-19 with preeclampsia (risk ratio, 2.16; 95% confidence interval, 1.63–2.86; risk ratio, 2.53; 95% confidence interval, 1.44–4.45; and risk ratio, 2.84; 95% confidence interval, 1.67–4.82, respectively). Similar findings were found for the composite adverse maternal outcome with risk ratios of 1.76 (95% confidence interval, 1.32–2.35), 2.07 (95% confidence interval, 1.20–3.57), and 2.77 (95% confidence interval, 1.66–4.63). The association between COVID-19 and gestational hypertension and the direction of the effects on preterm birth and adverse perinatal and maternal outcomes, were similar to preeclampsia, but confined to nulliparous women with lower risk ratios.
Conclusion
COVID-19 during pregnancy is strongly associated with preeclampsia, especially among nulliparous women. This association is independent of any risk factors and preexisting conditions. COVID-19 severity does not seem to be a factor in this association. Both conditions are associated independently of and in an additive fashion with preterm birth, severe perinatal morbidity and mortality, and adverse maternal outcomes. Women with preeclampsia should be considered a particularly vulnerable group with regard to the risks posed by COVID-19.
Key words: aspirin, cohort, gestational hypertension, hypertension, hypertensive disorders in pregnancy, infection, morbidity, mortality, obesity, overweight, preeclampsia, pregnancy, preterm birth, proteinuria, relative risk, renal disease, risk ratio, SARS-CoV 2, small for gestational age
Introduction
COVID-19, which is primarily a respiratory infection, can have marked multiorgan, vascular effects leading to hypertension, renal disease, thrombocytopenia, and hepatic injury. SARS-CoV-2 can produce direct endothelial damage, thromboinflammation, dysregulation of immune responses, and alterations in angiotensin-converting enzyme 2–related pathways.1 Preeclampsia, but not gestational hypertension (GH), causes endothelial damage, placental oxidative stress, and an antiangiogenic state leading to hypertension and proteinuria,2 and similar multiorgan effects as seen in severe cases of COVID-19.3
AJOG at a Glance.
Why was this study conducted?
It is unclear whether the suggested association between COVID-19 during pregnancy and preeclampsia is independent of common risk factors. This study aimed to quantify any independent association between COVID-19 during pregnancy and preeclampsia and to determine key related pregnancy outcomes and maternal and neonatal morbidity and mortality.
Key findings
After adjusting for risk factors, COVID-19 during pregnancy is independently associated with preeclampsia (risk ratio [RR), 1.77; 95% confidence interval [CI), 1.25–2.52 in all women, and RR, 1.89; 95% CI, 1.17–3.05 in nulliparous women) and, to a lesser degree, with gestational hypertension (RR, 1.53; 95% CI, 1.11–2.11). COVID-19 and preeclampsia are associated independently of and in an additive fashion with an increased risk for preterm birth (RR, 4.05; 95 % CI, 2.99–5.49), small for gestational age neonates (RR, 2.32; 95% CI, 1.50–3.58), severe perinatal morbidity and mortality (RR, 2.84; 95% CI, 1.67–4.82), and composite maternal morbidity and mortality (RR, 2.51; 95% CI, 1.72–3.67).
What does this add to what is known?
Women with preeclampsia should be considered a particularly vulnerable group for COVID-19.
Because COVID-19 has been shown to increase the risk for adverse pregnancy outcomes, including preeclampsia,4, 5, 6 the concept of a COVID-19–associated preeclampsia-like syndrome, which includes similar placental pathology, has been proposed.7, 8, 9 However, it is important to note that COVID-19 during pregnancy and preeclampsia share the same set of risk factors, namely preexisting hypertension, obesity, and diabetes.10, 11, 12 Thus, the association between COVID-19 and preeclampsia could be confounded by common underlying risk factors.
A genuine association could manifest in the following 3 ways: (1) COVID-19 could cause symptoms and signs that meet the diagnostic criteria for preeclampsia, although these are separate conditions; (2) preeclampsia, which has pathophysiological changes already apparent early in pregnancy,13, 14, 15 could constitute an additional risk factor for COVID-19, or (3) COVID-19 could be on an etiologic pathway toward preeclampsia, which in itself has been related etiologically to infectious diseases.16, 17, 18
In this secondary analysis of the INTERCOVID multinational study,19 we explored these possibilities including the independent association between the 2 conditions with severe adverse maternal and neonatal outcomes.
Materials and Methods
INTERCOVID was a large, longitudinal, prospective, multinational observational study assessing the effect of COVID-19 during pregnancy on mothers and neonates.19 , 20 A total of 43 institutions in Argentina, Brazil, Egypt, France, Ghana, India, Indonesia, Italy, Japan, Mexico, Nigeria, North Macedonia, Pakistan, Russia, Spain, Switzerland, the United Kingdom, and the United States contributed to the study.
Between March 2, 2020, and February 2, 2021, we enrolled women aged ≥18 years with a diagnosis of COVID-19 at any stage during their current pregnancy (diagnosed group) based on (1) a laboratory confirmation of COVID-19 or radiological pulmonary findings suggestive of COVID-1921 or (2) ≥2 predefined COVID-19 symptoms. Of note, 2 immediately concomitant pregnant women aged ≥18 years without any of these diagnostic criteria (not-diagnosed group) were enrolled for each diagnosed woman to create an unbiased sample representative of all not-diagnosed pregnant women in these institutions. Women in the diagnosed and not-diagnosed samples were followed until hospital discharge of the newborn.
When a woman was diagnosed antenatally, 2 not-diagnosed women of a similar gestational age (±2 weeks), receiving standard antenatal care, were enrolled that day. If not possible or if those not-diagnosed women were lost to follow-up, we enrolled 2 not-diagnosed women who delivered immediately after the diagnosed woman. The same selection strategy was employed when a diagnosed woman was identified at hospital admission and delivery was likely to occur during that admission. If a not-diagnosed woman declined participation, the next woman was approached until 2 not-diagnosed women were enrolled for each diagnosed case. We sought confirmation from a biweekly, random 10% sample that the 2 not-diagnosed women were appropriately chosen; we excluded 5 diagnosed women and the corresponding not-diagnosed women without such confirmation. Live and stillborn and singleton and multiple gestation pregnancies in addition to those with congenital anomalies were included.
For the secondary analysis, we considered preeclampsia as a primary co-exposure in addition to COVID-19 status; GH was considered a secondary co-exposure. Preeclampsia was defined by (1) blood pressure of 140/90 mm Hg or greater or an increase of 30 mm Hg systolic or 15 mm Hg diastolic above baseline values on at least 2 occasions 6 hours or more apart with proteinuria that developed after 20 weeks’ gestation in a previously normotensive pregnancy; or (2) blood pressures of ≥160 mm Hg systolic or ≥110 mm Hg diastolic on 2 occasions, at least 4 hours but not more than 168 hours apart, or if the first measurement was immediately followed by treatment with an antihypertensive, either of these scenarios being associated with the presence of proteinuria; (3) eclampsia, defined as the occurrence of convulsions or coma unrelated to cerebral conditions in a woman with symptoms and signs of preeclampsia; or (4) evidence of hemolysis, elevated liver enzymes, and low platelet count syndrome. GH was defined as blood pressure of >140/90 mm Hg without proteinuria after 20 weeks’ gestation in a previously normotensive woman.
Outcomes
The neonatal outcomes of interest were preterm birth (<37 weeks’ gestation), frequency of small for gestational age (SGA) neonates (birthweight below the 10th percentile for gestational age and sex according to the INTERGROWTH-21st Newborn Size Standards),22 and an unweighted index, namely the severe perinatal morbidity and mortality index, which includes (1) fetal death; or (2) at least 1 of the following severe complications: bronchopulmonary dysplasia, hypoxic-ischemic encephalopathy, sepsis, anemia requiring transfusion, patent ductus arteriosus requiring treatment or surgery, intraventricular hemorrhage, necrotizing enterocolitis or retinopathy of prematurity diagnosed before hospital discharge; or (3) admission to the neonatal intensive care unit for ≥7 days; or (4) neonatal death before hospital discharge. We also examined a composite adverse maternal outcome, defined as the presence of (1) at least 1 of the following pregnancy-related morbidities: vaginal bleeding during pregnancy, preterm labor, and infection requiring antibiotics; or (2) any other pregnancy-related conditions requiring treatment or referral; or (3) maternal admission to the intensive care unit (ICU) or referral to a higher level of care; or (4) death.
Gestational age at delivery was estimated based on the earliest ultrasound scan (<14 weeks’ gestation using the international INTERGROWTH-21st standards).23 If early ultrasound dating was not carried out, the best obstetrical estimate was used based on all clinical and ultrasound data available.24
Data management and analysis
We used the same centrally coordinated data management system developed for the INTERGROWTH-21st Project (MedSciNet, London, United Kingdom).25 All data were entered locally into the on-line system with its extensive, built-in quality control facility. Queries can be dispatched immediately to the study sites, which provides continuously clean datasets for intermediate analysis.
Statistical methodology
We first compared the baseline demographic characteristics, and gynecologic, obstetrical, and medical histories of the 4 study groups, namely COVID-19 diagnosis without preeclampsia, preeclampsia without COVID-19, COVID-19 diagnosis with preeclampsia, and neither condition diagnosed. Second, we explored the association between preeclampsia and COVID-19 by adjusting for potential confounders that were selected a priori to be on the casual pathway using a directed acyclic graph. We further considered effect modification by parity or aspirin use during pregnancy and performed sensitivity analyses of multiple pregnancies and age of <20 years and, for the main outcome of COVID-19 and preeclampsia, adjustment by study site as a covariate and using mixed-effects models with random slopes by site.
Associations of preeclampsia and COVID-19 with the binary outcomes were assessed using generalized linear models with a Poisson distribution and log link function. Estimates are expressed as risk ratios (RRs) and 95% confidence intervals (CIs). We used robust standard errors (SEs) to account for model misspecification and a clustered estimator of variance in models with perinatal outcomes to account for the lack of independence of multiple births. We assessed the joint effects of COVID-19 and preeclampsia on neonatal outcomes using an interaction term in the models.
In all regression models, we adjusted first for potential sociodemographic confounders, such as maternal age, parity, and tobacco use during pregnancy, and second for a set of risk factors know to be associated with both COVID-19 and preeclampsia such as overweight and obesity, a history of diabetes, cardiac disease, hypertension, or renal disease. Finally, we adjusted for pregnancy history that included a preterm birth, miscarriage, or low birthweight neonate.
We plotted Kaplan-Meier curves with the percentage of women who remained pregnant according to the gestational age to compare the distributions among the 4 groups. We also plotted Kaplan-Meier curves with the gestational age at the time of COVID-19 diagnosis as the dependent variable and the diagnosis of preeclampsia as the independent binary variable. Hazard ratios with 95% CIs were estimated using a Cox proportional hazards model.26
Ethics approval was obtained from the Oxford Tropical Research Ethics Committee (OxTREC), reference 526-20, and all participating medical institutions obtained local approval from their corresponding ethics committees or institutional review boards. Written or verbal consent was obtained according to local practices. The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization-Good Clinical Practice guidelines and did not interfere with the clinical management of the women enrolled. The protocol was published on the study website before starting the study.20
Results
We enrolled a total of 2184 pregnant women. Of these, 725 were diagnosed with COVID-19 during pregnancy on the basis of a laboratory confirmation (real-time polymerase chain reaction [RT-PCR) test, n=672, 92.7%), radiological confirmation (n=4, 0.6%), or ≥2 predefined COVID-19 symptoms (n=49, 6.8%) if no laboratory results were available. The remaining 1459 pregnant women constituted the not-diagnosed COVID-19 group enrolled concomitantly using the rigorous methodology described above. In the not-diagnosed COVID-19 group, 730 of 1459 (50.0%) women were tested; of these, 698 had a negative RT-PCR test and 32 had a negative COVID-19 antibody test.
The contribution to the total study population ranged from 19.9% (Italy, n=434) to 0.1% (Macedonia, n=3). The other sites contributed to the study data as follows: Argentina contributed 10.6% (n=231), the United States contributed 10.2% ( n=222), Pakistan contributed 9.6% (n=210), Mexico contributed 8.0% (n=174), France contributed 6.7% (n=147), India contributed 6.7% (n=147), the United Kingdom contributed 5.3% (n=116), Russia contributed 5.0% (n=108), Spain contributed 4.7% (n=102), Brazil contributed 4.3% (n=93), Nigeria contributed 3.6% (n=78), Indonesia contributed 2.6% (n=57), Ghana contributed 1.7% (n=36), Japan contributed 0.6% (n=12), Egypt contributed 0.4% (n=9), and Switzerland contributed 0.2% (n=5).
Overall, 123 women had preeclampsia; of these, 8.1% (59 of 725) of the women were in the COVID-19 diagnosed group and 4.4% (64 of 1459) were in the COVID-19 not-diagnosed group, (RR, 1.77; 95% CI, 1.25–2.52). There were 143 women with GH; 8.4% (61 of 725) of the women were in the COVID-19 diagnosed group and 5.6% (82 of 1459) were in the COVID-19 not-diagnosed group (RR, 1.53; 95% CI, 1.11–2.11). Of the 725 women with COVID-19, 292 (40.3%) were asymptomatic and 433 (59.7%) were symptomatic; 22 cases of preeclampsia occurred in the asymptomatic group and 37 cases of preeclampsia occurred in the symptomatic group.
The baseline demographic characteristics and gynecologic, obstetrical, and medical histories across these 4 groups are shown in Table 1 . The median gestational age at COVID-19 diagnosis was 37.6 (interquartile range, 34.3–39.1) weeks, and 71.3% of women were diagnosed <10 days before giving birth.
Table 1.
Baseline characteristics among women according to preeclampsia and COVID-19 diagnosis
| Demographic and socioeconomic characteristics | Preeclampsia (n=123) |
No preeclampsia (n=2061) |
||
|---|---|---|---|---|
| COVID-19 diagnosed (n=59) | COVID-19 not-diagnosed (n=64) | COVID-19 diagnosed (n=666) | COVID-19 not-diagnosed (n=1395) | |
| Maternal age (y) | 29.5±7.1 | 30.7±6.3 | 30.0±6.0 | 30.3±6.1 |
| Maternal height (cm) | 158.8±7.3 | 159.9±7.1 | 161.4±7.6 | 161.2±7.9 |
| Maternal weight (kg) | 72.1±23.2 | 69.7±17.2 | 66.8±14.9 | 64.9±15.8 |
| Body mass index (kg/m2) | 28.5±8.5 | 27.2±6.0 | 25.6±5.4 | 24.9±5.7 |
| Married or cohabiting (%) | 82.5 | 85.0 | 88.2 | 89.0 |
| University education (%) | 19.2 | 22.6 | 32.3 | 33.9 |
| Worked outside the home (%) | 39.2 | 42.6 | 52.4 | 51.0 |
| Smoker during index pregnancy (%) | 3.4 | 6.3 | 2.8 | 3.7 |
| Alcohol ≥1 units/wk (%) | 0.0 | 0.0 | 2.1 | 2.3 |
| Gynecologic and obstetrical history | ||||
| Previous pregnancy (%) | 62.7 | 64.1 | 72.0 | 66.9 |
| Previous miscarriage (%) | 32.2 | 32.8 | 31.6 | 30.9 |
| Previous birth (%) | 47.5 | 50.0 | 61.3 | 55.0 |
| Previous baby <2.5 kg or >4.5 kg (%) | 11.8 | 16.1 | 8.7 | 7.3 |
| Previous baby <37 wk’s gestation (%) | 7.8 | 14.3 | 7.1 | 5.6 |
| Previous stillbirth or neonatal death (%) | 5.9 | 3.6 | 5.3 | 3.2 |
| Previous adverse pregnancy outcomea (%) | 39.0 | 45.3 | 38.9 | 36.7 |
| Maternal medical history | ||||
| Diabetes (%) | 8.5 | 3.1 | 4.2 | 1.3 |
| Thyroid and other endocrine disease (%) | 3.4 | 4.7 | 11.4 | 9.2 |
| Cardiac disease (%) | 8.5 | 1.6 | 1.2 | 1.2 |
| Hypertension (%) | 22.0 | 15.6 | 2.0 | 1.5 |
| Chronic respiratory disease (%) | 3.7 | 6.5 | 3.4 | 2.0 |
| Kidney disease (%) | 1.7 | 4.7 | 0.6 | 0.9 |
| Malaria (%) | 5.1 | 0.0 | 1.2 | 1.5 |
| Tuberculosis (%) | 0.0 | 0.0 | 0.5 | 0.2 |
| ≥2 of the above conditions (%) | 10.2 | 3.1 | 2.7 | 1.7 |
Papageorghiou et al. Preeclampsia and COVID-19. Am J Obstet Gynecol 2021.
Includes previous miscarriage, stillbirth or neonatal death, or infant born preterm or at a low birthweight.
Association between COVID-19 and preeclampsia
Table 2 presents the preeclampsia risk for the COVID-19 diagnosed and not-diagnosed groups. After initial adjustment for maternal age, parity (nulliparous vs parous), tobacco use, and history of adverse pregnancy outcomes, the RR for preeclampsia remained statistically significant for nulliparous (RR, 2.14; 95% CI, 1.33–3.44) and parous women (RR, 1.75; 95% CI, 1.06–2.88), however, the interaction was not significant (P=.60). Further adjustment for conditions known to be associated with preeclampsia (overweight and obesity, history of diabetes, cardiac disease, hypertension, or renal disease) led to a relatively small reduction in the RR, which remained statistically significant for nulliparous women (RR, 1.89; 95% CI, 1.17–3.05) (Table 2). These results strongly suggest that the association between COVID-19 and preeclampsia is independent of the main confounding variables. The results were also very similar after adjusting for study site (Supplemental Table 3).
Table 2.
Associations between COVID-19 diagnosis and preeclampsia
| Adjustments | All women (n=2075) | Nulliparous (n=901) | Parous (n=1174) |
|---|---|---|---|
| Unadjusted | |||
| No COVID-19 diagnosis | Ref | Ref | Ref |
| COVID-19 diagnosis | 1.95 (1.38–2.75)a | 2.20 (1.37–3.55)a | 1.78 (1.08–2.94)a |
| No COVID-19 diagnosis | Ref | Ref | Ref |
| COVID-19 diagnosis, asymptomatic | 1.82 (1.14–2.91)a | 2.23 (1.21–4.12)a | 1.46 (0.71–3.02) |
| COVID-19 diagnosis with symptoms | 2.04 (1.37–3.02)a | 2.18 (1.24–3.82)a | 1.98 (1.14–3.46)a |
| Demographic adjustment onlyb | |||
| No COVID-19 diagnosis | Ref | Ref | Ref |
| COVID-19 diagnosis | 2.00 (1.41–2.83)a | 2.14 (1.33–3.44)a | 1.75 (1.06–2.88)a |
| No COVID-19 diagnosis | Ref | Ref | Ref |
| COVID-19 diagnosis, asymptomatic | 1.84 (1.15–2.94)a | 2.14 (1.17–3.93)a | 1.53 (0.74–3.16) |
| COVID-19 diagnosis with symptoms | 2.11 (1.42–3.14)a | 2.14 (1.22–3.76)a | 1.88 (1.08–3.27)a |
| Full modelc | |||
| No COVID-19 diagnosis | Ref | Ref | Ref |
| COVID-19 diagnosis | 1.77 (1.25–2.52)a | 1.89 (1.17–3.05)a | 1.64 (0.99–2.73)d |
| No COVID-19 diagnosis | Ref | Ref | Ref |
| COVID-19 diagnosis, asymptomatic | 1.70 (1.07–2.72)a | 1.99 (1.07–3.71)a | 1.46 (0.71–3.00) |
| COVID-19 diagnosis with symptoms | 1.81 (1.22–2.70)a | 1.81 (1.04–3.16)a | 1.75 (0.99–3.08)d |
The total numbers reflect the number of participants with complete outcome and covariate data in the final models.
Ref, reference group.
Papageorghiou et al. Preeclampsia and COVID-19. Am J Obstet Gynecol 2021.
P<.05
Adjusted for maternal age, previous parity (nulliparous vs parous), tobacco use during pregnancy, and history of adverse pregnancy outcomes
Adjusted for maternal age, previous parity (nulliparous vs parous), tobacco use during pregnancy, overweight status (normal, underweight, overweight, or obese), or history of diabetes, cardiac disease, hypertension, kidney disease, or adverse pregnancy outcomes
P<.1.
Aspirin was used by 9.6% of women, but no evidence of effect modification by aspirin use was seen (P value for interaction, .42 for the model adjusting for demographic and risk factors). Sensitivity analyses excluding subgroups of women with maternal age <20 years and those with multiple pregnancy did not change the results. Similarly, a sensitivity analysis that excluded women diagnosed early in the pandemic based on 2 or more symptoms (n=54), led to a small reduction in the association (for all women: from RR, 1.77; 95% CI, 1.25–2.52; to RR, 1.57; 95% CI, 1.08–2.28 and for nulliparous women from RR, 1.89; 95% CI, 1.17–3.05; to RR, 1.76; 95% CI, 1.06–2.90).
We explored 5 characteristics to better understand the association. First, as described above, the effect was mostly observed among nulliparous women (a main feature of preeclampsia). Second, there was no evidence of a biologically or clinically substantive difference in the strength of the association regardless of whether the women had COVID-19 symptoms or not (RR, 1.81; 95% CI, 1.22–2.70 and RR, 1.70; 95% CI, 1.07–2.72 for symptomatic and asymptomatic affected women, respectively) (Table 2). Hence, COVID-19 severity was not a factor in the observed association (Table 2) when assuming that symptom occurrence is indicative of worse disease.
Third, there was no association among women (32.5%) in whom COVID-19 was diagnosed >7 days from delivery (RR, 0.99; 95% CI, 0.55–1.79) (Table 3 ), suggesting that the independent association mostly relates to women who may have developed preeclampsia already by the time of their COVID-19 diagnosis (RR, 2.12; 95% CI, 1.44–3.11), particularly among nulliparous women (RR, 2.36; 95% CI, 1.40–3.98) (Table 3).
Table 3.
Association between COVID-19 and preeclampsia according to time elapsed between COVID-19 diagnosis and birth
| Time between COVID-19 diagnosis and birth | n (%) | All women | Nulliparous | Parous |
|---|---|---|---|---|
| Unadjusted | ||||
| No COVID-19 diagnosis | 1402 (68.1) | Ref | Ref | Ref |
| COVID-19 diagnosis, within 7 d of birth or postnatally | 426 (20.7) | 2.28 (1.57–3.32)a | 2.51 (1.51–4.18)a | 2.10 (1.21–3.62)a |
| COVID-19 diagnosis ≥7 d before birth | 232 (11.3) | 1.07 (0.57–2.01) | 0.96 (0.35–2.66) | 1.20 (0.54–2.68) |
| Adjustedb | ||||
| No COVID-19 diagnosis | 1402 (68.1) | Ref | Ref | Ref |
| COVID-19 diagnosis, within 7 d of birth or postnatally | 426 (20.7) | 2.12 (1.44–3.11)a | 2.36 (1.40–3.98)a | 1.83 (1.04–3.21)a |
| COVID-19 diagnosis ≥7 d before birth | 232 (11.3) | 0.99 (0.55–1.79) | 0.74 (0.31–1.80) | 1.21 (0.55–2.66) |
The total numbers reflect the number of participants with complete outcome and covariate data in the final models.
Ref, reference group.
Papageorghiou et al. Preeclampsia and COVID-19. Am J Obstet Gynecol 2021.
P<.05
Adjusted for maternal age, previous parity (nulliparous vs parous), tobacco use during pregnancy, overweight status (normal, underweight, overweight, or obese), or history of diabetes, cardiac disease, hypertension, kidney disease, or history of adverse pregnancy outcomes.
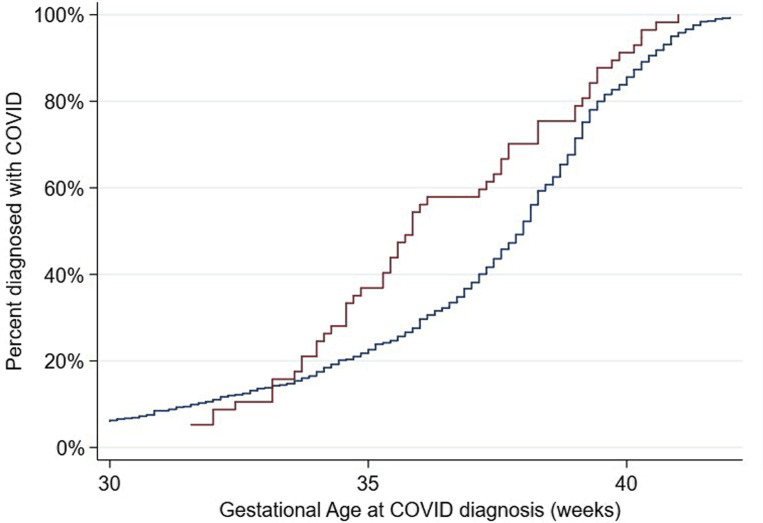
Fourth, as seen in Figure 1 , there was a gradual and constant increase in COVID-19 diagnoses across gestational age among women without preeclampsia, whereas women with preeclampsia were mostly diagnosed with COVID-19 from 33 to 37 weeks’ gestation; thereafter, both curves remained parallel (Cox model hazard ratio, 1.49; 95% CI, 1.12–1.97 for COVID-19 among women with preeclampsia).
Figure 1.
Kaplan-Meier curves
For gestational age at diagnosis of COVID-19, stratified by preeclampsia status during pregnancy. Blue represents no preeclampsia; red represents preeclampsia. Cox model hazard ratio, 1.49 (95% CI, 1.12–1.97). One woman was diagnosed with COVID-19 at ≤13 weeks’ gestation; 34 were diagnosed from >13 to ≤26 weeks’ gestation; 636 were diagnosed at >26 weeks’ gestation; for 35 women the information about the gestational age at diagnosis was not available.
Papageorghiou et al. Preeclampsia and COVID-19. Am J Obstet Gynecol 2021.
Finally (Table 4 ), there was a statistically significant association between COVID-19 and GH (RR, 1.53; 95%CI, 1.11–2.11) mostly seen among nulliparous women (RR, 1.79; 95% CI, 1.13–2.85) (Table 4).
Table 4.
Associations between COVID-19 diagnosis and hypertensive disorders in pregnancya
| COVID-19 diagnosis according to parity | Hypertensive disorders in pregnancyb (n=266) |
Gestational hypertension (n=143) |
||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| All mothers | ||||
| No COVID-19 diagnosis | Ref | Ref | Ref | Ref |
| COVID-19 diagnosis | 1.67 (1.32–2.12)c | 1.56 (1.23–1.98)c | 1.61 (1.17–2.22)c | 1.53 (1.11–2.11)c |
| Nulliparous | ||||
| No COVID-19 diagnosis | Ref | Ref | Ref | Ref |
| COVID-19 diagnosis | 2.09 (1.50–2.91)c | 1.80 (1.28–2.52)c | 2.09 (1.33–3.29)c | 1.79 (1.13–2.85)c |
| Parous | ||||
| No COVID-19 diagnosis | Ref | Ref | Ref | Ref |
| COVID-19 diagnosis | 1.38 (0.98–1.95)d | 1.36 (0.97–1.91)d | 1.29 (0.82–2.03) | 1.32 (0.84–2.06) |
The total numbers reflect the number of participants with complete outcome and covariate data in the final models.
Ref, reference group.
Papageorghiou et al. Preeclampsia and COVID-19. Am J Obstet Gynecol 2021.
Adjusted for maternal age, previous parity (nulliparous vs parous), tobacco use during pregnancy, overweight status (normal, underweight, overweight, or obese), or history of diabetes, cardiac disease, hypertension, kidney disease, or adverse pregnancy outcomes
Includes preeclampsia or gestational hypertension
P<.05
P<.1.
Impact of COVID-19 and preeclampsia on the outcomes
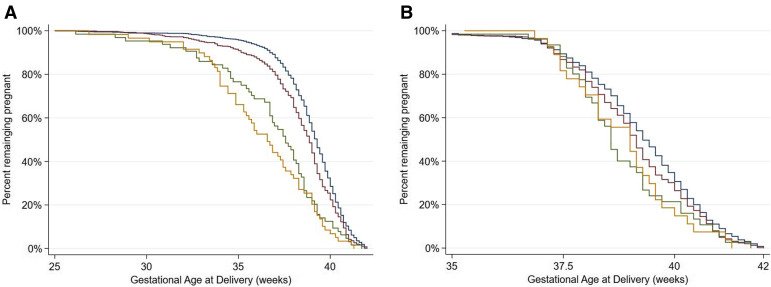
As we have previously shown,19 the COVID-19 group had an almost 2-fold higher risk for preterm birth, even after adjusting for a previous preterm birth. In Figure 2 , A, we show that there was an additional negative effect of preeclampsia, namely women with both conditions had a RR for preterm birth of more than 4 (above 6 for nulliparous women) (Table 5 ). In women with COVID-19 and preeclampsia, 97% of the preterm births were medically indicated, similar to the 93% in those with preeclampsia without COVID-19. However, 80% were medically indicated in those with COVID-19 without preeclampsia and 61% were medically indicated in those with neither condition (Figure 2, B).
Figure 2.
Kaplan-Meier curves
A, For gestational age at delivery, stratified by preeclampsia and COVID-19 diagnosis and (B) for gestational age at spontaneous birth, treating medically-indicated births as censored; the spontaneous preterm birth rate was 4.4%. Blue represents no preeclampsia and no COVID-19 diagnosis; red represents no preeclampsia with a COVID-19 diagnosis; green represents preeclampsia without a COVID-19 diagnosis; orange represents preeclampsia with a COVID-19 diagnosis.
Papageorghiou et al. Preeclampsia and COVID-19. Am J Obstet Gynecol 2021.
Table 5.
Associations between preeclampsia and COVID-19 diagnosis with adverse pregnancy and neonatal outcomesa
| Outcomes | n (%) with the outcome | All women | Nulliparous | Parous |
|---|---|---|---|---|
| Preterm birth | ||||
| No preeclampsia, no COVID-19 diagnosis | 163 (12.2) | Ref | Ref | Ref |
| No preeclampsia, with COVID-19 diagnosis | 119 (19.6) | 1.57 (1.27–1.95)b | 1.66 (1.16–2.38)b | 1.50 (1.14–1.96)b |
| Preeclampsia, no COVID-19 diagnosis | 28 (45.2) | 3.48 (2.54–4.76)b | 4.14 (2.72–6.30)b | 2.49 (1.58–3.93)b |
| Preeclampsia, with COVID-19 diagnosis | 33 (56.9) | 4.05 (2.99–5.49)b | 6.26 (4.35–9.00)b | 3.01 (1.92–4.72)b |
| Small for gestational age | ||||
| No preeclampsia, no COVID-19 diagnosis | 162 (12.6) | Ref | Ref | Ref |
| No preeclampsia, with COVID-19 diagnosis | 69 (11.6) | 0.98 (0.75–1.28) | 1.17 (0.83–1.65) | 0.77 (0.50–1.16) |
| Preeclampsia, no COVID-19 diagnosis | 11 (17.5) | 1.48 (0.80–2.71) | 1.65 (0.81–3.34) | 0.98 (0.33–2.88) |
| Preeclampsia, with COVID-19 diagnosis | 17 (29.3) | 2.32 (1.50–3.58)b | 2.61 (1.55–4.40)b | 2.33 (1.06–5.13)b |
| Severe perinatal morbidity and mortality indexc | ||||
| No preeclampsia, no COVID-19 diagnosis | 100 (7.3) | Ref | Ref | Ref |
| No preeclampsia, with COVID-19 diagnosis | 100 (15.9) | 2.16 (1.63–2.86)b | 2.41 (1.54–3.77)b | 1.91 (1.33–2.74)b |
| Preeclampsia, no COVID-19 diagnosis | 15 (22.4) | 2.53 (1.44–4.45)b | 3.22 (1.51–6.88)b | 1.76 (0.77–4.02) |
| Preeclampsia, with COVID-19 diagnosis | 16 (27.1) | 2.84 (1.67–4.82)b | 3.88 (1.89–7.96)b | 2.25 (1.06–4.80)b |
| Composite maternal morbidity and mortality indexd | ||||
| No preeclampsia, no COVID-19 diagnosis | 188 (14.1) | Ref | Ref | Ref |
| No preeclampsia, with COVID-19 diagnosis | 157 (25.8) | 1.84 (1.52–2.22)b | 1.76 (1.32–2.35)b | 1.85 (1.45–2.38)b |
| Preeclampsia, no COVID-19 diagnosis | 16 (25.8) | 1.74 (1.11–2.71)b | 2.07 (1.20–3.57)b | 1.31 (0.62–2.77) |
| Preeclampsia, with COVID-19 diagnosis | 23 (39.7) | 2.51 (1.72–3.67)b | 2.77 (1.66–4.63)b | 2.35 (1.32–4.18)b |
The unadjusted models are presented in Supplemental Table 1. The total numbers reflect the number of participants with complete outcome and covariate data in the final models.
Ref, reference group.
Papageorghiou et al. Preeclampsia and COVID-19. Am J Obstet Gynecol 2021.
Adjusted for maternal age, previous parity (nulliparous vs parous), tobacco use during pregnancy, overweight status (normal, underweight, overweight, or obese), or history of diabetes, cardiac disease, hypertension, kidney disease, or history of adverse pregnancy outcomes
P<.05
Severe perinatal morbidity and mortality index includes at least 1 of the following morbidities: bronchopulmonary dysplasia, hypoxic-ischemic encephalopathy, sepsis, anemia requiring transfusion, patent ductus arteriosus, intraventricular hemorrhage, necrotizing enterocolitis, retinopathy of prematurity, intrauterine or neonatal death, or neonatal intensive care unit stay of ≥7 days
Composite maternal morbidity and mortality index includes at least 1 of the following morbidities: third trimester vaginal bleeding, preterm labor, infections requiring antibiotics, maternal admission to the intensive care unit, referral to a higher level of care, or maternal death.
The SGA rate was 29.3% in women with COVID-19 and preeclampsia with an RR of 2.32 (95% CI, 1.50–3.58) when compared with the group with neither condition. The rate in those with preeclampsia without COVID-19 was 17.5%; however, COVID-19 without preeclampsia was not associated with SGA (11.6%), suggesting that preeclampsia was affecting fetal growth before COVID-19 infection.
The RR for the severe perinatal morbidity and mortality index increased stepwise, particularly among nulliparous women; the RRs were 2.41 (95% CI, 1.54–3.77), 3.22 (95% CI, 1.51–6.88), and 3.88 (95% CI, 1.89–7.96) for women with COVID-19 without preeclampsia, preeclampsia without COVID-19, and COVID-19 with preeclampsia, respectively (Table 5; unadjusted results are given in Supplemental Table 1). Similar results were observed in those 3 groups for the composite adverse maternal outcome; the RRs were 1.76 (95% CI, 1.32–2.35), 2.07 (95% CI, 1.20–3.57), and 2.77 (95% CI, 1.66–4.63), respectively. However, we did not observe a significant interaction between COVID-19 exposure and preeclampsia on these neonatal outcomes.
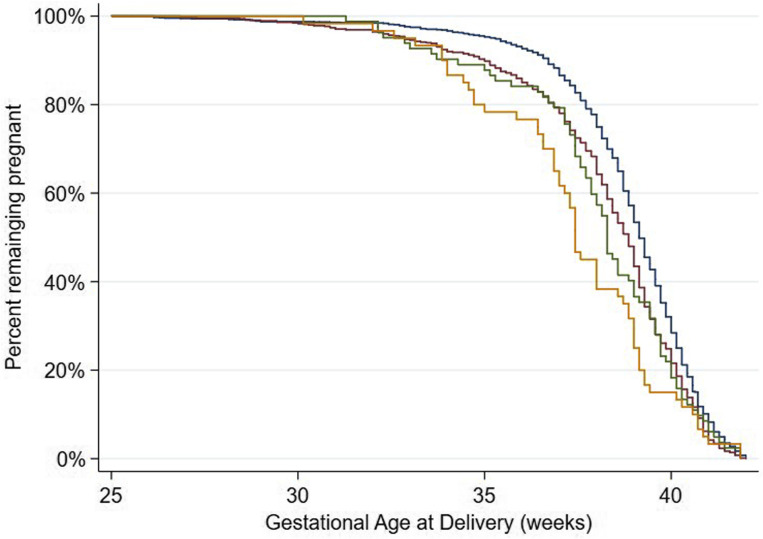
Overall, the direction of the effects of GH on preterm birth (Supplemental Figure), SGA, the severe perinatal morbidity and mortality index, and composite adverse maternal outcome (Table 6 ; unadjusted results are given in Supplemental Table 2) were similar to what was observed for preeclampsia and were confined to nulliparous women, albeit with lower RRs. There was also a stepwise increase in the risk for preterm birth among women with COVID-19 without GH, women with GH without COVID-19, and women with both conditions.
Supplemental Figure.
Kaplan-Meier curves
For gestational age at delivery, stratified by gestational hypertension (GH) and COVID-19 diagnosis. The blue curve indicates no GH, no COVID-19 diagnosis; the red curve indicates no GH with COVID-19 diagnosis; the green curve indicates GH, no COVID-19 diagnosis; and the orange curve indicates GH with COVID-19 diagnosis.
Papageorghiou et al. Preeclampsia and COVID-19. Am J Obstet Gynecol 2021.
Table 6.
Associations between gestational hypertension and COVID-19 diagnosis with adverse pregnancy and neonatal outcomesa
| Outcomes | n (%) with the outcome | All women | Nulliparous | Parous |
|---|---|---|---|---|
| Preterm delivery | ||||
| No GH, no COVID-19 diagnosis | 174 (13.2) | Ref | Ref | Ref |
| No GH, with COVID-19 diagnosis | 130 (21.5) | 1.56 (1.27–1.92)b | 1.76 (1.27–2.49)b | 1.46 (1.12–1.90)b |
| GH, no COVID-19 diagnosis | 17 (21.5) | 1.43 (0.90–2.27) | 1.62 (0.82–3.21) | 1.27 (0.68–2.36) |
| GH, with COVID-19 diagnosis | 22 (36.1) | 2.34 (1.62–3.37)b | 2.53 (1.47–4.37)b | 2.11 (1.25–3.54)b |
| Small for gestational age | ||||
| No GH, no COVID-19 diagnosis | 156 (12.2) | Ref | Ref | Ref |
| No GH, with COVID-19 diagnosis | 74 (12.4) | 1.08 (0.83–1.40) | 1.25 (0.90–1.74) | 0.90 (0.60–1.34) |
| GH, no COVID-19 diagnosis | 17 (21.8) | 2.06 (1.27–3.33)b | 1.51 (0.77–2.98) | 2.59 (1.30–5.15)b |
| GH, with COVID-19 diagnosis | 12 (20.3) | 1.74 (1.01–3.01)b | 1.87 (1.00–3.51)c | 1.37 (0.46–4.07) |
| Severe perinatal morbidity and mortality indexd | ||||
| No GH, no COVID-19 diagnosis | 102 (7.5) | Ref | Ref | Ref |
| No GH, with COVID-19 diagnosis | 103 (16.4) | 2.14 (1.62–2.82)b | 2.46 (1.60–3.77)b | 1.88 (1.31–2.69)b |
| GH, no COVID-19 diagnosis | 13 (15.9) | 1.66 (0.83–3.31) | 2.02 (0.78–5.24) | 1.45 (0.55–3.80) |
| GH, with COVID-19 diagnosis | 13 (20.6) | 2.19 (1.24–3.87)b | 2.15 (0.97–4.80)c | 2.24 (1.00–5.02)c |
| Composite maternal morbidity and mortality indexe | ||||
| No GH, no COVID-19 diagnosis | 191 (14.5) | Ref | Ref | Ref |
| No GH, with COVID-19 diagnosis | 166 (27.2) | 1.86 (1.54–2.23)b | 1.73 (1.30–2.29)b | 1.93 (1.51–2.47)b |
| GH, no COVID-19 diagnosis | 14 (17.7) | 1.16 (0.71–1.92) | 0.97 (0.45–2.08) | 1.25 (0.64–2.45) |
| GH, with COVID-19 diagnosis | 17 (27.9) | 1.80 (1.17–2.77)b | 1.94 (1.13–3.35)b | 1.38 (0.66–2.91) |
Unadjusted models are shown in Supplemental Table 2. The total numbers reflect the number of participants with complete outcome and covariate data in the final models.
GH, gestational hypertension; Ref, reference group.
Papageorghiou et al. Preeclampsia and COVID-19. Am J Obstet Gynecol 2021.
Models adjusted for maternal age, previous parity (nulliparous vs parous) tobacco use during pregnancy, overweight status (normal, underweight, overweight, or obese), or history of diabetes, cardiac disease, hypertension, kidney disease, or history of adverse pregnancy outcomes
P<.05
P<.1
Severe perinatal morbidity and mortality index includes at least 1 of the following morbidities: bronchopulmonary dysplasia, hypoxic-ischemic encephalopathy, sepsis, anemia requiring transfusion, patent ductus arteriosus, intraventricular hemorrhage, necrotizing enterocolitis, retinopathy of prematurity, intrauterine or neonatal death, or neonatal intensive care unit stay of ≥7 days
Composite maternal morbidity and mortality index includes at least 1 of the following morbidities: third trimester vaginal bleeding, preterm labor, infections requiring antibiotics, maternal admission to the intensive care unit, referral to a higher level of care, or maternal death.
Comment
Principal findings
COVID-19 during pregnancy and preeclampsia are strongly associated with each other, especially among nulliparous women. This association is independent of risk factors and preexisting conditions. COVID-19 severity does not affect the association. In an additive fashion, these conditions increase the risks for preterm birth, severe perinatal morbidity and mortality, and adverse maternal outcomes. An effect on SGA frequency was seen only among women with preeclampsia.
Hypothesized mechanism of action and further research
COVID-19 and preeclampsia share many common risk factors such as obesity and underlying hypertension. Is it possible that these explain the observed association by a process of confounding instead of a biological interaction or causal relationship?
There is certainly evidence emerging in support of a biological explanation. COVID-19 causes endothelial dysfunction either directly or indirectly, leading to hyperinflammation and aberrant antiviral responses.27 , 28 In addition, coagulopathy and disseminated intravascular coagulation–like massive intravascular clot formation are seen in the most severely ill, nonpregnant patients.29 During pregnancy, COVID-19 induces specific vascular pathology that is similar to the changes seen in preeclampsia. In fact, Mendoza et al7 have introduced the concept of a “preeclampsia-like syndrome” associated with COVID-19. In other words, it may be difficult to clinically distinguish this syndrome from “true” preeclampsia because they both share characteristics of the severe endothelial dysfunction seen in nonpregnant patients.7 , 30
However, 2 observations from our study discourage this line of thinking. The first is that the severity of COVID-19 symptoms did not increase the association with preeclampsia, although we acknowledge that, in a recent cohort study, hypertensive disorders in pregnancy were more frequent in those women with severe COVID-19.31 Secondly, the association was also present with GH, a hypertensive condition that does not present with the “preeclampsia-like syndrome.”
We believe that the most likely explanation for the observed association is that preeclampsia and GH are vascular conditions, preceding infection with SARS-CoV-2, which increase the risk for COVID-19 in the same way essential hypertension does. This is supported by the relationship being mostly seen in nulliparous women; if COVID-19 led to preeclampsia (rather than the other way round), the association should also have been seen in parous women. Furthermore, the risk was highest when COVID-19 was diagnosed in the last 7 days of pregnancy; if COVID-19 were on the etiologic pathway, an earlier diagnosis would likely have had a stronger association with preeclampsia. The same conclusion emerges from Figure 1, namely there was a sharp increase in COVID-19 diagnoses among women with preeclampsia between 33 to 37 weeks’ gestation when the condition typically manifests itself clinically. Conversely, in women without preeclampsia, COVID-19 diagnoses were proportionally distributed throughout pregnancy. Lastly, the excess risk for preeclampsia in women with COVID-19 persisted (RR, 1.77; 95% CI, 1.25–2.52) even when we controlled for maternal sociodemographic and risk factors for preeclampsia.
Clinical implications
COVID-19 during pregnancy is known to increase severe maternal morbidity and death, particularly respiratory dysfunction requiring invasive mechanical ventilation or admission to the ICU.32 Our data provide additional information about the additive negative effects of COVID-19 and preeclampsia during pregnancy. Women who are at high risk for preeclampsia should also be considered at higher risk for COVID-19 and should be included in all preventive strategies during the pandemic. Whether aspirin administration reduces this risk further is not known, but effect modification by aspirin treatment in our study did not show any changes to the strength of the association.
Strengths and limitations of the study
We used data from a large-scale, prospective, multinational study that was specifically conducted to assess the symptoms and effects of COVID-19 during pregnancy on maternal and neonatal outcomes when compared with pregnant women not-diagnosed with COVID-19 who were carefully enrolled concomitantly to minimize selection bias. Rigorous data monitoring was applied to record severe morbidity markers including preeclampsia and GH.
Our study has expected limitations. The circumstances for the diagnosis of maternal infection changed over time, from testing for severe symptoms of COVID-19 only to screening on admission. Hence, we cannot exclude the possibility that women admitted to hospital with a diagnosis of preeclampsia, or those with risk factors, were more likely to be tested for, or diagnosed with COVID-19. Nor did we examine the placentas of the women enrolled, which might have helped to determine whether the severity of infection correlates with the extent of vasculitis or whether different patterns of inflammation are seen in the 3 diagnostic groups.33 However, this should not reduce the impact of our results because preeclampsia is a purely clinical diagnosis. We assessed the association between COVID-19 and preeclampsia in symptomatic and asymptomatic women; however, we could not conduct stratified analyses according to the COVID-19 disease severity classification proposed by the National Institutes of Health, because we did not have the complete information required by these relatively recent criteria.31 Because this was an observational study, the clinical management of pregnancy complications, such as COVID-19 and preeclampsia, was based on local practice and therefore not standardized. Finally, it is also possible that the not-diagnosed COVID-19 cohort may have included a small number of asymptomatic infected women who were not identified, either because routine testing was not available at that stage of the pandemic or because they became infected after they were included in the not-diagnosed cohort. We do not consider this a strong source of bias because it would have led to more conservative estimates from the analyses using that group as reference.
Conclusions
Preeclampsia (and to a lesser degree GH) is independently associated with COVID-19 during pregnancy; both conditions are associated independently of and in an additive fashion with increased risks for preterm birth, the severe perinatal morbidity and mortality index, and composite adverse maternal outcome. Hence, preeclampsia (and GH) seem to be a strong risk factor for SARS-CoV-2 infection and its related complications. There was no evidence to support that COVID-19 is etiologically associated with preeclampsia or GH. Clinicians caring for women with preeclampsia should be aware of the additional risks that COVID-19 poses.
Acknowledgments
We are very grateful to the contributing institutions and local researchers involved in the study. The Appendix contains their details and the details of the study committees.
Footnotes
A.T.P. and P.D. contributed equally.
S.H.K. and J.V. contributed equally.
The authors report no conflict of interest.
The study was supported by the COVID-19 Research Response Fund from the University of Oxford (Ref 0009083). A.T.P. is supported by the Oxford Partnership Comprehensive Biomedical Research Centre with funding from the National Institute for Health Research (NIHR) Biomedical Research Centre funding scheme. The funding organization had no involvement in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
The opinions expressed in this article are those of the authors and not necessarily reflect the views of the National Health Service, NIHR, Department of Health, or any of the other funders.
J.V. and A.T.P. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Cite this article as: Papageorghiou AT, Deruelle P, Gunier RB, et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gyneco 2021;225:289.e1-17.
Appendix
Contributors and members of the international study on the effects of COVID-19 in pregnancy on maternal and newborn outcomes in the INTERGROWTH-21st global network (The INTERCOVID Study)
Contributors
J.V., S.H.K., and A.T.P. conceptualized and designed the INTERCOVID Study. J.V., S.H.K., and A.T.P. prepared the original protocol, with later input from A.W. and R.C., and coordinated the project’s overall undertaking with invaluable input from Ana Langer, Cesar Victora, Jose Cordero, B.E., E.F., Z.A.B., Julian Robinson, and J.G.T. R.B.G., S.R., Carmen Condon, R.Cr., and A.W. undertook the data management and analysis in collaboration with J.V. and A.T.P. J.G.T. and A.T.P. undertook the ongoing literature review and interpretation of published data. The principal investigators at each study site implemented the protocol at their respective institutions. Carmen Condon, A.W., and R.Cr. led the quality control of data. J.V., S.H.K., and A.T.P. wrote the manuscript with input from all co-authors. All co-authors read the manuscript and made suggestions on its content.
Members of the international study on the effects of COVID-19 in pregnancy on maternal and newborn outcomes in the INTERGROWTH-21st global network (The INTERCOVID Study) and its committees
Scientific Advisory Committee: Ana Langer (chair), Cesar Victora, Jose Cordero, B.E., E.F., Z.A.B., Julian Robinson, and J.G.T.
Coordinating Unit: J.V., A.T.P., A.W., R.C., Montserrat Izquierdo Renault, S.H.K., R.N., A.R., and Josephine Agyeman-Duah.
Data Management Group: Carmen Condon, RB.G., S.R., R.C., and A.W.
Participating institutions and investigators
Sanatorio Otamendi, Buenos Aires, Argentina: Carmen Vecchiarelli (principal investigator), Cristina Osio, Cecilia Baston, Marcela Volpe, Zenith Guzman, Hernan Jensen, Fernando Tami, and Federico Crispin.
Hospital Materno Infantil Ramón Sarda, Buenos Aires, Argentina: R.N. and C.P.S.C. (co-principal investigators), Eugenia Luque, Jimena Melisa Vargas, Marta Isabel Lopez, and Karen Zelada.
Hospital Universitario Austral, Buenos Aires, Argentina: Maria Carola Capelli and M.R. (co-Principal Investigators), and Josefina Maria Bran.
Hospital Magdalena V de Martinez, Buenos Aires, Argentina: E.A.D. (principal investigator).
Hospital Nacional Profesor Alejandro Posadas, Buenos Aires, Argentina: R.Ca. (principal investigator), Silvia Garcia, Alberto Ferreiros, Lucio Ribola, Grethelm Ferrufino, Karell Rojas, Dafne Sidiropulos, and Silvana Varela.
Hospital Municipal O. B de Lavignole, Buenos Aires, Argentina: M.S. (principal investigator), Silvana Aguirre, and Maria Rocio Tozzini.
Hospital Universitário da Universidade Federal do Maranhão, São Luís, Brazil: M.S.D.V. (principal investigator), Ana Claudia Garcia Marques, Patricia Franco Marques, and Rebeca Aranha Arrais Santos Almeida.
Tanta University Hospital, Tanta, Egypt: S.A.E. (principal investigator), Mohamed Elbahnasawy, Mai Khalaf, Mohamed Samir Abd El Ghafar, and Eslam Saber Esmail.
Bordeaux University Hospital, Bordeaux, France: L.S. (principal investigator), Amaury Brot, Aurélien Mattuizzi, and Clémence Houssin.
Hôpital Necker-Enfants Malades (Hôpital AP-HP), Paris, France: Laurent J. Salomon (principal investigator), J.S., and Laurence Bussières.
Hôpitaux Universitaires de Strasbourg, Strasbourg, France: P.D. (principal investigator), Fanny De Marcillac, Mary Pontvianne, Georges-Emmanuel Roth, Charlotte Jouffrieau, Sylvain Hufschmitt-Henry, Valentine Bergthold, Mathilde Airoldi, Olivier Behra, Julie Delplanque, and Coraline Schutz.
Fr. Thomas Alan Rooney Memorial Hospital, Asankragua, Ghana: V.B.N. (principal investigator), Bright Sandow, Priscilla Baffour Kyeremeh, Rita Akpene Kumi, Thomas Asechaab, and Grace Hanson
National Catholic Health Service, Directorate, Ghana: George Adjei, Anita Appiah, Roberta Ama Asiedu, and Ivan Essegbey.
Holy Family Hospital, Nkawkaw, Ghana: E.B. (principal investigator) and Genevieve Insaidoo.
Translational Health Science and Technology Institute, Faridabad, India, Employee's State Insurance Corporation Medical College and Hospital, Faridabad, India, and affiliated Badshah Khan Civil Hospital, Haryana, India: R.T. and Shinjini Bhatnagar (co-principal investigators), Vandita Bhartia, Mudita Gosain, Anil K. Pandey, Jagadish Chandra Sharma, Rajesh Dhiman, and GARBH-Ini- Department of Biotechnology India Consortium for COVID-19 Research Collaboration.
Medical Faculty, Universitas Airlangga, Dr Soetomo General Academic Hospital, Surabaya, Indonesia: E.E. (principal investigator), Hendy Hendarto, Erry Gumilar, and Aditiawarman.
Spedali Civili and University of Brescia, Brescia, Italy: F.P. (principal investigator), Roberta Castellani, and Marta Papaccio.
Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy: Paola Roggero and Enrico Ferrazzi (co-principal investigators), Camilla Menis, Michela Perrone, and Enrico Iurlaro.
Ospedale Luigi Sacco, Milan, Italy: V.S. (principal investigator), Silvia Corti, and Francesca Rana.
IRCCS San Raffaele Hospital and University, Milan, Italy: P.I.C. (principal investigator), Massimo Candiani, and Giulia Bonavina.
Ospedale Vittore Buzzi, Milan, Italy: I.C. (principal investigator), Alice Zavatta, and Stefania Livio.
University of Pavia and Fondazione IRCCS Policlinico San Matteo, Pavia, Italy: Hellas Cena and R.M.C. (co-principal investigators), Debora Porri, Rachele De Giuseppe, Arsenio Spinillo, Annachiara Licia Scatigno, Francesca Perotti, and Marco Zecca.
Ospedale Infantile Regina Margherita-Sant'Anna, Città della Salute e della Scienza di Torino, Turin, Italy: F.G. and Manuela Oberto (co-principal investigators), Enrico Bertino, Pietro Gaglioti, Cristian Romolo Macchione, and Francesca Carpano Maglioli.
The Jikei University School of Medicine, Tokyo, Japan: K.T. (principal investigator) and Osamu Samura.
Keio University School of Medicine, Tokyo, Japan: Mamoru Tanaka and S.I. (co-principal investigators), Daigo Ochiai, Yoshifumi Kasuga, and Miho Iida.
General Hospital Borka Taleski, Prilep, North Macedonia: G.T. (principal investigator).
General Hospital Kumanovo, Kumanovo, North Macedonia: Aleksandra Hristova (principal investigator).
Instituto Nacional de Perinatología Isidro Espinosa de los Reyes, Mexico City, Mexico: J.A.C.P. (principal investigator), Sandra Acevedo-Gallegos, Irma Alejandra Coronado-Zarco, Brenda Ivette Frías, Addy Cecilia Helguera-Repetto, Maria José Rodriguez Sibaja, Isabel Villegas-Mota, and Maria Yolotzin Valdespino.
Lic. Adolfo López Mateos ISSSTE, Mexico City, Mexico: P.K.G.M. (principal investigator), Alberto Almanza Aguilar, Sanjuanita Gonzalez Garza, Lorena Gomez Aldape, Alejandra Loera Olvera, and Paulina Soriano Cabelo.
Abubakar Tafawa Balewa University Teaching Hospital, Bauchi, Nigeria: M.B.A. (principal investigator) and Tiamiyu Ismail.
University of Calabar Teaching Hospital, Calabar, Nigeria: S.E. (principal investigator), Chinyere Akpanika, Komommo Okpebri, Etim Ekanem, and Ubong Akpan.
Federal Teaching Hospital Gombe, Gombe, Nigeria: B.B. (principal investigator) and Yahaya Musa Suleiman.
University College Hospital, Ibadan, Nigeria: Adejumoke Idowu Ayede and Yetunde John-Akinola (co-principal investigators), Oladapo OlayemI, and Olufisayo Christopher Ologunore.
Mainland Hospital Yaba, Lagos, Nigeria: A.B. (principal investigator) and Tope Ogunniyan.
Aminu Kano Teaching Hospital, Kano State, Nigeria: Hadiza Galadanci and F.H.H. (co-principal investigators) and Mahmoud Magashi.
Muhammad Abdullahi Wase Teaching Hospital, Kano State, Nigeria: M.A.U. (principal investigator), Iman Haruna, Maryam Sulaiman, Rahila Garba, Badiyya Sayyidi, Hanifa Datti, Frank Akabudu, Abdurrahman Ali Bunawa, and Amira Aminu.
The Aga Khan University Hospital, Karachi, Pakistan: Shabina Ariff (principal investigator), G.Z., Lumaan Shaikh, and Khalil Ahmed.
National Medical Research Center for Obstetrics, Gynecology & Perinatology, Moscow, Russia: A.K. (principal investigator), Irina Yakovleva, Aleksander Gus, Alexander Sencha, Roman Shmakov, and Gennady Sukhikh.
Hospital Universitari Vall d’Hebron, Barcelona, Spain: N.M. (principal investigator), Berta Serrano, Ester del Baco, Marta Miguez, Ana Perestelo, Lidia Barberán, Cristina Tusquets, Montserrat Capell, Clementina de Antonio, and Judit Gil.
Hospital Clínico Universitario Lozano Blesa Zaragoza, Zaragoza, Spain: D.O. (principal investigator), Sara Ruiz-Martinez, Marta Fabre, and Cristina Paules.
Hôpitaux Universitaires de Genève, Geneva, Switzerland: A.C.B. (principal investigator), Martinez de Tejada Weber, Véronique Othenin-Girard, Monia Moreau, Dominique Deletraz, and David Baud.
St George’s University Hospitals NHS Foundation Trust, London, United Kingdom: A.T.P. (principal investigator), B.L., Matthew Cauldwell, Yaa Acheampong, Danielle Hake, Sophie Robinson, and Rosemary Nyamboya.
University College Hospitals NHS Foundation Trust, London, United Kingdom: R.N. (principal investigator), Laura Salazar, Rohit Atre, Alex Fry, and Hilary Hewitt.
Oxford University Hospitals NHS Foundation Trust, Oxford, United Kingdom: J.V., S.H.K., S.D. (co-principal investigators), Angelika Capp, Lotoyah Carty, Kate Dixon, Yvonne Nsiah, and Fenella Roseman.
Brigham and Women's Hospital, Boston, Massachusetts: S.R.E. (principal investigator).
Tufts Medical Center, Boston, Massachusetts: M.M. (principal investigator), Jenny Koenig, and Arome Obende.
Mercy Hospital and Medical Center, Chicago, Illinois: Jagjit Singh Teji (principal investigator) and M.L.F.
University of Washington, Seattle, Washington: Michael Gravett (principal investigator) and L.E.S.
Supplemental Table 1.
Unadjusted associations between preeclampsia and COVID-19 diagnosis with adverse pregnancy and neonatal outcomes
| Adverse outcomes | n (%) with the outcome | All women | Nulliparous | Parous |
|---|---|---|---|---|
| Preterm delivery | ||||
| No preeclampsia, no COVID-19 diagnosis | 163 (12.2) | Ref | Ref | Ref |
| No preeclampsia, with COVID-19 diagnosis | 119 (19.6) | 1.60 (1.29–1.99)a | 1.68 (1.17–2.41)a | 1.53 (1.16–2.00)a |
| Preeclampsia, no COVID-19 diagnosis | 28 (45.2) | 3.69 (2.71–5.03)a | 4.43 (2.87–6.86)a | 3.19 (2.04–5.00)a |
| Preeclampsia, with COVID-19 diagnosis | 33 (56.9) | 4.65 (3.57–6.07)a | 5.99 (4.18–8.58)a | 3.69 (2.44–5.57)a |
| Small for gestational age | ||||
| No preeclampsia, no COVID-19 diagnosis | 162 (12.6) | Ref | Ref | Ref |
| No preeclampsia, with COVID-19 diagnosis | 69 (11.6) | 0.92 (0.70–1.21) | 1.15 (0.81–1.62) | 0.76 (0.50–1.17) |
| Preeclampsia, no COVID-19 diagnosis | 11 (17.5) | 1.39 (0.76–2.53) | 1.63 (0.82–3.27) | 0.94 (0.31–2.83) |
| Preeclampsia, with COVID-19 diagnosis | 17 (29.3) | 2.33 (1.53–3.55)a | 2.48 (1.49–4.10)a | 2.01 (0.97–4.15)b |
| Severe perinatal morbidity and mortality indexc | ||||
| No preeclampsia, no COVID-19 diagnosis | 100 (7.3) | Ref | Ref | Ref |
| No preeclampsia, with COVID-19 diagnosis | 100 (15.9) | 2.17 (1.64–2.86)a | 2.53 (1.63–3.92)a | 1.94 (1.36–2.78)a |
| Preeclampsia, no COVID-19 diagnosis | 15 (22.4) | 3.06 (1.83–5.13)a | 3.63 (1.79–7.35)a | 2.53 (1.17–5.45)a |
| Preeclampsia, with COVID-19 diagnosis | 16 (27.1) | 3.7 (2.32–5.91)a | 4.35 (2.30–8.23)a | 3.15 (1.57–6.36)a |
| Composite adverse maternal outcomed | ||||
| No preeclampsia, no COVID-19 diagnosis | 188 (14.1) | Ref | Ref | Ref |
| No preeclampsia, with COVID-19 diagnosis | 157 (25.8) | 1.84 (1.53–2.22)a | 1.78 (1.33–2.39)a | 1.89 (1.48–2.42)a |
| Preeclampsia, no COVID-19 diagnosis | 16 (25.8) | 1.83 (1.17–2.84)a | 2.17 (1.25–3.77)a | 1.43 (0.67–3.00) |
| Preeclampsia, with COVID-19 diagnosis | 23 (39.7) | 2.81 (1.99–3.96)a | 3.01 (1.91–4.74)a | 2.56 (1.51–4.35)a |
Ref, reference group.
Papageorghiou et al. Preeclampsia and COVID-19. Am J Obstet Gynecol 2021.
P<.05
P<.1
Severe perinatal morbidity and mortality index includes at least 1 of the following morbidities: bronchopulmonary dysplasia, hypoxic-ischemic encephalopathy, sepsis, anemia requiring transfusion, patent ductus arteriosus, intraventricular hemorrhage, necrotizing enterocolitis, retinopathy of prematurity, intrauterine or neonatal death, or neonatal intensive care unit stay of ≥7 days
Composite maternal morbidity and mortality index includes at least 1 of the following morbidities: third trimester vaginal bleeding, preterm labor, infections requiring antibiotics, maternal admission to the intesive care unit, referral to a higher level of care, or maternal death.
Supplemental Table 2.
Unadjusted associations between gestational hypertensiona and COVID-19 diagnosis with adverse pregnancy and neonatal outcomes
| Adverse outcomes | n (%) with the outcome | All women | Nulliparous | Parous |
|---|---|---|---|---|
| Preterm delivery | ||||
| No GH, no COVID-19 diagnosis | 174 (13.2) | Ref | Ref | Ref |
| No GH, with COVID-19 diagnosis | 130 (21.5) | 1.58 (1.26–1.99)b | 1.72 (1.17–2.51)b | 1.48 (1.11–1.97)b |
| GH, no COVID-19 diagnosis | 17 (21.5) | 2.5 (1.88–3.42)b | 3.09 (2.00–4.79)b | 2.18 (1.44–3.29)b |
| GH, with COVID-19 diagnosis | 22 (36.1) | 3.63 (2.79–4.73)b | 4.18 (2.83–6.16)b | 3.30 (2.29–4.76)b |
| Small for gestational age | ||||
| No GH, no COVID-19 diagnosis | 156 (12.2) | Ref | Ref | Ref |
| No GH, with COVID-19 diagnosis | 74 (12.4) | 0.90 (0.68–1.21) | 1.12 (0.77–1.63) | 0.77 (0.50–1.20) |
| GH, no COVID-19 diagnosis | 17 (21.8) | 1.61 (1.08–2.39)b | 1.57 (0.95–2.60)c | 1.62 (0.86–3.05) |
| GH, with COVID-19 diagnosis | 12 (20.3) | 2.05 (1.41–2.99)b | 2.11 (1.35–3.28)b | 1.82 (0.92–3.59)c |
| Severe perinatal morbidity and mortality indexd | ||||
| No GH, no COVID-19 diagnosis | 102 (7.5) | Ref | Ref | Ref |
| No GH, with COVID-19 diagnosis | 103 (16.4) | 2.30 (1.73–3.07)b | 2.82 (1.80–4.45)b | 2.00 (138–2.91)b |
| GH, no COVID-19 diagnosis | 13 (15.9) | 2.59 (1.66–4.03)b | 2.90 (1.52–5.56)b | 2.33 (1.26–4.31)b |
| GH, with COVID-19 diagnosis | 13 (20.6) | 3.04 (1.97–4.68)b | 2.98 (1.59–5.61)b | 3.13 (1.72–5.68)b |
| Composite maternal morbidity and mortality indexe | ||||
| No GH, no COVID-19 diagnosis | 191 (14.5) | Ref | Ref | Ref |
| No GH, with COVID-19 diagnosis | 166 (27.2) | 1.89 (1.55–2.29)b | 1.78 (1.31–2.42)b | 1.96 (1.52–2.52)b |
| GH, no COVID-19 diagnosis | 14 (17.7) | 1.46 (1.01–2.09)b | 1.67 (1.03–2.71)b | 1.26 (0.73–2.17) |
| GH, with COVID-19 diagnosis | 17 (27.9) | 2.22 (1.62–3.03)b | 2.50 (1.67–3.75)b | 1.88 (1.13–3.10)b |
GH, gestational hypertension; Ref, reference group.
Papageorghiou et al. Preeclampsia and COVID-19. Am J Obstet Gynecol 2021.
Includes preeclampsia or gestational hypertension
P<.05
P<.1
Severe perinatal morbidity and mortality index includes at least 1 of the following morbidities: bronchopulmonary dysplasia, hypoxic-ischemic encephalopathy, sepsis, anemia requiring transfusion, patent ductus arteriosus, intraventricular hemorrhage, necrotizing enterocolitis, retinopathy of prematurity, intrauterine or neonatal death, or neonatal intensive care unit stay of ≥7 days
Composite maternal morbidity and mortality index includes at least 1 of the following morbidities: third trimester vaginal bleeding, preterm labor, infections requiring antibiotics, maternal admission to the intensive care unit, referral to a higher level of care, or maternal death.
Supplemental Table 3.
Sensitivity analyses incorporating study site in associations between COVID-19 diagnosis and preeclampsiaa
| Model | All women | Nulliparous | Parous |
|---|---|---|---|
| Primary model | 1.77 (1.25–2.52)b | 1.89 (1.17–3.05)b | 1.64 (0.99–2.73)c |
| Adjust for study site | 1.87 (1.33–2.61)b | 1.95 (1.21–3.15)b | 1.95 (1.21–3.15)b |
| Mixed-effects with random intercept | 1.85 (1.36–2.53)b | 1.90 (1.53–2.37)b | 1.88 (1.06–3.35)b |
Papageorghiou et al. Preeclampsia and COVID-19. Am J Obstet Gynecol 2021.
Models adjusted for maternal age, previous parity (nulliparous vs parous), tobacco use during pregnancy, overweight status (normal, underweight, overweight, or obese), or history of diabetes, cardiac disease, hypertension, kidney disease, or adverse pregnancy outcomes and country of enrollment
P<.05
P<.1.
References
- 1.Gupta A., Madhavan M.V., Sehgal K., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 2.Rana S., Burke S.D., Karumanchi S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.10.022. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turpin C.A., Sakyi S.A., Owiredu W.K.B.A., Ephraim R.K.D., Anto E.O. Association between adverse pregnancy outcome and imbalance in angiogenic regulators and oxidative stress biomarkers in gestational hypertension and preeclampsia. BMC Pregnancy Childbirth. 2015;15:189. doi: 10.1186/s12884-015-0624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dashraath P., Wong J.L.J., Lim M.X.K., et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222:521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Mascio D., Khalil A., Saccone G., et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2:100107. doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBolt C.A., Bianco A., Limaye M.A., et al. Pregnant women with severe or critical coronavirus disease 2019 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2021;224:510.e1–510.e12. doi: 10.1016/j.ajog.2020.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendoza M., Garcia-Ruiz I., Maiz N., et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020;127:1374–1380. doi: 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prabhu M., Cagino K., Matthews K.C., et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127:1548–1556. doi: 10.1111/1471-0528.16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenbloom J.I., Raghuraman N., Carter E.B., Kelly J.C. Coronavirus disease 2019 infection and hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.03.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartsch E., Medcalf K.E., Park A.L., Ray J.G. High Risk of Pre-eclampsia Identification Group. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. 2016;353:i1753. doi: 10.1136/bmj.i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yap M., Debenham L., Kew T., et al. Clinical manifestations, prevalence, risk factors, outcomes, transmission, diagnosis and treatment of COVID-19 in pregnancy and postpartum: a living systematic review protocol. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-041868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandt J.S., Hill J., Reddy A., et al. Epidemiology of coronavirus disease 2019 in pregnancy: risk factors and associations with adverse maternal and neonatal outcomes. Am J Obstet Gynecol. 2021;224:389.e1–389.e9. doi: 10.1016/j.ajog.2020.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Gorman N., Wright D., Syngelaki A., et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11-13 weeks gestation. Am J Obstet Gynecol. 2016;214:103.e1–103.e12. doi: 10.1016/j.ajog.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 14.Staff A.C., Fjeldstad H.E., Fosheim I.K., et al. Failure of physiological transformation and spiral artery atherosis: their roles in preeclampsia. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.09.026. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Brosens I., Puttemans P., Benagiano G. Placental bed research: I. The placental bed: from spiral arteries remodeling to the great obstetrical syndromes. Am J Obstet Gynecol. 2019;221:437–456. doi: 10.1016/j.ajog.2019.05.044. [DOI] [PubMed] [Google Scholar]
- 16.Kell D.B., Kenny L.C. A dormant microbial component in the development of preeclampsia. Front Med (Lausanne) 2016;3:60. doi: 10.3389/fmed.2016.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Easter S.R., Cantonwine D.E., Zera C.A., Lim K.H., Parry S.I., McElrath T.F. Urinary tract infection during pregnancy, angiogenic factor profiles, and risk of preeclampsia. Am J Obstet Gynecol. 2016;214:387.e1–387.e7. doi: 10.1016/j.ajog.2015.09.101. [DOI] [PubMed] [Google Scholar]
- 18.Algarroba G.N., Hanna N.N., Rekawek P., et al. Confirmatory evidence of the visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am J Obstet Gynecol. 2020;223:953–954. doi: 10.1016/j.ajog.2020.08.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villar J, Papageorghiou AT, Shabina A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;e211050. [DOI] [PMC free article] [PubMed]
- 20.Essential study documents. INTERGROWTH-21st. 2020. https://intergrowth21.tghn.org/intercovid/intercovid-study-documents/ Available at:
- 21.Shi H., Han X., Jiang N., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villar J., Cheikh Ismail L., Victora C.G., et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 23.Papageorghiou A.T., Kennedy S.H., Salomon L.J., et al. International standards for early fetal size and pregnancy dating based on ultrasound measurement of crown-rump length in the first trimester of pregnancy. Ultrasound Obstet Gynecol. 2014;44:641–648. doi: 10.1002/uog.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papageorghiou A.T., Kemp B., Stones W., et al. Ultrasound-based gestational-age estimation in late pregnancy. Ultrasound Obstet Gynecol. 2016;48:719–726. doi: 10.1002/uog.15894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohuma E.O., Hoch L., Cosgrove C., et al. Managing data for the international, multicentre INTERGROWTH-21st Project. BJOG. 2013;120(Suppl2):64–70. doi: 10.1111/1471-0528.12080. v. [DOI] [PubMed] [Google Scholar]
- 26.Cox D.R. Regression models and life-tables. J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 27.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernard I., Limonta D., Mahal L.K., Hobman T.C. Endothelium infection and dysregulation by SARS-CoV-2: evidence and caveats in COVID-19. Viruses. 2020;13:29. doi: 10.3390/v13010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID-19. J Thromb Haemost. 2020;18:2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dap M., Morel O. Proteinuria in Covid-19 pregnant women: preeclampsia or severe infection? Eur J Obstet Gynecol Reprod Biol. 2020;252:612. doi: 10.1016/j.ejogrb.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metz T.D., Clifton R.G., Hughes B.L., et al. Disease severity and perinatal outcomes of pregnant patients With coronavirus Disease 2019 (COVID-19) Obstet Gynecol. 2021;137:571–580. doi: 10.1097/AOG.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sentilhes L., De Marcillac F., Jouffrieau C., et al. Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth. Am J Obstet Gynecol. 2020;223:914.e1–914.e15. doi: 10.1016/j.ajog.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patberg E.T., Adams T., Rekawek P., et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am J Obstet Gynecol. 2021;224:382.e1–382.e18. doi: 10.1016/j.ajog.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]