Abstract
This current study aims to optimize, characterize, and observe the stability of the self-nano emulsifying drug delivery system (SNEDDS) of propolis extract (PE) for improving the immune response. Optimization of the selected composition of SNEDDS was conducted using a D-optimal mixture design. SNEDDS was prepared by loading 150 mg/mL of PE in oil, surfactant, and cosurfactant phases. The thermodynamic stability test was carried out with phase separation parameters followed by the robustness to dilution and accelerated stability test. The immunostimulant activity was examined in vitro and in vivo by determining the phagocytic activity, cell proliferation, production of nitrite oxide levels of RAW 264.7 cells, phagocytic activity of macrophages, and the number of leukocytes, neutrophils, and lymphocytes. The formula optimization showed that the formula containing Capryol-90, Cremophor RH40, and PEG 400 at a ratio of 30: 34: 36 was optimum. The verification response of the optimum formula with drug loading showed that the transmittance, droplet size, and zeta potential were 96.90 ± 0.00%, 28.7 ± 1.20 nm, and −56.5 ± 2.05 mV, respectively. The thermodynamic stability test and robustness to dilution did not find any separation phase. The accelerated stability test results were classified as stable. The in vitro and in vivo immunostimulant activity test showed that PE-loaded SNEDDS exhibited a higher immunostimulant effect than PE. In conclusion, the optimum and stable composition of PE loaded SNEDDS was found with a simple and accurate method using the D-Optimal mixture design and demonstrated an immunostimulant activity.
Keywords: Propolis extract, SNEDDS, Capryol-90, D-optimal mixture design, Immunostimulant
1. Introduction
Propolis is a natural compound produced by bees originating from a resin substrate of the leaf shoots and bark of plants mixed with the honeycomb's enzymes and waxes (Takashima et al., 2019). Propolis is a thick substance like resin, with a yellowish to light brown color (Iqbal et al., 2019). Propolis gives various health benefits through its essential roles as an immunomodulatory, antibacterial, antiviral, antifungal, antiparasitic, anti-inflammatory, and antitumor agent (Gargouri et al., 2019). Recent research also reports other benefits of propolis in the pharmaceutical field. Standardized ethanol extract of Indian propolis shows its potential as anti-carcinogenic (Kapare et al., 2019). Propolis extract (PE) shows a significant reduction in oxidative damage from oxidative stress and a significant protective effect against hepatotoxicity (Chaa et al., 2019). Oral administration of micellar propolis nanoformulation is promising as an oral delivery system of propolis against oxidative stress injury in the liver cells (Tzankova et al., 2019). A previous study reports that PE has an immunomodulatory effect and boosts the immune response with an increase in the phagocytic index, NO production, and the production of IgG antibodies (Kalsum, 2017).
Propolis contains numerous nutrients, such as polyphenols (flavonoids, phenolic acids, and esters), terpenoids, steroids, and amino acids, which are active ingredients commonly found in propolis (Bankova et al., 2000). The active components responsible for the pharmacological effects of propolis include rutin, caffeic acid phenethyl ester (CAPE), quercetin, p-coumaric acid, benzoic acid, galangin, pinocembrin, chrysin, and pinobankasin. CAPE is also known as an immunomodulating agent and should be considered as an alternative to help reduce an exaggerated inflammatory response (Berretta et al., 2020, Orsatti et al., 2010). However, several studies report that the flavonoid aglycones in propolis cause it to have low solubility and bioavailability (Di Pierro et al., 2016).
It is possible to overcome the poor solubility and bioavailability of propolis by preparing it into a self-nano emulsifying drug delivery system (SNEDDS). SNEDDS has a droplet size on a nanometer scale and has proved to increase bioavailability and maintain stability (Anton and Vandamme, 2011, Syukri et al., 2017). SNEDDS has more advantages than other lipid carriers, such as being more stable in storage, more practical, and quickly produced on a large scale. SNEDDS can be absorbed through the lymphatic pathway to avoid the first-pass effect, dissolve hydrophobic compounds, and turn droplets into phases that are more easily absorbed in the body fluid (Kassem et al., 2016). SNEDDS formulations have been widely used and shown to increase the solubility of embelin (Parmar et al., 2015), nystatin (Kassem et al., 2016), and adefovir dipivoxil (Gupta et al., 2011). Meanwhile, andrographolide in self-nano emulsifying is reported effective to prevent diabetes by lowering blood glucose levels, ameliorating islet beta cells, and inhibiting lipid formation in adipocyte cells (Syukri et al., 2021b).
Towards this goal, a PE-loaded SNEDDS is formulated to improve the immunostimulant effect. Selected oil, surfactant, and co-surfactant are used as a vehicle to prepare the SNEDDS using a D-optimal mixture design. Hence, this study aims to carry out optimization, characterization, and stability study of PE in SNEDDS formulation using D-optimal mixture design to improve the immunostimulant activity.
2. Materials and methods
2.1. Materials
PE was obtained from the Bee House in East Java as a supplier and standardized source based on previous research (Syukri et al., 2020). Labrafac, Labrasol, and Capryol-90 were taken from Gattefose, France. Cremophor RH40, Kollisolv, and Kolliphor were received from BASF Indonesia. Castor oil, sunflower oil, sesame oil, virgin coconut oil, Tween 20 and PEG 400 were obtained from Brataco Indonesia Ltd. Distilled water was produced by the Research Laboratory of Universitas Islam Indonesia. RAW 264.7 cells, DMEM (Dulbecco's Modified Eagle Medium), and MTT (3-(4,5-dimethylthiazol-2-yl-2,5-diphenyl-tetrazolium bromide) were obtained from the Parasitology Laboratory of Gadjah Mada University. Fetal bovine serum (PBS) and Giemsa were from Merck, and latex beads polystyrene was bought from Sigma.
2.2. SNEDDS preparation and construction of ternary phase diagram
Oil, surfactant, and co-surfactant were selected as carriers based on their ability to provide the highest solubility for the propolis extract. PE (100 mg) was dissolved in each carrier, beginning with the lowest volume sequentially from 0.1, 0.25, 0.5, 0.75, 1.0, 1.25, to 1.5 mL. Carriers with the smallest amount but most significant ability to dissolve propolis extract became the chosen carriers. The ternary phase diagram for the selected carriers obtained through a solubility test without the addition of propolis extract was constructed by plotting the test results in a ternary phase diagram to identify the nanoemulsion region of three-carrier combinations. Formulations of oil, surfactant, and co-surfactant were prepared in SNEDDS by mixing each chosen oil (10–50%), surfactant (10–80%), and co-surfactant (10–40%) based on the comparison of the determined compositions. Then, it was homogenized and diluted with double distilled water (1: 100). Visual observations were conducted on the formation of nanoemulsions with clarity parameters. The transmittance value was analyzed using a UV–Visible spectrophotometer (Shimadzu 1800, Japan) at a wavelength of 650 nm. The transmittance of more than 80% was considered to be selected for the construction of the ternary phase diagram using Triplot® software.
2.3. SNEDDS optimization design
The optimization design was conducted using the Design Expert Software. The component formulations in this study were designed based on the three components as the independent variables, namely oil phase (X1), surfactant (X2), and co-surfactant (X3) with the total concentration of the three components being 100%. Meanwhile, the response was the dependent variable, namely % transmittance (Y1), droplet size (Y2), and zeta potential (Y3).
2.4. Determination and verification of design optimization
The responses obtained from previous “run” experiments were analyzed using ANOVA in the D-optimal mixture design. The appropriate mixture model was determined based on the ANOVA results to obtain the optimum formula with the target of transmittance value, droplet size, and zeta potential. The mixture model was verified by comparing the experimental results with the target values of transmittance, droplet size, and zeta potential with less than 10% bias.
2.5. Preparation of the optimum PE in SNEDDS formulation
The formulation of PE-loaded SNEDDS was carried out for the optimum formulation, which was determined through the D-optimal mixture design with 150 mg/mL of drug loading.
2.6. Characterization of the optimum PE in SNEDDS formulation
2.6.1. Transmittance
The transmittance of the optimum PE in SNEDDS was determined by 100-fold dilution in double-distilled water as a blank and measurement of the transmittance using a UV–vis spectrophotometer (Shimadzu UV 1800, Japan) at a wavelength of 650 nm.
2.6.2. Droplet size and zeta potential
The droplet size and zeta potential of the optimum PE in SNEDDS formulation were also obtained by 100-fold dilution in double-distilled water and measurement of the dispersed particles using a laser dynamic light scattering (DLS) method with a particle size analyzer (PSA) designed explicitly for measuring nanometer-sized particles (Horiba SZ 100, Japan) (Syukri et al., 2018).
2.7. Stability studies of the optimum PE in SNEDDS formulation
2.7.1. Thermodynamic stability test
The thermodynamic stability test performed included the centrifugation test, heating–cooling cycle, and freeze–thaw cycle. The SNEDDS formulation was diluted 25 times in double-distilled water. The centrifugation test was carried out in centrifugation at 4000 rpm for 30 min. The heating–cooling cycle was analyzed in three repetitions at a temperature range of 4–45 °C and a minimum of 48-h storage. Each period was repeated for 8 h at a temperature of 4 °C and 8 h at 45 °C. The freeze–thaw test was performed three times between −20 °C and +25 °C with a minimum of 48 h of storage duration. Each repetition was conducted for 8 h at a temperature of 4 °C and 8 h at 45 °C. The formulations were selected based on the condition without signs of creaming, phase separation, drug precipitation, and cracking. The passed formula in the thermodynamic stability test was selected to be continued with the robustness to dilution test (Syukri et al., 2019a).
2.7.2. Robustness to dilution test
The optimum formula of PE-loaded SNEDDS was diluted 25 times, 50 times, 100 times, and 250 times using double-distilled water. Afterwards, it was evaluated by determining the droplet size. The formulations with droplet size values that did not change significantly were selected for the accelerated stability test (Chabib et al., 2017).
2.7.3. Accelerated stability test
The accelerated stability test was conducted according to ICH guidelines by storing the optimum formula in a climatic chamber with the conditions of 40 °C ± 2 °C/75% RH ± 5% RH for three months. The transmittance and droplet size were measured in 0, 1, 2, and 3 months (Bhagwat et al., 2021).
2.8. In vitro immunostimulant study
2.8.1. Effects on RAW 264.7 cell viability
The RAW 264.7 cells are a macrophage-like transformed cell line derived from BALB/c mice. These cells were seeded into three 96-well cell culture plates at a density of 105 cells per well and then treated with different concentrations of 6.25, 12.5, and 25 µg/mL of PE and PE-loaded SNEDDS. After the treatment with PE and PE-loaded SNEDDS, the cells were incubated for 24 h at 37 °C, 5% CO2. MTT (2 mg/ml) in phosphate-buffered saline (PBS) was added to each well followed by incubation at 37 °C for 3 h. The medium with MTT was removed with the addition of 100 μL DMSO that solubilized the formed formazan crystals, and the absorbance was read at 540 nm using a microplate reader.
2.8.2. Phagocytic activity on RAW 264.7 cells
Macrophage monolayers were established by the addition of 6.25, 12.5, and 25 µg/mL of PE and PE-loaded SNEDDS into a sterile microplate. After 1 h of incubation, non-adherent cells were washed away. The remaining macrophage monolayers were then co-incubated at 37 °C in a humidified 5% CO2 atmosphere for 60 min with a 1.0 mL suspension of latex added to each dish. After the non-internalized latex was washed away, the coverslips were then stained with Giemsa stain. A total of 100 activated macrophages were then scored for phagocytosis. The phagocytic index was determined by counting the total number of latex engulfed/macrophages divided by 100 activated macrophages.
2.8.3. Measurement of nitric oxide (NO) levels
The RAW 264.7 cultures (105 cells/well) were designed into four groups. Each group was treated with 6.25, 12.5, and 25 µg/mL of PE and PE-loaded SNEDDS, and one became the control (containing culture media only). After incubation in 5% CO2 at 37° C for 48 h, the cultured media were discarded, and the latex was added to each sample. The cells were then incubated in 5% CO2 at 37° C for 4 h. The NO produced by the cells was determined using Griess reaction by adding 100 µL of Griess A and B (1:1) in each sample, and then its absorbance was measured at 595 nm through a microplate reader. The nitrite concentration was then calculated using the standard curve of sodium nitrite.
2.9. In vivo immunostimulant study
2.9.1. Animals
Thirty adults male Wistar rats with a bodyweight ranging from 150 g to 200 g at the beginning of the experiment were used in this current study. All of the experimental animals were placed in a standard-size cage at a temperature of 25 °C under the conditions of 12 h of light and 12 h of dark with the natural food and distilled water provided ad libitum. This study received an approval from the Animal Care Committee of the Islamic University of Indonesia, Yogyakarta, Indonesia (No. 3/Ka.Kom.Et/70/KE/II/2020), and was performed by referring to the European Community guidelines for studies on experimental animals. The experimental design to assess the immunostimulant activity is presented in Table 1.
Table 1.
Experimental design of immunostimulant study (N = 6).
| Group | Treatment |
|---|---|
| Normal control | The rats received 2 mL/day distilled water orally for 14 days |
| Placebo SNEDDS | The rats received 2 mL/day placebo SNEDDS for 14 days |
| Positive control (levamisole) | The rats received 2 mL/day levamisole (0.9 mg/200 g) for 14 days. |
| PE | The rats received 2 mL/day propolis extract (200 mg/200 g) for 14 days |
| PE-loaded SNEDDS | The rats received 2 mL/day propolis SNEDDS (200 mg/200 g) for 14 days |
The formula was administered at 2 mL/day for 14 days to the rats. The experimental design included the standard control (receiving distilled water), placebo SNEDDS (acquiring SNEDDS vehicle), positive control (receiving levamisole), PE (receiving PE), and PE-loaded SNEDDS (receiving propolis SNEDDS).
2.9.2. Measurement of neutrophils, lymphocytes, and leukocytes
The measurement of the number of leukocytes, neutrophils, and lymphocytes was conducted using blood as the sample. The blood was collected from the orbital sinus of rats under anesthetic conditions. Before this procedure, the rats were injected with ketamine-xylazine at a dose of 100 mg/kgBW. The blood samples were transferred into microtubes filled with EDTA 0.1% to prevent coagulation. The blood plasma was then counted for the number of neutrophils, lymphocytes, and leucocytes using an automated cell counter (hematology analyzer) for the samples of day 0 and day 14.
2.10. Statistical analysis
All of the statistical analyses were performed using Microsoft Office Excel 2010. The quantitative data calculations for the phagocytic activity, measurement of NO levels, neutrophils, lymphocytes, and leukocytes were expressed as a mean ± SEM. The one-way analysis of variance was used for the statistical analysis. A p-value of less than 0.05 was considered as indicating a significant difference.
3. Results
3.1. 1 SNEDDS preparation and construction of ternary phase diagram
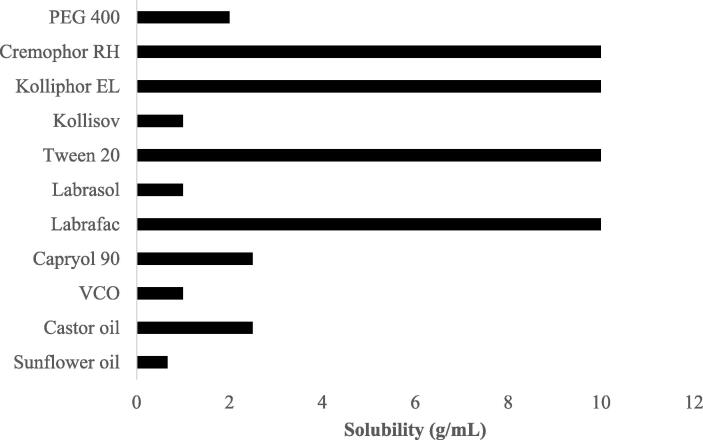
The results of solubility test for propolis extract with the carriers comprising oil, emulsifier, and co-emulsifier are presented in Fig. 1. Fig. 1 shows that PE has the best solubility in Capryol-90 (2.5 g/mL), virgin coconut oil (VCO) (1 g/mL), and sunflower oil (0.67 g/mL). PE also has the best solubility in Cremophor RH 40, Kolliphor, Tween 20, and Labrafac as the surfactant, at 10 g/mL. Meanwhile, the ability to dissolve PE in co-surfactant is found in 1 g/mL of Kollisov and Labrasol and 2 mg/mL of PEG 400. Therefore, the selected oil, surfactant, and cosurfactant phases were Capryol-90, Cremophor RH 40, and PEG 400, respectively.
Fig. 1.
Solubility study of PE in various oils, surfactants, and cosurfactants (n = 3).
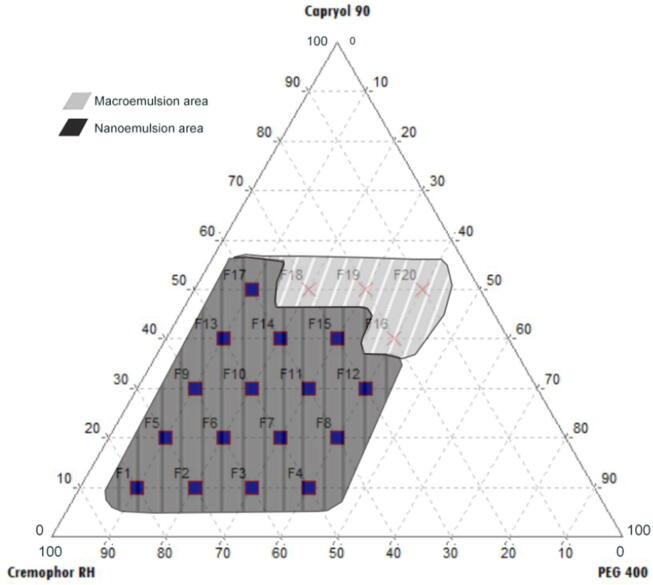
The phase compositions of each oil, surfactant, and co-surfactant with the ability to self-emulsify when dropped in water to form a nanoemulsion are presented in the ternary phase diagram in Fig. 2. The bright and non-separating nanoemulsions are illustrated with shaded areas. Meanwhile, illustrations with black areas show macroemulsion formulations that are cloudy. Formulas with oil content (10–50%), surfactants (40–80%), and co-surfactants (10–40%) produced nanoemulsions that were clear, homogeneous, and spontaneously formed.
Fig. 2.
Ternary phase diagram of PE attributed to the region of o/w nanoemulsion in Capryol 90, Cremophor RH40, and PEG 400 as the oil, surfactant, and co-surfactant.
3.2. Formula optimization using D-optimal mixture design
The optimum formula was obtained based on a numerical analysis of 3 responses, including transmittance, droplet size, and zeta potential. The D-optimal mixture design was also equipped with ANOVA to assist the statistical calculation. The results of the response (Table 3) were analyzed, and the statistical model was selected.
Table 3.
Optimal formulation of PE-loaded SNEDDS using D-optimal design.
| Formula (F) | Capryol-90 (%) | Cremophor RH40 (%) | PEG 400 (%) | Transmittance (%) | Droplet size (nm) |
|---|---|---|---|---|---|
| F1 | 30 | 34 | 36 | 97.36 | 29.42 |
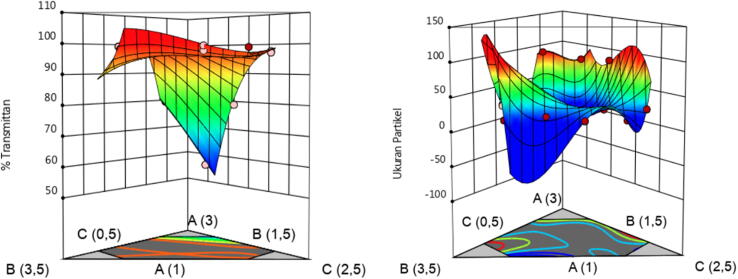
Fig. 3 presents a significant relationship between the droplet size response and the percentage of each component of Capryol-90 (A), Cremophor RH40 (B), and PEG 400 (C). The smaller the amount of Capryol-90 (A), the more modest the droplet size. In Cremophor RH40 (B), the percentage rated midway between the upper and lower limits can create a better droplet size. PEG 400 (C) can form smaller droplet sizes when used with increasing concentrations. The zeta potential value showed a range from −12.23 mV to −58.73 mV. The p-value > 0.05 indicated that the response value did not show a significant change in the D-optimal mixture design's variation of component compositions.
Fig. 3.
3D graphic model of transmittance (left) and droplet size (right) of optimum PE-loaded SNEDDS formula.
Afterwards, the expected response criteria were selected and included in the D-optimal mixture design to determine the optimum formula for PE-loaded SNEDDS. The expected value of transmittance had a lower limit value of 80% and an upper limit of 100% to maximize the criteria. The maximizing rules were chosen to produce the optimum formula with the best transmittance value being close to 100%. Droplet size parameters were selected, with the lower limit used for the droplet size being 20 nm and the upper limit of 200 nm. For the zeta potential value, the desired criteria needed not be determined because it had a non-significant response value. In the desired criteria column, the “none” option was selected.
Furthermore, the target criteria results, the predetermined lower and upper limits, were processed, and the optimum formula for D-optimal recommendations was produced. The chosen formulations were obtained from the D-optimal mixture design suggestions, thus ensuring that the method was verified. Table 2 is the results of the optimum formula and predicted target.
Table 2.
Formula optimization and D-optimal mixture design response.
| Run | Components |
Response |
||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | Y3 | |
| 1 | 28 | 32 | 40 | 97.70 | 23.00 | −25.53 |
| 2 | 28 | 38 | 32 | 96.86 | 21.23 | −21.17 |
| 3 | 42 | 30 | 26 | 84.91 | 73.50 | –33.67 |
| 4 | 28 | 32 | 40 | 97.20 | 22.23 | −12.23 |
| 5 | 36 | 54 | 10 | 97.68 | 19.40 | −45.37 |
| 6 | 22 | 60 | 18 | 99.10 | 14.80 | −47.37 |
| 7 | 34 | 42 | 22 | 98.12 | 20.67 | −52.53 |
| 8 | 20 | 48 | 32 | 97.74 | 15.93 | −41.13 |
| 9 | 22 | 60 | 18 | 99.07 | 15.27 | −21.80 |
| 10 | 42 | 30 | 26 | 78.89 | 82.45 | −38.87 |
| 11 | 50 | 32 | 18 | 56.51 | 78.97 | −42.30 |
| 12 | 42 | 44 | 14 | 100.12 | 24.17 | −18.30 |
| 13 | 20 | 48 | 32 | 99.41 | 15.10 | −53.63 |
| 14 | 50 | 38 | 12 | 79.61 | 90.90 | −52.90 |
| 15 | 20 | 40 | 40 | 98.94 | 15.80 | –22.53 |
| 16 | 24 | 52 | 24 | 99.24 | 16.93 | −58.73 |
| 17 | 36 | 54 | 10 | 98.58 | 21.27 | −43.83 |
3.3. Characterization of the optimum formula of PE-loaded SNEDDS
3.3.1. Transmittance
The optimum formula of PE-loaded SNEDDS showed that the transmittance of 96.90 ± 0.00% (Table 4) had equivalent clarity with double-distilled water and was an indication that the finished droplet size had a nanometer-scale (Halnor et al., 2018). After PE was loaded into the SNEDDS formulation, the bias value was 0.47% (Table 4). This value showed an excellent compatibility with the target value before PE loading.
Table 4.
Characterization of the optimal formula of PE-loaded SNEDDS.
| Transmittance (%) |
Droplet size (nm) |
Zeta Potential (mV) |
||
|---|---|---|---|---|
| Data obtained | Bias (%) | Data obtained | Bias (%) | Data obtained |
| 96,90 ± 0,00 | 0.47 | 28.7 ± 1.20 | 2.44 | −56.5 ± 2.05 |
3.3.2. Droplet size
The propolis extract loaded SNEDDS had a droplet size that met the SNEDDS preparation requirement of less than 200 nm. The optimum formula had a droplet size of 28.7 ± 1.20 nm (Table 4). The bioavailability of drugs in the body will increase with the decreasing droplet size (Date et al., 2010). Drug loading with PE was carried out for SNEDDS carriers, resulting in a bias value of 2.44%.
3.3.3. Zeta potential
The results (Table 4) showed that the optimum formula for PE-loaded SNEDDS had an excellent zeta potential value of −56.5 ± 2.05 mV. These results were considered capable of keeping PE-loaded SNEDDS droplets more stable during storage and preventing precipitation.
3.4. Stability studies
3.4.1. Thermodynamic stability test
This test aims to determine SNEDDS preparation stability against creaming, cracking, and precipitation during prolonged storage. The thermodynamic stability test also seeks to assess the level of stability of SNEDDS preparations for temperature changes and even against extreme condition (Kanwal et al., 2019).
The centrifugation test results, the heating–cooling cycle test, and the freeze–thaw cycle test showed that all of the optimum formulae of PE-loaded SNEDDS experienced no phase separation, creaming, or cracking.
3.4.2. Robustness to dilution test
The robustness to dilution test was done by diluting PE-loaded SNEDDS with multilevel dilutions of 25 times, 50 times, 100 times, and 250 times using double-distilled water. The dilution was measured in a droplet size analyzer to determine the uniform size of the droplets produced. Table 5 presents the robustness to dilution test of the optimum formula of PE-loaded SNEDDS.
Table 5.
Robustness to dilution test of the optimal formula of PE-loaded SNEDDS.
| Droplet size (nm) | |||
|---|---|---|---|
| 1: 25 | 1: 50 | 1: 100 | 1: 250 |
| 29.3 ± 0.3 | 30.3 ± 0.6 | 28.7 ± 1.20 | 27.9 ± 0.2 |
The data in Table 5 indicated that the optimum formula of PE-loaded SNEDDS did not increase the droplet size to greater than 200 nm. The results obtained at 25, 50, 100, and 250 times of dilution did not experience significant changes. The resulting solution looked clear and was not cloudy. The results of the optimum formula for PE-loaded SNEDDS indicated a stable condition at each dilution.
3.4.3. Accelerated stability test
The optimum formula for PE-loaded SNEDDS was stored in a climatic chamber with storage conditions of 40 °C ± 2 °C/75%±5% of RH for three months. The SNEDDS were then evaluated for the droplet size and % transmittance values in 0, 1, 2, and 3 months (Gupta et al., 2011). Table 6 shows the accelerated stability study of propolis extract-loaded SNEDDS.
Table 6.
Accelerated stability study of PE-loaded SNEDDS.
| Parameters | Month |
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Droplet size (nm) | 29.3 ± 0.35 | 31.2 ± 0.20 | 24.8 ± 0.05 | 29.6 ± 1.71 |
| Transmittance (%) | 96.0 ± 0.00 | 94.3 ± 0.01 | 93.3 ± 0.01 | 93.4 ± 0.00 |
The droplet size in the optimum formula of PE-loaded SNEDDS is explained in Table 6. The results did not indicate a significant change. The resulting droplet size had a value of less than 200 nm, indicating a relatively good result. The % transmittance value produced from the optimum formula of PE-loaded SNEDDS was also good. The range of the % transmittance value for the one-month period was 96.0–102.7%. The results were better if they approached 100%. The data also indicated that the globule produced was measured by nanoparticles. When the stability test was accelerated for three months, the optimum formula for PE-loaded SNEDDS was classified as stable.
3.5. In vitro immunostimulant study
3.5.1. Effects on RAW 264.7 cell viability
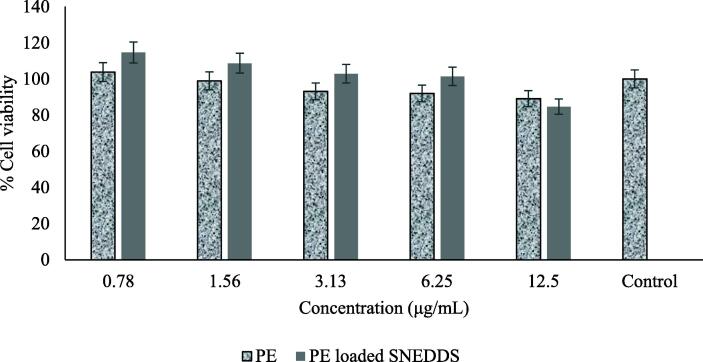
The cytotoxicity of PE and PE-loaded SNEDDS in RAW 264.7 cells was investigated to find the optimal concentration, which effectively provided an immunostimulant activity with minimum toxicity. As shown in Fig. 4, the cell survival is reduced by ~6% after 24-h treatment with PE-loaded SNEDDS at a concentration of 12.5 μg/mL. At a concentration less than 12.5 μg/mL, PE-loaded SNEDDS maintained the cell viability and showed no toxicity on the cells. On the other hand, the treatment with PE at a concentration range of 1.56–12.5 μg/mL on the cells showed an increased cell death rate by ~ 7%. Another study with a similar result concludes that no toxicity of nanoemulsion is observed at an experimental concentration tested on RAW 264.7 cells (da Silva et al., 2018).
Fig. 4.
Percentage of viable cells obtained by MTT assay on macrophages of RAW 264.7.
3.5.1.1. Phagocytic activity on RAW 264.7 cells
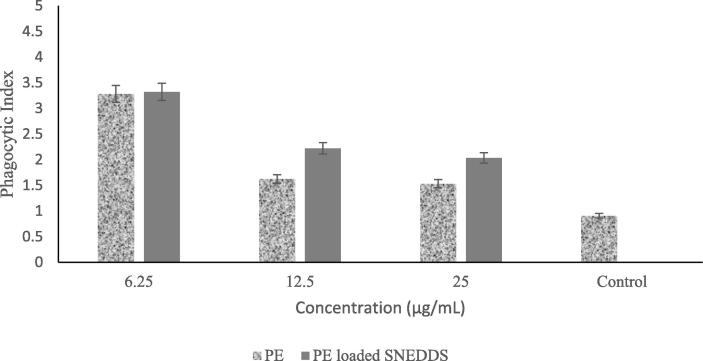
Fig. 5 suggests that the phagocytosis activity of RAW 264.7 cells is dose-dependent. PE-loaded SNEDDS and PE showed no significant difference in inducing the phagocytosis activity of macrophage cells, which was shown by its phagocytic index. Phagocytic index was calculated according to the total number of engulfed cells divided by the total number of counted macrophages, but both of them still gave a higher phagocytic index value compared to the control. The content of complex secondary metabolites in plant-derived propolis may play an essential role in providing this effect (Kim et al., 2019).
Fig. 5.
Effect of PE and PE-loaded SNEDDS on phagocytic activity of macrophages of RAW 264.7.
3.5.1.2. Measurement of nitric oxide (NO) levels
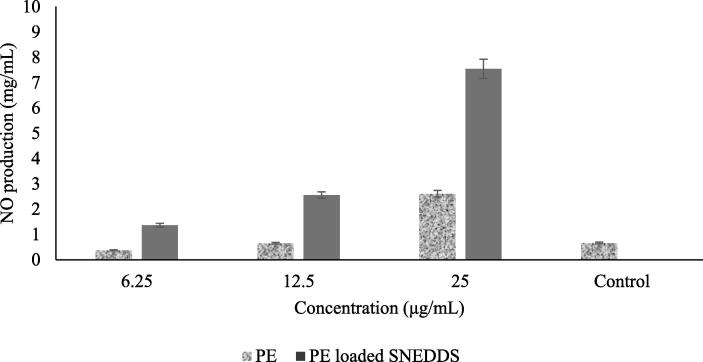
The relative production of NO was markedly increased upon exposure to PE-loaded SNEDDS in a dose-dependent manner as shown in Fig. 6. The results showed that the 24 h treatment of PE-loaded SNEDDS on cells at 12.5 mg/mL and 25 mg/mL triggered a 3-fold increase in nitrate concentration compared to that of the PE. These results indicated that PE-loaded SNEDDS and PE effects on NO production in the cells at those concentrations were caused by cellular toxicity, as shown in the cell viability test result in Fig. 4.
Fig. 6.
Effect of PE-loaded SNEDDS on the production of NO on macrophages of RAW 264.7.
3.6. In vivo immunostimulant study
3.6.1. Measurement of neutrophils, lymphocytes, and leukocytes
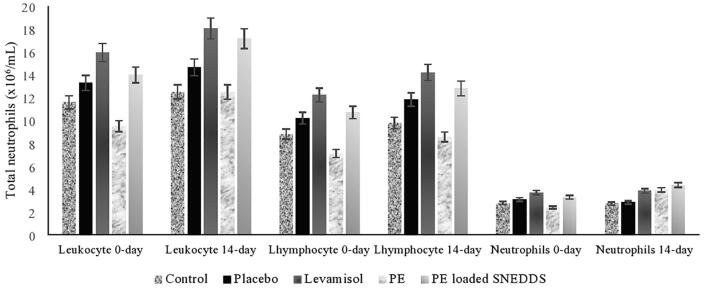
The immunostimulant in vivo investigation of PE and PE-loaded SNEDDS showed an effect at a dose of 200 mg. In the practical orientation, stimulation of the proliferation of neutrophil cells and leukocytes in the treatment group treated with PE and PE-loaded SNEDDS showed an increase in the number of cells starting from a week to two weeks, and observations were therefore made by comparing the results on 0 day and 14th day. PE-loaded SNEDDS showed an effect of lymphocyte, leucocyte, and neutrophil cell proliferation, demonstrated by the total number of cells, which differed significantly between day 0 and day 14 of the response to this preparation. PE-loaded SNEDDS was also able to show better performance than the control and PE in increasing the number of these three cells. A significant increase in neutrophils on the 14th day also occurred in the treatment group compared to the control. The data showed different responses between the cells after exposure to PE and PE-loaded SNEDDS. PE-loaded SNEDDS demonstrated the most significant effect of stimulating neutrophil proliferation, which showed similar results to that of levamisole. The total neutrophils, lymphocytes, and leukocytes after treatment with PE-loaded SNEDDS are demonstrated in Fig. 7.
Fig. 7.
Total neutrophils, lymphocytes, and leukocytes after treatment with PE-loaded SNEDDS.
4. Discussion
PE solubility test in carriers is essential to obtain a stable formulation. It is required to guarantee that the formed nanoemulsion does not precipitate out in the digestive tract (Syukri et al., 2019b). The generally recognized as safe (GRAS) category has become the main criterion to consider when selecting emulsifier and co-emulsifier since these materials have to be pharmaceutically acceptable for oral administration. Another consideration is that the required hydrophilic-lipophilic balance (HLB) value to form o/w emulsion has to be more than 10 (Agarwal et al., 2018). Capryol-90 has an HLB value of 6, which is lower than those of virgin coconut oil (HLB: 8) and sunflower oil (HLB: 7), indicating that a lower HLB in carriers enables better dissolution of PE (Alayoubi et al., 2018).
The ternary phase diagram construction is used to determine the nanoemulsion formation area and select the concentration of oil, surfactants, and co-surfactants as the appropriate carriers of SNEDDS. The nanoemulsion area is determined based on visual observations of clarity and transmittance during dilution (Garg et al., 2017). The phase diagram consisting of Capryol-90 (oil), Cremophor RH40 (surfactant), and PEG 400) (co-surfactant) was prepared to facilitate the researchers in identifying nanoemulsion regions that separated and coalesced to form clear solutions. The nanoemulsion area in a ternary diagram demonstrates the concentration of oil, surfactants, and co-surfactants as the appropriate carriers of SNEDDS. Adsorption of surfactants and co-surfactants on oil and water surfaces can reduce surface tension and cohesion forces in the emulsion system to support the formation of more stable nanoemulsions (Sriamornsak et al., 2015).
SNEDDS formulations consist of the oil phase, surfactant, and co-surfactant (Syukri et al., 2019a). Capryol-90 has been widely used in the formulation of SNEDDS preparations and is proven to be the best solvent for substances that are less soluble in water (Syukri et al., 2018). Cremophor RH40 (HLB, 14–16) is an emulsifier of oil in water with a high loading capacity (Sriamornsak et al., 2015). PEG 400 is used to obtain the most optimum mixture of preparations and to streamline the use of Cremophor RH40 as a surfactant (Weerapol et al., 2014). The mixture composition among Capryol-90, Cremophor RH40, and PEG 400 was varied statistically by the D-optimal mixture design in the Design Expert. The Design Expert software helps determine formulas, optimize formulation variables, identify the number of runs, keep the total concentration constant, and obtain optimum formulation predictions (Dash et al., 2015).
The response of transmittance and droplet size is influenced by the constituent components of the D-optimal mixture design. The data in Table 3 are significant, with a p-value of < 0.05. The transmittance response lacks a fit value of 0.17 > 0.05, which indicates that the transmittance response has no error significance between the cubic model and the response data. The lack of fit value is used to test the model's suitability with the response data (Maheshwari and Chandan, 2013).
The data show an r-squared value of 0.9812. The value of r-squared, which is closer to number 1, indicates that the response results are formed by the model used. The following coefficient value result indicates the equation formula between the component and the response data based on the model: Y1 = −30.57 X1 + 98.17 X2 + 102.28 X3 + 270.51 X1X2.
Fig. 3 shows a significant relationship between the transmittance response and the percentage of each component of Capryol-90 (A), Cremophor RH40 (B), and PEG 400 (C). The smaller proportion of Capryol-90 (A) and PEG 400 (C) can produce a higher transmittance value, whereas the RH40 (B) Cremophor appears to provide a high transmittance value in each variation of concentration.
The p-value obtained for a response to droplet size was 0.0001 < 0.05. The data show that droplet size values are influenced by the composition of oil components, surfactants, and co-surfactants found in the D-optimal mixture design. The range of droplet size values obtained was from 14.8 nm to 90.9 nm. Meanwhile, the earned value of the r-squared obtained was 0.9965, indicating that the response value was formed by the model used. The modified mixture was chosen as the model used in analyzing the response results. The following coefficient value result indicates the equation formula between the component and the response data based on the model: Y2 = +438.26 X1 + 265.85 X2-436.38 X3-1699.72 X1X2.
The optimum formula of PE-loaded SNEDDS showed a transmittance of 96.90%. Transmittance was measured to evaluate the stability of the optimized nanoemulsion of SNEDDS. It also gave a proposal about the features of formulation, such as the droplet's size and uniformity. It was found, which confirmed its clarity after dispersion into distilled water. Also, it confirmed that there are no chances of drug precipitation and optimized formulation had good solubilization capacity after dispersion (Verma and Kaushik, 2020).
The optimum formula of PE-loaded SNEDDS shows excellent compatibility with the target value, and it has a droplet size of less than 200 nm. The optimum formula had a droplet size of 28.7 ± 1.20 nm. Droplet size is of the primary essential qualities of nanoemulsion for stability assessment and a fundamental advance in improving assimilation of medicament. Its smaller size results in greater interfacial surface area for assimilation of medicament and enhanced bioavailability and pharmacological effect (Verma and Kaushik, 2020). The higher the ratio of surfactant and co-surfactant concentration, the lower the droplet size produced (Kadu et al., 2011). The bioavailability of drugs in the body will increase correspondingly with a decreasing droplet size (Date et al., 2010). The addition of surfactants and co-surfactants to the drug delivery system can increase dissolution and spread the drug in the digestive tract. In the dilution process, surfactants and co-surfactants form stable bonds with their water carriers (Parmar et al., 2011). The surfactants act to break and reduce droplet size in the emulsion, while co-surfactants help cover small gaps between surfactants.
PE-loaded SNEDDS had an excellent zeta potential value of −56.5 ± 2.05 mV. The zeta potential value represents the level of inherent stability in a colloidal system. Droplets with a large zeta charge with a positive or negative potential will repel each other to produce no coalescence. In comparison, a load that is too small will not produce sufficient force. An excellent zeta potential value is higher than + 30 mV or lower than −30 mV. Zeta has the potential to help prevent droplets from attracting each other, which results in precipitation. The formation of the resisting force also prevents enlarged droplet sizes (Chaudhary et al., 2019). Zeta potential values determine the potential stability of the SNEDDS in the aqueous system. Zeta potential is the charge that develops at the interface between a solid surface and its liquid medium. If the dispersed particles of aqueous media have high positive or negative zeta potential values, they will repel each other to create dispersion stability. On the other hand, substantially low zeta potential values of the dispersant cannot prevent the particles from coming together and become unstable (Kazi et al., 2021).
The thermodynamic stability studies showed that the optimum formula of PE-loaded SNEDDS remained transparent and homogeneous. Kinetic strength is one of the required characteristics of PE-loaded SNEDDS because stability can distinguish a nanoemulsion from an emulsion. PE-loaded SNEDDS spontaneously forms nanoemulsions when dissolved in a solvent without exhibiting phase separation and precipitation during storage (Kassem et al., 2016). The thermodynamic studies were done to investigate the dispersion efficiency and stability of SNEDDS in gastrointestinal fluids. Thermodynamic stability is an indicator of dispersion's kinetic stability and is generally used to study the chemical reactions occurring between the components of dispersion. Poor stability of dispersion can lead to precipitation or phase separation, which could affect drug absorption and therapeutic efficacy (Buya et al., 2020, Usmani et al., 2019).
The robustness to dilution test aims to determine the character's resistance and uniformity from several dilution levels. This test also assesses the level of drug release and the possible dilution factor that causes precipitation, which can disrupt the drug's absorption rate (Date et al., 2010). The formation of emulsions at various dilutions is essential because it guarantees emulsion uniformity after the dilution process. Formulations passing the stability test are subjected to the robustness to dilution test to examine whether uniform emulsions are spontaneously formed at different dilution rates (Kassem et al., 2016). The data of robustness to dilution demonstrated that the solution did not experience significant changes. The resulting solution looked clear and was not cloudy. The results of the optimum formula for PE-loaded SNEDDS indicated a stable condition at each dilution.
The accelerated stability test is conducted to determine and evaluate the effect of storage conditions on the stability of SNEDDS preparations. Nanoemulsions that do not experience significant changes from their original state and do not exceed the ideal criteria of nanoemulsion can be declared stable. The proper size of nanoemulsion droplet has a value of less than 200 nm, while the excellent transmittance has a value between 80 and 100%. The closer it is to 100%, the better the clarity that the nanoemulsion has (Porter et al., 2008, Senapati et al., 2016). The data have also proved that in the accelerated stability test for three months, the optimum formula for PE-loaded SNEDDS was stable.
In determining the immunostimulant activity, the cells that play a major role in the immune response, such as macrophages, neutrophils, and basophils, can be measured as the indicators to determine the immunostimulant effect (Trinh et al., 2020). The ability of SNEDDS to activate macrophages was analyzed through the phagocytic activity on RAW 264.7 cells. NO is an essential molecule against various pathogens for the defense response of the host. Macrophage's produce short-lived free radicals of NO during the occurrence of infection. The measurement of nitric oxide cellular production in RAW 264.7 stimulated by latex was carried out to evaluate its phagocytosis activity after the treatment with PE and PE-loaded SNEDDS preparations.
The in vitro immunostimulant study showed that PE-loaded SNEDDS at a concentration of 6.25 effectively induced macrophage's phagocytic activity and generated these cells to produce higher NO compared to PE. This result indicates that PE loaded SNEDDS has immunostimulatory effects against macrophages. Based on these results and the relationship between the phagocytosis activity against latex, we assumed that the immunostimulating effect of PE-loaded SNEDDS might trigger the RAW 264.7 cells through the activation of NO production.
The in vivo immunostimulant investigation demonstrated that although PE-loaded SNEDDS did not significantly affect the in vitro, the in vivo results showed that PE-loaded SNEDDS offered a better absorption activity than the extract. In this animal model, propolis will transform into self-nano emulsifying particles after a release and contact with the body fluid. A different environment including a sufficient amount of fluid and its composition in the in vivo study gives a better result of PE-loaded SNEDDS than in the in vitro study. PE-loaded SNEDDS in nanoparticles consisting of oil, surfactant, and co-surfactant phases can increase the dissolution, absorption, and surface area in contact with cells, thereby increasing the bioavailability and effects of propolis (Syukri et al., 2021a). The penetration power of a compound mainly influences the cell proliferation activity into the cell membrane. In this study, it was shown that PE-loaded SNEDDS was able to produce better penetration power compared to its extract form, thus enabling it to pass through the body barrier (Kalsum et al., 2017). The ability of SNEDDS to produce emulsions as well as physical and chemical stability has a dominant influence on the resulting immunostimulant activity.
5. Conclusion
The optimum formulation of propolis extract SNEDDS was the formula containing Capryol-90 (30%), Cremophor RH40 (34%), and PEG 400 (36%). The SNEDDS formulation containing 150 mg/mL of propolis extract resulted in 96.90% transmittance with 0.47% bias, 28.7 nm droplet size with 2.44% bias, and −56.5 zeta potential. This formulation has proved to have an excellent stability as marked by the absence of precipitation or phase separation during the stability test and the presence of strength during the accelerated stability study. This study shows that propolis formulated in SNEDDS exhibits an immunostimulant activity in vitro and in vivo.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to the Minister of Research and the Higher Education Republic of Indonesia for funding and facilitating the research (grant numbers: 042/DirDPPM/70/DPPM/PTUPT-KEMRISTEKDIKTI/II/2018).
Footnotes
Peer review under responsibility of King Saud University.
References
- Agarwal V.K., Amresh G., Chandra P. Pharmacodynamic evaluation of self micro-emulsifying formulation of standardized extract of Lagerstroemia speciosa for antidiabetic activity. J. Ayurveda Integ. Med. 2018;9:38–44. doi: 10.1016/j.jaim.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alayoubi A., Aqueel M.S., Cruz C.N., Ashraf M., Zidan A.S. Application of in vitro lipolysis for the development of oral self-emulsified delivery system of Nimodipine. Int. J. Pharm. 2018;553:441–453. doi: 10.1016/j.ijpharm.2018.10.066. [DOI] [PubMed] [Google Scholar]
- Anton N., Vandamme T.F. Nano-emulsions and Micro-emulsions: Clarifications of the Critical Differences. Pharm. Res. 2011;28:978–985. doi: 10.1007/s11095-010-0309-1. [DOI] [PubMed] [Google Scholar]
- Bankova V., De Castro S., Marcucci M. Propolis: recent advances in chemistry and plant origin. Apidologie. 2000;31:3–15. Springer Verlag. [Google Scholar]
- Berretta A.A., Silveira M.A.D., Cóndor Capcha J.M., De Jong D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease. Biomed. Pharmacother. 2020;131:110622. doi: 10.1016/j.biopha.2020.110622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwat D.A., Swami P.A., Nadaf S.J., Choudhari P.B., Kumbar V.M., More H.N., Killedar S.G., Kawtikwar P.S. Capsaicin loaded solid SNEDDS for enhanced bioavailability and anticancer activity: in-vitro, in-silico, and in-vivo characterization. J. Pharm. Sci. 2021;110:280–291. doi: 10.1016/j.xphs.2020.10.020. [DOI] [PubMed] [Google Scholar]
- Buya A.B., Beloqui A., Memvanga P.B., Préat V. Self-nano-emulsifying drug-delivery systems: from the development to the current applications and challenges in oral drug delivery. Mole. Struct. 2020;55 doi: 10.3390/pharmaceutics12121194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaa S., Boufadi M.Y., Keddari S., Benchaib A.H., Soubhye J., Van Antwerpen P., Riazi A. Chemical composition of propolis extract and its effects on epirubicin-induced hepatotoxicity in rats. Revista Brasileira de Farmacognosia. 2019;29:294–300. doi: 10.1016/j.bjp.2019.01.005. [DOI] [Google Scholar]
- Chabib L., Muhtadi W., Ikawati Z., Martien R., Ismail H. Stability study of gamavuton (GVT-0) self-nanoemulsifiying drug delivery system (SNEDDS) myritol as the oil phase. Int. J. Curr. Inovat. Res. 2017;3:590–594. [Google Scholar]
- Chaudhary S., Aqil M., Sultana Y., Kalam M.A. Self-nanoemulsifying drug delivery system of nabumetone improved its oral bioavailability and anti-inflammatory effects in rat model. J. Drug Delivery Sci. Technol. 2019;51:736–745. doi: 10.1016/j.jddst.2018.04.009. [DOI] [Google Scholar]
- Dash R.N., Mohammed H., Humaira T., Ramesh D. Design, optimization and evaluation of glipizide solid self-nanoemulsifying drug delivery for enhanced solubility and dissolution. Saudi Pharmac. J. 2015;23:528–540. doi: 10.1016/j.jsps.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date A.A., Desai N., Dixit R., Nagarsenker M. Self-nanoemulsifying drug delivery systems: formulation insights, applications and advances. Nanomedicine. 2010;5:1595–1616. doi: 10.2217/nnm.10.126. [DOI] [PubMed] [Google Scholar]
- Di Pierro F., Zanvit A., Colombo M. Role of a proprietary propolis-based product on the wait-and-see approach in acute otitis media and in preventing evolution to tracheitis, bronchitis, or rhinosinusitis from nonstreptococcal pharyngitis. Int. J. Gen. Med. 2016;9:409–414. doi: 10.2147/IJGM.S118967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg V., Kaur P., Singh S.K., Kumar B., Bawa P., Gulati M., Yadav A.K. Solid self-nanoemulsifying drug delivery systems for oral delivery of polypeptide-k: formulation, optimization, in-vitro and in-vivo antidiabetic evaluation. Eur. J. Pharm. Sci. 2017;109:297–315. doi: 10.1016/j.ejps.2017.08.022. [DOI] [PubMed] [Google Scholar]
- Gargouri W., Osés S.M., Fernández-Muiño M.A., Sancho M.T., Kechaou N. Evaluation of bioactive compounds and biological activities of Tunisian propolis. LWT. 2019;111:328–336. doi: 10.1016/j.lwt.2019.05.044. [DOI] [Google Scholar]
- Gupta S., Chavhan S., Sawant K.K. Self-nanoemulsifying drug delivery system for adefovir dipivoxil: Design, characterization, in vitro and ex vivo evaluation. Colloids Surf., A. 2011;392:145–155. doi: 10.1016/j.colsurfa.2011.09.048. [DOI] [Google Scholar]
- Halnor V.V., Pande V.V., Borawake D.D., Nagare H.S. Nanoemulsion: a novel platform for drug delivery system. J Mat Sci Nanotechol. 2018;6:104. [Google Scholar]
- Iqbal M., Fan T., Watson D., Alenezi S., Saleh K., Sahlan M. Preliminary studies: the potential anti-angiogenic activities of two Sulawesi Island (Indonesia) propolis and their chemical characterization. Heliyon. 2019;5:e01978. doi: 10.1016/j.heliyon.2019.e01978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadu P.J., Kushare S.S., Thacker D.D., Gattani S.G. Enhancement of oral bioavailability of atorvastatin calcium by self-emulsifying drug delivery systems (SEDDS) Pharm. Dev. Technol. 2011;16:65–74. doi: 10.3109/10837450903499333. [DOI] [PubMed] [Google Scholar]
- Kalsum N. Preliminary studies of the immunomodulator effect of the Propolis Trigona spp. extract in a mouse model. IOSR JAVS. 2017;10:75–80. doi: 10.9790/2380-1002027580. [DOI] [Google Scholar]
- Kalsum N., Sulaeman A., Setiawan B., Wibawan I.W.T. Preliminary studies of the immunomodulator effect of the propolis trigona spp. extract in a mouse model. IOSR JAVS. 2017;10:75–80. doi: 10.9790/2380-1002027580. [DOI] [Google Scholar]
- Kanwal T., Kawish M., Maharjan R., Ghaffar I., Ali H.S., Imran M., Perveen S., Saifullah S., Simjee S.U., Shah M.R. Design and development of permeation enhancer containing self-nanoemulsifying drug delivery system (SNEDDS) for ceftriaxone sodium improved oral pharmacokinetics. J. Mol. Liq. 2019;289:1–11. [Google Scholar]
- Kapare H., Lohidasan S., Sinnathambi A., Mahadik K. Standardization, anti-carcinogenic potential and biosafety of Indian propolis. J. Ayurveda Integ. Med. 2019;10:81–87. doi: 10.1016/j.jaim.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem A.A., Mohsen A.M., Ahmed R.S., Essam T.M. Self-nanoemulsifying drug delivery system (SNEDDS) with enhanced solubilization of nystatin for treatment of oral candidiasis: Design, optimization, in vitro and in vivo evaluation. J. Mol. Liq. 2016;218:219–232. doi: 10.1016/j.molliq.2016.02.081. [DOI] [Google Scholar]
- Kazi M., Alqahtani A., Ahmad A., Noman O.M., Aldughaim M.S., Alqahtani A.S., Alanazi F.K. Development and optimization of sitagliptin and dapagliflozin loaded oral self-nanoemulsifying formulation against type 2 diabetes mellitus. Drug Delivery. 2021;28:100–114. doi: 10.1080/10717544.2020.1859001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Jo E., Kim N.-Y., Hwang K.W., Park S.-Y. Immune-enhancing screening of fourteen plants on murine macrophage RAW 264.7 cells. Trop. J. Pharm. Res. 2019;18:85. doi: 10.4314/tjpr.v18i1.13. [DOI] [Google Scholar]
- Maheshwari R., Chandan C. Mixed solvency concept in reducing surfactant concentration of self-emulsifying drug delivery systems of candesartan cilexetil using D-optimal mixture design. Asian J. Pharmac. 2013;7:83. doi: 10.4103/0973-8398.115960. [DOI] [Google Scholar]
- Orsatti C.L., Missima F., Pagliarone A.C., Bachiega T.F., Búfalo M.C., Araújo J.P., Sforcin J.M. Propolis immunomodulatory action in vivo on Toll-like receptors 2 and 4 expression and on pro-inflammatory cytokines production in mice: propolis action on toll-like receptors and cytokines. Phytother. Res. 2010;24:1141–1146. doi: 10.1002/ptr.3086. [DOI] [PubMed] [Google Scholar]
- Parmar K., Patel J., Sheth N. Self nano-emulsifying drug delivery system for Embelin: design, characterization and in-vitro studies. Asian J. Pharm. Sci. 2015;10:396–404. doi: 10.1016/j.ajps.2015.04.006. [DOI] [Google Scholar]
- Parmar N., Singla N., Amin S., Kohli K. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) self nanoemulsifying drug delivery system. Colloids Surf., B. 2011;86:327–338. doi: 10.1016/j.colsurfb.2011.04.016. [DOI] [Google Scholar]
- Porter C.J.H., Pouton C.W., Cuine J.F., Charman W.N. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv. Drug Deliv. Rev. 2008;60:673–691. doi: 10.1016/j.addr.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Senapati P.C., Sahoo S.K., Sahu A.N. Mixed surfactant based (SNEDDS) self-nanoemulsifying drug delivery system presenting efavirenz for enhancement of oral bioavailability. Biomed. Pharmacother. 2016;80:42–51. doi: 10.1016/j.biopha.2016.02.039. [DOI] [PubMed] [Google Scholar]
- Sriamornsak P., Limmatvapirat S., Piriyaprasarth S., Mansukmanee P., Huang Z. A new self-emulsifying formulation of mefenamic acid with enhanced drug dissolution. Asian J. Pharm. Sci. 2015;10:121–127. doi: 10.1016/j.ajps.2014.10.003. [DOI] [Google Scholar]
- Syukri Y., Afetma D.W., Sirin M., Fajri R., Ningrum A.D.K., Setiawan S.D., Wibowo A. Validation of a simple HPLC-UV method for the quantification of andrographolide in self-nano emulsifying drug delivery system (Snedds) for dissolution study. IJDDT. 2017;7 doi: 10.25258/ijddt.v7i04.10646. [DOI] [Google Scholar]
- Syukri Y., Fitria A., Hanifah S., Idrati M. Development of new Indonesian propolis extract-loaded selfemulsifying: characterization, stability and antibacterial activity. Adv Pharm Bull. 2021;11:120–129. doi: 10.34172/apb.2021.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syukri Y., Fitriani H., Pandapotan H., Nugroho B.H. Formulation, characterization and stability of ibuprofen-loaded self-nano emulsifying drug delivery system (SNEDDS) Indonesian J. Pharm. 2019;30:105–113. [Google Scholar]
- Syukri Y., Martien R., Lukitaningsih E., Nugroho A.E. Novel self-nano emulsifying drug delivery system (SNEDDS) of andrographolide isolated from Andrographis paniculata Nees: characterization, in-vitro and in-vivo assessment. J. Drug Delivery Sci. Technol. 2018;47:514–520. doi: 10.1016/j.jddst.2018.06.014. [DOI] [Google Scholar]
- Syukri Y., Purwati R., Hazami N., Anshory Tahmid H., Fitria A. Standardization of specific and non-specific parameters of propolis extract as raw material for herbal product. J. Sci. Data Anal. 2020;20:36–43. doi: 10.20885/EKSAKTA.vol1.iss1.art6. [DOI] [Google Scholar]
- Syukri Y., Taher M., Martien R., Lukitaningsih E., Nugroho A.E., Zakaria Z.A. Self-nanoemulsifying delivery of andrographolide: ameliorating islet beta cells and inhibiting adipocyte differentiation. Adv. Pharm. Bull. 2021;11:171–180. doi: 10.34172/apb.2021.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima M., Ichihara K., Hirata Y. Neuroprotective effects of Brazilian green propolis on oxytosis/ferroptosis in mouse hippocampal HT22 cells. Food Chem. Toxicol. 2019;132:110669. doi: 10.1016/j.fct.2019.110669. [DOI] [PubMed] [Google Scholar]
- Trinh, T.A., Park, J., Oh, J.H., Park, J.S., Lee, D., Kim, C.E., Choi, H.-S., Kim, S.-B., Hwang, G.S., Koo, B.A., Kang, K.S., 2020. Effect of Herbal Formulation on Immune Response Enhancement in RAW 264.7 Macrophages 16. [DOI] [PMC free article] [PubMed]
- Tzankova V., Aluani D., Yordanov Y., Kondeva-Burdina M., Petrov P., Bankova V., Simeonova R., Vitcheva V., Odjakov F., Apostolov A., Tzankov B., Yoncheva K. Micellar propolis nanoformulation of high antioxidant and hepatoprotective activity. Revista Brasileira de Farmacognosia. 2019;29:364–372. doi: 10.1016/j.bjp.2018.12.006. [DOI] [Google Scholar]
- Usmani A., Mishra A., Arshad M., Jafri A. Development and evaluation of doxorubicin self nanoemulsifying drug delivery system with Nigella Sativa oil against human hepatocellular carcinoma. Artif. Cells Nanomed. Biotechnol. 2019;47:933–944. doi: 10.1080/21691401.2019.1581791. [DOI] [PubMed] [Google Scholar]
- Verma R., Kaushik D. Design and optimization of candesartan loaded self-nanoemulsifying drug delivery system for improving its dissolution rate and pharmacodynamic potential. Drug Delivery. 2020;27:756–771. doi: 10.1080/10717544.2020.1760961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerapol Y., Limmatvapirat S., Nunthanid J., Sriamornsak P. Self-nanoemulsifying drug delivery system of nifedipine: impact of hydrophilic-lipophilic balance and molecular structure of mixed surfactants. AAPS PharmSciTech. 2014;15:456–464. doi: 10.1208/s12249-014-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Zanela, Marques T., Santos-Oliveira R., de Oliveira Betzler, de Siqueira L., da Silva Cardoso V., Faria Maria, de Freitas Z., Barros R.C.S.A., Vazquez Villa A.L., Monteiro M.S.S.B., Pereira dos Santos E., Ricci-Junior E. Development and characterization of a nanoemulsion containing propranolol for topical delivery. IJN. 2018;13:2827–2837. doi: 10.2147/IJN.S164404. [DOI] [PMC free article] [PubMed] [Google Scholar]