Abstract
We evaluated the SARS-CoV-2-inactivation activity of ozonated glycerol (OG). When a viral solution with 1% fetal bovine serum (FBS) was mixed with test solutions at a ratio of 1:19 and incubated for 20 s, OG with ozone concentrations of over 1000 ppm inactivated ≥ 94.38% of the virus. Extension of the reaction time to 1 h led to the inactivation of ≥ 99.82% of the virus (the viral titer was below the detection limit). Extension to 24 h resulted in concentrations over 200 ppm OG inactivating ≥ 99.87% of the virus (the viral titers were below the detection limit). Next, viral solutions with 1, 20, and 40% FBS were mixed with test solutions at a ratio of 1:19 and incubated for 5 min. Whereas the virucidal activity of 500 ppm OG was very limited in the presence of 1% FBS (79.47% inactivation), it increased in the presence of 20 and 40% FBS (95.13 and 97.95% inactivation, respectively; the viral titers were not below the detection limit). Meanwhile, over 1000 ppm OG inactivated ≥ 99.44% of the virus regardless of the FBS concentration (the viral titers were below the detection limit). Extension of the reaction time to 1 h led to 500 ppm OG inactivating ≥ 99.91 and ≥ 99.95% of the virus with 20 and 40% FBS, respectively (the viral titers were below the detection limit). These results suggested that OG might be useful as a virucidal agent against SARS-CoV-2.
Keywords: Ozonated glycerol, Severe acute respiratory syndrome coronavirus 2, Antiviral material, Hand hygiene
Introduction
There has been a rapid worldwide increase in studies regarding control of the novel coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (WHO, 2021a). Since the main infection route of SARS-CoV-2 is droplet transmission, appropriate implementation of measures against it is needed to prevent COVID‐19 transmission. However, it is also possible to contract infections from the hands and environmental surfaces (Choi et al., 2021; Meyerowitz et al., 2021). The WHO recommends cleaning environmental surfaces and keeping hands clean, in addition to measures against droplet transmission (WHO, 2021b). Concomitantly, the need for antiviral agents for hand and environmental hygiene has been increasing. In this study, we focused on the biological activity of ozone. Ozone is a triatomic molecule consisting of 3 oxygen atoms, and has a short half-life, quickly converting into oxygen (Burns, 1997). Ozone is an oxidant that is highly corrosive to metals. High concentrations of ozone gas are harmful to humans and animals, and have been shown to impair the respiratory system when inhaled (Bartlett et al., 1974). However, low concentrations of ozone gas are not toxic, and an ozone and oxygen gas mixture has been used therapeutically (Bocci et al., 2011). Dissolution of ozone in water, creating ozonated water (OW), has been reported to improve both the effectiveness and safety of ozone (Hirai et al., 2019). However, ozone in OW has a shorter half-life than ozone gas (Rice & Gomez-Taylor, 1986). In particular, OW is known as a powerful inactivating agent against bacteria, fungi, protozoa, and viruses (Kim et al., 1999), and has been suggested to exert antimicrobial effects in running water (Nakamura et al., 2018). More recently, low concentrations of OW were shown to reduce viral infectivity upon exposure to SARS-CoV-2 for 1 min (Martins et al., 2021). In addition, it was reported that high ozone concentrations in OW rapidly inactivated SARS-CoV-2 (Inagaki et al., 2021).
As its name implies, ozonated glycerol (OG) is a glycerol gel containing ozone (Niinomi et al., 2004). More specifically, OG has been reported to have a long half-life (Niinomi et al., 2004), be safe for the skin, eyes, and oral mucosa (Fukui et al., 2015; Wang et al., 2011), as well as exert antibacterial (Niinomi et al., 2004; Fukui et al., 2014) and hemostatic (Sakai et al., 2014) effects.
In this study, we evaluated the ability of various concentrations of ozone in OG to inactivate SARS-CoV-2. Furthermore, we compared the viral-inactivating activity of OG in the presence of various fetal bovine serum (FBS) concentrations, which were used as a potentially interfering organic substance, and translated the obtained results for hand hygiene.
Materials and Methods
Test Samples
Glycerol (concentration ≥ 95%) was purchased from Sakamoto Yakuhin Kogyo Co., Ltd. (Osaka, Japan). For the preparation of OG samples, glycerol was ozonated using an Ozonizer (Mediplus Co., Tokyo, Japan), which produces ozone from medical grade oxygen. The maximal ozone concentration in OG was 2000 ppm. Glycerol (undiluted) was used as the solvent control for OG (2000 ppm). Then, OG samples (20, 200, 500, and 1000 ppm) were prepared by diluting the prepared OG (2000 ppm) with ultrapure water. Glycerol (100-times-, 10-times-, 4-times-, and 2-times-diluted) was also prepared by diluting glycerol with ultrapure water, and used as the solvent control for the OG sample (20, 200, 500, and 1000 ppm).
Virus and Cells
SARS-CoV-2 (JPN/TY/WK-521 strain) was kindly provided by the National Institute of Infectious Diseases (Tokyo, Japan). VeroE6/TMPRSS2 cells (cell number: JCRB1819) (Nao et al., 2019) were purchased from the Japanese Collection of Research Bioresources (Osaka, Japan). The virus growth medium (VGM) was Dulbecco’s modified Eagle’s medium (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) supplemented with 1% FBS, 2 mM l-glutamine (Fujifilm Wako Pure Chemical Co., Ltd., Osaka, Japan), 100 μg/mL kanamycin (Meiji Seika Pharma Co., Ltd., Tokyo, Japan), and 2 μg/mL amphotericin B (Bristol-Myers Squibb Co., New York, NY).
Evaluation of Virucidal Activities of Test Samples
SARS-CoV-2-containing VGM with a viral titer of ~ 7.0 log10 50% tissue culture infective dose (TCID50)/mL was supplemented with 1, 20, or 40% (v/v) FBS and mixed with test samples at viral-to-test sample ratios of 1:19. These mixtures were incubated for 20 s to 24 h at 22 °C and then inoculated into cells cultured in VGM containing 10 mM sodium thiosulfate, which quenches the ozone by reducing it. Then, the inoculated mixtures were diluted ten-fold in the cell culture medium (VGM) in the presence of sodium thiosulfate. After incubation for 3 d at 37 °C, the cytopathic effect was observed, and the viral titer (TCID50/mL) in each sample group was calculated using the Behrens–Kärber method (Kärber, 1931). Then the reduction in viral titer was calculated as log10 TCID50/mL of the control group subtract log10 TCID50/mL of the OG group. The means of multiple samples are presented in the Results section. The detection limit of the viral titer in each test sample group was determined based on the cytotoxicity of each test sample. The detection limit was set higher in the group treated with higher cytotoxicity test samples. As the 20 ppm OG sample did not show any cytotoxicity, the detection limit of the viral titer in the group treated with this sample was set to 1.25 log10 TCID50/mL, according to our viral titer calculation. Whereas, the rest of the OG samples (200, 500, 1000, and 2000 ppm) demonstrated cytotoxicity. The detection limit in the groups treated with these samples was set to 2.25 log10 TCID50/mL.
Statistical Analysis
The Student’s t test was performed to analyze the statistical significance of the differences in viral titer between the solvent control and OG groups. Statistical significance was set at p < 0.05.
Results
Evaluation of Virucidal Activity of Ozonated Glycerol
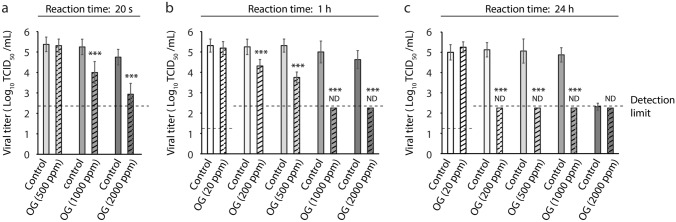
First, we evaluated the SARS-CoV-2-inactivating activity of OG samples (ozone concentrations: 500–2000 ppm) after 20 s of reaction time. We mixed the 1% FBS-containing viral solution with either solvent control glycerol or OG samples at a viral-to-test solution ratio of 1:19. We observed that, whereas, the viral titer in the 500 ppm OG group was comparable to that in the control group, the viral titers in the 1000 ppm and 2000 ppm OG-treated groups were lower than those of the control [OG (1000 ppm): 1.25 log10 TCID50/mL reduction compared with the control: 94.38% virus inactivation; OG (2000 ppm): ≥ 1.81 log10 TCID50/mL reduction: ≥ 98.46% virus inactivation] (Fig. 1a). We subsequently evaluated the virucidal activities of OG samples (20–2000 ppm) after 1 h of reaction time. Whereas the viral titer in the 20 ppm OG group was comparable to that in the control group, the 200 to 2000 ppm OG-treated groups showed concentration-dependent virucidal activity [OG (200 ppm): 0.94 log10 TCID50/mL reduction: 88.45% virus inactivation; OG (500 ppm): 1.56 log10 TCID50/mL reduction: 97.26% virus inactivation; OG (1000 ppm): ≥ 2.75 log10 TCID50/mL reduction: ≥ 99.82% virus inactivation; OG (2000 ppm): ≥ 2.28 log10 TCID50/mL reduction: ≥ 99.58% virus inactivation]. We further found that the viral titers in the 1000 ppm and 2000 ppm OG groups were below the detection limit (Fig. 1b). After 24 h of reaction time, we noticed that the viral titer in the 20 ppm OG group was comparable to that in the control group, whereas the viral titers in the 200 to 1000 ppm OG-treated groups were below the detection limit [OG (200 ppm): ≥ 2.88 log10 TCID50/mL reduction: ≥ 99.87% virus inactivation; OG (500 ppm): ≥ 2.81 log10 TCID50/mL reduction: ≥ 99.85% virus inactivation; OG (1000 ppm): ≥ 2.63 log10 TCID50/mL reduction: ≥ 99.76% virus inactivation]. As the viral titers in both the glycerol control, of which the virucidal activity is known (Cameron et al., 2000), and the 2000 ppm OG-treated groups were almost below the detection limit, we did not observe any statistically significant difference between these two groups (Fig. 1c).
Fig. 1.
Evaluation of virucidal activity of ozonated glycerol (OG). (a–c) Severe acute respiratory syndrome coronavirus 2 solution containing 1% fetal bovine serum was mixed with OG samples (ozone concentration: 20, 200, 500, 1000, and 2000 ppm) or solvent control glycerol at a viral-to-test sample ratio of 1:19. The viral titer of each reaction mixture was measured after 20 s (a), 1 h (b), or 24 h (c) reaction time. Results are presented as the mean ± SD (n = 8 per group). The detection limits of the viral titer were 1.25 log10 50% tissue culture infective dose (TCID50)/mL in the 20 ppm OG-treated groups, whereas it was 2.25 log10 TCID50/mL in the 200, 500, 1000, and 2000 ppm OG-treated groups. ND stands for not detected, indicating that the viral titer was below the detection limit. Student’s t test was performed to analyze the statistical significance between the solvent control and OG groups; ***p < 0.001
Impact of Fetal Bovine Serum Content in Viral Solution on Virucidal Activity of Ozonated Glycerol
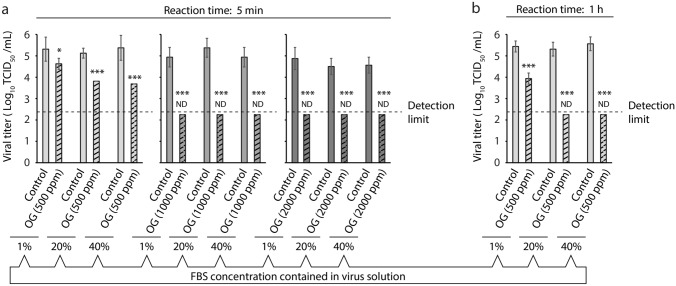
Next, we tested the virucidal activity of OG samples against SARS-CoV-2 solutions in different FBS concentrations (1, 20, and 40%). Viral solutions were mixed with either solvent control glycerol or OG samples at a viral-to-test solution ratio of 1:19. After 5 min of reaction time, we observed that the reduction in viral titer by the 500 ppm OG samples was proportional to the FBS content in the viral solution (1% FBS: 0.69 log10 TCID50/mL reduction compared with the control: 79.47% virus inactivation; 20% FBS: 1.31 log10 TCID50/mL reduction: 95.13% virus inactivation; 40% FBS: 1.69 log10 TCID50/mL reduction: 97.95% virus inactivation). In contrast, the viral titers in the 1000 ppm and 2000 ppm OG-treated groups were below the detection limit regardless of the FBS content in the viral solution (≥ 2.25 log10 TCID50/mL reduction: ≥ 99.44% virus inactivation) (Fig. 2a). We further found that after 1 h of reaction time, the viral titer in the 500 ppm OG-treated groups was not below the detection limit when the viral solution contained 1% FBS (1.50 log10 TCID50/mL reduction: 96.84% virus inactivation). We noticed that the viral titer in this group was below the detection limit when it contained 20 or 40% FBS (20% FBS: ≥ 3.06 log10 TCID50/mL reduction: ≥ 99.91% virus inactivation; 40% FBS: ≥ 3.31 log10 TCID50/mL reduction: ≥ 99.95% virus inactivation) (Fig. 2b).
Fig. 2.
Evaluation of the impact of fetal bovine serum (FBS) content in the viral solution on virucidal activity of ozonated glycerol (OG). (a, b) Severe acute respiratory syndrome coronavirus 2 solution containing 1, 20, or 40% FBS was mixed with OG samples (ozone concentration: 500, 1000, and 2000 ppm), or solvent control glycerol at a viral-to-test sample ratio of 1:19. The viral titer of each reaction mixture was measured after 5 min (a) or 1 h (b) reaction time. Results are indicated as the mean ± SD (n = 8 per group). The detection limit of the viral titer was 2.25 log10 50% tissue culture infective dose (TCID50)/mL. ND stands for not detected, indicating that the viral titer was below the detection limit. Student’s t test was performed to analyze the statistical significance between the solvent control and OG groups; *p < 0.05, ***p < 0.001
Discussion
The mechanism of inactivation of viruses by ozone is known to involve the destruction of viral spikes, envelope proteins, and lipids through the oxidation of viral proteins, lipoproteins, lipids, glycolipids, and glycoproteins (Rowen & Robins, 2020), resulting in potential inhibition of the attachment of the virus to the host receptor (Tizaoui, 2020). In the case of SARS-CoV-2 and other coronaviruses, the nucleocapsid is surrounded by an envelope consisting of a lipid bilayer, with the spike protein (S protein) being expressed on the surface of the viral particle. The S protein consists of 2 subunits: the S1 and S2 subunits. SARS-CoV-2 has been reported to initiate invasion into host cells after binding of the S1 subunit to the viral receptor (ACE2) on the cell membrane. The S protein is then cleaved into S1 and S2 subunits by a protease, thought to be furin, derived from host cells. The S2 subunit is further cleaved and primed by transmembrane serine protease 2 (TMPRSS2), a serine protease on the surface of host cells, resulting in the fusion of the viral envelope with the host cell membrane (Hoffmann et al., 2020). Recently, ozone was proposed to act on the S protein of coronavirus, preventing membrane fusion with host cells (Manjunath et al., 2021).
OW has recently been shown to reduce the infectivity of SARS-CoV-2 following exposure of the virus to low concentrations of OW for 1 min (Martins et al., 2021). In addition, 10 ppm OW, which is a high ozone concentration for OW, rapidly inactivates SARS-CoV-2 (Inagaki et al., 2020). While the ozone in OW is rapidly lost, OG maintains its bactericidal activity over a longer period. Almost no decrease was observed in its virucidal activity even when OG was left at 25 °C (room temperature) for 2 months after production (Niinomi et al., 2004). The viscosity of glycerol has been suggested to impede the release of ozone from OG into the air, and delay its decomposition owing to its stabilization by glycerol.
Disinfectants are generally affected by the presence of organic substances, such as blood and saliva (Kampf et al., 2005). In case the object to be sterilized contains a large amount of protein, such as blood, it is recommended to remove the protein by wiping or prewashing before exposure to OW (Hirai et al., 2019). In this study, we added excess FBS as an organic contaminant in the viral solution. We found that OG with high ozone concentrations strongly inactivated SARS-CoV-2 contaminated with 1–40% FBS. Notably, at high concentrations of 20 or 40% FBS, viral inactivation by 500 ppm OG samples was stronger than that with 1% FBS. It has been shown that the oxidizing ability of ozone is improved when used in combination with iron, ultraviolet rays, and hydrogen peroxide (Aziz & Amr, 2015). As the activity of ozone is known to be increased by activation factors such as iron ions contained in FBS, the inactivation activities of OG might have increased with increases in the concentration of FBS. As OG can contain much higher concentration of ozone than OW, which has been previously shown to exert SARS-CoV-2-inactivation activity, a sufficient amount of ozone would have remained even after reaction with excess amounts of organic substances. Hence, such a high concentration of residual ozone might have shown potent virucidal activities through not only a single function but also an additive function with an activation factor in FBS. Saliva contains lactoferrin, which contains iron ions. Therefore, OG is expected to potentially have the same inactivation activity on SARS-CoV-2 coexisting with saliva on environmental surfaces as that observed in this experiment.
It has been reported that SARS-CoV-2 is active on most contactable surfaces (Wu et al., 2020). It has also been pointed out that SARS-CoV-2 might survive for 9 h on the skin (Hirose et al., 2020). Although the main route of SARS-CoV-2 infection is droplet transmission, it is also possible to contract the infection from hands and environmental surfaces (Choi et al., 2021; Meyerowitz et al., 2021). Therefore, healthcare professionals need to encourage handwashing procedures to achieve robust SARS-CoV-2 control measures during the COVID-19 pandemic (The Lancet, 2020). Specifically, disinfection with 80% ethanol for 15 s was shown to completely inactivate SARS-CoV-2 on the skin, demonstrating the importance of hand hygiene for SARS-CoV-2 (Hirose et al., 2020). Most recently, a disinfectant containing ethyl alcohol, chloroxylenol, and quaternary ammonium compounds has been reported to inactivate SARS-CoV-2 (Ijaz et al., 2020). However, these disinfectants have been shown to cause skin irritation, and as handwashing measures have now been strengthened, healthcare professionals were reported to exhibit increased skin irritation (Erdem et al., 2020; Guertler et al., 2020; Lan et al., 2020). Recent studies have shown that OW is less irritating to the skin than traditional disinfectants, raising interest in OW as an alternative handwashing treatment (Breidablik et al., 2019; Kashiwazaki et al., 2020). Likewise, OG is also less irritating to the skin and has a longer ozone half-life than OW (Fukui et al., 2015; Wang et al., 2011).
We believe that OG, which showed an antiviral effect on SARS-CoV-2 even in the presence of high concentrations of organic substances, is a good candidate for safe disinfection procedures. OG is slower to inactivate viruses than ethanol. Thus, at this point, it is difficult to use OG as a substitute for ethanol. However, OG is less harmful to the skin than ethanol, and its effects are sustainable. Therefore, applying OG to the hands after washing may prevent infectious viruses from subsequently attaching to the hands for a long time. OG is also less irritating to mucous membranes than ethanol. To further expand the use of OG, we are currently investigating its application to mucous membranes such as the nasal and oral cavities.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by the Japanese Association for Dental Science, Sponsored Research 2020-A-1.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
The authors declared no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yohei Takeda, Email: ytakeda@obihiro.ac.jp.
Dulamjav Jamsransuren, Email: duuya.dj@gmail.com.
Yoshimasa Makita, Email: makita@cc.osaka-dent.ac.jp.
Akihiro Kaneko, Email: kaneko@ikegamihosp.jp.
Sachiko Matsuda, Email: chaka@obihiro.ac.jp.
Haruko Ogawa, Email: hogawa@obihiro.ac.jp.
Hourei Oh, Email: ohoh@cc.osaka-dent.ac.jp.
References
- Aziz HA, Amr SSA. Performance of combined ozone and Fenton process in treating different leachate concentrations. IAFOR Journal of Sustainability, Energy and the Environment. 2015;2(1):3–20. doi: 10.22492/ijsee.2.1.01. [DOI] [Google Scholar]
- Bartlett D, Jr, Faulkner CS, 2nd, Cook K. Effect of chronic ozone exposure on lung elasticity in young rats. Journal of Applied Physiology. 1974;37(1):92–96. doi: 10.1152/jappl.1974.37.1.92. [DOI] [PubMed] [Google Scholar]
- Bocci V, Zanardi I, Travagli V. Has oxygen-ozonetherapy a future in medicine? Journal of Experimental and Integrative Medicine. 2011;1(1):5–11. doi: 10.5455/jeim.161210.ir.002. [DOI] [Google Scholar]
- Breidablik HJ, Lysebo DE, Johannessen L, Skare Å, Andersen JR, Kleiven OT. Ozonized water as an alternative to alcohol-based hand disinfection. The Journal of Hospital Infection. 2019;102(4):419–424. doi: 10.1016/j.jhin.2019.01.026. [DOI] [PubMed] [Google Scholar]
- Burns D. Early problems in the analysis and the determination of ozone. Fresenius’ Journal of Analytical Chemistry. 1997;357:178–183. doi: 10.1007/s002160050133. [DOI] [Google Scholar]
- Cameron PU, Pagnon JC, van Baare J, Reece JC, Vardaxis NJ, Crowe SM. Efficacy and kinetics of glycerol inactivation of HIV-1 in split skin grafts. Journal of Medical Virology. 2000;60(2):182–188. doi: 10.1002/(SICI)1096-9071(200002)60:2<182::AID-JMV13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Choi H, Chatterjee P, Coppin JD, Martel JA, Hwang M, Jinadatha C, Sharma VK. Current understanding of the surface contamination and contact transmission of SARS-CoV-2 in healthcare settings. Environmental Chemistry Letters. 2021;19:1935–1944. doi: 10.1007/s10311-021-01186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem Y, Altunay IK, Aksu Çerman A, Inal S, Ugurer E, Sivaz O, Kaya HE, Gulsunay IE, Sekerlisoy G, Vural O, Özkaya E. The risk of hand eczema in healthcare workers during the COVID-19 pandemic: Do we need specific attention or prevention strategies? Contact Dermatitis. 2020;83(5):422–423. doi: 10.1111/cod.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui T, Masuno K, Makita Y, Fujiwara S, Shiota G, Imamura Y, Shiba A, Wang PL. Evaluation of oral mucosa irritation produced by ozone gel. Journal of Hard Tissue Biology. 2015;24(1):104–106. doi: 10.2485/jhtb.24.104. [DOI] [Google Scholar]
- Fukui T, Masuno K, Makita Y, Fujiwara S, Shiota G, Imamura Y, Shiba A, Wang P. Antimicrobial effects of ozone gel against periodontal bacteria. Journal of Hard Tissue Biology. 2014;23(4):445–448. doi: 10.2485/jhtb.23.445. [DOI] [Google Scholar]
- Guertler A, Moellhoff N, Schenck TL, Hagen CS, Kendziora B, Giunta RE, French LE, Reinholz M. Onset of occupational hand eczema among healthcare workers during the SARS-CoV-2 pandemic: Comparing a single surgical site with a COVID-19 intensive care unit. Contact Dermatitis. 2020;83(2):108–114. doi: 10.1111/cod.13618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K, Ando N, Komada H, Sounai A, Murakami A, Nakayama H. Investigation of the effective concentration of ozonated water for disinfection in the presence of protein contaminants. Biocontrol Science. 2019;24(3):155–160. doi: 10.4265/bio.24.155. [DOI] [PubMed] [Google Scholar]
- Hirose R, Ikegaya H, Naito Y, Watanabe N, Yoshida T, Bandou R, Daidoji T, Itoh Y, Nakaya T. Survival of SARS-CoV-2 and influenza virus on the human skin: Importance of hand hygiene in COVID-19. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz MK, Whitehead K, Srinivasan V, McKinney J, Rubino JR, Ripley M, Jones C, Nims RW, Charlesworth B. Microbicidal actives with virucidal efficacy against SARS-CoV-2. American Journal of Infection Control. 2020;48(8):972–973. doi: 10.1016/j.ajic.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki H, Saito A, Sudaryatma PE, Sugiyama H, Okabayashi T, Shouichi F. Rapid inactivation of SARS-CoV-2 with ozonated water. Ozone Science & Engineering. 2021;43(3):208–212. doi: 10.1080/01919512.2021.1889318. [DOI] [Google Scholar]
- Kampf G, Grotheer D, Steinmann J. Efficacy of three ethanol-based hand rubs against feline calicivirus, a surrogate virus for norovirus. The Journal of Hospital Infection. 2005;60(2):144–149. doi: 10.1016/j.jhin.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Kärber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn-Schmiedebergs Archiv Für Experimentelle Pathologie Und Pharmakologie. 1931;162:480–483. doi: 10.1007/BF01863914. [DOI] [Google Scholar]
- Kashiwazaki J, Nakamura K, Hara Y, Harada R, Wada I, Kanemitsu K. Evaluation of the cytotoxicity of various hand disinfectants and ozonated water to human keratinocytes in a cultured epidermal model. Advances in Skin & Wound Care. 2020;33(6):313–318. doi: 10.1097/01.ASW.0000658592.51430.ea. [DOI] [PubMed] [Google Scholar]
- Kim JG, Yousef AE, Dave S. Application of ozone for enhancing the microbiological safety and quality of foods: A review. Journal of Food Protection. 1999;62(9):1071–1087. doi: 10.4315/0362-028x-62.9.1071. [DOI] [PubMed] [Google Scholar]
- Lan J, Song Z, Miao X, Li H, Li Y, Dong L, Yang J, An X, Zhang Y, Yang L, Zhou N, Yang L, Li J, Cao J, Wang J, Tao J. Skin damage among health care workers managing coronavirus disease-2019. Journal of the American Academy of Dermatology. 2020;82(5):1215–1216. doi: 10.1016/j.jaad.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath SN, Sakar M, Katapadi M, Geetha Balakrishna R. Recent case studies on the use of ozone to combat coronavirus: Problems and perspectives. Environmental Technology & Innovation. 2021;21:101313. doi: 10.1016/j.eti.2020.101313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins RB, Castro IA, Pontelli M, Souza JP, Lima TM, Melo SR, Siqueira JPZ, Caetano MH, Arruda E, de Almeida MTG. SARS-CoV-2 inactivation by ozonated water: a preliminary alternative for environmental disinfection. Ozone Science & Engineering. 2021;43(2):108–111. doi: 10.1080/01919512.2020.1842998. [DOI] [Google Scholar]
- Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: A review of viral, host, and environmental factors. Annals of Internal Medicine. 2021;174(1):69–79. doi: 10.7326/M20-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Saito K, Kashiwazaki J, Aoyagi T, Arai K, Hara Y, Kobari S, Mori H, Ohashi K, Takano Y, Kaku M, Kanemitsu K. Evaluation of ozonated water using ASTM E1174 for standardized testing of handwash formulations for healthcare personnel. The Journal of Hospital Infection. 2018;100(2):211–213. doi: 10.1016/j.jhin.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Nao N, Sato K, Yamagishi J, Tahara M, Nakatsu Y, Seki F, Katoh H, Ohnuma A, Shirogane Y, Hayashi M, Suzuki T, Kikuta H, Nishimura H, Takeda M. Consensus and variations in cell line specificity among human metapneumovirus strains. PLoS ONE. 2019;14(4):e0215822. doi: 10.1371/journal.pone.0215822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinomi K, Kanaishi A, Akihiko S. The bactericidal effects of ozone gel. The Journal of Showa University Dental Society. 2004;24(2):103–109. [Google Scholar]
- Rice RG, Gomez-Taylor M. Occurrence of by-products of strong oxidants reacting with drinking water contaminants–scope of the problem. Environmental Health Perspectives. 1986;69:31–44. doi: 10.1289/ehp.866931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowen RJ, Robins H. A plausible “penny” costing effective treatment for corona virus: Ozone therapy. Journal of Infectious Diseases and Epidemiology. 2020;6(2):113. [Google Scholar]
- Sakai D, Makita Y, Masuno K, Fujiwara S, Okazaki J, Wang P. Local hemostatic effect of aqueous ozone in cutting wound surface. Journal of Hard Tissue Biology. 2014;23(2):245–248. doi: 10.2485/jhtb.23.245. [DOI] [Google Scholar]
- The Lancet COVID-19: Protecting health-care workers. Lancet (london, England) 2020;395(10228):922. doi: 10.1016/S0140-6736(20)30644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizaoui C. Ozone: a potential oxidant for COVID-19 virus (SARS-CoV-2) Ozone Science & Engineering. 2020;42(5):378–385. doi: 10.1080/01919512.2020.1795614. [DOI] [Google Scholar]
- Wang PL, Shiota G, Shiba A. Safety evaluation of ozone gel for skin and eye on animal experiments. Journal of Hard Tissue Biology. 2011;20(4):313–318. doi: 10.2485/jhtb.20.313. [DOI] [Google Scholar]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Retrieved April 20, 2021a, from http://covid19.who.int/
- World Health Organization. (2020, October 20). Coronavirus disease (COVID-19): How is it transmitted? Retrieved April 20, 2021b, from https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-how-is-it-transmitted
- Wu S, Wang Y, Jin X, Tian J, Liu J, Mao Y. Environmental contamination by SARS-CoV-2 in a designated hospital for coronavirus disease 2019. American Journal of Infection Control. 2020;48(8):910–914. doi: 10.1016/j.ajic.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.