Abstract
In this issue, Aboulouard et al. (2021) use spatially resolved shot-gun proteomics to characterize the sentinel lymph nodes (SLNs) of endometrial cancer patients at different pathological grades. They identify biomarkers that predict prognosis as well as tumor grade.
In this issue, Aboulouard et al. (2021) use spatially resolved shot-gun proteomics to characterize the sentinel lymph nodes (SLNs) of endometrial cancer patients at different pathological grades. They identify biomarkers that predict prognosis as well as tumor grade.
Main text
Endometrial cancer constitutes one of the most prevalent gynecological malignancies worldwide. In the United States, endometrial cancer is rising in both incidence and mortality.1 In this issue of Cell Reports Medicine, Aboulouard et al. use spatially-resolved shot-gun proteomics to offer a proteomic landscape of sentinel lymph nodes (SLNs) in patients with endometrial cancer at different pathological grades.2
The standardized treatment for endometrial cancer primarily involves surgical removal and chemotherapy for high-risk patients. Surgical procedures have evolved for endometrial cancer and now include SLN biopsy, along with hysterectomy and removal of fallopian tubes and ovaries. As the surgical area of SLN biopsy is much smaller and more specific compared with systemic lymphadenectomy, use of SLN biopsy has dramatically decreased the incidence of surgical complications, including severe damage of major vessels, destruction of important nerves, and occurrence of lymphocele. It also offers greater safety for those with internal complications or for elderly patients, as SLN biopsy remarkably shortens surgical time. According to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for Uterine Neoplasms 2020,3 SLN mapping should be considered for surgical staging of obvious uterine–confined tumors with no evidence of metastasis or extra-uterine disease, which includes primary endometrial cancer that also invades into the extra-uterine space. For low-risk subgroups, SLN biopsy is a feasible diagnostic means for surgical staging. While macro-metastasis of lymph nodes for patients with endometrial cancer is clearly associated with poor prognosis, the clinical significance of identified low-volume lymph node metastasis (isolated tumor cells and micro-metastasis) is relatively unclear. Moreover, the molecular feature of SLNs for endometrial cancer patients has not been characterized.
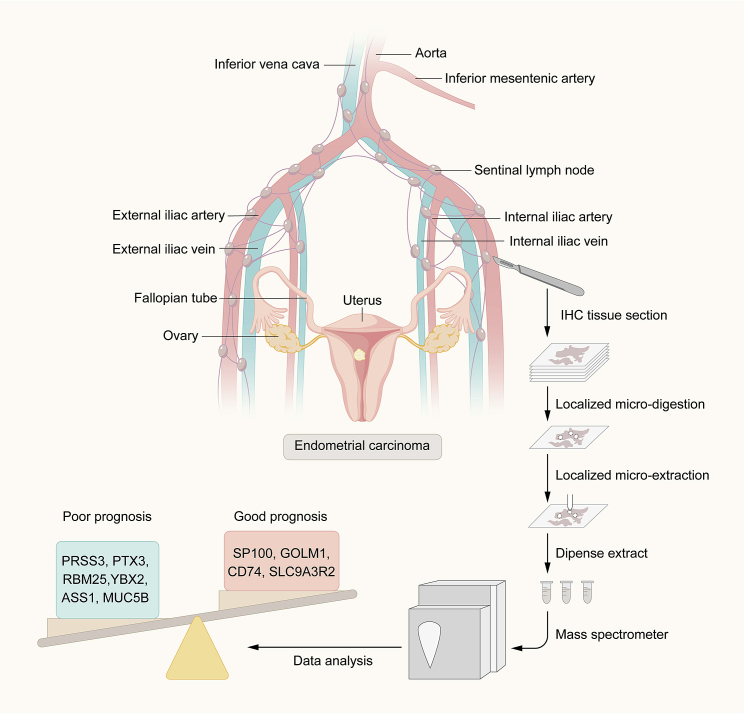
In this study, researchers perform spatially resolved shot-gun proteomics on selected regions of SLN samples from endometrial cancer patients with pathological grades ranging from I to III.2 Expression of specific markers (including PRSS3, PTX3, ASS1, YBX2, RBM25, and MUC5B) in endometrial cancer SLN specimens is associated with poor prognosis. SLN biomarkers associated with good prognosis and improved overall survival include CD74, GOLM1, SLC9A3R2, and SP100 proteins in endometrial cancer patients (Figure 1). The authors validated these biomarkers in an external TCGA cohort and by immunohistochemistry analysis using tissue microarray (TMA). In addition, they identify specific mutated proteins and ghost proteins translated from the non-coding region of mRNA or from non-coding RNA. The study further identifies biomarkers specific for endometrial cancer and SLN cancer-grading and prognosis. In summary, PRSS3, PTX3, and ASS1 are associated with a poor prognosis, and ALDH2 and ANX1 are correlated with a positive outcome (Figure 1). This study represents a significant advance for endometrial cancer, as it reports biomarkers that enable stratification of SLN-positive endometrial cancer patients into prognostic groups.
Figure 1.
A schematic model for spatially resolved proteomics to dissect SLNs of endometrial cancer and specific biomarkers associated with clinical prognosis identified
Currently, the majority of investigations of molecular biomarkers of endometrial cancer SLNs are limited to a one-step nucleic acid amplification (OSNA) assay to detect known cancer-related proteins, such as CK19.4 The current investigation provides the first global proteomic view of endometrial cancer SLNs and validates their clinical significance. In addition, the authors have applied a pioneering technology, termed “spatially resolved proteomics,” to globally characterize the proteome of endometrial cancer SLNs with different pathological grades. Spatially resolved proteomics enables characterization of the proteome in situ preserving spatial information, thus offering a powerful tool to dissect intra-tumoral heterogeneity.5
Apart from this study, there have been several previous investigations using spatially resolved proteomics to explore cancer biology. For instance, Xu et al. combined laser capture microdissection (LCM) and integrated the proteomics sample preparation technology SISPROT to dissect specific cell types in tumor samples with single-cell resolution and process for high-sensitivity proteome profiling.6 They identified over 500 proteins from only 0.1 mm2 and 10 μm thickness colon cancer tissue sections and obtained proteome profiling of four cell types from one colon tumor and surrounding normal tissue. In another study, Ombrato and colleagues used a labeling system in which metastatic cancer cells releasing a cell-penetrating fluorescent protein to enable spatial proteomic identification of the local metastatic cellular environment.7 Using spatially resolved proteomics, they studied the proteome hallmarks of different cellular components of metastatic breast cancer cells in the lung and found the presence of cancer-associated parenchymal cells with stem-cell-like features. Thus, one could anticipate this fluorescent labeling proteomics analysis strategy could be applied to contexts beyond cancer.
Even though SLN biopsy has entered the central stage in the treatment of gynecological malignancies, including endometrial cancer, major questions remain. There are prospective cohort study results (e.g., the SENTOR study)8 that indicate that SLN biopsy has an acceptable diagnostic accuracy for patients with high-grade endometrial cancer at increased risk of nodal metastases and also enhances the detection of node-positive cases compared with lymphadenectomy. However, future randomized clinical trials are needed to evaluate whether SLN biopsy is a viable option for all patients with endometrial cancer who need surgical staging. Explicit biomarkers for prognosis prediction and therapy stratification (either SLN biopsy or systematic lymphadenectomy) will make SLN biopsy evaluation trials more informative. The question of whether additional chemo- or radio-therapy could benefit those with positive SLN biopsy results remains unanswered. Finding specific biomarkers predicative of successful additional treatment for SLN-positive endometrial cancer patients should be a future research focus for gynecological oncologists, pathologists, and translational biomedicine scientists.
References
- 1.Lu K.H., Broaddus R.R. Endometrial Cancer. N. Engl. J. Med. 2020;383:2053–2064. doi: 10.1056/NEJMra1514010. [DOI] [PubMed] [Google Scholar]
- 2.Aboulouard S., Wisztorski M., Duhamel M., Saudemont P., Cardon T., Narducci F., Lemaire A.-S., Kobeissy F., Leblanc E., Fournier I. Sentinel lymph node and endometrial cancer grade in-depth proteomic analyses: a novel approach for molecular diagnosis. Cell Rep. Med. 2021:100318-1–100318-14. doi: 10.1016/j.xcrm.2021.100318. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network . 2020. Uterine neoplasms, version 1.https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf [DOI] [PubMed] [Google Scholar]
- 4.Fanfani F., Monterossi G., Di Meo M.L., La Fera E., Dell’Orto F., Gioè A., Lamanna M., Ferrari D., De Ponti E., Perego P. Standard ultra-staging compared to one-step nucleic acid amplification for the detection of sentinel lymph node metastasis in endometrial cancer patients: a retrospective cohort comparison. Int. J. Gynecol. Cancer. 2020;30:372–377. doi: 10.1136/ijgc-2019-000937. [DOI] [PubMed] [Google Scholar]
- 5.Crosetto N., Bienko M., van Oudenaarden A. Spatially resolved transcriptomics and beyond. Nat. Rev. Genet. 2015;16:57–66. doi: 10.1038/nrg3832. [DOI] [PubMed] [Google Scholar]
- 6.Xu R., Tang J., Deng Q., He W., Sun X., Xia L., Cheng Z., He L., You S., Hu J. Spatial-resolution cell type proteome profiling of cancer tissue by fully integrated proteomics technology. Anal. Chem. 2018;90:5879–5886. doi: 10.1021/acs.analchem.8b00596. [DOI] [PubMed] [Google Scholar]
- 7.Ombrato L., Nolan E., Kurelac I., Mavousian A., Bridgeman V.L., Heinze I., Chakravarty P., Horswell S., Gonzalez-Gualda E., Matacchione G. Metastatic-niche labelling reveals parenchymal cells with stem features. Nature. 2019;572:603–608. doi: 10.1038/s41586-019-1487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cusimano M.C., Vicus D., Pulman K., Maganti M., Bernardini M.Q., Bouchard-Fortier G., Laframboise S., May T., Hogen L.F., Covens A.L. Assessment of sentinel lymph node biopsy vs lymphadenectomy for intermediate- and high-grade endometrial cancer staging. JAMA Surg. 2021;156:157–164. doi: 10.1001/jamasurg.2020.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]