Abstract
Background: The burden and impact of premenstrual syndrome (PMS) and premenstrual dysphoric disorder (PMDD) is not well characterised among Indian population. Therefore, we conducted this systematic review and meta-analysis to estimate the prevalence of PMS and PMDD among females of reproductive age group living in India.
Methods: We searched PubMed, Cochrane Library, Scopus and IndMed for studies reporting the prevalence of PMS and/ or PMDD from any part of India, published from 2000 up to Aug 2020. We performed random-effects meta-analyses evaluated using I2 statistic, subgroup analyses, sensitivity analyses and assessed study quality. Estimated prevalence along with 95% confidence intervals (CIs) were reported for each outcome of interest. The quality of each study was evaluated using modified Newcastle Ottawa Scale (NOS). This review was conducted following the standard of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines. The protocol was registered prospectively in PROSPERO (CRD42020199787).
Results: Our search identified 524 citations in total, of which 25 studies (22 reported PMS, and 11 reported PMDD) with 8542 participants were finally included. The pooled prevalence of PMS and PMDD were 43% (95% CI: 0.35-0.50) and 8% (95% CI: 0.60-0.10) respectively. The estimated prevalence of PMS in adolescence was higher and account to be 49.6% (95% CI: 0.40-0.59). The heterogeneity for all the estimates was very high and could be explained through several factors involved within and between studies.
Conclusion: This study identified a substantially high prevalence of PMS and PMDD in India. To identify potentially related factors, more focused epidemiological research is warranted. However, noticing the fact of significant prevalence and its potential impact on the population, stakeholders and policymakers need to address this problem at the community and individual level.
Keywords: Premenstrual syndrome, Premenstrual dysphoric disorder, Prevalence, Systematic review, Meta-Analysis
Introduction
Premenstrual disorders occur during the luteal phase of the menstrual cycle and resolve shortly following menstruation. The luteal phase lasts from ovulation to the start of menstruation. Premenstrual disorders are diagnosed depending upon whether the diagnostic criteria followed are from the American College of Obstetricians and Gynaecologists (ACOG)1 or from the Diagnostic and Statistical Manual – fifth edition (DSM-5) of the American Psychiatric Association (APA).2 Whereas ACOG requires the presence of at least one affective symptom (e.g. angry outbursts, anxiety, confusion, depression, irritability or social withdrawal) and one somatic symptom (e.g. abdominal bloating, breast tenderness or swelling, headache, joint or muscle pain, swelling of extremities or weight gain) for a diagnosis of premenstrual syndrome (PMS)1, APA requires only the presence of somatic symptoms.2 As for premenstrual dysphoric disorder (PMDD), DSM-5 requires the presence of five symptoms in total, with at least one affective symptom (mood swings, marked irritability, marked depressed mood or marked anxiety) along with other symptoms, which may include somatic symptoms. Certain duration criteria for the symptoms need to be met too.2 Symptoms may appear anytime between menarche and menopause. Premenstrual disorders cause significant distress or interfere with work, school or usual social activities and lower quality of life.3 These disorders are treatable – selective serotonin reuptake inhibitors (SSRIs) such as sertraline, paroxetine, fluoxetine and escitalopram have been shown to treat both the psychiatric as well as physical symptoms4; other medications that have shown benefit include quetiapine (as an adjunct to an SSRI),5 oral contraceptives6 and calcium supplementation.7 Among non-pharmacological treatments, evidence suggests that cognitive behaviour therapy may be helpful.8
Although premenstrual disorders are not culture-bound syndromes, cultural factors play an important role in the frequency, intensity and expressivity of symptoms and help-seeking patterns.2
The reported prevalence estimates of PMS in India have ranged from 14.3%9 to 74.4%.10 Similarly, the reported prevalence of PMDD in India has varied widely between 3.7%11 to 65.7%.12 Factors influencing prevalence estimates include diagnostic criteria or tools used as well as socio-demographic and sub-cultural differences within a diverse country such as India that impact expressivity of symptoms.
Owing to the more or less taboo nature of menstruation in conservative societies as in India, coupled with the traditional gender role subscribed to by females, awareness regarding premenstrual disorders and/or help-seeking behaviour for these disorders has been sub-optimal. Given the treatability of these conditions, proper health policy formulation and implementation to address premenstrual disorders can go a long way in reducing the treatment gap. However, a prerequisite for appropriate policy formulation includes availability of high quality information on which to base effective policy. A meta-analysis of existing studies is a proven method of providing high quality scientific evidence.
To the best of our knowledge, this is the first meta-analysis of prevalence studies of premenstrual disorders in India. The purpose of this study is to provide a better understanding of the epidemiology of PMS and PMDD in India, highlighting inter-regional differences in prevalence, so as to sensitise practising health professionals as well as enabling health planners to allocate scarce health resources commensurate to the scale of the problem.
Materials and Methods
This systematic review has been conducted to know the estimated prevalence of PMS and PMDD among females of reproductive age group in India. The whole review process has been conducted and narrated in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA),13 and Meta-Analysis of Observational Studies in Epidemiology (MOOSE)14 guidelines. The protocol was registered prospectively in International prospective register of systematic reviews (PROSPERO), the Centre for Reviews and Dissemination, University of York (CRD42020199787),15 prior to the commencement of the study.
Search strategy
Four electronic databases namely, PubMed (US National Library of Medicine, National Institutes of Health), Cochrane library, Scopus and IndMed were searched for potential studies from inception up to August 2020. The search was not restricted to the language or publication date. Final search strategy was decided by consensus among the authors.
The following search strategy was used to identify studies in PubMed: (“Premenstrual Syndrome”[Title/Abstract] OR premenstrual syndrome[MeSH Terms] OR premenstrual syndromes[MeSH Terms] OR premenstrual tension[MeSH Terms] OR syndrome, premenstrual[MeSH Terms] OR “prevalence of Premenstrual Syndrome”[Title/Abstract] OR Premenstrual dysphoric disorder[Title/Abstract]) AND (prevalence[Title/Abstract] OR prevalence[MeSH Terms] OR analysis, cross sectional[MeSH Terms] OR “Cross sectional”[Title/Abstract] OR Observational[Title/Abstract] OR “Case control”[Title/Abstract] OR Cohort[Title/Abstract]) AND (Indi*[Title/Abstract] OR “Indian state”[Title/Abstract]). Similar search terms were used in combination for Cochrane library. For Scopus and IndMed, only free text searches with or without truncations were used. We did not impose any language or date restrictions and filters while running the search. Major Indian journals on the concerned topic were also consulted to identify any additional, relevant studies. Finally, the snow balling method of search was adopted through the screening of bibliographic list of relevant papers for any additional, relevant studies.
Selection of studies
Two reviewers (AD & AS) were independently involved in screening of the retrieved studies for titles and abstracts. After the preliminary screening, full texts of the relevant studies were examined according to the following eligibility criteria:
Studies primarily reporting extractable prevalence data of PMS or PMDD
Studies conducted in any part of India and not on mixed population.
Study types included cross-sectional studies, case-control studies or cohort studies (only baseline data were analysed)
Studies published after the year 2000 and reporting the population being studied adequately.
We excluded editorials, letters, commentaries, studies with inadequate data, reviews, posters, and interventional studies, preprint documents and grey literature (including theses).
Any confusion or discrepancy regarding the selection of studies was resolved by consensus between the authors. If a single study was reported in multiple publications, then only the one with comparatively better quality and larger sample size was included for analysis.
Quality assessment
All the retrieved studies were evaluated for methodological quality by two reviewers (AD & AS) independently, using a modified version of Newcastle Ottawa Scale (NOS).16 We considered five domains (1 point each) for assessing quality of each study: “sample representativeness,” “sample size,” “ascertainment of Premenstrual syndrome,” “comparability between respondents and non-respondents,” and “statistical quality”.
According to the resultant number of points assigned, each study was judged to be at high (≥ 3 points) or low (< 3 points) quality. Any discrepancies concerning the author’s judgments were resolved by consensus.
Data extraction
One reviewer extracted the data (AD), which was cross-checked by the other (AS). Following data were extracted in a standardised excel sheet for each eligible study: Author, year of publication, study region, time frame of data collection, age range of the study participants, study population, diagnosis, diagnosis criteria or tools used, sample size and reported prevalence. In case of incomplete or incomprehensible data, the concerned author was contacted for clarification and non-response was considered as drop-out. Any disagreements between the authors were resolved by discussion.
Statistical analysis
Meta-analysis was carried out to estimate the prevalence pooled from individual studies. Summary estimates were reported along with their 95% CIs, for both PMS and PMDD. Heterogeneity between studies was quantified with the I2 statistic. In general, I2 values of 25%, 50% and 75% are considered as belonging to, low, moderate and high category of heterogeneity respectively.17 According to the presence of heterogeneity between prevalence studies, we used fixed or random effect model following DerSimonian and Laird’s method.
Subgroup analyses according to the States/UTs, quality of the studies (high vs. low), and population (adolescent vs. mixed population) were carried out. We also ran sensitivity analysis to evaluate the effect of individual studies on the pooled estimates. All the analyses were done using ‘metafor’ and‘metaprop’ package in ‘R’ software (https://cran.r-project.org/). Presence of publication bias was evaluated through visual inspection and rank correlation test for any asymmetry present in the funnel plot.
Results
Search results and study characteristics
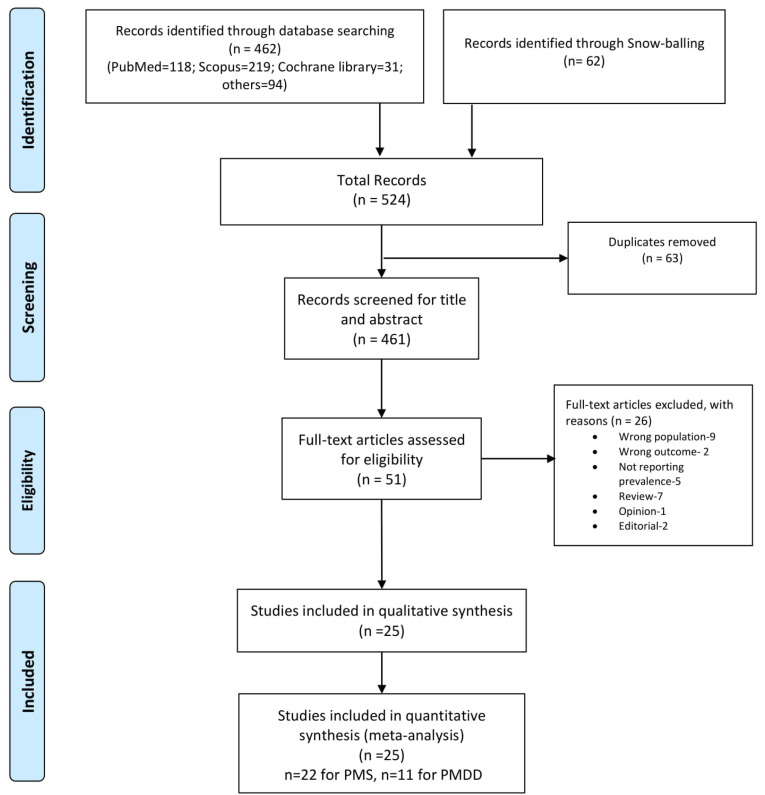
The final search yielded 524 citations including snowballing; following the removal of 63 duplicate records, 431 abstracts were screened for potential eligibility. Of remaining 51 studies (full-text screening), 26 were excluded on the basis of specific article types and eligibility. Finally 25 studies were included for the systematic review and meta-analysis. The study selection process is depicted in detailed manner in Figure 1.
Figure 1.
Study selection process.
Among 25 included studies, all were of cross-sectional design and reported from different states at varied number and time points. Very few studies reported community-based prevalence, mostly reporting prevalence from school or college settings. Collective number population was 8542; sample size ranged from 60 to 1281, with a median of n=224. We found only three studies published before 2011, and four studies on adolescence age group (if we strictly consider adolescents up to the age of 19). Three studies exclusively reported PMDD, whereas eight evaluated both PMS and PMDD; 22 were exclusively on the prevalence of PMS. A detailed description of included studies is provided in Table 1.
Table 1. Summary of included studies .
| Author | Year | Place (State/UT) | Data collection period | Age (y) | Population | Diagnosis | Scale used/criteria | Population studied (N) |
PMS
No. (%) |
PMDD
No. (%) |
| Sharma et al18 | 2008 | Delhi | - | 13-19 | Adolescents | PMS | Pre-tested, semi-structured questionnaire | 198 | 125 (63.13) | - |
| Sharma et al19 | 2008 | Delhi | - | 20-23 | Unmarried undergraduate medical students | PMS | 12 Symptoms of PMS | 100 | 67 (67) | - |
| Ray et al20 | 2010 | West Bengal | - | 10-19 | Adolescents | PMS | Pre-tested questionnaire | 715 | 303 (42.38) | - |
| Sharma et al21 | 2013 | Himachal Pradesh | - | 20–50 | Adult women | PMS | PMS (A,D,C,H,O) | 105 | 48 (45.71) | - |
| Brahmbhatt et al22 | 2013 | Gujrat | - | Medical and nursing students, teaching and non-teaching staffs |
PMS | Structured questionnaire developed for PMS | 100 | 42 (42) | - | |
| Mandal et al23 | 2015 | West Bengal | Jul-Aug, 2014 | 13-19 | Adolescents | PMS | ACOG criteria | 278 | 150 (53.96) | - |
| Sarkar et al24 | 2015 | West Bengal | Jul-Aug, 2015 | 13–21 | Adolescents | PMS | ACOG criteria | 244 | 150 (61.48) | - |
| Mishra et al25 | 2015 | Gujrat | - | 19–28 | UG and PG Medical students | PMDD | SPAF; SSQ-PMDD | 100 | - | 37 (37) |
| Kavitha et al10 | 2015 | Tamil Nadu | Feb- Apr, 2015 | - | Medical students | PMS | DSM-IV | 90 | 67 (74.44) | - |
| Joseph et al26 | 2016 | Kerala | - | 18-20 | Nursing students | PMS | Modified standardized premenstrual syndrome scale | 60 | 9 (15) | - |
| Raval et al11 | 2016 | Gujrat | Jan- Aug, 2012 | 17–30 | College students | PMS and PMDD | PSST; SCID-PMDD | 489 | 72 (14.72) | 18 (3.68) |
| Rumana et al27 | 2017 | Karnataka | - | up to 25 | Medical students | PMS | PEQ | 270 | 84 (31.11) | - |
| Abirami and Ambika28 | 2017 | Tamil Nadu | Jan 5-21, 2015 | 17-26 | Nursing students | PMS | Structured questionnaire developed for PMS | 100 | 74 (74) | - |
| Negi et al29 | 2018 | Himachal Pradesh | - | 13-19 | Adolescents | PMS | 470 | 190 (40.43) | - | |
| Shenuka et al30 | 2018 | Tamil Nadu | May- Oct, 2017 | 17-27 | Healthcare and non-healthcare students | PMS and PMDD | PMS (A,D,C,H,O) and PSST | 478 | 204 (42.68) | 19 (3.97) |
| Badkur et al31 | 2018 | Madhya Pradesh | Dec- Jun, 2015 | 18-25 | College students | PMS | ACOG criteria | 101 | 40 (39.6) | - |
| Budarapu et al32 | 2018 | Andhra Pradesh | - | - | Medical students | PMS and PMDD | PSST | 635 | 177 (27.87) | 88 (13.86) |
| Kamat et al33 | 2019 | Gujrat | - | 10–23 | College and school students | PMS AND PMDD | PSST-A | 1281 | 243 (18.97) | 64 (5) |
| Bhuvaneswari et al12 | 2019 | Puducherry | - | 18-22 | College students | PMS and PMDD | SPAF for PMS | 300 | 188 (62.67) | 197 (65.67) |
| Laxmi et al34 | 2019 | Andhra Pradesh | - | 17-24 | Nursing students | PMS | Structured questionnaire containing 47 questions of PMS | 133 | 61 (45.86) | - |
| Durairaj et al9 | 2019 | Tamil Nadu | - | 17-25 | Medical and engineering students |
PMS and PMDD | PSST | 1047 | 150 (14.33) | 39 (3.72) |
| Srikanth and Nandini35 | 2019 | Andhra Pradesh | - | Medical students | PMS and PMDD | PSST | 100 | 30 (30) | 5 (5) | |
| Bansal et al36 | 2019 | Karnataka | Sept 2017- Aug 2018 | 16-30 | College students | PMS and PMDD | PSST | 571 | 205 (35.9) | 58 (10.16) |
| Koganti et al37 | 2020 | Telengana | Sept- Dec 2019 | 18-25 | Medical students | PMDD | DSR | 180 | - | 20 (11.11) |
| Gupta et al38 | 2020 | Chandigarh | - | 11–20 | School Students | PMDD | PMDD scale | 397 | - | 19 (4.79) |
UT: Union Territory; ACOG: American College of Obstetricians and Gynaecologists; DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision; DSR: Penn State Daily Symptom Report; PEQ: PMS self-Evaluation Questionnaire; PMS (A, D, C, H, O): Premenstrual Syndrome Symptoms (Anxiety, Cravings, Heaviness, Hydration, Headaches, Depression, Others); PSST: Premenstrual Symptoms Screening Tool; PSST-A: Premenstrual Symptoms Screening Tool-Adolescents; SCID-PMDD: Structured Clinical Interview for DSM-IV-TR Defined PMDD; SPAF: Shortened Premenstrual Assessment Form; SSQ-PMDD: Self-screening quiz for PMDD as per the DSM-IV-TR criteria.
Quality of included studies
Owing to all included studies being of cross-sectional design, we used modified version of NOS for evaluation of the quality of each included study. The majority of studies (n=14) are rated as high,9,11,12,18-28 while the remaining 11 studies were evaluated as low quality.10,29-38 Studies were mostly at low risk of bias regarding assessment of outcome and statistical tests. However, the studies were at high risk of bias in terms of sample representativeness, sample size and response rate/reporting (Table 2).
Table 2. Quality assessment of studies using modified Newcastle Ottawa Scale .
| Author | Selection | Outcome | Total score | Quality | |||
| Sample representativeness | Sample size | Response rate | Assessment of outcome | Statistical tests | |||
| Sharma et al18 | * | - | * | - | * | 3 | High |
| Sharma et al19 | * | * | - | - | * | 3 | High |
| Ray et al20 | * | * | * | - | * | 4 | High |
| Sharma et al21 | - | - | - | * | * | 2 | Low |
| Brahmbhatt et al22 | - | - | - | - | * | 1 | Low |
| Mandal et al23 | - | - | - | * | * | 2 | Low |
| Sarkar et al24 | * | - | - | * | * | 3 | High |
| Mishra et al25 | - | - | - | * | * | 2 | Low |
| Kavitha et al10 | - | - | - | - | * | 1 | Low |
| Joseph et al26 | * | - | - | - | * | 2 | Low |
| Raval et al11 | - | * | * | * | * | 4 | High |
| Rumana et al27 | - | - | - | * | * | 2 | Low |
| Abirami and Ambika28 | - | - | - | - | * | 1 | Low |
| Negi et al29 | - | * | - | - | * | 2 | Low |
| Shenuka et al30 | * | * | * | * | * | 5 | High |
| Badkur et al31 | * | - | - | * | * | 3 | High |
| Budarapu et al32 | - | * | * | * | * | 4 | High |
| Kamat et al33 | * | * | * | * | - | 4 | High |
| Bhuvaneswari et al12 | - | * | - | * | * | 3 | High |
| Laxmi et al34 | - | - | - | * | * | 2 | Low |
| Durairaj et al9 | - | * | * | * | * | 3 | High |
| Srikanth and Nandini35 | - | - | - | * | - | 1 | Low |
| Bansal et al36 | - | * | * | * | * | 4 | High |
| Koganti et al37 | - | - | * | * | * | 3 | High |
| Gupta et al38 | * | * | - | * | * | 4 | High |
Factors related with PMS and PMDD
The influence of the presence of various risk factors on PMS or PMDD were explained or analysed in 13 of 25 studies. The increased prevalence of PMS or PMDD was associated with the consumption of tea, coffee, sweet or sweetened beverages, junk food and food intake under stress.12,25 Several studies also reported positive correlation with the lack of physical activity or leading a sedentary lifestyle.12,25,29,33 Bhuvaneswari et al12 reported that a positive family history of PMS/PMDD was associated with higher prevalence, while Laxmi et al34 found no significant association, whereas Badkur et al31 reported negative association with the history of menstrual problems in mother. The bitter receptor gene TAS2R38 was found to be correlated with severity and susceptibility of PMS.21 Among psychological variables, increased stress33,38 was the most important followed by anxiety, depression.38 However, socio-demographic variables like age, body mass index (BMI), amount of blood flow during menstruation, dysmenorrhoea, age of menarche and place of residence were reported as having either positive20,24,27,33,37 or no correlation.10
Prevalence of PMS
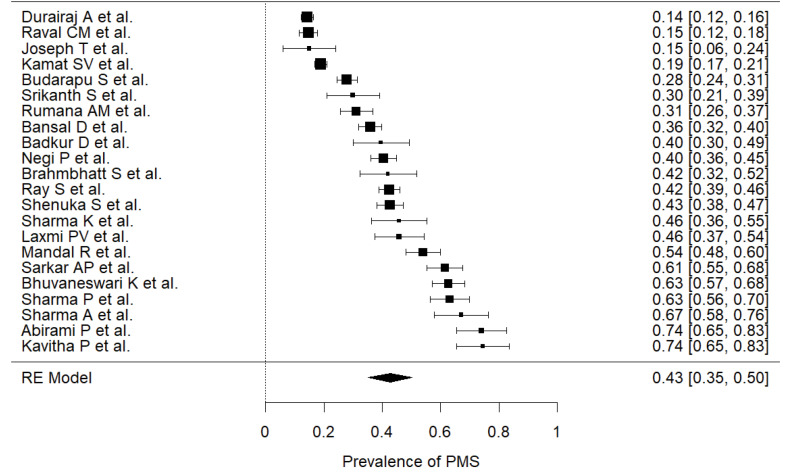
The prevalence of PMS was reported in 22 studies,9-12,18-24,26-36 including n=7865 participants (median=257), across different states. Participants were recruited from various sampling frame using different sampling methods, which has a very low chance of overlapping and overweighing of results. The estimated prevalence (Figure 2), pooled from all included studies was found to be 43% (95% CI: 0.35-0.50) with a high level of heterogeneity (I2=98%). The highest prevalence was found by pooling studies reported from Delhi, estimated at 64.4% (95% CI: 0.59-0.70, I2=0) and lowest was for Kerala at 15.3% (95% CI: 0.69-0.25).
Figure 2.
Pooled Prevalence of premenstrual syndrome. Random-Effect model; Heterogeneity statistics: I2=98.26%, Q=1205.976, P < 0.001.
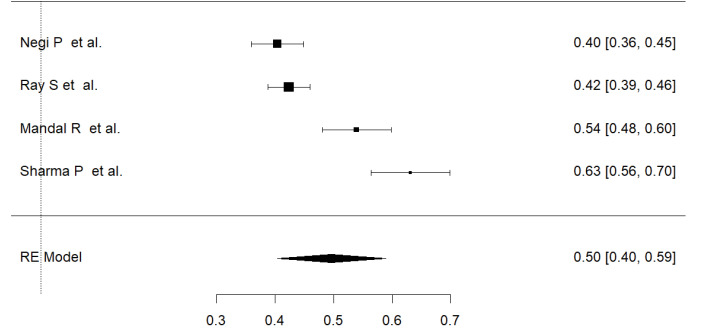
We also estimated the prevalence of PMS among adolescents (considering the age limit 10-19 years) from data extracted from four studies (Figure 3). The overall estimated prevalence of PMS among Indian adolescents was 49.6% (95% CI: 0.40-0.59, I2=93%).
Figure 3.
Pooled prevalence of premenstrual syndrome among adolescents. Random-Effect model; Heterogeneity statistics: I2=92.84%, Q=41.899, P < 0.001.
Sensitivity analysis was performed to check the effect of individual study on the overall estimate. No study had significant effect on changing the estimate more than 2%, thus confirming the robustness of the estimated prevalence.
Prevalence of PMDD
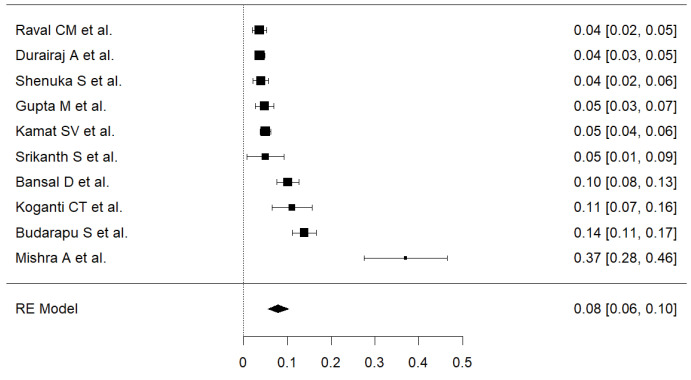
A total of 11 studies9,11,12,25,30,32,33,35-38 with 5578 participants (median=478) were included in the meta-analysis. Prevalence reported by individual studies were ranged from 3.7% to 65.67%. The pooled estimate of PMDD was 14% (95% CI: 0.10-0.21) with a heterogeneity, I2=98.32%.
In sensitivity analysis, exclusion of a study by Bhuvneswari et al,12 dropped the overall prevalence significantly. The estimated prevalence of rest of the 10 studies was 8% (95% CI: 0.06-0.10), but still the heterogeneity remained high (I2=98.32). Further analysis did not show sensitivity of the overall estimate for any study more than 2%. Therefore, the latter estimate was more robust and taken into consideration (Figure 4). A sensitivity analysis using double arcsine transformation showed similar results.
Figure 4.
Pooled prevalence of premenstrual dysphoric disorder. Random-Effect model; Heterogeneity statistics: I2=92.28%, Q=116.541, P < 0.001.
Subgroup analysis
A further subgroup analysis was carried out in terms of prevalence from individual State/UT, and quality of the included studies (Tables 3 and 4). We found studies from 10 States/UTs of India reporting prevalence of PMS, of these, a very small number of studies were conducted at the community level. Studies from Delhi showed the highest prevalence of PMS [64.4% (95% CI: 58.9-69.7, I2=0%)], whereas Kerala showed the lowest [(15.3% (95% CI: 6.9-25.3)]. The estimated prevalence of PMDD was maximum at Puducherry, at 65.7% (95% CI: 0.60-0.71) and minimum for Chandigarh, 4.8% (95% CI: 0.03-0.07). Notably, both the estimates for PMDD were based on the single studies. However, prevalence estimate of PMDD for Tamil Nadu based on two studies, was most robust in our study, at 3.8% (95% CI: 0.03-0.05) with heterogeneity, I2=0%.
Table 3. Subgroup analysis of PMS studies .
| Category | Groups | Prevalence |
| Study Quality | High | 40.6% (95% CI: 30.9-50.3, I2=99%) |
| Low | 45.2% (95% CI: 35.0-55.4, I2=95%) | |
| State or UT | Andhra Pradesh | 34.1% (95% CI: 23.3-45.5, I2=87%) |
| Delhi | 64.4% (95% CI: 58.9-69.7, I2=0%) | |
| Gujrat | 23.2% (95% CI: 14.2-33.2, I2=94%) | |
| Himachal Pradesh | 41.4% (95% CI: 37.4-45.5, I2=0%) | |
| Karnataka | 33.9% (95% CI: 29.4-38.6, I2=46%) | |
| Kerala | 15.3% (95% CI: 6.9-25.3) | |
| Madhya Pradesh | 39.6% (95% CI: 30.3-49.4) | |
| Puducherry | 62.7% (95% CI: 57.1-68.1) | |
| Tamil Nadu | 50.4% (95% CI: 20.1-80.7, I2=99%) | |
| West Bengal | 52.4% (95% CI: 40.6-64.2, I2=93%) |
Table 4. Subgroup analysis of PMDD studies .
| Category | Groups | Prevalence |
| Study Quality | High | 5.2% (95% CI: 0.04-0.09, I2=92%) |
| Low | 18.4% (95% CI: -0.00-0.58, I2=97%) | |
| State or UT | Andhra Pradesh | 9.5% (95% CI: 0.02-0.19, I2=87%) |
| Gujrat | 11.6% (95% CI: 0.02-0.24, I2=97%) | |
| Karnataka | 10.2% (95% CI: 0.08-0.13, I2=96%) | |
| Telengana | 11.1% (95% CI: 0.07-0.16) | |
| Puducherry | 65.7% (95% CI: 0.60-0.71) | |
| Tamil Nadu | 3.7% (95% CI: 0.03-0.05, I2=0%) |
The difference of pooled estimates between high and low quality studies were different. For PMS, the difference between low and high quality of studies was 4.6%. But, in case of PMDD, prevalence in ‘low quality subgroup’ was 7.7% higher than the ‘high quality’ subgroup. However, even after excluding the ‘low quality studies,’ the overall estimate of PMDD did not change much.
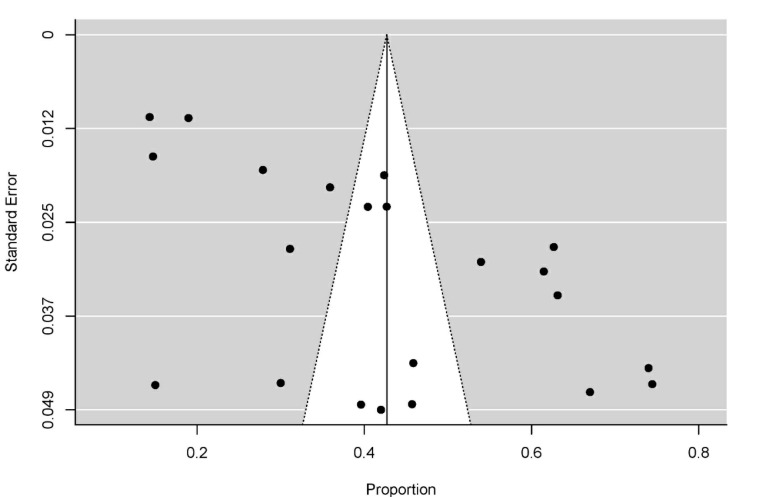
Publication bias was not indicated through the visual inspection of the funnel plot (Figure 5) and ‘rank correlation test for Funnel plot asymmetry’ (Kendall’s Tau = 0.186; P = 0.239)
Figure 5.
Funnel plot.
Discussion
This is the first systematic review and meta-analysis that has comprehensively searched and synthesised studies reporting prevalence of PMS and/or PMDD in the Indian population. In this study, we found that both the conditions are common among Indian females. The pooled prevalence estimates of PMS and PMDD among Indian females were 43% (95% CI: 0.35-0.50) and 8% (95% CI: 0.06-0.10), respectively. The prevalence varied with the geographic region, with the highest prevalence of PMS being reported in Delhi, whereas the lowest prevalence was reported in Kerala.
We observed a substantial heterogeneity in meta-analyses of both the estimates, which might be explained by age group, geographical region, study settings, residence in a rural or urban area, type of diagnostic tool and cut-off points used, or the study quality. We surmise from the report of different studies that substantial presence of factors such as socioeconomic conditions, diet, genetic pattern, family history, custom could have some role.
It may be instructive to look at prevalence estimates of premenstrual disorders in other low- and middle-income countries. A meta-analysis reported the pooled prevalence of PMS in Iran39 was 70.8% (95% CI: 63.8-77.7) which is much higher than the present findings for India. Similarly, Chandraratne and Gunawardena40 found that 65.7% of adolescents from Sri Lanka experienced PMS, where the commonest somatic symptom was fatigue, and a study on Pakistani women41 reported a prevalence of 79.9%. Our findings are quite similar to a cross-sectional study conducted in Taiwan which found a prevalence of PMS at 39.85% in female university students.42 Other studies from neighbouring countries showed lower prevalence of PMS – prevalence of 37.3% was reported in a study on the reproductive aged women of Myanmar43 and 21.1% in a community-based sample in China.44
The pooled prevalence of PMDD was also higher in India than a community-based study from China44 which reported 2.1%, and in a study from Pakistan41 prevalence was 5.5%. However, a recent meta-analysis of studies on PMDD among adolescents of Ethiopia45 reported a prevalence of 54.5%, which is much higher than our estimate.
This systematic review and meta-analysis is the first of its kind to estimate the national burden of PMS and PMDD in Indian population. This study is significant in terms of rigorous search in national and international databases along with snowballing through hand-search within different relevant articles minimising chances of missing important relevant studies. A systematised protocol was registered beforehand in the international prospective register of systematic reviews (PROSPERO) to maintain transparency throughout the study process. However, we included PMDD along with PMS as the topic of interest as they lie in the same continuum, which was not or faintly decided during protocol stage, but found important in the course of the study. The standard nature of this review was maintained by adhering to the PRISMA and MOOSE guidelines throughout different steps. An important caveat while interpreting the results is the presence of substantial amount of heterogeneity. However, this type of heterogeneity is expected due to the demographic variation, cultural differences and other factors in a country as diverse as India. The reason for such heterogeneity was attempted to be explained by means of sensitivity analysis and subgroup analysis. We assessed the quality of each study with a modified version of NOS and ran a subgroup analysis to assess any influence of it on the results. Most of the included studies were not conducted in community settings which might have affected reaching a precise estimate. Different institutional studies are expected to have included a mixed population from various states or regions. In sensitivity analysis, we confirmed the robustness of the overall obtained estimate which might have increased the generalizability of the results. The study by Bhuvaneswari et al12 influenced the overall estimate of PMDD to a greater degree, probably due to false high estimate, as reported, so we calculated the overall prevalence after excluding the study. Owing to the small number of studies belonging to each region, the state-wise prevalence must be interpreted with caution.
Conclusion
We found a high prevalence of PMS and PMDD among Indian females of reproductive age group. A comparatively high prevalence was found among adolescents which affects the quality of life adversely. These are largely contributed by various socio-demographic, genetic and psychological factors. Owing to the less number of studies and sharp variation of estimates between regions, large scale studies (preferably nationwide) are required to find out more robust estimates of disease burden and potentially associated factors. However depending on the present findings, we recommend the development of governmental policies and guidelines to address this problem at individual and community level to increase the health status and productivity of this population.
Funding
This study received no external funding.
Competing interests
None.
Ethical approval
This study only used published secondary data and did not involve any participants directly. Therefore, permission from the human ethics committee was not applicable.
Authors’ contributions
AD, Conceptualization, Project administration, Supervision, Validation, Literature Search, Methodology, Data curation, Appraisal, Formal analysis, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. AS, Clinical input, Project administration, Supervision, Validation, Interpretation, Writing – review & editing.
Disclaimer
No part of this paper is copied from other source.
References
- 1. American College of Obstetricians and Gynaecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynaecologists; 2014. p. 607-13.
- 2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
- 3.Mishell DR Jr. Premenstrual disorders: epidemiology and disease burden. Am J Manag Care. 2005;11(16 Suppl):S473–9. [PubMed] [Google Scholar]
- 4.Marjoribanks J, Brown J, O’Brien PM, Wyatt K. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;2013(6):CD001396. doi: 10.1002/14651858.CD001396.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson C, Pearson B, Girdler S, Johnson J, Hamer RM, Killenberg S. et al. Double-blind, placebo-controlled pilot study of adjunctive quetiapine SR in the treatment of PMS/PMDD. Hum Psychopharmacol. 2015;30(6):425–34. doi: 10.1002/hup.2494. [DOI] [PubMed] [Google Scholar]
- 6.Freeman EW, Halbreich U, Grubb GS, Rapkin AJ, Skouby SO, Smith L. et al. An overview of four studies of a continuous oral contraceptive (levonorgestrel 90 mcg/ethinyl estradiol 20 mcg) on premenstrual dysphoric disorder and premenstrual syndrome. Contraception. 2012;85(5):437–45. doi: 10.1016/j.contraception.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Thys-Jacobs S, Starkey P, Bernstein D, Tian J. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual symptoms. Premenstrual Syndrome Study Group. Am J Obstet Gynecol. 1998;179(2):444–52. doi: 10.1016/s0002-9378(98)70377-1. [DOI] [PubMed] [Google Scholar]
- 8.Lustyk MK, Gerrish WG, Shaver S, Keys SL. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12(2):85–96. doi: 10.1007/s00737-009-0052-y. [DOI] [PubMed] [Google Scholar]
- 9.Durairaj A, Ramamurthi R. Prevalence, pattern and predictors of premenstrual syndrome (PMS) and premenstrual dysphoric disorder (PMDD) among college girls. New Indian J OBGYN. 2019;5(2):93–8. [Google Scholar]
- 10.Kavitha P, Shanmughavadivu R. A study on the prevalence of premenstrual syndrome and its relation with anthropometric indices. Int J Pharmacol Physiol. 2015;1(1):27–32. [Google Scholar]
- 11.Raval CM, Panchal BN, Tiwari DS, Vala AU, Bhatt RB. Prevalence of premenstrual syndrome and premenstrual dysphoric disorder among college students of Bhavnagar, Gujarat. Indian J Psychiatry. 2016;58(2):164–70. doi: 10.4103/0019-5545.183796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhuvaneswari K, Rabindran P, Bharadwaj B. Prevalence of premenstrual syndrome and its impact on quality of life among selected college students in Puducherry. Natl Med J India. 2019;32(1):17–9. doi: 10.4103/0970-258x.272109. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15. Dutta A. Prevalence of premenstrual syndrome in Indian population: A systematic review and meta-analysis. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020199787. Accessed 18 October 2020.
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 17. Deeks JJ, Higgins JP, Altman DG, Jpt H. Cochrane Handbook for Systematic Reviews of Interventions. 2019. Available from: https://training.cochrane.org/handbook. Accessed 18 October 2020.
- 18.Sharma P, Malhotra C, Taneja DK, Saha R. Problems related to menstruation amongst adolescent girls. Indian J Pediatr. 2008;75(2):125–9. doi: 10.1007/s12098-008-0018-5. [DOI] [PubMed] [Google Scholar]
- 19.Sharma A, Taneja DK, Sharma P, Saha R. Problems related to menstruation and their effect on daily routine of students of a medical college in Delhi, India. Asia Pac J Public Health. 2008;20(3):234–41. doi: 10.1177/1010539508316939. [DOI] [PubMed] [Google Scholar]
- 20.Ray S, Mishra SK, Roy AG, Das BM. Menstrual characteristics: a study of the adolescents of rural and urban West Bengal, India. Ann Hum Biol. 2010;37(5):668–81. doi: 10.3109/03014460903563442. [DOI] [PubMed] [Google Scholar]
- 21.Sharma K, Kansal A, Chopra S. Premenstrual syndrome, body fat and bitter taste receptor gene TAS2R38 among adult Kullu females of Himachal Pradesh, India. Anthropol Anz. 2013;70(2):203–19. doi: 10.1127/0003-5548/2013/0295. [DOI] [PubMed] [Google Scholar]
- 22.Brahmbhatt S, Sattigeri BM, Shah H, Kumar A, Parikh D. A prospective survey study on premenstrual syndrome in young and middle aged women with an emphasis on its management. Int J Res Med Sci. 2013;1(2):69–72. [Google Scholar]
- 23.Mandal R, Sarkar AP, Ghorai S. A study on premenstrual syndrome among adolescent girl students in an urban area of West Bengal. Int J Reprod Contracept Obstet Gynecol. 2015;4(4):1012–5. doi: 10.18203/2320-1770.ijrcog20150417. [DOI] [Google Scholar]
- 24.Sarkar AP, Mandal R, Ghorai S. Premenstrual syndrome among adolescent girl students in a rural school of West Bengal, India. Int J Med Sci Public Health. 2016;5(3):408–11. doi: 10.5455/ijmsph.2016.2407201566. [DOI] [Google Scholar]
- 25.Mishra A, Banwari G, Yadav P. Premenstrual dysphoric disorder in medical students residing in hostel and its association with lifestyle factors. Ind Psychiatry J. 2015;24(2):150–7. doi: 10.4103/0972-6748.181718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joseph T, Nandini M, Sabira KA. Prevalence of premenstrual syndrome (PMS) among adolescent girls. J Nurs Health Sci. 2016;5:24–7. [Google Scholar]
- 27.Rumana A, Sudharani M, Kallupurackal S, Ramya V, Nagendra G, Suryakantha A. Prevalence of premenstrual syndrome among medical students. Natl J Community Med. 2017;8(6):292–4. [Google Scholar]
- 28.Abirami P, Ambika S. Assess the prevalence of premenstrual syndrome among adolescent girls at SRM College of Nursing, SRM University, Kattankulathur. Asian J Pharm Clin Res. 2017;10(5):202–5. doi: 10.22159/ajpcr.2017.v10i5.13332. [DOI] [Google Scholar]
- 29.Negi P, Mishra A, Lakhera P. Menstrual abnormalities and their association with lifestyle pattern in adolescent girls of Garhwal, India. J Family Med Prim Care. 2018;7(4):804–8. doi: 10.4103/jfmpc.jfmpc_159_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shenuka S, Vijayalakshmi R, Sambath Kumar R. Prevalence, severity and coping behaviour of premenstrual syndrome and premenstrual dysphoric disorder among female students in a private institution in India. Int Res J Pharm. 2018;9(9):193–7. doi: 10.7897/2230-8407.099212. [DOI] [Google Scholar]
- 31.Badkur D, Singh S, Chauhan D, Sinha A. Premenstrual syndrome and its association with menstrual profile among female students of colleges in Ujjain city, Madhya Pradesh, India. Int J Res Med Sci. 2018;6(8):2726–31. doi: 10.18203/2320-6012.ijrms20183259. [DOI] [Google Scholar]
- 32.Budarapu S, Sadam H, Harshitha K, Nageswari DM, Reddy HK, Dhanekula G. A study to assess the prevalence of premenstrual syndrome and premenstrual dysphoric disorder and various coping strategies used by students in a Womens Medical College from South India. Int J Contemp Med Res. 2018;5(11):K1–5. doi: 10.21276/ijcmr.2018.5.11.18. [DOI] [Google Scholar]
- 33.Kamat SV, Nimbalkar A, Phatak AG, Nimbalkar SM. Premenstrual syndrome in Anand district, Gujarat: a cross-sectional survey. J Family Med Prim Care. 2019;8(2):640–7. doi: 10.4103/jfmpc.jfmpc_302_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laxmi P, Vasundhara R, Miryani J. Prevalence of premenstrual syndrome among the females in reproductive age group. Int J Obstet Gynaecol Nurs. 2019;1(2):6–9. [Google Scholar]
- 35.Srikanth S, Nandini S. Menstrual hygienic practices and prevalence of premenstrual syndrome in medical students. Indian J Appl Res. 2019;9(8):55–7. [Google Scholar]
- 36.Bansal D, Raman R, Rao TSS. Premenstrual dysphoric disorder: ranking the symptoms and severity in Indian college students. Journal of Psychosexual Health. 2019;1(2):159–63. doi: 10.1177/2631831819827183. [DOI] [Google Scholar]
- 37.Koganti CT, Bobba NS. A study on the prevalence of premenstrual dysphoric disorder in medical students. Acad J Med. 2020;3(1):74–7. doi: 10.47008/ajm.2020.3.1.15. [DOI] [Google Scholar]
- 38.Gupta M, Dua D, Kaur H, Grover S. Prevalence of premenstrual dysphoric disorder among school-going adolescent girls. Ind Psychiatry J. 2019;28(2):198–202. doi: 10.4103/ipj.ipj_79_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ranjbaran M, Omani Samani R, Almasi-Hashiani A, Matourypour P, Moini A. Prevalence of premenstrual syndrome in Iran: a systematic review and meta-analysis. Int J Reprod Biomed. 2017;15(11):679–86. [PMC free article] [PubMed] [Google Scholar]
- 40.Chandraratne NK, Gunawardena NS. Premenstrual syndrome: the experience from a sample of Sri Lankan adolescents. J Pediatr Adolesc Gynecol. 2011;24(5):304–10. doi: 10.1016/j.jpag.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Pal SA, Dennerstein L, Lehert P. Premenstrual symptoms in Pakistani women and their effect on activities of daily life. J Pak Med Assoc. 2011;61(8):763–8. [PubMed] [Google Scholar]
- 42.Cheng SH, Shih CC, Yang YK, Chen KT, Chang YH, Yang YC. Factors associated with premenstrual syndrome - a survey of new female university students. Kaohsiung J Med Sci. 2013;29(2):100–5. doi: 10.1016/j.kjms.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Oo HH, Sein MT, Mar O, Aung A. Assessment of premenstrual syndrome among reproductive aged Myanmar women. Asian J Med Sci. 2016;7(4):39–43. [Google Scholar]
- 44.Qiao M, Zhang H, Liu H, Luo S, Wang T, Zhang J. et al. Prevalence of premenstrual syndrome and premenstrual dysphoric disorder in a population-based sample in China. Eur J Obstet Gynecol Reprod Biol. 2012;162(1):83–6. doi: 10.1016/j.ejogrb.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 45.Duko B, Mekuriaw B, Molla A, Ayano G. The prevalence of premenstrual dysphoric disorder among adolescents in Ethiopia: a systematic review and meta-analysis. Ir J Med Sci. 2021;190(1):419–27. doi: 10.1007/s11845-020-02275-7. [DOI] [PubMed] [Google Scholar]