Abstract
Src family kinases (SFKs) are key regulators of cell proliferation, differentiation, and survival. The expression of these non-receptor tyrosine kinases is strongly correlated with cancer development and tumor progression. Thus, this family of proteins serves as an attractive drug target. The activation of SFKs can occur via multiple signaling pathways, yet many of them are poorly understood. Here, we summarize the current knowledge on G protein-coupled receptor (GPCR)-mediated regulation of SFKs, which is of considerable interest because GPCRs are among the most widely used pharmaceutical targets. This type of activation can occur through a direct interaction between the two proteins or be allosterically regulated by arrestins and G proteins. We postulate that a rearrangement of binding motifs within the active conformation of arrestin-3 mediates Src regulation by comparison of available crystal structures. Therefore, we hypothesize a potentially different activation mechanism compared to arrestin-2. Furthermore, we discuss the probable direct regulation of SFK by GPCRs and investigate the intracellular domains of exemplary GPCRs with conserved polyproline binding motifs that might serve as scaffolding domains to allow such a direct interaction. Large intracellular domains in GPCRs are often understudied and, in general, not much is known of their contribution to different signaling pathways. The suggested direct interaction between a GPCR and a SFK could allow for a potential immediate allosteric regulation of SFKs by GPCRs and thereby unravel a novel mechanism of SFK signaling. This overview will help to identify new GPCR–SFK interactions, which could serve to explain biological functions or be used to modulate downstream effectors.
Keywords: G protein-coupled receptors, GPCR, SFK, Src kinases, G proteins, arrestin, allosteric regulation, biased signaling, non-receptor tyrosine kinases, SH3 domains, polyproline motifs, kinase activation, signaling
1. Introduction
Src family kinases (SFKs) are non-receptor protein-tyrosine kinases that regulate essential processes such as cell proliferation, differentiation, survival, migration, and metabolism [1].
SFKs are upregulated in malignancies, and their expression levels as well as specific activity are elevated in brain, breast, colon, lung, and pancreatic carcinomas [2,3,4,5,6,7,8,9]. For acute myeloid leukemia and colorectal cancer, a direct correlation between expression level of some SFK family members and patient survival was observed [10,11].
The human SFKs consist of eight typical family members (Src, Fyn, Yes, Fgr, Hck, Blk, Lck, and Lyn) and three atypical family members (Brk, Frk, Srms) based on sequence similarity. In some nomenclatures, atypical family members are considered a separate family also called the Frk family [12,13]. Src, Fyn, and Yes are expressed ubiquitously, while the other family members show tissue-specific expression [14]. Most of the SFKs, such as Yes, Fgr, Blk, Hck, Lck, and Lyn have an important regulatory role in signaling pathways of hematopoietic cells. A majority of SFKs are essential in immune response; whereas Fyn and Lck are activated immediately after T-cell receptor stimulation, expression of Fgr, Hck, Lyn is induced in stimulated mature monocytes and macrophages [15].
SFKs represent drug targets with great therapeutic potential, especially in cancer treatment, since SFKs are involved in cancer progression in various stages (reviewed in [16,17]). Approved drugs for cancer treatment targeting Src family kinases, such as Dasatinib, show a high level of toxicity [18] due to their unselective inhibition of SFKs in cancer and healthy cells. In-depth structural, functional, and mechanistic knowledge of each single SFK in combination with a detailed understanding of their expression regulation can be the basis for a more specific therapeutic approach with limited side effects.
2. Structural Hallmarks of SFKs
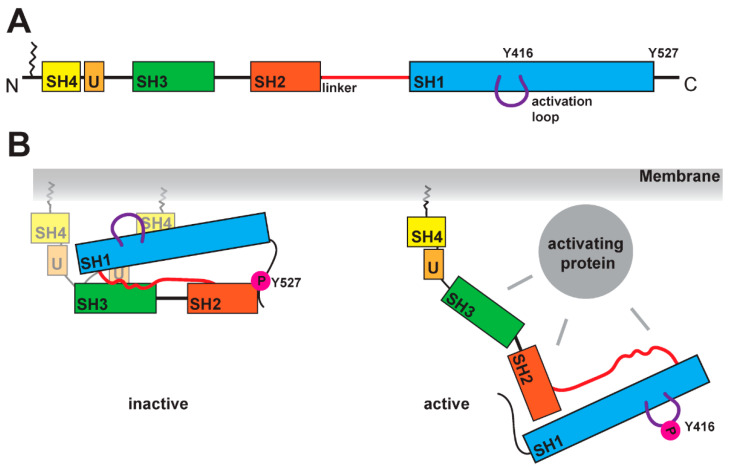
SFKs are composed of distinct domains (Figure 1A). The N-terminal region, also called the SH4 domain, contains a myristoylation or palmitoylation site, which acts as a membrane anchor and is a key element for the localization of SFKs [19,20]. The unique domain, which is located after the SH4 domain, has a regulatory function for membrane localization and can form a fuzzy intramolecular complex with the neighboring SH3 domain [21,22,23]. SH3 domains serve as binding elements and are known to interact with a variety of polyproline motifs (reviewed in [24]). After a linker, SFKs contain the SH2 domain, known to interact with phosphorylated tyrosine residues, and following a longer linker region, the kinase domain, containing an N-lobe and a C-lobe. This domain entails two regulatory phosphorylation sites (Y-416 and Y-527 for Src as a representative example, Figure 1B) [25,26]. The first regulatory site is the activating autophosphorylation site, and the second one the negative regulatory site. The phosphorylation and dephosphorylation of these tyrosine residues cause dramatic structural changes and affect the activity of the kinase. In the inactive structure, Y-527 is phosphorylated by CSK (C-terminal Src kinase) or CHK (CSK homologous kinase) [27,28], which results in an interaction of the kinase domain with the SH2 domain [25,29,30]. This inactive conformation is further stabilized by the binding of the SH3 domain with the polyproline motif in the linker region between SH2 domain and kinase domain [30,31]. A recent finding showed a possible involvement of the SH4 domain, which binds in the inactive conformation to the kinase domain [32]. This compact state results in a closed conformation of the N- and C-lobes in the kinase domain, which results in a shielding of the Y-416 in the active site. In this closed conformation, the binding of ATP and substrates is blocked. In the active conformation, the interactions of SH2 and SH3 domains are displaced by other binding partners, which results in an open conformation (Figure 1B). This grants accessibility of the active site and allows for autophosphorylation of Y-416.
Figure 1.
Schematic of SFK domains and activation mechanism. (A) SFKs are organized in several domains: the SH4 domain (in yellow), a unique domain (in light orange), the SH3 domain (in green), the SH2 domain (in dark orange), the SH1 domain (in blue). At the N-terminal end, SFKs contain a lipid anchor, which is localizing the kinase at the membrane. The linker region between the SH2 and SH1 domain is crucial for the activation mechanism. A further structural feature is the activation loop within the SH1 domain, which contains a tyrosine residue (for Src Y416) that is phosphorylated in the active state of the kinase. (B) Comparison of inactive and active states of SFK. In the inactive conformation, the tyrosine in the activation loop is not phosphorylated, while the tyrosine (Y527 for Src) at the C-terminus carries a phosphate residue as it binds to the SH2 domain. The closed conformation is stabilized by the linker region binding to the SH1 and SH3 domains simultaneously. SH4 and unique domain seem to be more flexible, and recent studies found binding of the SH4 domain to the SH1 domain [32] (two possible conformations are shown). The open conformation is induced by the binding of an activating protein, which can interact with the SH3, SH2, SH1 domains and the linker. This active conformation shows phosphorylation of the tyrosine (for Src Y416) in the activation loop and dephosphorylation of the tyrosine in the C-terminus (for Src 527).
3. Modes of SFK Activation
In general, SFKs are activated by several different growth factor receptor tyrosine kinases. For example, the SH2 domains interact with SHP-1 protein tyrosine phosphatase, CRK-associated substrate, or protein tyrosine phosphatase-1B [33,34,35]. Proteins with typical polyproline motifs such as cyclin-dependent kinase-5, KCNB1, p21-acitvated kinase-2, vinculin and GRB2 have also been shown to induce the active conformation [36,37,38,39,40,41].
Additionally, for a number of G protein-coupled receptors (GPCRs), SFK activation was shown (Table 1). However, the exact activation mechanism of this interaction is poorly understood. It has been postulated that there are three ways of GPCR-mediated SFK activation: through arrestins, G proteins, or direct binding. A detailed understanding of the mechanism is highly desirable due to the potential druggability of GPCRs and the crucial role of SFKs in cancer development and progression.
Table 1.
Overview of GPCRs that regulate or bind SFKs. Summarized are most of the GPCRs known to activate SFKs in a G protein- or arrestin-dependent manner. Some studies showed a direct interaction between the receptors and the SFKs, while in other studies, activation of the SFK was observed, but a mechanism was not defined.
| GPCR | G-Protein | Arrestin | Direct | Other | References |
|---|---|---|---|---|---|
| α2AR | Arrestin-2/3 | [42] | |||
| β2AR | Arrestin-2 | [43,44] | |||
| β3AR | 3rd ICL | [44] | |||
| D1R | Arresin-3 | unknown | [45,46] | ||
| D2R | Arrestin-2 | unknown | [47,48] | ||
| D4R | 3rd ICL | [49] | |||
| V1bR | unknown | [50] | |||
| V2R | Indication of direct | [50] | |||
| GnRH-a | Indication of Gβγ protein | [51,52] | |||
| M1R | Indication of Gαq protein | [53] | |||
| M2R | Arrestin-2 | [43,54] | |||
| M3R | Arrestin | [55] | |||
| M4R | unknown | [54] | |||
| B1R | Gαi | unknown | [56,57] | ||
| ETAR | unknown | [58] | |||
| ATR2 | unknown | [59,60] | |||
| Latrophilin-2 | unknown | [61] | |||
| GPR56 (ADGRG1) | unknown | [62] |
4. Src Activation through a GPCR–Arrestin Complex
Until now, the best understood GPCR-mediated activation of SFK is arrestin-based. As early as 1999, arrestin-2-mediated Src activation by beta-2 adrenergic receptor (β2AR) stimulation was detected [63]. Later on, this was observed for multiple other receptors (Table 1). Until recently, there was no evidence of how this interaction could take place.
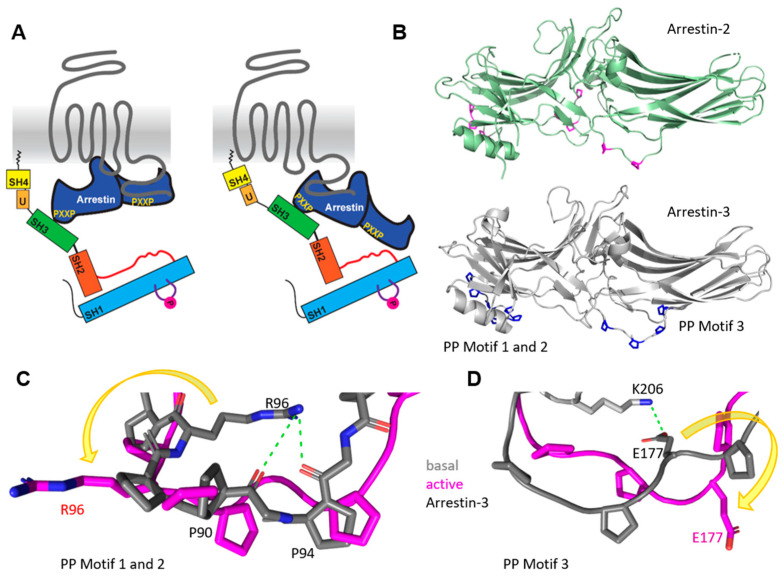
Arrestins have two major functions in GPCR regulation. First, receptor desensitization and internalization through recruitment of clathrin-coated pits [64,65] and second, the recruitment and activation of effectors such as MAPK and SFKs ([63] and reviewed in [66,67]). However, the concept of purely arrestin-based signaling has been recently challenged [68]. The active state of arrestins can be induced through their binding to the phosphorylated C-terminus (‘tail’ conformation) or the hydrophobic intracellular pocket between the helices of a GPCR (‘core’ conformation) [69,70] (Figure 2A). Activation by other regulatory molecules such as IP6 or a C-tail phosphopeptide has also been described [71,72,73]. The ‘core’ conformation is essential for the desensitization of G-protein signaling, while arrestin in the ‘tail’ conformation loses its desensitization ability [74]. Nevertheless, in the ‘tail’ conformation, arrestin internalization and signaling are still possible. The most dominant conformational change during the activation of arrestin is the rotation of the N- and C-domains towards each other. With this domain rotation, multiple small conformational changes appear (also called switch regions for arrestin-3) [75]. It is predicted that at least one of the previously described switch regions in arrestin-3 could be unique for this protein. This regulatory element contains a polyproline motif, which is a classical binding motif for SH3 domains. The interaction of SFK SH3 domains with polyproline motifs in arrestin are substantial for the activation of SFKs [76].
Figure 2.
Binding motifs in arrestin-3 but not in arrestin-2 show structural rearrangement with activation. (A) Cartoon of arrestin-mediated SFK activation in the ‘core’ (left) and the ‘tail’ conformation (right) of arrestin. The activating receptor is shown in grey, arrestin is colored in dark blue with yellow polyproline motifs, and SFK color scheme was described earlier. (B) Structure of basal arrestin-2 in green with polyproline motifs in magenta (PDB file 1JSY [77]) and arrestin-3 with highlighted polyproline motifs in blue that are surface-accessible in a receptor-bound state (PDB file 3P2D [78]). (C) The comparison of polyproline motifs 1 and 2 in the basal (grey) and active (magenta) arrestin-3 conformations shows a large structural rearrangement with a 180° rotation of the R96 (indicated by a yellow arrow). In the basal state of arrestin-3, R96 forms electrostatic interactions with the backbone of the polyproline motif 1. (D) The comparison of polyproline motif 3 between basal (grey) and active (magenta) states shows an 180° outward movement of E177 in the active state (indicated by yellow arrow). For comparison of basal and active arrestin-3, PDB files 3P2D and 5TV1 were used [71,78].
Yang et al showed that the receptor phospho-tail allosterically regulates the different conformations within the polyproline motifs in arrestin-2, which subsequently allows for the binding of the SFK SH3 domain. leading to the adoption of an open active conformation of the kinase [76]. A further recent study verified that receptor-bound arrestin-2, but not free arrestin-2, is able to activate Src [43]. Here, the binding of the receptor phospho-tail to arrestin-2 was shown to be sufficient to activate arrestin-2 and therefore Src (Figure 2A). There are only a few activation studies for arrestin-3-mediated Src activation. For example, in the case of the alpha 2 adrenergic receptor, arrestin-3 acts like a molecular switch, resulting in Src-mediated ERK activation [42]. For dopamine D1 receptor, activation of Src in the presence of arrestin-3 was shown [45].
Interestingly, for PAR-1 (protease-activated receptor-1), arrestin-3 showed opposite effects compared to arrestin-2 [79]. While arrestin-3 appeared to mediate the degradation of Src with the activation of PAR-1, arrestin-2 was crucial for Src activation. Arrestin-2 and -3 have each three polyproline motifs, PXXP, that differ slightly (88PPAP, 121PNLP and 178PERP for arrestin-2 and 89PPVP, 94PPRPPTR, 175PEKP for arrestin-3, Figure 2B). Most the polyproline motifs do not contain a positively charged arginine, which could contribute to high-affinity binding of SFKs [80,81,82]. The exemptions are R180 in arrestin-2 and R96 and R100 in arrestin-3. By comparing the active and basal crystal structures of arrestin-3 (PDB 3P2D for basal and 5TV1 for active arrestin-3) [71,78], we found that in the basal structure of arrestin-3, R96 stabilizes the polyproline loop in a potential inactive conformation through electrostatic interactions with the backbone of the amid bond of P92 and N93 (Figure 2C). Polyproline motif 3 indicated a similar stabilization of the basal conformation by an electrostatic interaction between K206 and the highly conserved E177 (Figure 2D). By comparison with the active arrestin-3 structure, we found that the side chains of R96 (Figure 2C) and E177 (Figure 2D) are rotated 180° outward, which could allow the rearrangement of the polyproline motif. This structural reorganization of the polyproline binding motif of arrestin-3 might potentially have a regulatory effect on the SH3 domain interaction of SFKs with arrestin-3. Even though arrestin-2 harbors an arginine in motif 3 (R180), no electrostatic interactions were found by comparing crystal structures of basal and active arrestin-2 (PDB 1JSY for basal arrestin-2, 6UP7 and 6U1N for active arrestin-2) [77,83,84], which could significantly alter the polyproline motif conformation within the different activation stages. This could result in different affinities for SH3 domains and, therefore, explain the observed different roles of arrestin-2 and -3 in PAR1 activation.
5. Src Activation by G Proteins
G proteins (heterotrimeric guanine nucleotide-binding regulatory proteins) contain α, ß, and γ subunits. The α subunits can be classified into four families based upon sequence similarity: Gαs, Gαi, Gαq, and Gα12 [85]. The ß and γ subunits form a signaling complex due to their strong interaction. Agonist-bound GPCRs activate G proteins by facilitating the exchange of GDP to GTP at the α subunit. This active state causes the dissociation of the Gα subunit from the membrane-anchored ßγ subunit. Activation of SFKs by G proteins can be achieved through either the α subunit or the ßγ subunit. The interaction with the α subunit was shown by in vitro studies using Y-530-phosphorylated Src, with Gαs or Gαi resulting in the activation of Src. The interaction is believed to be mediated through the kinase domain of the SFK and the switch II region of the Gα subunit [86]. The described two switch regions in G proteins are defined regions crucial for binding of effectors such as Ras protein or adenylyl cyclase [87,88,89]. The activation of Src through the ßγ subunit was found for the CRF1 receptor by using a ßγ subunit inhibitor which caused downregulation of Src activation [90]. For carvedilol-stimulated β1 adrenergic receptor, Src-dependent ERK activation was shown [91]. Here, it was suggested that the activation of Src also involved the Gßγ subunits of the G protein, whereas this complex formation was arrestin-dependent [92].
Src, contrastingly, is able to phosphorylate Gα subunits in vitro, whereas the highest efficiency is shown for the GDP-bound inactive subunit. The two sites of phosphorylation are Y37 and Y377 [93,94], and both promote GTP hydrolysis. The different regulatory mechanisms by G protein phosphorylation are reviewed elsewhere [95]. In transducin, an additional phosphorylation site, Y142, was found [96]. Furthermore, Gßγ subunits are possibly phosphorylated, but it is not known if SFKs are involved. Overall, an arrestin-independent G protein-mediated activation of Src is still not fully understood and requires further investigation.
6. Src Family Kinases as Direct Effectors of GPCRs
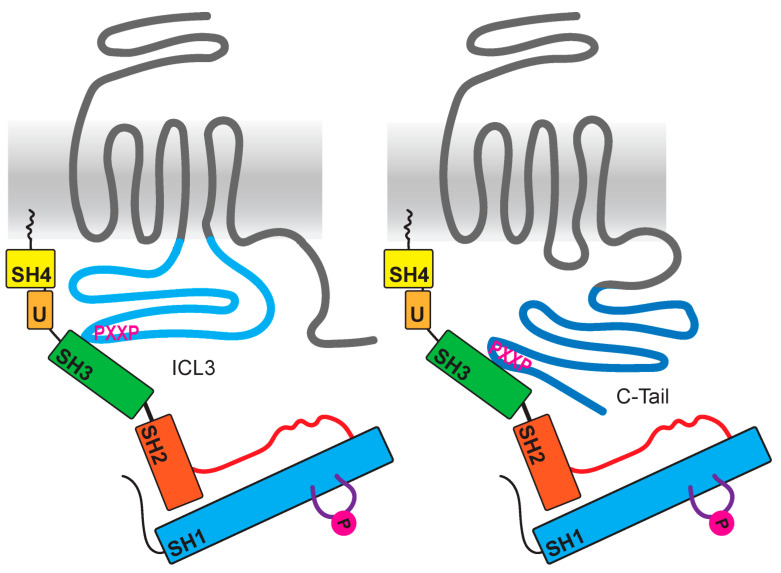
The existence of protein binding motifs within the intracellular structures of GPCRs is well known; however, the impact on GPCR signaling remains poorly understood. A variety of binding motifs are located in the intracellular loops and C-termini for, e.g., PDZ proteins as well as SH2 or SH3 binding motifs [97]. Seventy-two out of 825 human GPCRs contain the classical polyproline SH3 domain-binding motif [76]. Most of these GPCRs have polyproline motifs within the third intracellular loop or the C-terminus (Figure 3), and for some of these receptors, an interaction with Src SH3 domains is predicted and was shown. For example, the beta-3 adrenergic receptor (β3AR) has typical SH3 binding sites in the third intracellular loop (Table 2), while it neither contains any GRK phosphorylation sites nor does it bind to arrestin [44]. β3AR mutations in Src binding sites inhibited the activation of Src or MAPKs. Nevertheless, β3AR Src activation is also Gαi-dependent. Further, the purinergic P2Y2 receptor entails polyproline motifs in the C-terminus, and Src binding, as well as its activation, was verified (Table 2) [98]. In most of these studies, the impact of arrestin was not taken into consideration.
Figure 3.
Direct interaction of GPCRs with SFKs through intracellular domains. Some GPCRs (colored in grey) encode polyproline motifs (shown in magenta) either in their third intracellular loop (light blue) or in their C-tail (colored in dark blue). For several receptors, an SFK SH3 domain (shown in green) interaction was verified.
Table 2.
Comparison of SFK binding motifs in the 3rd intracellular loop and C-tail of GPCRs. Shown are the amino acid Scheme 2. Y2 receptor, serotonin receptor type 6, and the subfamily of dopamine receptors, with highlighted polyproline motifs for SFKs in red and for other kinases in blue. Using a software to predict SH3 domain interactions, different SFK family members appeared to likely interact with individual domains [105].
| 3ICL | C-Terminus | Predicted SFK SH3 Domain Interactions | |
|---|---|---|---|
| b1AR | REAQKQVKKIDSCERRFLGGPARPPSPSPSPVPAPAPPPGPPRPAAAAATAPLANGRAGKRRPSRLVALRE | CRSPDFRKAFQRLLCCARRAARRRHATHGDRPRASGCLARPGPPPSPGAASDDDDDDVVGATPPARLLEPWAGCNGGAAADSDSSLDEPCRPGFASESKV | FGR, LYN |
| b2AR | RVFQEAKRQLQKIDKSEGRFHVQNLSQVEQDGRTGHGLRRSSKFCLKEHKALKT | PDFRIAFQELLCLRRSSLKAYGNGYSSNGNTGEQSGYHVEQEKENKLLCEDLPGTEDFVGHQGTVPSDNIDSQGRNCSTNDSLL | - |
| b3AR | RVFVVATRQLRLLRGELGRFPPEESPPAPSRSLAPAPVGTCAPPEGVPACGRRPARLLPLREHRALC | RSPDFRSAFRRLLCRCGRRLPPEPCAAARPALFPSGVPAARSSPAQPRLCQRLDGASWGVS | SRC, FGR, LYN, HCK, LCK, FYN |
| P2Y2 | MARRLLKPAYGTSGGLPRAKRKSVRT | GQRLVRFARDAKPPTGPSPATPARRRLGLRRSDRTDMQRIEDVLGSSEDSRRTESTPAGSENTKDIRL | FGR |
| 5HT6 | CRILLAARKQAVQVASLTTGMASQASETLQVPRTPRPGVESADSRRLATKHSRKALK | PLFMRDFKRALGRFLPCPRCPRERQASLASPSLRTSHSGPRPGLSLQQVLPLPLPPDSDSDSDAGSGGSSGLRLTAQLLLPGEATQDPPLPTRAAAAVNFFNIDPAEPELRPHPLGIPTN | LYN |
| D1R | RIAQKQIRRIAALERAAVHAKNCQTTTGNGKPVECSQPESSFKMSFKRETKVLK | RKAFSTLLGCYRLCPATNNAIETVSINNNGAAMFSSHHEPRGSISKECNLVYLIPHAVGSSEDLKKEEAAGIARPLEKLSPALSVILDYDTDVSLEKIQPITQNGQHPT | - |
| D2R | IVLRRRRKRVNTKRSSRAFRAHLRAPLKGNCTHPEDMKLCTVIMKSNGSFPVNRRRVEAARRAQELEMEMLSSTSPPERTRYSPIPPSHHQLTLPDPSHHGLHSTPDSPAKPEKNGHAKDHPKIAKIFEIQTMPNGKTRTSLKTMSRRKLSQQKEKKATQ | EFRKAFLKILHC | - |
| D3R | RIYVVLKQRRRKRILTRQNSQCNSVRPGFPQQTLSPDPAHLELKRYYSICQDTALGGPGFQERGGELKREEKTRNSLSPTIAPKLSLEVRKLSNGRLSTSLKLGPLQPRGVPLREKKATQ | NIEFRKAFLKILSC | LYN |
| D4R | ATFRGLQRWEVARRAKLHGRAPRRPSGPGPPSPTPPAPRLPQDPCGPDCAPPAPGLPRGPCGPDCAPAAPSLPQDPCGPDCAPPAPGLPPDPCGSNCAPPDAVRAAALPPQTPPQTRRRRRAKITGRERKAMR | NAEFRNVFRKALRACC | SRC, FGR, HCK, LYN, |
| D5R | RIYRIAQVQIRRISSLERAAEHAQSCRSSAACAPDTSLRASIKKETKVLK | FNADFQKVFAQLLGCSHFCSRTPVETVNISNELISYNQDIVFHKEIAAAYIHMMPNAVTPGNREVDNDEEEGPFDRMFQIYQTSPDGDPVAESVWELDCEGEISLDKITPFTPNGFH | - |
Next to the typical interaction with polyproline motifs, another or an additional possibility is the interaction with phosphorylated tyrosine residues through the SH2 domain of SFKs. This could be shown for β2AR through mutation of residue Y350 in the C-terminal tail, which resulted in the decrease of Src phosphorylation and also impaired the desensitization of the receptor [99].
Dopamine receptors are a classical receptor family in which many family members contain polyproline motifs (Table 2). Multiple studies have confirmed the binding of SH3 domains to dopamine D2, D3, and D4 receptor [49,100,101]. However, it is unclear if there are additional adaptor or scaffold proteins involved in the activation mechanism. For dopamine D4 receptor, it could be shown that it directly activates the Src/SHC/Ras/ERK pathway [102]. The inhibition of Src by PP2 blocked ERK phosphorylation, which indicates signaling through Src for D2 and D4 receptors [103]. Recently, it was found that Fyn interacts with serotonin 5-HT6 receptor (5-HT6R) (Table 2) directly as well as in an arrestin-dependent manner to activate ERK1/2 [104].
7. GPCR-Mediated Src Signaling with Undefined Mediators
For several GPCRs, activation of an SFK was shown, but the exact regulation mechanism of the SFK is unknown. Some examples are the muscarinic M4 receptor (M4R), bradykinin receptor B1 (B1R), angiotensin type 2 receptor (ATR2), and A-type endothelin receptor (ETAR) (Table 1) [54,56,57,58,59,60]. For the V1b vasopressin receptor (V1bR), Src activation was shown, and a potential interaction of the SH2 domain with intracellular domains of the receptor as well as an arrestin-mediated activation of Src was discussed [50]. Src activation was also shown for two adhesion GPCRs, Latrophilin-2 (ADGRL2) and GPR56 (ADGRG1) [61,62]. For Latrophilin-2, Src activation was observed, which could be either independent of or dependent on CDK5 [61]. For GPR56, overexpression of the receptor in 295T cells resulted in Src–Fak activation, which is RhoA-independent [62]. The C-terminus of Latrophilin-2 is exceptionally long, with 375 amino acids, which suggests that it could act as an adaptor for downstream effectors. GPR56, on the other hand, displays a rather short C-terminus, with only 35 amino acids. However, this C-terminus entails several potential phosphorylation sites, which hints at an arrestin-mediated activation of the Src-kinase.
In general, not many studies are available that address the direct interaction of GPCRs with SFKs and subsequent SFK activation. For a more detailed understanding, additional studies are needed to shed light on the multiple ways in which SFKs transduce GPCR-mediated signals. Similar to arrestin SFKs can provide an additional signaling option through a GPCR that contributes to the physiological roles of this receptor. Deciphering the pathways that are mediated specifically through the SFKs will add to our understanding of the physiological functions of even known and established GPCRs. Being able to attribute intercellular signals and subsequent cellular functions specifically to the SFK opens the opportunity for a so far untapped biased signaling approach that could be exploited by pharmaceutical interventions.
Funding
Our research is funded by the German Research Foundation (CRC1423 project number 421152132 B05, FOR2149 project number 246212759, P5). We acknowledge support from the Leipzig University for Open Access Publishing.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tice D.A., Biscardi J.S., Nickles A.L., Parsons S.J. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc. Natl. Acad. Sci. USA. 1999;96:1415–1420. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cartwright C.A., Kamps M.P., Meisler A.I., Pipas J.M., Eckhart W. pp60c-src activation in human colon carcinoma. J. Clin. Investig. 1989;83:2025–2033. doi: 10.1172/JCI114113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talamonti M.S., Roh M.S., Curley S.A., Gallick G.E. Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J. Clin. Investig. 1993;91:53–60. doi: 10.1172/JCI116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guarino M. Src signaling in cancer invasion. J. Cell. Physiol. 2010;223:14–26. doi: 10.1002/jcp.22011. [DOI] [PubMed] [Google Scholar]
- 5.Luttrell D.K., Lee A., Lansing T.J., Crosby R.M., Jung K.D., Willard D., Luther M., Rodriguez M., Berman J., Gilmer T.M. Involvement of pp60c-src with two major signaling pathways in human breast cancer. Proc. Natl. Acad. Sci. USA. 1994;91:83–87. doi: 10.1073/pnas.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garmendia I., Pajares M.J., Hermida-Prado F., Ajona D., Bértolo C., Sainz C., Lavín A., Remírez A.B., Valencia K., Moreno H., et al. YES1 Drives Lung Cancer Growth and Progression and Predicts Sensitivity to Dasatinib. Am. J. Respir. Crit. Care Med. 2019;200:888–899. doi: 10.1164/rccm.201807-1292OC. [DOI] [PubMed] [Google Scholar]
- 7.Staley C.A., Parikh N.U., Gallick G.E. Decreased tumorigenicity of a human colon adenocarcinoma cell line by an antisense expression vector specific for c-Src. Cell Growth Differ. 1997;8:269–274. [PubMed] [Google Scholar]
- 8.Stettner M.R., Wang W., Nabors L.B., Bharara S., Flynn D.C., Grammer J.R., Gillespie G.Y., Gladson C.L. Lyn kinase activity is the predominant cellular SRC kinase activity in glioblastoma tumor cells. Cancer Res. 2005;65:5535–5543. doi: 10.1158/0008-5472.CAN-04-3688. [DOI] [PubMed] [Google Scholar]
- 9.Alcalá S., Mayoral-Varo V., Ruiz-Cañas L., López-Gil J.C., Heeschen C., Martín-Pérez J., Sainz B. Targeting SRC Kinase Signaling in Pancreatic Cancer Stem Cells. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21207437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel R.K., Weir M.C., Shen K., Snyder D., Cooper V.S., Smithgall T.E. Expression of myeloid Src-family kinases is associated with poor prognosis in AML and influences Flt3-ITD kinase inhibitor acquired resistance. PLoS ONE. 2019;14:e0225887. doi: 10.1371/journal.pone.0225887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han N.M., Curley S.A., Gallick G.E. Differential activation of pp60(c-src) and pp62(c-yes) in human colorectal carcinoma liver metastases. Clin. Cancer Res. 1996;2:1397–1404. [PubMed] [Google Scholar]
- 12.Qiu H., Miller W.T. Regulation of the nonreceptor tyrosine kinase Brk by autophosphorylation and by autoinhibition. J. Biol. Chem. 2002;277:34634–34641. doi: 10.1074/jbc.M203877200. [DOI] [PubMed] [Google Scholar]
- 13.Robinson D.R., Wu Y.M., Lin S.F. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- 14.Stein P.L., Vogel H., Soriano P. Combined deficiencies of Src, Fyn, and Yes tyrosine kinases in mutant mice. Genes Dev. 1994;8:1999–2007. doi: 10.1101/gad.8.17.1999. [DOI] [PubMed] [Google Scholar]
- 15.Kovács M., Németh T., Jakus Z., Sitaru C., Simon E., Futosi K., Botz B., Helyes Z., Lowell C.A., Mócsai A. The Src family kinases Hck, Fgr, and Lyn are critical for the generation of the in vivo inflammatory environment without a direct role in leukocyte recruitment. J. Exp. Med. 2014;211:1993–2011. doi: 10.1084/jem.20132496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Summy J.M., Gallick G.E. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/A:1023772912750. [DOI] [PubMed] [Google Scholar]
- 17.Biscardi J.S., Tice D.A., Parsons S.J. c-Src, receptor tyrosine kinases, and human cancer. Adv. Cancer Res. 1999;76:61–119. doi: 10.1016/s0065-230x(08)60774-5. [DOI] [PubMed] [Google Scholar]
- 18.Brunner A.M., Costa D.B., Heist R.S., Garcia E., Lindeman N.I., Sholl L.M., Oxnard G.R., Johnson B.E., Hammerman P.S. Treatment-related toxicities in a phase II trial of dasatinib in patients with squamous cell carcinoma of the lung. J. Thorac. Oncol. 2013;8:1434–1437. doi: 10.1097/JTO.0b013e3182a47162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koegl M., Zlatkine P., Ley S.C., Courtneidge S.A., Magee A.I. Palmitoylation of multiple Src-family kinases at a homologous N-terminal motif. Biochem. J. 1994;303:749–753. doi: 10.1042/bj3030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Resh M.D. Fatty acylation of proteins: New insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta. 1999;1451:1–16. doi: 10.1016/S0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 21.Pérez Y., Gairí M., Pons M., Bernadó P. Structural characterization of the natively unfolded N-terminal domain of human c-Src kinase: Insights into the role of phosphorylation of the unique domain. J. Mol. Biol. 2009;391:136–148. doi: 10.1016/j.jmb.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Arbesú M., Maffei M., Cordeiro T.N., Teixeira J.M.C., Pérez Y., Bernadó P., Roche S., Pons M. The Unique Domain Forms a Fuzzy Intramolecular Complex in Src Family Kinases. Structure. 2017;25:630–640.e4. doi: 10.1016/j.str.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Teixeira J.M.C., Fuentes H., Bielskutė S., Gairi M., Żerko S., Koźmiński W., Pons M. The Two Isoforms of Lyn Display Different Intramolecular Fuzzy Complexes with the SH3 Domain. Molecules. 2018;23 doi: 10.3390/molecules23112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurochkina N., Guha U. SH3 domains: Modules of protein-protein interactions. Biophys. Rev. 2013;5:29–39. doi: 10.1007/s12551-012-0081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper J.A., Gould K.L., Cartwright C.A., Hunter T. Tyr527 is phosphorylated in pp60c-src: Implications for regulation. Science. 1986;231:1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- 26.Smart J.E., Oppermann H., Czernilofsky A.P., Purchio A.F., Erikson R.L., Bishop J.M. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src) Proc. Natl. Acad. Sci. USA. 1981;78:6013–6017. doi: 10.1073/pnas.78.10.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nada S., Okada M., MacAuley A., Cooper J.A., Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991;351:69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- 28.Hamaguchi I., Yamaguchi N., Suda J., Iwama A., Hirao A., Hashiyama M., Aizawa S., Suda T. Analysis of CSK homologous kinase (CHK/HYL) in hematopoiesis by utilizing gene knockout mice. Biochem. Biophys. Res. Commun. 1996;224:172–179. doi: 10.1006/bbrc.1996.1003. [DOI] [PubMed] [Google Scholar]
- 29.Sicheri F., Moarefi I., Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385:602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- 30.Williams J.C., Weijland A., Gonfloni S., Thompson A., Courtneidge S.A., Superti-Furga G., Wierenga R.K. The 2.35 A crystal structure of the inactivated form of chicken Src: A dynamic molecule with multiple regulatory interactions. J. Mol. Biol. 1997;274:757–775. doi: 10.1006/jmbi.1997.1426. [DOI] [PubMed] [Google Scholar]
- 31.Xu W., Harrison S.C., Eck M.J. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 32.Ahler E., Register A.C., Chakraborty S., Fang L., Dieter E.M., Sitko K.A., Vidadala R.S.R., Trevillian B.M., Golkowski M., Gelman H., et al. A Combined Approach Reveals a Regulatory Mechanism Coupling Src’s Kinase Activity, Localization, and Phosphotransferase-Independent Functions. Mol. Cell. 2019;74:393–408.e20. doi: 10.1016/j.molcel.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Somani A.K., Bignon J.S., Mills G.B., Siminovitch K.A., Branch D.R. Src kinase activity is regulated by the SHP-1 protein-tyrosine phosphatase. J. Biol. Chem. 1997;272:21113–21119. doi: 10.1074/jbc.272.34.21113. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg G.S., Alexander D.B., Pellicena P., Zhang Z.-Y., Tsuda H., Miller W.T. Src phosphorylates Cas on tyrosine 253 to promote migration of transformed cells. J. Biol. Chem. 2003;278:46533–46540. doi: 10.1074/jbc.M307526200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjorge J.D., Pang A., Fujita D.J. Identification of protein-tyrosine phosphatase 1B as the major tyrosine phosphatase activity capable of dephosphorylating and activating c-Src in several human breast cancer cell lines. J. Biol. Chem. 2000;275:41439–41446. doi: 10.1074/jbc.M004852200. [DOI] [PubMed] [Google Scholar]
- 36.Kato G., Maeda S. Neuron-specific Cdk5 kinase is responsible for mitosis-independent phosphorylation of c-Src at Ser75 in human Y79 retinoblastoma cells. J. Biochem. 1999;126:957–961. doi: 10.1093/oxfordjournals.jbchem.a022540. [DOI] [PubMed] [Google Scholar]
- 37.Tiran Z., Peretz A., Attali B., Elson A. Phosphorylation-dependent regulation of Kv2.1 Channel activity at tyrosine 124 by Src and by protein-tyrosine phosphatase epsilon. J. Biol. Chem. 2003;278:17509–17514. doi: 10.1074/jbc.M212766200. [DOI] [PubMed] [Google Scholar]
- 38.Renkema G.H., Pulkkinen K., Saksela K. Cdc42/Rac1-mediated activation primes PAK2 for superactivation by tyrosine phosphorylation. Mol. Cell. Biol. 2002;22:6719–6725. doi: 10.1128/MCB.22.19.6719-6725.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z., Izaguirre G., Lin S.-Y., Lee H.Y., Schaefer E., Haimovich B. The phosphorylation of vinculin on tyrosine residues 100 and 1065, mediated by SRC kinases, affects cell spreading. Mol. Biol. Cell. 2004;15:4234–4247. doi: 10.1091/mbc.e04-03-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones D.A., Benjamin C.W. Phosphorylation of growth factor receptor binding protein-2 by pp60c-src tyrosine kinase. Arch. Biochem. Biophys. 1997;337:143–148. doi: 10.1006/abbi.1996.9789. [DOI] [PubMed] [Google Scholar]
- 41.Luttrell L.M., Hawes B.E., van Biesen T., Luttrell D.K., Lansing T.J., Lefkowitz R.J. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gbetagamma subunit-mediated activation of mitogen-activated protein kinases. J. Biol. Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- 42.Wang Q., Lu R., Zhao J., Limbird L.E. Arrestin serves as a molecular switch, linking endogenous alpha2-adrenergic receptor to SRC-dependent, but not SRC-independent, ERK activation. J. Biol. Chem. 2006;281:25948–25955. doi: 10.1074/jbc.M605415200. [DOI] [PubMed] [Google Scholar]
- 43.Pakharukova N., Masoudi A., Pani B., Staus D.P., Lefkowitz R.J. Allosteric activation of proto-oncogene kinase Src by GPCR-beta-arrestin complexes. J. Biol. Chem. 2020;295:16773–16784. doi: 10.1074/jbc.RA120.015400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao W., Luttrell L.M., Medvedev A.V., Pierce K.L., Daniel K.W., Dixon T.M., Lefkowitz R.J., Collins S. Direct binding of activated c-Src to the beta 3-adrenergic receptor is required for MAP kinase activation. J. Biol. Chem. 2000;275:38131–38134. doi: 10.1074/jbc.C000592200. [DOI] [PubMed] [Google Scholar]
- 45.Kaya A.I., Perry N.A., Gurevich V.V., Iverson T.M. Phosphorylation barcode-dependent signal bias of the dopamine D1 receptor. Proc. Natl. Acad. Sci. USA. 2020;117:14139–14149. doi: 10.1073/pnas.1918736117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao L.-M., Wang J.Q. Dopaminergic and cholinergic regulation of Fyn tyrosine kinase phosphorylation in the rat striatum in vivo. Neuropharmacology. 2015;99:491–499. doi: 10.1016/j.neuropharm.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang M., Zhang H., Voyno-Yasenetskaya T., Ye R.D. Requirement of Gbetagamma and c-Src in D2 dopamine receptor-mediated nuclear factor-kappaB activation. Mol. Pharmacol. 2003;64:447–455. doi: 10.1124/mol.64.2.447. [DOI] [PubMed] [Google Scholar]
- 48.Hattori K., Uchino S., Isosaka T., Maekawa M., Iyo M., Sato T., Kohsaka S., Yagi T., Yuasa S. Fyn is required for haloperidol-induced catalepsy in mice. J. Biol. Chem. 2006;281:7129–7135. doi: 10.1074/jbc.M511608200. [DOI] [PubMed] [Google Scholar]
- 49.Oldenhof J., Vickery R., Anafi M., Oak J., Ray A., Schoots O., Pawson T., von Zastrow M., van Tol H.H. SH3 binding domains in the dopamine D4 receptor. Biochemistry. 1998;37:15726–15736. doi: 10.1021/bi981634+. [DOI] [PubMed] [Google Scholar]
- 50.Perkovska S., Méjean C., Ayoub M.A., Li J., Hemery F., Corbani M., Laguette N., Ventura M.-A., Orcel H., Durroux T., et al. V1b vasopressin receptor trafficking and signaling: Role of arrestins, G proteins and Src kinase. Traffic. 2018;19:58–82. doi: 10.1111/tra.12535. [DOI] [PubMed] [Google Scholar]
- 51.Kraus S., Levy G., Hanoch T., Naor Z., Seger R. Gonadotropin-releasing hormone induces apoptosis of prostate cancer cells: Role of c-Jun NH2-terminal kinase, protein kinase B, and extracellular signal-regulated kinase pathways. Cancer Res. 2004;64:5736–5744. doi: 10.1158/0008-5472.CAN-04-1156. [DOI] [PubMed] [Google Scholar]
- 52.Kraus S., Benard O., Naor Z., Seger R. C-Src is Activated by the EGF Receptor in a Pathway that Mediates JNK and ERK Activation by Gonadotropin-Releasing Hormone in COS7 Cells. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21228575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tian M., Xu J., Lei G., Lombroso P.J., Jackson M.F., MacDonald J.F. STEP activation by Gαq coupled GPCRs opposes Src regulation of NMDA receptors containing the GluN2A subunit. Sci. Rep. 2016;6:36684. doi: 10.1038/srep36684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mao L.-M., Faris H.J., Wang J.Q. Muscarinic Acetylcholine Receptors Inhibit Fyn Activity in the Rat Striatum In Vivo. J. Mol. Neurosci. 2018;64:523–532. doi: 10.1007/s12031-018-1053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pronin A.N., Wang Q., Slepak V.Z. Teaching an Old Drug New Tricks: Agonism, Antagonism, and Biased Signaling of Pilocarpine through M3 Muscarinic Acetylcholine Receptor. Mol. Pharmacol. 2017;92:601–612. doi: 10.1124/mol.117.109678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng C.-Y., Tseng H.-C., Yang C.-M. Bradykinin-mediated cell proliferation depends on transactivation of EGF receptor in corneal fibroblasts. J. Cell. Physiol. 2012;227:1367–1381. doi: 10.1002/jcp.22849. [DOI] [PubMed] [Google Scholar]
- 57.Yu H.-S., Lin T.-H., Tang C.-H. Bradykinin enhances cell migration in human prostate cancer cells through B2 receptor/PKCδ/c-Src dependent signaling pathway. Prostate. 2013;73:89–100. doi: 10.1002/pros.22544. [DOI] [PubMed] [Google Scholar]
- 58.Simo-Cheyou E.R., Vardatsikos G., Srivastava A.K. Src tyrosine kinase mediates endothelin-1-induced early growth response protein-1 expression via MAP kinase-dependent pathways in vascular smooth muscle cells. Int. J. Mol. Med. 2016;38:1879–1886. doi: 10.3892/ijmm.2016.2767. [DOI] [PubMed] [Google Scholar]
- 59.Mima A., Matsubara T., Arai H., Abe H., Nagai K., Kanamori H., Sumi E., Takahashi T., Iehara N., Fukatsu A., et al. Angiotensin II-dependent Src and Smad1 signaling pathway is crucial for the development of diabetic nephropathy. Lab. Investig. 2006;86:927–939. doi: 10.1038/labinvest.3700445. [DOI] [PubMed] [Google Scholar]
- 60.Touyz R.M., Wu X.-H., He G., Salomon S., Schiffrin E.L. Increased angiotensin II-mediated Src signaling via epidermal growth factor receptor transactivation is associated with decreased C-terminal Src kinase activity in vascular smooth muscle cells from spontaneously hypertensive rats. Hypertension. 2002;39:479–485. doi: 10.1161/hy02t2.102909. [DOI] [PubMed] [Google Scholar]
- 61.Lee C.-S., Cho H.-J., Lee J.-W., Son H., Chai J., Kim H.-S. Adhesion GPCR Latrophilin-2 Specifies Cardiac Lineage Commitment through CDK5, Src, and P38MAPK. Stem Cell Rep. 2021;16:868–882. doi: 10.1016/j.stemcr.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chatterjee T., Zhang S., Posey T.A., Jacob J., Wu L., Yu W., Francisco L.E., Liu Q.J., Carmon K.S. Anti-GPR56 monoclonal antibody potentiates GPR56-mediated Src-Fak signaling to modulate cell adhesion. J. Biol. Chem. 2021;296:100261. doi: 10.1016/j.jbc.2021.100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luttrell L.M., Ferguson S.S., Daaka Y., Miller W.E., Maudsley S., Della Rocca G.J., Lin F., Kawakatsu H., Owada K., Luttrell D.K., et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 64.Gurevich V.V., Benovic J.L. Mechanism of phosphorylation-recognition by visual arrestin and the transition of arrestin into a high affinity binding state. Mol. Pharmacol. 1997;51:161–169. doi: 10.1124/mol.51.1.161. [DOI] [PubMed] [Google Scholar]
- 65.Goodman O.B., Krupnick J.G., Santini F., Gurevich V.V., Penn R.B., Gagnon A.W., Keen J.H., Benovic J.L. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 66.Gurevich V.V., Chen Q., Gurevich E.V. Arrestins: Introducing Signaling Bias into Multifunctional Proteins. Prog. Mol. Biol. Transl. Sci. 2018;160:47–61. doi: 10.1016/bs.pmbts.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peterson Y.K., Luttrell L.M. The Diverse Roles of Arrestin Scaffolds in G Protein-Coupled Receptor Signaling. Pharmacol. Rev. 2017;69:256–297. doi: 10.1124/pr.116.013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grundmann M., Merten N., Malfacini D., Inoue A., Preis P., Simon K., Rüttiger N., Ziegler N., Benkel T., Schmitt N.K., et al. Lack of beta-arrestin signaling in the absence of active G proteins. Nat. Commun. 2018;9:1–16. doi: 10.1038/s41467-017-02661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kang Y., Zhou X.E., Gao X., He Y., Liu W., Ishchenko A., Barty A., White T.A., Yefanov O., Han G.W., et al. Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature. 2015;523:561–567. doi: 10.1038/nature14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shukla A.K., Westfield G.H., Xiao K., Reis R.I., Huang L.-Y., Tripathi-Shukla P., Qian J., Li S., Blanc A., Oleskie A.N., et al. Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature. 2014;512:218–222. doi: 10.1038/nature13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Q., Perry N.A., Vishnivetskiy S.A., Berndt S., Gilbert N.C., Zhuo Y., Singh P.K., Tholen J., Ohi M.D., Gurevich E.V., et al. Structural basis of arrestin-3 activation and signaling. Nat. Commun. 2017;8:1427. doi: 10.1038/s41467-017-01218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shukla A.K., Manglik A., Kruse A.C., Xiao K., Reis R.I., Tseng W.-C., Staus D.P., Hilger D., Uysal S., Huang L.-Y., et al. Structure of active β-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature. 2013;497:137–141. doi: 10.1038/nature12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee Y., Warne T., Nehmé R., Pandey S., Dwivedi-Agnihotri H., Chaturvedi M., Edwards P.C., García-Nafría J., Leslie A.G.W., Shukla A.K., et al. Molecular basis of β-arrestin coupling to formoterol-bound β1-adrenoceptor. Nature. 2020;583:862–866. doi: 10.1038/s41586-020-2419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cahill T.J., Thomsen A.R.B., Tarrasch J.T., Plouffe B., Nguyen A.H., Yang F., Huang L.-Y., Kahsai A.W., Bassoni D.L., Gavino B.J., et al. Distinct conformations of GPCR-β-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc. Natl. Acad. Sci. USA. 2017;114:2562–2567. doi: 10.1073/pnas.1701529114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Q., Iverson T.M., Gurevich V.V. Structural Basis of Arrestin-Dependent Signal Transduction. Trends Biochem. Sci. 2018;43:412–423. doi: 10.1016/j.tibs.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang F., Xiao P., Qu C.-X., Liu Q., Wang L.-Y., Liu Z.-X., He Q.-T., Liu C., Xu J.-Y., Li R.-R., et al. Allosteric mechanisms underlie GPCR signaling to SH3-domain proteins through arrestin. Nat. Chem. Biol. 2018;14:876–886. doi: 10.1038/s41589-018-0115-3. [DOI] [PubMed] [Google Scholar]
- 77.Milano S.K., Pace H.C., Kim Y.-M., Brenner C., Benovic J.L. Scaffolding functions of arrestin-2 revealed by crystal structure and mutagenesis. Biochemistry. 2002;41:3321–3328. doi: 10.1021/bi015905j. [DOI] [PubMed] [Google Scholar]
- 78.Zhan X., Gimenez L.E., Gurevich V.V., Spiller B.W. Crystal structure of arrestin-3 reveals the basis of the difference in receptor binding between two non-visual subtypes. J. Mol. Biol. 2011;406:467–478. doi: 10.1016/j.jmb.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuo F.-T., Lu T.-L., Fu H.-W. Opposing effects of beta-arrestin1 and beta-arrestin2 on activation and degradation of Src induced by protease-activated receptor 1. Cell. Signal. 2006;18:1914–1923. doi: 10.1016/j.cellsig.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 80.Cesareni G., Panni S., Nardelli G., Castagnoli L. Can we infer peptide recognition specificity mediated by SH3 domains? FEBS Lett. 2002;513:38–44. doi: 10.1016/S0014-5793(01)03307-5. [DOI] [PubMed] [Google Scholar]
- 81.Feng S., Kasahara C., Rickles R.J., Schreiber S.L. Specific interactions outside the proline-rich core of two classes of Src homology 3 ligands. Proc. Natl. Acad. Sci. USA. 1995;92:12408–12415. doi: 10.1073/pnas.92.26.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grabs D., Slepnev V.I., Songyang Z., David C., Lynch M., Cantley L.C., de Camilli P. The SH3 domain of amphiphysin binds the proline-rich domain of dynamin at a single site that defines a new SH3 binding consensus sequence. J. Biol. Chem. 1997;272:13419–13425. doi: 10.1074/jbc.272.20.13419. [DOI] [PubMed] [Google Scholar]
- 83.Huang W., Masureel M., Qu Q., Janetzko J., Inoue A., Kato H.E., Robertson M.J., Nguyen K.C., Glenn J.S., Skiniotis G., et al. Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature. 2020;579:303–308. doi: 10.1038/s41586-020-1953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Staus D.P., Hu H., Robertson M.J., Kleinhenz A.L.W., Wingler L.M., Capel W.D., Latorraca N.R., Lefkowitz R.J., Skiniotis G. Structure of the M2 muscarinic receptor-β-arrestin complex in a lipid nanodisc. Nature. 2020;579:297–302. doi: 10.1038/s41586-020-1954-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simon M.I., Strathmann M.P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 86.Ma Y.C., Huang J., Ali S., Lowry W., Huang X.Y. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102:635–646. doi: 10.1016/S0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 87.Berlot C.H., Bourne H.R. Identification of effector-activating residues of Gs alpha. Cell. 1992;68:911–922. doi: 10.1016/0092-8674(92)90034-A. [DOI] [PubMed] [Google Scholar]
- 88.Tesmer J.J., Sunahara R.K., Gilman A.G., Sprang S.R. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha.GTPgammaS. Science. 1997;278:1907–1916. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- 89.Syrovatkina V., Alegre K.O., Dey R., Huang X.-Y. Regulation, Signaling, and Physiological Functions of G-Proteins. J. Mol. Biol. 2016;428:3850–3868. doi: 10.1016/j.jmb.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Parra-Mercado G.K., Fuentes-Gonzalez A.M., Hernandez-Aranda J., Diaz-Coranguez M., Dautzenberg F.M., Catt K.J., Hauger R.L., Olivares-Reyes J.A. CRF1 Receptor Signaling via the ERK1/2-MAP and Akt Kinase Cascades: Roles of Src, EGF Receptor, and PI3-Kinase Mechanisms. Front. Endocrinol. 2019;10:869. doi: 10.3389/fendo.2019.00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang F., Steinberg S.F. S49G and R389G polymorphisms of the β₁-adrenergic receptor influence signaling via the cAMP-PKA and ERK pathways. Physiol. Genom. 2013;45:1186–1192. doi: 10.1152/physiolgenomics.00087.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brovkovych V., Zhang Y., Brovkovych S., Minshall R.D., Skidgel R.A. A novel pathway for receptor-mediated post-translational activation of inducible nitric oxide synthase. J. Cell. Mol. Med. 2011;15:258–269. doi: 10.1111/j.1582-4934.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moyers J.S., Linder M.E., Shannon J.D., Parsons S.J. Identification of the in vitro phosphorylation sites on Gs alpha mediated by pp60c-src. Biochem. J. 1995;305:411–417. doi: 10.1042/bj3050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hausdorff W.P., Pitcher J.A., Luttrell D.K., Linder M.E., Kurose H., Parsons S.J., Caron M.G., Lefkowitz R.J. Tyrosine phosphorylation of G protein alpha subunits by pp60c-src. Proc. Natl. Acad. Sci. USA. 1992;89:5720–5724. doi: 10.1073/pnas.89.13.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chakravorty D., Assmann S.M. G protein subunit phosphorylation as a regulatory mechanism in heterotrimeric G protein signaling in mammals, yeast, and plants. Biochem. J. 2018;475:3331–3357. doi: 10.1042/BCJ20160819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bell M.W., Desai N., Guo X.X., Ghalayini A.J. Tyrosine phosphorylation of the alpha subunit of transducin and its association with Src in photoreceptor rod outer segments. J. Neurochem. 2000;75:2006–2019. doi: 10.1046/j.1471-4159.2000.0752006.x. [DOI] [PubMed] [Google Scholar]
- 97.Romero G., von Zastrow M., Friedman P.A. Role of PDZ proteins in regulating trafficking, signaling, and function of GPCRs: Means, motif, and opportunity. Adv. Pharmacol. 2011;62:279–314. doi: 10.1016/B978-0-12-385952-5.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu J., Liao Z., Camden J., Griffin K.D., Garrad R.C., Santiago-Pérez L.I., González F.A., Seye C.I., Weisman G.A., Erb L. Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J. Biol. Chem. 2004;279:8212–8218. doi: 10.1074/jbc.M312230200. [DOI] [PubMed] [Google Scholar]
- 99.Fan G., Shumay E., Malbon C.C., Wang H. c-Src tyrosine kinase binds the beta 2-adrenergic receptor via phospho-Tyr-350, phosphorylates G-protein-linked receptor kinase 2, and mediates agonist-induced receptor desensitization. J. Biol. Chem. 2001;276:13240–13247. doi: 10.1074/jbc.M011578200. [DOI] [PubMed] [Google Scholar]
- 100.Oldenhof J., Ray A., Vickery R., van Tol H.H. SH3 ligands in the dopamine D3 receptor. Cell. Signal. 2001;13:411–416. doi: 10.1016/S0898-6568(01)00157-7. [DOI] [PubMed] [Google Scholar]
- 101.Nair V.D., Sealfon S.C. Agonist-specific transactivation of phosphoinositide 3-kinase signaling pathway mediated by the dopamine D2 receptor. J. Biol. Chem. 2003;278:47053–47061. doi: 10.1074/jbc.M303364200. [DOI] [PubMed] [Google Scholar]
- 102.Zhen X., Zhang J., Johnson G.P., Friedman E. D(4) dopamine receptor differentially regulates Akt/nuclear factor-kappa b and extracellular signal-regulated kinase pathways in D(4)MN9D cells. Mol. Pharmacol. 2001;60:857–864. [PubMed] [Google Scholar]
- 103.Oak J.N., Lavine N., van Tol H.H. Dopamine D(4) and D(2L) Receptor Stimulation of the Mitogen-Activated Protein Kinase Pathway Is Dependent on trans-Activation of the Platelet-Derived Growth Factor Receptor. Mol. Pharmacol. 2001;60:92–103. doi: 10.1124/mol.60.1.92. [DOI] [PubMed] [Google Scholar]
- 104.Liu P., Yin Y.-L., Wang T., Hou L., Wang X.-X., Wang M., Zhao G.-G., Shi Y., Xu H.E., Jiang Y. Ligand-induced activation of ERK1/2 signaling by constitutively active Gs-coupled 5-HT receptors. Acta Pharmacol. Sin. 2019;40:1157–1167. doi: 10.1038/s41401-018-0204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kundu K., Costa F., Backofen R. A graph kernel approach for alignment-free domain-peptide interaction prediction with an application to human SH3 domains. Bioinformatics. 2013;29:i335–i343. doi: 10.1093/bioinformatics/btt220. [DOI] [PMC free article] [PubMed] [Google Scholar]