Abstract
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has changed the lives of people around the world. Fortunately, sufficient vaccines are now available. Local reactions with ipsilateral lymphadenopathy are among the most common side effects. We investigated the impact of lymphadenopathy after COVID-19 vaccination on the value of ultrasound in tumour patients.
Patients and methods
Patients with melanoma or Merkel cell carcinoma were included who underwent lymph node excision and received COVID-19 vaccination within 6 weeks before surgery. The consistency of the preoperative ultrasound findings with the histopathologic findings was investigated.
Results
Eight patients were included (two Merkel cell carcinoma and six melanoma patients) who underwent lymph node excision between 16th April 2021 and 19th May 2021 and had previously received COVID-19 vaccination. In three of the eight patients (one Merkel cell carcinoma and two melanoma patients), lymph node metastases were erroneously diagnosed preoperatively during tumour follow-up with physical examination, ultrasound, and or fluorodeoxyglucose (FDG)–positron emission tomography (PET)/computed tomography (CT). In these three patients, the suspected lymph node metastases were located in the left axilla after COVID-19 vaccination in the left upper arm, which resulted in selective lymph node removal in two patients and complete lymphadenectomy in one patient.
Conclusion
COVID-19 vaccine–associated lymphadenopathy is expected to be observed much more frequently in the near future because of increasing vaccination rates. This cause of lymphadenopathy, which may in ultrasound as well as in FDG PET/CT resemble lymph node metastases, must be considered, especially in oncologic patients undergoing tumour follow-up. In addition, COVID-19 vaccination should be given as far away as possible from an underlying primary on the contralateral side to avoid oncologic misdiagnosis followed by malpractice.
Keywords: Melanoma, Merkel cell carcinoma, COVID-19 vaccine, Ultrasound, Lymphadenopathy
1. Introduction
The introduction of modern systemic therapies such as immune checkpoint blockade has led to a significant improvement in the prognosis of tumour patients with advanced skin tumours, for example, melanoma, Merkel cell carcinoma, and squamous cell carcinoma [[1], [2], [3]]. However, early detection of metastases is of crucial importance for patient outcome. Currently, computed tomographic and magnetic resonance techniques are used as standard of care in suspected distant metastases and in the follow-up of skin cancer patients [[4], [5], [6]]. As another important method lymph node ultrasound with transducers of 7.5–18 MHz has been successfully used for many years for the detection of metastases in primary spread diagnostics as well as in the follow-up of aggressive tumours, such as melanoma [7]. Ultrasound has a higher sensitivity and specificity with regard to the question of lymph node metastases than the other radiological imaging procedures [8]. The coronavirus disease 2019 (COVID-19) pandemic has been raging worldwide since late 2019, with more than 160 million people infected and more than 3.5 million people who have died so far [9]. Fortunately, several vector-based and messenger RNA (mRNA) vaccines have become available in recent months that provide adequate protection against infection [10]. All COVID-19 vaccines licensed in Europe and the United States show good patient safety. Patients often experience modest systemic side effects after COVID-19 vaccination, such as fatigue, fever, chills, headache, or local side effects, such as swelling at the injection site. Younger patients seem to be more affected by side effects [11]. Another common side effect after COVID-19 vaccination is ipsilateral lymph node swelling, with recent reports of increased fluorodeoxyglucose (FDG) uptake on positron emission tomography (PET)-computed tomography (CT) being observed in patients up to 6 weeks after vaccination [12]. Furthermore, sonographically detectable lymphadenopathy after COVID-19 vaccination has already been reported in a recent case report [13]. The following work aims at investigating the impact of COVID-19 vaccination on the validity of lymph node ultrasound in primary spread diagnosis and follow-up in skin cancer patients.
2. Patients and methods
2.1. Patients
The study included patients with histologically confirmed melanoma or Merkel cell carcinoma who underwent sentinel lymph node biopsy, selective lymph node extirpation, or complete lymph node dissection. Only patients who have received COVID-19 vaccination within the past 6 weeks were included. Patient characteristics were obtained from the clinical database of Essen University Hospital. These characteristics included tumour type, tumour stage, preoperative sonographic findings, further preoperative radiological imaging, clinical symptoms, type of surgery, location of lymph node removal, and pathologic findings of the removed lymph nodes. The study was approved by the ethics committee of Duisburg-Essen University and conducted in accordance with the Declaration of Helsinki (BO 21-10115). All surgical procedures were performed according to the German guideline recommendations after discussion in the interdisciplinary tumour conference.
2.2. Lymph node ultrasound
Ultrasound examinations of lymph nodes were performed by specially trained dermatologists (according to the German Society for Ultrasound in Medicine/DEGUM) using a high-resolution real-time scanner with a 13 MHz transducer (MyLab Xvision, Esaote, Cologne, Germany). Ultrasound scanning of the regional lymph nodes was standardised. The lymph node regions were examined with the patient lying down. To examine the cervical region, the patient's head was turned to the opposite side. Initially, the parotid gland was examined in two directions. The transducer was then moved to the retroauricular area. After the cervical vessels, the complete neck was examined, including the surrounding tissue from the insertion of the sternocleidomastoid muscle to the upper thoracic aperture. Evaluation of the submandibular and, finally, the supraclavicular and the infraclavicular regions followed. Axillary examination was performed with the patient's arm abducted to 120°. We used the axillary artery as a ‘leading structure’ for scanning the upper axillary region, moving toward the brachial artery and the proximal third of the upper arm heading toward the opposite side to the subclavian artery. Finally, the area between the musculus latissimus dorsi (posterior axillary line), the pectoral muscles (anterior axillary line), and the proximal parts of the upper arm were completely investigated.
To examine the inguinal lymph nodes, we followed the inguinal ligament from the anterior superior iliac spine to the symphysis crossing the inguinal and femoral vessels. The transducer followed the vessels in a horizontal plane 5–10 cm above the inguinal ligament. Inferior to the inguinal ligament, the adductor canal was completely scanned. The upper third of the thigh, lateral and medial to the femoral vessels, was examined in two directions. The investigations were performed in longitudinal and transversal sections. All suspect lymph nodes were documented using a longitudinal and transverse documentation system. Ultrasound assessment was based on morphological criteria (size, shape, echogenicity of the centre, and cortex of the lymph node). To interpret the two-dimensional shape of lymph nodes, we adhered to the criteria proposed by Solbiati et al. and Vassallo et al. [14,15]. The respective index (ratio of maximal and minimal lymph node diameters) was calculated for suspicious lymph nodes. Lymph nodes were considered suspicious of malignancy when at least one of the following criteria was applied: Solbiati−Vasallo index less than two, predominance of low echogenicity of the whole lymph node structure, lymph node centre with low echogenicity, or asymmetric regions with low echogenicity in the lymph node margin. The Doppler technique was only used to distinguish blood vessels and other low echogenic structures.
2.3. Histopathological analysis
For histopathological examination, standard staining consisting of haematoxylin and eosin staining was performed. In addition, immunohistochemical workup of lymph node tissue was performed according to the underlying tumour type on an automated platform (Ventana Benchmark Ultra, Ventana Medical Systems, AZ, USA) adherent to manufacturer's protocols as follows: Melan-A (Clone: A103, ready to use kit; Ventana Medical Systems) and MITF (Clone: D5, 1:100; Agilent Dako, CA, USA) for melanoma and CK20 (Clone: Ks20.8, 1:1000; Agilent Dako) and Synaptophysin (Clone: MRQ-40, ready to use kit; Ventana Medical Systems) for Merkel cell carcinoma. Histopathologic evaluation of the lymph nodes was performed by an experienced pathologist from the Institute of Pathology or by an experienced dermatopathologist from the Department of Dermatology, Essen University Hospital. Histopathological lymph node specimens were digitised with the Aperio AT2 whole-slide scanner (Leica, Wetzlar, Germany) at 20× resolution.
2.4. Statistical analysis
Statistical analysis was performed with SPSS Statistics (Version 27; IBM Corp.; Armonk, NY). For statistical testing, the chi-square test and Mann–Whitney U test were performed. A P value of <0.05 was considered statistically significant.
3. Results
3.1. Patients
Between 16th April 2021 and 19th May 2021, a total of eight patients with melanoma or Merkel cell carcinoma were identified in whom the indication for lymph node surgery and at least one COVID-19 vaccination had previously taken place in the Department of Dermatology at the University Hospital Essen. Vaccination was performed in the left upper arm in all patients. The mean age was 52.5 years (28-91 years), with equal numbers of male and female patients. Six patients had melanoma as their underlying disease, and two patients had Merkel cell carcinoma, with two patients having received prior systemic therapy, one with targeted BRAF/MEK inhibition and one with anti-PD1–based immune checkpoint blockade. Five patients underwent sentinel lymph node excision, two patients underwent targeted lymph node extirpation, and one patient underwent complete lymphadenectomy. Half of the patients had previously received one COVID-19 vaccination, and the other half of the patients had already received two vaccinations. All patients received mRNA-based vaccines; seven patients received BioNTech and Pfizer vaccine and one patient received CureVac vaccine within the scope of an approved study. On average, patients had received the last vaccination 23 days (7–50 days) before surgery (Table 1 ).
Table 1.
Patient characteristics.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 54 | 28 | 58 | 77 | 91 | 44 | 43 | 84 |
| Sex | Female | Female | Male | Male | Male | Male | Female | Female |
| Primary tumour | Melanoma | Melanoma | MCC | Melanoma | MCC | Melanoma | Melanoma | Melanoma |
| Previous tumour therapy | None | None | None | None | None | Dabrafenib and trametinib | None | |
| Type of vaccine | CureVac | BioNTech/Pfizer | BioNTech/Pfizer | BioNTech/Pfizer | BioNTech/Pfizer | BioNTech/Pfizer | BioNTech/Pfizer | BioNTech/Pfizer |
| Number of vaccines | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 2 |
| Time between vaccine and ultrasound (days) | 30 | 28 | 7 | 11 | 16 | 15 | 50 | 12 |
| Performed surgery | Sentinel lymph node excision | Selective lymph node excision | Selective lymph node excision | Sentinel lymph node excision | Sentinel lymph node excision | Sentinel lymph node excision | Complete lymph-adenectomy | Sentinel lymph node excision |
MCC, Merkel cell carcinoma.
3.2. Impact of COVID-19 vaccination on preoperative significance of lymph node ultrasound
In the present study, five patients underwent routine ultrasound examination before sentinel lymph node sampling; here, three patients showed unremarkable lymph nodes preoperatively, and histopathological evaluation did not reveal evidence of metastases. In the other two patients, malignancy could not be excluded with certainty on ultrasound, and targeted lymph node removal was performed in addition to sentinel lymph node sampling, each of which showed evidence of metastases. Thus, five of eight patients (62.5%) showed a concordant result on preoperative ultrasound and histology. In the remaining three patients (37.5%), ultrasound examination of the lymph nodes was performed as part of tumour follow-up. All patients showed enlarged lymph nodes suspected of metastasis in the left axilla with partial marginal vascularisation and decreased echogenicity. After lymph node removal, histopathology showed no evidence of metastasis in these patients.
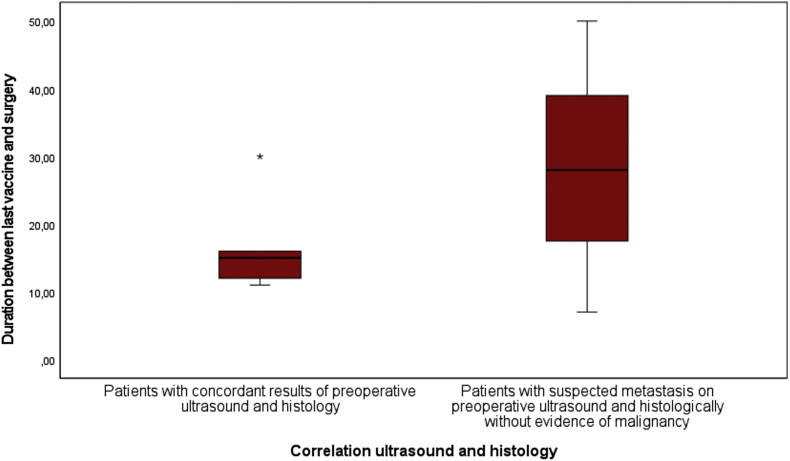
Subsequently, various patient characteristics were examined as influencing factors that may have contributed to the misdiagnosis. Patients in whom the sonographic and histologic results of the lymph nodes matched had received the vaccine a mean of 16.8 days (11.8–30 days) before surgery. In contrast, patients with different sonographic and histologic results had received the vaccine on average 28.3 days (7-50 days) before surgery, whereas the difference was not statistically significant (P = 0.65; Fig. 1 ). Regarding surgical localisation, it was found that all sonographically classified false-positive lymph nodes were localised in the left axilla. With a small number of cases, the statistical difference was not significant (P = 0.091; Fig. 2 ). Regarding gender, two women and one man showed false-positive lymph nodes preoperatively. Regarding the number of vaccinations, two patients had received only one vaccination, and one patient had received two vaccinations.
Fig. 1.
Boxplots show the time between the last COVID-19 vaccination and ultrasound examination for patients.
Fig. 2.
The bar graph show the distribution of surgical locations of sonographically correct and false estimation regarding metastasis.
3.3. Selected patient cases demonstrating that COVID-19 vaccination is a potential pitfall in the diagnosis of lymph node metastasis by ultrasound
3.3.1. Case 1
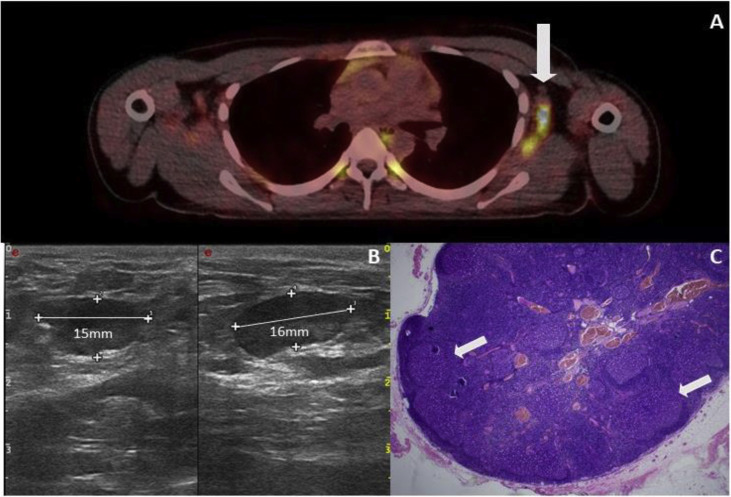
A 27-year-old female patient had an initial diagnosis of a 0.4-mm-thick melanoma on the left scapula in September 2020, followed by removal of a melanoma in situ on the left lower leg in January 2021. On 29th March 2021, the first vaccination with BioNTech's vaccine took place. On 15th April 2021, an FDG PET/CT scan ordered by the primary care physician showed highly malignant lymph nodes in the left axilla (Fig. 3 A). One week later, the patient presented to our clinic for the first time. A lymph node ultrasound was performed, which showed multiple lymph nodes, some of which were echo-deficient, with increased marginal vascularisation and a maximum diameter of 1.6 cm in the left axilla, so there was a high suspicion of lymph node metastases (Fig. 3B). Together with the patient, the performance of a diagnostic lymph node removal was determined. A total of four intraoperatively conspicuous lymph nodes were removed from the left axilla. Histopathologically, there was no evidence of infiltration by melanoma but marked lymphofollicular hyperplasia in all lymph nodes (Fig. 3C). The patient's postoperative clinical course was without complications.
Fig. 3.
Case 1. (A) PET-CT examination of the patient, which shows a pronounced FDG accumulation in the area of the lymph nodes of the left axilla ( ). (B) Lymph node ultrasound shows pronounced enlargement with a clear decrease in echogenicity. (C) Histological picture of the lymph node showing follicular hyperplasia (
). (B) Lymph node ultrasound shows pronounced enlargement with a clear decrease in echogenicity. (C) Histological picture of the lymph node showing follicular hyperplasia ( ). Metastases from the melanoma cannot be seen (HE).
). Metastases from the melanoma cannot be seen (HE).
3.3.2. Case 2
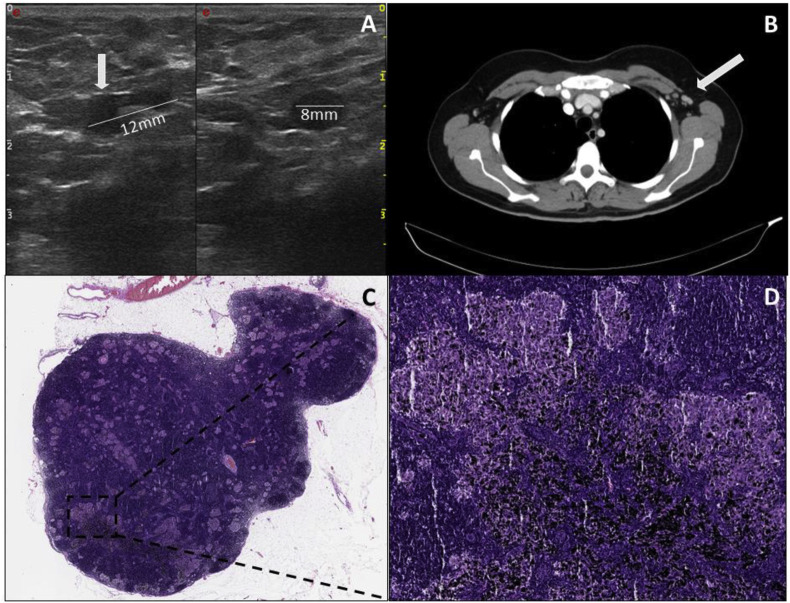
A 43-year-old woman was first diagnosed with melanoma with a tumour thickness of 1.4 mm in the left mammillary area in June 2020. Sentinel lymph node excision was performed in the left axilla, which showed a metastasis with a maximum diameter of 0.8 mm. Consecutive tumour staging by CT and magnetic resonance imaging showed no evidence of metastasis. Subsequently, with the BRAF V600E mutation present, adjuvant therapy with dabrafenib and trametinib was initiated in August 2020. On 2nd March 2021, the first vaccination and on 23rd March 2021, the second vaccination with BioNTech's vaccine took place. In late April 2021, lymph node metastases were suspected sonographically in the left axilla during tumour follow-up (Fig. 4 A). Consecutive radiologic imaging with CT scanning also revealed suspicious lymph nodes for metastasis in the left axilla (Fig. 4B). Therefore, the indication for complete lymphadenectomy was decided in the interdisciplinary tumour conference. In mid-May 2021, a complete lymph node removal of the left axilla was performed. Intraoperatively, there were several black coloured lymph nodes measuring a maximum of 1 cm. Histologically, there was no evidence of melanoma metastasis in the 15 harvested lymph nodes; instead, there were histologic signs of a sarcoid-like reaction. The black discolouration of the lymph nodes was a pigment deposit from a long-standing tattoo on the patient's left scapula (Fig. 4C and D). Postoperatively, the patient showed marked lymphorrhea, requiring multiple sclerotherapy with ethoxysclerol. The patient was hospitalised for a total of 14 days.
Fig. 4.
Case 2. (A) (Left) Ultrasound shows an enlarged lymph node with preserved vascular hilus and unilaterally widened, echo-poor margin. (Right) In the other plane, the enlarged area is echo-poor and rounded. (B) CT scan shows multiple lymph nodes suspicious for malignancy in the left axilla ( ). (C) Histopathologic image of the lymph node. (D)The sarcoidosis-like granulomas/lesions are visible in addition to distinct pigmentary deposits associated with the patient's underlying tattooing.
). (C) Histopathologic image of the lymph node. (D)The sarcoidosis-like granulomas/lesions are visible in addition to distinct pigmentary deposits associated with the patient's underlying tattooing.
4. Discussion
In this study, we describe for the first time to the best of our knowledge sonographically detectable lymph node changes after COVID-19 vaccination in skin cancer patients during tumour follow-up. Lymph node changes resembling lymph node metastases in terms of enlargement and peripheral vascularisation occurred relatively frequently in patients, especially after inoculation into the left upper arm in the area of the left axilla. The time interval between vaccination and ultrasound did not appear to play a significant role, although in one patient vaccination had occurred 50 days before the abnormal ultrasound. This observation is consistent with the results of a recent radiological study, which showed that increased FDG uptake on PET/CT was still detectable in ipsilateral lymph node stations in patients vaccinated with COVID-19 up to 70 days after vaccination [12]. Histologically, one of the patients whose lymph nodes were misjudged to be malignant preoperatively showed marked follicular hyperplasia in all lymph nodes. Marked reactive follicular hyperplasia of the lymph nodes is otherwise frequently observed in the context of infections, such as syphilis, toxoplasmosis, HIV, infectious mononucleosis, or in the context of autoimmune diseases, such as rheumatoid arthritis or Sjögren's syndrome [16]. Interestingly, another patient whose lymph node was misclassified as malignant showed histopathologically a lymphofollicular hyperplasia and pronounced sarcoidosis-like lymphadenopathy. The granulomatous changes can be triggered by activated T-lymphocytes and macrophages through their secretion of interferon gamma, tumour necrosis factor α, and interleukin 12 [17]. Granulomatous lymph node changes have also been described extensively in patients under immune checkpoint blockade therapy [18,19]. Our patient was under adjuvant therapy with BRAF and MEK inhibition, and granulomatous changes are not typical for this therapy. It should be noted that in rare cases, sarcoidosis is also induced by targeted therapy with BRAF and MEK inhibition, in which case mainly the hilar lung lymph nodes are affected [20].
Of course, our study has some limitations, such as the small number of cases. Furthermore, it is not a controlled randomised trial, and it covers a very short observation period of only one month. Nevertheless, because of the high clinical relevance with COVID-19 vaccination rates continuing to increase rapidly in the future, we believe it is important to report the sonographic and histopathologic observations of patients after COVID-19 vaccination in the context of tumour follow-up.
In conclusion, lymph node enlargements suspicious for malignancy are likely to be detected more frequently during follow-up of tumour patients with imaging techniques, such as ultrasound and or FDG PET/CT after COVID-19 vaccination. Patients should be asked about previous COVID-19 vaccinations if lymph node enlargement is unclear, especially axillary. In these cases, a repeat ultrasound examination of the affected lymph node region should be considered after 6–8weeks. As part of the preoperative ultrasound diagnosis before sentinel lymph node removal, the suspicion of metastasis, especially axillary, should also be critically questioned in a condition after COVID-19 vaccination. In these cases, the indication for lymphadenectomy should not be rushed. Finally, in tumour patients, the vaccinating physician should perform the vaccination contralateral to the primary to avoid oncologic misdiagnosis and malpractice because of unnecessary lymph node surgery.
Authors’ contributions
J.M.P. and J.K. analysed and interpreted the patient data and were major contributors to writing the article. H.R. and E.H. performed the histological examinations. A.R., D.S., and I.S. helped discuss the clinical findings and provided critical feedback and helped design the research and analysis and approved the final version of the article.
Consent for publication
Consent for publication is available on request.
Availability of data and materials
The data sets used and/or analysed during the present study are available from the corresponding author on reasonable request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Conflict of interest statement
J.M.P. served as consultant and/or has received honoraria from Bristol-Myers Squibb and Novartis and has received travel support from Bristol-Myers Squibb, Novartis, and Therakos. J.K. reported grants and or personal fees from Novartis, LaVision Bio Tec, and SastoMed. A.R. reported grants from Novartis, Bristol Myers Squibb, and ADTEC; personal fees from Merck Sharp & Dohme; and nonfinancial support from Amgen, Roche, Merck Sharp & Dohme, Novartis, Bristol Myers Squibb, and Teva. D.S. received grants and other support from Bristol-Myers Squibb, personal fees from Bristol-Myers Squibb during the conduct of the study; personal fees from Amgen; personal fees from Boehringer Ingelheim; personal fees from InFlarX; personal fees and other support from Roche; grants, personal fees, and other support from Novartis; personal fees from Incyte; personal fees and other support from Regeneron; personal fees from 4SC; personal fees from Sanofi; personal fees from NeraCare; personal fees from Pierre-Fabre; personal fees and other support from Merck-EMD; personal fees from Pfizer; personal fees and other support from Philogen; personal fees from Array; and personal fees and other support from MSD Sharp & Dohme, outside the submitted work. H.R. is on the advisory board of Bristol-Myers Squibb, received honoraria from Roche and Bristol-Myers Squibb, received travel support from Philips, Roche, and Bristol-Myers Squibb, received grants from Bristol-Myers Squibb, and holds shares of Bayer. I.S. and E.H. declared that they have no conflicts of interest with respect to the authorship or the publication of this article.
Acknowledgements
The authors are indebted to all patients and their relatives.
References
- 1.D'Angelo S.P., et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol. 2018;4(9) doi: 10.1001/jamaoncol.2018.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J., et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 3.Migden M.R., et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 4.DeRose E.R., et al. Utility of 3-year torso computed tomography and head imaging in asymptomatic patients with high-risk melanoma. Melanoma Res. 2011;21(4):364–369. doi: 10.1097/CMR.0b013e3283471086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hausmann D., et al. Comparison of the diagnostic accuracy of whole-body MRI and whole-body CT in stage III/IV malignant melanoma. J Dtsch Dermatol Ges. 2011;9(3):212–222. doi: 10.1111/j.1610-0387.2011.07614.x. [DOI] [PubMed] [Google Scholar]
- 6.Schadendorf D., et al. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Canc. 2017;71:53–69. doi: 10.1016/j.ejca.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Bafounta M.-L., et al. Ultrasonography or palpation for detection of melanoma nodal invasion: a meta-analysis. Lancet Oncol. 2004;5(11):673–680. doi: 10.1016/S1470-2045(04)01609-2. [DOI] [PubMed] [Google Scholar]
- 8.Xing Y., et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. JNCI: J Natl Cancer Inst. 2011;103(2):129–142. doi: 10.1093/jnci/djq455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO, World Health Organization Coronavirus (COVID-19) Dashboard; https://covid19.who.int. 2021, May 29.
- 10.Forni G., et al. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28(2):626–639. doi: 10.1038/s41418-020-00720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh E.E., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eshet Y., et al. Prevalence of increased FDG PET/CT axillary lymph node uptake beyond 6 Weeks after mRNA COVID-19 vaccination. Radiology. 2021:210886. doi: 10.1148/radiol.2021210886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiller N., et al. Lymphadenopathy associated with the COVID-19 vaccine. Cureus. 2021;13(2):e13524. doi: 10.7759/cureus.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vassallo P., et al. Differentiation of benign from malignant superficial lymphadenopathy: the role of high-resolution US. Radiology. 1992;183(1):215–220. doi: 10.1148/radiology.183.1.1549675. [DOI] [PubMed] [Google Scholar]
- 15.Solbiati L., Cova L. Springer Milan; Milano: 2005. Improved characterization of reactive and malignant lymph nodes using contrast-enhanced ultrasound. [Google Scholar]
- 16.Weiss L.M., O'Malley D. Benign lymphadenopathies. Mod Pathol. 2013;26(1):S88–S96. doi: 10.1038/modpathol.2012.176. [DOI] [PubMed] [Google Scholar]
- 17.Sneller M.C. Granuloma formation, implications for the pathogenesis of vasculitis. Cleve Clin J Med. 2002;69(Suppl 2):Sii40–S43. doi: 10.3949/ccjm.69.suppl_2.sii40. [DOI] [PubMed] [Google Scholar]
- 18.Gkiozos I., et al. Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. 2018;13(8):1076–1082. doi: 10.1016/j.jtho.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 19.Luke J.J., et al. Antitumor granuloma formation by CD4+ T cells in a patient with rapidly progressive melanoma experiencing spiking fevers, neuropathy, and other immune-related toxicity after treatment with ipilimumab. J Clin Oncol. 2015;33(6):e32–e35. doi: 10.1200/JCO.2013.49.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubio-Rivas M., Moreira C., Marcoval J. Sarcoidosis related to checkpoint and BRAF/MEK inhibitors in melanoma. Autoimmun Rev. 2020;19(8):102587. doi: 10.1016/j.autrev.2020.102587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analysed during the present study are available from the corresponding author on reasonable request.