Abstract
Objectives
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – also known as the coronavirus disease (COVID-19) – pandemic has led to the swift introduction of population testing programmes in many countries across the world, using testing modalities such as drive-through, walk-through, mobile and home visiting programmes. Here, we provide an overview of the literature describing the experience of implementing population testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Study design
Scoping review.
Methods
We conducted a scoping review using Embase, Medline and the Cochrane library in addition to a grey literature search. We identified indicators relevant to process, quality and resource outcomes related to each testing modality.
Results
In total, 2999 titles were identified from the academic literature and the grey literature search, of which 22 were relevant. Most studies were from the USA and the Republic of Korea. Drive-through testing centres were the most common testing modality evaluated and these provided a rapid method of testing whilst minimising resource use.
Conclusions
The evidence base for population testing lacks high quality studies, however, the literature provides evaluations of the advantages and limitations of different testing modalities. There is a need for robust evidence in this area to ensure that testing is deployed in a safe and effective manner in response to the COVID-19 pandemic.
Keywords: Mass testing, Population testing, SARS-CoV-2, COVID-19
Introduction
In response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, many countries implemented population testing programmes as part of countermeasures to contain the spread of infection and mitigate its health and economic impacts. Population testing provides disease surveillance required to inform broader policy decisions, target resource utilisation and, when twinned with timely case isolation and contact tracing, more effective containment of the virus.1 Worldwide, population testing programmes are diverse, depending on the population eligible for testing, the technology used to sample and analyse specimens, as well as the timing and frequency of testing. In this article, we have defined population testing as any testing programme which uses an antigen or antibody test to identify coronavirus disease 2019 (COVID-19) in a group of symptomatic and/or asymptomatic individuals. In the UK, there are two distinct testing programmes. The UK National Health Service (NHS) Test and Trace system [NHSTT] tests self-reported, symptomatic individuals using quantitative polymerase chain reaction (Q-PCR) assays. More recently, mass testing of asymptomatic individuals using cheaper, faster lateral flow devices has also been introduced.
Population testing seeks to identify people infected with SARS-CoV-2 in a predefined group such as healthcare workers. By identifying cases of infection through testing, action can be taken to limit infection spread by isolating infected individuals and their contacts during their infectious period. As SARS-CoV-2 may be spread by asymptomatic individuals, including these individuals in testing programmes could help reduce viral transmission. Cumulatively, these actions help control the spread of infection and create conditions that would enable the relatively normal functioning of society.
Modalities used for COVID-19 population testing include drive-in, walk-in, mobile sites, postal testing and home visits. The UK, for example, adopted a testing strategy with five pillars, each pillar pertaining to a population subgroup and focussing on either diagnosis, detecting past infection or for surveillance purposes to estimate population prevalence. Testing is co-ordinated centrally and delivered from satellite centres and via postal testing.2 South Korea adopted walk-in and drive-through testing,3 , 4 whilst some areas in Scotland have instituted home-testing to reach more vulnerable groups who cannot access test facilities easily.5 , 6 There is also increasing political and societal concern of the socio-economic impact of blunt strategies such as national ‘lockdowns’ to combat COVID-19,7 and their effect on health inequalities. There may therefore be value in studying the different approaches used worldwide and to learn from successful programmes from other countries.
What is currently unclear is whether any modality of population testing is more robust and efficacious for containing the virus. There is a need to identify population testing programmes that are more accessible and effective at containing the spread of infection, together with the determinants of success. This can help inform national testing policies as part of the pandemic response efforts to minimise the health, social and economic harms. We conducted a scoping review to describe the volume and type of evidence reporting on the experience of implementing population testing for SARS-CoV-2 in high and upper-middle income countries during the pandemic.
Methods
Scoping review methodology
Scoping reviews aim to rapidly map the key concepts underpinning a research area, by comprehensively summarising evidence to inform practice and policy and provide direction for future research.8 , 9 Scoping reviews use rigorous and transparent literature searching methods but differ from systematic reviews as the quality of included studies are not routinely assessed, nor do they provide a synthesised answer to a particular research question.10
This scoping review followed the framework proposed by Arksey and O'Malley8 and refined by Levac et al.11 briefly comprising: identification of the research question, identification of studies, selection of studies, charting of data and collation of results. This review was commissioned by Public Health England who were consulted on the interim outputs of the study. The trial protocol was published on PROSPERO, number CRD42020186506.
Identification of the research question
The purpose of this review was to assess the volume of published literature describing the experience of implementing population testing for COVID-19 and to identify the nature and characteristics of the testing programmes. We sought to elucidate what data were available to assess the outcomes of these testing programmes in terms of processes, participants, quality and/or resource-use. We developed these broad aims in order to generate breadth of coverage and map the literature on this topic so that key concepts and gaps could be identified to inform further practice and policy.
Table 1 outlines the inclusion and exclusion criteria for this review. Studies were included if they described the process of providing or accessing a population testing point for symptomatic and/or asymptomatic individuals for COVID-19, using an antigen or antibody test in any setting, using any testing modality. In order to prioritise research relevant to high-income countries such as the UK, we included literature relating to comparable health services from high and upper-middle income countries only.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Studies of laboratory aspects of testing (including diagnostic accuracy), commentaries, opinion pieces and modelling studies were excluded. Studies that described screening where samples were not taken, or that described the testing of passengers at ports or borders, were also considered out of scope and excluded.
Literature identification and selection
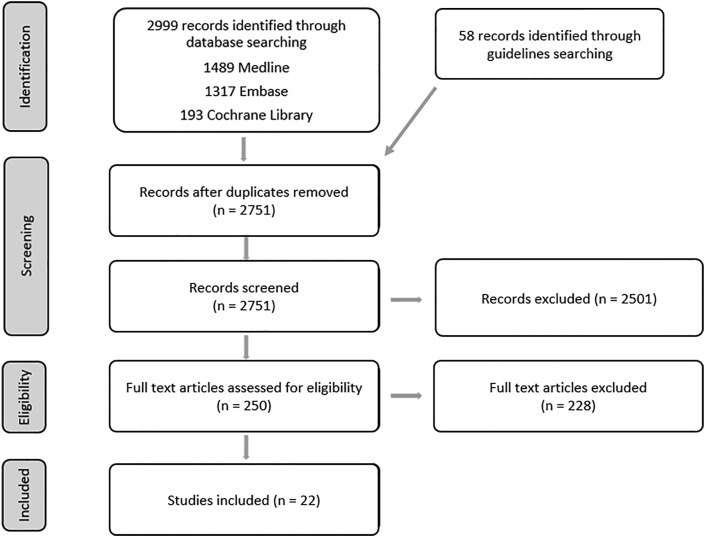
A search strategy (see Appendix 1) was developed to retrieve studies that had evaluated or described the process of providing or accessing a testing point for population testing for COVID-19. An information specialist (AC) searched the electronic databases Medline, Embase and the Cochrane Library (Fig. 1 ). Searches were originally conducted in May 2020 and updated in August 2020. The search was limited to studies in English and published between January and August 2020.
Fig. 1.
PRISMA flow diagram.
Extracted titles and abstracts were screened by at least two reviewers (CF, FC, LB). A total of 250 full text articles were reviewed to clarify whether the article met the inclusion criteria given in Table 1, either because no abstract was available or because it was unclear from the title and abstract alone whether the study met the inclusion criteria. Abstracts were often unavailable due to the large number of commentaries and opinion pieces found by the search strategy. A formal quality appraisal of the evidence was not conducted, given the remit of the scoping review.
In addition, a grey literature search for national and international clinical guidelines was conducted during May 2020. The World Health Organization (WHO) and the European Centre for Disease Prevention and Control (ECDC) websites were searched, plus websites in the English language from the UK, USA, Canada, Australia, New Zealand, South Korea, China and Taiwan. This search identified 58 potentially relevant guidelines. On further review, only one guideline was relevant to population testing and detailed an approach to drive-through screening implemented in South Korea.12 A further 21 guidelines looked at wider aspects of screening.
Charting of data and collation of results
After the screening was completed, relevant content in the included studies was extracted into a spreadsheet. Data extraction was verified by a second reviewer who checked data extraction from a random sample of four articles. The mode of testing was categorised into one of five different types: drive-through testing, home visiting testing, indoor walk-through centre, outdoor walk-through centre and mobile testing. For outcome data, a thematic framework was used which categorised any quantitative outcomes indicators into one of four groups: process outcomes, participant outcomes, quality outcomes and resource use outcomes.
Results
The database search returned 2999 results (Fig. 1). After automated and manual deduplication, 2751 unique references were screened for relevance to the question. On first screening of titles, 250 references were identified as potentially relevant, and on further reading 22 were categorised as relevant.
Study characteristics
A summary of the studies’ characteristics is presented in Table 2 .
Table 2.
Characteristics of included articles.
| First Author | Country | Mode of Testing |
|---|---|---|
| Appa14 | USA | Drive-through |
| Brammer13 | USA | Drive-through |
| Chang16 | Republic of Korea | Drive-through and walk-through |
| Drees17 | USA | Drive-through |
| Flynn18 | USA | Drive-through and walk-through |
| Goldberg19 | USA | Home visiting testing |
| Halalau20 | USA | Drive-through |
| Hill5 | Scotland | Drive-through and home visiting testing |
| Kim21 | Israel | Drive-through and home visiting testing |
| Kim22 | Republic of Korea | Indoor walk-through centre |
| Kwon4 | Republic of Korea | Drive-through |
| Kwon23 | Republic of Korea | Outdoor walk-through centre |
| Lee15 | Malaysia | Drive-through |
| Lin24 | Taiwan | Outdoor walk-through centre |
| Lindholm25 | USA | Drive-through |
| Manauis26 | Singapore | Indoor walk-through centre |
| Ministry of Foreign Affairs12 | Republic of Korea | Drive-through |
| Mark6 | Scotland | Drive-through and home visiting testing |
| Rivkees27 | USA | Drive-through, walk-through and mobile testing |
| Seo28 | Republic of Korea | Mobile testing |
| Shah29 | USA | Drive-through |
| Ton30 | USA | Drive-through |
Most of the published literature on this topic is difficult to assign to a study type. Studies were often referred to as brief reports or short communications. The articles typically comprise a description of the testing modality of interest, often with a diagram of the layout of the testing centre and useful operational details followed by an evaluation of its advantages and disadvantages. In some articles, a comparison group was described; however, there were no published randomised controlled trials (RCTs). One article was a qualitative interview study of early experiences of drive-through testing centres.13
All the eligible studies described population testing programmes where samples were taken from symptomatic and occasionally asymptomatic individuals. There were no eligibility studies of mass testing, defined as regular and/or large-scale testing of individuals from defined populations regardless of symptom status using lateral flow tests.
Many articles describing testing programmes were from the USA (43% of studies) and the Republic of Korea (29% of studies). Most articles described testing programmes in high income countries (95% of articles), with only one originating from an upper-middle income country (Malaysia).15
Testing modalities
Several different testing modalities were described, which were categorised into five main categories: drive-through, home visiting, mobile testing, indoor walk-through centres and outdoor walk-through centres.
Sometimes described as off-site COVID-19 testing centres (OSCTCs),13 drive-through testing centres were by far the most common testing modality evaluated (in 72% of articles), in Israel, Malaysia, Korea, Scotland and the USA. This testing modality enabled the use of a vehicle as a self-contained unit, which can reduce the spread of infection. Most were in car parks, stadiums and parks, and one was in an open-air area of a hospital.26 Some centres enabled individuals without a car to walk in for testing13 in order to increase accessibility. Six articles described drive-through testing in combination with either home visiting testing, mobile testing and/or walk-through testing, enabling a greater proportion of the population to access testing.
Home visiting testing (18% of articles) typically involved a small number of healthcare workers visiting the home of an individual to perform a test. This enabled individuals who are home-bound, frail or have no means of private transportation to access testing without having to use an ambulance, visit a hospital or rely on the assistance of others to access a drive-through site. These schemes were often used as an alternative to local drive-through testing facilities. Home visiting testing took place in Israel, Scotland and the USA.
Two articles described mobile testing, in which testing staff visited populations rather than expecting the participants to travel to a testing centre. In Korea, testing staff visited workers onsite at their workplaces28 and in Florida, mobile polymerase chain reaction (PCR) testing laboratories were used to provide point-of-care testing in different cities.27
Indoor walk-through centres based in healthcare facilities were used in Singapore and Korea. There were several different designs for walk-through centres, which were located inside hospitals or other healthcare facilities: screening centres,22 , 26 negative pressure booths22 and negative pressure tents.16 Screening centres permit individuals to access testing inside a building. The Singapore Screening Centre was designed to minimise the movement of patients around the building.26 Patients were assigned a seat number and tagged with a tracker to facilitate contact tracing; staff visited patients in their seats to further reduce contact amongst patients.
Negative pressure booths and tents have been designed to minimise the opportunity for viral spread in an indoor setting. Negative pressure booths22 were used for sample collection and medical examination procedures in Korea. The booths were inspired by the design of biosafety cabinets and contain a ‘glove wall’ separating the patient and the medical staff member, who communicate using an interphone. Patients complete registration, questionnaires and payment outside the booths in other sections of the screening centre. Negative pressure booth systems aimed to protect healthcare staff, reduce personal protective equipment (PPE) use and increase throughput compared to other walk-through systems. Negative pressure tents,16 also located in Korea, were similar to negative pressure booths, but the whole tent is under negative pressure. Staff working in the tent wore full personal protective equipment (PPE) and most of the tent required sterilisation between people tested that took at least 30 min.
The final testing modality described was outdoor walk-through centres. These were located outside the hospitals in Korea and Taiwan.23 , 24 The Korean clinic23 screened all patients and visitors to the hospital with the aim of minimising ward closures due to COVID-19 outbreaks. In Taiwan, a ‘multifunctional sampling station’ was built outside an emergency department, using a 2-cm thick clear acrylic board to separate emergency department patients and medical personnel, with inbuilt gloves used to conduct sampling.24
Populations tested
Table 3 summarises details of the populations tested and the types of test used. Fifty-five percent of studies provided information regarding the population that was eligible to be tested. Forty-one percent of articles described accepting both symptomatic and asymptomatic individuals, and 14% accepted those with symptoms only. Some testing centres had an algorithm for testing eligibility involving symptoms, epidemiological links, occupational risk factors and/or potential for community exposure. For some, the testing criteria changed over time as the pandemic progressed.20 , 29
Table 3.
Populations eligible to be tested and types of testing used.
| First Author | Population tested | Type of test |
|---|---|---|
| Appa14 | Symptomatic and asymptomatic | Oropharyngeal/midturbinate swab for PCR testing, finger prick blood test for antibody testing |
| Brammer13 | N/A | N/A |
| Chang16 | Symptomatic and asymptomatic | RT-PCR tests of 'specimens' |
| Drees17 | Symptomatic only | Not specified |
| Flynn18 | Unclear/unspecified | ‘In-house COVID-19 test' of nasopharyngeal swabs |
| Goldberg19 | Symptomatic and/or epidemiological link and/or risk factors and/or potential for community exposure | Nasopharyngeal swab collection for RT-PCR analysis |
| Halalau20 | Unclear/unspecified | Nasopharyngeal swab collection for RT-PCR analysis |
| Hill5 | Unclear/unspecified | Combined nose and throat swab |
| Kim21 | Symptomatic only | Swab testing |
| Kim22 | Symptomatic only | Not specified |
| Kwon4 | Symptomatic and asymptomatic | Naso- and oropharyngeal swabs, sputum specimen |
| Kwon23 | Symptomatic and/or epidemiological link and/or risk factors and/or potential for community exposure | Pretesting questionnaire |
| Lee15 | Symptomatic and asymptomatic | Temperature measurements, nasopharyngeal and oral swabs |
| Lin24 | Unclear/unspecified | Nasopharyngeal and oral swab, sputum collection, blood testing for antibodies |
| Lindholm25 | Symptomatic and/or epidemiological link and/or risk factors and/or potential for community exposure | Screening questionnaire followed by nasopharyngeal swab collection for RT-PCR analysis for those with symptoms or epidemiological link |
| Manauis26 | Symptomatic and/or epidemiological link and/or risk factors and/or potential for community exposure | Swab testing |
| Mark6 | Unclear/unspecified | Not specified |
| Ministry of Foreign Affairs12 | Unclear/unspecified | Upper respiratory tract sample (all), lower respiratory tract sample (only if participant can expectorate sputum alone into a container) |
| Rivkees27 | Symptomatic and asymptomatic | PCR and antibody testing |
| Seo28 | Unclear/unspecified | Questionnaire followed by sample collection for RT-PCR testing |
| Shah29 | Symptomatic and/or epidemiological link and/or risk factors and/or potential for community exposure | Originally naso- and oropharyngeal swab, later solely nasopharyngeal swab for reverse transcription (RT)-PCR testing (following Centre for Disease Prevention and Control [CDC] advice) |
| Ton30 | Unclear/unspecified | Nasal swab |
Types of testing used
The most common method for sampling was through nasal or throat swabs. 45% of articles described the use of nasal swabbing, involving either nasal, nasopharyngeal or mid-turbinate sampling. 27% described the use of throat/oropharyngeal swabbing. 27% stated that swabbing was used but did not specify whether these were nasal and/or throat swabs. Less commonly described testing procedures included sputum sampling (14% of articles) and blood sampling for antibody testing (14% of articles) and one included the use of temperature measurements. 9% of publications described the use of prescreening questionnaires before sampling took place. 18% of articles did not specify the method of testing and 41% of articles described the use of a combination of the above methods.
Outcomes of interest
Outcomes of interest were divided into three categories: process outcomes, quality outcomes and resource use outcomes. No participant outcomes were measured quantitatively in any of the included studies; however, the discussion section of many articles contained rich qualitative data describing participant outcomes such as staff and participant safety and well-being and service equity.
Process outcomes
The process outcomes described comprise throughput, duration of test, decontamination time, time to don/doff PPE and waiting time.
Seventy-seven percent of the articles reported the number of people tested in a specified time period (Table 4 ). These figures were used to calculate the mean number of people tested each day.a Although the different studies are not directly comparable due to factors such as the different sizes of populations served and staff employed, this section gives a broad overview of the types of throughput that may be experienced for different testing modalities.
Table 4.
Number of people tested per day.
| Number tested per day (calculated) | Prescreening (Questionnaire/Temperature only) | Testing (Samples taken) | Ref |
|---|---|---|---|
| Drive-through | 22∗ | 6 | |
| 107 | 45 | 20 | |
| 60 | 12 | ||
| 192 | 30 | ||
| >100 | 4 | ||
| 163 | 122 | 25 | |
| 200 (max 400) | 29 | ||
| 242 | 16 | ||
| 460 | 14 | ||
| 539 | 17 | ||
| Drive-through and home visiting | 2000 country-wide | 21 | |
| Drive-through and Walk-through | 65 (range 11–127) | 18 | |
| Indoor Walk-through centres | 9–10 (no negative pressure booths) >70 (with negative pressure booths) |
22 | |
| 41 | 16 | ||
| 50–500 | 26 | ||
| Home visiting testing | 6 (max 11)∗ | 6 | |
| 15 | 5 | ||
| 15 | 19 | ||
| Outdoor Walk-through centre | 300 | 23 |
Drive-through testing centres tested between 22 and 539 individuals per day. Indoor walk-through centres tested 9–500 people per day. One outdoor walk-through centre tested 300 people per day. Home visiting testing teams tested 6–15 people per day.
Some studies compared the throughput of different testing modalities (Table 5). Three studies compared drive-through testing with walk-through or home visiting testing.5 , 6 , 16 For similar settings, a higher throughput of individuals could be achieved in a drive-through setting compared to walk-through testing16 or home visiting testing.5 , 6 For the same time period, one indoor walk-through screening centre using negative pressure booths tested more patients than a walk-through centre with no negative pressure booths (>70 people per day compared to 9–10 people per day).22 Multiple booths could be installed and decontamination time between individuals could be reduced to 3–5 min from over 30 min. It is difficult to compare different studies, as several variables other than the testing modality can affect the number of people tested per day, such as the number of staff present, the procedures used and the number of individuals who could be tested concurrently.
Table 5.
Differences in process outcomes when comparing different testing modalities.
| Ref | Testing Modality | PROCESS OUTCOMES |
|||
|---|---|---|---|---|---|
| Throughput (mean number tested per day) | Duration of test (mean time per test) | Decontamination time (time to disinfect room between individuals) | Time to don/doff PPE | ||
| Chang16 | Drive-through | 242 | 5–7 min | N/A | |
| Walk-through (negative pressure tent) | 41 | 30 min | At least 30 min between patients | ||
| Hill5 | Drive-through | 92 patients in 18 h | |||
| Home visiting testing | 15 patients per three staff members per day | ||||
| Kwon4 | Drive-through | 10 min | |||
| Screening centre | 30 min | ||||
| Lin24 | Outdoor walk-through centre (multifunctional sample collection station) | 2 min | 10 min per patient | 1 min | |
| Traditional sample collection (single negative-pressure isolation room) | 5 min | 35 min per patient | 21 min | ||
| Mark6 | Home visiting testing | 79 in 2 weeks | 30 min + <1 h travel time | 0 | |
| Drive-through | 313 in 2 weeks | 0 | |||
| Specialist ambulance and hospital sampling | Not specified | <1 h + overnight stay may be required | <6 h | ||
| Kim22 | Indoor walk-through screening (negative pressure booths) | >70 per day | 3–5 min | ||
| Indoor walk-through screening (no negative pressure booths) | 9–10 per day | >30 min | |||
The mean duration of a drive-through test was between 3 and 15 min. One study reported a median time per test of 28 min (interquartile range [IQR] 17–44 min).20 Some centres allowed multiple people to be tested per vehicle, whereas others allowed only one person per vehicle. The layout of drive-through testing sites can allow several individuals to be tested at one time, for example one drive- and walk-through centre could test two patients every 5 min.18
Twenty-seven percent of articles compared testing modalities, calculating a range of indicators for process outcomes (Table 5 ). Drive-through testing was found to be faster than walk-through testing using a negative pressure tent16 or a screening centre.4 Testing using an outdoor walk-through centre (2 min per test) was faster than using traditional sample collection in a single negative-pressure isolation room (5 min per test).24 Home visiting testing (30 min per test) was also quicker than transporting patients to hospital for tests with a specialist ambulance (<1 h).6 However, waiting times, defined as the time between arrival at the drive-through centre and testing, was reported to be as high as 7 h at peak volume.20
Testing in an outdoor walk-through centre dramatically reduced time to don/doff PPE compared to traditional sample collection in a single negative-pressure isolation room (1 min per patient compared to 21 min per patient).24
Several studies measured the time to disinfect equipment between individuals (decontamination time). The use of drive-through testing eliminated the need for a 30 min decontamination time between patients in a walk-through centre using a negative pressure tent.16 Drive-through and home visiting testing also required no decontamination time, compared to up to 6 h of decontamination time needed if a patient was tested in the emergency department.6 Decontamination time was much shorter when using an outdoor walk-through centre (10 min per patient) compared to a single negative-pressure isolation room (35 min per patient). The use of negative pressure booths reduced decontamination time from >30 min with no negative pressure booths to 3–5 min between patients.31
Quality outcomes
Two different indicators were used to describe quality outcomes: the median time from referral to test and the test turnaround time. Only one study of home visiting testing calculated the median time from referral to test, which was 1 day with a maximum of 3 days.6 One drive-through testing study calculated the test turnaround time, defined as the time between testing and communication of results. This was found to be 25 h (IQR 21–29) in-house and 221 h (IQR 161–269) if outsourced.25
Resource use outcomes
Resource use outcomes were measured using cost per patient, use of PPE and impact on hospital closure. Home visiting testing reportedly costed much less (£55 per patient) than the use of a specialist ambulance and hospital sampling (£768 per patient)6 (Table 6 ).
Table 6.
Resource use outcomes.
| Ref | Testing modality | Resource use outcomes |
|
|---|---|---|---|
| Cost per patient | PPE use | ||
| Lin24 | Outdoor walk-through centre (multifunctional sample collection station) | 0 PPE items used∗ | |
| Traditional sample collection (single negative-pressure isolation room) | 24 PPE items used | ||
| Mark6 | Home visiting testing | £55 | |
| Drive-through | Not specified | ||
| Specialist ambulance and hospital sampling | £768 | ||
| Ton30 | Drive-through | 1152 masks, 960 gowns/pairs of gloves for 192 patients | |
| Emergency department testing | 42 masks, 24 gowns, 504 pairs of gloves for 192 patients | ||
Another study reported that staff in an outdoor walk-through centre used fewer items of PPE than staff working in negative-pressure isolation rooms.24 Similarly, drive-through testing can reduce PPE use (96% reduction in mask use, 97% reduction in gown use and 47% reduction in glove use) compared to emergency department based testing.30
One study reported the effect of a screening and testing clinic on maintaining the functioning of a tertiary hospital.23 Before the clinic was opened, an average of 36 beds per day were closed due to COVID-19 patients entering the hospital, whereas after the clinic was open and operating well, there was only one closure event (25 beds).
Discussion
This scoping review provides an overview of the literature describing the experience of implementing population testing for SARS-CoV-2 in high and upper-middle income countries. Whilst a range of modalities were reported, the most commonly evaluated were drive-through services using naso- and/oropharyngeal swabbing. Drive-through testing provided a rapid and scalable method of testing for COVID-19, reducing the risk of exposure to staff and patients within healthcare settings and minimising PPE use. However, this approach raises questions regarding equity of access for those who do not have access to a private vehicle or are not well enough to drive. The addition of other testing options such as home visiting, mobile testing or walk-through services may help address this issue. Recently, home-based diagnostic and screening tests for SARS-CoV-2 have become much more widely available, which may reduce the need for large scale testing facilities in the future.
However, the evidence base for population testing lacks robust studies and the heterogeneous nature of the testing programmes described in the literature makes it difficult to compare between studies. Many were simply an evaluation of a testing programme with a discussion of its advantages and limitations rather than robust research studies with control groups. Prospective randomised controlled trials (RCTs) of testing centres would give higher quality data, however the researchers would need to overcome challenges such as adversely impacting the expediency and evolution of site practices in real time, and keeping pace with the rapid development of testing methods in response to the pandemic. Conversely, the studies described in this review are rich in qualitative data which could be synthesised to produce valuable insights into the lessons that have been learned in a variety of different settings. Such a review may be a better use of public health resources to identify translatable and implementable best practices.
There is a paucity of published literature on the implementation of mass testing for SARS-CoV-2, defined as regular and/or large-scale testing of individuals from defined populations regardless of symptom status, using lateral flow tests. There were no studies on mass testing that fit the inclusion criteria of this review, although a few articles have described mass testing of residents in facilities such as care homes and prisons.32 , 33 A recent review by the European Centre for Disease Prevention and Control (ECDC) highlights the need for further studies on the assessment and impact of mass testing.34 Indeed, some countries have rushed to adopt mass testing before the benefits, risks and costs of this approach is fully understood.35 It is therefore pertinent to draw on the international literature on population testing to inform decision making in order to ensure that testing is deployed in a safe and effective way as part of the overall COVID response.36 It is imperative that future studies assess the cost-effectiveness, specificity and sensitivity of home-based testing, in conjunction with assessing possible scenarios for ending or reducing access to home-testing in the future.
A distinction needs to be made between population testing and screening for COVID-19. As COVID-19 is a new disease, it is unsurprising that definitions for screening and testing in this context have not yet been standardised and often appear to be arbitrary. We found the term ‘screening’ is loosely used in different ways in the literature, including the testing of symptomatic and/or asymptomatic individuals, assessment of risk factors via a questionnaire, and temperature measurement of individuals travelling past a screening post.37, 38, 39
Our literature search aimed to be comprehensive but timely and expedient. Whilst we used rigorous and transparent search methods, we had to limit our search to articles published in English. Therefore it is possible that some relevant studies have not been included. Although we did not formally assess the quality of included articles, much of the literature was not robust as stated earlier. Any preference for one testing modality over another cannot be extrapolated from the data collected in this review, as there is considerable heterogeneity between studies and outcomes will reflect factors such as the local population, geography and site protocols.
Further exploration is needed of population testing using different SARS-CoV-2 tests as the strengths and limitations of the various SARS-CoV-2 tests could influence the yield, cost-effectiveness and viability of the population testing programmes. Additionally, research into the wider consequences of testing programmes is needed especially on population behaviours as a result of testing. Finally, further study of the cost-effectiveness of population testing compared to other pandemic control measures is also required. As with most public health interventions, there is no ‘one-size-fits-all’ approach, and whatever population testing approach is adopted it will need to be tailored to the local context and target population.
Author statements
Ethical approval
Not required.
Funding
This research was part-funded by Public Health England.
Competing interests
AL is the co-editor for the journal, but had no involvement in the peer review and editorial decision for this article.
Footnotes
∗2 studies reported the time period per ‘week’ but did not state which days of the week were available for testing; a 7-day week was assumed for calculations.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.puhe.2021.06.012.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Walensky R.P., Del Rio C. From mitigation to containment of the COVID-19 pandemic: putting the SARS-CoV-2 genie back in the bottle. JAMA – J Am Med Assoc. 2020;323:1889–1890. doi: 10.1001/jama.2020.6572. [DOI] [PubMed] [Google Scholar]
- 2.Department of Health and Social Care . 2020. Coronavirus (COVID-19): scaling up our testing programmes. [Google Scholar]
- 3.Choi S., Han C., Lee J., Kim S.I., Kim I.B. Innovative screening tests for COVID-19 in South Korea. Clin Exp Emerg Med. 2020;7:73–77. doi: 10.15441/ceem.20.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon K.T., Ko J.-H., Shin H., Sung M., Kim J.Y. Drive-through screening center for COVID-19: a safe and efficient screening system against massive community outbreak. J Kor Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill K., Campbell R., Mutch C., Koch O., Mackintosh C. Drive-through testing in COVID-19: experience from NHS Lothian. Clin Med. 2020;20:290–291. doi: 10.7861/clinmed.2020-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mark K., Steel K., Stevenson J., Evans C., McCormick D., Willocks L., et al. Coronavirus disease (COVID-19) community testing team in Scotland: a 14-day review, 6 to 20 February 2020. Euro Surveill. 2020;25:2000217. doi: 10.2807/1560-7917.ES.2020.25.12.2000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Health Foundation . 2020. Will COVID-19 be a watershed moment for health inequalities? [Google Scholar]
- 8.Arksey H., O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. [Google Scholar]
- 9.Colquhoun H.L., Levac D., O'Brien K.K., Straus S., Tricco A.C., Perrier L., et al. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol. 2014;67:1291–1294. doi: 10.1016/j.jclinepi.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Munn Z., Peters M.D.J., Stern C., Tufanaru C., McArthur A., Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143. doi: 10.1186/s12874-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levac D., Colquhoun H., O'Brien K.K. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Republic of Korea Ministry of Foreign Affairs . 2020. Standard operating model for “drive-thru” screening clinics.http://www.mofa.go.kr/eng/brd/m_22592/view.do?seq=1&srchFr=&srchTo=&srchWord=&srchTp=&multi_itm_seq=0&itm_seq_1=0&itm_seq_2=0&company_cd=&company_nm= Available from: [Google Scholar]
- 13.Brammer C., Donohue S., Elwell T., Fishbein E., Forschino D.A., Horne D., et al. Qualitative review of early experiences of off-site COVID-19 testing centers and associated considerations. Healthcare. 2020;8:100449. doi: 10.1016/j.hjdsi.2020.100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appa A., Chamie G., Sawyer A., Baltzell K., Dippel K., Ribeiro S., et al. medRxiv : the preprint server for health sciences; 2020. SARS-CoV-2 PCR and antibody testing for an entire rural community: methods and feasibility of high-throughput testing procedures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee E., Mohd Esa N.Y., Wee T.M., Soo C.I. Bonuses and pitfalls of a paperless drive-through screening and COVID-19: a field report. J Microbiol, Immunol, Infect = Wei mian yu gan ran za zhi. 2021 Feb;54(1):85–88. doi: 10.1016/j.jmii.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang M.C., Seo W.-S., Park D., Hur J. Analysis of SARS-CoV-2 screening clinic (including drive-through system) data at a single university hospital in South Korea from 27 January 2020 to 31 March 2020 during the COVID-19 outbreak. Healthcare (Basel, Switzerland) 2020;8 doi: 10.3390/healthcare8020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drees M.L., Papas M.A., Corbo T.E., Williams K.D., Kurfuerst S.T. Identifying community spread of COVID-19 via a free drive-through screening event. Infect Contr Hosp Epidemiol. 2020:1–3. doi: 10.1017/ice.2020.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn E.F., Kuhn E., Shaik M., Tarr E., Scattolini N., Ballantine A. Drive-through COVID-19 testing during the 2020 pandemic: a safe, efficient, and scalable model for pediatric patients and health care workers. Acad Pediatr. 2020;20:753–755. doi: 10.1016/j.acap.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg S.A., Bonacci R.A., Carlson L.C., Pu C.T., Ritchie C.S. Home-based testing for SARS-CoV-2: leveraging prehospital resources for vulnerable populations. West J Emerg Med. 2020;21:813–816. doi: 10.5811/westjem.2020.5.47769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halalau A., Ditkoff J., Hamilton J., Sharrak A., Vanood A., Abbas A., et al. Emergency center curbside screening during the COVID-19 pandemic: retrospective cohort study. JMIR Publ Health Surveill. 2020;6 doi: 10.2196/20040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim E. Drawing on Israel's experience organizing volunteers to operationalize drive-through Coronavirus testing centers. Disaster Med Public Health Prep. 2020:1–3. doi: 10.1017/dmp.2020.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S.I., Lee J.Y. Walk-through screening center for COVID-19: an accessible and efficient screening system in a pandemic situation. J Kor Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon Y.S., Park S.H., Kim H.J., Lee J.Y., Hyun M.R., Kim H.A., et al. Screening clinic for Coronavirus disease 2019 to prevent intrahospital spread in Daegu, Korea: a single-center report. J Kor Med Sci. 2020;35:e246. doi: 10.3346/jkms.2020.35.e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin P.-T., Ni T.-Y., Chen T.-Y., Su C.-P., Sun H.-F., Chen M.-K., et al. Reducing the consumption of personal protective equipment by setting up a multifunctional sampling station in the emergency department to screen for COVID-19 infection in Taiwan. Environ Health Prev Med. 2020;25:34. doi: 10.1186/s12199-020-00874-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindholm D.A., Kiley J.L., Jansen N.K., Hoard R.T., Bondaryk M.R., Stanley E.M., et al. Outcomes of Coronavirus disease 2019 drive-through screening at an academic military medical center. Open Forum Infect Dis. 2020;7:ofaa306. doi: 10.1093/ofid/ofaa306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manauis C.M., Loh M., Kwan J., Chua Mingzhou J., Teo H.J., Teng Kuan Peng D., et al. Bracing for impact: operational upshots from the national centre for infectious diseases screening centre (Singapore) during the COVID-19 outbreak. J Am Coll Emerg Phys Open. 2020 Jun 19;1(4):549–556. doi: 10.1002/emp2.12134. Epub ahead of print. PMID: 32838378; PMCID: PMC7323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivkees S.A., Roberson S. The Florida department of health STEPS public health approach: the COVID-19 response plan and outcomes through may 31, 2020. Publ Health Rep. 2020;135(5):560–564. doi: 10.1177/0033354920946785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seo E., Mun E., Kim W., Lee C. Fighting the COVID-19 pandemic: onsite mass workplace testing for COVID-19 in the Republic of Korea. Ann Occup Environ Med. 2020;32:e22. doi: 10.35371/aoem.2020.32.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah A., Challener D., Tande A.J., Mahmood M., O'Horo J.C., Berbari E., et al. Drive-through testing: a unique, efficient method of collecting large volume of specimens during the SARS-CoV-2 (COVID-19) pandemic. Mayo Clin Proc. 2020;95:1420–1425. doi: 10.1016/j.mayocp.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ton A.N., Jethwa T., Waters K., Speicher L.L., Francis D. COVID-19 drive through testing: an effective strategy for conserving personal protective equipment. Am J Infect Contr. 2020;48:731–732. doi: 10.1016/j.ajic.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim E. Comparing the operational strategies of South Korea and Israel's Coronavirus drive-through testing centers and the implications on testing capacity. Risk Manag Healthc Pol. 2020;13:821–823. doi: 10.2147/RMHP.S259347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatfield K.M., Reddy S.C., Forsberg K., Korhonen L., Garner K., Gulley T., et al. Facility-wide testing for SARS-CoV-2 in nursing homes - seven U.S. Jurisdictions, March-June 2020. MMWR Morbidity and mortality weekly report. 2020;69:1095–1099. doi: 10.15585/mmwr.mm6932e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagan L.M., Williams S.P., Spaulding A.C., Toblin R.L., Figlenski J., Ocampo J., et al. Mass testing for SARS-CoV-2 in 16 prisons and jails - six jurisdictions, United States, April-may 2020. MMWR Morbidity and mortality weekly report. 2020;69:1139–1143. doi: 10.15585/mmwr.mm6933a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.European Centre for Disease Prevention and Control . 2020. Population-wide testing of SARS-CoV-2: country experiences and potential approaches in the EU/EEA and the United Kingdom.https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-population-wide-testing-country-experiences.pdf Available from: [Google Scholar]
- 35.Gill M., Gray M. Mass testing for covid-19 in the UK. BMJ. 2020;371:m4436. doi: 10.1136/bmj.m4436. [DOI] [PubMed] [Google Scholar]
- 36.Association of Directors of Public Health and the Faculty of Public Health . 2020. Joint statement from the association of directors of public health and the faculty of public health on targeted community testing.https://www.fph.org.uk/news-events/fph-news/joint-statement-from-the-association-of-directors-of-public-health-and-the-faculty-of-public-health-on-targeted-community-testing/ [cited 2020 04/12/2020]; Available from: [Google Scholar]
- 37.Viswanathan M., Kahwati L., Jahn B., Giger K., Dobrescu A.I., Hill C., et al. Universal screening for SARS-CoV-2 infection: a rapid review. Cochrane Database Syst Rev. 2020 Sep 15;9:CD013718. doi: 10.1002/14651858.CD013718. PMID: 33502003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.David N., Mash R. Community-based screening and testing for Coronavirus in Cape Town, South Africa: short report. Afr J Prim Health Care Fam Med. 2020;12:e1–e3. doi: 10.4102/phcfm.v12i1.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seddighi H. The performance of the Iranian red crescent by launching testing centers for the Coronavirus disease. Disaster Med Public Health Prep. 2020:1–2. doi: 10.1017/dmp.2020.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.