Graphical abstract
Keywords: SARS-CoV-2, COVID-19, Natural immunity, Vaccination, Antibody testing, Resource allocation
Abstract
Objective
To assess the value of using SARS-CoV-2 specific antibody testing to prioritize the vaccination of susceptible individuals as part of a COVID-19 vaccine distribution plan when vaccine supply is limited.
Methods
An extended susceptible-infected-recovered (SIR) compartmental model was used to simulate COVID-19 spread when considering diagnosis, isolation, and vaccination of a cohort of 1 million individuals. The scenarios modeled represented 4 pandemic severity scenarios and various times when the vaccine becomes available during the pandemic. Eligible individuals have a probability p of receiving antibody testing prior to vaccination (p = 0, 0.25, 0.5, 0.75, and 1). The vaccine was modeled as a single dose vaccine with 90% and 70% efficacy. The value of serology testing was evaluated by comparing the infection attack rate, peak infections, peak day, and deaths.
Results
The use of antibody testing to prioritize the allocation of limited vaccines reduces infection attack rates and deaths. The size of the reduction depends on when the vaccine becomes available relative to the infection peak day. The largest percentage reduction in cases and deaths occurs when the vaccine is deployed before and close to the infection peak day. The reduction in the number of cases and deaths diminishes as vaccine deployment is delayed.
Conclusions
Antibody testing as part of the vaccination plan is an effective method to maximize the benefit of a COVID-19 vaccine. Decision-makers need to consider relative timing between the infection peak day and when the vaccine becomes available.
1. Introduction
The effective deployment of safe and effective vaccines is a key intervention to control the spread of the coronavirus disease 2019 (COVID-19) pandemic by establishing herd immunity via immunization in a shorter time and without additional deaths and burden on healthcare systems. As of December 2020, there are 61 vaccine candidates against SARS-CoV-2, the novel coronavirus causing COVID-19, in the clinical development phase using different platforms (i.e., genetic, viral vector, protein-based, inactivated virus), efficacy, doses required, and storage considerations [1]. In the U.S., the vaccines developed by manufacturers Pfizer-BioNTech, Moderna, and Johnson & Johnson were given first emergency use authorization (EUA) which allows their distribution in the country [2], [3], [4].
The number of vaccines available to countries in the next months will be limited due to manufacturing and logistic constraints. Due to the limited amount of vaccine supply, as well as that their distribution will be staggered, vaccine allocation guidelines are extremely important to ensure vaccine equity and effective distribution worldwide especially in resource-limited settings.
Various guidelines have been developed by governing organizations ahead of the vaccine distribution. The National Academies of Science, Engineering, and Medicine has developed a framework to assist policymakers when planning for vaccine allocation. This framework includes a phased allocation of vaccines that prioritizes essential workers and high-risk individuals while ensuring equity in the distribution [5]. Similarly, states in the U.S. are developing plans for vaccine distribution which includes vaccine storage, distribution, administration, community communication, safety, and capacity considerations. While these plans address and consider various aspects of the vaccine supply chain and administration, they do not consider the potential benefit that serologic testing and assessing SARS-CoV-2 specific antibody response can have while allocating vaccines.
Serologic tests can help determine the individuals who were previously infected with SARS-CoV-2 [6], [7], [8], [9]. Although the duration of immunity to and the rate of SARS-CoV-2 reinfection is still to be determined, in the majority of the COVID-19 cases, serum antibodies developed against SARS-CoV-2 specific proteins raise within 2-3 weeks of infection and are detectable for at least three to six months after exposure [10], [11], [12]. Research suggests that the antibodies developed following natural infection are protective similar to immunity provided when getting a COVID-19 vaccine, although how long protection will last still remains to be seen both for natural infection and the vaccination [13]. Even if previous infection does not provide an individual full immunity, prioritizing the limited number of vaccines to those who have not acquired any natural immunity could be beneficial. Due to this, the identification of individuals who have been previously infected including those who have not developed the distinctive symptoms, so recovered without knowing they have had a SARS-CoV-2 infection, could be a promising policy for a more effective allocation of vaccines while supply is limited. In addition, it is estimated that only 1 in every 7.7 cases or 13% of the total cases have been detected and reported with the majority of the true cases remaining undiagnosed [14]. Thus, it is vital to deploy mass serology testing to identify individuals who have recovered from the symptomatic or asymptomatic SARS-CoV-2 infection.
In this paper, we used a compartmental model to quantify the benefit of using serology testing to prioritize the vaccination of individuals who are susceptible as they have never been infected with COVID-19 before. We evaluated 5 scenarios with respect to the degree to which serology is used during vaccination. To model serology test utilization, we assigned a probability p (0, 0.25, 0.5, 0.75, and 1) that an individual receives serology testing before vaccination. We tested vaccine administration under different disease spread intensity rates and time of vaccine availability and compared the infection attack rate (IAR), peak infections, peak day, and deaths.
2. Materials and methods
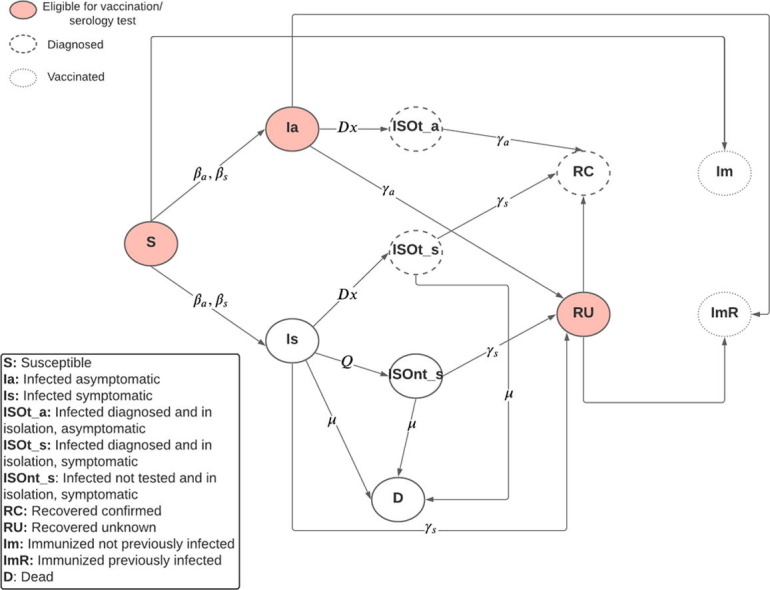
We used an extended version of the susceptible-infected-recovered (SIR) compartmental model to capture the epidemiology and the natural history of the SARS-CoV-2 infection, diagnosis and isolation of infected individuals, and serology testing and vaccine allocation, as seen in Fig. 1 . The movement from and to the susceptible, infected, diagnosed, isolated, dead, and recovered states are modeled using a system of differential equations. A detailed description of the model formulation is found in the Supplementary Materials. The model parameters are shown in Table 1 . The parameters were obtained from literature and guidelines from the US Centers for Disease Control and Prevention [15]. The simulated cohort size was 1 million individuals. The simulation starts on January 1st, 2020 with 1 infected symptomatic individual. We ran 10 replications of the simulation. The simulation was run for 550 days (approximately 1.5 years). The simulation and modeling were done using the statistical software R [16].
Fig. 1.
Compartmental model diagram, βa, βs: Transmission rate due to contact between susceptible and asymptomatic or symptomatic individual; Dx: Rate of diagnosis and isolation of infected individuals; Q: Rate of self isolation of symptomatic infected individuals who are undiagnosed; μ: Infection fatality rate among symptomatic infected indiviuals; γa, γs: Rate of recovery of an asymptomatic or symptomatic individual.
Table 1.
Model parameters.
| Parameter | Description | Value | Sources |
|---|---|---|---|
| The transmission rate due to contact between susceptible and symptomatic infected individual. The transmission rate for asymptomatic infected subjects is 75% of ( |
High: 0.233 Medium-high: 0.203 Medium: 0.187 Low: 0.177 |
Estimated [15] | |
| Po | Proportion of infected individuals who develop symptoms | 0.6 | [15], [17] |
| γs | Rate of recovery of a symptomatic infected individual | 0.053 | [20] |
| γa | Rate of recovery of an asymptomatic infected individual | 0.125 | [20] |
| μ | Infection fatality rate among symptomatic infected individuals. | 0.001 | Estimated from [18], [19] |
| Dx | Rate of diagnosis and isolation of infected individuals | 0.015 | Estimated from [14] |
| Q | Rate of self-isolation of symptomatic infected individuals who are undiagnosed. | 0.05 | Assumption |
2.1. Infection
We extended the infected compartment of the basic SIR model to distinguish between symptomatic and asymptomatic infected individuals (Is and Ia) as they have different transmission rates. The infectiousness of asymptomatic infected individuals relative to symptomatic individuals is estimated to be 75% and 60% of infections are symptomatic [15], [17]. Susceptible individuals move to the Is and Ia compartments given the symptomatic and asymptomatic transmission rate (, . We considered 4 scenarios to illustrate a high, medium–high, medium, and low severity of the epidemic. The base reproductive number (R0) in the absence of interventions are 3.17, 2.76, 2.54, and 2.4 for the high, medium–high, medium, and low severity cases, respectively.
2.2. Diagnosis, isolation, death, and recovery
We added isolation compartments for the symptomatic and asymptomatic infected individuals who were diagnosed (ISOt_s and ISOt_a) and for the symptomatic infected individuals who decided to isolate voluntarily (ISOnt_s). In the isolation compartment, infected individuals have no contact with susceptible individuals, thus they do not transmit the disease. We assumed that infected individuals are diagnosed at a rate of 0.015 per day (Dx). This is equivalent to roughly 11.9% of the total cases to be diagnosed and confirmed by the end of the simulation, which follows practice [14]. Additionally, we assumed that symptomatic infected individuals would self-isolate at a rate of 0.05 per day (Q). We assumed 100% compliance for isolation. The infection fatality rate among symptomatic individuals was 0.001 (μ), which was equivalent to a symptomatic infection fatality ratio of approximately 1.6% by the end of the simulation [18], [19]. We only considered mortality for infected symptomatic individuals. Infected individuals who recover move to the recovered compartment given the symptomatic and asymptomatic recovery rate (γs, γa) [20]. We stratified this compartment to indicate if the infection was diagnosed (i.e., recovered and known infection, RC) or not (i.e., recovered and unknown infection, RU). We assumed that recovered individuals are immune, thus they can interact with infected individuals without risk of re-infection. When considering isolation, the effective reproductive number R0 becomes 1.68, 1.46, 1.35, and 1.27 for the high, medium–high, medium, and low epidemic severity cases, respectively.
2.3. Serology testing and vaccine distribution
We considered various times for when vaccines become available (within 7 months before and after the peak infection date during the pandemic in the baseline scenarios where vaccines are not available). For the base scenario, we assumed that vaccines were available for 50% of the population (500,000 vaccine doses) and that the supply was uniformly distributed across 6 months which was equivalent to a daily supply of 2,748 units. Additionally, we reported results for when vaccines were available for 25% of the population. We assumed that the serology tests employed were 98% sensitive and specific, which corresponds to tests that employ chemiluminescent immunoassays (CLIAs) [21]. Starting the day that the vaccines become available, we used the results of the compartmental model at the end of the day before to perform serology testing and vaccine administration. Eligible individuals for vaccination included the susceptible (S), infected asymptomatic (Ia), and recovered unknown (RU) populations. Individuals who are eligible for vaccination receive a serology test with probability p until vaccine capacity for the day is exhausted. We considered 5 values for p (0, 0.25, 0.5, 0.75, and 1) which indicates various degrees of serology testing usage from none to universal testing. If a serology test is given to the individual, they received a vaccine if they had a negative serology test result. Individuals in the recovered unknown (RU) compartment who had a positive serology test result do not receive a vaccine and they are moved to the recovered known (RC) compartment. If a serology test is not given, individuals received a vaccine independently of their status. Vaccine efficacy is modeled as the probability that a susceptible individual who is vaccinated transitions to the immunized and not previously infected compartment (Im) [22]. We assumed that vaccine protection is conferred immediately. Infected asymptomatic (Ia) and recovered unknown (RU) individuals who receive a vaccine are moved to the immunized but previously infected (ImR) compartment. After vaccines are allocated for the day, the movement among the infected, isolated, dead, and recovered compartments occurred. We considered a vaccine efficacy of 90% [23] as the base scenario and an efficacy of 70% for the alternative scenario [24].
2.4. Outcomes
The outcomes used to evaluate the scenarios and quantify the benefits of incorporating serology testing during vaccination plans include:
-
•
Infection attack rate (IAR): Cumulative percentage of the population infected.
-
•
Peak infections: Maximum number of new daily symptomatic and asymptomatic infections.
-
•
Peak day: The day when the daily new symptomatic and asymptomatic infections are the highest.
-
•
Deaths: Total number of deaths due to complications of COVID-19.
3. Results
The model outcomes of the scenarios where no vaccines were available are displayed in Table 2 . The results presented in this section correspond to the scenarios where the vaccine supply covers 50% of the population and the vaccine efficacy is 90%.
Table 2.
Simulation output for the scenario where vaccines were not available.
| Transmission Rate | Base R0 | Effective R0 under isolation | Peak Day | IAR (%) | Peak Infections | Deaths |
|---|---|---|---|---|---|---|
| High | 3.17 | 1.68 | June 2nd, 2020 | 68.63 | 12,980 | 7,678 |
| Medium-High | 2.76 | 1.46 | August 2nd, 2020 | 56.05 | 7,350 | 6,271 |
| Medium | 2.54 | 1.35 | October 1st, 2020 | 46.86 | 4,679 | 5,242 |
| Low | 2.40 | 1.27 | December 1st, 2020 | 39.85 | 3,192 | 4,449 |
3.1. Infection attack rate (IAR)
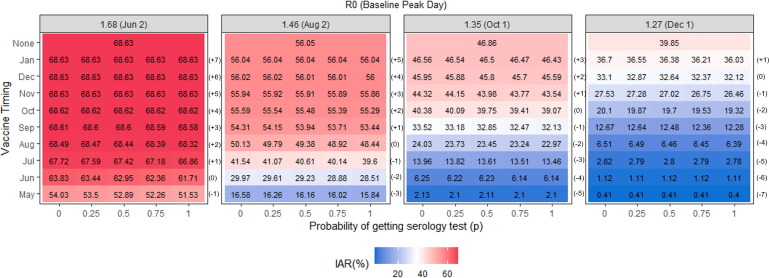
Fig. 2 shows the IAR for the scenarios modeled. The highest IAR happens when the R0 is the highest and vaccination becomes available after the peak. The earlier the vaccine is deployed, the largest the reduction in the IAR. When vaccines are deployed too late (5 months or later after the peak), vaccine usage does not reduce the IAR compared to when vaccines are not available. The reduction in the IAR as a consequence of using serology tests to prioritize vaccination of susceptible individuals is the largest when the vaccines are deployed before and close to the infection peak day and depends on the value of p. When comparing scenarios with the same R0s and vaccine timing, we observe that as the value of p increases (i.e., the number of serology tests increases), IAR decreases, except for the scenarios where the vaccines become available 5 months ahead of the peak (Fig. 2). The largest percentage reduction in infections compared to when serology testing is not available happens when the vaccine becomes available before the peak. In the scenario where the effective R0 is 1.68 (high), the largest reduction in infections occurs when the vaccine becomes available one month ahead of the peak, followed by the same month as the peak. When R0s are 1.46 (medium–high) and 1.35 (medium), the largest reductions happen when the vaccine is available two or three months ahead of the peak, depending on the value of p. When the R0 is 1.27 (low), the largest reduction occurs the vaccine is available one or two months ahead of the peak. When vaccines are deployed too early or too late (5 months or more months before or after the peak) using serology testing does not significantly affect the IAR.
Fig. 2.
Infection attack rate for the scenarios evaluated when the vaccine is available for 50% of the population and the vaccine efficacy is 90%.
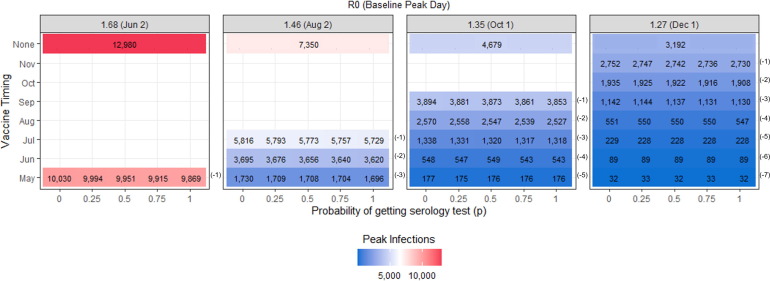
3.2. Peak infections and peak day
The timing of the vaccine availability has a significant effect on reducing the peak infections as seen in Fig. 3 . When vaccines are deployed before the baseline peak day it pushes the peak back (peak day is now earlier). When the vaccine is deployed after the baseline peak day, the peak infections and peak day remain unchanged. The use of serology testing helps to reduce the peak infections when the vaccine is deployed one or two months before the peak. The largest reduction of 161 new daily cases occurs when employing universal serology testing (p = 1), the R0 is 1.68 (high) and the vaccine becomes available one month before the peak. The peak day does not significantly change when serology testing is used and we evaluate scenarios with the same R0 and vaccine timing.
Fig. 3.
Peak infections for the scenarios evaluated when the vaccine is available for 50% of the population and the vaccine efficacy is 90%.
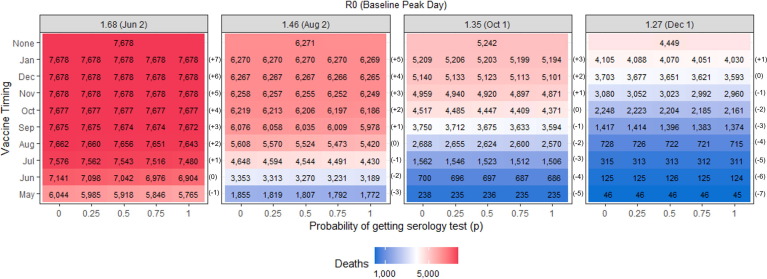
3.3. Deaths
The earlier vaccines become available, the largest the reduction in deaths, as displayed in Fig. 4 . The use of serology testing has the largest percentage reduction in deaths when the time of vaccine deployment is before and close to the baseline peak day, following the same pattern as when evaluating IAR (Table 4 ). In the scenarios where the vaccine becomes available before or during the peak, the use of serology reduces up to 279 deaths compared to when serology is not used, which occurs when employing universal serology testing (p = 1), the R0 is 1.68 (high) and the vaccine is available one month before the baseline peak.
Fig. 4.
Total deaths for the scenarios evaluated when the vaccine is available for 50% of the population and the vaccine efficacy is 90%.
Table 4.
Reduction in the cumulative deaths when a person receives a serology test with probability 0.25, 0.5, 0.75, and 1 relative to when serology test is not used (p = 0), when the vaccine is available for 50% of the population and the vaccine efficacy is 90%. The largest percentage reduction is highlighted.
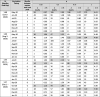 |
3.4. Alternative scenarios
The results under alternative scenarios of vaccine supply and efficacy can be found in the Supplementary Materials. The base scenario consists of a vaccine supply that covers 50% of the population with a vaccine efficacy of 90%. When the vaccine supply covers 25% of the population and the vaccine efficacy is 90%, we observed a similar pattern as to the base scenario. The use of serology testing reduces the IAR and deaths when the vaccines are deployed before the infection peak. A distinction to the base scenario is that when supply is limited, serology testing utilization has significant results even if the vaccine becomes available 5 months ahead of the peak.
When the vaccine supply covers 50% of the population and has an efficacy of 70%, the number of cases and deaths increases compared to the base scenario, however employing serology testing is still beneficial to reduce the IAR and deaths. The percentage reduction in cumulative infections and deaths is comparable to when vaccine efficacy is higher at 90%. Similar to what is observed in the base scenario, when the vaccine is available 5 months ahead of the peak, serology testing does not significantly reduce the number of cases and deaths.
4. Discussion
The deployment of vaccines is an effective intervention to reduce the spread of a pandemic by limiting the number of susceptible individuals who can get infected by the disease safely and timely. Vaccine supply will be limited as the pandemic affects countries worldwide. Therefore, it is important to evaluate policies that can provide us with effective and fair allocation guidelines for vaccines. In this paper, we evaluated the use of serology testing to prioritize the allocation of the limited supply of vaccines to susceptible individuals, as opposed to individuals who have previously contracted the disease and are immune to some extent but are unaware of their status. The value of using serology testing comes from the information that it provides regarding the status of the individuals concerning the existence of a previous infection at the time of vaccination.
Serology testing programs have been already deployed in large-scale geographic surveys as well at the local level to estimate the true prevalence of COVID-19 [25], [26], [27], [28], [29]. Additionally, serological testing has been previously explored as a way to deploy a non-medical intervention called “shield immunity” where recovered individuals substitute susceptible individuals to avoid shutting down businesses and services while limiting the spread of the disease [30].
The simulation results show that vaccination has the largest reduction in IAR and deaths when it is deployed as early as possible. When the vaccine is deployed too late, after 5 months or later, vaccines do not significantly reduce the IAR and deaths. Employing serology testing to prioritize administering the vaccines to those who are fully susceptible reduces the IAR and the deaths. The largest reductions occur when the vaccine becomes available before and close to the peak day of the pandemic. The benefit gained by using serology testing diminishes as the vaccines are deployed too early or late (5 months or more months before or after the peak). In the base scenario where vaccine supply covers 50% of the population and vaccine efficacy is 90% and we compare the impact of serology testing among scenarios with the same R0, the largest reduction in IAR and deaths occurs when the vaccines become available one to three months before the peak day, depending on the transmission rate and probability of getting a serology test (Table 3, Table 4). When the supply of the vaccine is lower (i.e., supply covers 25% of the population instead of 50%) or the vaccine efficacy is lower (i.e., 70% instead of 90%) serology testing offers similar benefits regarding the reduction in IAR and deaths, with the distinction that when the supply covers 25% of the population, the use of serology testing significantly reduces the IAR and deaths even when the vaccine becomes available 5 months ahead of the peak (Tables S1, S2). Ideally, vaccine deployment should occur as early as possible, and the use of serology testing should be evaluated depending on how early or late the vaccine becomes available relative to the disease progression.
Table 3.
Reduction in the cumulative infections when a person receives a serology test with probability 0.25, 0.5, 0.75, and 1 relative to when serology test is not used (p = 0), when the vaccine is available for 50% of the population and the vaccine efficacy is 90%. The largest percentage reduction is highlighted.
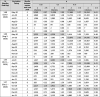 |
We tested scenarios where serology testing was deployed at various intensities. The results of the model show that when universal serology testing is not viable (i.e., p = 1), adopting an approach where only a fraction of the eligible individuals receive serology tests is still effective.
The earliest the vaccine is introduced, the largest the reduction will be observed in the peak infections. A reduction in peak infections implies a decrease in the number of daily new cases. This is important to consider since access to healthcare services can become impaired if the number of infected individuals seeking medical attention is greater than the available capacity. The use of serology testing reduces the peak infections when the vaccine is deployed one or two months before the peak day (Fig. 3). In all other scenarios, serology testing does not significantly affect the infection peak. Similarly, serology testing does not significantly affect the resulting peak day.
The results of the modeling show that when vaccine supply is limited, using serology testing to decide vaccine allocation priority groups would ensure targeted utilization and higher impact of vaccines to reduce the IAR and deaths. Since the magnitude of the reduction depends on the number of serology tests employed and the relative time between the infection peak day and when vaccination becomes available, policymakers must consider the trade-off between the cost of the serology testing and the marginal benefit of its implementation given the state of the pandemic. While predicting the exact timing of the peak might be challenging, decision-makers could use data-driven tools to project the disease spread geographically and over time. If a region is projected to be close to or has passed the peak, these projections (along with other factors, such as cost and capacity available for testing) could inform the decisions as to whether to utilize serology testing during the vaccine rollout.
Costs associated with the implementation of pre-vaccination serology testing include the direct costs of testing, as well as the indirect logistical burden related to running a testing site, which includes coordination of supply chains for timely delivery of results and vaccination processes, robust appointment systems to avoid delays in vaccination, among others; therefore, strategic logistical planning is essential for effective implementation. While the implementation of pre-vaccination serology testing might pull resources away from other interventions, we have demonstrated that its use reduces infection and death counts. Other benefits of implementing serology testing to identify recovered individuals not discussed in this research include positive public health (i.e., determine the level of herd immunity in the community, understand the level of symptomatic vs asymptomatic infection) [31] and societal (i.e., individuals’ return to work, improved mental health) impact, as well reducing resource utilization of diagnostic testing.
4.1. Limitations
The results presented are limited by the restriction of our model and the assumptions made for the parameters explained in the methodology section. We use an extended SIR model which assumes homogenous mixing of the population. We do not stratify the population by age and therefore cannot account for age-dependent model parameters, such as the infection fatality ratio [15]. The infection transmission rates used in the model are static. In reality, these rates change over time depending on the interventions put in place and community compliance [32], [33]. We decided to use a simpler SIR model to directly evaluate the impact of serology testing without confounding the effects of other interventions. We modeled vaccine distribution as a one-dose vaccine. Most vaccines currently available in the market require two doses separated by 21–28 days [34], [35]. In our model assuming a one-dose vaccine is equivalent to simulating a perfect administration of a two-dose vaccine (e.g., nobody misses the second dose).
We assumed that serology tests are administered daily and that results are available immediately. In practice, the turnaround times for serology testing results range from 24 to 48 h in general. Even though our assumption simplifies the serology testing process, it does not significantly change the results. In practice, serology testing can be done reasonably ahead of time which can provide logistical flexibility to the implementation process. Changes in the status of the individual (i.e., susceptible individual at the time of serology test who become infected after or infected individual who become recovered) are unlikely to be captured through a serology test as results are delivered and can be mitigated if participants are asked to isolate while they wait for their serology results. Although the limitations listed, the model proposed captures the pandemic dynamics and the allocation of vaccines using serology testing. The model provides us with a simple and accurate representation of the benefits of using serology as part of the vaccination process.
5. Conclusions
The use of serology testing as part of the vaccine implementation process is an effective method to maximize the benefit of a COVID-19 vaccine. It provides a tool to identify and deliver the limited supply of the vaccine to a larger portion of the population that may be more susceptible to SARS-CoV-2 infection and as a result, increases the likelihood of success of COVID-19 vaccination efforts. Identifying and prioritizing vaccination of those individuals who are susceptible to infection reduces the IAR and deaths. The magnitude of the reduction depends on the relative timing between the infection peak day and the time when vaccines become available. Policymakers need to evaluate the cost-effectiveness of deploying serology tests based on the current spread of the pandemic in their community and the timeline for the vaccine distribution.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Yildirim reported being a member of the mRNA-1273 Study Group. Dr. Yildirim has received funding to her institution to conduct clinical research from BioFire, MedImmune, Regeneron, PaxVax, Pfizer, GSK, Merck, Novavax, Sanofi-Pasteur, and Micron. Dr. Keskinocak and Ms. Fujimoto do not have conflict of interest to disclose.
Acknowledgments
Acknowledgments
The authors are grateful to representatives from the Georgia Department of Public Health for sharing information and expertise regarding vaccine distribution. The authors are also thankful to the anonymous Vaccine reviewers and editors whose constructive comments and suggestions helped significantly improve the manuscript.
Funding
This work was supported in part by the RADx-UP NIH/NIDDK grant (P30DK111024-05S1), the Cooperative Agreement number NU38OT000297 from The Centers for Disease Control and Prevention (CDC) and CSTE, and the following Georgia Tech benefactors: William W. George, Andrea Laliberte, Joseph C. Mello, Richard “Rick” E. & Charlene Zalesky, and Claudia & Paul Raines. This work is solely the responsibility of the research team and does not necessarily represent the official views of the NIH, CDC, and CSTE.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.06.067.s
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.World Health Organization. The COVID-19 candidate vaccine landscape, https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines, 2020. Updated December 22, 2020. Accessed December 28, 2020.
- 2.US Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine, https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine, 2020. Updated December 23, 2020. Accessed December 28, 2020.
- 3.US Food and Drug Administration. Moderna COVID-19 Vaccine, https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine, 2020. Updated December 21, 2020. Accessed December 28, 2020.
- 4.US Food and Drug Administration. Janssen COVID-19 Vaccine, https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine, 2021. Updated May 5 2021. Accessed May 17 2021.
- 5.Gayle H., Foege W., Brown L., Kahn B. The National Academy Press; Washington, DC: 2020. Framework for equitable allocation of COVID-19 vaccine; p. 25917. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. What COVID-19 Seroprevalence Surveys Can Tell Us, https://www.cdc.gov/coronavirus/2019-ncov/covid-data/seroprevalance-surveys-tell-us.html, 2020. Updated July 8, 2020. Accessed October 30, 2020.
- 7.Okba N.M., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., et al. Severe acute respiratory syndrome coronavirus 2− specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H., Chromikova V., McMahon M., et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;1–4 doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueiredo-Campos P., Blankenhaus B., Mota C., Gomes A., Serrano M., Ariotti S., et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to six months post disease onset. Eur J Immunol. 2020;50:2025–2040. doi: 10.1002/eji.202048970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isho B., Abe K.T., Zuo M., Jamal A.J., Rathod B., Wang J.H., et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvey R.A., Rassen J.A., Kabelac C.A., Turenne W., Leonard S., Klesh R., et al. Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Internal Med. 2021;181:672–679. doi: 10.1001/jamainternmed.2021.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reese H., Iuliano A.D., Patel N.N., Garg S., Kim L., Silk B.J., et al. Estimated incidence of COVID-19 illness and hospitalization—United States, February–September, 2020. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Centers for Disease Control and Prevention. COVID-19 Pandemic Planning Scenarios, https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html, 2020. Updated September 10, 2020. Accessed December 1, 2020.
- 16.R Core Team. R: A Language and Environment for Statistical Computing. R version 3.6.3 ed. Vienna, Austria; 2018.
- 17.Oran D.P., Topol E.J. Prevalence of Asymptomatic SARS-CoV-2 Infection: A Narrative Review. Ann Intern Med. 2020 doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basu A. Estimating The Infection Fatality Rate Among Symptomatic COVID-19 Cases In The United States: Study estimates the COVID-19 infection fatality rate at the US county level. Health Aff (Millwood) 2020;39:1229–1236. doi: 10.1377/hlthaff.2020.00455. [DOI] [PubMed] [Google Scholar]
- 19.Ioannidis J. Bull World Health Organ; 2020. Infection fatality rate of COVID-19 inferred from seroprevalence data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang R., Gui X., Xiong Y. Comparison of clinical characteristics of patients with asymptomatic vs symptomatic coronavirus disease 2019 in Wuhan, China. JAMA Network Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.10182. e2010182-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastos M.L., Tavaziva G., Abidi S.K., Campbell J.R., Haraoui L.-P., Johnston J.C., et al. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel M.D., Rosenstrom E., Ivy J.S., Mayorga M.E., Keskinocak P., Boyce R.M., et al. Association of Simulated COVID-19 Vaccination and Nonpharmaceutical Interventions With Infections, Hospitalizations, and Mortality. JAMA Network Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.10782. e2110782-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H.L., Yoon S.K., Meece J., et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight US locations, December 2020–March 2021. Morb Mortal Wkly Rep. 2021;70:495. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee Meeting February 26, 2021 https://www.fda.gov/media/146217/download, 2021. Updated February 26, 2021. Accessed May 10, 2021.
- 25.US Centers for Disease Control and Prevention. Large-scale Geographic Seroprevalence Surveys, https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/geographic-seroprevalence-surveys.html, 2020. Updated October 2, 2020. Accessed December 15, 202.
- 26.Bendavid E., Mulaney B., Sood N., Shah S., Ling E., Bromley-Dulfano R., et al. COVID-19 Antibody Seroprevalence in Santa Clara County, California. MedRxiv. 2020 doi: 10.1101/2020.04.14.20062463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajema K.L., Wiegand R.E., Cuffe K., Patel S.V., Iachan R., Lim T., et al. Estimated SARS-CoV-2 Seroprevalence in the US as of September 2020. JAMA Internal Med. 2020 doi: 10.1001/jamainternmed.2020.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sood N., Simon P., Ebner P., Eichner D., Reynolds J., Bendavid E., et al. Seroprevalence of SARS-CoV-2–Specific Antibodies Among Adults in Los Angeles County, California, on April 10–11, 2020. JAMA. 2020 doi: 10.1001/jama.2020.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lerner A.M., Eisinger R.W., Lowy D.R., Petersen L.R., Humes R., Hepburn M., et al. The COVID-19 serology studies workshop: recommendations and challenges. Immunity. 2020;53:1–5. doi: 10.1016/j.immuni.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weitz J.S., Beckett S.J., Coenen A.R., Demory D., Dominguez-Mirazo M., Dushoff J., et al. Modeling shield immunity to reduce COVID-19 epidemic spread. Nat Med. 2020 doi: 10.1038/s41591-020-0895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nadoushan M.J., Ahmadi S., Nadoushan P.J. Serology Testing for SARS-CoV-2: Benefits and Challenges. Iranian J Pathol. 2020;15:154. doi: 10.30699/IJP.2020.39841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeffrey B., Walters C.E., Ainslie K.E., Eales O., Ciavarella C., Bhatia S., et al. Anonymised and aggregated crowd level mobility data from mobile phones suggests that initial compliance with COVID-19 social distancing interventions was high and geographically consistent across the UK. Wellcome Open Res. 2020;5 doi: 10.12688/wellcomeopenres.15997.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao S., Rao J., Kang Y., Liang Y., Kruse J. Mapping county-level mobility pattern changes in the United States in response to COVID-19. SIGSPATIAL Special. 2020;12:16–26. doi: 10.1145/3404820.3404824. [DOI] [Google Scholar]
- 34.US Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 Vaccine, https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html, 2020. Updated December 22, 2020. Accessed December 15, 2020.
- 35.Centers for Disease Control and Prevention. Moderna COVID-19 Vaccine, https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html, 2020. Updated December 22, 2020. Accessed December 28, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.