Abstract
Purpose
To measure the degree of fatty liver using non-contrast enhanced chest computed tomography (CT) and investigate its relationship with the severity and prognosis of coronavirus disease 2019 (COVID-19) in adult patients.
Methods
This retrospective study included consecutive patients who had been diagnosed with COVID-19 using real-time reverse-transcription polymerase chain reaction (RT-PCR) and subsequently underwent non-contrast enhanced chest CT between October 10 and December 10, 2020. Hepatic attenuation values were measured from Couinaud segments 2, 4, and 8 based on the CT images and the relationships between these values and the Pneumonia Severity Score (PSS), requirement of hospitalization, and the length of hospital and intensive care unit (ICU) stay were analyzed.
Results
The study included 414 patients (182 were female, 43.96%), among whom 106 (25.6%) were diagnosed with hepatosteatosis (HS). In the patients with HS, the PSS scores were higher (10.8 ± 4.96 vs. 8.07 ± 5.12; p < 0.001), and 69 (65%) received inpatient care. Moreover, the number of HS patients who received inpatient care was 1.99 (95% confidence interval (CI) 1.26–3.15, p < 0.003) times higher than that of the non-HS patients. No significant difference was found between the HS and non-HS patients with regard to the length of hospital or ICU stay.
Conclusion
HS can be easily evaluated using non-contrast enhanced chest CT in COVID-19 patients and can be used as a prognostic marker to determine the requirement of hospitalization.
Keywords: Hepatosteatosis, COVID-19, Computed tomography, Hounsfield unit
Abbreviations
- HS
Hepatosteatosis
- COVID-19
Coronavirus disease 2019
- CT
Computed tomography
- HU
Hounsfield unit
- PSS
Pneumonia Severity Score
- RT-PCR
Reverse-transcription polymerase chain reaction
- WHO
World Health Organization
- ROI
Region-of-interest
- CTL/S
hepatic-to-splenic attenuation ratio
- CTL-S
Hepatic-to-splenic attenuation difference
1. Introduction
Coronavirus disease 2019 (COVID-19) is a serious infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 emerged in the city of Wuhan, China, in December 2019, and rapidly spread around the world.1 The term COVID-19 was officially declared in February 2020, and the disease was declared a pandemic by the World Health Organization (WHO) in March 2020. Real-time reverse-transcription polymerase chain reaction (RT-PCR) testing for SARS-CoV-2 is the gold standard for the diagnosis of COVID-19.2 Additionally, computed tomography (CT) has been shown to be highly useful in the clinical diagnosis and prediction of the prognosis.3
COVID-19 can manifest as an asymptomatic or mildly symptomatic disease and may cause severe pneumonia, respiratory failure, and subsequent death. Mortality rates can be high, particularly in elderly patients and those with comorbidities, such as metabolic syndrome or cerebrovascular disease.4 Additionally, metabolic risk factors have been shown to be strongly associated with increased severity of the disease.5 , 6 Fatty liver disease (FLD), also known as hepatosteatosis (HS), affects up to 30% of the general population.7 , 8 FLD is known to cause significant impairment in the liver functions that play a role in the immune system.9., [10], [11] Additionally, FLD has also been associated with increased mortality in recurrent bacterial infections and community-acquired pneumonia.12 , 13 To our knowledge, there have been very few studies conducted on the measurement of the degree of fatty liver using CT and its relation with COVID-19.9 , 14 , 15 The aim of this study was to measure the degree of fatty liver using CT and investigate its relationship with the severity and prognosis of COVID-19 in adult patients.
2. Methods
This study was approved by the local ethics committee, and the requirement for informed consent was waived by the same committee (Approval No.: 2021/01-18).
2.1. Patient selection
This retrospective study included consecutive patients who had been diagnosed with COVID-19 using RT-PCR and subsequently underwent non-contrast enhanced chest CT between October 10 and December 10, 2020. Exclusion criteria included being under 18 years of age; CT images with artifacts and images that did not involve Couinaud segments 2, 4, and 8; the presence of heterogeneous patchy hepatosteatosis; and prior splenectomy or hepatectomy. Non-contrast enhanced chest CT images that were obtained at the time of the first admission to the hospital were evaluated for each patient. The patients included in the study had not yet begun treatment.
2.2. Computed tomography procedure
All of the CT scans were performed using a 16-slice multidetector CT scanner (MX16, Philips Medical System, Koninklijke, Netherlands) with the patient at deep inspiration in the supine position. After each scan, the CT device and the entire room were sanitized. Scanning and reconstruction parameters included 16 × 0.75-mm beam collimation, 0.75-s rotation time, 1-mm slice thickness, 1-mm slice reconstructions, 90–120-kV tube voltage, and 50–110-mAs effective tube current-time product. The scanning boundaries extended from the apex to the lung base. The field of view was 250–300 mm. The lung and abdomen window settings were adjusted to a length of 600 and 400 Hounsfield units (HU) and a width of 1500 and 40 HU, respectively. The average effective dose amounts varied between 4 and 6 mSv.
2.3. Image interpretation
All of the CT images were analyzed using the institutional database system (Oracle database V1.10.43.134). The images were evaluated by two radiologists who had 6 (M.Ç.) and 3 years (C.O.) of experience in radiology and decisions were reached via consensus. In cases of discrepancies between the two radiologists, the final decision was made by the third radiologist (H.T.B.), who had 16 years of experience in radiology.
The Pneumonia Severity Score (PSS), which was developed by Chung et al.16 and verified by Li et al.,17 is a semiquantitative CT scoring system used for estimating the extent of lobar involvement in COVID-19 pneumonia. The PSS was calculated by summing the involvement percentage scores of five lung lobes and ranged from 0 to 20 (Fig. 1 ). The scores were classified as follows: ‘0’ no pneumonia (0%), ‘1’ minimal involvement (1%–25%), ‘2’ mild involvement (26%–50%), ‘3’ moderate involvement (51%–75%), and ‘4’ severe involvement (76%–100%).16 , 17
Fig. 1.
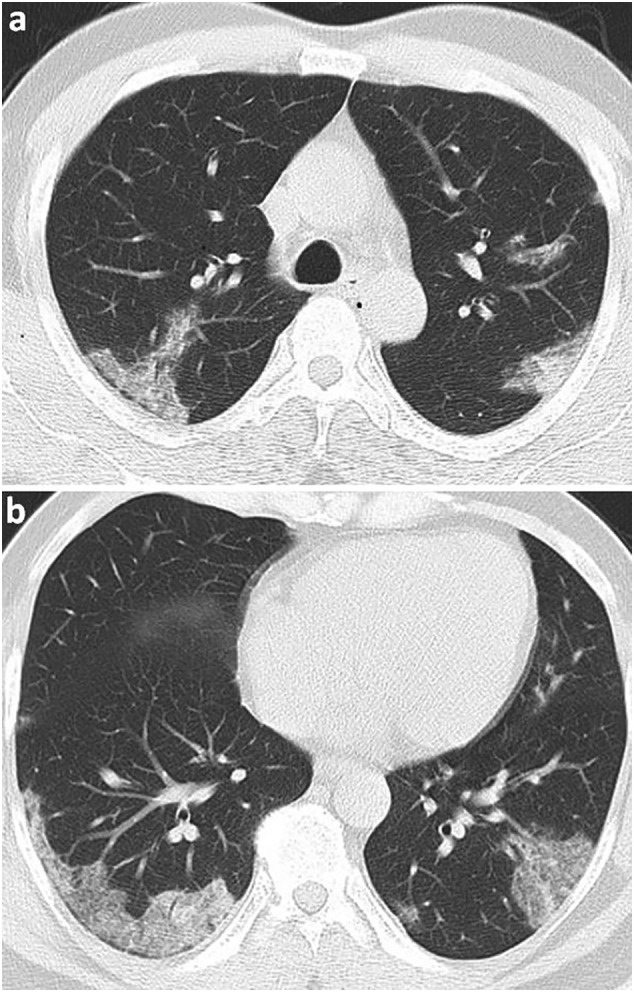
Axial chest CT lung parenchyma imaging of a 54-year-old male patient, whose diagnosis of COVID-19 was confirmed by RT-PCR test. There are peripheral ground glass opacity and consolidation areas in the upper and lower lobes of both lungs, which showed an involvement of approximately 0%–25% of each lobe. There is no involvement in the middle lobe of the right lung. The total PSS was 4.
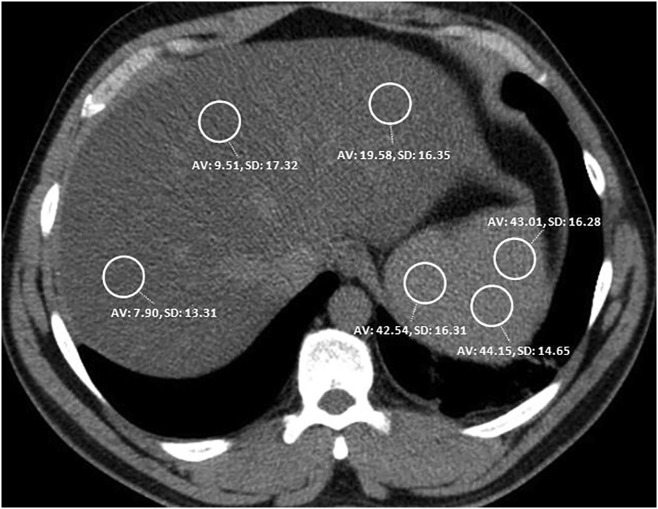
The hepatic-to-splenic attenuation ratio (CTL/S) defined by Park et al. was employed for the hepatosteatosis measurement.18 When the cutoff for the CTL/S was considered to be <0.8 according to this method, 100% specificity was reported for moderate to severe HS, defined as fatty liver over 30%.18 The CTL/S for each patient was determined by using the 1.5 cm2 circular region-of-interest (ROI) measurements of the hepatic and splenic attenuation values HU. The hepatic attenuation values were calculated from Couinaud segments 2, 4, and 8. The splenic attenuation values were measured from the anterior, middle, and posterior regions of the spleen (Fig. 2 ). All of the hepatic and splenic attenuation values were homogenized by averaging the measurements and then the CTL/S were calculated. When performing the measurements, the ROIs were determined at distant locations from large vascular structures. Patients visually showing heterogeneous steatosis in the liver were excluded from the study to avoid measurement errors. None of the patients included in this study were receiving amiodarone therapy or had a history of hemosiderosis or hemochromatosis that could affect liver attenuation.
Fig. 2.
Measurement of attenuation in the three liver and in the three splenic regions.
2.4. Clinical examination
Indications for hospitalization were determined based on the Guidelines for the Diagnosis of COVID-19 published by the Turkish Health Ministry, which comprised: 1) mild-to-moderate pneumonia (bilateral <50% radiological involvement), respiratory rate ≥ 24, and SpO2 ≤ 93%; 2) mild-to-moderate pneumonia and poor prognostic criteria in blood tests performed on admission (blood lymphocyte count <800/μL or serum C-reactive protein (CRP) >10 × upper limit of normal value or ferritin >500 ng/mL or D-dimer >1000 ng/mL); 3) severe pneumonia (change of consciousness, respiratory distress, respiratory rate ≥ 24, SpO2 90% on room air, diffuse bilateral involvement [>50%] on chest CT); 4) hypotension (<65 mmHg); 5) tachycardia (>100); 6) sepsis; 7) septic shock; 8) myocarditis; 9) acute coronary syndrome; 10) arrhythmia; and 11) acute kidney injury. Patients meeting at least one of these criteria were hospitalized.19
2.5. Statistical analysis
Data were analyzed using IBM SPSS for Windows 23.0 (IBM Corp., Armonk, NY, USA). Patients with and without HS were compared using the HU values. Descriptive statistics were expressed as frequencies (n) and percentages (%) for the categorical variables and as the mean standard deviation (SD) and minimum-maximum values for the continuous variables. The normal distribution of data was assessed using the Kolmogorov-Smirnov test. Age was compared using the independent samples t-test, and the remaining continuous variables were non-parametric and therefore compared using the Mann-Whitney U test. Categorical variables were compared using Pearson's chi-square test. p < 0.05 was accepted as statistically significant.
3. Results
A total of 550 patients had a positive RT-PCR test and underwent chest CT at the time of the first admission during the study period. Of these, 136 patients were excluded based on the following exclusion criteria: prior hepatectomy (n = 3), prior splenectomy (n = 4), heterogeneous steatosis (n = 27), age under 18 years (n = 56), images with artifacts and/or poor quality (n = 33), and a diagnosis of COVID-19 established by contrast-enhanced CT (n = 13). As a result, a total of 414 patients were included in the study (Fig. 3 ).
Fig. 3.
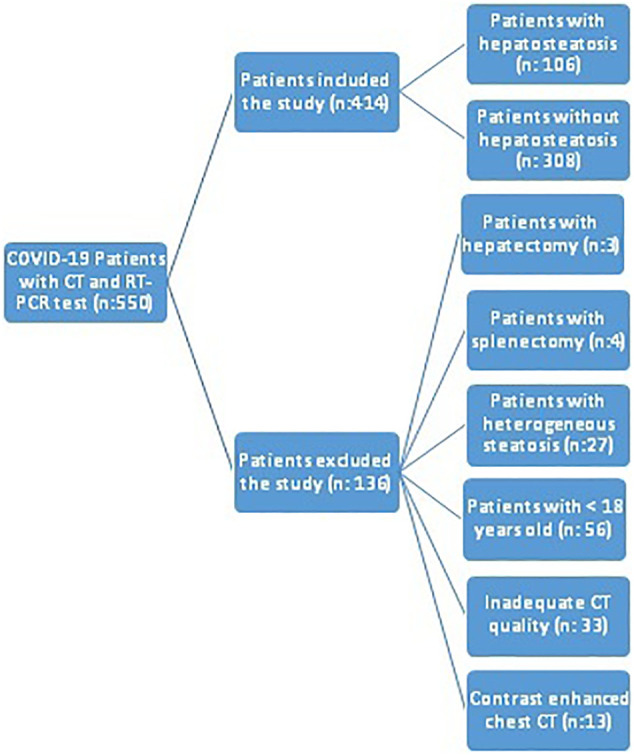
Flow-chart for patient selection.
HS was detected in 106 (25.6%) patients (CTL/S < 0.8) and was not detected in the remaining 308 (74.39%) patients (CTL/S ≥ 0.8). There was no statistically significant difference in the age and gender distribution between the two groups (age: p = 0.330, gender: p = 0.054), but the prevalence of HS was higher in the men than in the women (29.31% vs. 20.87%). Mean hepatic attenuation was significantly lower in the HS group (26.35 ± 9.26 versus 50.18 ± 7.74; p < 0.001). The mean splenic attenuation value was 45.73 ± 4.41 in the HS group and was 44.33 ± 5.53 in the non-HS group. No significant difference was found between the two groups with regard to splenic attenuation (p = 0.089). The CTL/S values ranged between 0.8 and 2.5 in the non-HS group and between 0 and 7.9 in the HS group (Table 1 ).
Table 1.
Relationship between the demographic characteristics, attenuation values, and hepatosteatosis.
| CTL/S ≥ 0.8 (n = 308) |
CTL/S < 0.8 (n = 106) |
p value | |
|---|---|---|---|
| Age (years) | 55.89 ± 15.51 (18–94) | 54.25 ± 13 (25–92) | 0.330 |
| Genderb | |||
| Female, n (%) | 144 (53.2) | 38 (35.8) | 0.054 |
| Male, n (%) | 164 (46.8) | 68 (64.2) | |
| Liver/spleen ratioa | 1.14 ± 0.18 (0.80–2.50) | 0.54 ± 0.19 (0–0.79) | <0.001 |
| Liver HUa | 50.18 ± 7.74 (30–69) | 26.35 ± 9.26 (0–43) | <0.001 |
| Spleen HUa | 44.33 ± 5.53 (32–64) | 45.73 ± 4.41 (39–61) | 0.089 |
HU: Hounsfield units, CTL/S: hepatic-to-splenic attenuation ratio.
Mann-Whitney U test was used.
Pearson's chi-square test was used.
Mean PSS was 10.81 ± 4.96 in the HS group and 8.07 ± 5.12 in the non-HS group, and the difference was statistically significant (p < 0.001). Inpatient care was received by 65% of HS patients as opposed to 48% of non-HS patients, and outpatient care was received by 35% of HS patients as opposed to 52% of non-HS patients. The odds ratio of COVID-19 positive patients with HS compared to non-HS patients for inpatients care requirement was 1.99 (95% CI 1.26–3.15, p < 0.003). However, no significant difference was found between the two groups in the length of hospitalization in the general ward and ICU (Table 2 ).
Table 2.
Relationship between PSS, type of patient care and hepatosteatosis
| CTL/S ≥ 0.8 (n = 308) |
CTL/S < 0.8 (n = 106) |
p value | |
|---|---|---|---|
| PSSa | 8.07 ± 5.12 (1−20) | 10.81 ± 4.96 (1–20) | <0.001 |
| Patient careb | |||
| Outpatient, n (%) | 159 (52%) | 37 (35%) | 0.003 |
| Inpatient, n (%) | 149 (48%) | 69 (65%) | |
| General ward (days)a | 9.07 ± 5.78 (1–40) | 9.12 ± 5.89 (1−32) | 0.934 |
| ICU (days)a | 11.22 ± 8.17 (1–49) | 10.51 ± 7.06 (1–40) | 0.497 |
PSS: Pneumonia Severity Score, ICU: intensive care unit, CTL/S: hepatic-to-splenic attenuation ratio.
Mann-Whitney U test was used.
Pearson's chi-square test was used.
4. Discussion
Our study demonstrated a significantly higher PSS score in COVID-19 positive patients with HS as defined by a CTL/S < 0.8 when compared to patients without HS. Furthermore, patients with HS had a 1.99 times increased risk of being admitted as an inpatient during the course of treatment.
Thoracic CT is widely used in the follow-up and diagnosis of COVID-19. HS can be easily evaluated in addition to pulmonary parenchymal findings, since the upper abdominal region is covered within the standard chest protocol. There are 3 different methods that have been defined in the evaluation performed with unenhanced CT, which comprise measuring liver attenuation, evaluation of the difference in liver and spleen attenuation (CTL—S), and evaluation of the CTL/S.20 The HU values of the tissues may differ depending on the device and the exposure parameters applied. The spleen is considered as a good internal control measure, since it is not affected by many pathological processes.21 Therefore, in this study, the CTL/S method defined by Park et al.18 was used for the diagnosis of HS and a cutoff value of 0.8 for moderate-severe hepatosteatosis. In a study conducted by Limanond et al.,22 using the CTL-S method, results similar to those in the study of Park et al.18 were found when a cutoff value of −10 HU was used. In the current study, the CTL-S was also tested, and no significant difference was found between the findings of either method.
This was the first study in the literature to calculate the CTL/S and demonstrate the relationship between HS, PSS, and the prognosis of COVID-19. Medeiros et al.14 reported a higher prevalence of HS in COVID-19 patients when compared to the control subjects. They also found a relationship between the HS, metabolic syndrome, and obesity, and noted that this relationship had a role in the pathogenesis of COVID-19 and the increased prevalence of infection. However, the effect of HS on the severity of pneumonia and patient prognosis was not examined in the study of Medeiros et al.,14 in which they defined hepatosteatosis as patients who had a liver attenuation value of ≤40 HU. This may have resulted in an erroneous HS evaluation due to the differences in the device and scan parameters.
In the study conducted by Palomar-Lever et al.15 on 213 patients diagnosed with COVID-19, it was revealed that HS increased the severity of COVID-19 pneumonia as an independent risk factor. However, unlike the current study, they examined the severity of pneumonia in two categories according to the severity level using the chest CT severity index (≥20/40, as severe COVID-19). On the other hand, in the present study, the severity of pneumonia in the HS group was quantified with the PSS, and similar to the study of Palomar-Lever et al.,15 a statistically significant difference was found in the HS group. Additionally, the follow-up and treatment processes of the patients were also examined in the current study, and the rate of hospitalization was found to be statistically significantly higher in patients with HS than in those without HS.
It was revealed in a meta-analysis of COVID-19 patients that cardiometabolic risk factors, such as obesity, are potential risk factors for mortality.23 HS is regarded as a hepatic manifestation of the metabolic syndrome, including insulin resistance, diabetes, obesity, and dyslipidemia.24 Therefore, HS, as an indicator of these intertwined pathologies, can be considered as a predisposing factor in severe COVID-19. The current study also supported this result. HS occurs as a result of the accumulation of lipids consisting mostly of triglycerides in hepatocytes, and is the basic first form of FLD, which is a complex entity.25 The liver plays a pivotal role in host defense against bacteria and also plays a role in most systemic infections, since it receives a dual blood supply from the hepatic portal vein and hepatic arteries.26 Although the underlying factors contributing to the severe course of infections in FLD remain unclear, the accumulated evidence thus far has suggested that hepatic and circulating interleukin 6 (IL-6) levels are increased in these patients. Moreover, it has also been reported that the production of proinflammatory cytokines, such as TNF-a from Kupffer cells, is increased in these patients and that this increase has a negative impact on infectious diseases.[27], [28], [29] With many other mechanisms in addition to these factors, HS negatively affects the functions of the liver on the immune system and makes the patient more susceptible to infections.30 , 31
This study was limited in several ways. First, the study was a single-center retrospective study. Second, the degree of fatty liver was evaluated using CT, and the presence of mild fatty liver (<30%) was not evaluated using other imaging modalities or biopsies. Additionally, the patients were divided only based on the presence of HS. Therefore, further studies are needed to investigate the correlation between the severity of HS and the disease severity by classifying the patients according to the severity of HS. Third, the patients did not have a homogeneous distribution, and no evaluation was performed for the comorbidities that could have affected their prognosis. Moreover, the steatohepatitis and fibrosis status of the patients could not be evaluated since no liver function tests or parenchymal biopsies were performed. Finally, the effect of HS on the course of COVID-19 could not be investigated since the evaluations were performed based on the chest CT images obtained at the time of the first admission.
5. Conclusion
HS and PSS were significantly associated with the outcomes of adult COVID-19 patients. The study showed a significant relationship between HS and a more severe clinical course with increased need of hospitalization. HS can be easily evaluated using chest CT in COVID-19 patients and can be used in daily practice to determine the prognosis of the disease without the requirement of additional examinations.
Declaration of competing interest
None.
References
- 1.Wang F.S., Zhang C. What to do next to control the 2019-nCoV epidemic? Lancet. 2020;395(10222):391–393. doi: 10.1016/S0140-6736(20)30300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ufuk F., Savaş R. Chest CT features of the novel coronavirus disease (COVID-19) Turk J Med Sci. 2020;50 doi: 10.3906/sag-2004-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed]
- 6.Cai Q., Chen F., Wang T., et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43(7):1392–1398. doi: 10.2337/dc20-0576. 07. [DOI] [PubMed] [Google Scholar]
- 7.Chen VL, Hawa F, Berinstein JA, Reddy CA, Kassab I, Platt KD, et al. Hepatic steatosis is associated with increased disease severity and liver injury in coronavirus disease-19. Dig Dis Sci doi: 10.1007/s10620-020-06618-3. [DOI] [PMC free article] [PubMed]
- 8.Le M.H., Devaki P., Ha N.B., et al. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0173499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao F, Zheng KI, Wang X-B, Yan H-D, Sun Q-F, Pan K-H, et al. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J Gastroenterol Hepatol.n/a(n/a). doi: 10.1111/jgh.15112. [DOI] [PMC free article] [PubMed]
- 10.Ji D., Qin E., Xu J., et al. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefere S., Tacke F. Macrophages in obesity and non-alcoholic fatty liver disease: crosstalk with metabolism. JHEP Rep. 2019;1(1):30–43. doi: 10.1016/j.jhepr.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nseir W., Taha H., Khateeb J., Grosovski M., Assy N. Fatty liver is associated with recurrent bacterial infections independent of metabolic syndrome. Dig Dis Sci. 2011;56(11):3328–3334. doi: 10.1007/s10620-011-1736-5. [DOI] [PubMed] [Google Scholar]
- 13.Nseir W., Mograbi J., Amara A., Abu Elheja O., Mahamid M. Non-alcoholic fatty liver disease and 30-day all-cause mortality in adult patients with community-acquired pneumonia. QJM. 2019;112(2):95–99. doi: 10.1093/qjmed/hcy227. [DOI] [PubMed] [Google Scholar]
- 14.Medeiros A.K., Barbisan C.C., Cruz I.R., et al. Higher frequency of hepatic steatosis at CT among COVID-19-positive patients. Abdom Radio. 2020;45(9):2748–2754. doi: 10.1007/s00261-020-02648-7. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palomar-Lever A, Barraza G, Galicia-Alba J, Echeverri-Bolaños M, Escarria-Panesso R, Padua-Barrios J, et al. Hepatic steatosis as an independent risk factor for severe disease in patients with COVID-19: a computed tomography study. JGH Open. doi: 10.1002/jgh3.12395. [DOI] [PMC free article] [PubMed]
- 16.Chung M., Bernheim A., Mei X., et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230. 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li K., Fang Y., Li W., et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30(8):4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park S.H., Kim P.N., Kim K.W., et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239(1):105–112. doi: 10.1148/radiol.2391050361. Apr. [DOI] [PubMed] [Google Scholar]
- 19.Republic of TurkeyMinistry of Healhty Hospitalization indications on COVID-19. 2020. https://covid19.saglik.gov.tr/Eklenti/39061/0/COVID19rehberieriskinhastatedavisipdf.pdf
- 20.Ma X., Holalkere N.S., Kambadakone R.A., Mino-Kenudson M., Hahn P.F., Sahani D.V. Imaging-based quantification of hepatic fat: methods and clinical applications. Radiographics. 2009;29(5):1253–1277. doi: 10.1148/rg.295085186. Sep–Oct. [DOI] [PubMed] [Google Scholar]
- 21.Bydder G.M., Chapman R.W., Harry D., Bassan L., Sherlock S., Kreel L. Computed tomography attenuation values in fatty liver. J Comput Tomogr. 1981;5(1):33–35. doi: 10.1016/0149-936X(81)90054-0. Mar. [DOI] [PubMed] [Google Scholar]
- 22.Limanond P., Raman S.S., Lassman C., et al. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology. 2004;230(1):276–280. doi: 10.1148/radiol.2301021176. Jan. [DOI] [PubMed] [Google Scholar]
- 23.Sachdeva S, Khandait H, Kopel J, Aloysius MM, Desai R, Goyal H. NAFLD and COVID-19: a pooled analysis. SN Compr Clin Med. doi: 10.1007/s42399-020-00631-3. [DOI] [PMC free article] [PubMed]
- 24.Hashimoto E., Taniai M., Tokushige K. Characteristics and diagnosis of NAFLD/NASH. J Gastroenterol Hepatol. 2013 Dec;28(suppl 4):64–70. doi: 10.1111/jgh.12271. [DOI] [PubMed] [Google Scholar]
- 25.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–1231. doi: 10.1056/NEJMra011775. Apr 18. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z., Xu M., Yi J.Q., et al. Clinical characteristics and mechanism of liver damage in patients with severe acute respiratory syndrome. Hepatobiliary Pancreat Dis Int. 2005;4:60–63. [PubMed] [Google Scholar]
- 27.Ji D., Qin E., Xu J., et al. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020;8:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Poorten D., Milner K.L., Hui J., et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449–457. doi: 10.1002/hep.22350. [DOI] [PubMed] [Google Scholar]
- 29.Wieckowska A., Papouchado B.G., Li Z., Lopez R., Zein N.N., Feldstein A.E. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:1372–1379. doi: 10.1111/j.1572-0241.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 30.Petrakis D., Margină D., Tsarouhas K., et al. Obesity - a risk factor for increased COVID-19 prevalence, severity and lethality (review) Mol Med Rep. 2020;22(1):9–19. doi: 10.3892/mmr.2020.11127. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kassir R. Risk of COVID-19 for patients with obesity. Obes Rev. 2020;21(6) doi: 10.1111/obr.13034. (06) [DOI] [PMC free article] [PubMed] [Google Scholar]