Abstract
Objective
High-dose intravenous vitamin C (HIVC) is a major concern when treating patients with coronavirus disease 2019 (COVID-19). The aim of this study was to assess the clinical efficacy of HIVC on hyperinflammation in patients with severe COVID-19.
Methods
This retrospective cohort study included hospitalized patients with severe COVID-19, a subset of whom was treated with HIVC. The medical records were screened for demographic data, laboratory findings, and medications, as well as initial and repeated values of multiple inflammatory markers for analysis.
Results
A high percentage of patients presented with hyperinflammation based on inflammatory marker levels above the upper limit of normal (high-sensitivity C-reactive protein, 80.1%; interleukin-6, 91.5%; and tumor necrosis factor-α, 67.4%). Eighty-five (36%) patients received HIVC therapy. After treatment with HIVC, the levels of inflammatory markers displayed a significant decrease compared with those of patients without HIVC. Furthermore, the percentages of reduction in inflammatory marker levels were higher in patients receiving HIVC compared with those in patients treated without HIVC. Stepwise multiple linear regression analysis revealed that HIVC was independently associated with percentages of reduction in levels of inflammatory markers.
Conclusions
HIVC has the potential benefit of attenuating hyperinflammation by reducing inflammatory marker levels in patients with severe COVID-19.
Keywords: COVID-19, SARS-CoV-2, Vitamin C, Inflammation
Introduction
Patients who have severe coronavirus disease 2019 (COVID-19) with hyperinflammation often progress to acute respiratory failure and multiorgan dysfunction [1]. However, to our knowledge, no optimal strategy is currently available to work well against hyperinflammation caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), and symptomatic supportive care is the mainstay of treatment [2]. Therefore, there is an urgent need for an effective pharmaceutical approach to the grim situation.
High-dose intravenous vitamin C (HIVC) is a major concern when treating patients with COVID-19 due to its powerful biological properties. Considerable debate arises regarding the clinical efficacy of HIVC therapy in patients with COVID-19. Some clinical evidence indicates that HIVC infusion is a promising adjuvant therapeutic target for the epidemic [3]. However, there are cases in which HIVC showed no beneficial effect in treating sepsis, similar to SARS-CoV-2 infection [4]. Thus, the therapeutic efficacy of HIVC is worth investigation. The present study detailed an analysis of the clinical data to assess the effect of short-term IV infusion of high-dose vitamin C on multiple inflammatory markers in patients with severe COVID-19.
Methods
Patients
In this cohort study, we retrospectively reviewed patients admitted to Tongji Hospital of Huazhong University of Science and Technology in Wuhan, China, between February 1 and March 10, 2020. All inpatients were tested positive for SARS-CoV-2 viral RNA by use of real-time reverse-transcriptase polymerase chain reaction on throat swab specimens [5]. Severe illness was diagnosed if any of the following conditions was met:
-
•
Respiratory distress with respiratory rate >30 times/min;
-
•
Oxygen saturation ≤93% while breathing ambient air;
-
•
Arterial blood oxygen partial pressure/fraction of inspired oxygen ≤300 mm Hg.
The exclusion criteria included hospital length of stay (LOS) of <21 d, incomplete medical records, and pregnancy.
This study was approved by the Research Ethics Committee of Tongji Hospital of Huazhong University of Science and Technology and written informed consent was waived duo to the high volume of admissions during the pandemic.
Data extraction
The demographics, laboratory findings, and medication data were extracted from the electronic medical records. Additionally, we screened availability of initial and repeated values of multiple inflammatory markers including high-sensitivity C-reactive protein (hs-CRP), interleukin (IL)-6, and tumor necrosis factor (TNF)-α at baseline and during hospitalization.
Inflammatory biomarker measurements were completed in the clinical laboratory in Tongji Hospital. Blood samples were processed according to hospital's standard procedures. Levels of hs-CRP were determined by immunoturbidimetry method according to the manufacturer's instructions. Levels of IL-6 and TNF-α were detected using chemiluminescence immunoassay performed on a fully automated analyzer (Cobas e602, Roche, Germany).
Treatment protocol of HIVC
According to the Fifth Revised Trial Version of the Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance published by the National Health Commission of China [6], all patients received symptomatic supportive care at the appropriate situation by the medical group in Tongji Hospital on admission. In addition to receiving symptomatic supportive care, a subset of patients with consent were also administered HIVC within 24 h of admission. The HIVC protocol was administered as described elsewhere [4]. Briefly, high-dose vitamin C was intravenously administered under 6-d course in excess dosage of 100 mg/kg of body weight vitamin C diluted in 50 mL of saline solution to infuse within 30 min every 6 h on day 1 plus 100 mg/kg of body weight vitamin C diluted in 50 mL of saline solution to infuse within 30 min every 12 h for the next 5 d.
Statistical analysis
The sample size was calculated according to hs-CRP from the study regarding the effect of HIVC on hs-CRP in COVID-19 patients [7]. We used a non-inferiority test for the sample size calculation based on the following parameters: α = 0.025 (two-sided), power: 1-β = 0.8, a withdrawal rate of 10% in each group, 2:1 ratio of the control group to the intervention group. The resulting total sample size was fixed at 135, with 90 patients in the control group and 45 patients in the intervention group. Continuous variables were presented as the median with interquartile range (25th and 75th percentiles) and categorical variables as numbers with percentages. Normality of the data was tested using the Shapiro–Wilk test. The differences for clinical characteristics were compared between the two groups using two-sample independent group t test for continuous variables, and χ2 test or Fisher's exact test for categorical variables where appropriate. Because the values of inflammatory markers levels and the percentages of reduction in levels of hs-CRP, IL-6, and TNF-α, were not normally distributed, between-group differences were compared using Mann–Whitney U test. Multiple linear regression analysis with a stepwise variable selection was used to evaluate the relationship between medications including HIVC therapy and percentages of reduction in levels of each inflammatory marker after treatment. The formula for calculating the percentage of reduction was as follows: Percentage of reduction = (Levels of inflammatorymarkers at baseline - Levels of inflammatorymarkers after treatment) / (Levels of inflammatorymarkers at baseline) × 100%.
Two-tailed P < 0.05 was considered statistically significant. All analysis was performed using SPSS version 12 (SPSS, Inc., Chicago, IL, USA). Figures were drawn using GraphPad Prism version 6 (GraphPad Software, La Jolla, CA, USA).
Results
Clinical characteristics
The database contained information on 236 inpatients. The patients’ clinical characteristics under analysis are listed in Table 1 . To assess the balance between patients treated with and without HIVC, comparisons of the parameters of clinical characteristics were undertaken. No statistically significant difference was detected.
Table 1.
Clinical characteristics of patients
| Characteristics | No HIVC (n = 151) | HIVC (n = 85) | P-value |
|---|---|---|---|
| Demographic characteristics | |||
| Age, y | 66 (57–73) | 68 (60–76) | 0.115 |
| Men, n (%) | 73 (48.3) | 33 (38.8) | 0.158 |
| Current smoker, n (%) | 29 (19.2) | 16 (18.8) | 0.943 |
| Heart rate, beats per min | 88 (78–100) | 89 (80–108) | 0.274 |
| Systolic blood pressure, mm Hg | 134 (121–146) | 138 (121–150) | 0.518 |
| Diastolic blood pressure, mm Hg | 78 (72–87) | 78 (75–87) | 0.491 |
| Hypertension, n (%) | 26 (17.2) | 11 (12.9) | 0.386 |
| Coronary heart disease, n (%) | 18 (11.9) | 10 (11.8) | 0.972 |
| Diabetes, n (%) | 25 (16.6) | 13 (15.3) | 0.800 |
| Laboratory findings on admission | |||
| Leukocyte counts, cells × 109/L | 6.74 (5.04–10.39) | 9.14 (5.49–11.43) | 0.304 |
| Erythrocyte counts, cells × 1012/L | 3.94 (3.53–4.46) | 3.90 (3.49–4.38) | 0.399 |
| Platelet counts, cells × 109/L | 208 (147–258) | 209 (148–252) | 0.767 |
| Alanine aminotransferase, U/L | 23 (16–38) | 31 (17–42) | 0.930 |
| Aspartate aminotransferase, U/L | 33 (22–45) | 36 (25–51) | 0.297 |
| Urea nitrogen, mmol/L | 6.1 (4–8.4) | 7.5 (5.4–9) | 0.139 |
| Serum creatinine, μmol/L | 73 (56–93) | 85 (64–106) | 0.106 |
| Medications | |||
| Antiviral, n (%) | 98 (64.9) | 45 (52.9) | 0.071 |
| Antibiotics, n (%) | 54 (35.8) | 20 (23.5) | 0.052 |
| Glucocorticoid, n (%) | 59 (39.1) | 23 (27.1) | 0.063 |
| Immunoglobin, n (%) | 28 (18.5) | 12 (14.1) | 0.384 |
| Biologics, n (%) | 8 (5.3) | 4 (4.7) | 0.554 |
| Noninvasive ventilation, n (%) | 15 (9.9) | 11 (12.9) | 0.479 |
| Renal replacement therapy, n (%) | 6 (4) | 3 (3.5) | 0.584 |
| Antihypertensives, n (%) | 13 (8.6) | 6 (7.1) | 0.674 |
| Antidiabetics, n (%) | 21 (13.9) | 7 (8.2) | 0.196 |
HIVC, high-dose intravenous vitamin C; IQR, interquartile range
Continuous data were expressed as median (IQR), and categorical data were presented as number (%), respectively
Hyperinflammatory state
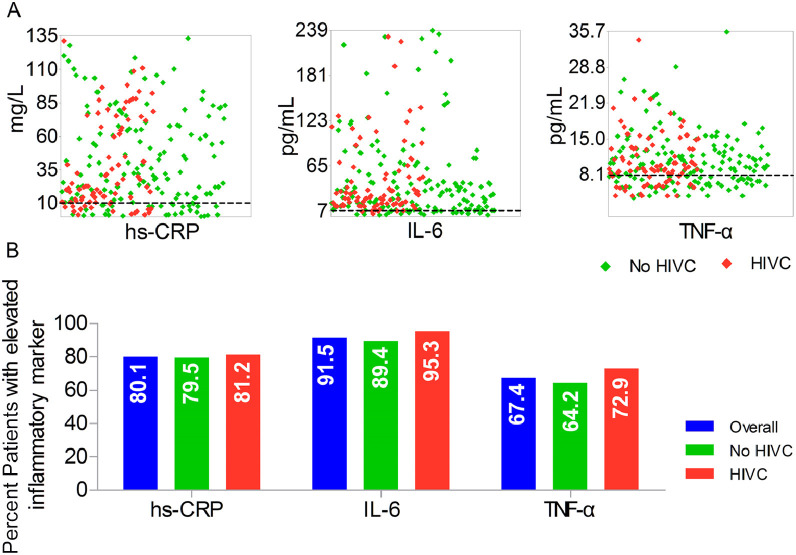
We identified hyperinflammation with an increase of multiple inflammatory markers levels [8]. As shown in Figure 1 A, most of patients were in a hyperinflammatory state with inflammatory markers levels above upper limit of normal (hs-CRP >10 mg/L, IL-6 >7 pg/mL, and TNF-α >8.1 pg/mL) at baseline. Overall, hs-CRP was substantially elevated in patients with 27.9 mg/L in conjunction with IL-2 27.8 pg/mL and TNF-α 9.6 pg/mL at baseline. The percentages of patients with elevated concentrations of hs-CRP, IL-6, and TNF-α above upper limit of normal were 80.1%, 91.5%, and 67.4%, respectively (Fig. 1B).
Fig. 1.
Hyperinflammation in patients with severe COVID-19. (A) Distribution of inflammatory marker levels on admission in patients with or without HIVC. (B) Percentages of patients with elevated levels of inflammatory markers at baseline above ULN. Dashed line represents the ULN. HIVC, high-dose intravenous vitamin C; hs-CRP, high-sensitivity C-reactive protein; IL, interleukin; TNF, tumor necrosis factor; ULN, upper limit of normal.
Changes in inflammatory markers
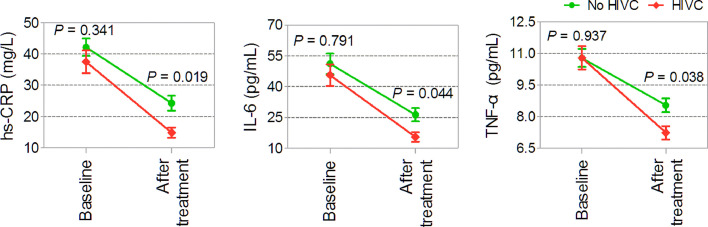
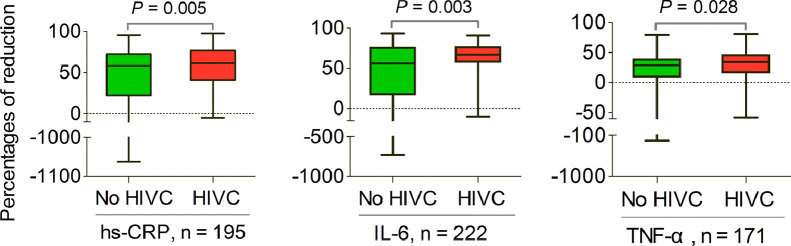
Eighty-five (36%) patients received HIVC therapy. Changes in levels of inflammatory markers from baseline to 21 d during hospitalization are shown in Figure 2 . No significant differences were observed in the levels of inflammatory markers at baseline between patients with and without HIVC administration (hs-CRP, P = 0.341; IL-6, P = 0.791; TNF-α, P = 0.937). However, the concentrations of inflammatory markers displayed a significant decrease after treatment with HIVC related to those among patients without HIVC infusion (hs-CRP, P = 0.019; IL-6, P = 0.044; TNF-α, P = 0.038). If only those patients with concentrations of inflammatory markers at baseline and 21 d during hospitalization below upper limit of normal were excluded, most patients with or without HIVC therapy experienced reduction in inflammatory marker levels. Furthermore, the percentages of reduction in inflammatory markers levels were higher in patients receiving HIVC therapy than those in those not receiving HIVC infusion (hs-CRP, P = 0.005, n = 195; IL-6, P = 0.003, n = 222; TNF-α, P = 0.028, n = 171; Fig. 3 ). Correspondingly, the negative values reflected inflammation progression, and the positive values implied alleviation of inflammation.
Fig. 2.
Changes in levels of inflammatory markers at baseline and after treatment (n = 236). HIVC, high-dose intravenous vitamin C; hs-CRP, high-sensitivity C-reactive protein; IL, interleukin; TNF, tumor necrosis factor.
Fig. 3.
Percentages of reduction in inflammatory markers levels after treatment (hs-CRP, n = 195; IL-6, n = 222; TNF-α, n = 171; respectively). Boxes represent the 25th to 75th percentiles, and horizontal lines within the box represent median value. Whiskers represent the minimum and maximum value, respectively. HIVC, high-dose intravenous vitamin C; hs-CRP, high-sensitivity C-reactive protein; IL, interleukin; TNF, tumor necrosis factor.
Additionally, we performed stepwise multiple linear regression analysis to evaluate the association between whether to treat with HIVC and percentages of reduction in levels of each inflammatory marker after treatment. As shown in Table 2 , HIVC therapy was independently associated with percentages of reduction in levels of inflammatory markers (hs-CRP, P = 0.032; IL-6, P = 0.005; TNF-α, P = 0.015).
Table 2.
Stepwise multiple linear regression analysis on the relationship between HIVC and percentages of reduction in inflammatory marker levels
| Variable | Unstandardized coefficient (β) | Standard error (SE) | Standardized coefficient (β) | 95% CI for β | t value | P-value |
|---|---|---|---|---|---|---|
| Reduction in hs-CRP levels, % | 0.328 | 0.152 | 0.153 | 0.028–0.628 | 2.156 | 0.032 |
| Reduction in IL-6 levels, % | 0.315 | 0.111 | 0.188 | 0.097–0.533 | 2.853 | 0.005 |
| Reduction in TNF-α levels, % | 0.140 | 0.057 | 0.185 | 0.028–0.252 | 2.463 | 0.015 |
HIVC, high-dose intravenous vitamin C; hs-CRP, high-sensitivity C-reactive protein; IL, interleukin; R, receptor; TN-, tumor necrosis factor
Discussion
COVID-19 epidemic is a rapidly developing medical disorder with high fatality rate [1]. Clinical investigations in hospitalized patients have shown an overwhelming increase of numerous inflammatory mediators induced by SARS-CoV-2 infection, which results in violent systemic hyperinflammatory response [9,10]. Accumulating evidence shows that hyperinflammation is a relatively common issue in some viral diseases such as SARS and Middle East respiratory syndrome (MERS), and contributes to acute respiratory failure and multiorgan dysfunction [9]. It is partially responsible for high mortality rates [11,12]. Hyperinflammation can facilitate disease development and is believed to be the main mechanism in the deterioration of SARS-CoV-2 action on humans [11]. Several studies indicate that full-blown inflammation is probably linked to the severity of the disease and affects clinical outcomes [1,13,14]. Herein, severe COVID-19 may be regarded as a SARS-CoV-2–induced hyperinflammatory condition with multiorgan involvement [14]. We identified multiple inflammatory agents including hs-CRP, IL-6, and TNF-α as the variables reflecting hyperinflammation. Amazingly, we found that hyperinflammatory status appeared in severely ill patients due to levels of hs-CRP, IL-6, and TNF-α that were markedly above the upper limit of normal. In the same context, consistent results demonstrated that high blood levels of several inflammatory parameters have been detected in some conditions such as sepsis [9]. These proinflammatory mediators trigger hyperinflammatory response in severe cases of COVID-19, similar to SARS-CoV and MERS-CoV infections [9]. Moreover, elevated levels of inflammatory markers identify patients at risk for progression to refractory hypoxemia and death.
To our knowledge, there are no therapeutic options available for SARS-CoV-2, and symptomatic supportive care is the most common treatment. Hyperinflammation may be a potential therapeutic target for severe COVID-19 disease, and treatments of hyperinflammation usually focus on this environment: intensive care of hemodynamic parameters and uphold of damaged organs. However, the results of the present study indicated that decreasing highly elevated levels of inflammatory markers may be the optimal strategy for treating patients with severe COVID-19. Additionally, suppressing hyperinflammation directed to severe and critically ill cases may prevent fatal deterioration. Therefore, the identification of existing approved therapies with proven safety to treat hyperinflammation is worth investigation to improve severity and reduce mortality.
Considerable debate arises concerning the possible clinical efficacy of HIVC on COVID-19. A case report showed that use of HIVC (200 mg/kg every 6 h) successfully treated a 20-y-old woman with virus-induced acute respiratory distress syndrome (ARDS) and she made a rapid recovery after receiving extracorporeal membrane oxygen [15]. This was further strengthened by the fact that HIVC has been used in the treatment of sepsis and has shown beneficial efficacy in reduction of systemic inflammation by inhibiting the cytokine surge [16,17]. However, another study reported no difference in the primary outcome of organ dysfunction scores and biomarkers of inflammation and vascular injury in patients receiving vitamin C (50 mg/kg every 6 h for 96 h) compared with placebo [4]. We asked whether HIVC acts as an anti-inflammatory agent to influence systematic inflammatory markers and prevent hyperinflammatory response. Vitamin C, ascorbic acid, is essential nutrient for immune cells. In addition to its important role in protecting the body against oxidative challenges, vitamin C has anti-inflammatory properties against pathogens, particularly when it is administered intravenously at high dose. First, vitamin C can inhibit the activation of nuclear factor-κB, which is primary proinflammatory transcription factor, and plays a pivotal role in hyperinflammatory response [18]. Second, vitamin C can inhibit the production of IL-6 and TNF-a, and this effect appears to be dose-dependent [19]. Third, vitamin C can inhibit oxidative stress, which may play a role in the pathogeny of COVID-19. It has been reported that vitamin C can modulate reactive oxygen species generation and inflammatory expression [20]. In this context, HIVC was recommended for treatment of SARS-CoV-2 infection by the Chinese Center for Disease Control and Prevention and Chinese Nutrition Society [6]. HIVC has been proven to be safe and therapeutic in critical care medicine, primarily as an adjunct to the treatment of sepsis and multiple organ failure, where it has shown promise in shortening the duration of mechanical ventilation and the intensive care unit (ICU) LOS [16]. The findings are also confirmed in a randomized clinical trial involving 167 patients with sepsis-related ARDS who received administration of 15 g/d of HIVC for 4 d [4]. In the retrospective cohort study, the effect of HIVC on inflammatory markers including hs-CRP, IL-6, and TNF-α was evaluated. In the patients receiving HIVC in combination with other symptomatic supportive care, the inflammatory marker concentrations appeared to be substantially reduced, while chronically decreased in patients with symptomatic supportive care alone.
Several clinical trials have been announced to investigate the therapeutic effect of HIVC on patients with COVID-19. How the dose ranges were established in these different studies is not always clear. The previous study reported that inflammatory biomarkers levels decreased significantly in patients receiving HIVC compared with those treated with low-dose vitamin C [21] .Therefore, the therapeutic efficacy of HIVC was much better than that of low-dose vitamin C in alleviating hyperinflammation.
Based on the above data, the present study provided evidence that treatment with vitamin C at high doses for 6 d reduced multiple inflammatory markers levels in patients with severe COVID-19. We also found the benefit of decrease in inflammatory marker levels among patients receiving symptomatic supportive care, which indicated HIVC in combination with other available medicines was potentially beneficial in alleviating hyperinflammation. Moreover, stepwise multiple linear regression analysis demonstrated that HIVC was independently related to percentages of reduction in levels of inflammatory markers after treatment. Together with the evidence from the study, we suggest that HIVC may be warranted in suppression of hyperinflammation as an adjuvant therapy for patients with severe COVID-19 lung disease.
Our results were not consistent with the findings from a pilot trial, in which HIVC (12 g every 12 h for 7 d) ameliorated inflammation by reducing IL-6 in ICU patients critically ill with COVID-19 [22]. However, CRP levels did not significantly change in the patients receiving HIVC compared with the placebo group, as seen in the present study [22]. The inconsistency between our study and this trial was mainly due to the trial design, and the enrolled patients. Importantly, the trial only collected blood samples at day 3 and day 7 during treatment, it was thus uncertain whether HIVC may have a potential signal of benefit for CRP at day 21 during hospitalization. The measurements of inflammatory markers at additional time points after treatment might produce different results.
Limitations
The number of patients, lack of a placebo control group, and the retrospective nature of the study are obvious limitations. The measurements of inflammatory markers were not very detailed during hospitalization. Additionally, a relevant number of patients received glucocorticoid therapy, a potential confounder affecting inflammatory marker levels. The pathogenic mechanism of COVID-19 is not yet clearly understood, and the study of specific protections against SARS-CoV-2 of HIVC is very limited. Based on the findings from the present analysis, we highlighted the fact that HIVC reduces multiple inflammatory marker levels. The results may not comprehensively illustrate how this benefit would help immune system defense from infections, but it demonstrated the potential clinical efficacy of HIVC. Furthermore, the dose and time of administration of HIVC were not taken into consideration in the current work.
Conclusions
The strength of the present study lies in the promise of short-term HIVC in reducing multiple inflammatory marker levels including hs-CRP, IL-6, TNF-α, and thereby attenuating hyperinflammation. The results suggested that HIVC can be considered potentially beneficial as an adjuvant therapy in the management of hyperinflammation for patients with severe COVID-19. Meanwhile, the present study showed the importance of further investigation in the form of large-scale randomized clinical trials about the application of HIVC targeting SARS-CoV-2 infection to accurately assess its therapeutic efficacy in patients with severe COVID-19.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophsiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Carr AS, Rowe S. The emerging role of vitamin C in the prevention and treatment of COVID-19. Nutrients. 2020;12:3286. doi: 10.3390/nu12113286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler AA, 3rd, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322:1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia G, Fan D, Ma C, He Y, Wang M, Zhu Y, et al. Hyper-inflammatory response involves in cardiac injury among patients with coronavirus disease 2019 [Epub ahead of print] Am J Med Sci. 2021 doi: 10.1016/j.amjms.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Fifth Revised Trial Version of the Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance. Available at: http://www.gov.cn/zhengce/zhengceku/2020-02/05/content_5474791.htm. Accessed 4 Feb 2020.
- 7.Gao D, Xu M, Wang G, Lv J, Ma X, Guo Y, et al. The efficiency and safety of high-dose vitamin C in patients with COVID-19: a retrospective cohort study. Aging (Albany NY) 2021;13:7020–7034. doi: 10.18632/aging.202557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerlach M, Gerlach H. Markers of hyperinflammation. Minerva Anestesiol. 2002;68:252–256. [PubMed] [Google Scholar]
- 9.Lipworth B, Chan R, Lipworth S, RuiWen Kuo C. Weathering the cytokine storm in susceptible patients with severe SARS-CoV-2 infection. J Allergy Clin Immunol Pract. 2020;8:1798–1801. doi: 10.1016/j.jaip.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 11.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau SKP, Lau CCY, Chan KH, Li CPY, Chen H, Jin DY, et al. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J Gen Virol. 2013;94(Pt 12):2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler AA, 3rd, Kim C, Lepler L, Malhotra R, Debesa O, Debesa O, et al. Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World J Crit Care Med. 2017;6:85–90. doi: 10.5492/wjccm.v6.i1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr AC, Rosengrave PC, Bayer S, Chambers S, Mehrtens J, Shaw GM. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care. 2017;21:300. doi: 10.1186/s13054-017-1891-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler 3rd AA, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32. doi: 10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Luo G, Yuan J, Wang Y, Yang X, Wang X, et al. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/426740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mousavi S, Bereswill S, Heimesaat MM. Immunomodulatory and antimicrobial effects of vitamin C. Eur J Microbiol Immunol. 2019;9:73–79. doi: 10.1556/1886.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Härtel C, Strunk T, Bucsky P, Schultz C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine. 2004;27:101–106. doi: 10.1016/j.cyto.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Du WD, Yuan ZR, Sun J, Tang JX, Cheng AQ, Shen DM, et al. Therapeutic efficacy of high-dose vitamin C on acute pancreatitis and its potential mechanisms. World J Gastroenterol. 2003;9:2565–2569. doi: 10.3748/wjg.v9.i11.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Rao X, Li Y, Zhu Y, Liu F, Guo G, et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care. 2021;11:5. doi: 10.1186/s13613-020-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]