Abstract
The alternation of the stimulatory action of the tachykinin neurokinin B (NKB) and the inhibitory action of dynorphin within arcuate (ARH) Kiss1 neurons has been proposed as the mechanism behind the generation of gonadotropin-releasing hormone (GnRH) pulses through the pulsatile release of kisspeptin. However, we have recently documented that GnRH pulses still exist in gonadectomized mice in the absence of tachykinin signaling. Here, we document an increase in basal frequency and amplitude of luteinizing hormone (LH) pulses in intact male mice deficient in substance P, neurokinin A (NKA) signaling (Tac1KO), and NKB signaling (Tac2KO and Tacr3KO). Moreover, we offer evidence that a single bolus of the NKB receptor agonist senktide to gonad-intact wild-type males increases the basal release of LH without changing its frequency. Altogether, these data support the dispensable role of the individual tachykinin systems in the generation of LH pulses. Moreover, the increased activity of the GnRH pulse generator in intact KO male mice suggests the existence of compensation by additional mechanisms in the generation of kisspeptin/GnRH pulses.
Keywords: tachykinins, kisspeptin, GnRH, hypothalamus, gonadotropin, reproduction
Pulsatile gonadotropin-releasing hormone (GnRH) release is essential for the attainment and maintenance of reproductive function (1). While this notion has been well-described for decades, the identification of the nature of the GnRH “pulse generator” has only emerged in recent years (2). Our lab and others have proposed a model in which the coordinated action of the stimulatory effect of neurokinin B (NKB) and the inhibitory action of dynorphin (Dyn), acting autosynaptically within arcuate (ARH) Kiss1 (Kiss1ARH) neurons, leads to pulses of kisspeptin that are then mirrored by GnRH and luteinizing hormone (LH) pulses (3, 4). Compelling evidence substantiating this model was presented by Clarkson and colleagues in 2017 (5). However, this model has been challenged by the observations that mouse models deficient in any of the individual tachykinin systems, ie, Tac1 (encoding substance P and NKA), Tacr1 (substance P receptor, NK1R), Tacr2 (NKA receptor, NK2R), Tac2 (NKB) and Tacr3 (NKB receptor, NK3R), present normal overall reproductive capabilities (6-10), suggesting that additional mechanisms are at play, likely involving cross-reactivity between the tachykinin ligand-receptor systems (11-14) that sustain GnRH pulsatility in the absence of NKB signaling. In this vein, previous studies have documented the involvement of all tachykinin receptors (ie, NK1R, NK2R, and NK3R) in the activation of Kiss1ARH neurons in the mouse (13) and in the induction of LH pulses in the rat (14). In both cases, only the simultaneous antagonization of all 3 receptors prevented the stimulatory action of tachykinins. Along these lines, we have documented that the absence of all tachykinin systems in a double Tac1/Tac2 knockout (KO) model leads to reduced pulsatile release of LH compared with controls (6) in a more robust manner than in the individual absence of Tac1 or Tac2KO models. However, Tac1/Tac2KO mice still display a rudimentary basal activity of the GnRH pulse generator that is sufficient to maintain reproduction in male subjects (6). The source of this basal activity of GnRH pulses remains to be elucidated.
In humans, inactivating mutations in the genes encoding NKB or its receptor (NK3R), TAC3, and TACR3, respectively, lead to hypogonadotropic hypogonadism (15, 16) although a number of the patients bearing these mutations have been shown to reverse their hypogonadal phenotype (17), highlighting the similarities with the overall fertile phenotype in rodents. To date, there have not been any identified mutations in the remaining tachykinin genes leading to reproductive alterations in humans. Nonetheless, understanding the precise mechanisms underlying the shaping of GnRH pulses, mostly in the absence of tachykinin stimulation, will inform new potential pathways to treat fertility impairments.
In this study, we characterized the profile of LH pulses (frequency and amplitude) in males in a series of mouse models (Tac1KO, Tac2KO, and Tacr3KO). In addition, we sought to determine the time course of NK3R activation in LH pulsatility in intact and castrated wild-type (WT) male mice.
Materials and Methods
Mice
WT male C57Bl/6 mice were purchased from Charles River Laboratories International, Inc. The Tac1 knockout (KO) mice were purchased from the Jackson Laboratories (stock No. 004103) and Tac2KO and Tac3rKO mice were obtained from Dr. Stephanie Seminara (Massachusetts General Hospital, Boston, MA) (9). All animal studies were approved by the Brigham and Women’s Hospital Institutional Animal Care and Use Committee. Mice were group housed, maintained in a 12:12 hour light/dark cycle and were fed standard rodent chow diet and water ad libitum. Genotyping was conducted by polymerase chain reaction analyses on isolated genomic DNA from tail biopsies.
Reagents
The NK3R agonist (senktide) was purchased from Tocris Bioscience (Minneapolis, MN). Senktide was dissolved in saline (0.9% sodium chloride). The dose of senktide was chosen based on preliminary studies investigating the effect of a single intraperitoneal (ip) injection of senktide on LH release.
Experimental Design
Characterization of the profile of pulsatile secretion of LH in gonad-intact male Tac1KO, Tac2KO, and Tac3rKO mouse models
To assess the profile of LH pulse secretion, gonad-intact (4 months old) WT (n = 10), Tac1KO (n = 7), Tac2KO (n = 7), and Tac3rKO (n = 6) mice were handled daily to allow acclimation to sampling conditions for 3 weeks prior to the experiment. Pulsatile measurements of LH secretion were assessed by repeated blood collection through a single incision at the tip of the tail. The tail was cleaned with saline and 4 μL of blood was taken at each time point from the cut tail with a pipette. We collected sequential blood samples every 10 minutes over a 180-minute sampling period.
Effect of NK3R agonist on the pulsatile secretion of LH in gonad-intact WT male mice
To study the effect of NK3R stimulation on the profile of LH pulse secretion, gonad-intact (4-6 months old) WT male mice (n = 10) were handled daily to allow acclimation to sampling conditions for 3 weeks prior to the experiment. Four μL of whole blood was collected every 10 minutes for 120 minutes to assess the LH baseline secretion profile. At 120 minutes of sampling, mice were injected with senktide (5 nmol/100 uL of saline, ip) and blood samples were taken for an additional 240 minutes (360 minutes in total).
Effect of NK3R agonist on LH pulsatile secretion in gonadectomized WT male mice
To study the effect of the NK3R agonist (senktide) on the pattern of LH pulsatility in gonadectomized (GDX) mice, the adult male WT mice used in the previous experiment were bilaterally castrated under isoflurane anesthesia. Analgesics (0.5 mg/kg buprenex and 5 mg/kg meloxicam, subcutaneously) were administered pre- and post-surgery. Briefly, the ventral skin was shaved and cleaned, and a single small incision was made in the abdominal musculature to remove both testes. After gonads were excised, the incision was sutured, and the skin was closed with surgical clips. Mice were allowed a 1-week recovery before the onset of experiments and were handled daily 2 weeks before and 1 week after surgery to allow acclimation to sampling conditions. Pulsatile measurements of LH secretion were assessed by repeated blood collection and 4 μL of whole blood was collected every 10 minutes for 120 minutes, then mice were injected with senktide (5 nmol/100 uL of saline, ip) and blood samples were taken for an additional 180 minutes (300 minutes in total).
Blood Samples and LH Measurements
In all cases, blood samples for LH measurements were obtained after a single excision of the tip of the tail. The tip was cleaned with saline and 4 μL whole blood was immediately diluted in 116 μL of 0.05% PBST (phosphate buffer saline [Boston Bio Products, Cat. No. BM220] containing Tween-20 [Sigma, Cat. No. P2287]), vortexed, and frozen on dry ice. Samples were stored at −80 °C until analyzed with a LH enzyme-linked immunosorbent assay (ELISA) as previously described (18).
Data Analysis
LH pulses
LH pulses in mice were analyzed using a custom-made MATLAB-based algorithm. The MATLAB code includes a loop that determines LH pulses as any LH peak: (i) whose height is 20% greater than the heights of the 2 previous values; (ii) 10% greater than the height of the following value; and (iii) the peak at the second time interval needs to be 20% greater than the single value that comes before it to be considered a pulse (6).
LH pulsatility
LH pulsatility was assessed by measuring the following parameters: (1) total number of pulses throughout the sampling period, unless stated otherwise; (2) pulse amplitude, calculated by averaging the 4 highest LH values in the samples collection period for each animal; (3) basal LH, calculated by averaging the 4 lowest LH values in the samples collection period for each animal; and (4) total secretory mass, assessed by area under the curve (AUC).
Statistical Analysis
All data are expressed as the mean ± SEM for each group. A 2-tailed unpaired Student t test was used to assess variation among experimental groups. Significance level was set at P < 0.05. All analyses were performed with GraphPad Prism Software, Inc.
Results
Absence of an individual tachykinin system signaling (Tac1KO, Tac2KO, and Tacr3KO) in gonad-intact male mice increases frequency and amplitude of LH pulses.
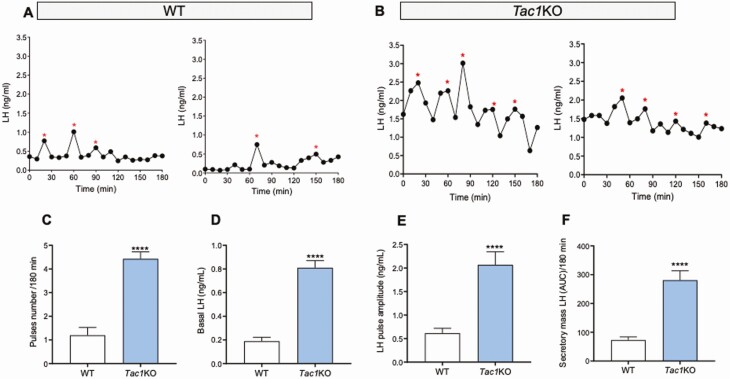
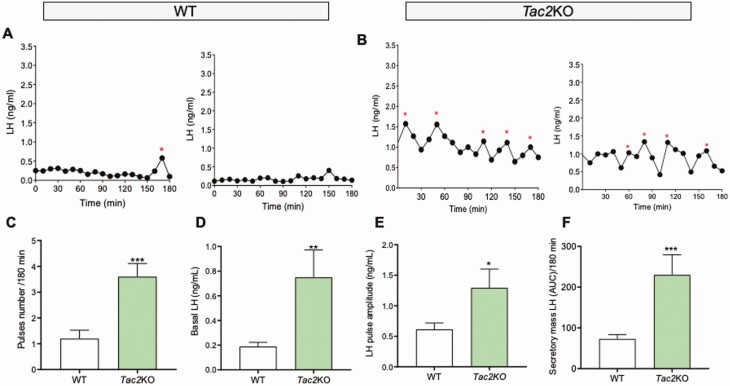
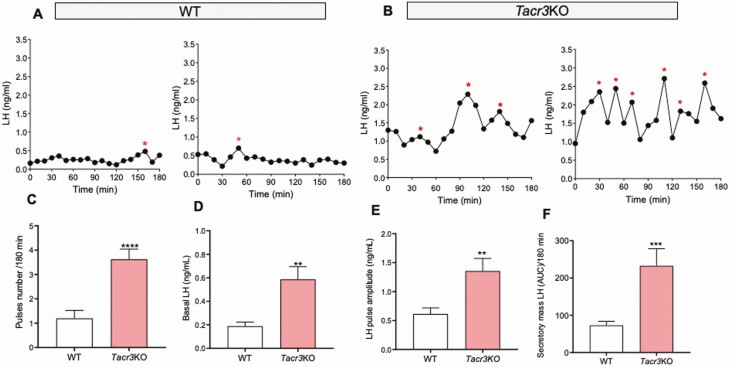
In order to assess the pulsatile pattern of LH release, adult male mice of each genotype and corresponding WT counterparts were sampled through the tail vein every 10 minutes for 180 minutes, and LH measurements were determined through ELISA. Adult gonad-intact WT male mice displayed 1 to 2 LH pulses in 180 minutes (WT LH pulses = 1.20 ± 0.32), in line with previous studies. However, the number of LH pulses observed in littermate knockout (KO) mice for the genes encoding Tac1 (4.42 ± 0.29, P = 0.0001, Figure 1), Tac2 (3.60 ± 0.50, P = 0.0004, Fig. 2), and Tacr3 (3.62 ± 0.41, P = 0.0001 Fig. 3) was significantly higher than in controls, indicating a higher frequency of LH pulses in all KO models. Similarly, the amplitude, total secretory mass, and baseline were significantly elevated in all KO models compared with WT controls: (i) Amplitude: WT = 0.61 ± 0.10 ng/mL; Tac1KO = 2.06 ± 0.27 ng/mL, P < 0.0001; Tac2KO = 1.29 ± 0.30 ng/mL, P < 0.05; Tacr3KO = 1.35 ± 0.21 ng/mL, P < 0.01; (ii) Secretory mass: WT = 73.04 ± 10.67 ng/mL; Tac1KO = 281.1 ± 32.18 ng/mL, P < 0.0001; Tac2KO = 230.0 ± 49.53 ng/mL, P < 0.001; Tacr3KO = 232.7 ± 45.85 ng/mL, P < 0.001; (iii) Baseline: WT = 0.18 ± 0.03 ng/mL; Tac1KO = 0.81 ± 0.05 ng/mL, P < 0.0001; Tac2KO = 0.75 ± 0.22 ng/mL, P < 0.01; Tacr3KO = 0.59 ± 0.11 ng/mL, P < 0.01 (Figs. 1-3).
Figure 1.
LH pulses profile in gonad-intact WT and Tac1KO mice. LH samples were collected every 10 minutes for 180 minutes from gonad-intact WT and Tac1KO male mice. (A, B) are 2 representative examples of pulsatile LH secretion in WT (A) and Tac1KO (B) male mice. Pulses number/180 min (C), Basal LH (D), LH pulse amplitude (E) and total secretory mass assessed by area under the curve (AUC) (F) were all significantly different in Tac1KO vs WT mice. ****P = 0.0001, Student t test. *represents LH pulses following the protocol described in the methods.
Figure 2.
LH pulses profile in gonad-intact WT and Tac2KO mice. LH samples were collected every 10 minutes for 180 minutes from gonad-intact WT and Tac2KO male mice. (A, B) are 2 representative examples of pulsatile LH secretion in WT (A) and Tac2KO (B) male mice. Pulses number/180 min (C), Basal LH (D), LH pulse amplitude (E) and total secretory mass assessed by area under the curve (AUC) (F) were all significantly different in Tac2KO vs WT mice. *P < 0.05, **P < 0.01, *** P < 0.001, Student t test. * represents LH pulses following the protocol described in the methods.
Figure 3.
LH pulses profile in gonad-intact WT and Tacr3KO mice. LH samples were collected every 10 minutes for 180 minutes from gonad-intact WT and Tacr3KO male mice. (A, B) are 2 representative examples of pulsatile LH secretion in WT (A) and Tacr3KO (B) male mice. Pulses number/180 min (C), Basal LH (D), LH pulse amplitude (E) and total secretory mass assessed by area under the curve (AUC) (F) were all significantly different in Tacr3KO vs WT mice. **P < 0.01, *** P < 0.001, ****P < 0.0001, Student t test. * represents LH pulses following the protocol described in the methods.
NK3R Stimulation in Gonad-Intact WT Male Mice Elevates LH Baseline Without Changing LH Pulse Frequency
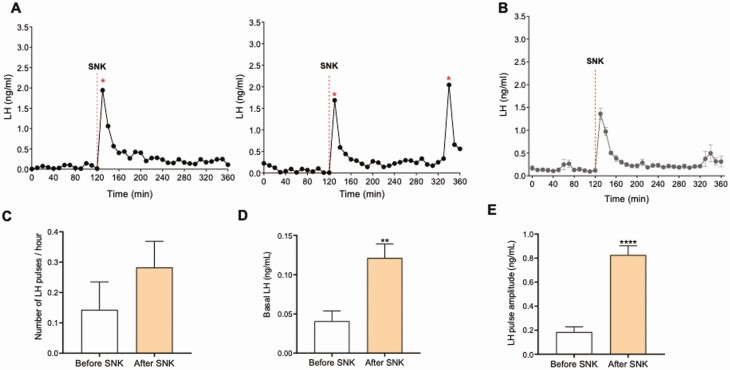
Among tachykinins, the NKB/NK3R system has been described to have the most prominent role in reproduction (19). Therefore, we set out to investigate the effect of NK3R activation in LH pulsatility under different gonadal conditions. First, in this experiment, we assessed the effect of the exogenous administration of the NK3R agonist senktide on LH pulsatility in gonad-intact adult male mice. We observed that the peripheral administration of senktide was sufficient to induce a robust increase in LH release in all animals tested within 10 minutes (Fig. 4A). However, this action did not induce further activation of the GnRH pulse generator, as observed by the same number of LH pulses/hour before and after senktide administration (Fig. 4C): number of pulses before senktide = 0.14 ± 0.09 pulses/h, after senktide = 0.28 ± 0.08 pulses/h; P = 0.28. Nonetheless, this one-time activation of NK3R was sufficient to increase amplitude and basal LH levels for, at least, 4h after senktide administration (Fig. 4D-4E) Amplitude before senktide was 0.1854 ± 0.04343 ng/mL; after senktide it was 0.8263 ± 0.07645 ng/mL (P < 0.0001). Baseline LH before senktide was 0.04 ± 0.01 ng/mL and after senktide it was 0.12 ± 0.02 ng/mL (P < 0.01). This indicates that the mechanisms behind the regulation of LH pulse frequency and basal LH release may follow different pathways.
Figure 4.
The effect of a single peripheral injection of senktide on the LH pulses profile in gonad-intact WT male mice. A) Two representative examples of LH pulses 120 minutes before and 180 minutes after senktide (SNK) injection. B) LH pulses (mean ± SEM) 120 minutes before and 240 minutes after SNK injection. SNK injection is indicated by red dashed lines. C) represents the number of LH pulses/hour, D) Basal LH, and E) LH pulses amplitude. P > 0.05, **P < 0.01, ****P < 0.0001. Student t test. * represents LH pulses following the protocol described in the methods.
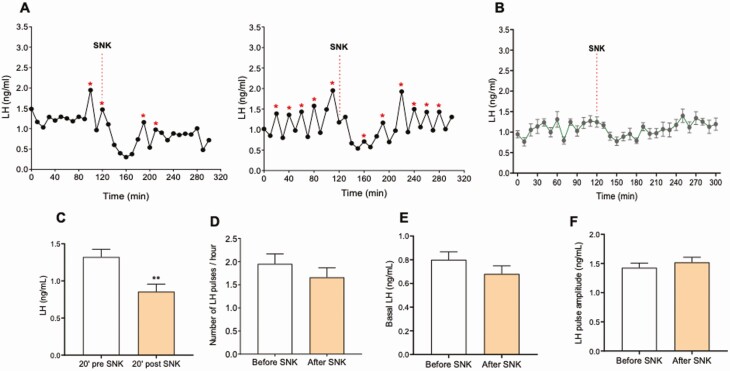
NK3R Stimulation in Castrated WT Male Mice Inhibits LH Pulse Frequency Without Affecting LH Baseline
Next, we investigated the effect of senktide action on LH pulsatility in a model of high frequency and amplitude of LH pulses, such as GDX. Adult WT males were GDX for a week. Basal LH pulses were monitored every 10 minutes for 2 hours. At this point, a single ip bolus of 5 nmol senktide was administered and LH pulses monitored for 3 additional hours. Senktide was able to significantly inhibit LH pulses and significantly suppress LH levels 20 to 40 minutes after treatment (Fig. 5A and 5B): LH pulses 20 minutes before senktide were 1.32 ± 0.10 ng/mL, after senktide 0.85 ± 0.10 ng/mL (P = 0.0054); LH pulses/hour before senktide were 1.950 ± 0.2167 pulses/h and after senktide were 1.660 ± 0.2061 pulses/h (P = 0.345). However, overall LH basal levels were not affected by senktide: LH baseline levels before senktide = 0.80 ± 0.07 ng/mL, after senktide = 0.68 ± 0.07 ng/mL (P = 0.22).
Figure 5.
The effect of a single peripheral injection of senktide on the LH pulses profile in castrated WT male mice. A) Two representative examples of LH pulses 120 minutes before and 180 minutes after SNK injection. B) LH pulses (mean ± SEM) 120 minutes before and 180 minutes after SNK injection. SNK injection is indicated by red dashed lines. C) represents LH levels 20 minutes pre- and 40 minutes posttreatment, D) the number of LH pulses/hour, E) Basal LH, and F) LH pulses amplitude. **P < 0.01, Student t test. * represents LH pulses following the protocol described in the methods.
Discussion
The role of Kiss1ARH neurons as the master GnRH pulse generator has been well documented in rodent species (2, 5). Episodic bursts of activity in Kiss1ARH neurons lead to concomitant LH pulses. These studies have been a major contribution to the field of neuroendocrinology because they filled a conceptual void that was present since the identification of the pulsatile nature of GnRH release and its fundamental role for reproduction (20). However, the rather simplistic idea that every kisspeptin pulse is the consequence of just the stimulatory effect of NKB followed by the inhibitory effect of dynorphin, has been challenged by studies in rodents and humans showing pulsatile LH activity in the absence of some (or all) of the components of this so-called GnRH pulse generator (6, 7, 21). For example, patients bearing inactivating mutations in the NKB/NK3R system often present with a reversal of the hypogonadotropic hypogonadism that characterizes this genetic deficit (17). Similarly, their rodent counterparts (Tac2KO and Tacr3KO) are fertile (9, 10), indicating that NKB signaling is dispensable for the pulsatile release of GnRH. Nonetheless, our studies in Tac2KO female mice have shown that their LH pulses in response to gonadectomy (where the removal of sex steroids leads to LH pulses of high frequency and amplitude) are impaired, leading to a lower frequency and amplitude of LH pulses (21). This defect leads to a slower response to gonadectomy, however, this rudimentary central activity that generates LH pulses was enough to eventually induce compensatory rises after gonadectomy in KO mice, similar to WT controls.
Tachykinins present a high degree of cross-reactivity between their ligand-receptor systems (11, 13, 14). Therefore, it was speculated that compensation by other tachykinins (namely substance P and neurokinin A [NKA]) could take over the stimulatory action of NKB in its absence as both (encoded by the same gene, Tac1) have been demonstrated to significantly induce LH release (7, 8, 22-29). However, we have recently demonstrated that in the absence of all tachykinin signaling (Tac1/Tac2KO), mice still retain a level of basal LH pulsatility in their gonadectomized state similar to the individual Tac1KO and Tac2KO models (6). Altogether, compelling evidence shows that while the action of tachykinins on Kiss1ARH neurons is critical for the normal pulsatile release of kisspeptin/GnRH (ie, the rapid response to changing sex steroid levels with appropriate frequency and amplitude of pulses), additional factors are at play keeping the system functional at a basal level. In light of these previous studies, we set out to further characterize the pattern of pulsatile release in gonad-intact male mice of 3 different tachykinin deficient models (Tac1KO, Tac2KO, Tacr3KO). Unexpectedly, the basal activity of the GnRH pulse generator was significantly increased in all 3 KO models, which displayed higher frequency of LH pulses and higher basal LH level compared with control littermates. These KO models are fertile (8-10, 30) and males display normal testosterone levels (9, 30), suggesting that the increase in the activity of LH pulses observed in gonad-intact conditions is not a compensatory mechanism due to hypogonadism.
Nonetheless, these data suggest that in the absence of a single tachykinin system (ie, NKB/NK3R and SP/NK1R, NKA/NK2R), an increase in the activity of the GnRH pulse generator is possible and likely driven by the coordinated action of the remaining tachykinin systems and dynorphin, as indicated in previous studies in Kiss1ARH neurons (13). Still, these mice fail to reach the frequency and amplitude of pulses of WT mice after gonadectomy (6, 7, 21), suggesting that NKB signaling is needed for the full activation of Kiss1ARH neurons.
The mechanism behind the activation of Kiss1ARH neurons by NKB is not completely understood. In this study, we investigated whether a single bolus of NKB (or the NK3R-selective agonist senktide), mimicking the higher release of NKB per pulse in the GDX state, would be sufficient to increase the frequency and amplitude of LH pulses in gonad-intact male mice. At the dose selected, senktide induced a conspicuous burst of LH that peaked 10 minutes after administration and lasted for ~60 minutes. This treatment did not induce any consequent change in the frequency of LH pulses, suggesting that constant NKB pulses are required to induce kisspeptin pulses and that a one-time activation of NK3R does not accelerate kisspeptin pulses. However, the nadirs of basal LH levels were consistently higher after senktide treatment for the duration of the experiment (4 hours posttreatment). This finding suggests that the mechanisms underlying pulse frequency and basal LH release are differently regulated and that the activation of NK3R is sufficient to increase GnRH and/or LH output in a continuous fashion, which determines LH basal levels in the absence of kisspeptin pulses. Whether this effect is mediated at the level of Kiss1ARH neurons, at the level of GnRH neurons, or at the level of the gonadotrope, remains to be determined.
The action of NKB on LH is dependent on the level of sex steroids. In their presence, NKB (or senktide) stimulates LH release as documented in several species (4, 22-24, 31-35) as well as in this study; however, in their absence, NKB inhibits LH release in gonadectomized female and male rodents (22, 36-38). Thus, we assessed the effect of senktide on pulsatile LH during a state of high LH pulse frequency and amplitude, ie, gonadectomy. We observed the opposite effect to the one obtained in gonad-intact males, that is a robust inhibition of LH release and complete ablation of LH pulses for around 60 minutes after treatment, when pulses and basal LH levels returned to pretreatment levels. The ablation of LH pulsatility during this time is in line with the inhibition of LH detected in previous gonadectomized models (37, 38). It has been proposed that an increase in dynorphin tone mediates this inhibitory effect (37). However, an alternative possibility is that excessively high levels of NK3R activation, and therefore hyperstimulation of Kiss1ARH neurons, leads to the collapse of the GnRH pulse generator, as elegantly demonstrated recently through a model of Kiss1ARH hyperstimulation in the mouse using optogenetic approaches (39). Interestingly, LH basal levels after senktide treatment tend to be below those during pretreatment, which adds additional support to a role of NKB in the maintenance of a tonic minimum level of LH release.
Overall, our present study further documents the complexity of the neuroendocrine mechanisms that govern GnRH pulsatility. The paradoxical finding that in the absence of the stimulatory component of the Kiss1ARH neuron pulse generator, an increase in basal frequency and amplitude of LH pulses is observed in 3 individual tachykinin models, indicates that additional factors intervene in the control of kisspeptin release. It is possible that cross-activation through the remaining tachykinin systems in these mice is sufficient to maintain kisspeptin pulses in the basal (gonad-intact) state. Moreover, our data in WT mice treated with senktide demonstrate that the underlying mechanisms controlling LH pulse amplitude and frequency and those controlling basal LH levels are different and that the NKB/NK3R system is able to induce a sustained increase in basal levels after a single bolus of an NK3R agonist. Because this effect was only observed in gonad-intact mice, we can speculate that it is dependent on circulating sex steroids.
Acknowledgments
Financial Support: This work was supported by Grants R01HD090151, R21HD095383 by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and National Institute of Health (NIH) to V.M.N., F32HD097963 by the NIH to A.E.M. and the International Brain Research Organization (IBRO) Fellowship and the Lalor Foundation Research Fellowship to R.T.
Glossary
Abbreviations
- ARH
arcuate
- ELISA
enzyme-linked immunosorbent assay
- GDX
gonadectomized
- GnRH
gonadotropin-releasing hormone
- ip
intraperitoneal
- KO
knockout
- NKA
neurokinin A
- NKB
neurokinin B
- NK3R
NKB receptor
- LH
luteinizing hormone
- WT
wild-type
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Herbison AE. Physiology of the GnRH neuronal network. In: Knobil JNaE, ed. Physiology of Reproduction. San Diego, CA: Academic Press; 2006:1415-1482. [Google Scholar]
- 2. Plant TM. The neurobiological mechanism underlying hypothalamic GnRH pulse generation: the role of kisspeptin neurons in the arcuate nucleus. F1000Res. 2019;8:F1000 Faculty Rev-982. Published June 28, 2019. doi:10.12688/f1000research.18356.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859-11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clarkson J, Han SY, Piet R, et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A. 2017;114(47):E10216-E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leon S, Fergani C, Talbi R, et al. Tachykinin signaling is required for the induction of the preovulatory LH surge and normal LH pulses. Neuroendocrinology. 2020. Published online ahead of print June 8, 2020. doi:10.1159/000509222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. León S, Fergani C, Talbi R, et al. Characterization of the role of NKA in the control of puberty onset and gonadotropin release in the female mouse. Endocrinology. 2019;160(10):2453-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simavli S, Thompson IR, Maguire CA, et al. Substance p regulates puberty onset and fertility in the female mouse. Endocrinology. 2015;156(6):2313-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. True C, Nasrin Alam S, Cox K, Chan YM, Seminara S. Neurokinin B is critical for normal timing of sexual maturation but dispensable for adult reproductive function in female mice. Endocrinology. 2015; 156(4):1386-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang JJ, Caligioni CS, Chan YM, Seminara SB. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology. 2012;153(3):1498-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parnet P, Mitsuhashi M, Turck CW, Kerdelhue B, Payan DG. Tachykinin receptor cross-talk. Immunological cross-reactivity between the external domains of the substance K and substance P receptors. Brain Behav Immun. 1991;5(1):73-83. [DOI] [PubMed] [Google Scholar]
- 12. Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014;94(1):265-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154(8):2750-2760. [DOI] [PubMed] [Google Scholar]
- 14. Noritake K, Matsuoka T, Ohsawa T, et al. Involvement of neurokinin receptors in the control of pulsatile luteinizing hormone secretion in rats. J Reprod Dev. 2011;57(3):409-415. [DOI] [PubMed] [Google Scholar]
- 15. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Young J, Bouligand J, Francou B, et al. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab. 2010;95(5):2287-2295. [DOI] [PubMed] [Google Scholar]
- 17. Gianetti E, Tusset C, Noel SD, et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95(6):2857-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939-4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fergani C, Navarro VM. Expanding the role of tachykinins in the neuroendocrine control of reproduction. Reproduction. 2016;153(1):R1-R14. [DOI] [PubMed] [Google Scholar]
- 20. Knobil E. On the control of gonadotropin secretion in the rhesus monkey. Recent Prog Horm Res. 1974;30(0):1-46. [DOI] [PubMed] [Google Scholar]
- 21. Lippincott MF, León S, Chan YM, et al. Hypothalamic reproductive endocrine pulse generator activity independent of neurokinin B and dynorphin signaling. J Clin Endocrinol Metab. 2019;104(10):4304-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Navarro VM, Bosch MA, León S, et al. The integrated hypothalamic tachykinin-kisspeptin system as a central coordinator for reproduction. Endocrinology. 2015;156(2):627-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruiz-Pino F, Garcia-Galiano D, Manfredi-Lozano M, et al. Effects and interactions of tachykinins and dynorphin on FSH and LH secretion in developing and adult rats. Endocrinology. 2015;156(2):576-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruiz-Pino F, Navarro VM, Bentsen AH, et al. Neurokinin B and the control of the gonadotropic axis in the rat: developmental changes, sexual dimorphism, and regulation by gonadal steroids. Endocrinology. 2012;153(10):4818-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coiro V, Volpi R, Capretti L, et al. Luteinizing hormone response to an intravenous infusion of substance P in normal men. Metabolism. 1992;41(7):689-691. [DOI] [PubMed] [Google Scholar]
- 26. Hidalgo-Díaz C, Castaño JP, López-Pedrera R, Malagón MM, García-Navarro S, Gracia-Navarro F. A modulatory role for substance P on the regulation of luteinizing hormone secretion by cultured porcine gonadotrophs. Biol Reprod. 1998;58(3):678-685. [DOI] [PubMed] [Google Scholar]
- 27. Ohtsuka S, Miyake A, Nishizaki T, Tasaka K, Aono T, Tanizawa O. Substance P stimulates gonadotropin-releasing hormone release from rat hypothalamus in vitro with involvement of oestrogen. Acta Endocrinol (Copenh). 1987;115(2):247-252. [DOI] [PubMed] [Google Scholar]
- 28. Shamgochian MD, Leeman SE. Substance P stimulates luteinizing hormone secretion from anterior pituitary cells in culture. Endocrinology. 1992;131(2):871-875. [DOI] [PubMed] [Google Scholar]
- 29. Traczyk WZ, Pau KY, Kaynard AH, Spies HG. Modulatory role of substance P on gonadotropin and prolactin secretion in the rabbit. J Physiol Pharmacol. 1992;43(3):279-297. [PubMed] [Google Scholar]
- 30. Maguire CA, Song YB, Wu M, et al. Tac1 signaling is required for sexual maturation and responsiveness of GnRH neurons to kisspeptin in the male mouse. Endocrinology. 2017;158(7):2319-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garcia JP, Keen KL, Kenealy BP, Seminara SB, Terasawa E. Role of kisspeptin and neurokinin B signaling in male rhesus monkey puberty. Endocrinology. 2018;159(8):3048-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151(9):4494-4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fergani C, Mazzella L, Coolen LM, et al. Do substance P and neurokinin A play important roles in the control of LH secretion in ewes? Endocrinology. 2016;157(12):4829-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goodman RL, Hileman SM, Nestor CC, et al. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Porter KL, Hileman SM, Hardy SL, Nestor CC, Lehman MN, Goodman RL. Neurokinin-3 receptor activation in the retrochiasmatic area is essential for the full pre-ovulatory luteinising hormone surge in ewes. J Neuroendocrinol. 2014;26(11):776-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fergani C, Leon S, Padilla SL, Verstegen AM, Palmiter RD, Navarro VM. NKB signaling in the posterodorsal medial amygdala stimulates gonadotropin release in a kisspeptin-independent manner in female mice. Elife. 2018;7:e40476. Published online December 19, 2018. doi:10.7554/eLife.40476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grachev P, Li XF, Kinsey-Jones JS, et al. Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism. Endocrinology. 2012;153(10):4894-4904. [DOI] [PubMed] [Google Scholar]
- 38. Kinsey-Jones JS, Grachev P, Li XF, et al. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153(1):307-315. [DOI] [PubMed] [Google Scholar]
- 39. Han SY, Cheong I, McLennan T, Herbison AE. Neural determinants of pulsatile luteinizing hormone secretion in male mice. Endocrinology. 2020;161(2):bqz045. Published online January 7, 2020. doi:10.1210/endocr/bqz045 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.