Abstract
Inflammation is increasingly recognized as a critical mediator of angiogenesis, and unregulated angiogenic responses often involve human diseases. The importance of regulating angiogenesis in inflammatory diseases has been demonstrated through some successful cases of anti-angiogenesis therapies in related diseases, including arthritis, but it has been reported that some synthetic types of antiangiogenic drugs have potential side effects. In recent years, the importance of finding alternative strategies for regulating angiogenesis has begun to attract the attention of researchers. Therefore, identification of natural ingredients used to prevent or treat angiogenesis-related diseases will play a greater role. Isookanin is a phenolic flavonoid presented in Bidens extract, and it has been reported that isookanin possesses some biological properties, including antioxidative and anti-inflammatory effects, anti-diabetic properties, and an ability to inhibit α-amylase. However, its antiangiogenic effects and mechanism thereof have not been studied yet. In this study, our results indicate that isookanin has an effective inhibitory effect on the angiogenic properties of microvascular endothelial cells. Isookanin shows inhibitory effects in multiple stages of PGE2-induced angiogenesis, including the growth, proliferation, migration, and tube formation of microvascular endothelial cells. In addition, isookanin induces cell cycle arrest in S phase, which is also the reason for subsequent inhibition of cell proliferation. The mechanism of inhibiting angiogenesis by isookanin is related to the inhibition of PGE2-mediated ERK1/2 and CREB phosphorylation. These findings make isookanin a potential candidate for the treatment of angiogenesis-related diseases.
Keywords: isookanin, anti-angiogenesis, HMEC-1 cell, prostaglandin E2 (PGE2), cell cycle arrest, ERK1/2 and CREB signaling pathway
1. Introduction
Angiogenesis is the formation of new capillaries from existing vasculature and is an integral biological process that occurs throughout life in a number of physiological and pathological processes [1]. It is known that angiogenesis is essential for reproduction, development, and wound repair, where it is highly regulated. However, many diseases are caused by unregulated angiogenesis, including cancer, rheumatoid arthritis, inflammatory bowel disease (IBD), and diabetic retinopathy [2,3]. In addition, angiogenesis is also involved in various skin diseases including psoriasis, telangiectasias, and rosacea, where various types of blood vessel growth can be observed in diseased skin [4].
Many potent proangiogenic factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), angiogenin, and transforming growth factor-β (TGF-β) have been found to be involved in angiogenesis [2]. Abnormal angiogenesis caused by these stimulants is accompanied by a series of endothelial cell behavioral changes associated with increased cell proliferation, cell migration and invasion, and the formation of new tubular structures [5]. Angiogenesis inhibitors targeting this set of mechanisms have been studied and applied in clinical trials, where the data have strongly suggested that suppressing aberrant angiogenesis may be a promising treatment for angiogenesis-related diseases [6,7].
Meanwhile, recent clinical and basic scientific studies indicate that angiogenic inducers involve (many) other factors, including inflammatory mediators [8]. Among the many inflammatory mediators, prostaglandins (PGs) are representative and are naturally occurring lipid compounds derived from the cyclooxygenase (COX)-mediated metabolism of arachidonic acid [9]. Prostaglandins link the interactions between various immune regulatory cells and are thought to play a key role in regulating the inflammatory response [10]. Prostaglandin E2 (PGE2) is the most abundant prostanoid in humans and the most important COX-2 mediated product found to date. PGE2 is involved in regulating many different fundamental biological functions, and its role in inflammation and immune response has been well established in previous studies [11,12].
To date, PGE2 has been the most researched inflammatory mediator in immune cells, and in recent years, a lot of attention has been paid to the other roles of PGE2 [13]. However, the mechanisms and effects of PGE2 on endothelial cells and angiogenesis have been less studied [14]. PGE2 affects target cells by binding four cognate receptors called EP1, EP2, EP3, and EP4, which belong to a large family of G protein-coupled receptors (GPCRs). Ligand binding of different EP receptors leads to different actions through activation of separate downstream signaling pathways [15]. Some prior studies have confirmed that PGE2 and EP receptors have a direct role in angiogenic gene expression and angiogenic cell behavior [14,16,17,18,19].
The importance of angiogenesis in inflammatory disease processes has been demonstrated by successful cases of antiangiogenic therapeutic trials for diseases such as asthma [20], rheumatic diseases [21], and dermatosis [22]. These treatments are commonly used FDA-approved drugs that target angiogenic factors or angiogenic factor signaling cascades [6]; however, these drugs are known to have some clinical limitations and some side effects associated with their administration [23,24].
Therefore, the importance of finding alternative strategies for controlling angiogenesis has begun to attract people’s attention, and the identification of natural compounds will play an important role in finding therapeutics for the prevention and treatment of angiogenesis-related diseases [25]. Isookanin (C15H12O6, molecular weight: 288.3) is a phenolic flavonoid presented in Bidens extract [26]. It has been reported that isookanin possesses some biological properties, including antioxidative [27,28] and anti-diabetic properties [27], anti-inflammatory effects [29] and an ability to inhibit α-amylase [26]. However, the antiangiogenic effects and mechanism thereof have not been studied yet.
In this study, we investigated the effect of isookanin on PGE2-induced angiogenesis in human dermal microvascular endothelial cells (HMEC-1). Our results show that PGE2 led to proliferation of HMEC-1 cells and that isookanin administration suppressed the proliferation, migration, and tube formation ability of HMEC-1 cells. Additionally, the results from the mechanism study showed that isookanin exerted its inhibitory effect on angiogenesis through the induction of cell cycle arrest and the regulation of the PGE2 receptor and its downstream ERK1/2 and CREB phosphorylation.
2. Results
2.1. Effects of Isookanin on PGE2-Induced Endothelial Cell Proliferation and Cytotoxicity
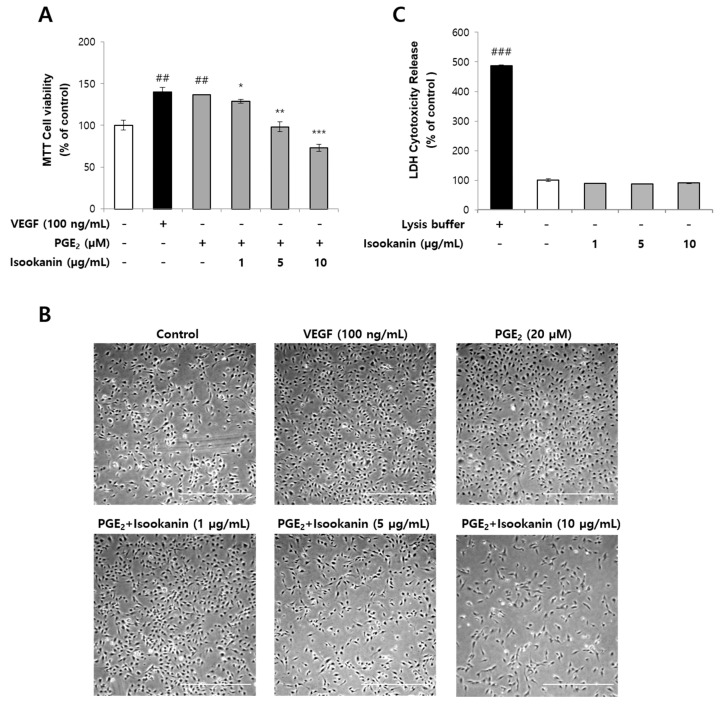
Activation of endothelial cell proliferation is one common feature of angiogenesis [30]. To explore the inhibitory effect of isookanin on PGE2-induced endothelial cell proliferation in vitro, we performed an MTT assay. HMEC-1 cells were pretreated with isookanin (1, 5, 10 µg/mL) for 2 h and then stimulated with 20 µM PGE2 for 48 h prior to evaluation for cell viability. VEGF (100 ng/mL) was used as an angiogenic positive control. Treatment with PGE2 alone increased the levels of HMEC-1 cell proliferation, whereas the addition of isookanin inhibited HMEC-1 cell proliferation in a dose-dependent manner (Figure 1A). We also observed via microscopy that treatment with PGE2 alone increased the density of endothelial cells, while the addition of isookanin decreased the density of endothelial cells after 48 h, and no cell damage was observed in cell morphology (Figure 1B).
Figure 1.
The effect of isookanin on cell proliferation in PGE2-induced HMEC-1 cells. Cells were pretreated with the indicated concentrations of isookanin for 2 h before stimulation with PGE2 (20 µM) or VEGF (100 ng/mL) for 48 h. (A) Cell viability was measured using the MTT assay. (B) The density of endothelial cells was observed under a microscope; scale bars are 80 μm. (C) Cytotoxicity was measured using the lactate dehydrogenase (LDH) cytotoxicity assay. The results are mean ± standard deviation (SD) (n = 3). ## p < 0.01, ### p < 0.001 vs. control. * p < 0.05, ** p < 0.01, and *** p < 0.001 vs. PGE2-treated control.
Next, we examined the effect of isookanin on cytotoxicity in HMEC-1 cells to determine whether the antiangiogenic effect was caused by toxicity. Cell cytotoxicity was assessed using an LDH assay, and a significant increase in LDH activity was observed in cells treated with lysis buffer as a high control, showing that this assay system was reliable. In contrast, isookanin showed no increase in LDH activity at concentrations ranging from 1 to 10 μg/mL (Figure 1C) compared with the control. These results indicate that the antiangiogenic activity of isookanin was not caused by cytotoxic actions.
2.2. Effects of Isookanin on PGE2-Induced Endothelial Cell Migration
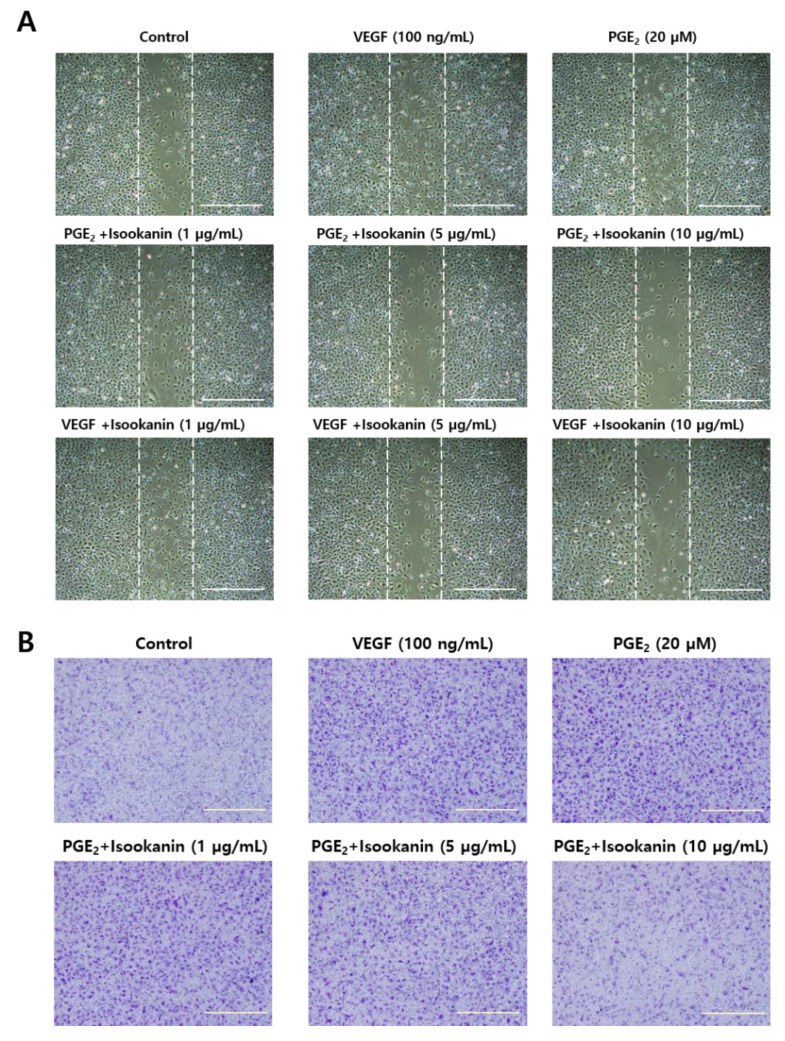
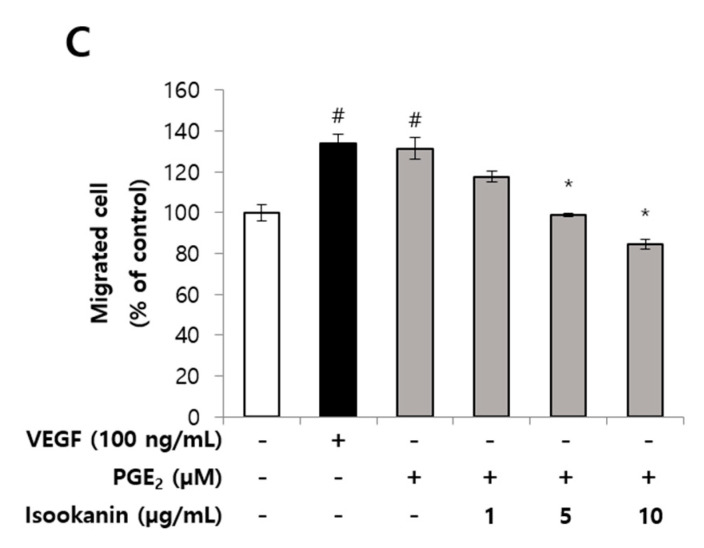
Endothelial cell migration is a key process during the formation of new capillaries [31]. Thus, we next investigated the effect of isookanin on PGE2-induced endothelial cell migration using a scratch migration assay and a transwell migration assay. The results revealed that the endothelial cells treated only with PGE2 showed increased migration, while the cells pretreated with isookanin before PGE2 stimulation showed a significant and dose-dependent decrease in cell migration (Figure 2A,B). In the scratch migration assay, endothelial cells stimulated with PGE2 increased the cell migration area compared with the non-stimulated group, while treatment with isookanin significantly reduced the cell migration area compared with the group stimulated only with PGE2 (Figure 2A). In the transwell migration assay, as shown in Figure 2B, isookanin obviously suppressed endothelial cell migration from the upper chamber to the lower chamber. The inhibitory effect reached 46.9% with 10 µg/mL isookanin compared with the group treated only with PGE2 (Figure 2C).
Figure 2.
The effect of isookanin on cell migration in PGE2-induced HMEC-1 cells. Cells were pretreated with the indicated concentrations of isookanin for 2 h before stimulation with PGE2 (20 µM) or VEGF (100 ng/mL) for 24 h. (A) Cell migration was measured using the scratch migration assay, and cells were photographed under a microscope; scale bars are 80 µm. (B) Cell migration was measured using the transwell migration assay, and cells were photographed under a microscope; scale bars are 200 µm. (C) Cells were extracted with 10% acetic acid, the absorbance at 600 nm was used to calculate the percentage of migrated cells. The results are mean ± standard deviation (SD) (n = 3). # p < 0.05 vs. control. * p < 0.05 vs. PGE2-treated control.
2.3. Effect of Isookanin on PGE2-Induced Endothelial Cell Tube Formation
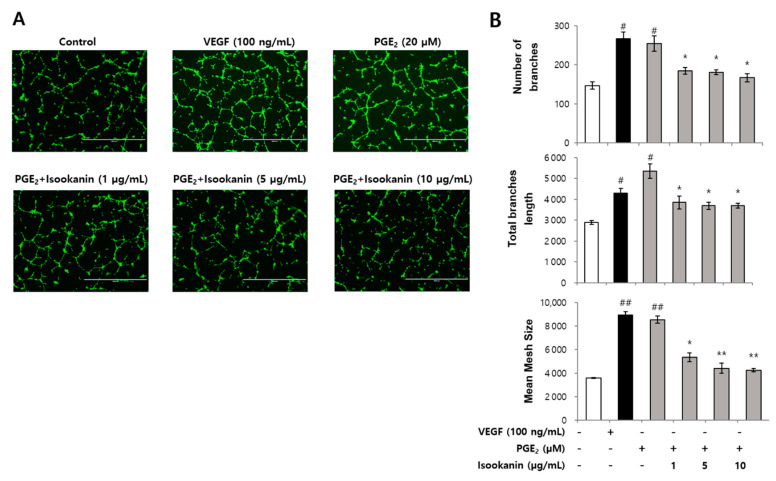
In order to further evaluate the antiangiogenic activities of isookanin, we detected the effect of isookanin on the tube formation ability of endothelial cells via tube formation assay, which mimics the angiogenic process. Following seeding onto Matrigel, endothelial cells quickly attached to the matrix and morphologically differentiated to a capillary-like network [32]. As shown in Figure 3A, PGE2 could promote the formation of capillary/tube-like networks of HMEC-1 cells, similar VEGF treatment. On the other hand, isookanin-treated HMEC-1 cells formed relatively incomplete and narrow tube-like structures, and this effect became more pronounced with increasing concentration of treatment. In addition, the antiangiogenic activities of isookanin on tube formation were quantified by analyzing the total branch number, length, and mesh size of the tube-like structures using Image-J software. The analysis showed that isookanin at concentrations ranging from 1 to 10 µg/mL significantly reduced the extent of tubular formation of HMEC-1 in a dose-dependent manner (Figure 3A,B).
Figure 3.
The effect of isookanin on tube formation in PGE2-induced HMEC-1 cells. Cells were pretreated with the indicated concentrations of isookanin for 2 h before stimulation with PGE2 (20 µM) or VEGF (100 ng/mL) for 24 h. HMEC-1 cells tube formation was measured using the tube formation assay. (A) Calcein AM dye-stained cells were photographed under a microscope; scale bars are 1000 μm. (B) Tube formation was evaluated by analyzing the total branch number, length, and mesh size of the tube-like structures using Image-J software. The results are mean ± standard deviation (SD) (n = 3). # p < 0.05, ## p < 0.01 vs. control. * p < 0.05, ** p < 0.01 vs. PGE2-treated control.
2.4. Effect of Isookanin on HMEC-1 Proliferation Inhibition
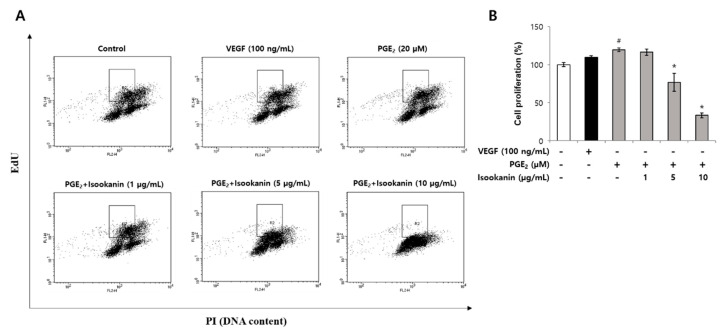
To evaluate the anti-proliferative effect of isookanin on PGE2-induced HMEC-1 cells, Click-iT EdU incorporation assays were performed, and EdU and DNA contents were analyzed by flow cytometry. Bivariate distributions of EdU incorporation (y axis) vs. DNA content (x axis) were plotted. The boxes indicate EdU-incorporated cells in the S-phase, and cell proliferations are expressed as percentages of EdU-labeled S-phase populations to total cell numbers. Flow cytometric analysis showed EdU-labeled S phase HMEC-1 cells increased to 19% by PGE2 treatment compared with the control. However, the proportion of EdU-labeled cells decreased in a dose-dependent manner after pre-treatment with isookanin and significantly decreased by 85.5% at a concentration of 10 µg/mL (Figure 4A,B). These results once again indicate that isookanin inhibited the proliferation of HMEC-1 cells.
Figure 4.
The anti-proliferative effect of isookanin on PGE2-induced HMEC-1 cells. Cells were pretreated with the indicated concentrations of isookanin for 2 h before stimulation with PGE2 (20 µM) for 24 h. Then, cell proliferation was measured using the Click-iT EdU Cytometry Assay Kit and propidium iodide dual staining to determine cell proliferation. (A) EdU and DNA contents were analyzed by flow cytometry. Bivariate distributions of EdU incorporation (y axis) vs. DNA content (x axis) were plotted. The boxes indicate EdU-incorporated cells in the S-phase. (B) Cell proliferations are expressed as percentages of EdU-labeled S-phase populations to total cell numbers. The results are mean ± standard deviation (SD) (n = 3). # p < 0.05 vs. control. * p < 0.05 vs. PGE2-treated control.
2.5. Effects of Isookanin on the Regulation of Cell Cycle and the Expression Level of Cell Cycle Regulatory Proteins
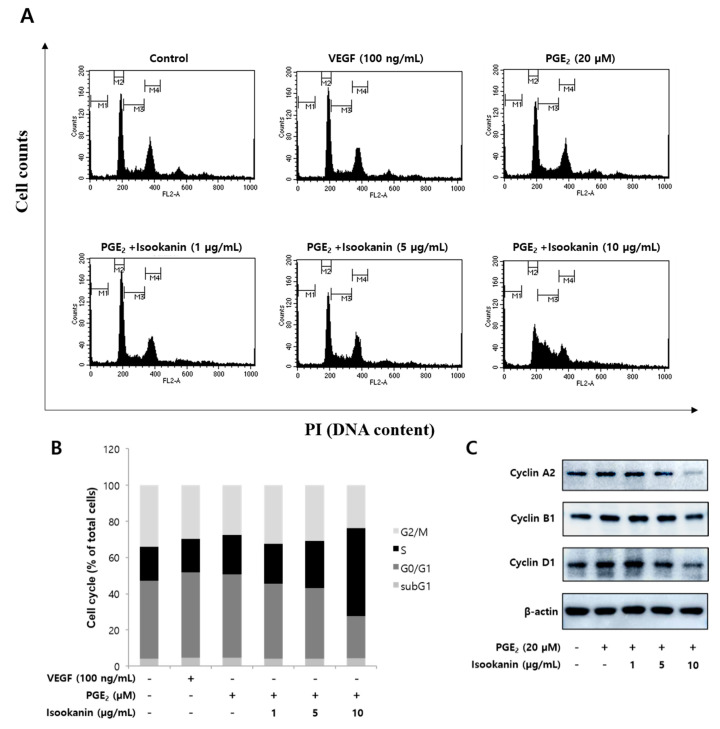
To clarify the relevant mechanism of isookanin inhibiting cell proliferation, we examined the effect of isookanin on the HMEC-1 cell cycle by propidium iodide staining (Figure 5A). The results show that in the group treated only with PGE2, there were 46.2% cells in G0/G1 phase, 21.4% cells in S phase, and 27.7% in G2/M phase (Figure 5B). However, in the presence of isookanin, the majority of cells stayed in the S phase and thus did not enter the G2/M phase. These results suggest that the antiangiogenic effect of isookanin was associated with cell cycle control. Furthermore, the flow cytometric data did not detect the increase of sub-G1 cells after isookanin treatment compared with the control group; thus, the possibility of cell death induced by isookanin under this experimental condition was ruled out again. In addition, we performed Western blot analysis for cell cycle-related proteins (Cyclin A2, Cyclin B1, Cyclin D1). Figure 5C shows that treatment with isookanin significantly decreased the expression of Cyclin A2 and Cyclin D1 and that treatment slightly reduced the expression of Cyclin B1. These results indicate that isookanin inhibited cell proliferation by downregulating the expression of cell cycle-related proteins to arrest the S phase cell.
Figure 5.
Effects of isookanin on the regulation of cell cycle and the expression level of cell cycle regulatory proteins on PGE2-induced HMEC-1 cells. Cells were pretreated with the indicated concentrations of isookanin for 2 h before stimulation with PGE2 (20 µM). (A) Total DNAs were labeled with propidium iodide and analyzed by flow cytometry. (B) The cell cycle distribution was analyzed with BD CellQuest™ Pro Software. (C) Cell cycle-related proteins (Cyclin A2, Cyclin B1, Cyclin D1) and β-actin (a loading control) were analyzed by Western blotting.
2.6. Effects of Isookanin on Phophorylation of ERK1/2 and CREB in PGE2-Induced HMEC-1 Cells
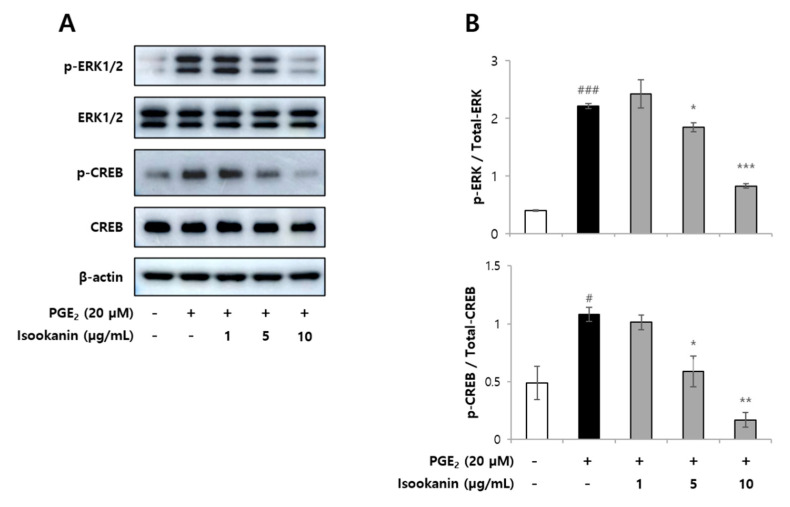
Mitogen-activated protein kinase (MAPK) pathways constitute a large modular network that regulates a variety of physiological processes, including cell growth and differentiation. Extracellular signal regulated kinases (ERK1/2) are responsible for stimulating numerous downstream effectors, many of which are transcription factors [33]. Cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB), is one of the transcription factors that plays an important role in cell proliferation and the cell cycle [34]. To characterize whether isookanin acts on the PGE2-mediated angiogenesis signaling pathway, activation of ERK1/2 and CREB was investigated. Figure 6 shows that the phosphorylation of ERK1/2 and CREB was induced by PGE2 (20 µM), and phosphorylation of both ERK1/2 and CREB was inhibited by isookanin. These results indicate that isookanin inhibited ERK1/2 phosphorylation, which may further suppress CREB activation. Additionally, since CREB activation can be achieved by cAMP/PKA activation in an ERK-independent manner [35], the possibility that isookanin directly inhibits CREB phosphorylation via ERK-independent pathway cannot be excluded. In this regard, it is necessary to confirm through additional experiments whether isookanin inhibits CREB phosphorylation directly.
Figure 6.
Effects of isookanin on the phosphorylation of ERK and CREB in PGE2-induced HMEC-1 cells. The HMEC-1 cells were cultured for 24 h and then treated with PGE2 (20 µM) in the presence or absence of isookanin. Cell lysates were prepared at 20 min and were then subjected to Western blot analysis. (A) The bands for phospho-ERK1/2 and phospho-CREB were detected and normalized to their total forms of ERK1/2 and CREB. (B) The scanning densitometric values of each band were analyzed with Image J. The results are mean ± standard deviation (SD) (n = 3). # p < 0.05, ### p < 0.001 vs. control. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. PGE2-treated control.
3. Discussion
Inflammatory mediators are well known for their role in inducing an appropriate immune response to external stimuli, and recently, they have also been shown to play an important role in the body’s response to vascular disease [36]. Previous studies have shown that the inflammatory response enhances angiogenesis and tumor growth [37]. Prostaglandins, especially PGE2, have been demonstrated to play an important role in immune regulation, and according to recent reports, PGE2 also plays a role in promoting the assembly of new blood vessels in angiogenesis [14]. The development of anti-angiogenesis agents has attracted great interest due to the high prevalence of angiogenesis-related diseases. The importance of antiangiogenic agents in the treatment of cancer is well established [38], and their role in the treatment of chronic inflammatory disorders is also gaining more support. In cases such as rheumatoid arthritis [39] and human IBD [40], it has been demonstrated that angiogenesis plays an important role in the pathophysiology of these chronic inflammatory diseases. There are several types of synthetic drugs for anti-angiogenesis therapy, but considering their side effects, it is worth investigating bioactive active compounds derived from natural products for the prevention and treatment of angiogenesis-related diseases [41]. Currently, a large variety of polyphenolic compounds from natural sources have drawn considerable attention for their multiplex biologic properties. Many natural compounds—for example, resveratrol, curcumin, or quercetin—can target a wide variety of molecules, implying that natural products might be effective inhibitors [42]. Isookanin is a phenolic flavonoid component contained in some plants, such as Asteraceae [26] and Leguminosae [28], and its anti-inflammatory efficacy and mechanism of action have already been revealed in our previous research [29].
In this study, we investigated the role that isookanin plays in the process of PGE2-induced angiogenesis. The results of the LDH experiment show that isookanin has no cytotoxicity, and the results of MTT cell viability confirm that isookanin can reduce the proliferation of PGE2-induced HMEC-1 cells in a concentration-dependent manner, while not causing cell damage. In addition, we confirmed that isookanin inhibited the migration and tube formation of HMEC-1 cells induced by PGE2. These results support the potential of isookanin as an antiangiogenic agent. In addition, isookanin induces the S phase arrest of HMEC-1 cells through downregulating the expression of cell cycle-related proteins. Moreover, the flow cytometric data of cell cycle assay did not detect the increase of sub-G1 cells after isookanin treatment compared with the control group. This indicates that the antiangiogenic activity of isookanin is due to the inhibition of cell proliferation, not the induction of cell death.
PGE2 affects target cells by activating cognate receptors EP1-4 that belong to the G protein-coupled receptor superfamily of cell surface-expressed receptors [15]. The receptors demonstrate distinct, as well as opposing, effects on intracellular signaling events. The EP1 receptor couples to Gq and mediates a rise in intracellular calcium concentration, and the EP3 receptor couples to Gi, reducing cAMP concentration. Both EP2 and EP4 couple to Gs, leading to increased synthesis of cAMP and consequent activation of PKA [14]. Subsequent PKA activation is involved in multiple signaling, including activation of the cAMP response element (CREB) [35]. The transcription factor CREB plays a key role in controlling cell growth, survival, and cell cycle progression, and it plays an important role in the development of many cell types, including vascular smooth muscle cells [43], adipocytes [44], glioma cell [45], and vascular endothelial cells [46]. Multiple intracellular signaling kinases can induce the phosphorylation of CREB, including extracellular signal regulated kinases (ERKs) [45,47]. Moreover, the ERK signaling cascade is a central MAPK pathway that plays a role in the regulation of various cellular processes, such as proliferation, differentiation, survival, and apoptosis under some conditions [48]. For example, Pozzi et al. [49] demonstrated an EP4-mediated PI3K/ERK signaling pathway in mouse colon carcinoma cells. Activation of the ERK1/2 pathway is required for the growth, proliferation, and migration of endothelial cells, and this pathway is considered an important target for antiangiogenic agents [50,51].
In recent studies, it is known that PGE2 can promote cell growth by activating multiple signaling pathways involved in cell proliferation and that both MAPK and cAMP/PKA pathways are required for PGE2-induced cell proliferation [45,52]. In this study, PGE2 was shown to increase phosphorylation of ERK1/2 and CREB; therefore, PGE2-induced proliferation is associated with activation of ERK1/2 and CREB in vascular endothelial cells. In addition, isookanin exhibits the efficacy of inhibiting phosphorylation of both ERK 1/2 and CREB induced by PGE2. These results suggest that the mechanism by which isookanin inhibits the proliferation of vascular endothelial cells is by inhibition of ERK1/2 and CREB phosphorylation. However, these results should be confirmed by further experiments to determine whether isookanin inhibits CREB activation through ERK1/2 dependence or acts directly on CREB.
PGE2 may induce angiogenesis by acting indirectly on a variety of cell types to produce proangiogenic factors. Indeed, PGE2 stimulation of airway smooth muscle, endometrium, or colon cancer cells leads to increased production of VEGF, bFGF, or CXCL1 that, in turn, act on target endothelial cells to promote the angiogenesis [53,54,55]. In addition, previous studies have reported that PGE2 acts directly on endothelial cells, promoting the creation of new blood vessels through activation of G protein-coupled receptor EP4 and its downstream pathway [14]. Of all the PGE2 receptors, the roles of the EP4 receptor in health and disease have received much attention [56]. Chell et al. [57] reported that EP4 receptor protein expression was increased in colorectal cancers, as well as in adenomas when compared with normal colonic epithelium. EP receptor overexpression—and thus upregulation of various signaling cascades associated with angiogenic tumorigenesis, PGE2 modification of the tumor microenvironment, and evasion of the immune system—is regulated through the EP receptors [58]. Therefore, the direct effect of isookanin on the expression of the EP receptor was confirmed and is presented in Supplementary Materials. The result shows that isookanin effectively inhibited the expression of EP4 (Figure S1 in Supplementary Materials). These results suggest that isookanin possibly regulates downstream signaling by inhibiting the expression of EP4, and thereby inhibiting angiogenesis. However, to confirm the effect between the suppression of EP4 expression and downstream signaling, additional experiments are required. In addition, recent studies have reported that several specific receptors may be involved in the angiogenesis of PGE2. Finetti et al. [59] reported that PGE2 primes the angiogenic switch through a synergistic interaction with the fibroblast growth factor-2 pathway. There is also a report claiming that EP2 receptor agonists stimulate angiogenesis to promote adipogenesis [60]. Based on these studies, it can be expected that several specific receptors, including EP4, are the major receptors responsible for PGE2-induced angiogenesis. To identify the role of specific receptors for PGE2 in an angiogenesis model, further studies using specific EP antagonists or gene silencing are required.
4. Materials and Methods
4.1. Reagents
MCDB 131 medium, fetal bovine serum, L-glutamine, and epidermal growth factor (EGF) were purchased from Gibco (Grand Island, NY, USA). Prostaglandin E2 (PGE2), hydrocortisone, dimethyl sulfoxide (DMSO), and propidium iodide (PI) were purchased from Sigma-Aldrich (St. Louis, MO, USA), and human recombinant VEGF was obtained from ProSpec (Rehobot, Israel). Isookanin (purity ≥ 98%) was obtained from ChemFaces Biochemical Co. Ltd. (Wuhan, China) and dissolved in DMSO. Antibodies for phospho CREB and total CREB, phospho ERK1/2 and total ERK1/2, and cell cycle-related antibodies (Cyclin A2, Cyclin B1, Cyclin D1) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA), while β-actin and secondary antibodies were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
4.2. Cell Culture
The human dermal microvascular endothelial cell line (HMEC-1) was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in MCDB 131 medium supplemented with 10% fetal bovine serum, 10 mM L-glutamine, 10 ng/mL epidermal growth factor (EGF), and 1 µg/mL hydrocortisone. Cells were maintained at 37 °C in a humidified atmosphere of 5% CO2.
4.3. Cell Proliferation Assay
The effect of isookanin on cell proliferation was determined via MTT (Duchefa, Haarlem, The Netherlands) assay. HMEC-1 cells were plated at a density of 4 × 104 cells/well in 24-well plates and incubated overnight. Then, the cells were pre-treated 2 h in the presence or absence of isookanin (1, 5, and 10 µg/mL) in serum-free medium and stimulated by PGE2 (20 µM) for 48 h. VEGF (100 ng/mL) was used as a positive control to support that PGE2 is a potent proangiogenic factor, and an untreated group was used as a control. After treatment, microscopic observation was performed using an inverted microscope (Nikon Inc., Japan), and then, MTT reagent (1 mg/mL) was added to each well and incubated for 3 h before the supernatants were discarded followed by the addition of 200 μL DMSO. The absorbance at 570 nm was measured using a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). The results are expressed as a percentage of control.
4.4. Lactate Dehydrogenase (LDH) Cytotoxicity Assay
The LDH release assay was performed using a LDH cytotoxicity detection kit (Enzo Life Sciences, Inc., Farmingdale, NY, USA) according to the manufacturer’s instructions. In brief, HMEC-1 cells were seeded in 24-well plates at a density of 5 × 104 cells per well. The cells were then incubated with various concentrations of isookanin (1, 5, and 10 µg/mL) for 48 h, or they were incubated with lysis buffer as a high cytotoxicity control. The cell supernatants were collected and analyzed. The absorbance of formed formazan was read at 490 nm on a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA), and the results are expressed as a percentage of control.
4.5. Scratch Migration Assay
To assess the effect of isookanin on HMEC-1 growth following angiogenic stimuli, a migration assay was performed as described previously [61]. Briefly, HMEC-1 cells were seeded in 12-well plates at a density of 2 × 105 cells/well in 10% serum containing MCDB 131 medium for 24 h. After the cells reached 90% confluence, the cells were scratched with a sterile pipette tip and washed with phosphate buffered saline (PBS) subsequently to eliminate the impaired cells. The cells were then incubated in 1% fetal bovine serum medium containing different concentrations of isookanin (1, 5, and 10 µg/mL) and stimulated with PGE2 (20 µM) or VEGF (100 ng/mL) for 24 h, and an untreated group was used as a control. The migration of HMEC-1 cells across the demarcation line was monitored using an inverted microscope (Nikon Inc., Japan).
4.6. Transwell Migration Assay
A transwell analysis was performed to observe cell migration in 3D models through a transwell Boyden Chamber (8.0 μM pore size, Corning Costar Corporation, NY, USA), which were coated with 1% gelatin. The HMEC-1 cell suspensions were seeded at a density of 1 × 105 cells/well into the upper chambers of the Boyden system and cultured in 1% FBS medium containing different concentrations of isookanin (1, 5, 10 μg/mL) or the solvent control [61,62]. The lower chamber added 700 μL serum-free medium containing 20 µM PGE2 or 100 ng/mL VEGF to induce angiogenesis, and the untreated group was used as a control. Twenty-four hours later, all non-migrant cells in the upper chamber were removed using a cotton swab. The chambers were washed twice with Dulbecco’s phosphate-buffered saline (DPBS) and fixed with 4% paraformaldehyde for 30 min. After washing twice with DPBS again, the chambers were stained with 5% crystal violet stain for 20 min, and then the stained cells were photographed under a microscope. Cells were subsequently extracted with 10% acetic acid; the absorbance at 600 nm was used to calculate the percentage of migrated cells.
4.7. Matrigel Endothelial Cell Tube Formation Assay
Tube formation assay was performed, as previously described, to analyze in vitro angiogenesis [61]. Briefly, a pre-cooled 24-well plate was coated with Matrigel (Corning Costar Corporation, NY, USA) and allowed to solidify at 37 °C for 1 h. After coagulation, HMEC-1 cells (1.5 × 104 cells/well) were seeded on the Matrigel and cultured in 1% FBS medium containing different concentrations of isookanin (1, 5, and 10 μg/mL), and 20 µM PGE2 or 100 ng/mL VEGF was added to stimulate endothelial cell tube formation, while control wells were not treated with any test agent. The angiogenesis assay plates were then incubated for 24 h at 37 °C in a 5% CO2 atmosphere.
Following incubation and removal of medium from the plates, Calcein AM dye diluted to 8 μg/mL concentration in Hanks Balanced Salt Solution (HBSS) was added to each well, and the plates were incubated at 37 °C with 5% CO2 for 40 min to label the cells. After incubation, the plates were photographed using an EVOS fluorescent microscope (Advanced Microscopy Group, Bothell, WA, USA). Tube formation was evaluated by analyzing the total branch number, length, and mesh size of the tube-like structures using Image-J software (National Institutes of Health, Bethesda, MD, USA).
4.8. Flow Cytometry Analysis of Edu Cell Proliferation Assay and Cell Cycle Arrest
Cell proliferation was measured using the Click-iT EdU Cytometry Assay Kit (Invitrogen, Carlsbad, CA, USA) and propidium iodide dual staining according to the manufacturer’s instructions. HMEC-1 cells were seeded into 6-well plates at 1.2 × 105 per well and incubated overnight. The cells were pretreated with or without isookanin (1, 5, and 10 μg/mL) for 2 h and stimulated with 20 µM PGE2 or 100 ng/mL VEGF in each well for 24 h under serum-free conditions. The cells were then labeled with EdU (10 μM) for 3 h, fixed for 15 min with 4% paraformaldehyde, and permeabilized for 5 min at room temperature using a Triton X-100 solution. The fixed cells were then stained with the Click-iT reaction mixture for 30 min at room temperature. Afterwards, propidium iodide-staining was performed to determine cell proliferation and cell cycle distribution, and the cells were analyzed with BD FACS Calibur™ flow cytometry and BD CellQuest™ Pro Software (Becton Dickinson, San Jose, CA, USA).
4.9. Western Blotting
Western blotting was performed to measure the levels of proteins associated with the cell cycle and angiogenic signaling pathways. HMEC-1 cells were pretreated with or without isookanin (1, 5, and 10 μg/mL) for 2 h prior to induction with 20 µM PGE2. The cells were then harvested and lysed by protein extraction solution, PRO-PREP™ (iNtRON Biotechnology, Seongnam, Korea). The protein extracts (20 μg) from lysed cells were loaded onto a NuPAGE Novex 10% Bis-Tris Gel 1.0 mm, 15-well (Invitrogen, Waltham, MA, USA), and transferred to a nitrocellulose membrane by using an iBlot™ Gel Transfer Device (Invitrogen, Waltham, MA, USA). The membranes were blocked with 5% bovine serum albumin (BSA) for 1 h. Membranes were then incubated with primary antibodies (1:1000 dilution of stock) in 10 mL of blocking buffer, followed by incubation with horseradish peroxidase-conjugated secondary antibody (1:3000 dilution of stock). The protein bands were detected by enhanced chemiluminescence detection reagents (Invitrogen, Waltham, MA, USA) and visualized using ImageQuant™ LAS 500 (Cytiva, Marlborough, MA, USA).
4.10. Statistical Analyses
All experimental data are expressed as mean ± standard deviation. Differences between the control and the treatment group were evaluated by one-way ANOVA (SPSS, IBM, Armonk, NY, USA). Values of p < 0.05, 0.01, or 0.001 were considered statistically significant.
5. Conclusions
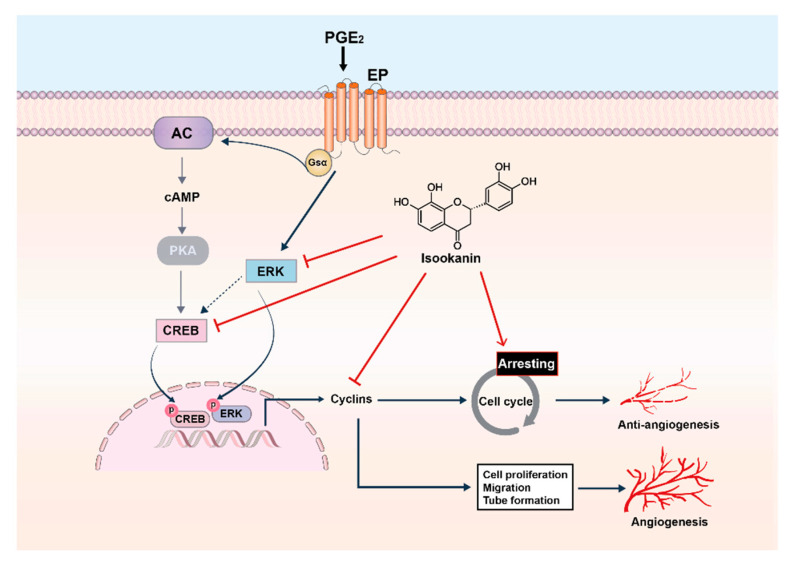
In this study, we demonstrated that isookanin has an effective inhibitory effect on the angiogenic properties of microvascular endothelial cells. Isookanin exerts inhibitory effects at multiple stages of PGE2-induced angiogenesis, including the growth, proliferation, migration, and tube formation of microvascular endothelial cells. In addition, isookanin induces cell cycle arrest in S phase, which is also the reason for subsequent inhibition of cell proliferation. Our results also suggest that the mechanism of inhibiting angiogenesis by isookanin is derived from the inhibition of PGE2-mediated phosphorylation of ERK1/2 and CREB (Figure 7). Based on these results, isookanin shows its potential as a candidate for the treatment of angiogenesis-related diseases, and it is necessary to further determine the therapeutic effect of isookanin as an antiangiogenic agent in established animal models and clinical trials.
Figure 7.
The mechanism of isookanin inhibiting angiogenesis is associated with suppressing PGE2-mediated ERK1/2 and CREB phosphorylation and with inducing cell cycle arrest.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22126466/s1.
Author Contributions
Y.X. designed the research; Y.X. performed the bioactivities experiments; Y.X., K.R., E.C., W.W., D.P. and E.J. analyzed data; Y.X. wrote the manuscript and prepared figures. All authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adair T.H., Montani J.-P. Angiogenesis, Colloquium Series on Integrated Systems Physiology: From Molecule to Function. Morgan & Claypool Life Sciences; San Rafael, CA, USA: 2010. pp. 1–84. [Google Scholar]
- 2.Folkman J., Shing Y. Angiogenesis. J. Biol. Chem. 1992;267:10931–10934. doi: 10.1016/S0021-9258(19)49853-0. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 4.Velasco P., Lange-Asschenfeldt B. Dermatological aspects of angiogenesis. Br. J. Dermatol. 2002;147:841–852. doi: 10.1046/j.1365-2133.2002.05073.x. [DOI] [PubMed] [Google Scholar]
- 5.van Hinsbergh V.W., Collen A., Koolwijk P. Angiogenesis and anti-angiogenesis: Perspectives for the treatment of solid tumors. Ann. Oncol. 1999;10:S60–S63. doi: 10.1093/annonc/10.suppl_4.S60. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerbel R., Folkman J. Clinical translation of angiogenesis inhibitors. Nat. Rev. Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Korbecki J., Baranowska-Bosiacka I., Gutowska I., Chlubek D. Cyclooxygenase pathways. Acta Biochim. Pol. 2014;61 doi: 10.18388/abp.2014_1825. [DOI] [PubMed] [Google Scholar]
- 10.Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Atertio. Thromb. Vasc. Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dey I., Lejeune M., Chadee K. Prostaglandin E2 receptor distribution and function in the gastrointestinal tract. Br. J. Pharmacol. 2006;149:611–623. doi: 10.1038/sj.bjp.0706923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park J.Y., Pillinger M.H., Abramson S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006;119:229–240. doi: 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Legler D.F., Bruckner M., Uetz-von Allmen E., Krause P. Prostaglandin E2 at new glance: Novel insights in functional diversity offer therapeutic chances. Int. J. Biochem. Cell Biol. 2010;42:198–201. doi: 10.1016/j.biocel.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y., Daaka Y. PGE2 promotes angiogenesis through EP4 and PKA Cγ pathway. Blood J. Am. Soc. Hematol. 2011;118:5355–5364. doi: 10.1182/blood-2011-04-350587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugimoto Y., Narumiya S. Prostaglandin E receptors. J. Biol. Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 16.Hatazawa R., Tanigami M., Izumi N., Kamei K., Tanaka A., Takeuchi K. Prostaglandin E 2 stimulates VEGF expression in primary rat gastric fibroblasts through EP4 receptors. Inflammopharmacology. 2007;15:214–217. doi: 10.1007/s10787-007-1595-z. [DOI] [PubMed] [Google Scholar]
- 17.Rao R., Redha R., Macias-Perez I., Su Y., Hao C., Zent R., Breyer M.D., Pozzi A. Prostaglandin E2-EP4 receptor promotes endothelial cell migration via ERK activation and angiogenesis in vivo. J. Biol. Chem. 2007;282:16959–16968. doi: 10.1074/jbc.M701214200. [DOI] [PubMed] [Google Scholar]
- 18.Muller M., Sales K.J., Katz A.A., Jabbour H.N. Seminal plasma promotes the expression of tumorigenic and angiogenic genes in cervical adenocarcinoma cells via the E-series prostanoid 4 receptor. Endocrinology. 2006;147:3356–3365. doi: 10.1210/en.2005-1429. [DOI] [PubMed] [Google Scholar]
- 19.Yanni S.E., Barnett J.M., Clark M.L., Penn J.S. The role of PGE2 receptor EP4 in pathologic ocular angiogenesis. Investig. Ophthalmol. Vis. Sci. 2009;50:5479–5486. doi: 10.1167/iovs.09-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olivieri D., Chetta A. Therapeutic perspectives in vascular remodeling in asthma and chronic obstructive pulmonary disease. Angiogenesis Lymphangiogenesis Clin. Implic. 2014;99:216–225. doi: 10.1159/000353307. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W., Li F., Gao W. Tripterygium wilfordii inhibiting angiogenesis for rheumatoid arthritis treatment. J. Natl. Med. Assoc. 2017;109:142–148. doi: 10.1016/j.jnma.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Arbiser J.L., Govindarajan B., Battle T.E., Lynch R., Frank D.A., Ushio-Fukai M., Perry B.N., Stern D.F., Bowden G.T., Liu A. Carbazole is a naturally occurring inhibitor of angiogenesis and inflammation isolated from antipsoriatic coal tar. J. Investig. Dermatol. 2006;126:1396–1402. doi: 10.1038/sj.jid.5700276. [DOI] [PubMed] [Google Scholar]
- 23.Chen H.X., Cleck J.N. Adverse effects of anticancer agents that target the VEGF pathway. Nat. Rev. Clin. Oncol. 2009;6:465. doi: 10.1038/nrclinonc.2009.94. [DOI] [PubMed] [Google Scholar]
- 24.Des Guetz G., Uzzan B., Chouahnia K., Morère J.-F. Cardiovascular toxicity of anti-angiogenic drugs. Target. Oncol. 2011;6:197–202. doi: 10.1007/s11523-011-0204-7. [DOI] [PubMed] [Google Scholar]
- 25.Rajasekar J., Perumal M.K., Vallikannan B. A critical review on anti-angiogenic property of phytochemicals. J. Nutr. Biochem. 2019;71:1–15. doi: 10.1016/j.jnutbio.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Yang X.-W., Huang M.-Z., Jin Y.-S., Sun L.-N., Song Y., Chen H.-S. Phenolics from Bidens bipinnata and their amylase inhibitory properties. Fitoterapia. 2012;83:1169–1175. doi: 10.1016/j.fitote.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed D., Kumar V., Sharma M., Verma A. Target guided isolation, in-vitro antidiabetic, antioxidant activity and molecular docking studies of some flavonoids from Albizzia Lebbeck Benth. bark. BMC Complement. Altern. Med. 2014;14:155. doi: 10.1186/1472-6882-14-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W., Chen W., Yang Y., Liu T., Yang H., Xin Z. New phenolic compounds from Coreopsis tinctoria Nutt. and their antioxidant and angiotensin I-converting enzyme inhibitory activities. J. Agric. Food Chem. 2015;63:200–207. doi: 10.1021/jf504289g. [DOI] [PubMed] [Google Scholar]
- 29.Xin Y.-J., Choi S., Roh K.-B., Cho E., Ji H., Weon J.B., Park D., Whang W.K., Jung E. Anti-Inflammatory Activity and Mechanism of Isookanin, Isolated by Bioassay-Guided Fractionation from Bidens pilosa L. Molecules. 2021;26:255. doi: 10.3390/molecules26020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng J., Zhang Y., Xing D. Low-power laser irradiation (LPLI) promotes VEGF expression and vascular endothelial cell proliferation through the activation of ERK/Sp1 pathway. Cell. Signal. 2012;24:1116–1125. doi: 10.1016/j.cellsig.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 32.Montesano R., Orci L., Vassalli P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J. Cell Biol. 1983;97:1648–1652. doi: 10.1083/jcb.97.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao J., Zhu H., Wan H., Zou X., Ma X., Gao G. Harmine suppresses the proliferation and migration of human ovarian cancer cells through inhibiting ERK/CREB pathway. Oncol. Rep. 2017;38:2927–2934. doi: 10.3892/or.2017.5952. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal S., Kim S.-W., Cheon K., Tabassam F.H., Yoon J.-H., Koo J.S. Nonclassical action of retinoic acid on the activation of the cAMP response element-binding protein in normal human bronchial epithelial cells. Mol. Biol. Cell. 2006;17:566–575. doi: 10.1091/mbc.e05-06-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.New D.C., Wong Y.H. Molecular mechanisms mediating the G protein-coupled receptor regulation of cell cycle progression. J. Mol. Signal. 2007;2:1–15. doi: 10.1186/1750-2187-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szekanecz Z., Koch A.E. Mechanisms of disease: Angiogenesis in inflammatory diseases. Nat. Clin. Pract. Rheumatol. 2007;3:635–643. doi: 10.1038/ncprheum0647. [DOI] [PubMed] [Google Scholar]
- 37.Kofler S., Nickel T., Weis M. Role of cytokines in cardiovascular diseases: A focus on endothelial responses to inflammation. Clin. Sci. 2005;108:205–213. doi: 10.1042/CS20040174. [DOI] [PubMed] [Google Scholar]
- 38.Rajabi M., Mousa S.A. The role of angiogenesis in cancer treatment. Biomedicines. 2017;5:34. doi: 10.3390/biomedicines5020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szekanecz Z., Gáspár L., Koch A.E. Angiogenesis in rheumatoid arthritis. Front Biosci. 2005;10:1739–1740. doi: 10.2741/1657. [DOI] [PubMed] [Google Scholar]
- 40.Pousa I., Maté J., Gisbert J. Angiogenesis in inflammatory bowel disease. Eur. J. Clin. Investig. 2008;38:73–81. doi: 10.1111/j.1365-2362.2007.01914.x. [DOI] [PubMed] [Google Scholar]
- 41.EL-Meghawry E., Rahman H., Abdelkarim G., Najda A. Natural products against cancer angiogenesis. Tumor Biol. 2016;37:14513–14536. doi: 10.1007/s13277-016-5364-8. [DOI] [PubMed] [Google Scholar]
- 42.Ji H.F., Li X.J., Zhang H.Y. Natural products and drug discovery: Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 2009;10:194–200. doi: 10.1038/embor.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klemm D.J., Watson P.A., Frid M.G., Dempsey E.C., Schaack J., Colton L.A., Nesterova A., Stenmark K.R., Reusch J.E.-B. cAMP response element-binding protein content is a molecular determinant of smooth muscle cell proliferation and migration. J. Biol. Chem. 2001;276:46132–46141. doi: 10.1074/jbc.M104769200. [DOI] [PubMed] [Google Scholar]
- 44.Reusch J.E., Colton L.A., Klemm D.J. CREB activation induces adipogenesis in 3T3-L1 cells. Mol. Cell. Biol. 2000;20:1008–1020. doi: 10.1128/MCB.20.3.1008-1020.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daniel P., Filiz G., Brown D., Hollande F., Gonzales M., D’Abaco G., Papalexis N., Phillips W., Malaterre J., Ramsay R.G. Selective CREB-dependent cyclin expression mediated by the PI3K and MAPK pathways supports glioma cell proliferation. Oncogenesis. 2014;3:e108. doi: 10.1038/oncsis.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayo L.D., Kessler K.M., Pincheira R., Warren R.S., Donner D.B. Vascular endothelial cell growth factor activates CRE-binding protein by signaling through the KDR receptor tyrosine kinase. J. Biol. Chem. 2001;276:25184–25189. doi: 10.1074/jbc.M102932200. [DOI] [PubMed] [Google Scholar]
- 47.Zhang C.-J., Liu C., Wang Y.-X., Zhu N., Hu Z.-Y., Liao D.-F., Qin L. Long non-coding RNA-SRA promotes neointimal hyperplasia and vascular smooth muscle cells proliferation via MEK-ERK-CREB pathway. Vascul. Pharmacol. 2019;116:16–23. doi: 10.1016/j.vph.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Shaul Y.D., Seger R. The MEK/ERK cascade: From signaling specificity to diverse functions. Biochim. Biophys. Acta BBA Mol. Cell Res. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Pozzi A., Yan X., Macias-Perez I., Wei S., Hata A.N., Breyer R.M., Morrow J.D., Capdevila J.H. Colon carcinoma cell growth is associated with prostaglandin E2/EP4 receptor-evoked ERK activation. J. Biol. Chem. 2004;279:29797–29804. doi: 10.1074/jbc.M313989200. [DOI] [PubMed] [Google Scholar]
- 50.Kim G.D. Hesperetin inhibits vascular formation by suppressing of the PI3K/AKT, ERK, and p38 MAPK signaling pathways. Prev. Nutr. Food Sci. 2014;19:299. doi: 10.3746/pnf.2014.19.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song Y., Dai F., Zhai D., Dong Y., Zhang J., Lu B., Luo J., Liu M., Yi Z. Usnic acid inhibits breast tumor angiogenesis and growth by suppressing VEGFR2-mediated AKT and ERK1/2 signaling pathways. Angiogenesis. 2012;15:421–432. doi: 10.1007/s10456-012-9270-4. [DOI] [PubMed] [Google Scholar]
- 52.Ansari K.M., Rundhaug J.E., Fischer S.M. Multiple Signaling Pathways Are Responsible for Prostaglandin E2–Induced Murine Keratinocyte Proliferation. Mol. Cancer Res. 2008;6:1003–1016. doi: 10.1158/1541-7786.MCR-07-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bradbury D., Clarke D., Seedhouse C., Corbett L., Stocks J., Knox A. Vascular endothelial growth factor induction by prostaglandin E2 in human airway smooth muscle cells is mediated by E prostanoid EP2/EP4 receptors and SP-1 transcription factor binding sites. J. Biol. Chem. 2005;280:29993–30000. doi: 10.1074/jbc.M414530200. [DOI] [PubMed] [Google Scholar]
- 54.Battersby S., Sales K., Williams A., Anderson R., Gardner S., Jabbour H. Seminal plasma and prostaglandin E2 up-regulate fibroblast growth factor 2 expression in endometrial adenocarcinoma cells via E-series prostanoid-2 receptor-mediated transactivation of the epidermal growth factor receptor and extracellular signal-regulated kinase pathway. Hum. Reprod. 2007;22:36–44. doi: 10.1093/humrep/del328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang D., Wang H., Brown J., Daikoku T., Ning W., Shi Q., Richmond A., Strieter R., Dey S.K., DuBois R.N. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J. Exp. Med. 2006;203:941–951. doi: 10.1084/jem.20052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Konya V., Marsche G., Schuligoi R., Heinemann A. E-type prostanoid receptor 4 (EP4) in disease and therapy. Pharmacol. Ther. 2013;138:485–502. doi: 10.1016/j.pharmthera.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chell S.D., Witherden I.R., Dobson R.R., Moorghen M., Herman A.A., Qualtrough D., Williams A.C., Paraskeva C. Increased EP4 receptor expression in colorectal cancer progression promotes cell growth and anchorage independence. Cancer Res. 2006;66:3106–3113. doi: 10.1158/0008-5472.CAN-05-3702. [DOI] [PubMed] [Google Scholar]
- 58.Musser M.L., Viall A.K., Phillips R.L., Hostetter J.M., Johannes C.M. Gene expression of prostaglandin EP4 receptor in three canine carcinomas. BMC Vet. Res. 2020;16:1–10. doi: 10.1186/s12917-020-02431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finetti F., Donnini S., Giachetti A., Morbidelli L., Ziche M. Prostaglandin E2 primes the angiogenic switch via a synergic interaction with the fibroblast growth factor-2 pathway. Circ. Res. 2009;105:657–666. doi: 10.1161/CIRCRESAHA.109.203760. [DOI] [PubMed] [Google Scholar]
- 60.Tsuji T., Yamaguchi K., Kikuchi R., Itoh M., Nakamura H., Nagai A., Aoshiba K. Promotion of adipogenesis by an EP2 receptor agonist via stimulation of angiogenesis in pulmonary emphysema. Prostaglandins Other Lipid Mediat. 2014;112:9–15. doi: 10.1016/j.prostaglandins.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Rafiee P., Heidemann J., Ogawa H., Johnson N.A., Fisher P.J., Li M.S., Otterson M.F., Johnson C.P., Binion D.G. Cyclosporin A differentially inhibits multiple steps in VEGF induced angiogenesis in human microvascular endothelial cells through altered intracellular signaling. Cell Commun. Signal. 2004;2:1–22. doi: 10.1186/1478-811X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koyama S., Takagi H., Otani A., Suzuma K., Nishimura K., Honda Y. Tranilast inhibits protein kinase C-dependent signalling pathway linked to angiogenic activities and gene expression of retinal microcapillary endothelial cells. Br. J. Pharmacol. 1999;127:537–545. doi: 10.1038/sj.bjp.0702564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.