Abstract
Globally, Alzheimer’s disease (AD) is one of the most prevalent age-related neurodegenerative disorders associated with cognitive decline and memory deficits due to beta-amyloid deposition (Aβ) and tau protein hyperphosphorylation. To date, approximately 47 million people worldwide have AD. This figure will rise to an estimated 75.6 million by 2030 and 135.5 million by 2050. According to the literature, the efficacy of conventional medications for AD is statistically substantial, but clinical relevance is restricted to disease slowing rather than reversal. Withaferin A (WA) is a steroidal lactone glycowithanolides, a secondary metabolite with comprehensive biological effects. Biosynthetically, it is derived from Withania somnifera (Ashwagandha) and Acnistus breviflorus (Gallinero) through the mevalonate and non-mevalonate pathways. Mounting evidence shows that WA possesses inhibitory activities against developing a pathological marker of Alzheimer’s diseases. Several cellular and animal models’ particulates to AD have been conducted to assess the underlying protective effect of WA. In AD, the neuroprotective potential of WA is mediated by reduction of beta-amyloid plaque aggregation, tau protein accumulation, regulation of heat shock proteins, and inhibition of oxidative and inflammatory constituents. Despite the various preclinical studies on WA’s therapeutic potentiality, less is known regarding its definite efficacy in humans for AD. Accordingly, the present study focuses on the biosynthesis of WA, the epidemiology and pathophysiology of AD, and finally the therapeutic potential of WA for the treatment and prevention of AD, highlighting the research and augmentation of new therapeutic approaches. Further clinical trials are necessary for evaluating the safety profile and confirming WA’s neuroprotective potency against AD.
Keywords: withaferin A, Alzheimer’s disease, beta-amyloid, mevalonate, non-mevalonate pathway
1. Introduction
Dementia is a progressive or chronic syndrome associated with reduced cognitive ability beyond the considerable consequence of normal aging. Approximately 50 brain diseases can cause dementia, of which Alzheimer’s disease (AD) is perhaps the most frequent among elderly patients and is likely to account for 50–70% of all cases of dementia [1]. AD is a chronic, progressive, irreversible, neurodegenerative disorder that is seen in age-dependent dementia. Early illness shows short-term memory loss, mood swings, inability to learn new information, difficulty in finding words, difficulty in solving problems, and disorientation in time and space [2,3,4,5,6]. Patients in severe stages suffer from serious memory loss, hallucinations, disorientation, and lack of self-sufficiency [5,7,8]. AD eventually leads to ample incapability and death of patients within 3 to 9 years after diagnosis [9]. To date, AD is the sixth leading cause of death [10,11]. Since 2014, approximately 36 million people worldwide have been affected by AD. This figure will significantly increase to approximately 75.6 million and 135.5 million by 2030 and 2050, respectively. Today, someone develops AD every 67 s in America. According to the center for disease control (CDC), there are now 5.3 million people who have AD in the United States (US), and by 2050, the total number of patients is expected to grow to 13.2 million [1,12,13]. In addition, the World Health Organization (WHO) estimates that 5% of men worldwide and 6% of women over 60 years of age are largely characterized by Alzheimer’s dementia [3,10,14,15].
The key pathological features of AD include beta-amyloid (Aβ) plaques, hyperphosphorylated tau proteins, known as neurofibrillary tangles, and neuronal loss, followed by cerebrovascular amyloidosis, inflammation, and other major synaptic changes in the brain [5,6]. AD is a genetically heterogeneous disorder with an early-onset familial form (FAD), which is autosomal dominant and a late-onset sporadic form (SAD) [16]. Mutation in the Aβ precursor protein (APP) and presenilin (PSEN1 and PSEN2) genes are responsible for FAD, while SAD has a complex etiology including genetic, viral, environmental, and metabolic factors, among others. In this regard, apolipoprotein E is a polymorphic protein of three isoforms (APOE2, APOE3, and APOE4) in which APOE4 is the most solid genetic risk factor for SAD [4,9,16,17,18]. Globally, AD has become more and more prevalent among aging populations. In this respect, identification of treatment options will need a better understanding of the pathological mechanisms involved and different ideas for drug design and assessment [19]. Despite the enormous progress made in AD research over the last few decades, the actual cause and pathogenesis of AD are not fully understood, so that there are only a few effective drugs available that can slow the progress rate of the disease [5,12]. Currently, available FDA-approved drugs for the treatment of AD are acetylcholinesterase inhibitors (AChEI) such as rivastigmine, donepezil, and galantamine, and N-methyl-D-aspartate (NMDA) antagonists including memantine. Moreover, just fewer than 20% of patients respond moderately to these drugs with an average betterment in 6 to 12 months. However, approved drugs cannot treat AD without high side effects. There is, therefore, a strong need to identify and evaluate more appropriate medications with fewer side effects. Within this context, numerous herbal molecules have proven effective to prevent and manage various neurodegenerative disorders [12]. Withaferin A (WA), a steroidal glycowithanolide lactone obtained from the Ayurvedic medicinal plant Withania somnifera has the potential to be a therapeutic agent for AD. WA is a major biologically active withanolides with a wide range of activities, such as anti-inflammatory, antitumor, anti-angiogenic, and antioxidant activities, and it also inhibits mitochondrial apoptosis [20,21]. According to published research, WA seems to have inhibitory effects against the development of a pathological marker associated with AD. In addition, the underlying protective effect of WA has been studied in a variety of cellular and animal models of AD [22,23,24,25,26,27]. Results indicated that the neuroprotective effects of WA in AD are mediated by its ability to reduce beta-amyloid plaque aggregation, tau protein accumulation, inhibition of acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) activities, regulation of heat shock proteins, and inhibition of oxidative and inflammatory components. Because there is now no pharmacological therapy that can be efficiently employed for treating AD, the leading hypothesis might be a more recent viewpoint on medication therapy for the disease in the future. Based on the preceding discussion, the present review focuses on the antioxidant, immunomodulatory, anticholinesterase, and anti-inflammatory properties of WA and its derivatives as potential medications for the treatment and prevention of AD [28,29,30].
2. Biosynthesis of Withaferin A (WA)
WA is a steroidal lactone belonging to the withanolide group, which was first isolated from the familiar medicinal plant W. somnifera in 1965 by Lavie. It is formed from naturally occurring withanolides, which are C-28 steroids with an ergostane-based skeleton functionalized o at C-26 and C-22 to form a δ lactone. Furthermore, the ketone and hydroxyl groups functionally oxidize the C-1 group to form a 1-one [31,32]. WA was also biosynthetically isolated from Acnistus breviflorus [33]. Chemoinformatic differentiation sterols, triterpenes, and withanolides indicated that withanolides may have originated from 24-methylene lophenol, lanosterol, campesterol, 24-methylene cholesterol metabolite analogs, or from the triterpene pathway’s upstream intermediates [34]. Although conversion of the 24-methylene cholesterol into withanolides involves a complicated metabolic pathway, which includes glycosylation, hydroxylation, chain elongation, desaturation, cyclization, and epoxidation, that is not fully understood, several researchers have specified the biosynthesis of the responsible gene. However, the triterpenoid pathway occurs by the isoprenogenesis process, which is a biogenetical metabolic course for the determination of isoprene units such as dimethylallyl pyrophosphate and isopentenyl diphosphate [35,36,37,38].
Similarly, isoprenogenesis arises in two separate ways in plants. The first is the classical mevalonate (MVA) pathway, which occurs in the cytosol, and the other is the non-mevalonate pathway, also known as the deoxyxylulose pathway (DOXP) or the newly discovered methyl erythreitol pathway (MEP) in plastids [39,40]. In the MVA route, endophyte inoculation up-regulates the expression of HMGR with the concerned isopentenyl pyrophosphate (IPP), which is the predominant pathway [41]. In this case, the enzymes responsible for gene encoding in the transcription process have no effect on 1-deoxy-d-xylulose-5-phosphate reductase (DXR) and 1-deoxy-d-xylulose-5-phosphate synthase (DXS) and may be the primary reason for the maximum productivity of withanolide components [41]. Biosynthetically, isopentenyl diphosphate (IPP) is a five-carbon precursor, a common compound in both MVA and MEP processes [42]. In the MVA pathway, cholesterol is the pertinent starting point for the biosynthesis of withanolides. Acetyl co-enzyme A obtained from activation of acetate is the first step of biotransformation of cholesterol. Two units of acetyl-CoA then convert to mevalonate through metabolization, and by losing one carbon atom, are metamorphosed into isopentenyl pyrophosphate (IPP). It is worth mentioning that the S-form of mevalonate is inert metabolically, while the R-form can produce terpenes in the living system [42].
In contrast, pyruvate integrates with glyceraldehyde-3-phosphate in the MEP pathway, and the presence of DXS enzyme produces 1-deoxy-d-xylulose-5-phosphate (DOXP). Furthermore, DOXP is converted into 2-methylerythritol-4-phosphate (MEP) in the presence of DXR catalyst and finally turns into IPP [42,43,44]. Head-to-tail condensation of IPP with 3,3-dimethylallyl pyrophosphate (DMAPP) produces geranyl pyrophosphate (GPP). Further, farnesyl pyrophosphate (FPP) is obtained by the condensation of trans-geranyl pyrophosphate with another IPP molecule. Similarly, in a head-to-head manner, condensation of two molecules of farnesyl pyrophosphate catalyzed by the enzyme squalene synthase in the presence of NADPH yields squalene [45,46]. In turn, squalene 2,3-epoxide is formed by atmospheric oxygen through an oxidation process that is catalyzed by NADPH-linked oxide. Then, ring closure leads to the formation of lanosterol, which can be converted into a few skeletons of obtusifoliol to steroidal triterpenoid (Delta 8, 14 sterol).
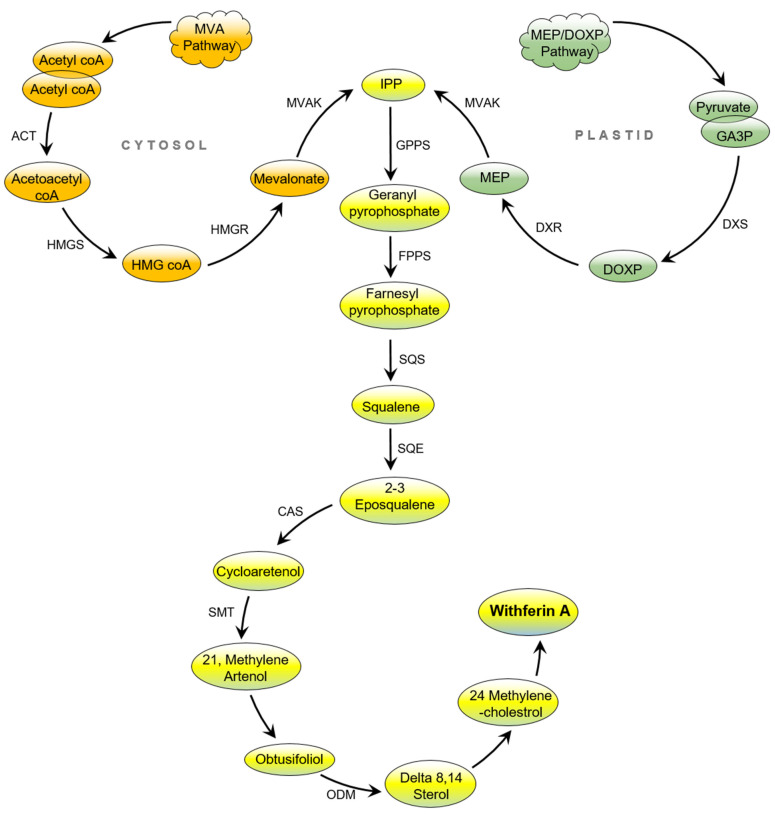
Furthermore, the 24-methylenecholesterol biotransformation from lanosterol is not yet fully understood. However, 24-methylenecholestrol may be a biosynthetic precursor to steroidal lactones. In this regard, it has been suggested that WA forms from δ-lactonization between C22 and C26 of 24-methylenecholestrol and the hydroxylation of C22. It has also been proposed that WA is the α,β-unsaturated ketone in ring A of common withanolides and may be produced through the sequence 20–23 [45]. Three sites are prone to Michael addition alkylation reactions and nucleophilic attacks, namely the unsaturated A-ring at C3, the epoxide structure at C5, and the E-ring at the C24 position [47]. Depicted in Figure 1 is the biosynthesis of WA.
Figure 1.
Biosynthesis of WA. Abbreviations: ACT: acetyltransferase; HMGS: hydroxymethyl glutaryl CoA synthase; HMG-CoA: 3-hydroxy-3-methylglutaryl-co enzyme; HMGR: 3-hydroxy-3-methylglutaryl-coenzyme A reductase; MVAK: mevalonate kinase; IPP: 3-isopentenyl pyrophosphate; GPPS: geranyl pyrophosphate synthase; FPPS: farnesyl diphosphate synthase; SQS: squalene synthase; SQE: squalene epoxidase; CAS: cycloartenol synthase; SMT: sterol methyl transferase; ODM: obtusifoliol-14-demethylase; DOXP: deoxy xylulose pathway; MEP: methyl erythreitol pathway; DXS: 1-deoxy-d-xylulose-5-phosphate synthase; DXR: 1-deoxy-d-xylulose-5-phosphate reductase; WA: withaferin A.
3. Pathogenesis of AD
AD was first mentioned by Alois Alzheimer at a conference in Germany in 1906 as a “distinctive extreme cerebral cortex disease phase.” Recently, AD is believed to be a persistent or progressive condition, marked by diminished cognitive performance beyond what may be considered the result of normal aging, influencing memory, orientation, thinking, awareness, listening, judgment, and vocabulary [48]. Over a hundred years have passed since the very first pathophysiological dimensions of AD were mentioned. Over the last decades, developments in molecular biology have been critical to the interpretation of the molecular pathways behind AD. Therefore, it is widely understood that the molecular pathogenesis of AD is complex and includes a variety of theories or explanations where several different variables interrelate [49,50]. Nevertheless, neither of these postulates alone is capable of clarifying the whole pathology component, and further research is required [2].
Areas including the original factors of the disorder, the abnormal development of Aβ and the pathways through which it disrupts nicotinic acetylcholine receptors and neurons, and the association between cholinergic disorders and AD cognitive impairment are still not well known [48]. In this context, new findings that lead to the explanation and interaction of AD pathogenic mechanisms are important because they may allow the development of new treatment methods of the disease where current drug therapy cannot inhibit its incidence and growth [2]. Neurobiological pathways underpinning AD have been a key factor in pathological interpretation [51]; specifically, the most popular found alteration can be clarified by the cholinergic hypothesis, the amyloid peptides hypothesis, the tau protein hypothesis, the metal imbalance hypothesis, and the presence of oxidative stress (OS) [2,52].
3.1. The Amyloid Hypothesis
John Hardy and David Allsop introduced the amyloid hypothesis in 1991 [4,53]. In this respect, the amyloid β-peptide, which is prevalent in the brain plaques that define Alzheimer’s disease (AD), was decoded from the meningeal blood vessels of AD patients and those with Down syndrome about 20 years ago. The same peptide was shown to be the main component of senile plaques in the brain tissue of an AD patient. These results marked the beginning of the current phase of research into this prevalent, and possibly lethal, neurodegenerative disease [4,53,54]. Amyloid β peptide is a transmembrane protein generated via the amyloidogenic pathway by hydrolysis of the Aβ precursor protein (APP). According to published research, APP generates C-terminal fragments via three routes when hydrolyzed by α-, β-, γ-, and η-secretases [55]. Under normal conditions, the first non-amyloidogenic pathological route results in products that have neurotrophic and neuroprotective properties for nerve cells, such as the soluble ectodomain of APP-α (sAPPα), the C-terminal fragment (CTF)-α, and other smaller fragments, via the participation of α- and γ-secretases. On the other hand, the amyloidogenic pathological pathway is the second route where cleavage of APP by β-secretase results in the formation of CTF-β, whereas cleavage by γ-secretase leads to various lengths of Aβ peptides including Aβ42 [56,57]. In addition, η-secretase regulates the third pathway, which is the alternative processing route under physiological conditions. In this hypothesis, the situation is analyzed as a sequence of anomalies in the development and secretion of the amyloid precursor protein (APP), where the difference between the development and clearance of amyloid β is the starting event and the most important factor responsible for the additional irregularities reported in AD [58]. In this regard, amyloid β is a highly susceptible peptide to proteolytic degradation [59]. Aβ contains 37–43 amino acids, the most common of which are isoforms 1–40 and 1–42 [60,61]. The amyloid peptide isoform 1–42 is the most hydrophobic and has the highest toxicity [1,62]. Due to its physical characteristics, the β-plated sheet is often configured, showing a strong tendency to accumulate and form the amyloid plaque nucleus [58]. It is, therefore, the vital ingredient of neuritic amyloid plaques [2].
3.1.1. Nonamyloidogenic Pathway
APP is a glycoprotein that is produced in the brain and central nervous system as an integral membrane glycoprotein [63]. APP consists of 770 amino acids, where Aβ includes 28 residues just outside the membrane in addition to 14 residues from the APP transmembrane domain [64]. It can be processed sequentially by two proteolytic pathways, namely the non-amyloidogenic and the amyloidogenic route [63]. The non-amyloidogenic route leads α secretase-mediated cleavage of full-length APP. The cleavage removes the sAPP ectodomain from the cell membrane and leaves an 83-amino-acid-long C-terminal APP fragment (α-CTF) inside the plasma membrane. The α-CTF will then be cleaved further by γ-secretase, releasing a tiny p3 fragment into the extracellular area while the rest of APP intracellular domain remains in the cytoplasm [65]. The major α-secretases are ADAM10 and ADAM17, which are the metalloproteases in neurons. A small p3 hydrophobic fragment is generated by α-secretase, which plays a role in normal synaptic signaling and is further sliced at residue 711 by γ-secretase; however, its exact functions still need to be clarified. Endocytosis of cell-surface APP leads to endosome growth of Aβ that contributes to extracellular discharge and accumulation of Aβ [66].
3.1.2. Amyloidogenic Pathway
The amyloidogenic process involves the proteolytic cleavage of APP by β-secretase (BACE1) and the γ-secretase complex in a sequential manner [63,67]. In this regard, BACE1 is a membrane-spanning aspartyl protease [63], whereas γ-secretase is an intramembrane aspartyl protease composed of four proteins: PEN2, nicastrin, presenilin, and anterior pharynx defective 1 (APH-1) complexed together [68,69]. γ-Secretase converts the complex into insoluble and neurotoxic Aβ fragments [70]. The first and rate-limiting step in this process is the β-secretase cleavage [66,71]. β-Cleavage releases the sAPP ectodomain, and a 99 amino acid APP carboxy-terminal fragment (C99) can be cleaved further at numerous sites by γ-secretase. APP cleavage by γ-secretase can result in amyloid peptides with varying chain lengths, such as Aβ37, 38, 39, 40, 42, and 43 [72]. These peptides form Aβ oligomers, whereas aggregated plaques are produced by further polymerization [63]. In this respect, Aβ42 and Aβ40 are the two primary Aβ species found in the brain. Although soluble, Aβ40 is substantially more prevalent than soluble Aβ42; Aβ42 has a greater tendency for aggregation because of the hydrophobicity within its two terminal residues. In addition, Aβ42 is the key element of amyloid plaques, which has been found to be neurotoxic [66,73,74]. As a result, Aβ42 is assumed to be a critical participant in plaque development and AD pathogenesis [75]. Furthermore, it has been demonstrated that the levels of Aβ38, Aβ42, and the Aβ42/Aβ38 ratio in cerebral spinal fluid may be utilized to differentiate AD from other dementias [3,76]. On the other hand, fibrillar Aβ has been demonstrated to enhance cytokine and nitric oxide generation in CD14, TLR2, and TLR4-dependent microglia [77,78]. Stewart et al. discovered yet another signaling mechanism via which Aβ may cause inflammation [79]. These authors discovered that Aβ causes inflammatory signaling by forming heterodimers of Toll-like receptors 4 and 6. Interaction of Aβ with the scavenger receptor CD36 regulates the assembly of this heterodimer. In this context, participation of CD14, CD36, and Toll-like receptors in Aβ-induced microglia activation clearly demonstrates that innate immunity is associated with AD pathogenesis [80,81].
3.2. The Tau (τ) Hypothesis
The amyloid cascade concept has been the prevailing hypothesis in AD research, although it does not fully explain the etiopathogenesis of the disease. According to this hypothesis, the τ protein appears as a secondary pathogenic event that leads to neurodegeneration [2,82]. Tau is a microtubule-associated protein that is generated via the MAPT gene’s alternative splicing [83]. It is mostly present in neuronal axons of the brain where it interacts with microtubules (MTs) [84]. Tau was first separated from plaques in the brains of AD patients by Claude Wischik in 1988 and demonstrated for the first time that it may be the cause of dementia [58,85]. It is a highly soluble protein related to microtubules, and its role is to stabilize microtubules [86]. These microtubules provide support for axonal transport, structural improvement, and neuronal development. In normal circumstances, Tau has a few phosphorylation sites and adversely regulates the attachment of τ to microtubules. However, tau phosphorylation can become saturated under pathological conditions. Development of tau pathology is a complex multifactorial process, and dysfunctions arise in the phosphorylation mechanism of the τ protein in AD, resulting in the enzyme’s hyperphosphorylation. In the brains of AD patients, hyperphosphorylated tau induces configuration alterations and tubulin polymerization capacity reduction [87], culminating in microtubule dysfunction [88]. Increased cytosolic tau levels cause tau–tau interactions and polymerization, resulting in insoluble PHFs and straight filaments (SFs), which form intraneuronal fibrillary deposits called neurofibrillary tangles (NFTs) [87]. In this respect, AD is often distinguished by the inclusion of NFTs and hyperphosphorylation of the microtubule-associated τ proteins that form these tangles [89]. NFTs are fragments of combined, as well as helical, protein filaments in neuron cytoplasm cells [63]. When the τ protein interacts with kinases produced as a result of the increasing amount of Aβ in the system, it is hyperphosphorylated [86]. Due to the separation of the subunits of the tubules, the tubule becomes unstable, breaks apart, and then turns into large parts of the τ filaments, which are further consolidated into NFTs [63]. Such NFTs are fibrillary, smooth, and highly insoluble patches in neuronal cytoplasms and systems, due to abnormal neuron connectivity, signal processing, and, finally, neuron apoptosis [58,90]. Research findings indicated that cleavage and phosphorylation of τ are regulated by soluble Aβ for the generation of NFT [66,71]. In summary, the pathophysiology of pathogenic tau is still clearly unknown due to its intricacy and needs further study [91].
3.3. Oxidative Stress Hypothesis
Both normal and pathological processes in the human body produce reactive oxygen and nitrogen species (ROS and RNS). The brain is more vulnerable to oxidative stress (OS), as it requires 20% more oxygen than other mitochondrial respiratory tissues, so that the intake of oxygen in the brain is high [92]. It has long been understood that the association between neuronal apoptosis and OS is one of the common features of neurodegenerative diseases [93]. ROS interact chemically with biological molecules including lipids and proteins, nucleic acids, and cell organelles [50,94]. Moreover, the known pathology of senile plaques and neurofibrillary tangles in the presence of substantial OS is typical of the AD brain [95]. Accumulation of damaging free radicals and alteration of the action of antioxidant enzymes such as superoxide catalase and dismutase are also found in AD. In AD pathogenesis, OS is an essential factor, but the causes of abnormal free radicals production and the mechanisms by which redox equilibrium is changed are not clearly understood [67,96]. Published research showed that abnormal aggregation of amyloid β can assist the generation of ROS via a process involving activation of NMDA receptors, and that OS can increase the development and aggregation of amyloid β along with the promotion of τ phosphorylation and polymerization, producing a vicious circle that facilitates the development and progression of AD [97].
3.4. The Cholinergic Hypothesis
The cholinergic hypothesis was the first molecular approach to the pathophysiology of AD. This hypothesis states that AD is a primary degenerative process capable of selectively damaging groups of cholinergic neurons in the amygdala, hippocampus, nucleus basalis, frontal cortex, and medial septum regions, which play important roles in attention, awareness, learning, memory, and other mnemonic processes [59]. Furthermore, ROS may be responsible for the formation of Aβ plaques, resulting in a decrease in the production of the cholinergic neurotransmitter acetylcholine (Ach) in the cortical and basal ganglia of the brain. This might alter synaptic communication, which may lead to inflammatory processes, memory loss, and cell death [23]. ACh is immediately hydrolyzed after discharge at the synapse by AChE to prevent the negative consequences of excessive cholinergic stimulation. On the other hand, BuChE is a related enzyme with the same functionality. ACh does not hydrolyze as fast when cholinesterase is blocked, and ACh levels increase [2,98]. As a result, AchE and BuChE inhibitors are potential targets for AD.
Interestingly, apolipoproteins are major players in regulating Aβ pathology. The ApoE gene encodes three alleles, namely ApoE2, ApoE3, and ApoE4It, where ApoE4 is the major genetic risk factor for sporadic AD and is associated with cognitive deficits [99]. The effect of ApoE on AD was studied in the context of the “cholinergic hypothesis” in 1976, by recognizing that cholinergic neurons are not the crucial target of AD. In specific areas of the brain, cholinergic receptor binding decreases with minor to severe AD, which is associated with neuropsychiatric symptoms [59]. In vivo binding of the cholinergic receptor may reveal links to other key changes in the brain connected with aging and AD and may provide a potential target for molecular treatment. In this case, a significant cholinergic neuron loss in the forebrain nucleus leads to a decrease in the acetylcholine-mediated neurotransmission. In addition, drugs that contribute to the regulation of acetylcholine transmitter levels, such as donepezil and cholinesterase inhibitors (ChEIs) have laid the foundation for symptomatic AD therapy for more than 20 years [10].
3.5. Inflammatory Hypothesis
Advances in the field of AD research seem to indicate that the pathogenesis of the illness does not exist only in the neuronal compartment but has a significant interplay with immune systems in the brain [100]. Neuroinflammation in AD is not a passive system triggered with the emergence of senile plaques and neurofibrillary tangles, but rather is due as much to pathogenesis as plaques and tangles themselves [101]. Numerous findings indicating genes encoding immune receptors, such as TREM22 and CD33, linked to AD, highlight the importance of neuroinflammation [102,103]. A critical step in AD is believed to be the ability of microglia to attach to soluble Aβ oligomers and fibrils through cell-surface receptors such as α6β1 integrin, CD36, CD14, SCARA1, CD47, and Toll-like receptors, namely TLR2, TLR4, TLR6, and TLR9 [79,104,105]. Activation of microglia leads to the production of proinflammatory cytokines and chemokines, which cause binding of Aβ with CD36, TLR4, and TLR6 [79,106,107]. In this respect, genetic deletion of these three genes (CD36, TLR4, and TLR6) limits Aβ-induced cytokine production in vitro and blocks the aggregation of amyloid proteins and the activation of inflammasomes [107,108,109]. Following the binding of Aβ to this receptor complex leads to intracellular signaling results in the signal transduction of the nuclear factor-κB (NF-κB) from the cytoplasm to the nucleus [110]. In addition, this leads to the initiation of protein kinase A, response element-binding protein [111,112], which results in the transcription of numerous pro-inflammatory cytokines, cyclooxygenase-2 (COX-2), and NO-synthase [111,113]. Recently, Aβ has been reported to induce NLRP3 inflammasome, high-molecular-weight protein complexes that are involved in several inflammatory pathophysiologies in microglial cells in vitro and in vivo, thus identifying a novel mechanism that could contribute to the development of AD [111,112]. As a result, stimulation of NLRP3 inflammasome by Aβ in microglia is essential for IL-1β maturation and subsequent inflammatory events [114]. Increasing our understanding of the ways that these immune processes affect AD might lead to future therapeutic or preventative approaches.
4. Epidemiology and Clinical Epidemiology
AD is a slowly progressive neurodegenerative disease. In the USA, there were approximately 4.5 million persons with AD in 2000. However, as the age of the population in the next 50 years increases, the number of persons who will have AD will increase by threefold in the next 50 years, and there will be about 13.2 AD cases in 2050 [115]. In this respect, age and gender are the potential risk factor for AD. Statistics showed that AD cases were 66/100,000 per year at age 60–69, 409/100,000 per year at age 70–79, and 1479/100,000 per year age 80 and older in 1960–1975. In 2014, WHO reported that 4000 Alzheimer’s death annually occurred in Iran [115]. The epidemiological study of AD with other risk factors of AD was conducted in five cities of Khuzestan Province in 2016–2018. Results indicated that smoking has a significant role in AD, where 26.9% of Alzheimer’s patients had a history of smoking, 34.8% a history of diabetes, and 26.3% had Alzheimer’s family history [116]. Additionally, research findings showed that women have a higher risk of AD than men. Statistics revealed that AD has a prevalence of approximately 1 percent among those 65 to 69 years of age, and increases 40 to 50 percent among persons 95 years of age and over [117]. Finally, family history is one of the significant risk factors for AD. In this regard, when family history is compared with age in AD patients, about 1% developed AD by age; in contrast, 20% developed their AD from their family [118].
Clinical Epidemiology
In the last 30 years, the criteria for AD have been changed in several circumstances; however, neuropathologic diagnosis has been considered as the gold standard. In this respect, the National Institute on Aging (NIA)-sponsored Alzheimer disease centers (ADCs) collected the accuracy rate of AD from 2005 to 2010. Data revealed that the clinical diagnosis of AD with other dementia varied depending on several criteria, which were conducted by epidemiologic studies, clinical trials, and governmental healthcare analyses [118]. Clinical studies on 1075 Yoruba population members of Ibadan in Iran demonstrated that with increasing levels of cholesterol, APOE-ε4 increased the risk of AD [119]. In another study, the amplitude of AD was investigated depending on age. Results revealed that 10.3% of those over 65 years of age had AD. Additionally, results showed that 3% of those aged 65–74, 18.7% of those aged 75–84, and 47.2% of those over 85 years of age had AD [116].
5. Therapeutic Implications of WA in AD
WA is a secondary metabolite that is used to treat AD. An in vitro study demonstrated that W. somnifera root contains WA, which has a significant effect on H2O2 and Aβ amyloid-induced neurotoxicity [120]. In an AD model, cognitive defects induced by ibotenic acid that was significantly reversed by WA isolated from Ashwagandha root [121]. Oral administration of WA to mice caused a boost of AChE activity with improving muscarinic receptor binding abilities in the cortical area but did not affect the GABA, benzodiazepine, NMDA, or AMPA receptor in the globus pallidus and the lateral septum area of the brain. In contrast, WA reversed cholinergic marker reduction and enhanced binding affinity with the muscarinic receptor, suggesting that WA could be a potential compound for AD therapy [122]. On the other hand, the pro-inflammatory mediator iNOS (a) moderated Aβ nitration that ameliorated peptides inclination to aggregate nitration of Aβ, (b) enhanced the peptide’s propensity to formed seeding core and aggregated Aβ plaques, (c) inhibited iNOS expression and NF-κB signaling pathway, and (d) diminished aggregation of Aβ plaques in the cerebral area and flourished cognitive performance [123,124].
Similarly, WA inhibited NF-κB and iNOS markers in astrocytes [125]. Furthermore, Hsp90 accelerates aberrant accumulation and aggregation of τ protein [126] and induces Aβ toxicity in AD [126]. In addition, WA inhibited Hsp90 [127] and induced Hsp 27 and Hsp70 expressions, and reduced τ aggregation in a mouse model of tauopathies [128,129]. Cholinesterase enzyme develops AD, which was inhibited by WA obtained from the water extract of Ashwagandha [130]. It inhibited nuclear factor NF-κB activation, which is responsible for the expression of oxidation and inflammation gene, thus prevented the accumulation of Aβ. WA also increased the neuro-protective protein heme oxygenase-1, which is beneficial to AD prevention [131,132]. WA additionally enhances memory [133], prevents Aβ production, reconstructs synapses, and regenerates axons [134]. AD is often related to the hyperactivity of microglial cells. In this respect, WA modulated their action to prevent brain tissue damage [135]. In this respect, an in vitro study demonstrated that WA inhibits Aβ production in microglial and SH-SY5Y cells overexpressing amyloid precursor protein (SH-APP cells) [135] and suppresses NF-κB mediated neuro-inflammation [136]. Multidomain protein LRRK2 remains often mutated, which elevates kinase expression to enhance AD, regulated by WA [137,138]. Berghe et al. (2012) demonstrated that the TAR DNA-binding protein-43, a pathological hallmark of AD, is alleviated by WA and boosted autophagic marker LC3BII level in the brain, while it suppressed NF-κB-dependent neuro-inflammation in frontotemporal lobar degeneration (FTLD) in a mice model [27].
5.1. Role of WA and Its Derivatives
WA is a C-28-steroidal lactone withanolide that is found in W. somnifera [139,140]. WA and its derivatives, at the molecular level, extracted from W. somnifera root, exhibit beneficial effects in AD by blocking Aβ production, inhibiting NF-κB activation, preserving synaptic function, decreasing apoptotic cell death, reversing the reduction in cholinergic markers, and improving antioxidant effects via the migration of Nrf2 to the nucleus. Similarly, Nrf2 improves the activation of antioxidant enzymes. It is recommended that the Nrf2 translocation to the nucleus is triggered by WA, where the transcription factor increases the expression of neuroprotective heme oxygenase-1 proteins [141]. The benefits of W. somnifera root constituents, especially WA in neurodegenerative diseases, could be attributed to their anti-inflammation, neuritis-promoting, anti-apoptotic, antioxidant, and anxiolytic activities, and also to their capability to restore energy levels, mitochondrial dysfunction, and increase antioxidant defenses like reduction of glutathione [141,142]. The possible underlying mechanism of the neuroprotective activities of W. somnifera could be controlled through several pathways. In this respect, studies have indicated the neuroprotective effect of WA [143,144], withanolide A [110], and other derivatives along with the synergistic action of multiple compounds that contribute towards the beneficial effect in the pathogenesis of neurodegenerative diseases [21].
5.1.1. WA and Its Derivatives Aβ Plaque Formation Inhibitors
Published research indicated that Aβ deposition in the brain is one of the main factors in neurodegeneration [145]. As a result, amyloid β is one of the therapeutic targets for the development of possible AD treatments [146]. By limiting the production of this peptide, compounds that may aid in their accumulation and aggregation are sought for both the prevention and advancement of AD. However, research findings showed that these strategies have failed to provide therapeutic benefits in large clinical trials for those with mild to severe AD. As a result, molecular species called Aβ oligomers are currently the primary focus, and numerous monoclonal antibodies have provided promising outcomes when directed against these species [147]. Because Aβ oligomers are in equilibrium with both monomeric and aggregated species, earlier medications that effectively eliminated monomeric Aβ or Aβ plaques should have shown therapeutic advantages as well.
Tiwari and coworkers conducted the first study, which showed that WA could protect the brain from the toxic effects of amyloid-beta plaques. These researchers demonstrated that amyloid-beta severely affects neuronal functions and structures, and WA decreased the secretion of amyloid β and induced neurotoxicity in SH-SY5Y cells (SH-APP) in which the amyloid precursor protein (APP)-plasmid was transfected. When SH-APP cells were exposed to neurotoxic HIV-1 Tat and cocaine, the level of Aβ40 rose. Both neurotoxic protein HIV-1 Tat-induced and cocaine-induced Aβ aggregates in SH-APP were significantly reduced by employing WA with a concentration of 2 μM. Additionally, WA diminishes the amount of beading on the cell membrane and the occurrence of cytoplasmic vacuoles, two indices of neurotoxicity. However, further research is required to prove WA effectiveness in vivo [24]. A follow-up investigation examined the role of WA on Aβ formation by using congo red (CR) staining to highlight the cells. Results showed that WA treated SH-APP cell cultures display less staining with the toxic Aβ peptide than dimethyl sulfoxide (DMSO)-treated SH-APP cell cultures. DMSO-treated SH-APP cells expressed larger quantities of Aβ, which is stained red after being CR tagged. As SH-APP cells treated with WA had lower amounts of Aβ, there was less staining in these WA-treated cells [148].
Published work demonstrated that derivatives such as withanolide A increases α-secretase expression and decrease β-secretase expression in cultured normal rat cortical neurons. When APP is treated with ADAM10, production of Aβ is reduced due to alternative production of soluble and non-toxic APPα. As a result, withanolide A enhanced production of soluble APPα in cultured neurons. It was also shown that withanolide A enhances the expression of a significant proteolytic insulin-degrading enzyme (IDE), which is associated with Aβ degradation [4,146,149,150]. These findings indicate that withanolide A may decrease Aβ by enhancing the production of soluble APPα and Aβ. In light of these reports, withanolide A is a significant candidate for a multifunctional Alzheimer’s disease drug [4,146,149,150]. Furthermore, neuronal cell death caused by amyloid plaques was blocked by withanamide. Molecular modeling studies have shown that withanamide A and C attach uniquely to the active beta-amyloid pattern (Aβ 25–35) and block fibril formation [7,151].
5.1.2. WA Acts as Antioxidant in AD
AD etiology often starts with the accumulation of β-amyloid fibrils, accompanied by tau pathology and then neuronal cell death. In this respect, the free radical-triggered harm of the brain tissues is also one of the vital factors considered in this disease. Furthermore, increased levels of Al, Fe, Hg, and Ca decreased polyunsaturated fatty acids, increased oxidation of protein and DNA and lipid peroxidation, and the presence of oxidation products such as carbonyls, malondialdehyde, heme oxygenase-1, and peroxynitrite, which are initiators of free radicals, could trigger AD [146].
In addition, WA was evaluated for antioxidant activity using major free-radical scavenging enzymes such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPX), and NADPH dehydrogenase levels in the frontal cortex and striatum of the rat brain [28]. It exhibits anti-oxidative activity by increasing the levels of various antioxidant enzymes, which reduce the free radicals or stress formed in the affected cells and provide a healthy mitochondrial function. This function is demonstrated in several recent studies by simple testing of treated and untreated mouse models and human cell lines. Treated cells with WA have significantly reduced cellular oxidative damage [152,153]. Accordingly, these findings indicate that W. somnifera extracts, especially WA, reduce oxidative stress and mitochondrial dysfunction, and their beneficial role appears to be primarily based on their antioxidant properties [6]. Withanamides are a subset of these components that have been proven to scavenge free radicals produced at the time of the initiation and progression of AD [7,151].
5.1.3. WA Inhibits AChE and BuChE Activities
The first unsuccessful research that investigated cholinesterase inhibition laid the groundwork for other treatments that were developed based on cholinesterase inhibition [154]. The current treatment of AD comprises drugs such as AChE inhibitors and N-methyl-D-aspartate receptor antagonists [155]. It is important to note that AChE inhibitors only temporarily relieve some of the disease’s cognitive symptoms and do not stop the patient’s cognitive loss. According to a literature assessment, the efficacy of conventional medications is statistically substantial, but clinical relevance is restricted to disease slowing rather than reversal [156]. In addition, adverse effects such as disorientation, falls, dizziness, and fatigue may occur with these medications and should be used only as recommended [156]. The limited clinical effectiveness of existing AChE inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists has sparked a search for therapeutic approaches that target the causes of neurodegeneration that are closer to the source.
Within this context, WA exhibited beneficial properties for the prevention of AD. WA has an important role in AD by reversing the reduction in cholinergic markers such as choline acetyltransferase (ChAT) and acetylcholine [28,150,151]. In an in vitro assay, the AChE and BuChE enzyme activity was blocked by WA [21]. Based on sub-acute toxicity findings, Withania somnifera extract (containing 4.5% of WA) up to a dose of 2000 mg/kg/day did not cause adverse effects when administered orally [157]. These actions of WA could illustrate the well-known cognition enhancement effects due to preferential activity on cholinergic neurotransmission to the brain areas like the basal and cortical forebrain, which is involved in cognitive function [122,153]. It is, however, unknown how this effect occurs, although it may be related to the way cholinergic neurotransmission is controlled. The neurotransmitter systems in the brain were monitored after the administration of an extract containing WA. Findings demonstrated that the extract increases cholinesterase activity in the globus pallidus and lateral septum parts of the brain and enhances muscarinic M1 receptor binding in cortical regions. Additionally, WA increased the level of ACh, the amount of choline acetyltransferase (ChAT), and other cholinergic indicators such as choline acetyltransferase (ChAT) in rats [158]. The preferred influence on cholinergic neurotransmission in the cortical and basal forebrain, brain regions that are important in cognitive function, may be able to explain the reported cognition-enhancing benefits of WA. Another advantage of WA is that it has neither effect on GABAA, NMDA, or glutamate receptor subtypes, nor does it interact with benzodiazepine receptors [159]. WA might have future value in the treatment of AD, but additional research is needed to confirm this assumption [98,158,159].
On the other hand, the neuroprotective potential of WA (50 mg/kg b.w.) revealed a recurrence of homo vanillic acid (HVA) and dopamine (DA) in striatum and substantia nigra. Reduction of these catecholamines results in motor deficits. The neuroprotective potential of WA is also indicated by the elevated level of DA and HVA in the treatment of AD [21]. Within this context, a powerful neurotoxicant, ibotenic acid, has been used to study the effect of brain lesions. When it is injected into the brains of rats, mice, or monkeys, it results in reflective destruction of the basal cholinergic neurons of the forebrain. Recent studies showed that WA and sitoindosides significantly reverse ibotenic acid-induced cognitive impairment in an AD model [15,17,28,152,160,161].
5.1.4. WA Blocks the Neuroinflammation in AD
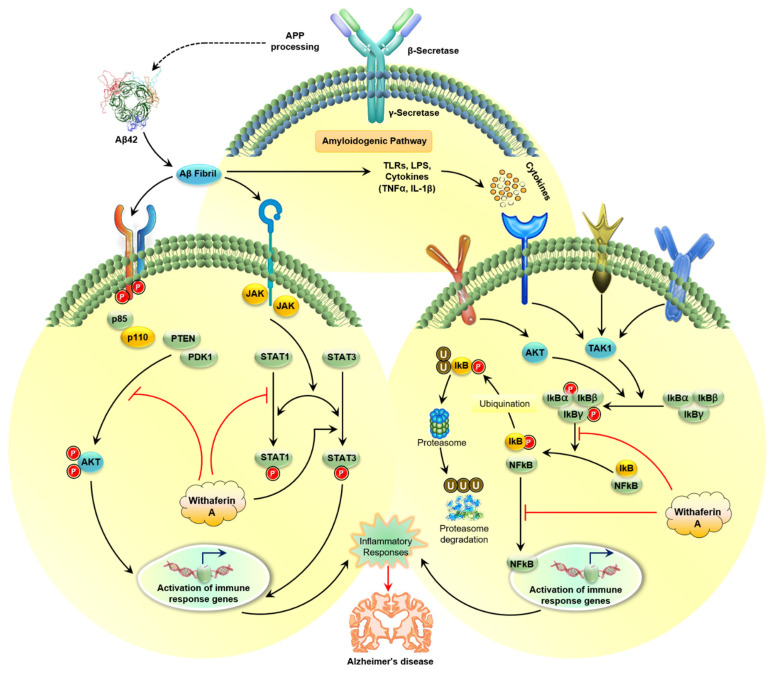
In AD, microglia activation is achieved through the interaction between Aβ fibrils and Aβ oligomers that initiate inflammatory reactions by promoting NLRP3 inflammasome and nuclear factor NF-κB, which is responsible for the release of pro-inflammatory chemokines and cytokines [114]. Microglia regulate Aβ fibrils by the process of phagocytosis, and then neprilysin and insulin-degrading enzymes destroy these fibrils. In AD, the NF-κB pathway and expression of the NLRP3 block the phagocytosis of Aβ fibrils, which results in increasing the buildup of Aβ fibrils in the brain, creating a self-perpetuating loop and finally leads to neuroinflammation [114]. In addition, WA inhibits the expression of NF-κB, which plays an important part in the cascade of inflammatory cytokines. Additionally, reduced expression of STAT and JAK, and increased expression of IKBKG and IKBKB have been observed (Figure 2) [162]. By targeting critical inflammatory pathways like NF-κB and nuclear factor erythroid 2 related factors 2 signaling, WA defeats inflammatory disease in numerous in vitro and preclinical in vivo models of neurodegenerative disorders like AD and PD, cystic fibrosis, and osteoarthritis (Table 1) [20].
Figure 2.
Schematic representation showing the target sites of WA action in amyloidogenic pathway that leads to AD. WA inhibits NF-κB signaling (right side). WA regulates several kinase-signaling pathways such as AKT and JAK/STAT. Abbreviations: APP: amyloid precursor protein; Aβ: β-amyloid; TLR: Toll-like receptor; LPS: lipopolysaccharides; IKK: IκB kinase; TNF-α: tumor necrosis factor-α; IL-1β: interleukin-1β; MAP3K7)/TAK1: mitogen-activated protein kinase 7 (MAP3K7)/(TAK1); NF-κB: nuclear factor kappa B; U: ubiquinone; JAK: Janus kinase; STAT: signal transducers and activators of transcription; PTEN: phosphatase and tensin homolog; PDK1: phosphoinositide-dependent kinase-1; AD: Alzheimer disease; WA: withaferin A.
Table 1.
Preclinical trials of WA.
| Plant Extract | Method | Subject | WA Mediated Protective Effect | Possible Mechanism | Up/Down Regulation | References |
|---|---|---|---|---|---|---|
| Aqueous methanol extract of Withania somnifera roots | In vivo | Mice | Reversed anti-AChE activity | Enhance ACh, choline acetyltransferase; ChAT activity in globus pallidus and lateral septum |

|
[22] |
| Aqueous chloroform extract of Withania somnifera roots | In vivo | Rat | Anti-cholinergic activity | Reduced cholinergic marker activity |

|
[23] |
| Withania somnifera root extract | In vitro | SH-SY5Y cells (SHAPP) | Anti-amyloidogenic | Aβ40 |

|
[24] |
| Withania somnifera root extract | In vitro | SHAPP cells and CHME5 microglial cell line | Anti-inflammatory | NF-κB, JUN and STAT gene, IL-1β |

|
[24,148] |
| Withania somnifera extract | In vitro | SK-N-SH cells | Anti-oxidant and anti-cholinergic | ROS, Ache, Aβ peptide toxicity |

|
[25] |
| Aqueous root extract of Withania somnifera | In vitro | Rat pheochromocytoma (PC12) cell line | Anti-Alzheimer activity | H2O2- and Aβ-induced toxicity |

|
[26] |
| Plant extract of withania somnifera | In vivo | Male Wistar rats | Anti-Alzheimer activity | Reduced acetyl cholinesterase |

|
[27] |
| Plant extract of withania somnifera | In vitro | Amyloid-β marker thioflavin-T | Anti-amyloidogenic | Reduced amyloid beta |

|
[184] |
| WA | In vivo | HFD-induced obese mice | Anti-obesity | COX2, NF-κB, TNF-α, inflammation, oxidative stress, and insulin resistance |

|
[185] |
| WA | In vitro, in vivo | Human umbilical vein endothelial cells (HUVECs), mouse | Anti-inflammatory | Inhibit phorbol-12-myristate-3-acetate (PMA), TNF-a, (IL)-1β, PMA-stimulated phosphorylation of p38, extracellular regulated kinases (ERK)-1/2, and c-Jun N-terminal kinase (JNK) |

|
[186] |
| WA | In vitro | Murine fibrosarcoma | Anti-inflammatory | p38, ERK-1/2, C-Jun (JNK) |

|
[136] |
| WA | In vitro | Cellular models of cystic fibrosis inflammation (KKLEB cells) | Anti-inflammatory | NFk-β and IL-8 |

|
[187] |
| WA | In vitro | Human melanoma cells (M14, Lu1205, and Sk28) | Anti-cancer | ROS-induced apoptosis increased by lowering the Bax/Bcl2 and Bcl2/Bim ratio |

|
[188] |
| WA | In vitro | Breast cancer cells (MDA-MB-231 and MCF-7) | Anti-cancer | Caspase-9 and 3 and PARP |

|
[189] |
| WA | In vitro, in vivo | Breast tumor progression in xenograft and transgenic mouse models | Anti-cancer | ERK/RSK axis, death receptor 5 (DR-5), ETS domain containing protein-1 (Elk1), and CAAT/enhancer-binding protein-homologous protein (CHOP) |

|
[190] |
| WA | In vitro | Human laryngeal carcinoma Hep2 cells | Anti-cancer | Cell cycle arrest with concomitant blockade of angiogenesis |

|
[191] |
| WA | In vitro | Renal cancers (Caki cells) | Anti-cancer | STAT-3 pathway |

|
[192] |
| WA | In vitro | Renal cancers (Caki cells) | Anti-cancer | GRP-78 and CHOP |

|
[193] |
| Extract of Whitania aristata | In vivo | Male albino Sprague-Dawley rats and male and female albino Swiss mice | Diuretic effect | Diuretic activity, excretion of sodium and potassium ions |

|
[194] |
| WA | In vitro | H. pylori-induce bone marrow-derived dendritic cells (BMDCs) | Anti-gastric cancer | NF-κB, IL-1β, NLRP3 |

|
[195] |
| Aqueous root extract of Withania somnifera | In vitro | Nicotine induced conditioned place reference in male albino mice | Anti-addictive | Nicotine efficacy |

|
[196] |
| WA | In vitro | Microglial cells | Anti-inflammatory | STAT1/3, interferon-gamma activated sequence (GAS)-promoter activity |

|
[197] |
| WA | In vitro | Mouse model of FTLD | Neuroprotective | TAR DNA-binding protein-43, NF-κB activity and neuroinflammation |

|
[198] |
| WA | In vivo and ex vivo | TNF-stimulated human umbilical vein-endothelial cells | Anti-coagulant | Plasminogen activator inhibitor type 1 (PAI-1/t), tissue-type plasminogen activator (t-PA) |

|
[21] |
| WA | In vivo | Swiss albino mice | Anti-diabetic | Hyperglycemia | [199] |
WA Inhibits the NF-κB Pathway
In normal and pathophysiological immune responses, the transcription factor NF-κB and its activation signaling pathways play a critical role, making it an interesting therapeutic target. Therefore, WZA has been extensively studied to investigate its effect on the signaling NF-κB activation [163,164,165].
WA decreases inflammatory mediators including TNF-α and IL-1 in plaque formation and neurodegeneration [152,161,166,167]. W. somnifera is anti-inflammatory due to the presence of WA. Research findings indicated that WA effectively prevents the activation of NF-κB by stopping phosphorylation and deterioration by inhibiting stimulation of IκB kinase, while other W. somnifera derived steroidal lactones, like withanolide A, are far less effective [168]. Moreover, WA inhibits NF-κB activation by attacking cysteine 179 located at the IKKβ catalytic site [165]. Furthermore, nitric oxide and COX-2 production are inhibited by WA by inhibiting nitric oxide synthase (iNOS) [152]. WA also inhibits vascular cell adhesion molecule (VCAM)-1 and intracellular adhesion molecule (ICAM)-1, which is induced by TNF-α along with inducible iNOS expression and NO production induced by lipopolysaccharide (LPS) by reducing Akt and NF-κB activation [169]. Moreover, WA inhibits microglial inflammatory response through inhibition of LPS-induced COX-2 mRNA and protein expression and production of prostaglandin E2, one of the most important products of COX-2. As a result, WA controls the microglial inflammatory response by decreasing COX-2 expression and PGE2 construction and blocking LPS-induced STAT1 and STAT3 phosphorylation [170]. There were no significant effects of WA on LPS-induced ERK and Akt phosphorylation, but WA reduced slightly the JNK-pathway and p38 phosphorylation (Figure 2).
Several molecular docking studies also revealed that WA is capable of interfering with the NF-κB production pathway through different mechanisms. Among these mechanisms is in silico analysis, which has shown a solid probable intermolecular interaction between WA and IKKγ that interrupts the development of the IKK complex and inhibits IκB depletion [171]. Another suggested mechanism of WA-mediated inhibition of NF-κB is by straight interaction of WA with NF-κB itself [172] or its IκB inhibitor [20,172]. Thus, it was concluded that pure WA has important NF-κB inhibitor activity, which makes it a novel anti-inflammatory agent for the treatment of AD [163].
WA Affects Inflammasome Activation
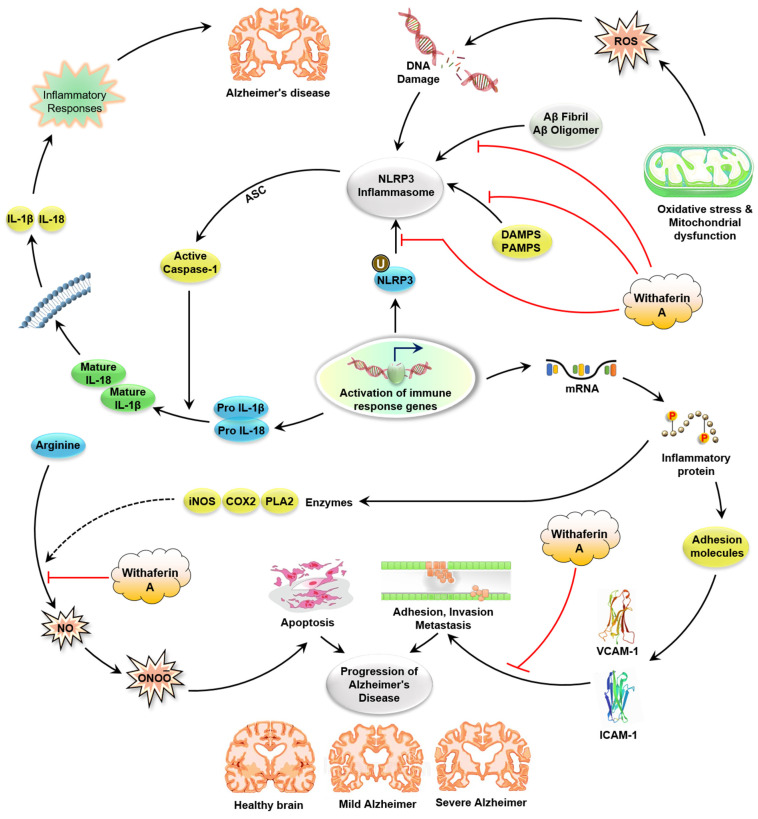
Inflammasomes, a collection of multiprotein complexes, are gathered inside the cytosol after sensing pattern-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) [173]. Once gathered, they function as a scaffold for recruitment of the inactive pro-caspase-1 zymogen that contributes to the oligomerization of the pro-caspase-1 protein and triggers their autoproteolytic cleavage into the bioactive enzyme caspase-1. The corresponding cleavage of pro-IL-18 and pro-IL-1β allows the discharge of such pro-inflammatory cytokines into the extracellular space. In this respect, two main groups of inflammasomes have been recognized based on the presence of their intracellular receptors, AIM2-like receptor inflammasomes (ALR) and Nod-like receptor inflammasomes (NLR) [174]. Numerous studies using inflammatory disease models showed that WA interferes with the development of a crucial subtype of the NLR class inflammasome NLRP3 (Figure 3). Stimulation of the NLRP3 inflammasome is induced by protein aggregations such as β-amyloids, toxins, pathogens, and crystals [175,176,177]. Abnormal activation of NLRP3 has been known to be involved in the initiation and progression of inflammation [178]. Though the precise biological mechanism of WA-mediated blocking of NLRP3 inflammasome development needs further exploration, medicinal use of WA may provide a chance to control cytokine activation in inflammatory diseases [177]. It is essential to know that the impact of WA on other inflammasome subtypes also needs to be examined, since some studies have shown that WA treatment increases the expression and initiation of AIM2 inflammasomes of M2 macrophages, resulting in a pro-inflammatory instead of the anti-inflammatory response in immune cells [20,179].
Figure 3.
Overview of inflammatory signaling pathways altered by WA through direct molecular targets. Fibrillary Aβ, oxidative stress, DAMP, and PAMP can contribute to the activation of the inflammasome. Aβ fibrils trigger the activation of microglial cells and thus give signal 1 via NF-κB transcription of pro IL-1β and NLRP3. Intracellular aggregation of soluble Aβ and lysosomal rupture by phagocytosis Aβ fibrils may perform another signal, and oxidative stress contributes to the formation of an active NLRP3 inflammasome. Active caspase-1, released from active NLRP3, converts IL-1β pro to active IL-1β, which is released into extracellular space and leads to neuroinflammation and finally AD. WA prevents NLRP3 inflammasome formation and activation by blocking several steps of this pathway. Abbreviations: ROS: reactive oxygen species; NLEP3: NOD-like receptor protein 3; DAMP: damage-associated molecular pattern; PAMP: pathogen-associated molecular pattern; COX-2: cyclooxygenase-2; IκB: inhibitory subunit of NF-κB; IL-18: interleukin-18; VCAM-1: vascular cell adhesion molecule 1; ICAM-1: intercellular adhesion molecule 1; AD: Alzheimer disease; WA: withaferin A.
WA Regulates Heat Shock Proteins
WA may control the activity of heat shock proteins (HSPs) that are highly conserved molecular chaperones associated with the transporting, folding, maintenance, and assembly of main regulatory protein kinases [180,181]. In addition, WA controls the target proteins of HSP90 such as AKT and IKKK-complex by attacking and disassociating the CDC37–HSP90 complex, by either preventing the protein cleavage of CDC37 [182] or by straight binding of HSP90 itself. The therapeutic significance of WA in CDC37–HSP90 blockage has already been proven in N9 microglial cells, where one specific target of HSP90 kinase is significantly reduced [182]. On the other hand, LRRK2 is a multi-domain unidentified protein often mutated in patients with sporadic and familial AD. Most mutations in this protein kinase are linked to functional activities such as enhanced protein expression, which is essential for the etiology of inflammatory diseases [20]. Interestingly, a more helpful anti-inflammatory function of WA was proven in TAR–DNA binding protein transgenic mice (TDP43) [183]. Altogether, these data verify that WA has tremendous potential as a natural neurotherapeutic agent for improving cognitive deficits associated with AD. Thus, its application in other neurodegenerative disease models needs to be investigated [21].
6. Structural Modifications of WA for Further Neuroprotective Activity
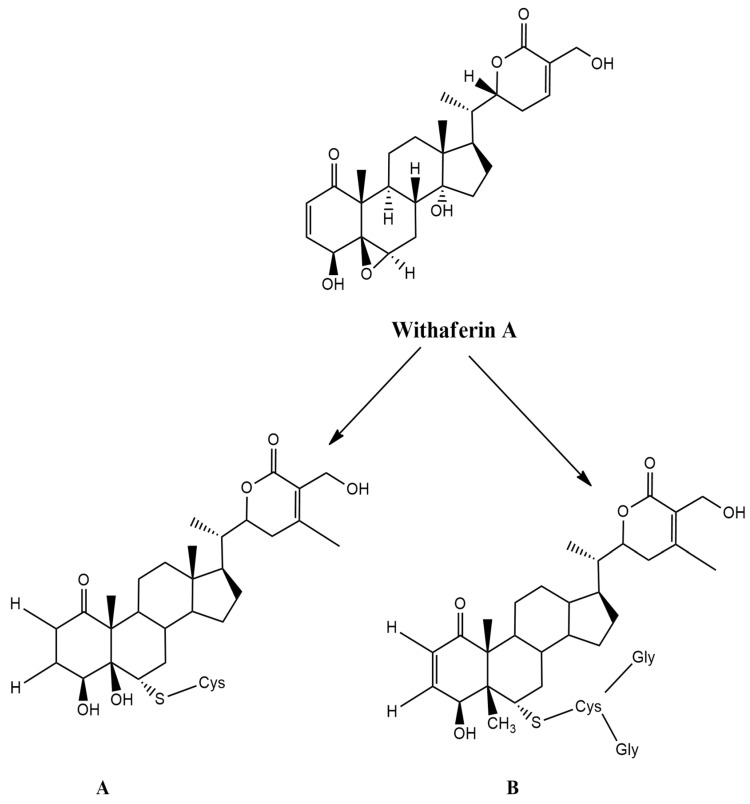
One of the methods that could enhance the pharmacological activity of a bioactive compound is through modification of its structure. In this respect, reverse pharmacology approaches have shown that WA has the highest pharmacological potential as a bioactive substance in W. somnifera [200]. Evaluation of the WA molecular structure shows three positions that could interact with target proteins. Modification of its structure involves nucleophilic site binding and alkylation reactions at C3 of A-ring at place C3 and the epoxide system at C24 (Figure 4). These sites are most vulnerable to nucleophilic attacks and alkylation reactions.
Figure 4.
Metabolites of WA: (A) cysteine conjugate of WA; (B) glutathione conjugate of WA. Here, WA: withaferin A.
Published research revealed that the two WA conjugates, glutathione (CR-777) and cysteine (CR-591), exhibit neuroprotective properties in various neurodegenerative disorders. In this respect, WA glutathione (CR-777) conjugate at a nanomolar dose reversed mesencephalic neuron damage caused by alpha-synuclein (α-Syn), 6-hydroxydopamine (6-OHDA), and 1-methyl-4-phenylpyridinium (MPP+). In addition, the WA glutathione conjugate retains neuritis integrity and decreases the overexpression of α-Syn induced by 6-OHDA. These substances activate the PI3K/mTOR pathway, which reduces oxidative stress and inhibits TAU phosphorylation, aggregation of α-Syn, and caspase 3 expression, demonstrating neuroprotective properties [21].
7. Conclusions and Future Perspectives
Since ancient times, humans have relied on nature as the source of medicine to treat complex diseases, which were managed by primary and secondary metabolites of Ayurvedic, herbal, and medicinal foods. There have been various endeavors to treat AD by reducing Aβ, tau protein level, oxidative stress, and neuroinflammation in the brain. In this context, WA has several specific mechanisms targeting receptors and enzymes in AD. Data collected from this literature review highlight WA as a promising neuroprotective agent to treat AD. It is associated with protein, enzymes, and oxidative and inflammatory markers of AD. Specific markers of AD including amyloid-beta accumulation, tau protein hyperphosphorylation, synaptic changes in the brain, oxidative stress, and inflammatory marker may be treated with WA. Considering the readily available literature pertaining to WA potentiality on AD, future clinical trials should be initiated depending on specific targets.
Structurally, the transformation of WA in nucleophilic sites and alkylation can be speculated as mechanistic insights in neuro-protection. Although in-depth research on several molecular targets of WA has been identified, the pharmacological efficacy of CNS to treat AD has not been yet discovered in a dose-dependent manner. In addition, even though there is an abundance of in vitro and in vivo studies related to the ability of WA as a potent therapeutic agent to alleviate AD, lack of clinical trials is a critical limitation of WA in AD. In particular, WA exerts anti-Alzheimer activity through various mechanisms of action at cellular and molecular levels. WA exhibits antioxidant or free radical scavenging potential associated with AD. One area that is worth mentioning is the encapsulation of WA in nanoparticles, as means to enhance its delivery into the brain, to improve cognitive disorders; this work is in preclinical and clinical trials. Further mechanistic studies of drug delivery and in vivo efficacy are indispensable parts to analyze the therapeutic role of WA in AD. In summary, we have systematically analyzed the therapeutic efficacy of WA in various animal AD models that furnished unbiased evidence for further execution of WA as a promising therapeutic candidate in AD clinical treatment. WA has actions on multiple targets; however, it can cross the BBB through liposomal nanoformulations, which will be a potent therapeutic candidate to combat AD and AD-like diseases. Since several WA analogs can be important in combatting AD, WA and its analogs is an interesting field of research of therapeutic potential for the maintenance of neuronal health and treatment of AD.
Author Contributions
Conceptualization, R.D., A.R., S.A. and T.B.E.; investigation and resources, R.D., S.A., T.B.E., S.M., I.N.K. and M.S.M.; writing—original draft preparation, R.D., A.R., S.A., M.N.I. and T.B.E.; writing—review and editing, A.R., S.M., T.B.E. and M.S.M.; visualization and supervision, M.N.I., T.B.E. and M.S.M.; project administration, A.R., T.B.E., S.M. and M.S.M.; funding acquisition, M.S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li C., Zhao R., Gao K., Wei Z., Yaoyao Yin M., Ting Lau L., Chui D., Cheung Hoi Yu A. Astrocytes: Implications for Neuroinflammatory Pathogenesis of Alzheimers Disease. Curr. Alzheimer Res. 2011;8:67–80. doi: 10.2174/156720511794604543. [DOI] [PubMed] [Google Scholar]
- 2.Sanabria-Castro A., Alvarado-Echeverría I., Monge-Bonilla C. Molecular pathogenesis of alzheimer’s disease: An update. Ann. Neurosci. 2017;24:46–54. doi: 10.1159/000464422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tiwari S., Venkata A., Kaushik A., Adriana Y., Nair M. Alzheimer’s Disease Diagnostics and Therapeutics Market. Int. J. Nanomed. 2019;2019:5541–5554. doi: 10.2147/IJN.S200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan L., Mao C., Hu X., Zhang S., Yang Z., Hu Z., Sun H., Fan Y., Dong Y., Yang J., et al. New Insights Into the Pathogenesis of Alzheimer’s Disease. Front. Neurol. 2020;10:1312. doi: 10.3389/fneur.2019.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo T., Zhang D., Zeng Y., Huang T.Y., Xu H., Zhao Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020;15:40. doi: 10.1186/s13024-020-00391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohd Sairazi N.S., Sirajudeen K.N.S. Natural Products and Their Bioactive Compounds: Neuroprotective Potentials against Neurodegenerative Diseases. Evid. Based Complement. Altern. Med. 2020;2020:6565396. doi: 10.1155/2020/6565396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao R.V., Descamps O., John V., Bredesen D.E. Ayurvedic medicinal plants for Alzheimer’s disease: A review. Alzheimer’s Res. Ther. 2012;4:22. doi: 10.1186/alzrt125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh A.P. The Role of Natural Products in Pharmacotherapy of Alzheimer’s Disease. Ethnobot. Leafl. 2005;2005:46. [Google Scholar]
- 9.Reddy P.H., Beal M.F. Are mitochondria critical in the pathogenesis of Alzheimer’s disease? Brain Res. Rev. 2005;49:618–632. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Kumar Thakur A., Kamboj P., Goswami K., Ahuja K. Pathophysiology and management of alzheimer’s disease: An overview. J. Anal. Pharm. Res. 2018;7:226–235. doi: 10.15406/japlr.2018.07.00230. [DOI] [Google Scholar]
- 11.Maccioni R.B., Rojo L.E., Fernández J.A., Kuljis R.O. The role of neuroimmunomodulation in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2009;1153:240–246. doi: 10.1111/j.1749-6632.2008.03972.x. [DOI] [PubMed] [Google Scholar]
- 12.Brown T.L. Alzheimer’s Disease Prevention and Treatment Using Herbal Agents. J. Anal. Pharm. Res. 2016;2:2–5. doi: 10.15406/japlr.2016.02.00027. [DOI] [Google Scholar]
- 13.Hamill R.W., Pilgrim D.M. Advances in Alzheimer’s disease. Contemp. Intern. Med. 1995;7 doi: 10.1096/fasebj.5.3.2001787. [DOI] [PubMed] [Google Scholar]
- 14.Reddy C.R.M., Raju E.V.N., Reddy M.S., Manjunatha P.M. Role of medicinal plants in the treatment of Alzheimer’s disease: A review. Sch. Acad. J. Pharm. 2013;2:21–26. [Google Scholar]
- 15.Patel K.C., Pramanik S., Patil V.C. Ayurvedic approach with a propective to treat and prevent Alzheimer’s and other cognitive diseases: A review. World J. Pharm. Pharm. Sci. 2014;3:234–252. [Google Scholar]
- 16.Kalra J., Khan A. Reducing Aβ load and tau phosphorylation: Emerging perspective for treating Alzheimer’s disease. Eur. J. Pharmacol. 2015;764:571–581. doi: 10.1016/j.ejphar.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 17.Sahoo A.K., Dandapat J., Dash U.C., Kanhar S. Features and outcomes of drugs for combination therapy as multi-targets strategy to combat Alzheimer’s disease. J. Ethnopharmacol. 2018;215:42–73. doi: 10.1016/j.jep.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Iqbal M.N., Ansarn A.A., Khan K.Z. Approaches for the Treatment of Alzheimer ’ s disease in Unani Medicine: A Review. J. Integr. Community Health. 2017;2012:25–30. [Google Scholar]
- 19.Mucke L. Neuroscience: Alzheimer’s disease. Nature. 2009;461:895–897. doi: 10.1038/461895a. [DOI] [PubMed] [Google Scholar]
- 20.Logie E., Berghe W. Vanden Tackling chronic inflammation with withanolide phytochemicals—A withaferin a perspective. Antioxidants. 2020;9:1107. doi: 10.3390/antiox9111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behl T., Sharma A., Sharma L., Sehgal A., Zengin G., Brata R., Fratila O., Bungau S. Exploring the multifaceted therapeutic potential of withaferin a and its derivatives. Biomedicines. 2020;8:571. doi: 10.3390/biomedicines8120571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uddin M.S., Al Mamun A., Kabir M.T., Jakaria M., Mathew B., Barreto G.E., Ashraf G.M. Nootropic and Anti-Alzheimer’s Actions of Medicinal Plants: Molecular Insight into Therapeutic Potential to Alleviate Alzheimer’s Neuropathology. Mol. Neurobiol. 2019;56:4925–4944. doi: 10.1007/s12035-018-1420-2. [DOI] [PubMed] [Google Scholar]
- 23.Omar F., Tareq A.M., Alqahtani A.M., Dhama K., Sayeed M.A., Emran T.B., Simal-Gandara J. Plant-based Indole Alkaloids: A Comprehensive Overview from a Pharmacological Perspective. Molecules. 2021;26:2297. doi: 10.3390/molecules26082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiwari S., Atluri V.S.R., Yndart Arias A., Jayant R.D., Kaushik A., Geiger J., Nair M.N. Withaferin a Suppresses Beta Amyloid in APP Expressing Cells: Studies for Tat and Cocaine Associated Neurological Dysfunctions. Front. Aging Neurosci. 2018;10:291. doi: 10.3389/fnagi.2018.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh M., Ramassamy C. In vitro screening of neuroprotective activity of Indian medicinal plant Withania somnifera. J. Nutr. Sci. 2017;6 doi: 10.1017/jns.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S., Seal C.J., Howes M.J.R., Kite G.C., Okello E.J. In vitro protective effects of Withania somnifera (L.) Dunal root extract against hydrogen peroxide and β-amyloid(1-42)-induced cytotoxicity in differentiated PC12 cells. Phyther. Res. 2010;24:1567–1574. doi: 10.1002/ptr.3261. [DOI] [PubMed] [Google Scholar]
- 27.Vanden Berghe W., Sabbe L., Kaileh M., Haegeman G., Heyninck K. Molecular insight in the multifunctional activities of Withaferin A. Biochem. Pharmacol. 2012;84:1282–1291. doi: 10.1016/j.bcp.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Das T., Das T., Sloan M., Cancer K., Shad K.F. Potential of glycowithanolides from Withania somnifera (ashwagandha) as therapeutic agents for the treatment of Alzheimer’s disease. World J. Pharm. Res. 2015;4:16–38. [Google Scholar]
- 29.Patel S.B., Rao N.J., Hingorani L.L. Safety assessment of Withania somnifera extract standardized for Withaferin A: Acute and sub-acute toxicity study. J. Ayurveda Integr. Med. 2016;7:30–37. doi: 10.1016/j.jaim.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahaman M.M., Rakib A., Mitra S., Tareq A.T., Emran T.B., Ud-Daula S.A.F.M., Amin M.N., Simal-Gandara J. The Genus Curcuma and Inflammation: Overview of the Pharmacological Perspectives. Plants. 2021;10:63. doi: 10.3390/plants10010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang B.Y., Xia Y.G., Pan J., Liu Y., Wang Q.H., Kuang H.X. Phytochemistry and biosynthesis of δ-lactone withanolides. Phytochem. Rev. 2016;15:771–797. doi: 10.1007/s11101-015-9420-6. [DOI] [Google Scholar]
- 32.Chen L.X., He H., Qiu F. Natural withanolides: An overview. Nat. Prod. Rep. 2011;28:705–740. doi: 10.1039/c0np00045k. [DOI] [PubMed] [Google Scholar]
- 33.Monteagudo E.S., Veleiro A.S., Burton G., Gros E.G. Biosynthesis of Withanolides in Acnistus breviflorus Chemical Degradation of 14C-Labelled Jaborosalactone A and Withaferin A. Z. Naturforsch. Sect. B. 1987;42:1471–1475. doi: 10.1515/znb-1987-1117. [DOI] [Google Scholar]
- 34.Sangwan R.S., Das Chaurasiya N., Lal P., Misra L., Tuli R., Sangwan N.S. Withanolide a is inherently de novo biosynthesized in roots of the medicinal plant Ashwagandha (Withania somnifera) Physiol. Plant. 2008;133:278–287. doi: 10.1111/j.1399-3054.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- 35.Gupta N., Sharma P., Santosh Kumar R.J., Vishwakarma R.K., Khan B.M. Functional characterization and differential expression studies of squalene synthase from Withania somnifera. Mol. Biol. Rep. 2012;39:8803–8812. doi: 10.1007/s11033-012-1743-4. [DOI] [PubMed] [Google Scholar]
- 36.Razdan S., Bhat W.W., Rana S., Dhar N., Lattoo S.K., Dhar R.S., Vishwakarma R.A. Molecular characterization and promoter analysis of squalene epoxidase gene from Withania somnifera (L.) Dunal. Mol. Biol. Rep. 2013;40:905–916. doi: 10.1007/s11033-012-2131-9. [DOI] [PubMed] [Google Scholar]
- 37.Dhar N., Rana S., Razdan S., Bhat W.W., Hussain A., Dhar R.S., Vaishnavi S., Hamid A., Vishwakarma R., Lattoo S.K. Cloning and functional characterization of three branch point oxidosqualene cyclases from Withania somnifera (L.) Dunal. J. Biol. Chem. 2014;289:17249–17267. doi: 10.1074/jbc.M114.571919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel N., Patel P., Kendurkar S.V., Thulasiram H.V., Khan B.M. Overexpression of squalene synthase in Withania somnifera leads to enhanced withanolide biosynthesis. Plant. Cell. Tissue Organ. Cult. 2015;122:409–420. doi: 10.1007/s11240-015-0778-3. [DOI] [Google Scholar]
- 39.Rohmer M. Mevalonate-independent methylerythritol phosphate pathway for isoprenoid biosynthesis. Elucidation and distribution. Pure Appl. Chem. 2003;75:375–387. doi: 10.1351/pac200375020375. [DOI] [Google Scholar]
- 40.Bhat W.W., Lattoo S.K., Razdan S., Dhar N., Rana S., Dhar R.S., Khan S., Vishwakarma R.A. Molecular cloning, bacterial expression and promoter analysis of squalene synthase from Withania somnifera (L.) Dunal. Gene. 2012;499:25–36. doi: 10.1016/j.gene.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Singh S., Pal S., Shanker K., Chanotiya C.S., Gupta M.M., Dwivedi U.N., Shasany A.K. Sterol partitioning by HMGR and DXR for routing intermediates toward withanolide biosynthesis. Physiol. Plant. 2014;152:617–633. doi: 10.1111/ppl.12213. [DOI] [PubMed] [Google Scholar]
- 42.Thirugnanasambantham P., Roy I.M., Charles S.N., Senthil K. Ontogenetic assessment of withanolide biogenesis and expression of selected pathway genes in Withania somnifera, a traditional medicinal herb. J. Pharm. Res. 2014;8:1344–1351. [Google Scholar]
- 43.Senthil K., Wasnik N.G., Kim Y.J., Yang D.C. Generation and analysis of expressed sequence tags from leaf and root of Withania somnifera (Ashwgandha) Mol. Biol. Rep. 2010;37:893–902. doi: 10.1007/s11033-009-9696-y. [DOI] [PubMed] [Google Scholar]
- 44.Pandey S.S., Singh S., Pandey H., Srivastava M., Ray T., Soni S., Pandey A., Shanker K., Babu C.S.V., Banerjee S., et al. Endophytes of Withania somnifera modulate in planta content and the site of withanolide biosynthesis. Sci. Rep. 2018;8:5450. doi: 10.1038/s41598-018-23716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirjalili M.H., Moyano E., Bonfill M., Cusido R.M., Palazón J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules. 2009;14:2373–2393. doi: 10.3390/molecules14072373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yousefian Z., Hosseini B., Rezadoost H., Palazón J., Mirjalili M.H. Production of the anticancer compound withaferin a from genetically transformed hairy root cultures of Withania somnifera. Nat. Prod. Commun. 2018;13:943–948. doi: 10.1177/1934578X1801300806. [DOI] [Google Scholar]
- 47.Kabir M.T., Uddin M.S., Jeandet P., Emran T.B., Mitra S., Albadrani G.M., Sayed A.A., Abdel-Daim M.M., Simal-Gandara J. Anti-Alzheimer’s Molecules Derived from Marine Life: Understanding Molecular Mechanisms and Therapeutic Potential. Mar. Drugs. 2021;19:251. doi: 10.3390/md19050251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holmes C., Cotterell D. Role of Infection in the Pathogenesis of Alzheimer’s Disease. CNS Drugs. 2009;23:993–1002. doi: 10.2165/11310910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 49.Eikelenboom P., Van Exel E., Hoozemans J.J.M., Veerhuis R., Rozemuller A.J.M., Van Gool W.A. Neuroinflammation-An early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegener. Dis. 2010;7:38–41. doi: 10.1159/000283480. [DOI] [PubMed] [Google Scholar]
- 50.Agostinho P., A Cunha R., Oliveira C. Neuroinflammation, Oxidative Stress and the Pathogenesis of Alzheimer ’ s Disease. Curr. Pharm. Des. 2010;16:2766–2778. doi: 10.2174/138161210793176572. [DOI] [PubMed] [Google Scholar]
- 51.Holmes C. Inflammation in Alzheimer’s Disease. Volume 21. Taylor & Francis (CRC Press); Boca Raton, FL, USA: 2017. [Google Scholar]
- 52.Chakrabarti S., Khemka V.K., Banerjee A., Chatterjee G., Ganguly A., Biswas A. Metabolic risk factors of sporadic Alzheimer’s disease: Implications in the pathology, pathogenesis and treatment. Aging Dis. 2015;6:282–299. doi: 10.14336/AD.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mannangatti P., Naidu K.N. Indian herbs for the treatment of neurodegenerative disease. Adv. Neurobiol. 2016;12:323–336. doi: 10.1007/978-3-319-28383-8_17. [DOI] [PubMed] [Google Scholar]
- 54.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 55.Uddin M.Z., Rana M.S., Hossain S., Dutta E., Ferdous S., Dutta M., Emran T.B. In vivo neuroprotective, antinociceptive, anti-inflammatory potential in Swiss albino mice and in vitro antioxidant and clot lysis activities of fractionated Holigarna longifolia Roxb. bark extract. J. Complement. Integr. Med. 2019;17:1–10. doi: 10.1515/jcim-2019-0102. [DOI] [PubMed] [Google Scholar]
- 56.Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T.D., Hardy J., Hutton M., Kukull W., et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat. Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 57.Di Carlo M., Giacomazza D., San Biagio P.L. Alzheimers disease: Biological aspects, therapeutic perspectives and diagnostic tools. J. Phys. Condens. Matter. 2012;24 doi: 10.1088/0953-8984/24/24/244102. [DOI] [PubMed] [Google Scholar]
- 58.Sajjad R., Arif R., Shah A.A., Manzoor I., Mustafa G. Pathogenesis of Alzheimer’s disease: Role of amyloid-β and hyperphosphorylated tau protein. Indian J. Pharm. Sci. 2018;80:581–591. doi: 10.4172/pharmaceutical-sciences.1000397. [DOI] [Google Scholar]
- 59.Parihar M.S., Hemnani T. Alzheimer’s disease pathogenesis and therapeutic interventions. J. Clin. Neurosci. 2004;11:456–467. doi: 10.1016/j.jocn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Carr D.B., Goate A., Phil D., Morris J.C. Current concepts in the pathogenesis of Alzheimer’s disease. Am. J. Med. 1997;103:3S–10S. doi: 10.1016/S0002-9343(97)00262-3. [DOI] [PubMed] [Google Scholar]
- 61.Morgan B.P. Complement in the pathogenesis of Alzheimer’s disease. Semin. Immunopathol. 2018;40:113–124. doi: 10.1007/s00281-017-0662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osborn L.M., Kamphuis W., Wadman W.J., Hol E.M. Astrogliosis: An integral player in the pathogenesis of Alzheimer’s disease. Prog. Neurobiol. 2016;144:121–141. doi: 10.1016/j.pneurobio.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Suzhen D., Yale D., Feng G., Yinghe H., Zheng Z. Advances in the pathogenesis of Alzheimer’s disease: A re-evaluation of amyloid cascade hypothesis. Transl. Neurodegener. 2012;1:18. doi: 10.1186/2047-9158-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar A., Singh A. Ekavali A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015;67:195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y.W., Thompson R., Zhang H., Xu H. APP processing in Alzheimer’s disease. Mol. Brain. 2011;4:3. doi: 10.1186/1756-6606-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yesmin S., Paul A., Naz T., Rahman A.B.M.A., Akhter S.F., Wahed M.I.I., Emran T.B., Siddiqui S.A. Membrane stabilization as a mechanism of the anti-inflammatory activity of ethanolic root extract of Choi (Piper chaba) Clin. Phytosci. 2020;6:59. doi: 10.1186/s40816-020-00207-7. [DOI] [Google Scholar]
- 67.Polis B., Samson A.O. A New Perspective on Alzheimer’s Disease as a Brain Expression of a Complex Metabolic Disorder. Alzheimer’s Dis. 2019:1–22. doi: 10.15586/alzheimersdisease.2019.ch1. [DOI] [PubMed] [Google Scholar]
- 68.Kim D.H., Yeo S.H., Park J.M., Choi J.Y., Lee T.H., Park S.Y., Ock M.S., Eo J., Kim H.S., Cha H.J. Genetic markers for diagnosis and pathogenesis of Alzheimer’s disease. Gene. 2014;545:185–193. doi: 10.1016/j.gene.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 69.Hutton M., Hardy J. The presenilins and Alzheimer’s disease. Hum. Mol. Genet. 1997;6:1639–1646. doi: 10.1093/hmg/6.10.1639. [DOI] [PubMed] [Google Scholar]
- 70.Roberts G.W., Gentleman S.M., Lynch A., Murray L., Landon M., Graham D.I. β3 Amyloid protein deposition in the brain after severe head injury: Implications for the pathogenesis of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 1994;57:419–425. doi: 10.1136/jnnp.57.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jarrett J.T., Berger E.P., Lansbury P.T. The Carboxy Terminus of the β Amyloid Protein Is Critical for the Seeding of Amyloid Formation: Implications for the Pathogenesis of Alzheimer’s Disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 72.Bibl M., Mollenhauer B., Lewczuk P., Esselmann H., Wolf S., Trenkwalder C., Otto M., Stiens G., Rüther E., Kornhuber J., et al. Validation of amyloid-β peptides in CSF diagnosis of neurodegenerative dementias. Mol. Psychiatry. 2007;12:671–680. doi: 10.1038/sj.mp.4001967. [DOI] [PubMed] [Google Scholar]
- 73.Iwatsubo T., Odaka A., Suzuki N., Mizusawa H., Nukina N., Ihara Y. Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: Evidence that an initially deposited species is Aβ42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 74.Chakraborty A.J., Mitra S., Tallei T.E., Tareq A.M., Nainu F., Cicia D., Dhama K., Simal-Gandara J., Emran T.B., Capasso R. Bromelain a Potential Bioactive Compound: A Comprehensive Overview from a Pharmacological Perspective. Life. 2021;11:317. doi: 10.3390/life11040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jan A., Gokce O., Luthi-Carter R., Lashuel H.A. The ratio of monomeric to aggregated forms of Aβ40 and Aβ42 is an important determinant of amyloid-β aggregation, fibrillogenesis, and toxicity. J. Biol. Chem. 2008;283:28176–28189. doi: 10.1074/jbc.M803159200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang W., Huang Q., Wang Y., Wang Z.Y., Yao Y.Y. Assessment of CSF Aβ42 as an aid to discriminating Alzheimer’s disease from other dementias and mild cognitive impairment: A meta-analysis of 50 studies. J. Neurol. Sci. 2014;345:26–36. doi: 10.1016/j.jns.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 77.Jana M., Palencia C.A., Pahan K. Fibrillar Amyloid-β Peptides Activate Microglia via TLR2: Implications for Alzheimer’s Disease. J. Immunol. 2008;181:7254–7262. doi: 10.4049/jimmunol.181.10.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reed-Geaghan E.G., Savage J.C., Hise A.G., Landreth G.E. CD14 and toll-like receptors 2 and 4 are required for fibrillar Aβ-stimulated microglial activation. J. Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stewart C.R., Stuart L.M., Wilkinson K., Van Gils J.M., Deng J., Halle A., Rayner K.J., Boyer L., Zhong R., Frazier W.A., et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jimenez S., Baglietto-Vargas D., Caballero C., Moreno-Gonzalez I., Torres M., Sanchez-Varo R., Ruano D., Vizuete M., Gutierrez A., Vitorica J. Inflammatory response in the hippocampus of PS1M146L/APP 751SL mouse model of Alzheimer’s disease: Age-dependent switch in the microglial phenotype from alternative to classic. J. Neurosci. 2008;28:11650–11661. doi: 10.1523/JNEUROSCI.3024-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heneka M.T., O’Banion M.K., Terwel D., Kummer M.P. Neuroinflammatory processes in Alzheimer’s disease. J. Neural Transm. 2010;117:919–947. doi: 10.1007/s00702-010-0438-z. [DOI] [PubMed] [Google Scholar]
- 82.Wang H., Lin S., Allen J.P., Williams J.C., Blankert S., Laser C., Woodbury N.W. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 83.Neve R.L., Harris P., Kosik K.S., Kurnit D.M., Donlon T.A. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Mol. Brain Res. 1986;1:271–280. doi: 10.1016/0169-328X(86)90033-1. [DOI] [PubMed] [Google Scholar]
- 84.Rakib A., Ahmed S., Islam M.A., Haye A., Uddin S.N., Uddin M.M.N., Hossain M.K., Paul A., Emran T.B. Antipyretic and hepatoprotective potential of Tinospora crispa and investigation of possible lead compounds through in silico approaches. Food Sci. Nutr. 2020;8:547–556. doi: 10.1002/fsn3.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wischik C.M., Novak M., Edwards P.C., Klug A., Tichelaar W., Crowther R.A. Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1988;85:4884–4888. doi: 10.1073/pnas.85.13.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duan Y., Dong S., Gu F., Hu Y., Zhao Z. Advances in the Pathogenesis of Alzheimer ’ s Disease: Focusing on Tau-Mediated Neurodegeneration. Transl. Neurodegener. 2012;1:24. doi: 10.1186/2047-9158-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grundke-Iqbal I., Iqbal K., Tung Y.C., Quinlan M., Wisniewski H.M., Binder L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jara C., Aránguiz A., Cerpa W., Tapia-Rojas C., Quintanilla R.A. Genetic ablation of tau improves mitochondrial function and cognitive abilities in the hippocampus. Redox Biol. 2018;18:279–294. doi: 10.1016/j.redox.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Braak H., Alafuzoff I., Arzberger T., Kretzschmar H., Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mocanu M.M., Nissen A., Eckermann K., Khlistunova I., Biernat J., Drexler D., Petrova O., Schönig K., Bujard H., Mandelkow E., et al. The potential for β-structure in the repeat domain of tau protein determines aggregation, synaptic decay, neuronal loss, and coassembly with endogenous Tau in inducible mouse models of tauopathy. J. Neurosci. 2008;28:737–748. doi: 10.1523/JNEUROSCI.2824-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mitra S., Rauf A., Tareq A.M., Jahan S., Emran T.B., Shahriar T.G., Dhama K., Alhumaydhi F.A., Aljohani A.S.M., Rebezov M., et al. Potential health benefits of carotenoid lutein: An updated reviews. Food Chem. Toxicol. 2021;154:112328. doi: 10.1016/j.fct.2021.112328. [DOI] [PubMed] [Google Scholar]
- 92.Wang X., Su B., Zheng L., Perry G., Smith M.A., Zhu X. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2009;109:153–159. doi: 10.1111/j.1471-4159.2009.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Axelsen P.H., Komatsu H., Murray I.V.J. Oxidative stress and cell membranes in the pathogenesis of Alzheimer’s disease. Physiology. 2011;26:54–69. doi: 10.1152/physiol.00024.2010. [DOI] [PubMed] [Google Scholar]
- 94.Bezprozvanny I., Mattson M.P. Neuronal Calcium Mishandling and the Pathogenesis of Alzheimer’s Disease. Trends Neurosci. 2009;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eskici G., Axelsen P.H. Copper and oxidative stress in the pathogenesis of Alzheimer’s disease. Biochemistry. 2012;51:6289–6311. doi: 10.1021/bi3006169. [DOI] [PubMed] [Google Scholar]
- 96.Sharma A., Kumar Y. Nature’s Derivative(s) as Alternative Anti-Alzheimer’s Disease Treatments. J. Alzheimer’s Dis. Rep. 2019;3:279–297. doi: 10.3233/ADR-190137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iova A., Micle O., Vicaş L., Micle L., Iova S., Mureşan M., Ioniţǎ C.A. Oxidative stress in Alzheimer’s dementia. Farmacia. 2014;62:538–546. [Google Scholar]
- 98.Houghton P.J., Howes M.J. Natural products and derivatives affecting neurotransmission relevant to Alzheimer’s and Parkinson’s disease. Neurosignals. 2005;14:6–22. doi: 10.1159/000085382. [DOI] [PubMed] [Google Scholar]
- 99.Guerreiro R., Wojtas A., Bras J., Carrasquillo M., Rogaeva E., Majounie E., Cruchaga C., Sassi C., Kauwe J.S.K., Younkin S., et al. TREM2 Variants in Alzheimer’s Disease. N. Engl. J. Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heneka M.T. Neuroinfl ammation in Alzheimer’s disease Michael. Lancet. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [Google Scholar]
- 101.Tran L., Melquist S., Fluder E., Zhu J., Gudnason V., Stone D.J., Zhang B., Lamb J.R., MacDonald M.E., Narayanan M., et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153:707–720. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bradshaw E.M., Chibnik L.B., Keenan B.T., Ottoboni L., Raj T., Tang A., Rosenkrantz L.L., Imboywa S., Lee M., Von Korff A., et al. CD33 Alzheimer’s disease locus: Altered monocyte function and amyloid biology. Nat. Neurosci. 2013;16:848–850. doi: 10.1038/nn.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Griciuc A., Serrano-Pozo A., Parrado A.R., Lesinski A.N., Asselin C.N., Mullin K., Hooli B., Choi S.H., Hyman B.T., Tanzi R.E. Alzheimer’s disease risk gene cd33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bamberger M.E., Harris M.E., McDonald D.R., Husemann J., Landreth G.E. A cell surface receptor complex for fibrillar β-amyloid mediates microglial activation. J. Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Banu N., Alam N., Islam M.N., Islam S., Sakib S.A., Hanif N.B., Chowdhury M.R., Tareq A.M., Chowdhury K.H., Jahan S., et al. Insightful Valorization of the Biological Activities of Pani Heloch Leaves through Experimental and Computer-Aided Mechanisms. Molecules. 2020;25:5153. doi: 10.3390/molecules25215153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.El Khoury J.B., Moore K.J., Means T.K., Leung J., Terada K., Toft M., Freeman M.W., Luster A.D. CD36 mediates the innate host response to β-amyloid. J. Exp. Med. 2003;197:1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jyoti M.A., Barua N., Hossain M.S., Hoque M., Bristy T.A., Mahmud S., Kamruzzaman, Adnan M., Chy M.N.U., Paul A., et al. Unravelling the biological activities of the Byttneria pilosa leaves using experimental and computational approaches. Molecules. 2020;25:4737. doi: 10.3390/molecules25204737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sheedy F.J., Grebe A., Rayner K.J., Kalantari P., Ramkhelawon B., Carpenter S.B., Becker C.E., Ediriweera H.N., Mullick A.E., Golenbock D.T., et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., et al. Inflammation and Alzheimer ’s Disease-Neuroinflammation Working Group. Volume 21. Frontiers Media SA; Lausanne, Switzerland: 2000. [Google Scholar]
- 110.Cunningham C. Microglia and neurodegeneration: The role of systemic inflammation. Glia. 2013;61:71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- 111.Gold M., El Khoury J. β-amyloid, microglia, and the inflammasome in Alzheimer’s disease. Semin. Immunopathol. 2015;37:607–611. doi: 10.1007/s00281-015-0518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cho S., Kim Y., Cruz M.O., Park E.M., Chu C.K., Song G.Y., Joh T.H. Repression of proinflammatory cytokine and inducible nitric oxide synthase (NOS2) gene expression in activated microglia by N-acetyl-O-methyldopamine: Protein kinase A-Dependent mechanism. Glia. 2001;33:324–333. doi: 10.1002/1098-1136(20010315)33:4<324::AID-GLIA1031>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 113.Yates S.L., Burgess L.H., Kocsis-Angle J., Antal J.M., Dority M.D., Embury P.B., Piotrkowski A.M., Brunden K.R. Amyloid β and amylin fibrils induce increases in proinflammatory cytokine and chemokine production by THP-1 cells and murine microglia. J. Neurochem. 2000;74:1017–1025. doi: 10.1046/j.1471-4159.2000.0741017.x. [DOI] [PubMed] [Google Scholar]
- 114.Heneka M.T., Kummer M.P., Stutz A., Delekate A., Saecker A., Griep A., Axt D., Remus A., Tzeng T., Gelpi E., et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hebert L.E., Scherr P.A., Bienias J.L., Bennett D.A., Evans D.A. Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Arch. Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 116.Madmoli M., Kord Z., Bandani A., Sedighi N., Shandiz M.R., Darabiyan P., AfsharNia A. Epidemiological and clinical study of patients with Alzheimer’s in Five Cities of Khuzestan Province in 2016–2018. Med. Sci. 2019;23:1–5. [Google Scholar]
- 117.Nussbaum R.L., Ellis C.E. Alzheimer’s Disease and Parkinson’s Disease. N. Engl. J. Med. 2003;348:2588. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- 118.Wang X.P., Ding H.L. Alzheimer’s disease: Epidemiology, genetics, and beyond. Neurosci. Bull. 2008;24:105–109. doi: 10.1007/s12264-008-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hall K., Murrell J., Ogunniyi A., Deeg M., Baiyewu O., Gao S., Gureje O., Dickens J., Evans R., Smith-Gamble V., et al. Cholesterol, APOE genotype, and Alzheimer disease: An epidemiologic study of Nigerian Yoruba. Neurology. 2006;66:223–227. doi: 10.1212/01.wnl.0000194507.39504.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barua N., Aziz M.A.I., Tareq A.M., Sayeed M.A., Alam N., ul Alam N., Uddin M.A., Lyzu C., Emran T.B. In vivo and in vitro evaluation of pharmacological activities of Adenia trilobata (Roxb.) Biochem. Biophys. Rep. 2020;23:100772. doi: 10.1016/j.bbrep.2020.100772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Singh N., Bhalla M., De Jager P., Gilca M. An Overview on Ashwagandha: A Rasayana (Rejuvenator) of Ayurveda. Afr. J. Tradit. Complement. Altern. Med. 2011;8 doi: 10.4314/ajtcam.v8i5S.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Al Mahmud Z., Emran T.B., Qais N., Bachar S.C., Sarker M., Uddin M.M.N. Evaluation of analgesic, anti-inflammatory, thrombolytic and hepatoprotective activities of roots of Premna esculenta (Roxb) J Basic Clin. Physiol. Pharmacol. 2016;27:63–70. doi: 10.1515/jbcpp-2015-0056. [DOI] [PubMed] [Google Scholar]
- 123.McGeer E.G., McGeer P.L. Innate Immunity in Alzheimer’s Disease. Mol. Interv. 2001;1:22–29. [PubMed] [Google Scholar]
- 124.Hoshino T., Murao N., Namba T., Takehara M., Adachi H., Katsuno M., Sobue G., Matsushima T., Suzuki T., Mizushima T. Suppression of Alzheimer’s disease-related phenotypes by expression of heat shock protein 70 in mice. J. Neurosci. 2011;31:5225–5234. doi: 10.1523/JNEUROSCI.5478-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martorana F., Guidotti G., Brambilla L., Rossi D. Withaferin A Inhibits Nuclear Factor- B-Dependent Pro-Inflammatory and Stress Response Pathways in the Astrocytes. Neural Plast. 2015;2015 doi: 10.1155/2015/381964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Blair L.J., Nordhues B.A., Hill S.E., Scaglione K.M., O’Leary J.C., Fontaine S.N., Breydo L., Zhang B., Li P., Wang L., et al. Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J. Clin. Investig. 2013;123:4158–4169. doi: 10.1172/JCI69003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ansar S., Burlison J.A., Hadden M.K., Yu X.M., Desino K.E., Bean J., Neckers L., Audus K.L., Michaelis M.L., Blagg B.S.J. A non-toxic Hsp90 inhibitor protects neurons from Aβ-induced toxicity. Bioorg. Med. Chem. Lett. 2007;17:1984–1990. doi: 10.1016/j.bmcl.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 128.Sinadinos C., Quraishe S., Sealey M., Samson P.B., Mudher A., Wyttenbach A. Low endogenous and chemical induced heat shock protein induction in a 0N3Rtau-Expressing drosophila larval model of Alzheimer’s disease. J. Alzheimer’s Dis. 2013;33:1117–1133. doi: 10.3233/JAD-2012-121534. [DOI] [PubMed] [Google Scholar]
- 129.Blair L.J., Sabbagh J.J., Dickey C.A. Targeting Hsp90 and its co-chaperones to treat Alzheimer’s disease. Expert Opin. Ther. Targets. 2014;18:1219–1232. doi: 10.1517/14728222.2014.943185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nile S.H., Nile A., Gansukh E., Baskar V., Kai G. Subcritical water extraction of withanosides and withanolides from ashwagandha (Withania somnifera L.) and their biological activities. Food Chem. Toxicol. 2019;132 doi: 10.1016/j.fct.2019.110659. [DOI] [PubMed] [Google Scholar]
- 131.Sandhir R., Sood A. Neuroprotective potential of Withania somnifera (ashwagandha) in neurological conditions. Sci. Ashwagandha Prev. Ther. Potentials. 2017:373–387. doi: 10.1007/978-3-319-59192-6_18. [DOI] [Google Scholar]
- 132.Sun G.Y., Li R., Cui J., Hannink M., Gu Z., Fritsche K.L., Lubahn D.B., Simonyi A. Withania somnifera and Its Withanolides Attenuate Oxidative and Inflammatory Responses and Up-Regulate Antioxidant Responses in BV-2 Microglial Cells. Neuromol. Med. 2016;18:241–252. doi: 10.1007/s12017-016-8411-0. [DOI] [PubMed] [Google Scholar]
- 133.Ahmer S., Khan S.A. Alzheimer’s disease in perspective of Unani system of medicine. Int. Hum. Resour. J. 2015;3:2347–7067. [Google Scholar]
- 134.Tareq A.M., Farhad S., Uddin A.N., Hoque M., Nasrin M.S., Uddin M.M.R., Hasan M., Sultana A., Munira M.S., Lyzu C. Chemical profiles, pharmacological properties, and in silico studies provide new insights on Cycas pectinata. Heliyon. 2020;6:e04061. doi: 10.1016/j.heliyon.2020.e04061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ahmed S., Rakib A., Islam M.A., Khanam B.H., Faiz F.B., Paul A., Chy M.N.U., Bhuiya N.M.A., Uddin M.M.N., Ullah S.A. In vivo and in vitro pharmacological activities of Tacca integrifolia rhizome and investigation of possible lead compounds against breast cancer through in silico approaches. Clin. Phytosci. 2019;5:36. doi: 10.1186/s40816-019-0127-x. [DOI] [Google Scholar]
- 136.Heyninck K., Lahtela-Kakkonen M., Van Der Veken P., Haegeman G., Berghe W. Vanden Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKβ. Biochem. Pharmacol. 2014;91:501–509. doi: 10.1016/j.bcp.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 137.Wallings R.L., Tansey M.G. LRRK2 regulation of immune-pathways and inflammatory disease. Biochem. Soc. Trans. 2019;47:1581–1595. doi: 10.1042/BST20180463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Narayan M., Zhang J., Braswell K., Gibson C., Zitnyar A., Lee D., Varghese-Gupta S., Jinwal U. Withaferin A Regulates LRRK2 Levels by Interfering with the Hsp90- Cdc37 Chaperone Complex. Curr. Aging Sci. 2015;8:259–265. doi: 10.2174/1874609808666150520111109. [DOI] [PubMed] [Google Scholar]
- 139.Al Mahmud Z., Qais N., Bachar S.C., Hasan C.M., Emran T.B., Uddin M.M.N. Phytochemical investigations and antioxidant potential of leaf of Leea macrophylla (Roxb.) BMC Res. Notes. 2017;10:245. doi: 10.1186/s13104-017-2503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dembitsky V.M., Dzhemileva L., Gloriozova T., D’yakonov V. Natural and synthetic drugs used for the treatment of the dementia. Biochem. Biophys. Res. Commun. 2020;524:772–783. doi: 10.1016/j.bbrc.2020.01.123. [DOI] [PubMed] [Google Scholar]
- 141.Farooqui A.A., Farooqui T., Madan A., Ong J.H.J., Ong W.Y. Ayurvedic Medicine for the Treatment of Dementia: Mechanistic Aspects. Evid. Based Complement. Altern. Med. 2018;2018 doi: 10.1155/2018/2481076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dutta T., Paul A., Majumder M., Sultan R.A., Emran T.B. Pharmacological evidence for the use of Cissus assamica as a medicinal plant in the management of pain and pyrexia. Biochem. Biophys. Rep. 2020;21:100715. doi: 10.1016/j.bbrep.2019.100715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Batumalaie K., Amin M.A., Murugan D.D., Sattar M.Z.A., Abdullah N.A. Withaferin A protects against palmitic acid-induced endothelial insulin resistance and dysfunction through suppression of oxidative stress and inflammation. Sci. Rep. 2016;6:27236. doi: 10.1038/srep27236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Palliyaguru D.L., Chartoumpekis D.V., Wakabayashi N., Skoko J.J., Yagishita Y., Singh S.V., Kensler T.W. Withaferin A induces Nrf2-dependent protection against liver injury: Role of Keap1-independent mechanisms. Free Radic. Biol. Med. 2016;101:116–128. doi: 10.1016/j.freeradbiomed.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Emran T.B., Rahman M.A., Uddin M.M.N., Rahman M.M., Uddin M.Z., Dash R., Layzu C. Effects of organic extracts and their different fractions of five Bangladeshi plants on in vitro thrombolysis. BMC Complement. Altern. Med. 2015;15:128. doi: 10.1186/s12906-015-0643-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jang M.H., Piao X.L., Kim J.M., Kwon S.W., Park J.H. Inhibition of cholinesterase and amyloid-&bgr; aggregation by resveratrol oligomers from Vitis amurensis. Phyther. Res. 2008;22:544–549. doi: 10.1002/ptr. [DOI] [PubMed] [Google Scholar]
- 147.Panza F., Lozupone M., Logroscino G., Imbimbo B.P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019;15:73–88. doi: 10.1038/s41582-018-0116-6. [DOI] [PubMed] [Google Scholar]
- 148.Atluri V.S.R., Tiwari S., Rodriguez M., Kaushik A., Yndart A., Kolishetti N., Yatham M., Nair M. Inhibition of Amyloid-Beta Production, Associated Neuroinflammation, and Histone Deacetylase 2-Mediated Epigenetic Modifications Prevent Neuropathology in Alzheimer’s Disease in vitro Model. Front. Aging Neurosci. 2020;11:342. doi: 10.3389/fnagi.2019.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kuboyama T., Tohda C., Komatsu K. Effects of Ashwagandha (Roots of Withania somnifera) on neurodegenerative diseases. Biol. Pharm. Bull. 2014;37:892–897. doi: 10.1248/bpb.b14-00022. [DOI] [PubMed] [Google Scholar]
- 150.White P.T., Subramanian C., Motiwala H.F., Cohen M.S. Natural Withanolides in the Treatment of Chronic Diseases. Volume 928. Springer Nature; Gewerbestrasse, Switzerland: 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Roy A. Role of medicinal plants against Alzheimer’s disease. Int. J. Complement. Altern. Med. 2018;11 doi: 10.15406/ijcam.2018.11.00398. [DOI] [Google Scholar]
- 152.Jain R., Mathur K. An insight to curative effects of Ashwagandha (Withania somnifera), an Ayurveda herb. J. Med. Plants. 2020;8:227–235. [Google Scholar]
- 153.Singh P.N., Rao C. V Ethnopharmacological review of native traditional medicinal plants for brain disorders Kalmegh Project View project Ayurvedic Phytopharmacology View project. Pharmacog. Rev. 2007;1:20–28. [Google Scholar]
- 154.Summers W.K., Majovski L.V., Marsh G.M., Tachiki K., Kling A. Oral Tetrahydroaminoacridine in Long-Term Treatment of Senile Dementia, Alzheimer Type. N. Engl. J. Med. 1986;315:1241–1245. doi: 10.1056/NEJM198611133152001. [DOI] [PubMed] [Google Scholar]
- 155.Hardy J. A Hundred Years of Alzheimer’s Disease Research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 156.Lanctôt K.L., Rajaram R.D., Herrmann N. Therapy for Alzheimer’s disease: How effective are current treatments? Ther. Adv. Neurol. Disord. 2009;2:163–180. doi: 10.1177/1756285609102724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Islam M.N., Rauf A., Fahad F.I., Emran T.B., Mitra S., Olatunde A., Shariati M.A., Rebezov M., Mubarak M.S. Superoxide dismutase: An updated review on its pharmaceutical and industrial applications. Crit. Rev. Food Sci. Nutr. 2021;2021:1–19. doi: 10.1080/10408398.2021.1913400. [DOI] [PubMed] [Google Scholar]
- 158.Bhattacharya S.K., Kumar A., Ghosal S. Effects of glycowithanolides from Withania somnifera on an animal model of Alzheimer’s disease and perturbed central cholinergic markers of cognition in rats. Phyther. Res. 1995;9:110–113. doi: 10.1002/ptr.2650090206. [DOI] [Google Scholar]
- 159.Schliebs R., Liebmann A., Bhattacharya S.K., Kumar A., Ghosal S., Bigl V. Systemic administration of defined extracts from Withania somnifera (Indian Ginseng) and Shilajit differentially affects cholinergic but not glutamatergic and gabaergic markers in rat brain. Neurochem. Int. 1997;30:181–190. doi: 10.1016/S0197-0186(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 160.Roy A. A Review on Medicinal Plants for Alzheimers Disease. SciFed J. Herb. Med. 2017;1:1–6. [Google Scholar]
- 161.Howes M.R., Perry N.S.L., Houghton P.J. Plants with Traditional Uses and Activities, Relevant to the Management of Alzheimer’s Disease and Other Cognitive Disorders. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003;18:1–18. doi: 10.1002/ptr.1280. [DOI] [PubMed] [Google Scholar]
- 162.Rakib A., Ahmed S., Islam M.A., Uddin M.M.N., Paul A., Chy M.N.U., Emran T.B., Seidel V. Pharmacological studies on the antinociceptive, anxiolytic and antidepressant activity of Tinospora crispa. Phytother. Res. 2020;34:2978–2984. doi: 10.1002/ptr.6725. [DOI] [PubMed] [Google Scholar]
- 163.Kalra R., Kaushik N. Withania somnifera (Linn.) Dunal: A Review of Chemical and Pharmacological Diversity. Volume 16. Springer; Dordrecht, The Netherlands: 2017. [Google Scholar]
- 164.Bristy T.A., Barua N., Tareq A.M., Sakib S.A., Etu S.T., Chowdhury K.H., Jyoti M.A., Aziz M., Ibn A., Reza A. Deciphering the pharmacological properties of methanol extract of Psychotria calocarpa leaves by in vivo, in vitro and in silico approaches. Pharmaceuticals. 2020;13:183. doi: 10.3390/ph13080183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rahman J., Tareq A.M., Hossain M.M., Sakib S.A., Islam M.N., Uddin A.B.M.N., Hoque M., Nasrin M.S., Ali M.H., Caiazzo E., et al. Biological evaluation, DFT calculations and molecular docking studies on the antidepressant and cytotoxicity activities of Cycas pectinata Buch.-Ham. Compounds. Pharmaceuticals. 2020;13:232. doi: 10.3390/ph13090232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sudhir S., Budhiraja R.D., Miglani G.P., Arora B., Gupta L.C., Garg K.N. Pharmacological studies on leaves of Withania somnifera. Planta Med. 1986;1:61–63. doi: 10.1055/s-2007-969072. [DOI] [PubMed] [Google Scholar]
- 167.Zhou Z., Xiang W., Jiang Y., Tian N., Wei Z., Wen X., Wang W., Liao W., Xia X., Li Q., et al. Withaferin A alleviates traumatic brain injury induced secondary brain injury via suppressing apoptosis in endothelia cells and modulating activation in the microglia. Eur. J. Pharmacol. 2020;874:172988. doi: 10.1016/j.ejphar.2020.172988. [DOI] [PubMed] [Google Scholar]
- 168.Kaileh M., Vanden Berghe W., Heyerick A., Horion J., Piette J., Libert C., De Keukeleire D., Essawi T., Haegeman G. Withaferin A strongly elicits IκB kinase β hyperphosphorylation concomitant with potent inhibition of its kinase activity. J. Biol. Chem. 2007;282:4253–4264. doi: 10.1074/jbc.M606728200. [DOI] [PubMed] [Google Scholar]
- 169.Oh J.H., Kwon T.K. Withaferin A inhibits tumor necrosis factor-α-induced expression of cell adhesion molecules by inactivation of Akt and NF-κB in human pulmonary epithelial cells. Int. Immunopharmacol. 2009;9:614–619. doi: 10.1016/j.intimp.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 170.Min K.J., Choi K., Kwon T.K. Withaferin A down-regulates lipopolysaccharide-induced cyclooxygenase-2 expression and PGE2 production through the inhibition of STAT1/3 activation in microglial cells. Int. Immunopharmacol. 2011;11:1137–1142. doi: 10.1016/j.intimp.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 171.Grover A., Shandilya A., Punetha A., Bisaria V.S., Sundar D. Inhibition of the NEMO/IKKβ association complex formation, a novel mechanism associated with the NF-κB activation suppression by Withania somnifera’s key metabolite withaferin A. BMC Genom. 2010;11:S25. doi: 10.1186/1471-2164-11-S4-S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Purushotham P.M., Kim J.M., Jo E.K., Senthil K. Withanolides against TLR4-Activated Innate Inflammatory Signalling Pathways: A Comparative Computational and Experimental Study. Phyther. Res. 2017;31:152–163. doi: 10.1002/ptr.5746. [DOI] [PubMed] [Google Scholar]
- 173.Christopher A.M.L.S.M. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Physiol. Behav. 2016;176:100–106. doi: 10.1038/nm.3893.Inflammasomes. [DOI] [Google Scholar]
- 174.Place D.E., Kanneganti T.-D. Recent Advances In Inflammasome Biology. Physiol. Behav. 2016;176:100–106. doi: 10.1016/j.coi.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lamkanfi M., Dixit V.M. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 176.Tiruveedi V.L., Bale S., Khurana A., Godugu C. Withaferin A, a novel compound of Indian ginseng (Withania somnifera), ameliorates Cerulein-induced acute pancreatitis: Possible role of oxidative stress and inflammation. Phyther. Res. 2018;32:2586–2596. doi: 10.1002/ptr.6200. [DOI] [PubMed] [Google Scholar]
- 177.Dubey S., Yoon H., Cohen M.S., Nagarkatti P., Nagarkatti M., Karan D. Withaferin a associated differential regulation of inflammatory cytokines. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang Z., Zhang S., Xiao Y., Zhang W., Wu S., Qin T., Yue Y., Qian W., Li L. NLRP3 Inflammasome and Inflammatory Diseases. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/4063562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ngoungoure F.P., Owona B.A. Withaferin A modulates AIM2 inflammasome and caspase-1 expression in THP-1 polarized macrophages. Exp. Cell Res. 2019;383:111564. doi: 10.1016/j.yexcr.2019.111564. [DOI] [PubMed] [Google Scholar]
- 180.Karnitz L.M., Felts S.J. Cdc37 regulation of the kinome: When to hold ’em and when to fold ’em. Sci. STKE. 2007;2007:pe22. doi: 10.1126/stke.3852007pe22. [DOI] [PubMed] [Google Scholar]
- 181.Khan S., Rammeloo A.W., Heikkila J.J. Withaferin A Induces Proteasome Inhibition, Endoplasmic Reticulum Stress, the Heat Shock Response and Acquisition of Thermotolerance. PLoS ONE. 2012;7:e50547. doi: 10.1371/journal.pone.0050547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Grover A., Shandilya A., Agrawal V., Pratik P., Bhasme D., Bisaria V.S., Sundar D. Withaferin A Targets Heat Shock Protein 90 in Pancreatic Cancer Cells. BMC Bioinform. 2011;12:S30. doi: 10.1186/1471-2105-12-S1-S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kumar S., Phaneuf D., Julien J.P. Withaferin-A Treatment Alleviates TAR DNA-Binding Protein-43 Pathology and Improves Cognitive Function in a Mouse Model of FTLD. Neurotherapeutics. 2020:15–17. doi: 10.1007/s13311-020-00952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Witter S., Samoson A., Vilu R., Witter R. Screening of Nutraceuticals and Plant Extracts for Inhibition of Amyloid-β Fibrillation. J. Alzheimer’s Dis. 2020;73:1003–1012. doi: 10.3233/JAD-190758. [DOI] [PubMed] [Google Scholar]
- 185.Abu Bakar M.H., Azmi M.N., Shariff K.A., Tan J.S. Withaferin A Protects Against High-Fat Diet–Induced Obesity Via Attenuation of Oxidative Stress, Inflammation, and Insulin Resistance. Appl. Biochem. Biotechnol. 2019;188:241–259. doi: 10.1007/s12010-018-2920-2. [DOI] [PubMed] [Google Scholar]
- 186.Ku S.K., Han M.S., Bae J.S. Withaferin A is an inhibitor of endothelial protein C receptor shedding in vitro and in vivo. Food Chem. Toxicol. 2014;68:23–29. doi: 10.1016/j.fct.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 187.Maitra R., Porter M.A., Huang S., Gilmour B.P. Inhibition of NFB by the natural product withaferin a in cellular models of cystic fibrosis inflammation. J. Inflamm. 2009;6 doi: 10.1186/1476-9255-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mayola E., Gallerne C., Esposti D.D., Martel C., Pervaiz S., Larue L., Debuire B., Lemoine A., Brenner C., Lemaire C. Withaferin A induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2. Apoptosis. 2011;16:1014–1027. doi: 10.1007/s10495-011-0625-x. [DOI] [PubMed] [Google Scholar]
- 189.Stan S.D., Zeng Y., Singh S.V. Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr. Cancer. 2008;60:51–60. doi: 10.1080/01635580802381477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nagalingam A., Kuppusamy P., Singh S.V., Sharma D., Saxena N.K. Mechanistic elucidation of the antitumor properties of withaferin a in breast cancer. Cancer Res. 2014;74:2617–2629. doi: 10.1158/0008-5472.CAN-13-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mathur R., Gupta S.K., Singh N., Mathur S., Kochupillai V., Velpandian T. Evaluation of the effect of Withania somnifera root extracts on cell cycle and angiogenesis. J. Ethnopharmacol. 2006;105:336–341. doi: 10.1016/j.jep.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 192.Choi M.J., Park E.J., Min K.J., Park J.W., Kwon T.K. Endoplasmic reticulum stress mediates withaferin A-induced apoptosis in human renal carcinoma cells. Toxicol. In Vitro. 2011;25:692–698. doi: 10.1016/j.tiv.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 193.Um H.J., Min K.J., Kim D.E., Kwon T.K. Withaferin A inhibits JAK/STAT3 signaling and induces apoptosis of human renal carcinoma Caki cells. Biochem. Biophys. Res. Commun. 2012;427:24–29. doi: 10.1016/j.bbrc.2012.08.133. [DOI] [PubMed] [Google Scholar]
- 194.Benjumea D., Martín-Herrera D., Abdala S., Gutiérrez-Luis J., Quiñones W., Cardona D., Torres F., Echeverri F. Withanolides from Whitania aristata and their diuretic activity. J. Ethnopharmacol. 2009;123:351–355. doi: 10.1016/j.jep.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 195.Kim J.-E., Jeong Y.-J., Choi J.-A., Oh S.-M., Lee K.-B., Lee J.-Y., Kang M.-J., Park J.-H. Withaferin A Inhibits Helicobacter pylori-induced Production of IL-1β in Dendritic Cells by Regulating NF-κB and NLRP3 Inflammasome Activation. Immune Netw. 2015;15:269–277. doi: 10.4110/in.2015.15.6.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dumore N.G., Umekar M.J., Taksande B.G., Aglawe M.M., Kotagale N.R. Effects of Withania somnifera nicotine induced conditioned place preference in mice. Pharmacogn. J. 2019;11:43–47. doi: 10.5530/pj.2019.1.8. [DOI] [Google Scholar]
- 197.Bahbah E.I., Ghozy S., Attia M.S., Negida A., Emran T.B., Mitra S., Albadrani G.M., Abdel-Daim M.M., Uddin M.S., Simal-Gandara J. Molecular Mechanisms of Astaxanthin as a Potential Neurotherapeutic Agent. Mar. Drugs. 2021;19:201. doi: 10.3390/md19040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jahan I., Tona M.R., Sharmin S., Sayeed M.A., Tania F.Z., Paul A., Chy M., Uddin N., Rakib A., Emran T.B. GC-MS phytochemical profiling, pharmacological properties, and in silico studies of Chukrasia velutina leaves: A novel source for bioactive agents. Molecules. 2020;25:3536. doi: 10.3390/molecules25153536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tekula S., Khurana A., Anchi P., Godugu C. Withaferin-A attenuates multiple low doses of Streptozotocin (MLD-STZ) induced type 1 diabetes. Biomed. Pharmacother. 2018;106:1428–1440. doi: 10.1016/j.biopha.2018.07.090. [DOI] [PubMed] [Google Scholar]
- 200.Hassannia B., Wiernicki B., Ingold I., Qu F., Van Herck S., Tyurina Y.Y., Bayir H., Abhari B.A., Angeli J.P.F., Choi S.M., et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J. Clin. Investig. 2018;128:3341–3355. doi: 10.1172/JCI99032. [DOI] [PMC free article] [PubMed] [Google Scholar]