Abstract
The Ventana PD-L1 SP142 immunohistochemical (IHC) assay is the FDA-approved companion diagnostic assay for atezolizumab therapy selection for patients with PD-L1-positive locally-advanced or metastatic triple-negative breast carcinoma (TNBC). We aimed to elucidate clinical, pathologic and molecular features associated with PD-L1 expression in TNBCs. Clinical, pathologic and next-generation sequencing (NGS)-based molecular data for TNBCs tested with PD-L1 (SP142) IHC at our institution between 11/2018-12/2019 were retrieved and reviewed. PD-L1 positivity was defined as ≥1% IC staining. Patients with metastatic TNBC treated at first-line with atezolizumab regimens were evaluated for treatment response and for time to treatment failure (TTF). Among 156 TNBCs, PD-L1 was positive in 47.4% of cases. Primary TNBCs were significantly more frequently PD-L1 positive, compared to recurrent/metastatic samples (P = .002). PD-L1-positive TNBCs had increased stromal IC, compared to PD-L1-negative samples (P < .001). The repertoire of somatic genetic alterations of PD-L1-positive and PD-L1-negative TNBCs was similar. Matched primary and recurrent/metastatic TNBC samples were available for 8 patients, in whom 4 had discordant results. Thirty patients with metastatic TNBC were treated with atezolizumab regimens, with treatment failure occurring in 16 patients and a median TTF of 5.1 months in this early evaluation. The findings of this study show stromal ICs in primary TNBCs are more likely to show PD-L1 positivity than in recurrent or metastatic samples. This information should guide selection of samples suitable for testing. Further studies are needed to identify other features associated with PD-L1-positive breast carcinomas and clinical benefit of treatment.
Keywords: breast cancer, PD-L1 immunohistochemistry assay, programmed death ligand 1, triple negative
Triple-negative breast carcinomas (TNBCs) lack expression of estrogen receptor (ER) and progesterone receptor (PR) and do not overexpress human epidermal growth factor receptor 2 (HER2). The frequency of TNBC ranges from 13 to 40% of all breast carcinoma cases and depends on the patient population studied.1–4 TNBCs are associated with more aggressive clinical behavior and poorer prognosis, compared to other types of breast carcinoma.5, 6 For patients with metastatic TNBC (mTNBC), current first-line systemic treatment is chemotherapy;7 however, duration of response to therapy is short, and median overall survival for patients with mTNBC remains 18 months or less.8, 9 Attempts to improve outcomes led to investigations into the tumor microenvironment, where multiple studies have demonstrated the prognostic and predictive value of tumor-infiltrating immune cells.10, 11
Development of immune checkpoint inhibitors created novel therapeutic approaches in many malignancies.12–15 Programmed death protein 1 (PD-1), a transmembrane protein receptor, suppresses T-cell function and hinders tumor cell destruction through its interaction with programmed death ligand 1 (PD-L1).16, 17 As reported by Schmid et al.,18 the IMpassion130 trial found that in patients with PD-L1-positive mTNBC, the combination of atezolizumab, a PD-L1 inhibitor, and chemotherapy prolonged progression-free survival when compared to chemotherapy alone. The United States Food and Drug Administration (FDA) approved atezolizumab in combination with nanoparticle albumin-bound (nab) paclitaxel for patients with locally advanced or mTNBC with PD-L1-positive immune cells. The Ventana PD-L1 SP142 immunohistochemistry (IHC) assay (Ventana Medical Systems, Tuscan AZ, USA) is the FDA-approved companion diagnostic assay for atezolizumab therapy selection and has been widely implemented.19
The purpose of this retrospective study was to assess the clinical, pathologic and molecular features of TNBCs that were associated with PD-L1 (SP142) IHC expression in the stromal tumor-infiltrating immune cells at a single large academic tertiary-care cancer center.
MATERIALS AND METHODS
Study Population
At our institution, PD-L1 IHC is performed at the request of the treating physician. Following approval from the institutional review board, we identified all patients with TNBC whose primary, recurrent or metastatic breast carcinoma was subjected to PD-L1 (SP142) IHC between November 1, 2018 to December 31, 2019, through a retrospective search of our pathology database. Specimens that underwent decalcification process and cytology specimens were not suitable for the assay, as validation studies in these sample types have not been performed. In total, 164 samples from 156 patients were included in this study. For patients with tumor samples from both primary and recurrent or metastatic sites, the primary tumor sample was included in the overall analysis, and the results of the matched samples were analyzed and reported separately.
PD-L1 SP142 Immunohistochemistry
PD-L1 (SP142) IHC was conducted using 4 μm-thick full sections. IHC staining was performed on a Benchmark Ultra System (Ventana Medical Systems, Tucson, AZ, USA) with antibody detection using the OptiView DAB IHC Detection Kit (Ventana Medical Systems), according to the manufacturer’s manual.20
Slide Review
PD-L1 IHC slides and available corresponding hematoxylin and eosin (H&E)-stained slides were reviewed. PD-L1 IHC was assessed in all cases (n = 164) by a dedicated study pathologist with training in breast cancer diagnosis (R.S.H.) following the IMpassion130 trial criteria,18 with PD-L1 positive cases showing PD-L1 expression on stromal tumor-infiltrating immune cells occupying ≥1% of the tumor area. Quantification of stromal tumor-infiltrating immune cells was assessed in all cases (n = 164) based on the recommendations by the International Tumor-Infiltrating Lymphocytes Working Group21 and was performed by a study pathologist (R.S.H.), blinded to the PD-L1 status and following a washout period of at least one week from the date of PD-L1 assessment. The following parameters were retrieved from the pathology reports: patient age at breast cancer diagnosis, primary tumor size, lymph node status, histologic subtype, tumor grade and status of estrogen receptor (ER), progesterone receptor (PR), androgen receptor (AR) and human epidermal growth factor receptor 2 (HER2). ER, PR and HER2 IHC were assessed using the most recent American Society of Clinical Oncology/College of American Pathologists guideline.22, 23 AR status was assessed using two cutoffs. Nuclear expression of AR IHC in ≥1% or ≥10% of tumor cells was considered positive.
Clinical Review
Patient follow-up data, including treatment regimens, imaging studies and clinical status, and patient self-reported race or ethnicity were retrieved from the electronic medical record. To assess clinical benefit of anti-PD-L1 therapy, electronic medical records for patients who received atezolizumab were reviewed by a medical oncologist with experience in breast cancer (C.D.A.). Patients who fulfilled the following criteria were included in the benefit analysis: (1) had TNBC; (2) received full prescribed treatment regimen of atezolizumab as first-line therapy for metastatic disease; and (3) therapy was administered at our institution. Treatment response was assessed based on the findings of computed tomography (CT) scans and/or clinical examination. To assess clinical benefit, time to treatment failure (TTF) was calculated for patients, who met the inclusion criteria. TTF was defined as time from treatment initiation to treatment discontinuation for any reason, including disease progression, treatment toxicity and death.
Next-Generation Sequencing Analysis
Tumor and matched normal DNA of cases with available material were subjected to a FDA-approved targeted next-generation sequencing (NGS) using the Memorial Sloan Kettering Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) assay, targeting 468 key cancer-related genes, as previously described.24, 25 Microsatellite instability was determined by using MSIsensor, with a score of 10 or greater defined as MSI high, score between 3 and 10 as MSI indeterminate and score of 3 or less as MSI stable, as previously reported.26, 27 Somatic mutation and gene copy number results and MSIsensor score derived from MSK-IMPACT testing were recorded. We performed an exploratory, hypothesis-generating analysis of the repertoire of somatic genetic alterations between PD-L1-positive and PD-L1-negative TNBCs. We reasoned that given that primary and post-therapy TNBCs differ in terms of mutation load and somatic mutations,28 these analyses were separately performed for primary TNBCs and for metastatic/recurrent TNBCs separately.
Statistical Analyses
Differences between groups in categorical variables were calculated by χ2 test and Fisher exact test, where applicable, and in continuous variables by using the Student t test. Statistical significance was established at P < .05. Multiple comparisons adjustment was performed using the Benjamini–Hochberg procedure with a corrected false discovery rate cut-off of .05.
RESULTS
Study Cohort
During the study period, 252 breast carcinoma samples from 234 patients were tested with PD-L1 (SP142) IHC at our institution. These samples included 91 primary tumor specimens, 43 recurrent tumor specimens and 118 metastatic tumor specimens. Eighty-eight samples from 78 patients were excluded from the analysis for the following reasons: ER-positive and/or PR-positive, HER2-negative disease (n = 69 patients), ER-negative, HER2-amplified disease and (n = 5 patients) and ER-positive, HER2-amplified disease (n = 4 patients). Patients with hormone-receptor-positive, HER2-negative breast carcinomas may be tested for eligibility in clinical trials or for compassionate use of anti-PD-L1 therapy. One hundred sixty-four TNBC samples from 156 patients fulfilled the study inclusion criteria for clinical, pathologic and molecular analysis. Of these 156 study patients, 155 were women. Study tumor samples included 58 (37.2%) primary samples, 31 (19.9%) locally recurrent samples and 67 (42.9%) metastatic samples. Eight study patients had PD-L1 IHC performed on a primary tumor samples and a metastatic (n = 7 patients) or locally recurrent (n = 1 patient) tumor sample.
Therapy with anti-PD-L1 was administered to 48 of 234 patients with breast carcinoma tested with PD-L1 IHC. For assessment of clinical benefit, 18 patients were excluded from the analysis for the following reasons: anti-PD-L1 treatment as other than first line therapy (n = 12); ER-positive, HER2-negative disease (n = 2); therapy administration at another institution (n = 2); treatment in the neoadjuvant setting (n = 1); and full treatment regimen not given due to infusion reaction (n = 1). Thirty patients fulfilled the study inclusion criteria for clinical benefit analysis.
Clinicopathologic Features Associated with PD-L1 (SP142) Expression in Stromal Immune Cells of Triple-Negative Breast Carcinoma
Clinical and pathologic findings in the patients with TNBC are summarized in Table 1. Of the 156 patients with TNBC, PD-L1 IHC was positive in 74 (47.4%) patients, and negative in 82 (52.6%). The median age at primary breast cancer diagnosis of patients with PD-L1-positive TNBC was 51 years (mean = 51.5 years; range = 21-87 years), similar to that of patients with PD-L1-negative TNBC (median = 51.5 years; mean = 51.5 years; range = 32-82 years; P = .93). There was no significant correlation between patient-identified race or ethnicity and PD-L1 status (Table 1). Primary tumor size for PD-L1-positive TNBCs ranged from 0.1 to 18.0 cm in maximum dimension, with a median size of 2.5 cm (mean = 3.3 cm), comparable to that of PD-L1-negative TNBC (median = 2.1 cm; mean = 3.0 cm; range = 0.1—18.0 cm; P = .46). Invasive lobular carcinoma, both classic and pleomorphic types, tended to be PD-L1-negative; however, overall, there was no significant correlation in tumor histology and PD-L1 status. PD-L1-positive and PD-L1-negative TNBCs did not differ significantly in histologic grade (P = .99), AR status using both 1% cutoff (P = .59) and 10% cutoff (P = .40) or involvement of axillary lymph nodes (P = .18).
Table 1.
Comparison of clinicopathologic features of 156 triple-negative breast carcinoma samples from 156 patients by PD-L1 (SP142) immunohistochemical expression status.
| Characteristic, n | PD-L1 positive (n=74) | PD-L1 negative (n=82) | P |
|---|---|---|---|
| Gender, n (%) | 1.0 | ||
| Female | 74 (100) | 81 (98.8) | |
| Male | 0 | 1 (1.2) | |
| Age at primary diagnosis, median (range), years | 51 (21-87) | 51.5 (32-82) | .93 |
| Race/ethnicity, n (%) | .48 | ||
| White: Non-Spanish; Non-Hispanic | 42 (56.8) | 52 (63.4) | |
| Black or African American | 12 (16.2) | 11 (13.4) | |
| Asian: Far East/Indian Subcontinent | 10 (13.5) | 10 (12.2) | |
| Spanish NOS; Hispanic NOS; Latinx NOS | 10 (13.5) | 5 (6.1) | |
| Data not available | 0 | 4 (4.9) | |
| Sample tested, n (%) | .002* | ||
| Primary tumor | 36 (48.6) | 22 (26.8) | |
| Metastatic tumor | 21 (28.4) | 46 (56.1) | |
| Locoregional recurrence | 17 (23.0) | 14 (17.1) | |
| Primary tumor size, median (range), cm | 2.5 (0.1-18.0) | 2.0 (0.1-18.0) | .46 |
| Histologic grade, n (%) | .91 | ||
| 1 | 0 | 1 (1.2) | |
| 2 | 12 (16.2) | 12 (14.6) | |
| 3 | 57 (77.0) | 62 (75.6) | |
| Data not available | 5 (6.8) | 7 (8.5) | |
| Primary tumor histology, n (%) | .22 | ||
| Invasive carcinoma NST | 53 (71.6) | 52 (63.4) | |
| Metaplastic carcinoma | 7 (9.5) | 9 (11.0) | |
| Invasive lobular carcinoma, classic or | 1 (1.4) | 7 (8.5) | |
| pleomorphic | |||
| Other† | 10 (13.5) | 11 (13.4) | |
| Data not available | 3 (4.1) | 3 (3.7) | |
| Androgen receptor status, 1% cutoff, n (%) | .59 | ||
| Positive | 23 (31.1) | 35 (42.7) | |
| Negative | 28 (37.8) | 35 (42.7) | |
| Data not available | 23 (31.1) | 12 (14.6) | |
| Androgen receptor status, 10% cutoff, n (%) | .40 | ||
| Positive | 18 (24.3) | 30 (36.6) | |
| Negative | 33 (44.6) | 40 (48.8) | |
| Data not available | 23 (31.1) | 12 (14.6) | |
| Lymph node status, n (%) | .18 | ||
| Involved | 41 (55.4) | 41 (50.0) | |
| Uninvolved | 18 (24.3) | 26 (31.7) | |
| Data not available | 15 (20.3) | 15 (18.3) | |
| Microsatellite instability status, n (%) | .37 | ||
| Stable | 46/48 (95.8) | 52/60 (86.7) | |
| Indeterminate | 2/48 (4.2) | 7/60 (11.7) | |
| Instable | 0 | 1/60 (1.7) | |
| Treatment with anti-PD-L1 therapy, n (%) | <.001* | ||
| Yes | 45 (60.8) | 3 (3.7) | |
| No | 29 (39.2) | 79 (96.3) |
Abbreviations: NS, not significant; NST, no special type
denotes statistical significance P<.05 by χ2 test, Fisher exact test or Student t test, where applicable.
Other category encompasses cases of invasive ductal carcinoma with special histologic features, including apocrine, micropapillary and mucinous features, and cases of invasive mammary carcinoma, wherein carcinoma shared features of both ductal and lobular carcinoma.
Triple-Negative Breast Carcinomas with Increased Stromal Tumor-Infiltrating Immune Cells Are Associated with PD-L1 Positivity
Increased stromal tumor-infiltrating immune cells was significantly associated with PD-L1 (SP142) expression of TNBCs. As shown in Figure 1, PD-L1-positive primary, recurrent and metastatic TNBCs had a median of 20% of the tumor infiltrating immune cells (mean, 27.6%; standard deviation, 22.4%; range, 5—90%), whereas PD-L1 negative TNBCs showed significantly lower level of tumor infiltrating immune cells, with a median of 5% (mean, 14.4%; standard deviation, 17.0%; range, 0—80%; P < .001). Examining only primary TNBC samples, the difference in stromal-tumor infiltrating immune cells between PD-L1-positive (n = 36) and PD-L1-negative (n = 22) tumor samples remained significant (P = .04); PD-L1-positive primary TNBCs displayed a higher percentage of stromal tumor-infiltrating immune cells (median, 20%; mean, 29.0%; standard deviation, 22.8%; range, 5—80%), compared to PD-L1-negative primary TNBCs (median, 10%; mean, 17.0%; standard deviation, 17.8%; range, 0—80%).
Figure 1.
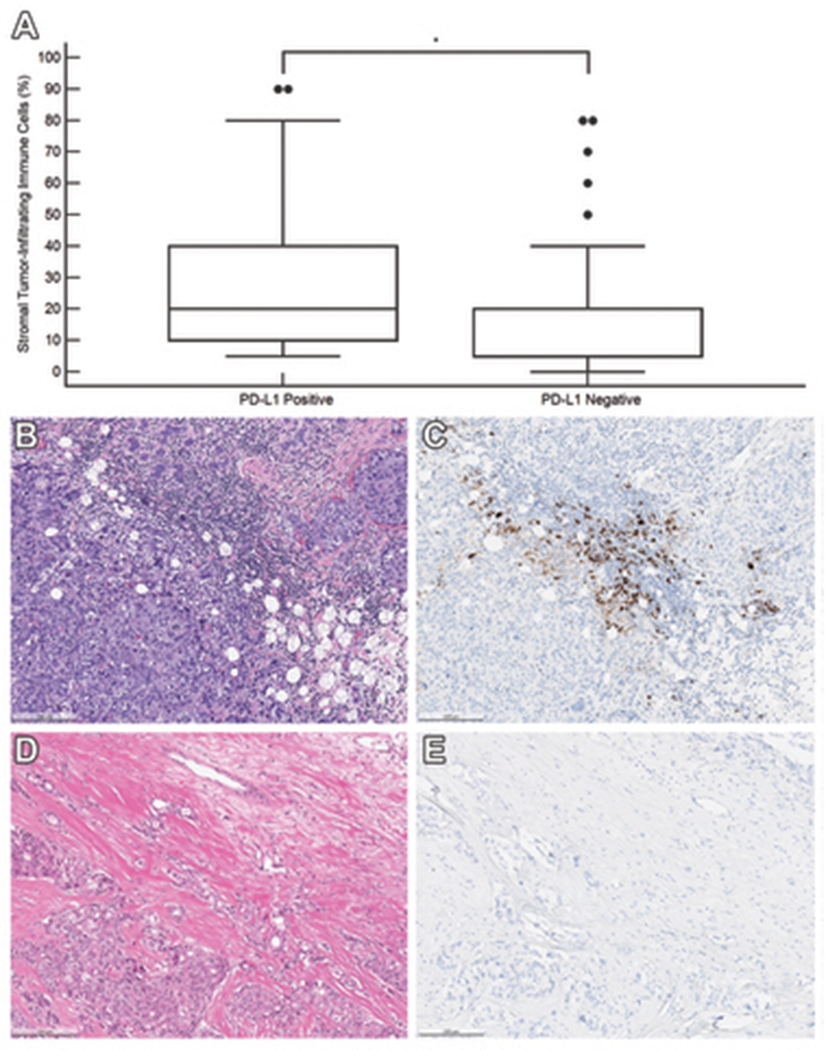
Association between stromal tumor-infiltrating immune cells and PD-L1 (SP142) immunohistochemistry result in 156 triple-negative breast carcinoma samples. (A) Box and whisker plots of the percentage of stromal area occupied by immune cells demonstrates a significantly higher percentage in PD-L1 positive tumors than in PD-L1 negative tumors (P < .001). * denotes significant P < .05. (B) Case of a 32-year-old woman with primary invasive carcinoma of no special type, harboring stromal-infiltrating immune cells that occupy 60% of the stroma and (C) express PD-L1 [PD-L1 (SP142) immunohistochemical stain]. (D) Case of a 55-year-old woman with primary invasive carcinoma of no special type, status-post neoadjuvant chemotherapy. The stromal area is densely collagenous with 5% of the stroma occupied by immune cells with (E) no expression of PD-L1 [PD-L1 (SP142) immunohistochemical stain].
Primary Triple-Negative Breast Carcinoma Samples Are More Likely to Be PD-L1 Positive Compared to Recurrent and Metastatic Specimens
Primary TNBCs were significantly more frequently PD-L1 positive than recurrent or metastatic TNBCs (Figure 2, P = .002). Table 2 details sites of metastasis and PD-L1 IHCs results. Most common sites of metastases tested included liver (n = 13), lung or pleura (n = 12), lymph node (n = 9) and soft tissue (n = 9). Matched TNBC samples of the primary tumor (n = 8) and metastatic (n = 7) or recurrent (n = 1) sites were available for 8 patients (Table 3). Discordant PD-L1 (SP142) IHC results were found in 4 (50%) cases. In each discordant case, the primary tumor expressed PD-L1 in the stromal tumor-infiltrating immune cells, while the metastatic tumor harbored PD-L1-negative stromal tumor-infiltrating immune cells or minimal stromal tumor-infiltrating immune cells.
Figure 2.
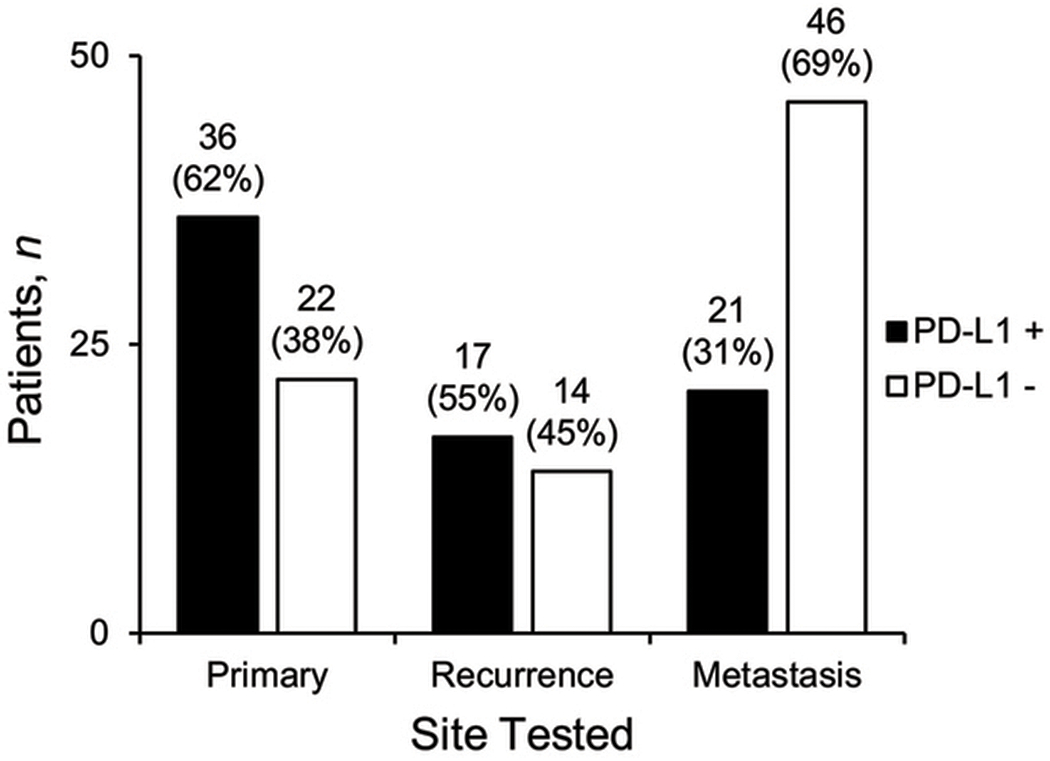
Site of 156 triple-negative breast carcinoma samples tested with PD-L1 (SP142) immunohistochemistry by PD-L1 result. There is significant association with positive PD-L1 status and primary tumor samples, compared to recurrent and metastatic tumor samples (P = .002, by χ2 test).
Table 2.
Summary of 67 metastatic triple-negative breast carcinoma samples from 67 patients by PD-L1 (SP142) immunochemical expression status.
| Site of metastasis | PD-L1 positive (n = 21) | PD-L1 negative (n = 46) |
|---|---|---|
| Liver | 2 | 11 |
| Lung/pleura | 5 | 7 |
| Lymph node | 5 | 4 |
| Soft tissue | 2 | 7 |
| Bone (non-decalcified) | 2 | 6 |
| Skin | 3 | 3 |
| Brain | 1 | 3 |
| Contralateral breast | 0 | 2 |
| Ovary | 1 | 0 |
| Colon | 0 | 1 |
| Mediastinum | 0 | 1 |
| Stomach | 0 | 1 |
Table 3.
Summary of PD-L1 (SP142) immunohistochemistry results for 8 patients with paired primary and recurrent/metastatic triple-negative breast carcinoma samples.
| Patient | Age at primary diagnosis, years | Primary tumor histology | Second site tested | Primary tumor PD-L1 status | Second site PD-L1 status |
|---|---|---|---|---|---|
| 1 | 60 | Invasive carcinoma NST | Liver | Positive | Negative |
| 2 | 47 | Invasive carcinoma NST | Liver | Positive | Negative |
| 3 | 51 | Invasive carcinoma NST | Liver | Positive | Negative |
| 4 | 51 | Invasive carcinoma NST | Bone* | Positive | Negative |
| 5 | 68 | Invasive carcinoma NST | Lung | Positive | Positive |
| 6 | 43 | Invasive carcinoma NST | Lung | Positive | Positive |
| 7 | 67 | Invasive carcinoma NST | Soft tissue | Negative | Negative |
| 8 | 64 | Ductal, apocrine | Breast (recurrent) | Negative | Negative |
Abbreviations: NST, no special type
The bone specimen did not undergo decalcification.
Two patients with discordant PD-L1 results in the matched primary and metastatic samples received anti-PD-L1 therapy and are worth special mention. A 60-year-old woman with a 1.7 cm triple-negative, poorly differentiated invasive carcinoma of no special type (IC-NST) underwent breast-conserving surgery, adjuvant dose-dense doxorubicin, cyclophosphamide and paclitaxel (ACT) and radiation therapy. Figure 3 (A–F) shows the pertinent pathologic and radiologic findings of the patient. Thirty-seven months following her initial diagnosis, she developed numerous metastatic lesions involving both hepatic lobes on CT scan. Biopsy of a liver nodule showed metastatic adenocarcinoma, morphologically similar to the patient’s breast primary. PD-L1 IHC was performed on both the primary breast and metastatic samples. The primary breast carcinoma showed PD-L1 positivity in the stromal tumor-infiltrating immune cells, while the hepatic metastasis yielded negative PD-L1 staining. The patient was started on atezolizumab and nab-paclitaxel. Follow-up CT scan performed seven months after initiation of treatment showed the hepatic lesions to be responding to the treatment, with marked decrease in size.
Figure 3.
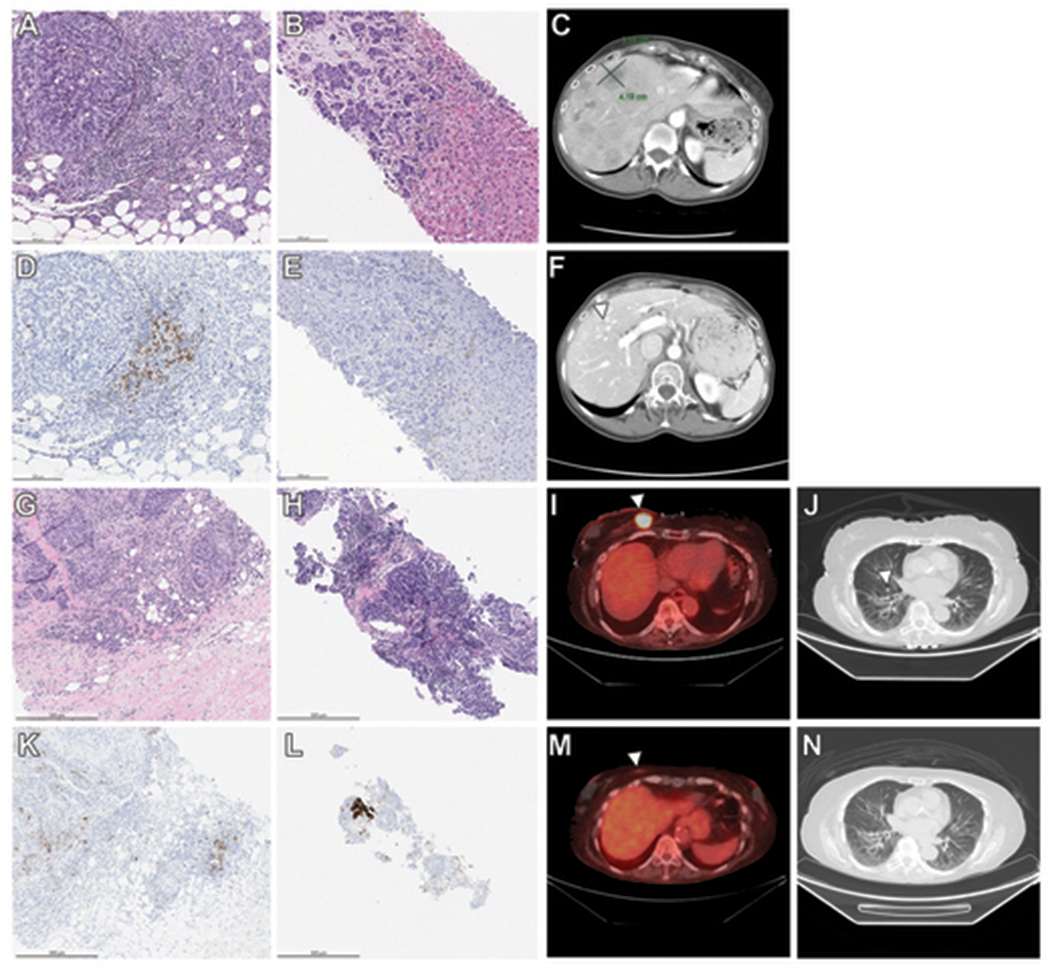
A-F. Discordant PD-L1 results between primary and metastatic tumor in the case of a 60-year-old woman with cT1N0, triple-negative, PD-L1-positive breast carcinoma and PD-L1 negative metastatic disease to the liver three years following her initial diagnosis. (A) The breast excision specimen shows a 1.7 cm poorly differentiated invasive carcinoma of no special type with prominent stromal-infiltrating immune cells. (B) Expression of PD-L1 is demonstrated in the stromal-infiltrating immune cells of the primary tumor [PD-L1 (SP142) immunohistochemical stain]. (C) Biopsy of the liver lesion reveals metastatic poorly differentiated adenocarcinoma with (D) no expression of PD-L1 in stromal-infiltrating immune cells. (E) Computed tomography (CT) scan shows number bilobar low-attenuation hepatic lesions. (F) Seven months later, follow-up CT scan reveals marked decrease in size of previously noted hepatic lesions (arrowhead) on atezolizumab and nab-paclitaxel. G-N. Complete response to atezolizumab and nab-paclitaxel in the case of an 81-year-old woman with triple-negative breast carcinoma and metastatic disease at presentation. (G) Core-needle biopsy shows a poorly differentiated invasive carcinoma of no special type. (H) Expression of PD-L1 is demonstrated in the stromal-infiltrating immune cells of the primary tumor [PD-L1 (SP142) immunohistochemical stain]. (I) Biopsy of the lung lesion reveals metastatic carcinoma with (J) expression of PD-L1 in stromal-infiltrating immune cells. (K) Positron emission tomography-computed tomography (PET-CT) scan shows a 2.5 cm hypermetabolic mass within the right breast (arrowhead). (L) Four months later, follow-up PET-CT scan reveals resolution of previously noted breast mass, denoted by biopsy marker (arrowhead), after 4 cycles of atezolizumab and nab-paclitaxel. (M) CT scan of the lung prior to therapy shows scattered subcentimeter pulmonary nodules (arrowhead). (N) Pulmonary nodules are no longer seen on follow-up CT scan of the lung after 4 cycles of anti-PD-L1 therapy.
The other patient is a 51-year-old woman with a 3.9 cm triple-negative, poorly differentiated IC-NST, for which she underwent neoadjuvant treatment with dose-dense doxorubicin, cyclophosphamide, followed by paclitaxel. Breast-conserving surgery and axillary lymph node dissection after completion of neoadjuvant treatment yielded 1.4 cm residual invasive carcinoma with negative surgical margins and two involved lymph nodes. She then received adjuvant capecitabine and radiation therapy. Twenty months later, positron emission tomography–computed tomography (PET-CT) scan revealed 18F-fluorodeoxyglucose (FDG)–avid lesions in the patient’s adrenal gland, brain, liver, lungs, pelvis, rib and thyroid. Biopsy of a hepatic lesion demonstrated metastatic adenocarcinoma, morphologically similar to the patient’s breast primary. PD-L1 IHC was performed on both the primary breast and metastatic tumors, the former showing PD-L1 positive, and the latter revealing PD-L1-negative staining. The patient was started on atezolizumab and nab-paclitaxel. Follow-up PET-CT scan performed three months after initiation of treatment showed no evidence of residual FDG-avid disease, with resolution of all prior hypermetabolism foci at all sites.
Molecular Features Associated with PD-L1 (SP142) Expression in Stromal Tumor-Infiltrating Immune Cells of Triple-Negative Breast Carcinoma
MSK-IMPACT sequencing was performed for 114 TNBCs, of which 51 were PD-L1-positive and 63 were PD-L1-negative. Among the 37 primary TNBCs with NGS results, 21 (56.8%) were PD-L1 positive, and 16 (43.2%) were PD-L1 negative. Among the 11 locally recurrent TNBCs and 66 metastatic TNBCs with NGS results, 30 (39.0%) were PD-L1 positive, and 47 (61.0%) were PD-L1 negative. As expected, TP53 was the most commonly mutated gene across the primary and recurrent/metastatic TNBCs studied (Figures 4 and 5). The frequency of genes altered by somatic mutations or gene copy number alterations was similar between PD-L1-positive and PD-L1-negative primary TNBCs (Figure 4). Of the 468 genes tested, only somatic mutations in the core-binding factor subunit beta gene (CBFB) were found to be significantly more frequent in primary TNBCs with PD-L1-negative stromal tumor-infiltrating immune cells (4/16; 25%) than in PD-L1-positive cases (0%; P = .03, Fisher’s exact test; Figure 4). After Benjamini-Hochberg adjustment for multiple testing, however, the association was no longer significant (P = .32). Of the 4 breast carcinomas with CBFB mutations, 2 cases demonstrated pleomorphic lobular carcinoma with apocrine features (Cases 57 and 59) on histology, 1 case displayed metaplastic carcinoma (Case 53) and 1 case showed IC-NST (Case 54). The cases of pleomorphic lobular carcinoma with CBFB mutations harbored low percentage of stromal tumor-infiltrating immune cells (10% and 5% for Cases 57 and 59, respectively) and positive for AR on IHC (80% and 99% for Cases 57 and 59, correspondingly). The cases of metaplastic carcinoma and IC-NST showed variably higher percentage of stromal tumor-infiltrating immune cells (80% and 15% for Cases 53 and 54, respectively) and negative AR IHC, where performed (<1% for Case 53, and data not available in Case 54).
Figure 4.
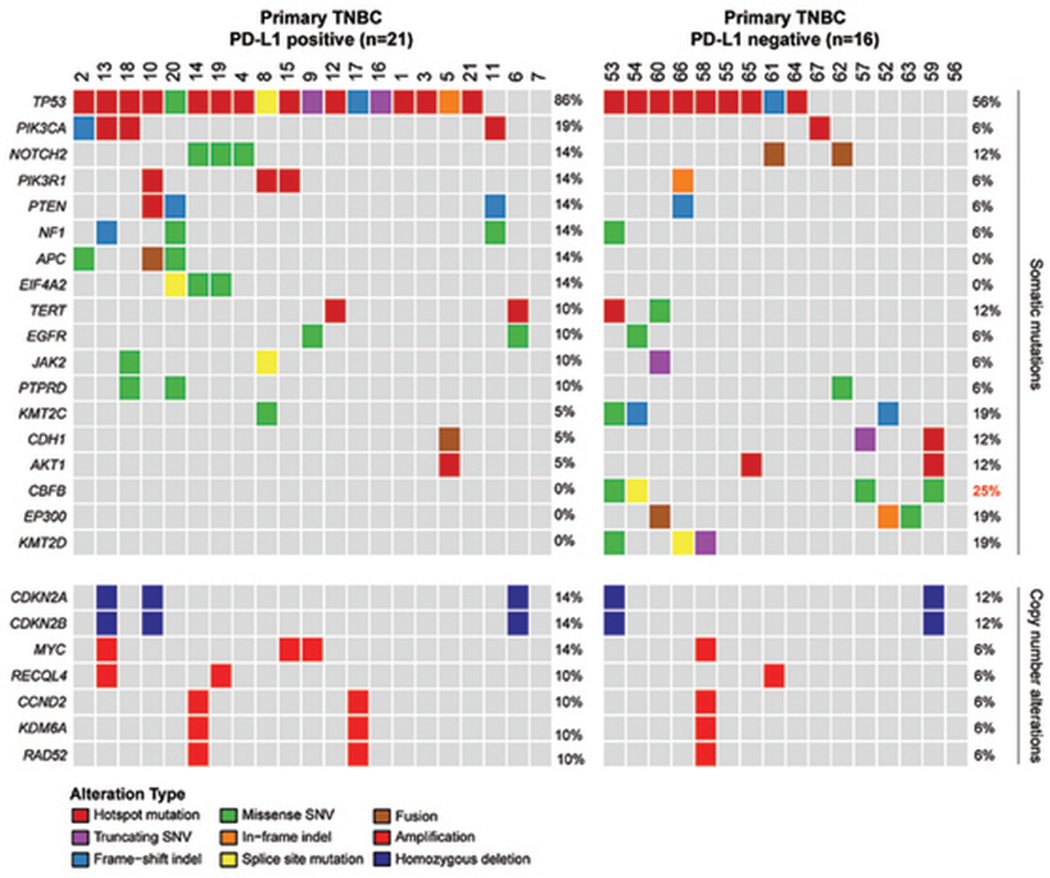
Somatic mutations and copy number alterations identified by Memorial Sloan Kettering Mutation Profiling of Actionable Cancer Targets next-generation sequencing assay in 37 primary triple-negative breast carcinomas by PD-L1 status of stromal tumor-infiltrating immune cells. Cases are represented in columns, and genes are displayed in rows. Alteration types are color-coded according the legend. Somatic mutations in CBFB were significantly associated with PD-L1-negative stromal tumor-infiltrating immune cells in primary triple-negative breast carcinoma on univariate analysis with Fisher exact test (P = .03), but no longer significant after adjusting for multiple comparisons (P = .32). Values written in red denote significant P < .05.
Figure 5.

Somatic mutations and copy number alterations identified by Memorial Sloan Kettering Mutation Profiling of Actionable Cancer Targets next-generation sequencing assay in 77 recurrent or metastatic triple-negative breast carcinomas by PD-L1 status of stromal tumor-infiltrating immune cells. Cases are represented in columns, and genes are displayed in rows. The type of tumor site tested is shown in phenotype bars (top). Alteration types are color-coded according the legend. There were no significant differences in somatic mutations or copy number alterations between recurrent and metastatic triple-negative breast carcinoma with PD-L1 positive or negative stromal tumor-infiltrating immune cells.
The repertoire of genetic alterations between recurrent/metastatic TNBCs with PD-L1-positive or PD-L1-negative was similar, and no statistically significant differences in either their somatic mutations or gene copy number alterations were found (Figure 5).
The mutational burden of PD-L1-positive and PD-L1-negative TNBCs did not differ [median 4 non-synonymous somatic mutations/Mb (range, 0—20) and median 5 non-synonymous somatic mutations/Mb (range, 1—37), respectively; P = .73]. Microsatellite instability, as defined by MSIsensor,26, 27 was rare in the TNBCs studied, and not statistically significantly different between PD-L1-positive and PD-L1-negative TNBCs (P = .37). Only 1 PD-L1 negative metastatic TNBC was classified as MSI high and showed 14 non-synonymous somatic mutations/Mb. Seven PD-L1-negative and 2 PD-L1-positive TNBCs were classified as MSI indeterminate by MSIsensor.
Assessment of Clinical Benefit of Anti-PD-L1 Therapy in Patients with Triple-Negative Breast Carcinoma
Of the 30 patients with TNBC treated with anti-PD-L1 therapy, 28 patients had PD-L1-positive tumors. Patients with PD-L1-negative TNBCs who received atezolizumab were either enrolled in a clinical trial (n = 1) or had a positive PD-L1 IHC result at the referring institution (n = 1). Eight out of 30 patients were treated for de-novo mTNBC. Twenty-one patients had received previous neoadjuvant or adjuvant treatment for primary disease, including 10 patients in whom the interval between the end of systemic perioperative treatment and the initiation of first line metastatic treatment was inferior to 12 months. Twenty-seven patients received atezolizumab combined with nab-paclitaxel, 2 patients received atezolizumab combined with chemotherapies other than nab-paclitaxel and 1 patient was treated with single-agent atezolizumab.
Median clinical follow-up time for these patients was 4.3 months (range: 1.3—14.3 months). At present, 14 patients remain on anti-PD-L1 treatment, and 16 patients have failed first-line therapy (15 due to disease progression, and one due to toxicity). Median time to treatment failure (TTF) with atezolizumab regimen was 5.1 months. Based on radiologic findings and clinical evaluation, 1 patient had complete response to therapy, and 7 had partial response; 11 patients showed disease progression, while 7 had stable disease and 4 had non-measurable lesions.
The patient with complete response was an 81-year-old woman with a history of right T1N0 IC-NST in 2000, who presented with a palpable 2.5 cm mass in a different quadrant of the ipsilateral breast in 2019. Figure 3 (G–H) displays the relevant pathologic and radiologic findings of the patient. Staging PET-CT scan four months later showed scattered bilateral hypermetabolic pulmonary nodules, biopsy of which demonstrated metastatic carcinoma, morphologically similar to the patient’s breast primary. PD-L1 IHC was positive in the metastatic lesion. The patient was started on atezolizumab and nab-paclitaxel. Restaging PET-CT scan after 4 cycles of therapy showed complete resolution of breast mass and bilateral pulmonary nodules.
DISCUSSION
Our study demonstrates PD-L1 (SP142) IHC expression is significantly more frequent in primary tumor samples than in recurrent tumor samples and in samples of distant metastasis and is significantly associated with increased percentage of stromal tumor-infiltrating immune cells. A hypothesis-generating genomic analysis has suggested that the differences between TNBC with PD-L1-positive or -negative stromal tumor-infiltrating immune cells are likely minimal, but somatic mutations in CBFB may be associated with TNBC with PD-L1-negative stromal tumor-infiltrating immune cells.
Other groups have previously reported on clinical and pathologic associations with PD-L1 expression in breast carcinoma.29–39 We caution, however comparison of our results with prior publications with these publications, as early studies primarily focused on PD-L1 expression in the tumor cells themselves, rather than on the tumor-infiltrating immune cells.29–35 Furthermore, prior studies also used PD-L1 antibodies other than the Ventana SP142 clone, such as 5H129 or E1L3N,32, 37 which are assays used in more commonly in other malignancies.
Our study found a significant association between increased stromal tumor-infiltrating immune cells and positive PD-L1 status. This finding has been previously noted.29 Mittendorf et al.29, 30 observed the association of PD-L1 expression in tumor cells and higher number of CD8-positive tumor-infiltrating lymphocytes in their tissue microarray-based study. Park and colleagues34 found that PD-L1 expression on hormone-receptor-positive breast carcinoma cells was significantly associated with higher degree of stromal tumor-infiltrating lymphocytes. Kurozumi et al.35 analyzed features associated with PD-L1 (SP142) IHC positivity in HER2-amplified breast carcinomas and found increased percentage of tumor-infiltrating lymphocytes was significantly correlated with PD-L1 expression on tumor cells.
Other clinical and pathologic parameters have shown conflicting results. In our experience, there was no association with patient age, tumor size, histologic grade or lymph node status with PD-L1 expression; however, Muenst et al.30 demonstrated PD-L1 expression in tumor cells was significantly associated with those particular features. Baptista and colleagues33 reported association with younger patient age at diagnosis with PD-L1 expression in tumor cells. Higher histologic grade has also been reported to be associated with positive PD-L1 status. Zeng et al.37 found patients with higher grade tumors were significantly associated with PD-L1 (E1L3N) expression within tumor-associated immune cells. In HER2-amplified breast carcinomas, higher histologic grade was also noted to be associated with PD-L1 expression in tumor cells.35
Our study identified a significantly higher proportion of PD-L1 expression within primary TNBC tumor samples, compared to recurrent and metastatic samples. Other studies have demonstrated similar findings, but it should be noted that these groups utilize PD-L1 antibodies other than SP142, such as E1L3N,39 SP26340 or B7-H1.41 A PD-L1 negative result in a metastatic sample, however, may not be indicative of clinical benefit from anti-PD-L1 therapy. In our study cohort, discordant PD-L1 results for patients with matched primary and recurrent or metastatic TNBC samples were often the result of a PD-L1 positive primary sample and additional sample demonstrating PD-L1 negativity with the SP142 antibody. In such cases, if the patient received anti-PD-L1 therapy, clinical benefit was seen in the recurrent or metastatic site, despite the PD-L1 negative result. One contributing factor may be that metastatic sites are more likely to be sampled by core needle biopsy, rather than resection. Therefore, a larger area of tumor and stroma than is provided by biopsy may be necessary to properly assess for PD-L1 IHC expression. Additionally, these data may suggest that potential differences in the immune microenvironment between primary and recurrence or metastatic sites that result in the absence of PD-L1-staining stromal tumor-infiltrating immune cells.42
We performed a hypothesis-generating, exploratory analysis utilizing an FDA-approved targeted NGS assay (i.e. MSK-IMPACT™) and found that the mutational landscape of the TNBCs analyzed was similar to those commonly reported in TNBC,43 with TP53 and PIK3CA being the most frequently mutated genes in our study. A significantly higher frequency of somatic mutations in CBFB were identified in primary TNBCs with PD-L1-negative stromal tumor-infiltrating immune cells than in PD-L1-positive primary TNBCs. Typically associated with acute myeloid leukemia,44–46 CBFB mutations in breast cancer, in particular TNBCs, are rare.47 A cBioPortal re-analysis of The Cancer Genome Atlas study48 and of the data from Razavi et al.47 revealed that CBFB mutations co-occur with mutations affecting genes altered in luminal cancers (e.g. AKT1 and CDH1, data not shown), consistent with the results of this cohort. Given the relative low-frequency of CBFB mutations in TNBCs, their co-occurrence with genes mutated in luminal breast cancers and the fact that TNBCs lacking PD-L1 expression in immune cells more frequently harbor CBFB mutations, one could hypothesize that the higher frequency of CBFB mutations found in this subset would be due to an enrichment in Luminal Androgen Receptor TNBCs, which have lower levels of lymphocytic infiltrate and are enriched for mutations affecting AKT1 and CDH1.49 Two cases of 4 cases in our study cohort with CBFB mutations (none of which are included in the Razavi et al.47 study cohort) showed pleomorphic lobular histology, which are enriched in Luminal Androgen Receptor TNBCs, positive AR IHC, and low numbers of stromal tumor-infiltrating immune cells. The significance of CBFB gene and its potential role in stromal tumor-infiltrating immune cells, as well as the importance of TNBC molecular subtypes in relation to PD-L1 expression, warrant further investigation.
Other studies revealing molecular insights involved with PD-L1 expression remain limited. Zeng and colleagues37 reported significant correlation of PD-L1 expression in tumor-infiltrating lymphocytes and p53 expression by immunohistochemistry. Barroso-Sousa et al.50 found in their study of 64 patients with TNBC that higher tumor mutational burden was associated with longer survival and alterations in PTEN were associated with poorer outcomes.
Our study is limited by its retrospective nature. Additionally, study cases included in our cohort were evaluated for PD-L1 expression at the request of the treating physician in patients with locally advanced or metastatic breast cancer; thus, non-random selection of cases places this study at risk of sampling bias. Furthermore, this study is limited by the number of patients who received atezolizumab and nab-paclitaxel, and the short clinical follow-up time of these patients, further restricting the conclusions one can derive from the cohort. Due to this limited clinical experience, further analyses of this patient cohort could not be performed. Subsequent reporting of clinical outcomes and factors associated with clinical benefit may be performed with longer follow-up. Moreover, approximately two-thirds of our study cases had undergone molecular testing with next-generation sequencing analysis using a select panel of 468 genes. Further testing with whole exome or genome sequencing in this regard in a prospective setting may reveal associations with genetic alterations not found in this study. Despite these limitations, our cohort provides unique insights into the distribution of PD-L1 staining in primary versus non-primary breast carcinomas, and preliminary information on the molecular characteristics of tumors associated with PD-L1-positive stromal tumor-infiltrating immune cells.
Herein we have highlighted the pathologic findings associated with PD-L1 expression. We observed that 47.4% of triple-negative breast carcinomas tested with PD-L1 IHC were positive. Triple-negative breast carcinoma was more likely to be positive for PD-L1 in the setting of primary breast tumor samples and higher degree of stromal tumor-infiltrating immune cells. Our data suggest PD-L1 testing of the primary TNBC sample should be considered. Mutations in CBFB gene were significantly associated with primary TNBCs with PD-L1-negative stromal tumor-infiltrating immune cells. Further investigation with immunologic and molecular studies is warranted to identify additional findings predictive of response to atezolizumab therapy.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the Molecular Diagnostics Service in the Department of Pathology and the Marie-Josee and Henry R. Kravis Center for Molecular Oncology.
Source of Funding Support:
BW, JSR-F, TT, MER and LN are funded in part by the Breast Cancer Research Foundation. This work was supported in part by a National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30CA008748).
Footnotes
The abstract for this paper was presented at the 109th annual meeting of the United States and Canadian Academy of Pathology on March 3, 2020 in Los Angeles, California, United States.
Declaration of Conflicting Interests: The authors declare no conflicts of interest.
REFERENCES
- 1.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–8. [DOI] [PubMed] [Google Scholar]
- 2.Brown M, Tsodikov A, Bauer KR, Parise CA, Caggiano V. The role of human epidermal growth factor receptor 2 in the survival of women with estrogen and progesterone receptor-negative, invasive breast cancer: the California Cancer Registry, 1999-2004. Cancer. 2008;112:737–47. [DOI] [PubMed] [Google Scholar]
- 3.Parise CA, Bauer KR, Caggiano V. Variation in breast cancer subtypes with age and race/ethnicity. Crit Rev Oncol Hematol. 2010;76:44–52. [DOI] [PubMed] [Google Scholar]
- 4.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. [DOI] [PubMed] [Google Scholar]
- 5.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.den Brok WD, Speers CH, Gondara L, Baxter E, Tyldesley SK, Lohrisch CA. Survival with metastatic breast cancer based on initial presentation, de novo versus relapsed. Breast Cancer Res Treat. 2017;161:549–56. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network clinical practice guidelines in oncology — breast cancer. V3: National Comprehensive Cancer Network; 2019. [Google Scholar]
- 8.Andre F, Zielinski CC. Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Ann Oncol. 2012;23 Suppl 6:vi46–51. [DOI] [PubMed] [Google Scholar]
- 9.Yardley DA, Coleman R, Conte P, Cortes J, Brufsky A, Shtivelband M, et al. nab-Paclitaxel plus carboplatin or gemcitabine versus gemcitabine plus carboplatin as first-line treatment of patients with triple-negative metastatic breast cancer: results from the tnAcity trial. Ann Oncol. 2018;29:1763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. 2016;13:228–41. [DOI] [PubMed] [Google Scholar]
- 11.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. [DOI] [PubMed] [Google Scholar]
- 12.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. [DOI] [PubMed] [Google Scholar]
- 14.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol. 2019;12:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069–86. [DOI] [PubMed] [Google Scholar]
- 18.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018;379:2108–21. [DOI] [PubMed] [Google Scholar]
- 19.Marra A, Viale G, Curigliano G. Recent advances in triple negative breast cancer: the immunotherapy era. BMC Med. 2019;17:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reis H, Serrette R, Posada J, Lu V, Chen YB, Gopalan A, et al. PD-L1 Expression in Urothelial Carcinoma With Predominant or Pure Variant Histology: Concordance Among 3 Commonly Used and Commercially Available Antibodies. Am J Surg Pathol. 2019;43:920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36:2105–22. [DOI] [PubMed] [Google Scholar]
- 23.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010;134:e48–72. [DOI] [PubMed] [Google Scholar]
- 24.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middha S, Zhang L, Nafa K, Jayakumaran G, Wong D, Kim HR, et al. Reliable Pan-Cancer Microsatellite Instability Assessment by Using Targeted Next-Generation Sequencing Data. JCO Precis Oncol. 2017;2017:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu B, Ye K, Zhang Q, Lu C, Xie M, McLellan MD, et al. MSIsensor: microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics. 2014;30:1015–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertucci F, Ng CKY, Patsouris A, Droin N, Piscuoglio S, Carbuccia N, et al. Genomic characterization of metastatic breast cancers. Nature. 2019;569:560–4. [DOI] [PubMed] [Google Scholar]
- 29.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droeser RA, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin T, Zeng YD, Qin G, Xu F, Lu JB, Fang WF, et al. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget. 2015;6:33972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wimberly H, Brown JR, Schalper K, Haack H, Silver MR, Nixon C, et al. PD-L1 Expression Correlates with Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancer Immunol Res. 2015;3:326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baptista MZ, Sarian LO, Derchain SF, Pinto GA, Vassallo J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016;47:78–84. [DOI] [PubMed] [Google Scholar]
- 34.Park IH, Kong SY, Ro JY, Kwon Y, Kang JH, Mo HJ, et al. Prognostic Implications of Tumor-Infiltrating Lymphocytes in Association With Programmed Death Ligand 1 Expression in Early-Stage Breast Cancer. Clin Breast Cancer. 2016;16:51–8. [DOI] [PubMed] [Google Scholar]
- 35.Kurozumi S, Inoue K, Matsumoto H, Fujii T, Horiguchi J, Oyama T, et al. Clinicopathological values of PD-L1 expression in HER2-positive breast cancer. Sci Rep. 2019;9:16662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M, Sun H, Zhao S, Wang Y, Pu H, Wang Y, et al. Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget. 2017;8:31347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng Y, Wang CL, Xian J, Ye Q, Qin X, Tan YW, et al. Positive correlation between programmed death ligand-1 and p53 in triple-negative breast cancer. Onco Targets Ther. 2019;12:7193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarty KS Jr., Szabo E, Flowers JL, Cox EB, Leight GS, Miller L, et al. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res. 1986;46:4244s–8s. [PubMed] [Google Scholar]
- 39.Szekely B, Bossuyt V, Li X, Wali VB, Patwardhan GA, Frederick C, et al. Immunological differences between primary and metastatic breast cancer. Ann Oncol. 2018;29:2232–9. [DOI] [PubMed] [Google Scholar]
- 40.Manson QF, Schrijver W, Ter Hoeve ND, Moelans CB, van Diest PJ. Frequent discordance in PD-1 and PD-L1 expression between primary breast tumors and their matched distant metastases. Clin Exp Metastasis. 2019;36:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y, Meeker A, et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol. 2016;47:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cancer Genome Atlas N Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Wang F, Chen X, Zhang Y, Wang M, Liu H, et al. CSF3R Mutations are frequently associated with abnormalities of RUNX1, CBFB, CEBPA, and NPM1 genes in acute myeloid leukemia. Cancer. 2018;124:3329–38. [DOI] [PubMed] [Google Scholar]
- 45.Cameron ER, Neil JC. The Runx genes: lineage-specific oncogenes and tumor suppressors. Oncogene. 2004;23:4308–14. [DOI] [PubMed] [Google Scholar]
- 46.Shigesada K, van de Sluis B, Liu PP. Mechanism of leukemogenesis by the inv(16) chimeric gene CBFB/PEBP2B-MHY11. Oncogene. 2004;23:4297–307. [DOI] [PubMed] [Google Scholar]
- 47.Razavi P, Chang MT, Xu G, Bandlamudi C, Ross DS, Vasan N, et al. The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell. 2018;34:427–38 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bailey MH, Tokheim C, Porta-Pardo E, Sengupta S, Bertrand D, Weerasinghe A, et al. Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell. 2018;173:371–85 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bareche Y, Venet D, Ignatiadis M, Aftimos P, Piccart M, Rothe F, et al. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann Oncol. 2018;29:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barroso-Sousa R, Keenan TE, Pernas S, Exman P, Jain E, Garrido-Castro AC, et al. Tumor mutational burden and PTEN alterations as molecular correlates of response to PD-1/L1 blockade in metastatic triple-negative breast cancer. Clin Cancer Res. 2020:[Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


