Abstract
Thyroid hormones are necessary for the normal functioning of physiological systems. Therefore, knowledge of any factor (whether genetic, environmental or intrinsic) that alters the levels of thyroid-stimulating hormone (TSH) and thyroid hormones is crucial. Genetic factors contribute up to 65% of interindividual variations in TSH and thyroid hormone levels, but many environmental factors can also affect thyroid function. This review discusses studies that have analyzed the impact of environmental factors on TSH and thyroid hormone levels in healthy adults. We included lifestyle factors (smoking, alcohol consumption, diet and exercise) and pollutants (chemicals and heavy metals). Many inconsistencies in the results have been observed between studies, making it difficult to draw a general conclusion about how a particular environmental factor influences TSH and thyroid hormone levels. However, lifestyle factors that showed the clearest association with TSH and thyroid hormones were smoking, body mass index (BMI) and iodine (micronutrient taken from the diet). Smoking mainly led to a decrease in TSH levels and an increase in triiodothyronine (T3) and thyroxine (T4) levels, while BMI levels were positively correlated with TSH and free T3 levels. Excess iodine led to an increase in TSH levels and a decrease in thyroid hormone levels. Among the pollutants analyzed, most studies observed a decrease in thyroid hormone levels after exposure to perchlorate. Future studies should continue to analyze the impact of environmental factors on thyroid function as they could contribute to understanding the complex background of gene–environment interactions underlying the pathology of thyroid diseases.
Keywords: thyroid hormones, TSH, environmental factors, lifestyle factors, pollutants, diet, chemicals
1. Introduction
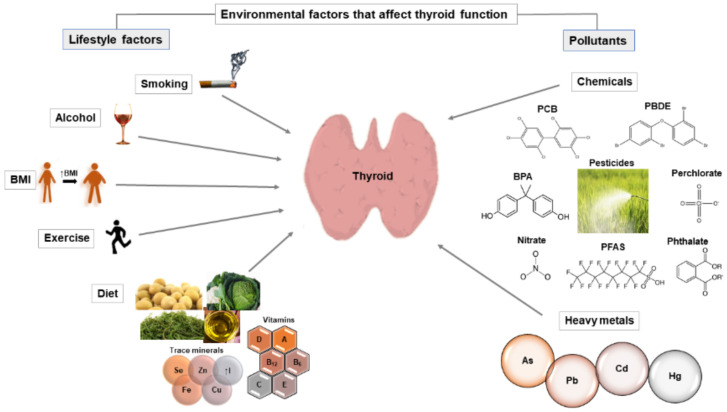
Thyroid hormones are crucial for normal development and necessary for the proper functioning of physiological systems. Thyroid hormone synthesis is regulated by feedback mechanisms mediated by the hypothalamus–pituitary–thyroid (HPT) axis. Decreased thyroid hormone levels lead to increased synthesis of hypothalamic thyrotropin-releasing hormone (TRH) which increases the secretion of thyroid-stimulating hormone (TSH) from the anterior pituitary. TSH stimulates the production of thyroid hormones from thyrocytes [1]. Thyroid hormone synthesis requires active iodide uptake through sodium/iodide symporter (NIS), thyroglobulin (Tg) production and Tg iodination by thyroid peroxidase (TPO) enzyme. Thyroid hormones, thyroxine (T4) and triiodothyronine (T3), are released by Tg proteolysis. T4 is released from the thyroid gland in a much larger amount (in a ratio of approximately 14:1) [2]. However, most T4 is converted to T3 in target tissues (by the action of type 1 and type 2 iodothyronine deiodinases (Dio1 and Dio2)) [3]. When secreted in plasma, thyroid hormones are bound to plasma proteins (more than 99.7%) and only a small amount of thyroid hormones are in unbound (free) form (fT4 and fT3). The unbound form of thyroid hormones is biologically active [4]. Variation in the TSH and thyroid hormone levels may indicate that normal thyroid function has been altered. Since the prevalence of thyroid diseases is very high (it is estimated that 12% of the U.S. population will develop a thyroid condition during their lifetime [5]), understanding the mechanisms underlying the variations in TSH and thyroid hormone levels is crucial. Genetic factors account for up to 65% of interindividual variations in TSH and thyroid hormone levels [6,7], but many other factors can also influence thyroid function. Such factors include demographic factors (age and sex [8,9]), intrinsic factors (microbiota [10], stress [11]), usage of medicaments [12] and various environmental factors [13,14,15,16]. The purpose of this review is to provide a comprehensive insight into the literature discussing the impact of environmental factors (such as lifestyle factors and pollutants) on TSH and thyroid hormone levels (Figure 1). Knowledge of any factors that could affect TSH and thyroid hormone levels is especially important for vulnerable groups, such as people with thyroid diseases and pregnant women. However, the focus of this review will be on the general population without thyroid diseases.
Figure 1.
Environmental factors (lifestyle factors and pollutants) that affect thyroid function. As, arsenic; BMI, body mass index; BPA, bisphenol A; Cd, cadmium; Cu, copper; Fe, iron; Hg, mercury; I, iodine; Pb, lead; PBDE, polybrominated diphenyl ether; PCB, polychlorinated biphenyl; PFAS, perfluoroalkyl substance; Se, selenium; Zn, zinc.
2. Short Overview of Genetic Factors That Influence TSH and Thyroid Hormone Levels
Twin studies have shown that genetic factors underlie 45–65% of interindividual variations in TSH and thyroid hormone levels [6,7]. Many of these genetic variants have been identified in genome-wide association studies (GWAS) [17,18,19,20]. Genes that contribute to interindividual variations in TSH and thyroid hormone levels are divided into the following groups: genes encoding proteins involved in the synthesis (TG, TPO, CAPZB), metabolism (AADAT, DIO1, DIO2, DIO3OS) and transport (SLC17A4, OATP1B1, MCT8) of thyroid hormones; genes for proteins involved in TSH receptor signaling cascade (TSHR, PDE10A, PDE8B, GNAS, ITPK1); genes encoding growth factors and growth factor binding proteins (FOXA2, IGF2BP2, VEGFA, IGFBP2/IGFBP5, FGF7, INSR, SASH1); genes for transcription factors and proteins involved in the development of HPT axis (SOX9, NCOR1, FOXE1, TTF1/MBIP, GLIS3, LHX3, NFIA); and genes for proteins with unknown thyroid function (reviewed in [20]). Although major progress has been made in researching the genetic basis of thyroid function, many new potential genetic factors affecting TSH and thyroid hormone levels have yet to be discovered.
3. Environmental Factors That Influence TSH and Thyroid Hormone Levels
3.1. Lifestyle Factors
3.1.1. Smoking
Most studies investigating the influence of smoking on TSH and thyroid hormone levels have observed a decrease in TSH levels and an increase in T3 and T4 levels in smokers [16,21,22,23]. Large population-based studies have confirmed these results [24,25,26,27,28] (Table 1). In the majority of studies, a decrease in TSH levels was followed by an increase in thyroid hormone levels (Table 1). Recently, in a large cohort of 5766 White North European subjects, Gruppen et al., observed that cigarette smoking leads to a decrease in TSH levels and an increase in fT3 and fT4 levels [16]. Kim et al., even noticed a dose-dependent relationship between cigarette smoking (measured by serum cotinine levels, which is an objective measure of smoke exposure) and TSH levels (study included 4249 participants). They observed that every 10 ng/mL increase in serum cotinine resulted in a 1.4% decrease in TSH levels [28]. It was also observed that TSH levels gradually increased after smoking cessation [27]. The mechanism through which cigarette smoking affects TSH and thyroid hormone levels is still unclear. This is not surprising since there are more than 4000 components in tobacco. One of the proposed mechanisms is that thiocyanate, which is transformed from cyanide in tobacco, inhibits iodide transport and iodine organification (incorporation of iodine into Tg) [29]. Since the transport of iodide is a rate-limiting step in the synthesis of thyroid hormones, exposure to thiocyanate results in decreased thyroid hormone synthesis. However, thiocyanate has been observed to decrease protein-bound T4 levels and consequently increase fT4 levels [30] (which could explain the increase in fT4 levels in smokers). Additionally, several studies have suggested that smoking reduces autoimmune processes in the thyroid gland [24,31], resulting in alterations in TSH and thyroid hormone levels. It has also been suggested that an increase in thyroid hormone levels and a consequent decrease in TSH levels [32] is the result of increased sympathetic nervous activity in smokers [33]. Alarming results were obtained in the study of Filis et al., showing that maternal smoking disrupts fetal thyroid development [34].
Table 1.
Effect of lifestyle factors on thyroid-stimulating hormone, thyroid hormone and thyroglobulin levels in healthy individuals.
| Factor | Effect on Hormone Levels | Number of Participants | Reference | ||
|---|---|---|---|---|---|
| Smoking | ↓TSH, ↑fT4, ↑fT3 | 5766 | [16] | ||
↓TSH,  fT4, fT4,  fT3 fT3 |
4585 | [146] | |||
| ↓TSH, ↑fT4 | 895 | [21] | |||
↓TSH,  fT4 fT4 |
4357 | [28] | |||
| ↓TSH | 15,181 | [27] | |||
↓TSH,  fT4 fT4 |
3404 | [251] | |||
| ↓TSH | 5639 | [252] | |||
| ↓TSH | 4427 | [253] | |||
| ↓TSH, ↓tT4, ↑Tg | 1409 | [208] | |||
↓TSH, ↓tT4,  fT4, fT4,  tT3, ↑fT3, ↑Tg tT3, ↑fT3, ↑Tg |
1540 | [254] | |||
| ↓TSH | 1581 | [255] | |||
 TSH, TSH,  fT4, ↑fT3 fT4, ↑fT3 |
931 | [256] | |||
↓TSH,  fT4 fT4 |
3399 | [26] | |||
| ↓TSH, ↓tT4, ↓tT3 | 237 | [257] | |||
↓TSH,  fT4 fT4 |
1853 | [64] | |||
| ↓TSH, ↑fT4, ↑fT3 | 7799 | [22] | |||
| ↓TSH | 30,834 | [25] | |||
| ↓TSH, ↑fT4, ↑fT3 | 6085 | [258] | |||
↓TSH,  T4 T4 |
15,592 | [24] | |||
| ↓TSH, ↑T4 | 4462 | [259] | |||
↓TSH,  T4, T4,  fT4, fT4,  T3, T3,  fT3 fT3 |
1154 | [260] | |||
↓TSH,  T3 T3 |
4100 | [261] | |||
 TSH, TSH,  T4, ↑T3 T4, ↑T3 |
50 | [262] | |||
↓TSH,  T4, T4,  T3, ↑Tg T3, ↑Tg |
219 | [263] | |||
↓TSH, ↑T4,  T3 T3 |
181 | [264] | |||
 TSH, ↓T4, ↓T3 TSH, ↓T4, ↓T3 |
200 | [265] | |||
↓TSH,  T4, ↑T3, ↓rT3, ↑Tg T4, ↑T3, ↓rT3, ↑Tg |
441 | [266] | |||
| Alcohol consumption | ↑TSH, ↓fT3 | 5766 | [16] | ||
 TSH, ↓fT4 TSH, ↓fT4 |
549 (men) | [40] | |||
 TSH, ↓fT4, ↓fT3 TSH, ↓fT4, ↓fT3 |
67 | [39] | |||
 TSH, TSH,  T4, T4,  T3 T3 |
100 | [38] | |||
 TSH, TSH,  T4, ↑T3 T4, ↑T3 |
30 | ||||
 TSH, TSH,  fT4, ↓T4, fT4, ↓T4,  fT3, fT3,  T3 T3 |
55 | [37] | |||
| ↑fT4 | 21 | [43] | |||
 TSH, TSH,  T4, T4,  fT4, ↓T3, ↓fT3 fT4, ↓T3, ↓fT3 |
70 | [36] | |||
↑TSH,  T4, T4,  fT4, ↓fT3, ↓T3 fT4, ↓fT3, ↓T3 |
80 | [41] | |||
 TSH, TSH,  T4, T4,  T3, ↑Tg T3, ↑Tg |
111 | [44] | |||
 TSH, TSH,  fT4, ↓tT4, ↓tT3 fT4, ↓tT4, ↓tT3 |
38 | [42] | |||
| Increased body mass index |
 TSH, ↓fT4 TSH, ↓fT4 |
90 | [267] | ||
| ↑TSH (BMI higher than 25.3 kg/m2) | 11,224 | [268] | |||
| ↓TSH (BMI lower than 25.3 kg/m2) | |||||
| ↑TSH | 75 | [269] | |||
| ↑TSH | 2789 | [15] | |||
 TSH, TSH,  fT4, fT4,  fT3 fT3 |
34 | [270] | |||
| ↑TSH, ↓fT4, ↑fT3 | 77,991 | [9] | |||
 TSH TSH |
88 | [271] | |||
| ↓fT4, ↑fT3, ↑fT3/ fT4 | 16,975 | [65] | |||
 TSH, TSH,  fT4, ↓fT3 fT4, ↓fT3 |
36,655 (all subjects) | [272] | |||
| ↓fT4 | 18,746 (men) | ||||
| ↑TSH | 80 | [273] | |||
 TSH, ↓fT4 TSH, ↓fT4 |
7693 | [274] | |||
 TSH, ↓fT4 TSH, ↓fT4 |
1100 | [275] | |||
| ↑TSH | 140 | [276] | |||
 TSH, TSH,  fT4, ↑fT3 fT4, ↑fT3 |
940 | [277] | |||
↑TSH, ↓fT4,  fT3 fT3 |
26,719 | [56] | |||
 TSH, TSH,  fT4, fT4,  fT3 fT3 |
1275 | [278] | |||
 TSH TSH |
162 | [279] | |||
| ↑TSH, ↓fT4 | 9402 | [8] | |||
| ↑TSH | 800 | [280] | |||
 TSH TSH |
1097 | [281] | |||
↑TSH,  fT4, fT4,  tT4, tT4,  fT3, ↑tT3, ↑Tg fT3, ↑tT3, ↑Tg |
746 (men) | [208] | |||
| ↑TSH | 1044 (men) | [282] | |||
 TSH, ↓fT4, ↑fT3, ↑tT3, ↑fT3/ fT4 TSH, ↓fT4, ↑fT3, ↑tT3, ↑fT3/ fT4 |
2315 | [60] | |||
 TSH, ↓fT4 TSH, ↓fT4 |
6241 (all subjects) | [283] | |||
| ↓TSH | 2837 (women) | ||||
 TSH, TSH,  fT4, ↑tT4, fT4, ↑tT4,  fT3, ↑tT3 fT3, ↑tT3 |
736 | [67] | |||
| ↑TSH | 417 | [284] | |||
| ↑TSH | 5918 | [285] | |||
↑TSH,  fT4, fT4,  fT3 fT3 |
60 | [286] | |||
 fT4, ↑fT3 fT4, ↑fT3 |
865 | [287] | |||
↑TSH,  fT4, ↑fT3 fT4, ↑fT3 |
3114 | [288] | |||
↑TSH,  fT4 fT4 |
778 | [289] | |||
| ↑TSH | 1084 | [290] | |||
| ↑TSH | 15,020 | [291] | |||
↑TSH,  fT4 fT4 |
581 | [292] | |||
↑TSH,  fT4, ↑fT3 fT4, ↑fT3 |
520 | [59] | |||
 TSH, TSH,  fT4, ↑T3, ↑T3/fT4 fT4, ↑T3, ↑T3/fT4 |
275 | [293] | |||
↑TSH,  fT4, fT4,  tT3 tT3 |
27,097 | [53] | |||
 TSH, ↓fT4 TSH, ↓fT4 |
44,196 | [294] | |||
 TSH, ↓fT4 TSH, ↓fT4 |
1853 | [64] | |||
↑TSH,  fT4, ↑fT3 fT4, ↑fT3 |
152 | [242] | |||
 TSH, ↓fT4 TSH, ↓fT4 |
1572 | [295] | |||
↑TSH,  fT4, fT4,  fT3 fT3 |
265 | [296] | |||
↑TSH,  fT4 fT4 |
86 | [297] | |||
 TSH, TSH,  fT4, ↑fT3, ↑fT3/ fT4 fT4, ↑fT3, ↑fT3/ fT4 |
201 | [57] | |||
| ↑TSH | 1725 | [23] | |||
↑TSH,  fT4, fT4,  fT3 fT3 |
87 | [298] | |||
↑TSH, ↓fT4,  fT3 fT3 |
4082 | [55] | |||
| Diet | Soy food or soy isoflavones | ↑TSH,  fT4, fT4,  fT3 fT3 |
Meta-analysis | [14] | |
↑TSH, ↓fT4,  fT3, ↑rT3 fT3, ↑rT3 |
400 | [96] | |||
↑TSH, ↓fT4,  fT3 fT3 |
200 | [95] | |||
 TSH, TSH,  fT4, fT4,  fT3 fT3 |
47 | [93] | |||
 TSH, TSH,  fT4 fT4 |
505 | [299] | |||
 TSH, TSH,  fT4, ↓fT3 fT4, ↓fT3 |
43 | [100] | |||
 TSH, TSH,  fT4 fT4 |
403 | [94] | |||
 TSH, TSH,  fT4, fT4,  fT3 fT3 |
93 | [300] | |||
 TSH, TSH,  fT4 fT4 |
63 | [301] | |||
 TSH, TSH,  fT4, fT4,  fT3 fT3 |
389 | [92] | |||
 TSH, TSH,  T4 T4 |
Meta-analysis | [302] | |||
 TSH TSH |
77 | [303] | |||
 TSH, TSH,  fT4, fT4,  tT4, tT4,  fT3, fT3,  tT3, tT3,  Tg Tg |
147 | [91] | |||
 TSH, TSH,  fT4, fT4,  tT4, tT4,  fT3, fT3,  tT3 tT3 |
35 | [90] | |||
 TSH, TSH,  T4, T4,  T3, T3,  fT4 fT4 |
25 | [143] | |||
 TSH TSH |
89 | [304] | |||
 TSH, TSH,  T4, T4,  T3 T3 |
38 | [89] | |||
 TSH, ↓fT4, TSH, ↓fT4,  fT3 fT3 |
32 | [98] | |||
| ↑TSH, ↑T4, ↑T3 | 73 | [97] | |||
 TSH TSH |
76 | [305] | |||
 TSH, TSH,  fT4, ↓fT3, fT4, ↓fT3,  T4, T4,  T3 T3 |
14 | [306] | |||
 TSH, TSH,  fT4, fT4,  tT4, tT4,  fT3, fT3,  tT3 tT3 |
18 | [99] | |||
| Brassica vegetables | Sulforaphane (natural product present in cruciferous vegetables like broccoli) |
 TSH, TSH,  T4, T4,  Tg Tg |
45 | [109] | |
| Roots of cruciferous plant Lepidium peruvianum Chacon |
 TSH, TSH,  T4, T4,  T3 T3 |
20 | [108] | ||
| Brussels sprouts |
 TSH, TSH,  tT4, tT4,  fT4, fT4,  tT3 tT3 |
10 | [107] | ||
| Other food | Seaweed | ↑TSH,  fT3, fT3,  fT4 fT4 |
19 | [144] | |
| Seaweed | ↑TSH (returned to normal after several days),  fT4 fT4 |
9 | [145] | ||
| Seaweed | ↑TSH (returned to normal after several days), ↓fT4, ↓fT3 (returned to normal after several days) | 13 | [141] | ||
| Seaweed | ↑TSH,  T4, T4,  T3, T3,  fT4 fT4 |
25 | [143] | ||
| Kelp | ↑TSH,  fT4, ↓fT3 fT4, ↓fT3 |
36 | [142] | ||
| Kelp, vegans vs. omnivores | ↑TSH | 101 | [307] | ||
| Full-fat cheese, cottage cheese, hard cheese | ↓fT4 | 4585 | [146] | ||
| Pasta and rice | ↑fT4 | ||||
| Whole-grain bread | ↓fT4 | ||||
| White bread | ↑fT4 | ||||
| White fish, blue fish, dried fish, seafood, squid | ↑fT4, ↑fT3 | ||||
| Fruit juices, cedevita, nonalcoholic drinks | ↓TSH, ↑fT4 | ||||
| Pork, beef, eggs | ↓fT4 | ||||
| Bacon, sausages | ↑fT4, ↑fT3 | ||||
| Butter, animal fat | ↓fT4 | ||||
| Canned vegetables, mushrooms | ↓fT4, ↓fT3 | ||||
| Powder soups, vegetable juices | ↑fT4 | ||||
| Venison, fish derivates | ↓TSH | ||||
| Non-home-made meal | ↑T4 | 100 | [147] | ||
| Whole grains, green tea | ↓T3 | ||||
| Pasta | ↑fT4 | ||||
| Olive oil | Mediterranean diet |
 TSH, ↓fT4, ↓fT3 TSH, ↓fT4, ↓fT3 |
324 | [120] | |
| Olive oil |
 TSH, ↓fT4, ↓fT3 TSH, ↓fT4, ↓fT3 |
||||
| Vegetables cooked with olive oil | ↑T3 | 100 | [147] | ||
| Food associated with the development of endemic goiter | Cassava | ↓T4, ↓T3 | 20 | [308] | |
| Beverages | Coffee |
 TSH, ↑fT4 TSH, ↑fT4 |
9408 | [164] | |
| Coffee |
 TSH, TSH,  T3 T3 |
Not reported | [163] | ||
| Micronutrients | ↑ Vitamin D |
 TSH, ↑fT4, TSH, ↑fT4,  fT3 fT3 |
123 | [309] | |
| ↑ Vitamin D |
 TSH, ↓T4, ↓T3 TSH, ↓T4, ↓T3 |
300 | [184] | ||
| ↓ Vitamin D |
 TSH, TSH,  fT4 fT4 |
2006 | [310] | ||
| ↓ Vitamin D | ↑TSH | 294 | [182] | ||
| ↑ Vitamin D | ↓TSH | 1424 | [183] | ||
| ↑ Vitamin D | ↓TSH | 2582 | [175] | ||
| ↑Selenium |
 TSH, ↓fT4, TSH, ↓fT4,  fT3 fT3 |
69 | [207] | ||
 TSH, ↑fT4 TSH, ↑fT4 |
184 (women) | [229] | |||
 TSH, TSH,  T4, T4,  fT4, fT4,  T3, T3,  rT3 rT3 |
387 | [311] | |||
↓TSH, ↓fT4,  fT3 fT3 |
361 | [206] | |||
 TSH, TSH,  fT4, fT4,  fT3, fT3,  Tg Tg |
1383 | [208] | |||
 TSH, ↓fT4, ↓fT3 TSH, ↓fT4, ↓fT3 |
1144 | [205] | |||
 TSH, ↑fT4 TSH, ↑fT4 |
140 | [312] | |||
 TSH, TSH,  tT4, ↑T3, tT4, ↑T3, |
28 | [313] | |||
 TSH, TSH,  fT4, fT4,  fT3, fT3,  Tg Tg |
88 | [314] | |||
 TSH, TSH,  T4, T4,  T3 T3 |
42 | [315] | |||
 TSH, ↓fT4, TSH, ↓fT4,  fT3 fT3 |
52 | [316] | |||
 TSH, ↓fT4, TSH, ↓fT4,  tT4, tT4,  fT3, fT3,  tT3, ↑fT3/fT4 tT3, ↑fT3/fT4 |
368 | [204] | |||
| ↑TSH, ↓T3 | 12 | [317] | |||
 TSH, TSH,  fT4, fT4,  fT3, fT3,  fT3/fT4 fT3/fT4 |
44 | [219] | |||
↓T4,  Tg Tg |
52 | [203] | |||
 TSH, ↓T4, TSH, ↓T4,  T3, ↑T3/T4 T3, ↑T3/T4 |
109 | [202] | |||
 TSH, ↓T4, ↓fT4, TSH, ↓T4, ↓fT4,  T3, ↓rT3 T3, ↓rT3 |
52 | [201] | |||
| ↑Zinc |
 TSH, TSH,  fT4, fT4,  fT3 fT3 |
98 | [217] | ||
 TSH, ↓tT4, ↓fT4, TSH, ↓tT4, ↓fT4,  tT3, tT3,  fT3 fT3 |
746 (men) | [208] | |||
 TSH, TSH,  fT4, ↑fT3 fT4, ↑fT3 |
64 | [220] | |||
 TSH TSH |
219 | [318] | |||
↓tT4,  tT3 tT3 |
178 | [218] | |||
 TSH, TSH,  fT4, ↑fT3, ↑fT3/fT4 fT4, ↑fT3, ↑fT3/fT4 |
44 | [219] | |||
 TSH, TSH,  tT4, tT4,  fT4, fT4,  tT3 tT3 |
109 | [202] | |||
| Iron deficiency |
 TSH, ↓fT4, ↓fT3 TSH, ↓fT4, ↓fT3 |
3846 | [228] | ||
| Iron deficiency anemia |
 TSH, ↓tT4, TSH, ↓tT4,  fT4, ↓tT3, fT4, ↓tT3,  fT3 fT3 |
128 | [227] | ||
| Anemia |
 TSH, TSH,  fT4, fT4,  tT4, ↑fT3 tT4, ↑fT3 |
50 | [226] | ||
| Iron supplements |
 TSH, TSH,  fT4, ↑tT4, ↑tT3, ↓rT3 fT4, ↑tT4, ↑tT3, ↓rT3 |
94 | [319] | ||
| Anemia | ↓fT4, ↓fT3 | 20 | [320] | ||
| ↑Copper |
 TSH, ↑fT4 TSH, ↑fT4 |
417 | [229] | ||
 TSH, ↑fT4, ↑tT4, TSH, ↑fT4, ↑tT4,  fT3, fT3,  tT3, tT3,  Tg Tg |
746 (men) | [208] | |||
 TSH, TSH,  fT4, ↑tT4, fT4, ↑tT4,  fT3, ↑tT3, fT3, ↑tT3,  Tg Tg |
663 (women) | ||||
| Iodine excess | ↑TSH | Meta-analysis | [240] | ||
| ↑TSH | 78,470 | [241] | |||
↑TSH,  fT4, ↓fT3 fT4, ↓fT3 |
854 | [239] | |||
| ↑TSH, ↓fT4, ↓fT3 | 236 | [321] | |||
| ↑TSH, ↓fT4, ↓fT3 | 256 | [238] | |||
| ↑TSH, ↓fT4, ↑Tg | 10 | [237] | |||
↓T4,  T3 T3 |
30 | [235] | |||
| ↑TSH, ↓T4, ↓T3 | 32 | [236] | |||
| Exercise |
 TSH, TSH,  fT4 fT4 |
2470 | [250] | ||
| ↓TSH, ↑T4, ↑T3 | 36 | [248] | |||
 TSH, ↑fT4, ↑fT3 TSH, ↑fT4, ↑fT3 |
9 | [247] | |||
| ↑TSH, ↑fT4, ↑T4, ↓fT3, ↓T3 | 60 | [249] | |||
 TSH, TSH,  fT4, fT4,  T4, T4,  fT3, fT3,  T3 T3 |
26 | [246] | |||
| ↑fT4, ↓fT3, ↓T3, ↑rT3 | 27 | [322] | |||
 TSH TSH |
6 | [323] | |||
 fT4, fT4,  T4, T4,  fT3, fT3,  T3, T3,  rT3 rT3 |
46 | [324] | |||
| ↑TSH, ↑fT4 | 14 | [325] | |||
 T4, ↑T3, ↑rT3 T4, ↑T3, ↑rT3 |
12 | [326] | |||
| ↑T4, ↓T3, ↑rT3 | 4 | [327] | |||
| ↑TSH | 8 | [328] | |||
Studies involving pregnant women, infants, children and individuals with a history of thyroid diseases were not included in this table. rT3, reverse triiodothyronine; T3, triiodothyronine; T4, thyroxine; tT3, total T3; tT4, total T4; Tg, thyroglobulin; TSH, thyroid-stimulating hormone.
3.1.2. Alcohol Consumption
Alcohol has been shown to have a toxic effect on thyroid cells, which is considered to be the cause of decreased thyroid volume in alcoholics [35]. A recent study investigating the influence of alcohol consumption on thyroid hormone levels reported an increase in TSH levels and a decrease in fT3 levels [16]. However, other studies reported conflicting results, with TSH levels being unchanged [36,37,38,39,40] or increased [16,41] in alcoholics, while levels of thyroid hormones were decreased [16,39,40,42], unchanged [36] or increased [38,43] (Table 1). Nevertheless, most of these studies were underpowered, and the study that included the largest number of participants (5766 individuals) detected an increase in TSH levels and a decrease in fT3 levels [16]. Serum Tg levels were increased in patients with chronic alcoholic cirrhosis [44]. Many studies have measured levels of TSH and thyroid hormones during alcohol withdrawal (reviewed in [35]); however, inconsistencies have been reported between studies. Aoun and collaborators even detected a positive correlation between fT3 and alcohol-seeking behaviors in alcoholics [45]. On the other hand, many studies have consistently shown blunted TSH response after TRH stimulation [43,46,47,48]. It has been experimentally proven that chronic ethanol treatment increases TRH levels [49], which could consequently lead to a decrease in pituitary TRH receptors [50] and blunted TSH response after TRH stimulation. Hermann et al., hypothesized that this could be the mechanism by which alcohol leads to alteration in TSH and thyroid hormone levels [51]. They suggested that a decrease in thyroid hormone levels in alcoholics induces an increase in TRH release [51]. Other authors proposed that thyroid dysfunction in alcoholics may be caused by euthyroid sick syndrome (ESS). This syndrome is characterized by decreased levels of T3 and increased levels of thyroid hormone metabolite reverse T3 (rT3). However, the results of many studies did not support this hypothesis (reviewed in [35]). In addition, there is evidence from in vitro and in vivo studies that additional compounds in some alcoholic beverages, such as resveratrol (a natural polyphenol found in red wine) also have a thyroid-disrupting effect (reviewed in [52]).
3.1.3. Body Mass Index
The majority of studies that investigated the influence of body mass index (BMI) on TSH and thyroid hormone levels reported a positive correlation between BMI values and TSH [9,15,23,53,54,55,56] and fT3 levels [57,58,59,60,61] (Table 1). Even high maternal BMI has been shown to be associated with increased fetal TSH levels and increased fetal thyroid weight [34]. However, the results of studies investigating the association between fT4 and BMI were contradictory. Most studies have not observed an association between fT4 and BMI [54,57,59,62,63], although there are studies that have reported negative [9,55,56,60,61,64,65] and even positive association between fT4 and BMI in the general population [66,67,68] (Table 1). Many studies that investigated the influence of BMI on TSH and thyroid hormone levels involved a large number of participants, so sufficient statistical power was reached in these studies. Although for TSH and fT3, there was some consistency of results between studies, this was not observed for fT4. When only studies with a large number of participants (above 1000) were considered, most studies reported a negative correlation between BMI values and fT4 levels (Table 1). The relationship between thyroid hormone levels and weight is well understood in autoimmune disorders. Hyperthyroidism is accompanied by weight loss while hypothyroidism is associated with weight gain [69]. However, the reason for variation in TSH and thyroid hormone levels in euthyroid individuals after an increase in their BMI is not very well understood. Several hypotheses have been proposed. Adipose tissue secretes the hormone leptin which is also involved in the production of hypothalamic TRH (and consequently the production of pituitary TSH) [70]. There is a positive correlation between leptin levels and BMI [71], so this could be a good explanation for why TSH levels increase with increasing BMI. However, some authors think that changes in TSH levels and levels of thyroid hormones are the cause, not the consequence, of an increase in BMI. They propose that lower thyroid function can lead to obesity, probably as a result of a lower metabolic rate [53]. In fact, thyroid hormones have even been used to treat obesity in the past [72], although due to numerous side effects, this weight loss method has been discarded. It has also been speculated that the increase in TSH levels in obesity is a consequence of hormone resistance [73]. This hypothesis could explain why both TSH and T3 levels are increased in obesity. Since T3 receptors are reduced in obesity [74], this could lead to decreased negative feedback between TSH and thyroid hormones and consequently an increase in both TSH and T3 levels. It has also been hypothesized that alterations in TSH and thyroid hormone levels in obesity are due to the process of adjustment to weight gain or subclinical hypothyroidism [73].
3.1.4. Diet
In this section, we discuss how diet can alter TSH and thyroid hormone levels. We do not discuss the well-known dietary iodine deficiency considered to be the most common cause of hypothyroidism in the world [75], as iodine deficiency has decreased dramatically due to the salt iodization programs [76]. We discuss other components in diet that can change the levels of TSH and thyroid hormones, such as soy, brassica vegetables, food associated with the development of endemic goiter, beverages (coffee and tea), other food (junk food, seaweed, spices) and micronutrients (vitamins, trace minerals and macrominerals). It is important to point out that cyanogenic glucosides (reviewed in [77]) and flavonoids (reviewed in [78]) found in a wide range of plant-based food can alter TSH and thyroid hormone levels. Although there are many indications that a particular type of food affects thyroid function, an insufficient number of studies on this issue have been conducted so far. The majority of the studies have investigated the influence of soy-based food on TSH and thyroid hormone levels.
Soy
The possible antithyroid effect of soy-based food (soy, tofu, edamame, miso and soy milk) has long been of scientific concern. The goitrogenic compounds found in soy are soy isoflavones, a subclass of flavonoids. Isoflavones are also found in red clover and linseeds. Isoflavones inhibit TPO, an enzyme involved in the synthesis of thyroid hormones [79]. Many in vitro [79,80] and in vivo studies [81,82,83,84,85] have shown that soy isoflavones have a negative effect on thyroid function. Human studies have shown that soy-fed infants developed goiter [86,87,88]. A recent meta-analysis of human studies showed that soy supplementation did not affect thyroid hormone levels and only modestly raised TSH levels [14]. Most human studies have not shown the effect of soy food and goitrogenic compounds found in soy on TSH and thyroid hormone levels [89,90,91,92,93,94]. Some studies however have noticed an increase in TSH levels after consuming soy food or soy isoflavones [95,96,97]. Moreover, an increase [97] and a decrease [95,96,98] in fT4 levels and an increase [97] and a decrease [99,100] in fT3 levels after consumption of soy food or soy isoflavones were observed (Table 1). However, the majority of these studies were underpowered, and additional studies with a larger number of participants are needed to elucidate the influence of soy food and soy isoflavones on TSH and thyroid hormone levels. De Souza dos Santos et al., hypothesized that compared to the other species, the bioavailability of flavonoids to the human thyroid gland may be limited (due to less intestinal absorption and greater hepatic metabolism) [78]. This could explain the less deleterious effect of soy isoflavones on thyroid function in humans compared to experimental animals [78]. However, these authors [78] and others [101] pointed out that soy food and soy isoflavones may have a possible negative effect on thyroid in vulnerable groups, such as people with subclinical hypothyroidism, with iodine deficiency (for example during pregnancy) and with thyroid disorders.
Brassica Vegetables
There is much evidence that compounds found in brassica vegetables (also known as cruciferous vegetables) can have a goitrogenic effect [102,103]. Brassica vegetables include broccoli, cabbage, cauliflower, rutabaga, choy sum and turnip. Two compounds identified in brassica vegetables with the potential to have a goitrogenic effect are thiocyanate and goitrin [102]. Thiocyanate and goitrin are produced by myrosinase-induced degradation of indole glucosinolates [104] and progoitrin [105], respectively. Goitrin inhibits iodine uptake by the thyroid gland [30,102,103]. Thiocyanate also reduces iodine uptake by the thyroid since thiocyanate is a competitive inhibitor of NIS [106]. However, human studies have shown no effect of brassica vegetables on TSH and thyroid hormone levels so far [107,108,109] (Table 1).
Olive Oil
Many experimental studies have shown that olive derivatives, especially olive oil, have a stimulating effect on the thyroid gland (reviewed in [110]). The experimental studies were performed on various animal models: rats [111,112,113,114], chicken [115,116], lambs [117], goats [118] and calves [119]. The mechanism by which olive derivatives and olive oil affect the TSH and thyroid hormone levels is still unclear [110]. To our knowledge, only one study in humans has shown the effect of olive oil consumption on thyroid hormone levels [120]. Zupo et al., showed that people consuming olive oil have lower levels of fT3 and fT4 [120]. They also observed that the Mediterranean diet, rich in olive oil, slightly inhibits the production of fT3 and fT4 without significantly affecting thyroid function [120].
Food Associated with the Development of Endemic Goiter
Millet. The flavonoid C-glycosylflavone found in the pearl millet (Pennisetum glaucum) inhibited 85% of the TPO enzyme [121]. Pearl millet is a staple food for many people in rural areas of Asia and Africa. Animal studies [121,122,123,124] and epidemiological evidence [123,125,126] suggested that pearl millet may contribute to the development of endemic goiter in areas where this nutrient is consumed. Sartelet et al., proposed that flavonoids present in fonio millet (Digitaria exilis) (apigenin and luteolin) also have an antithyroid effect [127].
Cassava. Consumption of cassava (Manihot esculenta) has been reported to have contributed to the development of endemic goiter in tropical areas where its starchy tuberous root is consumed as staple food [128,129,130,131,132,133]. Linamarin, a cyanogenic glucoside, is thought to be metabolized from cassava to thiocyanate [132,134,135], which reduces iodine uptake by the mechanism previously described.
Bamboo Shoots. Consumption of bamboo shoots contributes to the development of endemic goiter [136,137]. Cyanogenic glycosides present in bamboo shoots are metabolized to toxic thiocyanate. Additionally, the in vitro study of Sarkar et al., showed that cyanogenic constituents in bamboo shoots generate reactive oxidative species that contribute to oxidative DNA damage and cell cycle disruption. This is followed by the inhibition of regulatory elements that synthesize thyroid hormones [138,139].
Other Food
Seaweeds. Seaweeds are frequently used in cuisine in Asian countries. They include different types of algae (green, red and brown) that are accumulators of iodine from the ocean. For example, kelps (the largest of the brown seaweeds) are the main accumulators of iodine in the ocean [140]. While iodine deficiency causes hypothyroidism, iodine excess can cause both hyperthyroidism and hypothyroidism [141]. Studies in euthyroid humans have observed an increase in TSH levels after seaweed consumption [142,143,144] (Table 1). However, Noahsen et al., observed a transient 150% increase in TSH levels in euthyroid individuals after consumption of seaweed (while fT4 levels remained unchanged) that returned to normal within three days [145]. Consumption of seaweed also increased urinary iodine excretion [145]. Miyai et al., also observed an increase in TSH levels after consumption of seaweed “Kombu”, but these levels returned to normal after some time [141]. Additionally, our recent study showed that consumption of seafood (different types of fish and squid) leads to an increase in fT4 and fT3 levels [146] (Table 1).
Junk Food. There is evidence that intake of non-home-made meals [147] alters thyroid hormone levels. Consumption of such food increases weight (BMI) and insulin levels [148]. The alteration in TSH and thyroid hormone levels after an increase in BMI is discussed in the previous section. An increase in thyroid hormone levels was also observed after consumption of white bread [146] and pasta [147], while consumption of whole-grain bread [146] leads to a decrease in fT4 levels. Additionally, it was showed that consumption of bacon and sausages lead to an increase in fT3 and fT4 levels [146].
Spices. There are proofs from animal studies that some spices can alter thyroid hormone levels. Thus, piperine (the main alkaloid found in black pepper) has been shown to reduce thyroid hormone levels in mice [149]. Cinnamon has also been shown to reduce tT3 levels in rats [150].
Beverages
Tea. Chandra et al., showed that green and black tea extracts have antithyroid potential [151,152]. It has been shown that catechins (flavonoids found in abundance in tea) have a goitrogenic effect in rats [151,153,154]. An in vitro study showed that exposure to catechins affected thyroid hormone synthesizing enzymes, leading to a decrease in the activity of TPO and 5′-deiodinase I [136]. However, Hu et al., pointed out that there was insufficient evidence of a possible antithyroid effect of catechins in humans [155]. Although perchlorate (a chemical that interferes with thyroid hormone production) was detected in tea samples [156,157,158], it was concluded that exposure to perchlorate due to tea consumption was too low to have a negative health outcome [159].
Coffee. Evidence indicates that caffeine blocks the absorption of thyroid hormone replacement therapy (levothyroxine) in patients with hypothyroidism [160]. Although these results have suggested a possible interaction of caffeine with the thyroid hormone system, few studies have been conducted to date on this issue. The results of studies on the effect of caffeine on thyroid hormone levels in experimental animals were inconsistent [161,162]; generally, the existence of a transitory effect of caffeine on the thyroid hormone system with a possible tolerance-related outcome was observed. So far, few human studies have been conducted. Spindel et al., observed no effect of coffee consumption on TSH and T3 levels [163], while Friedrich et al., observed a positive association between urinary trigonelline levels (a marker of coffee consumption) and fT4 levels [164]. The concentration of 3,5-T2, which is a metabolic product of T4 degradation, was positively associated with trigonelline levels [165]. In addition, 3,5-T2 levels were associated with levels of other compounds in plasma that indicate coffee consumption (caffeine, theophylline, paraxanthine and 1-methylxanthine [166]; trigonelline, pyroglutamate and hippurate [167]).
Micronutrients (Vitamins, Trace Minerals, Macrominerals)
Many micronutrients have been shown to have an effect on TSH and thyroid hormone levels. Among vitamins, vitamin D has been the most studied. Moreover, many trace minerals have been shown to have an effect on thyroid function, including selenium, zinc, iron, copper and iodine. Many studies have investigated the effect of micronutrients on TSH and thyroid hormone levels (Table 1). However, it is difficult to draw a general conclusion about how certain micronutrient affects thyroid function due to a high degree of variation between results. These inconsistencies between the results are likely due to the majority of the studies involved being underpowered. Additional studies involving larger cohorts should be conducted.
Vitamin D. Vitamin D exerts its effect by binding to the vitamin D receptor (VDR) [168]. It is mainly synthesized in the skin when exposed to sunlight (95%), and only a small amount is taken from food (5%) [169]. VDRs are detected in the pituitary gland, and it is thought that in addition to other pituitary hormones [170,171,172], vitamin D also regulates TSH secretion [173]. In addition, VDRs were detected in cultured rat-derived thyrocytes [174]. The decrease in TSH levels present in the higher status of vitamin D is thought to be the result of an increase in thyroid hormone levels (that is the result of the stimulatory effect of vitamin D on thyrocytes) [175]. Many studies have shown an association between vitamin D deficiency and autoimmune thyroid diseases [176,177,178,179,180]. Additionally, a positive correlation between TSH and vitamin D levels was observed in a study including pregnant women, while fT3 and fT4 levels were negatively correlated with vitamin D levels [181]. These authors suggested that pregnant women diagnosed with transient hyperthyroidism should also be tested for possible vitamin D deficiency [181]. Interestingly, Barchetta et al., suggested that vitamin D influences circannual variation in TSH levels and that seasonal variability in TSH levels in euthyroid individuals depends on vitamin D levels [182]. Studies on the association between vitamin D levels and TSH and thyroid hormone levels in euthyroid individuals have generally observed a negative correlation between vitamin D levels and TSH [175,182,183] and thyroid hormone levels [184] (Table 1).
Other vitamins. A deficiency of other vitamins such as vitamin A [185,186], vitamin B12 [187], vitamin B6 [188,189] and vitamin E [190] has also been observed in thyroid diseases. Supplementation with vitamin C and E [191,192], vitamin A [193], vitamin B12 [194] and vitamin B6 (reviewed in [195]) has been suggested to improve thyroid health. The influence of a deficiency of these vitamins on TSH and thyroid hormone levels in euthyroid individuals has not been well studied.
Selenium. Selenium is an essential trace element that is crucial for the normal functioning of many proteins and enzymes [196]. It is taken from food, mainly meat, grains and seafood. The content of selenium in food is determined by its content in the soil. Thus, some regions with low selenium content in the soil use selenium-rich fertilizers to increase the selenium content in the soil and consequently the intake of selenium by the plants [197]. Selenium is important for the functioning of many enzymes (selenoproteins) involved in the synthesis and metabolism of thyroid hormones and protection against oxidative damage (such as iodothyronine deiodinases, thioredoxin reductases and glutathione peroxidases) [196]. In fact, compared to other organs, the thyroid gland has a high concentration of selenium [198]. Many studies have observed selenium deficiency among patients with benign thyroid diseases [196,199,200]. Thus, selenium supplementation is used to treat various autoimmune thyroid diseases (reviewed in [196]). Most studies in healthy individuals have observed an inverse relationship between selenium concentration and fT4 levels [201,202,203,204,205,206,207]. Regarding TSH and fT3, most studies did not observe significant changes in these hormones after selenium intake (Table 1). It is important to point out that in addition to selenium deficiency, selenium excess is also not good for health [208]. Exposure to high levels of selenium can cause selenosis (when selenium intake is above 850 μg/day) [209].
Zinc. Zinc is the second most abundant trace element in the human body and has structural, catalytic and regulatory roles [210]. Zinc is found in meat, milk and fish. Zinc is involved in the synthesis of TSH (since it participates in the synthesis of TRH (as part of zinc-dependent enzyme carboxypeptidase that converts pre-TRH to pro-TRH) [211,212]) and the synthesis of thyroid hormones (as a cofactor of Dio1 and Dio2 [213] and also as part of thyroid transcription factor 2 (zinc-finger protein) [214] that is involved in the transcription of Tg and TPO genes). Zinc is also important for the proper functioning of T3 because T3 nuclear receptors contain zinc ions [215]. Significantly lower zinc levels have been reported in patients with hypothyroidism [216], and some studies have shown a beneficial effect of zinc supplementation on thyroid hormone levels (reviewed in [217]). Studies on the association between zinc levels and TSH and thyroid hormone levels in euthyroid individuals mostly observed no association between zinc and TSH levels (Table 1), negative correlation [208,218] or no association [202,217,219,220] between T4 and zinc levels or positive correlation [219,220] or no association [202,208,217,218] between T3 and zinc levels.
Iron. Iron is the most abundant trace element in the human body and is crucial for various cellular functions. Red meat, poultry, fish, leafy greens vegetables, lentils and beans are all rich in iron. Iron is involved in the synthesis of thyroid hormones, and its deficiency can alter thyroid hormone levels in several ways: (1) iron deficiency can reduce TPO activity [221]; (2) iron deficiency can increase rT3 deiodination, leading to thyroid hormone metabolism by inactivating pathway [222]; and (3) iron deficiency can lead to inefficient erythropoiesis, consequently causing a decrease in oxygen transport to tissues. Oxygen is crucial for various enzymatic reactions (including thyroid hormone synthesis) [223]. In a study conducted on 42,162 individuals, Wopereis et al., observed a higher chance of anemia in patients with hypothyroidism and hyperthyroidism [224]. In their meta-analysis, Talebi et al., observed decreased iron levels in patients with subclinical hypothyroidism [216]. Consistent with this finding, another study conducted on 1764 pregnant women showed an increase in TSH levels and a decrease in fT4 levels in the iron deficiency group [225]. Although few, studies in euthyroid individuals mainly observed a decrease in thyroid hormone levels [226,227,228] and no change in TSH levels [226,227,228] in patients with anemia or iron deficiency (Table 1).
Copper. Copper is crucial for the normal functioning of many body functions and is an important component of many enzymes. It is also involved in the normal functioning of the thyroid gland and the production of thyroid hormones [229]. Reducing copper levels can increase oxidative stress in thyrocytes because copper is a component of superoxide dismutase that protects cells from oxidative stress [230]. It has also been observed that blood copper levels can change according to thyroid function [229]. Although some studies have indicated a link between copper imbalance and benign thyroid diseases [231,232], a recent meta-analysis by Talebi et al., showed that there was no significant difference in the copper levels between hypothyroid patients and healthy controls [216]. Although few, studies in euthyroid individuals have observed a positive correlation between copper levels and T4 [208,229] and tT3 levels [208].
Iodine excess. It is well known that iodine deficiency disrupts the normal functioning of the thyroid gland, but, on the other hand, high iodine intake can also cause thyroid problems. Although most healthy people tolerate high iodine intake well, in vulnerable individuals, it can lead to the development of hyperthyroidism and even hypothyroidism (reviewed in [233]). Causes of excess iodine are the consumption of overiodized salt, seaweeds (as already mentioned) [141,144,145], consumption of excess iodine through water and milk and taking diary supplements that contain iodine [234]. Most studies investigating the effect of high iodine intake on TSH and thyroid hormone levels in healthy adults observed an increase in TSH levels and a decrease in the levels of thyroid hormones after excess iodine [141,144,145,235,236,237,238,239,240,241]. Although only a few studies have been conducted, the pattern of TSH and thyroid hormone levels observed in these studies has shown consistency: an increase in TSH levels followed by a decrease in thyroid hormone levels (Table 1). This hormone profile is a characteristic of hypothyroidism.
Magnesium. Magnesium is an essential mineral involved in the functioning of more than 300 enzymes, among which are those important for the synthesis of thyroid hormones [242]. Magnesium is absorbed mainly from magnesium-rich food such as leafy greens, nuts, whole grains and seeds. Some studies have shown a link between magnesium imbalance and benign thyroid diseases [243,244]. However, the meta-analysis of Talebi et al., showed that there was no significant difference in magnesium levels between hypothyroid patients and healthy controls [216]. The effect of magnesium deficiency on TSH and thyroid hormone levels in euthyroid individuals has not been well studied.
3.1.5. Exercise
Exercise affects the homeostasis of the body, the regulation of which involves the HPT axis. Thus, alterations in TSH and thyroid hormone levels were observed after exercise. Additionally, thyroid hormones are involved in the normal functioning of skeletal muscles and pulmonary, cardiac and vascular systems whose activity is significantly altered during the exercise [245]. Many studies have measured TSH and thyroid hormone levels after exercise in healthy individuals, but it is difficult to draw any conclusions due to inconsistencies between studies ([246,247,248,249]; Table 1). A recent study involving 2740 healthy individuals observed no changes in TSH and fT4 levels after exercise [250]. Factors contributing to inconsistencies between studies are the physical status of the subjects, the intensity, duration and type of exercise, differences in age and gender among the subjects and even the ambient temperature [245].
3.2. Pollutants
3.2.1. Chemicals
Many industrial chemicals and pesticides can alter the normal functioning of the thyroid gland. These chemicals are classified as endocrine-disrupting compounds (EDCs) [13]. Because thyroid hormones are crucial in normal brain development [329], any compound that could potentially affect normal thyroid function should be thoroughly investigated. In fact, many studies on the impact of potential EDCs on normal thyroid function have included pregnant women [330,331], infants [332,333] and young children [334,335]. However, these studies are not discussed in this section, which only considers studies including a general healthy population. Although so far many studies have been conducted on the influence of different types of chemicals on TSH and thyroid hormone levels, there is still a high degree of variation between the results. The majority of conducted studies were underpowered, not including a sufficient number of participants (Table 2). In addition, exposure of participants to different subtypes and doses of chemicals could contribute to the differences between the results. Therefore, it is difficult to draw a general conclusion about whether or how a particular chemical affects thyroid function.
Table 2.
Effect of pollutants on thyroid-stimulating hormone, thyroid hormone and thyroglobulin levels in healthy individuals.
| Factor | Compounds Used in the Study | Effect on Hormone Levels | Number of Participants | Reference | ||
|---|---|---|---|---|---|---|
| Chemicals | Polychlorinated biphenyls and polybrominated biphenyls | PBB |
 TSH, ↓fT4, ↑fT3, ↑tT3, ↑fT3/fT4 TSH, ↓fT4, ↑fT3, ↑tT3, ↑fT3/fT4 |
715 | [349] | |
| PCB |
 TSH, ↑fT4, TSH, ↑fT4,  fT3, ↑fT3/fT4 fT3, ↑fT3/fT4 |
|||||
| PCBs and hydroxylated PCBs |
 TSH, TSH,  fT4, fT4,  tT4, tT4,  fT3, fT3,  tT3 tT3 |
79 | [348] | |||
| PCB |
 TSH, ↑fT4, ↑tT4, ↑fT3, ↑tT3 TSH, ↑fT4, ↑tT4, ↑fT3, ↑tT3 |
551 | [347] | |||
| PCB |
 TSH, ↓fT4, ↓fT3 TSH, ↓fT4, ↓fT3 |
122 | [346] | |||
| PCB |
 TSH, ↓fT4, ↑tT4, TSH, ↓fT4, ↑tT4,  tT3 tT3 |
87 | [345] | |||
| PCB | ↓TSH, ↑fT4, ↑tT4, ↑fT3, ↑tT3 | 67 | [353] | |||
| PCB | ↓tT3 | 114 | [435] | |||
| PCB x BDE | ↑tT3 | |||||
| PCB |
 TSH, TSH,  fT4, ↓tT3 fT4, ↓tT3 |
623 | [344] | |||
| PCB | ↑TSH, ↓T4, ↓T3 | 211 | [351] | |||
| PCB | ↑TSH, ↓fT4,  tT4, tT4,  tT3 tT3 |
232 | [423] | |||
| PCB |
 TSH, ↑fT4, ↑tT3 TSH, ↑fT4, ↑tT3 |
2042 | [484] | |||
| PCB |
 TSH, TSH,  fT4, ↓tT3 fT4, ↓tT3 |
341 | [343] | |||
| PCB |
 TSH, ↑fT4 TSH, ↑fT4 |
2045 | [422] | |||
| PCB | ↓tT4 | 2445 | [356] | |||
| Dioxin-like toxic equivalents | ↑TSH, ↓tT4 | |||||
| PCB | ↓TSH | 454 | [418] | |||
| PCB |
 TSH, TSH,  fT4 fT4 |
196 | [341] | |||
| PCB | ↓TSH, ↓tT4, ↓tT3 | 66 | [352] | |||
| Dioxin-like toxic equivalents | ↓TSH | |||||
| PCB |
 TSH, TSH,  fT4, fT4,  tT4, tT4,  fT3, fT3,  tT3 tT3 |
110 | [354] | |||
| PCB |
 TSH, TSH,  fT4, fT4,  tT4, tT4,  fT3, ↓tT3 fT3, ↓tT3 |
182 | [339] | |||
| PCB | ↓T4 | 229 | [355] | |||
| PCB |
 TSH, TSH,  fT4, fT4,  tT4 tT4 |
192 | [421] | |||
| PCB |
 TSH, TSH,  fT4, fT4,  tT4, tT4,  fT3 fT3 |
173 | [340] | |||
| PCB |
 tT4 tT4 |
111 | [350] | |||
| Polybrominated diphenyl ethers | PBDE | ↑TSH,  fT4, fT4,  tT4, ↑fT3, ↓tT3 tT4, ↑fT3, ↓tT3 |
85 | [362] | ||
| PBDE |
 TSH, TSH,  fT4, ↑tT4, fT4, ↑tT4,  fT3, ↑tT3 fT3, ↑tT3 |
79 | [348] | |||
| PBDE |
 TSH, TSH,  fT4, ↓tT4, fT4, ↓tT4,  tT3 tT3 |
52 | [361] | |||
| PBDE |
 TSH, TSH,  fT4, ↑tT3 fT4, ↑tT3 |
623 | [344] | |||
| PBDE | ↑TSH | 49 | [363] | |||
| PBDE |
 TSH, TSH,  fT4, fT4,  tT4, tT4,  tT3 tT3 |
36 | [360] | |||
| PBDE | ↓TSH, ↑tT4, ↓tT3, ↑rT3 | 308 | [364] | |||
| PBDE (BDE-47) | ↓TSH,  tT4, tT4,  fT4, fT4,  tT3, tT3,  fT3 fT3 |
110 | [354] | |||
| Bisphenol A | BPA |
 TSH, TSH,  tT4, ↑tT3 tT4, ↑tT3 |
90 | [368] | ||
| BPA | ↓TSH,  tT4, tT4,  tT3 tT3 |
6003 | [372] | |||
| BPA | ↑TSH,  fT4 fT4 |
194 | [370] | |||
| BPA |
 TSH, ↓fT4 TSH, ↓fT4 |
2340 | [367] | |||
| BPA | ↓TSH,  fT4, ↑fT3 fT4, ↑fT3 |
3394 | [371] | |||
| BPA |
 TSH, TSH,  fT4, fT4,  tT4, tT4,  tT3, tT3,  Tg Tg |
1346 | [369] | |||
| BPA | ↓TSH,  fT4, fT4,  tT3 tT3 |
167 | [373] | |||
| Phthalates | DEHP metabolites and MEHHP | ↓tT4 | Meta-analysis (included studies on pregnant women and children) | [382] | ||
| MEOHP | ↓fT4 | |||||
| MEHHP, DEHP metabolite |
 TSH, TSH,  fT4, ↓T4, fT4, ↓T4,  T3 T3 |
279 | [379] | |||
| MEHP, MEOHP |
 TSH, ↓fT4, TSH, ↓fT4,  T4, T4,  T3 T3 |
|||||
| Monoethyl phthalate |
 TSH, ↑fT4, TSH, ↑fT4,  T4, T4,  T3 T3 |
|||||
| MEOHP | ↑TSH,  tT4, tT4,  tT3 tT3 |
6003 | [372] | |||
| DEHP metabolites |
 TSH, ↓tT4, TSH, ↓tT4,  tT3 tT3 |
|||||
| MnBP |
 TSH, TSH,  tT4, ↓tT3 tT4, ↓tT3 |
|||||
| MnBP | ↑TSH,  fT4 fT4 |
43 (all subjects) | [380] | |||
| MnBP, 5Cx-MEP, 5Oxo-MEHP, MBzP | ↑TSH,  fT4 fT4 |
30 (women) | ||||
| MEHHP | ↓tT4 | 1877 (all subjects) | [381] | |||
| MEOHP | ↑tT4 | 907 (women) | ||||
| DEHP | ↑TSH, ↓fT4, ↓tT4, ↓tT3, ↓Tg | 1346 | [369] | |||
| MEHP |
 TSH, ↓fT4, ↓tT3 TSH, ↓fT4, ↓tT3 |
408 | [378] | |||
| Perchlorate | Perchlorate |
 TSH, ↓fT4 TSH, ↓fT4 |
2702 | [388] | ||
| ↓fT4 | 564 | [387] | ||||
 TSH, ↓fT4, ↓tT4 TSH, ↓fT4, ↓tT4 |
4023 | [386] | ||||
↓fT4, ↓tT4, ↓fT3,  tT3 tT3 |
1877 | [381] | ||||
| ↑TSH, ↓tT4 | 1111 | [385] | ||||
| Perfluoroalkyl substances | PFAS |
 TSH, TSH,  fT4, fT4,  fT3 fT3 |
3297 | [399] | ||
| PFOS, PFNA, PFAS, PFHxS |
 TSH, ↑fT4, TSH, ↑fT4,  tT4, tT4,  fT3, fT3,  tT3 tT3 |
1325 | [400] | |||
| PFOA |
 TSH, ↓tT4, ↓fT4, TSH, ↓tT4, ↓fT4,  tT3 tT3 |
3070 | [398] | |||
| PFOS |
 TSH, ↓tT4, TSH, ↓tT4,  fT4, fT4,  tT3 tT3 |
|||||
| PFNA, PFDeA | ↓TSH,  tT4, tT4,  fT4, fT4,  tT3 tT3 |
|||||
| PFOA, PFNA | ↑TSH,  tT4, tT4,  fT4, fT4,  tT3, tT3,  fT3 fT3 |
85 | [406] | |||
| PFNA | ↑tT3, ↑fT3 | 47 (women) | ||||
| PFNA | ↓tT3, ↓fT3 | 38 (men) | ||||
| PFOS | ↑fT3 | 47 (women) | ||||
| PFOS | ↓fT3 | 38 (men) | ||||
| PFOS | ↑TSH, ↓tT4, ↑fT4, ↓tT3 | Meta-analysis (including pregnant women) | [401] | |||
| PFOA |
 TSH, ↓tT4, TSH, ↓tT4,  tT3 tT3 |
|||||
| PFNA |
 TSH, TSH,  T4, ↑fT4, ↑T3 T4, ↑fT4, ↑T3 |
99 | [397] | |||
| PFOA |
 TSH, TSH,  T4, T4,  fT4, ↑T3 fT4, ↑T3 |
|||||
| PFOA |
 TSH, TSH,  fT4, fT4,  tT4, tT4,  tT3, ↑fT3 tT3, ↑fT3 |
1012 | [396] | |||
| PFOS |
 TSH, ↑fT4, ↑tT4, TSH, ↑fT4, ↑tT4,  tT3 tT3 |
87 | [345] | |||
| PFOS, PFNA | ↓TSH,  tT4, tT4,  fT4, fT4,  tT3, tT3,  fT3 fT3 |
158 (male adolescents) | [402] | |||
| PFOA | ↓TSH,  tT4, tT4,  fT4, fT4,  tT3, tT3,  fT3 fT3 |
145 (female adolescents) | ||||
| PFOA, PFOS, PFNA |
 TSH, TSH,  tT4, ↑fT4, tT4, ↑fT4,  tT3, tT3,  fT3 fT3 |
257 (women 20-40 years old) | ||||
| PFOA |
 TSH, TSH,  tT4, tT4,  fT4, ↑tT3, ↑fT3 fT4, ↑tT3, ↑fT3 |
199 (women 60-80 years old) | ||||
| PFNA |
 TSH, ↑fT4 TSH, ↑fT4 |
567 | [403] | |||
| PFOA | ↑TSH,  tT4, tT4,  fT4, ↑tT3, fT4, ↑tT3,  fT3, fT3,  Tg Tg |
1540 | [254] | |||
| PFHxS |
 TSH, ↑tT4, TSH, ↑tT4,  fT4, fT4,  tT3, tT3,  fT3, fT3,  Tg Tg |
|||||
| PFOA |
 TSH, TSH,  tT4, tT4,  fT4, ↑tT3, fT4, ↑tT3,  fT3 fT3 |
509 (women) | [395] | |||
| PFHxS |
 TSH, ↑tT4, TSH, ↑tT4,  fT4, ↑tT3, fT4, ↑tT3,  fT3 fT3 |
509 (women) | ||||
 TSH, TSH,  tT4, ↓fT4, tT4, ↓fT4,  tT3, tT3,  fT3 fT3 |
672 (men) | |||||
| PFTrDA | ↑TSH, ↓tT4 | 633 | [405] | |||
| PFOS, PFOA |
 TSH, ↑tT4 TSH, ↑tT4 |
50,113 | [394] | |||
| PFC |
 TSH, TSH,  fT4 fT4 |
31 | [393] | |||
| PFOS | ↓TSH, ↑fT4, ↓tT3 | 623 | [344] | |||
| PFOA |
 TSH, ↓fT4, TSH, ↓fT4,  T4, ↑T3 T4, ↑T3 |
506 | [392] | |||
| PFOA |
 TSH TSH |
371 | [391] | |||
| PFOS |
 TSH, TSH,  fT4, fT4,  T4, ↑T3 T4, ↑T3 |
255 | [404] | |||
| Pesticides | Conventional farmers that use insecticides, herbicides and fungicides in comparison to organic farmers | ↑TSH, ↓fT4, ↑T4, ↑fT3, ↑T3 | 438 | [410] | ||
| Organophosphate insecticides |
 TSH, TSH,  fT4, fT4,  tT4, tT4,  fT3, fT3,  tT3 tT3 |
41 | [432] | |||
| Rural workers exposed to pesticides in comparison to controls | ↓TSH, ↑fT4, ↑tT3 | 73 | [420] | |||
| 3-PBA (metabolite of pyrethroid insecticide) |
 TSH, ↓tT4, ↓tT3 TSH, ↓tT4, ↓tT3 |
6208 | [429] | |||
| Insecticides and pyrethroids for >20 years | ↓fT4, ↓tT3 | 106 | [436] | |||
| TCPY (a metabolite of chlorpyrifos) |
 TSH, ↓tT4, TSH, ↓tT4,  fT4, fT4,  tT3, tT3,  fT3, ↓Tg fT3, ↓Tg |
2015 | [427] | |||
| Mancozeb (fungicide) |
 TSH, TSH,  fT4, ↓T4, ↑fT3, fT4, ↓T4, ↑fT3,  T3, ↓Tg T3, ↓Tg |
63 | [428] | |||
| 3-PBA |
 TSH, TSH,  fT4, fT4,  tT4, tT4,  fT3, fT3,  tT3, tT3,  Tg Tg |
2015 | [430] | |||
| p,p′-DDE (a stable metabolite of DDT) |
 TSH, ↑tT4, ↑tT3 TSH, ↑tT4, ↑tT3 |
136 | [434] | |||
| Pesticide sprayers exposed to organophosphate and organochlorine pesticides | ↑TSH,  T4, ↓T3 T4, ↓T3 |
60 | [417] | |||
| DDT+DDE | ↑tT4, ↑tT3 | 48 (women) | [435] | |||
| DDT+DDE + PCB | ↑tT4 | |||||
| DDT+DDE + PCB | ↓tT3 | 66 (men) | ||||
| Exposure to organophosphate and carbamate pesticides |
 TSH, TSH,  fT4 fT4 |
99 | [425] | |||
| High exposure pesticide season |
 TSH, ↓fT4, TSH, ↓fT4,  tT3 tT3 |
91 | [426] | |||
| HCH | ↑TSH, ↓fT4 | 303 (men) | [416] | |||
| HCB, heptachlor, o,p′-DDT and p,p′-DDT | ↑fT4 | 305 (women) | ||||
| Endosulphan 2 | ↓tT3 | 303 (men) | ||||
| Alpha-chlordane, p,p′- DDT, endosulphan 2 and methoxychlor | ↑tT3 | 305 (women) | ||||
| TCPY (a metabolite of chlorpyrifos) | ↓TSH, ↑tT4 | 1589 (men) | [415] | |||
| ↑TSH | 218 (women) | |||||
| Insecticide fipronil sulfone metabolite | ↓TSH,  fT4, fT4,  tT4 tT4 |
155 | [419] | |||
| DAP | ↑TSH, ↑tT4 | 215 | [414] | |||
| DMP | ↑TSH, ↑tT4, ↓tT3 | |||||
| Organochlorine pesticides | ↓tT3 | 623 | [344] | |||
| Hexachlorobenzene | ↓fT4 | |||||
| cis-DCCA (pyrethroid metabolite) |
 TSH, TSH,  fT4, fT4,  tT3 tT3 |
161 | [424] | |||
| 3-PBA and trans-DCCA (pyrethroid metabolites) |
 TSH, TSH,  fT4, fT4,  tT3 tT3 |
|||||
| HCB |
 TSH, TSH,  fT4, ↓tT4, fT4, ↓tT4,  tT3 tT3 |
232 | [423] | |||
| DDE |
 TSH, ↓fT4, ↑tT3 TSH, ↓fT4, ↑tT3 |
2045 | [422] | |||
| HCB | ↑TSH, ↓fT4,  tT3 tT3 |
|||||
| p,p′-DDE | ↓TSH, ↑fT4, ↑tT3 | 341 | [343] | |||
| HCB | ↓tT3 | |||||
| p,p′-DDE |
 TSH, TSH,  tT4 tT4 |
2445 | [356] | |||
| PCB + DDE + HCB | ↓TSH | 454 | [418] | |||
| DDE |
 TSH, TSH,  tT4, tT4,  tT3 tT3 |
66 | [352] | |||
| p,p′-DDE | ↑TSH,  fT4 fT4 |
196 | [341] | |||
| High exposure pesticide season |
 TSH, ↓fT4, TSH, ↓fT4,  fT3, fT3,  tT3 tT3 |
122 | [433] | |||
| In the fall in comparison to the spring season (people are exposed to higher levels of pesticides in fall) | ↓TSH, ↑fT4, ↑fT3, ↑tT3 | |||||
| TCPY (a metabolite of chlorpyrifos) | ↑TSH,  fT4, fT4,  tT3 tT3 |
322 | [413] | |||
| 1N (a metabolite of carbaryl and naphthalene) |
 TSH, TSH,  fT4, fT4,  tT3 tT3 |
|||||
| EBDC fungicides |
 TSH TSH |
131 | [431] | |||
| HCB |
 T4 T4 |
66 | [485] | |||
| High exposure pesticide season | ↑TSH, ↑fT4, ↑tT4 | 193 | [412] | |||
| DDT, HCB | ↓T3 | 16 | [438] | |||
| DDE |
 TSH, TSH,  T4, T4,  T3 T3 |
51 | [355] | |||
| HCB |
 TSH, TSH,  fT4, ↓tT4 fT4, ↓tT4 |
192 | [421] | |||
| Exposure to organophosphates and organochlorine pesticides |
 TSH, TSH,  T4, ↓T3 T4, ↓T3 |
50 | [437] | |||
| EBDC fungicides | ↑TSH,  T4 T4 |
94 | [411] | |||
| Nitrate | Nitrate |
 T4, T4,  T3 T3 |
30 | [445] | ||
↑TSH,  fT4 fT4 |
41 | [443] | ||||
| ↓fT4 | 307 | [387] | ||||
 TSH, ↓tT4 TSH, ↓tT4 |
1111 | [385] | ||||
 TSH, TSH,  T4, T4,  T3 T3 |
20 | [444] | ||||
| ↓TSH, ↑T4 | 60 | [442] | ||||
| Heavy metals | Studies determining multiple metals | Pb, Cd, As |
 TSH TSH |
102 | [454] | |
| Pb | ↑TSH,  fT4, fT4,  fT3 fT3 |
100 | [460] | |||
| Cd |
 TSH, TSH,  fT4, fT4,  fT3 fT3 |
|||||
| Pb | ↑TSH,  tT4, tT4,  tT3 tT3 |
5628 | [461] | |||
| Cd |
 TSH, TSH,  tT4, tT4,  tT3 tT3 |
|||||
| Cd |
 TSH, TSH,  fT4, fT4,  tT4, tT4,  fT3, fT3,  tT3, tT3,  Tg Tg |
1391 | [459] | |||
| Pb |
 TSH, TSH,  fT4, fT4,  tT4, tT4,  fT3, fT3,  tT3, tT3,  Tg Tg |
|||||
| Cd |
 TSH, ↑fT4, TSH, ↑fT4,  tT4, tT4,  fT3, fT3,  tT3, ↑Tg tT3, ↑Tg |
6231 (all subjects) | [458] | |||
 TSH, TSH,  fT4, fT4,  tT4, tT4,  fT3, ↑tT3, ↑Tg fT3, ↑tT3, ↑Tg |
3231 (men) | |||||
| Pb |
 TSH, TSH,  fT4, fT4,  tT4, ↑fT3, tT4, ↑fT3,  tT3, tT3,  Tg Tg |
6231 (all subjects) | ||||
 TSH, TSH,  fT4, ↓tT4, fT4, ↓tT4,  fT3, fT3,  tT3, tT3,  Tg Tg |
3231 (men) | |||||
 TSH, ↑fT4, TSH, ↑fT4,  tT4, tT4,  fT3, fT3,  tT3, tT3,  Tg Tg |
3000 (women) | |||||
| Hg |
 TSH, TSH,  fT4, ↓tT4, ↓fT3, ↓tT3, fT4, ↓tT4, ↓fT3, ↓tT3,  Tg Tg |
4409 | [457] | |||
| Cd |
 TSH, TSH,  fT4, ↑tT4, ↑fT3, ↑tT3, ↑Tg fT4, ↑tT4, ↑fT3, ↑tT3, ↑Tg |
|||||
| Pb |
 TSH, TSH,  fT4, fT4,  tT4, tT4,  fT3, fT3,  tT3, tT3,  Tg Tg |
|||||
| Hg |
 TSH, TSH,  fT4, ↓T4, ↓fT3, ↓T3 fT4, ↓T4, ↓fT3, ↓T3 |
1587 | [456] | |||
| Cd | ↓TSH, ↑fT4, ↑T4, ↑fT3, ↑T3 | |||||
| Pb | ↓TSH | 219 | [318] | |||
| As | ↑TSH | |||||
| Hg, Cd |
 TSH TSH |
|||||
| Pb | ↓TSH,  T4, ↑T3 T4, ↑T3 |
211 | [351] | |||
| Hg | ↑TSH,  T4, T4,  T3 T3 |
|||||
| Pb |
 TSH, TSH,  fT4, fT4,  tT4, ↑T3 tT4, ↑T3 |
232 | [423] | |||
| Hg |
 TSH, TSH,  fT4, fT4,  tT4, tT4,  T3 T3 |
|||||
| Studies determining single metal | Arsenic | UDMA | ↑TSH, ↓fT4, ↓tT4,  fT3, fT3,  tT3, tT3,  Tg Tg |
4126 | [453] | |
| UAAS |
 TSH, TSH,  fT4, ↓tT4, fT4, ↓tT4,  fT3, fT3,  tT3, tT3,  Tg Tg |
|||||
| UAS, UAB | ↓TSH,  fT4, fT4,  tT4, tT4,  fT3, fT3,  tT3, ↓Tg tT3, ↓Tg |
|||||
↑TSH,  fT4, fT4,  fT3 fT3 |
38 | [452] | ||||
| ↑TSH, ↓fT4, ↓fT3, ↑Tg | 185 | [451] | ||||
| Cadmium |
 TSH, ↓fT4 TSH, ↓fT4 |
1972 | [463] | |||
 TSH, ↑fT4, ↑fT3 TSH, ↑fT4, ↑fT3 |
1724 | [462] | ||||
| ↑TSH, ↓fT4, ↓fT3 | 277 | [455] | ||||
| ↓fT4, ↑tT3 | 105 | [464] | ||||
| Mercury | ↑TSH, ↑fT4, ↓T4,  fT3, fT3,  T3 T3 |
Meta-analysis | [480] | |||
↑TSH,  fT4, fT4,  fT3, fT3,  tT3 tT3 |
110 | [479] | ||||
 TSH, ↓T4, ↓T3 TSH, ↓T4, ↓T3 |
137 | [482] | ||||
 TSH, ↑fT4, ↓fT3, ↑fT4/fT3 TSH, ↑fT4, ↓fT3, ↑fT4/fT3 |
82 | [481] | ||||
| ↑rT3, ↑fT4/fT3 | 94 | [483] | ||||
| Lead | ↓TSH, ↑fT4, ↑fT3 | 87 | [468] | |||
 TSH, TSH,  fT4, fT4,  T4, T4,  fT3, fT3,  T3 T3 |
Meta-analysis | [475] | ||||
 TSH, TSH,  T4, T4,  T3 T3 |
195 | [474] | ||||
 T4, ↓fT4 T4, ↓fT4 |
220 | [478] | ||||
| ↑T3, ↑T4 | 76 | [477] | ||||
| ↑TSH | 125 | [467] | ||||
 TSH, ↓fT4, TSH, ↓fT4,  fT3 fT3 |
97 | [473] | ||||
 TSH, TSH,  fT4, fT4,  T4, ↓fT3, ↓T3 T4, ↓fT3, ↓T3 |
157 | [472] | ||||
 TSH, TSH,  fT4, fT4,  fT3 fT3 |
103 | [471] | ||||
↑TSH,  fT4, ↓T4, ↓T3 fT4, ↓T4, ↓T3 |
75 | [465] | ||||
↑TSH,  fT4, fT4,  fT3 fT3 |
93 | [466] | ||||
 TSH, ↑fT4, ↑tT4, ↑fT3, TSH, ↑fT4, ↑tT4, ↑fT3,  tT3 tT3 |
57 | [476] | ||||
 TSH, TSH,  T4 T4 |
151 | [469] | ||||
 TSH, ↓fT4, ↓tT4, TSH, ↓fT4, ↓tT4,  tT3 tT3 |
176 | [470] | ||||
Studies in pregnant women, infants, kids and individuals with a history of thyroid diseases were not included in this table. 1N, 1-naphthol; 3-PBA, phenoxybenzoic acid; 5Cx-MEP, mono-ethyl phthalate; 5Oxo-MEHP, mono-(2-ethylhexyl) phthalate; As, arsenic; BPA, bisphenol-A; Cd, cadmium; DAP, dialkylphosphate; cis and trans-DCCA, cis and trans-3-2,2-dichlorovinyl-2,2-dimethylcyclopropane carboxylic acid; DDE, p,p′-diphenyldichloroethene; DDT, p,p′-dichlorodiphenyltrichloroethane; DEHP, di-(2-ethylhexyl) phthalate; DMP, dimethyl metabolites; EBDC, ethylene bisdithiocarbamates; fT3, free triiodothyronine; fT4, free thyroxine; HCB, hexachlorobenzene; HCH, beta-hexachlorocyclohexane; Hg, mercury; MBzP, mono-benzyl phthalate; MEHP, mono(2-ethylhexyl) phthalate; MEHHP, mono (2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono-(2-ethyl-5-oxohexyl) phthalate; MnBP, mono-n-butyl phthalate; PBB, polybrominated biphenyls; PBDE, polybrominated diphenyl ethers; PCB, polychlorinated biphenyls; PFAS, perfluoroalkyl substances; PFC, perfluorinated compounds; PFDeA, perfluorodecanoate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFTrDA; perfluorotridecanoic acid; rT3, reverse triiodothyronine; T3, triiodothyronine; T4, thyroxine; tT3, total T3; tT4, total T4; TCPY, trichloro-2-pyridinol; Tg, thyroglobulin; TSH, thyroid-stimulating hormone; UAAS, arsenic adjusted for arsenobetaine; UAB, arsenobetaine; UAS, total arsenic; UDMA, dimethylarsinic acid.
Polychlorinated Biphenyls and Polybrominated Biphenyls
Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) are EDCs. Due to their structural similarities to thyroid hormones, PCBs and PBBs interfere with thyroid hormone signaling [13]. An in vitro study showed that PCBs bind to thyroid hormone receptors [336], and PBBs could affect iodide intake by the thyroid gland [329,337]. PCBs have been widely used as electrical insulating fluids and in carbonless copy paper, inks, paints and other industrial and consumer products. They were banned in the United States in 1979 and again by the Stockholm Convention on Persistent Organic Pollutants in 2001 [338]. However, these chemicals are persistent organic pollutants (POPs) that can accumulate in the environment and body fat and thus can still have detrimental effects on health [13]. PBBs are also POPs and although they are still used as flame retardants (chemicals added to materials used to prevent potential ignition of products), their use is controlled by the 2003 Restriction of Hazardous Substances Directive. Many studies have investigated the influence of PCBs on thyroid hormone levels in healthy adults, but many inconsistencies have been observed between studies. Some studies have not observed the effect of PCBs on TSH [339,340,341,342,343,344,345,346,347,348,349] and thyroid hormone levels [339,340,341,348,350]. However, other studies have shown an increase [351] and a decrease [352,353] in TSH, an increase [347,353] and a decrease [344,346,351,352,354] in T3 and an increase [342,347,349,353] and a decrease [343,346,351,352,354,355,356] in T4 after exposure to PCBs. Although previous studies have investigated the effect of PBBs on the development of thyroid diseases [357,358], we have found only one study investigating the effect of PBBs on TSH and thyroid hormone levels in euthyroid individuals [349].
Polybrominated Diphenyl Ethers
Polybrominated diphenyl ethers (PBDEs) are used as flame retardants. Although, to date, the use of most types of PBDEs has been banned or restricted, these chemicals continue to pose a threat to human health because the Stockholm Convention on Persistent Organic Pollutants considers them to be POPs. These chemicals are also EDCs and share structural similarities with T4 [359]. The results of the studies on the influence of PBDEs on TSH and thyroid hormone levels in healthy adults were inconsistent. Some studies did not show an effect of PBDEs on TSH [360,361], T3 [348,360,361] and T4 levels [360,362], while the others observed an increase in TSH [362,363], T3 [344,348,362] and T4 [348,364] levels and a decrease in TSH [364], T3 [364] and T4 [361] levels after PBDE exposure.
Bisphenol A
Bisphenol A (BPA) is one of the world’s most commonly used chemical in food packaging, food can lining, toys, tubes, cosmetics, etc. Because BPA is not chemically bound to the material, it can easily diffuse into food or beverages after repeated use, physical manipulation or under high heat [13]. BPA inhibits thyroid hormone synthesis in several ways: it reduces thyroid iodide intake and TPO activity and alters gene expression for proteins involved in thyroid hormone synthesis (reviewed in [365]). In addition, BPA is an antagonist of thyroid hormone receptors [366]. Studies on the influence of BPA on TSH and thyroid hormone levels in healthy adults have yielded inconsistent results. TSH levels were not affected [367,368,369], increased [370] or decreased [371,372,373] after BPA exposure. T3 levels were also unaffected [369,372,373] or increased [368,371] by BPA exposure, while T4 levels were not affected [368,369,370,371,372,373] or decreased [367] by BPA exposure.
Phthalates
Phthalates are among the most produced chemicals in the world. They are used as plasticizers and softeners in products such as food packaging, food can lining, toys, tubes, cosmetics, etc. Because phthalates are not chemically bound to the material, they can easily diffuse into food, water and air [374]. In vitro studies have shown that di-(2-ethylhexyl) phthalate (DEHP) has an antagonistic effect on thyroid hormone action [375,376]. In addition, studies in rats have shown that DEHP causes histopathologic changes in the thyroid gland and increases the level of liver enzymes involved in the degradation of thyroid hormones (resulting in a decrease in thyroid hormone levels) [377]. Studies on the influence of phthalates on TSH and thyroid hormone levels in healthy adults have yielded inconsistent results. TSH levels were not affected [372,378,379] or increased [369,372,380] by exposure to phthalates. T3 levels were also not affected [372,379] or decreased [369,372,378] after phthalate exposure, while T4 levels were not affected [372,379,380], increased [379] or decreased [369,372,378,379,381,382] after phthalate exposure (Table 2).
Perchlorate
Perchlorate is a chemical substance used in the production of propellants, pyrotechnics, airbags and fertilizers and is approved as a food contact substance (therefore, it can be released into various foods, milk and water) [383]. Perchlorate reduces the intake of iodine in the thyroid because it is an inhibitor of NIS [384]. Studies in healthy adults generally observed a decrease in T4 [381,385,386,387,388] and T3 levels [381] after exposure to perchlorate, while TSH levels were either not affected [386,388] or increased [385] (Table 2). Although only a few studies have been conducted so far, they have all included a sufficient number of participants. In addition, a similar pattern of TSH and thyroid hormone levels could be observed among studies: a decrease in thyroid hormone levels, with TSH levels remaining unchanged (Table 2). This indicates that perhaps perchlorate first exerts its effect on thyroid hormones.
Perfluoroalkyl Substances
Perfluoroalkyl substances (PFASs) can resist both water and oil and are therefore used as surfactants in products such as textiles, paints, food packaging, cookware and cosmetics [389]. PFASs inhibit the synthesis and increase the metabolic excretion of thyroid hormones [390]. Many studies have tested the effect of PFASs on thyroid hormone homeostasis in healthy adults. The results were inconsistent and showed no effect [254,345,391,392,393,394,395,396,397,398,399,400,401,402,403,404], increase [254,401,405,406] or decrease [344,398] in TSH levels after exposure to PFASs. No effect [254,345,398,399], increase [254,392,395,396,397,402,404,406] or decrease [344,401,406] in T3 levels and no effect [254,393,395,396,397,399,404,406], increase [254,344,345,394,397,400,402] or decrease [392,398,401,405] in T4 levels were observed after exposure to PFASs (Table 2).
Pesticides
Pesticides are EDCs, and various in vitro and in vivo studies have shown that pesticides, including insecticides, fungicides and herbicides, alter normal thyroid function (reviewed in [407,408]). Pesticides affect the metabolism and production of thyroid hormones (reviewed in [409]). The effect of various pesticides on TSH and thyroid hormone levels in healthy adults was tested (phenoxybenzoic acid (3-PBA) (metabolite of pyrethroid insecticide), trichloro-2-pyridinol (TCPY) (a metabolite of chlorpyrifos), cis and trans-3-2,2-dichlorovinyl-2,2-dimethylcyclopropane carboxylic acid (cis and trans-DCCA) (pyrethroid metabolites), 1-napththol (1N) (a metabolite of carbaryl and naphthalene), ethylene bisdithiocarbamate (EBDC) fungicides, insecticide fipronil sulfone metabolite, dithiocarbamate fungicides, lambda-cyhalothrin (pyrethroid), paraquat (herbicide), p,p′-dichlorodiphenyltrichloroethane (DDT), p,p′-diphenyldichloroethene (DDE), hexachlorobenzene (HCB), alpha-chlordane, endosulphan 2, methoxychlor, beta-hexachlorocyclohexane (HCH) and mancozeb (fungicide)) (Table 2). Some studies have compared TSH and thyroid hormone levels between conventional farmers (who use pesticides) and organic farmers [410]. Studies have yielded inconsistent results. Pesticide use increased [341,410,411,412,413,414,415,416,417], decreased [415,418,419,420] or had no effect [352,355,356,413,421,422,423,424,425,426,427,428,429,430,431,432] on TSH levels. T4 levels either increased [343,410,412,414,415,416,420,433,434,435], decreased [344,410,416,421,422,423,426,427,428,429,433,436] or did not change [341,352,355,356,411,413,417,421,423,424,425,427,428,430] after pesticide use. The same was observed with T3: studies reported an increase [343,410,416,420,433,434,435], decrease [343,344,414,416,417,429,436,437,438] or no change [352,355,413,423,424,426,427,428,430,433] after pesticide use. Such variations in results are expected since different types of pesticides were analyzed.
Nitrate
Nitrate can occur naturally in vegetables grown in soil and in surface water and groundwater. However, due to excessive use of fertilizers, septic systems in rural areas, food processing waste and industrial waste, nitrate levels in the food and water can increase. Nitrate ion competitively binds to NIS, resulting in low iodine intake in the thyroid gland (reviewed in [439]). Higher exposure to nitrate has even been associated with a higher risk of developing hypothyroidism [440,441]. Studies examining the effect of nitrate on TSH and thyroid hormone levels in healthy adults have yielded inconsistent results. Some studies have observed a decrease [442], an increase [443] or no change [385] in TSH levels as a result of higher nitrate exposure. Moreover, a decrease [385,387], an increase [442] or no change [443,444,445] in T4 levels was observed as a result of higher nitrate exposure, while T3 levels did not correlate with nitrate levels [444,445].
3.2.2. Heavy Metals
Heavy metals such as arsenic (As), cadmium (Cd), lead (Pb) and mercury (Hg) are environmental toxins that interfere with the normal functioning of the thyroid gland. Arsenic has been shown to inhibit TPO activity [446]. Cadmium affects TPO activity [329,447] and alters thyroid hormone metabolism [329,448]. Lead affects the intake of iodide in the thyroid gland [329] and alters the metabolism of thyroid hormones [449]. Mercury affects TPO activity [329,447] and inhibits deiodinases involved in the metabolism of thyroid hormones [450]. Various studies examining the effect of heavy metals on TSH and thyroid hormone levels in healthy adults have yielded inconsistent results. Arsenic exposure leads to an increase [318,451,452,453], decrease [453] or no change [453,454] in TSH levels. T4 levels decreased [451,453], while T3 levels either decreased [451] or did not change [453] after arsenic exposure. After cadmium exposure, an increase [455], a decrease [456] or no change [318,457,458,459,460,461,462,463] in TSH levels was observed. T4 levels increased [456,457,458,462], decreased [455,463,464] or did not change [423,457,458,459,460,461] after cadmium exposure. The same was observed for T3; an increase [456,457,458,462,464], decrease [455,463] or no change [458,459,460,461] after cadmium exposure. Lead exposure caused an increase [460,461,465,466,467], a decrease [318,351,468] or no change [423,454,457,458,459,469,470,471,472,473,474,475] in TSH levels. Likewise, an increase [458,468,476,477], a decrease [318,458,465,470,473,478] or no change [351,423,457,459,460,461,465,466,469,471,472,474,475,478] in T4 levels and an increase [351,423,458,468,477], a decrease [465,472] or no change [423,457,459,460,461,466,470,471,473,474,475] in T3 levels were observed after lead exposure. TSH levels were increased [479,480] or unchanged [318,423,456,457,481,482] after exposure to mercury. T4 levels were also increased [480,481], decreased [456,457,480,482] or unchanged [351,423,456,457,479] after exposure to mercury. Inconsistent results for T3 were also observed: studies reported an increase [483], a decrease [456,457,481,482] or no change [479] after mercury exposure (Table 2). The cause of variability between the studies was probably due to the fact that the participants were exposed to different doses of heavy metals. Moreover, the majority of conducted studies were underpowered. Therefore, additional studies with a larger number of participants are needed to elucidate the influence of heavy metals on TSH and thyroid hormone levels.
4. Conclusions
The scope of this review was to provide a comprehensive insight into the literature discussing the influence of environmental factors on TSH and thyroid hormone levels in healthy adults. We included lifestyle factors (smoking, alcohol consumption, diet and exercise) and pollutants (chemicals and heavy metals) (Figure 1). After analyzing the literature, we conclude that there is still a large variability in results between studies. The pollutant that showed the clearest relationship with thyroid hormones was perchlorate; most studies have noticed a decrease in thyroid hormone levels after exposure to perchlorate (Table 2). Lifestyle factors that showed the highest consistency in results between studies were smoking, BMI and iodine (micronutrient taken from the diet). Smoking leads to a decrease in TSH levels and an increase in T3 and T4 levels (Table 1). There was a positive correlation between BMI levels and TSH and fT3 levels (Table 1). In addition, an increase in TSH levels and a decrease in thyroid hormone levels were observed after excess iodine (Table 1). Future studies should continue to analyze the influence of environmental factors on thyroid function. Studies should involve a large number of participants and meta-analyses should also be conducted. More studies in this area will provide researchers with valuable information needed to understand the complex background of gene–environment interactions that underlie the development of thyroid disease.
Abbreviations
| 1N | 1-naphthol |
| 3-PBA | phenoxybenzoic acid |
| 5Cx-MEP | mono-ethyl phthalate |
| 5Oxo-MEHP | mono-(2-ethylhexyl) phthalate |
| As | arsenic |
| BMI | body mass index |
| BPA | bisphenol A |
| Cd | cadmium |
| DAP | dialkyl phosphate |
| cis and trans-DCCA | cis and trans-3-2,2-dichlorovinyl-2,2-dimethylcyclopropane carboxylic acid |
| DDE | p,p′-diphenyldichloroethene |
| DDT | p,p′-dichlorodiphenyltrichloroethane |
| DEHP | di-(2-ethylhexyl) phthalate |
| Dio1 | type 1 iodothyronine deiodinase |
| Dio2 | type 2 iodothyronine deiodinase |
| DMP | dimethyl metabolite |
| EBDC | ethylene bisdithiocarbamate |
| EDC | endocrine-disrupting compound |
| ESS | euthyroid sick syndrome |
| fT3 | free triiodothyronine |
| fT4 | free thyroxine |
| GWAS | genome-wide association studies |
| HCB | hexachlorobenzene |
| HCH | beta-hexachlorocyclohexane |
| Hg | mercury |
| HPT axis | hypothalamus–pituitary–thyroid axis |
| MBzP | mono-benzyl phthalate |
| MEHP | mono(2-ethylhexyl) phthalate |
| MEHHP | mono (2-ethyl-5-hydroxyhexyl) phthalate |
| MEOHP | mono-(2-ethyl-5-oxohexyl) phthalate |
| MnBP | mono-n-butyl phthalate |
| NIS | sodium/iodide symporter |
| PBB | polybrominated biphenyl |
| PBDE | polybrominated diphenyl ether |
| PCB | polychlorinated biphenyl |
| PFAS | perfluoroalkyl substance |
| PFC | perfluorinated compound |
| PFDeA | perfluorodecanoate |
| PFHxS | perfluorohexane sulfonate |
| PFNA | perfluorononanoic acid |
| PFOA | perfluorooctanoic acid |
| PFOS | perfluorooctane sulfonic acid |
| PFTrDA | perfluorotridecanoic acid |
| POP | persistent organic pollutant |
| rT3 | reverse triiodothyronine |
| T3 | triiodothyronine |
| T4 | thyroxine |
| tT3 | total T3 |
| tT4 | total T4 |
| TCPY | trichloro-2-pyridinol |
| Tg | thyroglobulin |
| TPO | thyroid peroxidase |
| TRH | thyrotropin-releasing hormone |
| TSH | thyroid-stimulating hormone |
| UAAS | arsenic adjusted for arsenobetaine |
| UAB | arsenobetaine |
| UAS | total arsenic |
| UDMA | dimethylarsinic acid |
| VDR | vitamin D receptor |
Author Contributions
T.Z. and M.B.L. conceived the review. M.B.L. wrote the first draft of the manuscript. I.G., N.P. and T.Z. critically revised and edited the manuscript with important intellectual content. Conceptualization, T.Z. and M.B.L.; validation, T.Z., I.G. and N.P.; investigation, M.B.L.; resources, T.Z.; writing—original draft preparation, M.B.L.; writing—review and editing, T.Z., I.G. and N.P.; visualization, T.Z., I.G., M.B.L. and N.P.; supervision, T.Z.; project administration, T.Z.; funding acquisition, T.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the Croatian Science Foundation under the project “Regulation of Thyroid and Parathyroid Function and Blood Calcium Homeostasis” (No. 2593).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable. No new data were created or analysed in this study.
Conflicts of Interest
Authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fekete C., Lechan R.M. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr. Rev. 2014;35:159–194. doi: 10.1210/er.2013-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dayan C., Panicker V. Management of hypothyroidism with combination thyroxine (T4) and triiodothyronine (T3) hormone replacement in clinical practice: A review of suggested guidance. Thyroid Res. 2018;11:1. doi: 10.1186/s13044-018-0045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianco A., Kim B. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Investig. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoermann R., Midgley J.E.M., Larisch R., Dietrich J.W. Relational stability in the expression of normality, variation, and control of thyroid function. Front. Endocrinol. 2016;7:142. doi: 10.3389/fendo.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Thyroid Association. [(accessed on 30 March 2021)]; Available online: https://www.thyroid.org/media-main/press-room/
- 6.Hansen P.S., Brix T.H., Sørensen T.I.A., Kyvik K.O., Hegedüs L. Major genetic influence on the regulation of the pituitary-thyroid axis: A study of healthy danish twins. J. Clin. Endocrinol. Metab. 2004;89:1181–1187. doi: 10.1210/jc.2003-031641. [DOI] [PubMed] [Google Scholar]
- 7.Panicker V., Wilson S.G., Spector T.D., Brown S.J., Falchi M., Richards J.B., Surdulescu G.L., Lim E.M., Fletcher S.J., Walsh J.P. Heritability of serum TSH, free T4 and free T3 concentrations: A study of a large UK twin cohort. Clin. Endocrinol. 2008;68:652–659. doi: 10.1111/j.1365-2265.2007.03079.x. [DOI] [PubMed] [Google Scholar]
- 8.Chaker L., Korevaar T.I.M., Medici M., Uitterlinden A.G., Hofman A., Dehghan A., Franco O.H., Peeters R.P. Thyroid function characteristics and determinants: The Rotterdam study. Thyroid. 2016;26:1195–1204. doi: 10.1089/thy.2016.0133. [DOI] [PubMed] [Google Scholar]
- 9.Song Q., Chen X., Su Y., Xie Z., Wang S., Cui B. Age and gender specific thyroid hormones and their relationships with body mass index in a large chinese population. Int. J. Endocrinol. Metab. 2019;17:e66450. doi: 10.5812/ijem.66450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knezevic J., Starchl C., Berisha A.T., Amrein K. Thyroid-gut-axis: How does the microbiota influence thyroid function? Nutrients. 2020;12:1769. doi: 10.3390/nu12061769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helmreich D.L., Parfitt D.B., Lu X.Y., Akil H., Watson S.J. Relation between the Hypothalamic-Pituitary-Thyroid (HPT) axis and the Hypothalamic-Pituitary-Adrenal (HPA) axis during repeated stress. Neuroendocrinology. 2005;81:183–192. doi: 10.1159/000087001. [DOI] [PubMed] [Google Scholar]
- 12.Montanelli L., Benvenga S., Hegedüs L., Vitti P., Latrofa F., Duntas L.H. Drugs and other substances interfering with thyroid function. In: Vitti P., Hegedüs L., editors. Thyroid Diseases. Endocrinology. Springer; Cham, Switzerland: 2018. pp. 733–761. [Google Scholar]
- 13.Gore A.C., Chappell V.A., Fenton S.E., Flaws J.A., Nadal A., Prins G.S., Toppari J., Zoeller R.T. EDC-2: The endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 2015;36:E1–E150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otun J., Sahebkar A., Östlundh L., Atkin S.L., Sathyapalan T. Systematic review and meta-analysis on the effect of soy on thyroid function. Sci. Rep. 2019;9:3964. doi: 10.1038/s41598-019-40647-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai H., Zhang L., Han X., Zhao H., Guo J., Li Z., Yang A. Body mass index (BMI) is associated with serum thyroid stimulating hormone (TSH) level in infertile women: A cross-sectional study. Endocr. J. 2020;67:923–928. doi: 10.1507/endocrj.EJ19-0441. [DOI] [PubMed] [Google Scholar]
- 16.Gruppen E.G., Kootstra-Ros J., Kobold A.M., Connelly M.A., Touw D., Bos J.H.J., Hak E., Links T.P., Bakker S.J.L., Dullaart R.P.F. Cigarette smoking is associated with higher thyroid hormone and lower TSH levels: The PREVEND study. Endocrine. 2020;67:613–622. doi: 10.1007/s12020-019-02125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medici M., Visser T.J., Peeters R.P. Genetics of thyroid function. Best Pract. Res. Clin. Endocrinol. Metab. 2017;31:129–142. doi: 10.1016/j.beem.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Gunjača I., Matana A., Boutin T., Torlak V., Punda A., Polašek O., Boraska Perica V., Hayward C., Zemunik T., Barbalić M. Genome-wide association meta-analysis for total thyroid hormone levels in Croatian population. J. Hum. Genet. 2019;64:473–480. doi: 10.1038/s10038-019-0586-4. [DOI] [PubMed] [Google Scholar]
- 19.Popović M., Matana A., Torlak V., Boutin T., Brdar D., Gunjača I., Kaličanin D., Kolčić I., Boraska Perica V., Punda A., et al. Genome-wide meta-analysis identifies novel loci associated with free triiodothyronine and thyroid-stimulating hormone. J. Endocrinol. Investig. 2019;42:1171–1180. doi: 10.1007/s40618-019-01030-9. [DOI] [PubMed] [Google Scholar]
- 20.Kus A., Chaker L., Teumer A., Peeters R.P., Medici M. The genetic basis of thyroid function: Novel findings and new approaches. J. Clin. Endocrinol. Metab. 2020;105:dgz225. doi: 10.1210/clinem/dgz225. [DOI] [PubMed] [Google Scholar]
- 21.Kadkhodazadeh H., Amouzegar A., Mehran L., Gharibzadeh S., Azizi F., Tohidi M. Smoking status and changes in thyroid-stimulating hormone and free thyroxine levels during a decade of follow-up: The Tehran thyroid study. Casp. J. Intern. Med. 2020;11:47–52. doi: 10.22088/cjim.11.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vejbjerg P., Knudsen N., Perrild H., Carlé A., Laurberg P., Pedersen I.B., Rasmussen L.B., Ovesen L., Jørgensen T. The impact of smoking on thyroid volume and function in relation to a shift towards iodine sufficiency. Eur. J. Epidemiol. 2008;23:423–429. doi: 10.1007/s10654-008-9255-1. [DOI] [PubMed] [Google Scholar]
- 23.Nyrnes A., Jorde R., Sundsfjord J. Serum TSH is positively associated with BMI. Int. J. Obes. 2006;30:100–105. doi: 10.1038/sj.ijo.0803112. [DOI] [PubMed] [Google Scholar]
- 24.Belin R.M., Astor B.C., Powe N.R., Ladenson P.W. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the Third National Health and Nutrition Examination Surve. J. Clin. Endocrinol. Metab. 2004;89:6077–6086. doi: 10.1210/jc.2004-0431. [DOI] [PubMed] [Google Scholar]
- 25.Åsvold B.O., Bjøro T., Nilsen T.I.L., Vatten L.J. Tobacco smoking and thyroid function: A population-based study. Arch. Intern. Med. 2007;167:1428–1432. doi: 10.1001/archinte.167.13.1428. [DOI] [PubMed] [Google Scholar]
- 26.Cho N.H., Choi H.S., Kim K.W., Kim H.-L., Lee S.-Y., Choi S.H., Lim S., Park Y.J., Park D.J., Jang H.C., et al. Interaction between cigarette smoking and iodine-intake and their impact on thyroid function. Clin. Endocrinol. 2010;73:264–270. doi: 10.1111/j.1365-2265.2010.03790.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., Shi L., Zhang Q., Peng N., Chen L., Lian X., Liu C., Shan Z., Shi B., Tong N., et al. The association between cigarette smoking and serum thyroid stimulating hormone, thyroid peroxidase antibodies and thyroglobulin antibodies levels in Chinese residents: A cross-sectional study in 10 cities. PLoS ONE. 2019;14:e0225435. doi: 10.1371/journal.pone.0225435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim S.J., Kim M.J., Yoon S.G., Myong J.P., Yu H.W., Chai Y.J., Choi J.Y., Lee K.E. Impact of smoking on thyroid gland: Dose-related effect of urinary cotinine levels on thyroid function and thyroid autoimmunity. Sci. Rep. 2019;9:4213. doi: 10.1038/s41598-019-40708-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukayama H., Nasu M., Murakami S., Sugawara M. Examination of antithyroid effects of smoking products in cultured thyroid follicles: Only thiocyanate is a potent antithyroid agent. Acta Endocrinol. 1992;127:520–525. doi: 10.1530/acta.0.1270520. [DOI] [PubMed] [Google Scholar]
- 30.Langer P. Extrathyroidal effect of thiocyanate and propylthiouracil: The depression of the protein-bound iodine level in intact and thyroidectomized rats. J. Endocrinol. 1971;50:367–372. doi: 10.1677/joe.0.0500367. [DOI] [PubMed] [Google Scholar]
- 31.Wiersinga W.M. Smoking and thyroid. Clin. Endocrinol. 2013;79:145–151. doi: 10.1111/cen.12222. [DOI] [PubMed] [Google Scholar]
- 32.Cryer P.E., Haymond M.W., Santiago J.V., Shah S.D. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N. Engl. J. Med. 1976;295:573–577. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- 33.Melander A., Westgren U., Ericson L.E., Sundler F. Influence of the sympathetic nervous system on the secretion and metabolism of thyroid hormone. Endocrinology. 1977;101:1228–1237. doi: 10.1210/endo-101-4-1228. [DOI] [PubMed] [Google Scholar]
- 34.Filis P., Hombach-Klonisch S., Ayotte P., Nagrath N., Soffientini U., Klonisch T., O’Shaughnessy P., Fowler P.A. Maternal smoking and high BMI disrupt thyroid gland development. BMC Med. 2018;16:194. doi: 10.1186/s12916-018-1183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balhara Y.S., Deb K. Impact of alcohol use on thyroid function. Indian J. Endocrinol. Metab. 2013;17:580–587. doi: 10.4103/2230-8210.113724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hegedüs L., Rasmussen N., Ravn V., Kastrup J., Krogsgaard K., Aldershvile J. Independent effects of liver disease and chronic alcoholism on thyroid function and size: The possibility of a toxic effect of alcohol on the thyroid gland. Metabolism. 1988;37:229–233. doi: 10.1016/0026-0495(88)90100-X. [DOI] [PubMed] [Google Scholar]
- 37.Heinz A., Bauer M., Kuhn S., Krüger F., Gräf K.J., Rommelspacher H., Schmidt L.G. Long-term observation of the hypgthalamic-pituitary-thyroid (HPT) axis in alcohol-dependent patients. Acta Psychiatr. Scand. 1996;93:470–476. doi: 10.1111/j.1600-0447.1996.tb10679.x. [DOI] [PubMed] [Google Scholar]
- 38.Liappas I., Piperi C., Malitas P., Tzavellas E., Zisaki A., Liappas A., Kalofoutis C., Boufidou F., Bagos P., Rabavilas A., et al. Interrelationship of hepatic function, thyroid activity and mood status in alcohol-dependent individuals. In Vivo. 2006;20:293–300. [PubMed] [Google Scholar]
- 39.Ozsoy S., Esel E., Izgi H.B., Sofuoglu S. Thyroid function in early and late alcohol withdrawal: Relationship with aggression, family history, and onset age of alcoholism. Alcohol Alcohol. 2006;41:515–521. doi: 10.1093/alcalc/agl056. [DOI] [PubMed] [Google Scholar]
- 40.Valeix P., Faure P., Bertrais S., Vergnaud A.C., Dauchet L., Hercberg S. Effects of light to moderate alcohol consumption on thyroid volume and thyroid function. Clin. Endocrinol. 2008;68:988–995. doi: 10.1111/j.1365-2265.2007.03123.x. [DOI] [PubMed] [Google Scholar]
- 41.Hegedüs L. Decreased Thyroid gland volume in Alcoholic cirrhosis of the liver. J. Clin. Endocrinol. Metab. 1984;58:930–933. doi: 10.1210/jcem-58-5-930. [DOI] [PubMed] [Google Scholar]
- 42.Geurts J., Demeester-Mirkine N., Glinoer D., Prigogine T., Fernandez-Deville M., Corvilain J. Alterations in circulating thyroid hormones and thyroxine binding globulin in chronic alcoholism. Clin. Endocrinol. 1981;14:113–118. doi: 10.1111/j.1365-2265.1981.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 43.Anderson D.L., Nelson J.C., Haviland M.G., MacMurray J.P., Cummings M.A., McGhee W.H., Hubbard R.W. Thyroid stimulating hormone and prolactin responses to thyrotropin releasing hormone in nondepressed alcoholic inpatients. Psychiatry Res. 1992;43:121–128. doi: 10.1016/0165-1781(92)90126-N. [DOI] [PubMed] [Google Scholar]
- 44.Hegedüs L., Kastrup J., Feldt-Rasmussen U., Petersen P. Serum thyroglobulin in acute and chronic liver disease. Clin. Endocrinol. 1983;19:231–237. doi: 10.1111/j.1365-2265.1983.tb02985.x. [DOI] [PubMed] [Google Scholar]
- 45.Aoun E.G., Lee M.R., Haass-Koffler C.L., Swift R.M., Addolorato G., Kenna G.A., Leggio L. Relationship between the thyroid axis and alcohol craving. Alcohol Alcohol. 2015;50:24–29. doi: 10.1093/alcalc/agu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Välimäki M., Pelkonen R., Härkönen M., Ylikahri R. Hormonal changes in noncirrhotic male alcoholics during ethanol withdrawal. Alcohol Alcohol. 1984;19:235–242. [PubMed] [Google Scholar]
- 47.Müller N., Hoehe M., Klein H.E., Nieberle G., Kapfhammer H.P., May F., Müller O.A., Fichter M. Endocrinological studies in alcoholics during withdrawal and after abstinence. Psychoneuroendocrinology. 1989;14:113–123. doi: 10.1016/0306-4530(89)90060-7. [DOI] [PubMed] [Google Scholar]
- 48.Pienaar W., Roberts M., Emsley R., Aalbers C., Taljaard F. The thyrotropin releasing hormone stimulation test in alcoholism. Alcohol Alcohol. 1995;30:661–667. [PubMed] [Google Scholar]
- 49.Zoeller R.T., Fletcher D.L., Simonyi A., Rudeen P.K. Chronic ethanol treatment reduces the responsiveness of the hypothalamic-pituitary-thyroid axis to central stimulation. Alcohol. Clin. Exp. Res. 1996;20:954–960. doi: 10.1111/j.1530-0277.1996.tb05277.x. [DOI] [PubMed] [Google Scholar]
- 50.Sheppard M.C., Shennan K.I.J. Desensitization of rat anterior pituitary gland to thyrotrophin releasing hormone. J. Endocrinol. 1984;101:101–105. doi: 10.1677/joe.0.1010101. [DOI] [PubMed] [Google Scholar]
- 51.Hermann D., Heinz A., Mann K. Dysregulation of the hypothalamic-pituitary-thyroid axis in alcoholism. Addiction. 2002;97:1369–1381. doi: 10.1046/j.1360-0443.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 52.Oliveira K.J., Chiamolera M.I., Giannocco G., Pazos-Moura C.C., Ortiga-Carvalho T.M. Thyroid function disruptors: From nature to chemicals. J. Mol. Endocrinol. 2019;62:R1–R19. doi: 10.1530/JME-18-0081. [DOI] [PubMed] [Google Scholar]
- 53.Åsvold B.O., Bjøro T., Vatten L.J. Association of serum TSH with high body mass differs between smokers and never-smokers. J. Clin. Endocrinol. Metab. 2009;94:5023–5027. doi: 10.1210/jc.2009-1180. [DOI] [PubMed] [Google Scholar]
- 54.Lundbäck V., Ekbom K., Hagman E., Dahlman I., Marcus C. Thyroid-stimulating hormone, degree of obesity, and metabolic risk markers in a cohort of swedish children with obesity. Horm. Res. Paediatr. 2017;88:140–146. doi: 10.1159/000475993. [DOI] [PubMed] [Google Scholar]
- 55.Knudsen N., Laurberg P., Rasmussen L.B., Bülow I., Perrild H., Ovesen L., Jørgensen T. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J. Clin. Endocrinol. Metab. 2005;90:4019–4024. doi: 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- 56.Wolffenbuttel B.H.R., Wouters H.J.C.M., Slagter S.N., van Waateringe R.P., Muller Kobold A.C., van Vliet-Ostaptchouk J.V., Links T.P., van der Klauw M.M. Thyroid function and metabolic syndrome in the population-based LifeLines cohort study. BMC Endocr. Disord. 2017;17:65. doi: 10.1186/s12902-017-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Pergola G., Ciampolillo A., Paolotti S., Trerotoli P., Giorgino R. Free triiodothyronine and thyroid stimulating hormone are directly associated with waist circumference, independently of insulin resistance, metabolic parameters and blood pressure in overweight and obese women. Clin. Endocrinol. 2007;67:265–269. doi: 10.1111/j.1365-2265.2007.02874.x. [DOI] [PubMed] [Google Scholar]
- 58.Grandone A., Santoro N., Coppola F., Calabrò P., Perrone L., del Giudice E.M. Thyroid function derangement and childhood obesity: An Italian experience. BMC Endocr. Disord. 2010;10:8. doi: 10.1186/1472-6823-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marras V., Casini M.R., Pilia S., Carta D., Civolani P., Porcu M., Uccheddu A.P., Loche S. Thyroid function in obese children and adolescents. Horm. Res. Paediatr. 2010;73:193–197. doi: 10.1159/000284361. [DOI] [PubMed] [Google Scholar]
- 60.Roef G.L., Rietzschel E.R., Van Daele C.M., Taes Y.E., De Buyzere M.L., Gillebert T.C., Kaufman J.M. Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid. 2014;24:223–231. doi: 10.1089/thy.2013.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor P.N., Richmond R., Davies N., Sayers A., Stevenson K., Woltersdorf W., Taylor A., Groom A., Northstone K., Ring S., et al. Paradoxical relationship between body mass index and thyroid hormone levels: A study using mendelian randomization. J. Clin. Endocrinol. Metab. 2016;101:730–738. doi: 10.1210/jc.2015-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aypak C., Turedi Ö., Yüce A., Görpelioǧlu S. Thyroid-stimulating hormone (TSH) level in nutritionally obese children and metabolic co-morbidity. J. Pediatr. Endocrinol. Metab. 2013;26:703–708. doi: 10.1515/jpem-2012-0384. [DOI] [PubMed] [Google Scholar]
- 63.Habib A., Molayemat M., Habib A. Elevated serum TSH concentrations are associated with higher BMI Z-scores in southern Iranian children and adolescents. Thyroid Res. 2020;13:9. doi: 10.1186/s13044-020-00084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Makepeace A.E., Bremner A.P., O’Leary P., Leedman P.J., Feddema P., Michelangeli V., Walsh J.P. Significant inverse relationship between serum free T4 concentration and body mass index in euthyroid subjects: Differences between smokers and nonsmokers. Clin. Endocrinol. 2008;69:648–652. doi: 10.1111/j.1365-2265.2008.03239.x. [DOI] [PubMed] [Google Scholar]
- 65.Xu R., Huang F., Zhang S., Lv Y., Liu Q. Thyroid function, body mass index, and metabolic risk markers in euthyroid adults: A cohort study. BMC Endocr. Disord. 2019;19:58. doi: 10.1186/s12902-019-0383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tarcin O., Abanonu G.B., Yazici D., Tarcin O. Association of metabolic syndrome parameters with TT3 and FT3/FT4 ratio in obese Turkish population. Metab. Syndr. Relat. Disord. 2012;10:137–142. doi: 10.1089/met.2011.0098. [DOI] [PubMed] [Google Scholar]
- 67.Milionis A., Milionis C. Correlation between body mass index and thyroid function in euthyroid individuals in Greece. ISRN Biomark. 2013;2013:651494. doi: 10.1155/2013/651494. [DOI] [Google Scholar]
- 68.Ghergherehchi R., Hazhir N. Thyroid hormonal status among children with obesity. Ther. Adv. Endocrinol. Metab. 2015;6:51–55. doi: 10.1177/2042018815571892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanyal D., Raychaudhuri M. Hypothyroidism and obesity: An intriguing link. Indian J. Endocrinol. Metab. 2016;20:554–557. doi: 10.4103/2230-8210.183454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Flier J.S., Harris M., Hollenberg A.N. Leptin, nutrition, and the thyroid: The why, the wherefore, and the wiring. J. Clin. Investig. 2000;105:859–861. doi: 10.1172/JCI9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paul R., Hassan M., Nazar H., Gillani S., Afzal N., Qayyum I. Effect of body mass index on serum leptin levels. J. Ayub Med. Coll Abbottabad. 2011;23:40–43. [PubMed] [Google Scholar]
- 72.Krotkiewski M. Thyroid hormones in the pathogenesis and treatment of obesity. Eur. J. Pharmacol. 2002;440:85–98. doi: 10.1016/S0014-2999(02)01420-6. [DOI] [PubMed] [Google Scholar]
- 73.Reinehr T. Obesity and thyroid function. Mol. Cell. Endocrinol. 2010;316:165–171. doi: 10.1016/j.mce.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 74.Burman K.D., Latham K.R., Djuh Y.Y., Smallridge R.C., Tseng Y.C.L., Lukes Y.G., Maunder R., Wartofsky L. Solubilized nuclear thyroid hormone receptors in circulating human mononuclear cells. J. Clin. Endocrinol. Metab. 1980;51:106–116. doi: 10.1210/jcem-51-1-106. [DOI] [PubMed] [Google Scholar]
- 75.Biban B.G., Lichiardopol C. Iodine deficiency, still a global problem? Curr. Health Sci. J. 2017;43:103–111. doi: 10.12865/CHSJ.43.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sullivan K.M. The challenges of implementing and monitoring of salt iodisation programmes. Best Pract. Res. Clin. Endocrinol. Metab. 2010;24:101–106. doi: 10.1016/j.beem.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 77.Román G.C. Autism: Transient in utero hypothyroxinemia related to maternal flavonoid ingestion during pregnancy and to other environmental antithyroid agents. J. Neurol. Sci. 2007;262:15–26. doi: 10.1016/j.jns.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 78.De Souza dos Santos M.C., Gonçalves C.F.L., Vaisman M., Ferreira A.C.F., de Carvalho D.P. Impact of flavonoids on thyroid function. Food Chem. Toxicol. 2011;49:2495–2502. doi: 10.1016/j.fct.2011.06.074. [DOI] [PubMed] [Google Scholar]
- 79.Divi R.L., Chang H.C., Doerge D.R. Anti-thyroid isoflavones from soybean. Isolation, characterization, and mechanisms of action. Biochem. Pharmacol. 1997;54:1087–1096. doi: 10.1016/S0006-2952(97)00301-8. [DOI] [PubMed] [Google Scholar]
- 80.Divi R.L., Doerge D.R. Inhibition of thyroid peroxidase by dietary flavonoids. Chem. Res. Toxicol. 1996;9:16–23. doi: 10.1021/tx950076m. [DOI] [PubMed] [Google Scholar]
- 81.Chang H.C., Doerge D.R. Dietary genistein inactivates rat thyroid peroxidase in vivo without an apparent hypothyroid effect. Toxicol. Appl. Pharmacol. 2000;168:244–252. doi: 10.1006/taap.2000.9019. [DOI] [PubMed] [Google Scholar]
- 82.Tahboub R., Arafah B.M. Sex steroids and the thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2009;23:769–780. doi: 10.1016/j.beem.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 83.Silverstein M.G., Kaplan J.R., Appt S.E., Register T.C., Shively C.A. Effect of soy isoflavones on thyroid hormones in intact and ovariectomized cynomolgus monkeys (Macaca fascicularis) Menopause. 2014;21:1136–1142. doi: 10.1097/GME.0000000000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Šošić-Jurjević B., Filipović B., Wirth E.K., Živanović J., Radulović N., Janković S., Milošević V., Köhrle J. Soy isoflavones interfere with thyroid hormone homeostasis in orchidectomized middle-aged rats. Toxicol. Appl. Pharmacol. 2014;278:124–134. doi: 10.1016/j.taap.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 85.Doerge D.R., Woodling K.A., Churchwell M.I., Fleck S.C., Helferich W.G. Pharmacokinetics of isoflavones from soy infant formula in neonatal and adult rhesus monkeys. Food Chem. Toxicol. 2016;92:165–176. doi: 10.1016/j.fct.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 86.Hydovitz J.D. Occurrence of goiter in an infant on a soy diet. N. Engl. J. Med. 1960;262:351–353. doi: 10.1056/NEJM196002182620707. [DOI] [PubMed] [Google Scholar]
- 87.Shepard T.H., Pyne G.E., Kirschvink J.F., McLean M. Soybean goiter. N. Engl. J. Med. 1960;262:1099–1103. doi: 10.1056/NEJM196006022622201. [DOI] [Google Scholar]
- 88.Ripp J.A. Soybean-induced goiter. Am. J. Dis. Child. 1961;102:106–109. doi: 10.1001/archpedi.1961.02080010108017. [DOI] [PubMed] [Google Scholar]
- 89.Bruce B., Messina M., Spiller G.A. Isoflavone supplements do not affect thyroid function in iodine-replete postmenopausal women. J. Med. Food. 2003;6:309–316. doi: 10.1089/109662003772519859. [DOI] [PubMed] [Google Scholar]
- 90.Dillingham B.L., McVeigh B.L., Lampe J.W., Duncan A.M. Soy protein isolates of varied isoflavone content do not influence serum thyroid hormones in healthy young men. Thyroid. 2007;17:131–137. doi: 10.1089/thy.2006.0206. [DOI] [PubMed] [Google Scholar]
- 91.Khaodhiar L., Ricciotti H., Li L., Pan W., Schickel M., Zhou J., Blackburn G. Daidzein-rich isoflavone aglycones are potentially effective in reducing hot flashes in menopausal women. Menopause. 2008;15:125–132. doi: 10.1097/gme.0b013e31805c035b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bitto A., Polito F., Atteritano M., Altavilla D., Mazzaferro S., Marini H., Adamo E.B., D’Anna R., Granese R., Corrado F., et al. Genistein aglycone does not affect thyroid function: Results from a three-year, randomized, double-blind, placebo-controlled trial. J. Clin. Endocrinol. Metab. 2010;95:3067–3072. doi: 10.1210/jc.2009-2779. [DOI] [PubMed] [Google Scholar]
- 93.Lazarevic B., Boezelijn G., Diep L.M., Kvernrod K., Ogren O., Ramberg H., Moen A., Wessel N., Berg R.E., Egge-Jacobsen W., et al. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: A randomized, placebo-controlled, double-blind phase 2 clinical trial. Nutr. Cancer. 2011;63:889–898. doi: 10.1080/01635581.2011.582221. [DOI] [PubMed] [Google Scholar]
- 94.Steinberg F.M., Murray M.J., Lewis R.D., Cramer M.A., Amato P., Young R.L., Barnes S., Konzelmann K.L., Fischer J.G., Ellis K.J., et al. Clinical outcomes of a 2-y soy isoflavone supplementation in menopausal women. Am. J. Clin. Nutr. 2011;93:356–367. doi: 10.3945/ajcn.110.008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sathyapalan T., Rigby A.S., Bhasin S., Thatcher N.J., Kilpatrick E.S., Atkin S.L. Effect of soy in men with type 2 diabetes mellitus and subclinical hypogonadism: A randomized controlled study. J. Clin. Endocrinol. Metab. 2017;102:425–433. doi: 10.1210/jc.2016-2875. [DOI] [PubMed] [Google Scholar]
- 96.Sathyapalan T., Köhrle J., Rijntjes E., Rigby A.S., Dargham S.R., Kilpatrick E.S., Atkin S.L. The effect of high dose isoflavone supplementation on serum reverse T3 in euthyroid men with type 2 diabetes and post-menopausal women. Front. Endocrinol. 2018;9:698. doi: 10.3389/fendo.2018.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Persky V.W., Turyk M.E., Wang L., Freels S., Chatterton R., Barnes S., Erdman J., Sepkovic D.W., Bradlow H.L., Potter S. Effect of soy protein on endogenous hormones in postmenopausal women. Am. J. Clin. Nutr. 2002;75:145–153. doi: 10.1093/ajcn/75.1.145. [DOI] [PubMed] [Google Scholar]
- 98.Jayagopal V., Albertazzi P., Kilpatrick E.S., Howarth E.M., Jennings P.E., Hepburn D.A., Atkin S.L. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002;25:1709–1714. doi: 10.2337/diacare.25.10.1709. [DOI] [PubMed] [Google Scholar]
- 99.Duncan A.M., Underhill K.E.W., Xu X., LaValleur J., Phipps W.R., Kurzer M.S. Modest hormonal effects of soy isoflavones in postmenopausal women. J. Clin. Endocrinol. Metab. 1999;84:3479–3484. doi: 10.1210/jc.84.10.3479. [DOI] [PubMed] [Google Scholar]
- 100.Mittal N., Hota D., Dutta P., Bhansali A., Suri V., Aggarwal N., Marwah R.K., Chakrabarti A. Evaluation of effect of isoflavone on thyroid economy & autoimmunity in oophorectomised women: A randomised, double-blind, placebo-controlled trial. Indian J. Med. Res. 2011;133:633–640. [PMC free article] [PubMed] [Google Scholar]
- 101.Hüser S., Guth S., Joost H.G., Soukup S.T., Köhrle J., Kreienbrock L., Diel P., Lachenmeier D.W., Eisenbrand G., Vollmer G., et al. Effects of isoflavones on breast tissue and the thyroid hormone system in humans: A comprehensive safety evaluation. Arch. Toxicol. 2018;92:2703–2748. doi: 10.1007/s00204-018-2279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Felker P., Bunch R., Leung A.M. Concentrations of thiocyanate and goitrin in human plasma, their precursor concentrations in brassica vegetables, and associated potential risk for hypothyroidism. Nutr. Rev. 2016;74:248–258. doi: 10.1093/nutrit/nuv110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Babiker A., Alawi A., Atawi M., Alwan I. The role of micronutrients in thyroid dysfunction. Sudan. J. Paediatr. 2020;20:13–19. doi: 10.24911/SJP.106-1587138942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Agerbirk N., De Vos M., Kim J.H., Jander G. Indole glucosinolate breakdown and its biological effects. Phytochem. Rev. 2009;8:101–120. doi: 10.1007/s11101-008-9098-0. [DOI] [Google Scholar]
- 105.Bischoff K.L. Nutraceuticals: Efficacy, Safety and Toxicity. Elsevier Inc.; Amsterdam, The Netherlands: 2016. Glucosinolates; pp. 551–554. [Google Scholar]
- 106.Tonacchera M., Pinchera A., Dimida A., Ferrarini E., Agretti P., Vitti P., Santini F., Crump K., Gibbs J. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid. 2004;14:1012–1019. doi: 10.1089/thy.2004.14.1012. [DOI] [PubMed] [Google Scholar]
- 107.Mcmillan M., Spinks E.A., Fenwick G.R. Preliminary observations on the effect of dietary brussels sprouts on thyroid function. Hum. Exp. Toxicol. 1986;5:15–19. doi: 10.1177/096032718600500104. [DOI] [PubMed] [Google Scholar]
- 108.Meissner H.O., Reich-Bilinska H., Mscisz A., Kedzia B. Therapeutic effects of pre-gelatinized maca (Lepidium Peruvianum Chacon) used as a non-hormonal alternative to HRT in perimenopausal women—Clinical pilot study. Int. J. Biomed. Sci. 2006;2:143–159. [PMC free article] [PubMed] [Google Scholar]
- 109.Chartoumpekis D.V., Ziros P.G., Chen J.G., Groopman J.D., Kensler T.W., Sykiotis G.P. Broccoli sprout beverage is safe for thyroid hormonal and autoimmune status: Results of a 12-week randomized trial. Food Chem. Toxicol. 2019;126:1–6. doi: 10.1016/j.fct.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pang K.L., Lumintang J.N., Chin K.Y. Thyroid-modulating activities of olive and its polyphenols: A systematic review. Nutrients. 2021;13:529. doi: 10.3390/nu13020529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Al-Qarawi A.A., Al-Damegh M.A., ElMougy S.A. Effect of freeze dried extract of Olea europaea on the pituitary-thyroid axis in rats. Phyther. Res. 2002;16:286–287. doi: 10.1002/ptr.855. [DOI] [PubMed] [Google Scholar]
- 112.Mahmoudi A., Ghorbel H., Feki I., Bouallagui Z., Guermazi F., Ayadi L., Sayadi S. Oleuropein and hydroxytyrosol protect rats’ pups against bisphenol A induced hypothyroidism. Biomed. Pharmacother. 2018;103:1115–1126. doi: 10.1016/j.biopha.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 113.Mekircha F., Chebab S., Gabbianelli R., Leghouchi E. The possible ameliorative effect of Olea europaea L. oil against deltamethrin-induced oxidative stress and alterations of serum concentrations of thyroid and reproductive hormones in adult female rats. Ecotoxicol. Environ. Saf. 2018;161:374–382. doi: 10.1016/j.ecoenv.2018.05.086. [DOI] [PubMed] [Google Scholar]
- 114.Quitete F.T., Lisboa P.C., de Moura E.G., de Oliveira E. Different oils used as supplement during lactation causes endocrine-metabolic dysfunctions in male rats. J. Funct. Foods. 2018;48:43–53. doi: 10.1016/j.jff.2018.06.027. [DOI] [Google Scholar]
- 115.Oke O.E., Emeshili U.K., Iyasere O.S., Abioja M.O., Daramola J.O., Ladokun A.O., Abiona J.A., Williams T.J., Rahman S.A., Rotimi S.O., et al. Physiological responses and performance of broiler chickens offered olive leaf extract under a hot humid tropical climate. J. Appl. Poult. Res. 2017;26:376–382. doi: 10.3382/japr/pfx005. [DOI] [Google Scholar]
- 116.Ahmed M., El-Saadany A., Shreif E., El-Barbary A. Effect of dietary olive leaves extract (oleuropein) supplementation on productive, physiological and immunological parameters in bandarah chickens 2-during production period. Egypt. Poult. Sci. J. 2018;37:277–292. [Google Scholar]
- 117.Abd-Alla O., Abdel-Samee A., EL-Adawy S. Effect of acacia saligna and olive pulp on growth, biochemical and hormonal status in lambs under heat stress in Sinai province. SCVMJ. 2007;7:129–138. [Google Scholar]
- 118.Farooq M., Ali S., Zubair M., Ullah Q., Jamil H., Haroon M., Ghaffar A. Effect of feed supplementation with olive oil on serum testosterone, triiodothyronine, thyroxine and some biochemical metabolites in teddy goat bucks. Asian J. Agric. Biol. 2019;7:116–121. doi: 10.1530/endoabs.50.P365. [DOI] [Google Scholar]
- 119.Abdalla E., El-Masry K.A., Khalil F.A., Teama F.E., Emara S.S. Alleviation of oxidative stress by using olive pomace in crossbred (Brown Swiss X Baladi) calves under hot environmental conditions. Arab J. Nucl. Sci. Appl. 2015;47:88–99. [Google Scholar]
- 120.Zupo R., Castellana F., Panza F., Lampignano L., Murro I., Di Noia C., Triggiani V., Giannelli G., Sardone R., De Pergola G. Adherence to a mediterranean diet and thyroid function in obesity: A cross-sectional Apulian survey. Nutrients. 2020;12:3173. doi: 10.3390/nu12103173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gaitan E., Lindsay R.H., Reichert R.D., Ingbarf S.H., Cooksey R.C., Legan J., Meydrech E.F., Hill J., Kubota K. Antithyroid and goitrogenic effects of millet: Role of C-Glycosylflavones. J. Clin. Endocrinol. Metab. 1989;68:707–714. doi: 10.1210/jcem-68-4-707. [DOI] [PubMed] [Google Scholar]
- 122.Gaitan E., Cooksey R.C., Legan J., Lindsay R.H. Antithyroid effects in vivo and in vitro of vitexin: A C-glucosylflavone in millet. J. Clin. Endocrinol. Metab. 1995;80:1144–1147. doi: 10.1210/jcem.80.4.7714083. [DOI] [PubMed] [Google Scholar]
- 123.Elnour A., Liedén S.Å., Bourdoux P., Eltom M., Khalid S.A., Hambraeus L. Traditional fermentation increases goitrogenic activity in pearl millet. Ann. Nutr. Metab. 1998;42:341–349. doi: 10.1159/000012754. [DOI] [PubMed] [Google Scholar]
- 124.Abdel Gadir W., Adam S. Effects of pearl millet (Pennisetum typhoides), and fermented and processed fermented millet on Nubian goats. Vet. Hum. Toxicol. 2000;42:133–136. [PubMed] [Google Scholar]
- 125.Moreno-Reyes R., Boelaert M., El Badawi S., Eltom M., Vanderpas J.B. Endemic juvenile hypothyroidism in a severe endemic goitre area of Sudan. Clin. Endocrinol. 1993;38:19–24. doi: 10.1111/j.1365-2265.1993.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 126.Brahmbhatt S., Fearnley R., Brahmbhatt R., Eastman C., Boyages S. Study of biochemical prevalence indicators for the assessment of iodine deficiency disorders in adults at field conditions in Gujarat (India) Asia Pac. J. Clin. Nutr. 2001;10:51–57. doi: 10.1046/j.1440-6047.2001.00197.x. [DOI] [PubMed] [Google Scholar]
- 127.Sartelet H., Serghat S., Lobstein A., Ingenbleek Y., Anton R., Petitfrère E., Aguie-Aguie G., Martiny L., Haye B. Flavonoids extracted from fonio millet (Digitaria exilis) reveal potent antithyroid properties. Nutrition. 1996;12:100–106. doi: 10.1016/0899-9007(96)90707-8. [DOI] [PubMed] [Google Scholar]
- 128.Delange F., Ermans A.M. Role of a dietary goitrogen in the etiology of endemic goiter on Idjwi Island. Am. J. Clin. Nutr. 1971;24:1354–1360. doi: 10.1093/ajcn/24.11.1354. [DOI] [PubMed] [Google Scholar]
- 129.Ermans A., Mbulamoko N., Delange F., Ahluwalia R. Role of Cassava in the Etiology of Endemic Goitre and Cretinism. IDRC; Ottawa, ON, Canada: 1980. [Google Scholar]
- 130.Delange F., Iteke F.B., Ermans A.M. Nutritional factors involved in the goitrogenic action of cassava. J. Nucl. Med. 1983;24:90. [Google Scholar]
- 131.Hershman J.M., Due D.T., Sharp B., My L., Kent J.R., Binh L.N., Reed A.W., Phuc L.D., Herle A.J.V., Thai N.A., et al. Endemic goiter in Vietnam. J. Clin. Endocrinol. Metab. 1983;57:243–249. doi: 10.1210/jcem-57-2-243. [DOI] [PubMed] [Google Scholar]
- 132.Cliff J., Lundquist P., Rosling H., Sörbo B., Wide L. Thyroid function in a cassava-eating population affected by epidemic spastic paraparesis. Acta Endocrinol. 1986;113:523–528. doi: 10.1530/acta.0.1130523. [DOI] [PubMed] [Google Scholar]
- 133.Thilly C.H., Swennen B., Bourdoux P., Ntambue K., Moreno-Reyes R., Gillies J., Vanderpas J.B. The epidemiology of iodine-deficiency disorders in relation to goitrogenic factors and thyroid-stimulating-hormone regulation. Am. J. Clin. Nutr. 1993;57:267S–270S. doi: 10.1093/ajcn/57.2.267S. [DOI] [PubMed] [Google Scholar]
- 134.Bourdoux P., Delange F., Gerard M., Mafuta M., Hanson A., Ermans A.M. Evidence that cassava ingestion increases thiocyanate formation: A possible etiologic factor in endemic goiter. J. Clin. Endocrinol. Metab. 1978;46:613–621. doi: 10.1210/jcem-46-4-613. [DOI] [PubMed] [Google Scholar]
- 135.Kittivachra R. Effects of cassava on thyroid gland in rats. Thai J. Pharm. Sci. 2007;30:57–62. [Google Scholar]
- 136.Chandra A.K., Singh L.H., Ghosh S., Pearce E.N. Role of bamboo-shoot in the pathogenesis of endemic goiter in Manipur, north east India. Endocr. Pract. 2013;19:36–45. doi: 10.4158/EP12162.OR. [DOI] [PubMed] [Google Scholar]
- 137.Singh L.H., Chandra A.K., Yumnam S.D., Sarkar D., Manglem R.K., Dhabali T., Mookerjee S., Ray I. Thiocyanate in excess develops goiter followed by auto immune thyroid diseases even after effective salt iodization in a rural community of north east India. Ecotoxicol. Environ. Saf. 2021;208:111711. doi: 10.1016/j.ecoenv.2020.111711. [DOI] [PubMed] [Google Scholar]
- 138.Sarkar D., Chandra A.K., Chattopadyay S., Biswas M., Das S., Singh L.H., Ray I. Possible mechanism of bamboo shoots (Bambusa balcooa) induced thyroid disruption—An in vitro study. Hum. Exp. Toxicol. 2020;40:483–496. doi: 10.1177/0960327120958037. [DOI] [PubMed] [Google Scholar]
- 139.Sarkar D., Chandra A.K., Chakraborty A., Ghosh S., Chattopadhyay S., Singh L.H., Ray I. Effects of bamboo shoots (Bambusa balcooa) on thyroid hormone synthesizing regulatory elements at cellular and molecular levels in thyrocytes. J. Ethnopharmacol. 2020;250:112463. doi: 10.1016/j.jep.2019.112463. [DOI] [PubMed] [Google Scholar]
- 140.Xu D., Brennan G., Xu L., Zhang X.W., Fan X., Han W.T., Mock T., McMinn A., Hutchins D.A., Ye N. Ocean acidification increases iodine accumulation in kelp-based coastal food webs. Glob. Chang. Biol. 2019;25:629–639. doi: 10.1111/gcb.14467. [DOI] [PubMed] [Google Scholar]
- 141.Miyai K., Tokushige T., Kondo M., Kondo M., Takahashi M., Watanabe R. Suppression of thyroid function during ingestion of seaweed “Kombu” (Laminaria japonoca) in normal Japanese adults. Endocr. J. 2008;55:1103–1108. doi: 10.1507/endocrj.K08E-125. [DOI] [PubMed] [Google Scholar]
- 142.Clark C.D., Bassett B., Burge M.R. Effects of kelp supplementation on thyroid function in euthyroid subjects. Endocr. Pract. 2003;9:363–369. doi: 10.4158/EP.9.5.363. [DOI] [PubMed] [Google Scholar]
- 143.Teas J., Braverman L.E., Kurzer M.S., Pino S., Hurley T.G., Hebert J.R. Seaweed and soy: Companion foods in Asian cuisine and their effects on thyroid function in American women. J. Med. Food. 2007;10:90–100. doi: 10.1089/jmf.2005.056. [DOI] [PubMed] [Google Scholar]
- 144.Aakre I., Evensen L.T., Kjellevold M., Dahl L., Henjum S., Alexander J., Madsen L., Markhus M.W. Iodine status and thyroid function in a group of seaweed consumers in Norway. Nutrients. 2020;12:3483. doi: 10.3390/nu12113483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Noahsen P., Kleist I., Larsen H.M., Andersen S. Intake of seaweed as part of a single sushi meal, iodine excretion and thyroid function in euthyroid subjects: A randomized dinner study. J. Endocrinol. Investig. 2020;43:431–438. doi: 10.1007/s40618-019-01122-6. [DOI] [PubMed] [Google Scholar]
- 146.Brdar D., Gunjača I., Pleić N., Torlak V., Knežević P., Punda A., Polašek O., Hayward C., Zemunik T. The effect of food groups and nutrients on plasma thyroid hormone levels in healthy individuals. Nutrition. 2021 doi: 10.1016/j.nut.2021.111394. in press. [DOI] [PubMed] [Google Scholar]
- 147.Lambrinakou S., Katsa M.E., Zyga S., Ioannidis A., Sachlas A., Panoutsopoulos G., Pistikou A.M., Magana M., Kougioumtzi Dimoligianni D.E., Kolovos P., et al. Advances in Experimental Medicine and Biology. Volume 987. Springer LLC; New York, NY, USA: 2017. Correlations between nutrition habits, anxiety and metabolic parameters in Greek healthy adults; pp. 23–34. [DOI] [PubMed] [Google Scholar]
- 148.Cheryl H. Thyroid disease and diet—Nutrition plays a part in maintaining thyroid health. Today’s Dietit. 2012;14:40. [Google Scholar]
- 149.Panda S., Kar A. Piperine lowers the serum concentrations of thyroid hormones, glucose and hepatic 5′D activity in adult male mice. Horm. Metab. Res. 2003;35:523–526. doi: 10.1055/s-2003-42652. [DOI] [PubMed] [Google Scholar]
- 150.Gaique T.G., Lopes B.P., Souza L.L., Paula G.S.M., Pazos-Moura C.C., Oliveira K.J. Cinnamon intake reduces serum T3 level and modulates tissue-specific expression of thyroid hormone receptor and target genes in rats. J. Sci. Food Agric. 2016;96:2889–2895. doi: 10.1002/jsfa.7460. [DOI] [PubMed] [Google Scholar]
- 151.Chandra A.K., De N. Goitrogenic/antithyroidal potential of green tea extract in relation to catechin in rats. Food Chem. Toxicol. 2010;48:2304–2311. doi: 10.1016/j.fct.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 152.Chandra A.K., De N., Choudhury S.R. Effect of different doses of un-fractionated green and black tea extracts on thyroid physiology. Hum. Exp. Toxicol. 2011;30:884–896. doi: 10.1177/0960327110382563. [DOI] [PubMed] [Google Scholar]
- 153.Sakamoto Y., Mikuriya H., Tayama K., Takahashi H., Nagasawa A., Yano N., Yuzawa K., Ogata A., Aoki N. Goitrogenic effects of green tea extract catechins by dietary administration in rats. Arch. Toxicol. 2001;75:591–596. doi: 10.1007/s00204-001-0286-6. [DOI] [PubMed] [Google Scholar]
- 154.Satoh K., Sakamoto Y., Ogata A., Nagai F., Mikuriya H., Numazawa M., Yamada K., Aoki N. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem. Toxicol. 2002;40:925–933. doi: 10.1016/S0278-6915(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 155.Hu J., Webster D., Cao J., Shao A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018;95:412–433. doi: 10.1016/j.yrtph.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 156.Zhao Y.G., Zhang Y., Wang F.L., Zhou J., Zhao Q.M., Zeng X.Q., Hu M.Q., Jin M.C., Zhu Y. Determination of perchlorate from tea leaves using quaternary ammonium modified magnetic carboxyl-carbon nanotubes followed by liquid chromatography-tandem quadrupole mass spectrometry. Talanta. 2018;185:411–418. doi: 10.1016/j.talanta.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 157.Ji Y., Shi Z., Liu M., Liu S., Liu S., Wang J. Association between the COMTVal158Met genotype and Alzheimer’s disease in the han chinese population. Dement. Geriatr. Cogn. Dis. Extra. 2014;4:14–21. doi: 10.1159/000357161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang C., Chen H., Zhu L., Liu X., Lu C. Accurate, sensitive and rapid determination of perchlorate in tea by hydrophilic interaction chromatography-tandem mass spectrometry. Anal. Methods. 2020;12:3592–3599. doi: 10.1039/D0AY00811G. [DOI] [PubMed] [Google Scholar]
- 159.Centre for Food Safety of the Food and Environmental Hygiene, Department (FEHD) of the Government of the Hong Kong Special Administrative Region . Chemical Hazard Evaluation, Report No. 59. Perchlorate in Tea and Tea Beverages. Centre for Food Safety of the Food and Environmental Hygiene, Department (FEHD) of the Government of the Hong Kong Special Administrative Region; Hong Kong: 2018. p. 35. [Google Scholar]
- 160.Benvenga S., Bartolone L., Pappalardo M.A., Russo A., Lapa D., Giorgianni G., Saraceno G., Trimarchi F. Altered intestinal absorption of L-thyroxine caused by coffee. Thyroid. 2008;18:293–301. doi: 10.1089/thy.2007.0222. [DOI] [PubMed] [Google Scholar]
- 161.Clozel M., Branchaud C.L., Tannenbaum G.S., Dussault J.H., Aranda J.V. Effect of caffeine on thyroid and pituitary function in newborn rats. Pediatr. Res. 1983;17:592–595. doi: 10.1203/00006450-198307000-00015. [DOI] [PubMed] [Google Scholar]
- 162.Bartsch W., Dasenbrock C., Ernst H., Kamino K., Mohr U. Absence of effect of caffeine on the thyroid in the Syrian golden hamster: Results of a 90-day study. Food Chem. Toxicol. 1996;34:153–159. doi: 10.1016/0278-6915(95)00095-X. [DOI] [PubMed] [Google Scholar]
- 163.Spindel E.R., Wurtman R.J., McCall A., Carr D.B., Conley L., Griffith L., Arnold M.A. Neuroendocrine effects of caffeine in normal subjects. Clin. Pharmacol. Ther. 1984;36:402–407. doi: 10.1038/clpt.1984.195. [DOI] [PubMed] [Google Scholar]
- 164.Friedrich N., Pietzner M., Cannet C., Thuesen B.H., Hansen T., Wallaschofski H., Grarup N., Skaaby T., Budde K., Pedersen O., et al. Urinary metabolomics reveals glycemic and coffee associated signatures of thyroid function in two population-based cohorts. PLoS ONE. 2017;12:e0173078. doi: 10.1371/journal.pone.0173078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pietzner M., Homuth G., Budde K., Lehmphul I., Völker U., Völzke H., Nauck M., Köhrle J., Friedrich N. Urine metabolomics by 1H-NMR spectroscopy indicates associations between serum 3,5-T2 Concentrations and intermediary metabolism in euthyroid humans. Eur. Thyroid J. 2015;4:92–100. doi: 10.1159/000381308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pietzner M., Köhrle J., Lehmphul I., Budde K., Kastenmüller G., Brabant G., Völzke H., Artati A., Adamski J., Völker U., et al. A thyroid hormone-independent molecular fingerprint of 3,5-Diiodothyronine suggests a strong relationship with coffee metabolism in humans. Thyroid. 2019;29:1743–1754. doi: 10.1089/thy.2018.0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Köhrle J., Lehmphul I., Pietzner M., Renko K., Rijntjes E., Richards K., Anselmo J., Danielsen M., Jonklaas J. 3,5-T2—A janus-faced thyroid hormone metabolite exerts both canonical T3-mimetic endocrine and intracrine hepatic action. Front. Endocrinol. 2020;10:787. doi: 10.3389/fendo.2019.00787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Berg J.P., Liane K.M., Bjørhovde S.B., Bjøro T., Torjesen P.A., Haug E. Vitamin D receptor binding and biological effects of cholecalciferol analogues in rat thyroid cells. J. Steroid Biochem. Mol. Biol. 1994;50:145–150. doi: 10.1016/0960-0760(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 169.Grundmann M., von Versen-Höynck F. Vitamin D—Roles in women’s reproductive health? Reprod. Biol. Endocrinol. 2011;9:146. doi: 10.1186/1477-7827-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Haug E., Pedersen J.I., Gautvik K.M. Effects of vitamin D3 metabolites on production of prolactin and growth hormone in rat pituitary cells. Mol. Cell. Endocrinol. 1982;28:65–79. doi: 10.1016/0303-7207(82)90041-7. [DOI] [PubMed] [Google Scholar]
- 171.Wark J.D., Tashjian A.H. Vitamin D stimulates prolactin synthesis by GH4C1 cells incubated in chemically defined medium. Endocrinology. 1982;111:1755–1757. doi: 10.1210/endo-111-5-1755. [DOI] [PubMed] [Google Scholar]
- 172.Stumpf W.E., Sar M., O’Brien L.P. Vitamin D sites of action in the pituitary studied by combined autoradiography-immunohistochemistry. Histochemistry. 1987;88:11–16. doi: 10.1007/BF00490160. [DOI] [PubMed] [Google Scholar]
- 173.Sar M., Stumpf W.E., DeLuca H.F. Thyrotropes in the pituitary are target cells for 1,25 dihydroxy vitamin D3. Cell Tissue Res. 1980;209:161–166. doi: 10.1007/BF00219932. [DOI] [PubMed] [Google Scholar]
- 174.Clinckspoor I., Gérard A.-C., Van Sande J., Many M.-C., Verlinden L., Bouillon R., Carmeliet G., Mathieu C., Verstuyf A., Decallonne B. The Vitamin D receptor in thyroid development and function. Eur. Thyroid J. 2012;1:168–175. doi: 10.1159/000342363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chailurkit L.O., Aekplakorn W., Ongphiphadhanakul B. High vitamin D status in younger individuals is associated with low circulatingthyrotropin. Thyroid. 2013;23:25–30. doi: 10.1089/thy.2012.0001. [DOI] [PubMed] [Google Scholar]
- 176.Mackawy A.M.H., Al-Ayed B.M., Al-Rashidi B.M. Vitamin D deficiency and its association with thyroid disease. Int. J. Health Sci. 2013;7:267–275. doi: 10.12816/0006054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bizzaro G., Shoenfeld Y. Vitamin D and autoimmune thyroid diseases: Facts and unresolved questions. Immunol. Res. 2015;61:46–52. doi: 10.1007/s12026-014-8579-z. [DOI] [PubMed] [Google Scholar]
- 178.Wang J., Lv S., Chen G., Gao C., He J., Zhong H., Xu Y. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. 2015;7:2485–2498. doi: 10.3390/nu7042485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.D’Aurizio F., Villalta D., Metus P., Doretto P., Tozzoli R. Is vitamin D a player or not in the pathophysiology of autoimmune thyroid diseases? Autoimmun. Rev. 2015;14:363–369. doi: 10.1016/j.autrev.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 180.Talaei A., Ghorbani F., Asemi Z. The effects of Vitamin D supplementation on thyroid function in hypothyroid patients: A randomized, double-blind, placebo-controlled trial. Indian J. Endocrinol. Metab. 2018;22:584–588. doi: 10.4103/ijem.IJEM_603_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pan Y., Zhong S., Liu Q., Wang C.-B., Zhu W.-H., Shen X.-A., Lu B., Shen L.-W., Zeng Y. Investigating the relationship between 25-hydroxyvitamin D and thyroid function in second-trimester pregnant women. Gynecol. Endocrinol. 2018;34:345–348. doi: 10.1080/09513590.2017.1393659. [DOI] [PubMed] [Google Scholar]
- 182.Barchetta I., Baroni M.G., Leonetti F., De Bernardinis M., Bertoccini L., Fontana M., Mazzei E., Fraioli A., Cavallo M.G. TSH levels are associated with vitamin D status and seasonality in an adult population of euthyroid adults. Clin. Exp. Med. 2015;15:389–396. doi: 10.1007/s10238-014-0290-9. [DOI] [PubMed] [Google Scholar]
- 183.Zhang Q., Wang Z., Sun M., Cao M., Zhu Z., Fu Q., Gao Y., Mao J., Li Y., Shi Y., et al. Association of high vitamin D status with low circulating thyroid-stimulating hormone independent of thyroid hormone levels in middle-aged and elderly males. Int. J. Endocrinol. 2014;2014:631819. doi: 10.1155/2014/631819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mansorian B., Attari M.M.A., Vahabzadeh D., Mohebbi I. Serum vitamin D level and its relation to thyroid hormone, blood sugar and lipid profiles in Iranian sedentary work staff. Nutr. Hosp. 2018;35:1107–1114. doi: 10.20960/nh.1719. [DOI] [PubMed] [Google Scholar]
- 185.Zimmermann M.B., Wegmüller R., Zeder C., Chaouki N., Torresani T. The effects of vitamin A deficiency and vitamin A supplementation on thyroid function in goitrous children. J. Clin. Endocrinol. Metab. 2004;89:5441–5447. doi: 10.1210/jc.2004-0862. [DOI] [PubMed] [Google Scholar]
- 186.Hess S.Y. The impact of common micronutrient deficiencies on iodine and thyroid metabolism: The evidence from human studies. Best Pract. Res. Clin. Endocrinol. Metab. 2010;24:117–132. doi: 10.1016/j.beem.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 187.Orzechowska-Pawilojc A., Sworczak K., Lewczuk A., Babinska A. Homocysteine, folate and cobalamin levels in hypothyroid women before and after treatment. Endocr. J. 2007;54:471–476. doi: 10.1507/endocrj.K06-112. [DOI] [PubMed] [Google Scholar]
- 188.Delitala G., Rovasio P., Lotti G. Suppression of thyrotropin (TSH) and prolactin (PRL) release by pyridoxine in chronic primary hypothyroidism. J. Clin. Endocrinol. Metab. 1977;45:1019–1022. doi: 10.1210/jcem-45-5-1019. [DOI] [PubMed] [Google Scholar]
- 189.Ren S.G., Melmed S. Pyridoxal phosphate inhibits pituitary cell proliferation and hormone secretion. Endocrinology. 2006;147:3936–3942. doi: 10.1210/en.2005-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Singer E. Effects of vitamin E deficiency on the thyroid gland of the rat. J. Physiol. 1936;87:287–290. doi: 10.1113/jphysiol.1936.sp003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Deshpande U., Joseph L., Patwardhan U., Samuel A. Effect of antioxidants (vitamin C, E and turmeric extract) on methimazole induced hypothyroidism in rats. Indian J. Exp. Biol. 2002;40:735–738. [PubMed] [Google Scholar]
- 192.Guerra L.N., Ríos De Molina M.D.C., Miler E.A., Moiguer S., Karner M., Burdman J.A. Antioxidants and methimazole in the treatment of Graves’ disease: Effect on urinary malondialdehyde levels. Clin. Chim. Acta. 2005;352:115–120. doi: 10.1016/j.cccn.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 193.Zimmermann M.B., Jooste P.L., Mabapa N.S., Schoeman S., Biebinger R., Mushaphi L.F., Mbhenyane X. Vitamin A supplementation in iodine-deficient African children decreases thyrotropin stimulation of the thyroid and reduces the goiter rate. Am. J. Clin. Nutr. 2007;86:1040–1044. doi: 10.1093/ajcn/86.4.1040. [DOI] [PubMed] [Google Scholar]
- 194.Aktaş H.Ş. Vitamin B12 and Vitamin D levels in patients with autoimmune hypothyroidism and their correlation with anti-thyroid peroxidase antibodies. Med. Princ. Pract. 2020;29:364–370. doi: 10.1159/000505094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sworczak K., Wiśniewski P. The role of vitamins in the prevention and treatment of thyroid disorders. Endokrynol. Pol. 2011;62:340–344. [PubMed] [Google Scholar]
- 196.Winther K.H., Rayman M.P., Bonnema S.J., Hegedüs L. Selenium in thyroid disorders—Essential knowledge for clinicians. Nat. Rev. Endocrinol. 2020;16:165–176. doi: 10.1038/s41574-019-0311-6. [DOI] [PubMed] [Google Scholar]
- 197.Johnson C.C., Fordyce F.M., Rayman M.P. Symposium on “Geographical and geological influences on nutrition”: Factors controlling the distribution of selenium in the environment and their impact on health and nutrition. Proc. Nutr. Soc. 2010;69:119–132. doi: 10.1017/S0029665109991807. [DOI] [PubMed] [Google Scholar]
- 198.Köhrle J., Jakob F., Contempré B., Dumont J.E. Selenium, the thyroid, and the endocrine system. Endocr. Rev. 2005;26:944–984. doi: 10.1210/er.2001-0034. [DOI] [PubMed] [Google Scholar]
- 199.Schomburg L. Selenium, selenoproteins and the thyroid gland: Interactions in health and disease. Nat. Rev. Endocrinol. 2012;8:160–171. doi: 10.1038/nrendo.2011.174. [DOI] [PubMed] [Google Scholar]
- 200.Stuss M., Michalska-Kasiczak M., Sewerynek E. The role of selenium in thyroid gland pathophysiology. Endokrynol. Pol. 2017;68:440–465. doi: 10.5603/EP.2017.0051. [DOI] [PubMed] [Google Scholar]
- 201.Contempré B., Duale N.L., Dumont J.E., Ngo B., Diplock A.T., Vanderpas J. Effect of selenium supplementation on thyroid hormone metabolism in an iodine and selenium deficient population. Clin. Endocrinol. 1992;36:579–583. doi: 10.1111/j.1365-2265.1992.tb02268.x. [DOI] [PubMed] [Google Scholar]
- 202.Olivieri O., Girelli D., Stanzial A.M., Rossi L., Bassi A., Corrocher R. Selenium, zinc, and thyroid hormones in healthy subjects: Low T3/T4 ratio in the elderly is related to impaired selenium status. Biol. Trace Elem. Res. 1996;51:31–41. doi: 10.1007/BF02790145. [DOI] [PubMed] [Google Scholar]
- 203.Duffield A.J., Thomson C.D., Hill K.E., Williams S. An estimation of selenium requirements for New Zealanders. Am. J. Clin. Nutr. 1999;70:896–903. doi: 10.1093/ajcn/70.5.896. [DOI] [PubMed] [Google Scholar]
- 204.Rayman M.P., Thompson A.J., Bekaert B., Catterick J., Galassini R., Hall E., Warren-Perry M., Beckett G.J. Randomized controlled trial of the effect of selenium supplementation on thyroid function in the elderly in the United Kingdom. Am. J. Clin. Nutr. 2008;87:370–378. doi: 10.1093/ajcn/87.2.370. [DOI] [PubMed] [Google Scholar]
- 205.Hoeg A., Gogakos A., Murphy E., Mueller S., Köhrle J., Reid D.M., Glüer C.C., Felsenberg D., Roux C., Eastell R., et al. Bone turnover and bone mineral density are independently related to selenium status in healthy euthyroid postmenopausal women. J. Clin. Endocrinol. Metab. 2012;97:4061–4070. doi: 10.1210/jc.2012-2121. [DOI] [PubMed] [Google Scholar]
- 206.Winther K.H., Bonnema S.J., Cold F., Debrabant B., Nybo M., Cold S., Hegedüs L. Does selenium supplementation affect thyroid function? Results froma randomized, controlled, double-blinded trial in a Danish population. Eur. J. Endocrinol. 2015;172:657–667. doi: 10.1530/EJE-15-0069. [DOI] [PubMed] [Google Scholar]
- 207.Fontenelle L.C., Feitosa M.M., Freitas T.E.C., Severo J.S., Morais J.B.S., Henriques G.S., Oliveira F.E., Moita Neto J.M., do Nascimento Marreiro D. Selenium status and its relationship with thyroid hormones in obese women. Clin. Nutr. ESPEN. 2021;41:398–404. doi: 10.1016/j.clnesp.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 208.Jain R.B. Thyroid function and serum copper, selenium, and zinc in general U.S. population. Biol. Trace Elem. Res. 2014;159:87–98. doi: 10.1007/s12011-014-9992-9. [DOI] [PubMed] [Google Scholar]
- 209.Goldhaber S.B. Trace element risk assessment: Essentiality vs. toxicity. Regul. Toxicol. Pharmacol. 2003;38:232–242. doi: 10.1016/S0273-2300(02)00020-X. [DOI] [PubMed] [Google Scholar]
- 210.Bhattacharya P.T., Misra S.R., Hussain M. Nutritional aspects of essential trace elements in oral health and disease: An extensive review. Scientifica. 2016;2016:5464373. doi: 10.1155/2016/5464373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pekary A.E., Lukaski H.C., Mena I., Hershman J.M. Processing of TRH precursor peptides in rat brain and pituitary is zinc dependent. Peptides. 1991;12:1025–1032. doi: 10.1016/0196-9781(91)90055-T. [DOI] [PubMed] [Google Scholar]
- 212.Osterman A.L., Grishin N.V., Smulevitch S.V., Matz M.V., Zagnitko O.P., Revina L.P., Stepanov V.M. Primary structure of carboxypeptidase T: Delineation of functionally relevant features in Zn-carboxypeptidase family. J. Protein Chem. 1992;11:561–570. doi: 10.1007/BF01025034. [DOI] [PubMed] [Google Scholar]
- 213.Kralik A., Eder K., Kirchgessner M. Influence of zinc and selenium deficiency on parameters relating to thyroid hormone metabolism. Horm. Metab. Res. 1996;28:223–226. doi: 10.1055/s-2007-979169. [DOI] [PubMed] [Google Scholar]
- 214.Civitareale D., Saiardi A., Falasca P. Purification and characterization of thyroid transcription factor 2. Biochem. J. 1994;304:981–985. doi: 10.1042/bj3040981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Freake H.C., Govoni K.E., Guda K., Huang C., Zinn S.A. Actions and interactions of thyroid hormone and zinc status in growing rats. J. Nutr. 2001;131:1135–1141. doi: 10.1093/jn/131.4.1135. [DOI] [PubMed] [Google Scholar]
- 216.Talebi S., Ghaedi E., Sadeghi E., Mohammadi H., Hadi A., Clark C.C.T., Askari G. Trace element status and hypothyroidism: A systematic review and meta-analysis. Biol. Trace Elem. Res. 2020;197:1–14. doi: 10.1007/s12011-019-01963-5. [DOI] [PubMed] [Google Scholar]
- 217.Beserra J.B., Morais J.B.S., Severo J.S., Cruz K.J.C., De Oliveira A.R.S., De Oliveira F.E., De Matos Neto E.M., Henriques G.S., Marreiro D.D.N. No association between zinc and thyroid activity in obese women. Int. J. Vitam. Nutr. Res. 2021;91:40–47. doi: 10.1024/0300-9831/a000600. [DOI] [PubMed] [Google Scholar]
- 218.Meunier N., Beattie J.H., Ciarapica D., O’Connor J.M., Andriollo-Sanchez M., Taras A., Coudray C., Polito A. Basal metabolic rate and thyroid hormones of late-middle-aged and older human subjects: The ZENITH study. Eur. J. Clin. Nutr. 2005;59:S53–S57. doi: 10.1038/sj.ejcn.1602299. [DOI] [PubMed] [Google Scholar]
- 219.Ravaglia G., Forti P., Maioli F., Nesi B., Pratelli L., Savarino L., Cucinotta D., Cavalli G. Blood micronutrient and thyroid hormone concentrations in the oldest-old. J. Clin. Endocrinol. Metab. 2000;85:2260–2265. doi: 10.1210/jcem.85.6.6627. [DOI] [PubMed] [Google Scholar]
- 220.Ertek S., Cicero A.F.G., Caglar O., Erdogan G. Relationship between serum zinc levels, thyroid hormones and thyroid volume following successful iodine supplementation. Hormones. 2010;9:263–268. doi: 10.14310/horm.2002.1276. [DOI] [PubMed] [Google Scholar]
- 221.Hess S.Y., Zimmermann M.B., Arnold M., Langhans W., Hurrell R.F. Iron deficiency anemia reduces thyroid peroxidase activity in rats. J. Nutr. 2002;132:1951–1955. doi: 10.1093/jn/132.7.1951. [DOI] [PubMed] [Google Scholar]
- 222.Smith S.M., Johnson P.E., Lukaski H.C. In vitro hepatic thyroid hormone deiodination in iron-deficient rats: Effect of dietary fat. Life Sci. 1993;53:603–609. doi: 10.1016/0024-3205(93)90268-8. [DOI] [PubMed] [Google Scholar]
- 223.Surks M.I. Effect of thyrotropin on thyroidal iodine metabolism during hypoxia. Am. J. Physiol. 1969;216:436–439. doi: 10.1152/ajplegacy.1969.216.2.436. [DOI] [PubMed] [Google Scholar]
- 224.Wopereis D.M., Du Puy R.S., Van Heemst D., Walsh J.P., Bremner A., Bakker S.J.L., Bauer D.C., Cappola A.R., Ceresini G., Degryse J., et al. The relation between thyroid function and anemia: A pooled analysis of individual participant data. J. Clin. Endocrinol. Metab. 2018;103:3658–3667. doi: 10.1210/jc.2018-00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Fu J., Yang A., Zhao J., Zhu Y., Gu Y., Xu Y., Chen D. The relationship between iron level and thyroid function during the first trimester of pregnancy: A cross-sectional study in Wuxi, China. J. Trace Elem. Med. Biol. 2017;43:148–152. doi: 10.1016/j.jtemb.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 226.Isguven P., Arslanoglu I., Erol M., Yildiz M., Adal E., Erguven M. Serum levels of ghrelin, leptin, IGF-I, IGFBP-3, insulin, thyroid hormones and cortisol in prepubertal children with iron deficiency. Endocr. J. 2007;54:985–990. doi: 10.1507/endocrj.K07-031. [DOI] [PubMed] [Google Scholar]
- 227.Ipek I.O., Kacmaz E., Bozaykut A., Sezer R.G., Seren L., Paketci C. The effect of iron deficiency anemia on plasma thyroid hormone levels in childhood/Cocukluk caginda demir eksikligi anemisinin plazma tiroid hormonlari uzerine etkisi. Turk. Pediatr. Arch. 2011;46:129–133. doi: 10.4274/tpa.46.59. [DOI] [Google Scholar]
- 228.Maldonado-Araque C., Valdés S., Lago-Sampedro A., Lillo-Muñoz J.A., Garcia-Fuentes E., Perez-Valero V., Gutierrez-Repiso C., Goday A., Urrutia I., Peláez L., et al. Iron deficiency is associated with Hypothyroxinemia and Hypotriiodothyroninemia in the Spanish general adult population: Di@bet.es study. Sci. Rep. 2018;8:6571. doi: 10.1038/s41598-018-24352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kim M.J., Kim S.C., Chung S., Kim S., Yoon J.W., Park Y.J. Exploring the role of copper and selenium in the maintenance of normal thyroid function among healthy Koreans. J. Trace Elem. Med. Biol. 2020;61:126558. doi: 10.1016/j.jtemb.2020.126558. [DOI] [PubMed] [Google Scholar]
- 230.Uriu-Adams J.Y., Keen C.L. Copper, oxidative stress, and human health. Mol. Aspects Med. 2005;26:268–298. doi: 10.1016/j.mam.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 231.Kazi T.G., Kandhro G.A., Afridi H.I., Kazi N., Baig J.A., Arain M.B., Shah A.Q., Syed N., Kumar S., Kolachi N.F., et al. Interaction of copper with iron, iodine, and thyroid hormone status in goitrous patients. Biol. Trace Elem. Res. 2010;134:265–279. doi: 10.1007/s12011-009-8478-7. [DOI] [PubMed] [Google Scholar]
- 232.Rasic-Milutinovic Z., Jovanovic D., Bogdanovic G., Trifunovic J., Mutic J. Potential influence of selenium, copper, zinc and cadmium on l-thyroxine substitution in patients with hashimoto thyroiditis and hypothyroidism. Exp. Clin. Endocrinol. Diabetes. 2017;125:79–85. doi: 10.1055/s-0042-116070. [DOI] [PubMed] [Google Scholar]
- 233.Katagiri R., Yuan X., Kobayashi S., Sasaki S. Effect of excess iodine intake on thyroid diseases in different populations: A systematic review and meta-analyses including observational studies. PLoS ONE. 2017;12:e0173722. doi: 10.1371/journal.pone.0173722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Farebrother J., Zimmermann M.B., Andersson M. Excess iodine intake: Sources, assessment, and effects on thyroid function. Ann. N. Y. Acad. Sci. 2019;1446:44–65. doi: 10.1111/nyas.14041. [DOI] [PubMed] [Google Scholar]
- 235.Gardbeer D.F., Centor R.M., Utiger R.D. Effects of low dose oral iodide supplementation on thyroid function in normal men. Clin. Endocrinol. 1988;28:283–288. doi: 10.1111/j.1365-2265.1988.tb01214.x. [DOI] [PubMed] [Google Scholar]
- 236.Paul T., Meyers B., Witorsch R.J., Pino S., Chipkin S., Ingbar S.H., Braverman L.E. The effect of small increases in dietary iodine on thyroid function in euthyroid subjects. Metabolism. 1988;37:121–124. doi: 10.1016/S0026-0495(98)90004-X. [DOI] [PubMed] [Google Scholar]
- 237.Namba H., Yamashita S., Kimura H., Yokoyama N., Usa T., Otsuru A., Izumi M., Nagataki S. Evidence of thyroid volume increase in normal subjects receiving excess iodide. J. Clin. Endocrinol. Metab. 1993;76:605–608. doi: 10.1210/jcem.76.3.8445017. [DOI] [PubMed] [Google Scholar]
- 238.Sang Z., Wang P.P., Yao Z., Shen J., Halfyard B., Tan L., Zhao N., Wu Y., Gao S., Tan J., et al. Exploration of the safe upper level of iodine intake in euthyroid Chinese adults: A randomized double-blind trial. Am. J. Clin. Nutr. 2012;95:367–373. doi: 10.3945/ajcn.111.028001. [DOI] [PubMed] [Google Scholar]
- 239.Tan L., Sang Z., Shen J., Liu H., Chen W., Zhao N., Wei W., Zhang G., Zhang W. Prevalence of thyroid dysfunction with adequate and excessive iodine intake in Hebei Province, People’s Republic of China. Public Health Nutr. 2015;18:1692–1697. doi: 10.1017/S1368980014002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wan S., Jin B., Ren B., Qu M., Wu H., Liu L., Boah M., Shen H. The Relationship between high iodine consumption and levels of autoimmune thyroiditis-related biomarkers in a Chinese population: A meta-analysis. Biol. Trace Elem. Res. 2020;196:410–418. doi: 10.1007/s12011-019-01951-9. [DOI] [PubMed] [Google Scholar]
- 241.Zhao L., Teng D., Shi X., Li Y., Ba J., Chen B., Du J., He L., Lai X., Li Y., et al. The effect of increased iodine intake on serum thyrotropin: A cross-sectional, Chinese nationwide study. Thyroid. 2020;30:1810–1819. doi: 10.1089/thy.2019.0842. [DOI] [PubMed] [Google Scholar]
- 242.Reinehr T., Isa A., de Sousa G., Dieffenbach R., Andler W. Thyroid hormones and their relation to weight status. Horm. Res. 2008;70:51–57. doi: 10.1159/000129678. [DOI] [PubMed] [Google Scholar]
- 243.Jones J.E., Desper P.C., Shane S.R., Flink E.B. Magnesium metabolism in hyperthyroidism and hypothyroidism. J. Clin. Investig. 1966;45:891–900. doi: 10.1172/JCI105404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wang K., Wei H., Zhang W., Li Z., Ding L., Yu T., Tan L., Liu Y., Liu T., Wang H., et al. Severely low serum magnesium is associated with increased risks of positive anti-thyroglobulin antibody and hypothyroidism: A cross-sectional study. Sci. Rep. 2018;8:9904. doi: 10.1038/s41598-018-28362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ylli D., Wartofsky L. Can we link thyroid status, energy expenditure, and body composition to management of subclinical thyroid dysfunction? J. Clin. Endocrinol. Metab. 2019;104:209–212. doi: 10.1210/jc.2018-01997. [DOI] [PubMed] [Google Scholar]
- 246.Huang W.S., Yu M.D., Lee M.S., Cheng C.Y., Yang S.P., Chin H.M.L., Wu S.Y. Effect of treadmill exercise on circulating thyroid hormone measurements. Med. Princ. Pract. 2004;13:15–19. doi: 10.1159/000074045. [DOI] [PubMed] [Google Scholar]
- 247.Benso A., Broglio F., Aimaretti G., Lucatello B., Lanfranco F., Ghigo E., Grottoli S. Endocrine and metabolic responses to extreme altitude and physical exercise in climbers. Eur. J. Endocrinol. 2007;157:733–740. doi: 10.1530/EJE-07-0355. [DOI] [PubMed] [Google Scholar]
- 248.Altaye K.Z., Mondal S., Legesse K., Abdulkedir M. Effects of aerobic exercise on thyroid hormonal change responses among adolescents with intellectual disabilities. BMJ Open Sport Exerc. Med. 2019;5:524. doi: 10.1136/bmjsem-2019-000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ciloglu F., Peker I., Pehlivan A., Karacabey K., Ilhan N., Sayglin O., Ozmerdivenli R. Exercise intensity and its effects on thyroid hormones. Neuro Endocrinol. Lett. 2005;26:830–834. [PubMed] [Google Scholar]
- 250.Roa Dueñas O.H., Koolhaas C., Voortman T., Franco O.H., Ikram M.A., Peeters R.P., Chaker L. Thyroid function and physical activity: A population-based cohort study. Thyroid. 2020;31:870–875. doi: 10.1089/thy.2020.0517. [DOI] [PubMed] [Google Scholar]
- 251.Kang J., Kong E., Choi J. Associations of urinary cotinine-verified active and passive smoking with thyroid function: Analysis of population-based nationally representative data. Thyroid. 2018;28:583–592. doi: 10.1089/thy.2017.0567. [DOI] [PubMed] [Google Scholar]
- 252.Park S., Kim W.G., Jeon M.J., Kim M., Oh H.S., Han M., Kim T.Y., Shong Y.K., Kim W.B. Serum thyroid-stimulating hormone levels and smoking status: Data from the Korean National Health and Nutrition Examination Survey VI. Clin. Endocrinol. 2018;88:969–976. doi: 10.1111/cen.13606. [DOI] [PubMed] [Google Scholar]
- 253.Brown S.J., Bremner A.P., Hadlow N.C., Feddema P., Leedman P.J., O’Leary P.C., Walsh J.P. The log TSH-free T4 relationship in a community-based cohort is nonlinear and is influenced by age, smoking and thyroid peroxidase antibody status. Clin. Endocrinol. 2016;85:789–796. doi: 10.1111/cen.13107. [DOI] [PubMed] [Google Scholar]
- 254.Jain R.B. Association between thyroid profile and perfluoroalkyl acids: Data from NHNAES 2007–2008. Environ. Res. 2013;126:51–59. doi: 10.1016/j.envres.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 255.Mehran L., Amouzgar A., Delshad H., Azizi F. The Association of cigarette smoking with serum TSH concentration and thyroperoxidase antibody. Exp. Clin. Endocrinol. Diabetes. 2012;120:80–83. doi: 10.1055/s-0031-1285910. [DOI] [PubMed] [Google Scholar]
- 256.De Pergola G., Ciampolillo A., Alò D., Sciaraffia M., Guida P. Free triiodothyronine is associated with smoking habit, independently of obesity, body fat distribution, insulin, and metabolic parameters. J. Endocrinol. Investig. 2010;33:815–818. doi: 10.1007/BF03350348. [DOI] [PubMed] [Google Scholar]
- 257.Soldin O.P., Goughenour B.E., Gilbert S.Z., Landy H.J., Soldin S.J. Thyroid hormone levels associated with active and passive cigarette smoking. Thyroid. 2009;19:817–823. doi: 10.1089/thy.2009.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jorde R., Sundsfjord J. Serum TSH levels in smokers and non-smokers. The 5th Tromsø study. Exp. Clin. Endocrinol. Diabetes. 2006;114:343–347. doi: 10.1055/s-2006-924264. [DOI] [PubMed] [Google Scholar]
- 259.Fisher C.L., Mannino D.M., Herman W.H., Frumkin H. Cigarette smoking and thyroid hormone levels in males. Int. J. Epidemiol. 1997;26:972–977. doi: 10.1093/ije/26.5.972. [DOI] [PubMed] [Google Scholar]
- 260.Petersen K., Lindstedt G., Lundberg P.-A., Bengtsson C., Lapidus L., Nyström E. Thyroid disease in middle-aged and elderly Swedish women: Thyroid-related hormones, thyroid dysfunction and goitre in relation to age and smoking. J. Intern. Med. 1991;229:407–413. doi: 10.1111/j.1365-2796.1991.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 261.Ericsson U.-B., Lindgärde F. Effects of cigarette smoking on thyroid function and the prevalence of goitre, thyrotoxicosis and autoimmune thyroiditis. J. Intern. Med. 1991;229:67–71. doi: 10.1111/j.1365-2796.1991.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 262.Karakaya A., Tunçel N., Alptuna G., Koçer Z., Erbay G. Influence of cigarette smoking on thyroid hormone levels. Hum. Exp. Toxicol. 1987;6:507–509. doi: 10.1177/096032718700600610. [DOI] [PubMed] [Google Scholar]
- 263.Hegedüs L., Karstrup S., Veiergang D., Jacobsen B., Skovsted L., Feldt-Rasmussen U. High frequency of goitre in cigarette smokers. Clin. Endocrinol. 1985;22:287–292. doi: 10.1111/j.1365-2265.1985.tb03242.x. [DOI] [PubMed] [Google Scholar]
- 264.Edén S., Jagenburg R., Lindstedt G., Lundberg P.-A., Mellström D. Thyroregulatory changes associated with smoking in 70-year-old men. Clin. Endocrinol. 1984;21:605–610. doi: 10.1111/j.1365-2265.1984.tb01402.x. [DOI] [PubMed] [Google Scholar]
- 265.Sepkovic D., Haley N., Wynder E. Thyroid activity in cigarette smokers. Arch. Intern. Med. 1984;144:501–503. doi: 10.1001/archinte.1984.00350150089027. [DOI] [PubMed] [Google Scholar]
- 266.Christensen S.B., Ericsson U.B., Janzon L., Tibblin S., Melander A. Influence of cigarette smoking on goiter formation, thyroglobulin, and thyroid hormone levels in women. J. Clin. Endocrinol. Metab. 1984;58:615–618. doi: 10.1210/jcem-58-4-615. [DOI] [PubMed] [Google Scholar]
- 267.Layegh P., Asadi A., Jangjoo A., Layegh P., Nematy M., Salehi M., Shamsian A., Ranjbar G. Comparison of thyroid volume, TSH, free t4 and the prevalence of thyroid nodules in obese and non-obese subjects and correlation of these parameters with insulin resistance status. Casp. J. Intern. Med. 2020;11:278–282. doi: 10.22088/cjim.11.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Diniz M.F.H.S., Beleigoli A.M.R., Benseñor I.M., Lotufo P.A., Goulart A.C., Barreto S.M. Association between TSH levels within the reference range and adiposity markers at the baseline of the ELSA–Brasil study. PLoS ONE. 2020;15:e0228801. doi: 10.1371/journal.pone.0228801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Priya R., Patel S., Dubey S.S., Nanda R., Mohapatra E. Association of thyroid function with body mass index in adolescent girls. Indian J. Med. Biochem. 2020;24:55–58. doi: 10.5005/jp-journals-10054-0158. [DOI] [Google Scholar]
- 270.Kouidrat Y., Diouf M., Desailloud R., Louhou R. Effects of a diet plus exercise program on thyroid function in patients with obesity. Metab. Open. 2019;2:100008. doi: 10.1016/j.metop.2019.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ranabir S., Archana N., Ipsita R., Naorem S., Prasad L. Is there a correlation between body mass index and thyroid stimulating hormone? Endocrinol. Int. J. 2019;7:151–154. doi: 10.15406/emij.2019.07.00262. [DOI] [Google Scholar]
- 272.Kwon H., Cho J.H., Lee D.Y., Park S.E., Park C.Y., Lee W.Y., Oh K.W., Park S.W., Rhee E.J. Association between thyroid hormone levels, body composition and insulin resistance in euthyroid subjects with normal thyroid ultrasound: The Kangbuk Samsung Health study. Clin. Endocrinol. 2018;89:649–655. doi: 10.1111/cen.13823. [DOI] [PubMed] [Google Scholar]
- 273.Rajini B., Haragopal R. Study on the Association between the serum thyroid stimulating hormone levels and the body mass index. Physiol. Int. J. Contemp. Med. Res. 2018;5:2393–2915. doi: 10.21276/ijcmr.2018.5.12.13. [DOI] [Google Scholar]
- 274.Chen J., Zhou W., Pan F., Cui W., Li M., Hu Y. Age-related change in thyroid-stimulating hormone: A cross-sectional study in healthy euthyroid population. Endocr. J. 2018;65:1075–1082. doi: 10.1507/endocrj.EJ18-0113. [DOI] [PubMed] [Google Scholar]
- 275.Abdi H., Kazemian E., Gharibzadeh S., Amouzegar A., Mehran L., Tohidi M., Rashvandi Z., Azizi F. Association between thyroid function and body mass index: A 10-year follow-up. Ann. Nutr. Metab. 2017;70:338–345. doi: 10.1159/000477497. [DOI] [PubMed] [Google Scholar]
- 276.Reza Rahbar A., Kalantarhormozi M., Izadi F., Arkia E., Rashidi M., Pourbehi F., Daneshifard F., Rahbar A. Relationship between body mass index, waist-to-hip ratio, and serum lipid concentrations and thyroid-stimulating hormone in the euthyroid adult population. Iran. J. Med. Sci. 2017;42:301–305. [PMC free article] [PubMed] [Google Scholar]
- 277.Ferrannini E., Iervasi G., Cobb J., Ndreu R., Nannipieri M. Insulin resistance and normal thyroid hormone levels: Prospective study and metabolomic analysis. Am. J. Physiol. Metab. 2017;312:E429–E436. doi: 10.1152/ajpendo.00464.2016. [DOI] [PubMed] [Google Scholar]
- 278.Temizkan S., Balaforlou B., Ozderya A., Avci M., Aydin K., Karaman S., Sargin M. Effects of thyrotrophin, thyroid hormones and thyroid antibodies on metabolic parameters in a euthyroid population with obesity. Clin. Endocrinol. 2016;85:616–623. doi: 10.1111/cen.13095. [DOI] [PubMed] [Google Scholar]
- 279.Bieler B.M., Gaughan J., Khan M., Rao G., Hunter K., Morgan F.H. Lack of an association between BMI and TSH in treated hypothyroid patients and euthyroid controls. Endocr. Pract. 2016;22:555–560. doi: 10.4158/EP15946.OR. [DOI] [PubMed] [Google Scholar]
- 280.Bétry C., Challan-Belval M.A., Bernard A., Charrié A., Drai J., Laville M., Thivolet C., Disse E. Increased TSH in obesity: Evidence for a BMI-independent association with leptin. Diabetes Metab. 2015;41:248–251. doi: 10.1016/j.diabet.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 281.Bakiner O., Bozkirli E., Cavlak G., Ozsahin K., Ertorer E. Are plasma thyroid-stimulating hormone levels associated with degree of obesity and metabolic syndrome in euthyroid obese patients? A Turkish cohort study. Int. Sch. Res. Not. 2014;2014:803028. doi: 10.1155/2014/803028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Sakurai M., Nakamura K., Miura K., Yoshita K., Takamura T., Nagasawa S.Y., Morikawa Y., Ishizaki M., Kido T., Naruse Y., et al. Association between a serum thyroid-stimulating hormone concentration within the normal range and indices of obesity in Japanese men and women. Intern. Med. 2014;53:669–674. doi: 10.2169/internalmedicine.53.1387. [DOI] [PubMed] [Google Scholar]
- 283.Shin J.A., Mo E.Y., Kim E.S., Moon S.D., Han J.H. Association between lower normal free thyroxine concentrations and obesity phenotype in healthy euthyroid subjects. Int. J. Endocrinol. 2014;2014:104318. doi: 10.1155/2014/104318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Solanki A., Bansal S., Jindal S., Saxena V., Shukla U. Relationship of serum thyroid stimulating hormone with body mass index in healthy adults. Indian J. Endocrinol. Metab. 2013;17:167. doi: 10.4103/2230-8210.119560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ittermann T., Thamm M., Schipf S., John U., Rettig R., Völzke H. Relationship of smoking and/or passive exposure to tobacco smoke on the association between serum thyrotropin and body mass index in large groups of adolescents and children. Thyroid. 2013;23:262–268. doi: 10.1089/thy.2012.0110. [DOI] [PubMed] [Google Scholar]
- 286.Muscogiuri G., Sorice G.P., Mezza T., Prioletta A., Lassandro A.P., Pirronti T., Della Casa S., Pontecorvi A., Giaccari A. High-normal tsh values in obesity: Is it insulin resistance or adipose tissue’s guilt? Obesity. 2013;21:101–106. doi: 10.1002/oby.20240. [DOI] [PubMed] [Google Scholar]
- 287.Ren R., Jiang X., Zhang X., Guan Q., Yu C., Li Y., Gao L., Zhang H., Zhao J. Association between thyroid hormones and body fat in euthyroid subjects. Clin. Endocrinol. 2014;80:585–590. doi: 10.1111/cen.12311. [DOI] [PubMed] [Google Scholar]
- 288.Kitahara C.M., Platz E.A., Ladenson P.W., Mondul A.M., Menke A., Berrington de González A. Body fatness and markers of thyroid function among U.S. men and women. PLoS ONE. 2012;7:e34979. doi: 10.1371/journal.pone.0034979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Díez J.J., Iglesias P. Relationship between thyrotropin and body mass index in euthyroid subjects. Exp. Clin. Endocrinol. Diabetes. 2011;119:144–150. doi: 10.1055/s-0030-1265133. [DOI] [PubMed] [Google Scholar]
- 290.De Moura Souza A., Sichieri R. Relationship between body mass index and thyrotropin in euthyroid women: Differences by smoking, race and menopausal status. Obes. Facts. 2011;4:175–179. doi: 10.1159/000327843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Svare A., Nilsen T.I.L., Bjøro T., Åsvold B.O., Langhammer A. Serum TSH related to measures of body mass: Longitudinal data from the HUNT Study, Norway. Clin. Endocrinol. 2011;74:769–775. doi: 10.1111/j.1365-2265.2011.04009.x. [DOI] [PubMed] [Google Scholar]
- 292.Ambrosi B., Masserini B., Iorio L., Delnevo A., Malavazos A.E., Morricone L., Sburlati L.F., Orsi E. Relationship of thyroid function with body mass index and insulin-resistance in euthyroid obese subjects. J. Endocrinol. Investig. 2010;33:640–643. doi: 10.1007/BF03346663. [DOI] [PubMed] [Google Scholar]
- 293.Alevizaki M., Saltiki K., Voidonikola P., Mantzou E., Papamichael C., Stamatelopoulos K. Free thyroxine is an independent predictor of subcutaneous fat in euthyroid individuals. Eur. J. Endocrinol. 2009;161:459–465. doi: 10.1530/EJE-09-0441. [DOI] [PubMed] [Google Scholar]
- 294.Kim B.-J., Kim T.Y., Koh J.-M., Kim H.-K., Park J.-Y., Lee K.-U., Shong Y.K., Kim W.B. Relationship between serum free T4 (FT4) levels and metabolic syndrome (MS) and its components in healthy euthyroid subjects. Clin. Endocrinol. 2009;70:152–160. doi: 10.1111/j.1365-2265.2008.03304.x. [DOI] [PubMed] [Google Scholar]
- 295.Shon H.S., Jung E.D., Kim S.H., Lee J.H. Free T4 is negatively correlated with body mass index in euthyroid women. Korean J. Intern. Med. 2008;23:53–57. doi: 10.3904/kjim.2008.23.2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Bastemir M., Akin F., Alkis E., Kaptanoglu B. Obesity is associated with increased serum TSH level, independent of thyroid function. Swiss Med. Wkly. 2007;137:431–434. doi: 10.4414/smw.2007.11774. [DOI] [PubMed] [Google Scholar]
- 297.Chikunguwo S., Brethauer S., Nirujogi V., Pitt T., Udomsawaengsup S., Chand B., Schauer P. Influence of obesity and surgical weight loss on thyroid hormone levels. Surg. Obes. Relat. Dis. 2007;3:631–635. doi: 10.1016/j.soard.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 298.Iacobellis G., Ribaudo M.C., Zappaterreno A., Iannucci C.V., Leonetti F. Relationship of thyroid function with body mass index, leptin, insulin sensitivity and adiponectin in euthyroid obese women. Clin. Endocrinol. 2005;62:487–491. doi: 10.1111/j.1365-2265.2005.02247.x. [DOI] [PubMed] [Google Scholar]
- 299.Li J., Teng X., Wang W., Chen Y., Yu X., Wang S., Li J., Zhu L., Li C., Fan C., et al. Effects of dietary soy intake on maternal thyroid functions and serum anti-thyroperoxidase antibody level during early pregnancy. J. Med. Food. 2011;14:543–550. doi: 10.1089/jmf.2010.1078. [DOI] [PubMed] [Google Scholar]
- 300.Tousen Y., Ezaki J., Fujii Y., Ueno T., Nishimuta M., Ishimi Y. Natural S-equol decreases bone resorption in postmenopausal, non-equol-producing Japanese women: A pilot randomized, placebo-controlled trial. Menopause. 2011;18:563–574. doi: 10.1097/gme.0b013e3181f85aa7. [DOI] [PubMed] [Google Scholar]
- 301.Zhou Y., Alekel D.L., Dixon P.M., Messina M., Reddy M.B. The effect of soy food intake on mineral status in premenopausal women. J. Women’s Health. 2011;20:771–780. doi: 10.1089/jwh.2010.2491. [DOI] [PubMed] [Google Scholar]
- 302.Hooper L., Ryder J.J., Kurzer M.S., Lampe J.W., Messina M.J., Phipps W.R., Cassidy A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: A systematic review and meta-analysis. Hum. Reprod. Update. 2009;15:423–440. doi: 10.1093/humupd/dmp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Ryan-Borchers T., Chew B., Park J.S., McGuire M., Fournier L., Beerman K. Effects of dietary and supplemental forms of isoflavones on thyroid function in healthy postmenopausal women. Top. Clin. Nutr. 2008;23:13–22. doi: 10.1097/01.TIN.0000312076.38329.55. [DOI] [Google Scholar]
- 304.Lydeking-Olsen E., Beck-Jensen J.E., Setchell K.D.R., Holm-Jensen T. Soymilk or progesterone for prevention of bone loss: A 2 year randomized, placebo-controlled trial. Eur. J. Nutr. 2004;43:246–257. doi: 10.1007/s00394-004-0497-8. [DOI] [PubMed] [Google Scholar]
- 305.Mackey R., Ekangaki A., Eden J.A. The effects of soy protein in women and men with elevated plasma lipids. BioFactors. 2000;12:251–257. doi: 10.1002/biof.5520120138. [DOI] [PubMed] [Google Scholar]
- 306.Duncan A.M., Merz B.E., Xu X., Nagel T.C., Phipps W.R., Kurzer M.S. Soy isoflavones exert modest hormonal effects in premenopausal women1. J. Clin. Endocrinol. Metab. 1999;84:192–197. doi: 10.1210/jcem.84.1.5387. [DOI] [PubMed] [Google Scholar]
- 307.Key T.J.A., Thorogood M., Keenan J., Long A. Raised thyroid stimulating hormone associated with kelp intake in British vegan men. J. Hum. Nutr. Diet. 1992;5:323–326. doi: 10.1111/j.1365-277X.1992.tb00171.x. [DOI] [Google Scholar]
- 308.Osman B., Ng M., Bakar A., Khalid B. The effect of cassava leave intake on thyroid hormone and urinary iodine. East. Afr. Med. J. 1993;70:314–315. [PubMed] [Google Scholar]
- 309.Verrusio W., Magro V.M., Renzi A., Casciaro B., Andreozzi P., Cacciafesta M. Thyroid hormones, metabolic syndrome and Vitamin D in middle-aged and older euthyroid subjects: A preliminary study. Aging Clin. Exp. Res. 2019;31:1337–1341. doi: 10.1007/s40520-018-1071-1. [DOI] [PubMed] [Google Scholar]
- 310.Zhou P., Cai J., Markowitz M. Absence of a relationship between thyroid hormones and Vitamin D levels. J. Pediatr. Endocrinol. Metab. 2016;29:703–707. doi: 10.1515/jpem-2015-0210. [DOI] [PubMed] [Google Scholar]
- 311.Beukhof C.M., Medici M., Van Den Beld A.W., Hollenbach B., Hoeg A., Visser W.E., De Herder W.W., Visser T.J., Schomburg L., Peeters R.P. Selenium status is positively associated with bone mineral density in healthy aging European men. PLoS ONE. 2016;11:e0152748. doi: 10.1371/journal.pone.0152748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Alissa E.M., Ahmed W.H., Al-ama N., Ferns G.A.A. Selenium status and cardiovascular risk profile in healthy adult Saudi males. Molecules. 2009;14:141–159. doi: 10.3390/molecules14010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Combs G.F., Midthune D.N., Patterson K.Y., Canfield W.K., Hill A.D., Levander O.A., Taylor P.R., Moler J.E., Patterson B.H. Effects of selenomethionine supplementation on selenium status and thyroid hormone concentrations in healthy adults. Am. J. Clin. Nutr. 2009;89:1808–1814. doi: 10.3945/ajcn.2008.27356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Thomson C.D., Campbell J.M., Miller J., Skeaff S.A., Livingstone V. Selenium and iodine supplementation: Effect on thyroid function of older New Zealanders. Am. J. Clin. Nutr. 2009;90:1038–1046. doi: 10.3945/ajcn.2009.28190. [DOI] [PubMed] [Google Scholar]
- 315.Hawkes W.C., Keim N.L., Diane Richter B., Gustafson M.B., Gale B., Mackey B.E., Bonnel E.L. High-selenium yeast supplementation in free-living North American men: No effect on thyroid hormone metabolism or body composition. J. Trace Elem. Med. Biol. 2008;22:131–142. doi: 10.1016/j.jtemb.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 316.Thomson C.D., McLachlan S.K., Grant A.M., Paterson E., Lillico A.J. The effect of selenium on thyroid status in a population with marginal selenium and iodine status. Br. J. Nutr. 2005;94:962–968. doi: 10.1079/BJN20051564. [DOI] [PubMed] [Google Scholar]
- 317.Hawkes W.C., Keim N.L. Dietary selenium intake modulates thyroid hormone and energy metabolism in men. J. Nutr. 2003;133:3443–3448. doi: 10.1093/jn/133.11.3443. [DOI] [PubMed] [Google Scholar]
- 318.Meeker J.D., Rossano M.G., Protas B., Diamond M.P., Puscheck E., Daly D., Paneth N., Wirth J.J. Multiple metals predict prolactin and thyrotropin (TSH) levels in men. Environ. Res. 2009;109:869–873. doi: 10.1016/j.envres.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Eftekhari M.H., Simondon K.B., Jalali M., Keshavarz S.A., Elguero E., Eshraghian M.R., Saadat N. Effects of administration of iron, iodine and simultaneous iron-plus-iodine on the thyroid hormone profile in iron-deficient adolescent Iranian girls. Eur. J. Clin. Nutr. 2006;60:545–552. doi: 10.1038/sj.ejcn.1602349. [DOI] [PubMed] [Google Scholar]
- 320.Beard J.L., Borel M.J., Derr J. Impaired thermoregulation and thyroid function in iron-deficiency anemia. Am. J. Clin. Nutr. 1990;52:813–819. doi: 10.1093/ajcn/52.5.813. [DOI] [PubMed] [Google Scholar]
- 321.Ren Y., Jia Q., Zhang X., Guo B., Wen X., Zhang F., Wang Y., Wang J. Epidemiological investigation on thyroid disease among fertile women in different iodine intake areas of Shanxi province. Zhonghua Liu Xing Bing Xue Za Zhi. 2014;35:45–48. [PubMed] [Google Scholar]
- 322.Loucks A.B., Heath E.M. Induction of low-T3 syndrome in exercising women occurs at a threshold of energy availability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1994;266:R817–R822. doi: 10.1152/ajpregu.1994.266.3.R817. [DOI] [PubMed] [Google Scholar]
- 323.Lehmann M., Knizia K., Gastmann U., Petersen K.G., Khalaf A.N., Bauer S., Kerp L., Keul J. Influence of 6-week, 6 days per week, training on pituitary function in recreational athletes. Br. J. Sports Med. 1993;27:186–192. doi: 10.1136/bjsm.27.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Loucks A.B., Callister R. Induction and prevention of low-T3 syndrome in exercising women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1993;264:R924–R930. doi: 10.1152/ajpregu.1993.264.5.R924. [DOI] [PubMed] [Google Scholar]
- 325.Liewendahl K., Helenius T., Näveri H., Tikkanen H. Fatty acid-induced increase in serum dialyzable free thyroxine after physical exercise: Implication for nonthyroidal illness. J. Clin. Endocrinol. Metab. 1992;74:1361–1365. doi: 10.1210/jcem.74.6.1592881. [DOI] [PubMed] [Google Scholar]
- 326.Premachandra B.N., Winder W.W., Hickson R., Lang S., Holloszy J.O. Circulating reverse triiodothyronine in humans during exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1981;47:281–288. doi: 10.1007/BF00422473. [DOI] [PubMed] [Google Scholar]
- 327.O’Connell M., Robbins D.C., Horton E.S., Sims E.A.H., Danforth E. Changes in serum concentrations of 3,3′,5′-triiodothyronine and 3,5,3′-triiodothyronine during prolonged moderate exercise. J. Clin. Endocrinol. Metab. 1979;49:242–246. doi: 10.1210/jcem-49-2-242. [DOI] [PubMed] [Google Scholar]
- 328.Mason J.W., Hartley L.H., Kotchen T.A., Wherry F.E., Pennington L.L., Jones L.G. Plasma thyroid-stimulating hormone response in anticipation of muscular exercise in the human. J. Clin. Endocrinol. Metab. 1973;37:403–406. doi: 10.1210/jcem-37-3-403. [DOI] [PubMed] [Google Scholar]
- 329.Howdeshell K.L. A model of the development of the brain as a construct of the thyroid system. Environ. Health Perspect. 2002;110:337–348. doi: 10.1289/ehp.02110s3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Jacobson J.L., Jacobson S.W. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N. Engl. J. Med. 1996;335:783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- 331.Kim Y., Ha E.H., Kim E.J., Park H., Ha M., Kim J.H., Hong Y.C., Chang N., Kim B.N. Prenatal exposure to phthalates and infant development at 6 months: Prospective mothers and children’s environmental health (MOCEH) study. Environ. Health Perspect. 2011;119:1495–1500. doi: 10.1289/ehp.1003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Koopman-Esseboom C., Weisglas-Kuperus N., de Ridder M.A.J., Van der Paauw C.G., Tuinstra L.G.M.T., Sauer P.J.J. Effects of polychlorinated biphenyl/dioxin exposure and feeding type on infants’ mental and psychomotor development. Pediatrics. 1996;97:700–706. [PubMed] [Google Scholar]
- 333.Gascon M., Fort M., Martínez D., Carsin A.E., Forns J., Grimalt J.O., Marina L.S., Lertxundi N., Sunyer J., Vrijheid M. Polybrominated diphenyl ethers (PBDEs) in breast milk and neuropsychological development in infants. Environ. Health Perspect. 2012;120:1760–1765. doi: 10.1289/ehp.1205266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Patandin S., Lanting C.I., Mulder P.G.H., Boersma E.R., Sauer P.J.J., Weisglas-Kuperus N. Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. J. Pediatr. 1999;134:33–41. doi: 10.1016/S0022-3476(99)70369-0. [DOI] [PubMed] [Google Scholar]
- 335.Gascon M., Vrijheid M., Martínez D., Forns J., Grimalt J.O., Torrent M., Sunyer J. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environ. Int. 2011;37:605–611. doi: 10.1016/j.envint.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 336.You S.H., Gauger K.J., Bansal R., Zoeller R.T. 4-Hydroxy-PCB106 acts as a direct thyroid hormone receptor agonist in rat GH3 cells. Mol. Cell. Endocrinol. 2006;257–258:26–34. doi: 10.1016/j.mce.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 337.Allen-Rowlands C.F., Castracane V.D., Hamilton M.G., Seifter J. Effect of polybrominated biphenyls (PBB) on the pituitary-thyroid axis of the rat. Proc. Soc. Exp. Biol. Med. 1981;166:506–514. doi: 10.3181/00379727-166-41099. [DOI] [PubMed] [Google Scholar]
- 338.Porta M., Zumeta E. Implementing the Stockholm treaty on persistent organic pollutants. Occup. Environ. Med. 2002;59:651–652. doi: 10.1136/oem.59.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Hagmar L., Rylander L., Dyremark E., Klasson-Wehler E., Erfurth E.M. Plasma concentrations of persistent organochlorines in relation to thyrotropin and thyroid hormone levels in women. Int. Arch. Occup. Environ. Health. 2001;74:184–188. doi: 10.1007/s004200000213. [DOI] [PubMed] [Google Scholar]
- 340.Steuerwald C., Weihe P., Jørgensen P.J., Bjerve K., Brock J., Heinzow B., Budtz-Jørgensen E., Grandjean P. Maternal seafood diet, methylmercury exposure, and neonatal neurologic function. J. Pediatr. 2000;136:599–605. doi: 10.1067/mpd.2000.102774. [DOI] [PubMed] [Google Scholar]
- 341.Rylander L., Wallin E., Jönssson B.A., Stridsberg M., Erfurth E.M., Hagmar L. Associations between CB-153 and p,p′-DDE and hormone levels in serum in middle-aged and elderly men. Chemosphere. 2006;65:375–381. doi: 10.1016/j.chemosphere.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 342.Langer P., Kočan A., Tajtaková M., Petrík J., Chovancová J., Drobná B., Jursa S., Rádiková Ž., Koška J., Kšinantová L., et al. Fish from industrially polluted freshwater as the main source of organochlorinated pollutants and increased frequency of thyroid disorders and dysglycemia. Chemosphere. 2007;67:S379–S385. doi: 10.1016/j.chemosphere.2006.05.132. [DOI] [PubMed] [Google Scholar]
- 343.Meeker J.D., Altshul L., Hauser R. Serum PCBs, p,p′-DDE and HCB predict thyroid hormone levels in men. Environ. Res. 2007;104:296–304. doi: 10.1016/j.envres.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Dallaire R., Dewailly É., Pereg D., Dery S., Ayotte P. Thyroid function and plasma concentrations of polyhalogenated compounds in inuit adults. Environ. Health Perspect. 2009;117:1380–1386. doi: 10.1289/ehp.0900633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Shrestha S., Bloom M.S., Yucel R., Seegal R.F., Wu Q., Kannan K., Rej R., Fitzgerald E.F. Perfluoroalkyl substances and thyroid function in older adults. Environ. Int. 2015;75:206–214. doi: 10.1016/j.envint.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Gaum P.M., Lang J., Esser A., Schettgen T., Neulen J., Kraus T., Gube M. Exposure to polychlorinated biphenyls and the thyroid gland—Examining and discussing possible longitudinal health effects in humans. Environ. Res. 2016;148:112–121. doi: 10.1016/j.envres.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 347.Jacobson M.H., Darrow L.A., Barr D.B., Howards P.P., Lyles R.H., Terrell M.L., Smith A.K., Conneely K.N., Marder M.E., Marcus M. Serum polybrominated biphenyls (PBBs) and polychlorinated biphenyls (PCBs) and thyroid function among michigan adults several decades after the 1973–1974 pbb contamination of livestock feed. Environ. Health Perspect. 2017;125:097020. doi: 10.1289/EHP1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Zheng J., He C.T., Chen S.J., Yan X., Guo M.N., Wang M.H., Yu Y.J., Yang Z.Y., Mai B.X. Disruption of thyroid hormone (TH) levels and TH-regulated gene expression by polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), and hydroxylated PCBs in e-waste recycling workers. Environ. Int. 2017;102:138–144. doi: 10.1016/j.envint.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 349.Curtis S.W., Terrell M.L., Jacobson M.H., Cobb D.O., Jiang V.S., Neblett M.F., Gerkowicz S.A., Spencer J.B., Marder M.E., Barr D.B., et al. Thyroid hormone levels associate with exposure to polychlorinated biphenyls and polybrominated biphenyls in adults exposed as children. Environ. Health Glob. Access Sci. Source. 2019;18:75. doi: 10.1186/s12940-019-0509-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Emmett E.A. Polychlorinated biphenyl exposure and effects in transformer repair workers. Environ. Health Perspect. 1985;60:185–192. doi: 10.1289/ehp.8560185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Abdelouahab N., Mergler D., Takser L., Vanier C., St-Jean M., Baldwin M., Spear P.A., Chan H.M. Gender differences in the effects of organochlorines, mercury, and lead on thyroid hormone levels in lakeside communities of Quebec (Canada) Environ. Res. 2008;107:380–392. doi: 10.1016/j.envres.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 352.Turyk M.E., Anderson H.A., Freels S., Chatterton R., Needham L.L., Patterson D.G., Steenport D.N., Knobeloch L., Imm P., Persky V.W. Associations of organochlorines with endogenous hormones in male Great Lakes fish consumers and nonconsumers. Environ. Res. 2006;102:299–307. doi: 10.1016/j.envres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 353.Eguchi A., Nomiyama K., Minh Tue N., Trang P.T.K., Hung Viet P., Takahashi S., Tanabe S. Residue profiles of organohalogen compounds in human serum from e-waste recycling sites in North Vietnam: Association with thyroid hormone levels. Environ. Res. 2015;137:440–449. doi: 10.1016/j.envres.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 354.Hagmar L., Björk J., Sjödin A., Bergman Å., Erfurth E.M. Plasma levels of persistent organohalogens and hormone levels in adult male humans. Arch. Environ. Health. 2001;56:138–143. doi: 10.1080/00039890109604065. [DOI] [PubMed] [Google Scholar]
- 355.Persky V., Turyk M., Anderson H.A., Hanrahan L.P., Falk C., Steenport D.N., Chatterton R., Freels S. The effects of PCB exposure and fish consumption on endogenous hormones. Environ. Health Perspect. 2001;109:1275–1283. doi: 10.1289/ehp.011091275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Turyk M.E., Anderson H.A., Persky V.W. Relationships of thyroid hormones with polychlorinated biphenyls, dioxins, furans, and DDE in adults. Environ. Health Perspect. 2007;115:1197–1203. doi: 10.1289/ehp.10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Bahn A.K., Mills J.L., Snyder P.J., Gann P.H., Houten L., Bialik O., Hollmann L., Utiger R.D. Hypothyroidism in workers exposed to polybrominated biphenyls. N. Engl. J. Med. 1980;302:31–33. doi: 10.1056/NEJM198001033020105. [DOI] [PubMed] [Google Scholar]
- 358.Yard E.E., Terrell M.L., Hunt D.R., Cameron L.L., Small C.M., McGeehin M.A., Marcus M. Incidence of thyroid disease following exposure to polybrominated biphenyls and polychlorinated biphenyls, Michigan, 1974–2006. Chemosphere. 2011;84:863–868. doi: 10.1016/j.chemosphere.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 359.Gorini F., Iervasi G., Coi A., Pitto L., Bianchi F. The role of polybrominated diphenyl ethers in thyroid carcinogenesis: Is it a weak hypothesis or a hidden reality? From facts to new perspectives. Int. J. Environ. Res. Public Health. 2018;15:1834. doi: 10.3390/ijerph15091834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Bloom M., Spliethoff H., Vena J., Shaver S., Addink R., Eadon G. Environmental exposure to PBDEs and thyroid function among New York anglers. Environ. Toxicol. Pharmacol. 2008;25:386–392. doi: 10.1016/j.etap.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 361.Makey C.M., McClean M.D., Braverman L.E., Pearce E.N., He X.M., Sjödin A., Weinberg J.M., Webster T.F. Polybrominated diphenyl ether exposure and thyroid function tests in North American adults. Environ. Health Perspect. 2016;124:420–425. doi: 10.1289/ehp.1509755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Byrne S.C., Miller P., Seguinot-Medina S., Waghiyi V., Buck C.L., Von Hippel F.A., Carpenter D.O. Associations between serum polybrominated diphenyl ethers and thyroid hormones in a cross sectional study of a remote Alaska Native population. Sci. Rep. 2018;8:2198. doi: 10.1038/s41598-018-20443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Yuan J., Chen L., Chen D., Guo H., Bi X., Ju Y., Jiang P., Shi J., Yu Z., Yang J., et al. Elevated serum polybrominated diphenyl ethers and thyroid-stimulating hormone associated with lymphocytic micronuclei in Chinese workers from an e-waste dismantling site. Environ. Sci. Technol. 2008;42:2195–2200. doi: 10.1021/es702295f. [DOI] [PubMed] [Google Scholar]
- 364.Turyk M.E., Persky V.W., Imm P., Knobeloch L., Chatterton R., Anderson H.A. Hormone disruption by PBDEs in adult male sport fish consumers. Environ. Health Perspect. 2008;116:1635–1641. doi: 10.1289/ehp.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Kim M.J., Park Y.J. Bisphenols and thyroid hormone. Endocrinol. Metab. 2019;34:340–348. doi: 10.3803/EnM.2019.34.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Moriyama K., Tagami T., Akamizu T., Usui T., Saijo M., Kanamoto N., Hataya Y., Shimatsu A., Kuzuya H., Nakao K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 2002;87:5185–5190. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- 367.Sriphrapradang C., Chailurkit L.O., Aekplakorn W., Ongphiphadhanakul B. Association between bisphenol A and abnormal free thyroxine level in men. Endocrine. 2013;44:441–447. doi: 10.1007/s12020-013-9889-y. [DOI] [PubMed] [Google Scholar]
- 368.Metwally F., Hasheesh A., Zaid M., Fattah El Sharkawy S., Abd Fattah F., Mazhar M. Bisphenol A levels among workers in plastic processing industry and their relations to thyroid hormones. J. Appl. Pharm. Sci. 2019;9:107–111. [Google Scholar]
- 369.Meeker J.D., Ferguson K.K. Relationship between urinary phthalate and bisphenol a concentrations and serum thyroid measures in U.S. adults and adolescents from the national health and nutrition examination survey (NHANES) 2007–2008. Environ. Health Perspect. 2011;119:1396–1402. doi: 10.1289/ehp.1103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Geens T., Dirtu A.C., Dirinck E., Malarvannan G., Van Gaal L., Jorens P.G., Covaci A. Daily intake of bisphenol A and triclosan and their association with anthropometric data, thyroid hormones and weight loss in overweight and obese individuals. Environ. Int. 2015;76:98–105. doi: 10.1016/j.envint.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 371.Wang T., Lu J., Xu M., Xu Y., Li M., Liu Y., Tian X., Chen Y., Dai M., Wang W., et al. Urinary bisphenol a concentration and thyroid function in Chinese adults. Epidemiology. 2013;24:295–302. doi: 10.1097/EDE.0b013e318280e02f. [DOI] [PubMed] [Google Scholar]
- 372.Park C., Choi W., Hwang M., Lee Y., Kim S., Yu S., Lee I., Paek D., Choi K. Associations between urinary phthalate metabolites and bisphenol A levels, and serum thyroid hormones among the Korean adult population—Korean National Environmental Health Survey (KoNEHS) 2012–2014. Sci. Total Environ. 2017;584–585:950–957. doi: 10.1016/j.scitotenv.2017.01.144. [DOI] [PubMed] [Google Scholar]
- 373.Meeker J.D., Calafat A.M., Hauser R. Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ. Sci. Technol. 2010;44:1458–1463. doi: 10.1021/es9028292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Guo Y., Zhang Z., Liu L., Li Y., Ren N., Kannan K. Occurrence and profiles of phthalates in foodstuffs from China and their implications for human exposure. J. Agric. Food Chem. 2012;60:6913–6919. doi: 10.1021/jf3021128. [DOI] [PubMed] [Google Scholar]
- 375.Shen O., Du G., Sun H., Wu W., Jiang Y., Song L., Wang X. Comparison of in vitro hormone activities of selected phthalates using reporter gene assays. Toxicol. Lett. 2009;191:9–14. doi: 10.1016/j.toxlet.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 376.Ghisari M., Bonefeld-Jorgensen E.C. Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol. Lett. 2009;189:67–77. doi: 10.1016/j.toxlet.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 377.Ye H., Ha M., Yang M., Yue P., Xie Z., Liu C. Di2-ethylhexyl phthalate disrupts thyroid hormone homeostasis through activating the Ras/Akt/TRHr pathway and inducing hepatic enzymes. Sci. Rep. 2017;7:1–12. doi: 10.1038/srep40153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Meeker J.D., Calafat A.M., Hauser R. Di(2-ethylhexyl) Phthalate metabolites may alter thyroid hormone levels in men. Environ. Health Perspect. 2007;115:1029–1034. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Huang H.-B., Pan W.H., Chang J.W., Chiang H.C., Guo Y.L., Jaakkola J.J.K., Huang P.C. Does exposure to phthalates influence thyroid function and growth hormone homeostasis? The Taiwan Environmental Survey for Toxicants (TEST) 2013. Environ. Res. 2017;153:63–72. doi: 10.1016/j.envres.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 380.Dirtu A.C., Geens T., Dirinck E., Malarvannan G., Neels H., Van Gaal L., Jorens P.G., Covaci A. Phthalate metabolites in obese individuals undergoing weight loss: Urinary levels and estimation of the phthalates daily intake. Environ. Int. 2013;59:344–353. doi: 10.1016/j.envint.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 381.Mendez W., Eftim S.E. Biomarkers of perchlorate exposure are correlated with circulating thyroid hormone levels in the 2007–2008 NHANES. Environ. Res. 2012;118:137–144. doi: 10.1016/j.envres.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 382.Kim M.J., Moon S., Oh B.-C., Jung D., Choi K., Park Y.J. Association between diethylhexyl phthalate exposure and thyroid function: A meta-analysis. Thyroid. 2019;29:183–192. doi: 10.1089/thy.2018.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Leung A.M., Pearce E.N., Braverman L.E. Environmental perchlorate exposure: Potential adverse thyroid effects. Curr. Opin. Endocrinol. Diabetes Obes. 2014;21:372–376. doi: 10.1097/MED.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Eskandari S., Loo D.D.F., Dai G., Levy O., Wright E.M., Carrasco N. Thyroid Na+/I− symporter. Mechanism, stoichiometry, and specificity. J. Biol. Chem. 1997;272:27230–27238. doi: 10.1074/jbc.272.43.27230. [DOI] [PubMed] [Google Scholar]
- 385.Blount B.C., Pirkle J.L., Osterloh J.D., Valentin-Blasini L., Caldwell K.L. Urinary perchlorate and thyroid hormone levels in adolescent and adult men and women living in the United States. Environ. Health Perspect. 2006;114:1865–1871. doi: 10.1289/ehp.9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Steinmaus C., Miller M.D., Cushing L., Blount B.C., Smith A.H. Combined effects of perchlorate, thiocyanate, and iodine on thyroid function in the National Health and Nutrition Examination Survey 2007–08. Environ. Res. 2013;123:17–24. doi: 10.1016/j.envres.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Suh M., Abraham L., Hixon J.G., Proctor D.M. The effects of perchlorate, nitrate, and thiocyanate on free thyroxine for potentially sensitive subpopulations o. The 2001–2002 and 2007–2008 National Health and Nutrition Examination Surveys. J. Expo. Sci. Environ. Epidemiol. 2014;24:579–587. doi: 10.1038/jes.2013.67. [DOI] [PubMed] [Google Scholar]
- 388.McMullen J., Ghassabian A., Kohn B., Trasande L. Identifying subpopulations vulnerable to the thyroid-blocking effects of perchlorateandthiocy anate. J. Clin. Endocrinol. Metab. 2017;102:2637–2645. doi: 10.1210/jc.2017-00046. [DOI] [PubMed] [Google Scholar]
- 389.Buck R.C., Franklin J., Berger U., Conder J.M., Cousins I.T., De Voogt P., Jensen A.A., Kannan K., Mabury S.A., van Leeuwen S.P.J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011;7:513–541. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Coperchini F., Awwad O., Rotondi M., Santini F., Imbriani M., Chiovato L. Thyroid disruption by perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) J. Endocrinol. Investig. 2017;40:105–121. doi: 10.1007/s40618-016-0572-z. [DOI] [PubMed] [Google Scholar]
- 391.Emmett E.A., Zhang H., Shofer F.S., Freeman D., Rodway N.V., Desai C., Shaw L.M. Community exposure to perfluorooctanoate: Relationships between serum levels and certain health parameters. J. Occup. Environ. Med. 2006;48:771–779. doi: 10.1097/01.jom.0000233380.13087.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Olsen G.W., Zobel L.R. Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. Int. Arch. Occup. Environ. Health. 2007;81:231–246. doi: 10.1007/s00420-007-0213-0. [DOI] [PubMed] [Google Scholar]
- 393.Bloom M.S., Kannan K., Spliethoff H.M., Tao L., Aldous K.M., Vena J.E. Exploratory assessment of perfluorinated compounds and human thyroid function. Physiol. Behav. 2010;99:240–245. doi: 10.1016/j.physbeh.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 394.Knox S.S., Jackson T., Frisbee S.J., Javins B., Ducatman A.M. Perfluorocarbon exposure, gender and thyroid function in the C8 health project. J. Toxicol. Sci. 2011;36:403–410. doi: 10.2131/jts.36.403. [DOI] [PubMed] [Google Scholar]
- 395.Wen L.L., Lin L.Y., Su T.C., Chen P.C., Lin C.Y. Association between serum perfluorinated chemicals and thyroid function in U.S. Adults: The national health and nutrition examination survey 2007–2010. J. Clin. Endocrinol. Metab. 2013;98:E1456–E1464. doi: 10.1210/jc.2013-1282. [DOI] [PubMed] [Google Scholar]
- 396.Webster G.M., Rauch S.A., Marie N.S., Mattman A., Lanphear B.P., Venners S.A. Cross-sectional associations of serum perfluoroalkyl acids and thyroid hormones in U.S. Adults: Variation according to TPOAb and iodine status (NHANES 2007–2008) Environ. Health Perspect. 2016;124:935–942. doi: 10.1289/ehp.1409589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Crawford N.M., Fenton S.E., Strynar M., Hines E.P., Pritchard D.A., Steiner A.Z. Effects of perfluorinated chemicals on thyroid function, markers of ovarian reserve, and natural fertility. Reprod. Toxicol. 2017;69:53–59. doi: 10.1016/j.reprotox.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Sohn Y.S., Moon S., Park Y.J. Effect of multiple exposure to perfluorinated chemicals on thyroid function among adults in the US: The National Health and Nutrition Examination Survey 2007–2008 and 2011–2012. Int. J. Thyroidol. 2020;13:19–29. doi: 10.11106/ijt.2020.13.1.19. [DOI] [Google Scholar]
- 399.Li Y., Xu Y., Fletcher T., Scott K., Nielsen C., Pineda D., Lindh C.H., Olsson D.S., Andersson E.M., Jakobsson K. Associations between perfluoroalkyl substances and thyroid hormones after high exposure through drinking water. Environ. Res. 2021;194:110647. doi: 10.1016/j.envres.2020.110647. [DOI] [PubMed] [Google Scholar]
- 400.Van Gerwen M., Alpert N., Alsen M., Ziadkhanpour K., Taioli E., Genden E. The impact of smoking on the association between perfluoroalkyl acids (Pfas) and thyroid hormones: A national health and nutrition examination survey analysis. Toxics. 2020;8:116. doi: 10.3390/toxics8040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Kim M.J., Moon S., Oh B.C., Jung D., Ji K., Choi K., Park Y.J. Association between perfluoroalkyl substances exposure and thyroid function in adults: A meta-analysis. PLoS ONE. 2018;13:e0197244. doi: 10.1371/journal.pone.0197244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Lewis R.C., Johns L.E., Meeker J.D. Serum biomarkers of exposure to perfluoroalkyl substances in relation to serum testosterone and measures of thyroid function among adults and adolescents from NHANES 2011–2012. Int. J. Environ. Res. Public Health. 2015;12:6098–6114. doi: 10.3390/ijerph120606098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Lin C.Y., Wen L.L., Lin L.Y., Wen T.W., Lien G.W., Hsu S.H.J., Chien K.L., Liao C.C., Sung F.C., Chen P.C., et al. The associations between serum perfluorinated chemicals and thyroid function in adolescents and young adults. J. Hazard. Mater. 2013;244–245:637–644. doi: 10.1016/j.jhazmat.2012.10.049. [DOI] [PubMed] [Google Scholar]
- 404.Olsen G.W., Burris J.M., Burlew M.M., Mandel J.H. Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J. Occup. Environ. Med. 2003;45:260–270. doi: 10.1097/01.jom.0000052958.59271.10. [DOI] [PubMed] [Google Scholar]
- 405.Ji K., Kim S., Kho Y., Paek D., Sakong J., Ha J., Kim S., Choi K. Serum concentrations of major perfluorinated compounds among the general population in Korea: Dietary sources and potential impact on thyroid hormones. Environ. Int. 2012;45:78–85. doi: 10.1016/j.envint.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 406.Byrne S.C., Miller P., Seguinot-Medina S., Waghiyi V., Buck C.L., Von Hippel F.A., Carpenter D.O. Exposure to perfluoroalkyl substances and associations with serum thyroid hormones in a remote population of Alaska Natives. Environ. Res. 2018;166:537–543. doi: 10.1016/j.envres.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Campos É., Freire C. Exposure to non-persistent pesticides and thyroid function: A systematic review of epidemiological evidence. Int. J. Hyg. Environ. Health. 2016;219:481–497. doi: 10.1016/j.ijheh.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 408.Leemans M., Couderq S., Demeneix B., Fini J.B. Pesticides with potential thyroid hormone-disrupting effects: A review of recent data. Front. Endocrinol. 2019;10:743. doi: 10.3389/fendo.2019.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.McKinlay R., Plant J.A., Bell J.N.B., Voulvoulis N. Endocrine disrupting pesticides: Implications for risk assessment. Environ. Int. 2008;34:168–183. doi: 10.1016/j.envint.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 410.Nankongnab N., Kongtip P., Kallayanatham N., Pundee R., Yimsabai J., Woskie S. Longitudinal study of thyroid hormones between conventional and organic farmers in Thailand. Toxics. 2020;8:82. doi: 10.3390/toxics8040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Steenland K., Cedillo L., Tucker J., Hines C., Sorensen K., Deddens J., Cruz V. Thyroid hormones and cytogenetic outcomes in backpack sprayers using ethylenebis(dithiocarbamate) (EBDC) fungicides in Mexico. Environ. Health Perspect. 1997;105:1126–1130. doi: 10.1289/ehp.971051126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Garry V.F., Holland S.E., Erickson L.L., Burroughs B.L. Male reproductive hormones and thyroid function in pesticide applicators in the Red River Valley of Minnesota. J. Toxicol. Environ. Health Part A. 2003;66:965–986. doi: 10.1080/15287390306399. [DOI] [PubMed] [Google Scholar]
- 413.Meeker J.D., Barr D.B., Hauser R. Thyroid hormones in relation to urinary metabolites of non-persistent insecticides in men of reproductive age. Reprod. Toxicol. 2006;22:437–442. doi: 10.1016/j.reprotox.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 414.Lacasaña M., López-Flores I., Rodríguez-Barranco M., Aguilar-Garduño C., Blanco-Muñoz J., Pérez-Méndez O., Gamboa R., Bassol S., Cebrian M.E. Association between organophosphate pesticides exposure and thyroid hormones in floriculture workers. Toxicol. Appl. Pharmacol. 2010;243:19–26. doi: 10.1016/j.taap.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 415.Fortenberry G.Z., Hu H., Turyk M., Barr D.B., Meeker J.D. Association between urinary 3, 5, 6-trichloro-2-pyridinol, a metabolite of chlorpyrifos and chlorpyrifos-methyl, and serum T4 and TSH in NHANES 1999–2002. Sci. Total Environ. 2012;424:351–355. doi: 10.1016/j.scitotenv.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Freire C., Koifman R.J., Sarcinelli P.N., Simões Rosa A.C., Clapauch R., Koifman S. Long-term exposure to organochlorine pesticides and thyroid status in adults in a heavily contaminated area in Brazil. Environ. Res. 2013;127:7–15. doi: 10.1016/j.envres.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 417.Farokhi F., Taravati A. Pesticide exposure and thyroid function in adult male sprayers. Int. J. Med. Investig. 2014;3:127–132. [Google Scholar]
- 418.Langer P., Tajtaková M., Kocan A., Vlcek M., Petrik J., Chovancova J., Drobna B., Jursa S., Pavuk M., Trnovec T., et al. Multiple organochlorine pollution and the thyroid. Endocr. Regul. 2006;40:46–52. [PubMed] [Google Scholar]
- 419.Herin F., Boutet-Robinet E., Levant A., Dulaurent S., Manika M., Galatry-Bouju F., Caron P., Soulat J.M. Thyroid function tests in persons with occupational exposure to fipronil. Thyroid. 2011;21:701–706. doi: 10.1089/thy.2010.0449. [DOI] [PubMed] [Google Scholar]
- 420.Bernieri T., Rodrigues D., Barbosa I.R., Ardenghi P.G., Basso da Silva L. Occupational exposure to pesticides and thyroid function in Brazilian soybean farmers. Chemosphere. 2019;218:425–429. doi: 10.1016/j.chemosphere.2018.11.124. [DOI] [PubMed] [Google Scholar]
- 421.Sala M., Sunyer J., Herrero C., To-Figueras J., Grimalt J. Association between serum concentrations of hexachlorobenzene and polychlorobiphenyls with thyroid hormone and liver enzymes in a sample of the general population. Occup. Environ. Med. 2001;58:172–177. doi: 10.1136/oem.58.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Langer P., Kočan A., Tajtáková M., Rádiková Ž., Petrík J., Koška J., Kšinantová L., Imrich R., Hučková M., Chovancová J., et al. Possible effects of persistent organochlorinated pollutants cocktail on thyroid hormone levels and pituitary-thyroid interrelations. Chemosphere. 2007;70:110–118. doi: 10.1016/j.chemosphere.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 423.Schell L.M., Gallo M.V., Denham M., Ravenscroft J., DeCaprio A.P., Carpenter D.O. Relationship of thyroid hormone levels to levels of polychlorinated biphenyls, lead, p,p′-DDE, and other toxicants in Akwesasne Mohawk Youth. Environ. Health Perspect. 2008;116:806–813. doi: 10.1289/ehp.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Meeker J.D., Barr D.B., Hauser R. Pyrethroid insecticide metabolites are associated with serum hormone levels in adult men. Reprod. Toxicol. 2009;27:155–160. doi: 10.1016/j.reprotox.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Miranda-Contreras L., Gómez-Pérez R., Rojas G., Cruz I., Berrueta L., Salmen S., Colmenares M., Barreto S., Balza A., Zavala L., et al. Occupational exposure to organophosphate and carbamate pesticides affects sperm chromatin integrity and reproductive hormone levels among venezuelan farm workers. J. Occup. Health. 2013;55:195–203. doi: 10.1539/joh.12-0144-FS. [DOI] [PubMed] [Google Scholar]
- 426.Khan D.A., Ahad K., Ansari W.M., Khan H. Pesticide exposure and endocrine dysfunction in the cotton crop agricultural workers of Southern Punjab, Pakistan. Asia-Pac. J. Public Health. 2013;25:181–191. doi: 10.1177/1010539511417422. [DOI] [PubMed] [Google Scholar]
- 427.Jain R. Association between thyroid function and urinary levels of 3,5,6-trichloro-2-pyridinol: Data from NHANES 2007–2008. Environ. Sci. Pollut. Res. 2017;24:2820–2826. doi: 10.1007/s11356-016-8007-0. [DOI] [PubMed] [Google Scholar]
- 428.Medda E., Santini F., De Angelis S., Franzellin F., Fiumalbi C., Perico A., Gilardi E., Mechi M.T., Marsili A., Citroni A., et al. Iodine nutritional status and thyroid effects of exposure to ethylenebisdithiocarbamates. Environ. Res. 2017;154:152–159. doi: 10.1016/j.envres.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 429.Hwang M., Lee Y., Choi K., Park C. Urinary 3-phenoxybenzoic acid levels and the association with thyroid hormones in adults: Korean National Environmental Health Survey 2012–2014. Sci. Total Environ. 2019;696:133920. doi: 10.1016/j.scitotenv.2019.133920. [DOI] [PubMed] [Google Scholar]
- 430.Jain R.B. Variability in the levels of 3-phenoxybenzoic acid by age, gender, and race/ethnicity for the period of 2001–2002 versus 2009–2010 and its association with thyroid function among general US population. Environ. Sci. Pollut. Res. 2016;23:6934–6939. doi: 10.1007/s11356-015-5954-9. [DOI] [PubMed] [Google Scholar]
- 431.Panganiban L.R., Cortes-Maramba N., Dioquino C., Suplido M.L., Ho H., Francisco-Rivera A., Manglicmot-Yabes A. Correlation between blood enthylenethiourea and thyroid gland disorders among banana plantation workers in the Philippines. Environ. Health Perspect. 2004;112:42–45. doi: 10.1289/ehp.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Rashidi M.A., Mahabadi H., Khavanin A. Evaluation of the effects of chronic exposure to organophosphorus pesticides on thyroid function. Asia Pac. J. Med. Toxicol. 2020;9:35–43. doi: 10.22038/APJMT.2020.16385. [DOI] [Google Scholar]
- 433.Toft G., Flyvbjerg A., Bonde J.P. Thyroid function in Danish greenhouse workers. Environ. Health Glob. Access Sci. Source. 2006;5:32. doi: 10.1186/1476-069X-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Blanco-Muñoz J., Lacasaña M., López-Flores I., Rodríguez-Barranco M., González-Alzaga B., Bassol S., Cebrian M.E., López-Carrillo L., Aguilar-Garduño C. Association between organochlorine pesticide exposure and thyroid hormones in floriculture workers. Environ. Res. 2016;150:357–363. doi: 10.1016/j.envres.2016.05.054. [DOI] [PubMed] [Google Scholar]
- 435.Bloom M.S., Jansing R.L., Kannan K., Rej R., Fitzgerald E.F. Thyroid hormones are associated with exposure to persistent organic pollutants in aging residents of upper Hudson River communities. Int. J. Hyg. Environ. Health. 2014;217:473–482. doi: 10.1016/j.ijheh.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Santos R., Piccoli C., Cremonese C., Freire C. Thyroid and reproductive hormones in relation to pesticide use in an agricultural population in Southern Brazil. Environ. Res. 2019;173:221–231. doi: 10.1016/j.envres.2019.03.050. [DOI] [PubMed] [Google Scholar]
- 437.Zaidi S.S., Bhatnagar V.K., Gandhi S.J., Shah M.P., Kulkarni P.K., Saiyed H.N. Assessment of thyroid function in pesticide formulators. Hum. Exp. Toxicol. 2000;19:497–501. doi: 10.1191/096032700677928536. [DOI] [PubMed] [Google Scholar]
- 438.Pelletier C., Doucet E., Imbeault P., Tremblay A. Associations between weight loss-induced changes in plasma organochlorine concentrations, serum T3 concentration, and resting metabolic rate. Toxicol. Sci. 2002;67:46–51. doi: 10.1093/toxsci/67.1.46. [DOI] [PubMed] [Google Scholar]
- 439.García Torres E., Pérez Morales R., González Zamora A., Ríos Sánchez E., Olivas Calderón E.H., Alba Romero J.d.J., Calleros Rincón E.Y. Consumption of water contaminated by nitrate and its deleterious effects on the human thyroid gland: A review and update. Int. J. Environ. Health Res. 2020:1–18. doi: 10.1080/09603123.2020.1815664. [DOI] [PubMed] [Google Scholar]
- 440.Ward M.H., Kilfoy B.A., Weyer P.J., Anderson K.E., Folsom A.R., Cerhan J.R. Nitrate intake and the risk of thyroid cancer and thyroid disease. Epidemiology. 2010;21:389–395. doi: 10.1097/EDE.0b013e3181d6201d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Aschebrook-Kilfoy B., Heltshe S.L., Nuckols J.R., Sabra M.M., Shuldiner A.R., Mitchell B.D., Airola M., Holford T.R., Zhang Y., Ward M.H. Modeled nitrate levels in well water supplies and prevalence of abnormal thyroid conditions among the Old Order Amish in Pennsylvania. Environ. Health Glob. Access Sci. Source. 2012;11:6. doi: 10.1186/1476-069X-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Van Maanen J.M.S., van Dijk A., Mulder K., de Baets M.H., Menheere P.C.A., van der Heide D., Mertens P.L.J.M., Kleinjans J.C.S. Consumption of drinking water with high nitrate levels causes hypertrophy of the thyroid. Toxicol. Lett. 1994;72:365–374. doi: 10.1016/0378-4274(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 443.Bivolarska A.V., Maneva A.I., Gatseva P.D., Katsarova M.N. Effect of nitrates, thiocyanates and selenium on the iron and iodine status of postpartum women. Folia Med. 2016;58:188–194. doi: 10.1515/folmed-2016-0024. [DOI] [PubMed] [Google Scholar]
- 444.Lambers A., Koppeschaar H., van Isselt J., Schothorst R., Mensinga T., Meulenbelt J. The Effect of Nitrate on the Thyroid Gland Function in Healthy Volunteers in a 4-Week Oral Toxicity Study. Rijksinst voor Volksgezond en Milieu. National Institute fot Public Health and the Environment; Utrecht, The Netherlands: 2000. p. 61. [Google Scholar]
- 445.Dos Santos Pinheiro V., Volino-Souza M., Vieira de Oliveira G., Adam Conte-Junior C., Silveira Alvares T. Effect of high-nitrate beetroot juice consumption on thyroid gland hormones and iodine levels in adults. Food Biosci. 2021;40:100869. doi: 10.1016/j.fbio.2020.100869. [DOI] [Google Scholar]
- 446.Palazzolo D.L., Jansen K.P. The minimal arsenic concentration required to inhibit the activity of thyroid peroxidase activity in vitro. Biol. Trace Elem. Res. 2008;126:49–55. doi: 10.1007/s12011-008-8183-y. [DOI] [PubMed] [Google Scholar]
- 447.Ghosh N., Bhattacharya S. Thyrotoxicity of the chlorides of cadmium and mercury in rabbit. Biomed. Environ. Sci. 1992:236–240. [PubMed] [Google Scholar]
- 448.Yoshida K., Sugihira N., Suzuki M., Sakurada T., Saito S., Yoshinaga K., Saito H. Effect of cadmium on T4 outer ring monodeiodination by rat liver. Environ. Res. 1987;42:400–405. doi: 10.1016/S0013-9351(87)80206-2. [DOI] [PubMed] [Google Scholar]
- 449.Chaurasia S.S., Gupta P., Kar A., Maiti P.K. Lead induced thyroid dysfunction and lipid peroxidation in the fish Clarias batrachus with special reference to hepatic type I-5′-monodeiodinase activity. Bull. Environ. Contam. Toxicol. 1996;56:649–654. doi: 10.1007/s001289900095. [DOI] [PubMed] [Google Scholar]
- 450.Soldin O.P., O’Mara D.M., Aschner M. Thyroid hormones and methylmercury toxicity. Biol. Trace Elem. Res. 2008;126:1. doi: 10.1007/s12011-008-8199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Ciarrocca M., Tomei F., Caciari T., Cetica C., Andrè J.C., Fiaschetti M., Schifano M.P., Scala B., Scimitto L., Tomei G., et al. Exposure to Arsenic in urban and rural areas and effects on thyroid hormones. Inhal. Toxicol. 2012;24:589–598. doi: 10.3109/08958378.2012.703251. [DOI] [PubMed] [Google Scholar]
- 452.Molin M., Ulven S.M., Dahl L., Lundebye A.K., Holck M., Alexander J., Meltzer H.M., Ydersbond T.A. Arsenic in seafood is associated with increased thyroid-stimulating hormone (TSH) in healthy volunteers—A randomized controlled trial. J. Trace Elem. Med. Biol. 2017;44:1–7. doi: 10.1016/j.jtemb.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 453.Jain R. Association between arsenic exposure and thyroid function: Data from NHANES 2007–2010. Int. J. Environ. Health Res. 2016;26:101–129. doi: 10.1080/09603123.2015.1061111. [DOI] [PubMed] [Google Scholar]
- 454.Jurdziak M., Gać P., Poręba M., Szymańska-Chabowska A., Mazur G., Poręba R. Concentration of thyrotropic hormone in persons occupationally exposed to lead, cadmium and arsenic. Biol. Trace Elem. Res. 2018;182:196–203. doi: 10.1007/s12011-017-1096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Rosati M.V., Montuori L., Caciari T., Sacco C., Marrocco M., Tomei G., Scala B., Sancini A., Anzelmo V., Bonomi S., et al. Correlation between urinary cadmium and thyroid hormones in outdoor workers exposed to urban stressors. Toxicol. Ind. Health. 2016;32:1978–1986. doi: 10.1177/0748233715602833. [DOI] [PubMed] [Google Scholar]
- 456.Christensen Y.K.L. Metals in blood and urine, and thyroid function among adults in the United States 2007–2008. Int. J. Hyg. Environ. Health. 2013;216:624–632. doi: 10.1016/j.ijheh.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 457.Chen A., Kim S.S., Chung E., Dietrich K.N. Thyroid hormones in relation to lead, mercury, and cadmium exposure in the national health and nutrition examination survey, 2007–2008. Environ. Health Perspect. 2013;121:181–186. doi: 10.1289/ehp.1205239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Luo J., Hendryx M. Relationship between blood cadmium, lead, and serum thyroid measures in US adults—The National Health and Nutrition Examination Survey (NHANES) 2007–2010. Int. J. Environ. Health Res. 2014;24:125–136. doi: 10.1080/09603123.2013.800962. [DOI] [PubMed] [Google Scholar]
- 459.Jain R.B., Choi Y.S. Interacting effects of selected trace and toxic metals on thyroid function. Int. J. Environ. Health Res. 2016;26:75–91. doi: 10.1080/09603123.2015.1020416. [DOI] [PubMed] [Google Scholar]
- 460.Mohammed Sherif M., Solyman Mohammed Y., Abd El-Moaty Zedan H., Abd El-hamid Kheder M., Hassan Abd EL-Salam Mohammed A., Author C. Toxic Effect of Some Heavy Metals (Cadmium and Lead) on Thyroid Function. Egypt. J. Hosp. Med. 2017;69:2512–2515. doi: 10.12816/0041703. [DOI] [Google Scholar]
- 461.Nie X., Chen Y., Chen Y., Chen C., Han B., Li Q., Zhu C., Xia F., Zhai H., Wang N., et al. Lead and cadmium exposure, higher thyroid antibodies and thyroid dysfunction in Chinese women. Environ. Pollut. 2017;230:320–328. doi: 10.1016/j.envpol.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 462.Akgöl E., Tutkun E., Yilmaz H., Yilmaz F., Gunduzoz M., Bal C., Ünlü A., Abusoglu S. Alterations of thyroid hormone levels in cadmium exposure. J. Clin. Anal. Med. 2017;8:202–206. doi: 10.4328/JCAM.4802. [DOI] [Google Scholar]
- 463.Chung S.M., Moon J.S., Yoon J.S., Won K.C., Lee H.W. Sex-specific effects of blood cadmium on thyroid hormones and thyroid function status: Korean nationwide cross-sectional study. J. Trace Elem. Med. Biol. 2019;53:55–61. doi: 10.1016/j.jtemb.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 464.Nishijo M., Nakagawa H., Morikawa Y., Tabata M., Senma M., Miura K., Tsuritani I., Honda R., Kido T., Teranishi H., et al. A study of thyroid hormone levels of inhabitants of the cadmium-polluted Kakehashi River basin. Jpn. J. Hyg. 1994;49:598–605. doi: 10.1265/jjh.49.598. [DOI] [PubMed] [Google Scholar]
- 465.López C.M., Piñeiro A.E., Núñez N., Avagnina A.M., Villaamil E.C., Roses O.E. Thyroid hormone changes in males exposed to lead in the Buenos Aires Area (Argentina) Pharmacol. Res. 2000;42:599–602. doi: 10.1006/phrs.2000.0734. [DOI] [PubMed] [Google Scholar]
- 466.Singh B., Chandran V., Bandhu H.K., Mittal B.R., Bhattacharya A., Jindal S.K., Varma S. Impact of lead exposure on pituitary-thyroid axis in humans. BioMetals. 2000;13:187–192. doi: 10.1023/A:1009201426184. [DOI] [PubMed] [Google Scholar]
- 467.Pekcici R., Kavlakoğlu B., Yilmaz S., Şahin M., Delibaşi T. Effects of lead on thyroid functions in lead-exposed workers. Cent. Eur. J. Med. 2010;5:215–218. doi: 10.2478/s11536-009-0092-8. [DOI] [Google Scholar]
- 468.Fahim Y.A., Sharaf N.E., Hasani I.W., Ragab E.A., Abdelhakim H.K. Assessment of thyroid function and oxidative stress state in foundry workers exposed to lead. J. Health Pollut. 2020;10:200903. doi: 10.5696/2156-9614-10.27.200903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Schumacher C., Brodkin C.A., Alexander B., Cullen M., Rainey P.M., Van Netten C., Faustman E., Checkoway H. Thyroid function in lead smelter workers: Absence of subacute or cumulative effects with moderate lead burdens. Int. Arch. Occup. Environ. Health. 1998;71:453–458. doi: 10.1007/s004200050305. [DOI] [PubMed] [Google Scholar]
- 470.Tuppurainen M., Wagar G., Kurppa K., Sakari W., Wambugu A., Froseth B., Alho J., Nykyri E. Thyroid function as assessed by routine laboratory tests of workers with long-term exposure. Scand. J. Work. Environ. Health. 1988;14:175–180. doi: 10.5271/sjweh.1934. [DOI] [PubMed] [Google Scholar]
- 471.Erfurth E.M., Gerhardsson L., Nilsson A., Rylander L., Schütz A., Skerfving S., Börjesson J. Effects of lead on the endocrine system in lead smelter workers. Arch. Environ. Health. 2001;56:449–455. doi: 10.1080/00039890109604481. [DOI] [PubMed] [Google Scholar]
- 472.Liang Q., Liao R., Su S., Huang S., Pan R., Huang J. Effects of lead on thyroid function of occupationally exposed workers. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2003;21:111–113. [PubMed] [Google Scholar]
- 473.Dundar B., Öktem F., Arslan M.K., Delibas N., Baykal B., Arslan Ç., Gultepe M., Ilhan I.E. The effect of long-term low-dose lead exposure on thyroid function in adolescents. Environ. Res. 2006;101:140–145. doi: 10.1016/j.envres.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 474.Soltani S., Sharifiyan A., Ghasemi M., Chavoshi F., Sadeghniiat K., Bahaedini L., Amlnian O., Meisami A.P. Assessment of thyroid function in male workers of battery recycling factory occupationally exposed to lead. J. Pharmacol. Toxicol. 2012;7:338–343. doi: 10.3923/jpt.2012.338.343. [DOI] [Google Scholar]
- 475.Krieg E.F. A meta-analysis of studies investigating the effects of occupational lead exposure on thyroid hormones. Am. J. Ind. Med. 2016;59:583–590. doi: 10.1002/ajim.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Dursun N., Tutus A. Chronic occupational lead exposure and thyroid function. J. Trace Elem. Exp. Med. 1999;12:45–49. doi: 10.1002/(SICI)1520-670X(1999)12:1<45::AID-JTRA5>3.0.CO;2-D. [DOI] [Google Scholar]
- 477.Saad-Hussein A., Hamdy H., Aziz H.M., Mahdy-Abdallah H. Thyroid functions in paints production workers and the mechanism of oxidative-antioxidants status. Toxicol. Ind. Health. 2011;27:257–263. doi: 10.1177/0748233710386409. [DOI] [PubMed] [Google Scholar]
- 478.Bledsoe M.L., Pinkerton L.E., Silver S., Deddens J.A., Biagini R.E. Thyroxine and free thyroxine levels in workers occupationally exposed to inorganic lead. Environ. Health Insights. 2011;5:55–61. doi: 10.4137/EHI.S7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Correia M.M., Chammas M.C., Zavariz J.D., Arata A., Martins L.C., Marui S., Pereira L.A.A. Evaluation of the effects of chronic occupational exposure to metallic mercury on the thyroid parenchyma and hormonal function. Int. Arch. Occup. Environ. Health. 2020;93:491–502. doi: 10.1007/s00420-019-01499-0. [DOI] [PubMed] [Google Scholar]
- 480.Hu Q., Han X., Dong G., Yan W., Wang X., Bigambo F.M., Fang K., Xia Y., Chen T., Wang X. Association between mercury exposure and thyroid hormones levels: A meta-analysis. Environ. Res. 2021;196:110928. doi: 10.1016/j.envres.2021.110928. [DOI] [PubMed] [Google Scholar]
- 481.Barregard L., Lindstedt G., Schutz A., Sallsten G. Endocrine function in mercury exposed chloralkali workers. Occup. Environ. Med. 1994;51:536–540. doi: 10.1136/oem.51.8.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Afrifa J., Ogbordjor W.D., Duku-Takyi R. Variation in thyroid hormone levels is associated with elevated blood mercury levels among artisanal small-scale miners in Ghana. PLoS ONE. 2018;13:e0203335. doi: 10.1371/journal.pone.0203335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Ellingsen D.G., Efskind J., Haug E., Thomassen Y., Martinsen I., Gaarder P.I. Effects of low mercury vapour exposure on the thyroid function in chloralkali workers. J. Appl. Toxicol. 2000;20:483–489. doi: 10.1002/1099-1263(200011/12)20:6<483::AID-JAT722>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 484.Rádiková Ž., Tajtáková M., Kočan A., Trnovec T., Šeböková E., Klimeš I., Langer P. Possible effects of environmental nitrates and toxic organochlorines on human thyroid in highly polluted areas in Slovakia. Thyroid. 2008;18:353–362. doi: 10.1089/thy.2007.0182. [DOI] [PubMed] [Google Scholar]
- 485.Bloom M.S., Weiner J.M., Vena J.E., Beehler G.P. Exploring associations between serum levels of select organochlorines and thyroxine in a sample of New York state sportsmen: The New York State Angler Cohort study. Environ. Res. 2003;93:52–66. doi: 10.1016/S0013-9351(02)00085-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable. No new data were created or analysed in this study.