Abstract
Gene therapy requires an effective and safe delivery vehicle for nucleic acids. In the case of non-viral vehicles, including cationic liposomes, the structure of compounds composing them determines the efficiency a lot. Currently, cationic amphiphiles are the most frequently used compounds in liposomal formulations. In their structure, which is a combination of hydrophobic and cationic domains and includes spacer groups, each component contributes to the resulting delivery efficiency. This review focuses on polycationic and disulfide amphiphiles as prospective cationic amphiphiles for gene therapy and includes a discussion of the mutual influence of structural components.
Keywords: polyamines, spermine, cationic amphiphiles, cationic liposomes, disulfide groups, gemini amphiphiles, gene delivery
1. Introduction
Gene therapy is a modern and promising method for treating severe hereditary and acquired diseases, including COVID-19 immunization, through the delivery of therapeutic nucleic acids (NAs) that can replace a damaged gene (pDNA), provide a new one, or block the expression of an unwanted protein (antisense oligonucleotides, siRNA) [1,2]. The direct administration of therapeutic NAs is an inefficient process due to multiple external and internal limiting factors [3]. External factors lead to the instability of NAs in biological fluids (degradation by nucleases or interaction with albumin or low-density lipoproteins, causing the aggregation and rapid clearance of NAs) and a low degree of interaction with target cells. Internal factors are determined by the presence of membrane barriers (plasma, endosomal, and nuclear membranes) that present a challenge to NAs as they attempt to reach the cytosol and nucleus [4].
Overcoming these factors requires the development of special delivery vehicles. At present, viruses [5,6], which are highly effective but present some serious disadvantages, primarily associated with the induction of inflammatory and immune responses in the body, fill this role. However, alternative non-viral delivery vehicles, such as cationic liposomes (CLs) based on cationic amphiphiles (CAs) [6,7,8,9], are being developed. The most recent success is development of an mRNA vaccine [10] against COVID-19, where lipid nanoparticles deliver nucleoside-modified mRNA encoding a mutated form of the spike protein of SARS-CoV-2 [11]. Generally, CA structure is a combination of hydrophobic and cationic domains linked together by various spacer groups [12]. The positive charge of CAs enables the “packing” of NAs due to electrostatic interactions with the formation of lipoplexes (complexes of NAs with liposomes) and facilitates their interaction with a negatively charged plasma membrane.
In addition to CAs, liposomes can include helper lipids (for example, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, DOPE) [13,14,15], which promote the formation of a certain lipid phase and favor cell transfection. Liposomes may also contain additional lipophilic molecules that permit them to target certain cells [16] or increase their circulation time in the bloodstream (for example, lipophilic derivatives of polyethylene glycol, PEG) [17]. If it is not stated below, CAs were used without additional components.
The CA structure significantly affects the efficiency of NA delivery to eukaryotic cells. In recent years, many monocationic amphiphiles have been obtained [18,19,20,21,22,23,24,25,26,27,28] that form liposomes for NA delivery. In this review, we will consider polycationic amphiphiles, which, compared to monocationic analogs, enable the more efficient transport of NAs into cells due to the formation of a system of distributed charges in the polyamine matrix and their ability to facilitate NA release from endosomes. This ability is strongly affected by the high H+ buffer capacity of polyamines containing titratable amines results in endosomal Cl− accumulation during acidification with presumed osmotic endosome disruption and enhanced lipoplex escape [29].
2. Cationic Amphiphiles Based on Polyamines or Amino Acids
2.1. Cationic Amphiphiles Based on Linear Polyamines
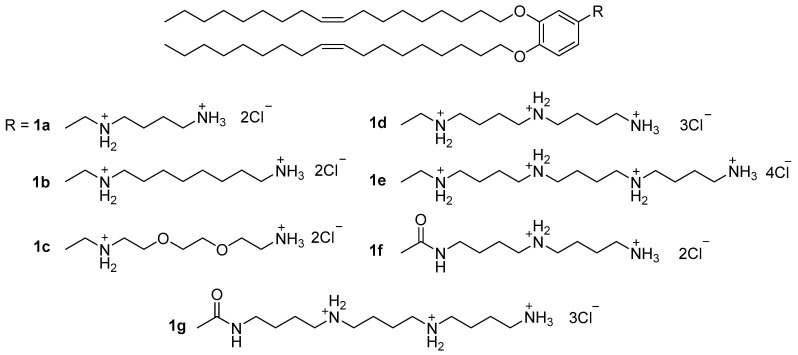
Enhancement of NA transport by polycationic amphiphiles may be related not only to distributed charges. On the cell surface, polyamine recognition sites—for example, PAT [30], a polyamine transporter—selectively transport both polyamines and their derivatives. Moreover, cancer cells have more such sites on their surfaces, which means that amphiphiles based on polyamines can transfect cancer cells more efficiently. Particularly important factors in NA delivery are the number and distribution of positive charges in the polyamine molecule. Transfection activity (TA) has been shown to increase as the number of amino groups in the polyamine structure increases. Compound 1e (Figure 1) exhibited the highest transfection efficiency among the synthesized lipopolyamines 1a–g [31], which suggests that 1e can use PAT and compete with other polyamines, for example, spermine, for binding to certain recognition sites on the cell surface (in particular, with the same PAT).
Figure 1.
Lipopolyamines with benzyl linker.
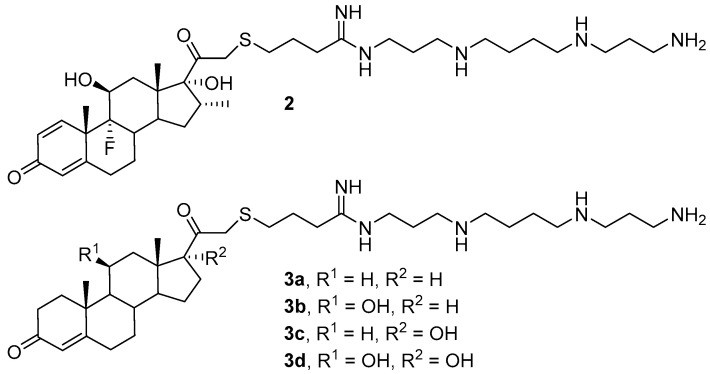
Another factor affecting the efficiency of transfection is the hydrophobicity of the amphiphilic molecule. A study of compounds 2 and 3a–d, which contain sterols (cortisol and its derivatives) as hydrophobic domains (Figure 2), revealed that TA increases with an increase in the hydrophobicity of the molecule [32]. While liposomes with compound 3d were shown to be incapable of delivering NAs, possibly due to the lower hydrophobicity of the amphiphile 3d and ineffective formation of lipoplexes, compounds 3b and 3c had the highest transfection efficiencies. Notably, the contribution of hydrophobicity to the efficiency of NA delivery also depends on other parameters, primarily the CA/DOPE (the last was a helper lipid) ratio and N/P ratio (the ratio of the number of CA amino groups to the number of NA phosphate groups).
Figure 2.
Cationic amphiphiles (CAs) based on cortisol and its derivatives.
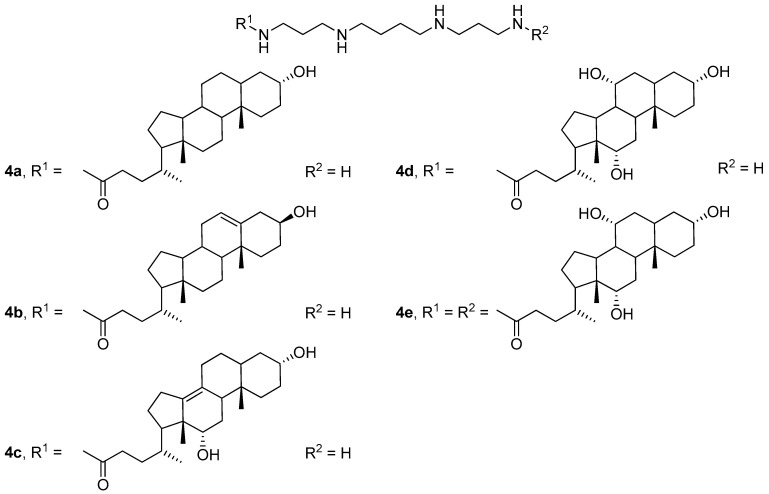
Subsequent studies have shown [33] that compounds 4b and 4c, which contain double bonds in the polycyclic hydrophobic domain (Figure 3), were the most effective. Three-component liposomes formed from compound 4d, its dimeric analog 4e, and DOPE delivered plasmid DNA (pDNA) more efficiently than two-component liposomes 4e/DOPE.
Figure 3.
CAs with different polycyclic hydrophobic domains.
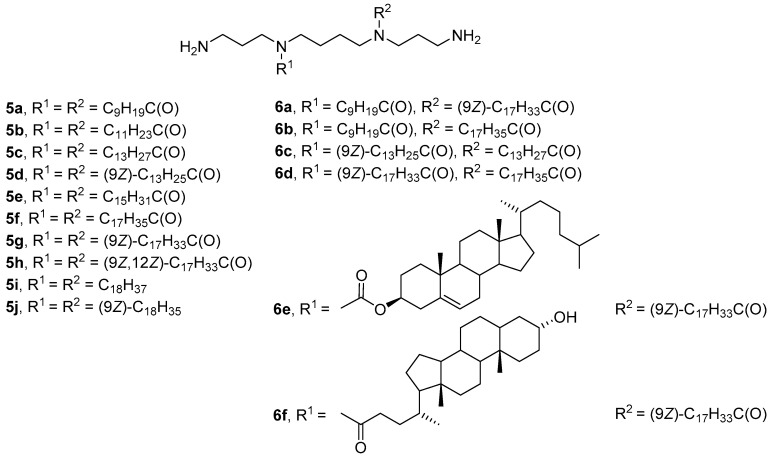
Hydrophobic substituents in the CA structure are mainly attached to primary amino groups of the polyamine. A different approach to the synthesis of CAs was proposed by Blagbrough et al. [34,35,36,37], who obtained N4,N9-disubstituted spermine derivatives 5a–j with acyl or alkyl residues of various lengths and degrees of unsaturation (Figure 4). All lipoplexes formed from acyl-substituted polyamines 5a–h exhibited high TA, excluding amphiphiles 5b and 5f. However, only compounds 5f and 5g with stearoyl and oleoyl residues had low toxicity toward FEK4 and HtTA cells. Notably, an increase in the degree of unsaturation of hydrocarbon chains increased both the efficiency of transfection and the cytotoxicity of the compounds. Alkyl derivatives of spermine 5i and 5j had comparable or slightly higher transfection efficiencies but were much more toxic than their acyl analogs [35].
Figure 4.
N4,N9-disubstituted spermine derivatives.
Asymmetric analogs 6a–f (Figure 4) were subsequently developed [38]. N4-myristoleoyl-N9-myristoylspermine (6c) and N4-oleoyl-N9-stearoylspermine (6d) showed the highest efficiency of siRNA delivery into FEK4 and HtTA cells, comparable to the efficiency of the commercial transfectant TransIT-TKO (Mirus Bio, Madison, WI, USA). Amphiphile 6f with a lithocholoyl residue effectively delivered NAs but caused cell death. The least effective was N4-cholesteryl-N9-oleoylspermine (6e).
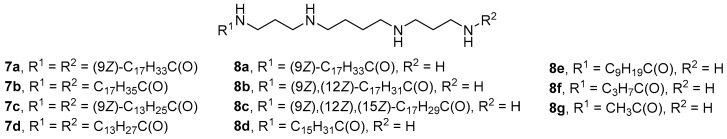
When N1,N12-substituted spermine derivatives 7a–d (Figure 5), structural isomers of amphiphiles 5c, 5d, 5f, 5g, were synthesized and studied [39], the efficiency of pDNA delivery into FEK4 and HtTA cells by complexes with amphiphiles 7a–d was lower, while the toxicity was higher than with amphiphile 5g. siRNA delivery efficiency mediated by compounds 7a and 7c was comparable to the efficiency of delivery using amphiphile 5g.
Figure 5.
Spermine derivatives with acyl-substituted terminal amino groups.
Multiple monosubstituted polyamine derivatives 8a–g (Figure 5) were obtained by modifying spermine with fatty acid residues of various lengths and degrees of unsaturation [40]. Although an increase in the length of the fatty acid residue increased toxicity, it positively affected the penetration of lipoplexes through the cell membrane in vitro. Experiments in vivo showed that the efficiency of NA delivery with N-butanoylspermine (8f) was higher than with N-decanoylspermine (8e).
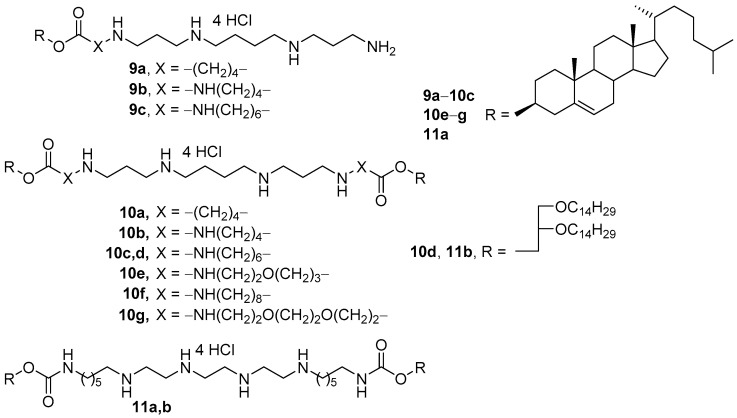
Mono- and disubstituted polycationic amphiphiles were developed based on spermine as a hydrophilic domain and cholesterol or 1,2-di-O-tetradecylglycerol as hydrophobic domains (Figure 6) [41,42,43]. The amphiphiles had different spacer lengths and linker types. CLs were prepared using these amphiphiles and DOPE (1:1 mol.). Among monosubstituted amphiphiles 9a–c, compound 9b showed the highest TA. While transfecting the same percentage of cells as their monomeric analogs 9a–c, however, dimeric polycationic amphiphiles 10a–c provided better expression of the green fluorescent protein. The highest transfection efficiency was exhibited by liposomes based on amphiphile 10c, which were superior to the efficiency of the commercial transfectant Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) for any type of NAs transferred [42,43]. Targeted liposomes based on CA 10c were also successfully employed in vivo [44,45,46,47,48,49].
Figure 6.
Mono- and dimeric polycationic amphiphiles based on spermine and triethylenetetramine.
Analogs 10e–g with ethoxypropylene, octamethylene, and ethoxyethoxyethylene spacers (Figure 6) permitted a greater TA increase than using 10c in vitro [50]. In contrast, the replacement of spermine with triethylenetetramine (TETA, 11a,b) led to a significant decrease in TA [51].
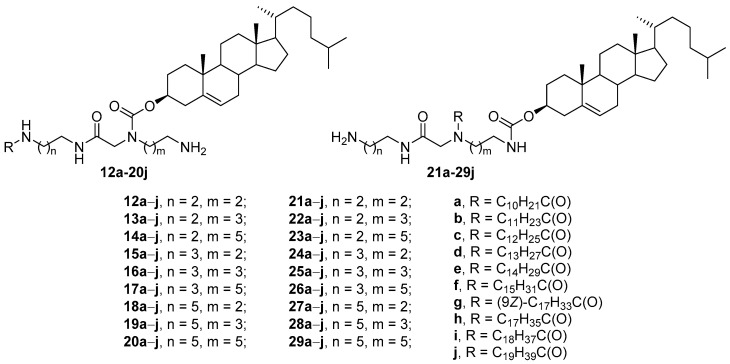
Extensive screening of CAs [52] revealed that in the structure of compounds 12a–j through 29a–j, both the polyamine matrix and the hydrophobic components changed (Figure 7). Among CLs formed from these amphiphiles and DOPE (1:2 weight ratio), the effective transfection of HEK293 cells was achieved only by liposomes with amphiphiles 12a–j through 20a–j containing an acyl substituent at the terminal amino group. Moreover, only eight compounds (12c, 12e, 13d, 14c, 16d, 16g, 17h, and 17j) were superior in TA to the commercial transfectant Effectene (Qiagen, Hilton, Germany). Subsequent transfection studies on HEK293, COLO 205, D17, HeLa, and PC3 cells showed that these compounds mediated more effective NA transport than did the commercial transfectants Effectene, DOTAP, and DC-Chol, while their toxicity was lower than that of commercial transfectants.
Figure 7.
CAs with asymmetric acyl hydrophobic tails.
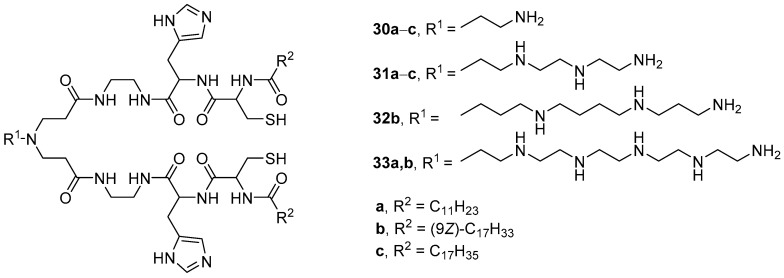
pH-Sensitive polycationic amphiphiles 30–33 (Figure 8) were obtained by subsequently coupling amino acids (l-histidine and l-cysteine) and fatty acids (lauric, oleic, and stearic) to polyamines [53,54]. The size of complexes of amphiphiles with siRNA was 160–210 nm, and the maximum TA on U87 cells was achieved using amphiphiles 30b–33b with oleic residues. Among them, TA decreased in the series 30b > 32b > 31b > 33b. A correlation was also established between the TA and the ability of compounds 30b–33b to disrupt the integrity of erythrocyte membranes. Leader compound 30b based on ethylenediamine exhibited the highest hemolytic activity at pH 5.4, which corresponds to the onset of endosomal acidification. Therefore, when using this amphiphile, one can expect effective NA release inside cells due to the disruption of endosomal membranes.
Figure 8.
First generation of pH-sensitive polycationic amphiphiles.
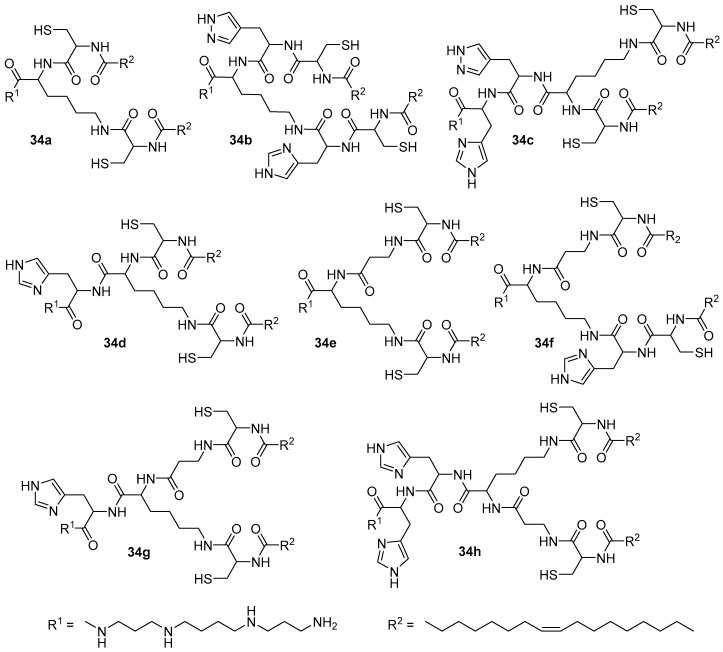
The second generation of pH-sensitive amphiphiles 34a–h based on spermine was subsequently obtained (Figure 9). In biological tests conducted on HeLa and U87 cells, the presence of an l-histidine in the amphiphile structure did not improve TA. In addition, no relationship was found between the efficiency of CAs and the distance between hydrophobic domains. Compound 34e exhibited the highest activity in the delivery of pDNA [55], while amphiphile 34f exhibited the highest activity in the delivery of siRNA [56,57]. The authors also noted that they did not utilize helper lipids in complex formation since the synthesized compounds were able to initiate a pH-dependent phase transition, which led to the destabilization of the complexes and the release of NAs.
Figure 9.
Second generation of pH-sensitive polycationic amphiphiles.
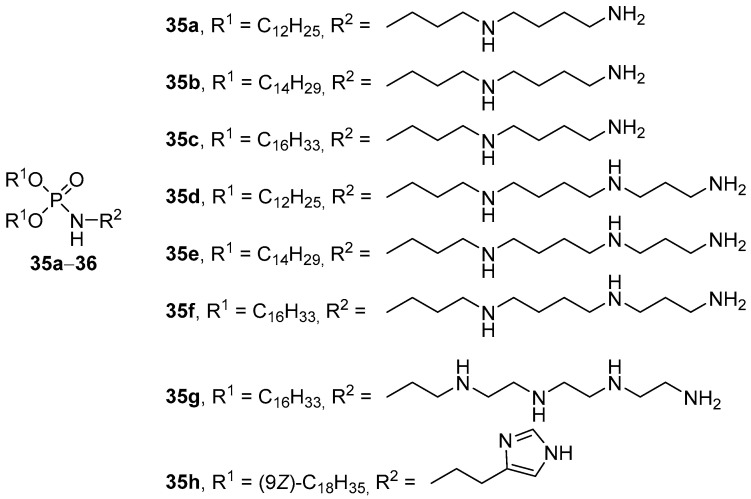
Multiple phosphamide derivatives containing long-chain alkyl substituents (dodecyl, tetradecyl, and hexadecyl) were obtained as hydrophobic fragments [58]. The transfection of COS-1 cells with lipoplexes formed by pDNA and micelles or liposomes based on amphiphiles 35a–d (Figure 10) showed that complexes based on micelles were only half as effective as complexes based on CA liposomes/lipid helper/Chol (1:1:1 mol.). DOPE and dipalmitoyl phosphatidylcholine (DPPC) have been used as helper lipids, but DPPC-containing liposomes have proven to be an ineffective delivery vehicle. TA on LLC and B16BL6 cells increased with an increase in the length of the alkyl chains and the number of amino groups in the polyamine. For LLC cells, the best compound was 35f based on spermine, and for B16BL6 cells, the best compound was amphiphile 35c based on spermidine.
Figure 10.
Phosphamide derivatives of polyamines.
An analog of compounds 35c and 35f was obtained based on a synthetic polyamine–tetraethylenepentamine (35g, Figure 10) [59]. Liposomes 35g/DOPE/DPPC/Chol (0.25:1:0.75:1 mol) efficiently delivered antisense oligonucleotides to eukaryotic cells. Here, the introduction of lipophilic derivatives of polyethylene glycol (PEG) and a cyclic analog of the peptide RGD ensured active targeting of liposomes to target cells and increased the efficiency of NA delivery [60,61].
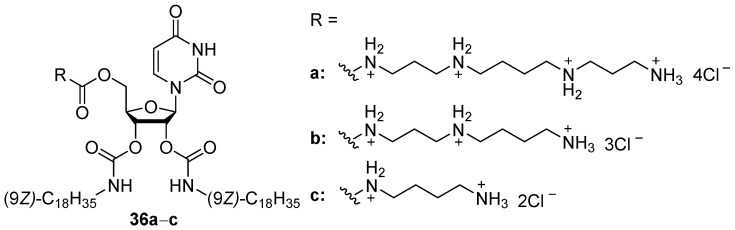
Cationic nucleoside amphiphiles may also be used for gene delivery. Thus, low-toxic uridine derivatives of various polyamines (36a–c, Figure 11) were synthesized and used for siRNA delivery. Their TA on HeLa cells is almost equal to that of Lipofectamine 2000 but was not affected by polyamine residue [62]. Notably, replacement of polyamine residue with L-arginine gave the same results, while l-lysine decreased TA [63].
Figure 11.
Cationic nucleoside amphiphiles.
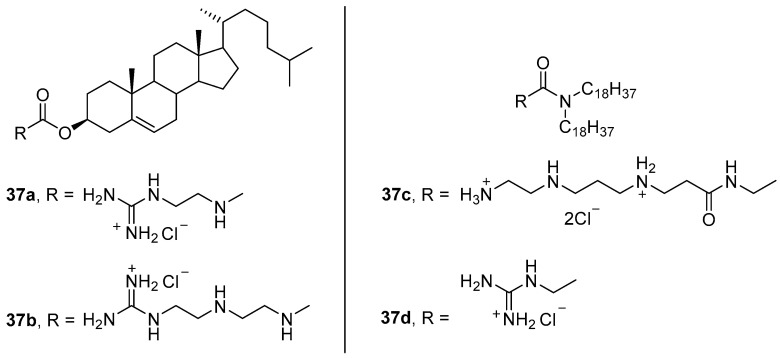
Amphiphiles 37a–d (Figure 12), in which the polyamine was bound to the hydrophobic domain via carbamoyl or amide linkers, formed liposomes with DOPE or compound 35h (Figure 11) and were used to deliver pDNA [64]. Protonation of the imidazolium residue of amphiphile 35h during endosomal acidification can induce rupture of the endosomal membrane and favors NA release [65,66]. Transfection of OVCAR-3, IGROV-1, and HeLa cells with complexes formed at different N/P ratios (4:1–12:1) showed that 37c/DOPE liposomes provided efficient pDNA delivery exceeding that of the commercial transfectant Lipofectamine 2000. Relative TA decreased in the series 37c > 37b > 37a >> 37d. It should also be noted that the use of amphiphile 36 as a helper lipid did not increase TA but did increase the cytotoxicity of lipoplexes.
Figure 12.
Amphiphiles based on polyamines and cholesterol.
In vivo delivery of pDNA by sterically stabilized liposomes 37c/DOPE/PEG4600-Chol (43:43:14 mol.) in a 4:1 (wt) ratio with pDNA led to a 33-fold increase in protein expression relative to unprotected DNA [67].
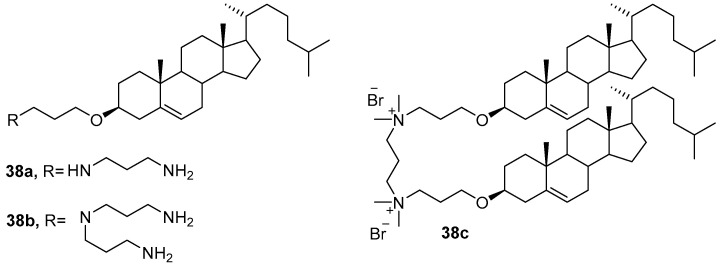
New CAs 38a–c (Figure 13), in which the cationic domain was linked to the cholesterol residue via an ether bond [68], formed liposomes with DOPE and were used for transfection of AGS and Huh-7 cells. The 38a/DOPE liposomes more efficiently delivered pDNA into AGS cells, while the 38b/DOPE liposomes provided effective transfection of Huh-7 cells. In both cases, their TA exceeded that of commercial transfectants [69]. Liposomes with dimeric gemini-amphiphile 38c also outperformed commercial agents in the transfection of COS-7 and Huh-7 cells [70].
Figure 13.
Ether-linked cationic amphiphiles.
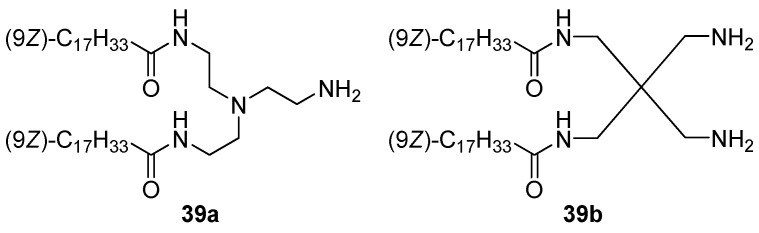
CAs 39a,b with different dicationic domains (Figure 14) formed liposomes, which facilitated the transport of siRNA into MB49 and K562 cells, while amphiphile 39a was superior in TA to amphiphile 39b [71].
Figure 14.
Branched cationic amphiphiles.
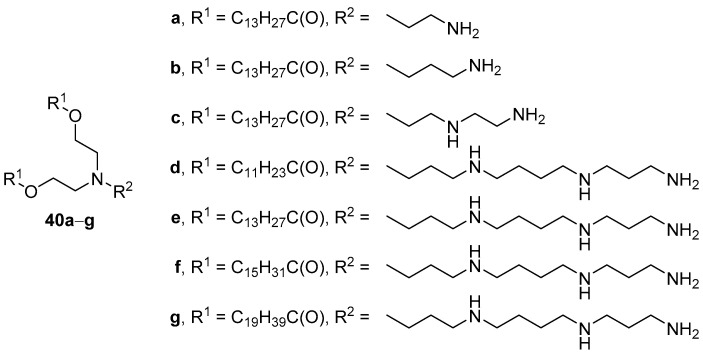
Comparing the TA of CAs that contained various polyamines in their structure (Figure 15) revealed that CLs composed of both phosphatidylcholine (Phospholipon 90G) and compounds 40d–g containing spermine (5:1 mol.) could deliver pDNA to HeLa cells, while other CLs showed no transfection [72]. Moreover, CAs with a shorter chain length of acyl substituents exhibited lower TA.
Figure 15.
CAs based on various polyamines.
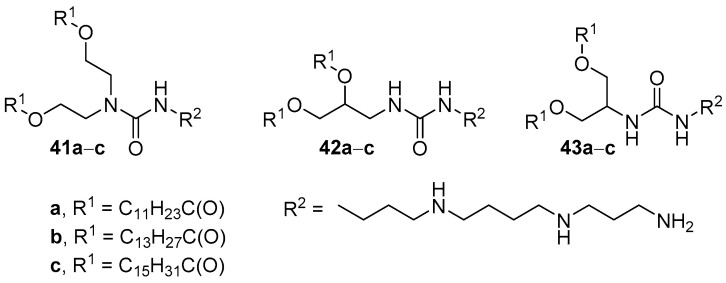
Analogs of compounds 40d–f based on spermine (compounds 41a–c, 42a–c, 43a–c) were obtained to study the influence of the structure core on TA (Figure 16) [73]. The efficiency of pDNA delivery mediated by liposomes based on DOPE and amphiphiles 42b,c, and 43a (1:1, weight ratio) was higher or comparable to that of Lipofectamine 2000. Moreover, unlike the other formulations, liposomes based on 43a with a core of 2-amino-1,3-propanediol retained their efficiency in the presence of serum. Investigation of the effect of hydrophobic domains on transfection revealed that myristoyl residues provided more effective TA.
Figure 16.
Spermine-based CAs with different cores.
A library of CAs (more than 1200 compounds) was developed using combinatorial chemistry methodology, in which both the hydrophobic (the length of the alkyl chain, the type of linker, and the presence of additional functional groups) and the cationic (the number of amino groups, the presence of cycles, and other functional groups) domains varied [74]. The results of in vitro experiments on HeLa cells revealed the following relationships: (1) TA increased in the presence of either two long-chain or several shorter alkyl substituents linked by an amide bond to the cationic domain (the optimal length was 8–12 carbon atoms); (2) high TA was achieved by compounds with two or more amino groups in the cationic domain, with TETA offering the best option; (3) the presence of a secondary amino group in the cationic domain positively affected TA (Figure 17).
Figure 17.
A combinatorial library of CAs.
Based on these findings, multiple CAs were selected for extended biological studies, which showed that the efficiency of NA delivery to primary macrophages exceeded that of commercial transfectants. In contrast, the transfection of HeLa or HepG2 cells by the selected amphiphiles was poor.
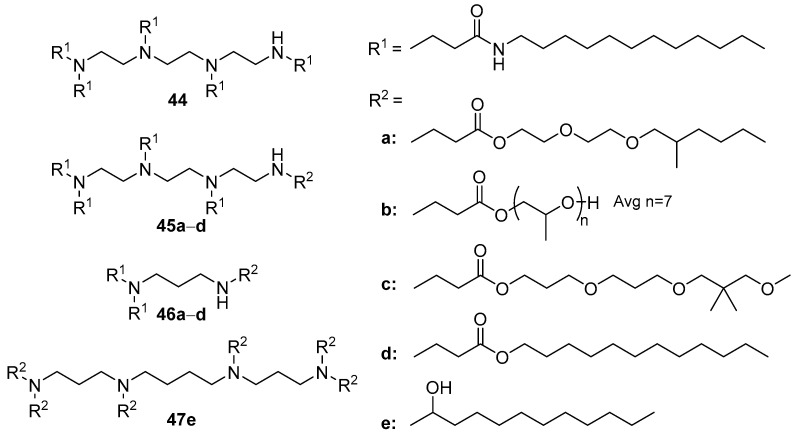
According to the results of in vitro tests, the 17 most effective compounds for in vivo siRNA delivery (siFVII and siApoB, which suppress the expression of blood coagulation factor VII and apolipoprotein B, respectively), were selected. For this, liposomes containing CA/PEG-lipid (mPEG2000-palmitoylceramide or mPEG2000-dimyristoylglycerol)/Chol (42:10:48 mol.) were formed. These CAs commonly contained various diamines or TETA. The most effective formulation (achieving more than 90% suppression of target gene expression) was based on compound 44 with TETA (Figure 17). The delivery of siRNAs in the lungs and macrophages of mice and macaque liver cells using these liposomes also significantly suppressed the target genes [74].
Subsequently, the library of compounds was expanded by synthesizing amphiphiles 45а–d and 46а–d with various hydrophobic domains (TETA and propylenediamine were chosen as cationic domains). Of these, the most effective were amphiphiles with a hydroxyl group in the hydrophobic domain, while the domain itself was bound to the polyamine with an ether or amide linker [75]. The hydrophobic domains based on oligoethylene glycol or octadecyl substituents led to an absence of TA. It should be noted that CAs 45c, 46a, and 46d were more effective than amphiphile 44 in vitro; however, they were inferior to amphiphile 44 in siRNA delivery in vivo.
In vitro screening of amphiphilic derivatives of spermine, spermidine, putrescine, and cadaverine showed that spermine derivative 47e facilitated the more efficient delivery of siRNA into human hepatocellular carcinoma Huh-7 cells [76]. Furthermore, liposomes 47e/Chol/DSPC/mPEG2000-palmitoylceramide/galactosylceramide delivered siRNA in vivo, which led to a significant decrease in hepatitis C virus replication in the hepatocyte cells of mice [76].
2.2. Cationic Amphiphiles Based on Cyclic Polyamines
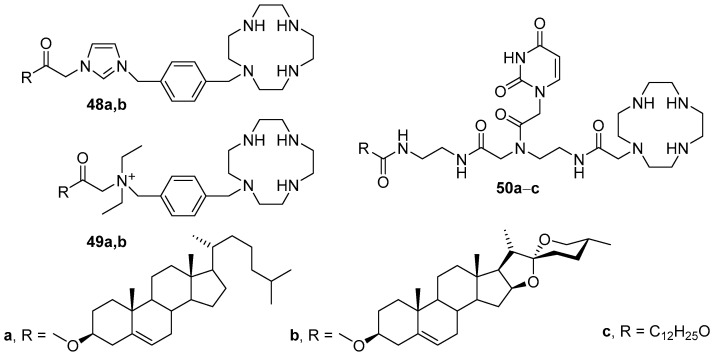
Lipophilic derivatives of macrocyclic polyamines can be used as liposomal components. Such compounds are less prone to self-packing, which improves binding to NAs. Two new cyclen-based amphiphiles 48a,b (Figure 18), which differed in the type of hydrophobic domain (cholesterol or diosgenin), were synthesized [77]. The results of in vitro biological studies showed that liposomes formed by amphiphiles 48a,b and DOPE had a low cytotoxicity but, because they bound easily to serum proteins, were inferior to Lipofectamine 2000 in delivering NAs to cells. The introduction of the quaternary ammonium group into the structure of the amphiphile (amphiphiles 49a,b) did not increase the TA; the liposomes remained inferior to Lipofectamine 2000 [78]. However, amphiphile 49b, which contained diosgenin as a hydrophobic domain, was more effective but also more toxic than amphiphile 49a, which contained cholesterol.
Figure 18.
Cyclen-based amphiphiles.
In order to increase the TA of liposomes, compounds 50a–c were synthesized (Figure 18), in which the cyclene and the hydrophobic domain were arranged by a peptide–nucleoside spacer [79]. Amphiphile 50b with a diosgenin residue (in liposomal composition with DOPE) demonstrated the highest efficiency, exceeding that of Lipofectamine 2000.
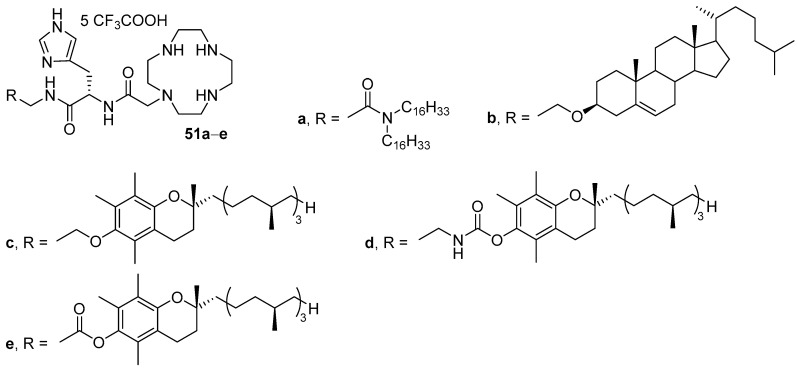
In the structure of CAs 51a–e, hydrophobic domains were linked to the cyclen using the l-histidine residue (Figure 19), as well as additional linkers (amide, ether, carbamoyl, and ester) [80]. All lipoplexes formed by amphiphiles 51a–e, DOPE and NAs were less toxic than Lipofectamine 2000, and amphiphile 51a with hexadecyl substituents demonstrated the lowest toxicity. The results of transfection studies revealed that only amphiphiles 51c–e, containing a tocopherol residue as a hydrophobic domain, were more effective than Lipofectamine 2000. Compound 51e with an ester linker exhibited the highest TA.
Figure 19.
Cyclen-based amphiphiles with l-histidine backbone.
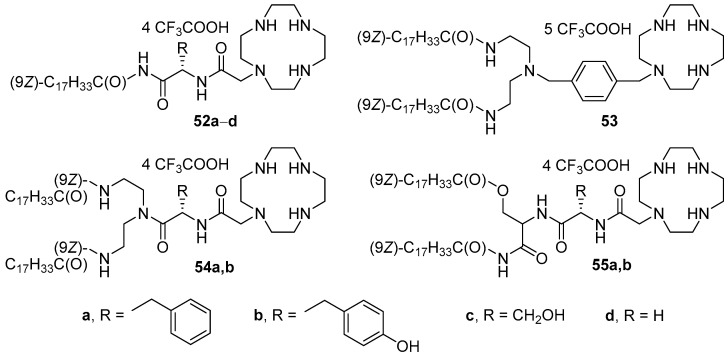
Cyclen derivatives containing oleoyl residues as a hydrophobic domain and amino acids (l-phenylalanine, l-tyrosine, l-serine, or glycine) as a backbone (Figure 20) were developed [81]. Transfection of HEK293 cells showed that liposomes based on DOPE and compounds 52a–d with one fatty acid residue (1:1 mol.) exhibited no TA. Among amphiphiles with two long-chain substituents, the compounds with phenyl (53) or phenylalanyl (55a) spacers demonstrated the highest activity (which was nevertheless lower than that of Lipofectamine 2000). Moreover, analogs with saturated hydrocarbon residues of various lengths were obtained based on amphiphile 53 (structures not shown). Among them, an amphiphile with tetradecyl substituents was the most effective, comparable to Lipofectamine 2000.
Figure 20.
Cyclen-based amphiphiles with different amino acid backbones.
2.3. Cationic Amphiphiles Based on Amino Acids
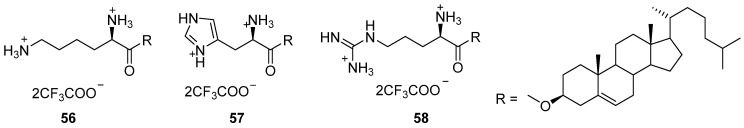
Amino acids are often employed as the hydrophilic domains of CAs, as they are natural compounds and convenient building blocks for creating a positive charge in molecules. To identify new compounds for CL formulation, a number of amphiphiles 56–58 (Figure 21) based on l-lysine, l-histidine, and l-arginine was synthesized and further used with DOPE (1:1 mol.) for liposomal preparations. Their efficiency of NA delivery in the presence of serum was studied [82].
Figure 21.
CAs based on amino acids and cholesterol containing no spacers.
TA is known to decrease in the presence of serum due to the interaction of negatively charged proteins with CAs, which inhibits NA delivery. The average size of lipoplexes formed by amphiphiles 56–58 increased in the presence of serum from 180 nm to 828 nm for amphiphile 56, up to 1710 nm for amphiphile 57, and up to 2345 nm for amphiphile 58. Amphiphile 56 was able to transfect eukaryotic cells at serum concentrations up to 40%. The TA of lipoplexes containing amphiphiles 56–58 was 2.8–3.5-fold higher than that of the commercial transfectant DOTAP (Roche Life Technologies, Switzerland), while amphiphile 57, with an L-histidine residue, exhibited the lowest activity.
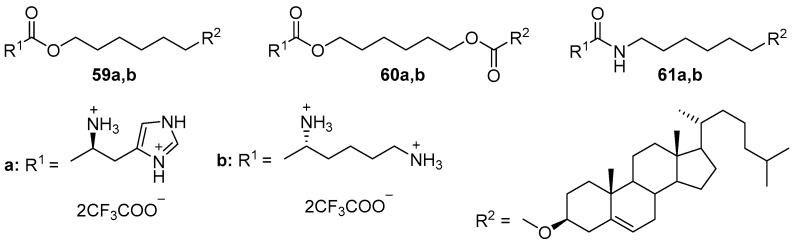
In the structure of CAs 59a,b and 60a,b (Figure 22), cholesterol was bound to l-lysine or l-histidine by various linker groups [83]. The best transfection of COS-7 cells was provided by amphiphiles 59b and 60b with l-lysine, which, however, demonstrated greater cytotoxicity than amphiphiles 59a and 60a containing l-histidine. The authors proposed that this greater toxicity was induced by the damaging electrostatic interaction of l-lysine amino groups with the negatively charged plasma membrane. Replacing the ester linker with an amide one (amphiphiles 61a,b) reduced the toxicity of the compounds. The l-lysine derivative 61b was still more effective than amphiphile 61a based on l-histidine [84].
Figure 22.
CAs based on amino acids and cholesterol with various linkers.
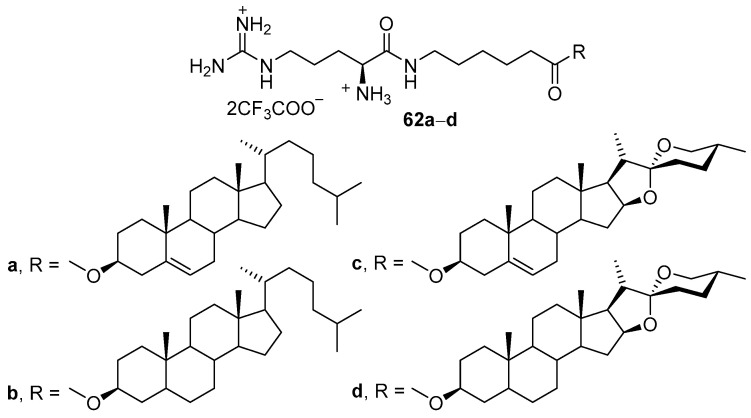
Amphiphiles 62a–d are based on l-arginine and certain sterols linked by amide and ester bonds (Figure 23) [85]. Cholesterol-based compound 62а exhibited the highest TA, forming lipoplexes with a particle size of approximately 100 nm. The size of lipoplexes formed by amphiphiles 62c,d was 200–300 nm, and they poorly penetrated cells. It should be noted that none of the amphiphiles 62a–d was able to outperform Lipofectamine 2000 in the presence of serum.
Figure 23.
CAs based on l-arginine and various sterols.
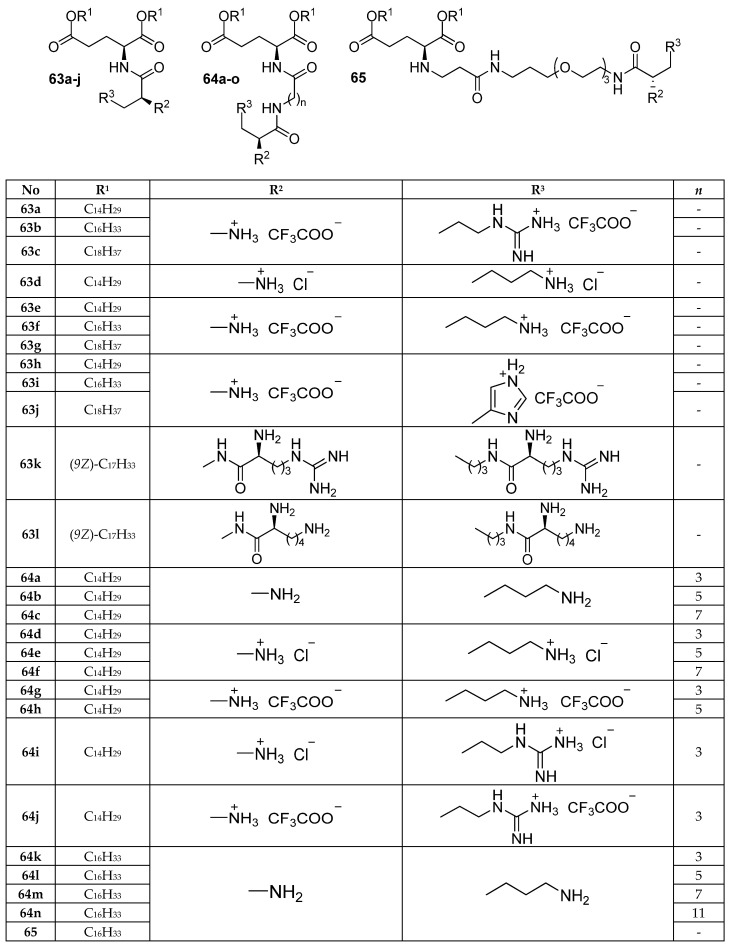
CAs 63a–c and 63e–j (Figure 24) were synthesized based on l-arginine, l-lysine, and l-histidine [86]. The efficiency of cell transfection decreased with an increase in the length of the alkyl chains, while the maximum efficiency was provided by amphiphiles 63a and 63e with tetradecyl substituents. The type of the cationic group insignificantly affected TA in the absence of serum. However, amphiphiles 63a–c based on L-arginine demonstrated the most efficient NA delivery in the presence of serum.
Figure 24.
CAs based on l-arginine, l-lysine, and l-histidine.
An increase in the number of positively charged groups in amphiphiles 63k,l provided an increase in the efficiency of pDNA delivery to HepG2 cells. Moreover, the resulting TA was superior to that of both Lipofectamine 2000 and polyethyleneimine [87]. For HEK293T cells, amphiphile 63k based on l-arginine was comparable to commercial transfectants, while the l-lysine derivative 63l demonstrated inferior efficiency. The results of pDNA delivery in vivo showed that CA 63l was 2.5-fold more effective than polyethyleneimine, while 63k was almost 10-fold more effective.
Low-toxicity lysine-containing CAs 63d, 64a–h (Figure 24) were synthesized to study the effect of spacer length and the type of counterion on TA [88]. CLs formed by DOPE and trifluoroacetates 64g and 64h delivered NAs more efficiently than other CAs, while CLs containing uncharged amphiphiles 64a,b demonstrated the lowest activity. An increase in the spacer length in CAs from 0 to 7 methylene units increased TA in both the presence and absence of serum, and all amphiphiles studied were more effective than Lipofectamine 2000 under both conditions.
A similar effect of spacer length on TA was observed in the amphiphile series 63f, 64k–m, and 65 based on l-lysine (Figure 24) [89]. Lipoplexes with amphiphile 63f, the structure of which lacks a spacer, were unable to deliver NA, but as the spacer length increased from 3 to 7 methylene units, TA increased. Notably, amphiphiles 64k–m with a hydrophobic oligomethylene spacer functioned as more active transfectants than amphiphiles 65 containing a hydrophilic oxyethylene spacer.
Amphiphiles 64d,g and 64i,j based on l-lysine and l-arginine (Figure 24) were studied to evaluate TA in PC-12, HeLa, and neuronal cells [90]. Lipoplexes containing amphiphiles 64i,j were more effective than both lipoplexes containing amphiphiles 64d,g and the commercial transfectant Lipofectamine 2000.
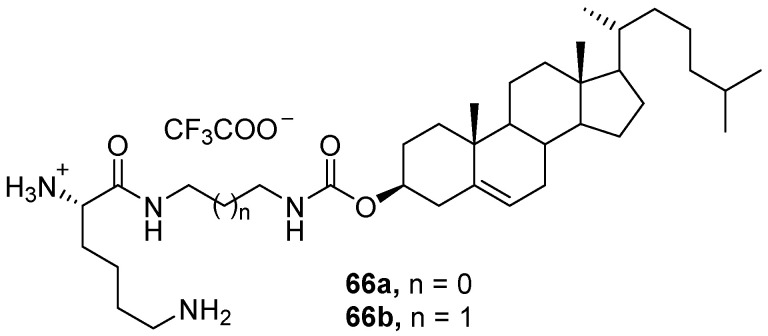
Cholesterol-containing CAs 66a,b (Figure 25) were recently developed based on l-lysine and diamines [91]. When transfecting HEK293, HeLa, PC-3, and HC-04 cells, the efficacy of liposomes 66b/DOPE (1:1, mol.) was comparable to that of Lipofectamine 2000, even in the presence of serum. Moreover, the toxicity of 66b/DOPE was significantly lower than that of Lipofectamine 2000.
Figure 25.
CAs based on l-lysine and diamines.
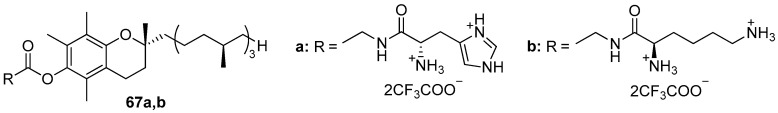
Polycationic amphiphiles 67а,b (Figure 26) consisting of the dipeptides glycylhistidine and glycyllysine as well as a tocopherol residue were synthesized [92]. While the TA of 67b/DOPE liposomes containing a lysine residue was higher on HEK293 and HeLa cells than that of 67a/DOPE liposomes containing a histidine residue, the 67b/DOPE liposomes did not achieve the results of Lipofectamine 2000. However, the complexes were more resistant to serum activity than was the commercial transfectant.
Figure 26.
CAs based on dipeptides.
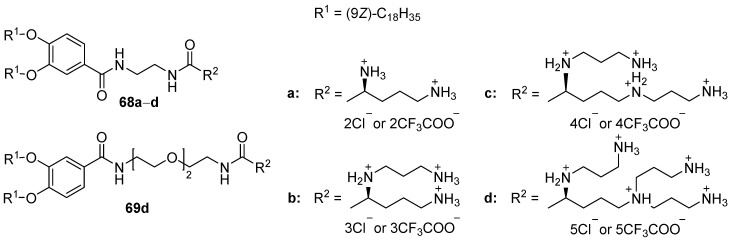
Polycationic amphiphiles 68a–d and 69d (Figure 27) were synthesized based on l -ornithine [93,94]. Liposomes CA/DOPC and complexes with pDNA were formed using different component ratios. The resulting efficiency of cell transfection, which was higher than for the commercial transfectant DOTAP (Avanti Polar Lipids, Alabaster, AL, USA), depended on the ratio of lipids used in the complex. Amphiphile 68d with three aminoethyl substituents exhibited the highest activity and was also used to deliver siRNA, which led to the specific suppression of the target gene [95].
Figure 27.
CAs based on L-ornithine.
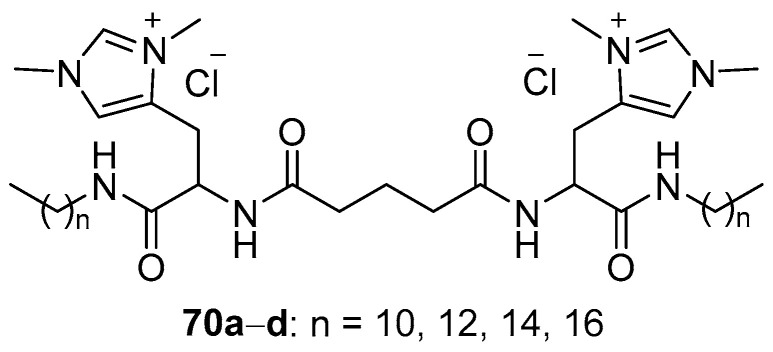
Amino acids are used to develop not only classical head-to-tail amphiphiles but also symmetric gemini-amphiphiles, as seen in the l -histidine derivatives 70a–d (Figure 28), which contain two alkyl substituents 10 to 16 carbon atoms in length [96,97]. CLs were formed with derivatives obtained and DOPE. With the exception of 70a with decyl residues, all CLs bound pDNA well. However, their TA was comparable to or lower than that of Lipofectamine 2000 [96].
Figure 28.
Gemini-amphiphiles based on L-histidine.
2.4. Disulfide Cationic Amphiphiles
Several special cell proteins and peptides (for example, glutathione and reductases) can reduce the disulfide bond [98]. Disruption of the disulfide bond in the CA molecule, in turn, can increase the degree of NA release from the lipoplex in the cell and thereby increase TA.
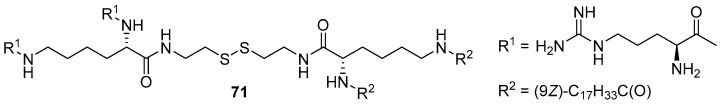
Disulfide amphiphile 71, based on l-lysine and l-arginine (Figure 29), formed CLs capable of transfecting HeLa and B16 cells in the absence or presence of serum, and its efficiency exceeded that of its analog lacking a disulfide bond [99]. In vivo studies on the delivery of the luciferase gene to xenograft mice showed a high expression of the reporter protein in isolated tumors in the case of CA 71, which exceeded that of the amphiphile without a disulfide bond (structure not shown) or polyethyleneimine.
Figure 29.
Disulfide amphiphiles based on l-lysine and l-arginine.
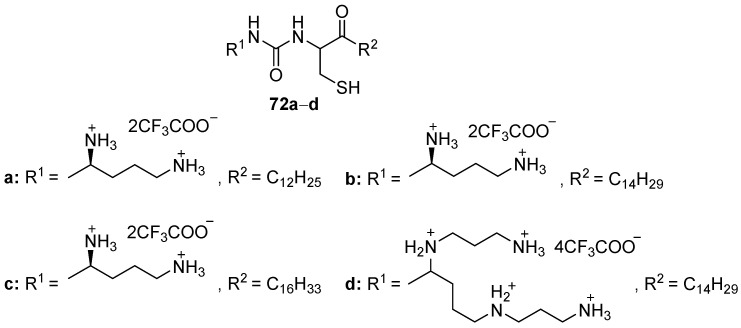
During the oxidation of thiol groups to disulfide groups, lipophilic thio compounds 72a–d (Figure 30) based on l-ornithine or spermine compacted pDNA molecules into small micellar complexes [100]. Their TA in 3T3 cells increased with increasing alkyl substituents. Spermine-based CA 72d was less effective than its ornithine-based analog 72b.
Figure 30.
Disulfide amphiphiles based on l-ornithine or spermine.
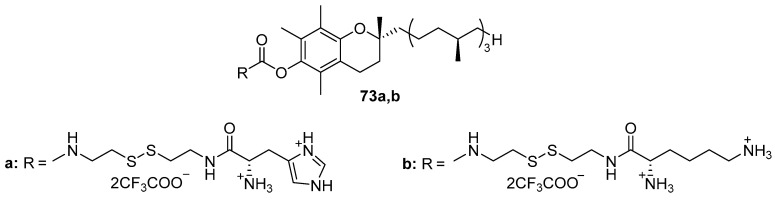
Disulfide amphiphiles 73a,b (Figure 31) containing an l-histidine or l-lysine residue were obtained based on tocopherol [101]. Liposomes CA/DOPC (1:1 mol) formed complexes with pDNA at various N/P ratios (from 1 to 8) with sizes ranging from 150 to 190 nm. The TA of liposomes 73b was lower than that of liposomes based on l-histidine derivative 73a and the commercial transfectant Lipofectamine 2000.
Figure 31.
Disulfide amphiphiles based on tocopherol.
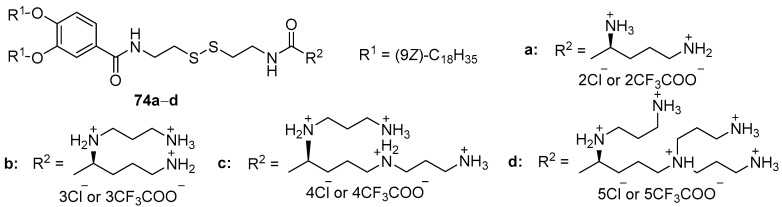
The introduction of a disulfide bond into the amphiphile structure also makes it possible to significantly reduce CAs toxicity while maintaining a comparable level of TA. CA/DOPC liposomes, based on biodegradable CAs 74a–d (Figure 32) with a disulfide bond, differed in the number of amino groups in the polyamine matrix had significantly lower toxicity (except for amphiphile 74a) than their analogs 68a–d, which did not contain a disulfide bond [102]. Moreover, compounds 74c and 74d demonstrated comparable or superior TA to amphiphile 68d. Thus, an increase in the number of amino groups in CAs increased TA, while the introduction of a disulfide group decreased the toxicity of lipoplexes, allowing for the use of higher CA dosages.
Figure 32.
Disulfide amphiphiles based on different polyamines.
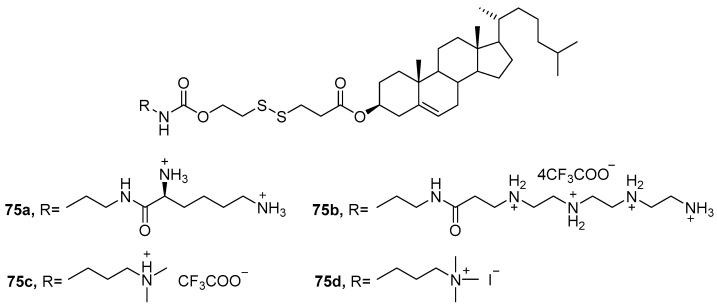
Biological testing on COS-7 cells of disulfide amphiphiles 75a,b (Figure 33), based on polyamines and amino acids [103], revealed low toxicity and rather high TA comparable to monocationic analogs 75c,d. Moreover, only amphiphile 75a retained its activity in the presence of serum.
Figure 33.
Disulfide amphiphiles based on polyamines, amino acids, and cholesterol.
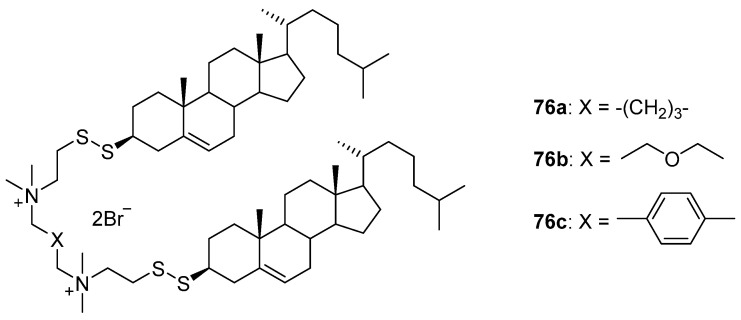
Synthesized gemini-amphiphiles 76a–c (Figure 34), which contained disulfide groups between the cationic and hydrophobic domains, differed in the spacer connecting the quaternary ammonium groups [104]. Liposomes 76a/DOPE and 76c/DOPE efficiently transfected HeLa and HT1080 cells. For PC3AR cells, liposomes 76b/DOPE proved to be the most efficient and were also capable of delivering NAs to difficult-to-transfect HaCaT cells.
Figure 34.
Disulfide gemini-amphiphiles with different spacers.
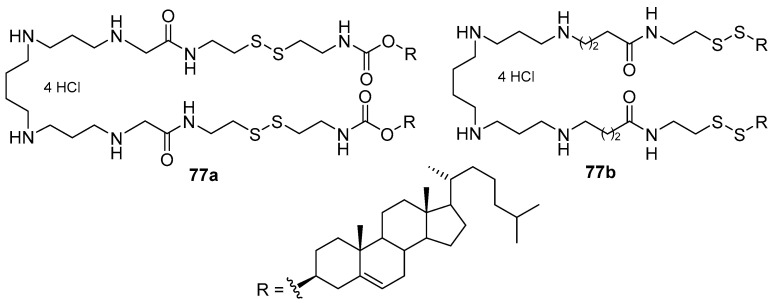
Disulfide polycationic amphiphiles 77a,b (Figure 35) were based on spermine and cholesterol. The disulfide group was located either in the spacer structure or as a linker connecting the cationic and hydrophobic domains [105,106]. CLs were formed with these amphiphiles and DOPE. The position of the disulfide group in the CA molecule affected both the sensitivity of the obtained liposomes to the action of reducing agents and their TA. Thus, introducing a disulfide group into the spacer structure (77a) led to a higher liposome sensitivity and more efficient siRNA delivery than that achieved by CA 10c (Figure 6), which was not sensitive to reduction. CA 77b with a disulfide group as a linker was less sensitive to reduction and delivered pDNA more efficiently than did its analogs [107].
Figure 35.
Disulfide polycationic amphiphiles based on spermine and cholesterol.
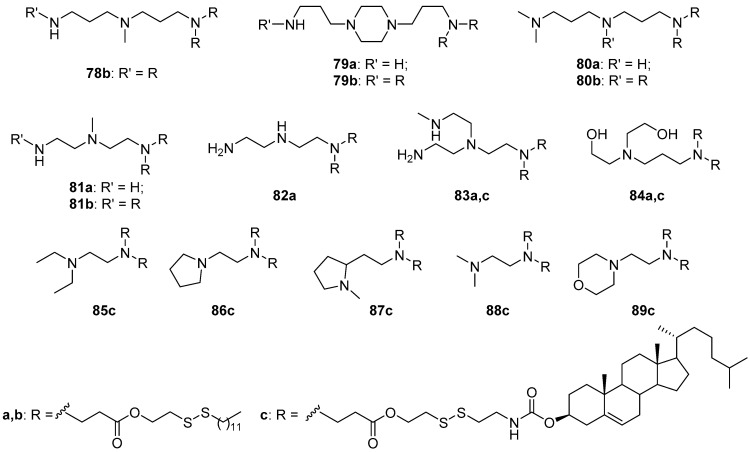
A library of disulfide CAs 78–89 (Figure 36) with various cationic domains was created. The first series of compounds, 79a–84a, contained a hydrophobic hexadecyl chain, including sulfur atoms [108]. CLs composed of disulfide CAs, cholesterol, DOPE and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (16:4:1:1, weight ratio) were used to deliver ribonucleoprotein complexes to GFP-HEK cells. Compounds 80a, 81a, 83a, 84a had the highest efficacy, comparable to or exceeding that of Lipofectamine 2000. An increase in the number of hydrophobic domains to three (78b–81b) increased the delivery efficiency of ribonucleoprotein complexes to GFP-HEK cells [109] and human mesenchymal stem cells [110]. Notably, liposomes based on compound 79a with two hydrophobic domains had practically no TA, while the use of an analog (79b) with three hydrophobic domains made it possible to transfect stem cells more efficiently than was possible with the commercial transfectant Lipofectamine 2000. An increase in the number of hydrophobic domains to four negatively affected TA regardless of the cationic domain. A study of the effect of the length of the hydrophobic domain demonstrated that shortening the chain length to 12 atoms significantly impaired TA, while including 14–18 atoms in the chain provided no efficiency difference [110].
Figure 36.
A library of disulfide CAs with various cationic domains.
Subsequently, the library of disulfide derivatives was expanded using a cholesterol residue in the structure of the hydrophobic domain, which reduced cytotoxicity relative to that of long-chain analogs 79a–84a [111]. Compounds 85c, 87c, 88c were highly efficient in delivering mRNA to all four investigated cell lines (B16F10, HEK, HeLa, NIH 3T3). Compound 86c was effective on all cells except NIH 3T3. The results of all four compounds were comparable or slightly inferior to that of Lipofectamine 2000. Compounds 84c and 89c demonstrated extremely low aggregation stability and delivery efficiency.
2.5. Influence of Structural Components of Cationic Amphiphiles on the Efficiency of Nucleic Acid Delivery
Each element of the CA structure performs a specific function and influences the TA. Hydrophobic domains are involved in the protection of NAs and promote the fusion of lipoplexes with cell membranes. Aliphatic hydrocarbon substituents usually represent these domains with a length of 10 to 18 carbon atoms, tocopherol, or sterols. The type of hydrophobic domain determines both the structure of the vesicles that a CA forms in the aqueous phase and its subsequent interaction with biological membranes. Liposomal formulations promote more efficient NA delivery than that accomplished by micelles or other types of nanoparticles [58]. Mostly, CAs used for transfection of eukaryotic cells are classic head-to-tail amphiphiles. Based on polyamines and amino acids, CAs synthesized with two hydrophobic domains (gemini-amphiphiles) can deliver NAs more efficiently than can their monosubstituted analogs [39,42,94,95].
An increase in the length of aliphatic hydrocarbon chains usually increases TA [32,35,40,53,58,88,89]. Notably, however, it may also increase the toxicity of compounds [40]. In contrast, for some spermine-based CAs, TA decreased with an increase in the length of aliphatic substituents [70,84]. Analysis of published data reveals that the optimal length of aliphatic substituents is 14–18 carbon atoms, while high TA is most often noted for CAs with myristoyl or tetradecyl substituents [71,79,84]. The degree of unsaturation of substituents also affects the efficiency of NA delivery: with an increase in unsaturation, TA increases but so does toxicity [20]. Thus, it is necessary to search for an optimal CA variant that effectively delivers NAs, while its toxicity remains within acceptable limits.
When sterol derivatives are used as hydrophobic domains, one should prefer natural compounds, which do not cause significant toxicity. In this case, it is optimal to use a common and widely available sterol such as cholesterol [38,78,85], although diosgenin derivatives are also capable of efficient NA delivery [75,76,77,83]. Tocopherol is also employed as a hydrophobic domain [80,92,101].
The positively charged CA domain is responsible for the electrostatic interactions of amphiphiles and/or liposomes with NAs, the formation of stable lipoplexes, and the interaction of complexes with cell membranes. An increase in the number of amino groups in the structure of the cationic domain leads to an increase in the TA [31,56,60,81,82,85]. The most effective are CAs with domains based on polyamines, with the number (more than two [74]) and distribution of amino groups in the polyamine chain [31,53,93,102] playing important roles. Many studies reveal that the most effective are polyamines with four amino groups, primarily a natural polyamine—spermine [58,72,76]. However, CAs based on synthetic polyamines (TETA, triethylenepentamine, tributylenepentamine) can be more efficient in delivering NAs than their natural counterparts [31,59,74]. The cationic domain can also be designed based on cyclen [77,78,79,80,81] or amino acids. In this case, it is advisable to use the residues of L-arginine or l-lysine [82,83,84,86,90,92] but not l-histidine, the presence of which in the CA structure did not improve the NA delivery [84].
Linkers, connecting hydrophobic and cationic domains, determine the stability and biocompatibility of the CAs and play a key role in the efficiency of NA delivery. The most commonly used are ether, ester, carbamate, amide, and disulfide linkers. Among the most effective linkers imparting low toxicity to CAs are carbamoyl ones [42,43,52,87]. Efficient delivery of NAs is also observed with the presence of ether bonds in the CA structure [68,82]. The introduction of a disulfide linker into a CA molecule makes it sensitive to the action of intracellular reducing agents, which can increase the efficiency of NA delivery and/or reduce the toxicity of compounds [101,102,104].
Multiple studies have proven that a close arrangement of the hydrophobic and cationic domains complicates both domain’s functioning and interferes with the formation of liposomes. The introduction of a spacer into the CA structure and an increase in its length increase NA delivery efficiency [42,50,88,89,91].
Low cytotoxicity is an important feature of CLs. First generation of CAs containing quaternary ammonium head was rather toxic, but numerous recently developed polycationic amphiphiles provided non-toxic transfection [8]. As mentioned above, toxicity may be increased with the length and degree of unsaturation of hydrophobic tails. In sterol derivatives use of diosgenin may cause toxicity [77,85] probably due to the inhibition of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. Linkers may also affect toxicity. Thus, the replacement of the ester linker with an amide one reduced the toxicity of the L-lysine based CAs [84]. Also, it should be noted that direct binding of both hydrophobic and cationic domains leads to a significant increase in toxicity of the CAs [35].
3. Conclusions and Perspectives
In this review, we focused on the cationic amphiphiles, which are the major but not sole component of CLs. Development of additional components, such as targeting [16] or stealth [17] lipids, is an actual trend in non-viral gene delivery.
Positive charge of CLs provides an effective binding and protection of NAs during transfection but also attracts serum proteins and other components during in vivo administration. Interaction with serum components may cause fast blood clearance of lipoplexes. Therefore, searching for possibilities to mask excessive CLs positive charge is required. One of such ways may be a use of PEG-lipids, which, however, decrease both lipoplex and NAs internalization into the cell. Alternatively, a coating of CLs or lipoplexes with additional lipid (polymer) shell may be also considered.
Sophistication of liposomal formulations turns its manufacturing procedure into novel challenges including scale-up and quality control stages. A possible solution to overcome existing difficulties might be a shift from the single large-scale to the multiple parallel small-scale production. For this purpose, microfluidic technologies become very attractive due to their ability to provide continuous and reproducible liposome preparation. Recent progress in microfluidics allows to use this technique for production of rather complex nanoparticles containing biopolymers and even whole cells [112].
In conclusion, the development of effective and safe CLs for the delivery of therapeutic NAs requires employment of the right combination of structural elements in the CA molecule to promote the formation of both the liposomes and their complexes with NAs while avoiding interference and overcoming biological barriers. Once within the target cell, the complexes must release NAs with a high efficiency to provide biological/therapeutic effect.
Author Contributions
Conceptualization, P.A.P., M.A.M.; literature review, P.A.P.; writing the manuscript, P.A.P.; manuscript revision, M.A.M., and supervision, M.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation, Grant No. 0706-2020-0019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study design, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Behr J.P. Synthetic gene transfer vectors II: Back to the future. Acc. Chem. Res. 2012;45:980–984. doi: 10.1021/ar200213g. [DOI] [PubMed] [Google Scholar]
- 2.Räty J.K., Pikkarainen J.T., Wirth T., Ylä-Herttuala S. Gene therapy: The first approved gene-based medicines, molecular mechanisms and clinical indications. Curr. Mol. Pharmacol. 2008;1:13–23. doi: 10.2174/1874467210801010013. [DOI] [PubMed] [Google Scholar]
- 3.Wiethoff C.M., Middaugh C.R. Barriers to nonviral gene delivery. J. Pharm. Sci. 2003;92:203–217. doi: 10.1002/jps.10286. [DOI] [PubMed] [Google Scholar]
- 4.Gottfried L.F., Dean D.A. Extracellular and intracellular barriers to non-viral gene transfer. In: Wei M., editor. Novel Gene Therapy Approaches. InTech; Rijeka, Croatia: 2013. pp. 75–88. [Google Scholar]
- 5.Konishi M., Kawamoto K., Izumikawa M., Kuriyama H., Yamashita T. Gene transfer into guinea pig cochlea using adeno-associated virus vectors. J. Gene Med. 2008;10:610–618. doi: 10.1002/jgm.1189. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y., Huang L. Lipid nanoparticles for gene delivery. In: Huang L., Liu D., Wagner E., editors. Advances in Genetics. Volume 88. Academic Press Inc.; San Diego, CA, USA: 2014. pp. 13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pahle J., Walther W. Vectors and strategies for nonviral cancer gene therapy. Expert Opin. Biol. Ther. 2016;16:443–461. doi: 10.1517/14712598.2016.1134480. [DOI] [PubMed] [Google Scholar]
- 8.Junquera E., Aicart E. Recent progress in gene therapy to deliver nucleic acids with multivalent cationic vectors. Adv. Colloid Interface Sci. 2016;233:161–175. doi: 10.1016/j.cis.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Nordling-David M.M., Golomb G. Gene delivery by liposomes. Isr. J. Chem. 2013;53:737–747. doi: 10.1002/ijch.201300055. [DOI] [Google Scholar]
- 10.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines-a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., et al. Safety and immunogenicity of two RNA-Based Covid-19 vaccine candidates. N. Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikheev A.A., Shmendel E.V., Zhestovskaya E.S., Nazarov G.V., Maslov M.A. Сationic liposomes as delivery systems for nucleic acids. Fine Chem. Technol. 2020;15:7–27. doi: 10.32362/2410-6593-2020-15-1-7-27. [DOI] [Google Scholar]
- 13.Mochizuki S., Kanegae N., Nishina K., Kamikawa Y., Koiwai K., Masunaga H., Sakurai K. The role of the helper lipid dioleoylphosphatidylethanolamine (DOPE) for DNA transfection cooperating with a cationic lipid bearing ethylenediamine. Biochim. Biophys. Acta Biomembr. 2013;1828:412–418. doi: 10.1016/j.bbamem.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Cheng X., Lee R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 2016;99:129–137. doi: 10.1016/j.addr.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Zidovska A., Evans H.M., Ahmad A., Ewert K.K., Safinya C.R. The role of cholesterol and structurally related molecules in enhancing transfection of cationic liposome-DNA complexes. J. Phys. Chem. B. 2009;113:5208–5216. doi: 10.1021/jp809000e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaffer D.V., Lauffenburger D.A. Targeted synthetic gene delivery vectors. Curr. Opin. Mol. Ther. 2000;2:155–161. [PubMed] [Google Scholar]
- 17.Amoozgar Z., Yeo Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology. 2012;4:219–233. doi: 10.1002/wnan.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hattori Y., Nakamura M., Takeuchi N., Tamaki K., Shimizu S., Yoshiike Y., Taguchi M., Ohno H., Ozaki K.I., Onishi H. Effect of cationic lipid in cationic liposomes on siRNA delivery into the lung by intravenous injection of cationic lipoplex. J. Drug Target. 2019;27:217–227. doi: 10.1080/1061186X.2018.1502775. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Z., Yao W., Wang N., Liu C., Zhou H., Chen H., Qiao W. Synthesis and evaluation of mono- and multi-hydroxyl low toxicity pH-sensitive cationic lipids for drug delivery. Eur. J. Pharm. Sci. 2019;133:69–78. doi: 10.1016/j.ejps.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Maslov M.A., Syicheva E.V., Morozova N.G., Serebrennikova G.A. Cationic amphiphiles of both lipid and nonlipid nature in gene therapy. Russ. Chem. Bull. 2000;49:385–401. doi: 10.1007/BF02494765. [DOI] [Google Scholar]
- 21.Bofinger R., Zaw-Thin M., Mitchell N.J., Patrick P.S., Stowe C., Gomez-Ramirez A., Hailes H.C., Kalber T.L., Tabor A.B. Development of lipopolyplexes for gene delivery: A comparison of the effects of differing modes of targeting peptide display on the structure and transfection activities of lipopolyplexes. J. Pept. Sci. 2018;24:e3131. doi: 10.1002/psc.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni J.A., Myhre J.L., Chen S., Tam Y.Y.C., Danescu A., Richman J.M., Cullis P.R. Design of lipid nanoparticles for in vitro and in vivo delivery of plasmid DNA. Nanomed. Nanotechnol. Biol. Med. 2017;13:1377–1387. doi: 10.1016/j.nano.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Ju J., Huan M.L., Wan N., Hou Y.L., Ma X.X., Jia Y.Y., Li C., Zhou S.Y., Zhang B. Le Cholesterol derived cationic lipids as potential non-viral gene delivery vectors and their serum compatibility. Bioorganic Med. Chem. Lett. 2016;26:2401–2407. doi: 10.1016/j.bmcl.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Cui S.H., Zhi D.F., Zhao Y.N., Chen H.Y., Meng Y., Zhang C.M., Zhang S.B. Cationic lioposomes with folic acid as targeting ligand for gene delivery. Bioorganic Med. Chem. Lett. 2016;26:4025–4029. doi: 10.1016/j.bmcl.2016.06.085. [DOI] [PubMed] [Google Scholar]
- 25.Hiwale A.A., Voshavar C., Dharmalingam P., Dhayani A., Mukthavaram R., Nadella R., Sunnapu O., Gandhi S., Naidu V.G.M., Chaudhuri A., et al. Scaling the effect of hydrophobic chain length on gene transfer properties of di-alkyl, di-hydroxy ethylammonium chloride based cationic amphiphiles. RSC Adv. 2017;7:25398–25405. doi: 10.1039/C7RA02271A. [DOI] [Google Scholar]
- 26.Li B., Guo W., Zhang F., Liu M., Wang S., Liu Z., Xiang S., Zeng Y. Synthesis and evaluation of L-arabinose-based cationic glycolipids as effective vectors for pDNA and siRNA in vitro. PLoS ONE. 2017;12:e0180276. doi: 10.1371/journal.pone.0180276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berchel M., Akhter S., Berthe W., Gonçalves C., Dubuisson M., Pichon C., Jaffrès P.A., Midoux P. Synthesis of α-amino-lipophosphonates as cationic lipids or co-lipids for DNA transfection in dendritic cells. J. Mater. Chem. B. 2017;5:6869–6881. doi: 10.1039/C7TB01080J. [DOI] [PubMed] [Google Scholar]
- 28.Medvedeva D.A., Maslov M.A., Serikov R.N., Morozova N.G., Serebrenikova G.A., Sheglov D.V., Latyshev A.V., Vlassov V.V., Zenkova M.A. Novel cholesterol-based cationic lipids for gene delivery. J. Med. Chem. 2009;52:6558–6568. doi: 10.1021/jm901022t. [DOI] [PubMed] [Google Scholar]
- 29.Stewart L., Manvell M., Hillery E., Etheridge C.J., Cooper R.G., Stark H., Van-Heel M., Preuss M., Alton E.W.F.W., Miller A.D. Physico-chemical analysis of cationic liposome-DNA complexes (lipoplexes) with respect to in vitro and in vivo gene delivery efficiency. J. Chem. Soc. Perkin Trans. 2001;2:624–632. doi: 10.1039/b005992g. [DOI] [Google Scholar]
- 30.Seiler N., Delcros J.G., Moulinoux J.P. Polyamine transport in mammalian cells. An update. Int. J. Biochem. Cell Biol. 1996;28:843–861. doi: 10.1016/1357-2725(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 31.Gardner R.A., Belting M., Svensson K., Phanstiel O., Iv O.P. Synthesis and Transfection Efficiencies of New Lipophilic Polyamines. J. Med. Chem. 2007;50:308–318. doi: 10.1021/jm0607101. [DOI] [PubMed] [Google Scholar]
- 32.Gruneich J.A., Diamond S.L. Synthesis and structure-activity relationships of a series of increasingly hydrophobic cationic steroid lipofection reagents. J. Gene Med. 2007;9:381–391. doi: 10.1002/jgm.1024. [DOI] [PubMed] [Google Scholar]
- 33.Randazzo R.A.S., Bucki R., Janmey P.A., Diamond S.L. A series of cationic sterol lipids with gene transfer and bactericidal activity. Bioorganic Med. Chem. 2009;17:3257–3265. doi: 10.1016/j.bmc.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed O.A.A., Adjimatera N., Pourzand C., Blagbrough I.S. N4,N9-Dioleoyl Spermine Is a Novel Nonviral Lipopolyamine Vector for Plasmid DNA Formulation. Pharm. Res. 2005;22:972–980. doi: 10.1007/s11095-005-4592-1. [DOI] [PubMed] [Google Scholar]
- 35.Ghonaim H.M., Ahmed O.A.A., Pourzand C., Blagbrough I.S. Varying the chain length in N4, N9-diacyl spermines: Non-viral lipopolyamine vectors for efficient plasmid DNA formulation. Mol. Pharm. 2008;5:1111–1121. doi: 10.1021/mp800062j. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed O.A.A., Pourzand C., Blagbrough I.S. Varying the unsaturation in N4,N9-dioctadecanoyl spermines: Nonviral lipopolyamine vectors for more efficient plasmid DNA formulation. Pharm. Res. 2006;23:31–40. doi: 10.1007/s11095-005-8717-3. [DOI] [PubMed] [Google Scholar]
- 37.Soltan M.K., Ghonaim H.M., El Sadek M., Kull M.A., El-Aziz L.A., Blagbrough I.S. Design and synthesis of N4,N9-disubstituted spermines for non-viral siRNA delivery—Structure-activity relationship studies of sifection efficiency versus toxicity. Pharm. Res. 2009;26:286–295. doi: 10.1007/s11095-008-9731-z. [DOI] [PubMed] [Google Scholar]
- 38.Blagbrough I.S., Metwally A.A., Ghonaim H.M. Asymmetrical N4,N9-diacyl spermines: SAR studies of nonviral lipopolyamine vectors for efficient siRNA delivery with silencing of EGFP reporter gene. Mol. Pharm. 2012;9:1853–1861. doi: 10.1021/mp200428d. [DOI] [PubMed] [Google Scholar]
- 39.Ghonaim H.M., Li S., Blagbrough I.S. N1,N12-diacyl spermines: SAR studies on non-viral lipopolyamine vectors for plasmid DNA and siRNA formulation. Pharm. Res. 2010;27:17–29. doi: 10.1007/s11095-008-9764-3. [DOI] [PubMed] [Google Scholar]
- 40.Viola J.R., Leijonmarck H., Simonson O.E., Oprea I.I., Frithiof R., Purhonen P., Moreno P.M.D., Lundin K.E., Strömberg R., Smith C.I.E. Fatty acid-spermine conjugates as DNA carriers for nonviral in vivo gene delivery. Gene Ther. 2009;16:1429–1440. doi: 10.1038/gt.2009.108. [DOI] [PubMed] [Google Scholar]
- 41.Petukhov I.A., Maslov M.A., Morozova N.G., Serebrennikova G.A. Synthesis of polycationic lipids based on cholesterol and spermine. Russ. Chem. Bull. 2010;59:260–268. doi: 10.1007/s11172-010-0071-x. [DOI] [Google Scholar]
- 42.Maslov M.A., Kabilova T.O., Petukhov I.A., Morozova N.G., Serebrennikova G.A., Vlassov V.V., Zenkova M.A. Novel cholesterol spermine conjugates provide efficient cellular delivery of plasmid DNA and small interfering RNA. J. Control. Release. 2012;160:182–193. doi: 10.1016/j.jconrel.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 43.Markov O.O., Mironova N.L., Maslov M.A., Petukhov I.A., Morozova N.G., Vlassov V.V., Zenkova M.A. Novel cationic liposomes provide highly efficient delivery of DNA and RNA into dendritic cell progenitors and their immature offsets. J. Control. Release. 2012;160:200–210. doi: 10.1016/j.jconrel.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 44.Markov O.V., Mironova N.L., Shmendel E.V., Serikov R.N., Morozova N.G., Maslov M.A., Vlassov V.V., Zenkova M.A. Multicomponent mannose-containing liposomes efficiently deliver RNA in murine immature dendritic cells and provide productive anti-tumour response in murine melanoma model. J. Control. Release. 2015;213:45–56. doi: 10.1016/j.jconrel.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 45.Kabilova T.O., Shmendel E.V., Gladkikh D.V., Chernolovskaya E.L., Markov O.V., Morozova N.G., Maslov M.A., Zenkova M.A. Targeted delivery of nucleic acids into xenograft tumors mediated by novel folate-equipped liposomes. Eur. J. Pharm. Biopharm. 2018;123:59–70. doi: 10.1016/j.ejpb.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 46.Shmendel E.V., Kabilova T.O., Morozova N.G., Zenkova M.A., Maslov M.A. Targeted delivery of nucleic acids by folate-containing liposomes into KB-3-1 and HEK 293 cells. Russ. J. Bioorganic Chem. 2019;45:719–725. doi: 10.1134/S1068162019060360. [DOI] [Google Scholar]
- 47.Shmendel E., Kabilova T., Morozova N., Zenkova M., Maslov M. Effects of spacers within a series of novel folate-containing lipoconjugates on the targeted delivery of nucleic acids. J. Drug Deliv. Sci. Technol. 2020;57:101609. doi: 10.1016/j.jddst.2020.101609. [DOI] [Google Scholar]
- 48.Kabilova T.O., Sen’kova A.V., Nikolin V.P., Popova N.A., Zenkova M.A., Vlassov V.V., Chernolovskaya E.L. Antitumor and antimetastatic effect of small immunostimulatory RNA against B16 melanoma in mice. PLoS ONE. 2016;11:e0150751. doi: 10.1371/journal.pone.0150751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markov O.V., Mironova N.L., Shmendel E.V., Maslov M.A., Zenkova M.A. Systemic delivery of complexes of melanoma RNA with mannosylated liposomes activates highly efficient murine melanoma-specific cytotoxic T cells in vivo. Mol. Biol. 2017;51:102–107. doi: 10.1134/S0026893317010137. [DOI] [PubMed] [Google Scholar]
- 50.Puchkov P.A., Kartashova I.A., Shmendel E.V., Luneva A.S., Morozova N.G., Zenkova M.A., Maslov M.A. Spacer structure and hydrophobicity influences transfection activity of novel polycationic gemini amphiphiles. Bioorganic Med. Chem. Lett. 2017;27:3284–3288. doi: 10.1016/j.bmcl.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 51.Puchkov P.A., Perevoshchikova K.A., Kartashova I.A., Luneva A.S., Kabilova T.O., Morozova N.G., Zenkova M.A., Maslov M.A. Polycationic amphiphiles based on triethylenetetramine and their transfection efficacy. Russ. J. Bioorganic Chem. 2017;43:561–569. doi: 10.1134/S1068162017050107. [DOI] [Google Scholar]
- 52.Radchatawedchakoon W., Krajarng A., Niyomtham N., Watanapokasin R., Yingyongnarongkul B. High transfection efficiency of cationic lipids with asymmetric acyl-Cholesteryl hydrophobic tails. Chem. A Eur. J. 2011;17:3287–3295. doi: 10.1002/chem.201001622. [DOI] [PubMed] [Google Scholar]
- 53.Wang X.L., Ramusovic S., Nguyen T., Lu Z.R. Novel polymerizable surfactants with pH-sensitive amphiphilicity and cell membrane disruption for efficient siRNA delivery. Bioconjug. Chem. 2007;18:2169–2177. doi: 10.1021/bc700285q. [DOI] [PubMed] [Google Scholar]
- 54.Wang X.L., Nguyen T., Gillespie D., Jensen R., Lu Z.R. A multifunctional and reversibly polymerizable carrier for efficient siRNA delivery. Biomaterials. 2008;29:15–22. doi: 10.1016/j.biomaterials.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 55.Xu R., Lu Z.R. Design, synthesis and evaluation of spermine-based pH-sensitive amphiphilic gene delivery systems: Multifunctional non-viral gene carriers. Sci. China Chem. 2011;54:359–368. doi: 10.1007/s11426-010-4198-2. [DOI] [Google Scholar]
- 56.Xu R.Z., Wang X.L., Lu Z.R. Intracellular siRNA delivery with novel spermine based surfactants. Chin. Sci. Bull. 2012;57:3979–3984. doi: 10.1007/s11434-012-5381-y. [DOI] [Google Scholar]
- 57.Malamas A.S., Gujrati M., Kummitha C.M., Xu R., Lu Z.R. Design and evaluation of new pH-sensitive amphiphilic cationic lipids for siRNA delivery. J. Control. Release. 2013;171:296–307. doi: 10.1016/j.jconrel.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dewa T., Asai T., Tsunoda Y., Kato K., Baba D., Uchida M., Sumino A., Niwata K., Umemoto T., Iida K., et al. Liposomal polyamine-dialkyl phosphate conjugates as effective gene carriers: Chemical structure, morphology, and gene transfer activity. Bioconjug. Chem. 2010;21:844–852. doi: 10.1021/bc900376y. [DOI] [PubMed] [Google Scholar]
- 59.Asai T., Matsushita S., Kenjo E., Tsuzuku T., Yonenaga N., Koide H., Hatanaka K., Dewa T., Nango M., Maeda N., et al. Dicetyl phosphate-tetraethylenepentamine-based liposomes for systemic siRNA delivery. Bioconjug. Chem. 2011;22:429–435. doi: 10.1021/bc1004697. [DOI] [PubMed] [Google Scholar]
- 60.Yonenaga N., Kenjo E., Asai T., Tsuruta A., Shimizu K., Dewa T., Nango M., Oku N. RGD-based active targeting of novel polycation liposomes bearing siRNA for cancer treatment. J. Control. Release. 2012;160:177–181. doi: 10.1016/j.jconrel.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Kenjo E., Asai T., Yonenaga N., Ando H., Ishii T., Hatanaka K., Shimizu K., Urita Y., Dewa T., Nango M., et al. Systemic delivery of small interfering RNA by use of targeted polycation liposomes for cancer therapy. Biol. Pharm. Bull. 2013;36:287–291. doi: 10.1248/bpb.b12-00817. [DOI] [PubMed] [Google Scholar]
- 62.Patil S.P., Yi J.W., Bang E.K., Jeon E.M., Kim B.H. Synthesis and efficient siRNA delivery of polyamine-conjugated cationic nucleoside lipids. Medchemcomm. 2011;2:505–508. doi: 10.1039/c1md00014d. [DOI] [Google Scholar]
- 63.Yang H.W., Yi J.W., Bang E.K., Jeon E.M., Kim B.H. Cationic nucleolipids as efficient siRNA carriers. Org. Biomol. Chem. 2011;9:291–296. doi: 10.1039/C0OB00580K. [DOI] [PubMed] [Google Scholar]
- 64.Mével M., Kamaly N., Carmona S., Oliver M.H., Jorgensen M.R., Crowther C., Salazar F.H., Marion P.L., Fujino M., Natori Y., et al. DODAG; a versatile new cationic lipid that mediates efficient delivery of pDNA and siRNA. J. Control. Release. 2010;143:222–232. doi: 10.1016/j.jconrel.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Mével M., Breuzard G., Yaouanc J.J., Clément J.C., Lehn P., Pichon C., Jaffrès P.A., Midoux P. Synthesis and transfection activity of new cationic phosphoramidate lipids: High efficiency of an imidazolium derivative. ChemBioChem. 2008;9:1462–1471. doi: 10.1002/cbic.200700727. [DOI] [PubMed] [Google Scholar]
- 66.Mével M., Neveu C., Gonçalves C., Yaouanc J.-J., Pichon C., Jaffrès P.-A., Midoux P. Novel neutral imidazole-lipophosphoramides for transfection assays. Chem. Commun. 2008:3124–3126. doi: 10.1039/b805226c. [DOI] [PubMed] [Google Scholar]
- 67.Aissaoui A., Chami M., Hussein M., Miller A.D. Efficient topical delivery of plasmid DNA to lung in vivo mediated by putative triggered, PEGylated pDNA nanoparticles. J. Control. Release. 2011;154:275–284. doi: 10.1016/j.jconrel.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 68.Kim B., Doh K., Hyeung J., Kang H., Park J., Moon I., Seu Y. Bioorganic & Medicinal Chemistry Letters Synthesis of novel cholesterol-based cationic lipids for gene delivery. Bioorg. Med. Chem. Lett. 2009;19:2986–2989. doi: 10.1016/j.bmcl.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 69.Kim B.K., Seu Y.B., Bae Y.U., Kwak T.W., Kang H., Moon I.J., Hwang G.B., Park S.Y., Doh K.O. Efficient delivery of plasmid DNA using cholesterol-based cationic lipids containing polyamines and ether linkages. Int. J. Mol. Sci. 2014;15:7293–7312. doi: 10.3390/ijms15057293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim B.K., Doh K.O., Bae Y.U., Seu Y.B. Synthesis and optimization of cholesterol-based diquaternary ammonium gemini surfactant (Chol-GS) as a new gene delivery vector. J. Microbiol. Biotechnol. 2011;21:93–99. doi: 10.4014/jmb.1008.08012. [DOI] [PubMed] [Google Scholar]
- 71.Sparks J., Slobodkin G., Matar M., Congo R., Ulkoski D., Rea-Ramsey A., Pence C., Rice J., McClure D., Polach K.J., et al. Versatile cationic lipids for siRNA delivery. J. Control. Release. 2012;158:269–276. doi: 10.1016/j.jconrel.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 72.Paecharoenchai O., Niyomtham N., Apirakaramwong A., Ngawhirunpat T., Rojanarata T., Yingyongnarongkul B., Opanasopit P. Structure relationship of cationic lipids on gene transfection mediated by cationic liposomes. AAPS PharmSciTech. 2012;13:1302–1308. doi: 10.1208/s12249-012-9857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Niyomtham N., Apiratikul N., Suksen K., Opanasopit P., Yingyongnarongkul B. Synthesis and in vitro transfection efficiency of spermine-based cationic lipids with different central core structures and lipophilic tails. Bioorg. Med. Chem. Lett. 2015;25:496–503. doi: 10.1016/j.bmcl.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 74.Akinc A., Zumbuehl A., Goldberg M., Leshchiner E.S., Busini V., Hossain N., Bacallado S.A., Nguyen D.N., Fuller J., Alvarez R., et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat. Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mahon K.P., Love K.T., Whitehead K.A., Qin J., Akinc A., Leshchiner E., Leshchiner I., Langer R., Anderson D.G. Combinatorial approach to determine functional group effects on lipidoid-mediated siRNA delivery. Bioconjug. Chem. 2010;21:1448–1454. doi: 10.1021/bc100041r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park H.-J., Jeon E.J., Lee J.S., Hong S.H., Cho A.-N., Lee J., Moon J.-S., Jung K.-E., Oh J.-W., Lee H., et al. Galactosylated lipidoid nanoparticles for delivery of small interfering RNA to inhibit Hepatitis C viral replication in vivo. Adv. Healthc. Mater. 2016:1–11. doi: 10.1002/adhm.201600416. [DOI] [PubMed] [Google Scholar]
- 77.Huang Q.D., Ou W.J., Chen H., Feng Z.H., Wang J.Y., Zhang J., Zhu W., Yu X.Q. Novel cationic lipids possessing protonated cyclen and imidazolium salt for gene delivery. Eur. J. Pharm. Biopharm. 2011;78:326–335. doi: 10.1016/j.ejpb.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 78.Huang Q.-D., Ren J., Chen H., Ou W.-J., Zhang J., Fu Y., Zhu W., Yu X.-Q. Cyclen-Based cationic lipids containing carbamate linkages as efficient gene delivery vectors with low toxicity. Chempluschem. 2012;77:584–591. doi: 10.1002/cplu.201200060. [DOI] [Google Scholar]
- 79.Liu J.L., Ma Q.P., Huang Q.D., Yang W.H., Zhang J., Wang J.Y., Zhu W., Yu X.Q. Cationic lipids containing protonated cyclen and different hydrophobic groups linked by uracil-PNA monomer: Synthesis and application for gene delivery. Eur. J. Med. Chem. 2011;46:4133–4141. doi: 10.1016/j.ejmech.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 80.Liu Q., Jiang Q.-Q., Yi W.-J., Zhang J., Zhang X.-C., Wu M.-B., Zhang Y.-M., Zhu W., Yu X.-Q. Novel imidazole-functionalized cyclen cationic lipids: Synthesis and application as non-viral gene vectors. Bioorg. Med. Chem. 2013;21:3105–3113. doi: 10.1016/j.bmc.2013.03.048. [DOI] [PubMed] [Google Scholar]
- 81.Chang D.-C., Zhang Y.-M., Zhang J., Liu Y.-H., Yu X.-Q. Cationic lipids with a cyclen headgroup: Synthesis and structure–activity relationship studies as non-viral gene vectors. RSC Adv. 2017;7:18681–18689. doi: 10.1039/C7RA00422B. [DOI] [Google Scholar]
- 82.Li L., Song H., Luo K., He B., Nie Y., Yang Y., Wu Y., Gu Z. Gene transfer efficacies of serum-resistant amino acids-based cationic lipids: Dependence on headgroup, lipoplex stability and cellular uptake. Int. J. Pharm. 2011;408:183–190. doi: 10.1016/j.ijpharm.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 83.Sheng R., Luo T., Li H., Sun J., Wang Z., Cao A. Cholesterol-based cationic lipids for gene delivery: Contribution of molecular structure factors to physico-chemical and biological properties. Colloids Surf. B Biointerfaces. 2014;116:32–40. doi: 10.1016/j.colsurfb.2013.12.039. [DOI] [PubMed] [Google Scholar]
- 84.Ju J., Huan M.L., Wan N., Qiu H., Zhou S.Y., Zhang B. Le Novel cholesterol-based cationic lipids as transfecting agents of DNA for efficient gene delivery. Int. J. Mol. Sci. 2015;16:5666–5681. doi: 10.3390/ijms16035666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sheng R., Wang Z., Luo T., Cao A., Sun J., Kinsella J. Skeleton-Controlled pDNA delivery of renewable steroid-based cationic lipids, the endocytosis pathway analysis and intracellular localization. Int. J. Mol. Sci. 2018;19:369. doi: 10.3390/ijms19020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Obata Y., Suzuki D., Takeoka S. Evaluation of cationic assemblies constructed with amino acid based lipids for plasmid DNA delivery. Bioconjug. Chem. 2008;19:1055–1063. doi: 10.1021/bc700416u. [DOI] [PubMed] [Google Scholar]
- 87.Jiang Q., Yue D., Nie Y., Xu X., He Y., Zhang S., Wagner E., Gu Z. Specially-Made lipid-based assemblies for improving transmembrane gene delivery: Comparison of basic amino acid residue rich periphery. Mol. Pharm. 2016;13:1809–1821. doi: 10.1021/acs.molpharmaceut.5b00967. [DOI] [PubMed] [Google Scholar]
- 88.Choi J.S., Lee E.J., Jang H.S., Park J.S. New cationic liposomes for gene transfer into mammalian cells with high efficiency and low toxicity. Bioconjug. Chem. 2001;12:108–113. doi: 10.1021/bc000081o. [DOI] [PubMed] [Google Scholar]
- 89.Sarker S.R., Arai S., Murate M., Takahashi H., Takata M., Kobayashi T., Takeoka S. Evaluation of the influence of ionization states and spacers in the thermotropic phase behaviour of amino acid-based cationic lipids and the transfection efficiency of their assemblies. Int. J. Pharm. 2012;422:364–373. doi: 10.1016/j.ijpharm.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 90.Sarker S.R., Aoshima Y., Hokama R., Inoue T., Sou K., Takeoka S. Arginine-based cationic liposomes for efficient in vitro plasmid DNA delivery with low cytotoxicity. Int. J. Nanomed. 2013;8:1361–1375. doi: 10.2147/IJN.S38903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Radchatawedchakoon W., Thongbamrer C., Konbamrung W., Khattawee P., Sakee U., Roobsoong W., Sattabongkot J., Opanasopit P., Yingyongnarongkul B.E. The effect of polar headgroups and spacer length on the DNA transfection of cholesterol-based cationic lipids. RSC Med. Chem. 2020;11:212–224. doi: 10.1039/C9MD00459A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yi W.J., Zheng L.T., Su R.C., Liu Q., Zhao Z.G. Amino acid-based cationic lipids with α-tocopherol hydrophobic tail for efficient gene delivery. Chem. Biol. Drug Des. 2015;86:1192–1202. doi: 10.1111/cbdd.12585. [DOI] [PubMed] [Google Scholar]
- 93.Ewert K., Ahmad A., Evans H.M., Schmidt H.W., Safinya C.R. Efficient synthesis and cell-transfection properties of a new multivalent cationic lipid for nonviral gene delivery. J. Med. Chem. 2002;45:5023–5029. doi: 10.1021/jm020233w. [DOI] [PubMed] [Google Scholar]
- 94.Ahmad A., Evans H.M., Ewert K., George C.X., Samuel C.E., Safinya C.R. New multivalent cationic lipids reveal bell curve for transfection efficiency versus membrane charge density: Lipid—DNA complexes for gene delivery. J. Gene Med. 2005;7:739–748. doi: 10.1002/jgm.717. [DOI] [PubMed] [Google Scholar]
- 95.Bouxsein N.F., McAllister C.S., Ewert K.K., Samuel C.E., Safinya C.R. Structure and gene silencing activities of monovalent and pentavalent cationic lipid vectors complexed with siRNA. Biochemistry. 2007;46:4785–4792. doi: 10.1021/bi062138l. [DOI] [PubMed] [Google Scholar]
- 96.Martínez-Negro M., Blanco-Fernández L., Tentori P.M., Pérez L., Pinazo A., de Ilarduya C.T., Aicart E., Junquera E. A gemini cationic lipid with histidine residues as a novel lipid-based gene nanocarrier: A biophysical and biochemical study. Nanomaterials. 2018;8:1061. doi: 10.3390/nano8121061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pinazo A., Pons R., Bustelo M., Manresa M.Á., Morán C., Raluy M., Pérez L. Gemini histidine based surfactants: Characterization; surface properties and biological activity. J. Mol. Liq. 2019;289 doi: 10.1016/j.molliq.2019.111156. [DOI] [Google Scholar]
- 98.Ostergaard H., Tachibana C., Winther J.R. Monitoring disulfide bond formation in the eukaryotic cytosol. J. Cell Biol. 2004;166:337–345. doi: 10.1083/jcb.200402120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen X., Yang J., Liang H., Jiang Q., Ke B., Nie Y. Disulfide modified self-assembly of lipopeptides with arginine-rich periphery achieve excellent gene transfection efficiency at relatively low nitrogen to phosphorus ratios. J. Mater. Chem. B. 2017;5:1482–1497. doi: 10.1039/C6TB02945K. [DOI] [PubMed] [Google Scholar]
- 100.Dauty E., Remy J.S., Blessing T., Behr J.P. Dimerizable cationic detergents with a low cmc condense plasmid DNA into nanometric particles and transfect cells in culture. J. Am. Chem. Soc. 2001;123:9227–9234. doi: 10.1021/ja015867r. [DOI] [PubMed] [Google Scholar]
- 101.Zheng L.-T., Yi W.-J., Su R.-C., Liu Q., Zhao Z.-G. Reducible amino acid based cationic lipids as highly efficient and serum-tolerant gene vectors. Chempluschem. 2016;81:125–134. doi: 10.1002/cplu.201500307. [DOI] [PubMed] [Google Scholar]
- 102.Shirazi R.S., Ewert K.K., Leal C., Majzoub R.N., Bouxsein N.F., Safinya C.R. Synthesis and characterization of degradable multivalent cationic lipids with disulfide-bond spacers for gene delivery. Biochim. Biophys. Acta Biomembr. 2011;1808:2156–2166. doi: 10.1016/j.bbamem.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sheng R., Luo T., Zhu Y., Li H., Sun J., Chen S., Sun W., Cao A. The intracellular plasmid DNA localization of cationic reducible cholesterol-disulfide lipids. Biomaterials. 2011;32:3507–3519. doi: 10.1016/j.biomaterials.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 104.Bajaj A., Kondaiah P., Bhattacharya S. Effect of the nature of the spacer on gene transfer efficacies of novel thiocholesterol derived gemini lipids in different cell lines: A structure-activity investigation. J. Med. Chem. 2008;51:2533–2540. doi: 10.1021/jm7010436. [DOI] [PubMed] [Google Scholar]
- 105.Puchkov P.A., Shmendel E.V., Luneva A.S., Morozova N.G., Zenkova M.A., Maslov M.A. Design, synthesis and transfection efficiency of a novel redox-sensitive polycationic amphiphile. Bioorganic Med. Chem. Lett. 2016;26:5911–5915. doi: 10.1016/j.bmcl.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 106.Puchkov P.A., Shmendel E.V., Andreeva V.D., Morozova N.G., Zenkova M.A., Maslov M.A. A novel disulfide-containing polycationic amphiphile: 1,28-di[(cholest-5-en-3β-yl)disulfanyl]-4,25-dioxo-3,8,12,17,21,26-hexaazaoctacosane tetrahydrochloride. Molbank. 2018;2018:M981. doi: 10.3390/M981. [DOI] [Google Scholar]
- 107.Puchkov P.A., Shmendel E.V., Luneva A.S., Zenkova M.A., Maslov M.A. Position of disulfide bond determines the properties of novel stimuli-responsive cationic lipids. ChemistrySelect. 2020;5:4509–4514. doi: 10.1002/slct.201904879. [DOI] [Google Scholar]
- 108.Li Y., Bolinger J., Yu Y., Glass Z., Shi N., Yang L., Wang M., Xu Q. Intracellular delivery and biodistribution study of CRISPR/Cas9 ribonucleoprotein loaded bioreducible lipidoid nanoparticles. Biomater. Sci. 2019;7:596–606. doi: 10.1039/C8BM00637G. [DOI] [PubMed] [Google Scholar]
- 109.Liu J., Chang J., Jiang Y., Meng X., Sun T., Mao L., Xu Q., Wang M. Fast and efficient CRISPR/Cas9 genome editing in vivo enabled by bioreducible lipid and messenger RNA nanoparticles. Adv. Mater. 2019;31:1–7. doi: 10.1002/adma.201902575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao X., Glass Z., Chen J., Yang L., Kaplan D.L., Xu Q. mRNA delivery using bioreducible lipidoid nanoparticles facilitates neural differentiation of human mesenchymal stem cells. Adv. Healthc. Mater. 2020;2000938:1–7. doi: 10.1002/adhm.202000938. [DOI] [PubMed] [Google Scholar]
- 111.Li Y., Jarvis R., Zhu K., Glass Z., Ogurlu R., Gao P., Li P., Chen J., Yu Y., Yang Y., et al. Protein and mRNA delivery enabled by cholesteryl-based biodegradable lipidoid nanoparticles. Angew. Chemie Int. Ed. 2020;59:14957–14964. doi: 10.1002/anie.202004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Terekhov S.S., Smirnov I.V., Stepanova A.V., Bobik T.V., Mokrushina Y.A., Ponomarenko N.A., Belogurov A.A., Rubtsova M.P., Kartseva O.V., Gomzikova M.O., et al. Microfluidic droplet platform for ultrahigh-throughput single-cell screening of biodiversity. Proc. Natl. Acad. Sci. USA. 2017;114:2550–2555. doi: 10.1073/pnas.1621226114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.