Abstract
As one of the nanostructures with enzyme-like activity, nanozymes have recently attracted extensive attention for their biomedical applications, especially for bacterial disinfection treatment. Nanozymes with high peroxidase activity are considered to be excellent candidates for building bacterial disinfection systems (nanozyme-H2O2), in which the nanozyme will promote the generation of ROS to kill bacteria based on the decomposition of H2O2. According to this criterion, a cerium oxide nanoparticle (Nanoceria, CeO2, a classical nanozyme with high peroxidase activity)-based nanozyme-H2O2 system would be very efficient for bacterial disinfection. However, CeO2 is a nanozyme with multiple enzyme-like activities. In addition to high peroxidase activity, CeO2 nanozymes also possess high superoxide dismutase activity and antioxidant activity, which can act as a ROS scavenger. Considering the fact that CeO2 nanozymes have both the activity to promote ROS production and the opposite activity for ROS scavenging, it is worth exploring which activity will play the dominating role in the CeO2-H2O2 system, as well as whether it will protect bacteria or produce an antibacterial effect. In this work, we focused on this discussion to unveil the role of CeO2 in the CeO2-H2O2 system, so that it can provide valuable knowledge for the design of a nanozyme-H2O2-based antibacterial system.
Keywords: nanozymes, cerium oxide nanoparticles, antibacterial, peroxidase, ROS scavenger
1. Introduction
Nanozymes, as a type of nanomaterial with enzyme-like activities, have recently garnered considerable researchers’ attention toward their biological and biomedical applications [1,2,3]. Thus far, various types of nanomaterials have been reported to have catalytic activities similar to those of peroxidase, catalase, superoxide dismutase, oxidase, and other natural enzymes, including different metal and metal oxide nanoparticles, carbon-based nanomaterials, as well as some organic nanostructures [4,5,6,7]. Compared with natural enzymes, nanozymes exhibit several advantages such as tunable size and activity, facile preparation, low cost, and high stability against denaturing [8,9]. These superior properties sufficiently meet the demands for diverse biological and biomedical applications. Indeed, nanozymes have recently been frequently used for disease treatment, such as cancer therapy, bacterial disinfection, as well as Alzheimer’s disease treatment [10,11,12].
Bacterial infection has long been a major threat for human health. Antibiotics treatment is currently a golden standard for bacterial disinfection. However, the overuse of antibiotics greatly promotes antibiotic resistance development in bacterial pathogens. Therefore, the exploration of new antimicrobial strategies is urgently demanded [13,14,15,16]. Nanozyme-based antibacterial strategies have been recently demonstrated to be a promising new approach for bacterial infection treatment [17,18,19,20,21,22,23,24]. For example, Qu et al. developed graphene quantum dots (GQDs) that can act as a peroxidase mimic to enhance the antibacterial capacity of H2O2 and can be used for wound disinfection [25]. Wang and coworkers reported Cu2WS4 nanocrystals (CWS NCs) with excellent antibacterial activity owing to their high intrinsic peroxidase activity that can catalyze the decomposition of H2O2 to form ·OH, so that it inhabits wound bacterial infection [26]. Besides, Qu and his team also proposed that MOF/Ce-based nanozymes with dual enzymatic activities can not only disperse biofilms ascribed to the Ce complex, but also kill bacteria on-site, thereby avoiding the proliferation of bacteria and the recurrence of biofilms [27]. Notably, most of these works were based on the peroxidase activity of nanozymes to promote the reactive oxygen species (ROS) generation of H2O2 to kill bacteria [28,29,30,31,32,33,34]. Thus, the nanozyme with high peroxidase activity can be regarded as an excellent candidate for building a bacterial disinfection nanozyme system.
The cerium oxide nanoparticle (Nanoceria, CeO2) is a classical nanozyme with high peroxidase activity [35,36]. According to the criterion that high peroxidase activity is more conducive to promoting the production of ROS, the CeO2-H2O2 nanozyme system would be very efficient for bacterial disinfection. However, CeO2 could show multiple enzyme-like activities owing to different shapes and sizes [8,37]. In addition to high peroxidase activity, CeO2 also possesses high superoxide dismutase activity and antioxidant activity, which can be used as a ROS scavenger. In view of the fact that CeO2 has both the activity of promoting ROS production and the opposite activity of ROS scavenging, it is meaningful to discuss which activity will play the dominating role in the CeO2-H2O2 system, and whether it will protect bacteria or produce an antibacterial effect (Scheme 1). Herein, we focus on this discussion to unveil the role of CeO2 in the CeO2-H2O2 system, which will offer valuable knowledge for the design of a nanozyme-H2O2 based antibacterial system.
Scheme 1.
A schematic representation of the question to be answered in this work.
2. Results
2.1. Preparation and Characterization of CeO2 Nanozyme
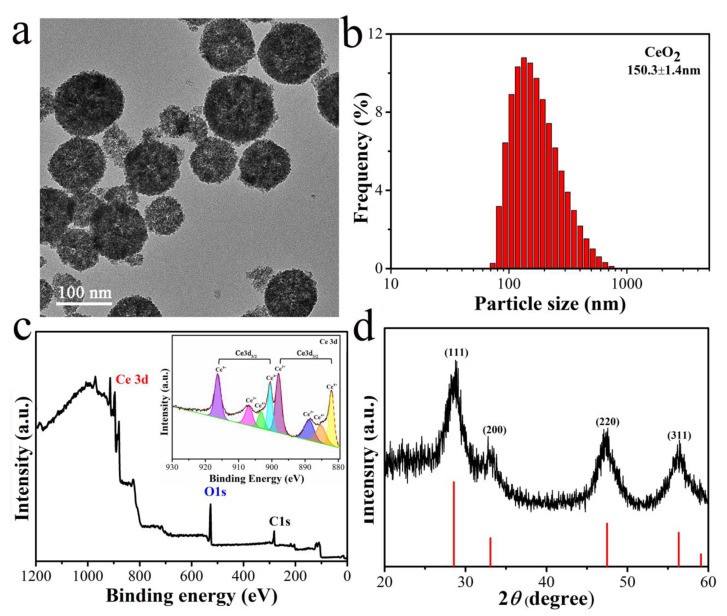
The CeO2 nanozyme was first synthesized according to a facile solvothermal method, of which the inorganic salt Ce (NO3)3·6H2O and organic acid C2H5COOH were selected as the initial materials without any surfactants [27]. As shown in Figure 1a, the transmission electron microscopy (TEM) images demonstrated the formation of CeO2 nanospheres with uniform dispersion. Dynamic light scattering (DLS) analysis was performed to determine the distribution of the hydration particle size of CeO2, which is placed at around 150 nm from Figure 1b. Furthermore, the X-ray photoelectron energy spectrum was employed to analyze the element and chemical value state of CeO2 nanospheres. We can observe from Figure 1c that there were main peaks such as Ce4+ 3d3/2 and Ce4+3d5/2 at around 914.2 and 896.0 eV in the Ce 3d core spectrum, and the Ce3+ 3d3/2 and Ce3+ 3d5/2 peaks are subscribed at 898.4 and 880.0 eV, respectively. In addition, the crystalline and phase information were implied by powder X-ray diffraction (XRD) patterns. As shown in Figure 1d, there were four clearly specific peaks, and the peaks at 2θ = 28.5°, 32.8°, 47.4°, and 56.4° can be subscribed to the characteristic (111), (200), (220), and (311) reflections of the face-centered cubic structure of CeO2 nanocrystals (JCPDS No. 43–1002, the red line in Figure 1d). All the above results demonstrate the successful synthesis of the CeO2 nanoparticles.
Figure 1.
The characterization of CeO2 nanozyme. (a) TEM images of CeO2; (b) the dynamic light scattering (DLS) analysis of CeO2; (c) the XPS spectra of the CeO2 nanozyme; the inset shows the high-resolution XPS spectra of Ce3d; (d) the XRD pattern of CeO2 (black curve).
2.2. The Peroxidase Activity of CeO2 Nanozyme
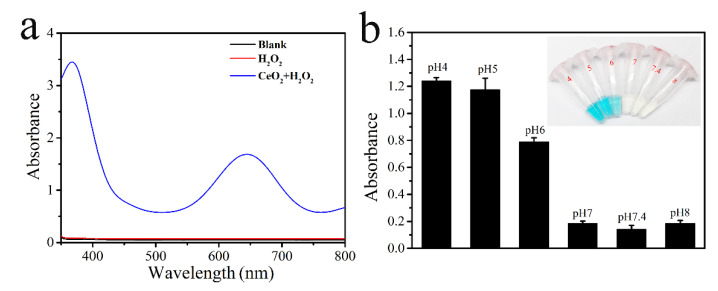
In this work, we utilized a classical colorimetric assay to estimate the peroxidase activity of CeO2 nanospheres. TMB (3,3’,5,5’-tetramethylbenzidine) was used as a substrate. In the presence of peroxidase mimics and H2O2, colorless TMB can be oxidized to blue oxTMB, which exhibits two specific absorbance peaks at 370 and 652 nm. As shown in Figure 2a, neither the nanozyme nor H2O2 alone could produce any color changes, but there appeared two sharp and strong peaks at 370 and 652 nm with the presence of both CeO2 and H2O2, which indicated that CeO2 nanospheres had intrinsic peroxidase enzymatic activity. Likewise, we investigated the effect of pH on its peroxidase activity, and the nanozyme was demonstrated to possess optimistic catalytic activity at a pH range from 4.0 to 6.0 (Figure 2b).
Figure 2.
The assay of peroxidase activity of CeO2. (a) The absorbance of TMB at 652 nm right in different reaction systems; (b) the effect of different pH on absorbance of TMB at 652 nm.
2.3. ROS Generation
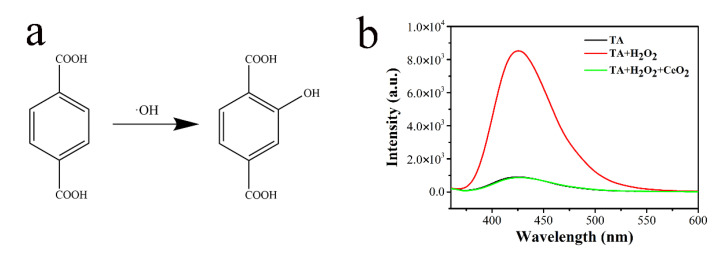
Considering that the catalytic capacity of most of the nanozymes with peroxidase activity may originate from the decomposition of H2O2 to generate ROS hydroxyl radicals (·OH), we further investigated the generation of ·OH in the CeO2-H2O2 system to verify the peroxidase activity of the CeO2 nanozyme. Terephthalic acid (TA), which could capture ·OH to generate fluorescent product 2-hydroxy terephthalic acid (TAOH) (Figure 3a), was used as a fluorescence probe for the tracing of ·OH. As shown in Figure 3b, compared with the fluorescent intensity of TA alone, there was a remarkable increase with the addition of H2O2. However, when we added the CeO2 nanozyme to the H2O2-TA system, the fluorescent intensity did not show any increase but sharply decreased to near the background intensity, which was contrary to what we predicted. This may be because CeO2 possesses certain antioxidant properties as obstacles to the decomposition of H2O2 to generate ·OH [37]. In other words, CeO2 shows considerable ROS scavenging capacity in the CeO2 nanozyme-H2O2 system.
Figure 3.
(a) Reaction between hydroxyl radical (OH) and terephthalic acid (TA); (b) fluorescence spectra of reaction system including only TA; TA and H2O2; TA, CeO2, and H2O2 after 2 h of reaction. [TA] = 0.5 mM, H2O2 = 1 mM, [CeO2] = 0.25 mg/mL.
2.4. The Evaluation of Antibacterial Activity of CeO2-H2O2 System
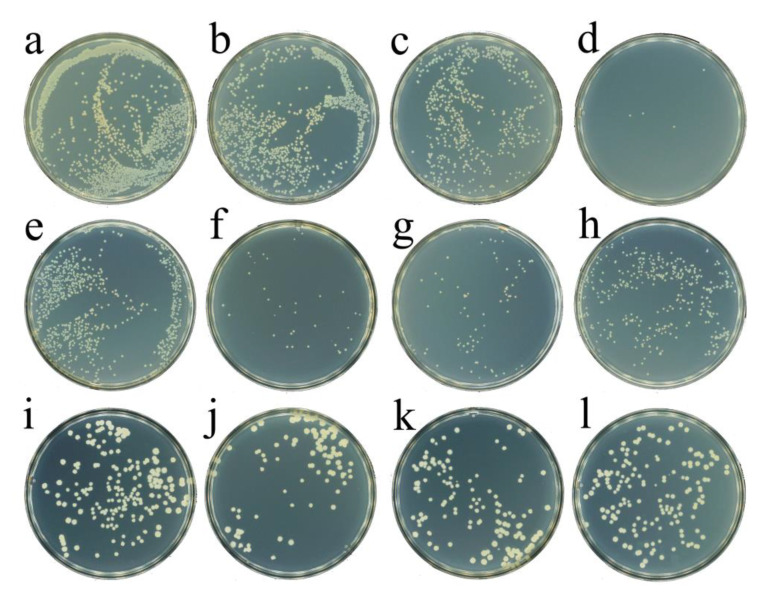
Finally, the antibacterial activities were investigated by using a Gram-negative bacterium (Escherichia coli, E. coli) as a model strain. In view of the effect of the pH value on the nanozyme activity, all the bacterial experiments were performed at pH 6.0 with PBS (phosphate-buffered saline) as the buffer. As shown in Figure 4b, CeO2 (0.25 mg/mL) alone caused a weak antibacterial effect. H2O2 showed concentration-dependent antibacterial activity. At a low concentration of 0.5 mM, H2O2 showed a weak antibacterial activity (Figure 4c). When the concentration of H2O2 was increased to 1.5 mM, almost all the bacteria were killed, indicating a significant high antibacterial activity (Figure 4d). Thereafter, the bacterial effect of the CeO2 nanozyme-H2O2 system was investigated. As shown in Figure 4e, compared with H2O2 (0.5 mM) alone, the presence of CeO2 (0.25 mg/mL) did not cause an obvious change in the number of bacteria, which demonstrated that the CeO2 nanozyme did not promote the killing effect of H2O2 against bacteria in this nanozyme-H2O2 system. Interestingly, compared with H2O2 (1.5 mM) alone, the presence of CeO2 (0.25 mg/mL) caused an obvious increase in the number of bacteria, which indicated that the CeO2 greatly inhibited the killing effect of H2O2 against bacteria in this nanozyme-H2O2 system (Figure 4g). This role was further found to be in a concentration-dependent manner. When we reduced the CeO2 concentration to 0.1 mg/mL, the effect of CeO2 on the bacterial killing activity of H2O2 decreased accordingly (Figure 4f). On the contrary, the effect of CeO2 on the bacterial killing activity of H2O2 was dramatically increased with the increase in the concentration of CeO2 to 0.4 mg/mL. The further study demonstrated that CeO2 played a similar protection role in the nanozyme-H2O2 system toward the Gram-positive bacterium (Bacillus subtilis, B. subtilis) (Figure 4f–i). All the above results manifested that the peroxidase activity of CeO2 did not contribute to the antibacterial effect of the nanozyme-H2O2 system. Instead, the ROS scavenging capacity of CeO2 protected the bacteria from being killed by H2O2. Although the phenomenon we observed may be different from other enzymes with multiple activities, our work would provide valuable new knowledge for the design of the nanozyme-H2O2-based antibacterial system.
Figure 4.
Photographs of bacterial colonies formed by E. coli and B. subtilis with different treatment at room temperature for 2 h. (a) E. coli; (b) E. coli + 0.25 mg/mL CeO2; (c) E. coli + 0.5 mM H2O2; (d) E. coli + 1.5 mM H2O2; (e) E. coli + 0.5 mM H2O2 + 0.25 mg/mL CeO2; (f) E. coli + 1.5 mM H2O2 + 0.1 mg/mL CeO2; (g) E. coli + 1.5 mM H2O2 + 0.25 mg/mL CeO2; (h) E. coli + 1.5 mM H2O2 + 0.4 mg/mL CeO2; (i) B. subtilis; (j) B. subtilis + 1 mM H2O2; (k) B. subtilis + 1 mM H2O2 + 0.25 mg/mL CeO2; (l) B. subtilis + 1 mM H2O2 + 0.4 mg/mL CeO2.
3. Materials and Methods
3.1. Chemicals and Instrument
All reagents and solvents were purchased from commercial sources. Hydrogen- peroxide is an AR reagent with a concentration of 30% in water. The degree of purity of other compounds was at least 98%, and they were used without any further treatment.
PL spectra were collected by a FL-970 Fluorescence Spectrometer (slit width of 2.5 nm and PMT voltage of 600 V). UV-vis adsorption spectra were measured by a UV-2600 spectrometer (SHIMADZU, Japan). The size of nanoparticles was monitored with a SZ-100V2 Nano Particle Analyzer. The X-ray diffraction (XRD) patterns of the products were determined on a DX-2700 X-ray diffractometer with Cu Kα radiation (λ = 1.5416 Å), with an operation voltage and current maintained at 35 kV and 25 mA, respectively. X-ray photoelectron spectroscopy (XPS) measurements were conducted on a Kratos AXIS Ultra DLD photoelectron spectrometer with Al Kα X-ray radiation as the X-ray source for excitation. The nanostructure of products was analyzed with a 120 kV JEM-1400Flash transmission electron microscope (TEM) with a Gatan Rio16 digital camera. Samples for TEM were prepared by dropping dilute solutions of nanoparticles onto carbon-coated copper grids and then maintained at 40 °C to wait for the solvent to evaporate.
3.2. Synthesis of CeO2 Nanozyme
The CeO2 nanozyme was synthesized by one simple solvothermal method right in this work. Briefly, 1 g of Ce(NO3)3·6H2O was dissolved in 1 mL of ultrapure water. Then, 1 mL of C2H5COOH and 30 mL of glycol were added in the above solution with stirring until the formation of a uniform solution. Following that, the mixture solution was transferred to an autoclave and heated to 180 °C for 200 min to obtain the initial product. The product was first centrifuged at 5000 rpm for 10 min to remove large particles; then, the supernatant was centrifuged at 10,000 rpm for 10 min, and the precipitation was washed with ethanol and ultrapure water twice. Ultimately, the product was redispersed into ultrapure water for later use.
3.3. Detection of Peroxidase Activity of CeO2
The peroxidase activity was investigated by a catalytic reaction of the TMB with the assistance of H2O2, and the catalytic performance was characterized by a specific peak at 652 nm. Typically, the reaction solution (200 μL) containing PBS buffer solution (0.2 M, 20 μL), TMB (80 mM, 2 μL), H2O2 (10 mM, 40 μL), and CeO2 nanozyme (40 μL) was incubated at room temperature for 10 min, and the blue product (oxTMB) was then monitored by a UV-spectrum at 652 nm.
3.4. Detection of OH
The detection of ·OH was dependent on the reaction of TA and ·OH. TA has weak fluorescence in the absence of ·OH, but it has unique fluorescence around 425 nm in the presence of ·OH ascribed to the generation of 2-hydroxy terephthalic acid.
The reaction solutions containing TA, TA + H2O2, and TA + H2O2 + nanozymes were reacted for 2 h and then centrifuged to separate the supernatant. The fluorescent intensity of the supernatant at around 430 nm was determined by a FL-970 fluorescence spectrometer. The concentrations of TA, H2O2, and CeO2 nanozyme were 0.5 mM, 1 mM, 0.25 mg/mL, respectively.
3.5. Antibacterial Experiment
The spread plate method was utilized to measure the bacterial number under different treatments. E. coli was coped with five different groups: (a) without any addition, (b) CeO2, (c) H2O2, and (d) CeO2 + H2O2 groups. In brief, the mono-colony of E. coli on a solid Luria–Bertani (LB) agar plate was diverted to 2 mL of liquid LB culture medium and grown at 37 °C for 5 h under 180 rpm rotation. After finishing this process, we chose 0.3 as the initial optical density of bacteria at OD600 nm. Then, 20 μL of as-prepared bacteria solution was mixed up with different groups we mentioned above with a 200 μL total volume and incubated in 24-cell culture plates at a reaction system (pH = 5, PBS buffer solution 20 mM) for 2 h. Thereafter, the bacteria solution was transferred from the 24-well plate to the solid medium by the spread plate method and was cultured at 37 °C for 12 h. A similar method was used in the B. subtilis experiment.
4. Conclusions
In summary, we successfully synthesized a CeO2 nanozyme with both the peroxidase and ROS scavenging activity. Based on this, we investigated the function between the CeO2-H2O2 system and bacteria. The results of the bacterial experiments demonstrated that the peroxidase activity of CeO2 did not contribute to the antibacterial effect in the CeO2-H2O2 system; instead, the ROS scavenging capacity of CeO2 could protect the bacteria from being killed by H2O2. Our present work not only unveils the role of CeO2 in the CeO2-H2O2 system toward bacteria but also provides valuable new knowledge for the design of nanozyme-H2O2-based antibacterial systems.
Author Contributions
Conceptualization, W.Z., Z.Z. and W.B.; methodology, W.Z. and Z.Z.; investigation, W.Z., L.W., Q.L., L.J., X.Y., X.G. and H.Q.; writing—original draft preparation, W.Z. and Z.Z.; writing—review and editing, W.Z., Z.Z. and W.B.; supervision, Z.Z.; funding acquisition, Z.Z. and W.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research’s financial support from the National Natural Science Foundation of China (NSFC) (No. 22007083, No. 52005049), Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ20B010010), Science Foundation of Zhejiang Sci-Tech University (ZSTU) under Grant No. 19062410-Y, and the Key Laboratory Fund of National Defense Science and Technology, China (Grant No. 6142005190201).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen Z., Wang Z., Ren J., Qu X. Enzyme Mimicry for Combating Bacteria and Biofilms. Acc. Chem. Res. 2018;51:789–799. doi: 10.1021/acs.accounts.8b00011. [DOI] [PubMed] [Google Scholar]
- 2.Lin Y., Ren J., Qu X. Nano-Gold as Artificial Enzymes: Hidden Talents. Adv. Mater. 2014;26:4200–4217. doi: 10.1002/adma.201400238. [DOI] [PubMed] [Google Scholar]
- 3.Wu J., Wang X., Wang Q., Lou Z., Li S., Zhu Y., Qin L., Wei H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II) Chem. Soc. Rev. 2019;48:1004–1076. doi: 10.1039/C8CS00457A. [DOI] [PubMed] [Google Scholar]
- 4.Huang Y., Su E., Ren J., Qu X. The recent biological applications of selenium-based nanomaterials. Nano Today. 2021;38:101205. doi: 10.1016/j.nantod.2021.101205. [DOI] [Google Scholar]
- 5.Jiang D., Ni D., Rosenkrans Z.T., Huang P., Yan X., Cai W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019;48:3683–3704. doi: 10.1039/C8CS00718G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng X., Zare I., Yan X., Fan K. Protein-protected metal nanoclusters: An emerging ultra-small nanozyme. WIREs Nanomed. Nanobiotechnol. 2020;12:e1602. doi: 10.1002/wnan.1602. [DOI] [PubMed] [Google Scholar]
- 7.Sun H., Zhou Y., Ren J., Qu X. Carbon Nanozymes: Enzymatic Properties, Catalytic Mechanism, and Applications. Angew. Chem. Int. Ed. 2018;57:9224–9237. doi: 10.1002/anie.201712469. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y., Ren J., Qu X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019;119:4357–4412. doi: 10.1021/acs.chemrev.8b00672. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q., Wei H., Zhang Z., Wang E., Dong S. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. TrAC Trends Anal. Chem. 2018;105:218–224. doi: 10.1016/j.trac.2018.05.012. [DOI] [Google Scholar]
- 10.Dong H., Fan Y., Zhang W., Gu N., Zhang Y. Catalytic Mechanisms of Nanozymes and Their Applications in Biomedicine. Bioconjugate Chem. 2019;30:1273–1296. doi: 10.1021/acs.bioconjchem.9b00171. [DOI] [PubMed] [Google Scholar]
- 11.Wang H., Wan K., Shi X. Recent Advances in Nanozyme Research. Adv. Mater. 2019;31:1805368. doi: 10.1002/adma.201805368. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z., Zhang Y., Ju E., Liu Z., Cao F., Chen Z., Ren J., Qu X. Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat. Commun. 2018;9:3334. doi: 10.1038/s41467-018-05798-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z., Ji H., Liu C., Bing W., Wang Z., Qu X. A Multinuclear Metal Complex Based DNase-Mimetic Artificial Enzyme: Matrix Cleavage for Combating Bacterial Biofilms. Angew. Chem. Int. Ed. 2016;55:10732–10736. doi: 10.1002/anie.201605296. [DOI] [PubMed] [Google Scholar]
- 14.Han Q., Lau J.W., Do T.C., Zhang Z., Xing B. Near-Infrared Light Brightens Bacterial Disinfection: Recent Progress and Perspectives. ACS Appl. Bio Mater. 2020;4:3937–3961. doi: 10.1021/acsabm.0c01341. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y., Shi L., Su L., van der Mei H.C., Jutte P.C., Ren Y., Busscher H.J. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem. Soc. Rev. 2019;48:428–446. doi: 10.1039/C7CS00807D. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z., Han Q., Lau J.W., Xing B. Lanthanide-Doped Upconversion Nanoparticles Meet the Needs for Cutting-Edge Bioapplications: Recent Progress and Perspectives. ACS Mater. Lett. 2020;2:1516–1531. doi: 10.1021/acsmaterialslett.0c00377. [DOI] [Google Scholar]
- 17.Cao F., Zhang L., Wang H., You Y., Wang Y., Gao N., Ren J., Qu X. Defect-Rich Adhesive Nanozymes as Efficient Antibiotics for Enhanced Bacterial Inhibition. Angew. Chem. Int. Ed. 2019;58:16236–16242. doi: 10.1002/anie.201908289. [DOI] [PubMed] [Google Scholar]
- 18.Gao F., Shao T., Yu Y., Xiong Y., Yang L. Surface-bound reactive oxygen species generating nanozymes for selective antibacterial action. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-20965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He X., Tian F., Chang J., Bai X., Yuan C., Wang C., Neville A. Haloperoxidase Mimicry by CeO2-x Nanorods of Different Aspect Ratios for Antibacterial Performance. ACS Sustain. Chem. Eng. 2020;8:6744–6752. doi: 10.1021/acssuschemeng.0c01113. [DOI] [Google Scholar]
- 20.Hu M., Korschelt K., Viel M., Wiesmann N., Kappl M., Brieger J., Landfester K., Therien-Aubin H., Tremel W. Nanozymes in Nanofibrous Mats with Haloperoxidase-like Activity to Combat Biofouling. ACS Appl. Mater. Interfaces. 2018;10:44722–44730. doi: 10.1021/acsami.8b16307. [DOI] [PubMed] [Google Scholar]
- 21.Liu X., Yan Z., Zhang Y., Liu Z., Sun Y., Ren J., Qu X. Two-Dimensional Metal-Organic Framework/Enzyme Hybrid Nanocatalyst as a Benign and m Self-Activated Cascade Reagent for in Vivo Wound Healing. ACS Nano. 2019;13:5222–5230. doi: 10.1021/acsnano.8b09501. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z., Zhao X., Yu B., Zhao N., Zhang C., Xu F.-J. Rough Carbon-Iron Oxide Nanohybrids for Near-Infrared-II Light-Responsive Synergistic Antibacterial Therapy. ACS Nano. 2021;15:7482–7490. doi: 10.1021/acsnano.1c00894. [DOI] [PubMed] [Google Scholar]
- 23.Wu R., Chong Y., Fang G., Jiang X., Pan Y., Chen C., Yin J.-J., Ge C. Synthesis of Pt Hollow Nanodendrites with Enhanced Peroxidase-Like Activity against Bacterial Infections: Implication for Wound Healing. Adv. Funct. Mater. 2018;28 doi: 10.1002/adfm.201801484. [DOI] [Google Scholar]
- 24.Xu B., Wang H., Wang W., Gao L., Li S., Pan X., Wang H., Yang H., Meng X., Wu Q., et al. A Single-Atom Nanozyme for Wound Disinfection Applications. Angew. Chem. Int. Ed. 2019;58:4911–4916. doi: 10.1002/anie.201813994. [DOI] [PubMed] [Google Scholar]
- 25.Sun H., Gao N., Dong K., Ren J., Qu X. Graphene Quantum Dots-Band-Aids Used for Wound Disinfection. ACS Nano. 2014;8:6202–6210. doi: 10.1021/nn501640q. [DOI] [PubMed] [Google Scholar]
- 26.Shan J., Li X., Yang K., Xiu W., Wen Q., Zhang Y., Yuwen L., Weng L., Teng Z., Wang L. Efficient Bacteria Killing by Cu2WS4 Nanocrystals with Enzyme-like Properties and Bacteria-Binding Ability. ACS Nano. 2019;13:13797–13808. doi: 10.1021/acsnano.9b03868. [DOI] [PubMed] [Google Scholar]
- 27.Liang X., Xiao J., Chen B., Li Y. Catalytically Stable and Active CeO2 Mesoporous Spheres. Inorg. Chem. 2010;49:8188–8190. doi: 10.1021/ic100795p. [DOI] [PubMed] [Google Scholar]
- 28.Wang L., Gao F., Wang A., Chen X., Li H., Zhang X., Zheng H., Ji R., Li B., Yu X., et al. Defect-Rich Adhesive Molybdenum Disulfide/rGO Vertical Heterostructures with Enhanced Nanozyme Activity for Smart Bacterial Killing Application. Adv. Mater. 2020;32:2005423. doi: 10.1002/adma.202005423. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y., Wu X., Ma L., He C., Cao S., Long Y., Huang J., Rodriguez R.D., Cheng C., Zhao C., et al. Bioinspired Spiky Peroxidase-Mimics for Localized Bacterial Capture and Synergistic Catalytic Sterilization. Adv. Mater. 2021;33:2005477. doi: 10.1002/adma.202005477. [DOI] [PubMed] [Google Scholar]
- 30.Yim G., Kim C.Y., Kang S., Min D.-H., Kang K., Jang H. Intrinsic Peroxidase-Mimicking Ir Nanoplates for Nanozymatic Anticancer and Antibacterial Treatment. ACS Appl. Mater. Interfaces. 2020;12:41062–41070. doi: 10.1021/acsami.0c10981. [DOI] [PubMed] [Google Scholar]
- 31.Yu Z.-H., Li X., Xu F., Hu X.-L., Yan J., Kwon N., Chen G.-R., Tang T., Dong X., Mai Y., et al. A Supramolecular-Based Dual-Wavelength Phototherapeutic Agent with Broad-Spectrum Antimicrobial Activity Against Drug-Resistant Bacteria. Angew. Chem. Int. Ed. 2020;59:3658–3664. doi: 10.1002/anie.201913506. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L., Zhang L., Deng H., Li H., Tang W., Guan L., Qiu Y., Donovan M.J., Chen Z., Tan W. In vivo activation of pH-responsive oxidase-like graphitic nanozymes for selective killing of Helicobacter pylori. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-22286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W., Ren X., Shi S., Li M., Liu L., Han X., Zhu W., Yue T., Sun J., Wang J. Ionic silver-infused peroxidase-like metal-organic frameworks as versatile "antibiotic" for enhanced bacterial elimination. Nanoscale. 2020;12:16330–16338. doi: 10.1039/D0NR01471K. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y., Li D., Tan J., Chang Z., Liu X., Ma W., Xu Y. Near-Infrared Regulated Nanozymatic/Photothermal/Photodynamic Triple-Therapy for Combating Multidrug-Resistant Bacterial Infections via Oxygen-Vacancy Molybdenum Trioxide Nanodots. Small. 2021;17:2005739. doi: 10.1002/smll.202005739. [DOI] [PubMed] [Google Scholar]
- 35.Liu Z., Wang F., Ren J., Qu X. A series of MOF/Ce-based nanozymes with dual enzyme-like activity disrupting biofilms and hindering recolonization of bacteria. Biomaterials. 2019;208:21–31. doi: 10.1016/j.biomaterials.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y., Li H., Wang Y., Wang Y., Huang Z., Su H., Liu J. CeO2 Nanoparticle Transformation to Nanorods and Nanoflowers in Acids with Boosted Oxidative Catalytic Activity. ACS Appl. Nano Mater. 2021;4:2098–2107. doi: 10.1021/acsanm.0c03387. [DOI] [Google Scholar]
- 37.Filippi A., Liu F., Wilson J., Lelieveld S., Korschelt K., Wang T., Wang Y., Reich T., Poeschl U., Tremel W., et al. Antioxidant activity of cerium dioxide nanoparticles and nanorods in scavenging hydroxyl radicals. RSC Adv. 2019;9:11077–11081. doi: 10.1039/C9RA00642G. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.