Abstract
Simple Summary
Beta-thalassemia (β-thalassemia) is an autosomal recessive inherited disorder that causes decreased production of hemoglobin. Ineffective erythropoiesis and excess iron deposition are the most significant pathophysiological problems. Chronic red blood cell transfusions along with control of iron overload are the main principles of treatment. Yet, the patients have a problematic quality of life. Recently, novel therapies have emerged based on better knowledge of the pathophysiology of the disease. Aiming at ineffective erythropoiesis through the TGF-β ligand traps, such as luspatercept, has been shown to reduce the transfusion burden. Therapeutic approaches aiming at the iron metabolism mechanisms as well as the pathway of the production of erythroid cyclic guanosine monophosphate are being used in clinical trials with encouraging results. Significant improvements in the technique of hemopoietic stem cell transplantation have been accomplished, with a focus on the improvement of the conditioning regimen and the donor selection. Gene therapy has exhibited remarkable advances using lentiviral β-globin gene insertion techniques or gene editing platforms that target the suppression of γ-globin repressors. All these approaches will have a positive result in the quality of life of thalassemia patients.
Abstract
The main characteristic of the pathophysiology of β-thalassemia is reduced β-globin chain production. The inevitable imbalance in the α/β-globin ratio and α-globin accumulation lead to oxidative stress in the erythroid lineage, apoptosis, and ineffective erythropoiesis. The result is compensatory hematopoietic expansion and impaired hepcidin production that causes increased intestinal iron absorption and progressive iron overload. Chronic hemolysis and red blood cell transfusions also contribute to iron tissue deposition. A better understanding of the underlying mechanisms led to the detection of new curative or “disease-modifying” therapeutic options. Substantial evolvement has been made in allogeneic hematopoietic stem cell transplantation with current clinical trials investigating new condition regimens as well as different donors and stem cell source options. Gene therapy has also moved forward, and phase 2 clinical trials with the use of β-globin insertion techniques have recently been successfully completed leading to approval for use in transfusion-dependent patients. Genetic and epigenetic manipulation of the γ- or β-globin gene have entered the clinical trial setting. Agents such as TGF-β ligand traps and pyruvate kinase activators, which reduce the ineffective erythropoiesis, have been tested in clinical trials with favorable results. One TGF-β ligand trap, luspatercept, has been approved for use in adults with transfusion-dependent β-thalassemia. The induction of HbF with the phosphodiesterase 9 inhibitor IMR-687, which increase cyclic guanosine monophosphate, is currently being tested. Another therapeutic approach is to target the dysregulation of iron homeostasis, using, for example, hepcidin agonists (inhibitors of TMPRSS6 and minihepcidins) or ferroportin inhibitors (VIT-2763). This review provides an update on the novel therapeutic options that are presently in development at the clinical level in β-thalassemia.
Keywords: thalassemia, clinical trial, ineffective erythropoiesis, iron metabolism, gene therapy
1. Introduction
Beta-thalassemia (β-thalassemia) is an autosomal recessive inherited disease characterized by decreased production of the β-globin chains of hemoglobin (Hb) A. The normal structure of HbA is two α- and two β-globin chains. Individuals with β-thalassemia are either homozygous or double heterozygotes for mutations in the β-globin gene. The severity of the disease depends on the type of mutation in the β-globin gene and the extent of impairment of β-globin chain production. The β+ allele correlates with decreased but not absent production, and the β0 with no production. β-thalassemia can be classified as transfusion-dependent thalassemia (TDT) and non-transfusion-dependent thalassemia (NTDT) according to the severity of anemia and the need for transfusions. The heterozygous β-thalassemia trait is a clinically asymptomatic state with a mild microcytic anemia [1].
The mutation in the β-globin gene on chromosome 11 is responsible for the impaired β-globin production and subsequently for the accumulation of unmatched a-globin precipitates. These iron-containing insoluble bodies induce the generation of reactive oxygen species that are deleterious to the cell membrane structures of erythroid cells. The oxidative stress leads to the premature apoptosis of the erythroblasts, which is called ineffective erythropoiesis [2,3]. The changes in membrane proteins of mature red blood cells (RBC), especially the increased expression of phosphatidylserine, lead to hemolysis from the macrophages of the reticuloendothelial system. Furthermore, the overactivation of transforming growth factor-β (TGF-β), which acts as an inhibitor of the final stage of erythropoiesis, has an important role in the process of ineffective erythropoiesis [4].
Ineffective erythropoiesis and peripheral hemolysis lead to severe anemia, tissue hypoxia, and a reactive production of erythropoietin (EPO) with a consequent compensatory increase of the number of bone marrow erythroblasts and extramedullary hematopoiesis with characteristic hepatosplenomegaly. The activated erythroblasts release several proteins, such as erythroferrone (ERFE), which inhibit hepcidin, the master regulator of iron homeostasis. The consequence of low hepcidin levels is the increased intestinal absorption of iron, which, along with chronic hemolysis and RBC transfusions, leads to progressive tissue iron deposition. Excessive free iron has a negative impact in erythropoiesis, creating a vicious cycle between ineffective erythropoiesis and increased iron absorption [5,6,7,8,9,10].
The management of β-thalassemia is optimized to each patient’s clinical course and profile and is primarily focused on the improvement of anemia and the regulation of iron overload and its complications. Endocrinological abnormalities (e.g., hypogonadism, hypothyroidism, insulin resistance/diabetes, growth impairment) are common and are attributable mainly to iron toxicity [11,12]. TDT patients require lifelong RBC transfusions, whereas NTDT patients are transfused upon certain indications, such as sudden Hb decrease, growth delay in children, splenomegaly, bone deformities, extramedullary hematopoiesis, and pulmonary hypertension. In cases of hypersplenism, splenectomy can be performed. Iron chelators, such as desferrioxamine, deferiprone, and deferasirox, are used for the management of excess iron deposition. In the case of severe iron deposition, the iron chelators can be used in combination [13,14,15]. The quality of life of thalassemic patients is affected due to the life-long need for chronic transfusions and iron chelation treatment and the relevant complications. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potential curative treatment for transfusion-dependent patients without iron-related complications, especially at a young age [16].
Recently, novel therapies have emerged which are based on the knowledge of the pathophysiological mechanisms of β-thalassemia. In order to correct the imbalance between the α- and non-α-chains of Hb, agents which promote the production of γ-chains, such as hydroxycarbamide, 5-azacytidine, short-chain fatty acids, and thalidomide, have been used in thalassemia patients and presented partial responses without long-term favorable outcomes [13,17,18]. Furthermore, the stimulation of erythropoiesis has been attempted with the use of EPO with variable results [19].
Further knowledge of the underlying pathways of ineffective erythropoiesis and hemosiderosis has resulted in the emergence of promising novel therapeutic agents, such as TGF-β ligand traps (i.e., luspatercept). Additionally, improvement of HSCT protocols concerning the conditioning regimen, the selection of the donor, and the source of the stem cells is currently under evaluation in clinical trials. Gene therapy with β-globin addition has been shown to be effective and safe in clinical trials and new experimental gene manipulation techniques, such as genome editing, have recently been developed and the first results from clinical trials are very encouraging. The aim of this review is to present the advances in the treatment of β-thalassemia by providing an update on the established and emerging curative and “disease-modifying” (non-curative) treatments (Table 1).
Table 1.
Clinical trials of novel treatment in β-thalassemia.
| Treatment Modality | Mechanism | Route | Phase | ClinicalTrials.gov (8 May 2021) | Status | Institution/ Developer |
|---|---|---|---|---|---|---|
| Luspatercept | Ligand trap TGF beta superfamily |
Subcutaneous | 2 | NCT01749540 | Completed | Acceleron Pharma, Celgene Corporation |
| 2, extension study 2 2 |
NCT02268409 NCT03342404 NCT04143724 |
Completed Open Not yet recruiting |
||||
| 3 | NCT02604433 | Completed | ||||
| Mitapivat | Pyruvate kinase activation | Oral | 2 | NCT03692052 | Open | Agios Pharmaceuticals |
| TMPRSS6-LRx | Matriptase-2 inhibition, hepcidin activation | Subcutaneous | 2 | NCT04059406 | Open | Ionis Pharmaceuticals |
| SLN124 | Matriptase-2 inhibition, hepcidin activation | Subcutaneous | 2 | NCT04718844 | Open | Silence Therapeutics plc |
| PTG-300 | Hepcidin analog | Subcutaneous | 2 | NCT04054921 | Completed | Protagonist Therapeutics, Inc. |
| VIT-2763 | Ferroportin inhibition | Oral | 2 | NCT04364269 | Open | Vifor (International) Inc. |
| IMR-687 | Phosphodiesterase 9 inhibition, HbF stimulation | Oral | 2 | NCT04411082 | Completed | Imara, Inc. |
| HSCT | Reduced intensity conditioning | Matched sibling donor | 1, 2 | NCT00920972, NCT01050855, NCT02435901 | Open Open Completed |
US, Canada US, Canada US, Canada |
| Family related matched donor or cord | 2 | NCT00408447 | Active not recruiting | Columbia University, USA | ||
| HSCT | Matched unrelated | 2 | NCT01049854 | Completed | New York Medical College, USA | |
| HSCT | Nonmyeloablative haploidentical | Haploidentical transplants | 1,2 | NCT00977691 | Active not recruiting | National Heart, Lung, and Blood Institute (NHLBI) |
| HSCT | Nonmyeloablative peripheral blood mobilized | Allogeneic peripheral blood stem cell | 1,2 | NCT02105766 | Open | National Heart, Lung, and Blood Institute (NHLBI) |
| HSCT | Umbilical cord blood | Umbilical cord stem cells | 1 | NCT02126046 | Open | Nanfang Hospital of Southern Medical University |
| HSCT CordIn™ | Umbilical cord blood-derived ex vivo stem cells | Ex vivo umbilical cord stem cells | 1 | NCT02504619 | Completed | Gamida Cell ltd |
| LentiGlobin BB305 vector | β-globin gene addition | Ex vivo autologous CD34+ stem cell transduction | 1 | NCT02151526 | Completed | bluebird bio, France |
| 1/2 3 |
NCT01745120 NCT03207009 |
Completed Open |
bluebird bio, Northstar Study |
|||
| TNS9.3.55 Lentiviral Vector | β-globin gene addition | Ex vivo autologous CD34+ stem cell transduction | 1 | NCT01639690 | Active not recruiting | Memorial Sloan Kettering Cancer Center, USA |
| GLOBE Lentiviral Vector | β-globin gene addition | Ex vivo autologous CD34+ stem cell transduction | 1 | NCT02453477 | Active not recruiting | IRCCS San Raffaele, Italy |
| CTX001 | BCL11A gene editing | Ex vivo autologous CD34+ stem cell transduction | 1/2 | NCT03655678 | Open | Vertex Pharmaceuticals Incorporated CRISPR Therapeutics |
| ST-400 | BCL11A gene editing | Ex vivo autologous CD34+ stem cell transduction | 1/2 | NCT03432364 | Active not recruiting | Sangamo Therapeutics Sanofi |
2. Curative Treatments
2.1. Allogeneic Hematopoietic Stem Cell Transplantation (Allo-HSCT)
Allo-HSCT is a potentially curative treatment for the TDT disease; however, the consequent toxicities and mortality are serious concerns. In TDT patients, the presence of good risk characteristics, according to the Pesaro criteria, is correlated to a successful outcome greater than 90%. The Pesaro criteria are applicable to patients below the age of 16 and include three variables related to iron toxicity: regularity of iron chelation, the presence of hepatomegaly, and the presence of liver fibrosis. The ideal candidates are mainly young children with no comorbidities and a human leukocyte antigen (HLA)-identical sibling donor. The standard myeloablative condition regimens are mainly based on chemotherapy alone, with the use of the alkylating agents busulfan and cyclophosphamide [20,21]. Allo-HSCT in thalassemic patients with high-risk criteria poses difficulties due to elevated rates of graft rejection and transplant-related mortality [16]. Adults with TDT will always be at high risk, but several new conditioning regimens are being evaluated in clinical trials as an effort to improve the transplant outcomes (NCT01050855, NCT00920972, NCT02435901). Favorable results have been reported using modified or reduced intensity conditioning (e.g., treosulfan/thiotepa/fludarabine) or even nonmyeloablative regimens. With these modern promising approaches, both age and Pesaro classification lose much of their predictive value [22,23,24].
The preferable source of stem cells is the bone marrow rather than peripheral blood, possibly due to lower risk of development of chronic graft-versus-host disease. Peripheral blood stem cells have also been used in an effort to decrease the possibility of graft rejection in high-risk thalassemic patients (NCT02105766). Optimally, fully matched sibling donors are preferred, but matched unrelated donors might also be considered (NCT01049854), as well as related cord blood transplants in patients with low-risk criteria. Unrelated umbilical cord blood cells and haploidentical transplants should ideally be performed in clinical trial setting (NCT02126046, NCT00977691, NCT00408447, NCT02504619).
2.2. Gene Therapy
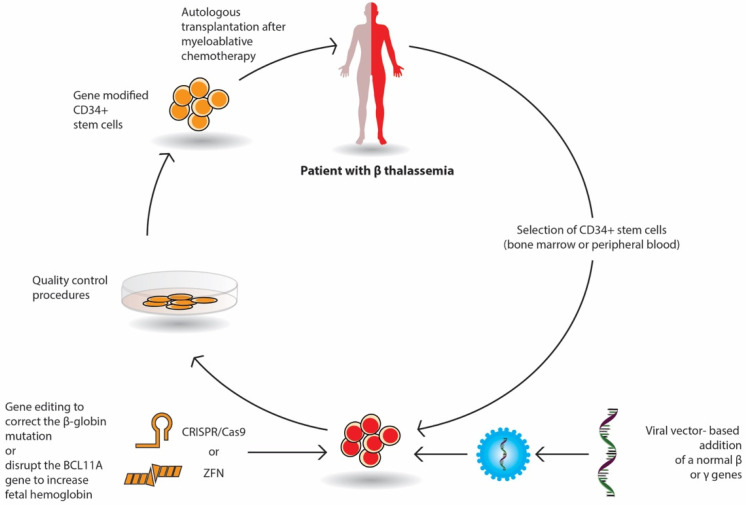
Gene therapy by autologous transplantation of genetically manipulated hematopoietic stem cells is a very promising curative option in β-thalassemia. Throughout the last decade, several gene transfer protocols have been comprehensively explored. Among them, lentivirus vectors have been used in thalassemic mouse models and in erythroid stem cells from thalassemic patients. These studies have focused on β or γ-globin addition; the increased expression of γ-globin-activating transcription factors; the silencing of DNA- or RNA-binding proteins that inhibit the expression of γ-globin repressors, such as BCL11A; and the genome editing of β-globin mutations or γ-globin repressors (Figure 1). Currently, several ongoing clinical trials have demonstrated promising results.
Figure 1.
Stepwise procedure of gene therapy by gene addition and by gene editing in β-thalassemia.
The selected CD34+ erythroid progenitor cells (bone marrow or peripheral cells) of the patient are genetically modified either by the viral vector-based addition of a normal β or γ gene or by gene editing with nucleases (Crisp/Cas9 or ZFN), which repair the β globin mutation or delete genomic regions of the BCL11A gene in order to reactivate fetal Hb (HbF) production. The genetically corrected CD34+ stem cells are prepared for autologous transplantation after strict quality control procedures. The patient receives proper myeloablative chemotherapy and then the stem cells are infused in the patient.
2.2.1. Gene Addition
In gene addition, a retroviral or lentiviral vector that encloses the regulatory elements and the β- or γ-globin gene area is inserted into previously collected autologous CD34+ erythroid progenitor cells ex vivo and then infused to the patient who has received a myeloablative busulfan conditioning. Following engraftment, it is expected that β-globin or γ-globin production will be restored, and the α/β imbalance will be reduced (Figure 1).
The outcomes of two phase 1/2 clinical trials (HGB-204/NCT01745120 and HGB-205/NCT02151526) using the LentiGlobin BB305 vector in 22 TDT patients (12–35 years of age) were published in 2018 [25]. The LentiGlobin BB305 vector inserts a functioning version of the HBB gene into the patient’s CD34+ erythroid progenitor cells, which encodes HbA with a new T87Q amino acid substitution (HbAT87Q). All patients tolerated the conditioning busulfan regimen with no serious toxicity, and no significant safety concerns regarding the infusion have been reported. Furthermore, regarding the safety of the viral vector, no oncogenic clonal dominance was noted. Hematopoietic engraftment was successful in all patients. Regarding the non–β0/β0 genotype, all but 1 of the 13 patients became transfusion independent after a median period of 2 years (range, 15–42 months) after the procedure. The Hb levels were between 8.2 and 13.7 g/dL, and the new hemoglobin HbAT87Q varied from 3.4 to 10.0 g/dL. An increase of Hb by approximately 5 g/dL was enough for the HbE/β-thalassemia or β0/β+ patients to become transfusion independent. As expected, the results were different in patients with the most severe genotype (β0/β0 or two copies of the IVS1-110 mutation), where transfusion independence requires significantly higher levels of Hb production. In these patients the median transfusion need per year was declined by two thirds, and three patients became transfusion independent.
In order to address this problem, especially in β0/β0 patients, the sponsor of the trial, bluebird bio, has significantly improved the protocol with the addition of small molecules to the transduction process. This change has a positive effect on the vector copy number in the ongoing phase 3 trials (HGB 207 and HGB-212). A rapid increase of HbAT87Q levels was noticed in most (18/20, 90%) non-β0/β0 patients, leading to transfusion independence with Hb levels > 9 g/d [26]. Based on these positive outcomes and satisfactory safety reports, the lentiglobin gene therapy (Zynteglo) received conditional EMA in June 2019 for TDT patients ≥ 12 years of age with a non-β0/β0 genotype who are eligible for stem cell transplantation but do not have a matching related donor.
The primary results of the HGB 212 phase 3 trial from 11 patients with either a β0 or IVS-I-110 mutation at both alleles of the HBB gene showed that three of four patients stopped transfusions for ≥ 6 months with Hb levels of 10.5–13.6 g/dL at the last visit [27]. The HbAT87Q levels stabilized at a range of 9.5 to 12.6 g/dL 6 months after infusion. After 2 years of follow-up in these four studies (HGB-204, HGB-205, HGB 207, and HGB-212), a long-term follow-up study (LTF-303, NCT02633943) was initiated. The interim results of 32 patients have shown that 64% of the patients from the HGB-204/HGB-205 studies and 90% of the patients of the HGB 207/HGB-212 studies achieved durable and stable transfusion independency [28]. Interim results in pediatric patients in the HGB-207 and HGB-212 showed that children achieved transfusion independence with comparable rates and safety as in adults [29].
Another lentiviral gene-insertion phase 1/2 trial (NCT02453477, SR-TIGET) was recently published [30]. Nine patients (three adults, six children) with β0 or severe β+ mutations received, with intrabone administration, transduced GLOBE lentiviral vector CD34+ cells. Satisfactory hematopoietic engraftment was achieved in all the patients, with no clonal dominance. Three children stopped transfusions, and the adults also exhibited a significant decrease in transfusion requirements.
A different gene insertion approach has been developed at Boston Children’s Hospital. The target is the Bcl11a gene with the use of RNA interference technology. A short hairpin RNA sequence that represses the Bcl11a gene is transduced into CD34+ erythroid progenitor cells using a lentivirus. A relevant clinical trial has been started for sickle cell disease patients [31].
2.2.2. Gene Editing
The Bcl11a gene located on chromosome 2 is a promising target for gene editing techniques. Two companies, Sangamo Therapeutics/Sanofi, Inc. and Crispr/Vertex, Inc., are studying the use of ZFN (NCT03432364) and CRISPR-Cas9 (NCT03655678) platforms, respectively, in TDT patients. The main idea is to produce minor deletions in the erythroid-specific enhancer region of the Bcl11a gene. The major advantage of this approach is that the HBB gene is not affected, allowing for the unceasing production of the γ-globin chains. These clinical trials are presently enlisting individuals with thalassemia. Sangamo Therapeutics/Sanofi, Inc. presented preliminary results at the 2019 ASH meeting (NCT03432364) from two patients. Both patients had rapid hematopoietic engraftments with elevated HbF levels [32]. The results of five patients treated with CRISPR-Cas9 gene editing (CTX001 product) were presented at the 2020 ASH meeting. The follow-up was at least 3 months post infusion as of July 2020. The neutrophil and platelet engraftment occurred at the end of the first month, and all patients stopped receiving transfusions after the second month. The first patient enrolled in the study has remained without the need for transfusion for over 15 months. The safety report after CTX001 treatment was similar to that with busulfan myeloablation [33,34].
3. “Disease-Modifying” (Non-Curative) Treatments
3.1. The TGF-β (Transforming Growth Factor-β) Superfamily Ligand Traps
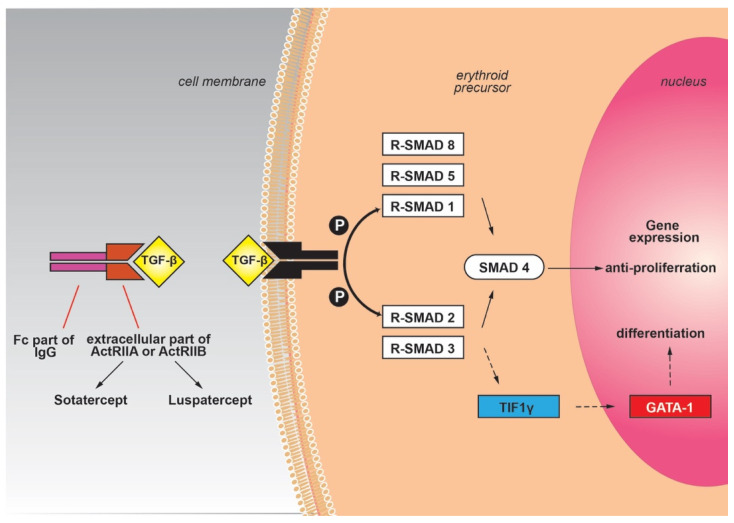
The TGF-β signaling is significant for the regulation of essential cellular pathways, especially in the bone and hematopoietic tissue [35], and comprises four similar protein groups: activins, TGF-β, GDFs (growth and differentiating factors), and BMP (bone morphogenetic proteins). The effect of these proteins on the erythroid lineage can be either inductive (TGF-β, BMP4) [36] or inhibitory (activin, GDFs) [37,38]. Their receptors are serine/threonine kinases that stimulate intracellular paths by recruiting the Sma and Mad related proteins (Smad). As a consequence, the Smad proteins (Smad2/3 or Smad1/5/8) in the cytoplasm are phosphorylated and create an oligomeric combination with Smad4 (co-Smad) and insert the nucleus to modify gene transcription. Activins, GDF8, and GDF11 act through the Smad2/3 pathway, whereas BMPs and other GDFs act through the Smad1/5/8 pathway [39]. The Smad2/3 pathway also has the ability to bypass the Smad4 and to alternatively bind to TIF1γ (transcription intermediary factor 1γ), according to the stage of cellular differentiation. TIF1γ stimulates the expression of the key erythroid transcription factor GATA-1 and promotes the differentiation of erythroid lineage [40] (Figure 2).
Figure 2.
TGF-β signaling pathway and its ligand traps.
Therefore, the Smad2/3 signaling has appeared as a significant regulatory mechanism of erythropoiesis with a balancing activity according to the implicated pathway. The Smad2/3-Smad4 pathway inhibits the proliferation of the erythrocyte progenitor cells, while the Smad2/3-TIF1γ promotes the differentiation. It seems that this orchestrated signaling is necessary for the regular differentiation and cessation of erythropoiesis. The overactivation or dysregulation of Smad2/3-Smad4 signaling has been implicated in illnesses with anemia caused by ineffective erythropoiesis, such as myelodysplastic syndromes or β-thalassemia [4]. Therefore, inhibitors of the TGF-β family that sequester the Smad2/3-Smad4 signaling could promote the end phase of erythropoiesis with a beneficial result in these diseases.
Altered receptors of activin (activin receptor-II trap ligands) have been derived from the colligation of the extracellular part of the activin receptor (ActRIIA or ActRIIB) with the Fc part of IgG immunoglobulin. The presence of the Fc domain not only stabilizes the fusion trap but also increases the half-life in the circulation through its interplay with the FcRn (neonatal Fc-receptor) recycling mechanism. This interaction is beneficial because it prolongs the effect of the drug [41]. In this form, the Fc-IgG fusion displays an increased binding tendency to activins or other TGF-β proteins and reduces the stimulation of the Smad pathway. ACE-011 (sotatercept) is an Fc-IgG fusion trap that derives from the combination of the extracellular fragment of ActRIIA with the Fc part of human IgG. ACE-536 (luspatercept) is a fusion of an altered extracellular domain of ActRIIB with the Fc region of human IgG1, while RAP-536 involves mouse IgG2a [42] (Figure 2). RAP-536 fusion proteins have been used in mouse models with thalassemia, with beneficial effects on the reduction of ineffective erythropoiesis, splenomegaly, and iron overload. The use of ACE-536 (luspatercept) reduced the activation of the Smad2/3-Smad4 pathway and promoted the differentiation of the erythroid lineage by reestablishing GATA-1 function, likely by favoring the Smad2/3-TIF1γ pathway over Smad2/3-Smad4. Furthermore, upregulation of HSP70 led to the amelioration of oxidative stress and the promotion of erythroid differentiation [43,44,45,46]. The exact mechanism of action of luspatercept is not fully defined, and further research is needed to reveal the cellular consequences of TGF-β inhibition.
The use of luspatercept in adult thalassemia subjects has been initially evaluated in a phase 2 trial (NCT01749540). In this study, the subcutaneous use of luspatercept improved Hb levels and reduced transfusion requirements [47]. In particular, luspatercept led to a mean increase in Hb of ≥ 1.5 g/dL for at least 2 weeks in more than half (58%) of the NTDT patients, while in 81% of TDT patients a ≥ 20% decrease in RBC transfusion requirements was recorded. The most common grade 1 to 2 adverse events were headache, bone pain, and myalgia [47]. This 24-week dose-finding study was followed by a 5-year extension phase, presently continuing (NCT02268409). Comparable results were obtained with sotatercept; however the drug was not selected for further phase 3 studies, mainly because, distinct to luspatercept, it is a less specific activin receptor-II ligand trap, may be less efficacious in treating anemia and have more off-target effects [48].
Regarding luspatercept, the encouraging results from the phase 2 trial led to the phase 3 BELIEVE trial (NCT02604433), which was recently completed. The trial was designed to define the efficacy and safety of luspatercept in TDT adults [49], who were randomized to obtain subcutaneously either luspatercept at an initial dose of 1.0 mg/kg with a gradual increase up to 1.25 mg/kg or a placebo every 3 weeks for ≥ 48 weeks. The primary endpoint was a ≥ 33% reduction in transfusions, with a reduction of ≥ 2 RBC units during weeks 13 to 24. The secondary endpoints comprised a ≥ 33% reduction in transfusion load and at least 2 RBC units at weeks 37 to 48, and a ≥ 50% reduction in transfusions and at least 2 RBC units at weeks 13 to 24. The median age of the patients was 30 years (range 18–66 years), and 58% were women. The β0/β0 genotype was observed in 30% patients in the luspatercept group and 31% patients in the placebo group. The primary endpoint was significantly achieved in the luspatercept group: 21.4% vs. 4.5% achieved a reduction of at least 33% in the transfusions during weeks 13 to 24 [49].
All the subgroups (β0/β0, β0/β+, β+/β+, HbE/β-thalassemia) benefited consistently from luspatercept treatment, even the difficult-to-treat patients, such as those with the β0/β0 genotype (although the magnitude of benefit was lower) or patients receiving more than 6 RBC units/12 weeks at baseline. While response rates were lower in patients with the most severe disease (β0/β0), clinically significant declines in transfusion load were observed across genotypes. A ≥ 33% decrease in the transfusions at weeks 37–48 was accomplished by 19% of the luspatercept group compared to 3.6% of the placebo group. Regarding the additional target of achieving a ≥ 33% decrease in transfusions throughout any successive 12 weeks of the trial, 70.5% of the luspatercept group reached this level compared to 29% of the placebo group, while for any 24-week period, the difference was 41.1% and 2.7%, respectively. Among patients who achieved a decline of at least 33% during any 12 weeks, 80.4% had at least two episodes of response, and 51.3% had at least four episodes of response. Treatment-emergent adverse effects were similar with data previously described in the phase 2 study. Adverse effects in the luspatercept arm included bone pain (19.7%), arthralgia (19.3%), dizziness (11.2%), hypertension (8.1%), and hyperuricemia (7.2%). Bone pain was more frequent during the first 12 weeks in both arms and could be alleviated with regular analgesia. Thromboembolic events occurred in eight patients (3.6%) in the luspatercept arm and in one patient (0.9%) in the placebo arm. All eight patients in the luspatercept group had undergone splenectomy and had at least one risk factor for thromboembolic events. The discontinuation rate due to an adverse event was 5.4% for the luspatercept arm and 0.9% for the placebo, and no deaths were reported in either treatment groups [49]. As expected, recent data on long-term follow up have shown that the addition of luspatercept in standard care had sustained long-term efficacy [50], reduced the risk of iron overload complications [51], and improved the health-related quality of life [52].
In order to study the safety and pharmacokinetics in TDT children, a phase 2 clinical trial (NCT04143724) has been planned but is not yet recruiting patients. A major concern in children is the potential toxicity of luspatercept in the developing hematopoietic system and other vital organs. Furthermore, interfering with the BMP pathway may compromise growth in an immature skeletal system.
Luspatercept could also have a place in the treatment of NTDT patients. The BEYOND trial is a phase 2 study to define the effectiveness and safety of luspatercept in adults with NTDT (NCT03342404) [53]. The primary objective is the increase of mean Hb without any transfusions over a 12-week period, from week 13 to 24, compared to the initial phase. The study was completed in September 2020, and the results are expected.
In summary, the subcutaneous use of luspatercept every 3 weeks demonstrated significant reductions in RBC transfusion burden in TDT adult patients and was granted FDA (Food and Drug Administration) and EMA (European Medicines Agency) approval for TDT patients. It seems that luspatercept will have a significant place in the real-life management of TDT patients.
3.2. Pyruvate Kinase Activation
Pyruvate kinase (PK) is the enzyme that plays a significant role in the last stage of glycolysis in the RBC, the conversion of phosphoenolpyruvate to pyruvate in order to generate ATP. Therefore, PK is necessary for the energy production in the RBC. In patients with PK deficiency, an autosomal recessive disease, the energy-depleted erythrocytes are prone to hemolysis. Additionally, the ATP-deficiency in the erythroblasts leads to ineffective erythropoiesis. Patients with PK deficiency have symptoms and signs due to ineffective erythropoiesis and chronic hemolysis [54] and the clinical picture resembles NTDT. The oral use of the PK activator AG-348 (mitapivat) in healthy subjects has been shown to increase ATP [55] and has been proven to be efficient and safe in PK deficiency patients [56].
The promising results of mitapivat in PK deficiency, a disease resembling NTDT, led to the use of mitapivat in NTDT. The results showed a significant decrease of reactive oxygen species levels and an improvement of ineffective erythropoiesis. Moreover, a reduction of liver iron deposition and an increase of hepcidin levels were observed [57]. The interim results from a phase 2 trial (NCT03692052) of mitapivat in 13 adults with NTDT (α- or β-thalassemia) were presented at the 2020 ASH annual meeting. Mitapivat was given orally twice daily for 6 weeks, with a starting dose of 50 mg and a possibility to increase to 100 mg at the 6th week. The majority of the patients (92.3%, 4/4 with α-thalassemia, 8/9 with β-thalassemia) achieved an Hb increase from baseline above 1.0 g/dL after a median of 3.1 weeks (range 1.4–7.1). During the period of 4–12 weeks, the mean Hb increase from baseline was 1.34 g/dL with concomitant amelioration of hemolysis indices. No serious adverse events were reported [58]. Phase 3 studies will assess the efficacy and safety of mitapivat in TDT and NTDT patients (α- or β-thalassemia) (NCT04770779, NCT04770753).
3.3. Phosphodiesterase 9 Inhibition
The alteration of intracellular cyclic guanosine monophosphate (cGMP) is a novel therapeutic objective for sickle cell disease and thalassemia. The cGMP-dependent pathway is significant for the production of HbF and has multiple roles in vascular biology. As phosphodiesterase (PDE) 9 selectively degrades cGMP in erythropoietic cells, the use of inhibitors of PDE9 can result in increased cGMP levels and the reactivation of HbF [59,60].
IMR-687 is a novel agent that has been developed for the inhibition of PDE 9. The oral use of IMR-687 in sickle cell disease patients has been recently completed (NCT03401112) and has shown to stimulate HbF production and to improve Hb levels and hemolysis indices [61]. A similar phase 2 study has been launched in order to evaluate the safety and tolerability of IMR-687 given once daily for 36 weeks in TDT and NTDT adult thalassemic patients (NCT04411082).
3.4. Iron Metabolism Manipulation
Hepcidin, a protein produced from the hepatocytes, is the key controller of iron metabolism. Hepcidin degrades ferroportin, the main iron exporter in intestinal cells; macrophages of the reticuloendothelial system; and the hepatocytes. In β-thalassemia, hepcidin production is inhibited through the action of ERFE, contributing to increased intestinal iron absorption and tissue deposition [7]. Restoration of hepcidin levels could improve iron overload and ineffective erythropoiesis [62].
Hepcidin production is mainly regulated by matriptase-2 (MT-2), a transmembrane serine protease, which is encoded by the TMPRSS6 gene. MT-2 inhibits hepcidin activation by cleaving membrane hemojuvelin [63]. In mouse models with NTDT, the inhibition of TMPRSS6 with silencing RNAs or antisense oligonucleotides caused a rise of hepcidin and an amelioration of anemia and iron deposition [64,65,66,67]. Phase 2 clinical trials are already recruiting NTDT patients in order to assess the efficacy, safety, and pharmacokinetics of the short interfering RNAs, SLN124 (NCT04718844), and IONIS TMPRSS6-LRx (NCT04059406).
The use of minihepcidins, which act as hepcidin agonists, has been proven to improve ineffective erythropoiesis and splenomegaly in a TDT mouse model [68]. Recent initial results of a phase 2 trial (NCT04054921) on the subcutaneous use of PTG-300, a hepcidin mimetic analog, have shown a reduction in serum iron parameters and a 20% or greater reduction in transfusions, with mild adverse effects [69]. Another approach is to restrict the availability of iron, targeting both the iron overload and the ineffective erythropoiesis, with the use of ferroportin inhibitors (VIT-2763). These agents could be beneficial in NTDT. In an NTDT mouse model, the oral use of VIT-2763 along with deferasirox reduced liver iron and improved anemia [70]. A phase 1 study in healthy volunteers has shown that the drug was well accepted with no significant safety concerns along with a transient decrease in mean serum iron levels and transferrin saturation [71]. A phase 2 study of VIT-2763 in NTDT (VITHAL, NCT04364269) is already recruiting patients.
4. Conclusions—Key Points
In β-thalassemia, the globin chain inequality, ineffective erythropoiesis, and iron tissue deposition are the main underlying pathophysiological mechanisms that lead to the complications of the disease. Recent research on these mechanisms has revealed novel curative and “disease-modifying” therapeutic approaches.
Clinically meaningful progress in the techniques of allo-HSCT has been accomplished mainly in the area of the conditioning regimen and donor selection. Gene manipulation studies have exhibited remarkable advances. The lentiglobin gene therapy (Zynteglo) has received conditional EMA approval for TDT patients with non-β0/β0 genotype who are ≥12 years of age and who are eligible for allo-HSCT but do not have a suitable donor. The need for a specialized center for the procedure as well as the high financial cost are barriers to the widespread application of gene therapy. Furthermore, long-term safety data are needed regarding toxicity due to the conditioning regimen and, potentially, to insertional mutagenesis. However, gene therapy has more a measurable and objective effect than luspatercept and may lead to transfusion independence.
A very recent and highly sophisticated approach is the application of epigenetic and genomic editing methods that target the silencing of γ-globin repressors, such as BCL11A, or the correction of β-globin gene. Remarkable results have been reported in preclinical studies, while the preliminary results from clinical trials are more than promising.
A phase 3 trial in TDT patients has shown that the subcutaneous administration of luspatercept, a TGF-β family member inhibitor, led to a substantial decrease in transfusions, with a favorable safety profile. These findings led to its approval by the FDA and the EMA for treatment of anemia in adult TDT patients. It seems that luspatercept will have a significant place in the treatment algorithm of TDT patients, and its real-world application is expected to offer further information. It is a pharmacological therapy that can be an additive to standard care with manageable side effects that allows the patient to discontinue the drug when needed or in cases of poor response, whereas this in not realistic in gene therapy. It can also be an alternative option in cases of young individuals above 18 years of age who do not have the criteria for transplantation or the availability of a matching related donor. The use of luspatercept is also being studied in NTDT patients, and clinical trials have been designed for children. Furthermore, the fact that luspatercept reduces the need for transfusions will be beneficial in health care systems that have a shortage of blood products, especially during this period of the SARS-CoV-2 pandemic.
Another agent that improves ineffective erythropoiesis is mitapivat, a PK activator. The oral administration of mitapivat in a small number of patients has shown promising results in ongoing clinical trials. An alternative way to improve anemia in thalassemic patients is to stimulate HbF production with the use of phosphodiesterase 9 inhibitors, such as IMR-687. This agent has shown encouraging results in sickle cell disease patients and is currently being tested in aduts with TDT and NTDT. A different therapeutic approach is to target the dysregulation of iron homeostasis and indirectly improve erythropoiesis, by using hepcidin agonists (inhibitors of TMPRSS6 and minihepcidins) or ferroportin inhibitors (VIT-2763).
To conclude, all these emerging treatment modalities require long-term experience in order to further establish their efficacy and safety. Another concern is the availability and the high cost, especially in low- and middle-income countries. Should all modalities be available, a patient-centered approach could be pursued. For individuals with NTDT, transfusions are given as needed in certain clinical settings. In TDT, the mainstream treatment is chronic transfusions to alleviate anemia and suppress extramedullary hematopoiesis, with parallel management of the excess free iron and the complications of the disease. The quality of life is affected, and some adult patients may consider the option of disease-modifying agents which improve erythropoiesis, such as luspatercept. If a curative and permanent treatment is considered, allo-HSCT can be offered with a good outcome, especially in children. Matched related transplantation is the preferable approach, but other alternatives can be considered in terms of donor selection and conditioning regimens. In the case of a non-suitable donor, gene therapy with β-gene addition is an option in certified transplant centers.
Author Contributions
Writing—original draft preparation, A.M. and E.H.; methodology and data curation, A.M., E.V., I.P. and E.H.; writing—review and editing, E.V. and I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nagar R., Sinha S., Raman R. Genotype-phenotype correlation and report of novel mutations in beta-globin gene in thalassemia patients. Blood Cells Mol. Dis. 2015;55:10–14. doi: 10.1016/j.bcmd.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Rund D., Rachmilewitz E. Beta-thalassemia. N. Engl. J. Med. 2005;353:1135–1146. doi: 10.1056/NEJMra050436. [DOI] [PubMed] [Google Scholar]
- 3.Musallam K.M., Rivella S., Vichinsky E., Rachmilewitz E.A. Non-transfusion-dependent thalassemias. Haematologica. 2013;98:833–844. doi: 10.3324/haematol.2012.066845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blank U., Karlsson S. The role of Smad signaling in hematopoiesis and translational hematology. Leukemia. 2011;25:1379–1388. doi: 10.1038/leu.2011.95. [DOI] [PubMed] [Google Scholar]
- 5.Gardenghi S., Marongiu M.F., Ramos P., Guy E., Breda L., Chadburn A., Liu Y., Amariglio N., Rechavi G., Rachmilewitz E.A., et al. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109:5027–5035. doi: 10.1182/blood-2006-09-048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kautz L., Jung G., Valore E.V., Rivella S., Nemeth E., Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014;46:678–684. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kautz L., Jung G., Du X., Gabayan V., Chapman J., Nasoff M., Nemeth E., Ganz T. Erythroferrone contributes to hepcidin suppression and iron overload in a mouse model of beta-thalassemia. Blood. 2015;126:2031–2037. doi: 10.1182/blood-2015-07-658419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Origa R., Cazzola M., Mereu E., Danjou F., Barella S., Giagu N., Galanello R., Swinkels D.W. Differences in the erythropoiesis-hepcidin-iron store axis between hemoglobin H disease and beta-thalassemia intermedia. Haematologica. 2015;100:e169–e171. doi: 10.3324/haematol.2014.115733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanno T., Bhanu N.V., Oneal P.A., Goh S.H., Staker P., Lee Y.T., Moroney J.W., Reed C.H., Luban N.L., Wang R.H., et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat. Med. 2007;13:1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 10.Tanno T., Porayette P., Sripichai O., Noh S.J., Byrnes C., Bhupatiraju A., Lee Y.T., Goodnough J.B., Harandi O., Ganz T., et al. Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood. 2009;114:181–186. doi: 10.1182/blood-2008-12-195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaudio A., Xourafa A., Rapisarda R., Zanoli L., Signorelli S.S., Castellino P. Hematological Diseases and Osteoporosis. Int. J. Mol. Sci. 2020;21:3538. doi: 10.3390/ijms21103538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Sanctis V., Soliman A.T., Elsedfy H., Pepe A., Kattamis C., El Kholy M., Yassin M. Diabetes and Glucose Metabolism in Thalassemia Major: An Update. Expert Rev. Hematol. 2016;9:401–408. doi: 10.1586/17474086.2016.1136209. [DOI] [PubMed] [Google Scholar]
- 13.Karimi M., Cohan N., De Sanctis V., Mallat N.S., Taher A. Guidelines for diagnosis and management of Beta-thalassemia intermedia. Pediatr. Hematol. Oncol. 2014;31:583–596. doi: 10.3109/08880018.2014.937884. [DOI] [PubMed] [Google Scholar]
- 14.Taher A.T., Radwan A., Viprakasit V. When to consider transfusion therapy for patients with non-transfusion-dependent thalassaemia. Vox Sang. 2015;108:1–10. doi: 10.1111/vox.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rachmilewitz E.A., Giardina P.J. How I treat thalassemia. Blood. 2011;118:3479–3488. doi: 10.1182/blood-2010-08-300335. [DOI] [PubMed] [Google Scholar]
- 16.Lucarelli G., Isgro A., Sodani P., Gaziev J. Hematopoietic stem cell transplantation in thalassemia and sickle cell anemia. Cold Spring Harb. Perspect. Med. 2012;2:a011825. doi: 10.1101/cshperspect.a011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elalfy M.S., Adly A.A., Ismail E.A., Elhenawy Y.I., Elghamry I.R. Therapeutic superiority and safety of combined hydroxyurea with recombinant human erythropoietin over hydroxyurea in young beta-thalassemia intermedia patients. Eur. J. Haematol. 2013;91:522–533. doi: 10.1111/ejh.12182. [DOI] [PubMed] [Google Scholar]
- 18.Karimi M. Hydroxyurea in the management of thalassemia intermedia. Hemoglobin. 2009;33(Suppl. 1):S177–S182. doi: 10.3109/03630260903351809. [DOI] [PubMed] [Google Scholar]
- 19.Bourantas K., Economou G., Georgiou J. Administration of high doses of recombinant human erythropoietin to patients with beta-thalassemia intermedia: A preliminary trial. Eur. J. Haematol. 1997;58:22–25. doi: 10.1111/j.1600-0609.1997.tb01405.x. [DOI] [PubMed] [Google Scholar]
- 20.Angelucci E., Matthes-Martin S., Baronciani D., Bernaudin F., Bonanomi S., Cappellini M.D., Dalle J.H., Di Bartolomeo P., de Heredia C.D., Dickerhoff R., et al. Hematopoietic stem cell transplantation in thalassemia major and sickle cell disease: Indications and management recommendations from an international expert panel. Haematologica. 2014;99:811–820. doi: 10.3324/haematol.2013.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goussetis E., Peristeri I., Kitra V., Vessalas G., Paisiou A., Theodosaki M., Petrakou E., Dimopoulou M.N., Graphakos S. HLA-matched sibling stem cell transplantation in children with beta-thalassemia with anti-thymocyte globulin as part of the preparative regimen: The Greek experience. Bone Marrow Transplant. 2012;47:1061–1066. doi: 10.1038/bmt.2011.219. [DOI] [PubMed] [Google Scholar]
- 22.Gaziev J., De Angelis G., Isgro A., Sodani P., Marziali M., Paciaroni K., Andreani M., Testi M., Gallucci C., Alfieri C., et al. Transplant Outcomes in High-Risk (Class 3) Patients with Thalassemia Treated with a Modified Protocol Are Equivalent to Low/Intermediate-Risk (Class 1/Class 2) Patients. Blood. 2015;126:620. doi: 10.1182/blood.V126.23.620.620. [DOI] [Google Scholar]
- 23.King A.A., Kamani N., Bunin N., Sahdev I., Brochstein J., Hayashi R.J., Grimley M., Abraham A., Dioguardi J., Wah Chan K., et al. Successful matched sibling donor marrow transplantation following reduced intensity conditioning in children with hemoglobinopathies. Am. J. Hematol. 2015;90:1093–1098. doi: 10.1002/ajh.24183. [DOI] [PubMed] [Google Scholar]
- 24.Mohanan E.P., Panetta J.C., Backia Royan S.S., Abraham A., Edison E.S., Lakshmi K.M., Abubacker F.N., Korula A., Abraham A., Viswabandya A., et al. Population Pharmacokinetics of Fludarabine and Treosulfan in Patients with Thalassemia Undergoing Hematopoietic Stem Cell Transplantation. Blood. 2015;126:3120. doi: 10.1182/blood.V126.23.3120.3120. [DOI] [Google Scholar]
- 25.Thompson A.A., Walters M.C., Kwiatkowski J., Rasko J.E.J., Ribeil J.A., Hongeng S., Magrin E., Schiller G.J., Payen E., Semeraro M., et al. Gene Therapy in Patients with Transfusion-Dependent beta-Thalassemia. N. Engl. J. Med. 2018;378:1479–1493. doi: 10.1056/NEJMoa1705342. [DOI] [PubMed] [Google Scholar]
- 26.Thompson A.A., Walters M.C., Kwiatkowski J.L., Hongeng S., Porter J.B., Sauer M.G., Thrasher A.J., Thuret I., Elliot H., Tao G., et al. Northstar-2: Updated Safety and Efficacy Analysis of Lentiglobin Gene Therapy in Patients with Transfusion-Dependent β-Thalassemia and Non-β0/β0 Genotypes. Blood. 2019;134:3543. doi: 10.1182/blood-2019-126046. [DOI] [Google Scholar]
- 27.Lal A., Locatelli F., Kwiatkowski J.L., Kulozik A.E., Yannaki E., Porter J.B., Thuret I., Sauer M.G., Elliot H., Chen Y., et al. Northstar-3: Interim Results from a Phase 3 Study Evaluating Lentiglobin Gene Therapy in Patients with Transfusion-Dependent β-Thalassemia and Either a β0 or IVS-I-110 Mutation at Both Alleles of the HBB Gene. Blood. 2019;134:815. doi: 10.1182/blood-2019-128482. [DOI] [Google Scholar]
- 28.Kwiatkowski J.L., Walters M.C., Hongeng S., Locatelli F., Rasko J.E.L., Cavazzana M., Chen Y., Colvin R.A., Thompson A.A. Long-Term Efficacy and Safety of Betibeglogene Autotemcel Gene Therapy for the Treatment of Transfusion-Dependent β-Thalassemia: Results in Patients with up to 6 Years of Follow-up; Proceedings of the 62nd ASH Annual Meeting; San Diego, CA, USA. 5–8 December 2020. [Google Scholar]
- 29.Thompson A.A., Kwiatkowski J.L., Porter J.B., Hongeng S., Yannaki E., Kulozik A.E., Sauer M.G., Thrasher A.J., Thuret I., Lal A., et al. Favorable Outcomes in Pediatric Patients in the Phase 3 Hgb-207 (Northstar-2) and Hgb-212 (Northstar-3) Studies of Betibeglogene Autotemcel Gene Therapy for the Treatment of Transfusion-Dependent β-Thalassemia; Proceedings of the 62nd ASH Annual Meeting; San Diego, CA, USA. 5–8 December 2020. [Google Scholar]
- 30.Marktel S., Scaramuzza S., Cicalese M.P., Giglio F., Galimberti S., Lidonnici M.R., Calbi V., Assanelli A., Bernardo M.E., Rossi C., et al. Intrabone hematopoietic stem cell gene therapy for adult and pediatric patients affected by transfusion-dependent ss-thalassemia. Nat. Med. 2019;25:234–241. doi: 10.1038/s41591-018-0301-6. [DOI] [PubMed] [Google Scholar]
- 31.Guda S., Brendel C., Renella R., Du P., Bauer D.E., Canver M.C., Grenier J.K., Grimson A.W., Kamran S.C., Thornton J., et al. miRNA-embedded shRNAs for Lineage-specific BCL11A Knockdown and Hemoglobin F Induction. Mol. Ther. 2015;23:1465–1474. doi: 10.1038/mt.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith A.R., Schiller G.J., Vercellotti G.M., Kwiatkowski J.L., Krishnamurti L., Esrick E.B., Williams D.A., Miller W.P., Woolfson A., Walters M.C. Preliminary Results of a Phase 1/2 Clinical Study of Zinc Finger Nuclease-Mediated Editing of BCL11A in Autologous Hematopoietic Stem Cells for Transfusion-Dependent Beta Thalassemia. Blood. 2019;134:3544. doi: 10.1182/blood-2019-125743. [DOI] [Google Scholar]
- 33.Frangoul H., Altshuler D., Cappellini M.D., Chen Y.-S., Domm J., Eustace B.K., Foell J., de la Fuente J., Grupp S., Handgretinger R., et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N. Engl. J. Med. 2020;384:252–260. doi: 10.1056/NEJMoa2031054. [DOI] [PubMed] [Google Scholar]
- 34.Frangoul H., Bobruff Y., Cappellini M.D., Corbacioglu S., Fernandez C.M., de la Fuente J., Grupp S.A., Handgretinger R., Ho T.W., Imren S., et al. Safety and Efficacy of CTX001 in Patients with Transfusion-Dependent β-Thalassemia and Sickle Cell Disease: Early Results from the Climb THAL-111 and Climb SCD-121 Studies of Autologous CRISPR-CAS9–Modified CD34+ Hematopoietic Stem and Progenitor Cells; Proceedings of the 62nd ASH Annual Meeting; San Diego, CA, USA. 5–8 December 2020. [Google Scholar]
- 35.Hinck A.P. Structural studies of the TGF-betas and their receptors—Insights into evolution of the TGF-beta superfamily. FEBS Lett. 2012;586:1860–1870. doi: 10.1016/j.febslet.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 36.Maguer-Satta V., Bartholin L., Jeanpierre S., Ffrench M., Martel S., Magaud J.P., Rimokh R. Regulation of human erythropoiesis by activin A, BMP2, and BMP4, members of the TGFbeta family. Exp. Cell Res. 2003;282:110–120. doi: 10.1016/S0014-4827(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 37.Tanno T., Noel P., Miller J.L. Growth differentiation factor 15 in erythroid health and disease. Curr. Opin. Hematol. 2010;17:184–190. doi: 10.1097/MOH.0b013e328337b52f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graham H., Peng C. Activin receptor-like kinases: Structure, function and clinical implications. Endocr. Metab. Immune Disord. Drug Targets. 2006;6:45–58. doi: 10.2174/187153006776056585. [DOI] [PubMed] [Google Scholar]
- 39.Wrana J.L. Signaling by the TGFbeta superfamily. Cold Spring Harb. Perspect. Biol. 2013;5:a011197. doi: 10.1101/cshperspect.a011197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuki R., Tatewaki T., Yamaguchi N., Aoyama K., Honda T., Kubota S., Morii M., Manabe I., Kuga T., Tomonaga T., et al. Desuppression of TGF-beta signaling via nuclear c-Abl-mediated phosphorylation of TIF1gamma/TRIM33 at Tyr-524, -610, and -1048. Oncogene. 2019;38:637–655. doi: 10.1038/s41388-018-0481-z. [DOI] [PubMed] [Google Scholar]
- 41.Rath T., Baker K., Dumont J.A., Peters R.T., Jiang H., Qiao S.W., Lencer W.I., Pierce G.F., Blumberg R.S. Fc-fusion proteins and FcRn: Structural insights for longer-lasting and more effective therapeutics. Crit. Rev. Biotechnol. 2015;35:235–254. doi: 10.3109/07388551.2013.834293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sako D., Grinberg A.V., Liu J., Davies M.V., Castonguay R., Maniatis S., Andreucci A.J., Pobre E.G., Tomkinson K.N., Monnell T.E., et al. Characterization of the ligand binding functionality of the extracellular domain of activin receptor type IIb. J. Biol. Chem. 2010;285:21037–21048. doi: 10.1074/jbc.M110.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suragani R.N., Cawley S.M., Li R., Wallner S., Alexander M.J., Mulivor A.W., Gardenghi S., Rivella S., Grinberg A.V., Pearsall R.S., et al. Modified activin receptor IIB ligand trap mitigates ineffective erythropoiesis and disease complications in murine beta-thalassemia. Blood. 2014;123:3864–3872. doi: 10.1182/blood-2013-06-511238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dussiot M., Maciel T.T., Fricot A., Chartier C., Negre O., Veiga J., Grapton D., Paubelle E., Payen E., Beuzard Y., et al. An activin receptor IIA ligand trap corrects ineffective erythropoiesis in beta-thalassemia. Nat. Med. 2014;20:398–407. doi: 10.1038/nm.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez P., Bhasin M., Li R., Pearsall S., Kumar R., Suragani R. RAP-536 (murine analog of ACE-536/Luspatercept) inhibits SMAD2/3 signaling and promotes erythroid diffentiation by restoring GATA-1 function in a murine model of b-thalassemia; Proceedings of the 21st Congress of the European Haematology Association; Copenhagen, Denmark. 9–12 June 2016. [Google Scholar]
- 46.Martinez P.A., Li R., Ramanathan H.N., Bhasin M., Pearsall R.S., Kumar R., Suragani R. Smad2/3-pathway ligand trap luspatercept enhances erythroid differentiation in murine beta-thalassaemia by increasing GATA-1 availability. J. Cell. Mol. Med. 2020;24:6162–6177. doi: 10.1111/jcmm.15243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piga A., Perrotta S., Gamberini M.R., Voskaridou E., Melpignano A., Filosa A., Caruso V., Pietrangelo A., Longo F., Tartaglione I., et al. Luspatercept improves hemoglobin levels and blood transfusion requirements in a study of patients with beta-thalassemia. Blood. 2019;133:1279–1289. doi: 10.1182/blood-2018-10-879247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cappellini M.D., Porter J., Origa R., Forni G.L., Voskaridou E., Galacteros F., Taher A.T., Arlet J.B., Ribeil J.A., Garbowski M., et al. Sotatercept, a novel transforming growth factor beta ligand trap, improves anemia in beta-thalassemia: A phase II, open-label, dose-finding study. Haematologica. 2019;104:477–484. doi: 10.3324/haematol.2018.198887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cappellini M.D., Viprakasit V., Taher A.T., Georgiev P., Kuo K.H.M., Coates T., Voskaridou E., Liew H.K., Pazgal-Kobrowski I., Forni G.L., et al. A Phase 3 Trial of Luspatercept in Patients with Transfusion-Dependent beta-Thalassemia. N. Engl. J. Med. 2020;382:1219–1231. doi: 10.1056/NEJMoa1910182. [DOI] [PubMed] [Google Scholar]
- 50.Taher A.T., Viprakasit V., Cappellini M.D., Hermine O., Georgiev P., Kuo K.H.M., Coates T., Voskaridou E., Liew H.K., Pazgal-Kobrowski I., et al. Assessment of longer-term efficacy and safety in the phase 3 BELIVE trial of luspatercept to treat anemia in patients (pts) with b-thalassemia; Proceedings of the 25th EHA Congress; virtual. 11–21 June 2020. [Google Scholar]
- 51.Hermine O., Cappellini M.D., Taher A.T., Coates T.D., Viprakasit V., Voskaridou E., Lal A., Liew H.K., Perrotta S., Khelif A., et al. Longitudinal Effect of Luspatercept Treatment on Iron Overload and Iron Chelation Therapy (ICT) in Adult Patients (Pts) with β-Thalassemia in the Believe Trial; Proceedings of the 62nd ASH Annual Meeting; San Diego, CA, USA. 5–8 December 2020. [Google Scholar]
- 52.Cappellini M.D., Taher A.T., Piga A., Shah F., Voskaridou E., Viprakasit V., Porter J.B., Hermine O., Neufeld E.J., Thompson A.A., et al. Health-Related Quality of Life Outcomes for Patients with Transfusion-Dependent Beta-Thalassemia Treated with Luspatercept in the Believe Trial; Proceedings of the 62nd ASH Annual Meeting; San Diego, CA, USA. 5–8 December 2020. [Google Scholar]
- 53.Piga A. Transfusion Dependent Beta (β)-Thalassemia (BEYOND) [(accessed on 8 May 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03342404.
- 54.Aizawa S., Harada T., Kanbe E., Tsuboi I., Aisaki K., Fujii H., Kanno H. Ineffective erythropoiesis in mutant mice with deficient pyruvate kinase activity. Exp. Hematol. 2005;33:1292–1298. doi: 10.1016/j.exphem.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 55.Yang H., Merica E., Chen Y., Cohen M., Goldwater R., Kim H., Kosinski P., Kung C., Goldwasser M., Silver B., et al. Phase 1 multiple ascending dose study of the safety, tolerability, and pharmacokinetics/pharmacodynamics of AG-348, a first-in-class allosteric activator of pyruvate kinase-R, in healthy subjects; Proceedings of the 20th Congress of the European Haematology Association; Vienna, Austria. 11–14 June 2015. [Google Scholar]
- 56.Grace R.F., Rose C., Layton D.M., Galactéros F., Barcellini W., Morton D.H., van Beers E.J., Yaish H., Ravindranath Y., Kuo K.H.M., et al. Safety and Efficacy of Mitapivat in Pyruvate Kinase Deficiency. N. Engl. J. Med. 2019;381:933–944. doi: 10.1056/NEJMoa1902678. [DOI] [PubMed] [Google Scholar]
- 57.Matte A., Beneduce E., Siciliano A., Kosinski P., Janin A., Lebouef C., Iolacson A., De Falco L., Dang L., Kung C., et al. The pyruvate kinase activator AG-348 improves murine b-thalassemic anemia and corrects ineffective erythropoiesis; Proceedings of the 21st Congress of the European Haematology Association; Copenhagen, Denmark. 9–12 June 2016. [Google Scholar]
- 58.Kuo K.H., Layton D.M., Lal A., Al-Samkari H., Tai F., Lynch M., Uhlig K., Vichinsky E.P. Proof of Concept for the Oral Pyruvate Kinase Activator Mitapivat in Adults with Non–Transfusion-Dependent Thalassemia: Interim Results from an Ongoing, Phase 2, Open-Label, Multicenter Study; Proceedings of the 62nd ASH Annual Meeting; San Diego, CA, USA. 5–8 December 2020. [Google Scholar]
- 59.Almeida C.B., Traina F., Lanaro C., Canalli A.A., Saad S.T., Costa F.F., Conran N. High expression of the cGMP-specific phosphodiesterase, PDE9A, in sickle cell disease (SCD) and the effects of its inhibition in erythroid cells and SCD neutrophils. Br. J. Haematol. 2008;142:836–844. doi: 10.1111/j.1365-2141.2008.07264.x. [DOI] [PubMed] [Google Scholar]
- 60.McArthur J.G., Svenstrup N., Chen C., Fricot A., Carvalho C., Nguyen J., Nguyen P., Parachikova A., Abdulla F., Vercellotti G.M., et al. A novel, highly potent and selective phosphodiesterase-9 inhibitor for the treatment of sickle cell disease. Haematologica. 2020;105:623–631. doi: 10.3324/haematol.2018.213462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bronte-Hall L., Andemariam B., Gershwin B., Lugthart S., Mant T., Howard J., Fok H., Eleftheriou P., Hagar R.W., Mason J., et al. Benefits and Safety of Long-Term Use of IMR-687 As Monotherapy or in Combination with a Stable Dose of Hydroxyurea (HU) in 2 Adult Sickle Cell Patients. Blood. 2020;136:29–30. doi: 10.1182/blood-2020-140540. [DOI] [Google Scholar]
- 62.Schmidt P.J., Fleming M.D. Modulation of hepcidin as therapy for primary and secondary iron overload disorders: Preclinical models and approaches. Hematol./Oncol. Clin. N. Am. 2014;28:387–401. doi: 10.1016/j.hoc.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silvestri L., Pagani A., Nai A., De Domenico I., Kaplan J., Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Casu C., Aghajan M., Oikonomidou P.R., Guo S., Monia B.P., Rivella S. Combination of Tmprss6- ASO and the iron chelator deferiprone improves erythropoiesis and reduces iron overload in a mouse model of beta-thalassemia intermedia. Haematologica. 2016;101:e8–e11. doi: 10.3324/haematol.2015.133348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmidt P.J., Racie T., Westerman M., Fitzgerald K., Butler J.S., Fleming M.D. Combination therapy with a Tmprss6 RNAi-therapeutic and the oral iron chelator deferiprone additively diminishes secondary iron overload in a mouse model of beta-thalassemia intermedia. Am. J. Hematol. 2015;90:310–313. doi: 10.1002/ajh.23934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stagg D.B., Whittlesey R.L., Li X., Lozovatsky L., Gardenghi S., Rivella S., Finberg K.E. Genetic loss of Tmprss6 alters terminal erythroid differentiation in a mouse model of beta-thalassemia intermedia. Haematologica. 2019;104:e442–e446. doi: 10.3324/haematol.2018.213371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmidt P.J., Liu K., Visner G., Fitzgerald K., Fishman S., Racie T., Hettinger J.L., Butler J.S., Fleming M.D. RNAi-mediated reduction of hepatic Tmprss6 diminishes anemia and secondary iron overload in a splenectomized mouse model of beta-thalassemia intermedia. Am. J. Hematol. 2018;93:745–750. doi: 10.1002/ajh.25079. [DOI] [PubMed] [Google Scholar]
- 68.Casu C., Chessa R., Liu A., Gupta R., Drakesmith H., Fleming R., Ginzburg Y.Z., MacDonald B., Rivella S. Minihepcidins improve ineffective erythropoiesis and splenomegaly in a new mouse model of adult beta-thalassemia major. Haematologica. 2020;105:1835–1844. doi: 10.3324/haematol.2018.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lai A., Voskaridou E., Flevari P., Taher A., Chew L., Valone F., Gupta S., Viprakasit V. A hepcidin mimetic, PTG-300, demonstrates, pharmacodynamic effects indicating reduced iron availability in transfusion-dependent beta-thalassemia subjects; Proceedings of the 25th EHA Congress; virtual. 11–21 June 2020. [Google Scholar]
- 70.Nyffenegger N., Flace A., Doucerain C., Durrenberger F., Manolova V. The Oral Ferroportin Inhibitor VIT-2763 Improves Erythropoiesis without Interfering with Iron Chelation Therapy in a Mouse Model of beta-Thalassemia. Int. J. Mol. Sci. 2021;22:873. doi: 10.3390/ijms22020873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Richard F., van Lier J.J., Roubert B., Haboubi T., Gohring U.M., Durrenberger F. Oral ferroportin inhibitor VIT-2763: First-in-human, phase 1 study in healthy volunteers. Am. J. Hematol. 2020;95:68–77. doi: 10.1002/ajh.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.