Abstract
Developing, regenerating, and repairing a lung all require interstitial resident fibroblasts (iReFs) to direct the behavior of the epithelial stem cell niche. During lung development, distal lung fibroblasts, in the form of matrix‐, myo‐, and lipofibroblasts, form the extra cellular matrix (ECM), create tensile strength, and support distal epithelial differentiation, respectively. During de novo septation in a murine pneumonectomy lung regeneration model, developmental processes are reactivated within the iReFs, indicating progenitor function well into adulthood. In contrast to the regenerative activation of fibroblasts upon acute injury, chronic injury results in fibrotic activation. In murine lung fibrosis models, fibroblasts can pathologically differentiate into lineages beyond their normal commitment during homeostasis. In lung injury, recently defined alveolar niche cells support the expansion of alveolar epithelial progenitors to regenerate the epithelium. In human fibrotic lung diseases like bronchopulmonary dysplasia (BPD), idiopathic pulmonary fibrosis (IPF), and chronic obstructive pulmonary disease (COPD), dynamic changes in matrix‐, myo‐, lipofibroblasts, and alveolar niche cells suggest differential requirements for injury pathogenesis and repair. In this review, we summarize the role of alveolar fibroblasts and their activation stage in alveolar septation and regeneration and incorporate them into the context of human lung disease, discussing fibroblast activation stages and how they contribute to BPD, IPF, and COPD.
Keywords: alveolar niche, bronchopulmonary dysplasia (BPD), chronic obstructive pulmonary disease (COPD), development, interstitial lung fibroblasts, idiopathic pulmonary fibrosis (IPF)
PDGFRα+ contractile myofibroblasts, AT2‐suppprting lipofibroblasts, and ECM‐producing matrix fibroblasts are all necessary to build the lung during alveolarization and rebuild the lung after acute or chronic injury. Highly sensitive to cues from the alveolar microenvironment, the PDGFRα+ population undergoes dynamic changes to function in disease or regeneration. We summarize the current knowledge of context‐dependent functional stages of interstitial fibroblasts.
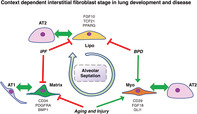
Significance statement.
This concise review summarizes the existing literature on alveolar fibroblasts and compares and contrasts findings in murine development and fibrosis resolution and human fibrotic lung disease. This article summarizes the fibroblast stages during lung development, regeneration, and repair, with a focus on their role in supporting the alveolar stem cell niche and their potential to function as stromal progenitor cells. The murine findings were extended and incorporated into the context of human disease, and the article also reports on the fibroblast activation stages and how they contribute to bronchopulmonary dysplasia, idiopathic pulmonary fibrosis, and chronic obstructive pulmonary disease.
1. INTRODUCTION
A diverse set of genes driven by various cell types are key to the regulation of alveolar septation and regeneration. Distal alveolar epithelial cells (AECs) (Alveolar Type 1 [AT1] and Type 2 [AT2]) facilitate proper gas exchange and maintain proper alveolar surface tension. An increase in alveolar surface area that occurs during the late phases of lung development is key to proper alveolar function. Throughout development, the epithelium requires constant and finely tuned molecular cues from neighboring fibroblasts to direct proper proliferation and differentiation. 1 Interruption of alveolar maturation by premature birth and exposure to hyperoxia can lead to bronchopulmonary dysplasia (BPD) in premature neonates, and repeated injury‐induced damage to the functional alveolar units in adults can cause chronic obstructive pulmonary disease (COPD), emphysema, and idiopathic pulmonary fibrosis (IPF). Chronic pulmonary diseases such as these are complex and involve a multitude of cell types beyond the epithelium. Supporting cell types within the alveolar niche, such as fibroblasts, influence the process of alveolar septation, regeneration, and fibrotic injury response. Successful alveolarization occurs when coordinated interactions between all cells in the alveolar niche generate secondary septa, subsequently refining their architecture to reflect their function. Within the mesenchyme, interstitial resident fibroblasts play diverse and temporally critical roles in both alveolarization and alveolar regeneration. In the mouse, these alveolar fibroblasts are a mixed population of platelet derived growth factor receptor alpha (PDGFRa)‐expressing fibroblasts. During alveolarization and alveolar regeneration, PDGFRa+ myofibroblasts generate the mechanical force to extend the septal tip, and PDGFRa+ matrix fibroblasts create ECM components to stabilize the newly formed septa, whereas PDGFRa+ lipofibroblasts support AT2 cell function during homeostasis. 2 , 3 , 4 , 5 , 6 Moreover, recently defined alveolar niche cells, marked by PDGFRa and Axin2/Wnt2/Lgr5 coexpression, support alveolar epithelial regeneration after injury. 7 All four of these cell populations arrive at precisely the right time to provide both the scaffold and the paracrine signals the epithelium needs to proliferate and differentiate. The advent of single‐cell RNA sequencing (scRNA‐seq) and the refinement of inducible mouse lineage‐tracing systems have yielded a plethora of data on the interstitial lung fibroblast during alveolarization, 6 , 7 , 8 , 9 , 10 , 11 , 12 but individually analyzing these data can be overwhelming. In this review, we summarize the role of alveolar fibroblasts in alveolar septation and regeneration and incorporate them into context of how they are modified in and contribute to human lung diseases like BPD, IPF, and COPD.
1.1. Fibroblast subpopulations or fibroblast activation stages
Since the mid‐1990s, the importance of the alveolar mesenchyme in directing alveolar epithelial proliferation and differentiation has become the focus of several studies. 13 , 14 Alveolar fibroblasts: (a) provide and modulate an ECM scaffold for epithelial cells to expand upon, (b) provide tensile forces to extend and thin the septal walls during secondary septation, and (c) provide paracrine cues to the surrounding epithelium and endothelium to initiate proliferation and differentiation. During development, alveolar fibroblasts can be defined as four functional populations: myofibroblasts, lipofibroblasts, matrixfibroblasts, and alveolar niche cells. 15 These four populations cover all functions of the alveolar fibroblast but have been described as several fibroblast lineages that partially overlap. Based on individual localization and PDGFRα expression they have been called interstitial resident fibroblasts, “iReF,” 2 , 15 or alveolar niche cells. 7 We will discuss interstitial fibroblasts in the formation of the alveolus during development, their role during reseptation after partial pneumonectomy (PNX), and their role in disease formation and progression.
1.2. Fibroblasts in development
Interstitial fibroblast function during alveolarization
Pulmonary interstitial fibroblasts are critical for the formation and extension of alveolar septa postnatally but already prepare for septation prenatally. The alveolus is architecturally conserved between the human and murine peripheral lung. 16 , 17 Mice are born in the saccular phase, and alveolarization is observed postnatally, whereas human alveolarization starts at around 36 weeks of gestation. Babies born before 36 weeks of gestation are therefore born in the saccular stage of lung development and are susceptible to barotrauma and hyperoxia‐induced damage, contributing to the chronic lung injury seen in BPD. 17 , 18 , 19 As alveolarization occurs postnatally in mice, and interventions like hyperoxia and pharmacological treatments are amenable in neonatal mice, the murine system is highly valuable to study alveolarization and BPD. 20 Here, we summarize the temporal role of myo‐, matrix, lipo‐, and alveolar niche cell fibroblasts in the process of alveolarization.
A temporal comparison of human and murine alveolarization and the functional roles of interstitial fibroblasts are illustrated in Figure 1. During sacculation, alveolar ducts that end in simple primary alveoli are formed. At the onset of alveolarization (murine: PN2‐PN3; human: 36 weeks of gestation until 1 month after birth), secondary crests/ridges bulge out from the walls of the primary septa (Figure 1.1). 1 , 10 , 11 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 The secondary crests are elongated by the contractile force of secondary‐crest myofibroblasts (Figure 1.2) (murine: PN3‐PN14, human: 1‐18 months after birth). 21 , 28 These myofibroblasts also produce a framework of elastin and tenascin, supporting the newly forming secondary crest. As the myofibroblast contracts, it is assumed to pull the ECM that the matrixfibroblast beneath is actively making. 2 , 10 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 As secondary crests form and elongate, endothelial cells proliferate and form a double‐capillary bed. During secondary septa maturation, the double‐capillary bed fuses to a single capillary (Figure 1.3) (murine: PN4‐PN21, human: 1‐36 months after birth). 22 , 26 , 27 , 31 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 During and after septation, lipofibroblasts align themselves with AT2 cells and support their proliferation and differentiation to AT1 cells. 34 , 49 , 50 , 51 , 52 , 53 , 54 , 55 As the septa mature, matrix and myofibroblasts secrete metalloproteinases and other ECM‐remodeling proteins to thin the septal tip ECM. 2 , 29 , 35 , 40 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 The secondary‐crest myofibroblast continues producing elastin, eventually undergoing apoptosis during adulthood. 5 , 31 , 32 , 34 , 64 , 65 , 66 Lipofibroblasts continue to support AT2 cell surfactant production. 53 , 55 , 67 , 68 At the end of alveolarization, adult stem cell niches become defined, consisting of the lipofibroblast‐like alveolar niche cell (also called mesenchymal alveolar niche cell, MANC) and the alveolar epithelial progenitor (AEP). 7 , 69 , 70 , 71 , 72 The process of alveolarization, depicted in Figure 1, requires temporal and coordinated activity of functionally distinct fibroblast stages. In order for fibroblasts to acquire specific functions, inductive and reciprocal autocrine and epithelial or endothelial‐derived paracrine signals are required. Selective signature genes of these cellular players are listed next to the cellular key in Figure 1 73 , 74 , 75 , 76 (LGEA: https://research.cchmc.org/pbge/lunggens/mainportal.html). 77
FIGURE 1.
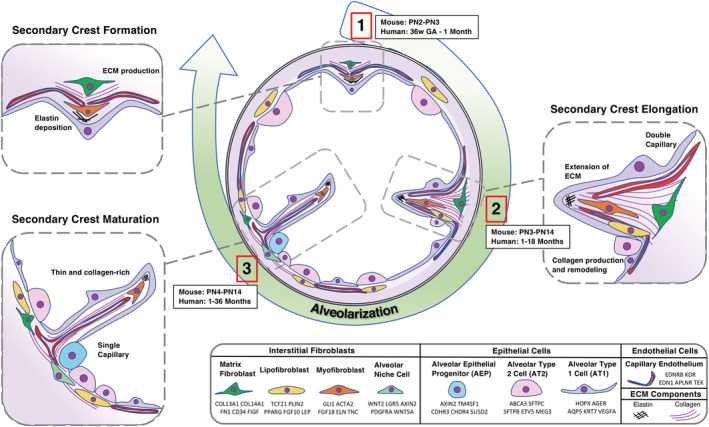
Interstitial fibroblasts and their role in alveolar septation and epithelial niche formation. Illustrations of mesenchymal subtypes in their spatial and temporal contributions to alveolarization. Three key stages of alveolarization are arranged in a clockwise fashion in the center of the illustration. (1) Secondary crest formation occurs in mice between PN2 and PN3 and in humans between 36 weeks' gestational age (GA) and 1 month. (2) Secondary crest elongation occurs in mice between PN3 and PN14 and in humans between 1 and 18 months. (3) Secondary crest maturation occurs in mice between PN4 and PN14 and in humans between 1 and 36 months. Major events, such as elastin deposition during primary septum formation, are labeled in the respective panels. The spatial location of each cell type is reflected, such as the myofibroblast that resides at the septal tip and the lipofibroblast sitting adjacent to the AT2 cell. Signature gene expression of each cell type, identified by developmental studies and scRNA‐seq, are given in the figure legend
Signaling pathways specify fibroblast subpopulations and control fibroblast function
Several conserved developmental signaling pathways are important for both fibroblast differentiation and signaling to the epithelium. PDGFRα signaling is critical for the activation of myofibroblasts in the septa as pdgfrα‐mutant mice fail to form septa during the initial phase of alveologenesis. 30 , 78 In addition to plateleted derived growth factor A (PDGFA, other signaling pathways such as retinoic acid (RA), fibroblast growth factor (FGF), sonic hedgehog (SHH), bone morphogenetic protein (BMP), and wingless‐related integration site (WNT) are indispensable for proper fibroblast differentiation and activation of their functional stages. RA induces PDGFA‐PDGFRα autocrine signaling in fibroblasts, and interruption during development by the genetic ablation of RA in mice causes alveolar simplification. 79 , 80 FGF10, produced in lipofibroblasts, directs epithelial proliferation and differentiation into AT1 cells during sacculation and alveolarization. 16 , 50 , 81 , 82 FGF18 marks a myofibroblast lineage, providing evidence that distinct FGF signaling is necessary to induce certain functional fibroblast stages. 5 , 67 , 83 , 84 The SHH ligand from the distal epithelium is required for the formation of Gli1+ secondary‐crest myofibroblasts as a lack of SHH signaling blocks myofibroblast differentiation. 3 , 32 , 85 , 86 , 87 The BMP/SMAD signaling pathway is reported to regulate alveolar stem cell proliferation and differentiation. PDGFRa+ myofibroblasts produce BMP4, which acts antagonistically to WNT and regulates AT2 cell renewal, differentiation, and regeneration. 69 Alveolar niche cells produce WNT ligands, which act mainly through canonical WNT signaling to replenish the AXIN2+ epithelial progenitor pool during development and repair. 7 , 70 , 71 , 88 Thus, all these fibroblast stages are integrated to form a fully functional alveolar niche. To aid in visualization, many of these signaling ligands that are specific to certain fibroblast subtypes during development are pictured in the legend of Figure 1. Further information about pulmonary fibroblast lineages during development can be found in a recent review that details the pathways and mice used for lineage‐tracing studies in the mesenchyme. 15
Besides these common and developmentally conserved pathways, hormones and steroids are emerging as significant modulators of fibroblast differentiation in the lung. 89 Recent findings identified that glucocorticoid receptor expression in pulmonary fibroblasts modulates alveolar maturation. 9 Glucocorticoid enhanced the differentiation of proliferative mesenchymal progenitor cells into matrixfibroblasts by a mechanism involving extracellular matrix‐associated target gene expression (including Fn1, Col16a4, and Eln) and by modulating vascular endothelial growth factor (VEGF), Janus kinase‐signal transducer and activator of transcription proteins (JAK‐STAT), and WNT signaling.
Based on scRNA‐seq data, there are two distinct types of matrixfibroblasts characterized by the expression of cell‐selective markers: MatrixFB‐1 and MatrixFB‐2. MatrixFB‐1 is known by the expression of molecular regulators such as WNT, FGF signaling, and T‐box binding domains. MatrixFB‐2 is significantly enriched for Sfrp2, an inhibitor of WNT signaling, and a family of insulin‐like growth factors. Although these matrixfibroblasts differ largely in their signature genes and main signaling pathways, some shared gene expression profiles suggest overlapping physiological functions such as ECM organization and collagen formation. Their spatial location, however, has not been defined thus far and might explain differences in paracrine signaling profiles. 11 Future scRNA‐seq studies and validation of their spatial and temporal localization will aid in understanding the differential and overlapping functions of various populations and cell stages of fibroblasts. Although interstitial fibroblasts play an important role in developmental alveolarization, they are equally important in injury and repair.
1.3. Fibroblasts in murine models of reseptation and repair
Reseptation
Although adult compensatory lung growth is restricted in humans, 90 unilateral left lobe PNX in mice initiates realveolarization in the remaining right lobes. PNX is an excellent model system to study molecular and cellular mechanisms of alveolar regeneration but does not recapitulate injury response. During realveolarization, myofibroblasts are critical to extend new septa (reseptation), and matrixfibroblasts are required for the production of new ECM to stabilize the septa. 29 The different fibroblast stages in reseptation after PNX are visualized in Figure 2A, along with their signature gene expression profiles. After PNX, septal tip myofibroblasts, which are PDGFRa‐GFP dim and alpha smooth muscle actin (αSMA) positive, increase in total number. PDGFRa‐GFPbright matrixfibroblasts subsequently expand and produce ECM required for the newly forming septal tip, defining the alveolar entry ring. A second wave of myofibroblast activation is observed toward the end of reseptation. 2 , 26 , 29 , 35 Taken together, these data demonstrate that dynamic PDGFRa activity controls temporal switches between myofibroblast and matrixfibroblast stages. 2 , 29 , 35
FIGURE 2.
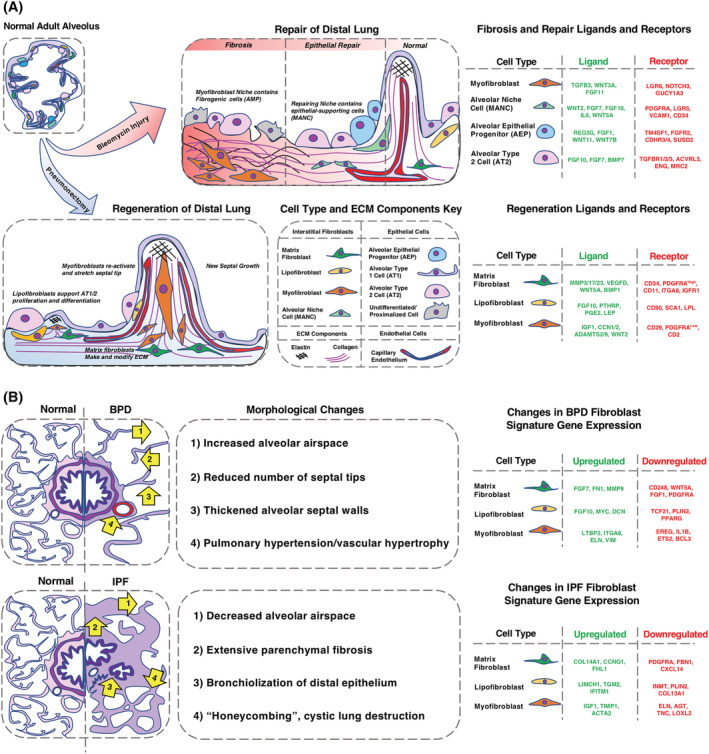
A, Role of resident interstitial fibroblasts in bleomycin‐induced fibrosis and realveolarization. Illustrations of spatial location and gene expression of the major fibroblast subtypes/stages in regeneration after partial pneumonectomy (PNX) and fibrosis/repair after bleomycin lung injury. To highlight critical epithelial‐mesenchymal crosstalk during reseptation or injury repair, signature ligands and receptors of epithelial and fibroblast subtypes are listed in tables next to the illustrations. Differences between the myofibroblast niche and epithelial repair niche in fibrosis are given within the schematic illustration of fibrosis. The bleomycin illustration follows a chronological progression from fibrosis to repair/fibrosis resolution to homeostasis (left to right). Similar illustration of alveolar regeneration after PNX. The figure legend shows each cell type and extra cellular matrix (ECM) components depicted in the figure. B, Context‐dependent interstitial fibroblast cell stage in IPF and BPD. Although lineage‐tracing experiments in murine injury models revealed some insight of the origin of the fibrotic fibroblast, we are limited to drawing any parallels to human diseases by reviewing single‐ cell RNA sequencing, bulk RNA sequencing, flow cytometry, and immunofluorescence from fibrotic human lung tissue. On the left are illustrations of altered morphology of the lung in idiopathic pulmonary fibrosis (IPF) and bronchopulmonary dysplasia (BPD). IPF has extensive regions of fibrosis, parenchymal honeycombing, and bronchiolization of the epithelium. BPD has alveolar simplification and septal wall thinning. Arrows point to hallmark morphological changes, described in the central portion of the figure. Signature genes and their expression changes for each fibroblast population in both diseases are listed on the right
Lipofibroblasts transfer neutral lipids to AT2 cells for surfactant phospholipid synthesis with the help of adipose differentiation‐related protein (ADRP) 91 and support AT2 proliferation and differentiation into new AT1 cells. 2 , 53 , 92 At a transcriptional level, activation of the transcription factor PPARγ via rosiglitazone treatment has been shown to induce lipofibroblast differentiation. 93 In the context of injury response, rosiglitazone treatment significantly reduced transforming growth factor beta (TGFβ)‐mediated myofibroblast differentiation. During regeneration following PNX, rosiglitazone treatment inhibited reseptation as myofibroblast activation in PDGFRa+ fibroblasts was blocked. 29 These data suggest PPARγ signaling as both an inhibitor of myofibroblast differentiation and an activator of lipofibroblast differentiation and support the theory that lipo‐ to myofibroblast differentiation is necessary to drive new septa formation. 94 These regeneration studies revealed molecular drivers and cell type‐specific roles of fibroblasts during reseptation. In the future, the use of transgenic mice with gene activation and inactivation before PNX surgery or during regeneration has great potential for discerning the molecular regulation of regeneration.
Age‐specific decline in alveolar regeneration has been investigated in murine models after PNX. Decreased fibroblast clonality and increased myofibroblast differentiation impair reseptation in aged mice compared with young mice. 95 , 96 , 97 In addition, perinatal hyperoxia exposure has recently been linked to the induction of senescence in the mesenchyme and is demonstrated to be a cause of BPD. 98 Priming aged mice with epigenetic modifiers to study gene silencing in the context of failed lung regeneration has translational application for initiating regeneration in human lungs. Although the murine PNX model gives insight into the role of fibroblasts during reseptation, a plethora of murine lung fibrosis models has been used to study the contribution of fibroblasts in chronic lung disease.
Injury and nonfibrotic repair
Bleomycin injury has been extensively used to study acute lung fibrosis and the contribution of various cell types to the fibrotic response. Mechanical and paracrine cues from the site of injury regulate controlled recruitment of fibroblasts to initiate repair in the bleomycin models. As each fibroblast population/stage has unique functions, murine lung models of fibrosis demonstrate that not all fibroblasts have the same injury response. 7 , 70 , 88 , 99 Different stages of fibroblasts contribute to either fibrosis or regeneration after injury and are visualized, along with their signature gene expression, in Figure 2A. A subset of myofibroblasts identified by Axin2+ responsiveness (AMPs) become pathologically deleterious myofibroblasts in the alveolar niche that proliferate and create extensive ECM components. 7 MANCs, identified by scRNA‐seq and spatial mapping analyses, 7 are Axin2 + PDGFRα+ fibroblast subtypes and contribute to regeneration. MANCs initiate reciprocal paracrine signaling with AT2 cells and AEPs to promote alveolar growth and regeneration after bleomycin injury. 7 , 70 The contribution of lipofibroblasts to bleomycin injury has been studied using ADRP as a lineage tracer. In response to bleomycin injury, lipofibroblasts transdifferentiate into αSMA‐expressing myofibroblasts and revert to lipofibroblasts as injury resolves. 100 These data support the hypothesis that a subset of lipofibroblasts give rises to myofibroblasts after lung injury and that lipofibroblast reconstitution is required for lung regeneration. 101 However, the existence of lipofibroblasts in human lungs remains to be further investigated. 47 , 102 , 103 Recent scRNA‐seq analysis on human lungs discerns a lipofibroblast signature that resembles the murine lipofibroblast but, in comparison with other stromal cells within the dataset, is a small population. 47
Single‐dose bleomycin and flu injury enable lung recovery to occur, whereas multidose bleomycin, surfactant protein C (SPC) mutant epithelium, and diphtheria toxin receptor transgenic mice are models for chronic lung fibrosis. These chronic fibrosis models have been used to study epithelial injury and repair; future studies focusing on the fibroblast response will shed more light on the epithelial mesenchymal crosstalk during injury and fibrosis initiation. 104 , 105 , 106
1.4. Fibroblasts in human disease
The functional significance of fibroblasts is not limited to development and regeneration in animal models but extends to human lung pathology. A significant increase, decrease, or shift in fibroblast functional stages/phenotypes changes homeostasis. Change in fibroblast activation stages affects the functionality of neighboring cells resulting in IPF, BPD, and COPD.
1.4.1. Idiopathic pulmonary fibrosis
IPF is characterized by extensive fibrosis, causing progressive respiratory decline and mortality, usually within 5 years of diagnosis. 107 , 108 , 109 Although the pathogenesis of IPF remains unclear, chronic alveolar epithelial cell injury and chronic fibrotic activation of fibroblasts are linked to the disorder. 110 Treatment regimens using pirfenidone and nintedanib showed effectiveness in reducing morbidity but not mortality. 111 , 112 Considerable effort has been taken to study the role and origin of fibroblasts as they are promising targets of antifibrotic therapy. At the tissue level, IPF is defined by a fibroblastic focus with an immature hyaluronic acid‐rich matrix underneath the epithelial layer, loss of alveolar type 1 cell differentiation, and increased αSMA+ myofibroblasts. 113 The presence of epithelial basal‐like cells that coexpress epithelial and mesenchymal markers has been reported in IPF lungs by scRNA‐seq. 6 These indeterminate alveolar type 2 cells were found to be located at the edge of myofibroblast foci in the IPF lung. 8 Despite its limitations, bleomycin injury in mice has been used to model and study IPF. In both human IPF samples and murine models of pulmonary fibrosis, the myofibroblast population expands considerably. 114 , 115 , 116 Myofibroblasts arise from both resident myofibroblasts and resident lipofibroblasts, 117 suggesting aberrant fibrotic activation in a variety of fibroblast populations. In the bleomycin injury model, interstitial lung fibroblasts, pericytes, and mesothelial cells are known to differentiate into myofibroblasts. Partial epithelial‐mesenchymal transition has also been reported using multiple reported systems and injury models. 118 , 119 , 120
Lipofibroblasts, whose existence was once questioned in the adult human lung, have recently been identified as a stable cell population during homeostasis using scRNA‐seq. 47 Studies of lipofibroblast function in the murine lung indicate a role in fibrosis. As previously mentioned, PDGFRα‐expressing lipofibroblasts differentiate into myofibroblasts upon injury and transdifferentiate back to lipofibroblasts during fibrosis resolution in the mouse lung. 78 , 100 , 117 In IPF, lipofibroblasts are prominent 121 ; however, the relative mRNA expression of canonical lipofibroblast markers PLIN2, PPARγ, and TCF21 is reduced. 117 Another study using freshly isolated PDGFRa‐expressing fibroblasts from IPF lungs showed that PDGFRa+ lipofibroblasts shift to a PDGFRa+ myofibroblast stage/activation. Moreover, PDGFRa+ matrixfibroblasts are significantly reduced in IPF lungs. 122 , 123 In a transgenic mouse model, expression of a constitutively active PDGFRa kinase mutation in PDGFRa+ cells significantly increased matrixfibroblast over myofibroblast differentiation. 2 , 29 , 122 The dynamic gene expression changes of myofibroblasts, lipofibroblasts, and matrixfibroblasts in IPF and mouse models of fibrosis are displayed in Figure 2B. Recent in vitro organoid studies demonstrated that fibroblasts from aged mice or adult human donors do not support alveolar type 2 to type 1 differentiation. In organoid cultures, young fibroblasts support alveolar type 1 cell differentiation, even in “indetermined” epithelial cells isolated from human IPF lungs. PDGF‐A treatment of human lung organoids, administered with aged and IPF fibroblasts, restored epithelial alveolar type 1 cell differentiation. These results suggest that restoration of the PDGFRa‐high matrixfibroblast stage may be beneficial for restoring epithelial differentiation to alveolar type 1 cells, which is lost in IPF. 122 “Activated myofibroblasts” identified by scRNA‐seq studies of IPF and other fibrotic lung diseases often share signature genes normally associated with the developmental matrixfibroblast. These studies suggest that, in IPF, not only epithelial cells but also fibroblasts can take on an indeterminate form, such as a “myo/matrix fibroblast” 63 , 114 , 115 , 116 (IPF cell atlas: https://p2med.shinyapps.io/IPFCellAtlas/). As myofibroblasts are the driver of IPF, and beneficial matrix function is lost, it is important for future studies to identify key pathways that trigger the activation of fibrotic matrix phenotypes over the beneficial regenerative matrix phenotype. Future studies using both in vivo lineage labeling and in vitro organoid cultures will shed more light on the plasticity of fibroblasts and their limitations to contribute to nonfibrotic repair after profibrotic stimulus.
1.4.2. Bronchopulmonary dysplasia
Hyperoxic and hyperbaric conditions due to supplemental oxygen in association with premature birth cause disruption of alveologenesis, resulting in permanent alveolar simplification (BPD). 124 Because of medical advances like antenatal steroid treatment and neonatal surfactant therapy, the fibrotic pathophysiology of “Old” BPD as described by Northway is rarely seen. 125 The “New” BPD, constituting alveolar simplification via arrest of lung development, remains a prominent comorbidity of premature birth today. 126 , 127 The injury is further characterized by damage to the lung epithelial cells that normally facilitate gas exchange and disruption in vascular development, particularly alveolar capillaries. 128 Unraveling the molecular mechanisms of fibroblast response to hyperoxia is essential to develop new strategies for the prevention of BPD. As previously mentioned, PDGFRα signaling is necessary for the normal functioning myofibroblasts that drive alveolar septation during distal lung development. 13 , 129 In mice, pharmacological or genetic ablation of PDGFRα signaling results in the loss of alveolar myofibroblasts and failure of alveolar septation. 24 , 130 In vitro studies suggest that failed alveolarization could be attributed in part to the loss of beneficial lipofibroblasts and activation of myofibroblasts. 131 Upon in vitro exposure to hyperoxia, lipofibroblasts rapidly transdifferentiate into a myogenic phenotype. 100 A lack of lipofibroblasts causes a decrease in the production of surfactants as they are the major providers of triglycerols to alveolar type 2 cells. Loss of lipofibroblasts and poor alveolar epithelial cell growth and differentiation result in failed alveolarization and BPD. 100 These preclinical observations implicate a critical role for PDGFRα fibroblasts in BPD. In humans, reduced PDGFRα expression was reported in neonatal mesenchymal stromal cells obtained from infants with BPD. 132 Recent scRNA‐seq data denote an extensive loss of PDGFRa+ fibroblasts in BPD and an increase of immature fibroblast subtypes accompanied by a reduction of mature matrix, lipo‐, and myofibroblasts. 133 , 134 The dynamic gene expression changes of fibroblasts in BPD and in vivo animal hyperoxia models are summarized in Figure 2B. BPD has recently been identified as a risk factor for COPD and severity of Coronavirus disease 2019 in both murine models and epidemiological studies, highlighting the importance of understanding the long‐term consequences of BPD. 135 , 136 , 137 , 138
Limitations and alternative models for BPD
In vitro studies are a reductionist approach that is inherently limited because of a lack of cellular microenvironmental context. On the other hand, the in vivo murine hyperoxia model is limited as mice are naturally born in the saccular phase. 139 To interrupt lung development during lung sacculation and simultaneously recapitulate BPD, rabbit kits can be delivered via c‐section and subjected to mechanical ventilation. 140 , 141 Modeling of BPD in sheep and nonhuman primates is expensive but provides excellent alternatives to better understand human lung development and BPD. 142 , 143 , 144 , 145 , 146
The recent development of organoid and precision‐cut lung slice (PCLS) models recapitulate certain aspects of hyperoxia exposure during alveolarization. Exposure of three‐dimensional organotypic coculture to hyperoxia mimics aspects of BPD pathogenesis, including activation of ACTA2 and COL1A1 expression in fibroblasts. 147 Ex vivo PCLS exposed to hyperoxia maintain some features of pulmonary architecture and facilitate live imaging studies to assess cellular migration, proliferation, and differentiation. 147 To advance the field of BPD, we will have to integrate findings from old and newly developing BPD model systems, with transcriptomic and proteomic analysis.
1.4.3. Chronic obstructive pulmonary disease
Irreversible airway obstruction 148 with emphysematous changes, such as loss of elastic fibers in the alveolar walls and subsequent destruction of the alveoli, define the pathology of COPD. 149 Reduced fibroblast proliferation and altered repair mechanisms contribute to the emphysematous lung. 150 A role for TGFβ1 has been reported in COPD patients compared with healthy control patients 151 and showed that COPD fibroblasts are less responsive to TGFβ1 in terms of proliferation and elastin production compared with normal fibroblasts. 152 Fibroblasts from moderate to severe COPD subjects show a secretory phenotype with upregulation of inflammatory molecules and increased soluble elastin. The formation of soluble elastin was inhibited by versican, an inflammatory matrix proteoglycan, which is predominately expressed in myofibroblasts. 153 Furthermore, studies on COPD fibroblasts also show less chemotactic activity and collagen contraction. 154 Although interstitial fibroblasts are important for septal tip formation and elastin deposition during development and repair, their role in COPD has not been studied. Investigating mechanisms and consequences to understand the association of functional fibroblast stages and ECM damage may pave the way for better outcomes.
2. CONCLUSION
The advent of scRNA‐seq has greatly strengthened the understanding of fibroblast heterogeneity within the lung field but, at the same time, has added to confusion over fibroblast nomenclature, fibroblast populations, and functional fibroblast stages. Although it is now possible to cluster similar cells based on gene expression in a total and unbiased manner, it becomes difficult to determine if fibroblasts exist as unique lineages or rather reside on a spectrum of activation and cell stages. Certain fibroblasts, like the myo‐ and lipofibroblasts, demonstrate clear functional, morphological, and lineage differences in development and disease. The matrixfibroblast and alveolar niche cell, however, remain mysterious and may be overlapping, nondistinct stages of the same fibroblast subtype. Both cell “types” aid in alveolar organoid formation and express genes required for ECM production, suggesting functionality somewhere between the contractile myofibroblast and secretory matrixfibroblast. Further studies on the origin of the alveolar niche cell, as it is currently defined, will clarify whether the niche cell is a quiescent stem cell or just a quiet matrixfibroblast. If lineage relationships exist, they might be revealed with the help of pseudo‐time analysis on developmental scRNA‐seq studies in fibroblasts. A recent study identified both the transcriptional signatures and locations of 58 cell types in the human lung, including nine stromal subtypes. 47 Pursing apart the heterogeneity and plasticity of the mesenchyme in the lung is necessary to develop effective therapies for BPD, IPF, COPD, and other human lung diseases.
As reflected in PNX studies, activation of proper fibroblast subtypes occurs in both a spatial and temporal manner, suggesting that treatment with pan‐inhibitors of signaling pathways to reduce fibrotic activation of one fibroblast's function would impede the regenerative function of another. This might be why anti fibrotic drugs like nintedanib and pirfenidone are not remarkably effective IPF therapies as they antagonize regenerative fibroblast activation. Future studies in murine models with combinatorial and time‐restricted treatment of multiple drugs, to target different aspects of fibrosis and subsequent repair, will likely yield a better understanding of how to treat human lung diseases. The fibroblast will remain a critical mediator in any of these processes.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
A.K.P., M.G.U. and M.R. wrote the manuscript. M.R. created the figures.
ACKNOWLEDGMENTS
The authors acknowledge R01 HL131661 (to M.U., M.R., and A.K.T.P.), T32 HL007752 (to M.R.), U01 HL122642 (A.K.T.P.) LungMAP, U01 HL 148856 (A.K.T.P.) LungMAP, U01 HL134745 (A.K.T.P.) PCTC, Translational Fibrosis Academic and Research Committee funding CCHMC and BPD Academic and Research Committee funding CCHMC for their support.
Ushakumary MG, Riccetti M, Perl A‐KT. Resident interstitial lung fibroblasts and their role in alveolar stem cell niche development, homeostasis, injury, and regeneration. STEM CELLS Transl Med. 2021;10:1021–1032. 10.1002/sctm.20-0526
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. McCulley D, Wienhold M, Sun X. The pulmonary mesenchyme directs lung development. Curr Opin Genet Dev. 2015;32:98‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Green J, Endale M, Auer H, Perl AK. Diversity of interstitial lung fibroblasts is regulated by platelet‐derived growth factor receptor alpha kinase activity. Am J Respir Cell Mol Biol. 2016;54:532‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kugler MC, Loomis CA, Zhao Z, Cushman JC, Liu L, Munger JS. Sonic hedgehog signaling regulates myofibroblast function during alveolar septum formation in murine postnatal lung. Am J Respir Cell Mol Biol. 2017;57:280‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li RB, Li XP, Hagood J, Zhu MS, Sun X. Myofibroblast contraction is essential for generating and regenerating the gas‐exchange surface. J Clin Invest. 2020;130:2859‐2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGowan SE, McCoy DM. Fibroblast growth factor signaling in myofibroblasts differs from lipofibroblasts during alveolar septation in mice. Am J Physiol Lung Cell Mol Physiol. 2015;309:L463‐L474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu Y, Mizuno T, Sridharan A, et al. Single‐cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight. 2016;1:e90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zepp JA, Zacharias WJ, Frank DB, et al. Distinct mesenchymal lineages and niches promote epithelial self‐renewal and myofibrogenesis in the lung. Cell. 2017;170:1134‐1148.e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adams TS, Schupp JC, Poli S, et al. Single‐cell RNA‐seq reveals ectopic and aberrant lung‐resident cell populations in idiopathic pulmonary fibrosis. Sci Adv. 2020;6:eaba1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bridges JP, Sudha P, Lipps D, et al. Glucocorticoid regulates mesenchymal cell differentiation required for perinatal lung morphogenesis and function. Am J Physiol Lung Cell Mol Physiol. 2020;319:L239‐L255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Endale M, Ahlfeld S, Bao E, et al. Temporal, spatial, and phenotypical changes of PDGFR alpha expressing fibroblasts during late lung development. Dev Biol. 2017;425:161‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo M, Du Y, Gokey JJ, et al. Single cell RNA analysis identifies cellular heterogeneity and adaptive responses of the lung at birth. Nat Commun. 2019;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Valenzi E, Bulik M, Tabib T, et al. Single‐cell analysis reveals fibroblast heterogeneity and myofibroblasts in systemic sclerosis‐associated interstitial lung disease. Ann Rheum Dis. 2019;78:1379‐1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Betsholtz C. Role of platelet‐derived growth factors in mouse development. Int J Dev Biol. 1995;39:817‐825. [PubMed] [Google Scholar]
- 14. Shannon JM. Induction of alveolar type II cell differentiation in fetal tracheal epithelium by grafted distal lung mesenchyme. Dev Biol. 1994;166:600‐614. [DOI] [PubMed] [Google Scholar]
- 15. Riccetti M, Gokey JJ, Aronow B, Perl AT. The elephant in the lung: integrating lineage‐tracing, molecular markers, and single cell sequencing data to identify distinct fibroblast populations during lung development and regeneration. Matrix Biol. 2020;91‐92:51‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al Alam D, El Agha E, Sakurai R, et al. Evidence for the involvement of fibroblast growth factor 10 in lipofibroblast formation during embryonic lung development. Development. 2015;142:4139‐4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Basil MC, Katzen J, Engler AE, et al. The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell. 2020;26:482‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mund SI, Stampanoni M, Schittny JC. Developmental alveolarization of the mouse lung. Dev Dyn. 2008;237:2108‐2116. [DOI] [PubMed] [Google Scholar]
- 19. Rawlins EL, Perl AK. The a“MAZE”ing world of lung‐specific transgenic mice. Am J Respir Cell Mol Biol. 2012;46:269‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berger J, Bhandari V. Animal models of bronchopulmonary dysplasia. The term mouse models. Am J Physiol Lung Cell Mol Physiol. 2014;307:L936‐L947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amy RW, Bowes D, Burri PH, Haines J, Thurlbeck WM. Postnatal growth of the mouse lung. J Anat. 1977;124:131‐151. [PMC free article] [PubMed] [Google Scholar]
- 22. Branchfield K, Li R, Lungova V, Verheyden JM, McCulley D, Sun X. A three‐dimensional study of alveologenesis in mouse lung. Dev Biol. 2016;409:429‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burri PH. The postnatal growth of the rat lung. 3. Morphology. Anat Rec. 1974;180:77‐98. [DOI] [PubMed] [Google Scholar]
- 24. Lindahl P, Karlsson L, Hellstrom M, et al. Alveogenesis failure in PDGF‐A‐deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development. 1997;124:3943‐3953. [DOI] [PubMed] [Google Scholar]
- 25. Moschopulos M, Burri PH. Morphometric analysis of fetal rat lung development. Anat Rec. 1993;237:38‐48. [DOI] [PubMed] [Google Scholar]
- 26. Rodriguez‐Castillo JA, Perez DB, Ntokou A, Seeger W, Morty RE, Ahlbrecht K. Understanding alveolarization to induce lung regeneration. Respir Res. 2018;19:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schittny JC. Development of the lung. Cell Tissue Res. 2017;367:427‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeltner TB, Burri PH. The postnatal development and growth of the human lung. II. Morphology. Respir Physiol. 1987;67:269‐282. [DOI] [PubMed] [Google Scholar]
- 29. Chen L, Acciani T, Le Cras T, Lutzko C, Perl AK. Dynamic regulation of platelet‐derived growth factor receptor alpha expression in alveolar fibroblasts during realveolarization. Am J Respir Cell Mol Biol. 2012;47:517‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lau M, Masood A, Yi M, Belcastro R, Li J, Tanswell AK. Long‐term failure of alveologenesis after an early short‐term exposure to a PDGF‐receptor antagonist. Am J Physiol Lung Cell Mol Physiol. 2011;300:L534‐L547. [DOI] [PubMed] [Google Scholar]
- 31. Li C, Lee MK, Gao F, et al. Secondary crest myofibroblast PDGFRalpha controls the elastogenesis pathway via a secondary tier of signaling networks during alveologenesis. Development. 2019;146:dev176354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li C, Li M, Li S, et al. Progenitors of secondary crest myofibroblasts are developmentally committed in early lung mesoderm. stem cells. 2015;33:999‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mitchell J, Woodcock‐Mitchell J, Reynolds S, et al. Alpha‐smooth muscle actin in parenchymal cells of bleomycin‐injured rat lung. Lab Invest. 1989;60:643‐650. [PubMed] [Google Scholar]
- 34. Ntokou A, Klein F, Dontireddy D, et al. Characterization of the platelet‐derived growth factor receptor‐alpha‐positive cell lineage during murine late lung development. Am J Physiol Lung Cell Mol Physiol. 2015;309:L942‐L958. [DOI] [PubMed] [Google Scholar]
- 35. Perl AK, Gale E. FGF signaling is required for myofibroblast differentiation during alveolar regeneration. Am J Physiol Lung Cell Mol Physiol. 2009;297:L299‐L308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Srisuma S, Bhattacharya S, Simon DM, et al. Fibroblast growth factor receptors control epithelial‐mesenchymal interactions necessary for alveolar elastogenesis. Am J Respir Crit Care Med. 2010;181:838‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vaccaro C, Brody JS. Ultrastructure of developing alveoli. I. The role of the interstitial fibroblast. Anat Rec. 1978;192:467‐479. [DOI] [PubMed] [Google Scholar]
- 38. Bell SE, Mavila A, Salazar R, et al. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G‐protein signaling. J Cell Sci. 2001;114:2755‐2773. [DOI] [PubMed] [Google Scholar]
- 39. Bland RD, Xu L, Ertsey R, et al. Dysregulation of pulmonary elastin synthesis and assembly in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1370‐L1384. [DOI] [PubMed] [Google Scholar]
- 40. Desmouliere A, Darby I, Costa AM, et al. Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab Invest. 1997;76:765‐778. [PubMed] [Google Scholar]
- 41. Hanson KP, Jung JP, Tran QA, et al. Spatial and temporal analysis of extracellular matrix proteins in the developing murine heart: a blueprint for regeneration. Tissue Eng Part A. 2013;19:1132‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hsia CC. Signals and mechanisms of compensatory lung growth. J Appl Physiol. 2004;97:1992‐1998. [DOI] [PubMed] [Google Scholar]
- 43. Kawanami O, Matsuda K, Yoneyama H, Ferrans VJ, Crystal RG. Endothelial fenestration of the alveolar capillaries in interstitial fibrotic lung diseases. Acta Pathol Jpn. 1992;42:177‐184. [DOI] [PubMed] [Google Scholar]
- 44. Li R, Herriges JC, Chen L, Mecham RP, Sun X. FGF receptors control alveolar elastogenesis. Development. 2017;144:4563‐4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith DG, Campbell G. The anatomy of the pulmonary vascular bed in the toad Bufo marinus . Cell Tissue Res. 1976;165:199‐213. [DOI] [PubMed] [Google Scholar]
- 46. Starcher BC. Lung elastin and matrix. Chest. 2000;117:229S‐234S. [DOI] [PubMed] [Google Scholar]
- 47. Travaglini KJ, Nabhan AN, Penland L, et al. A molecular cell atlas of the human lung from single‐cell RNA sequencing. Nature. 2020;587:619‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Warburton D, Schwarz M, Tefft D, Flores‐Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev. 2000;92:55‐81. [DOI] [PubMed] [Google Scholar]
- 49. Chen H, Jackson S, Doro M, McGowan S. Perinatal expression of genes that may participate in lipid metabolism by lipid‐laden lung fibroblasts. J Lipid Res. 1998;39:2483‐2492. [PubMed] [Google Scholar]
- 50. El Agha E, Herold S, Al Alam D, et al. Fgf10‐positive cells represent a progenitor cell population during lung development and postnatally. Development. 2014;141:296‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McGowan SE, Jackson SK, Doro MM, Olson PJ. Peroxisome proliferators alter lipid acquisition and elastin gene expression in neonatal rat lung fibroblasts. Am J Physiol. 1997;273:L1249‐L1257. [DOI] [PubMed] [Google Scholar]
- 52. McGowan SE, Torday JS. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol. 1997;59:43‐62. [DOI] [PubMed] [Google Scholar]
- 53. Park J, Ivey MJ, Deana Y, et al. The Tcf21 lineage constitutes the lung lipofibroblast population. Am J Physiol Lung Cell Mol Physiol. 2019;316:L872‐L885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tschanz SA, Damke BM, Burri PH. Influence of postnatally administered glucocorticoids on rat lung growth. Biol Neonate. 1995;68:229‐245. [DOI] [PubMed] [Google Scholar]
- 55. Yuan T, Volckaert T, Chanda D, Thannickal VJ, De Langhe SP. Fgf10 signaling in lung development, homeostasis, disease, and repair after injury. Front Genet. 2018;9:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hardie WD, Korfhagen TR, Sartor MA, et al. Genomic profile of matrix and vasculature remodeling in TGF‐alpha induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2007;37:309‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Matsui K, W KR, Hilbert SL, et al. Hyperplasia of type II pneumocytes in pulmonary lymphangioleiomyomatosis. Arch Pathol Lab Med. 2000;124:1642‐1648. [DOI] [PubMed] [Google Scholar]
- 58. McGowan PM, Duffy MJ. Matrix metalloproteinase expression and outcome in patients with breast cancer: analysis of a published database. Ann Oncol. 2008;19:1566‐1572. [DOI] [PubMed] [Google Scholar]
- 59. Ohbayashi H. Matrix metalloproteinases in lung diseases. Curr Protein Pept Sci. 2002;3:409‐421. [DOI] [PubMed] [Google Scholar]
- 60. Pardo A, Cabrera S, Maldonado M, Selman M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir Res. 2016;17:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schuliga MJ, See I, Ong SC, et al. Fibrillar collagen clamps lung mesenchymal cells in a nonproliferative and noncontractile phenotype. Am J Respir Cell Mol Biol. 2009;41:731‐741. [DOI] [PubMed] [Google Scholar]
- 62. Wert SE, Yoshida M, LeVine AM, et al. Increased metalloproteinase activity, oxidant production, and emphysema in surfactant protein D gene‐inactivated mice. Proc Natl Acad Sci U S A. 2000;97:5972‐5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xie T, Wang Y, Deng N, et al. Single‐cell deconvolution of fibroblast heterogeneity in mouse pulmonary fibrosis. Cell Rep. 2018;22:3625‐3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McGowan SE, McCoy DM. Fibroblasts expressing PDGF‐receptor‐alpha diminish during alveolar septal thinning in mice. Pediatr Res. 2011;70:44‐49. [DOI] [PubMed] [Google Scholar]
- 65. McGowan SE, McCoy DM. Platelet‐derived growth factor‐a regulates lung fibroblast S‐phase entry through p27(kip1) and FoxO3a. Respir Res. 2013;14:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Starcher B, d'Azzo A, Keller PW, Rao GK, Nadarajah D, Hinek A. Neuraminidase‐1 is required for the normal assembly of elastic fibers. Am J Physiol Lung Cell Mol Physiol. 2008;295:L637‐L647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hagan AS, Zhang B, Ornitz DM. Identification of an FGF18‐expressing alveolar myofibroblast that is developmentally cleared during alveologenesis. Development. 2020;147(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kheirollahi V, Wasnick RM, Biasin V, et al. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat Commun. 2019;10:2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chung MI, Bujnis M, Barkauskas CE, Kobayashi Y, Hogan BLM. Niche‐mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development. 2018;145:dev163014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lee JH, Tammela T, Hofree M, et al. Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell. 2017;170:1149‐1163.e1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ. Single‐cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018;359:1118‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zacharias WJ, Frank DB, Zepp JA, et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555:251‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gouveia L, Betsholtz C, Andrae J. PDGF‐A signaling is required for secondary alveolar septation and controls epithelial proliferation in the developing lung. Development. 2018;145:dev161976. [DOI] [PubMed] [Google Scholar]
- 74. Lazarus A, Del‐Moral PM, Ilovich O, Mishani E, Warburton D, Keshet E. A perfusion‐independent role of blood vessels in determining branching stereotypy of lung airways. Development. 2011;138:2359‐2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rafii S, Butler JM, Ding BS. Angiocrine functions of organ‐specific endothelial cells. Nature. 2016;529:316‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ramasamy SK, Kusumbe AP, Adams RH. Regulation of tissue morphogenesis by endothelial cell‐derived signals. Trends Cell Biol. 2015;25:148‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Du Y, Kitzmiller JA, Sridharan A, et al. Lung Gene Expression Analysis (LGEA): an integrative web portal for comprehensive gene expression data analysis in lung development. Thorax. 2017;72(5):481‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li R, Bernau K, Sandbo N, Gu J, Preissl S, Sun X. Pdgfra marks a cellular lineage with distinct contributions to myofibroblasts in lung maturation and injury response. Elife. 2018;7:e36865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liebeskind A, Srinivasan S, Kaetzel D, Bruce M. Retinoic acid stimulates immature lung fibroblast growth via a PDGF‐mediated autocrine mechanism. Am J Physiol Lung Cell Mol Physiol. 2000;279:L81‐L90. [DOI] [PubMed] [Google Scholar]
- 80. Snyder JM, Jenkins‐Moore M, Jackson SK, et al. Alveolarization in retinoic acid receptor‐beta‐deficient mice. Pediatr Res. 2005;57:384‐391. [DOI] [PubMed] [Google Scholar]
- 81. El Agha E, Al Alam D, Carraro G, et al. Characterization of a novel fibroblast growth factor 10 (Fgf10) knock‐in mouse line to target mesenchymal progenitors during embryonic development. PLoS One. 2012;7:e38452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. El Agha E, Kheirollahi V, Moiseenko A, Seeger W, Bellusci S. Ex vivo analysis of the contribution of FGF10(+) cells to airway smooth muscle cell formation during early lung development. Dev Dyn. 2017a;246:531‐538. [DOI] [PubMed] [Google Scholar]
- 83. Franco‐Montoya ML, Boucherat O, Thibault C, et al. Profiling target genes of FGF18 in the postnatal mouse lung: possible relevance for alveolar development. Physiol Genomics. 2011;43:1226‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hagan AS, Boylan M, Smith C, et al. Generation and validation of novel conditional flox and inducible Cre alleles targeting fibroblast growth factor 18 (Fgf18). Dev Dyn. 2019a;248:882‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu L, Kugler MC, Loomis CA, et al. Hedgehog signaling in neonatal and adult lung. Am J Respir Cell Mol Biol. 2013;48:703‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Moiseenko A, Kheirollahi V, Chao CM, et al. Origin and characterization of alpha smooth muscle Actin‐positive cells during murine lung development. stem cells. 2017;35:1566‐1578. [DOI] [PubMed] [Google Scholar]
- 87. Peng T, Tian Y, Boogerd CJ, et al. Coordination of heart and lung co‐development by a multipotent cardiopulmonary progenitor. Nature. 2013;500:589‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Frank DB, Peng T, Zepp JA, et al. Emergence of a wave of Wnt signaling that regulates lung alveologenesis by controlling epithelial self‐renewal and differentiation. Cell Rep. 2016;17:2312‐2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sathish V, Martin YN, Prakash YS. Sex steroid signaling: implications for lung diseases. Pharmacol Ther. 2015;150:94‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Butler JP, Loring SH, Patz S, Tsuda A, Yablonskiy DA, Mentzer SJ. Evidence for adult lung growth in humans. N Engl J Med. 2012;367:244‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schultz CJ, Torres E, Londos C, Torday JS. Role of adipocyte differentiation‐related protein in surfactant phospholipid synthesis by type II cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L288‐L296. [DOI] [PubMed] [Google Scholar]
- 92. McGowan SE, McCoy DM. Regulation of fibroblast lipid storage and myofibroblast phenotypes during alveolar septation in mice. Am J Physiol Lung Cell Mol Physiol. 2014;307:L618‐L631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rehan VK, Wang Y, Patel S, Santos J, Torday JS. Rosiglitazone, a peroxisome proliferator‐activated receptor‐gamma agonist, prevents hyperoxia‐induced neonatal rat lung injury in vivo. Pediatr Pulmonol. 2006;41:558‐569. [DOI] [PubMed] [Google Scholar]
- 94. Burgess HA, Daugherty LE, Thatcher TH, et al. PPARgamma agonists inhibit TGF‐beta induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1146‐L1153. [DOI] [PubMed] [Google Scholar]
- 95. Paxson JA, Gruntman A, Parkin CD, et al. Age‐dependent decline in mouse lung regeneration with loss of lung fibroblast clonogenicity and increased myofibroblastic differentiation. PLoS One. 2011;6:e23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Paxson JA, Gruntman AM, Davis AM, Parkin CM, Ingenito EP, Hoffman AM. Age dependence of lung mesenchymal stromal cell dynamics following pneumonectomy. Stem Cells Dev. 2013;22:3214‐3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Paxson JA, Parkin CD, Iyer LK, Mazan MR, Ingenito EP, Hoffman AM. Global gene expression patterns in the post‐pneumonectomy lung of adult mice. Respir Res. 2009;10:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sucre JMS, Plosa EJ. Ahead of their time: hyperoxia injury induces senescence in developing lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2019;317:L523‐L524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Adamson IY, Bowden DH. The pathogenesis of bloemycin‐induced pulmonary fibrosis in mice. Am J Pathol. 1974;77:185‐197. [PMC free article] [PubMed] [Google Scholar]
- 100. Rehan VK, Torday JS. The lung alveolar lipofibroblast: an evolutionary strategy against neonatal hyperoxic lung injury. Antioxid Redox Signal. 2014;21:1893‐1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Habiel DM, Hogaboam CM. Heterogeneity of fibroblasts and myofibroblasts in pulmonary fibrosis. Curr Pathobiol Rep. 2017;5:101‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ahlbrecht K, McGowan SE. In search of the elusive lipofibroblast in human lungs. Am J Physiol Lung Cell Mol Physiol. 2014;307:L605‐L608. [DOI] [PubMed] [Google Scholar]
- 103. Tahedl D, Wirkes A, Tschanz SA, Ochs M, Muhlfeld C. How common is the lipid body‐containing interstitial cell in the mammalian lung? Am J Physiol Lung Cell Mol Physiol. 2014;307:L386‐L394. [DOI] [PubMed] [Google Scholar]
- 104. Degryse AL, Tanjore H, Xu XC, et al. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;299:L442‐L452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nureki SI, Tomer Y, Venosa A, et al. Expression of mutant Sftpc in murine alveolar epithelia drives spontaneous lung fibrosis. J Clin Invest. 2018;128:4008‐4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sisson TH, Mendez M, Choi K, et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:254‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chilosi M, Poletti V, Murer B, et al. Abnormal re‐epithelialization and lung remodeling in idiopathic pulmonary fibrosis: the role of deltaN‐p63. Lab Invest. 2002;82:1335‐1345. [DOI] [PubMed] [Google Scholar]
- 108. Meltzer EB, Noble PW. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis. 2008;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Plantier L, Crestani B, Wert SE, et al. Ectopic respiratory epithelial cell differentiation in bronchiolised distal airspaces in idiopathic pulmonary fibrosis. Thorax. 2011;66:651‐657. [DOI] [PubMed] [Google Scholar]
- 110. Wolters PJ, Blackwell TS, Eickelberg O, et al. Time for a change: is idiopathic pulmonary fibrosis still idiopathic and only fibrotic? Lancet Respir Med. 2018;6:154‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hagmeyer L, Treml M, Priegnitz C, Randerath WJ. Successful concomitant therapy with pirfenidone and nintedanib in idiopathic pulmonary fibrosis: a case report. Respiration. 2016;91:327‐332. [DOI] [PubMed] [Google Scholar]
- 112. King TE Jr, Bradford WZ, Castro‐Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083‐2092. [DOI] [PubMed] [Google Scholar]
- 113. Selman M, Pardo A. Idiopathic pulmonary fibrosis: an epithelial/fibroblastic cross‐talk disorder. Respir Res. 2002;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Adams TS, Schupp JC, Poli S, et al. Single cell RNA‐seq reveals ectopic and aberrant lung resident cell populations in idiopathic pulmonary fibrosis. bioRxiv, 759902, 2019. [DOI] [PMC free article] [PubMed]
- 115. Neumark N, Cosme C Jr, Rose KA, Kaminski N. The idiopathic pulmonary fibrosis cell atlas. Am J Physiol Lung Cell Mol Physiol. 2020;319:L887‐L892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Reyfman PA, Walter JM, Joshi N, et al. Single‐cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med. 2019;199:1517‐1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. El Agha E, Moiseenko A, Kheirollahi V, et al. Two‐way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell. 2017b;20:571. [DOI] [PubMed] [Google Scholar]
- 118. Chen LJ, Ye H, Zhang Q, et al. Bleomycin induced epithelial‐mesenchymal transition (EMT) in pleural mesothelial cells. Toxicol Appl Pharmacol. 2015;283:75‐82. [DOI] [PubMed] [Google Scholar]
- 119. Kim KK, Kugler MC, Wolters PJ, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180‐13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tanjore H, Xu XC, Polosukhin VV, et al. Contribution of epithelial‐derived fibroblasts to bleomycin‐induced lung fibrosis. Am J Respir Crit Care Med. 2009;180:657‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Habermann AC, Gutierrez AJ, Bui LT, et al. Single‐cell RNA‐sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. bioRxiv, 753806, 2019. [DOI] [PMC free article] [PubMed]
- 122. Gokey JJ, Snowball J, Green J, et al. Pretreatment of aged mice with retinoic acid supports alveolar regeneration via upregulation of reciprocal PDGFA signalling. Thorax. 2021(thoraxjnl‐2020‐214986). 10.1136/thoraxjnl-2020-214986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gosens R, Ng‐Blichfeldt JP. Rejuvenating old lungs: ain't no tonic like a drop of retinoic. Thorax. 2021(horaxjnl‐2020‐216632). 10.1136/thoraxjnl-2020-216632. [DOI] [PubMed] [Google Scholar]
- 124. Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline‐membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276:357‐368. [DOI] [PubMed] [Google Scholar]
- 125. Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23:167‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res. 1999;46:641‐643. [DOI] [PubMed] [Google Scholar]
- 127. Thebaud B, Goss KN, Laughon M, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers. 2019;5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Toti P, Buonocore G, Tanganelli P, et al. Bronchopulmonary dysplasia of the premature baby: an immunohistochemical study. Pediatr Pulmonol. 1997;24:22‐28. [DOI] [PubMed] [Google Scholar]
- 129. Tallquist MD, Soriano P. Cell autonomous requirement for PDGFRalpha in populations of cranial and cardiac neural crest cells. Development. 2003;130:507‐518. [DOI] [PubMed] [Google Scholar]
- 130. Bostrom H, Gritli‐Linde A, Betsholtz C. PDGF‐A/PDGF alpha‐receptor signaling is required for lung growth and the formation of alveoli but not for early lung branching morphogenesis. Dev Dyn. 2002;223:155‐162. [DOI] [PubMed] [Google Scholar]
- 131. Torday JS, Torres E, Rehan VK. The role of fibroblast transdifferentiation in lung epithelial cell proliferation, differentiation, and repair in vitro. Pediatr Pathol Mol Med. 2003;22:189‐207. [DOI] [PubMed] [Google Scholar]
- 132. Popova AP. Mesenchymal cells and bronchopulmonary dysplasia: new insights about the dark side of oxygen. Am J Respir Cell Mol Biol. 2019;60:501‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hurskainen KM, Mižíková I, Cook DP, et al. Multiplexed single‐cell transcriptomic analysis of normal and impaired lung development in the mouse. bioRxiv, 868802, 2019.
- 134. Ribeiro Baptista B, Zysman M, Essari LA, et al. Lipogenic switch of fibroblast to lipofibroblast induce lung regeneration in a model of bronchopulmonary dysplasia. Eur Respir J. 2019;54:PA4119. [Google Scholar]
- 135. Benjamin JT, Plosa E, Sucre J, et al. Neutrophilic inflammation during lung development disrupts elastin assembly and predisposes adult mice to COPD. J Clin Invest. 2020;131(1):e139481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Moeller A, Thanikkel L, Duijts L, et al. COVID‐19 in children with underlying chronic respiratory diseases: survey results from 174 centres. ERJ Open Res. 2020;6:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015;385:899‐909. [DOI] [PubMed] [Google Scholar]
- 138. Yee M, David Cohen E, Haak J, Dylag AM, O'Reilly MA. Neonatal hyperoxia enhances age‐dependent expression of SARS‐CoV‐2 receptors in mice. Sci Rep. 2020;10:22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Nardiello C, Mizikova I, Morty RE. Looking ahead: where to next for animal models of bronchopulmonary dysplasia? Cell Tissue Res. 2017;367:457‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. D'Angio CT, Ryan RM. Animal models of bronchopulmonary dysplasia. The preterm and term rabbit models. Am J Physiol Lung Cell Mol Physiol. 2014;307:L959‐L969. [DOI] [PubMed] [Google Scholar]
- 141. Manzano RM, Mascaretti RS, Carrer V, et al. A hyperoxic lung injury model in premature rabbits: the influence of different gestational ages and oxygen concentrations. PLoS One. 2014;9:e95844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Albertine KH. Progress in understanding the pathogenesis of BPD using the baboon and sheep models. Semin Perinatol. 2013;37:60‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ardini‐Poleske ME, Clark RF, Ansong C, et al. LungMAP: the molecular atlas of lung development program. Am J Physiol Lung Cell Mol Physiol. 2017;313(5):L733‐L740. 10.1152/ajplung.00139.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Coalson JJ, Kuehl TJ, Escobedo MB, et al. A baboon model of bronchopulmonary dysplasia. II. Pathologic features. Exp Mol Pathol. 1982;37:335‐350. [DOI] [PubMed] [Google Scholar]
- 145. Escobedo MB, Hilliard JL, Smith F, et al. A baboon model of bronchopulmonary dysplasia. I. Clinical features. Exp Mol Pathol. 1982;37:323‐334. [DOI] [PubMed] [Google Scholar]
- 146. Jobe AH, Kramer BW, Moss TJ, Newnham JP, Ikegami M. Decreased indicators of lung injury with continuous positive expiratory pressure in preterm lambs. Pediatr Res. 2002;52:387‐392. [DOI] [PubMed] [Google Scholar]
- 147. Sucre JMS, Vickers KC, Benjamin JT, et al. Hyperoxia injury in the developing lung is mediated by mesenchymal expression of Wnt5A. Am J Respir Crit Care Med. 2020;201:1249‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435‐459. [DOI] [PubMed] [Google Scholar]
- 149. Black PN, Ching PS, Beaumont B, Ranasinghe S, Taylor G, Merrilees MJ. Changes in elastic fibres in the small airways and alveoli in COPD. Eur Respir J. 2008;31:998‐1004. [DOI] [PubMed] [Google Scholar]
- 150. Holz O, Zuhlke I, Jaksztat E, et al. Lung fibroblasts from patients with emphysema show a reduced proliferation rate in culture. Eur Respir J. 2004;24:575‐579. [DOI] [PubMed] [Google Scholar]
- 151. Togo S, Holz O, Liu X, et al. Lung fibroblast repair functions in patients with chronic obstructive pulmonary disease are altered by multiple mechanisms. Am J Respir Crit Care Med. 2008;178:248‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zhang J, Wu L, Feng MX, et al. Pulmonary fibroblasts from COPD patients show an impaired response of elastin synthesis to TGF‐beta1. Respir Physiol Neurobiol. 2011;177:236‐240. [DOI] [PubMed] [Google Scholar]
- 153. Zhang J, Wu L, Qu JM, Bai CX, Merrilees MJ, Black PN. Pro‐inflammatory phenotype of COPD fibroblasts not compatible with repair in COPD lung. J Cell Mol Med. 2012;16:1522‐1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Muller KC, Welker L, Paasch K, et al. Lung fibroblasts from patients with emphysema show markers of senescence in vitro. Respir Res. 2006;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


