Abstract
Data regarding the impact of infra-Hisian conduction disturbances leading to permanent pacemaker implantation (PPI) after transcatheter aortic valve implantation (TAVI) remain limited. The aim of this study was to determine the impact of right and/or left bundle branch block (RBBB/LBBB) on post-TAVI PPI. We performed a systematic literature review to identify studies reporting on RBBB and/or LBBB status and post-TAVI PPI. Study design, patient characteristics, and the presence of branch block were analyzed. Odds ratios (ORs) with 95% CI were extracted. The final analysis included 36 studies, reporting about 55,851 patients. Data on LBBB were extracted from 33 studies. Among 51,026 patients included, 5503 showed pre-implant LBBB (11.9% (10.4%–13.8%)). The influence of LBBB on post-TAVI PPI was not significant OR 1.1474 (0.9025; 1.4588), p = 0.2618. Data on RBBB were extracted from 28 studies. Among 46,663 patients included, 31,603 showed pre-implant RBBB (9.2% (7.3%–11.6%)). The influence of RBBB on post-TAVI PPI was significant OR 4.8581 (4.1571; 5.6775), p < 0.0001. From this meta-analysis, the presence of RBBB increased the risk for post-TAVI PPI, independent of age or LVEF, while this finding was not confirmed for patients experimenting with LBBB. This result emphasizes the need for pre-operative evaluation strategies in patient selection for TAVI.
Keywords: transcatheter aortic valve implantation, right bundle branch block, left bundle branch block, permanent pacemaker implantation
1. Introduction
Atrio-ventricular conduction disturbances and subsequent permanent pacemaker implantation (PPI) represent frequent complication after transcatheter aortic valve implantation (TAVI) [1,2]. Notably, infra-Hisian conductions’ desynchrony such as left bundle branch block (LBBB) and right bundle branch block (RBBB) remain an ongoing issue in TAVI [3,4], especially considering TAVI as well-established therapeutic approach for patients with aortic stenosis at high surgical risk [5], while considerable advances in procedural techniques tend to extend TAVI indications to patients with a lower surgical risk [6]. Mechanical stress on the aortic valve annulus, deterioration of the ventricular septum, and local edema may all injury the atrio-ventricular conduction during the TAVI procedures [1,3]. In patients with pre-existing conduction system impairments, such additional procedural-related factors may contribute to a higher post-TAVI PPI rate. Consequently, complications such as PPI thus remain a substantial barrier to extending this technique to operable patients who would otherwise undergo surgery [7]. Therefore, better patient selection and identification of pre-operative risk factors for progression of conduction disturbances, and subsequently PPI, are decisive [8]. Current data about the clinical impact of bundle branch block on post-TAVI PPI remain controversial [9,10]. Left bundle branch block (LBBB) occurs in 5 to 65% in TAVI patients and leads to PPI in 15 of 20% of cases [11]. Pre-operative right bundle branch block (RBBB) is present in 10 to 21% of patients and results in up to 40% of post-operative PPI, making pre-operative RBBB the most important patient-related factor [2,12]. However, the prognostic value on pre-existing infra-Hisian conduction disturbances on post-TAVI PPI remains unclear [10,11,12].
We aim to investigate the clinical impact of pre-operative RBBB/LBBB on PPI after TAVI.
2. Materials and Methods
2.1. Research Strategy
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the research strategy was developed according to available guidance from the Cochrane Collaboration. A broad, computerized literature search was performed to identify all relevant studies from Embase, Cochrane database, and PubMed exploring Medical Subject Heading (MeSH) terms related to pre-operative RBBB or pre-operative/new-onset LBBB in TAVI population. The PubMed database was searched entering the following key words: “Pacemaker, Artificial” [Mesh] OR pacemaker implantation AND “Transcatheter Aortic Valve Replacement” [Mesh] OR transcatheter aortic valve replacement AND “Bundle-Branch Block” [Mesh]. We restricted the research to English publications. Last access to the database was on 1 November 2020. The search was limited to studies in human.
2.2. Eligibility Criteria and Studies Selection
Studies were included in the final analyses if patients were >18 years (I); >250 patients were included in the main analysis, in order to provide data interpretation of the most consistent clinical series (II); and studies provided a description of pacemaker status of the population (III). Furthermore, articles with no possible extraction of the presence of RBBB/LBBB were excluded. Pre-operative RBBB/LBBB and new-onset RBBB/LBBB were both included in the present analysis. Systematic review and meta-analyses were not taken in account. Studies describing cardiac surgery procedures were also left out. The selected articles underwent extensive evaluation at title and abstract level by two independent researchers (J.R. and M.D.M.) to assess the potential inclusion in the meta-analysis. Discrepancies were solved by consensus with the intervention of a third reviewer (R.L.). There were no duplicate data.
2.3. Statistical Analysis
Calculation of an overall proportion from studies reporting a single proportion was performed using a meta-analytic approach by means of metaprop function of meta package in R. A logit-transformation was performed as suggested by Warton & Hui [13] to calculate confidence intervals (CIs) for individual study results. A Clopper–Pearson approach and a DerSimonian–Laird estimator were used to estimate the between-study variance [14]. Total proportion with 95% Cl was reported. Funnel plot and Egger’s test were used for estimation of publication bias. The primary endpoint was 30-day or in-hospital PPI after TAVI, so odds ratios (OR) with 95% CI were extracted from 36 studies. Statistical pooling of OR was performed using a random effect model with 95% CI. Forest plots were used to plot the effect size, either for each study or overall. We calculated the I2 statistics (0%~100%) to explain the between-study heterogeneity, with I2 ≤ 25% suggesting more homogeneity, 25% < I2 ≤ 75% suggesting moderate heterogeneity, and I2 > 75% suggesting high heterogeneity [15,16]. If the null hypothesis was rejected, a random effects model was used to calculate pooled effect estimations. If the null hypothesis was not rejected, a fixed effects model was used to calculate pooled effect estimations [14]; 95% CI was also reported. Forest plots were used to plot the effect size, either for each study or overall. Publication bias was evaluated by graphical inspection of funnel plot; estimation of publication bias was quantified by means of Egger’s linear regression test [17]. In the case of moderate or high heterogeneity, influence analysis was performed with different approaches: Baujat plot [18] and a leave-one-out sensitivity analysis were performed by iteratively removing one study at a time to confirm that our findings were not driven by any single study. Meta-regression analysis was performed, reporting results as regression coefficient (i.e., Beta) and p-value. One removed analysis was performed as a sensitivity analysis. “Meta package” in R-studio version 1.1.463 (2009–2018) was used. Because this study was a systematic review and meta-analysis based on published articles, ethical approval was waived by the institutional review board of the University Hospital of Maastricht.
3. Results
3.1. Study Inclusion
Our search yielded 877 records initially screened at the title and abstract level, with 222 papers fully reviewed for eligibility. There were no duplicate data. Ultimately, 36 studies were identified and provided data for the research analysis (Supplementary Figure S1).
3.2. Baseline Characteristics of Included Patients and Permanent Pacemaker Implantation Details
Table 1 shows the baseline characteristics of the included studies. The 36 studies included a total of 55,851 patients in the final analysis, from 2005 to 2018 [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. The mean age of the patients was 81.9 years, with a mean STS score of 8.3. Only five studies were prospective in nature [35,40,41,44,51]. The PPI details in the included studies are reported in Table 2. The overall incidence of PPI reached 15.2%, ranging from 4.3% to 32%.
Table 1.
Baseline characteristics of included studies (n = 36). TAVI, transcatheter aortic valve implantation.
| Study | Year | Study Design (Nr Centers) | Sample Size | Age (Years) | STS-Score (%) | Inclusion Period | Valve Type | Follow-Up (Months) * | Approach for TAVI | Mortality at 30 Days |
|---|---|---|---|---|---|---|---|---|---|---|
| De Carlo et al. [19] | 2012 | Retrospective (3) | 275 | 82.4 | na | Sep 2007–Jul 20120 | 100% MCV | 12 | na | 3% |
| Gensas et al. [20] | 2014 | Retrospective (18) | 353 | 82 | 14.4 | Jan 2008–Feb 2012 | 85.8% SE 14.2% BE |
60 | na | na |
| Mouillet et al. [21] | 2015 | Retrospective (29) | 833 | 82 | 14.1 | Jan 2010–Oct 2011 | 100% SE | 8 | na | 9.3% |
| Nazif et al. [22] | 2015 | Retrospective (21) |
1973 | 84.5 | 11.4 | May 2007–Sept 2011 | 100% BE | 12 | na | 6.6% |
| Rodriguez-Olivares et al. [23] | 2016 | Retrospective (1) | 302 | 81 | na | Nov 2005–Jan 2015 | 67.2% SE 21.2% BE 11.6% ME |
na | na | na |
| Gonska et al. [24] | 2017 | Retrospective (1) |
283 | 79.9 | 6.7 | na | 100% ES3 | na | na | na |
| Raelson et al. [25] | 2017 | Retrospective (1) |
578 | 85,5 | Na | Mar 2009–Dec 2014 | 21% SE 79% BE |
1 | na | na |
| Dumonteil et al. [26] | 2017 | Retrospective (14) |
250 | 84 | 6,3 | Oct 2012–May 2014 | 100% ME | 12 | 100% TF | 4% |
| Monteiro et al. [27] | 2017 | Retrospective (22) | 670 | 81.8 | 10.7 | Jan 2008–Jan 2015 | 74% MCV 26% ES |
na | 96% TF 4% others |
na |
| Pellegrini et al. [28] | 2018 | Retrospective (3) | 283 | 80.8 | 6 | Jan 2014–Jan 2016 | 100% SE | 12 | 100% TF | na |
| De-Torres-Alba et al. [29] | 2018 | Retrospective (1) | 606 | 81.6 | na | Jan 2014–Jan 2017 | 100% BE | na | na | na |
| Mangieri et al. [30] | 2018 | Retrospective (1) | 611 | 84.4 | 6.9 | Oct 2007–Jul 2015 | 51.7% BE 33.7% SE |
12 | na | na |
| Bhardwaj et al. [31] | 2018 | Retrospective (1) | 383 | 83 | 9 | Jan 2012–July 2016 | 82% BE 18% SE |
9 | 84% TF | na |
| Pellegrini et al. [32] | 2018 | Retrospective (3) | 709 | 81 | na | Jan 2014–Jan 2016 | 100% BE | na | 100% TF | 1.6% |
| Gaede et al. [33] | 2018 | Retrospective (1) |
1025 | 81.9 | na | 2010–2015 | na | 2.4 | na | na |
| Nadeem et al. [34] | 2018 | Retrospective (1) |
672 | 81.4 | 7.4 | 2011–2017 | na | 12 | na | na |
| Chamandi et al. [35] | 2018 | Prospective (9) |
1629 | 81.5 | 10.9 | May 2009–Feb 2015 | 50.3% BE 49.7% SE |
48 | na | 42.3% |
| Doshi et al. [36] | 2018 | Retrospective (na) | 8148 | 82.5 | na | Jan 2012–Dec 2014 | na | na | na | na |
| Vejpongsa et al. [37] | 2018 | Retrospective (na) | 18,400 | 81.2 | na | Jan 2012–Dec 2013 | na | na | TF 75.5% 24.5% TA |
na |
| Cresse et al. [38] | 2019 | Retrospective (1) | 386 | 83 | na | Apr 2008–Jun 2017 | na | na | na | na |
| Dolci et al. [39] | 2019 | Retrospective (1) |
266 | 80 | na | Feb 2014–Feb 2018 | 100% BE | 12 | 84% TF 16% TA |
na |
| Costa et al. [40] | 2019 | Prospective (1) |
1116 | 82 | 4.4 | June 2007–Feb 2018 | 61.8% SE 27.2% BE 0.5% ME 10.5% Others |
72 | 97% TF 3% others |
3.9% |
| Meduri et al. [41] | 2019 | Prospective (1) |
704 | 82.5 | 6.6 | na | 34% SE 66% ME |
12 | na | na |
| Du et al. [42] | 2020 | Retrospective (1) | 256 | 76.5 | 7.1 | Mar 2013–Oct 2018 | na | 12 | Na | 3.3% |
| Shivamurthy et al. [43] | 2020 | Retrospective (1) |
917 | 80 | na | Nov 2011–Feb 2017 | na | na | 89.7% TF 10.3% TA |
na |
| Studies reporting only on RBBB status in pacemaker population (n = 3) | ||||||||||
| Watanabe et al. [44] | 2016 | Prospective (9) | 749 | 85 | 6.9 | Oct 2013–Aug 2015 | 100% BE | 16.4 | 78.5% TF 18.8% TA 2.7% T iliac |
4% |
| Auffret et al. [45] | 2017 | Retrospective (na) | 3527 | 82 | na | na | 55.8% BE 44.2% SE |
23 | 79.6% TF 16.4% TA 1.9% T aortic 2.1% TS |
7.2% |
| Maeno et al. [46] | 2019 | Retrospective (1) | 659 | 83 | na | Jan 2013–Dec 2015 | 85% BE 15% SE |
19.1 | na | 2.6% |
| Studies reporting only on LBBB status in pacemaker population (n = 8) | ||||||||||
| Testa et al. [47] | 2013 | Retrospective (na) | 818 | 82 | na | Oct 2007–Apr 2011 | 100% SE | 9 | 88.6% TF 11.4% TS |
5.5% |
| Schymik et al. [48] | 2014 | Retrospective (1) | 624 | 82 | na | May 2008–Apr 2012 | 80.8% BE 19.2% SE |
12 | na | na |
| Urena et al. [49] | 2014 | Retrospective (4) | 668 | 79.5 | na | na | 100% BE | 13 | 2.8% TAortic 42.9% TA 54.3% TF |
na |
| Nazif et al. [50] | 2014 | Retrospective (21) | 1151 | 84 | 11.2 | Mar 2007–Mar 2009 | 100% BE | 12 | 57% TF 43% TA |
3.6% |
| Fischer et al. [51] | 2018 | Prospective (18) | 3404 | 81.5 | 5.9 | Feb 2005–Oct 2017 | 46% SE 54% BE |
22 | 82.2% TF 2.2% TS 14.2% TA 1.4% T aortic |
5.7% |
| Chamandi et al. [52] | 2019 | Retrospective (9) | 1020 | 80.5 | 6.6 | May 2007–Feb 2015 | 48% BE 52% SE |
36 | 84% TF 11% TA 2% Taortic 3% TS |
na |
| Nazif et al. [53] | 2019 | Retrospective (51) | 1179 | 81.2 | 5.5 | Dec 2011–Nov 2013 | 100% BE | 24 | 83.5% TF 16.5% Tthoracic |
1.3% |
| Hamandi et al. [54] | 2020 | Retrospective (1) | 424 | 82 | 7.6 | Jan 2012–Mar 2016 | 87% SE 13% BE |
12 | 85% TF 13% TA 3% TAortic |
1.3% |
Values are n (%), mean = SD, or median (interquartile range) as appropriate. * Follow-up is reported as mean or median as given by the authors. LBBB = left bundle branch block; RBBB = right bundle branch block; SE = self-expandable; BE = balloon-expandable; ME = mechanically-expandable; TF = trans-femoral; TS = trans-subclavian; TA = trans-apical; T iliac = trans-iliac; T aortic = trans-aortic.
Table 2.
Pacemaker-related details in included studies. PPI, pacemaker implantation.
| Studies Reporting on RBBB and LBBB Status in Pacemaker Population (n = 25) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| De Carlo et al. [19] | 70% AVB 3% SSS 27% others |
4 | 24% | 32 | 37 | 15 | 9 | * lower MCV implantation below aortic annulus * RBBB * left anterior hemiblock * longer PR interval |
na |
| Gensas et al. [20] | na | na | 25.2% | 41 | 50 | 22 | 10 | * pre-existing RBBB * balloon pre-dilatation * CoreValve use |
na |
| Mouillet et al. [21] | na | na | 30.3% | 115 | 106 | 60 | 24 | na | na |
| Nazif et al. [22] | 79% AVB 17.3% SSS |
3 | 8.8% | 312 | 174 | 82 | 12 | * Pre-existing RBBB * Prosthesis to LV outflow tract diameter ratio * LV-end diastolic diameter |
* longer duration of hospitalization * higher rates of repeat hospitalization and mortality or repeat hospitalization at 1 year |
| Rodriguez-Olivares et al. [23] | na | na | 22.5% | 28 | 32 | 13 | 5 | na | *more LVOT oversizing associated with higher PPI |
| Gonska et al. [24] | 94.2% AVB 3.8% B 2% others |
4,3 | 18.4% | 22 | 74 | 13 | 6 | * baseline AV1B * preprocedural complete RBBB |
na |
| Raelson et al. [25] | 82% AVB | 3 | 9% | 65 | 50 | 19 | 3 | na | na |
| Dumonteil et al. [26] | 88.9% AVB 5.9% others |
3 | 32% | 26 | 145 | 20 | 14 | * baseline RBBB * LV outflow tract overstretch >10% |
* trend lower PPI rate at 30 days with shallower (<=5mm) implant depth |
| Monteiro et al. [27] | na | na | 20.1% | 71 | 93 | 36 | 15 | * previous RBBB * mean aortic gradient >50mmHg * MCV |
na |
| Pellegrini et al. [28] | 71.5% AVB 3.5% SSS 25% B |
na | 10% | 22 | 25 | 6 | 4 | * higher EuroSCORE | na |
| De-Torres-Alba et al. [29] | 96% AVB 1.4% B 2.6% others |
na | 12.5% | 20 | 74 | 7 | 11 | na | na |
| Mangieri et al. [30] | 84% AVB 8.4% B |
0,3 | 8.8% | 37 | 61 | 7 | 5 | na | na |
| Bhardwaj et al. [31] | na | na | 11.5% | 50 | 39 | 11 | 3 | * PPI with short-term reduction in QoL without long-term implications | na |
| Pellegrini et al. [32] | 71.3% AVB 5.2% SSS 23.5% B |
na | 16.2% | 63 | 41 | 30 | 4 | * increase in prosthesis oversizing | na |
| Gaede et al. [33] | 90% AVB 8% SSS 2% B |
4 | 14.7% | 98 | 107 | 31 | 17 | * pre-existing RBBB * CoreValve prosthesis |
Predictors of lack of recovery of AVB * prior RBBB * higher mean aortic valve gradient * post-dilatation of the prosthesis |
| Nadeem et al. [34] | na | na | 21.7% | 113 | 51 | 65 | 4 | na | * PPI more likely to have heart failure admissions * PPI trend toward increased mortality |
| Chamandi et al. [35] | 76.7% AVB 5.6% SSS 3.1% B 14.6% others |
2 | 19.8% | 169 | 179 | 84 | 37 | na | * PPI higher rates of rehospitalization due to heart failure and combined endpoint of mortality or heart failure rehospitalization * PPI lesser improvement in LVEF over time, particularly in patients with reduced LVEF before TAVI |
| Doshi et al. [36] | na | na | 24% | 220 | 724 | 96 | 253 | * female sex * AF * LBBB * AVB |
na |
| Vejpongsa et al. [37] | na | 2 | 9.9% | 715 | 1670 | 265 | 390 | na | na |
| Cresse et al. [38] | na | 4 | 6.7% | 14 | 97 | 6 | 12 | na | * RBBB, LBBB, △PR >40 ms associated with PPI |
| Dolci et al. [39] | 80%AVB 11% B 9% others |
4 | 13% | 29 | 41 | 12 | 3 | * baseline RBBB * QRS width immediately after TAVI |
na |
| Costa et al. [40] | 84.8% AVB 4.1%SSS 11% Others |
na | 13% | 92 | 99 | 39 | 8 | na | * PPI associated with increased 6 years mortality * baseline RBBB higher chance of being dependent at follow-up |
| Meduri et al. [41] | 90% AVB 6% B 4% others |
2 | 28.4% | 85 | 56 | 68 | 20 | * baseline RBBB * mean depth of valve implantation |
* medically-treated diabetes mellitus in LOTUS valve patients |
| Du et al. [42] | 89.5% AVB | 8.7 | 14.8% | 20 | 19 | 6 | 3 | na | na |
| Shivamurthy et al. [43] | na | na | 9.8% | 130 | 79 | 38 | 9 | na | na |
| Studies reporting only on RBBB status in pacemaker population (n = 3) | |||||||||
| Watanabe et al. [44] | na | na | 4.9% | 108 | na | 18 | na | na | na |
| Auffret et al. [45] | na | na | 16.5% | 362 | na | 137 | na | na | na |
| Maeno et al. [46] | 77.9% AVB 11.5% SSS 10.6% |
na | 15.8% | 101 | na | 38 | na | na | na |
| Studies reporting only on LBBB status in pacemaker population (n = 8) | |||||||||
| Testa et al. [47] | na | na | 17.4% | na | 224 | na | 41 | na | * LBBB associated with higher short-term PPI |
| Schymik et al. [48] | na | na | 10.8% | na | 197 | na | 28 | * chronic AF * baseline RBBB * MCV |
na |
| Urena et al. [49] | 55.5% AVB SSS 20.7% 24.1% B |
365 | 4.3% | na | 128 | na | 11 | na | na |
| Nazif et al. [50] | na | na | 5.1% | na | 121 | na | 12 | na | * LBBB with higher PPI and failure of LVEF |
| Fischer et al. [51] | na | na | 15.5% | na | 398 | na | 84 | * pre-existing LBBB increase risk of death but not late PPI | * pre-existing LBBB associated with lower pre-operative LVEF |
| Chamandi et al. [52] | na | na | 7.2% | na | 212 | na | 29 | na | na |
| Nazif et al. [53] | na | na | 4.8% | na | 179 | na | 9 | na | * LBBB associated with PPI and repeat hospitalizations |
| Hamandi et al. [54] | na | na | 18% | na | 52 | na | 6 | na | na |
Values are n (%), mean = SD, or median (interquartile range) as appropriate. * Follow-up is reported as mean or median as given by the authors. PPI = permanent pacemaker implantation; AVB = atrio-ventricular block, SSS = Sick Sinus Syndrom; B = bradycardia; AV = atrio-ventricular; LVEF = left ventricle ejection fraction; VVI = single chamber device; DDD = dual chamber device; MCV = Medtronic CoreValve; RBBB = right bundle branch block; LVF = left ventricle function; BMI = body mass index; QoL = quality of life; AF = atrial fibrillation; LBBB = left bundle branch block; TAVI = trans-catheter aortic valve implantation; na = not available.
3.3. Influence of LBBB on PPI
Data on LBBB were extracted from 33 studies [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,47,48,49,50,51,52,53,54]; among the 51,026 patients included in the analysis, there were 5503 showing pre-implant LBBB. The cumulative proportion of LBBB was 11.9% (10.4%–13.8%) (Supplementary Figure S2). Heterogeneity was high (I2 96.4% [95.6%; 97.0%]). No publication bias was found (p = 0.2921). The cumulative proportion of LBBB in a subset of 7315 patients with post-TAVI PPI was 13.0% (10.6%–15.8%) with high heterogeneity (I2 87.7% [83.7%; 90.6%]) and no publication bias (p = 0.3856) (Supplementary Figure S3). The cumulative proportion of LBBB in a subset of 43,650 patients without post-TAVI PPI was 12.3% (10.5%–14.4%) with high heterogeneity (I2 96.5% [95.8%; 97.1%]) and no publication bias (p = 0.6200) (Supplementary Figure S4).
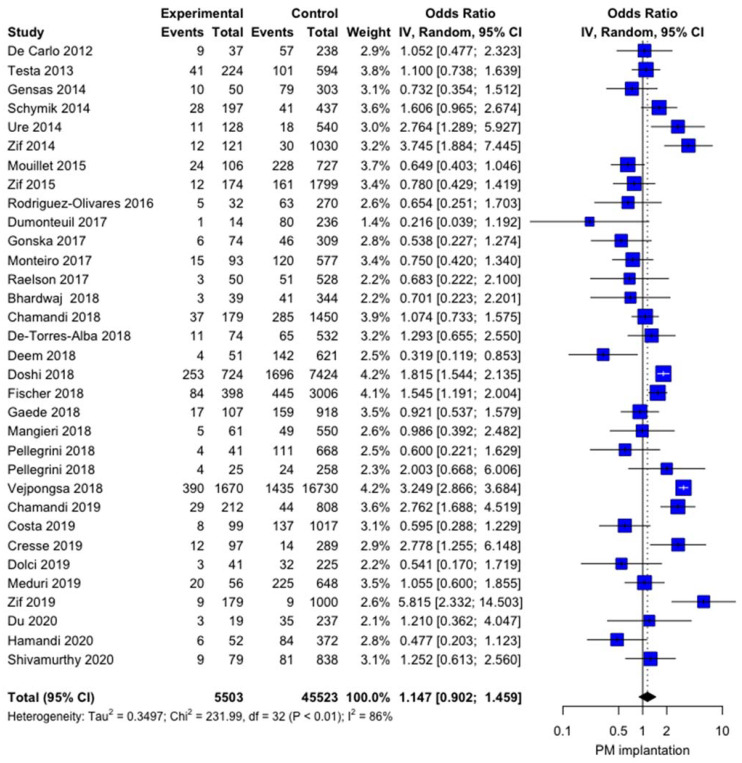
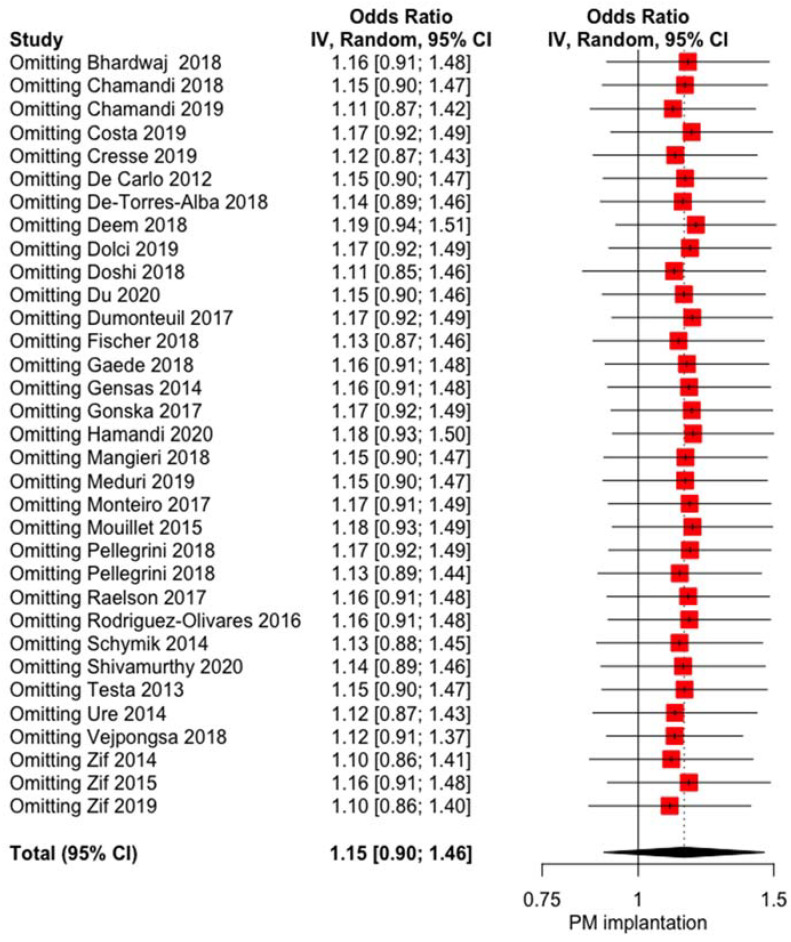
The influence of LBBB on post-TAVI PPI was not significant OR 1.1474 (0.9025; 1.4588, p = 0.2618) with high heterogeneity I2 = 86.2% [81.7%; 89.6%] and no publication bias (p = 0.7100) (Figure 1). The baujat plot (Supplementary Figure S5) shows that the study by Vejpongsa et al. [37] may impact high heterogeneity, even if the sensitivity analysis does not confirm this hypothesis, as no influence of LBBB on post-TAVI PPI rate was evidenced at the leave-one out analysis (Figure 2). Meta-regression failed to identify some modifiers: age (r = −0.0592, p = 0.4292), left ventricular ejection fraction (LVEF, r = 0.0754, p = 0.1741), and year of the study (r = −0.0008, p = 0.9893) did not show any influence on the meta-analytic results (Supplementary Figures S6–S8).
Figure 1.
Forest plot comparing the effect of LBBB on the rate of post-TAVI PPI. IV= interval variable; 95% CI = confidence interval.
Figure 2.
Forest plot leave one-out analysis comparing the effect of the presence of LBBB on the rate of post-TAVI PPI. IV= interval variable; 95% CI= confidence interval.
3.4. Influence of RBBB on PPI
Data on RBBB were extracted from 28 studies (19–46); among the 46,663 patients included in the analysis, there were 31,603 showing pre-implant RBBB. The cumulative proportion of RBBB was 9.2% (7.3%–11.6%) (Supplementary Figure S9). Heterogeneity was high (97.8% [97.3%; 98.1%]). No publication bias was found (p = 0.1112). The cumulative proportion of RBBB in a subset of 6932 patients with post-TAVI PPI was 24.7% (19.6%–30.6%) with high heterogeneity (94.6% [93.2%; 95.8%]) and no publication bias (p = 0.1023) (Supplementary Figure S10). The cumulative proportion of RBBB in a subset of 39,670 patients without post-TAVI PPI was 6.3% (4.9%–8.1%) with high heterogeneity (I2 96.6% [95.9%; 97.3%]) and no publication bias (p = 0.2659) (Supplementary Figure S11).
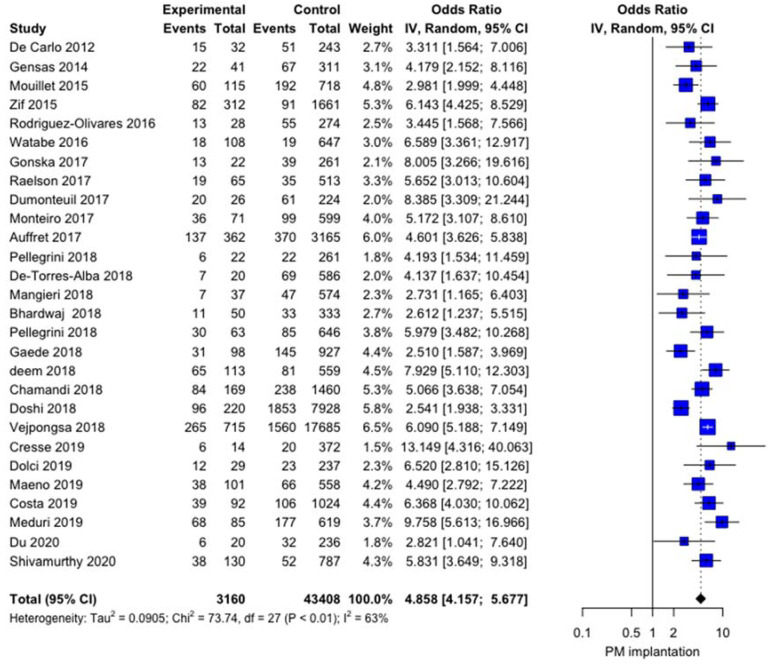
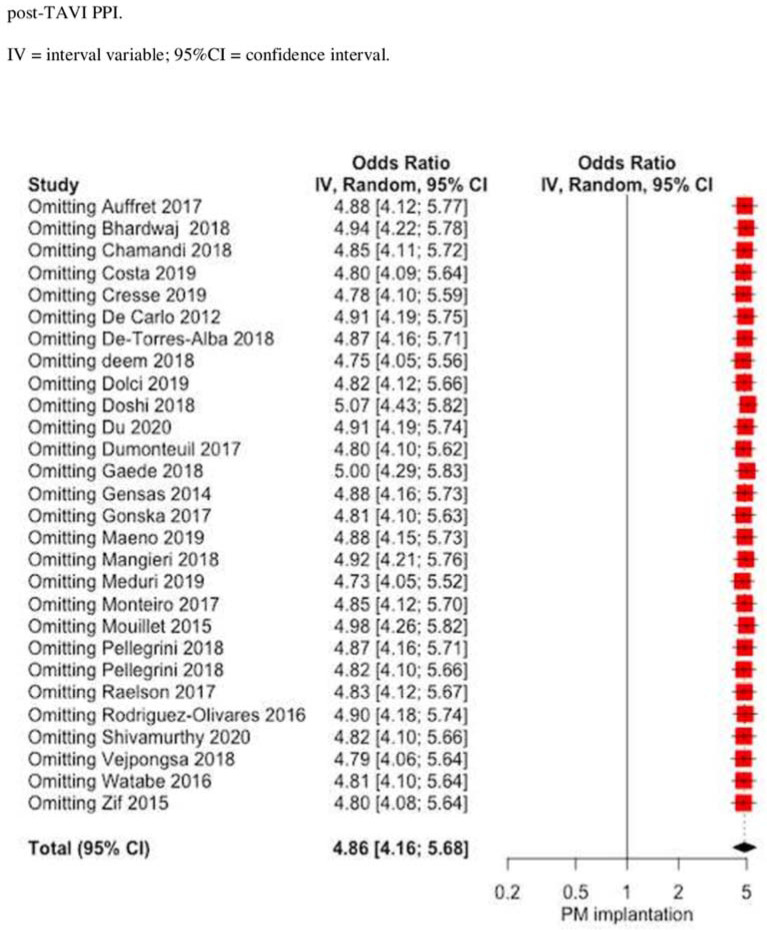
The influence of RBBB on post-TAVI PPI was significant, with OR 4.8581 (4.1571; 5.6775), p < 0.0001 with moderate heterogeneity I2 = 63.4% [45.1%; 75.6%] and no publication bias (p = 0.937) (Figure 3). The baujat plot (Supplementary Figure S12) shows that two studies [36,37] may impact the heterogeneity. Sensitivity analysis confirms the impact of RBBB on the post-TAVI PPI rate even at the leave-one out analysis (Figure 4). Meta-regression failed to identify some modifiers: age (r = −0.0592; p = 0.4292), left ventricular ejection fraction (LVEF, r = 0.0246, p = 0.3755), and year of the study (r = 0.0489, p = 0.3038) did not show any influence on meta-analytic results (Supplementary Figures S13–S15).
Figure 3.
Forest plot comparing the effect of RBBB on the rate of post-TAVI PPI.
Figure 4.
Forest plot leave one-out analysis comparing the effect of the presence of RBBB on the rate of post-TAVI PPI.
4. Discussion
Our meta-analysis demonstrates the impact of bundle branch block on post-TAVI PPI. Our study is derived from 36 studies reporting clinical outcomes in 55,851 patients receiving TAVI and presenting bundle branch conduction disturbances. The main results of this study can be assumed as follows: (i) the presence of LBBB does not influence post-TAVI PPI; (ii) the presence of RBBB has a significant impact on post-TAVI PPI.
The prevalence of LBBB in TAVI candidates is closed to 10% according to previous studies [45,55,56] and new-onset LBBB occurs in ≅30% after TAVI, depending on the type of prosthesis [9,57]. In the present study, the cumulative proportion of LBBB was 11.9%, including pre-operative LBBB and new-onset LBBB after TAVI. This rate is thus in accordance with previously published data [4,58]. The impact of LBBB on post-TAVI PPI remains controversial [49,50,59]. Data from the PARTNER experience [50,53] suggested that new-onset LBBB increases the rate of post-TAVI PPI. Moreover, an analysis at a national registry level as the prospective open TAVI Karlsruhe registry [48] identified slightly more PPI in patients with persistent new-onset LBBB. However, current data do not promote prophylactic PPI in patients presenting new-onset LBBB after TAVI [60] and some studies failed to identify LBBB as predictors for post-TAVI PPI [58,61]. In our study, the influence of LBBB on post-TAVI PPI was not significant. These controversial results can be related to various definitions of LBBB according to the degree of QRS prolongation defining the LBBB [58,61,62,63] or to the high heterogeneity among the current studies [64]. Moreover, only three studies [22,50,53] quantified LBBB as left anterior fascicular or left posterior fascicular block, which may underestimate the impact of LBBB on post-TAVI PPI. As development of high-degree atrio-ventricular block is shown to be a common complication in patients with pre-existing LBBB (or RBBB) after TAVI [65], we shared the view of Waksman and colleagues [66] emphasizing the need for routine electrophysiology evaluation in patients undergoing TAVI in order to limit post-TAVI PPI. Electrophysiology testing differentiates the supra-nodal conduction disturbances from the infra-nodal ones by analyzing the atrial-His and the His-ventricular intervals. By integrating these values into the baseline electrocardiogram, we may be able to predict the evolution of the atrio-ventricular conduction in high-grade atrio-ventricular block. Such data may help the clinicians to choose between an aggressive strategy of early post-TAVI PPI or, on the contrary, an expectative strategy, avoiding some unnecessary post-TAVI PPI. The results of current ongoing trials investigating the use of electrophysiology-based algorithmic approaches with HV measurements as a guide for PPI, particularly in patients with LBBB (Clinical Monitoring Strategy vs. EP-Guided Algorithmic in LBBB Patients Post-TAVR (NCT03303612); The MARE Study (NCT02153307); Prospective Validation of a Pre-Specified algorithm for the management of conduction disturbances following transcatheter aortic valve replacement PROMOTE (NCT04139616)), are expected with impatience.
Pre-existing RBBB has been demonstrated in 6.9% [67] up to 13.6% [44] of patients undergoing TAVI and is a common underlying conduction disturbance in TAVI patients [10]. In this study, the cumulative proportion of RBBB among the included studies reached 9.2%, also in accordance with the studies of Auffret and colleagues [45], reporting a prevalence of RBBB of 10.3% in TAVI candidates. Several studies have already highlighted the role of pre-existing RBBB in the development of atrioventricular conduction, leading to post-TAVI PPI [2,3,22,26,33,39,41]. Our findings confirm the impact of such a condition on post-TAVI PPI, without the influence of age or LVEF. As previous studies emphasized the higher all-cause mortality and the poorer clinical outcomes in patients with pre-existing RBBB [10,45], bradyarrhythmia events may participate in the poorer outcomes of these patients. However, this analysis does not provide the cause of such discrepancies between RBBB and LBBB. The fragility of the right bundle branch during minor trauma or procedures may partially explain its highest incidence in the general population and, consequently, the more important impact of RBBB in post-TAVI PPI [68]. We look forward to having definitive data and guidelines to determine the optimal management and monitoring of patients with RBBB undergoing TAVI [60].
The current guidelines with respect to post-TAVI PPI [69] do not specify the adequate timing for post-TAVI PPI. Previously, the 2013 European Society Guidelines [70] recommended a clinical rhythm observation period of 7 days in the presence of high-grade trio-ventricular block before proceeding to post-TAVI PPI. In the current meta-analysis, the range of timing for PPI varied from day 0 to 1 year, also emphasizing the need for PPI after discharge and the influence of atrio-ventricular conduction in the long term [49]. This hypothetical observation period may be shortened or lengthened according to patient-related factors and electrical findings. Once again, electrophysiological study may identify some high-risk patients for the development of high-grade atrio-ventricular disturbances and, thereby, adjust the observation rhythm period in such patients [71].
As the definitive impact of infra-Hisian conduction disturbances on post-TAVI PPI has not definitively been clarified yet, specific recommendations for the type of valves used in such patients must be done with caution. However, some studies investigated the impact of the valves used on post-TAVI conduction disturbances [3,44,49,61]. Indeed, Franzoni and colleagues [61] found a higher incidence of LBBB when using a Medtronic CoreRevalving System with respect to the Edwards Sapien Valve. A more intra-ventricular implantation of the stent valve frame may result in higher rate of post-TAVI PPI [49]. Furthermore, the gap between the lower edge of the coronary cusp and the end of the frame of the prosthetics valve has been shown to be greater in patients with new-onset LBBB than in patients with post-operative conduction disturbances [61], emphasizing the role of the prosthesis placement in the development of post-operative conduction disturbances. Nevertheless, the requirement for post-TAVI PPI is multifactorial and studies incorporating electrophysiological findings with procedural data are mandatory [72].
Study Limitations
This meta-analysis has several limitations to be acknowledged. First, this meta-analysis possesses all the inherent bias associated with this investigation technique. Second, including pre-operative LBBB and new-onset LBBB in the analysis may be considered as a limitation for the interpretation of the final results, as the pre-operative LBBB can be related to other cardiovascular conditions and the new-onset LBBB can be related to procedural/interventional aspects. However, as our primary outcome was 30-day or in-hospital PPI, we can afford to compare both LBBB as conduction disturbances present after TAVI, which can lead to PPI, independently of the chronic or acute apparition of the LBBB. Third, distinction of LBBB in left anterior fascicular or left posterior fascicular block was lacking in the majority of the included studies, thereby not providing enough information to perform in-depth analysis. Further research in this respect is currently needed. Finally, analysis of individual patient-level data may provide further understandings.
5. Conclusions
This meta-analysis found that patients with the presence of RBBB have higher risk for post-TAVI PPI, with no influence of age or LVEF. This finding has not been confirmed for patients experimenting with LBBB. Pre-operative evaluation strategies, including electrophysiological characteristics, are crucial in further extending patient selection for TAVI.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm10122719/s1, Figure S1. Flowsheet of the included studies, Figure S2. Forest plot pooling the proportion of LBBB in 33 studies, Figure S3. Forest plot pooling the proportion of LBBB in subset of 7315 patients with post-TAVI PPI. Figure S4. Forest plot pooling the proportion of LBBB in subset of 43,650 patients without post-TAVI PPI, Figure S5. Baujat plot: impact of the studies on overall heterogeneity in studies reporting on LBBB status, Figure S6. Bubble plots: influence of age on risk for post-TAVI PPI in patients with LBBB, Figure S7. Bubble plots: influence of LVEF on risk for post-TAVI PPI in patients with LBBB, Figure S8. Bubble plots: influence of the year of the study on risk for post-TAVI PPI in patients with LBBB, Figure S9. Forest plot pooling the proportion of RBBB in 28 studies, Figure S10. Forest plot pooling the proportion of RBBB in subset of 6932 patients with post-TAVI PPI, Figure S11. Forest plot pooling the proportion of RBBB in subset of 39,670 patients without post-TAVI PPI, Figure S12. Baujat plot: impact of the studies on overall heterogeneity in studies reporting on RBBB status, Figure S13. Bubble plots: influence of age on risk for post-TAVI PPI in patients with RBBB, Figure S14. Bubble plots: influence of LVEF on risk for post-TAVI PPI in patients with RBBB, Figure S15. Bubble plots: influence of the year of the study on risk for post-TAVI PPI in patients with RBBB.
Author Contributions
Conceptualization, J.M.R., M.D.M. and R.L.; methodology, J.M.R., M.D.M. and R.L.; software, M.D.M.; validation, K.V., S.M., A.W.V.H., S.K. and L.V.; formal analysis, J.M.R. and M.D.M.; investigation, J.M.R. and M.D.M.; resources, S.M., D.R. and J.S.; data curation, J.M.R. and M.D.M.; writing—original draft preparation, J.M.R. and M.D.M.; writing—review and editing, J.M.R., M.D.M. and R.L.; visualization, J.M.R., M.D.M., K.V., S.M., D.R., J.S., A.W.V.H., L.V., S.K., J.G.M. and R.L.; supervision, S.K. and R.L.; project administration, J.M.R., M.D.M. and R.L.; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Because this study was a systematic review and meta-analysis based on published articles, ethical approval was waived by the institutional review board of the University Hospital of Maastricht.
Informed Consent Statement
Patient consent was waived due to the systematic review and meta-analytic nature of this study.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Erkapic D., De Rosa S., Kelava A., Lehmann R., Fichtlscherer S., Hohnloser S.H. Risk for permanent pacemaker after transcatheter aortic valve implantation: A comprehensive analysis of the literature. J. Cardiovasc. Electrophysiol. 2012;23:391–397. doi: 10.1111/j.1540-8167.2011.02211.x. [DOI] [PubMed] [Google Scholar]
- 2.Siontis G.C., Jüni P., Pilgrim T., Stortecky S., Büllesfeld L., Meier B., Wenaweser P., Windecker S. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: A meta-analysis. J. Am. Coll. Cardiol. 2014;64:129–140. doi: 10.1016/j.jacc.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Van Gils L., Tchetche D., Lhermusier T., Abawi M., Dumonteuil N., Rodriguez-Olivares R., Molina-Martin D.N.J., Stella P.R., Carrié D., De Jaegere P.P., et al. Transcatheter Heart Valve Selection and Permanent Pacemaker Implantation in Patients with Pre-Existent Right Bundle Branch Block. J. Am. Heart Assoc. 2017;6:e005028. doi: 10.1161/JAHA.116.005028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez-Aguilera J., Saint-Gerons J.M.S., Bellido F.M., Suarez D.L.H.D.T.J., Pineda S.O., Alvarez-Ossorio M.P., Angel R.M.M., Pavlovic D., Suarez D.L.C.C.J. Effect of New-Onset Left Bundle Branch Block After Transcatheter Aortic Valve Implantation (CoreValve) on Mortality, Frequency of Re-Hospitalization, and Need for Pacemaker. Am. J. Cardiol. 2016;118:1380–1385. doi: 10.1016/j.amjcard.2016.07.057. [DOI] [PubMed] [Google Scholar]
- 5.Rodès-Cabau J. Transcatheter aortic valve implantation: Current and future approaches. Nat. Rev. Cardiol. 2012;9:15–29. doi: 10.1038/nrcardio.2011.164. [DOI] [PubMed] [Google Scholar]
- 6.Waksman R., Rogers T., Torguson R., Gordon P., Ehsan A., Wilson S.R., Goncalves J., Levitt R., Hahn C., Parikh P., et al. Transcatheter Aortic Valve Replacement in Low-Risk Patients with Symptomatic Severe Aortic Stenosis. J. Am. Coll. Cardiol. 2018;72:2095–2105. doi: 10.1016/j.jacc.2018.08.1033. [DOI] [PubMed] [Google Scholar]
- 7.Finkelstein A., Rozenbaum Z., Halkin A., Banai S., Bazan S., Barbash I., Segev A., Fefer P., Maor E., Danenberg H., et al. Outcomes of Transcatheter Aortic Valve Implantation in Patients with Low Versus Intermediate to High Surgical Risk. Am. J. Cardiol. 2019;123:644–649. doi: 10.1016/j.amjcard.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Voigtländer L., Seiffert M. Expanding TAVI to Low and Intermediate Risk Patients. Front. Cardiovasc. Med. 2018;5:92. doi: 10.3389/fcvm.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regueiro A., Abdul-Jawad A.O., Del Trigo M., Campelo-Parada F., Puri R., Urena M., Philippon F., Rodès-Cabau J. Impact of New-Onset Left Bundle Branch Block and Periprocedural Permanent Pacemaker Implantation on Clinical Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement: A systematic Review and Meta-Analysis. Circ. Cardiovasc. Interv. 2016;9:e003635. doi: 10.1161/CIRCINTERVENTIONS.115.003635. [DOI] [PubMed] [Google Scholar]
- 10.Croix G.R.S., Spencer C., Hrachian H.L., Beohar N. Clinical Impact of Preexisting Right Bundle Branch Block after Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. J. Interv. Cardiol. 2020;2020:1789516. doi: 10.1155/2020/1789516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massouillé G., Bordachar P., Ellenbogen K.A., Souteyrand G., Jean F., Combaret N., Vorilhon C., Clerfond G., Farhat M., Ritter P., et al. New-onset left bundle branch block induced by transcutaneous aortic valve implantation. Am. J. Cardiol. 2016;117:867–873. doi: 10.1016/j.amjcard.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Rivard L., Schram G., Asgar A., Khairy P., Andrade J.G., Bonan R., Dubuc M., Guerra P.G., Ibrahim R., Macle L., et al. Electrocardiographic and electrophysiological predictors of atrioventricular block after transcatheter aortic valve replacement. Heart Rhythm. 2015;12:321–329. doi: 10.1016/j.hrthm.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Warton D.I., Hui F.K.C. The arcsine is asinine: The analysis of proportions in ecology. Ecology. 2011;92:3–10. doi: 10.1890/10-0340.1. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Pt AContemp. Clin. Trials. 2015;45:139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowden J., Tierney J.F., Copas A.J., Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalized Q statistics. BMC Med. Res. Methodol. 2011;11:41–52. doi: 10.1186/1471-2288-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huedo-Medina T.B., Sanchez-Meca J., Marin-Martinez F., Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 17.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 18.Baujat B., Mahé C., Pignon J.-P., Hill C. A graphical method for exploring heterogeneity in meta-analyses: Application to a meta-analysis of 65 trials. Stat. Med. 2002;21:2641–2652. doi: 10.1002/sim.1221. [DOI] [PubMed] [Google Scholar]
- 19.De Carlo M., Giannini C., Bedogni F., Klugmann S., Brambilla N., De Marco F., Zuchelli G., Testa L., Oreglia J., Petronio A.S. Safety of a conservative strategy of permanent pacemaker implantation after transcatheter aortic CoreValve implantation. Am. Heart J. 2012;163:492–499. doi: 10.1016/j.ahj.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Gensas C.S., Caixeta A., Siqueira D., Carvalho L.A., Sarmento-Leite R., Mangione J.A., Lemos P.A., Colafranceschi A.S., Caramori P., Ferreira M.C., et al. Brazilian Registry in Transcatheter Aortic Valve Implantation Investigators. Predictors of permanent pacemaker requirement after transcatheter aortic valve implantation: Insights from a Brazilian registry. Int. J. Cardiol. 2014;175:248–252. doi: 10.1016/j.ijcard.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Mouillet G., Lellouche N., Yamamoto M., Oguri A., Dubois-Rande J.L., Van Belle E., Gilard M., Laskar M., Teiger E. Outcomes following pacemaker implantation after transcatheter aortic valve implantation with CoreValve (®) devices: Results from the FRANCE 2 Registry. Catheter. Cardiovasc. Interv. 2015;86:E158–E166. doi: 10.1002/ccd.25818. [DOI] [PubMed] [Google Scholar]
- 22.Nazif T.M., Dizon J.M., Hahn R.T., Xu K., Babaliaros V., Douglas P.S., El-Chami M.F., Herrmann H.C., Mack M., Makkar R.R., et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: The PARTNER (Placement of AoRtic TraNscathertER Valves) trial and registry. Pt AJACC Cardiovasc. Interv. 2015;8:60–69. doi: 10.1016/j.jcin.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Rodríguez-Olivaresa R., van Gils L., El Faquir N., Rahhab Z., Di Martino L.F.M., van Weenen S., de Vriesa J., Galema T.W., Geleijnse M.L., Budde R.P., et al. Importance of the left ventricular outflow tract in the need for pacemaker implantation after transcatheter aortic valve replacement. Int. J. Cardiol. 2016;216:9–15. doi: 10.1016/j.ijcard.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 24.Gonska B., Seeger J., Keßler M., Von Keil A., Rottbauer W., Wöhrle J. Predictors for permanent pacemaker implantation in patients undergoing transfemoral aortic valve implantation with the Edwards Sapien 3 valve. Clin. Res. Cardiol. 2017;106:590–597. doi: 10.1007/s00392-017-1093-2. [DOI] [PubMed] [Google Scholar]
- 25.Raelson C.A., Gabriels J., Ruan J., Ip J.E., Thomas G., Liu C.F., Cheung J.W., Lerman B.B., Patel A., Markowitz S.M. Recovery of atrioventricular conduction in patients with heart block after transcatheter aortic valve replacement. J. Cardiovasc. Electrophysiol. 2017;28:1196–1202. doi: 10.1111/jce.13291. [DOI] [PubMed] [Google Scholar]
- 26.Dumonteil N., Meredith I.T., Blackman D.J., Tchétché D., Hildick-Smith D., Spence M.S., Walters D.L., Harnek J., Worthley S.G., Rioufol G., et al. Insights into the need for permanent pacemaker following implantation of the repositionable LOTUS valve for transcatheter aortic valve replacement in 250 patients: Results from the REPRISE II trial with extended cohort. Euro Interv. 2017;13:796–803. doi: 10.4244/EIJ-D-16-01025. [DOI] [PubMed] [Google Scholar]
- 27.Monteiro C., Di Leoni F.A., Caramori P.R., Carvalho L.A.F., Siqueira D.A., Sao T.L.E.K., Perin M., De Lima V.C., Guérios E., Brito F.S.D., Jr., et al. Permanent Pacing After Transcatheter Aortic Valve Implantation: Incidence, Predictors and Evolution of Left Ventricular Function. Arq. Bras. Cardiol. 2017;109:550–559. doi: 10.5935/abc.20170170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellegrini C., Kim W.K., Holzamer A., Walther T., Mayr N.P., Michel J., Rheude T., Nuñez J., Kasel A.M., Trenkwalder T., et al. Multicenter Evaluation of Prosthesis Oversizing of the SAPIEN 3 Transcatheter Heart Valve Impact on Device Failure and New Pacemaker Implantations. Rev. Esp. Cardiol. 2019;72:641–648. doi: 10.1016/j.recesp.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 29.De-Torres-Alba F., Kaleschke G., Vormbrock J., Orwat S., Radke R., Feurle M., Diller G.P., Reinecke H., Baumgartner H. Delayed pacemaker requirement after transcatheter aortic valve implantation with a new-generation balloon expandable valve: Should we monitor longer? Int. J. Cardiol. 2018;273:56–62. doi: 10.1016/j.ijcard.2018.07.131. [DOI] [PubMed] [Google Scholar]
- 30.Mangieri A., Lanzillo G., Bertoldi L., Jabbour R.J., Regazzoli D., Ancona M.B., Tanaka A., Mitomo S., Garducci S., Montalto C., et al. Predictors of Advanced Conduction Disturbances Requiring a Late (≥48 h) Permanent Pacemaker Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2018;11:1519–1526. doi: 10.1016/j.jcin.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Bhardwaj A., Ramanan T., Sawant A.C., Sinibaldi E., Pham M., Khan S., Qureshi R., Agrawal N., Khalil C., Hansen R., et al. Quality of life outcomes in transcatheter aortic valve replacement patients requiring pacemaker implantation. J. Arrhythm. 2018;34:441–449. doi: 10.1002/joa3.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pellegrini C., Husser O., Kim W.K., Holzamer A., Walther T., Rheude T., Mayr N.P., Trenkwalder T., Joner M., Michel J., et al. Predictors of Need for Permanent Pacemaker Implantation and Conduction Abnormalities With a Novel Self-expanding Transcatheter Heart Valve. Rev. Esp. Cardiol. 2019;72:145–153. doi: 10.1016/j.recesp.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 33.Gaede L., Kim W.K., Liebetrau C., Dörr O., Sperzel J., Blumenstein J., Berkowitsch A., Walther T., Hamm C., Elsässer A., et al. Pacemaker implantation after TAVI: Predictors of AV block persistence. Clin. Res. Cardiol. 2018;107:60–69. doi: 10.1007/s00392-017-1158-2. [DOI] [PubMed] [Google Scholar]
- 34.Nadeem F., Tsushima T., Ladas T.P., Thomas R.B., Patel S.M., Saric P., Patel T., Lipinski J., Li J., Costa M.A., et al. Impact of Right Ventricular Pacing in Patients Who Underwent Implantation of Permanent Pacemaker After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2018;122:1712–1717. doi: 10.1016/j.amjcard.2018.07.046. [DOI] [PubMed] [Google Scholar]
- 35.Chamandi C., Barbanti M., Munoz-Garcia A., Latib A., Nombela-Franco L., Gutiérrez-Ibanez E., Veiga-Fernandez G., Cheema A.N., Cruz-Gonzalez I., Serra V., et al. Long-Term Outcomes in Patients with New Permanent Pacemaker Implantation Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2018;11:301–310. doi: 10.1016/j.jcin.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 36.Doshi R., Decter D.H., Meraj P. Incidence of arrhythmias and impact of permanent pacemaker implantation in hospitalizations with transcatheter aortic valve replacement. Clin. Cardiol. 2018;41:640–645. doi: 10.1002/clc.22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vejpongsa P., Zhang X., Bhise V., Kitkungvan D., Shivamurthy P., Anderson H.V., Balan P., Nguyen T.C., Estrera A.L., Dougherty A.H., et al. Risk Prediction Model for Permanent Pacemaker Implantation after Transcatheter Aortic Valve Replacement. Struct. Heart. 2018;2:328–335. doi: 10.1080/24748706.2018.1467067. [DOI] [Google Scholar]
- 38.Cresse S., Eisenberg T., Alfonso C., Cohen M.G., DeMarchena E., Williams D., Carrillo R. Cardiac conduction abnormalities associated with pacemaker implantation after transcatheter aortic valve replacement. Pacing Clin. Electrophysiol. 2019;42:846–852. doi: 10.1111/pace.13695. [DOI] [PubMed] [Google Scholar]
- 39.Dolci G., Vollema E.M., Van Der Kley F., De Weger A., Marsa N.A., Delgado V., Bax J.J. One-Year Follow-Up of Conduction Abdormalities After Transcatheter Aortic Valve Implantation with the SAPIEN 3 Valve. Am. J. Cardiol. 2019;124:1239–1245. doi: 10.1016/j.amjcard.2019.07.035. [DOI] [PubMed] [Google Scholar]
- 40.Costa G., Zappulla P., Barbanti M., Cirasa A., Todaro D., Rapisarda G., Picci A., Platania F., Tosto A., Di Grazia A., et al. Pacemaker dependency after transcatheter aortic valve implantation: Incidence, predictors and long-term outcomes. EuroIntervention. 2019;15:875–883. doi: 10.4244/EIJ-D-18-01060. [DOI] [PubMed] [Google Scholar]
- 41.Meduri C.U., Kereiakes D.J., Rajagopal V., Makkar R.R., O’Hair D., Linke A., Waksman R., Babliaros V., Stoler R.C., Mishkel G.J., et al. Pacemaker Implantation and Dependency After Transcatheter Aortic Valve Replacement in the REPRISE III Trial. J. Am. Heart Assoc. 2019;8:e012594. doi: 10.1161/JAHA.119.012594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du F., Zhu Q., Jiang J., Chen H., Liu X., Wang J. Incidence and Predictors of Permanent Pacemaker Implantation in Patients Who Underwent Transcatheter Aortic Valve Replacement: Observation of a Chinese Population. Cardioliology. 2019;145:27–34. doi: 10.1159/000502792. [DOI] [PubMed] [Google Scholar]
- 43.Shivamurthy P., Vejpongsa P., Gurung S., Jacob R., Zhao Y., Anderson H.V., Balan P., Nguyen T.C., Estrera A.L., Dougherty A.H., et al. Validation of Scoring System Predicting Permanent Pacemaker Implantation after Transcatheter Aortic Valve Replacement. Pacing Clin. Electrophysiol. 2020;43:479–485. doi: 10.1111/pace.13910. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe Y., Kozuma K., Hioki H., Kawashima H., Nara Y., Kataoka A., Nagura F., Nakashima M., Shirai S., Tada N., et al. Pre-Existing Right Bundle Branch Block Increases Risk for Death After Transcatheter Aortic Valve Replacement with a Balloon-Expandable Valve. JACC Cardiovasc. Interv. 2016;9:2210–2216. doi: 10.1016/j.jcin.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 45.Auffret V., Webb J.G., Eltchaninoff H., Munoz-Garcia A.J., Himbert D., Tamburino C., Nombela-Franco L., Nietlispach F., Moris C., Ruel M., et al. Clinical Impact of Baseline Right Bundle Branch Block in Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017;10:1564–1574. doi: 10.1016/j.jcin.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 46.Maeno Y., Abramowitz Y., Israr S., Yoon S.-H., Kubo S., Nomura T., Miyasaka H., Kawamori H., Kazuno Y., Takahashi N., et al. Prognostic impact of Permanent Pacemaker Implantation in Patients with Low Left Ventricular Ejection Fraction Following Transcatheter Aortic Valve Replacement. J. Invasive Cardiol. 2019;31:E15–E22. doi: 10.25270/jic/18.00142. [DOI] [PubMed] [Google Scholar]
- 47.Testa L., Latib A., De Marco F., De Carlo M., Agnifili M., Latino R.A., Petronio A.S., Ettori F., Poli A., De Servi S., et al. Clinical Impact of persistent left bundle-branch block after transcatheter aortic valve implantation with CoreValve Revalving System. Circulation. 2013;127:1300–1307. doi: 10.1161/CIRCULATIONAHA.112.001099. [DOI] [PubMed] [Google Scholar]
- 48.Schymik G., Tzamalis P., Bramlage P., Heimeshoff M., Würth A., Wondraschek R., Gonska B.-D., Posival H., Schmitt C., Schröfel H., et al. Clinical impact of a new left bundle branch block following TAVI implantation: 1-year results of the TAVIK cohort. Clin. Res. Cardiol. 2014;104:351–362. doi: 10.1007/s00392-014-0791-2. [DOI] [PubMed] [Google Scholar]
- 49.Urena M., Webb J.G., Cheema A., Serra V., Toggweiler S., Barbanti M., Cheung A., Ye J., Dumont E., DeLarochellière R., et al. Impact of New-Onset Persistent Left Bundle Branch Block on Late Clinical Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation with a Balloon-Expandable Valve. JACC Cardiovasc. Interv. 2014;7:128–136. doi: 10.1016/j.jcin.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 50.Nazif T.M., Williams M.R., Hahn R.T., Kapadia S., Babaliaros V., Rodés-Cabau J., Szeto W.Y., Jilaihawi H., Fearon W.F., Dvir D., et al. Clinical implications of new-onset left bundle branch block after transcatheter aortic valve replacement: Analysis of the PARTNER experience. Eur. Heart J. 2014;35:1599–1607. doi: 10.1093/eurheartj/eht376. [DOI] [PubMed] [Google Scholar]
- 51.Fischer Q., Himbert D., Webb J.G., Eltchaninoff H., Munoz-Garcia A.J., Tamburino C., Nombela-Franco L., Nietlispach F., Moris C., Ruel M., et al. Impact of Preexisting Left Bundle Branch Block in Transcatheter Aortic Valve Replacement Recipients. Circ. Cardiovasc. Interv. 2018;11:e006927. doi: 10.1161/CIRCINTERVENTIONS.118.006927. [DOI] [PubMed] [Google Scholar]
- 52.Chamandi C., Barbanti M., Munoz-Garcia A., Latib A., Nombela-Franco L., Guttiérrez-Ibanez E., Veiga-Fernandez G., Cheema A.N., Cruz-Gonzales I., Serra V., et al. Long-term Outcomes in Patients with New-Onset Persistent Left Bundle Branch Block Following TAVR. JACC Cardiovasc. Interv. 2019;12:1175–1184. doi: 10.1016/j.jcin.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 53.Nazif T.M., Chen S., George I., Dizon J.M., Hahn R.T., Crowley A., Alu M.C., Babaliaros V., Thourani V.H., Herrmann H.C., et al. New-onset left bundle branch block after transcatheter aortic valve replacement is associated with adverse long-term clinical outcomes in intermediate-risk patients: An analysis from the PARTNER II trial. Eur. Heart J. 2019;40:2218–2227. doi: 10.1093/eurheartj/ehz227. [DOI] [PubMed] [Google Scholar]
- 54.Hamandi M., Tabachnick D., Lanfear A.T., Baxter R., Shin K., Zingler B., Mack M.J., DiMaio J.M., Kindsvater S. Effect of new and persistent left bundle branch block after transcatheter aortic valve replacement on long-term need for pacemaker implantation. Bayl. Univ. Med. Cent. Proc. 2020;33:157–162. doi: 10.1080/08998280.2020.1717906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urena M., Webb J.G., Eltchaninoff H., Munoz-Garcia A.J., Bouleti C., Tamburino C., Nombela-Franco L., Nietlispach F., Moris C., Ruel M., et al. Late cardiac death in patients undergoing transcatheter aortic valve replacement: Incidence and predictors of advanced heart failure and sudden cardiac death. J. Am. Coll. Cardiol. 2015;65:437–448. doi: 10.1016/j.jacc.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 56.Erkapic D., Kim W.-K., Weber M., Möllmann H., Berkowitsch A., Zaltsberg S., Pajitnev D.J., Rixe J., Neumann T., Kuniss M., et al. Electrocardiographic and further predictors for permanent pacemaker requirement after transcatheter aortic valve implantation. Europace. 2010;12:1188–1190. doi: 10.1093/europace/euq094. [DOI] [PubMed] [Google Scholar]
- 57.Van der Boon R.M., Nuis R.J., Van Mieghem N.M., Jordaens L., Rodès-Cabau J., Van Domburg R.T., Seruys P.W., Anderson R.H., De Jaegere P.P.T. New conduction abnormalities after TAVI-frequency and causes. Nat. Rev. Cardiol. 2012;9:454–463. doi: 10.1038/nrcardio.2012.58. [DOI] [PubMed] [Google Scholar]
- 58.Sasaki K., Izumo M., Kuwata S., Ishibashi Y., Kamijima R., Watanabe M., Kaihara T., Okuyama K., Koga M., Nishikawa H., et al. Clinical Impact of New-Onset Left Bundle-Branch Block After Transcatheter Aortic Valve Implantation in the Japanese Population. Circ. J. 2020;84:1012–1019. doi: 10.1253/circj.CJ-19-1071. [DOI] [PubMed] [Google Scholar]
- 59.Keßler M., Gonska B., Seeger J., Rottbauer W., Wöhrle J. Long-term clinical outcome of persistent left bundle branch block after transfemoral aortic valve implantation. Catheter. Cardiovasc. Interv. 2018;93:538–544. doi: 10.1002/ccd.27850. [DOI] [PubMed] [Google Scholar]
- 60.Muntané-Carol G., Guimaraes L., Ferreira-Neto A.N., Wintzer-Wehekind J., Junquera L., Del Val D., Faroux L., Philippon F., Rodés-Cabau J. How does new-onset left bundle branch block affect the outcomes of transcatheter aortic valve repair? Expert Rev. Med. Devices. 2019;16:589–602. doi: 10.1080/17434440.2019.1624161. [DOI] [PubMed] [Google Scholar]
- 61.Franzoni I., Latib A., Maisano F., Costopoulos C., Testa L., Figini F., Giannini F., Basavarajaiah S., Mussardo M., Slavich M., et al. Comparison of Incidence and Predictors of Left Bundle Branch Block After Transcatheter Aortic Valve Implantation Using the CoreValve Versus the Edwards Valve. Am. J. Cardiol. 2013;112:554–559. doi: 10.1016/j.amjcard.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 62.Urena M., Mok M., Serra V., Dumont E., Nombela-Franco L., DeLarochellière R., Doyle D., Igual A., Larose E., Amat-Santos I., et al. Predictive factors and long-term clinical consequences of persistent left bundle branch block following transcatheter aortic valve implantation with a balloon expandable valve. J. Am. Coll. Cardiol. 2012;60:1743–1752. doi: 10.1016/j.jacc.2012.07.035. [DOI] [PubMed] [Google Scholar]
- 63.Vernooy K., Verbeek X.A., Peschar M., Crijns H.J.G.M., Arts T., Cornelussen R.N.M., Prinzen F.W. Left bundle branch block induces ventricular remodeling and functional septal hypoperfusion. Eur. Heart J. 2005;26:91–98. doi: 10.1093/eurheartj/ehi008. [DOI] [PubMed] [Google Scholar]
- 64.Shoar S., Batra S., Gulraiz I.W., Javed M., Hosseini F., Naderan M., Shoar N., John J., Modukuru V.R., Sharma S.K. Effect of pre-existing left bundle branch block on post-procedural outcomes of transcatheter aortic valve replacement: A meta-analysis of comparative studies. Am. J. Cardiovasc. Dis. 2020;10:294–300. [PMC free article] [PubMed] [Google Scholar]
- 65.Egger F., Nürnberg M., Rohla M., Weiss T.W., Unger G., Smetana P., Geppert A., Gruber S.C., Bambazek A., Falkensammer J., et al. High-degree atrioventricular block in patients with preexisting bundle branch block or bundle branch block occurring during transcatheter aortic valve implantation. Heart Rhythm. 2014;11:2176–2182. doi: 10.1016/j.hrthm.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 66.Waksman R., Khan J.M. Left Bundle Branch Block After TAVR. Buddle or Trouble? JACC Cardiovasc. Interv. 2019;12:1185–1187. doi: 10.1016/j.jcin.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 67.Husser O., Pellegrini C., Kim W.-K., Holzamer A., Pilgrim T., Toggweiler S., Schäfer U., Blumenstein J., Deuschl F., Rheude T., et al. Transcatheter Valve SELECTion in Patients with Right Bundle Branch Block and Impact on Pacemaker Implantation. J. Am. Coll. Cardiol. Interv. 2019;12:1781–1793. doi: 10.1016/j.jcin.2019.05.055. [DOI] [PubMed] [Google Scholar]
- 68.Kumpuris A.G., Casale T.B., Mokotoff D.M., Miller R.R., Luchi R.J. Right bundle-branch block. Occurrence following nonpenetrating chest trauma without evidence of cardiac contusion. JAMA. 1979;242:172–173. doi: 10.1001/jama.1979.03300020042025. [DOI] [PubMed] [Google Scholar]
- 69.Kusumoto F.M., Schoenfeld M.H., Barrett C., Edgerton J.R., Ellenbogen K.A., Gold M.R., Goldschlager N.F., Hamilton R.M., Joglar J.A., Kim R.J., et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients with Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140:e382–e482. doi: 10.1161/CIR.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 70.Brignole M., Auricchio A., Baron-Esquivias G., Bordachar P., Boriani G., Breithardt O.-A., Cleland J., Deharo J.-C., Delgado V., Elliott P.M., et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2013;34:2281–2329. doi: 10.15829/1560-4071-2014-4-5-63. [DOI] [PubMed] [Google Scholar]
- 71.Knecht S., Schaer B., Reichlin T., Spies F., Madaffari A., Vischer A., Fahrni G., Jeger R., Kaiser C., Osswald S., et al. Electrophysiology Testing to Stratify Patients with Left Bundle Branch Block After Transcatheter Aortic Valve Implantation. J. Am. Heart Assoc. 2020;9:e014446. doi: 10.1161/JAHA.119.014446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sandhu A., Tzou W., Ream K., Valle J., Tompkins C., Nguyen D.T., Sauer J.D., Messenger J., Aleong R.G. Heart Block After Discharge in Patients Undergoing TAVR with Latest-Generation Valves. J. Am. Coll. Cardiol. 2018;71:577–578. doi: 10.1016/j.jacc.2017.11.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.