Abstract
Four new chromones, phomochromenones D–G (1–4), along with four known analogues, diaporchromone A (5), diaporchromanone C (6), diaporchromanone D (7), and phomochromenone C (8), were isolated from the culture of Phomopsis asparagi DHS-48 from Chinese mangrove Rhizophora mangle. Their structures were elucidated on the basis of comprehensive spectroscopic analysis. The absolute configurations of 1 and 4 were assigned on the basis of experimental and calculated electronic circular dichroism (ECD) data, and those of enantiomers 2 and 3 were determined by a modified Mosher’s method and basic hydrolysis. To the best of our knowledge, phomochromenones D–F (1–4) possessing a 3-substituted-chroman-4-one skeleton are rarely found in natural sources. Diaporchromone A (5) showed moderate to weak immunosuppressive activity against T and/or B lymphocyte cells with IC50 of 34 μM and 117 μM.
Keywords: mangrove endophytic fungi, Phomopsis sp., chromenones, immunosuppressive activity
1. Introduction
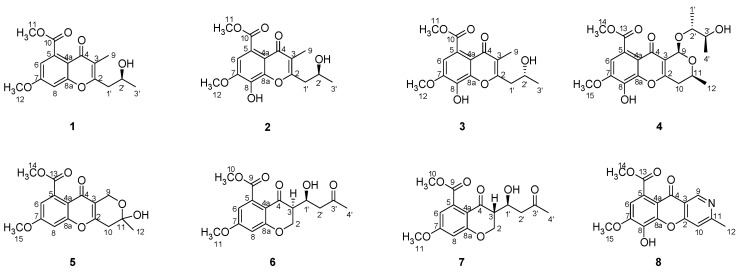
Marine-derived fungi are morphologically and physiologically adapted to harsh environmental stresses, such as high salinity, high temperature, extreme tides, oxygen pressure, high humidity, and light and air limitations, which have increasingly attracted the attention of both pharmaceutical and natural product chemists in recent decades [1,2]. Fungi colonized in mangrove forests, which comprise the second largest ecological group of marine fungi, have especially adapted their metabolic mechanisms to the unique properties of the marine environment via the generation of a large variety of structurally unprecedented and biologically interesting metabolites of pharmaceutical importance [3,4,5]. One fungal genus which is especially productive with regard to the accumulation of a diverse array of mostly bioactive compounds is Phomopsis. Chemical investigation of this fungal genus has resulted in the discovery of over 70 potentially bioactive secondary metabolites, such as subintestinal vessel plexus (SIV) accelerator phomopsis-H76 A [6], cytotoxic phomopchalasins B and C [7], mycoepoxydiene [8], dicerandrols [9], antibiotic phomoxanthone A [10], phomodiol [11], phompsichalasin [12], antimicrotubule phomosidin [13], and anti-inflammatory phomol [14]. As part of our ongoing investigation on bioactive metabolites from mangrove endophytic fungi [15,16,17,18,19], Phomopsis asparagi DHS-48 was isolated from a fresh root of the mangrove plant Rhizophora mangle. Four new chromones (1–4), and five known compounds, including diaporchromone A (5) [20], diaporchromanone C (6) [20], diaporchromanone D (7) [20], and phomochromenone C (8) [21] (Figure 1) were isolated from the EtOAc extract of P. asparagi after fermentation on a solid rice medium containing sea salt. Herein, we report the isolation, structural elucidation, and exploration on the biological activities of compounds 1–8.
Figure 1.
The chemical structures of compounds 1–8.
2. Results and Discussion
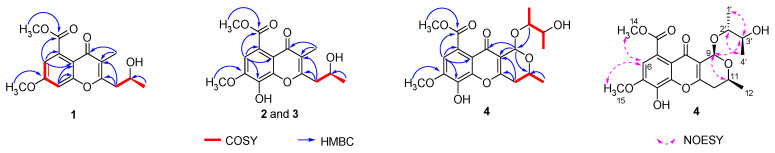
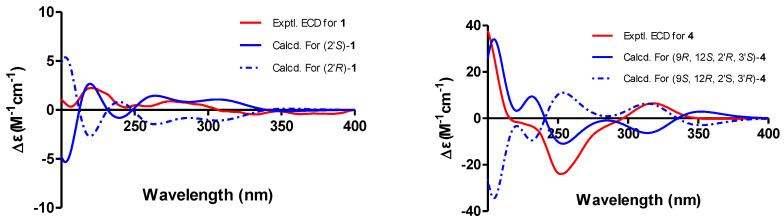
Phomochromenone D (1), a white amorphous powder, has the molecular formula C16H19O6, established by HR-ESIMS (m/z 307.1139, calcd. for [M+H]+ 307.1182), implying eight degrees of unsaturation. The UV absorption maxima at 219, 245, 295 nm indicated that 1 could be a chromone derivative. The 1D NMR data of 1 (Table 1) indicated that six of the eight units of unsaturation came from four carbon–carbon double bonds and two carbonyls. Therefore, the other two units of unsaturation come from two rings. The 1H NMR spectrum of 1 showed the presence of two meta-coupled aromatic protons at δH 6.92 (d, J = 2.4 Hz, H-6) and δH 7.10 (d, J = 2.4 Hz, H-8), one methine at δH 4.25 (m, H-2′), one methylene at δH 2.92 (dd, J = 14.0, 8.0 Hz, Ha-1′) and 2.60 (dd, J = 14.0, 5.1 Hz, Hb-1′), and two methyl at δH 2.02 (s, H3-9) and δH 1.29 (d, J = 6.3 Hz, H3-3′), and two methoxy at δH 3.917 (s, H3-11) and δH 3.924 (s, H3-12),. The 13C NMR and DEPT spectra showed 16 carbon resonances corresponding to two sp2 methine (δC 114.52 and 102.3), ten sp2 quaternary (δC 165.1, 164.8, 159.5, 135.9, 119.1, 114.49, one carbonyl at δC 171.4, one conjugated carbonyl at δC 178.4), one oxygenated methine (δC 67.1), two methoxy (δC 57.0, 53.5), one methylene (δC 42.6), and two methyl (δC 23.7, 10.3) carbons. The HMBC correlation (Figure 2) from H-6 to C-5, C-7, and C-10, and from H-8 to C-7, C-4a and C-8a indicated the presence of the chromone moiety. Moreover, HMBC correlations from H3-9 to C-2, C-3 and C-4; and from H2-1′ to C-2 and C-3, suggested that a 2-hydroxypropyl group was attached to C-2 of the chromone core. The absolute configuration of C-2′ of 1 was determined by the comparison of its experimental and time-dependent density functional theory (TDDFT)-calculated electronic circular dichroism spectrum. The experimental ECD spectrum (CH3OH) for 2′S-1 matched well with the calculated spectrum (Figure 3), which confirmed the unambiguous assignment of the absolute configuration of 1 as S, and the trivial name, phomochromenone D, was assigned.
Table 1.
1H (400 MHz) and 13C (125 MHz) NMR spectroscopic data for 1–4 in CD3OD.
| Position | 1 | 2/3 | 4 | |||
|---|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 2 | 164.8, C | 164.8, C | 166.7, C | |||
| 3 | 119.1, C | 118.2, C | 116.8, C | |||
| 4 | 178.4, C | 178.9, C | 176.8, C | |||
| 4a | 135.9, C | 115.4, C | 116.5, C | |||
| 5 | 114.5, C | 123.2, C | 123.0, C | |||
| 6 | 114.6, CH | 6.92, d, 2.4 | 110.0, CH | 7.07, s | 110.3, CH | 7.09, s |
| 7 | 165.1, C | 151.4, C | 152.6, C | |||
| 8 | 102.3, CH | 7.10, d, 2.4 | 138.2, C | 140.3, C | ||
| 8a | 159.5, C | 147.1, C | 147.2, C | |||
| 9 | 10.3, CH3 | 2.02, s | 10.2, CH3 | 2.02, s | 96.9, CH | 5.73, s |
| 10 | 171.4, C | 172.4, C | 35.2, CH2 | Ha 2.77, dd, 17.9, 3.7 Hb 2.68, dd, 17.9, 10.7 |
||
| 11 | 53.5, CH3 | 3.92, s | 53.5, CH3 | 3.89, s | 63.9, CH | 4.40, m |
| 12 | 57.0, CH3 | 3.92, s | 57.2, CH3 | 3.96, s | 20.8, CH3 | 1.37, d, 6.2 |
| 13 | 172.1, C | |||||
| 14 | 53.3, CH3 | 3.87, s | ||||
| 15 | 57.2, CH3 | 3.96, s | ||||
| 1′ | 42.6, CH2 | Ha 2.92, dd, 14.0, 8.0 Hb 2.83, dd, 14.0, 5.1 |
42.4, CH2 | Ha 2.95, dd, 14.1, 7.9 Hb 2.83, dd, 14.1, 5.1 |
18.8, CH3 | 1.19, d, 6.0 |
| 2′ | 67.1, CH | 4.25, m | 67.1, CH | 4.29, m | 83.4, CH | 3.60, m |
| 3′ | 23.7, CH3 | 1.29, d, 6.3 | 23.6, CH3 | 1.29, d, 6.3 | 73.4, CH | 3.54, m |
| 4′ | 18.3, CH3 | 1.13, d, 6.0 | ||||
Figure 2.
Selected 2D NMR of compounds 1–4.
Figure 3.
Experimental and calculated ECD spectra of 1 and 4.
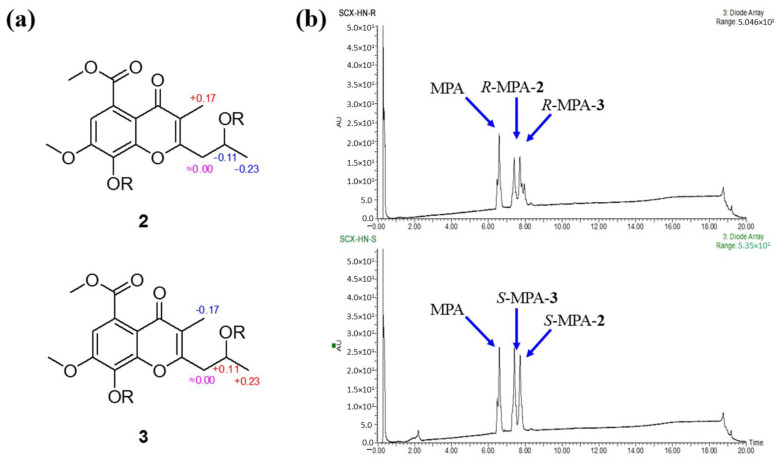
Phomochromenones E (2) and F (3) were isolated as a mixture of two enantiomers and shared the same NMR data, 1H-1H COSY and HMBC spectra. Based on the HR-ESIMS ion detected at m/z 321.0989 (calcd. for [M−H]−, 321.0974), the mixture (2/3) had the same molecular formula of C16H18O7 (i.e., differing from that of 1 by an additional hydroxyl group). The 1H and 13C NMR spectrum clearly indicated that this hydroxyl group was attached at C-6. Supporting evidence for this assignment was obtained from the downfield chemical shifts of C-8 (δC 138.2, s) and the absence of the proton signal of H-6 in 2/3 (δH 7.10, d, J = 2.4 Hz for H-8 and δC 102.3, d for C-8 in 1, respectively). Moreover, the presence of a hydroxyl group at C-8 was corroborated by the observed HMBC correlation from H-6 (δH 7.10, d, J = 2.4 Hz) to C-8, C-7 (δC 151.4), C-5 (δC 123.2), C-4a (δC 115.4), and C-10 (δC 172.4). However, the antipode rotation and ECD were detected, which suggested the mixture was not an optically pure compound. Since the chiral phase HPLC (CHIRALPAK IC) did not afford the separation of these two enantiomers, a modified Mosher’s experiment was performed to obtain its MPA esters. The products of Mosher’s reactions were subsequently analyzed by UPLC-ESI-MS through a RP-C18 chromatography column to afford two pairs of diastereomeric esters (R-MPA-2/R-MPA-3 and S-MPA-2/S-MPA-3) (Figure 4). The spectral non-equivalences in 1H NMR chemical shifts between (R)- and (S)-MPA esters (Δδ = δR − δS) indicated a 2′-S configuration for (+)-2 (positive for H-9 and negative for H-3′) and a 2′-R configuration for (−)-3 (negative for H-9 and positive for H-3′) (Figure 4). The (R)- and (S)-MPA esters of 2/3 was further separated by semi-prep. RP-C18 HPLC (40% CH3CN/H2O, 0.2% HCOOH) to afford R-MTPA-2/S-MTPA-3 (tR 18.8 min) and R-MPA-3/S-MPA-2 (tR 19.2 min). Compounds 2 and 3 were successfully obtained by chromatography after alkaline hydrolysis of R-MPA-2/S-MPA-2 and R-MPA-3/S-MPA-3, respectively. Therefore, enantiomers 2 and 3 were successfully isolated and assigned the names phomochromenone E and phomochromenone F, respectively.
Figure 4.
(a) Δδ (=δR − δS) values for (R)- and (S)- MPA esters of 2 and 3. (b) UPLC analysis profile of (R)- and (S)- MPA esters of 2 and 3 over a 20 min gradient as follows: T = 0.0, 5% B; T = 15.0, 95% B; T = 17.0, 100% B, T = 18.0, 100% B, and T = 18.1, 5% B, and T = 20.0, 5% B (A, MQ+0.2% HCOOH; B, MeOH+0.2% HCOOH).
Phomochromenone G (4) was obtained as a white amorphous powder and had a molecular formula of C20H24O9, as determined by its HR-ESIMS (m/z 431.1314, calcd. for [M+Na]+ 431.1318), indicating nine degrees of unsaturation. The 1H NMR spectrum showed resonances for one singlet aromatic proton at δH 7.09 (s, H-6); four methine protons at δH 5.73(s, H-9), δH 4.40 (m, H-11), δH 3.60 (m, H-1′), and δH 3.54 (m, H-2′); one methylene at δH 2.77 (dd, J = 17.9, 3.7 Hz, Ha-10) and 2.68 (dd, J = 17.9, 10.7 Hz, Hb-10); three methyl at δH 1.37 (d, J = 6.2 Hz, H3-12), δH 1.13 (d, J = 6.0 Hz, H3-3′), and δH 1.19 (d, J = 6.0 Hz, H3-4′); two methoxy groups at δH 3.96(s, H3-15) and δH 3.87(s, H3-14). The 13C NMR and DEPT spectra showed 20 carbon signals, including a keto group, an ester carbonyl group, eight olefinic carbon signals (including four oxygenated carbons), three oxy-methines, one methylene, two methoxy group, and three methyl group. Comparison of the NMR data of 4 with those of phomochromenone B [21], previously isolated from endophytic fungus Phomopsis sp. HNY29-2B derived from mangrove plant Acanthus ilicifolius Linn, revealed that both compounds differed with regard to the nature of the side chain at C-1, where the hydroxyl group of the latter was replaced by the 3-hydroxybutan-2-yloxyl group of 3. This was confirmed through 1H-1H COSY correlations of H3-4′/ H-1′/ H-2′/ H3-3′ and HMBC correlations (Figure 2) from H-9 to C-11(δC 63.9), C-4(δC 176.8), C-2(δC 166.7), and C-1′(δC 67.1). The relative configuration of 4 was based on the NOESY correlations as indicated in Figure 2. The NOESY correlations of H-9 to H-11, H3-1′ and H-3′; H-11 to H3-1′; and H3-4′ to H-2′ indicated that H-9, H-11, H3-1′, and H3-4′ were on the opposite side of the H3-12, H-2′, and H3-4′. The absolute configuration of 4 were also determined by comparing experimental and calculated electronic circular dichroism (ECD) spectra for the truncated model (9R, 12S, 2′R, 3′S)-4 and the truncated model (9S, 12R, 2′S, 3′R)-4 using time-dependent density functional theory (TDDFT). The theoretical spectrum of 4 showed an excellent fit with the experimental plot recorded in MeOH (Figure 3), which supported the absolute configuration to be 9R, 12S, 2′R, 3′S. Thus, the structure of 4 was determined and named phomochromenone F.
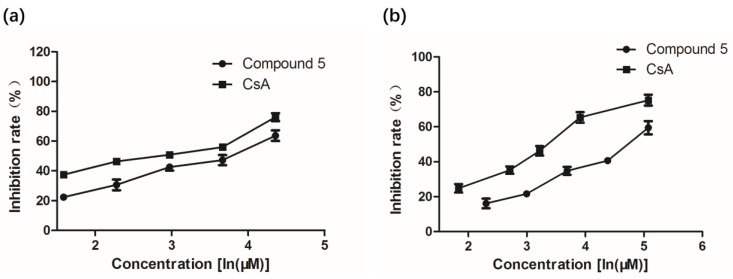
Our primary application of immunosuppressive activity screening indicated that a crude extract of P. asparagi DHS-48 showed strong inhibitory of splenic lymphocyte growth with IC50 of 6 μg/mL. An immunosuppressive assay showed that compound 5 exhibited moderate to weak inhibitory activity against ConA-induced T and LPS-induced B murine splenic lymphocytes in vitro with IC50 values of 34 and 117 μM, respectively (Table 2, Figure 5), whereas the other investigated compounds showed no obvious inhibitory effect. The cytotoxicity of 5 was tested in splenocyte cultures for 72 h using the tetrazolium salt-based CCK-8 assay. The results showed that it inhibited splenic lymphocyte growth with relatively lower toxicity (IC50 47μM), at which the survival of normal splenic cells was slightly influenced in comparison with that of CsA (IC50 11 μM). The results showed that compound 5 with a 1,3,4,10-tetrahydropyrano[4,3-b]chromene nucleus displayed significant immunosuppressive activity compared to compounds (1–3, 6, 7) with a chromone nucleus and compound 8 with a 10H-chromeno[3,2-c]pyridine nucleus. The additional 11-OH group in 5 is essential for its stimulated splenic lymphocyte inhibitory compared to compound 4.
Table 2.
Immunosuppressive activities of isolated compounds 1–8.
| Compound | Cytotoxicity a IC50 (μM) b |
ConA-Induced T-Cell Proliferation | LPS-Induced B-Cell Proliferation |
|---|---|---|---|
| IC50 (μM) b | IC50 (μM) b | ||
| 5 | 47 | 34 | 117 |
| 1–4, 6–8 | - | - | - |
| CsA c | 11 | 4 | 25 |
a Cell viability on murine splenocytes was tested by using CCK-8 method. b Data are presented as mean ± SD (n = 3) in μM. c Positive control.
Figure 5.
Effect of 5 on mouse splenocytes viability and proliferation. (a) Effect of 5 on the viability of T lymphocyte cells. (b) Effect of 5 on the viability of B lymphocyte cells. All values are expressed as mean ± SD. n = 3.
3. Materials and Methods
3.1. General Procedures
Specific rotations were obtained on a WYA-2S digital Abbe refractometer (Shanghai Physico-optical Instrument Factory, Shanghai, China). UV spectra were determined using a Shimadzu UV-2401 PC spectrophotometer (Shimadzu Corporation, Tokyo, Japan), while CD spectra were measured on a JASCO J-715 spectra polarimeter (Japan Spectroscopic, Tokyo, Japan). 1H, 13C and 2D NMR spectra were recorded on a Bruker AV 400 NMR spectrometer using TMS as an internal standard. High-resolution ESI-MS were performed on an LTQ Orbitrap XL instrument (Thermo Fisher Scientific, Bremen, Germany) using peak matching. TLC and column chromatography (CC) were carried out over silica gel (200–400 mesh, Qingdao Marine Chemical Inc., Qingdao, China), or a Sephadex-LH-20 (18−110 µm, Merck, Darmstadt, Germany), respectively. UPLC analysis (Waters Corporation, Milford, MA, USA) was recorded using a Waters system equipped in ESI mode on an Acquity UPLC H-Class connected to an SQ Detector 2 mass spectrometer using a BEH RP C18 column (2.1 × 50 mm, 1.7 µm, 0.5 mL/min). Semi-preparative HPLC was performed using a Waters equipped with a 2998 PDA detector (Waters Corporation, Milford, MA, USA) and a RP C18 column (YMC-Pack ODS-A, 10 × 250 mm, 5 μm, 3 mL/min).
3.2. Fungal Material
Endophytic fungus Phomopsis asparagi was isolated with PDA medium from the fresh root of the mangrove plant Rhizophora mangle, collected in October 2015 in Dong Zhai Gang-Mangrove Garden on Hainan Island, China. The fungus (strain no.DHS-8) was identified using a molecular biological protocol by DNA amplification and sequencing of the ITS region (GenBank Accession no.MT126606) [22]. A voucher strain was deposited at one of the authors’ laboratories (J.X.).
3.3. Extraction Isolation
The fungus was fermented onto auto autoclaved rice solid-substrate medium (thirty 1000 mL Erlenmeyer flasks, each containing 100 g of rice and 100 mL of 0.3% of saline water) and incubated for 28 days at 28 °C. In total, 140 flasks of culture were extracted three times with EtOAc and the filtrate was evaporated under reduced pressure to yield crude extract (65 g). The crude extract was partitioned with petroleum ether (PE), dichloromethane, ethyl acetate (EA), and n-butyl alcohol (BA). The dichloromethane fraction and ethyl acetate fraction were combined (30 g), then chromatographed on silica gel column chromatography using gradient elution with a CH2Cl2-MeOH mixture of increasing polarity (100:0–0:100, v/v) to afford 8 fractions (Fr. 1–Fr. 8). Fr. 2 was subjected to open silica gel CC using gradient elution with CH2Cl2-EtOAc (4:1–1:1, v/v) to yield fractions Fr. 2.1–2.6. Fr. 2.4 and Fr. 2.5 were purified by semi-preparative reversed-phase HPLC using MeOH-H2O (60:40, v/v) to afford 8 (5.0 mg) and 5 (4.8 mg), respectively. Fr. 3.4 was separated by silica gel CC using CH2Cl2-EtOAc (2:1, v/v) and was subsequently subjected to Sephadex LH-20 CC using MeOH as an eluent to give Fr. 3.4.5, followed by gradient elution MeOH-H2O (70:30–0:100, v/v) with semi-preparative reversed-phase HPLC to obtain 1 (1.2 mg) and a mixture of diaporchromanone C (6) and D (7) (5.1 mg). Purification of Fr. 4 was isolated using CC over silica gel CC using CH2Cl2-EtOAc (1:1, v/v) to afford Fr. 4.1–Fr. 4.6. Fr. 4.5 was purified using RP-18 with a MeOH−H2O (70:30, v/v) and then separated by semi-preparative reversed-phase HPLC with MeOH-H2O (50:50, v/v) to yield 2/3 (5.1 mg). Fr. 6.2, collected from Fr. 6, was subjected to silica gel CC with gradient elution of CH2Cl2-EtOAc (100:6–100:8, v/v) to give Fr. 6.2.1–Fr. 6.2.3. Fr. 6.2.2 was further separated by semi-preparative HPLC (MeOH-H2O, 55:45, v/v) to obtain 4 (3.0 mg).
3.4. Compound Characterization Data
Phomochromenone D (1): white amorphous powder (MeOH); [α]20D − 13 (c 0.001, MeOH); UV (MeOH) λmax 219, 245, 295 nm; 1H and 13C NMR data, see Table 1 and Table 2, respectively; HR-ESI-MS m/z 307.1179 [M+H]+ (calcd. for C16H19O6, 307.1182).
Phomochromenone E (2): yellow gum (MeOH); [α]20D +17 (c 0.001, MeOH); UV (MeOH) λmax 220, 245, 294 nm; 1H NMR data, see Table 1; HR-ESI-MS m/z 321.0989 [M−H]− (calcd. for C16H17O7, 321.0974).
Phomochromenone F (3): yellow gum (MeOH); [α]20D − 19 (c 0.0008, MeOH); UV (MeOH) λmax 220, 245, 294 nm; 1H NMR data, see Table 1; HR-ESI-MS m/z 321.0989 [M−H]− (calcd. for C16H17O7, 321.0974).
Preparation of MPA Esters of 2/3 by Mosher’s Method. The mixture of compounds 2 and 3 (1.5 mg) was treated with (R)- or (S)-MPA (1.0 mg) with DCC (1.0 mg) and DMAP (0.3 mg) in anhydrous CH2Cl2 (0.5 mL). After being stirred at room temperature for 4 h at 0 °C, the solvent from the reaction mixture was removed in vacuo to furnish a residue, which was then subjected to semi-preparative RP-HPLC eluting with CH3CN-H2O (40:60, 0.2% HCOOH) to obtain R-MTPA-2/S-MTPA-3 (tR 18.8 min) and R-MPA-3/S-MPA-2 (tR 19.2 min).
(R)-MPA ester of 2: yellow gum (MeOH); 1H NMR(400 MHz, CD3OD) δH 7.63–7.41 (m, 5H), 7.25–7.18 (m, 5H), 7.22 (s, 1H), 5.26 (s, 1H), 4.98 (m, 1H), 4.68 (s, 1H), 3.94 (s, 3H), 3.92 (s, 3H), 3.55 (s, 3H), 3.20 (s, 3H), 2.77 (m, 1H), 2.62 (m, 1H), 1.92 (s, 3H), 1.09 (d, J = 6.2, 3H); HR-ESI-MS m/z 619.2164 [M + H]+ (calcd. for C34H35O11, 619.2179).
(S)-MPA ester of 2: yellow gum (MeOH); 7.72–7.40 (m, 5H), 7.16 (s, 1H), 7.05–6.82 (m, 5H), 5.30 (s, 1H), 4.64 (s, 1H), 4.28 (m, 1H), 3.96 (s, 3H), 3.85 (s, 3H), 3.55 (s, 3H), 3.27 (s, 3H), 2.88 (m, 1H), 2.61 (m, 1H), 1.75 (s, 3H), 1.32 (d, J = 6.1, 3H); HR-ESI-MS m/z 619.2172 [M+H]+ (calcd. for C34H35O11, 619.2179).
(R)-MPA ester of 3: yellow gum (MeOH); 1H NMR(400 MHz, CD3OD) δH 7.65–7.42 (m, 5H), 7.16 (s, 1H), 7.05–6.82 (m, 5H), 5.30 (s, 1H), 4.64 (s, 1H), 4.28 (m, 1H), 3.96 (s, 3H), 3.85 (s, 3H), 3.55 (s, 3H), 3.27 (s, 3H), 2.88 (m, 1H), 2.62 (m, 1H), 1.75 (s, 3H), 1.32 (d, J = 6.2, 3H); HR-ESI-MS m/z 619.2184 [M+H]+ (calcd. for C34H35O11, 619.2179).
(S)-MPA ester of 3: yellow gum (MeOH); 1H NMR(400 MHz, CD3OD) δH 7.63–7.41 (m, 5H), 7.25–7.19 (m, 5H), 7.22 (s, 1H), 5.26 (s, 1H), 4.98 (m, 1H), 4.68 (s, 1H), 3.94 (s, 3H), 3.92 (s, 3H), 3.55 (s, 3H), 3.20 (s, 3H), 2.77 (m, 1H), 2.60 (m, 1H), 1.92 (s, 3H), 1.09 (d, J = 6.2, 3H); HR-ESI-MS m/z 619.2157 [M+H]+ (calcd. for C34H35O11, 619.2179).
Preparation of phomochromenones E(2) and F(3). (R)- and (S)-MPA ester of 2 were combined (1.2 mg) and dissolved in 10% NaOH (1.0 mL) and stirred for 1 h at room temperature (rt). The reaction mixture was extracted with EtOAc repeatedly and the organic layer was evaporated in vacuo to give optically pure (+)-2 (0.7 mg). Similarly, (−)-3 (0.8 mg) was prepared from the alkaline hydrolysis of (R)- and (S)-MPA ester of 3 (totally 1.0 mg) in the same manner.
Phomochromenone G (4): white amorphous powder (MeOH); [α]20D − 15 (c 0.001, MeOH); UV (MeOH) λmax 205, 241, 303 nm; 1H and 13C NMR data, see Table 1 and Table 2, respectively; HR-ESI-MS m/z 431.1314 [M+Na]+ (calcd. for C20H24O9Na, 431.1318).
Diaporchromone A (5): yellow gum (CHCl3); [α]20D − 40 (c 0.001, MeOH); UV (MeOH) λmax 219, 294 nm; 1H NMR(400 MHz, CDCl3) δH 6.89(1H, d, J = 2.3Hz, H-6), 6.87(1H, d, J = 2.3 Hz, H-8), 4.72(2H, m, H-9), 3.98(3H, s, H-14), 3.90(3H, s, H-15), 2.86(1H, dd, J = 17.4 Hz, J = 2.8 Hz, 10-Ha), 2.76(1H, dd, J = 17.4 Hz, J = 9.8 Hz, 10-Hb), 1.63(3H, s, H-12); 13C-NMR (100 MHz, CDCl3) δC 173.7 (C-4), 169.8 (C-13), 163.2 (C-7), 158.4 (C-2), 158.0 (C-8a), 134.7 (C-5), 115.6 (C-3), 114.4 (C-4a), 113.0 (C-6), 101.7 (C-8), 95.4 (C-11), 57.5 (C-9), 56.2 (C-15), 53.2 (C-14), 37.1 (C-10), 29.4 (C-12). ESI-MS m/z 321.09 [M+H]+.
Diaporchromanone C (6): yellow gum (CHCl3); UV (MeOH) λmax 215, 239, 275, 315 nm; 1H NMR(400MHz, CDCl3) δH 6.54(1H, d, J = 2.4 Hz, H = 6), 6.46(1H, d, J = 2.4 Hz, H = 8), 4.71(1H, dd, J = 11.6 Hz, J = 6.3 Hz, 2-Ha), 4.57(1H, dd, J = 11.6 Hz, J = 3.9 Hz, 2-Hb), 4.38 (1H, ddd, J = 8.5 Hz, J = 8.5 Hz, J = 2.9 Hz, H-1′), 3.94(3H, s, H-10), 3.84(3H, s, H-11), 2.66(1H, ddd, J = 8.5 Hz, J = 6.3 Hz, J = 3.9 Hz, H-3), 2.88(1H, dd, J = 18.1 Hz, J = 2.9 Hz, 2′-Ha), 2.71(1H, dd, J = 18.1 Hz, J = 8.5 Hz, 2′-Hb), 2.18(3H, s, H-4′); 13C NMR (125MHz, CDCl3) δC 210.0 (C-3′), 190.2 (C-4), 169.7 (C-9), 165.5 (C-8a), 164.0 (C-7), 136.3 (C-5), 111.7 (C-4a), 109.8 (C-6), 102.0 (C-8), 68.2 (C-2), 64.0 (C-1′), 56.1 (C-11), 53.1 (C-10), 50.6 (C-3), 47.7 (C-2′), 30.9 (C-4′). ESI-MS m/z 323.09 [M+H]+.
Diaporchromanone D (7): yellow gum (CHCl3); UV (MeOH) λmax 215, 239, 275, 315 nm; 1H NMR(400MHz, CDCl3) δH 6.56(1H, d, J = 2.4 Hz, H = 6), 6.46(1H, d, J = 2.4 Hz, H = 8), 4.60(1H, dd, J = 11.5 Hz, J = 5.1 Hz, 2-Ha), 4.51(1H, ddd, J = 8.7 Hz, J = 5.1 Hz, J = 3.6 Hz, H-1′), 4.47 (1H, dd, J = 11.5 Hz, J = 9.6 Hz, 2-Hb), 3.93(3H, s, H-10), 3.85(3H, s, H-11), 2.96(1H, ddd, J = 9.6 Hz, J = 5.1 Hz, J = 5.1 Hz, H-3), 2.80(1H, dd, J = 17.3 Hz, J = 8.7 Hz, 2′-Ha), 2.74(1H, dd, J = 17.3 Hz, J = 3.6 Hz, 2′-Hb), 2.18(3H, s, H-4′); 13C NMR (125MHz, CDCl3) δC 208.8 (C-3′), 190.9 (C-4), 169.7 (C-9), 165.6 (C-8a), 164.1 (C-7), 136.3 (C-5), 112.3 (C-4a), 109.8 (C-6), 102.2 (C-8), 68.6 (C-2), 66.4 (C-1′), 56.1 (C-11), 53.1 (C-10), 49.6 (C-3), 46.8 (C-2′), 31.0 (C-4′). ESI-MS m/z 323.09 [M+H]+.
Phomochromenone C (8): yellow gum (MeOH); UV (MeOH) λmax 206, 236, 308 nm; 1H NMR(400 MHz, CD3OD) δH 9.34(1H, s, H-1), 7.30(1H, s, H-4), 6.98(1H, s, H-8), 4.06(3H, s, H-14), 4.02(3H, s, H-13), 2.70(3H, s, H-3); 13C-NMR (125MHz, CD3OD) δC 176.3 (C-10), 171.7(C-12), 165.4 (C-3), 162.9 (C-4a), 153.9 (C-7), 150.1 (C-1), 146.6 (C-5a), 139.1 (C-6), 124.6 (C-9), 116.7 (C-10a), 115.4 (C-9a), 112.7 (C-4), 109.6 (C-8), 57.3 (C-14), 53.4 (C-13), 24.4 (C-11). ESI-MS m/z 316.12 [M+H]+.
3.5. Computational Analyses
Conformational analysis of the enantiomers of compounds 1 and 4 established by NOESY analyses were carried out using optimization and spectrum calculation. Conformational searches were carried out by means of the MM+ method in Hyper Chem 8.0 software (Hyperchem Release 8.0, Hypercube, Inc., Gainesville, FL, USA). The lowest energy conformers within 2 kcal/mol were subjected to further DFT calculations. The geometries of the conformers were optimized at the B3LYP/6-31+G(d,p) level in the gas phase using the Gaussian 09 program (Gaussian Inc., Wallingford, CT, USA). The theoretical calculation of ECD was conducted with IEFPCM solvent model for CH3OH using TDDFT at the B3LYP/6-31+G(d,p) level for all conformers of compounds (see Supplementary Materials). Boltzmann-weighted ECD spectra was obtained using SpecDis1.70.1 software (University of Würzburg, Würzburg, Germany).
3.6. Preparation of Spleen Lymphocytes
Female BALB/c mice (6–8 weeks old, 20 ± 2 g) were purchased from the Department of Laboratory Animal Science (Hainan Medicinal University, China). The mice were sacrificed by cervical dislocation, and their spleens were collected in complete RPMI 1640 medium, which was minced with surgical scissors in a germ-free condition. The suspension was then filtered through a sterile sieve mesh to obtain the single cell suspension. After centrifugation (1000 rpm at 4 °C for 5 min), the resulting cells were treated with erythrocytes lysis buffer, followed by washing twice with cold phosphate-buffered saline (PBS). Then, the cells were adjusted to the concentration of 5 × 106 cells/mL and resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS).
3.7. Cell Viability Assay
Cell viability of compounds 1–8 were measured using the tetrazolium salt-based CCK-8 assay according to a previously described protocol with some modifications [23]. Spleen lymphocytes were seeded into 96-well plates at a density of 2 × 106 cells/mL onto 96-well plates containing 100 μL of RPMI1640 complete medium (triplicate wells). Then, the cells were treated with 100 μL of various concentrations (3 μg/mL, 6 μg/mL, 12 μg/mL, 25 μg/mL, 50 μg/mL) of isolated compounds or cyclosporine (CsA) at 37 °C with a 5% CO2 incubator for 44 h. At the end of the culture, the cell culture plate was removed and observed under an inverted microscope followed by 20 μL of CCK-8 reagent (5 mg/mL). The cells were further incubated for 4 h at 37 °C with 5% CO2. The optical density was measured at 450 nm on a microplate reader.
3.8. Immunosuppressive Assay
Compounds 1–8 were evaluated for immunosuppressive activity against the proliferation of concanavalin A (ConA)-induced T and lipopolysaccharide (LPS)-induced B murine splenic lymphocyte in vitro using a CCK-8 method according to previously reported methods [24]. Cyclosporine A was used as a positive control.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/md19060348/s1. Figures S1–S36: Copies of HR-ESI-MS, 1D- and 2D-NMR spectra of 1–4. Tables S1–S4: Gibbs free energiesa and equilibrium populationsb of low-energy conformers of 1 and 4; Cartesian coordinates for the low-energy reoptimized MMFF conformers of 1 and 4 at B3LYP/6-31G(d,p) level of theory in gas.
Author Contributions
J.X. conceived and designed the experiments; C.W. and Z.F. isolated the metabolites; C.S. elucidated structures; X.Z. performed the bioactivity assays; J.X. and C.W. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Finance Science and Technology Project of Hainan Province (ZDKJ202018), the National Natural Science Foundation of China (No. 81973229/81660584), High-level Talents Programs of Hainan Province (2019RC006), and Key Project of the Education Department of Hainan Province (Hnky2019ZD-6).
Institutional Review Board Statement
All experiments conducted on animal material were approved by the Ethics Committee of the Hainan University (HNUAUCC-2021-00082, Feb. 19th, 2021), and all methods used were compliant with the regulations of the Ethics Committee.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xu J., Yi M., Ding L., He S. A Review of Anti-Inflammatory Compounds from Marine Fungi, 2000–2018. Mar. Drugs. 2019;17:636–660. doi: 10.3390/md17110636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bugni T.S., Ireland C.M. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004;21:143–163. doi: 10.1039/b301926h. [DOI] [PubMed] [Google Scholar]
- 3.Xu J. Bioactive natural products derived from mangrove-associated microbes. RSC Adv. 2015;5:841–892. doi: 10.1039/C4RA11756E. [DOI] [Google Scholar]
- 4.Xu J. Biomolecules Produced by Mangrove-Associated Microbes. Curr. Med. Chem. 2011;18:5224–5266. doi: 10.2174/092986711798184307. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J., Xu J. Mangrove Ecosystem Ecology and Function. InTechOpen Book Chapter: Chemistry and Biodiversity of Rhizophora-Derived Endophytic Fungi. Volume 8. InTechOpen; London, UK: 2018. pp. 165–184. [Google Scholar]
- 6.Yang J.X., Fang X., Huang C., Jing L., She Z., Zhong P., Lin Y. Metabolites from the mangrove endophytic fungus Phomopsis sp. Eur. J. Org. Chem. 2010;41:3692–3695. doi: 10.1002/ejoc.201000329. [DOI] [Google Scholar]
- 7.Yan B.C., Wang W.G., Hu D.B., Sun X.K., Ling M., Li X.N., Du X., Luo S.H., Liu Y., Li Y. Phomopchalasins a and b, two cytochalasans with polycyclic-fused skeletons from the endophytic fungus phomopsis sp shj2. Org. Lett. 2016;18:1108–1111. doi: 10.1021/acs.orglett.6b00214. [DOI] [PubMed] [Google Scholar]
- 8.Prachya S., Wiyakrutta S., Sriubolmas N., Ngamrojanavanich N., Mahidol C., Ruchirawat S., Kittakoop P. Cytotoxic mycoepoxydiene derivatives from an endophytic fungus phomopsis sp. isolated from hydnocarpus anthelminthicus. Planta Med. 2007;73:1418–1420. doi: 10.1055/s-2007-990240. [DOI] [PubMed] [Google Scholar]
- 9.Wagenaar M.M., Clardy J. Dicerandrols, new antibiotic and cytotoxic dimers produced by the fungus phomopsis l ongicolla isolated from an endangered mint. J. Nat. Prod. 2001;64:1006–1009. doi: 10.1021/np010020u. [DOI] [PubMed] [Google Scholar]
- 10.Elsässer B., Krohn K., Flörke U., Root N., Aust H.J., Draeger S., Schulz B., Antus S., Kurtán T. New oblongolides isolated from the endophytic fungus phomopsis sp. from melilotus dentata from the shores of the baltic sea. Eur. J. Org. Chem. 2005:4563–4570. doi: 10.1002/ejoc.200500265. [DOI] [Google Scholar]
- 11.Horn W.S., Schwartz R.E., Simmonds M.S.J., Blaney W.M. Isolation and characterization of phomodiol, a new antifungal from phomopsis. Tetrahedron Lett. 1994;35:6037–6040. doi: 10.1016/0040-4039(94)88068-9. [DOI] [Google Scholar]
- 12.Horn W.S., Simmonds M.S.J., Schwartz R.E., Blaney W.M. Phomopsichalasin, a Novel Antimicrobial Agent from an Endophytic Phomopsis sp. Tetrahedron Lett. 1995;51:3969–3978. doi: 10.1016/0040-4020(95)00139-Y. [DOI] [Google Scholar]
- 13.Kobayashi H., Meguro S., Yoshimoto T., Namikoshi M. Absolute structure, biosynthesis, and anti-microtubule activity of phomopsidin, isolated from a marine-derived fungus Phomopsis sp. Tetrahedron. 2003;59:455–459. doi: 10.1016/S0040-4020(02)01566-1. [DOI] [Google Scholar]
- 14.Weber D., Sterner O., Anke T., Gorzalczancy S., Martino V., Acevedo C. Phomol, a new antiinflammatory metabolite from an endophyte of the medicinal plant erythrina crista-galli. J. Antibiot. 2004;57:559–563. doi: 10.7164/antibiotics.57.559. [DOI] [PubMed] [Google Scholar]
- 15.Xu Z.Y., Xiong B.X., Xu J. Chemical investigation of secondary metabolites produced by mangrove endophytic fungus phyllosticta capitalensis. Nat. Prod. Res. 2019;35:1561–1565. doi: 10.1080/14786419.2019.1656624. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J., Li G., Deng Q., Zheng D.Y., Yang X.B., Xu J. Cytotoxic constituents from the mangrove endophytic Pestalotiopsis sp. induce G(0)/G(1) cell cycle arrest and apoptosis in human cancer cells. Nat. Prod. Res. 2018;32:2968–2972. doi: 10.1080/14786419.2017.1395431. [DOI] [PubMed] [Google Scholar]
- 17.Xu Z.Y., Wu X., Li G., Feng Z., Xu J. Pestalotiopisorin B, a new isocoumarin derivative from the mangrove endophytic fungus Pestalotiopsis sp. HHL101. Nat. Prod. Res. 2020;34:1002–1007. doi: 10.1080/14786419.2018.1539980. [DOI] [PubMed] [Google Scholar]
- 18.Hemberger Y., Xu J., Wray V., Proksch P., Wu J., Bringmann G. Pestalotiopens A and B: Stereochemically Challenging Flexible Sesquiterpene-Cyclopaldic Acid Hybrids from Pestalotiopsis sp. Chem. Eur. J. 2013;19:15556–15564. doi: 10.1002/chem.201302204. [DOI] [PubMed] [Google Scholar]
- 19.Deng Q., Li G., Sun M.Y., Yang X., Xu J. A new antimicrobial sesquiterpene isolated from endophytic fungus Cytospora sp. from the Chinese mangrove plant Ceriops tagal. Nat. Prod. Res. 2020;34:1404–1408. doi: 10.1080/14786419.2018.1512993. [DOI] [PubMed] [Google Scholar]
- 20.Cui H., Ding M., Huang D., Zhang Z., Liu H., Huang H., She Z. Chroman-4-one and pyrano[4,3-b]chromenone derivatives from the mangrove endophytic fungus diaporthe phaseolorum sks019. Rsc Advances. 2017;7:20128–20134. doi: 10.1039/C7RA03032K. [DOI] [Google Scholar]
- 21.Ding B., Wang Z.Y., Xia G.P., Huang X.S., Fang C. Three new chromone derivatives produced by phomopsis sp. hny29-2b from acanthus ilicifolius linn. Chin. J. Chem. 2017:1889–1893. doi: 10.1002/cjoc.201700375. [DOI] [Google Scholar]
- 22.Zhou J., Diao X., Wang T., Chen G., Lin Q., Yang X., Xu J. Phylogenetic diversity and antioxidant activities of culturable fungal endophytes associated with the mangrove species Rhizophora stylosa and R. mucronata in the South China Sea. PLoS ONE. 2018;13:e0197359. doi: 10.1371/journal.pone.0197359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cen J.R., Shi M.S., Yang Y.F., Fu Y.X., Zhou H.L., Wang M., Su Z., Wei Q., Mccormick D.L. Isogarcinol is a new immunosuppressant. PLoS ONE. 2013;8:e66503. doi: 10.1371/journal.pone.0066503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z.Y., Zhang X.X., Ma J.K., Yang Y., Zhou J., Xu J. Secondary metabolites produced by mangrove endophytic fungus Aspergillus fumigatus HQD24 with immunosuppressive activity. Biochem. Syst. Ecol. 2020;93:104166. doi: 10.1016/j.bse.2020.104166. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or Supplementary Materials.