Abstract
Antimicrobials are empirically used in COVID-19 patients resulting in increased antimicrobial resistance. Our objective was to assess antimicrobial use among suspected COVID-19 in-patients. From March to August 2020, we collected data from in-patients of 12 tertiary-level hospitals across Bangladesh. We identified suspected COVID-19 patients; collected information on antimicrobial received within 24 h before and on hospitalization; tested nasopharyngeal swab for SARS-CoV-2 using rRT-PCR. We used descriptive statistics and a regression model for data analysis. Among 1188 suspected COVID-19 patients, 69% were male, 40% had comorbidities, and 53% required oxygen. Antibiotics were used in 92% of patients, 47% within 24 h before, and 89% on admission. Patients also received antiviral (1%) and antiparasitic drugs (3%). Third-generation cephalosporin use was the highest (708; 60%), followed by macrolide (481; 40%), and the majority (853; 78%) who took antibiotics were SARS-CoV-2 negative. On admission, 77% mild and 94% moderately ill patients received antibiotics. Antibiotic use on admission was higher among severely ill patients (AOR = 11.7; 95% CI: 4.5–30.1) and those who received antibiotics within 24 h before hospital admission (AOR = 1.6; 95% CI: 1.0–2.5). Antimicrobial use was highly prevalent among suspected COVID-19 in-patients in Bangladesh. Initiating treatment with third-generation cephalosporin among mild to moderately ill patients was common. Promoting antimicrobial stewardship with monitoring is essential to prevent blanket antibiotic use, thereby mitigating antimicrobial resistance.
Keywords: COVID-19, suspected COVID-19, SARS-CoV-2, antimicrobial use, antibiotic, Bangladesh
1. Introduction
Different antimicrobials are frequently being administered to treat the novel coronavirus disease (COVID-19) even though it is a viral illness [1,2]. Due to the ambiguity of an effective treatment strategy, an empirical approach by the healthcare personnel (HCP) is to prescribe varieties of antibiotics for treating COVID-19 patients irrespective of the laboratory parameters [3]. The decision behind starting antibiotics is mostly based on clinical presentations that are similar to bacterial pneumonia [4,5]. Concern for community-acquired pneumonia, nosocomial bacterial superinfection in longer hospitalized patients, and high mortality due to COVID-19 among risk groups such as age over 60 years and underlying medical conditions has made the HCP consider antibiotics as a treatment strategy [3,6,7,8]. Uses of antibiotics for treating COVID-19 patients were found up to 72% around the globe [5,6,7,8,9], whereas bacterial co-infection was only 1–16% among those patients who received antibiotics [9,10,11,12,13]. Broad-spectrum antibiotics were commonly used in order to cover a wide range of bacteria [4,6,7,8,9]. Moreover, antivirals [8,14,15,16], antimalarial, such as hydroxychloroquine [17], and antiparasitic drugs, e.g., ivermectin [18,19], were also used for treating COVID-19. COVID-19 treatment has led long term and expanded use of antibiotics. Empiric antibiotic treatment is silently and gradually heading to higher antimicrobial resistance (AMR) and becoming a global threat [2,20,21,22,23]. As a result, an increased number of morbidity and mortality is likely to occur during and beyond the pandemic. World Health Organization (WHO) expressed concern that the AMR situation, which is already an urgent challenge of this time, will get worse due to the COVID-19 pandemic [24].
Bangladesh, a densely populated low-middle income country in Southeast Asia, reported 529,687 confirmed COVID-19 cases with 7950 deaths as of 20 January 2021, from the first detection on 8 March 2020 [25]. Most of the public hospitals lack microbiology laboratory and healthcare workforce but remain overcrowded with patients and their attendants [26,27]. During the early COVID-19 pandemic situation, very few facilities were equipped to test for SARS-CoV-2. It caused a long queue for testing and huge pressure on laboratory facilities, resulting in delayed test reports up to 5–10 days [28]. It became a barrier for testing and healthcare-seeking for people having symptoms [28,29]. To get rid of this illness, people with suspected COVID-19 took self-medication without performing any test [30,31]. These existing situations raised the concern for the overuse of antibiotics in the COVID-19 pandemic. Guidance on clinical management and treatment of COVID-19 cases by WHO is available [32]. Adopting this guidance and modifying considering the local context, Bangladesh introduced national guidelines on clinical management of COVID-19 [33]. To promote optimal use and reduce overprescription of antibiotics, WHO also recommends AWaRE (Access, Watch, Reserve)—a new tool on antibiotic classification [34]. However, it was uncertain that how closely these guidelines were followed. Moreover, there is a scarcity of data regarding antimicrobial use among hospital admitted suspected COVID-19 patients in Bangladesh. Available studies reported antibiotic use among confirmed COVID-19 patients, suggesting a wide range of antibiotic use during the COVID-19 pandemic [35,36]. Considering the scarcity of antimicrobial data in this overstressing COVID-19 pandemic situation in Bangladesh, we assessed the proportion of antimicrobial use and factors associated with antibiotic use among suspected COVID-19 patients.
2. Results
We included 1188 suspected COVID-19 patients in the analysis. Among them, 17% (n = 205) were from COVID-19 sentinel surveillance and 83% (n = 983) from hospital-based influenza surveillance.
2.1. Demographic and Clinical Characteristics
Among 1188 suspected COVID-19 patients, the median age was 34 years (IQR: 2–56), and the majority were between 19 and 59 years (41%), male (69%), and reported being non-smokers (79%) (Table 1). Only 1% were healthcare workers, and 0.7% were pregnant.
Table 1.
The use of antibiotics by demographic characteristics among the suspected COVID-19 patients admitted at 12 selected hospitals in Bangladesh, March–August 2020.
| Characteristics | Suspected COVID-19 Patients (n = 1188) n (%) |
Antibiotic Use n (%) |
||
|---|---|---|---|---|
| Overall (n = 1090) |
COVID-19 Sentinel Surveillance (n = 150) |
Hospital-Based Influenza Surveillance (n = 940) |
||
| Age in year | ||||
| ≤5 | 322 (27.1) | 312 (28.6) | 1 (0.7) | 311 (33.1) |
| 6–18 | 96 (8.1) | 89 (8.2) | 5 (3.3) | 84 (8.9) |
| 19–59 | 486 (40.9) | 436 (40.0) | 51 (34.0) | 385 (40.9) |
| ≥60 | 284 (23.9) | 253 (23.2) | 93 (62.0) | 160 (17.0) |
| Gender | ||||
| Male | 816 (68.7) | 755 (69.3) | 102 (68.0) | 653 (69.5) |
| Female | 372 (31.3) | 335 (30.7) | 48 (32.0) | 287 (30.5) |
| Type of ward | ||||
| Medicine | 676 (56.9) | 633 (58.1) | 94 (62.7) | 539 (57.3) |
| Pediatrics | 389 (32.7) | 375 (34.4) | 5 (3.3) | 370 (39.4) |
| ICU | 2 (0.2) | 2 (0.2) | 1 (0.7) | 1 (0.1) |
| CCU | 54 (4.5) | 43 (3.9) | 13 (8.7) | 30 (3.2) |
| COVID-19 Isolation | 67 (5.6) | 37 (3.4) | 37 (24.7) | 0 |
| COVID-19 test result | ||||
| Positive | 257 (21.6) | 237 (21.7) | 44 (29.3) | 193 (20.5) |
| Negative | 931 (78.4) | 853 (78.3) | 106 (70.7) | 747 (79.5) |
| Health care worker | ||||
| Yes | 14 (1.2) | 12 (1.1) | 0 | 12 (1.3) |
| No | 1174 (98.8) | 1078 (98.9) | 150 (100.0) | 928 (98.7) |
| Smoking | ||||
| Smoker | 250 (21.0) | 220 (20.2) | 53 (35.3) | 167 (17.8) |
| Non-smoker | 938 (79.0) | 870 (79.8) | 97 (64.7) | 773 (82.2) |
Table 2 shows the clinical characteristics of the enrolled patients. One or more comorbidities were found in 40% of patients, with 208 (17%) having diabetes, 182 (15%) having hypertension, and 169 (14%) having chronic lung diseases, including asthma, emphysema and chronic obstructive pulmonary disease (COPD). Overall, 628 (53%) patients required oxygen, and 7 (1%) required ICU or ventilation support after admission.
Table 2.
The use of antibiotics by clinical characteristics among the suspected COVID-19 patients admitted at 12 selected hospitals in Bangladesh, March–August 2020.
| Characteristics | Suspected COVID-19 Patients (n = 1188) n (%) |
Antibiotic Use n (%) |
||
|---|---|---|---|---|
| Overall (n = 1090) |
COVID-19 Sentinel Surveillance (n = 150) |
Hospital-Based Influenza Surveillance (n = 940) |
||
| Signs and symptoms on admission | ||||
| Cough (dry/productive) | 1097 (92.3) | 1027 (94.2) | 87 (58.0) | 940 (100.0) |
| Fever | 1063 (89.5) | 996 (91.4) | 56 (37.3) | 940 (100.0) |
| Shortness of breath | 845 (71.1) | 797 (73.1) | 67 (44.7) | 730 (77.7) |
| Runny nose | 405 (34.1) | 380 (34.9) | 9 (6.0) | 371 (39.5) |
| Headache | 382 (32.1) | 348 (31.9) | 29 (19.3) | 319 (33.9) |
| Sore throat | 225 (18.9) | 210 (19.3) | 18 (12.0) | 192 (20.4) |
| Loss of smell or taste (n = 205) | 69 (33.7) | 49 (4.5) | 49 (32.7) | 0 |
| Fever, cough and difficulty breathing | 765 (64.4) | 739 (67.8) | 9 (6.0) | 730 (77.7) |
| Comorbidities | ||||
| None | 708 (59.6) | 658 (60.4) | 48 (32.0) | 610 (64.9) |
| One or more | 480 (40.4) | 432 (39.6) | 102 (68.0) | 330 (35.1) |
| COVID-19 disease severity (WHO category) | ||||
| Mild (symptoms only) | 436 (36.7) | 357 (32.8) | 109 (72.7) | 248 (26.4) |
| Moderate (pneumonia) | 389 (32.7) | 374 (34.3) | 33 (22.0) | 341 (36.3) |
| Severe (severe pneumonia) | 326 (27.4) | 323 (29.6) | 6 (4.0) | 317 (33.7) |
| Critical (ARDS or Sepsis or septic shock or ICU/ventilation) | 37 (3.1) | 36 (3.3) | 2 (1.3) | 34 (3.6) |
| Care seeking before admission (n = 205) | ||||
| Visited any healthcare provider or facility within two weeks of hospital admission | 160 (78.0) | 114 (76.0) | 114 (76.0) | 0 |
| Death occurred in hospital | 69 (5.8) | 66 (6.1) | 13 (8.7) | 53 (5.6) |
2.2. Proportion of Antibiotic Use (before and on Hospital Admission)
The overall proportion of antibiotic use was 92% among suspected COVID-19 patients. Among the suspected COVID-19 patients, 33 (3%) reported taking antibiotics only within 24 h before admission, 528 (44%) received antibiotics only within 24 h after admission, and 529 (45%) received antibiotics at both points—before and after admission. The proportion was higher for patients aged 19–59 years, patients with cough, and moderately ill patients (pneumonia) (Table 1 and Table 2).
2.3. Healthcare Sought within Last Two Weeks Prior to Hospital Admission for Current Illness
Among the 205 patients from COVID-19 surveillance, the overall antibiotic use was 73% (n = 150); 40 (20%) patients reported taking antibiotics within 24 h before hospital admission. Of these 40 patients, 15 (37%) had a history of admission in a healthcare facility, 15 (37%) visited registered healthcare providers, 8 (20%) visited informal healthcare providers, such as non-medically trained traditional healthcare provider or local drug-sellers, and the remaining 2 (5%) had no history of seeking any healthcare but received a self-prescribed antibiotic.
2.4. Different Class of Antibiotic Used among SARS-CoV-2 Positive and Negative Patients before and on Hospital Admission
Among all suspected COVID-19 patients, the most frequently reported antibiotic class was cephalosporin (64%), particularly third-generation cephalosporin (60%), a Watch group antibiotic (Table 3). The second most frequently reported antibiotic class was macrolide (40%), where azithromycin (Watch group) was mostly (98%) used.
Table 3.
Antimicrobials used among suspected COVID-19 patients and SARS-CoV-2 positive and negative patients 24 h before and on hospital admission at 12 selected hospitals in Bangladesh, March–August 2020.
| Antimicrobials * | Suspected COVID-19 Patients (n = 1188) n (%) |
Within 24 h before Admission | On Admission | ||||
|---|---|---|---|---|---|---|---|
| SARS-CoV-2 Positive (n = 257) n (%) |
SARS-CoV-2 Negative (n = 931) n (%) |
p-Value | SARS-CoV-2 Positive (n = 257) n (%) |
SARS-CoV-2 Negative (n = 931) n (%) |
p-Value | ||
| Antibiotic | |||||||
| Antibiotics used in total | 1090 (91.7%) | 562 (47.3%) | 1057 (89.0%) | ||||
| Cephalosporin | 761 (64.1) | 25 (9.7) | 161 (17.3) | 0.003 | 127 (49.4) | 600 (64.4) | <0.001 |
| First-generation | 4 (0.3) | 1 (0.4) | 3 (0.3) | 0.870 | 0 | 0 | |
| Second-generation | 69 (5.8) | 5 (2) | 13 (1.4) | 0.523 | 7 (2.7) | 47 (5.1) | 0.113 |
| Third-generation | 708 (59.6) | 19 (7.4) | 145 (15.6) | 0.001 | 120 (46.7) | 554 (59.5) | <0.001 |
| Fourth-generation | 1 (0.1) | 0 | 0 | 0 | 0 | 1 (0.1) | 0.599 |
| Macrolide | 481 (40.5) | 104 (40.5) | 223 (24.0) | <0.001 | 85 (33.1) | 180 (19.3) | <0.001 |
| Penicillin | 200 (16.8) | 6 (2.3) | 27 (2.9) | 0.625 | 38 (14.8) | 140 (15.0) | 0.920 |
| Aminoglycoside | 114 (9.6) | 1 (0.4) | 6 (0.6) | 0.636 | 7 (2.7) | 102 (11.0) | <0.001 |
| Quinolones | 63 (5.3) | 3 (1.2) | 8 (0.9) | 0.648 | 11 (4.3) | 41 (4.4) | 0.932 |
| Tetracycline | 76 (6.4) | 10 (3.9) | 14 (1.5) | 0.016 | 17 (6.6) | 39 (4.2) | 0.104 |
| Carbapenems | 45 (3.8) | 0 | 3 (0.3) | 0.362 | 11 (4.3) | 33 (3.5) | 0.580 |
| Oxazolidinone | 12 (1.0) | 0 | 0 | 3 (1.2) | 9 (1.0) | 0.776 | |
| Glycopeptides | 10 (0.8) | 0 | 0 | 2 (0.8) | 8 (0.9) | 0.900 | |
| Nitroimidazoles | 13 (1.1) | 0 | 1 (0.1) | 0.599 | 3 (1.2) | 9 (1.0) | 0.776 |
| WHO AWaRe classification antibiotics | |||||||
| Access | 370 (31.1) | 68 (5.7) | 329 (27.7) | ||||
| Watch | 1016 (85.5) | 514 (43.3) | 949 (79.9) | ||||
| Reserve | 12 (1.0) | 0 | 12 (1.0) | ||||
| Antiviral drug | |||||||
| Antiviral used in total | 16 (1.4) | 0 | 16 (1.4) | ||||
| Acyclovir | 3 (0.3) | 0 | 0 | - | 0 | 3 (0.3) | 0.362 |
| Adefovir | 1 (0.1) | 0 | 0 | - | 0 | 1 (0.1) | 0.599 |
| Zidovudine | 1 (0.1) | 0 | 0 | - | 0 | 1 (0.1) | 0.599 |
| Valacyclovir | 3 (0.3) | 0 | 0 | - | 1 (0.4) | 2 (0.2) | 0.622 |
| Favipiravir | 8 (0.7) | 0 | 0 | - | 1 (0.4) | 7 (0.7) | 0.529 |
| Antiparasitic drug | |||||||
| Antiparasitic drug used in total | 33 (2.8) | 3 (0.3) | 30 (2.5) | ||||
| Ivermectin | 33 (2.8) | 1 (0.4) | 2 (0.2) | 0.622 | 9 (3.5) | 21 (2.7) | 0.260 |
* Multiple response.
Before admission, the most frequently used antibiotic class was macrolide (327; 27%), followed by cephalosporin (187; 16%). However, on admission, the antibiotic choice was the opposite; cephalosporin (727; 61%) followed by macrolide (265; 22%) (Table 3). Before admission, 32 (3%) suspected patients reported using two antibiotics, and on admission, 322 (27%) received two to four classes of antibiotics at the same time. Of those who received antibiotics either before or on admission, the majority (78%) were SARS-CoV-2 negative.
According to WHO AWaRe classification, the Watch group antibiotics were mostly used before (43%) as well as on admission (80%) (Table 3).
2.5. Antibiotic Use among Patients with Different COVID-19 Severity on Admission
Antimicrobial use among patients with four types of COVID-19 disease severity—mild–critical—on admission is shown in Table 4. The third-generation cephalosporin was mostly used among all four types of patients. In contrast, the first-generation cephalosporin was not used in any patient. The second most used antibiotic was macrolide among mild, moderate and severe patients and penicillin in critical patients. Among mild and moderately ill patients, 4% and 2%, respectively, received carbapenem, particularly meropenem (Watch group). All four categories of patients, with the majority from mild, reported receiving Reserve group antibiotics, particularly oxazolidinones (i.e., linezolid), on admission.
Table 4.
Antimicrobials used on admission among suspected COVID-19 patients according to disease severity at 12 selected hospitals in Bangladesh, March–August 2020.
| Antimicrobials | Disease Severity | ||||
|---|---|---|---|---|---|
| Mild (n = 436) n (%) |
Moderate (n = 389) n (%) |
Severe (n = 326) n (%) |
Critical (n = 37) n (%) |
Total (n = 1188) n (%) |
|
| Antibiotic | 334 (76.6) | 367 (94.3) | 321 (98.5) | 35 (94.6) | 1057 (89.0) |
| Cephalosporin | 197 (45.2) | 259 (66.6) | 250 (76.7) | 21 (56.8) | 727 (61.2) |
| First-generation | 0 | 0 | 0 | 0 | 0 |
| Second-generation | 10 (2.3) | 30 (7.7) | 10 (3.1) | 4 (10.8) | 54 (4.6) |
| Third-generation | 187 (42.9) | 231 (59.4) | 239 (73.3) | 17 (46.0) | 674 (56.7) |
| Fourth-generation | 0 (0) | 0 (0) | 1 (0.3) | 0 (0) | 1 (0.1) |
| Macrolide | 88 (20.2) | 98 (25.2) | 74 (22.7) | 5 (13.5) | 265 (22.3) |
| Penicilin | 48 (11) | 59 (15.2) | 63 (19.3) | 8 (21.6) | 178 (15.0) |
| Aminoglycoside | 15 (3.4) | 37 (9.5) | 51 (15.6) | 6 (16.2) | 109 (9.2) |
| Quinolones | 27 (6.2) | 11 (2.8) | 13 (4) | 1 (2.7) | 52 (4.4) |
| Tetracycline | 18 (4.1) | 16 (4.1) | 16 (4.9) | 6 (16.2) | 56 (4.7) |
| Carbapenems | 17 (3.9) | 6 (1.5) | 18 (5.5) | 3 (8.1) | 44 (3.7) |
| Oxazolidinones | 4 (0.9) | 3 (0.8) | 2 (0.6) | 3 (8.1) | 12 (1.0) |
| Glycopeptides | 4 (0.9) | 2 (0.5) | 2 (0.6) | 2 (5.4) | 10 (0.8) |
| Nitroimidazoles | 9 (2.1) | 2 (0.5) | 0 (0) | 1 (2.7) | 12 (1.0) |
| WHO AWaRe classification antibiotics | |||||
| Access | 82 (18.8) | 106 (27.2) | 122 (37.4) | 19 (51.3) | 329 (27.7) |
| Watch | 287 (65.8) | 341 (87.7) | 293 (89.9) | 28 (75.7) | 949 (79.9) |
| Reserve | 4 (0.9) | 3 (0.8) | 2 (0.6) | 3 (8.1) | 12 (1.0) |
| Antiviral drug | |||||
| Acyclovir | 2 (0.5) | 0 | 0 | 1 (2.7) | 3 (0.2) |
| Adefovir | 0 | 1 (0.3) | 0 | 0 | 1 (0.1) |
| Zidovudine | 1 (0.2) | 0 | 0 | 0 | 1 (0.1) |
| Valacyclovir | 3 (0.7) | 0 | 0 | 0 | 3 (0.2) |
| Favipiravir | 2 (0.5) | 0 | 6 (1.8) | 0 | 8 (0.7) |
| Antiparasitic drug | |||||
| Ivermectin | 3 (0.7) | 6 (1.5) | 16 (4.9) | 5 (13.5) | 30 (2.5) |
Antibiotics used among suspected COVID-19 patients in different wards and ICU on admission are shown in Table A1.
2.6. Antiviral Use
None of the suspected COVID-19 patients took antiviral before admission; rather, it was used among 16 (1.4%) patients on admission (Table 3). Among them, 14 (87%) were SARS-CoV-2 test negative. Half of the patients who received antiviral had a mild illness, whereas only one from the critical category received antiviral (Table 4). The most frequently used antiviral was favipiravir (1%).
2.7. Antiparasitic Drug Use
Overall ivermectin use was reported from 3% of the suspected COVID-19 patients; three patients used it before admission, and 30 received it on admission (Table 3). Ivermectin use was higher in severe COVID-19 patients on admission (Table 4).
2.8. Steroid Use (Other Medication)
A non-antimicrobial drug, steroid use was also reported before and on admission. Among the suspected COVID-19, a steroid was used in 105 (9%) patients; of those, 88 (84%) were SARS-CoV-2 test negative. Of those who received steroids, more than half (54%) had a mild illness, and about one-third (36%) had asthma, COPD, or sepsis. Before admission, only hydrocortisone was reported (n = 13), and on admission, hydrocortisone (n = 56) and dexamethasone (n = 37) were the most frequently used steroids among suspected COVID-19 patients.
2.9. Potential Factors Associated with Antibiotic Use on Hospital Admission
The factors associated with antibiotic use among suspected COVID-19 patients after admission are shown in Table 5. In multivariable analysis, disease severity and antibiotic use within 24 h before hospital admission were found to be significantly associated with antibiotic use among study patients. Patients who had a severe and moderate level of illness were 11.7 and 3.6 times more likely (95% CI: 4.5–30.1, 2.2–6.0) to use antibiotics than the mild category, respectively. Similarly, a higher probability of antibiotic use was found among those who received antibiotics within 24 h before hospital admission (AOR = 1.6; 95% CI: 1.0–2.5).
Table 5.
Independently associated factors with antibiotic use on hospital admission among suspected COVID-19 patients at 12 selected hospitals in Bangladesh, March–August 2020.
| UOR (95% CI) |
p-Value | AOR (95% CI) |
p-Value | |
|---|---|---|---|---|
| Disease severity 1 | ||||
| Mild | Reference | Reference | ||
| Moderate | 5.1 (3.1–8.3) | <0.001 | 3.6 (2.2–6.0) | <0.001 |
| Severe | 19.6 (7.9–48.8) | <0.001 | 11.7 (4.5–30.1) | <0.001 |
| Critical | 5.3 (1.3–22.6) | 0.023 | 3.4 (0.8–14.9) | 0.098 |
| Antibiotic use before hospital admission 2 | ||||
| No | Reference | Reference | ||
| Yes | 3.0 (2.0–4.5) | <0.001 | 1.6 (1.0–2.5) | 0.044 |
1 adjusted for age, sex, comorbid condition, smoking, antibiotic use before hospital admission, surveillance type; 2 adjusted for disease severity, surveillance type.
3. Discussion
This study assessed the proportion of antimicrobial use among patients with COVID-19 symptoms. Our study findings suggest that antibiotic use was as high as 92% in treating patients before the COVID-19 test report was available. A study conducted in the early pandemic period at Wuhan, China reported, all suspected COVID-19 hospitalized cases were treated with empirical antibiotics [14]. A similar treatment strategy was found in a recent Bangladeshi study where all confirmed COVID-19 patients received one broad-spectrum antibiotic on admission in a tertiary care hospital [35]. Our study also found a higher number of antibiotic uses among suspected COVID-19 patients. Of whom, three-fourths were SARS-CoV-2 negative. Clinicians empirically initiated antibiotics based on the patient’s clinical presentation on admission as the COVID-19 test report was not available due to the long turnaround time to receive the COVID-19 test result.
Our study findings show that most of the mild and moderately ill suspected COVID-19 patients received antibiotics on admission. This proportion was higher compared to a study conducted among adult hospitalized patients, where 24% mild and 60% moderate patients with COVID-19 received antibiotics on admission [6]. Prescribed antibiotic use found in our study was not adherent with the national [33] as well as the WHO guidelines on COVID-19 clinical management. In these guidelines, antibiotic use as prophylaxis for mild patients is strongly discouraged. However, for moderate patients, antibiotics are recommended for clinically suspected bacterial infection. Our study patients did not have any bacterial culture report at the time of initiating antibiotics on admission. This indicates non-compliance by the clinicians while prescribing antibiotics on admission. Complete adherence to guidelines among clinicians is highly required for optimal use of antibiotics.
WHO-AWaRe classification, developed in 2019 for selection and use of antibiotics, recommends using only Access antibiotics but not Watch or Reserve antibiotics [34,37]. In our study, we found Watch antibiotics were highly used. We found no use of first-generation cephalosporin (Access antibiotics) in any patient; rather, broad-spectrum antibiotics, particularly third-generation cephalosporin (Watch antibiotics), a critically important antimicrobial, was the major choice for empirical approach. This finding is consistent with previous studies where third-generation cephalosporin use was found higher for COVID-19 patients [1,6,10]. Other broad-spectrum antibiotics such as piperacillin/tazobactam, vancomycin, carbapenems, tigecycline, and combinations with fluoroquinolones were also used for treating COVID-19 patients [4,6,7,8,9], which are similarly found in our study. Reserve antibiotics such as linezolid were also found to be used even in mild and pediatric patients in our study. However, according to our study findings antibiotic use did not follow the WHO guideline, and it suggests an urgent requirement for strategies, such as frequent refresher training on the AWaRe tool. This might help clinicians prescribe safe and effective rational antibiotics with proper judgment. This widespread use of antibiotics during this COVID-19 pandemic is an alarming threat to antimicrobial resistance. Introducing antibiotic stewardship programs (ASP) with the formation of an active ASP committee in hospitals could be considered to reduce the irrational use of antibiotics and to improve rational antibiotic prescribing practices.
Antiviral use was found much lower compared to antibiotic use among suspected COVID-19 patients in our study. Previous studies reported similar lower use of antivirals such as oseltamivir, ganciclovir, remdesivir, lopinavir, favipiravir and ritonavir [8,14,15,16]. We found favipiravir was mostly used among all antivirals on admission. This finding goes against WHO-recommendation where it was mentioned not to administer favipiravir as treatment or prophylaxis for COVID-19, outside the context of clinical trials [32]. However, our national guideline on clinical management of COVID-19 recommends favipiravir for moderately-ill COVID-19 patients [33].
Ivermectin is a potential drug against COVID-19 infection yet to be approved and recommended only for clinical trial [18,19,38]. In our study, we observed using this anti-parasitic drug for COVID-19 treatment. One possible reason for using could be the antiviral property of this drug [38]. However, clinicians should hold back its use until it is fully recommended for the treatment of COVID-19 patients.
The application of steroids in COVID-19 is debated as its role is still unproven [39,40]. WHO recommends not to use systemic corticosteroids routinely for the treatment of viral pneumonia unless indicated, such as acute exacerbation of asthma, COPD, septic shock, or ARDS [32]. Despite the restriction, 9% of patients in our study reported receiving systemic corticosteroids; and two-third of them did not have asthma, COPD, septic shock or ARDS. The reason for using steroids could be that in our national guideline, oral methylprednisolone has been recommended in moderate COVID-19 cases in particular circumstances [33]. However, we observed that mild patients also received systemic corticosteroids on admission, which is not in accordance with the local guideline. Clinicians should strictly follow the guideline while prescribing COVID-19 patients, considering the threat that steroid use can impose [40].
We also found patients who received antibiotics within 24 h before hospital admission are two times more likely to receive antibiotics on hospital admission. It was likely because the physician wanted to complete the course or changed the antibiotic on admission.
In a subset of patients, we found 25% of patients who took antibiotics before admission reported visiting informal (unregistered) healthcare providers and having self-prescribed antibiotics—a common practice in Bangladesh [41]. People in Bangladesh, regardless of socio-economic status and education, took over-the-counter medicines and even antibiotics without consulting any qualified healthcare providers [41,42]. This practice of self-medication increased up to 88% during the COVID-19 pandemic [30,31]. Taking medicine without performing a COVID-19 test for symptoms, such as fever, sore throat, or cough, was found to be common; ivermectin (77%) and azithromycin (54%) were the most frequently used self-medicated drugs in Bangladesh during the COVID-19 pandemic [30]. Anxiety due to pandemic, avoiding going out during lockdown, high self-awareness, inability to access telemedicine, a suggestion from family members, friends, and local drug sellers, a low-cost alternate of treatment, low ratio between doctor and patient, and easy availability of drugs may have led to self-medication [30,43]. Educational campaigns immediately need to be strengthened to make the community aware of the consequences of self-medicated antibiotics. Adopting prescription-only status by all the pharmacies with strict monitoring by the government may reduce self-medication for antibiotics.
Our study had certain limitations. We only documented antimicrobial use among suspected COVID-19 patients within the first 24 h of hospital admission but not after 24 h of admission. Clinicians might have introduced, discontinued or switched to alternative antibiotics on a later date even after receiving the COVID-19 test results. Moreover, we did not collect information on ultimate diagnosis, including bacterial co-infection among patients. Therefore, we missed assessing the appropriateness of empirical antibiotic treatment. We also did not collect healthcare-seeking information before hospital admission from hospital-based influenza surveillance due to logistical challenges in a routine surveillance system.
However, this study findings documented an early assessment of the antimicrobial use among suspected COVID-19 patients 24 h before and 24 h after hospital admission. This study provides a first-hand picture with comprehensive information on antimicrobial treatment in patients at the suspected COVID-19 phase before receiving the COVID-19 test result. Our surveillance hospitals are situated in 12 different districts at diverse geographic locations across Bangladesh.
4. Materials and Methods
4.1. Study Site and Study Population
We collected data from two hospital-based surveillance platforms at 12 selected hospitals at different geographical locations across Bangladesh from 1 March to 31 August 2020 (Figure 1). Our study population was patients of all age groups admitted to the study wards. Both the surveillance systems were conducted in collaboration with the Institute of Epidemiology, Disease Control and Research (IEDCR) of the Government of Bangladesh (GoB).
Figure 1.
Location of the 12 surveillance hospitals across Bangladesh.
4.2. Surveillance Activities
4.2.1. COVID-19 Sentinel Surveillance
To identify COVID-19 among patients attending hospitals with an acute respiratory infection, we conducted the surveillance at medicine and pediatric wards, COVID-19 isolation unit, and intensive care unit (ICU) in 4 hospitals—3 district level public and 1 tertiary level private hospital. We enrolled patients from 10 June to 31 August 2020, from this platform.
4.2.2. Hospital-Based Influenza Surveillance (HBIS)
To identify the respiratory viruses among hospitalized patients with severe acute respiratory infections defined as a patient with history of fever and cough within the last 10 days (adopted from WHO), this nationally representative surveillance was initiated from May 2007 and still ongoing at 9 tertiary level (7 public and 2 private) hospitals [44,45]. We enrolled patients admitted to medicine and pediatric wards, COVID-19 isolation unit, and coronary care unit (CCU) from 1 March to 31 August 2020. One hospital was common in both the surveillance platforms, and we considered patients from that hospital once.
4.3. Patient Enrolment and Data Collection
We followed the Bangladesh government-defined case definition of suspected COVID-19: any patient having one or more of the symptoms, including fever, cough, sore throat, or difficulty breathing within the last 7 days [33]. We enrolled hospitalized patients who met the inclusion criteria and provided informed written consent by the patient or caregiver. For outcome, we collected a list of antimicrobials, e.g., antibiotic, antiviral, antimalarial, antifungal, antiparasitic drugs, and other medication prescribed on admission from the patient files within 24 h of admission. Trained field staff interviewed the patient or guardian on antimicrobial use within 24 h before hospital admission for current illness episode. They also collected data on relevant factors that may be associated with antibiotic use, such as demographics and clinical information, including vital signs, complete blood count report, and chest X-ray findings (if available); comorbid conditions and provisional diagnosis on admission. From patients of the COVID-19 surveillance platform, the staff additionally collected information on whether any type of healthcare was sought in two weeks preceding current hospital admission.
4.4. Sample Collection and Laboratory Testing
Followed by data collection, we collected a nasopharyngeal swab. The samples were kept in viral transport media (VTM) and shipped to virology laboratory at icddr,b in Dhaka city, maintaining cool chain for testing SARS-CoV-2 using a one-step real-time reverse transcription polymerase chain reaction (rRT-PCR).
4.5. Disease Severity Category among Suspected COVID-19 Patients
COVID-19 disease severity was categorized as mild, moderate, severe, and critical using WHO’s COVID-19 clinical management guideline (Table A2).
4.6. Data Analysis
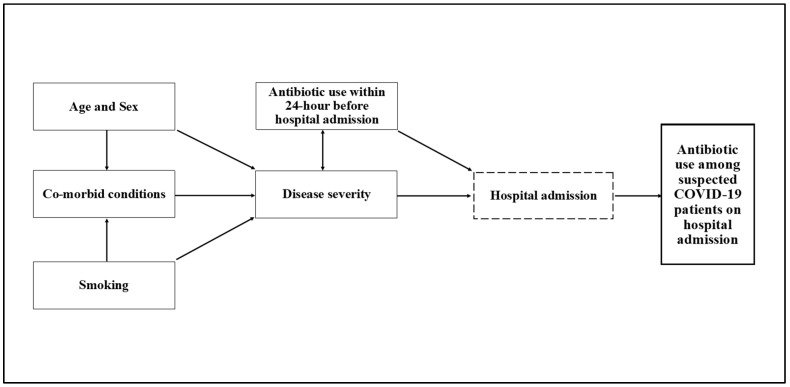
We conducted data analysis using Stata 15 software (Stata Corp. 2017. Stata Statistical Software: Release 13. College Station, TX, USA: Stata Corp LP.). We used frequency and percentage to summarize the background characteristics of the enrolled patients. We illustrated the use of antimicrobials among suspected COVID-19 patients by reporting frequency and percentage. We also performed bivariable binary logistic regression to estimate the crude relationship between exploratory variables and the outcome of interest. We reported output as the unadjusted odds ratio (OR) with 95% CI and p-value. We utilized the conceptual framework technique, which displayed the potential causal pathway and link between antibiotic use after admission and explanatory variables, including patient age, sex, comorbid conditions, smoking status, disease severity, and antibiotic use before hospital admission to delineate the causal relationship (Figure 2) [46]. Technical details of the conceptual framework technique have been explained in Jewell et al. [47]. Variables with missing values, such as healthcare-seeking behavior before admission, were excluded from the model. Multivariable logistic regression was utilized to calculate the association of each explanatory variable with the outcome of interest by adjusting confounders and reported as adjusted odds ratio (AOR) with 95% CI and p-value, where p < 0.05 was considered as the statistical significance level. All tests were two-tailed and were considered significant at an alpha value of 5%.
Figure 2.
Conceptual framework for the causal relationship between antibiotic use and explanatory variables, including patient age, sex, comorbid conditions, smoking status, disease severity, and antibiotic use within 24 h before hospital admission.
5. Conclusions
Antimicrobials, predominantly antibiotic use, was highly prevalent among hospital admitted suspected COVID-19 patients in Bangladesh. Initiating treatment with third-generation cephalosporin (Watch antibiotic) is common even in mild to moderate COVID-19 patients. These findings reflect that irrational use of antibiotics is being exercised without following the guideline. This blanket use of antibiotics in viral pandemic raises the concern for increasing long-term threat of AMR during and post COVID-19 pandemic period. It is of extreme importance to promote, standardize, and strengthen antimicrobial stewardship considering the key elements, including the adoption of WHO’s AWaRe tool with constant monitoring of adherence to the COVID-19 treatment guideline. Formative studies need to be conducted to better understand the drivers of prescribing antibiotics among clinicians.
Acknowledgments
We are grateful to our surveillance participants for providing their valuable time, sample, and information, collaborators of the surveillance hospitals, field staff for their hard work, and laboratory colleagues for the tests in the possible shortest period. COVID-19 sentinel surveillance was funded by Foreign, Commonwealth and Development Office (FCDO), grant number MD-0024 and Hospital-based Influenza surveillance was funded by Centers for Disease Control and Prevention (CDC), grant number 1 U01GH002259-01-00. icddr,b acknowledges with gratitude the commitment of FCDO and CDC to its research efforts. icddr,b is also grateful to the Governments of Bangladesh, Canada, Sweden, and the UK for providing core/unrestricted support.
Appendix A
Table A1.
Antimicrobials used for suspected COVID-19 patients on admission in different wards, CCUs, and ICUs of 12 selected hospitals in Bangladesh, March–August 2020.
| Antimicrobials | Type of Wards | |||||
|---|---|---|---|---|---|---|
| Medicine n = 676 n (%) |
Pediatrics n = 389 n (%) |
ICU n = 2 n (%) |
CCU n = 54 n (%) |
COVID-19 Isolation n = 67 n (%) |
Total n = 1188 n (%) |
|
| Antibiotics | ||||||
| Cephalosporin | 365 (54.0) | 334 (85.9) | 0 | 18 (33.3) | 10 (14.9) | 727 (61.2) |
| First-generation | 0 | 0 | 0 | 0 | 0 | 0 |
| Second-generation | 17 (2.5) | 35 (9.0) | 0 | 2 (3.7) | 0 | 54 (4.6) |
| Third-generation | 349 (51.6) | 299 (76.9) | 0 | 16 (29.6) | 10 (14.9) | 674 (56.7) |
| Fourth-generation | 0 | 1 (0.3) | 0 | 0 | 0 | 1 (0.1) |
| Macrolide | 238 (35.2) | 7 (1.8) | 0 | 11 (20.4) | 9 (13.4) | 265 (22.3) |
| Penicilin | 101 (14.9) | 53 (13.6) | 2 (100) | 19 (35.2) | 3 (4.5) | 178 (15.0) |
| Aminoglycoside | 4 (0.6) | 103 (26.5) | 1 (50.0) | 0 | 1 (1.5) | 109 (9.2) |
| Quinolones | 50 (7.4) | 2 (0.5) | 0 | 0 | 0 | 52 (4.4) |
| Tetracycline | 47 (6.9) | 0 | 0 | 2 (3.7) | 7 (10.4) | 56 (4.7) |
| Carbapenems | 26 (3.8) | 14 (3.6) | 1 (50.0) | 1 (1.8) | 2 (3.0) | 44 (3.7) |
| Oxazolidinone | 2 (0.3) | 10 (2.6) | 0 | 0 | 0 | 12 (1.0) |
| Glycopeptides | 0 | 10 (2.6) | 0 | 0 | 0 | 10 (0.8) |
| Nitroimidazoles | 11 (1.6) | 1 (0.3) | 0 | 0 | 0 | 12 (1.0) |
| WHO AWaRe classification antibiotics | ||||||
| Access | 145 (21.4) | 153 (39.3) | 2 (100.0) | 19 (35.2) | 10 (14.9) | 329 (27.7) |
| Watch | 547 (80.92) | 355 (91.3) | 1 (50.0) | 25 (46.3) | 21 (31.3) | 949 (79.9) |
| Reserve | 2 (0.3) | 10 (2.6) | 0 | 0 | 0 | 12 (1.0) |
| Antiviral | ||||||
| Acyclovir | 3 (0.4) | 0 | 0 | 0 | 0 | 3 (0.2) |
| Adefovir | 1 (0.1) | 0 | 0 | 0 | 0 | 1 (0.1) |
| Zidovudine | 1 (0.1) | 0 | 0 | 0 | 0 | 1 (0.1) |
| Valacyclovir | 1 (0.1) | 0 | 0 | 2 (3.7) | 0 | 3 (0.2) |
| Favipiravir | 6 (0.9) | 0 | 0 | 0 | 2 (3.0) | 8 (0.7) |
| Antiparasitic drug | ||||||
| Ivermectin | 28 (4.1) | 0 | 0 | 2 (3.7) | 0 | 30 (2.5) |
Table A2.
Algorithm for disease severity category in suspected COVID-19 patients.
| Classification | Clinical Syndromes | Definition | Absent | |
|---|---|---|---|---|
| Mild | COVID-19-like symptoms | Fever, cough, sore throat, fatigue, headache, muscle pain, anorexia, diarrhea, nausea or vomiting | Pneumonia or difficulty breathing | |
| Moderate | Pneumonia |
Adolescent or adult: (Any 1) 1. If x-ray conducted: Suggestive of pneumonia 2. If x-ray not conducted: Clinical sign of pneumonia: Fever (≥100.4 °F or ≥38 °C) + any respiratory symptom (cough or difficulty breathing) + decreased breath sound or rales 3. Physician diagnosed pneumonia |
Child (≤5 years): (Any 1) 1. If x-ray conducted: Suggestive of pneumonia 2. If x-ray not conducted: Clinical sign of pneumonia: Cough or difficulty breathing + fast breath (<2 m: ≥ 60; 2–11 m: ≥ 50; 1–5 y: ≥40) +/or chest indrawing 3. Physician diagnosed pneumonia |
Adolescent or adult: Respiratory rate: <30 Oxygen = 0 Child: Severe pneumonia |
| Severe | Severe pneumonia |
Adolescent or adult: (Any 1) 1. Pneumonia + fever + any one: Resp. rate (≥30 breath/min) or oxygen support 2. Physician diagnosed severe pneumonia |
Child (≤5 years): (Any 1) 1. Pneumonia + cough/difficulty breathing + any one danger sign: central cyanosis or oxygen support or stridor or inability to drink/lethergy/unconsciousness/convulsions 2. Physician diagnosed severe pneumonia |
|
| Critical | Sepsis |
Adult: (All 3) 1. Suspected or proven infection: Abnormal temp: Fever (low/high) + Neutrophil (high: >75) or ESR (high: >20) or WBC: (high: >12 k or low: < 4 k) or lymphocyte (low: <20) 2. Fast HR (pulse rate- ≥ 12 y: >100) 3. Altered mental status (2–5) or Oxygen support or Thrombocytopenia (Platelet: <50,000/cmm)or Physician diagnosed sepsis |
Child: (All 3) 1. Suspected or proven infection: Abnormal temp: Fever (low/high) + Neutrophil (high: >75) or ESR (high: >20) or WBC: (high: >12 k or low: <4 k) or lymphocyte (low: <20) 2. HR (pulse rate- 0–29 d: >180, 30 d-1 y: >160, 1–5 y: >140, 5–12 y: >90) 3. Altered mental status (3–5) or Oxygen support or Thrombocytopenia (Platelet: <50,000/cmm)or Physician diagnosed sepsis |
|
| ARDS | Physician diagnosed ARDS | |||
| Septic shock | Physician diagnosed septic shock | |||
| Required ICU or mechanical ventilation | ICU or Mechanical ventilation required | |||
Author Contributions
Conceptualization, S.M.-E.-M., M.Z.H., and F.C.; methodology, S.M.-E.-M. and M.Z.H.; software, S.M.-E.-M. and M.A.A.J.B.; validation, S.M.-E.-M. and M.Z.H.; formal analysis, S.M.-E.-M., F.R., and M.A.A.J.B.; investigation, Z.A., P.D., and M.A.I.; resources, S.M.-E.-M., Z.A., P.D., and M.A.I.; data curation, S.M.-E.-M., F.R., and M.A.A.J.B.; writing—original draft preparation, S.M.-E.-M.; writing—review and editing, M.Z.H., M.A.A.J.B., F.R., Z.A., P.D., M.A.I., and F.C.; visualization, S.M.-E.-M., M.Z.H., and M.A.A.J.B.; supervision, F.C.; project administration, S.M.-E.-M., Z.A., P.D., M.A.I., and F.C.; funding acquisition, F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foreign, Commonwealth and Development Office (FCDO) (former DFID), grant number MD-0024, and Centers for Disease Control and Prevention (CDC), grant number 1 U01GH002259-01-00.
Institutional Review Board Statement
The surveillances were conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board consisting of the Research Review Committee and Ethical Review Committee of icddr,b (protocol code PR-20032 and PR-2007-002).
Informed Consent Statement
Written informed consent was obtained from all patients and/or their guardian involved in the surveillance.
Data Availability Statement
The data presented in these surveillances are available on request from the corresponding author. The data are not publicly available due to privacy restrictions and icddr,b policy.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the surveillances; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nestler M.J., Godbout E., Lee K., Kim J., Noda A.J., Taylor P., Pryor R., Markley J.D., Doll M., Bearman G., et al. Impact of COVID-19 on pneumonia-focused antibiotic use at an academic medical center. Infect. Control Hosp. Epidemiol. 2020:1–3. doi: 10.1017/ice.2020.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clancy C.J., Nguyen M.H. Coronavirus Disease 2019, Superinfections, and Antimicrobial Development: What Can We Expect? Clin. Infect. Dis. 2020;71:2736–2743. doi: 10.1093/cid/ciaa524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huttner B., Catho G., Pano-Pardo J., Pulcini C., Schouten J. COVID-19: Don’t neglect antimicrobial stewardship principles! Clin. Microbiol. Infect. 2020;26:808–810. doi: 10.1016/j.cmi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beović B., Doušak M., Ferreira-Coimbra J., Nadrah K., Rubulotta F., Belliato M., Berger-Estilita J., Ayoade F., Rello J., Erdem H. Antibiotic use in patients with COVID-19: A ‘snapshot’ Infectious Diseases International Research Initiative (ID-IRI) survey. J. Antimicrob. Chemother. 2020;75:3386–3390. doi: 10.1093/jac/dkaa326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verroken A., Scohy A., Gerard L., Wittebole X., Collienne C., Laterre P.-F. Co-infections in COVID-19 critically ill and antibiotic management: A prospective cohort analysis. Crit. Care. 2020;24:1–3. doi: 10.1186/s13054-020-03135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei W., Ortwine J.K., Mang N.S., Joseph C., Hall B.C., Prokesch B.C. Limited Role for Antibiotics in COVID-19: Scarce Evidence of Bacterial Coinfection (6/4/2020) Available SSRN 3622388. 2020 doi: 10.2139/ssrn.3622388. [DOI] [Google Scholar]
- 7.Townsend L., Hughes G., Kerr C., Kelly M., O’Connor R., Sweeney E., Doyle C., O’Riordan R., Martin-Loeches I., Bergin C., et al. Bacterial pneumonia coinfection and antimicrobial therapy duration in SARS-CoV-2 (COVID-19) infection. JAC-Antimicrob. Resist. 2020;2:dlaa071. doi: 10.1093/jacamr/dlaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., Satta G., Cooke G., Holmes A. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R., Soucy J.-P.R., Daneman N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong W.H., Saha B.K., Ramani A., Chopra A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection. 2021:1–15. doi: 10.1007/s15010-021-01602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karaba S.M., Jones G., Helsel T., Smith L.L., Avery R., Dzintars K., Salinas A.B., Keller S.C., Townsend J.L., Klein E., et al. Prevalence of Co-infection at the Time of Hospital Admission in COVID-19 Patients, A Multicenter Study. Open Forum Infect. Dis. 2021;8:ofaa578. doi: 10.1093/ofid/ofaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L., Amin A.K., Khanna P., Aali A., McGregor A., Bassett P., Rao G.G. An observational cohort study of bacterial co-infection and implications for empirical antibiotic therapy in patients presenting with COVID-19 to hospitals in North West London. J. Antimicrob. Chemother. 2020;76:796–803. doi: 10.1093/jac/dkaa475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi S., Parkar J., Ansari A., Vora A., Talwar D., Tiwaskar M., Patil S., Barkate H. Role of favipiravir in the treatment of COVID-19. Int. J. Infect. Dis. 2021;102:501–508. doi: 10.1016/j.ijid.2020.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Babalola O.E., Bode C.O., Ajayi A.A., Alakaloko F.M., Akase I.E., Otrofanowei E., Salu O.B., Adeyemo W.L., Ademuyiwa A.O., Omilabu S.A. Ivermectin shows clinical benefits in mild to moderate Covid19 disease: A randomised controlled double blind dose response study in Lagos. QJM An Int. J. Med. 2021 doi: 10.1093/qjmed/hcab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed S., Karim M.M., Ross A.G., Hossain M.S., Clemens J.D., Sumiya M.K., Phru C.S., Rahman M., Zaman K., Somani J., et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int. J. Infect. Dis. 2021;103:214–216. doi: 10.1016/j.ijid.2020.11.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ. 2020;369:m1983. doi: 10.1136/bmj.m1983. [DOI] [PubMed] [Google Scholar]
- 21.Rossato L., Negrão F.J., Simionatto S. Could the COVID-19 pandemic aggravate antimicrobial resistance? Am. J. Infect. Control. 2020;48:1129–1130. doi: 10.1016/j.ajic.2020.06.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawson T.M., Moore L.S.P., Castro-Sanchez E., Charani E., Davies F., Satta G., Ellington M.J., Holmes A.H. COVID-19 and the potential long-term impact on antimicrobial resistance. J. Antimicrob. Chemother. 2020;75:1681–1684. doi: 10.1093/jac/dkaa194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.PEW . Could Efforts to Fight the Coronavirus Lead to Overuse of Antibiotics? The Pew Charitable Trusts; Philadelphia, PA, USA: 2021. [Google Scholar]
- 24.World Health Organisation . Record Number of Countries Contribute Data Revealing Disturbing Rates of Antimicrobial Resistance. World Health Organization; Geneva, Switzerland: 2020. [Google Scholar]
- 25.The Government of Bangladesh Bangladesh Corona Info. [(accessed on 11 November 2020)]; Available online: https://corona.gov.bd/
- 26.Islam M.S., Luby S.P., Sultana R., Rimi N.A., Zaman R.U., Uddin M., Nahar N., Rahman M., Hossain M.J., Gurley E.S. Family caregivers in public tertiary care hospitals in Bangladesh: Risks and opportunities for infection control. Am. J. Infect. Control. 2014;42:305–310. doi: 10.1016/j.ajic.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rimi N.A., Sultana R., Luby S.P., Islam M.S., Uddin M., Hossain M.J., Zaman R.U., Nahar N., Gurley E.S. Infrastructure and Contamination of the Physical Environment in Three Bangladeshi Hospitals: Putting Infection Control into Context. PLoS ONE. 2014;9:e89085. doi: 10.1371/journal.pone.0089085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahaman K.R., Mahmud S., Mallick B. Challenges of Testing COVID-19 Cases in Bangladesh. Int. J. Environ. Res. Public Health. 2020;17:6439. doi: 10.3390/ijerph17186439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anwar S., Nasrullah M., Hosen M.J. COVID-19 and Bangladesh: Challenges and How to Address Them. Front. Public Health. 2020;8:154. doi: 10.3389/fpubh.2020.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasir M., Chowdhury A.S.M.S., Zahan T. Self-medication during COVID-19 outbreak: A cross sectional online survey in Dhaka city. Int. J. Basic Clin. Pharmacol. 2020;9:1325. doi: 10.18203/2319-2003.ijbcp20203522. [DOI] [Google Scholar]
- 31.Ahmed I., Hasan M., Akter R., Sarkar B.K., Rahman M., Sarker S., Samad M.A. Behavioral preventive measures and the use of medicines and herbal products among the public in response to COVID-19 in Bangladesh: A cross-sectional study. PLoS ONE. 2020;15:e0243706. doi: 10.1371/journal.pone.0243706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization . COVID-19 Clinical Management: Living Guidance. World Health Organization; Geneva, Switzerland: 2021. p. 81. [Google Scholar]
- 33.Directorate General of Health Services . Bangladesh National Guidelines on Clinical Management of Coronavirus Disease 2019 (COVID-19) DGHS; Dhaka, Bangladesh: 2020. Version 7.0. [Google Scholar]
- 34.World Health Organisation . AWaRe Polcy Brief. WHO; Geneva, Switzerland: 2019. [Google Scholar]
- 35.Molla M.A., Yeasmin M., Islam K., Sharif M., Amin R., Nafisa T., Ghosh A.K., Parveen M., Arif M.H., Alam J.A.J., et al. Antibiotic Prescribing Patterns at COVID-19 Dedicated Wards in Bangladesh: Findings from a Single Center Study. Infect. Prev. Pract. 2021;3:100134. doi: 10.1016/j.infpip.2021.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parveen M., Molla M.A., Yeasmin M., Nafisa T., Barna A.A., Ghosh A.K. Evidences on Irrational Anti-Microbial Prescribing and Consumption among COVID-19 Positive Patients and Possible Mitigation Strategies: A Descriptive Cross Sectional Study. Bangladesh J. Infect. Dis. 2020;7:S3–S7. doi: 10.3329/bjid.v7i00.50155. [DOI] [Google Scholar]
- 37.World Health Organisation Adopt AWaRe: Handle Antibiotics with Care. [(accessed on 17 December 2020)]; Available online: https://adoptaware.org/assets/pdf/aware_brochure.pdf.
- 38.Kaur H., Shekhar N., Sharma S., Sarma P., Prakash A., Medhi B. Ivermectin as a potential drug for treatment of COVID-19: An in-sync review with clinical and computational attributes. Pharmacol. Rep. 2021:1–14. doi: 10.1007/s43440-020-00195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao J.P., Hu Y., Du R.H., Chen Z.S., Jin Y., Zhou M., Zhang J., Qu J.M., Cao B. Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia. Chin. J. Tuberc. Respir. Dis. 2020;43:183–184. doi: 10.3760/cma.j.issn.1001-0939.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biswas M., Roy M.N., Manik M.I.N., Hossain M.S., Tapu S.T.A., Moniruzzaman M., Sultana S. Self medicated antibiotics in Bangladesh: A cross-sectional health survey conducted in the Rajshahi City. BMC Public Health. 2014;14:847. doi: 10.1186/1471-2458-14-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alam N., Saffoon N., Uddin R. Self-medication among medical and pharmacy students in Bangladesh. BMC Res. Notes. 2015;8:763. doi: 10.1186/s13104-015-1737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmed S.A.K.S., Ajisola M., Azeem K., Bakibinga P., Chen Y.-F., Choudhury N.N., Fayehun O., Griffiths F., Harris B., Kibe P., et al. Impact of the societal response to COVID-19 on access to healthcare for non-COVID-19 health issues in slum communities of Bangladesh, Kenya, Nigeria and Pakistan: Results of pre-COVID and COVID-19 lockdown stakeholder engagements. BMJ Glob. Health. 2020;5:e003042. doi: 10.1136/bmjgh-2020-003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmed M., Aleem M.A., Roguski K., Abedin J., Islam A., Alam K.F., Gurley E.S., Rahman M., Azziz-Baumgartner E., Homaira N., et al. Estimates of seasonal influenza-associated mortality in Bangladesh, 2010–2012. Influenza Other Respir. Viruses. 2017;12:65–71. doi: 10.1111/irv.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaman R.U., Alamgir A.S.M., Rahman M., Azziz-Baumgartner E., Gurley E.S., Sharker M.A.Y., Brooks W.A., Azim T., Fry A.M., Lindstrom S., et al. Influenza in Outpatient ILI Case-Patients in National Hospital-Based Surveillance, Bangladesh, 2007–2008. PLoS ONE. 2009;4:e8452. doi: 10.1371/journal.pone.0008452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hassan Z., Monjur M.R., Biswas A.A.J., Chowdhury F., Kafi M.A.H., Braithwaite J., Jaffe A., Homaira N. Antibiotic use for acute respiratory infections among under-5 children in Bangladesh: A population-based survey. BMJ Glob. Health. 2021;6:e004010. doi: 10.1136/bmjgh-2020-004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jewell N.P. Statistics for Epidemiology. CRC Press; Boca Raton, FL, USA: 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in these surveillances are available on request from the corresponding author. The data are not publicly available due to privacy restrictions and icddr,b policy.