Abstract
Background
The lipid profile is reportedly related to peripheral blood pressure or pulse wave velocity. However, no studies have investigated the associations between lipid parameters, especially remnant lipoprotein cholesterol (RLP-C), and central systolic blood pressure (cSBP).
Methods
This study used baseline data of a community-based cohort in Beijing, China. Participants who had been treated with anti-hypertensive or lipid-lowering agents were excluded. RLP-C is equal to total cholesterol (TC) minus the sum of low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C). An Omron HEM-9000AI device was used to measure non-invasive cSBP. The associations between blood lipid profile and non-invasive cSBP were evaluated using multivariable regression models.
Results
The 5173 included participants were 55.0 ± 8.5 years old; 35.7% (1845) of participants were men. Increased cSBP was significantly associated with increased TC, LDL-C, non-high-density lipoprotein cholesterol (non-HDL-C), triglyceride (TG), and RLP-C but with decreased HDL-C, even after adjusting for possible covariates. When simultaneously entering individual pairs of RLP-C and other blood lipid parameters into the multivariable regression model, RLP-C remained significantly associated with cSBP, even after adjusting for other lipids. Compared with participants who had RLP-C levels in the first quartile (Q1), cSBP for those with RLP-C in Q4 was increased to 4.57 (95% confidence interval [CI]: 3.08–6.06) mmHg after adjusting for LDL-C, 4.50 (95%CI: 2.98–6.02) mmHg after adjusting for TC, 3.91 (95%CI: 1.92–5.89) mmHg after adjusting for TG, 5.15 (95%CI: 3.67–6.63) mmHg after adjusting for HDL-C, and 4.10 (95%CI: 2.36–5.84) mmHg after adjusting for non-HDL-C.
Conclusions
Increased blood RLP-C level was significantly associated with higher cSBP in a Chinese population, independently of other lipids, which indicates its importance in individual cardiovascular risk assessment.
Keywords: Lipids, Remnant lipoprotein cholesterol, Non-high-density lipoprotein cholesterol, Triglyceride, Cholesterol, Low-density lipoprotein cholesterol, Non-invasive central systolic blood pressure, Arterial stiffness
Background
Hypertension accounts for the largest proportion of the global disease burden and mortality in recent years [1]. In 2019, elevated systolic blood pressure (SBP) led to approximately 10.8 million deaths and the highest percentage of disability-adjusted life-years [2]. Hypertension is currently defined according to the peripheral blood pressure; however, pulse wave analysis (PWA) has enabled reproducible and non-invasive measurement of central hemodynamics. Numerous clinical studies have indicated that non-invasive central systolic blood pressure (cSBP) measurement may improve risk stratification for cardiovascular diseases owing to its stronger association with hypertension-mediated organ damage [3–7].
Previous studies have identified an association between blood lipids and blood pressure, although most such studies have focused on the relationships between hypercholesterolemia or hypertriglyceridemia and peripheral blood pressure [8–11]. Additionally, patients with increased remnant lipoprotein cholesterol (RLP-C) levels are more likely to develop incident cardiovascular diseases [12–14]. A 10-year longitudinal study in Japan showed associations between higher plasma RLP-C and incident hypertension [15]. To the best of our knowledge, the relationship between RLP-C and central blood pressure remains unknown. Hence, this retrospective study was conducted to investigate the associations between non-invasive cSBP and lipid profile levels, especially RLP-C.
Methods
Study design
This retrospective study used baseline data of a Beijing community-based cohort in China [16]. Among the initial 9540 participants recruited via phone calls or recruitment posters between December 2011 and April 2012, 8062 participants (84.5%) had effective data of cSBP and RLP-C at baseline. Those participants treated with anti-hypertensive agents (n = 2630) and/or lipid-lowering agents (n = 952) were excluded owing to therapeutic effects on cSBP and blood lipids of these drugs. Finally, 5173 eligible participants were included in the analysis.
Data collection
Trained research staff collected data by standards, including information on lifestyle, history of diseases and medications, and physical examination. Smoking status, drinking status, hypertension, and diabetes mellitus (DM) have been defined previously [17]. Calculations of estimated glomerular filtration rate (eGFR) and body mass index (BMI) were according to those in a previous publication [18].
After an overnight fast, blood samples were obtained from each participant via forearm venipuncture. Serum glucose, lipid, and creatinine levels were tested using previously published methods [19]. Non-high-density lipoprotein cholesterol (non-HDL-C) was calculated as subtraction of the high-density lipoprotein cholesterol (HDL-C) from the total cholesterol (TC), while the RLP-C was defined as TC minus the sum of low-density lipoprotein cholesterol (LDL-C) and HDL-C.
Before central blood pressure measurement, all participants were required to sit quietly for 5 min. Non-invasive cSBP was measured using the validated HEM-9000AI device (Omron Healthcare, Kyoto, Japan) [20–24]. Each participant underwent simultaneous measurement of left radial applanation tonometry and right brachial cuff oscillometric pressure. Radial waveforms were analyzed to obtain the peripheral systolic pressure peaks, and were then calibrated using the measured brachial blood pressure. A linear regression model with the late peak of the peripheral systolic pressure was used to calculate the non-invasive cSBP. This measurement was not repeated due to the tight research schedule.
Statistical analysis
Mean ± standard deviation was used to describe continuous variables with a normal distribution, and differences were determined with one-way analysis of variance. Median (interquartile range) was used to describe non-normally distributed variables, and differences were determined using the Kruskal-Wallis rank test. Number (%) was used to describe dichotomous variables, and differences were determined using the chi-square test.
Relationships between lipid parameters and cSBP level were examined with generalized additive models using a spline smoothing function. Associations between cSBP and different blood lipid parameters (as continuous variables and quartiles [Q]) were investigated using univariable and multivariable regression models. Age, sex, BMI, eGFR, smoking and drinking status, DM, antidiabetic drug use, myocardial infarction and stroke history were adjusted in multivariable regression analyses. Furthermore, independent associations of RLP-C with cSBP, adjusted for other lipid parameters, were determined by simultaneously entering the pairs of RLP-C and other lipid parameters into the multivariable regression models one at a time. With regard to possible collinearity, the variance inflation factor (VIF) was calculated for the included variables in each multivariable regression model. Empower(R) (www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA) and R (http://www.R-project.org) were used for data analyses, and two-sided P values < 0.05 were regarded as statistically significant.
Results
Baseline characteristics of enrolled participants
Table 1 summarizes the characteristics of enrolled participants according to quartiles of RLP-C. The 5173 included participants were 55.0 ± 8.5 years old, and 35.7% (1845) were men. Participants with a higher RLP-C level had increased levels of TC, triglyceride (TG), LDL-C, and non-HDL-C, as well as cSBP (P < 0.001). Despite no intergroup difference in the percentage of myocardial infarction or stroke history, participants with a higher RLP-C level tended to be older, male, more likely to smoke and drink, and to have higher BMI, worse renal function, a higher ratio of DM, hypertension (P < 0.001) and antidiabetic medication use (P = 0.013).
Table 1.
Baseline characteristics of all participants by RLP-C quartile
| Variables | Total | RLP-C, mmol/L | P Value | |||
|---|---|---|---|---|---|---|
| Q1 (< 0.39) | Q2 (0.39–< 0.54) | Q3 (0.54–< 0.73) | Q4 (≥0.73) | |||
| n | 5173 | 1269 | 1283 | 1318 | 1303 | |
| Age, y | 55.0 ± 8.5 | 54.2 ± 8.8 | 55.3 ± 9.1 | 55.5 ± 8.2 | 55.0 ± 7.9 | < 0.001 |
| Male, n (%) | 1845 (35.7%) | 365 (28.8%) | 444 (34.6%) | 476 (36.1%) | 560 (43.0%) | < 0.001 |
| BMI, kg/m2 | 25.5 ± 3.3 | 24.0 ± 3.0 | 25.3 ± 3.2 | 26.1 ± 3.2 | 26.7 ± 3.1 | < 0.001 |
| eGFR, mL/min·1.73m2 | 96.8 ± 12.0 | 98.7 ± 11.4 | 96.9 ± 11.9 | 96.1 ± 11.9 | 95.4 ± 12.5 | < 0.001 |
| cSBP, mmHg | 129.7 ± 17.3 | 126.1 ± 16.8 | 129.0 ± 17.3 | 130.2 ± 16.8 | 133.2 ± 17.4 | < 0.001 |
| TC, mmol/L | 5.4 ± 1.0 | 4.8 ± 0.8 | 5.1 ± 0.8 | 5.5 ± 0.9 | 6.0 ± 1.1 | < 0.001 |
| TG, mmol/L | 1.2 (0.9–1.8) | 0.8 (0.6–0.9) | 1.1 (0.9–1.3) | 1.4 (1.2–1.7) | 2.3 (1.8–3.1) | < 0.001 |
| HDL-C, mmol/L | 1.5 ± 0.4 | 1.8 ± 0.4 | 1.5 ± 0.3 | 1.4 ± 0.3 | 1.2 ± 0.3 | < 0.001 |
| LDL-C, mmol/L | 3.3 ± 0.8 | 2.7 ± 0.6 | 3.2 ± 0.6 | 3.5 ± 0.7 | 3.7 ± 1.0 | < 0.001 |
| RLP-C, mmol/L | 0.5 (0.4–0.7) | 0.3 (0.2–0.4) | 0.5 (0.4–0.5) | 0.6 (0.6–0.7) | 0.9 (0.8–1.1) | < 0.001 |
| Non-HDL-C, mmol/L | 3.9 ± 1.0 | 3.0 ± 0.6 | 3.6 ± 0.6 | 4.1 ± 0.7 | 4.8 ± 1.0 | < 0.001 |
| Smoking, n (%) | 1038 (20.1%) | 163 (12.8%) | 219 (17.1%) | 275 (20.9%) | 381 (29.2%) | < 0.001 |
| Drinking, n (%) | 1255 (24.3%) | 250 (19.7%) | 299 (23.3%) | 317 (24.1%) | 389 (29.9%) | < 0.001 |
| Prevalence of cardiovascular disease, n (%) | ||||||
| Diabetes mellitus | 953 (18.4%) | 159 (12.5%) | 208 (16.2%) | 253 (19.2%) | 333 (25.6%) | < 0.001 |
| Self-reported Myocardial Infarction | 21 (0.4%) | 0 (0.0%) | 7 (0.6%) | 7(0.5%) | 7 (0.5%) | 0.077 |
| Self-reported Stroke history | 97 (1.9%) | 21 (1.7%) | 30 (2.3%) | 28 (2.1%) | 18 (1.4%) | 0.261 |
| Hypertension | 1342 (25.9%) | 237 (18.7%) | 311 (24.2%) | 355 (26.9%) | 439 (33.7%) | < 0.001 |
| Antidiabetic medication use | 325 (6.3%) | 60 (4.7%) | 76 (5.9%) | 88 (6.7%) | 101 (7.8%) | 0.013 |
Data are presented as mean ± standard deviation for normally distributed continuous variables, median (interquartile range) for non-normally distributed continuous variables, and number (percentage) for dichotomous variables
Abbreviations: BMI body mass index, eGFR estimated glomerular filtration rate, cSBP central systolic blood pressure, TC total cholesterol, TG triglyceride, HDL-C high–density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, RLP-C remnant lipoprotein cholesterol, non-HDL-C non-high-density lipoprotein cholesterol
Associations of RLP-C and other lipid profiles with cSBP, considered individually
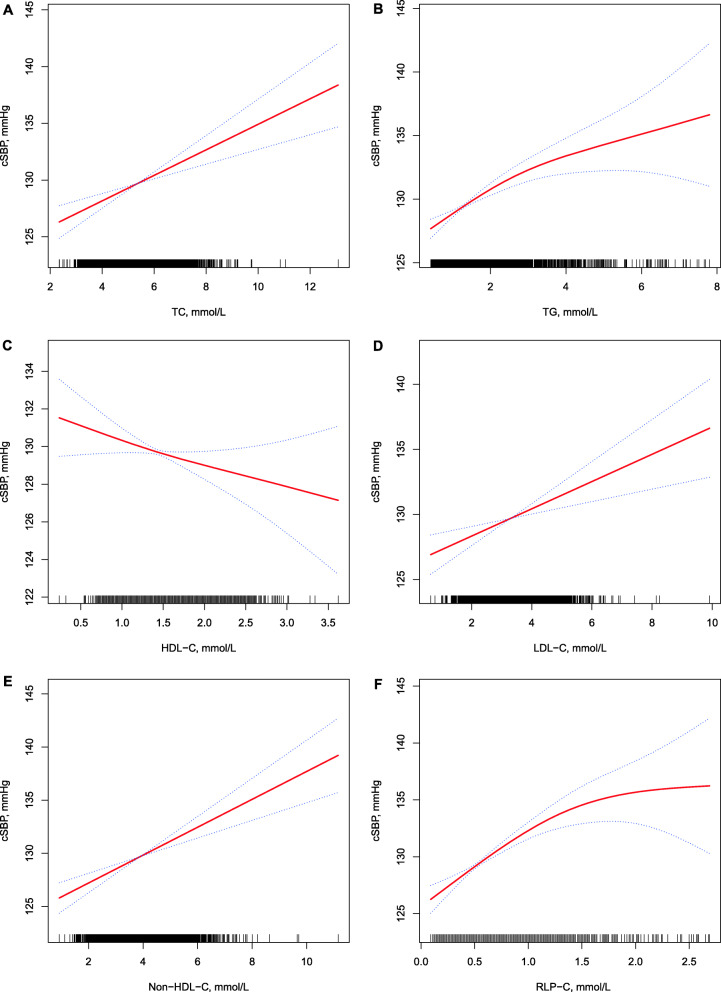
The smoothing curves of cSBP by lipid parameter after adjusting for possible covariates are shown in Fig. 1. The smoothing curves showed a positive linear association between most lipid parameters and cSBP, except that a negative linear association with the HDL-C level and a non-linear trend with RLP-C level were observed. Associations between the blood lipid profile and cSBP are summarized in Table 2. In the univariable analyses, cSBP showed a graded positive relationship with RLP-C, TC, TG, LDL-C, and non-HDL-C levels, but a graded negative relationship with the HDL-C level (P < 0.001). In the adjusted multivariable analyses, higher cSBP was significantly associated with increases in TC, TG, LDL-C, RLP-C, and non-HDL-C levels but decreased HDL-C level.
Fig. 1.
Smoothing curve of cSBP by lipid parameter. Adjusted for sex, age, body mass index, estimated glomerular filtration rate, smoking, alcohol use, diabetes mellitus, antidiabetic drug use, myocardial infarction and stroke. A: Association between TC and cSBP. B: Association between TG and cSBP. C: Association between HDL-C and cSBP. D: Association between LDL-C and cSBP. E: Association between non-HDL-C and cSBP. F: Association between RLP-C and cSBP. cSBP: central systolic blood pressure; TC: total cholesterol; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; non-HDL-C: non-high-density lipoprotein cholesterol; RLP-C: remnant lipoprotein cholesterol
Table 2.
Multivariable adjusted change in cSBP by quartiles of blood lipids, considered individually
| Variable | Change in cSBP, mmHg (95% CI) |
P value |
|---|---|---|
| TC, mmol/L | ||
| Continuous, per 1 mmol/L | 1.20 (0.73–1.67) | < 0.001 |
| Quartiles | ||
| Q1: < 4.70 | Ref. | |
| Q2: 4.70–< 5.28 | 0.72 (−0.57–2.00) | 0.274 |
| Q3: 5.28–< 5.95 | 1.99 (0.71–3.28) | 0.002 |
| Q4: ≥5.95 | 2.58 (1.28–3.88) | < 0.001 |
| TG, mmol/L | ||
| Continuous, per 1 mmol/L | 0.84 (0.49–1.19) | < 0.001 |
| Quartiles | ||
| Q1: < 0.87 | Ref. | |
| Q2: 0.87–< 1.24 | 1.51 (0.23–2.80) | 0.021 |
| Q3: 1.24–< 1.79 | 2.30 (0.98–3.61) | < 0.001 |
| Q4: ≥1.79 | 4.22 (2.87–5.57) | < 0.001 |
| HDL-C, mmol/L | ||
| Continuous, per 1 mmol/L | −1.43 (−2.76– − 0.10) | 0.035 |
| Quartiles | ||
| Q1: < 1.19 | Ref. | |
| Q2: 1.19–< 1.42 | −0.74 (−2.05–0.57) | 0.268 |
| Q3: 1.42–< 1.68 | −1.00 (−2.36–0.36) | 0.151 |
| Q4: ≥1.68 | −1.60 (−3.03– − 0.17) | 0.028 |
| LDL-C, mmol/L | ||
| Continuous, per 1 mmol/L | 1.00 (0.44–1.56) | < 0.001 |
| Quartiles | ||
| Q1: < 2.73 | Ref. | |
| Q2: 2.73–< 3.24 | 1.40 (0.12–2.68) | 0.032 |
| Q3: 3.24–< 3.78 | 1.35 (0.06–2.64) | 0.041 |
| Q4: ≥3.78 | 2.63 (1.34–3.93) | < 0.001 |
| Non-HDL-C, mmol/L | ||
| Continuous, per 1 mmol/L | 1.38 (0.91–1.85) | < 0.001 |
| Quartiles | ||
| Q1: < 3.22 | Ref. | |
| Q2: 3.22–< 3.83 | 2.33 (1.05–3.60) | < 0.001 |
| Q3: 3.83–< 4.49 | 2.14 (0.85–3.43) | 0.001 |
| Q4: ≥4.49 | 3.88 (2.57–5.18) | < 0.001 |
| RLP-C, mmol/L | ||
| Continuous, per 1 mmol/L | 3.94 (2.82–5.07) | < 0.001 |
| Quartiles | ||
| Q1: < 0.39 | Ref. | |
| Q2: 0.39–< 0.54 | 1.62 (0.33–2.91) | 0.014 |
| Q3: 0.54–< 0.73 | 2.19 (0.88–3.50) | 0.001 |
| Q4: ≥0.73 | 4.88 (3.53–6.23) | < 0.001 |
Model adjusted for age, sex, body mass index, estimated glomerular filtration rate, smoking status, drinking status, diabetes mellitus, antidiabetic drug use, history of myocardial infarction and history of stroke
Abbreviations: TC total cholesterol, TG triglyceride, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, non-HDL-C non-high-density lipoprotein cholesterol, RLP-C remnant lipoprotein cholesterol, cSBP central systolic blood pressure
Associations of RLP-C and other lipid profiles with cSBP, considered simultaneously
The VIF calculated for the included variables in each multivariable regression model did not show any collinearity (Supplementary materials). Table 3 shows the relationship of RLP-C level and other blood lipid parameters with non-invasive cSBP, with simultaneous entry of RLP-C and other lipid in pairs into the multivariable regression models, after adjusting for covariates. Compared with participants who had RLP-C in Q1, the cSBP for those with RLP-C in Q4 was increased to 4.57 (95% confidence interval [CI]: 3.08–6.06) mmHg after adjusting for LDL-C, 4.50 (95%CI: 2.98–6.02) mmHg after adjusting for TC, 3.91 (95%CI: 1.92–5.89) mmHg after adjusting for TG, 5.15 (95%CI: 3.67–6.63) mmHg after adjusting for HDL-C, and 4.10 (95%CI: 2.36–5.84) mmHg after adjusting for non-HDL-C. The other lipid parameters were not significantly associated with cSBP after adjusting for RLP-C.
Table 3.
Multivariable adjusted change in cSBP by quartiles of RLP-C and other lipid parameters, considered simultaneously
| Models | Change in cSBP, mmHg (95% CI) |
P value | Change in cSBP, mmHg (95% CI) |
P value | |
|---|---|---|---|---|---|
| Model I† | |||||
| RLP-C, mmol/L | LDL-C, mmol/L | ||||
| Q1: < 0.39 | Ref. | Q1: < 2.73 | Ref. | ||
| Q2: 0.39–< 0.54 | 1.43 (0.10–2.77) | 0.035 | Q2: 2.73–< 3.24 | 0.97 (−0.33–2.27) | 0.143 |
| Q3: 0.54–< 0.73 | 1.89 (0.48–3.31) | 0.009 | Q3: 3.24–< 3.78 | 0.43 (−0.92–1.79) | 0.529 |
| Q4: ≥0.73 | 4.57 (3.08–6.06) | < 0.001 | Q4: ≥3.78 | 0.93 (−0.50–2.37) | 0.202 |
| Model II† | |||||
| RLP-C, mmol/L | TC, mmol/L | ||||
| Q1: < 0.39 | Ref. | Q1: < 4.70 | Ref. | ||
| Q2: 0.39–< 0.54 | 1.47 (0.15–2.78) | 0.029 | Q2: 4.70–< 5.28 | 0.14 (−1.16–1.44) | 0.832 |
| Q3: 0.54–< 0.73 | 1.90 (0.51–3.29) | 0.007 | Q3: 5.28–< 5.95 | 0.92 (−0.42–2.26) | 0.178 |
| Q4: ≥0.73 | 4.50 (2.98–6.02) | < 0.001 | Q4: ≥5.95 | 0.66 (−0.80–2.12) | 0.374 |
| Model III† | |||||
| RLP-C, mmol/L | TG, mmol/L | ||||
| Q1: < 0.39 | Ref. | Q1: < 0.87 | Ref. | ||
| Q2: 0.39–< 0.54 | 1.22 (−0.17–2.62) | 0.087 | Q2: 0.87–< 1.24 | 0.96 (−0.41–2.32) | 0.170 |
| Q3: 0.54–< 0.73 | 1.53 (−0.07–3.13) | 0.061 | Q3: 1.24–< 1.79 | 1.02 (−0.57–2.61) | 0.209 |
| Q4: ≥0.73 | 3.91 (1.92–5.89) | < 0.001 | Q4: ≥1.79 | 1.47 (−0.50–3.43) | 0.144 |
| Model IV† | |||||
| RLP-C, mmol/L | HDL-C, mmol/L | ||||
| Q1: < 0.39 | Ref. | Q1: < 1.19 | Ref. | ||
| Q2: 0.39–< 0.54 | 1.72 (0.40–3.03) | 0.010 | Q2: 1.19–< 1.42 | 0.09 (−1.24–1.42) | 0.895 |
| Q3: 0.54–< 0.73 | 2.36 (0.99–3.73) | < 0.001 | Q3: 1.42–< 1.68 | 0.52 (−0.90–1.94) | 0.474 |
| Q4: ≥0.73 | 5.15 (3.67–6.63) | < 0.001 | Q4: ≥1.68 | 0.59 (−0.97–2.15) | 0.457 |
| Model V† | |||||
| RLP-C, mmol/L | Non-HDL-C, mmol/L | ||||
| Q1: < 0.39 | Ref. | Q1: < 3.22 | Ref. | ||
| Q2: 0.39–< 0.54 | 1.15 (−0.23–2.53) | 0.104 | Q2: 3.22–< 3.83 | 1.67 (0.31–3.03) | 0.016 |
| Q3: 0.54–< 0.73 | 1.54 (−0.00–3.08) | 0.050 | Q3: 3.83–< 4.49 | 0.79 (−0.70–2.28) | 0.300 |
| Q4: ≥0.73 | 4.10 (2.36–5.84) | < 0.001 | Q4: ≥4.49 | 1.52 (−0.16–3.20) | 0.077 |
†RLP-C and other blood lipid parameters were simultaneously entered into the multivariable regression model
Model adjusted for age, sex, body mass index, estimated glomerular filtration rate, smoking status, drinking status, diabetes mellitus, antidiabetic drug use, history of myocardial infarction and history of stroke
Abbreviations: cSBP central systolic blood pressure, RLP-C remnant lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, TC total cholesterol, TG triglyceride, HDL-C high-density lipoprotein cholesterol, non-HDL-C non-high-density lipoprotein cholesterol
Discussion
The present study shows associations between cSBP and blood lipid profiles, especially RLP-C, in a community-dwelling Chinese population. In further analyses by simultaneously entering RLP-C level and other lipid parameters in pairs, the RLP-C level showed a stronger association with non-invasive cSBP.
Although different physiological indices, blood pressure and lipid profiles have overlapping processes owing to common cardiovascular risk factors and complications. Both hypertriglyceridemia and hypercholesterolemia are associated with higher peripheral blood pressure, as reported in previous cross-sectional studies [8–11]. A cohort study further showed that adolescents with a higher TG to HDL-C ratio had a higher risk of developing adult hypertension in a 20-year follow-up [25]; similar results were also observed in another prospective cohort study among pregnant women [26]. Mechanisms such as endothelial cell dysfunction, neuroendocrine system activation, increased salt sensitivity and reduced sodium clearance by nephrons, and higher L-type calcium channel activities in the smooth myofibrils contributed to the relationship [9, 27–33]. However, previous studies focused on peripheral blood pressure, although central blood pressure has been more strongly associated with cardiovascular outcome and hypertension-mediated organ damage [3–7].
Arterial stiffness can be evaluated non-invasively using two-dimensional imaging techniques, pulse wave velocity (PWV), cardio-ankle vascular index (CAVI), and PWA, although carotid-femoral PWV is currently more reliable [34–36]. Associations between TG levels and arterial stiffness have been reported, using all measurements of the CAVI [37], brachial-ankle PWV [38], and carotid-femoral PWV [39]. Regarding cholesterol, aortic stiffness has been positively associated with TC, LDL-C, and non-HDL-C levels, and negatively associated with HDL-C level [8, 40–42]. Moreover, large artery stiffness and blood pressure control are improved with statin therapy in patients with hypertension [43], independently of changes in the LDL-C level [44]. The findings of the present cross-sectional study suggested that the blood lipid profile is associated with non-invasive cSBP, and this association might be explained by vascular impairment. Compared with PWV, central blood pressure is more than a tool to measure aortic stiffness; it is associated with a more complex set of determinants, including the physical properties of systemic arteries, PWV, the travel time of a forward wave and its reflection, and the distance to the major reflecting site of a pulse wave [45]. Relationships of these determinants and lipids have been discussed previously. Another cross-sectional study used PWA to show associations between the LDL-C level and wave reflection [46]. Hence, cSBP and PWV are both associated with the lipid profile owing to their shared determinant of aortic stiffness, whereas other determinants of cSBP may enhance this association.
Notably, RLP-C was more strongly associated with cSBP than the other lipid parameters analyzed in the present study, possibly because of its physiological characteristics. RLP-C is defined as the cholesterol content in TG-rich lipoproteins (TRLs), namely intermediate-density lipoprotein (IDL), very low-density lipoprotein (VLDL), and chylomicron remnants [47–49]. As TG contents are gradually degraded by lipoprotein lipase in the bloodstream, the cholesterol in RLP-C could also be involved in atherosclerosis plaque formation and subsequent cardiovascular events, and it has been recognized as more atherogenic and proinflammatory than the cholesterol in LDL-C [48–52]. A recent study indicated associations of plasma TGs and relatively small-sized LDL particles with the carotid-femoral PWV, despite no significant associations between VLDL/large IDL subclasses (the main carriers of TG in the blood) and the carotid-femoral PWV [53]. This raises the possibility that the TG content in TRLs may indirectly influence this process, considering greater small dense LDL generation in the presence of high VLDL-TG levels [54]. Another study conducted in Japan also linked higher serum RLP-C levels with increased intima-media thickness-defined carotid atherosclerosis and CAVI-defined aortic atherosclerosis [55]. However, an updated analysis of the Copenhagen General Population Study suggests that the cholesterol content in VLDL, but not the TG content, is the main contributor to atherosclerotic disease [56].
Some studies have focused on the influences of lipid-lowering agents on blood pressure. Statins could substantially reduce peripheral blood pressure (1 to 3 mmHg) [57, 58], improve arterial stiffness, and reduce central aortic pressure [59], despite the negative effects of atorvastatin on central hemodynamic parameters identified in the Conduit Artery Function Evaluation-Lipid-Lowering Arm study [60]. Considering the high comorbidity of hypertension and dyslipidemia possibly mediated by adiposity [61], the exact relationship between blood pressure and lipids should be investigated. A recent study showed that statin therapy could enhance blood pressure control in patients with hypertension, independently of the intensity of antihypertensive agents [62]. A mediation analysis also showed that LDL-C reduction after statin treatment is associated with better central blood pressure control [63]. The exact action of lipid-lowing agents on central blood pressure remains unknown, but clinicians should consider performing comprehensive measurement of central blood pressure and lipids profile in individuals with hypertension or dyslipidemia. To uncover the natural relationship of RLP-C with cSBP, participants taking lipid-lowing agents or antihypertensive medications were excluded in this study. Considering that these treated participants may have higher values of lipids or cSBP, analyses after including them were also performed, and the main findings were not substantially changed.
Study strengths and limitations
This study demonstrated the association between RLP-C and cSBP for the first time, which strengthens the association between blood lipid levels and vascular health from the viewpoint of cSBP. Among the analyzed lipid parameters, RLP-C showed the strongest association with cSBP, offering a novel research direction.
This study also has several limitations. First, central diastolic pressure and pulse pressure were not discussed because the Omron devices used in this study do not measure central diastolic pressure. Second, the enrolled participants were from one site; thus, generalizability of the findings to other populations is unclear. Third, other measures of central arterial stiffness, such as carotid-femoral PWV, were not available in this study. Fourth, despite finding no collinearity in the VIF calculations, the effects of RLP-C on cSBP might not be independent of other lipids because RLP-C has a linear relationship with other lipid fractions. Thus, routine lipid fraction tests and RLP-C assessment should be conducted simultaneously in both scientific research and clinical practice. Last, causality between RLP-C and cSBP cannot be judged because of the characteristics of a cross-sectional study; additional studies are warranted to determine whether a causal relationship exists between dyslipidemia and increased non-invasive cSBP or whether these are components of a single disease or pathological state, like metabolic syndrome.
Conclusions
Increased blood RLP-C level was significantly associated with higher cSBP in a Chinese community-based population, independently of other lipid levels. These findings emphasize the importance of RLP-C in comprehensive individual cardiovascular risk assessment. Further studies should focus on determining causality and potential mechanisms in this relationship.
Acknowledgments
We thank the entire study team for their participation and contributions. We also thank Kelly Zammit, BVSc, from Liwen Bianji (Edanz) (www.liwenbianji.cn/), for editing the English text of a draft of this manuscript.
Abbreviations
- cSBP
Central systolic blood pressure
- TRLs
Triglyceride-rich lipoproteins
- TC
Total cholesterol
- BMI
Body mass index
- TG
Triglyceride
- PWV
Pulse wave velocity
- CAVI
Cardio-ankle vascular index
- eGFR
Estimated glomerular filtration rate
- IDL
Intermediate-density lipoprotein
- VLDL
Very low-density lipoprotein
- HDL-C
High-density lipoprotein cholesterol
- DM
Diabetes mellitus
- PWA
Pulse wave analysis
- LDL-C
Low-density lipoprotein cholesterol
- RLP-C
Remnant lipoprotein cholesterol
- non-HDL-C
non-high-density lipoprotein cholesterol
- LDL
Low-density lipoprotein
- CI
confidence interval
Authors’ contributions
Y.Z. and J.Z. are responsible for the data resources and integrity. Y.Z., J.Z., B.Z. and Y.H. were in charge of the study concept and design. Y.Z., F.F., J.J., K.L., B.L., J.L. and C.C. conducted the entire study. F.F. and J.J. helped with data management and statistical analysis. K.L. analyzed the data and drafted the manuscript, and Y.Z., J.Z., Y.H., B.Z., F.F., J.J., B.L., J.L. and C.C. reviewed and revised the article. The authors read and approved the final manuscript.
Funding
University of Michigan Health Science -Peking University Health Science Centre (UMHS-PUHSC) Joint Institute for Translational and Clinical Research and the Fundamental Research Funds for the Central Universities (Grant No: BMU20110177 and BMU20160530); National Key Research and Development Project of China (Grant No. 2017YFC1307704); Key Laboratory of Molecular Cardiovascular Sciences (Peking University), Ministry of Education and National Health Commission Key Laboratory of Cardiovascular Molecular Biology and Regulatory Peptides; Chinese Cardiovascular Association- Access fund 2019-CCA-ACCESS-112.
Availability of data and materials
Data are available with reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by Peking University First Hospital Ethics Committee, and all participants provided written informed consent. The study was performed in accordance with the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Zhou and Yan Zhang contributed equally to this work.
Contributor Information
Jing Zhou, Email: zhoujing123@yahoo.com.
Yan Zhang, Email: drzhy1108@163.com.
References
- 1.Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Hypertension Lancet. 2015;386(9995):801–812. doi: 10.1016/S0140-6736(14)61468-9. [DOI] [PubMed] [Google Scholar]
- 2.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Moraes de Oliveira G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V, Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton A, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis JR, Catapano AL, Chugh S, Cooper LT, Coresh J, Criqui MH, DeCleene NK, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Sola J, Fowkes FGR, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum NJ, Koroshetz WJ, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Misganaw AT, Mokdad AH, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Oliveira GMM, Otto CM, Owolabi MO, Pratt M, Rajagopalan S, Reitsma MB, Ribeiro ALP, Rigotti NA, Rodgers A, Sable CA, Shakil SS, Sliwa K, Stark BA, Sundström J, Timpel P, Tleyjeh II, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke LJ, Abbasi-Kangevari M, Abdi A, Abedi A, Aboyans V, Abrha WA, Abu-Gharbieh E, Abushouk AI, Acharya D, Adair T, Adebayo OM, Ademi Z, Advani SM, Afshari K, Afshin A, Agarwal G, Agasthi P, Ahmad S, Ahmadi S, Ahmed MB, Aji B, Akalu Y, Akande-Sholabi W, Aklilu A, Akunna CJ, Alahdab F, al-Eyadhy A, Alhabib KF, Alif SM, Alipour V, Aljunid SM, Alla F, Almasi-Hashiani A, Almustanyir S, al-Raddadi RM, Amegah AK, Amini S, Aminorroaya A, Amu H, Amugsi DA, Ancuceanu R, Anderlini D, Andrei T, Andrei CL, Ansari-Moghaddam A, Anteneh ZA, Antonazzo IC, Antony B, Anwer R, Appiah LT, Arabloo J, Ärnlöv J, Artanti KD, Ataro Z, Ausloos M, Avila-Burgos L, Awan AT, Awoke MA, Ayele HT, Ayza MA, Azari S, B DB, Baheiraei N, Baig AA, Bakhtiari A, Banach M, Banik PC, Baptista EA, Barboza MA, Barua L, Basu S, Bedi N, Béjot Y, Bennett DA, Bensenor IM, Berman AE, Bezabih YM, Bhagavathula AS, Bhaskar S, Bhattacharyya K, Bijani A, Bikbov B, Birhanu MM, Boloor A, Brant LC, Brenner H, Briko NI, Butt ZA, Caetano dos Santos FL, Cahill LE, Cahuana-Hurtado L, Cámera LA, Campos-Nonato IR, Cantu-Brito C, Car J, Carrero JJ, Carvalho F, Castañeda-Orjuela CA, Catalá-López F, Cerin E, Charan J, Chattu VK, Chen S, Chin KL, Choi JYJ, Chu DT, Chung SC, Cirillo M, Coffey S, Conti S, Costa VM, Cundiff DK, Dadras O, Dagnew B, Dai X, Damasceno AAM, Dandona L, Dandona R, Davletov K, de la Cruz-Góngora V, de la Hoz FP, de Neve JW, Denova-Gutiérrez E, Derbew Molla M, Derseh BT, Desai R, Deuschl G, Dharmaratne SD, Dhimal M, Dhungana RR, Dianatinasab M, Diaz D, Djalalinia S, Dokova K, Douiri A, Duncan BB, Duraes AR, Eagan AW, Ebtehaj S, Eftekhari A, Eftekharzadeh S, Ekholuenetale M, el Nahas N, Elgendy IY, Elhadi M, el-Jaafary SI, Esteghamati S, Etisso AE, Eyawo O, Fadhil I, Faraon EJA, Faris PS, Farwati M, Farzadfar F, Fernandes E, Fernandez Prendes C, Ferrara P, Filip I, Fischer F, Flood D, Fukumoto T, Gad MM, Gaidhane S, Ganji M, Garg J, Gebre AK, Gebregiorgis BG, Gebregzabiher KZ, Gebremeskel GG, Getacher L, Obsa AG, Ghajar A, Ghashghaee A, Ghith N, Giampaoli S, Gilani SA, Gill PS, Gillum RF, Glushkova EV, Gnedovskaya EV, Golechha M, Gonfa KB, Goudarzian AH, Goulart AC, Guadamuz JS, Guha A, Guo Y, Gupta R, Hachinski V, Hafezi-Nejad N, Haile TG, Hamadeh RR, Hamidi S, Hankey GJ, Hargono A, Hartono RK, Hashemian M, Hashi A, Hassan S, Hassen HY, Havmoeller RJ, Hay SI, Hayat K, Heidari G, Herteliu C, Holla R, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Huang J, Humayun A, Iavicoli I, Ibeneme CU, Ibitoye SE, Ilesanmi OS, Ilic IM, Ilic MD, Iqbal U, Irvani SSN, Islam SMS, Islam RM, Iso H, Iwagami M, Jain V, Javaheri T, Jayapal SK, Jayaram S, Jayawardena R, Jeemon P, Jha RP, Jonas JB, Jonnagaddala J, Joukar F, Jozwiak JJ, Jürisson M, Kabir A, Kahlon T, Kalani R, Kalhor R, Kamath A, Kamel I, Kandel H, Kandel A, Karch A, Kasa AS, Katoto PDMC, Kayode GA, Khader YS, Khammarnia M, Khan MS, Khan MN, Khan M, Khan EA, Khatab K, Kibria GMA, Kim YJ, Kim GR, Kimokoti RW, Kisa S, Kisa A, Kivimäki M, Kolte D, Koolivand A, Korshunov VA, Koulmane Laxminarayana SL, Koyanagi A, Krishan K, Krishnamoorthy V, Kuate Defo B, Kucuk Bicer B, Kulkarni V, Kumar GA, Kumar N, Kurmi OP, Kusuma D, Kwan GF, la Vecchia C, Lacey B, Lallukka T, Lan Q, Lasrado S, Lassi ZS, Lauriola P, Lawrence WR, Laxmaiah A, LeGrand KE, Li MC, Li B, Li S, Lim SS, Lim LL, Lin H, Lin Z, Lin RT, Liu X, Lopez AD, Lorkowski S, Lotufo PA, Lugo A, M NK, Madotto F, Mahmoudi M, Majeed A, Malekzadeh R, Malik AA, Mamun AA, Manafi N, Mansournia MA, Mantovani LG, Martini S, Mathur MR, Mazzaglia G, Mehata S, Mehndiratta MM, Meier T, Menezes RG, Meretoja A, Mestrovic T, Miazgowski B, Miazgowski T, Michalek IM, Miller TR, Mirrakhimov EM, Mirzaei H, Moazen B, Moghadaszadeh M, Mohammad Y, Mohammad DK, Mohammed S, Mohammed MA, Mokhayeri Y, Molokhia M, Montasir AA, Moradi G, Moradzadeh R, Moraga P, Morawska L, Moreno Velásquez I, Morze J, Mubarik S, Muruet W, Musa KI, Nagarajan AJ, Nalini M, Nangia V, Naqvi AA, Narasimha Swamy S, Nascimento BR, Nayak VC, Nazari J, Nazarzadeh M, Negoi RI, Neupane Kandel S, Nguyen HLT, Nixon MR, Norrving B, Noubiap JJ, Nouthe BE, Nowak C, Odukoya OO, Ogbo FA, Olagunju AT, Orru H, Ortiz A, Ostroff SM, Padubidri JR, Palladino R, Pana A, Panda-Jonas S, Parekh U, Park EC, Parvizi M, Pashazadeh Kan F, Patel UK, Pathak M, Paudel R, Pepito VCF, Perianayagam A, Perico N, Pham HQ, Pilgrim T, Piradov MA, Pishgar F, Podder V, Polibin RV, Pourshams A, Pribadi DRA, Rabiee N, Rabiee M, Radfar A, Rafiei A, Rahim F, Rahimi-Movaghar V, Ur Rahman MH, Rahman MA, Rahmani AM, Rakovac I, Ram P, Ramalingam S, Rana J, Ranasinghe P, Rao SJ, Rathi P, Rawal L, Rawasia WF, Rawassizadeh R, Remuzzi G, Renzaho AMN, Rezapour A, Riahi SM, Roberts-Thomson RL, Roever L, Rohloff P, Romoli M, Roshandel G, Rwegerera GM, Saadatagah S, Saber-Ayad MM, Sabour S, Sacco S, Sadeghi M, Saeedi Moghaddam S, Safari S, Sahebkar A, Salehi S, Salimzadeh H, Samaei M, Samy AM, Santos IS, Santric-Milicevic MM, Sarrafzadegan N, Sarveazad A, Sathish T, Sawhney M, Saylan M, Schmidt MI, Schutte AE, Senthilkumaran S, Sepanlou SG, Sha F, Shahabi S, Shahid I, Shaikh MA, Shamali M, Shamsizadeh M, Shawon MSR, Sheikh A, Shigematsu M, Shin MJ, Shin JI, Shiri R, Shiue I, Shuval K, Siabani S, Siddiqi TJ, Silva DAS, Singh JA, Mtech AS, Skryabin VY, Skryabina AA, Soheili A, Spurlock EE, Stockfelt L, Stortecky S, Stranges S, Suliankatchi Abdulkader R, Tadbiri H, Tadesse EG, Tadesse DB, Tajdini M, Tariqujjaman M, Teklehaimanot BF, Temsah MH, Tesema AK, Thakur B, Thankappan KR, Thapar R, Thrift AG, Timalsina B, Tonelli M, Touvier M, Tovani-Palone MR, Tripathi A, Tripathy JP, Truelsen TC, Tsegay GM, Tsegaye GW, Tsilimparis N, Tusa BS, Tyrovolas S, Umapathi KK, Unim B, Unnikrishnan B, Usman MS, Vaduganathan M, Valdez PR, Vasankari TJ, Velazquez DZ, Venketasubramanian N, Vu GT, Vujcic IS, Waheed Y, Wang Y, Wang F, Wei J, Weintraub RG, Weldemariam AH, Westerman R, Winkler AS, Wiysonge CS, Wolfe CDA, Wubishet BL, Xu G, Yadollahpour A, Yamagishi K, Yan LL, Yandrapalli S, Yano Y, Yatsuya H, Yeheyis TY, Yeshaw Y, Yilgwan CS, Yonemoto N, Yu C, Yusefzadeh H, Zachariah G, Zaman SB, Zaman MS, Zamanian M, Zand R, Zandifar A, Zarghi A, Zastrozhin MS, Zastrozhina A, Zhang ZJ, Zhang Y, Zhang W, Zhong C, Zou Z, Zuniga YMH, Murray CJL, Fuster V. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng HM, Chuang SY, Wang TD, Kario K, Buranakitjaroen P, Chia YC, Divinagracia R, Hoshide S, Minh HV, Nailes J, Park S, Shin J, Siddique S, Sison J, Soenarta AA, Sogunuru GP, Sukonthasarn A, Tay JC, Teo BW, Turana Y, Verma N, Zhang Y, Wang JG, Chen CH. Central blood pressure for the management of hypertension: is it a practical clinical tool in current practice? J Clin Hypertens (Greenwich) 2020;22(3):391–406. doi: 10.1111/jch.13758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31(15):1865–1871. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 5.Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, Yin FCP, Chou P, Chen CH. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens. 2009;27(3):461–467. doi: 10.1097/HJH.0b013e3283220ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chi C, Yu X, Auckle R, Lu Y, Fan X, Yu S, Xiong J, Bai B, Teliewubai J, Zhou Y, Ji H, Li J, Zhang Y, Xu Y. Hypertensive target organ damage is better associated with central than brachial blood pressure: the northern Shanghai study. J Clin Hypertens (Greenwich) 2017;19(12):1269–1275. doi: 10.1111/jch.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kollias A, Lagou S, Zeniodi ME, Boubouchairopoulou N, Stergiou GS. Association of Central Versus Brachial Blood Pressure with Target-Organ Damage: systematic review and meta-analysis. Hypertension. 2016;67(1):183–190. doi: 10.1161/HYPERTENSIONAHA.115.06066. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson IB, Prasad K, Hall IR, Thomas A, MacCallum H, Webb DJ, Frenneaux MP, Cockcroft JR. Increased central pulse pressure and augmentation index in subjects with hypercholesterolemia. J Am Coll Cardiol. 2002;39(6):1005–1011. doi: 10.1016/S0735-1097(02)01723-0. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara LA, Guida L, Iannuzzi R, Celentano A, Lionello F. Serum cholesterol affects blood pressure regulation. J Hum Hypertens. 2002;16(5):337–343. doi: 10.1038/sj.jhh.1001388. [DOI] [PubMed] [Google Scholar]
- 10.Mancia G, Facchetti R, Bombelli M, Polo Friz H, Grassi G, Giannattasio C, et al. Relationship of office, home, and ambulatory blood pressure to blood glucose and lipid variables in the PAMELA population. Hypertension. 2005;45(6):1072–1077. doi: 10.1161/01.HYP.0000165672.69176.ed. [DOI] [PubMed] [Google Scholar]
- 11.Yan Z, Bi-Rong D, Hui W, Chang-Quan H. Serum lipid/lipoprotein and arterial blood pressure among Chinese nonagenarians/centenarians. Blood Press. 2011;20(5):296–302. doi: 10.3109/08037051.2011.572590. [DOI] [PubMed] [Google Scholar]
- 12.Castaner O, Pinto X, Subirana I, Amor AJ, Ros E, Hernaez A, et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J Am Coll Cardiol. 2020;76(23):2712–2724. doi: 10.1016/j.jacc.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Joshi PH, Khokhar AA, Massaro JM, Lirette ST, Griswold ME, Martin SS, et al. Remnant Lipoprotein Cholesterol and Incident Coronary Heart Disease: The Jackson Heart and Framingham Offspring Cohort Studies. J Am Heart Assoc. 2016;5(5). 10.1161/JAHA.115.002765. [DOI] [PMC free article] [PubMed]
- 14.Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Boren J, Catapano AL, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32(11):1345–1361. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasahara A, Adachi H, Hirai Y, Enomoto M, Fukami A, Yoshikawa K, Esaki E, Yokoi K, Ogata K, Tsukagawa E, Obuchi A, Yoshimura A, Nakamura S, Imaizumi T. High level of plasma remnant-like particle cholesterol may predispose to development of hypertension in normotensive subjects. Am J Hypertens. 2013;26(6):793–798. doi: 10.1093/ajh/hpt011. [DOI] [PubMed] [Google Scholar]
- 16.Fan F, Qi L, Jia J, Xu X, Liu Y, Yang Y, Qin X, Li J, Li H, Zhang Y, Huo Y. Noninvasive central systolic blood pressure is more strongly related to kidney function decline than peripheral systolic blood pressure in a Chinese community-based population. Hypertension. 2016;67(6):1166–1172. doi: 10.1161/HYPERTENSIONAHA.115.07019. [DOI] [PubMed] [Google Scholar]
- 17.Momin M, Fan F, Li J, Qin X, Jia J, Qi L, Zhang Y, Huo Y. Associations of plasma homocysteine levels with peripheral systolic blood pressure and noninvasive central systolic blood pressure in a community-based Chinese population. Sci Rep. 2017;7(1):6316. doi: 10.1038/s41598-017-06611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan F, Jia J, Li J, Huo Y, Zhang Y. White blood cell count predicts the odds of kidney function decline in a Chinese community-based population. BMC Nephrol. 2017;18(1):190. doi: 10.1186/s12882-017-0608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Zhou Z, Fan F, Qi L, Jia J, Sun P, Jiang Y, Kou M, Chen D, Zhang Y, Huo Y. Joint effect of non-invasive central systolic blood pressure and peripheral systolic blood pressure on incident hypertension in a Chinese community-based population. Sci Rep. 2018;8(1):3229. doi: 10.1038/s41598-018-21023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takazawa K, Kobayashi H, Kojima I, Aizawa A, Kinoh M, Sugo Y, Shimizu M, Miyawaki Y, Tanaka N, Yamashina A, Avolio A. Estimation of central aortic systolic pressure using late systolic inflection of radial artery pulse and its application to vasodilator therapy. J Hypertens. 2012;30(5):908–916. doi: 10.1097/HJH.0b013e3283524910. [DOI] [PubMed] [Google Scholar]
- 21.Takazawa K, Kobayashi H, Shindo N, Tanaka N, Yamashina A. Relationship between radial and central arterial pulse wave and evaluation of central aortic pressure using the radial arterial pulse wave. Hypertens Res. 2007;30(3):219–228. doi: 10.1291/hypres.30.219. [DOI] [PubMed] [Google Scholar]
- 22.Ding FH, Fan WX, Zhang RY, Zhang Q, Li Y, Wang JG. Validation of the noninvasive assessment of central blood pressure by the SphygmoCor and Omron devices against the invasive catheter measurement. Am J Hypertens. 2011;24(12):1306–1311. doi: 10.1038/ajh.2011.145. [DOI] [PubMed] [Google Scholar]
- 23.Wohlfahrt P, Krajcoviechova A, Seidlerova J, Mayer O, Filipovsky J, Cifkova R. Comparison of noninvasive assessments of central blood pressure using general transfer function and late systolic shoulder of the radial pressure wave. Am J Hypertens. 2014;27(2):162–168. doi: 10.1093/ajh/hpt166. [DOI] [PubMed] [Google Scholar]
- 24.Kips JG, Schutte AE, Vermeersch SJ, Huisman HW, Van Rooyen JM, Glyn MC, et al. Comparison of central pressure estimates obtained from SphygmoCor, Omron HEM-9000AI and carotid applanation tonometry. J Hypertens. 2011;29(6):1115–1120. doi: 10.1097/HJH.0b013e328346a3bc. [DOI] [PubMed] [Google Scholar]
- 25.Yeom H, Kim HC, Lee JM, Jeon Y, Suh I. Triglyceride to high density lipoprotein cholesterol ratio among adolescents is associated with adult hypertension: the Kangwha study. Lipids Health Dis. 2018;17(1):212. doi: 10.1186/s12944-018-0861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adank MC, Benschop L, Peterbroers KR, Smak Gregoor AM, Kors AW, Mulder MT, et al. Is maternal lipid profile in early pregnancy associated with pregnancy complications and blood pressure in pregnancy and long term postpartum? Am J Obstet Gynecol. 2019;221(2):150. doi: 10.1016/j.ajog.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Stancu CS, Toma L, Sima AV. Dual role of lipoproteins in endothelial cell dysfunction in atherosclerosis. Cell Tissue Res. 2012;349(2):433–446. doi: 10.1007/s00441-012-1437-1. [DOI] [PubMed] [Google Scholar]
- 28.Zheng XY, Liu L. Remnant-like lipoprotein particles impair endothelial function: direct and indirect effects on nitric oxide synthase. J Lipid Res. 2007;48(8):1673–1680. doi: 10.1194/jlr.R700001-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Doi H, Kugiyama K, Oka H, Sugiyama S, Ogata N, Koide SI, Nakamura SI, Yasue H. Remnant lipoproteins induce proatherothrombogenic molecules in endothelial cells through a redox-sensitive mechanism. Circulation. 2000;102(6):670–676. doi: 10.1161/01.CIR.102.6.670. [DOI] [PubMed] [Google Scholar]
- 30.Borghi C, Urso R, Cicero AF. Renin-angiotensin system at the crossroad of hypertension and hypercholesterolemia. Nutr Metab Cardiovasc Dis. 2017;27(2):115–120. doi: 10.1016/j.numecd.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Kurtel H, Rodrigues SF, Yilmaz CE, Yildirim A, Granger DN. Impaired vasomotor function induced by the combination of hypertension and hypercholesterolemia. J Am Soc Hypertens. 2013;7(1):14–23. doi: 10.1016/j.jash.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sposito AC. Emerging insights into hypertension and dyslipidaemia synergies. Eur Heart J Suppl. 2004;6(7):G8–G12. doi: 10.1016/j.ehjsup.2004.10.003. [DOI] [Google Scholar]
- 33.Ivanovic B, Tadic M. Hypercholesterolemia and hypertension: two sides of the same coin. Am J Cardiovasc Drugs. 2015;15(6):403–414. doi: 10.1007/s40256-015-0128-1. [DOI] [PubMed] [Google Scholar]
- 34.Kabutoya T, Kario K. Comparative assessment of cutoffs for the cardio-ankle vascular index and brachial-ankle pulse wave velocity in a Nationwide registry: a cardiovascular prognostic coupling study. Pulse (Basel) 2019;6(3–4):131–136. doi: 10.1159/000489604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salvi P, Scalise F, Rovina M, Moretti F, Salvi L, Grillo A, Gao L, Baldi C, Faini A, Furlanis G, Sorropago A, Millasseau SC, Sorropago G, Carretta R, Avolio AP, Parati G. Noninvasive estimation of aortic stiffness through different approaches. Hypertension. 2019;74(1):117–129. doi: 10.1161/HYPERTENSIONAHA.119.12853. [DOI] [PubMed] [Google Scholar]
- 36.Namba T, Masaki N, Takase B, Adachi T. Arterial Stiffness Assessed by Cardio-Ankle Vascular Index. Int J Mol Sci. 2019;20(15). 10.3390/ijms20153664. [DOI] [PMC free article] [PubMed]
- 37.Pavlovska I, Kunzova S, Jakubik J, Hruskova J, Skladana M, Rivas-Serna IM, Medina-Inojosa JR, Lopez-Jimenez F, Vysoky R, Geda YE, Stokin GB, González-Rivas JP. Associations between high triglycerides and arterial stiffness in a population-based sample: Kardiovize Brno 2030 study. Lipids Health Dis. 2020;19(1):170. doi: 10.1186/s12944-020-01345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhan B, Huang X, Wang J, Qin X, Zhang J, Cao J, Song Y, Liu L, Li P, Yang R, Wu Y, Wu Q, Zhang Y, Li J, Huo Y, Wang B, Xu X, Bao H, Cheng X. Association between lipid profiles and arterial stiffness in Chinese patients with hypertension: insights from the CSPPT. Angiology. 2019;70(6):515–522. doi: 10.1177/0003319718823341. [DOI] [PubMed] [Google Scholar]
- 39.Gottsater M, Ostling G, Persson M, Engstrom G, Melander O, Nilsson PM. Non-hemodynamic predictors of arterial stiffness after 17 years of follow-up: the Malmo diet and Cancer study. J Hypertens. 2015;33(5):957–965. doi: 10.1097/HJH.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang F, Ye P, Luo L, Xiao W, Qi L, Bian S, Wu H, Sheng L, Xiao T, Xu R. Association of serum lipids with arterial stiffness in a population-based study in Beijing. Eur J Clin Investig. 2011;41(9):929–936. doi: 10.1111/j.1365-2362.2011.02481.x. [DOI] [PubMed] [Google Scholar]
- 41.White DA, Anand GM, Qayum O, Ibezim CF, Sherman AK, Raghuveer G. Modifiable clinical correlates of vascular health in children and adolescents with dyslipidemia. Pediatr Cardiol. 2019;40(4):805–812. doi: 10.1007/s00246-019-02071-w. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Zhi F, Gao B, Ni J, Liu Y, Mo X, Huang J. Association between lipid profiles and arterial stiffness: a secondary analysis based on a cross-sectional study. J Int Med Res. 2020;48(7):300060520938188. doi: 10.1177/0300060520938188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrier KE, Muhlmann MH, Baguet JP, Cameron JD, Jennings GL, Dart AM, Kingwell BA. Intensive cholesterol reduction lowers blood pressure and large artery stiffness in isolated systolic hypertension. J Am Coll Cardiol. 2002;39(6):1020–1025. doi: 10.1016/S0735-1097(02)01717-5. [DOI] [PubMed] [Google Scholar]
- 44.Sahebkar A, Pecin I, Tedeschi-Reiner E, Derosa G, Maffioli P, Reiner Z. Effects of statin therapy on augmentation index as a measure of arterial stiffness: a systematic review and meta-analysis. Int J Cardiol. 2016;212:160–168. doi: 10.1016/j.ijcard.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Townsend RR, Rosendorff C, Nichols WW, Edwards DG, Chirinos JA, Fernhall B, Cushman WC. American Society of Hypertension position paper: central blood pressure waveforms in health and disease. J Am Soc Hypertens. 2016;10(1):22–33. doi: 10.1016/j.jash.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Choudhary MK, Eraranta A, Tikkakoski AJ, Koskela J, Hautaniemi EJ, Kahonen M, et al. LDL cholesterol is associated with systemic vascular resistance and wave reflection in subjects naive to cardiovascular drugs. Blood Press. 2019;28(1):4–14. doi: 10.1080/08037051.2018.1521263. [DOI] [PubMed] [Google Scholar]
- 47.Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev. 2019;40(2):537–557. doi: 10.1210/er.2018-00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salinas CAA, Chapman MJ. Remnant lipoproteins: are they equal to or more atherogenic than LDL? Curr Opin Lipidol. 2020;31(3):132–139. doi: 10.1097/MOL.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 49.Varbo A, Nordestgaard BG. Remnant lipoproteins. Curr Opin Lipidol. 2017;28(4):300–307. doi: 10.1097/MOL.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 50.Saeed A, Feofanova EV, Yu B, Sun W, Virani SS, Nambi V, Coresh J, Guild CS, Boerwinkle E, Ballantyne CM, Hoogeveen RC. Remnant-like particle cholesterol, low-density lipoprotein triglycerides, and incident cardiovascular disease. J Am Coll Cardiol. 2018;72(2):156–169. doi: 10.1016/j.jacc.2018.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vallejo-Vaz AJ, Fayyad R, Boekholdt SM, Hovingh GK, Kastelein JJ, Melamed S, Barter P, Waters DD, Ray KK. Triglyceride-rich lipoprotein cholesterol and risk of cardiovascular events among patients receiving statin therapy in the TNT trial. Circulation. 2018;138(8):770–781. doi: 10.1161/CIRCULATIONAHA.117.032318. [DOI] [PubMed] [Google Scholar]
- 52.Varbo A, Benn M, Nordestgaard BG. Remnant cholesterol as a cause of ischemic heart disease: evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol Ther. 2014;141(3):358–367. doi: 10.1016/j.pharmthera.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Hartz J, Krauss RM, Gottsater M, Melander O, Nilsson P, Mietus-Snyder M. Lipoprotein particle predictors of arterial stiffness after 17 years of follow up: the Malmo diet and Cancer study. Int J Vasc Med. 2020;2020:4219180. doi: 10.1155/2020/4219180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boren J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European atherosclerosis society consensus panel. Eur Heart J. 2020;41(24):2313–2330. doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taguchi M, Ishigami M, Nishida M, Moriyama T, Yamashita S, Yamamura T. Remnant lipoprotein-cholesterol is a predictive biomarker for large artery atherosclerosis in apparently healthy women: usefulness as a parameter for annual health examinations. Ann Clin Biochem. 2011;48(Pt 4):332–337. doi: 10.1258/acb.2011.010244. [DOI] [PubMed] [Google Scholar]
- 56.Balling M, Afzal S, Varbo A, Langsted A, Davey Smith G, Nordestgaard BG. VLDL cholesterol accounts for one-half of the risk of myocardial infarction associated with apoB-containing lipoproteins. J Am Coll Cardiol. 2020;76(23):2725–2735. doi: 10.1016/j.jacc.2020.09.610. [DOI] [PubMed] [Google Scholar]
- 57.Strazzullo P, Kerry SM, Barbato A, Versiero M, D'Elia L, Cappuccio FP. Do statins reduce blood pressure?: a meta-analysis of randomized, controlled trials. Hypertension. 2007;49(4):792–798. doi: 10.1161/01.HYP.0000259737.43916.42. [DOI] [PubMed] [Google Scholar]
- 58.Golomb BA, Dimsdale JE, White HL, Ritchie JB, Criqui MH. Reduction in blood pressure with statins: results from the UCSD statin study, a randomized trial. Arch Intern Med. 2008;168(7):721–727. doi: 10.1001/archinte.168.7.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanaki AI, Sarafidis PA, Georgianos PI, Kanavos K, Tziolas IM, Zebekakis PE, Lasaridis AN. Effects of low-dose atorvastatin on arterial stiffness and central aortic pressure augmentation in patients with hypertension and hypercholesterolemia. Am J Hypertens. 2013;26(5):608–616. doi: 10.1093/ajh/hps098. [DOI] [PubMed] [Google Scholar]
- 60.Williams B, Lacy PS, Cruickshank JK, Collier D, Hughes AD, Stanton A, Thom S, Thurston H, CAFE and ASCOT Investigators Impact of statin therapy on central aortic pressures and hemodynamics: principal results of the conduit artery function evaluation-lipid-lowering arm (CAFE-LLA) study. Circulation. 2009;119(1):53–61. doi: 10.1161/CIRCULATIONAHA.108.785915. [DOI] [PubMed] [Google Scholar]
- 61.Spannella F, Giulietti F, Di Pentima C, Sarzani R. Prevalence and control of dyslipidemia in patients referred for high blood pressure: the disregarded "double-trouble" lipid profile in overweight/obese. Adv Ther. 2019;36(6):1426–1437. doi: 10.1007/s12325-019-00941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spannella F, Filipponi A, Giulietti F, Di Pentima C, Bordoni V, Sarzani R. Statin therapy is associated with better ambulatory blood pressure control: a propensity score analysis. J Hypertens. 2020;38(3):546–552. doi: 10.1097/HJH.0000000000002276. [DOI] [PubMed] [Google Scholar]
- 63.Lamarche F, Agharazii M, Nadeau-Fredette AC, Madore F, Goupil R. Central and brachial blood pressures, statins, and low-density lipoprotein cholesterol: a mediation analysis. Hypertension. 2018;71(3):415–421. doi: 10.1161/HYPERTENSIONAHA.117.10476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available with reasonable request.