Abstract
The changes in brain function that perpetuate opiate addiction are unclear. In our studies of human narcolepsy, a disease caused by loss of immunohistochemically detected hypocretin (orexin) neurons, we encountered a control brain (from an apparently neurologically normal individual) with 50% more hypocretin neurons than other control human brains that we had studied. We discovered that this individual was a heroin addict. Studying five postmortem brains from heroin addicts, we report that the brain tissue had, on average, 54% more immunohistochemically detected neurons producing hypocretin than did control brains from neurologically normal subjects. Similar increases in hypocretin-producing cells could be induced in wild-type mice by long-term (but not short-term) administration of morphine. The increased number of detected hypocretin neurons was not due to neurogenesis and outlasted morphine administration by several weeks. The number of neurons containing melanin-concentrating hormone, which are in the same hypothalamic region as hypocretin-producing cells, did not change in response to morphine administration. Morphine administration restored the population of detected hypocretin cells to normal numbers in transgenic mice in which these neurons had been partially depleted. Morphine administration also decreased cataplexy in mice made narcoleptic by the depletion of hypocretin neurons. These findings suggest that opiate agonists may have a role in the treatment of narcolepsy, a disorder caused by hypocretin neuron loss, and that increased numbers of hypocretin-producing cells may play a role in maintaining opiate addiction.
One-sentence summary:
Heroin addiction is accompanied by an increase in detected hypocretin (orexin) neurons, and in narcoleptic mice deficient in these neurons, morphine can reverse cataplexy.
Editor’s Summary:
Opiate addiction and narcolepsy: Opposite sides of the same coin?
The neurological mechanisms that maintain opiate addiction and prevent long-term withdrawal are not well understood. In a new study, Thannickal et al. found that human heroin addicts have, on average, 54% more hypocretin-producing neurons than do neurologically normal control individuals. They show that a similar increase in hypocretin-producing neurons could be induced in mice through long-term morphine administration. This long-lasting increase in hypocretin neurons may be responsible for maintaining addiction. Narcolepsy is caused by a loss of hypocretin-producing neurons. Morphine administration restored the population of hypocretin neurons in hypocretin cell–depleted mice back to normal numbers and decreased cataplexy in narcoleptic animals. Induction of specific long-term changes in neuropeptide production, outlasting the half-life of the administered drugs, may be useful in treating diseases characterized by loss of neurons producing these neuropeptides.
INTRODUCTION
The annual rate of opiate overdose deaths in the United States has increased fourfold in the last 17 years, with more than 300,000 deaths since 2000. Addicts are unable to overcome opiate addiction, even as they realize the destructive effect it is having on them and their families (1). Prior work suggests that hypocretin cell activity is linked to pleasure-mediated arousal and, hence, may play a role in perpetuating addiction (2–4).
Previously, we reported that human narcolepsy is linked to, on average, a 90% loss of immunohistochemically identified hypocretin-producing cells (5). More recently, we reported a decreased number of immunohistochemically identified hypocretin neurons in Parkinson’s disease (6, 7). In analyzing postmortem brain tissue from neurologically normal control individuals, we unexpectedly encountered a brain with 50% more hypocretin-producing neurons than we had found in any of the other control human brain tissues we had studied. Further investigation revealed that this individual was a former heroin addict.
Here, we acquired postmortem brain tissue from four additional human heroin addicts. We saw a greatly elevated number of hypocretin-producing cells in the brain of each addict. We then explored whether administering morphine to wild-type mice would produce a similar increase in hypocretin-producing cells, what the effects of the dose and schedule of administration effects were on the magnitude of the increase, and how long the changes in hypocretin neuron number and cell size persisted after the cessation of administration. We then exploited the finding of an increase in hypocretin cells induced by opiate administration in mice to see whether morphine might have beneficial effects in a mouse model of narcolepsy where hypocretin cell number had been reduced to mimic the low number of hypocretin cells found in human narcolepsy.
RESULTS
An increased number of immunohistochemically positive hypocretin (orexin) neurons in the brains of human heroin addicts
There was an average increase of 54% in the number of detectable hypocretin-producing neurons in the postmortem brain tissue from five human heroin addicts relative to matched control brains [P = 0.0009, t = 8.89, df = 10 (t test); Table 1 and Fig. 1A]. The range of increase in the addicts was 36 to 74% above mean control values. Hypocretin-producing cells were found to be 22% smaller in the brains of addicts compared to controls [P < 0.01, t = 2.78, df = 10 (t test); Fig. 1, B and D]. About 90% of hypocretin-producing neurons in the brains of human control subjects also contained Narp (neuronal activity–regulated pentraxin), a secreted neuronal pentraxin that co-localizes with hypocretin in hypothalamic neurons (8). This percentage was similar in the larger number of hypocretin neurons observed in human heroin addicts (Fig. 1E).
Table 1. Characteristics of addicts, narcoleptic patients, and control subjects.
PMI, postmortem interval; avg, average; MI, myocardial infarction; N/A, not available; SLE, systemic lupus erythematosus.
| Subject | Sex | Age | Cause of death | Medications | PMI | pH | Years in fix | Estimated hypocretin cell no. | |
|---|---|---|---|---|---|---|---|---|---|
| Addicts (A) | |||||||||
| A-1 | M | 38 | Heroin intoxication | Oxycodone abuse, smoked 1 ½ packs per day | 20.0 | 6.84 | 3.5 | 121,173 | |
| A-2 | F | 26 | Asthma complicating Narc intoxication | Heroin, cocaine, methadone (110 g) for 8 months, nonsmoker | 4.0 | 6.63 | 3.5 | 130,719 | |
| A-3 | M | 72 | Cancer | Former heroin addict, morphine alcohol “comfort” 4 days before death | 19.0 | 3.6 | 111,466 | ||
| A-4 | M | 28 | Methadone intoxication and cocaine use | Heroin, cocaine, methadone | 13.0 | 5.52 | 3 | 123,810 | |
| A-5 | F | 19 | Methadone intoxication | High-dose drug abuse | 24.0 | 5.45 | 3 | 141,717 | Avg: 125,777 |
| Control subjects for addicts (C) | |||||||||
| C-1 | F | 42 | Breast cancer | 15.0 | N/A | 1.0 | 84,546 | ||
| C-2 | M | 32 | MI, aneurism | N/A | N/A | 8.0 | 89,652 | ||
| C-3 | M | 61 | Pneumonia, testicular tumor | N/A | N/A | 8.0 | 65,833 | ||
| C-4 | F | 44 | Breast cancer | N/A | N/A | 8.0 | 86,354 | ||
| C-5 | F | 27 | Mild hypoxia, arterial calcification, congenital heart defect, coronary artery atherosclerosis | N/A | N/A | 1.5 | 78,656 | ||
| C-6 | F | 33 | SLE, sepsis | N/A | N/A | 1.5 | 76,921 | ||
| C-7 | M | 29 | Leukemia, bacterial sepsis, hypoxia | N/A | N/A | 1.5 | 89,732 | Avg: 81,671 | |
| Control subjects for narcolepsy | |||||||||
| NBB-00-022 | F | 83 | Acute MI | 7.5 | 6.52 | 0.1 | 106,366 | ||
| NBB-00-072 | M | 78 | Kidney failure | 18.0 | 5.84 | 0.1 | 88,404 | ||
| NBB-00-142 | F | 82 | MI | 5.3 | 6.6 | 0.1 | 70,976 | Avg: 88,582 | |
| Narcolepsy with cataplexy | |||||||||
| NBB-08023 | F | 66 | Heart failure | Amphetamine, 5.45 mg, 1 time/day | |||||
| Modafinil, 100 mg, 3 times/day | 7.0 | 6.57 | 0.1 | 3276 | 3.6% control | ||||
| Sodium oxybate at 500 mg/ml, 2 times 6 ml/day | |||||||||
| Narcolepsy with cataplexy morphine-treated hypersomnia | |||||||||
| NBB-01064 | F | 85 | Chronic pain syndrome with palliative sedation | Morphine, 10 mg, 2 times/day (9 years) | 3.4 | 6.77 | 0.1 | 16,834 | 19% control |
| Modafinil, 200 mg, 1–2 times/day | |||||||||
Fig. 1. Postmortem brain tissue from heroin addicts shows an increased number of hypocretin-producing neurons.
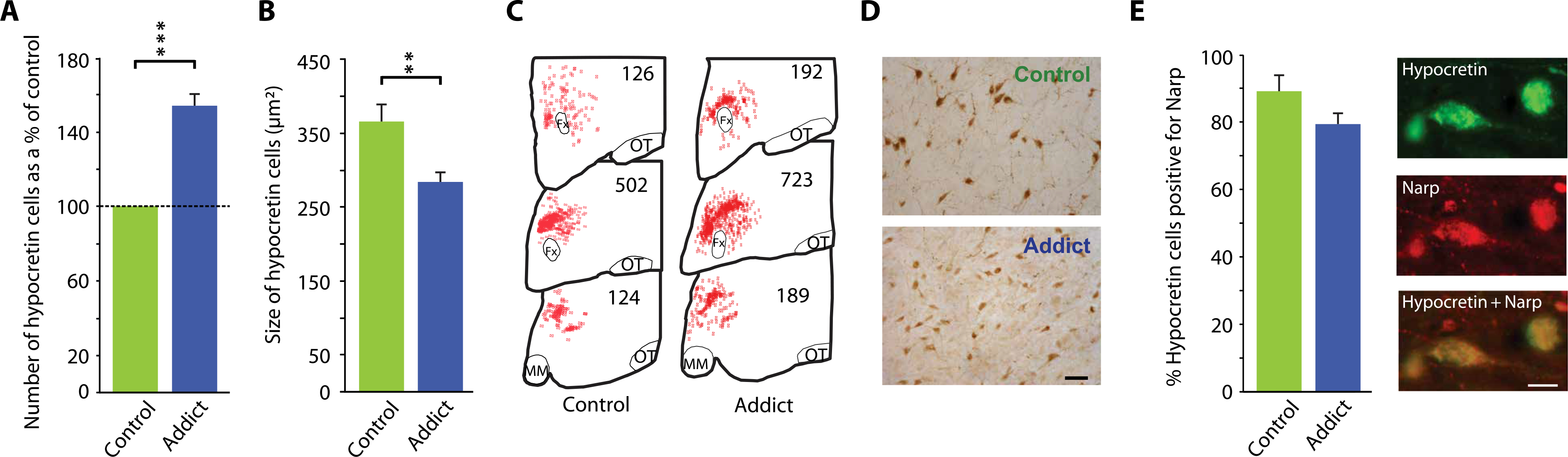
Subject characteristics are presented in Table 1. (A) Immunohistochemistry showed that there was a 54% increase in the number of detectable hypocretin neurons in hypothalamic brain tissue from human heroin addicts (n = 5) relative to hypothalamic tissue from human control subjects [n = 7; P = 0.0009, t = 8.89, df = 10 (t test)]. (B) Immunohistochemical staining of postmortem brain tissue showed that hypocretin cells were 22% smaller in brain tissue from heroin addicts compared to control subjects [P < 0.01, t = 2.78, df = 10 (t test)]. (C) Neurolucida mapping illustrates the distribution and increased number of hypocretin cells in brain tissue from heroin addicts relative to control subjects. Representative counts are given at three anterior-posterior positions: OT, optic tract; MM, mammillary bodies; Fx, fornix. (D) A representative example of immunohistochemical labeling of hypocretin cells in brain tissue from control individuals and heroin addicts is shown. Scale bar, 50 μm. (E) Left: Immunohistochemical staining showing that about 90% of hypocretin neurons in human brain tissue from control individuals also contained the neuropeptide Narp. This percentage did not significantly differ in brain tissue from human heroin addicts. Right: Representative images of immunohistochemical double staining for hypocretin (Hcrt) and Narp in hypothalamus of brain tissue from a heroin addict. ***P < 0.001, **P < 0.01, t test, compared to control.
Dose-dependent effects of morphine administration on hypocretin-producing cells in the brains of wild-type mice
To determine whether the differences in the number and size of hypocretin-producing neuron somas seen between human heroin addicts and controls might be induced by opiates, we administered morphine to mice at several doses for varying time periods. Subcutaneous injection of fixed and escalating doses of morphine for 7 days did not significantly change the number of hypocretin cells detected in the mouse brain. However, 14-day administration of either dose schedule increased hypocretin cell number relative to saline injection [P = 0.01, t = 4.23, df = 4 and P = 0.01, t = 4.52, df = 4 (t tests), respectively; Fig. 2A]. Fixed morphine doses of 10 mg/kg or greater for a single 2-week period increased hypocretin cell number [F7,16 = 8.1, P < 0.001, analysis of variance (ANOVA)] with a maximal average increase of 38% at the 50 mg/kg dose (Fig. 2B). With daily dosing for a 60-day period, the magnitude of changes in hypocretin cell number was smaller than that after 14 days of morphine administration (F3,12 = 13.5, P < 0.001, ANOVA, normality, variance tests passed between groups; Fig. 2C). The effect on hypocretin cell number was largest in the lateral hypothalamus (LH), but an increase in hypocretin cell number was also seen in the medial hypothalamus (MH) [LH: P = 0.001, t = 11.94, df = 7; MH: P = 0.05, t = 2.44, df = 7 (t tests); Fig. 2D]. After 14 days of administration of morphine (50 mg/kg), hypocretin cell number remained elevated for an additional 4 to 8 weeks [P = 0.001, t = 6.13, df = 8 (t test, ANOVA, normality, variance tests passed between groups), F5,22 = 9.4, P < 0.001; Fig. 2E]. After 60 days of administration of the same morphine dose (bottom), the elevation of hypocretin cell number persisted for an additional 2 weeks after the end of drug administration [F5,22 = 6.3, P < 0.001 (ANOVA, normality, variance tests passed between groups); Fig. 2E]. Figure 2F shows the time course of recovery from hypocretin cell shrinkage after daily administration of morphine (50 mg/kg) or saline to five experimental and five control mice for 2 weeks. Hypocretin cell size increased toward control values over the 4-week period after cessation of morphine administration [F5,58 = 5.3, P < 0.0005 (ANOVA); Fig. 2F]. The increased number of hypocretin-producing cells was within the regions containing hypocretin cells under baseline conditions [P = 0.001, t = 4.88, df = 10; Fig. 2G]. There was no change in the percentage of Narp-containing cells within the increased number of hypocretin-producing cells in mice treated with morphine (50 mg/kg) for 14 days (Fig. 2H).
Fig. 2. Dose-dependent effects of morphine administration on hypocretin cells in mouse brain.
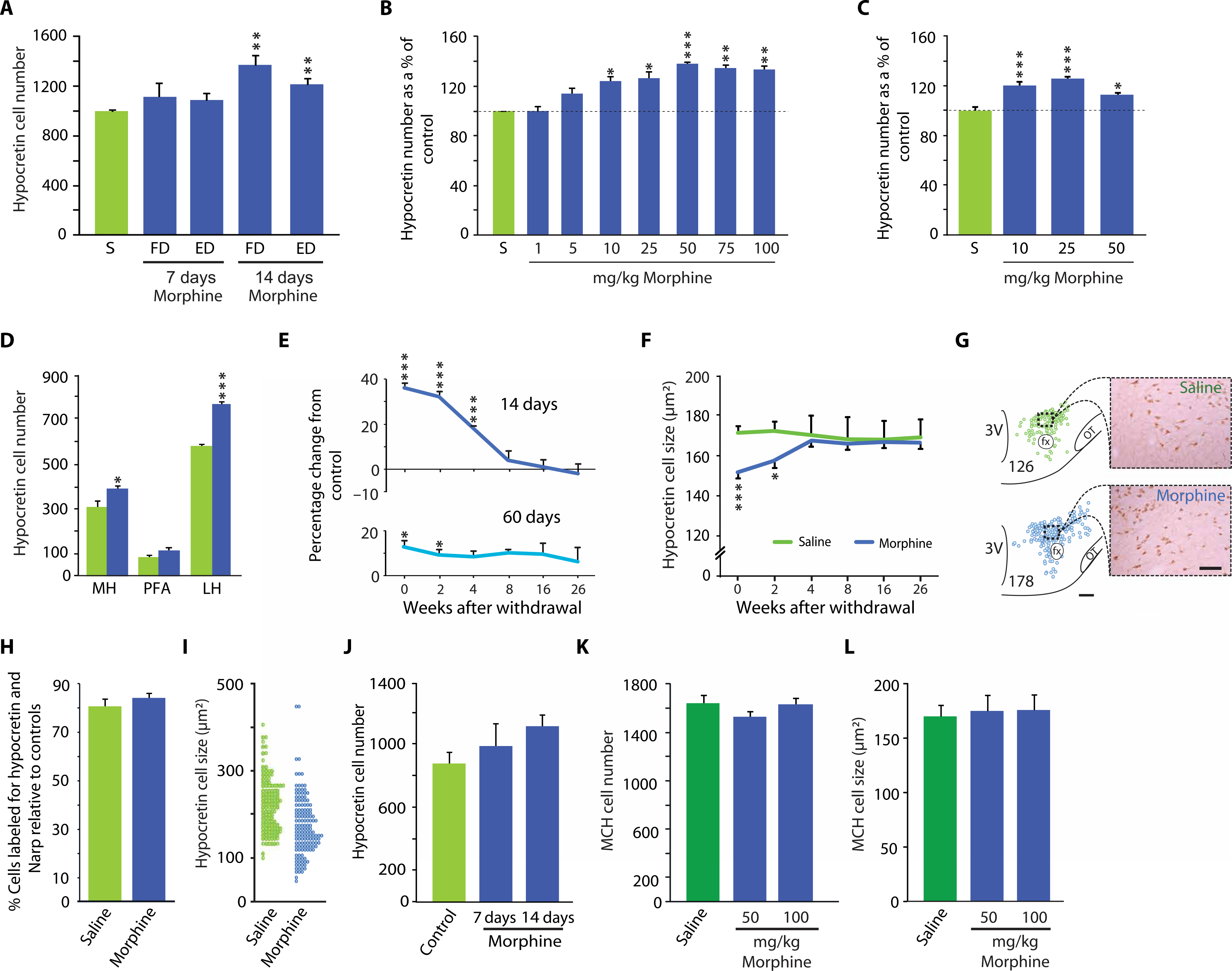
We administered morphine (blue) or saline (green) to wild-type littermate mice and then stained brain tissue for hypocretin-producing cells. (A) A fixed morphine dose (FD) of 100 mg/kg for 7 days, or an escalating morphine dose (ED) starting at 100 mg/kg for 3 days and increasing by 20% every third day for 7 days, did not significantly increase hypocretin cell number. However, a fixed dose of morphine (100 mg/kg) for 14 days and an escalating dose for 14 days (with a final dose of 180 mg/kg) both increased hypocretin cell number [P = 0.01, t = 4.23, df = 4 and +22%, P = 0.01, t = 4.52, df = 4 (t test), respectively). (B) Morphine doses of 10 mg/kg or higher all produced a significantly elevated number of hypocretin neurons compared to saline-injected mice. The elevation in hypocretin cell number at 50 mg/kg was 38%. Doses above 50 mg/kg produced no further increase [F7,16 = 8.1, P < 0.001 (ANOVA)]. (C) Shown are the effects of long-term (60 days) daily administration of morphine at doses of 10, 25, or 50 mg/kg. All three doses produced significant increases in hypocretin cell number as a percentage of control, with the largest increase (+26%) observed at a dose of 25 mg/kg [F3,12 = 13.5, P < 0.001 (ANOVA)]. (D) Shown is the mediolateral distribution of increased hypocretin cell number after 14 days of morphine administration at 50 mg/kg in wild-type mice. The elevation of hypocretin cell number was largest in the LH, but the effect was also significant in the MH [LH: P = 0.001, t = 11.94, df = 7; MH: P = 0.05, t = 2.44, df = 7 (t test)]. PFA, perifornical area. (E) Graph shows the persistence of the elevation of the number of hypocretin neurons after the termination of morphine administration to wild-type mice compared to mice injected with saline (control). Shown is the duration of hypocretin’s effects after termination of daily morphine (50 mg/kg) administration starting with the day after the final injection (day 0 of withdrawal) relative to control. After 14 days of morphine administration (top), hypocretin cell number remained significantly elevated for 4 weeks [F5,22 = 9.4, P < 0.001 (ANOVA)]. After 60 days of morphine administration (bottom), the significant elevation of hypocretin cell number lasted for 2 weeks [F5,22 = 6.3, P < 0.001 (ANOVA)]. (F) A 12.8 ± 2.8% decrease in hypocretin cell size was observed after administration of morphine (50 mg/kg) for 14 days to wild-type mice [significant interaction of treatment and withdrawal (F5,58 = 5.3, P < 0.0005)]. After 4 weeks of morphine withdrawal, hypocretin cell size returned to the size seen in saline-treated animals. (G) Representative neurolucida plots and photomicrographs illustrate the increased number and the reduced size of hypocretin cells after 2 weeks of morphine administration at 50 mg/kg fixed dose. Numbers indicate cell counts in section. Scale bars, 150 μm (neurolucida plots) and 50 μm (photomicrographs). 3V, third ventricle. (H) No change was observed in the percentage of cells double-labeled for both hypocretin and Narp in wild-type mice treated with morphine (50 mg/kg) for 14 days compared to control mice injected with saline. (I) We measured the effect of morphine pellet implantation on hypocretin cell number and size compared to control pellets in wild-type mice 72 hours after implantation. On average, cell size was decreased by 23% [P = 0.001 (Mann-Whitney U test), U = 5239]. n = 128 cells for control saline-injected mouse brain (green), and n = 149 cells for morphine-injected mouse brain (blue). (J) Shown are the effects of replacement of depleted morphine or control pellets at 3-day intervals for up to 7 or 14 days to mimic continuous opiate administration. (K and L) In contrast to hypocretin, MCH cell number and size were not affected by administration of morphine at 50 or 100 mg/kg for 14 days to wild-type mice. Dark green color in (K) and (L) distinguishes MCH neuron comparisons with saline control from hypocretin neuron comparisons with saline control. There was no change in MCH cell size compared to saline control. *P < 0.05, **P < 0.01, ***P < 0.001, t test with Bonferroni correction compared to saline control.
Because subcutaneous implantation of morphine pellets is commonly used in opiate research, we studied the effect of implantation of morphine pellets (25 mg) or control pellets in 14 mice. Figure 2I shows the effects of subcutaneously administered morphine pellets compared to control pellets on the distribution of hypocretin cell size 72 hours after implantation. On average, cell size was decreased by 23% in response to morphine pellet implantation [U = 5239, P < 0.01 (Mann-Whitney U test); Fig. 2I]. Thus, as with morphine injection, morphine pellet implantation resulted in a reduction in hypocretin cell size, shifting the entire population distribution downward. However, the continuous presence of morphine for 7 or 14 days, produced by replacing pellets every 3 days, did not produce a significant increase in hypocretin cell number (Fig. 2J). The number of melanin-concentrating hormone (MCH)–producing cells in the brains of mice was not affected by injection of morphine at 50 or 100 mg/kg for 14 days (Fig. 2K). There was no change in MCH cell size after 14 days of morphine administration to the mice (Fig. 2L).
Changes in preprohypocretin, Narp, preprodynorphin, and MCH in response to morphine
An escalating dose of morphine (100 mg/kg; Fig. 3, A to C) was given to wild-type mice for 14 days. Quantitative polymerase chain reaction (qPCR) was performed to measure mRNA amounts in mouse brains of preprohypocretin, Narp, and preprodynorphin (Fig. 3, A to C). Western blotting was used to assess the quantity of preprohypocretin and MCH peptide in mouse brain (Fig. 3, D and E, and fig. S1). The amounts of mRNAs encoding preprohypocretin, Narp, and preprodynorphin, all found in hypocretin-positive neurons, were found to be elevated [preprohypocretin: P = 0.03, t = 2.99, df = 5; Narp: P = 0.02, t = 3.36, df = 5 (t test); preprodynorphin: P = 0.01, t = 3.65, df = 5 (t test); Fig. 3, A to C]. Western blot quantitation showed that a 14-day administration of morphine to wild-type mice increased preprohypocretin by 79% [P = 0.05, t = 2.51, df = 6 in each group (t test)], which returned to baseline within 2 weeks of drug withdrawal (Fig. 3D). Figure 3E shows that there was no significant change in MCH in the same animals.
Fig. 3. Effect of morphine administration on preprohypocretin, preprodynorphin, Narp, and MCH expression in mouse brain.
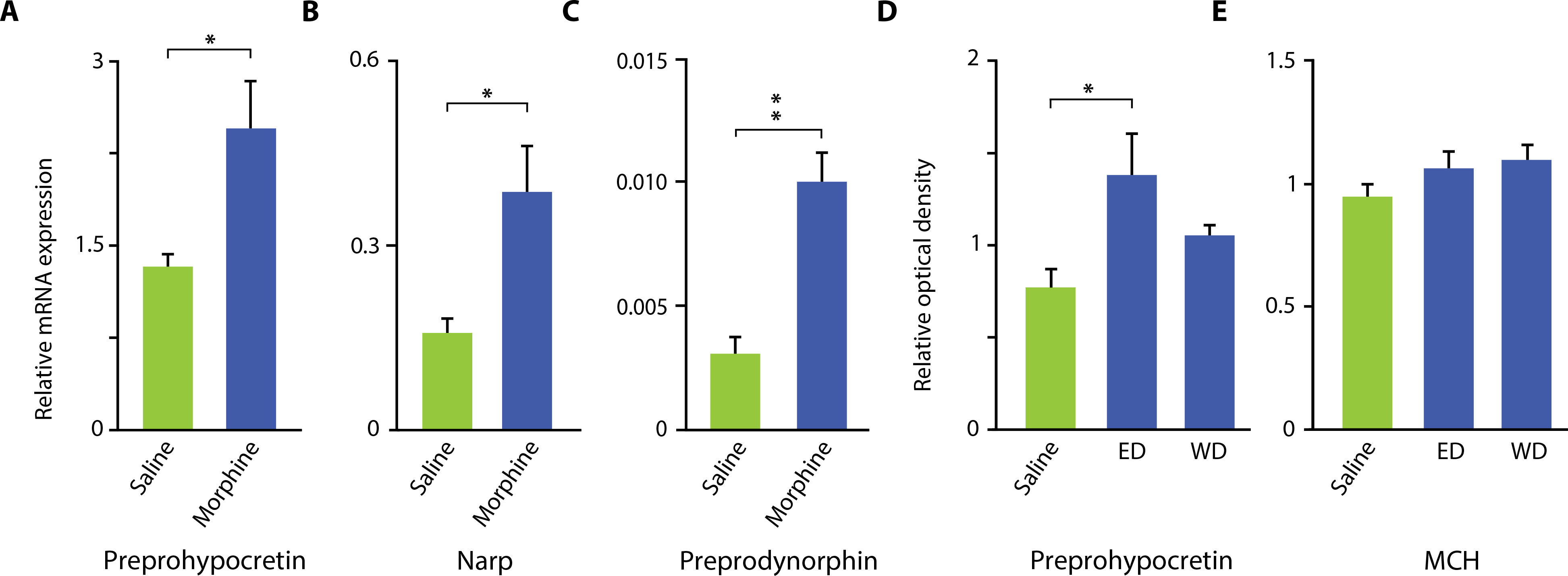
An escalating dose of morphine, starting at 100 mg/kg, was given for 14 days to wild-type mice. qPCR was performed to measure mRNA expression (A to C), and Western blotting was used to assess the amount of neuropeptide (D and E). (A to C) An increase in mRNA for preprohypocretin, Narp, and preprodynorphin was observed [preprohypocretin: P = 0.03, t = 2.99, df = 5; Narp: P = 0.02, t = 3.36, df = 5 (t test); preprodynorphin: P = 0.01, t = 3.65 df = 5 (t test)]. (D) Western blotting showed that 14 days of administration of morphine to wild-type mice increased preprohypocretin by 79% [P = 0.05, t = 2.51, df = 6 in each group (t test)] and returned to baseline within 2 weeks. (E) There was no significant change in MCH after 14 days of morphine administration. The difference remained nonsignificant from control after morphine withdrawal. WD, 2-week withdrawal. *P < 0.05, **P < 0.01, t test, compared to saline control.
Morphine-induced changes in hypocretin cell number are not due to neurogenesis
Morphine administration at 100 mg/kg for 14 days to wild-type mice increased the number of hypocretin-producing cells in mouse brains (Fig. 2). However, morphine administration to knockout mice (9) that cannot produce hypocretin did not result in immunohistochemical detection of hypocretin, indicating that the observed immunolabeling after morphine required the presence of hypocretin (Fig. 4A). There was no significant change in the number of 5-bromo-2′-deoxyuridine (BrdU)–labeled cells throughout the hypocretin neuron region after morphine administration to wild-type mice, indicating that the increased number of hypocretin neurons in response to morphine was not due to neurogenesis (Fig. 4B). Fourteen days of morphine (100 mg/kg) treatment also did not produce any change in doublecortin staining of neurons in the hypothalamus of mouse brains compared to saline treatment (Fig. 4C, left). Doublecortin staining validity was indicated by a normal labeling pattern in the dentate gyrus (Fig. 4C, right).
Fig. 4. The increase in hypocretin cell number after morphine administration was not due to neurogenesis.
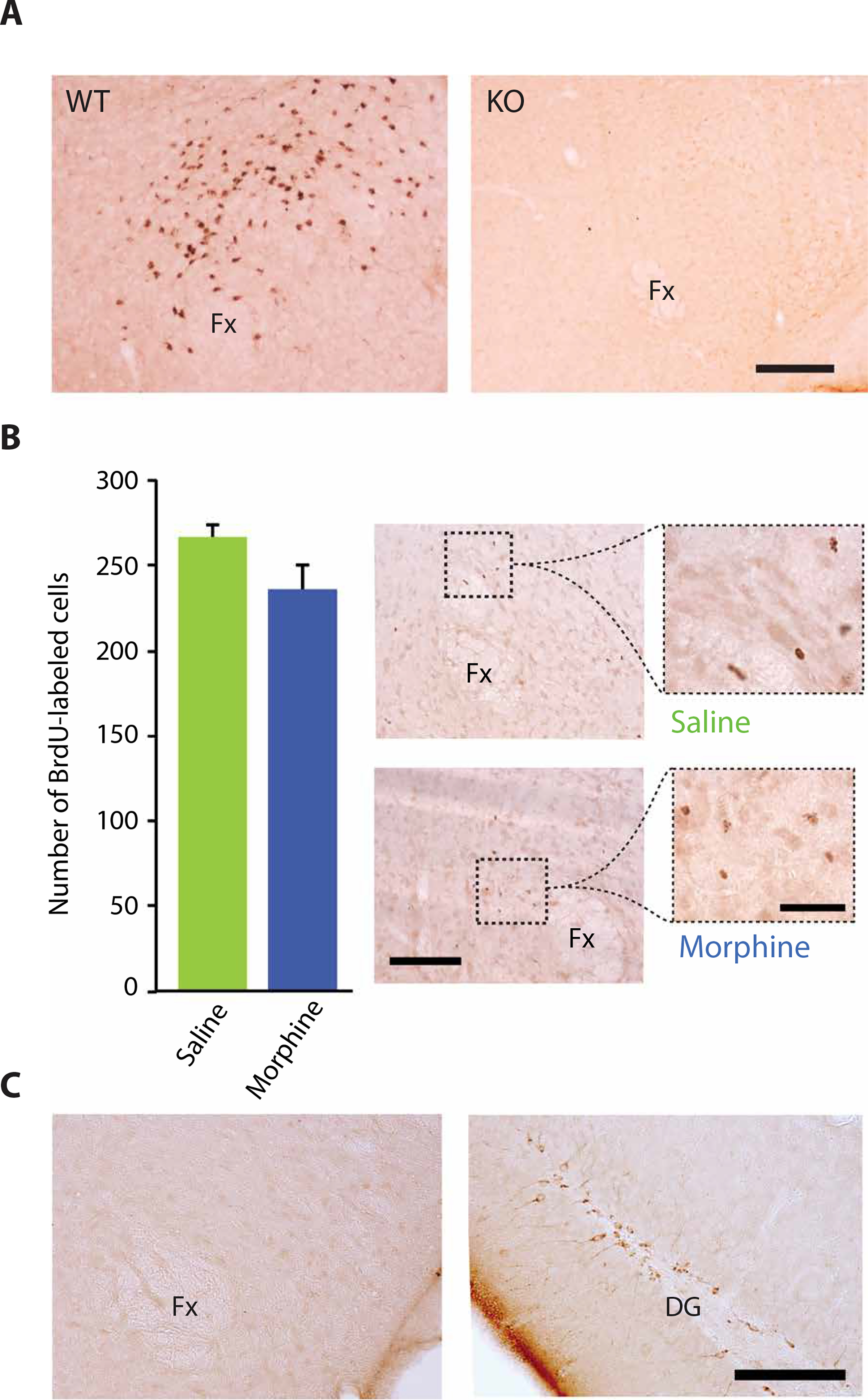
(A) Morphine administration at 100 mg/kg for 14 days resulted in labeling of hypocretin cells in wild-type (WT) mice, but not in hypocretin knockout (KO) mice, indicating that labeling required the presence of hypocretin in neurons. Scale bar, 200 μm. (B) The increased number of hypocretin neurons after morphine administration was not due to neurogenesis. BrdU labeling to identify new neurons showed no significant increase in the number of new hypocretin neurons after 14 days of morphine (100 mg/kg) treatment of wild-type mice compared to control mice given saline injections. Right: BrdU-labeled cells in the perifornical area of saline-treated (top) and morphine-treated (bottom) animals. Scale bars, 100 and 40 μm. (C) Shown is immunohistochemical staining in the hypothalamus for doublecortin after 14 days of morphine (100 mg/kg) treatment of wild-type mice. Left: Representative photomicrograph shows the absence of doublecortin staining in the hypothalamus of a morphine-treated animal. Right: Representative photomicrograph shows normal doublecortin staining of dentate gyrus (DG) of the same animal. Scale bar, 100 μm.
Effect of systemic morphine injections on the activity of hypocretin neurons in rats in vivo
A species-appropriate dose of morphine (15 mg/kg) resulted in an elevated action potential discharge rate in rat brain hypocretin neurons, accompanied by increased electroencephalographic (EEG) activation and increased electromyographic (EMG) activity in the freely moving rats (Fig. 5). Hypocretin neurons were identified using our previously published criteria (10). Figure 5A shows the discharge rate in rat hypocretin neurons during active waking, quiet waking, non–rapid eye movement (REM) sleep, and REM sleep and after morphine injection in waking animals (averaged across five neurons recorded in three rats). Hypocretin neurons displayed an elevated discharge rate after morphine administration comparable to that during active waking and greater than that during quiet waking, non-REM sleep, and REM sleep [one-way ANOVA for hypocretin neurons: n = 5, F4,16 = 18.2, P < 0.0001; post hoc comparisons with Tukey/Kramer procedure: active and quiet waking, P < 0.05; active waking and non-REM/REM sleep, P < 0.01; morphine administration: active waking, quiet waking, non-REM sleep, and REM sleep, all P < 0.01]. Figure 5B shows recordings of the discharge rate in discharges per second, muscle tone, and EEG data (expansions enable better visualization of the time course and an action potential waveform, used to aid in identification of hypocretin neurons; recording sites are indicated in Fig. 5C by black arrowheads).
Fig. 5. Effect of morphine administration on hypocretin cell activity in rats in vivo.
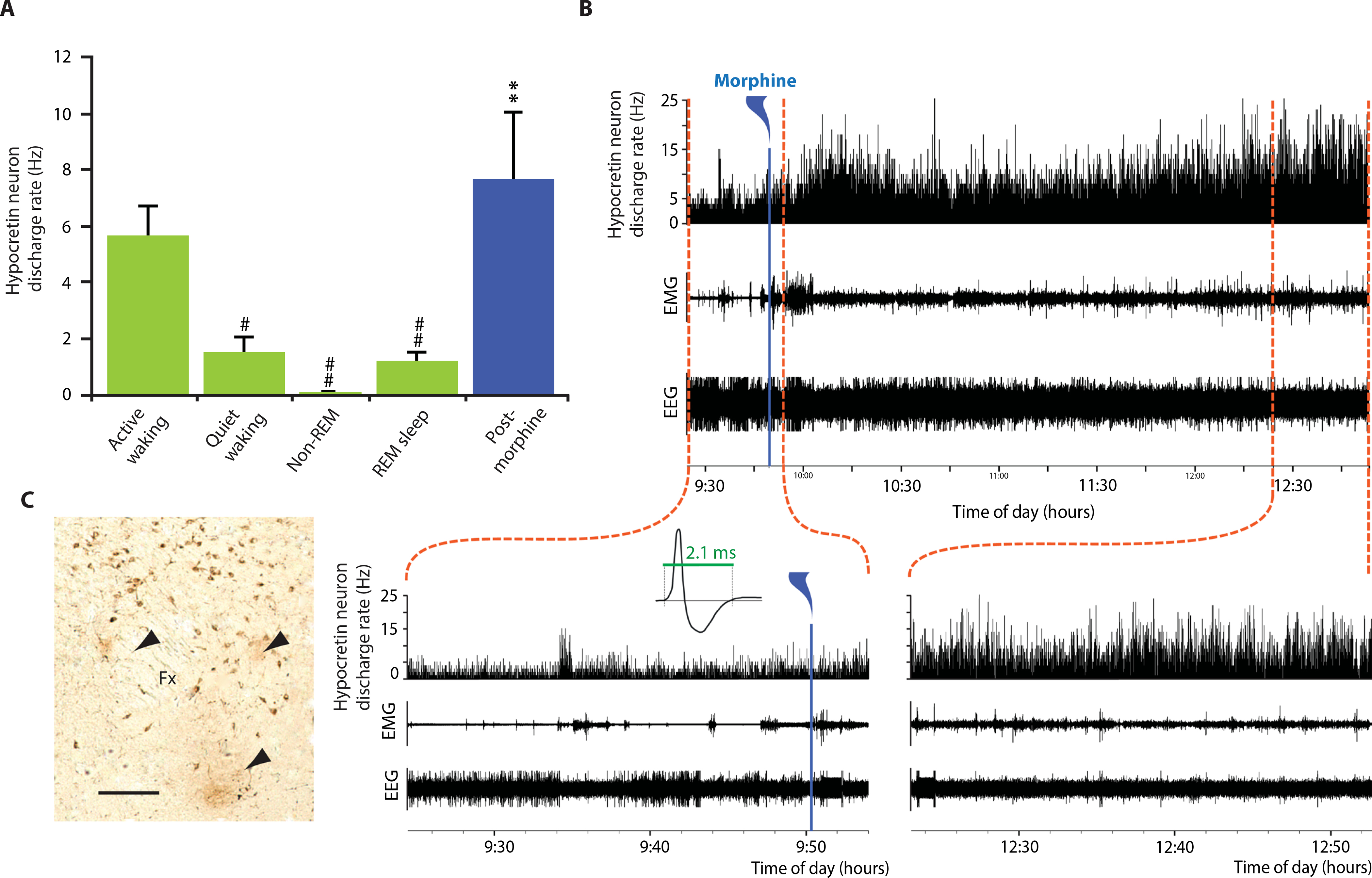
A species-appropriate dose of morphine (15 mg/kg) injected into three freely moving rats resulted in an elevated neuronal discharge rate lasting for 3 hours accompanied by an increase in EMG activity. (A) Sleep rates are averages of mean rate determined by five 10-s samples in each of five hypocretin neurons from three rats in each sleep state: active waking, quiet waking, non-REM sleep, and REM sleep. Post-morphine injection rate was based on five 10-s samples in each neuron taken 15 min after morphine injection. One-way ANOVA of hypocretin neurons (n = 5): F4,16 = 18.2, P < 0.0001. Post hoc comparisons with Tukey/Kramer procedure: active waking/quiet waking, P < 0.05; active waking/non-REM sleep, P < 0.01; active waking/REM, P < 0.01. (B) Discharge rate of rat hypocretin neurons after morphine administration. Bottom: Traces show EEG activation immediately after morphine injection (left) and 3 hours after injection (right). Increased rate of discharge in hypocretin neurons lasted for 3 or more hours after injection of morphine. Expanded traces show the characteristic long average waveform of hypocretin neurons. (C) Histology showing three recording sites of hypocretin neurons in rat brain, labeled with arrowheads. Scale bar, 150 μm. #P < 0.05, ##P < 0.01, compared to active waking; **P < 0.0001 compared to quiet waking, non-REM sleep, and REM sleep.
Reversal of decreased hypocretin cell number in narcoleptic mice after morphine administration
We used a newly developed transgenic mouse model in which hypocretin cell loss is triggered by doxycycline (DOX) withdrawal, the orexin-tTA; TetO diphtheria toxin A (DTA) mice (henceforth called DTA mice) (11). This transgenic mouse model allows induction of selective hypocretin cell loss and symptoms of narcolepsy with cataplexy in adult DTA mice after DOX withdrawal, resembling the syndrome that occurs in human narcolepsy. Resuming DOX administration stops further hypocretin cell loss in the DTA mice. Control mice were DTA mouse littermates maintained on DOX throughout the experiment. The dashed horizontal red line in Fig. 6A shows the number of hypocretin cells in DTA littermate control mice maintained on DOX and administered daily saline injections for 14 days. The green bar shows littermate DTA mice subjected to DOX withdrawal for 1.5 days, followed by restoration of DOX administration and saline injections for 14 days. A 30% reduction in hypocretin cells relative to the number of hypocretin cells in control DTA mice maintained on DOX was observed (Fig. 6A). However, when daily morphine (100 mg/kg) injections were given instead of saline injections for 14 days in the experimental group, the number of hypocretin cells detected by immunohistochemistry was restored to baseline [blue bar; P = 0.0003, t = 6.31, df = 4 (t test)]. Figure 6B shows the effect of morphine administration on cataplexy in two groups of four hypocretin cell–depleted DTA mice. DOX treatment was halted for 18 days, a duration that produces a 95% depletion of hypocretin neurons and cataplexy. Morphine treatment given at a dose of 50 mg/kg for 1 or 2 weeks reduced cataplexy relative to control untreated mice [treatment effect, F1,6 = 148.4, P = 0.0001 (ANOVA)]. Hypocretin knockout mice, in which the hypocretin neurons are present but the peptide is absent from birth, show much lower amounts of cataplexy than do DTA mice. Therefore, the effects of morphine administration on hypocretin knockout mice could not easily be tested. The extensive loss of most of the hypocretin cell bodies in the DTA mice more closely resembles the characteristics of human narcolepsy than does the lack of hypocretin neuron production in hypocretin knockout mice.
Fig. 6. Reversal of hypocretin cell loss and cataplexy in narcoleptic mice after morphine administration.
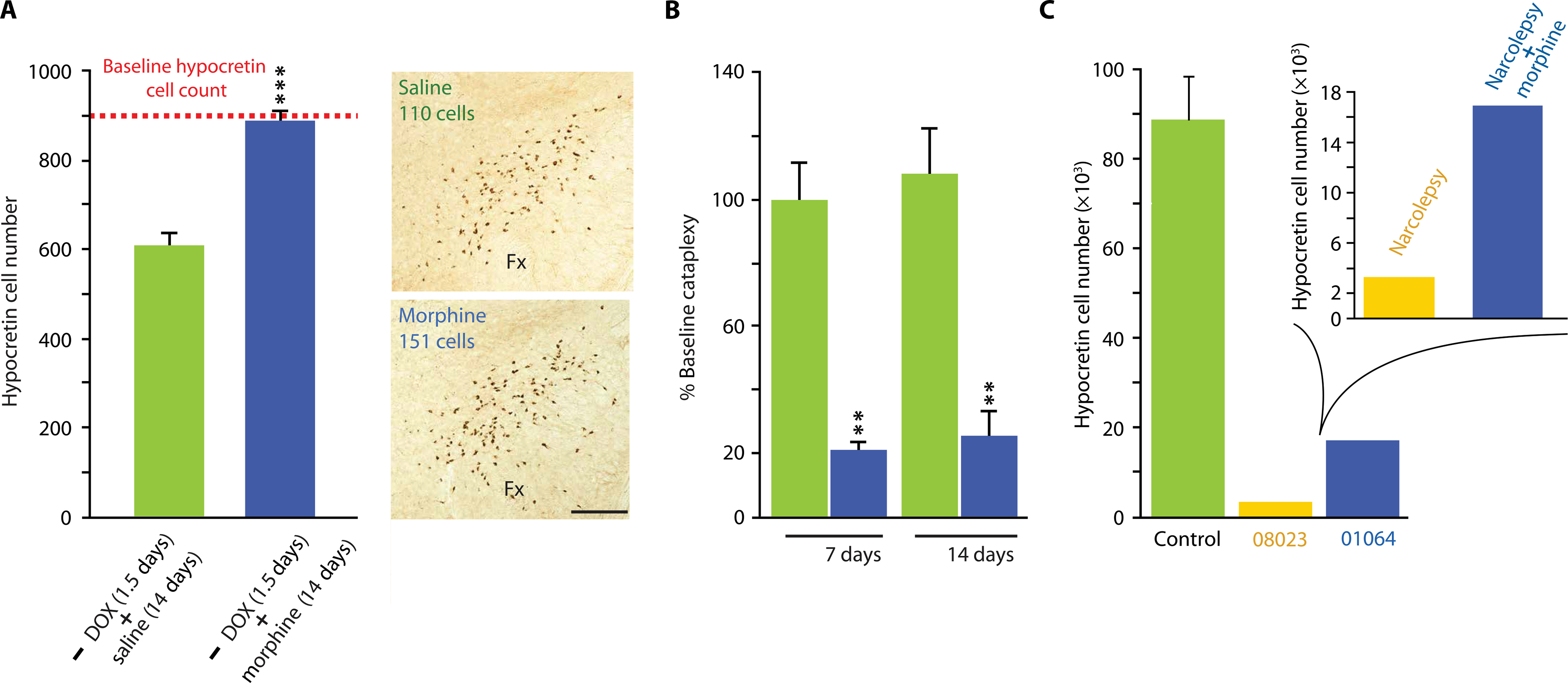
(A) The dashed horizontal red line shows the number of hypocretin cells in the brains of narcoleptic DTA mice maintained throughout the experiment on DOX, with 14 days of daily saline injections, followed by sacrifice. The green bar shows the number of hypocretin cells in the brains of DTA mice after DOX withdrawal for 1.5 days, followed by restoration of DOX administration and saline injections for 14 days. A 30% reduction of hypocretin cells relative to control was observed. When daily morphine (100 mg/kg) injections were given instead of saline for 14 days, the number of detected hypocretin cells was restored to baseline (blue bar). This difference was significant [***P = 0.003, t = 6.31, df = 4 (t test)]. Photomicrographs on the right show representative examples of hypocretin labeling in brains of DTA mice treated with saline (top) or morphine (bottom). Scale bar, 200 μm. (B) Morphine administration (blue) to DTA mice decreased cataplexy after 1 and 2 weeks of administration relative to control DTA mice receiving saline injections (green). Treatment effect: F1,6 = 148.4, P = 0.0001 (ANOVA). Changes after saline administration were not significant. Post hoc comparisons with Bonferroni correction revealed a significant difference at P < 0.01 between the saline-treated control DTA mice and the morphine-treated DTA mice at both 1 and 2 weeks of treatment. (C) Immunohistochemical staining shows that brain tissue from a human narcoleptic patient with cataplexy (01064, blue) treated for a long period with morphine has a higher number of hypocretin cells than does a case control narcoleptic patient with cataplexy (08023, yellow) not treated with morphine. The hypocretin cell counts in brain tissue from three control patients without narcolepsy or other identified neurological disorders are shown in green. The patients’ brains were willed to the Netherlands Brain Bank and preserved and analyzed using the same techniques (Table 1).
Figure 6C shows the number of hypocretin-producing cells in three human control subjects without narcolepsy (green) and in two patients with narcolepsy. In contrast to our prior studies of 40-μm sections of frozen human brain tissue analyzed by stereology (5), brain tissue of all five subjects was preserved in paraffin, was sectioned at 6-μm intervals, and was counted with an automated counting procedure. Both patient NBB-01064 and patient NBB-08023 were diagnosed as having narcolepsy with cataplexy. Patient NBB-01064 was chronically treated with morphine for relief of her pain resulting from discopathy after her initial narcolepsy diagnosis. Patient NBB-08023 was not treated with opiates. Eight years later, patient NBB-01064 was reclassified as having idiopathic hypersomnia without cataplexy, an unusual clinical course, suggesting that the extended period of morphine administration may have changed the trajectory of the disease. Figure 6C shows hypocretin cell counts in the diagnosed narcoleptic patients with cataplexy: without morphine treatment (NBB-08023) and with morphine treatment (NBB-01064). The morphine-treated patient (NBB-01064) had 16% the number of hypocretin cells found in healthy controls compared to 3% for the narcoleptic with cataplexy patient not treated with morphine (NBB-08023).
DISCUSSION
Cells with different neurotransmitter phenotypes have been characterized by various techniques since the early work of Falck et al. (12). Most current research studies use immunohistochemistry, the most sensitive technique, to identify and quantify these cell types in brain tissue representing normal and pathological human conditions. Hypocretin cell counts determined by immunohistochemistry (5) exceed those determined by genetic labeling (13) and RNA labeling (14). A relevant comparison is between our initial finding of an average of 71,000 hypocretin cells in human control brain tissue, with a 90% loss of these cells in brain tissue from patients with narcolepsy/cataplexy using immunohistochemistry (5, 15), and a contemporaneous report of 15,000 to 20,000 hypocretin cells in normal human control brain tissue and no detected hypocretin cells in patients with narcolepsy/cataplexy using in situ labeling (14). An independent study confirmed our immunohistochemistry work (Fig. 6C), finding surviving hypocretin cells in the brains of all human narcoleptic patients studied (16).
Immunohistochemistry can be combined with the measurement of immediate early gene expression (4) or with juxtacellular labeling and unit recording (10) to characterize the activity of identified cells in the brains of normal animals. Immunohistochemistry is also used to identify and study neuropathologies, including cell loss in narcolepsy, Huntington’s disease, Parkinson’s disease, Alzheimer’s disease, and other disorders. Our findings in the current study suggest that this approach may be missing an important aspect of brain function. Under some drug administration or disease conditions, the number of cells of a particular phenotype visualized with immunohistochemistry can change. A decrease in the number of cells can mistakenly be interpreted as representing neuronal death. However, increases of the sort we report here and that we and others have reported previously (17, 18) suggest that a portion of certain cell types do not produce immunohistochemically detectable amounts of their neurotransmitter under most conditions but can be induced to produce such amounts by drugs or perhaps by disease.
In recent work, we have shown that increasing hypocretin content by inhibiting microtubule polymerization with colchicine in mice increases the number of hypocretin cells detected by immunohistochemistry by as much as 40% in wild-type mice (19), similar to the amount we now see in mice after opiate administration. This raises the question of the extent to which the reduced number of hypocretin cells that characterizes human narcolepsy and other disorders may, at least partially, be due to reduced production of hypocretin in a subpopulation of hypocretin cells rather than the loss of hypocretin-producing cells through neuronal death. In mice given colchicine, the size of the hypocretin cells increases, possibly as a result of inhibition of axonal transport (16). One may speculate that the increased activity that we see in hypocretin neurons in mice after morphine administration has the opposite effect on cell size as colchicine administration. The action potentials associated with activation after morphine administration may cause hypocretin and other neuropeptides such as Narp and dynorphin to be transported out of the neurons and down axons faster than they can be synthesized, depleting the substrates and leading to shrinking of the cells.
Our work reported here shows that additionally recruited neurons after morphine administration in mice may not be uniformly distributed across the population of hypocretin cells. Further work will determine whether these neurons receive a different pattern of inputs, project to a different pattern of sites, or differ in other ways from the neuronal populations observed before opiate administration.
We show that the increased number of hypocretin neurons caused by morphine administration in mice was not due to neurogenesis. We also demonstrate that the increase in number lasted beyond the period when opiates would be readily detectable in the tissues of the mice. In mice, the time course of hypocretin cell number increase and cell size decrease differed. The increase required at least 2 weeks of high-dose morphine administration and returned to baseline by 8 weeks after administration. However, the cell size reduction in the same mice occurred within as little as 72 hours of morphine exposure, with hypocretin cell size returning to baseline by 4 weeks after morphine treatment. Morphine decreases the size of ventral tegmental area (VTA) neurons in rodents (20). Although a major increase (or decrease) in cell number of any neuronal group has not, to our knowledge, been reported with heroin or morphine administration in other studies, opiates have been shown to produce changes in dendritic field and spine morphology in the ventral tegmental field and nucleus accumbens, which may reflect altered release of dopamine from VTA cells (21).
We (4) and others (2) have demonstrated that increased hypocretin cell activity is linked to pleasurable but not to aversive tasks in mice and rats. We have found that hypocretin is released in nonaddicted humans when they are engaged in enjoyable tasks but not when they are aroused by pain or feeling sad (3). Conversely, human narcoleptic patients, who have, on average, a 90% loss of detectable hypocretin cells (5), show increased depression and are relatively resistant to drug addiction (22). In opiate addicts, elevating hypocretin production for long periods by self-administration of drug may create a positive mental state (affect), and a more negative affect is likely upon withdrawal of drug. This feedback loop may contribute to, or underlie, drug addiction.
Dopamine neurons, particularly those located in the VTA, have been strongly implicated in reinforcement in general and addiction in particular. Hypocretin and dopamine are evolutionarily linked from both a neurochemical and an anatomical perspective (23). The VTA receives a major hypocretin projection and projects to the nucleus accumbens. The amounts of dopamine and its major metabolites in the nucleus accumbens are markedly increased by the microinjection of hypocretin-1 and hypocretin-2 into the VTA. An intra-VTA injection of a selective hypocretin receptor-1 antagonist, SB334867A, suppresses morphine-induced place preference. Dopaminergic activation of neurons in the nucleus accumbens shell by morphine withdrawal requires the integrity of hypocretin receptor-1 (24).
An in vitro brain slice study found that opioids decrease the activity of hypocretin neurons and that blockade of μ-opioid receptors enhances the activity of hypocretin neurons. Morphine pretreatment inhibited subsequent excitatory responses to hypocretin in hypocretin neurons recorded in vitro (21). However, our current in vivo data show that systemic administration of morphine increased hypocretin neuron activity in rats, an effect presumably mediated at the neural circuit level, and therefore, this effect would not be seen in brain slices. Activation of hypocretin neurons reinstates an extinguished preference for morphine in a rodent study (25).
The VTA, nucleus accumbens, amygdala, locus coeruleus, and central gray matter all have been implicated in reward mediation (26). Hypocretin cells also contain and release glutamate (27), trigger glutamate release from adjacent cells, and show Narp, an immediate early gene encoding a protein involved in aggregating AMPA receptors and thought to have a role in addiction (8). Bingham et al. (28) found that hypocretin, like morphine, produces profound analgesia. Aston-Jones et al. (29) showed that hypocretin was required for learning a morphine-conditioned place preference task. Georgescu et al. (30) showed that hypocretin neurons, but not nearby MCH neurons, express μ-opioid receptors. Cyclic adenosine 5′-monophosphate response element–mediated transcription is induced in a subset of hypocretin cells, but not in MCH cells, after chronic exposure to morphine or induction of morphine withdrawal. In addition, c-Fos and the preprohypocretin gene are induced in hypocretin cells during morphine withdrawal. Constitutive hypocretin knockout mice develop attenuated morphine dependence, indicated by a less severe antagonist-precipitated withdrawal syndrome (24).
It has long been anecdotally noted that narcoleptic patients, who have, on average, a 90% loss of hypocretin neurons (5), show little, if any, evidence of drug abuse or addiction, despite their daily prescribed use of gamma hydroxybutyrate, methylphenidate, and amphetamines, drugs that are frequently abused. These data are consistent with our current finding of increased hypocretin cell populations in human heroin addicts, perhaps facilitating and maintaining addiction. The reduced number of hypocretin neurons in patients with narcolepsy may be related to the lack of drug addiction in these patients.
Our study has several limitations. The first is that we were unable to determine the difference in the dynamics of the hypocretin cell increase in humans and mice and the return to baseline after opiate withdrawal. Of particular interest were the duration of increase in the population of detected hypocretin neurons after cessation of opiate administration and whether it correlated with opiate craving in humans. Our discovery of tissues from a narcoleptic patient given morphine for pain management, a control narcoleptic patient not given such treatment, and neurologically normal control subjects, with all tissues preserved and analyzed in the same manner at the same brain bank, was unusual. This situation is highly unusual because there are so few postmortem brains from narcoleptic patients in brain banks and fewer still where the narcoleptic patients have been given long-term opiate treatment. Another limitation of our study is that we do not yet know the mechanisms responsible for the sustained increase in hypocretin cells that we see in the mice and in human heroin addicts. The DTA mouse model may make elucidating the mechanisms underlying this increase possible.
In 1981, a report of a narcoleptic patient given codeine (a natural isomer of methylated morphine) for the control of Crohn’s disease symptoms described a “disappearance of his narcolepsy, cataplexy, sleep paralysis, and hypnagogic hallucinations” (31). In a second case report, a narcoleptic patient, who could not continue taking stimulant drugs because of coronary artery disease and the necessity for kidney dialysis, urged his doctor to prescribe codeine for his narcolepsy because of the reversal of narcoleptic symptoms he had previously experienced when given codeine for pain. His physician published the results indicating a “dramatic improvement in alertness and substantial reduction of cataplexy,” the defining symptoms of narcolepsy (32). A third paper (33) tested codeine on eight narcoleptic patients. Sleep diaries and patient reports revealed consistent symptom improvement compared to placebo; however, there were no significant differences in the multiple sleep latency tests (cataplexy was not tested for). This ambiguous result from a 1-week trial in three human patients appears to have ended opiate use for treatment of narcolepsy. In human studies, separating the placebo effect from the drug effect and from drug-seeking behavior can be difficult. We now show (Fig. 6B) that opiate treatment is effective in reducing or eliminating cataplexy in a mouse model of narcolepsy.
It is possible that, with the appropriate schedule of administration and dosage, opiate agonists might be an effective treatment for human narcolepsy. However, tests to determine the dose of opiate agonist with the least addictive potential and maximal safety and effectiveness would be required before any recommendation for opiate use in human narcoleptic patients. The autonomic effects of effective opiate agonist doses would need to be determined. An alternate approach might be to develop agonists that more specifically increase the number of hypocretin-producing neurons and thereby increase hypocretin production without the risks of more widespread neuronal activation. The development of appropriate transgenic mice would be an important step in this direction. One may also speculate that reducing the number of neurons producing detectable amounts of hypocretin to the level seen in nonaddicted human controls or reducing hypocretin action pharmacologically using opiate receptor antagonists might be a potential strategy for treating opiate addiction in humans.
MATERIALS AND METHODS
Study design
Immunohistochemistry was used to stain for hypocretin in human brain tissue from neurologically normal control individuals, heroin addicts, and patients with narcolepsy (Table 1). Human brain tissue was obtained from the National Neurological Research Specimen Bank (Los Angeles), the Eunice Kennedy Shriver National Institute of Child Health and Human Development Brain Bank, and the Netherlands Brain Bank (Table 1). Parallel studies were done in wild-type mice and in a mouse model of narcolepsy in which selective hypocretin cell loss in mature animals was triggered by intracellular expression of diphtheria toxin (DTA mice) in response to removal of mice from a DOX diet. These mice simulate the consensus understanding of the cause, developmental timing, and symptoms of human narcolepsy. The amount of mRNA and neuropeptides and the activity of hypocretin neurons were measured in the mice. The age and sex of mice used in each study are described in table S1. Morphine and control saline injections were performed at the same time in experimental and control groups. All tissue analyses were performed by experimenters blinded to treatment conditions.
Human hypothalamic tissue
The hypothalamus of five addicts, one morphine-treated narcoleptic with cataplexy, one narcoleptic with cataplexy patient not given morphine, and nine control brains was examined for this study. Characteristics of addicts, narcoleptics, and control subjects are summarized in Table 1. Five heroin addict and seven control brains were fixed in 10% buffered formalin containing 0.1 M phosphate buffer (pH 7.4). The hypothalamus was cut into 40-μm sections. Sections were immunostained for hypocretin (orexin-A) and MCH. Adjacent sections were Nissl-stained. Human nuclear divisions are according Mai et al. (34). Two narcoleptic and three control brains were paraffin-embedded. Their sectioning and treatment are explained below.
Immunostaining for hypocretin and MCH in brain tissue from heroin addicts and control subjects
Immunostaining for hypocretin and MCH in 40-μm sections of frozen tissue was performed as in our earlier reports (5, 7). The sections were treated with 0.5% sodium borohydride in phosphate-buffered saline (PBS) for 30 min, washed with PBS, and then incubated for 30 min in 0.5% H2O2 for blocking of endogenous peroxidase activity. For antigen retrieval, sections were heated for 30 min at 80°C in a water bath with 10 mM sodium citrate (pH 8.5) solution. The sections were cooled to room temperature in sodium citrate and washed with PBS. After thorough washing with PBS, the sections were placed for 2 hours in 1.5% normal goat serum in PBS and incubated for 72 hours at 4°C with a 1:10,000 dilution of Hcrt-1 (Rabbit Anti-Orexin A, H-003-30, Phoenix Pharmaceuticals Inc.). Sections were then incubated in a secondary antibody (biotinylated goat anti-rabbit immunoglobulin G, Vector Laboratories), followed by avidinbiotin-peroxidase complex (ABC Elite Kit, Vector Laboratories), for 2 hours each at room temperature. The tissue-bound peroxidase was visualized by a diaminobenzidine (DAB) reaction (Vector Laboratories). Adjacent series of sections were immunostained for MCH (with a 1:20,000 polyclonal rabbit anti-MCH; H-070-47, Phoenix Pharmaceuticals Inc.). In all cases, the sectioning and staining were done blind to condition, with the same antibody lots used for all subgroups in each study.
Double labeling of neurons for hypocretin and Narp
After blocking using 3% normal donkey serum and 0.3% Triton X, sections were incubated with hypocretin antibody (orexin 1:1000, anti-goat; 8070, Santa Cruz Biotechnology) and Narp (1:1000; anti-rabbit, Worley laboratory, Johns Hopkins) for 72 hours. The secondary antibodies, Alexa Fluor 488 anti-goat (1:400) and Alexa Fluor 568 anti-rabbit (1:400) (Invitrogen, Life Technologies Corporation), were used.
Quantitative analysis
Hypocretin and MCH cell number, distribution, and size were determined in human brain tissue using stereological techniques on a 1-in-12 series of 40-μm sections through the complete hypothalamus. We used a Nikon E600 microscope with three-axis motorized stage, video camera, Neurolucida interface, and Stereo Investigator software (MicroBrightField Corp.). Quantification of hypocretin and Narp double labeling were carried out using Zeiss Axio Imager M2. In human subjects, the complete hypothalamic region of one-half of the human brain was cut into 40-μm-thick coronal sections using a one-in-six section interval for each series. One series of sections were stained with cresyl violet for the localization of anatomical regions. Adjacent series of sections were immunohistochemically stained for hypocretin. After staining, the sections were serially arranged and mounted on slides. Hypocretin cells were individually counted with the Neurolucida program. The final number reported is the number for the whole brain based on our systematic count. Sectioning, staining, and counting were done by investigators blind to condition. In our initial human studies (5, 7), we confirmed the results of stereological sampling with exhaustive counting of hypocretin neurons. In mice, we completely counted bilaterally the number of hypocretin neurons on a one-in-three series and reported the resulting number without multiplying by 3.
Hypocretin immunostaining in narcoleptic and control brain tissue (paraffin-fixed tissue)
Sections cut at 6 μm were incubated with rabbit anti-hypocretin A antibody (catalog no. H-003-30, Phoenix Pharmaceuticals Inc.) at 1:20,000 diluted in tris-buffered saline (TBS)–milk [5% (w/v) milk in 0.05 M tris and 0.15 M NaCl (pH 7.6)] for an hour at room temperature, followed by incubation overnight at 4°C. The next day, after rinsing in TBS, sections were incubated with goat anti-rabbit serum at 1:400 in TBS for 1 hour at room temperature. Antibody binding was visualized according to the ABC method at a 1:800 dilution of these complexes in TBS for 1 hour at room temperature. After rinsing in TBS, staining was developed by DAB and nickel ammonium sulfate for about 20 min. Reactions were stopped by washing sections in distilled water. Finally, slides were dehydrated in an ascending series of alcohol and coverslipped in xylene with Entellan.
Counting procedure for paraffin sections
Only positively stained neurons containing a nucleolus were included to prevent double counting. This counting procedure, which was judged to be the best for the thin (6 μm) sections used, is based on the principle that nucleoli can be considered as hard particles that will not be sectioned by a microtome knife but, instead, are pushed either in or out of the paraffin when hit by the knife (35). All the cell counts were from one side of the hypothalamus. Completeness of the cell counting was confirmed by graphically presenting the actual number of neurons counted in every section from rostral to caudal to review the distribution pattern. If the most rostral or caudal sections still showed positive cells, we cut the remaining tissue so as to have a complete series. Following hypocretin staining, the total number of neurons was counted at 600-μm intervals throughout the area. In each section, all hypocretin neurons with their typical cell profiles and a visible nucleolus serving as a unique marker for each neuron were counted using light microscopy at a magnification of 400×. Taking into account the interval distances between individual sections, the total number of hypocretin neurons was determined on the basis of the Cavalieri principle (36).
Animal study
All procedures were approved by the Institutional Animal Care and Use Committee of the University of California at Los Angeles and the Veterans Administration Greater Los Angeles Healthcare System.
Morphine pellet study in mice
Experiments were performed on male C57BL/6 mice weighing 25 to 30 g. Animals were housed on a 12-hour light-dark cycle. Food and water were available ad libitum. The characteristics in each group of the study are detailed in table S1. Morphine (25 mg) pellets [from the National Institute on Drug Abuse (NIDA)] and placebo pellets (NIDA) were subcutaneously implanted with isoflurane anesthesia. There were three groups for the pellet study: (1) pellet implanted for 3 days, (2) pellet implanted for 7 days, and (3) pellet implanted for 14 days. For groups 2 and 3, the initial pellet was replaced after 72 hours. All animals were sacrificed between 12:00 p.m. and 2:00 p.m. Animals were anesthetized intraperitoneally (i.p.) with Fatal-Plus solution and then perfused transcardially with PBS, followed by 4% paraformaldehyde in PBS. Brains were removed and post-fixed for 72 hours in 4% paraformaldehyde in PBS, followed by 30% sucrose in PBS. The sections were cut at 40 μm on a sliding microtome and stained for hypocretin as described earlier. Mouse nuclear divisions are as in McGregor et al. (4).
Morphine dose response in mice
A 14-day dose-response trial with daily administration of a fixed dose of morphine (morphine sulfate injection USP, Hospira Inc.) in each group of animals from 1 to 100 mg/kg body weight was conducted. The doses were 1, 5, 10, 25, 50, 75, and 100 mg/kg. Control groups received saline injections. The morphine escalating dose started with 100 mg/kg daily and was increased by 20% every 72 hours. For the 60-day dose-response trial, there were three doses: 10, 25, and 50 mg/kg body weight. Control groups received saline. Injections were done at 10:00 a.m. in mice on a 12-hour/12-hour cycle with light onset at 7:00 a.m. All animals were sacrificed between 12:00 p.m. and 2:00 p.m.
Morphine (50 mg/kg) for 14 days and withdrawal for up to 6 months
To study the effect of morphine withdrawal on hypocretin neurons, we administered 50 mg/kg for 14 days to the mice. The control group received saline. Two-week, 4-week, 8-week, 16-week, and 26-week withdrawal durations were used.
Morphine (50 mg/kg) for 60 days and withdrawal up to 6 months
To study the effect of long-duration administration of morphine, we gave 50 mg/kg for 60 days. The control groups received saline. Two-week, 4-week, 8-week, 16-week, and 26-week withdrawal durations were used.
Investigation of neurogenesis after morphine treatment in mice
To look for evidence of neurogenesis after morphine treatment, BrdU was given intraperitoneally at 50 mg/kg in sterile saline once daily for 2 weeks. BrdU injection was done in morphine-treated (100 mg/kg) and saline-treated animals. Morphine injection was done in the morning, and BrdU injection was done in the evening for the 2-week period. Animals were sacrificed 2 weeks after the initial injection or 4 weeks after the end of 2 weeks of injection period. There were three animals in each experimental group.
Immunohistochemistry (40-μm sections)
Brains were sectioned at 40 μm in the frontal plane through the hypothalamus. Immunohistochemistry for BrdU was performed on every fourth, free-floating section. Tissue was pretreated for BrdU immunostaining by DNA denaturation (2 M HCl at 37°C for 30 min), followed by 10 min in borate buffer (pH 8.5). Sections were then incubated with rat anti-BrdU monoclonal antibody (1:400; Novus Biologicals) for 72 hours. Sections were developed using the ABC and DAB methods (Vector Elite). Doublecortin staining was done using goat anti-doublecortin C-18 (DCX 1:1000; no. sc-8066, Santa Cruz Biotechnology). Homozygous male hypocretin-deficient mice were injected with morphine (100 mg/kg) subcutaneously. The control group received saline (n = 3 each group; table S1). The animals were sacrificed on day 14, and hypocretin immunohistochemistry was performed.
qPCR procedure
The morphine escalating dose started from 100 mg/kg and was escalated by 20% every 72 hours, ending up at 180 mg/kg for 2 weeks. Brain samples from mice (eight saline-injected mice versus eight morphine-injected mice) were snap-frozen by dry ice and stored in −80°C. They were cut into 200-μm sections in a cryostat at −18°C. The hypothalamus was bilaterally punched out by a prechilled 1.0-mm punching needle (Miltex Inc.). Brain tissue samples were pooled with two animals in each tube and immediately put back on dry ice. The tissue was homogenized with 1000 μl of QIAzol Lysis Reagent (QIAGEN Sciences) and 200 μl of chloroform (Merck). RNA was isolated according to the RNeasy Lipid Tissue Mini Kit (catalog no. 74804, QIAGEN) manufacturer’s protocol. RNA concentration and quality were measured by a Nanodrop TM ND-1000 spectrophotometer (Thermo Fisher Scientific). For each sample, 1000 ng of total RNA was used for synthesis of complementary DNA (cDNA), as described by the manufacturer’s protocol of the iScript cDNA Synthesis Kit (Bio-Rad Laboratories).
Primer sequences for preprohypocretin/orexin, preprodyn, and Narp GenBank accession code are indicated below. Primer sequences for glyceraldehyde-3-phosphate dehydrogenase, tubulin α-1A, ribosomal protein S28, actin-β, ubiquitin C, and tubulin β-4a (Tubb4a) were used as reference genes.
The qPCR procedures have been described previously (37). In short, qPCR was performed in a reaction volume of 20 μl using the SYBR Green PCR Kit (Promega) and a mixture of sense and antisense primers (2 pmol/μl). Reactions were run in a GeneAmp 7300 thermocycler under the following conditions: 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and, finally, 1 min at 60°C. Data were acquired and processed automatically by the Applied Biosystems Sequence Detection Software. Specificity of amplification was checked by melting curve analysis and electrophoresis of products on 1.5% agarose gel. Sterile water (nontemplate control) and omission of reverse transcriptase (non-RT control) during cDNA synthesis served as negative controls.
Amplification efficiency was determined by running qPCR reactions on a dilution series of pooled cDNA from all the subjects. Resulting cycle threshold values were plotted against the inverse log of each dilution, and the slope of this curve was then used to calculate the efficiency as follows: Efficiency (E) = 10−/slope. The normalization factor was based on the geometric mean of the following two most stable reference genes (that is, actin-β and Tubb4a) out of six candidate reference genes selected by geNorm analysis (38). To minimize handling variations, each gene was measured in triplicate. The relative absolute amount of target genes calculated (39) was divided by the normalization factor.
Western blotting
The mouse hypothalamus was sonicated in lysis buffer containing 50 mM tris-HCl, 50 mM MgCl2, 5 mM EDTA, and a protease inhibitor tablet (catalog no. 12482000, Roche) and was centrifuged at 800g (3000 rpm) for 30 min at 4°C. The protein concentration of the supernatant was determined using the DC Protein Assay Kit (catalog no. 500-0112, Bio-Rad). Protein (40 μg) was loaded on a 12% mini-protean TGX precast gel (catalog no. 456-1044, Bio-Rad) and separated at 50 V. The proteins were then transferred to a polyvinylidene difluoride membrane (catalog no. 162-0176, Bio-Rad) at 50 mA for 2 hours. The membrane was washed in 20 mM tris, 150 mM NaCl, and 0.1% Tween 20 (TBST) and then blocked in TBST containing 5% (w/v) nonfat dry milk (NFDM) for 1 hour. The membrane was incubated with rabbit anti–polyphenol oxidase (PPO) (sc-28935, Santa Cruz Biotechnology) and rabbit anti-MCH (sc-28931, Santa Cruz Biotechnology) at 1:1000 dilution in 2.5% NFDM in TBST overnight at 4°C. The next day, the membrane was washed in TBST before incubation with goat anti-rabbit horseradish peroxidase (HRP)–conjugated secondary antibody (catalog no. 31463, Thermo Fisher Scientific) at a dilution of 1:10,000 in 2.5% NFDM in TBST for 1 hour at room temperature. After washing in TBST, the antibody complex was visualized with SuperSignal West Femto (catalog no. 34094, Thermo Fisher Scientific). Anti–β-actin (MAB1501R, Millipore) was used as an internal normalizer, at a dilution of 1:10,000, with goat anti-mouse HRP-conjugated secondary antibody (catalog no. A2304, Sigma) at a dilution of 1:10,000. The densities of PPO, pro-MCH, and β-actin bands for each sample were measured using ImageJ software.
Morphine administration to DTA mice
To create a model of orexin/hypocretin deficiency with closer fidelity to human narcolepsy, DTA was expressed in orexin neurons under control of the Tet-off system (Fig. 6A) (11). Male orexin-tTA; TetO DTA mice were maintained from weaning to 10 weeks of age on DOX (+) chow. To reduce the number of orexin neurons without elimination of the entire cell population, DOX (+) chow was removed at 10 weeks of age and replaced with Labo MR stock food [DOX (−) condition] for 1.5 days, after which DOX (+) chow was reintroduced (1.5 days + restoration of DOX; n = 8). The experimental group (n = 4) then received morphine (100 mg/kg) subcutaneously for 14 days, and the control group received (n = 4) saline. Two hours after last day injections, mice were deeply anesthetized with pentobarbital (100 mg/kg, i.p.) and perfused sequentially with 15 ml of chilled saline and 15 ml of chilled 10% formalin solution (Wako). Brains were isolated and immersed in formalin solution for 72 hours at 4°C, followed by 30% sucrose. The sections were cut at 40 μm and stained for hypocretin as described earlier.
Cataplexy in DTA mice treated with morphine
Female orexin-tTA; TetO DTA mice (40), aged 4 to 6 months fed DOX food from birth (as were their mothers before the birth of the pups), were placed on regular laboratory rodent food for 18 days and then back on DOX food (catalog no. TD.130840, Teklad) for 1 to 2 months (Fig. 6B). The mice were singly housed (lights on at 6:00 a.m. and off at 6:00 p.m.). The mice were recorded from 5:00 p.m. to 6:00 a.m. the next day, via a LOREX DV700 recording system with a 1080p HD MPX DVR. Chocolate (“Hershey’s kisses,” milk chocolate) was given at 6:00 p.m. to enhance cataplexy attacks (11). Baseline cataplexy was recorded between 6:00 p.m. and 6:00 a.m. the next morning, 1 day before morphine treatment commenced. At 10:00 a.m., the mice were injected subcutaneously with either saline or morphine (50 mg/kg). Morphine or saline was administered every day at 10:00 a.m. for 2 weeks. Overnight video recording was repeated weekly, after 1 and 2 weeks of morphine or saline injections and for 3 weeks after the termination of morphine/saline treatment. The number of cataplexy bouts was scored for the first 2 hours (from 6:00 p.m. to 8:00 p.m.) of the dark phase. Cataplexy was scored on the basis of the criteria of Chemelli et al. (9) and Scammell et al. (41): an abrupt episode of nuchal atonia with immobility lasting at least 10 s, with at least 40 s of wakefulness preceding the episode. The number of cataplexy episodes was normalized with each subject’s baseline score and was expressed as the percentage of saline control on week 1.
Effect of morphine on hypocretin cell activity in vivo
Male Sprague-Dawley rats (Charles River Laboratories) weighing 250 to 300 g were used (n = 3). Hypocretin cells were recorded from the hypothalamus using microwire recording techniques as described previously (42). Under isoflurane anesthesia, microdrives containing 25-μm stainless steel microwires (California Fine Wire Co.) aimed at the lateral hypothalamic area were implanted, with the tip of the electrodes 0.5 mm above the target area. Stainless steel screw electrodes were placed over the sensorimotor cortex for EEG, and EMG activity was recorded from the dorsal neck muscles with Teflon-coated multistranded stainless steel wires (Cooner Wire). Animals were free to move around the recording chamber. Electrodes were moved in 80-μm steps until a cell or cells with signal-to-noise ratio of at least 2:1 were isolated. The activity of each cell was then characterized throughout sleep/waking states. Waking-specific cells that fit the profile of hypocretin cells (10) were studied after intraperitoneal injections of morphine (10 to 15 mg/kg) with continuous recording of neuronal activity for at least 3 hours.
Statistical analysis
The figure legends contain statistical details. The numbers of subjects in each human and animal study are indicated in Table 1 and table S1, respectively. Summary statistics are presented, with probability and df, in the figure legends. Unmatched two-tailed t tests or ANOVAs were used as appropriate. The number of subjects was determined in each group, generating data according to the formula: n = (z score)2 × SD × (1 − SD)/(margin of error)2 with α set at 0.05 and power set at 80%.
Supplementary Material
Funding:
This study was supported by NIDA grant DA034748, the Medical Research Service of the Department of Veterans Affairs grant BX001753 (J.M.S.), NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation (L.S.), and grant R35NS097966 (P.F.W.).
Footnotes
Competing interests: G.J.L. consults for Jazz Pharmaceuticals and Bioprojet. All other authors declare that they have no competing interests.
Data and materials availability: All data are in the paper and the Supplementary Materials.
REFERENCES AND NOTES
- 1.Centers for Disease Control and Prevention (CDC, 2017); www.cdc.gov/drugoverdose/epidemic/.
- 2.Baimel C, Bartlett SE, Chiou L-C, Lawrence AJ, Muschamp JW, Patkar O, Tung L-W, Borgland SL, Orexin/hypocretin role in reward: Implications for opioid and other addictions. Br. J. Pharmacol 172, 334–348 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blouin AM, Fried I, Wilson CL, Staba RJ, Behnke EJ, Lam HA, Maidment NT, Karlsson KÆ, Lapierre JL, Siegel JM, Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat. Commun 4, 1547 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGregor R, Wu M-F, Barber G, Ramanathan L, Siegel JM, Highly specific role of hypocretin (orexin) neurons: Differential activation as a function of diurnal phase, operant reinforcement vs. operant avoidance and light level. J. Neurosci 31, 15455–15467 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM, Reduced number of hypocretin neurons in human narcolepsy. Neuron 27, 469–474 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fronczek R, Overeem S, Lee SYY, Hegeman IM, van Pelt J, van Duinen SG, Lammers GJ, Swaab DF, Hypocretin (orexin) loss in Parkinson’s disease. Brain 130, 1577–1585 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Thannickal TC, Lai Y-Y, Siegel JM, Hypocretin (orexin) cell loss in Parkinson’s disease. Brain 130, 1586–1595 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blouin AM, Thannickal TC, Worley PF, Baraban JM, Reti IM, Siegel JM, Narp immunostaining of human hypocretin (orexin) neurons: Loss in narcolepsy. Neurology 65, 1189–1192 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M, Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell 98, 437–451 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Mileykovskiy BY, Kiyashchenko LI, Siegel JM, Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron 46, 787–798 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabuchi S, Tsunematsu T, Black SW, Tominaga M, Maruyama M, Takagi K, Minokoshi Y, Sakurai T, Kilduff TS, Yamanaka A, Conditional ablation of orexin/hypocretin neurons: A new mouse model for the study of narcolepsy and orexin system function. J. Neurosci 34, 6495–6509 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falck B, Hillarp N-Å, Torp A, A new type of chromaffin cells, probably storing dopamine. Nature 183, 267–268 (1959). [DOI] [PubMed] [Google Scholar]
- 13.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K.-i., Sugiyama F, Goto K, Yanagisawa M, Sakurai T, Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38, 701–713 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E, A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med 6, 991–997 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Thannickal TC, Moore RY, Aldrich M, Albin R, Cornford M, Siegel JM, Human narcolepsy is linked to reduced number, size and synaptic bouton density in hypocretin-2 labeled neurons. Abstr. Soc. Neurosci 26, 2061 (2000). [Google Scholar]
- 16.Crocker A, España RA, Papadopoulou M, Saper CB, Faraco J, Sakurai T, Honda M, Mignot E, Scammell TE, Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology 65, 1184–1188 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John J, Thannickal TC, McGregor R, Ramanathan L, Ohtsu H, Nishino S, Sakai N, Yamanaka A, Stone C, Cornford M, Siegel JM, Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann. Neurol 74, 786–793 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valko PO, Gavrilov YV, Yamamoto M, Reddy H, Haybaeck J, Mignot E, Baumann CR, Scammell TE, Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann. Neurol 74, 794–804 (2013). [DOI] [PubMed] [Google Scholar]
- 19.McGregor R, Shan L, Wu M-F, Siegel JM, Diurnal fluctuation in the number of hypocretin/orexin and histamine producing: Implication for understanding and treating neuronal loss. PLOS ONE 12, e0178573 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazei-Robison MS, Koo JW, Friedman AK, Lansink CS, Robison AJ, Vinish M, Krishnan V, Kim S, Siuta MA, Galli MA, Niswender KD, Appasani R, Horvath MC, Neve RL, Worley PF, Snyder SH, Hurd YL, Cheer JF, Han M-H, Russo SJ, Nestler EJ, Role for mTOR signaling and neuronal activity in morphine-induced adaptations in ventral tegmental area dopamine neurons. Neuron 72, 977–990 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, van den Pol AN, μ-Opioid receptor-mediated depression of the hypothalamic hypocretin/orexin arousal system. J. Neurosci 28, 2814–2819 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dauvilliers Y, Lopez R, Ohayon M, Bayard S, Hypersomnia and depressive symptoms: Methodological and clinical aspects. BMC Med 11,78 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefano GB, Kream RM, Endogenous morphine synthetic pathway preceded and gave rise to catecholamine synthesis in evolution (Review). Int. J. Mol. Med 20, 837–841 (2007). [PubMed] [Google Scholar]
- 24.Sharf R, Sarhan M, DiLeone RJ, Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol. Psychiatry 64, 175–183 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris GC, Wimmer M, Aston-Jones G, A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437, 556–559 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Sun W, Dopamine neurons in the ventral tegmental area: Drug-induced synaptic plasticity and its role in relapse to drug-seeking behavior. Curr. Drug Abuse Rev 4, 270–285 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Torrealba F, Yanagisawa M, Saper CB, Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience 119, 1033–1044 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Bingham S, Davey PT, Babbs AJ, Irving EA, Sammons MJ, Wyles M, Jeffrey P, Cutler L, Riba I, Johns A, Porter RA, Upton N, Hunter AJ, Parsons AA, Orexin-A, an hypothalamic peptide with analgesic properties. Pain 92, 81–90 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA, Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 1314, 74–90 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ, Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J. Neurosci 23, 3106–3111 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harper JM, Gelineau’s narcolepsy relieved by opiates. Lancet 317, 92 (1981). [DOI] [PubMed] [Google Scholar]
- 32.Benbadis SR, Effective treatment of narcolepsy with codeine in a patient receiving hemodialysis. Pharmacotherapy 16, 463–465 (1996). [PubMed] [Google Scholar]
- 33.Fry JM, Pressman MR, DiPhillipo MA, Forst-Paulus M, Treatment of narcolepsy with codeine. Sleep 9, 269–274 (1986). [DOI] [PubMed] [Google Scholar]
- 34.Mai JK, Assheuer J, Paxinos G, Atlas of the Human Brain (Elsevier Academic Press, 2004). [Google Scholar]
- 35.Fronczek R, Overeem S, Lee SYY, Hegeman IM, van Pelt J, van Duinen SG, Lammers GJ, Swaab DF, Hypocretin (orexin) loss and sleep disturbances in Parkinson’s disease. Brain 131, e88 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Verwer RWH, Raber-Durlacher JE, Efficient and unbiased estimation of volume and area of tissue components and cell number in gingival biopsies. J. Periodontal Res 28, 313–323 (1993). [DOI] [PubMed] [Google Scholar]
- 37.Shan L, Bossers K, Luchetti S, Balesar R, Lethbridge N, Chazot PL, Bao AM, Swaab DF, Alterations in the histaminergic system in the substantia nigra and striatum of Parkinson’s patients: A postmortem study. Neurobiol. Aging 33, 1488.e1–1488.e13 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F, Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamphuis W, Schneemann A, van Beek LM, Smit AB, Hoyng PFJ, Koya E, Prostanoid receptor gene expression profile in human trabecular meshwork: A quantitative real-time PCR approach. Invest. Ophthalmol. Vis. Sci 42, 3209–3215 (2001). [PubMed] [Google Scholar]
- 40.Inutsuka S, Tabuchi S, Tsunematsu T, Black SW, Kilduff TS, Tominaga A, Yamanaka A, New model mice for narcolepsy using timing controlled gene expression in transgenic mice which induces specific ablation of orexin/hypocretin neurons. Soc. Neurosci 799, 19 (2012). [Google Scholar]
- 41.Scammell TE, Willie JT, Guilleminault C, Siegel JM, A consensus definition of cataplexy in mouse models of narcolepsy. Sleep 32, 111–116 (2009). [PMC free article] [PubMed] [Google Scholar]
- 42.Wu M-F, Gulyani SA, Yau E, Mignot E, Phan B, Siegel JM, Locus coeruleus neurons: Cessation of activity during cataplexy. Neuroscience 91,1389–1399 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


