Abstract
Background
Plantaginis Semen has been widely used as folk medicine and health care food against hyperuricemia (HUA) and gout, but its pharmacological mechanism remains unclear. This study investigated the therapeutic mechanism of Plantaginis Semen extract on potassium oxonate -induced HUA rats based on a lipidomics approach.
Methods
A model of HUA was established by potassium oxonate intragastric administration. 42 Sprague-Dawley (SD) male rats were randomly divided into the control group, model group, benzbromarone group (10 mg/kg) and three Plantaginis Semen groups (n = 7). The Plantaginis Semen groups were treated orally with Plantaginis Semen, 0.9375, 1.875 or 3.75 g/kg for 28 days. The levels of serum uric acid (UA), creatinine (Cr), triacylglycerol (TG) and tumor necrosis factor-α (TNF-α) were measured using enzyme-linked immunosorbent assay kits. Ultra performance liquid chromatography quadrupole time of flight mass spectrometry (UPLC-Q-TOF/MS) was used for the serum lipidomics analysis, multivariate statistical analysis and independent samples t-test were carried out for the pattern recognition and characteristic metabolites identification. The relative levels of critical regulatory factors were determined by quantitative real-time polymerase chain reaction (RT-qPCR).
Results
Compared with the model group, the levels of serum UA, Cr, TG and TNF-α were significantly (p < 0.05) decreased in benzbromarone and three Plantaginis Semen groups. With lipidomics analysis, significant lipid metabolic perturbations were observed in HUA rats, 13 metabolites were identified as potential biomarkers and glycerophospholipid metabolism pathway was most affected. These perturbations were partially restored via treatment of benzbromarone and Plantaginis Semen. Additionally, the mRNA expression levels of urate anion transporter 1 (URAT1) and phosphatidylinositol 3-kinase/protein kinases B (PI3K/Akt) were significantly decreased (p < 0.01) after treatment with benzbromarone and high dose of Plantaginis Semen.
Conclusions
Plantaginis Semen had significant effects on anti-HUA, anti-inflammatory and renal protection. It attenuated potassium oxonate-induced HUA through regulation of lipid metabolism disorder.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-021-03350-x.
Keywords: Hyperuricemia, Lipidomics, Plantaginis Semen, Lipid metabolism disorder, Lowering uric acid
Background
Hyperuricemia (HUA), the cause of gout, and is associated with cardiovascular diseases and metabolic diseases such as diabetes hypertension and dyslipidemia [1]. HUA has been observed worldwide, placing a considerable public health burden on the society [2]. There are two categories of medications commonly used for the treatment of HUA: uricosuric agents such as probenecid and benzbromarone, and xanthine oxidase (XOD) inhibitors such as allopurinol [3]. However, allopurinol may produce severe cutaneous reactions and there are some unanswered questions about the pharmacological interactions of probenecid and the hepatotoxicity of benzbromarone [4, 5]. So alternative or complementary therapies are are needed to reduce the risk of HUA-induced attacks of gout.
Plantaginis Semen, the dried ripe seed of Plantago asiatica L. or Plantago depressa Willd., is a common traditional Chinese medicine which can be used to lower serum UA [6–13]. The URAT1 and PI3K/Akt pathway are two important potential targets of Plantaginis Semen in the treatment of HUA. URAT1 plays a central role in renal urate reabsorption and Plantaginis Semen can affect the expression of URAT1 in the kidney [14–17]. The PI3K/Akt pathway is able to trigger inflammatory and kidney injuries, impairing renal excretion of uric acid and HUA [18–24], the treatment of gouty nephropathy with Plantaginis Semen is mainly involved with the PI3K/Akt signaling pathway [25]. HUA also is related to disorders of lipid metabolism, about 60% of patients with HUA have abnormal lipid metabolism [26]. It has been proven that Plantaginis Semen could improve lipid metabolism [27–29]. Although there has been some research, it remains necessary to conduct in-depth studies on the pharmacological effects of Plantaginis Semen in treating HUA and its potential mechanism.
Lipidomics emphasizes the identification of lipid molecular composition in cells, biofluids, tissue, or whole organism and can reflect the changes of lipid metabolism in response to perturbations or stimulations [30, 31]. Lipidomics will assist us to more deeply understand the pathological process of HUA and could be a puissant tool to explore the anti-HUA mechanism of Plantaginis Semen.
In this study, we investigated pharmacological effects of Plantaginis Semen and explored molecular mechanisms of Plantaginis Semen in treating HUA. Our findings preliminarily interpreted the relationship between lipid metabolism and HUA and supplied evidences that Plantaginis Semen may be used for the treatment of HUA in the clinic.
Methods
Chemicals and reagents
Benzbromarone tablets (50 mg/tablet) were provided by Excella GmbH & Co.KG (Nurnberger, Germany). Potassium oxonate was acquired from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Pentobarbital sodium was provided by Tianjin Fuchen Chemical Technology Co., Ltd. (Tianjin, China). LC-MS grade acetonitrile, formic acid, ammonium formate, isopropyl alcohol, methanol were supplied by America Thermo Fisher Scientific Co., Ltd. (Massachusetts, America). Analytical grade ethanol was purchased from America Thermo Fisher Scientific Co., Ltd. (Massachusetts, America). Ultrapure water was made by America Millipore Co., Ltd. Milli-q ultra pure water machine (Massachusetts, America).
Preparation of plantaginis Semen extract
Plantaginis Semen was purchased from Tongrentang Chinese Medicine Company, (Beijing, China, catalogue number:800110551). 100 g Plantaginis Semen was taken and 65% ethanol of 8 times the amount of herbs was added to heat and reflux for 3 times, 2 h each time. The filtrate obtained from three reflux times was mixed and concentrated to 100 mL, containing crude drug content of 1.0 g·mL− 1. The filtrate was refrigerated for later use.
Animal care and experimental design
Specific pathogen free (SPF) grade SD 6 weeks male rats (200 ± 20 g) were bought from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and the certificate number is SCXK (Jing) 2016–0006. Rats were fed with a standard laboratory environment (humidity 40–70% and temperature 20–25 °C) and hold on a 12 h/12 h light/dark cycle during the whole period. All experimental protocols were approved by the ethics committee of Beijing University of Chinese Medicine (with Ethical code: BUCM-4-2,020,111,102-4061).
After the acclimation period, 42 rats were randomly separated into six group (n = 7), including control group (C), model group (M), benzbromarone group (Y), high dose group (CH), medium dose group (CM) and low dose group (CL) of Plantaginis Semen. Random numbers were generated using Microsoft Excel. Because of the high oral bioavailability, the rat HUA model was established by potassium oxonate intragastric administration. At 1 h before drug administration, the rats were given potassium oxonate by intragastric administration according to 1.5 g•kg− 1 dose, the C group was given the corresponding volume distilled water by intragastric administration. Then the CH, CM, and CL groups were orally administered three dosages of Plantaginis Semen (0.9375 g/kg, 1.875 g/kg, 3.75 g/kg). The clinical dosage of Plantaginis Semen in Chinese Pharmacopoeia (Ch P) is 9 ~ 15 g/day [32]. The dose of Plantaginis Semen in rat is calculated by multiplying the human dose (9 g/day = 0.15 g/Kg, 15 g/day = 0.25 g/Kg) by the conversion factor 6.25 [33], the result is 0.9375 ~ 1.5625 g/Kg. In pilot study, we studied the hypouricemic effect of Plantaginis Semen at various dose including 0.47, 0.9375, 1.875 and 3.75 g/Kg, and at these 4 concentrations, Plantaginis Semen all showed the effect of lowering UA. In consideration of cost, we used 0.9375, 1.875 and 3.75 g/kg in the study. The Y group was treated with benzbromarone (10 mg/kg) once a day for 28 days. Blood and kidney were collected and prepared for ELISA and RT-qPCR. Test time was between 10 am to 6 pm and testing order was randomized daily. After the experiment, the rats were sacrificed by dislocation of spine, which approved by the animal care and use committee.
Serum biochemistry analysis
On the 28th day of modeling, rats were anesthetized with 2% pentobarbital sodium (0.3 mL/100 g, intra-peritoneally). Blood samples were taken from the abdominal aorta by a vacuum blood collection tube, and centrifuged for 10 min (3000 rpm, 10 °C). Serum samples were stored at − 80 °C for analysis. The levels of serum UA, Cr, TG and TNF-α were measured using commercially available kits (Jiancheng, Nanjing, China) according to the manufacturer’s instructions.
Serum UPLC-Q-TOF/MS analysis
Serum samples were thawed at room temperature before data acquisition. Protein precipitation was performed by adding 320 μL solvent mixture (chloroform/methanol 3:1,V/V) to 80 μL serum in 1.5 mL eppendorf tubes. The mixture was centrifuged at 10,000 rpm for 10 min at room temperature. The supernatant was taken for UPLC-Q-TOF/MS analysis.
The UPLC column was ACQUITY CSH C18(2.1 × 100 mm,1.7 μm, Waters Corp., Milford, MA, USA) with the column temperature maintained at 40 °C and the flow rate was set at 0.3 mL•min− 1. The mobile phase A is 10 mM Ammonium acetate in acetonitrile/water/ formic acid (60:40:0.1, v/v/v), mobile phase B is 0.1% formic acid in isopropanol/acetonitrile (90:10, v/v). The gradient elution programme was as follows: 0.0 ~ 3.1 min 60 ~ 57% A, 3.1 ~ 4.1 min 57 ~ 30% A, 4.1 ~ 4.3 min 30 ~ 27% A, 4.3 ~ 8.0 min 27 ~ 23% A, 8.0 ~ 8.1 min 23–60% A, 8.1 ~ 10.0 min 60% A. Every 2 μl sample solution was injected for each run.
MS analysis was performed by Xevo G2-S Q/TOF MS (Waters Corp., Milford, MA, USA) system. The ionization mode is electrospray ionization and was set in positive modes. The mass conditions were as follows: the capillary and cone voltage were set at 2.5 KV and 30 V, the source temperature was 120 °C, the desolvated gas flow was 800 L/h at a temperature of 400 °C and the cone gas flow was 20 L/h. MS data were collected in the full scan mode from m/z 50–1200 amu. Both UPLC and MS conditions refer to the previous research of group [34].
Data processing
The raw data files acquired from the UPLC-Q/TOF-MS measurements were normalized using Micromass MarkerLynx Applications Manager version 4.1 software (Waters Corporation, MA, USA). This software can automatically complete noise filter, peak identification, peak matching and normalization under optimized parameters. The parameters of process were shown as follows: mass window is 20 mDa, retention time window is 0.50 min, marker intensity threshold is 10,000 counts, peak width at 5% height is 1.00s, noise elimination level is 6.00 [34]. Subsequently, an integrated metabolite data matrix as Excel spreadsheets composed of m/z, retention time and the corresponding peak area was generated. The normalized dataset was imported into SIMCA 13.0 (Umetrics, Sweden) for multivariate statistical analysis and SPSS Statistics 21.0 for univariate data analysis. The multivariate analysis results are expressed in the form of scores plot to observe the global clustering trends of various groups and visualize their distributions. The model parameters including R2 (goodness of fit) and Q2 (goodness of prediction) calculated from the PCA and OPLS-DA models were used to evaluate the quality of models. Finally, potential biomarkers were filtered by the results of variable importance for the projection (VIP) values (VIP > 1) and t-test values (p < 0.5). The above-mentioned biomarkers are deteced by Mass Fragment software combined with HMDB [35] and Lipid Maps [36] database. First, input the precise molecular mass, ionization method, and adduction information of potential biomarkers into HMDB and Lipid Maps, in accordance with the rule that the deviation of the mass-to-charge ratio (m/z) value does not exceed 0.02, the search results are verified by combining the exact number of charges and the ionization method that meet the experimental conditions. Secondly, compare the primary and secondary mass spectra of the potential biomarkers with the theoretical fragments of the HMDB search results, then infer the structure of the compound and the attribution of the fragments to obtain the HUA biomarkers. Finally, the pathway analysis of potential biomarkers and establishment of correlation metabolic networks were performed with Metabo Analyst [37] and KEGG database [38].
Measurement data are expressed by mean ± standard deviation (SD). SPSS 21.0 is used for data processing and statistical analysis. The comparison of means among multiple groups is performed by one-way analysis of variance (ANOVA). When the homogeneity of variance assumptions was satisfied, pairwise comparison between groups is performed by Tukey test, otherwise, nonparametric tests was used., P < 0.05 were considered as statistically significant. The graphs were drawn with GraphPad Prism 8.0.1.
RT-qPCR
RNA from kidney tissue was extracted by Hipure Total RNA Mini Kit (Magen, Guangzhou, China). RT-qPCR was carried out using TB Green Primix Ex TaqII kit (Takara, Kyoto-fu, Japan) in Bio-Rad CFX96 Real Time PCR System (BIO-RAD, Shanghai, China). The primers used in the study are: URAT1, Forward: 5′ − CTCTGCTGGTGTATGGAGTGG-3′, Reverse: 5′ − TTTCTGGATGTCTTGGATGGT-3′, PI3K, Forward:5′ − GGTTCTTGCGAAGTGAGATAGCCC-3, Reverse:5′ − ACCTGCTGCGTGAAGTCCTGTA-3′, Akt, Forward:5′ − TGTCTCGTGAGCGCGTGTTTT-3′, Reverse:5′ − CCGTTATCTTGATGTGCCCGTC-3′. Ct (cycle threshold) value was collected. Detected in triplicate and the relative expression levels of genes were determined by the 2−△△Ct method.
Results
The analyses of rat serum biochemistry were undertaken to evaluate the lowering UA, anti-inflammatory and renal protection effects of Plantaginis Semen. Lipidomics analysis was undertaken to evaluate the lipid metabolic profile of rats, and the mRNA expression of PI3K/Akt and URAT1 were determined to explore the UA lowering mechanism of Plantaginis Semen.
Plantaginis semen decreased the level of serum UA in HUA rats
After 28 days, rats fed potassium oxonate showed a significant change in the level of serum UA (Fig. 1a). As compared to the C group (95.79 ± 16.74 umol•L− 1), the level of serum UA in the M group (178.66 ± 8.24 umol•L− 1) increased significantly (p < 0.01), which represents the success of the model. Conversely, the level of serum UA in the Y (137.70 ± 15.74 umol•L− 1), CL (149.46 ± 11.23 umol•L− 1) and CH (139.62 ± 6.99 umol•L− 1) groups was significantly (p < 0.01) reduced except CM group (159.86 ± 9.02 umol•L-1, p>0.05), compared to the M group. The serum UA level of HUA rats could not be significantly (p>0.05) reduced in CM group, in addition, there were no significant differences of lowering UA effect between CL and CH group (p>0.05, supplementary material, Table 1.4). These results indicated that Plantaginis Semen could lower serum UA level.
Fig. 1.
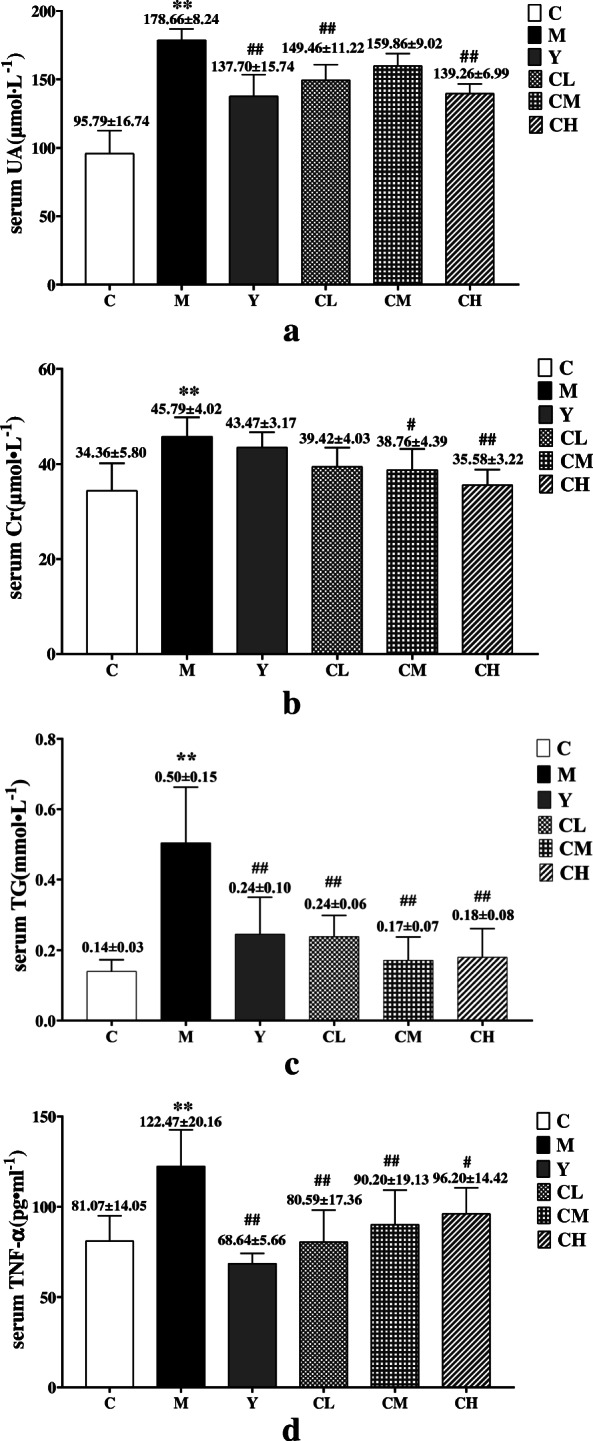
The results of serum biochemistry analysis. a UA level, b Cr level, c TG level, d TNF-α level. C, control group; M, model group; Y, benzbromarone group; CL, low dosage group; CM, medium dosage group; CH, high dosage group; values are given as the mean ± SD (n = 7), ANOVA, Tukey post hoc and nonparametric test were used for statistical analysis, **, P < 0.01 vs. control group. *, P < 0.05 vs. control group; ##, P < 0.01 vs. model group; #, P < 0.05 vs. model group
Plantaginis Semen exerted renal protection effect in HUA rats
Serum Cr was used to assess kidney function and reflects the extent of renal injury [39]. The results depicted in Fig. 1b show that the level of serum Cr in the M group (45.79 ± 4.02 μmol•L− 1) was significantly (p < 0.01) increased, as compared to that observed for the C group (34.36 ± 5.80 μmol•L− 1). After receiving treatment for 28 days, the serum Cr level in the CM (38.76 ± 4.39 μmol•L− 1) and CH (35.58 ± 3.22 μmol•L− 1) groups was significantly (p < 0.05) reduced, but the serum Cr level in the CL (39.42 ± 4.03 μmol•L− 1) and Y (45.79 ± 4.02 μmol•L− 1) groups were insignificantly (p>0.05) reduced compared with M group. The serum Cr level of HUA rats could not be significantly (p>0.05) reduced in CL group, in addition, there were no significant differences of lowering Cr effect between CM and CH group (p>0.05, supplementary material, Table 2.4). The above results indicated that the medium and high doses of Plantaginis Semen had good protective effect on renal.
Plantaginis Semen ameliorated serum TG accumulation in HUA rats
The level of Serum TG was remarkably (p < 0.01) elevation in the M group (0.50 ± 0.15 mmol/L) as compared with C group (0.14 ± 0.03 mmol/L). Then, over 28 days of treatment, the TG levels in the Y (0.25 ± 0.10 mmol/L), CH (0.18 ± 0.08 mmol/L), CM (0.17 ± 0.07 mmol/L), and CL (0.24 ± 0.06 mmol/L) groups all were significantly (p < 0.01) reduced compared to that in the M group. The results were depicted in Fig. 1c. The results of multiple comparisons showed that there were no significant differences in the serum TG level among CL, CM and CH groups (p>0.05, supplementary materia, Table 3.4). Plantaginis Semen had good effect on lowering blood lipids.
Plantaginis Semen reduced the level of TNF-α in HUA rats
The level of TNF-α in the serum was used to evaluate the anti- inflammatory effect of Plantaginis Semen in vivo. As shown in Fig. 1d, the level of TNF-α in the M group (122.47 ± 20.16 pg•mL− 1) significantly (p < 0.01) higher than that in the C group (81.07 ± 14.05 pg•mL− 1). The level of TNF-α in Y group (68.64 ± 5.66 pg•mL− 1), CL group (80.59 ± 17.63 pg•mL− 1), CM group (90.20 ± 19.13 pg•mL− 1) and CH group (96.20 ± 14.42 pg•mL− 1) were significantly decreased compared to M group (p < 0.05), and Tukey post hoc showed that there were no significantly differences in the level of TNF-α among CL, CM and CH groups (p>0.05, supplementary material, Table 4.4). These results indicated that Plantaginis Semen had good anti-inflammatory effect.
Metabolic perturbations and differential metabolites associated with HUA rats
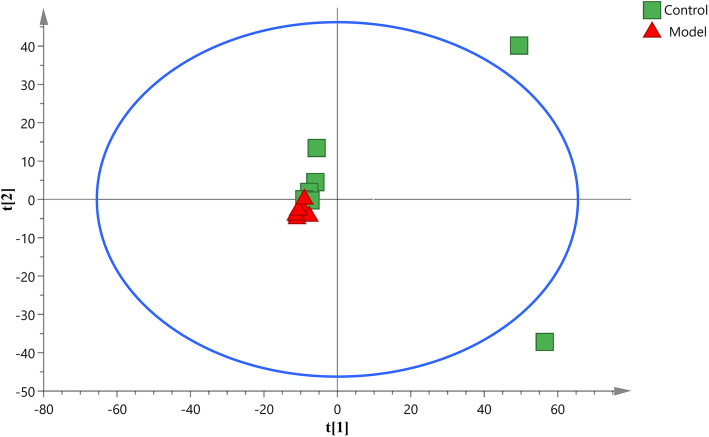
PCA is an unsupervised pattern recognition method that can be used to select different variables and find possible biomarkers, in order to investigate the effect of potassium oxonate on endogenous components changes, PCA was used to perform unsupervised data analysis on control and model groups (Fig. 2). These groups cannot be easily distinguished from each other. Therefore, we further used OPLS-DA to compare the serum samples obtained from the model and control groups. Import the normalized dataset of C and M group into the SIMCA, as shown in the OPLS-DA score plots of serum samples (Fig. 3a), a separation between the model group and the control group could be clearly seen, indicating that the HUA model was successful and had a completely different metabolic profiles compared with the healthy controls. The parameters of the OPLS-DA models were as follows: R2Y = 0.936 and Q2 = 0.737. The R2Y and Q2 values reflect excellent predictability and explain the differences between the control and model groups. In addition, 200-iteration permutation tests were also performed to assess the robustness of the OPLS-DA models (Fig. 3b). The validation plots showed that the original OPLS-DA models were not random and overfitted as both permutated Q2 and R2 values were lower than the corresponding original values along with the Y-intercepts of the regression lines of the Q2-points below zero.
Fig. 2.
The PCA score plot derived from UPLC-Q-TOF/MS profiles of serum sample from control group and model group
Fig. 3.
OPLS-DA score plots (a) and the corresponding validation plots (b) with 200 times permutation tests obtained
Potential biomarkers were identified from the interactions between control and model groups using the corresponding S-plot analysis under OPLS-DA model. The metabolites whose VIP values> 1 and p values< 0.05 of OPLS-DA were presumed as significant differences. Databases such as KEGG and HMDB were used to identify potential bio-markers, along with UPLC-Q-TOF/MS information. The results are shown in Table 1. 13 metabolites were identified, including 4 phosphatidylcholines (PCs), 4 haemolytic phosphatidylcholines (LPCs), 2 phosphatidylethanolamines (PEs), 2 TGs, 1 cholesterol ester (CE). Compared with C group, the metabolic perturbations occurring in serum of the HUA rats were mainly characterized by increased levels of these lipids.
Table 1.
Identification results of differential metabolites and their change trends
| No. | RT(min) | m/z | Formula | Metabolite | VIP | M vs. C | Y vs. M | CL vs. M | CM vs. M | CH vs. M |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.5875 | 898.7862 | C57H100O6 | TG(18:2/18:1/18:2) | 2.75 | ↑** | – | – | ↓# | ↓## |
| 2 | 8.9231 | 931.7526 | C55H92O6 | TG(20:4/14:0/18:3) | 2.55 | ↑** | – | – | – | – |
| 3 | 8.4467 | 925.7098 | C48H92NO8P | PC(24:1/16:1) | 2.54 | ↑** | – | – | ↓# | – |
| 4 | 2.6081 | 496.3364 | C50H94NO8P | PC(24:1/18:2) | 2.43 | ↑** | – | – | ↓## | ↓# |
| 5 | 9.0509 | 264.9627 | C41H82NO8P | PC(15:0/18:0) | 2.29 | ↑** | – | – | ↓# | – |
| 6 | 9.0937 | 326.9082 | C54H102NO8P | PC(24:1/22:2) | 2.70 | ↑** | ↓## | – | ↓## | ↓## |
| 7 | 1.8681 | 516.3129 | C24H48NO7P | LPC(16:1/0:0) | 2.35 | ↑** | – | – | – | – |
| 8 | 2.0389 | 610.3188 | C30H54NO7P | LPC(22:4/0:0) | 2.56 | ↑** | – | ↓# | – | – |
| 9 | 2.0460 | 542.3279 | C26H50NO7P | LPC(18:2/0:0) | 2.37 | ↑** | – | ↓## | – | – |
| 10 | 4.1085 | 273.6606 | C26H54NO7P | LPC(18:0/0:0) | 2.09 | ↑* | – | – | – | – |
| 11 | 9.3573 | 416.2983 | C45H76NO8P | PE(18:3/22:4) | 2.15 | ↑** | – | – | ↓# | ↓# |
| 12 | 9.0937 | 326.9082 | C53H102NO8P | PE(24:1/24:1) | 2.43 | ↑* | – | – | ↓## | ↓## |
| 13 | 9.0230 | 376.8943 | C51H90O2 | CE(24:1) | 2.60 | ↑* | ↓# | – | ↓## | ↓## |
C, control group; M, model group; Y, benzbromarone group; CL, low dosage group; CM, medium dosage group; CH, high dosage group; **, p < 0.01 vs. control group. *, p < 0.05 vs. control group; ##, p < 0.01 vs. model group; #, p < 0.05 vs. model group. (↑): up-regulated and (↓): down-regulated. (−):no statistically significant difference
Metabolic changes under the treatment of benzbromarone and Plantaginis Semen
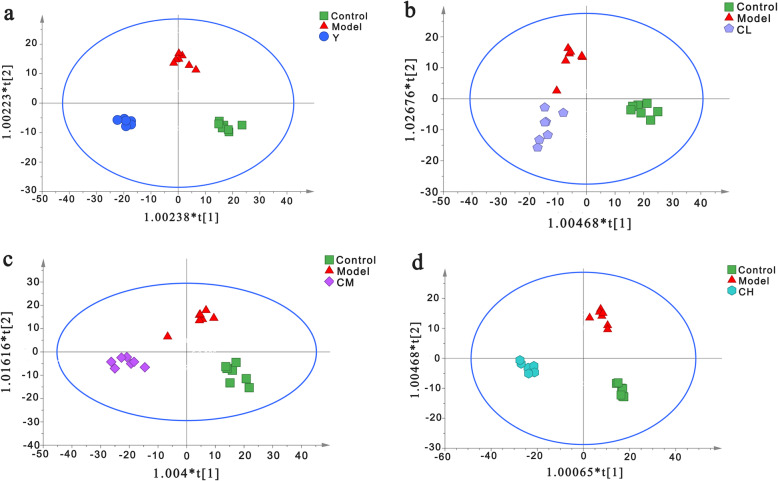
We import the normalized dataset of C group, M group and other 4 experimental groups into the SIMCA separately, Fig. 4 showed distinct metabolic profiles among different groups and there is a tendency to return to the normal group in benzbromarone-treated and different dose Plantaginis Semen-treated groups. The parameters of the OPLS-DA models were as follows: R2Y = 0.979 and Q2 = 0.830 (Fig. 4a), R2Y = 0.911 and Q2 = 0.709 (Fig. 4b), R2Y = 0.930 and Q2 = 0.781 (Fig. 4c), R2Y = 0.977 and Q2 = 0.829 (Fig. 4d). Heatmap analysis was produced to intuitively compare the relative content of 13 potential metabolites among 6 groups referring to Table 1 (Fig. 5). Control group and model group clearly distinguished, the color depth of benzbromarone group and Plantaginis Semen groups were close to control group, indicating that the performance on the callback of these metabolites is extraordinary obvious. 2, 2, 8 and 6 differential metabolites were significantly (p < 0.05) reversed by benzbromarone, low dose Plantaginis Semen, medium dose Plantaginis Semen, high dose Plantaginis Semen respectively (Fig. 6). Relative intensities of 13 differential metabolites in serum samples of 6 groups are listed in Table 2, the specific p-values are provided in the supplementary material Table 8-Table 20. These findings suggested that the metabolic perturbations induced by HUA could be normalized by benzbromarone and Plantaginis Semen treatment. Among them, the effect of normalizing differential metabolites of middle dose Plantaginis Semen group was the best.
Fig. 4.
Metabolic profiles of rat serum in the control, model, benzbromarone, and different dose Plantaginis semen groups. a Metabolic profiles of rat serum in C, M and Y group, b Metabolic profiles of rat serum in C, M and CL group, c Metabolic profiles of rat serum in C, M and CM group, d Metabolic profiles of rat serum in C, M and CH group. C, control group; M, model group; Y, benzbromarone group; CL, low dosage group; CM, medium dosage group; CH, high dosage group
Fig. 5.
Heat map analysis of relative contents of potential metabolites. (green through dark red corresponding to a progressive increase in concentration). C, control group; M, model group; Y, benzbromarone group; CL, low dosage group; CM, medium dosage group; CH, high dosage group
Fig. 6.
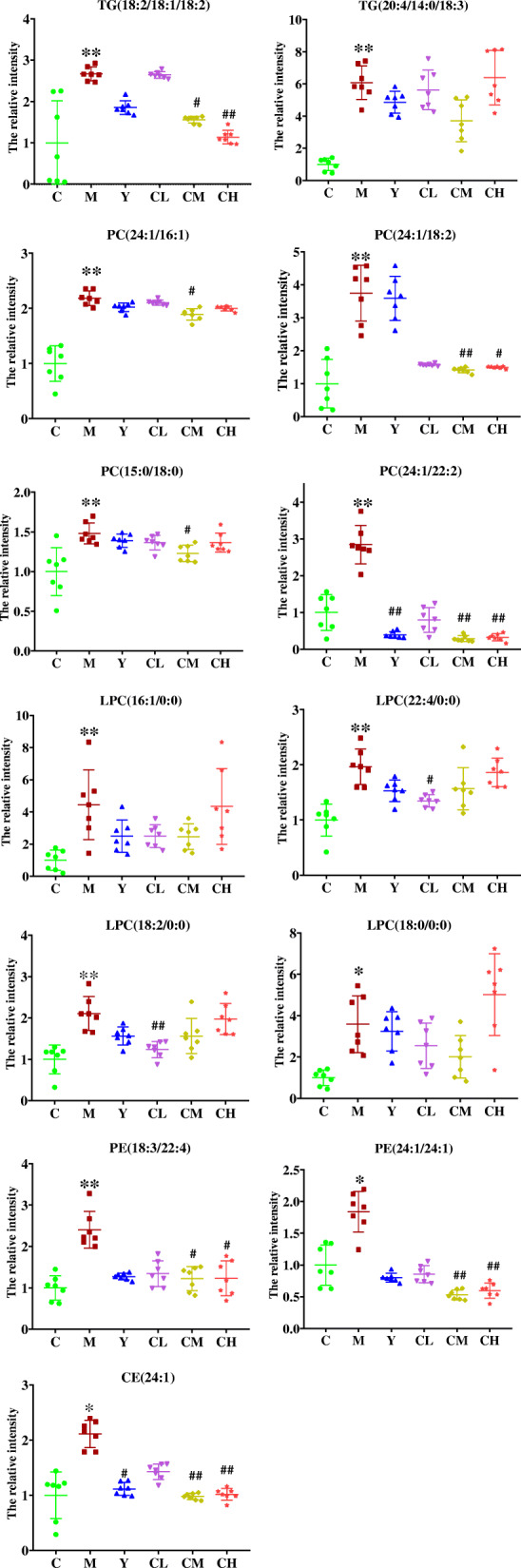
Comparison of 13 biomarkers peak relative signal intensities in 6 groups. C, control group; M, model group; Y, benzbromarone group; CL, low dosage group; CM, medium dosage group; CH, high dosage group; boxplots show the 25, 50 and 75% percentiles. ANOVA, Tukey post hoc and nonparametric test were used for statistical analysis, **, P < 0.01 vs. control group. *, P < 0.05 vs. control group; ##, P < 0.01 vs. model group; #, P < 0.05 vs. model group
Table 2.
Relative intensities of 13 differential metabolites in serum samples of 6 groups
| Differential metabolites | C | M | Y | CL | CM | CH |
|---|---|---|---|---|---|---|
| TG(18:2/18:1/18:2) | 1.00 ± 1.01 | 2.67 ± 0.17** | 1.85 ± 0.16 | 2.64 ± 0.08 | 1.56 ± 0.08# | 1.14 ± 0.17## |
| TG(20:4/14:0/18:3) | 1.00 ± 038 | 6.08 ± 1.05** | 4.86 ± 0.68 | 5.63 ± 1.23 | 3.71 ± 1.30 | 6.39 ± 1.68 |
| PC(24:1/16:1) | 1.00 ± 0.32 | 2.18 ± 0.13** | 2.02 ± 0.08 | 2.11 ± 0.45 | 1.89 ± 0.11# | 2.00 ± 0.04 |
| PC(24:1/18:2) | 1.00 ± 0.73 | 3.78 ± 0.85** | 3.59 ± 0.67 | 1.57 ± 0.34 | 1.41 ± 0.79## | 1.50 ± 0.03# |
| PC(15:0/18:0) | 1.00 ± 0.30 | 1.48 ± 0.13** | 1.39 ± 0.08 | 1.36 ± 0.09 | 1.23 ± 0.10# | 1.37 ± 0.12 |
| PC(24:1/22:2) | 1.00 ± 0.48 | 2.85 ± 0.52** | 0.39 ± 0.09## | 0.79 ± 0.33 | 0.29 ± 0.08## | 0.32 ± 0.10## |
| LPC(16:1/0:0) | 1.00 ± 0.62 | 4.45 ± 2.16** | 2.49 ± 1.00 | 2.49 ± 0.71 | 2.46 ± 0.80 | 4.34 ± 2.35 |
| LPC(22:4/0:0) | 1.00 ± 0.29 | 1.96 ± 0.32** | 1.53 ± 0.19 | 1.35 ± 0.11# | 1.57 ± 0.38 | 1.86 ± 0.26 |
| LPC(18:2/0:0) | 1.00 ± 0.35 | 2.11 ± 0.41** | 1.56 ± 0.22 | 1.23 ± 0.19## | 1.56 ± 0.43 | 1.97 ± 0.37 |
| LPC(18:0/0:0) | 1.00 ± 0.37 | 3.59 ± 1.37* | 3.24 ± 0.94 | 2.55 ± 1.09 | 2.01 ± 1.03 | 5.02 ± 0.97 |
| PE(18:3/22:4) | 1.00 ± 0.30 | 2.40 ± 0.44** | 1.27 ± 0.08 | 1.34 ± 0.31 | 1.22 ± 0.29# | 1.23 ± 0.42# |
| PE(24:1/24:1) | 1.00 ± 0.32 | 1.84 ± 0.32* | 0.80 ± 0.07 | 0.86 ± 1.13 | 0.53 ± 0.08## | 0.60 ± 0.12## |
| CE(24:1) | 1.00 ± 0.42 | 2.11 ± 0.25* | 1.11 ± 0.11# | 1.43 ± 0.14 | 0.98 ± 0.57## | 1.02 ± 0.11## |
The average peak area of potential biomarkers in the control group was set as 1. C, control group; M, model group; Y, benzbromarone group; CL, low dosage group; CM, medium dosage group; CH, high dosage group; ANOVA and nonparametric test were used for statistical analysis, **, p < 0.01 vs. control group. *, p < 0.05 vs. control group; ##, p < 0.01 vs. model group; #, p < 0.05 vs. model group
Metabolic pathways related to potential biomarker
The 13 potential biomarkers were found to be primarily involved in 6 disturbed metabolic pathways. Based on the impact value greater than 0.1 and p value less than 0.05, glycerophospholipid metabolism was considered as the most relevant pathways in potassium oxonate-induced HUA (Fig. 7) and a global metabolic network was mapped (Fig. 8).
Fig. 7.

six pathway related to changed biomarkers. a: Glycerophospholipid metabolism, b: Linoleic acid metabolism, c: Glycosylphosphatidylinositol (GPI)-anchor biosynthesis, d: alpha-Linolenic acid metabolism, e: Arachidonic acid metabolism, f: Steroid biosynthesis
Fig. 8.
KEGG global metabolic network related to changed biomarkers. The purple textboxes represented the pathways, the yellow and green textboxes represented the significant and no detection metabolites. The arrows in red represented the up regulated metabolites. The arrows in blue represented direct or indirect connections between two metabolites
Plantaginis Semen downregulated the mRNA expression of PI3k, Akt, URAT1 in HUA rats
The mRNA expressions of PI3K, Akt and URAT1 in rat renal were shown in Fig. 9. The contents of URAT1 and PI3K/Akt were significantly (P < 0.01) increased in M group compared with those in C group. After 28 days administration, the Y group and three Plantaginis Semen groups all showed significantly (P < 0.01) down-regulation in the expressions of URAT1 mRNA, and the ability of inhibiting URATI mRNA expression among different Plantaginis Semen groups had no significantly differences (p>0.05, supplementary material, Table 5.4). In addition, only CH group could significantly (P < 0.01) decrease the mRNA expressions of PI3K and Akt in HUA rat, low and medium doses of Plantaginis Semen had no obvious inhibitory effect on the mRNA expression of PI3k and Akt, and there were no significant differences between the two groups (p>0.05; supplementary material, Table 6.3 and 7.4). The experimental results showed that the high dose Plantaginis Semen could inhibt the mRNA expressions of URAT1 and PI3K/Akt pathway.
Fig. 9.

Effects of Plantaginis semen on PI3k/Akt and UTAR1 mRNA expression. a mRNA expression levels of URAT1, b mRNA expression levels of PI3k, c mRNA expression levels of Akt. C, control group; M, model group; Y, benzbromarone group; CL, low dosage group; CM, medium dosage group; CH, high dosage group; values are given as the mean ± SD(n = 7), ANOVA, Tukey post hoc and nonparametric test were used for statistical analysis, **, P < 0.01 vs. control group. *, P < 0.05 vs. control group; ##, P < 0.01 vs. model group; #, P < 0.05 vs. model group
Discussion
Serum biochemical indicators were used to confirm the effects of Plantaginis Semen on the metabolism of lipid and anti-HUA. The serum UA levels of rats were significantly increased after potassium oxonate administration for 4 weeks. While increasing the serum UA of the rats, the levels of serum TNF-α, Cr and TG also increased. Compared with M group, different dose of Plantaginis Semen treatment could achieve varying degrees of down-regulation in the levers of serum UA in HUA rats and also reduced their serum Cr, TG and TNF-α concentration HUA is characterized by high serum UA level, urate crystals deposit in the blood vessel walls and kidneys that causes chronic inflammatory damage, releasing large amounts of inflammatory factors, such as TNF-α, IL-6 [40]. Plantaginis Semen polysaccharides ameliorated renal damage and decreased the inflammatory response in gouty nephropathy rats through the down-regulation of the protein expression levels of NLRP3, ASC and caspase-1 and inhibit the release of downstream inflammatory factors [41]. HUA is closely related to hyperlipidemia (HPA) and lipid metabolic disorder [42, 43], Lan JP et al. found that Plantaginis Semen had anti-obesity effects and could decrease serum TG and effectively improve lipid metabolism in high-fat diet-induced obese mice [44]. The results of serum biochemistry analysis in this study also suggested the elevated serum UA levels may contribute to the development and progression of chronic kidney disease, inflammatory and disordered lipid metabolism, Plantaginis Semen can alleviate the pathological state and exhibits hypouricemic, nephroprotective, anti-inflammation and regulation of lipid disorders effects.
UPLC-Q/TOF-MS-based Lipidomics was performed to discover distinct lipid metabolites and metabolic pathways related to HUA. 1 CE,2 TGs, 4 PCs, 4 LPCs, 2PEs were identified as biomarkers and glycerophospholipid metabolism pathway was mostly affected.
The results of lipidomics showed that the CE (24:1) content in the serum of HUA rats was significantly increased. The liver contains the most abundant cholesteryl ester hydrolase (CEH), so it is the main organ of cholesterol ester metabolism [45]. The abnormal improvement of serum CE (24:1) levels in HUA rats suggests that cholesterol ester metabolism in the liver may be affected under high serum UA environment. Under physiological conditions UA presents antioxidant properties, and HUA has been linked to oxidative stress, chronic low-grade inflammation, and insulin resistance, basic signs of non-alcoholic fatty liver disease (NAFLD) [20, 46]. HUA combined with NAFLD is the result of HUA progression, up-regulated phosphatidic acid and CE (18:0) and down-regulated inosine in the serum have been identified as the potential biomarkers for the progression from HUA to HUA + NAFLD [47]. In this study, we have also observed an upward tendency of CE (24:1). CE is involved in Liver X receptor/retinoic X receptor (LXR/RXR) activation, which plays an important role in keeping the cholesterol balance outside and inside the cell [48]. Given the results of lipidomics, liver may be preferentially targeted in HUA besides kidney and elevated serum UA levels may impair liver function and possibly act through LXR/RXR to influence cholesterol metabolism. In addition, high level of UA causes inflammation in the body, inflammatory stress exacerbates hepatic cholesterol accumulation via disrupting cellular cholesterol export [49], which further indicated that elevated UA level would lead to liver function damage and affected lipid metabolism. The level of CE (24:1) in Plantaginis Semen groups decreased, which domonstrated that Plantaginis Semen can protect liver and improve cholesterol metabolism. Genipidic acid contained in Plantaginis semen is the main component to exert these pharmacological action [50, 51].
Lipid metabolism disorder is closely related to HUA. HUA patients with HPA are typical in the clinical setting [52]. An investigation on the efficacy of Plantaginis Semen based on UPLC-QTOF-MS metabolomics approach in HPA mice showed Plantaginis Semen can improve blood lipids. In their study, serum levels of TG in HPA mice significantly increased, TG (16:1/16:0/o-18:0) was considered to be a potential biomarker and its content in the serum can be reduced by Plantaginis Semen [11]. Similarly, both An Peng et al. and Renhao Chen et al. found the level of TG increased in HUA model and glycerol tributanoate and Sn-glycerol-3P could be recognized as biomarker of HUA [53, 54]. In this study, M group had higher serum TG (18:2/18:1/18:2) and TG (20:4/14:0/18:3) levels than C group (M vs. C, p < 0.01), the treatment with Plantaginis Semen (at medium/high dose) effectively reduced the serum TG (18:2/18:1/18:2) levels of the HUA rats (CM/CH vs. M, p < 0.05). The results demonstrated that potassium oxonate disturbed lipid metabolism while Plantaginis Semen could accelerate the process of fat decomposition to alleviate lipid metabolism. Lipid-lowering therapy may provide a supplementary role to slow the development of HUA.
PC, LPC and PE were mainly involved in glycerophospholipids metabolism and their levels were obviously increased in HUA rats. LPCs are biosynthesized from PCs through glycerophospholipid metabolism, this process is catalyzed by cytoplasmic phospholipase A2 (PLA2) and can be activated by UA [55]. UA can activate the phospholipid-remodeling enzymes LPCAT3 in vivo and in vitro, and LPCAT3 possesses primary LPC acyltransferase activity and catalyzes the production of PCs [56]. The enhancement of PLA2 and LPCAT3 activity results in accelerated glycerophospholipid metabolism, so the levels of PC and LPC were both up-regulated in HUA rats. In our previous studies, PEs are key biomarkers of potassium oxonate induced HUA rats [57]. Interestingly, we also found that PE(18:3/22:4) and PE(24:1/24:1) are the key biomarkers in the treatment of HUA with Plantaginis Semen this time. In another study, the metabolism of phospholipids is seriously disturbed in the HUA mice and hydrolysis of glycerophospholipids are restrained, causing the up-regulation in the concentration of glycerophospholipids like PCs and PEs and the down-regulation in lysophospholipids and free fatty acid [58]. The results of the two studies are not completely consistent, the effect of HUA on PE metabolism needs to be further studied.
The results of lipidomics indicated that lipid metabolism disorder occurred in HUA rats, whereas, the changes were reversed under the Plantaginis Semen treatment. From a holistic perspective, variations in the metabolite profiles of different groups showed that Plantaginis Semen could enhance the metabolism of endogenous substances in HUA rats, which may be the potential mechanism of Plantaginis Semen in the treatment of HUA.
URAT1 is a key target for the treatment of HUA, and the metabolism of UA in kidney is completed by glomerular filtration, proximal tubule absorption and secretion, and mainly depends on the uric acid transporter to achieve [15]. The activation of PI3K/Akt pathway releases TNF-α, which plays an important role in inflammation caused by HUA [59]. Different doses of Plantaginis Semen all significantly inhibited the mRNA expression of URAT1 in the renal tissue of HUA rats and high dose of Plantaginis Semen possesses inhibitory activity of PI3K/Akt pathway. The results of PCR indicated that Plantaginis Semen may achieve the effect of treating HUA by regulating the expression level of URAT1, promoting uric acid excretion and reducing body inflammation. However, the mRNA level is not necessarily correlate with protein expression and the effects of Plantaginis Semen on URAT1 and PI3K/Akt pathway still needs to be further verified.
Although the toxicity of Plantaginis Semen has not been reported yet, Plantaginis Semen may have potential side effects due to its no obvious dose-dependent trend in this study. This section will be conducted in our future research. In summary, Plantaginis Semen has significant effects in reducing uric acid, protecting the kidneys and regulating lipid metabolism, our study exhibits its applicability and superiority in the treatment of HUA.
Conclusions
Plantaginis Semen had significant anti-HUA, anti-inflammatory and renal protection effects and could attenuate potassium oxonate-induced HUA through regulation of lipid metabolism disorder. In addition, Plantaginis Semen could play anti-HUA effect through URAT1 and PI3K/Akt pathway, but the mechanism needs to be further studied.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- ANOVA
One-way analysis of variance
- CE
Cholesterol ester
- CEH
Cholesteryl ester hydrolase
- Cr
Creatinine
- HUA
Hyperuricemia
- HPA
Hyperlipidemia
- PLA2
Cytoplasmic phospholipase A2
- LPC
Hemolytic phosphatidylcholine
- NAFLD
Non-alcoholic fatty liver disease
- OPLS-DA
Orthogonal partial least squares discriminant analysis
- Ox-LDL
Oxidized low-density lipoprotein
- PCA
Principal component analysis
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- PI3K/Akt
Phosphatidylinositol 3-kinase/ protein kinases B
- RT
Retention time
- RT-qPCR
Quantitative real-time polymerase chain reaction
- SPF
Specific pathogen free
- SD
Sprague-Dawley
- TC
Total cholesterol
- TG
Triacylglycerol
- TNF-α
Tumor necrosis factor-α
- UA
Uric acid
- UPLC−Q-TOF/MS
Ultra performance liquid chromatography quadrupole time of flight mass spectrometry
- URAT1
urate anion transporter 1
- VIP
Variable importance in the projection
- XOD
Xanthine oxidase
Authors’ contributions
FY and XY established hyperuricemia rat model and collected serum samples. LTW collected serum samples and measured serum biochemical indicators. NKQ collected and analyzed UPLC-Q-TOF/MS data. CXW and JF used simca-p software for OPLS-DA analysis. YYG found and identified differential metabolites. GX conducted metabolic pathway enrichment analysis. WJS performed the RT-PCR examination of the kidney, and was a major contributor in writing the manuscript. QM conducted trial design and guidance. All authors have read and approved the final manuscript.
Funding
This research was funded by National Key R& Program of China, grant number 2018YFC1706800. The founding sponsor had no role in the study design, performance, data collection and analysis, decision to publish, or writing of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The experimental protocol was approved by the ethics committee of Beijing University of Chinese Medicine (Ethical code:BUCM-4-2020111102-4061).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fei Yang and Wenjun Shi contributed equally to this work.
References
- 1.Dalbeth N, Merriman TR, Stamp LK. Lancet. 2016;388(10055):2039–2052. doi: 10.1016/S0140-6736(16)00346-9. [DOI] [PubMed] [Google Scholar]
- 2.Smith E, March L. A systematic review of population-based epidemiological studies. Arthritis Rheumatol. 2015;67:3. doi: 10.1002/art.39048. [DOI] [Google Scholar]
- 3.Otani N, Ouchi M, Kudo H, Tsuruoka S, Hisatome I, Anzai N. Recent approaches to gout drug discovery: an update. Expert Opin Drug Discov. 2020;15(8):943–954. doi: 10.1080/17460441.2020.1755251. [DOI] [PubMed] [Google Scholar]
- 4.Strilchuk L, Fogacci F, Cicero AF. Safety and tolerability of available urate-lowering drugs: a critical review. Expert Opin Drug Saf. 2019;18(4):261–271. doi: 10.1080/14740338.2019.1594771. [DOI] [PubMed] [Google Scholar]
- 5.Stamp LK, Haslett J, Frampton C, White D, Gardner D, Stebbings S, Taylor G, Grainger R, Kumar R, Kumar S, Kain T, Porter D, Corkill M, Cathro A, Metcalfe S, Wyeth J, Dalbeth N. The safety and efficacy of benzbromarone in gout in Aotearoa New Zealand. Intern Med J. 2016;46(9):1075–1080. doi: 10.1111/imj.13173. [DOI] [PubMed] [Google Scholar]
- 6.Kong LD, Cai Y, Huang WW, Cheng CH, Tan RX. Inhibition of xanthine oxidase by some Chinese medicinal plants used to treat gout. J Ethnopharmacol. 2000;73(1–2):199–207. doi: 10.1016/S0378-8741(00)00305-6. [DOI] [PubMed] [Google Scholar]
- 7.Zhang XY, Zhang XY, Cheng J, Zhao P, Chen KL, Li J. Screening the best compatibility of Selaginella moellendorffii prescription on hyperuricemia and gouty arthritis and its mechanism. Evid Based Complement Alternat Med. 2019;2019:7263034. doi: 10.1155/2019/7263034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng JX, Wang J, Zhang SW, Zhu JX, Li M, Huang WH, et al. Antigout effects of Plantago asiatica: xanthine oxidase inhibitory activities assessed by electrochemical biosensing method. Evid Based Complement Alternat Med. 2018;2018:1364617. doi: 10.1155/2018/1364617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzeng TF, Liu WY, Liou SS, Hong TY, Liu IM. Antioxidant-rich extract from Plantaginis semen ameliorates diabetic retinal injury in a Streptozotocin-induced diabetic rat model. Nutrients. 2016;8:9. doi: 10.3390/nu8010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu YS, Lue SI, Lin SY, Luo CL, Chou CC, Weng CF. Plantago asiatica Seed Extracts Alleviated Blood Pressure in Phase I−Spontaneous Hypertension Rats. Molecules. 2019;24:9. doi: 10.3390/molecules24091734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, Lan J, Tong R, Zhang H, Sun S, Xiong A, Wang Z, Yang L. An integrative investigation on the efficacy of Plantaginis semen based on UPLC-QTOF-MS metabolomics approach in hyperlipidemic mice. Biomed Pharmacother. 2019;115:108907. doi: 10.1016/j.biopha.2019.108907. [DOI] [PubMed] [Google Scholar]
- 12.Ye CL, Hu WL, Dai DH. Extraction of polysaccharides and the antioxidant activity from the seeds of Plantago asiatica L. Int J Biol Macromol. 2011;49(4):466–470. doi: 10.1016/j.ijbiomac.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Son WR, Nam MH, Hong CO, Kim Y, Lee KW. Plantamajoside from Plantago asiatica modulates human umbilical vein endothelial cell dysfunction by glyceraldehyde-induced AGEs via MAPK/NF-κB. BMC Complement Altern Med. 2017;17(1):66. doi: 10.1186/s12906-017-1570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu L, Shi Y, Zhuang S, Liu N. Recent advances on uric acid transporters. Oncotarget. 2017;8(59):100852–100862. doi: 10.18632/oncotarget.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terkeltaub R, Bushinsky DA, Becker MA. Recent developments in our understanding of the renal basis of hyperuricemia and the development of novel antihyperuricemic therapeutics. Arthritis Res Ther. 2006;8 Suppl:S4. doi: 10.1186/ar1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng JX, Xu BB, Wang J, BI Y, Wang XY, Zhong GY, et al. Hypouricemic effects of acteoside and isoacteoside from Plantaginis semen on mice with acute hyperuricemia and their possible mechanisms. Chinese Tradit Patent Med. 2016;38(07):1449–1454. [Google Scholar]
- 17.Zeng JX, Wei J, Bi Y, Wang XY, Zhu JX, Zhu YY, et al. Research on Plantaginis semen extracts reduce level of uric acid in hyperuricemia mice and its Mechaism. Chin J Exp Tradit Med Formulae. 2013;19(09):173–177. [Google Scholar]
- 18.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Braga TT, Foresto-Neto O, Camara NOS. The role of uric acid in inflammasome-mediated kidney injury. Curr Opin Nephrol Hypertens. 2020;29(4):423–431. doi: 10.1097/MNH.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 20.Chen YL, Li H, Li S, Xu Z, Tian S, Wu J, Liang XY, Li X, Liu ZL, Xiao J, Wei JY, Ma CY, Wu KN, Ran L, Kong LQ. Prevalence of and risk factors for metabolic associated fatty liver disease in an urban population in China: a cross-sectional comparative study. BMC Gastroenterol. 2021;21(1):212. doi: 10.1186/s12876-021-01782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dragoș D, Manea MM, Timofte D, Ionescu D. Mechanisms of herbal Nephroprotection in diabetes mellitus. J Diabetes Res. 2020;2020:5710513. doi: 10.1155/2020/5710513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K, Zhang Y, Cao Y, Shi Z, Lin Y, Chen Y, Zhao H, Liu X. Glycyrrhetinic acid alleviates acute lung injury by PI3K/AKT suppressing macrophagic Nlrp3 inflammasome activation. Biochem Biophys Res Commun. 2020;532(4):555–562. doi: 10.1016/j.bbrc.2020.08.044. [DOI] [PubMed] [Google Scholar]
- 23.Dos Santos M, Veronese FV, Moresco RN. Uric acid and kidney damage in systemic lupus erythematosus. Clin Chim Acta. 2020;508:197–205. doi: 10.1016/j.cca.2020.05.034. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Chen C, Yan Y, Yuan Y, Wang KK, Chu C, Hu JW, Ma Q, Liao YY, Fu BW, Gao K, Sun Y, Lv YB, Zhu WJ, Yang L, Zhang J, Yang RH, Yang J, Mu JJ. Association of uric acid in serum and urine with subclinical renal damage: Hanzhong adolescent hypertension study. PLoS One. 2019;14(11):e0224680. doi: 10.1371/journal.pone.0224680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao H, Gao Q, Kong LZ, Tang WW, Jiao YY, Wang YL, Yu Z, Feng Y. Study on network pharmacological analysis and preliminary validation to understand the mechanisms of Plantaginis semen in treatment of gouty nephropathy. Evid Based Complement Alternat Med. 2020;2020:8861110. doi: 10.1155/2020/8861110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo LF, Chen X, Lei SS, Li B, Zhang NY, Ge HZ, et al. Effects and mechanisms of Dendrobium officinalis six nostrum for treatment of hyperuricemia with hyperlipidemia. Evid Based Complement Alternat Med. 2020;2020:2914019. doi: 10.1155/2020/2914019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Q, Qi M, Tong R, Wang D, Ding L, Li Z, et al. Plantago asiatica L. Seed Extract Improves Lipid Accumulation and Hyperglycemia in High-Fat Diet-Induced Obese Mice. Int J Mol Sci. 2017;18(7):1393. doi: 10.3390/ijms18071393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kho MC, Park JH, Han BH, Tan R, Yoon JJ, Kim HY, et al. Plantago asiatica L. Ameliorates Puromycin Aminonucleoside-Induced Nephrotic Syndrome by Suppressing Inflammation and Apoptosis Nutrients. Nutrients. 2017;9:4. doi: 10.3390/nu9040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung HY, Seo DW, Hong CO, Kim JY, Yang SY, Lee KW. Nephroprotection of plantamajoside in rats treated with cadmium. Environ Toxicol Pharmacol. 2015;39(1):125–136. doi: 10.1016/j.etap.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Alex BH. Lipidomics: when apocrypha becomes canonical. Curr Opin Chem Biol. 2012;16(1–2):221–226. doi: 10.1016/j.cbpa.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Yang L, Bai Y, Liu H. Analytical methods in lipidomics and their applications. Anal Chem. 2014;86(1):161–175. doi: 10.1021/ac403554h. [DOI] [PubMed] [Google Scholar]
- 32.Chinese Pharmacopoeia Commission . Chinese Pharmacopoeia, vol. 1. China Medical Science and Technology Press; 2020. [Google Scholar]
- 33.Wei W, WX LYJ. Experimental Methodology of Pharmmacology. People's Medical Publishing House; 2010. [Google Scholar]
- 34.Zhang S, Zhuang J, Yue G, Wang Y, Liu M, Zhang B, du Z, Ma Q. Lipidomics to investigate the pharmacologic mechanisms of ginkgo folium in the hyperuricemic rat model. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1060:407–415. doi: 10.1016/j.jchromb.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 35.HMDB. http://www.hmdb.ca/. Accessed 20 July 2019.
- 36.Lipid Maps. http://www.lipidmaps.org. Accessed 18 August 2019.
- 37.Metabo Analyst. http://www.metaboanalyst.ca/. Accessed 18 September 2019.
- 38.KEGG. http://www.genome.jp/kegg/. Accessed 22 October 2019.
- 39.Moore JF, Sharer JD. Methods for Quantitative Creatinine Determination. Curr Protoc Hum Genet. 2017;93:A.3O.1-A.3O.7. doi: 10.1002/cphg.38. [DOI] [PubMed] [Google Scholar]
- 40.Luis-Rodríguez D, Donate-Correa J, Martín-Núñez E, Ferri C, Tagua VG, Pérez Castro A, Mora-Fernández C, Navarro-González JF. Serum urate is related to subclinical inflammation in asymptomatic hyperuricaemia. Rheumatology (Oxford) 2021;60(1):371–379. doi: 10.1093/rheumatology/keaa425. [DOI] [PubMed] [Google Scholar]
- 41.Zhao H, Xu J, Wang R, Tang W, Kong L, Wang W, Wang L, Zhang Y, Ma W. Plantaginis semen polysaccharides ameliorate renal damage through regulating NLRP3 inflammasome in gouty nephropathy rats. Food Funct. 2021;12(6):2543–2553. doi: 10.1039/D0FO03143G. [DOI] [PubMed] [Google Scholar]
- 42.Bardin T, Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. 2017;15(1):123. doi: 10.1186/s12916-017-0890-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lippi G, Montagnana M, Luca Salvagno G, Targher G, Cesare GG. Epidemiological association between uric acid concentration in plasma, lipoprotein(a), and the traditional lipid profile. Clin Cardiol. 2010;33(2):E76–E80. doi: 10.1002/clc.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lan JP, Tong RC, Sun XM, Zhang HY, Sun S, Xiong AZ, et al. Comparison of main chemical composition of Plantago asiatica L and P depressa Willd seed extracts and their anti-obesity effects in high-fat diet-induced obese mice. Phytomedicine. 2021;81:153362. doi: 10.1016/j.phymed.2020.153362. [DOI] [PubMed] [Google Scholar]
- 45.Russo-Savage L, Schulman IG. Liver X receptors and liver physiology. Biochim Biophys Acta Mol basis Dis. 1867;2021(6):166121. doi: 10.1016/j.bbadis.2021.166121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toledo-Ibelles P, Gutiérrez-Vidal R, Calixto-Tlacomulco S, Delgado-Coello B, Mas-Oliva J. Hepatic Accumulation of Hypoxanthine: A Link Between Hyperuricemia and Nonalcoholic Fatty Liver Disease. Arch Med Res. 2021;S0188–4409(21):00111–00119. doi: 10.1016/j.arcmed.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Tan Y, Liu X, Zhou K, He X, Lu C, He B, Niu X, Xiao C, Xu G, Bian Z, Zu X, Zhang G, Zhang W, Lu A. The potential biomarkers to identify the development of steatosis in hyperuricemia. PLoS One. 2016;11(2):e0149043. doi: 10.1371/journal.pone.0149043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baranowski M. Biological role of liver X receptors. J Physiol Pharmacol. 2008;59(Suppl 7):31–55. [PubMed] [Google Scholar]
- 49.Chen Y, Chen Y, Zhao L, Chen Y, Mei M, Li Q, Huang A, Varghese Z, Moorhead JF, Ruan XZ. Inflammatory stress exacerbates hepatic cholesterol accumulation via disrupting cellular cholesterol export. J Gastroenterol Hepatol. 2012;27(5):974–984. doi: 10.1111/j.1440-1746.2011.06986.x. [DOI] [PubMed] [Google Scholar]
- 50.Chen H, Gao X, Zhao W, Yu H, Wang N, Mi S. Effect of geniposidic acid on SHP-LRH-1 signaling pathway in cholestasis rats. J Central South Univ Med Sci. 2019;44(6):605–613. doi: 10.11817/j.issn.1672-7347.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Chen H, Li J, Hu L, Zhao W, Yu H, Liu HZ, Ma ST. Effect of geniposidic acid on hepato-enteric circulation in cholestasis rats through Sirt1-FXR signaling pathway. China J Chinese Materia Med. 2019;44(4):787–795. doi: 10.19540/j.cnki.cjcmm.20181204.013. [DOI] [PubMed] [Google Scholar]
- 52.Qiu L, Cheng XQ, Wu J, Liu JT, Xu T, Ding HT, Liu YH, Ge ZM, Wang YJ, Han HJ, Liu J, Zhu GJ. Prevalence of hyperuricemia and its related risk factors in healthy adults from northern and northeastern Chinese provinces. BMC Public Health. 2013;13(1):664. doi: 10.1186/1471-2458-13-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng A, Lin L, Zhao M, Sun B. Identifying mechanisms underlying the amelioration effect of Chrysanthemum morifolium Ramat. 'Boju' extract on hyperuricemia using biochemical characterization and UPLC-ESI-QTOF/MS-based metabolomics. Food Funct. 2019;10(12):8042–8055. doi: 10.1039/C9FO01821B. [DOI] [PubMed] [Google Scholar]
- 54.Chen R, Wang Q, Li Z, Wang D, Yang S, Feng Y. Studies on effect of Tongfengxiaofang in HUM model mice using a UPLC-ESI-Q-TOF/MS metabolomic approach. Biomed Chromatogr. 2021. 10.1002/bmc.5118. [DOI] [PubMed]
- 55.Han HJ, Lim MJ, Lee YJ, Lee JH, Yang IS, Taub M. Uric acid inhibits renal proximal tubule cell proliferation via at least two signaling pathways involving PKC, MAPK, cPLA2, and NF-kappaB. Am J Physiol Renal Physiol. 2007;292(1):F373–F381. doi: 10.1152/ajprenal.00104.2006. [DOI] [PubMed] [Google Scholar]
- 56.Liu N, Sun Q, Xu H, Yu X, Chen W, Wei H, Jiang J, Xu Y, Lu W. Hyperuricemia induces lipid disturbances mediated by LPCAT3 upregulation in the liver. FASEB J. 2020;34(10):13474–13493. doi: 10.1096/fj.202000950R. [DOI] [PubMed] [Google Scholar]
- 57.Yang F, Liu M, Qin N, Li S, Yu M, Wang C, Ma Q. Lipidomics coupled with pathway analysis characterizes serum metabolic changes in response to potassium oxonate induced hyperuricemic rats. Lipids Health Dis. 2019;18(1):112. doi: 10.1186/s12944-019-1054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan Y, Wang L, Gao J, Ma J, Yu H, Zhang Y, Wang T, Han L. Multiomics integrative analysis for discovering the potential mechanism of Dioscin against hyperuricemia mice. J Proteome Res. 2021;20(1):645–660. doi: 10.1021/acs.jproteome.0c00584. [DOI] [PubMed] [Google Scholar]
- 59.Liu P, Xu H, Shi Y, Deng L, Chen X. Potential molecular mechanisms of plantain in the treatment of gout and hyperuricemia based on network pharmacology. Evid Based Complement Alternat Med. 2020;2020:3023127. doi: 10.1155/2020/3023127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.