Abstract
Background
Plasmodium falciparum (Pf) sporozoites (PfSPZ) can be administered as a highly protective vaccine conferring the highest protection seen to date. Sanaria® PfSPZ vaccines are produced using aseptically reared Anopheles stephensi mosquitoes. The bionomics of sporogonic development of P. falciparum in A. stephensi to fully mature salivary gland PfSPZ is thought to be modulated by several components of the mosquito innate immune system. In order to increase salivary gland PfSPZ infections in A. stephensi and thereby increase vaccine production efficiency, a gene knock down approach was used to investigate the activity of the immune deficiency (IMD) signaling pathway downstream effector leucine-rich repeat immune molecule 1 (LRIM1), an antagonist to Plasmodium development.
Methods
Expression of LRIM1 in A. stephensi was reduced following injection of double stranded (ds) RNA into mosquitoes. By combining the Gal4/UAS bipartite system with in vivo expression of short hairpin (sh) RNA coding for LRIM1 reduced expression of LRIM1 was targeted in the midgut, fat body, and salivary glands. RT-qPCR was used to demonstrate fold-changes in gene expression in three transgenic crosses and the effects on P. falciparum infections determined in mosquitoes showing the greatest reduction in LRIM1 expression.
Results
LRIM1 expression could be reduced, but not completely silenced, by expression of LRIM1 dsRNA. Infections of P. falciparum oocysts and PfSPZ were consistently and significantly higher in transgenic mosquitoes than wild type controls, with increases in PfSPZ ranging from 2.5- to tenfold.
Conclusions
Plasmodium falciparum infections in A. stephensi can be increased following reduced expression of LRIM1. These data provide the springboard for more precise knockout of LRIM1 for the eventual incorporation of immune-compromised A. stephensi into manufacturing of Sanaria’s PfSPZ products.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-021-03818-8.
Keywords: Anopheles stephensi, Mosquito, Immune system, Gene silencing, Plasmodium falciparum, Oocyst, Sporozoite, PfSPZ vaccine
Background
Malaria is responsible for over 400,000 deaths a year [1] and despite continued and sustained control efforts, infection rates have plateaued and elimination remains elusive, even in places where per capita spending on malaria control is high and advanced programmes are in place [2]. Indeed, the current COVID-19 pandemic is threatening to negate the significant advances that have been made over the last 15 years [3, 4]. New tools are needed in order to progress further towards control and elimination; a vaccine that prevents infection in the human host and thereby transmission to mosquitoes would be the ideal tool. With this specific goal in mind, Sanaria Inc., along with many collaborators, has demonstrated high level protection with two of its Plasmodium falciparum (Pf) sporozoite (SPZ)-based vaccines. In clinical trials in 6 countries in Africa, the Germany and the Netherlands in Europe and at 5 sites in the US, PfSPZ-based vaccines have been consistently safe and well tolerated. They have protected > 90% of recipients against controlled human malaria Infection (CHMI) in clinical trials conducted in the USA, Germany, Tanzania, and Mali [5–8] (Sissoko, unpublished) with protection lasting for at least 8 months against heterologous (P. falciparum strain 7G8) CHMI [9] and 14 months against homologous (P. falciparum strain NF54) CHMI [10]. Protective efficacy of approximately 50% lasting for at least 6 months against naturally transmitted malaria has been demonstrated in four independent clinical trials in Mali [5] (Sissoko and Halimatou unpublished) and Burkina Faso (Sirima, unpublished).
Sanaria Inc. has developed a platform technology for producing aseptic, purified, cryopreserved PfSPZ in compliance with Good Manufacturing Practices [11, 12]. Sanaria® PfSPZ vaccine (radiation-attenuated PfSPZ) [5, 9–11, 13–15], PfSPZ Challenge which is composed of infectious PfSPZ used for CHMI [16–25], PfSPZ-CVac (chemo-attenuated PfSPZ), which combines PfSPZ Challenge with anti-malarial drugs [7, 12, 26, 27], and PfSPZ-GA1 (genetically attenuated PfSPZ) [28] are all reliant on aseptically reared mosquitoes for their manufacture. For all of these products, infection intensity of PfSPZ (number of PfSPZ per mosquito) greatly influences their eventual cost of goods.
One factor controlling PfSPZ infection intensity is the innate immune system of the mosquito. The immune deficiency (IMD) pathway is one arm of the immune system that down-regulates Plasmodium infections at the oocyst and SPZ stages, and leucine-rich repeat (LRR) proteins are the downstream effector molecules in the IMD pathway [29]. One LRR, leucine-rich repeat immune molecule 1 (LRIM1), a member of the long LRIM subfamily found only in mosquitoes, is considered a strong suppressor of parasite development playing a role in both melanization and lysis [30–34] of Plasmodium ookinetes and oocysts. The current model suggests that LRIM1 functions in a complement-like pathway leading to the activation of a C3-like protein, TEP1, that localizes to the surface of the pathogen, targeting it for destruction [29, 35–37]. LRIM1 covalently binds intracellularly to APL1C forming a heterodimer that is secreted into the hemolymph. The LRIM1/APL1C complex then binds to a mature cleaved TEP1 molecule stabilizing it and promoting binding to the pathogen surface. LRIM1 expression in Anopheles gambiae is regulated by Plasmodium infection, with maximum expression coinciding with the movement of Plasmodium ookinetes across the midgut epithelium [38–40]. Silencing LRIM1 expression with dsRNA injected into the mosquito hemocoel increased the intensity of Plasmodium berghei oocyst infections 3–4.5 fold in A. gambiae [39].
The present study tested the hypothesis that knocking down A. stephensi LRIM1 would result in higher Plasmodium infection intensities at both oocyst and SPZ stages. To achieve this, a transgenic LRIM1 silencer line was produced by crossing a UAS-LRIM1 line to a line expressing the GAL4 transcription activator. LRIM1 expression was reduced but not eliminated and higher infections of P. falciparum oocysts and PfSPZ were observed, suggesting that transgenic mosquitoes carrying the knock-down mechanism could be an important approach to increasing the efficiency of manufacture and reducing cost of goods for all PfSPZ products.
Methods
Mosquitoes
SDA 500 is a laboratory strain of Anopheles stephensi selected for susceptibility to Plasmodium falciparum infection [41, 42]. Mosquitoes were maintained in a Conviron environmental chamber at 28 °C, 80% relative humidity and a 12 h:12 h light:dark cycle. Larvae were fed pulverized fish food (TetraMin Tropical Flakes) daily and adults were provided 10% sucrose ad libitum. For colony maintenance, seven-day old adult females were offered a blood meal of bovine blood in acid citrate dextrose (Lampire Biological Laboratories, Pipersville, PA) at 37 °C through a Parafilm membrane using a mosquito feeder (Chemglass Life Sciences, Vineland, NJ). Eggs were collected in 50 mL of deionized water in a 250 mL Biostor multipurpose container (Fisher Scientific, Rockville, MD), lined with Whatman (UK) filter paper.
Artificial feeding buffer composed of 150 mM NaCl; 10 mM NaHCO3; 1 mM Adenosine -5-triphosphate (ATP) [43, 44] was substituted for blood in experiments and fed through a Parafilm™ membrane as described above.
Infection of Anopheles stephensi with Plasmodium falciparum
Three separate cohorts of ~ 400 female A. stephensi were fed human blood containing stage V P. falciparum gametocytes (strain NF54), as described elsewhere [45] and unfed females were removed from the cage. Seven days post infection, midguts ~ 30 mosquitoes from each cohort were dissected and the oocyst intensity determined by microscopy. Fourteen days after blood feeding the salivary glands of ~ 20 mosquitoes were dissected and immediately flash frozen on dry ice for subsequent RNA extraction. The salivary glands of another ~ 30 mosquitoes were dissected, and PfSPZ intensity and prevalence determined.
Genomic DNA extraction and quantification
Mosquito tissues were homogenized in 50 µL of homogenization buffer (10 mM Tris–HCL pH 7.5, 10 mM EDTA, 5% sucrose [w/v], 0.15 mM spermine, 0.15 mM spermidine) and kept on ice. Fifty microlitres of lysis buffer (300 mM Tris–HCL pH 9.0, 100 mM EDTA, 0.625% SDS [w/v], 5% sucrose [w/v] were added to the homogenized mixture, mixed and incubated at 70 °C for 15 min. The mixture was then cooled to room temperature and 15 µL of 8 M potassium acetate were added, mixed thoroughly then placed on ice for 30 min after which it was centrifuged at 14,000 RPM for 10 min at RT. The supernatant was transferred to a fresh tube and 90 µL of phenol/chloroform/isoamylic alcohol were added. The mixture was centrifuged at 14,000 RPM, 4 °C and supernatant transferred to a new tube and DNA precipitated by adding two volumes of absolute ethanol. The mixture was centrifuged at 14,000 RPM for 5 min at RT, supernatant discarded, and the pellet washed in 70% ethanol. After centrifuging for 10 min at 14,000 RPM, the supernatant was discarded and the DNA pellet was vacuum dried then suspended in 1 × TE buffer, pH 7.4. The concentration of nucleic acids was determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop, Wilmington, USA) at 260 nm, and purity checked by measuring the absorption 230 nm and 280 nm.
RNA extraction and quantification
Total RNA was isolated from mosquito tissues using Ambion Trizol Reagent according to the manufacturer’s instructions. The concentration and purity were determined as above. At 24 h, 48 h and 72 h post feeding, midguts of 20 mosquitoes were dissected into cold phosphate buffered saline (PBS), then midguts and carcasses flash frozen immediately in RNase free tubes on dry ice for subsequent RNA extraction.
Cloning of Anopheles stephensi SDA 500 leucine rich immune molecule 1
LRIM1 was amplified from A. stephensi cDNA by PCR using primers AsLRIM1fw (5′-CCC GCC GGT ATA GCT TAT CAG-3′) and AsLRIM1rv (5′-CAA ATA GTG CTC GTC TGC GC-3′). The known A. gambiae LRIM1 sequence (AGAP0006348) was aligned using ApE-A Plasmid Editor to an assembled draft genome sequence of A. stephensi. Conserved regions between A. gambiae LRIM1 and A. stephensi LRIM1 [33] were identified and primers designed to amplify the full open reading frame. Phusion High-Fidelity polymerase (New England Biolabs, Ipswich, MA.) was employed for PCR. LRIM1 PCR product was purified by gel electrophoresis and gel extraction (QIAquick gel extraction kit, QIAGEN, Germantown, MD). Purified PCR product was inserted into Zero Blunt TOPO PCR Cloning vector (Thermo Fisher Scientific, Rockville, MD) according to the manufacturer’s instructions and transformed in Escherichia coli DH10B (Thermo Fisher Scientific, Rockville, MD). Positive colonies were digested with EcoRI and agarose gel electrophoresis was used to identify insertion of LRIM1 PCR product. Sequence identity was then confirmed by DNA sequencing (Macrogen Inc, Rockville, MD).
Real time reverse transcription PCR
To generate cDNA, 1–5 μg of total RNA were mixed with 1 µL of oligo(dT)20 primer (50 µM), 10 mM dNTP mix and RNase free water to a total volume of 10 μL. The mixture was heated to 65 °C for 5 min and then quickly chilled on ice. A master mix containing 2 μL of 10X reverse transcriptase (RT) buffer; 4 μL of 25 mM MgCl2; 2 μL of 0.1 M DTT; 1 µL of RNase OUT (40 U/µL); 1 µL of Superscript III RT (200 U/µL), was added, gently mixed and incubated at 50 °C for 50 min. The reaction was then inactivated by incubating at 85 °C for 5 min and then chilling on ice. After brief centrifugation, 1 µL of RNase H was added to the mixture and incubated at 37 °C for 20 min. Synthesized cDNA was diluted to 200 ng/µL and used for qPCR. All the samples to be compared were processed in parallel and in triplicate using was an ABI PRISM 7000 Sequence Detection System (Applied Biosystems). Reaction conditions are described in the Additional file 1.
Synthesis of dsRNA for LRIM1 silencing
A cDNA fragment of 500 bp of LRIM1 was amplified using the dsRNAfw and dsRNArv (Additional file 1: Table S3) primers using cDNA from 7-day old A. stephensi females as the template. The resulting PCR fragment was cloned into the pCR II-TOPO vector (Invitrogen, Carlsbad, CA) and transformed in E. coli DH10B (Thermo Fisher Scientific, Rockville, MD). High yield plasmid DNA was isolated using QIAGEN (Germantown, MD) Plasmid Maxi Kit. The T7 flanked DNA fragment used for dsRNA synthesized was removed from the plasmid by digestion with EcoRI and double stranded RNA was generated and purified using the MEGAscript kit (Ambion, Austin, TX).
Silencing Anopheles stephensi LRIM1 by dsRNA injection
Four day old A. stephensi females were anesthetized on ice for 5 min and held at 4 ˚C injection plate. Approximately 100 nL of LRIM1 dsRNA (3 ng/nL) or EGFP dsRNA control were injected into the thorax of the mosquitoes using a Pneumatic PicoPump PV820 (World Precision Instrument Inc., Sarasota, FL). After injection the mosquitoes were allowed to recover at RT for 1 h before being transferred to normal rearing conditions (see above). LRIM1 silencing was confirmed 4 days post dsRNA injection by qRT- PCR.
For bacterial infections a glass needle was dipped into a pellet of E. coli (DH10B) OD600 of 0.1 and injected into the thorax of the mosquito. For feeding experiments, artificial feeding buffer containing E. coli at 100 CFU/mL was fed to mosquitoes.
For survival studies, three cohorts of 50 four-day-old adult females were injected as above then held at 28 ˚C, 80% humidity, and 12 h:12 h light:dark cycle with 10% sucrose provided ad libitum. The number of dead mosquitoes were recorded each day. A cohort of 50 untreated mosquitoes served as a second control.
Vectors
All vectors used in this study are described in Additional file 1.
Generation of silencer lines
Anopheles stephensi preblastoderm embryos were injected with 150 ng/µL vector-containing plasmids and 300 ng/µL plasmids expressing piggyBac transposase [46], in 5 mM KCl, 0.1 mM NaPO4, pH 6.8. Insects that hatched and survived to adulthood were pooled according to sex and mated to wild type A. stephensi SDA 500. Progeny were screened as larvae for the expression of ECFP or nuclear localization sequence (nls)-EGFP, and transgenic individuals were used to establish lines. The piggyBac insertion sites were determined using splinkerette-PCR [47, 48] (Additional file 1: Tables S5, S6). For experiments that required analysis of genetically modified mosquitoes with both the Gal4 transgene and UAS::LRIM1silencer transgene, heterozygous individuals of the UAS::LRIM1silencer and MBL24 GAL4 line were mated to produce progeny with all four genotypes: wild type; MBL24-Gal4/+; UAS::LRIM1silencer/+ and MBL24-Gal4/UAS::LRIM1silencer. MBL24-Gal4/+, UAS::LRIM1silencer/+ and wild type mosquitoes were used as controls.
Survival comparison of transgenic mosquitoes
LRIM1-silencer/- lines were crossed with the MBL24 Gal4/- driver line. From the progeny, 100 female pupae of each genotype were identified using the fluorescence marker gene. Pupae were pooled, and placed in a 3.8 L mosquito cage. After emergence, the mosquitoes were maintained on a 10 percent sucrose solution. The number of dead mosquitoes were recorded each day.
Isolation of midgut microbiota for microbial load assessment
Individual A. stephensi SDA500 were surface-sterilized by washing three times with alternating 70% ethanol and sterile PBS washes. The midguts were then dissected in PBS using flame-sterilized forceps and homogenized in 200 µL PBS using a sterilized pestle. Each midgut homogenate was then serially diluted and inoculated on Luria–Bertani (LB) agar and incubated at 27 °C for 48 h after which individual colonies counted.
Statistical analysis
All relative expression data and sporozoite numbers were compared across multiple treatments by ANOVA followed by post-hoc Dunn’s test to identify differences between pairs of treatments. Oocyst data were compared using a Mann Whitney U test followed by Kruskal–Wallis test between pairs of treatments. Survival curves were compared using Mantel–Haenszel chi-squared tests to determine the Odds Ratio. Data were analysed using Graph Pad Prism software V9.1.
Results
Cloning of Anopheles stephensi leucine rich immune molecule 1
A 1.8 kb fragment amplified from A. stephensi cDNA showed 58% nucleotide sequence identity and 60% amino acid identity to the known A. gambiae LRIM1. The sequence contained nine LRR domains consisting of 19–41 amino acid residues, with two coiled coil domains in the region of amino acid residues 318 to 366 and 424 to 459, and a signal peptide region from residue 1 to 19 (Fig. 1). The sequence was identified as the predicted LRIM1 gene (ASTE000814) when compared to the A. stephensi genome (release version VB-2015-10, AsteS1) [49].
Fig. 1.

Amino acid sequence and predicted structural organization of Anopheles stephensi LRIM1. Grey—signal peptide; Red—leucine rich repeat regions; Blue—cysteine; Green—Coiled coil domains
Immune responses of Anopheles stephensi to Plasmodium falciparum infection
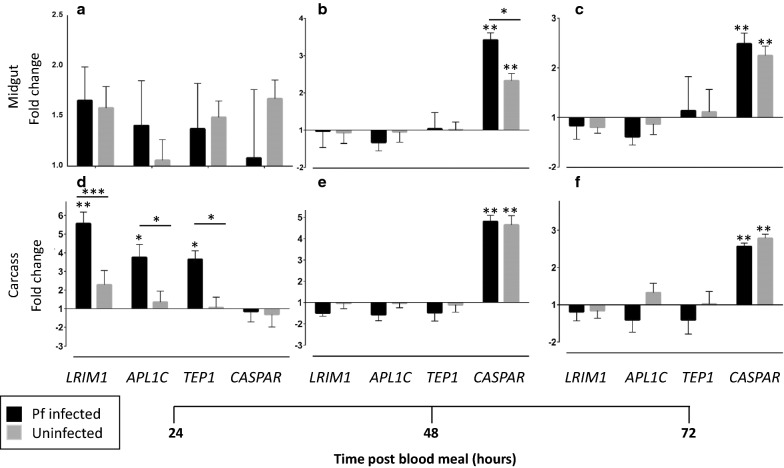
The midguts and carcasses (i. e. all other tissues) of female A. stephensi were assessed at 24, 48 and 72 h post blood meal (hpbm) of human blood alone or human blood containing P. falciparum gametocytes, for transcript levels of IMD effector genes LRIM1, APL1C and TEP1 and the IMD pathway negative regulator Caspar; mosquitoes maintained on 10% sucrose were used as controls against which expression levels were compared. LRIM1 was upregulated ~ 1.6 fold at 24 hpbm in the midgut independent of infection (Fig. 2a). In the carcass, expression was also upregulated at 24 hpbm, but there was a significant (p < 0.025), almost fivefold increase in LRIM1 expression associated with infection (Fig. 2d). There was no subsequent significant upregulation or down regulation after feeding in either tissue or in association with infection (Fig. 2b, c, e, f). TEP1 expression followed the same pattern as LRIM1 while APL1C additionally showed differential upregulation in the midgut associated with infection at 24 hpbm (Fig. 2a). Consistent with its role as a negative regulator of the IMD pathway, Caspar transcript levels were elevated in the midgut and the carcass only at 48 and 72 hpbm (Fig. 2).
Fig. 2.
Relative transcript levels of immune genes in adult female Anopheles stephensi. LRIM1, APL1C, TEP1 and Caspar in the midgut (a–c) and carcass (d–f) of female Anopheles stephensi 24 h (a, d), 48 h (b, e) and 72 h (c, f) after feeding on blood with (black bars) or without (gray bars) Plasmodium falciparum gametocytes. Bars represent mean of triplicate experiments ± standard error of mean. *p < 0.05; **p < 0.02; ***p < 0.01. Asterisks above each bar show comparison to baseline; asterisks above horizontal lines show comparison between treatments. Transcript levels are reported as a fold expression compared to naïve non-blood fed females
The salivary glands of infected females were assessed for IMD pathway responses fourteen days post P. falciparum infection. Both APL1C (p < 0.02) and TEP1 (p < 0.01) showed greater than twofold increase in average transcript levels when compared to mosquitoes that fed on non-infected blood, while LRIM1 showed a 1.8-fold (p < 0.02) increase when compared to controls. There was also a modest, but significant (p < 0.05), 1.3-fold increase in Caspar transcript levels when compared to non-infected blood fed females (Fig. 3).
Fig. 3.
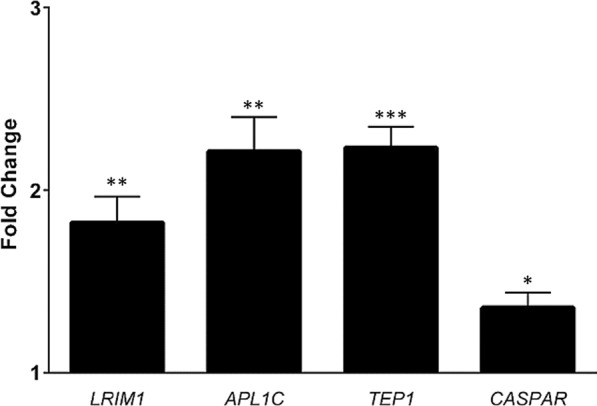
Relative transcript levels of LRIM1, APL1C, TEP1 and Caspar in salivary glands of female Anopheles stephensi 14 days post Plasmodium falciparum infection. Transcript levels are reported as a fold expression compared to non- infected blood fed females. Error bars indicate standard error of the mean of three independent replicates. *p < 0.05; **p < 0.02; ***p < 0.01. Asterisks above each bar show comparison to baseline
Silencing of A. stephensi LRIM1 by injection of dsRNA
dsRNA injections were used as a preliminary assessment for the silencing of LRIM1 in A. stephensi. Female mosquitoes were injected with dsAgLRIM1 or dsAsLRIM1 and LRIM1 expression compared to mosquitoes injected with dsEGFP, and silencing of LRIM1 expression in the whole body was assessed 4 days post injection. LRIM1 showed reduced transcript abundance of 78.8% and 53.5% after dsAgLRIM1 and dsAsLRIM1 injections respectively (Fig. 4a) indicating that AsLRIM1 could be used as the template for transgenic modification to down-regulate LRIM1. However, injecting A. stephensi females with dsAsLRIM1 caused 100% mortality in females by 10 days post injection compared to 66 percent and 68 percent fatality of dsEGFP and dsAgLRIM1 injected controls respectively (Fig. 4b).
Fig. 4.
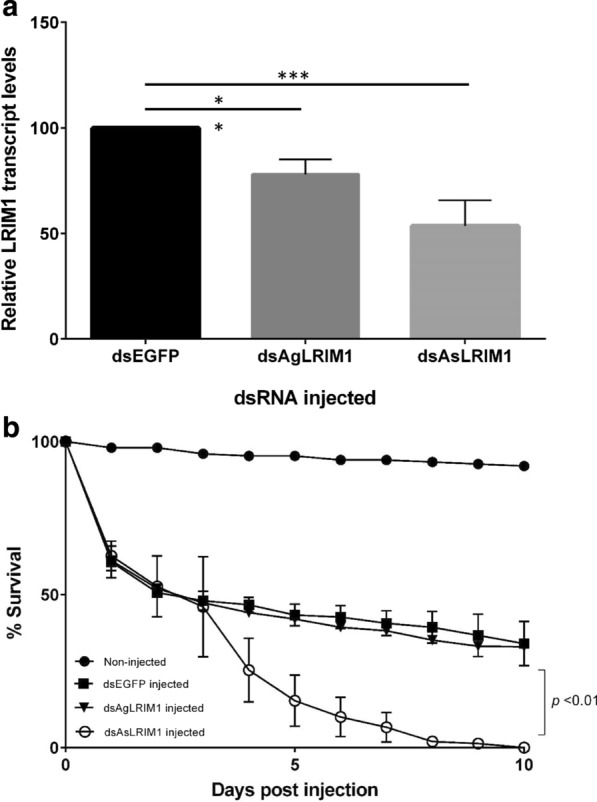
LRIM1 expression and survival of female Anopheles stephensi after dsRNA injection. a Expression of LRIM1 in whole mosquitoes 4 days after injection. Average transcript abundance is relative to control mosquitoes injected with dsEGFP. Transcript levels of ribosomal S7 gene were used as a calibrator. Bars indicate standard error of the mean of three independent replicates. **p < 0.02; ***p < 0.01. Asterisks above horizontal lines show comparison between treatments and control. b Survival of female Anopheles stephensi after injection of dsRNA. Fifty female mosquitoes were used for each treatment. Bars indicate standard error of the mean of three independent experiments
Characterization of LRIM1-silencer lines
After confirming the inhibition of AsLRIM1 following dsAsLRIM1 injections, the next step was to generate an LRIM1 silencer line. Three LRIM1 silencer lines, F2, M2 and M7 were created. The cytogenetic locations of the transgene insertion sites were determined using Splinkerette PCR [48] and the chromosomal location data of A. stephensi scaffolds. The integration site for F2 was in the intergenic region of scaffold KB664543 homologous to a locus on chromosome 3R in A. gambiae. For M2, the transgene was found in the intergenic region of scaffold KB664524 homologous to a locus on A. gambiae chromosome 2R, and the M7 transgene was located in the intergenic region of scaffold KB664832 homologous to a locus on A. gambiae chromosome 3L (Table 1).
Table 1.
Cytogenetic location of the LRIM1 silencing transgene in the Anopheles stephensi genome
The MBL24 Gal4 driver line expresses Gal4 in the midgut, fat body and salivary gland [50]. The progeny of the UAS:LRIM1-silencer lines were crossed with the driver line and progeny containing a copy of UAS::LRIM1-silencer and Gal4 were used to test for tissue specific silencing of LRIM1 expression using qRT-PCR. The abundance of LRIM1 transcript in each genotype was compared to transcript abundance in the wild type. Progeny that contained a single transgene element showed no statistically significant difference in LRIM1 transcript abundance in tissues examined compared to wild type. Progeny of the three silencer lines (F2, M2 and M7) that contained both the Gal4 and UAS::LRIM1-silencer elements showed reduction of LRIM1 transcript abundance in the midgut and carcass (midgut and salivary glands removed) (Fig. 5a–f), and M2 and M7 additionally in the salivary glands compared to wild type (Fig. 5h, i). In the midgut, the average transcript abundance was reduced to 72%, 65% and 52% for lines F2, M2 and M7 respectively (Fig. 5a–c). The mean carcass transcript levels in F2, M2 and M7 were reduced to 64%, 65% and 63%, respectively (Fig. 5d–f). For both the M2 and M7 lines, transcript reduction was highest in the salivary glands with average transcript abundance of 56% and 38% respectively (Fig. 5h, i). Based on these results, M7 was down-selected for infection studies.
Fig. 5.
Relative transcript abundance of LRIM1 in driver and silencer LRIM1 mosquito lines. LRIM1 expression in GAL4::LRIM1-silencer lines F2, M2, and M7 was determined in the midguts and carcasses 24 h post blood meal, and salivary glands 14 days post blood meal. For each genotype the midguts or carcasses of 10 females or salivary glands of 30 females were pooled and RNA extracted. Transcript abundance is shown relative to wild type mosquitoes using ribosomal S7 gene as a calibrator. Bars indicate mean and standard error of the mean of three independent experiments
Because injection of dsAsLRIM1 into female A. stephensi reduced their lifespan, it was necessary to check whether in vivo shRNA silencing with a smaller and more specific target site would have a reduced longevity phenotype. The life spans of the progeny generated from crossing LRIM1- silencer/- lines with the MBL24 Gal4/- driver line were not statistically different in three independent experiments (Fig. 6). In all lines and crosses, 60–70% of female mosquitoes were alive 21 days post emergence.
Fig. 6.
Survival of the progeny from crosses of LRIM1-silencer lines with the MLB24 Gal4 driver line. a F2, b M2, c M7 line. Fifty female pupae from each genotype were pooled and adults observed for 21 days post emergence. The cage was examined daily and dead individuals removed, the genotype determined. No statistical differences were observed among the genotypes examined
In addition, and because M7 demonstrated the greatest reduction in midgut expression of LRIM1, the bacterial load in the midgut of progeny from the cross of LRIM1 silencer M7 with MLB24-Gal4 driver was determined. No statistical difference was observed in bacterial load of the genotypes examined; the mean number of Colony Forming Units (CFU) in the female midguts ranged from 1.8 × 106 CFU/ml to 2.3 × 106 CFU/mL (Fig. 7).
Fig. 7.
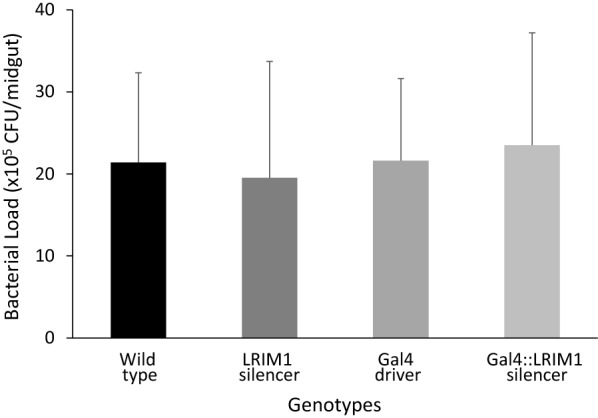
Midgut bacterial load in a cross of LRIM1-silencer M7 with MLB24 Gal4 driver Anopheles stephensi. Serial dilutions of midgut homogenate of 10 individual females of each genotype were plated on LB agar. CFUs were calculated after 48 h incubation at 27 °C. Error bars indicate the standard error of the mean of three independent experiments
Plasmodium falciparum infections
Heterozygous LRIM1-silencer M7 females were crossed with heterozygous MBL24/Gal4 driver males and the progeny were fed blood containing P. falciparum stage V gametocytes. Each resulting genotype was assessed for oocyst and PfSPZ infections. The mean P. falciparum oocyst infection intensity of the MBL24/Gal4 UAS::LRIM1-silencer mosquitoes expressing the hairpin silencing construct was 86.0 oocysts/midgut (mean of geometric means for three independent experiments) compared to 8.0 oocysts/midgut in wild type mosquitoes, and 35.7 oocysts/midgut or 35.3 oocysts/midgut in transgenic mosquitoes with only the GAL4 element or UAS LRIM1 silencer element, respectively (Fig. 8a). Mosquitoes expressing the LRIM1 silencer construct had 2.5–tenfold higher numbers of PfSPZ in the salivary glands compared to wild type. Intermediate PfSPZ intensities were seen in mosquitoes with only the MBL24 Gal4 transgene or the UAS::LRIM1 transgene (Fig. 8b) (Additional file 1: Table S1).
Fig. 8.
Plasmodium falciparum infections in progeny from a cross of LRIM1-silencer M7 with MLB24 Gal4 driver Anopheles stephensi. Oocyst infections were determined on day 7 post blood meal and PfSPZ infections on day 21–25 post blood meal. Circles represent the number of oocysts on a single midgut; horizontal black bars represent the median oocysts in each genotype. Three independent biological replicates were pooled, and significance was determined by a Kruskal–Wallis test followed by Dunn’s post-test in the case of multiple comparisons. For PfSPZ, circles represent the mean intensity in a pool of 30 salivary glands from each of three experiments
Discussion
The LRIM1 homolog of A. stephensi is a member of the LRIM family, containing the conserved double coiled coil C-terminal domain [30] that is thought to facilitate the protein/protein interactions of LRIM1 and APL1, the resulting heterodimer complex being the effector molecular of the complement-like immune response [35]. Mosquito LRIMs are characterized by a variable number of leucine-rich repeats (LRRs), which distinguishes the short (6–7 LRRs) and long (≥ 10 LRRs) subfamilies of LRIMs. AsLRIM1 possesses an N-terminal signal peptide indicating that it is a secreted protein; the AgLRIM1 monomer is secreted into the hemolymph only after formation of the LRIM1/APL1 complex. In both AsLRIM1 and AgLRIM1 between the C-terminal coiled coil domain and the LRRs is a conserved double cysteine motif implicated in the formation of the disulfide bond between LRIM1 and APL1 [30, 36].
LRIM1 in A. gambiae functions as a strong suppressor of P. berghei development [32–34], with highest expression observed 24 hpbm, the time at which ookinetes are traversing the mosquito midgut epithelium [39, 40]. Plasmodium falciparum infection of A. stephensi resulted in a similar transient but significant increase in expression of LRIM1 and other IMD effector molecules at 24 hpbm followed by downregulation at 48 hpbm [39, 40, 51]. The relationship between A. stephensi IMD pathway and Caspar expression is similar to that seen for Caspar and the IMD pathway response to Plasmodium in A. gambiae [31]. The IMD pathway is clearly induced in A. stephensi in response to parasite infections, specifically to P. falciparum ookinetes, and functions to limit parasite infections in the mosquito. The midgut immune response, specifically the IMD pathway, during P. falciparum infection of A. gambiae was infection intensity dependent [31, 52]. The experimental design of the present study did not allow for that relationship to be explored in A. stephensi.
The novel observation of a statistically significant increase in expression of IMD effector genes, including LRIM1, in the salivary glands fourteen days post P. falciparum infection, when PfSPZ are invading the salivary glands, could provide significant insight into mosquito defense against the parasite late in the sporogonic cycle. Mosquito humoral responses against Plasmodium are thought to be concentrated in the midgut, fat body and haemocoel; but these data suggest that, additionally, the salivary glands plus one or more of those tissues in the carcass, can and do mount an immune response against P. falciparum. However, it seems most likely that any effects of LRIM1 on PfSPZ may be prior to their full development in the oocyst or after sporulation and oocyst rupture, as parasites are exposed to the hemolymph for several days.
Reverse genetics is an important tool for dissecting aspects of mosquito biology and vector parasite interactions [53, 54]. Transient gene silencing by direct injection of dsRNA and stable expression of hairpin RNAs from transgenes integrated into the genome are two approaches for exploiting gene knockdown or transient silencing using RNAi in mosquitoes [54]. The efficacy of gene silencing by direct injection of dsRNA is severely limited [55–57], being a blunt instrument with which to inhibit tissue- and temporally-specific gene expression. LRIM1 expression in A. stephensi injected with AsLRIM1 or AgLRIM1 dsRNA was reduced by46.5% and 21.2%, respectively, demonstrating both the utility and weakness of the approach; while expression was indeed inhibited and the inhibition increased using the species-specific AsLRIM1, the inhibition was incomplete and short-lived. To address this, the bi-partite Gal4: UAS system was successfully adapted for control of tissue specific in vivo expression of hairpin RNAs in A. stephensi. LRIM1 expression was silenced in the midgut, carcass and salivary glands of A. stephensi throughout the entire sexual and sporogonic cycle of Plasmodium. However, silencing efficiency was only ~ 40% among the different tissues analysed in three separate LRIM1 silencing lines. Unlike the blunt instrument of injection, the more refined approach taken here is still imprecise, and optimizing the expression of dsRNA to specific localization, time and quantity of expression of the target gene would require numerous repeats of these experiments based on number and location of the inserted dsRNA. In contrast, the CRIPR/Cas9 gene editing system offers a more surgical, precise method for silencing gene function entirely by disrupting sequence fidelity. Silencing LRIM1 in A. stephensi using CRISPR/Cas9 results in a very different phenotype which will be described elsewhere (Inbar et al., unpublished).
Anopheles stephensi injected with AsLRIM1 dsRNA also had reduced life span [33, 39], a phenotype not observed in mosquitoes expressing in vivo dsRNA AsLRIM1. One explanation for this difference is the potential off-target or non-specific effects of the injected dsRNA which represents an overload to the mosquito. Short term high-level inhibition of LRIM1 expression after injection could also allow a transient increase in pathogenic microbiota in the mosquito in response to LRIM1 silencing, thereby increasing mortality. While the IMD pathway, and specifically TEP1, is considered to play an important role in mosquito defense against bacteria [30, 58–61]; the present results show that silencing LRIM1 by expression of dsRNA did not change the bacterial load in the midgut. These contrasting observations may explain the differential mortality observed in the two experimental approaches.
LRIM1 was identified originally as a strong antagonist of P. berghei, but not P. falciparum, oocysts developing in the midgut of A. gambiae [39, 62]. LRIM1 does in fact contribute to the anti-P. falciparum response, but at intermediate oocyst intensities with little effect at low intensities [31]. In the present study, A. stephensi expressed a transgene whose transcript formed a shRNA targeting the silencing of LRIM1; these mosquitoes had increased intensities of P. falciparum and P. berghei oocysts as well as PfSPZ and PbSPZ compared to wild type. However, some uncertainty remains concerning the mechanism of increased infections as transgenic mosquitoes containing only the Gal4 transgene or LRIM1 silencer transgene also had increased oocyst and SPZ intensities compared to wild type. It is possible that there was a position effect of the genomic region integrating the MBL24-GAL4 or LRIM1-silencer transgene, though it is unlikely that this would have the same phenotype in both lines. Determination of the insertion sites of the transgenes would provide information concerning the presence, absence or changes to another gene element at or near the insertion site; unfortunately the lines are no longer available for such analyses. Therefore, increases observed in mosquitoes with both transgenes in their genome could be interpreted as an additive effect of the transgenes and not necessarily just LRIM1 silencing. If increase in PfSPZ intensity is indeed a response to LRIM1 silencing, then the differences observed between the present data and published studies [31, 39, 62], is due to the approach used for silencing that allowed targeting of LRIM1 in organs directly involved in the parasite development cycle in the mosquito.
Conclusions
The survival of all lines of mosquitoes were identical for 21 days post-emergence, suggesting that LRIM1 knockdown does not affect responses to the main environmental microbial challenges faced by the mosquitoes. Indeed, this is supported by the lack of any effect on both the midgut bacterial population densities. These data, coupled with the more than five-fold average increase in PfSPZ intensities compared to wild type SDA500, suggest that immunocompromised mosquitoes can be developed that could significantly impact Sanaria’s technology platform, increasing manufacturing efficiency and ultimately reducing vaccine cost.
Supplementary Information
Additional file 1. The supplementary materials file contains methods for and results of mosquito infections with P. berghei, summary statistics and data for both P. falciparum and P. berghei infections of mosquitoes, additional molecular methods, and all primers used in the study.
Acknowledgements
Dr. Igor Sharakov, Virginia Polytechnic Institute and State University, kindly provided the chromosomal scaffolds of A. stephensi. The assembled draft genome sequence of A. stephensi was made available by Dr. Z. Tu (Virginia Polytechnic Institute and State University). We thank Ehud Inbar for his helpful comments on drafts and Ian McWilliams and Yonas Abebe for help with manuscript preparation. This work was funded by the National Institutes of Health Small Business Innovation Research grant R44AI077262.
Abbreviations
- AgLRIM1
Anopheles gambiae LRIM1
- APL1C
Anti-Plasmodium response leucine rich repeat 1
- AsLRIM1
Anopheles stephensi LRIM1
- ATP
Adenosine 5-triphosphate
- cDNA
Complementary DNA
- CFU
Colony forming units
- CHMI
Controlled human malaria infection
- DNA
Deoxyribonucleic acid
- ds
Double stranded
- dsRNA
Double stranded RNA
- ECFP
Enhanced cyan fluorescent protein
- EDTA
Ethylenediaminetetraacetic acid
- EGFP
Enhanced green fluorescent protein
- hpbm
Hours post blood meal
- IMD
Immune deficiency
- LRIM1
Leucine-rich immune protein 1
- PBS
Phosphate buffered saline
- PbSPZ
Plasmodium berghei sporozoite(s)
- PCR
Polymerase chain reaction
- RNA
Ribonucleic acid
- RPM
Rotations per minute
- RT
Room temperature
- SDS
Sodium dodecyl sulphate
- shRNA
Short hairpin RNA
- SPZ
Sporozoite(s)
- TE
Tris–EDTA
- TEP1
Thioester containing protein 1
- Tris–HCL
Trisaminomethane hydrochloride
- UAS
Upstream activation sequence(s)
Authors' contributions
PFB was principal investigator on the study, designed experiments, analysed data and prepared the manuscript; KIG performed most of the experimental work and prepared the initial write-up of the data, AGE helped with experimental design and analysis, and with preparation of the manuscript, RH performed and supervised mosquito egg injections and experimental design, RA selected transgenic strains and rear mosquitoes, TL prepared P. falciparum gametocytes for feeding, SC organized dissection and analysis of P. falciparum-infected mosquitoes, BKLS supervised P. falciparum-infection experiments, SLH oversaw project and data analysis, DAO’B supervised and designed molecular and genetic experiments, and data analysis. All authors read and approved the final manuscript.
Funding
This work was funded by a National Institute of Allergy and Infectious Diseases, National Institutes of Health, small business innovation research Grant, R44AI077262, Transgenic Mosquitoes for Improved Malaria Sporozoite Vaccine Manufacture.
Availability of data and materials
The data generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
There are no human subjects in this study so ethical approval and consent to participate are not needed. Protocol to feed adult female mosquitoes on mice infected with P. berghei and to support mosquito colony maintenance was approved by The University of Maryland Biotechnology institute IACUC committee under IACUC protocol DB09050401-01.
Consent for publication
Not applicable.
Competing interests
PFB, AGE, TL, SC, BKLS and SLH are employees of and shareholders (BKLS, SLH) in Sanaria Inc., manufacturers of PfSPZ vaccines.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO . World malaria report 2020. Geneva: World Health Organization; 2020. [Google Scholar]
- 2.Billingsley PF, Maas CD, Olotu A, Schwabe C, García GA, Rivas MR, et al. The Equatoguinean malaria vaccine initiative: from the launching of a clinical research platform to malaria elimination planning in central west Africa. Am J Trop Med Hyg. 2020;103:947–954. doi: 10.4269/ajtmh.19-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss DJ, Bertozzi-Villa A, Rumisha SF, Amratia P, Arambepola R, Battle KE, et al. Indirect effects of the Covid-19 pandemic on malaria intervention coverage, morbidity, and mortality in Africa: a geospatial modelling analysis. Lancet Infect Dis. 2020;21:59–69. doi: 10.1016/S1473-3099(20)30700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiodini J. Covid-19 and the impact on malaria. Travel Med Infect Dis. 2020;35:101758. doi: 10.1016/j.tmaid.2020.101758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sissoko MS, Healy SA, Katile A, Omaswa F, Zaidi I, Gabriel EE, et al. Safety and efficacy of PfSPZ vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis. 2017;17:498–509. doi: 10.1016/S1473-3099(17)30104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jongo SA, Shekalage SA, Church LWP, Ruben AJ, Schindler T, Zenklusen I, et al. Safety, immunogenicity, and protective efficacy against controlled human malaria infection of Plasmodium falciparum sporozoite vaccine in Tanzanian adults. Am J Trop Med Hyg. 2018;99:338–349. doi: 10.4269/ajtmh.17-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mordmüller B, Surat G, Lagler H, Chakravarty S, Ishizuka AS, Lalremruata A, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature. 2017;542:445–449. doi: 10.1038/nature21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jongo SA, Church LWP, Mtoro AT, Chakravarty S, Ruben AJ, Swanson Ii PA, et al. Increase of dose associated with decrease in protection against controlled human malaria infection by PfSPZ vaccine in Tanzanian adults. Clin Infect Dis. 2020;71:2849–2857. doi: 10.1093/cid/ciz1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyke KE, Ishizuka AS, Berry AA, Chakravarty S, Dezure A, Enama ME, et al. Attenuated PfSPZ vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci USA. 2017;114:2711–2716. doi: 10.1073/pnas.1615324114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishizuka AS, Lyke KE, Dezure A, Berry AA, Richie TL, Mendoza FH, et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med. 2016;22:614–623. doi: 10.1038/nm.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman SL, Billingsley PF, James E, Richman A, Loyevsky M, Li T, et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin. 2010;6:97–106. doi: 10.4161/hv.6.1.10396. [DOI] [PubMed] [Google Scholar]
- 12.Richie TL, Billingsley PF, Sim BK, Epstein JE, Lyke KE, Mordmuller B, et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine. 2015;33:7452–7461. doi: 10.1016/j.vaccine.2015.09.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein JE, Paolino KM, Richie TL, Sedegah M, Singer A, Ruben AJ, et al. Protection against Plasmodium falciparum malaria by PfSPZ vaccine. JCI Insight. 2017;2:e89154. doi: 10.1172/jci.insight.89154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+T cell immunity. Science. 2011;334:475–480. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 15.Olotu A, Urbano V, Hamad A, Eka M, Chemba M, Nyakarungu E, et al. Advancing global health through development and clinical trials partnerships: A randomized, placebo-controlled, double-blind assessment of safety, tolerability, and immunogenicity of Plasmodium falciparum sporozoite vaccine for malaria in healthy Equatoguinean men. Am J Trop Med Hyg. 2018;98:308–318. doi: 10.4269/ajtmh.17-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyke KE, Laurens MB, Strauss K, Adams M, Billingsley PF, James E, et al. Optimizing intradermal administration of cryopreserved Plasmodium falciparum sporozoites in controlled human malaria infection. Am J Trop Med Hyg. 2015;93:1274–1284. doi: 10.4269/ajtmh.15-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lell B, Mordmuller B, Dejon Agobe JC, Honkpehedji J, Zinsou J, Mengue JB, et al. Impact of sickle cell trait and naturally acquired immunity on uncomplicated malaria after controlled human malaria infection in adults in Gabon. Am J Trop Med Hyg. 2018;98:508–515. doi: 10.4269/ajtmh.17-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obiero JM, Shekalaghe S, Hermsen CC, Mpina M, Bijker EM, Roestenberg M, et al. Impact of malaria pre-exposure on anti-parasite cellular and humoral immune responses after controlled human malaria infection. Infect Immun. 2015;83:2185–2196. doi: 10.1128/IAI.03069-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodgson SH, Juma E, Salim A, Magiri C, Njenga D, Molyneux S, et al. Lessons learnt from the first controlled human malaria infection study conducted in Nairobi, Kenya. Malar J. 2015;14:182. doi: 10.1186/s12936-015-0671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mordmüller B, Supan C, Sim KL, Gómez-Pérez GP, Ospina Salazar CL, Held J, et al. Direct venous inoculation of Plasmodium falciparum sporozoites for controlled human malaria infection: a dose-finding trial in two centres. Malar J. 2015;14:117. doi: 10.1186/s12936-015-0628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodgson SH, Juma EA, Salim A, Magiri C, Kimani D, Njenga D, et al. Evaluating controlled human malaria infection in Kenyan adults with varying degrees of prior exposure to Plasmodium falciparum using sporozoites administered by intramuscular injection. Front Microbiol. 2014;5:686. doi: 10.3389/fmicb.2014.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roestenberg M, Bijker EM, Sim BK, Billingsley PF, James ER, Bastiaens GJ, et al. Controlled human malaria infections by intradermal injection of cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg. 2013;88:5–13. doi: 10.4269/ajtmh.2012.12-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheehy SH, Spencer AJ, Douglas AD, Sim BK, Longley RJ, Edwards NJ, et al. Optimising controlled human malaria infection studies using cryopreserved parasites administered by needle and syringe. PLoS ONE. 2013;8:e65960. doi: 10.1371/journal.pone.0065960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shekalaghe S, Rutaihwa M, Billingsley PF, Chemba M, Daubenberger CA, James ER, et al. Controlled human malaria infection of Tanzanians by intradermal injection of aseptic, purified, cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg. 2014;91:471–480. doi: 10.4269/ajtmh.14-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez-Perez GP, Legarda A, Munoz J, Sim BK, Ballester MR, Dobano C, et al. Controlled human malaria infection by intramuscular and direct venous inoculation of cryopreserved Plasmodium falciparum sporozoites in malaria-naive volunteers: effect of injection volume and dose on infectivity rates. Malar J. 2015;14:306. doi: 10.1186/s12936-015-0817-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bastiaens GJ, Van Meer MP, Scholzen A, Obiero JM, Vatanshenassan M, Van Grinsven T, et al. Safety, immunogenicity, and protective efficacy of intradermal immunization with aseptic, purified, cryopreserved Plasmodium falciparum sporozoites in volunteers under chloroquine prophylaxis: a randomized controlled trial. Am J Trop Med Hyg. 2016;94:663–673. doi: 10.4269/ajtmh.15-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mutapi F, Billingsley PF, Secor WE. Infection and treatment immunizations for successful parasite vaccines. Trends Parasitol. 2013;29:135–141. doi: 10.1016/j.pt.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roestenberg M, Walk J, Van Der Boor SC, Langenberg MCC, Hoogerwerf MA, Janse JJ, et al. A double-blind, placebo-controlled phase 1/2a trial of the genetically attenuated malaria vaccine PfSPZ-GA1. Sci Transl Med. 2020;12:eaaz5629. doi: 10.1126/scitranslmed.aaz5629. [DOI] [PubMed] [Google Scholar]
- 29.Fraiture M, Baxter RH, Steinert S, Chelliah Y, Frolet C, Quispe-Tintaya W, et al. Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe. 2009;5:273–284. doi: 10.1016/j.chom.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Waterhouse RM, Povelones M, Christophides GK. Sequence-structure-function relations of the mosquito leucine-rich repeat immune proteins. BMC Genomics. 2010;11:531. doi: 10.1186/1471-2164-11-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garver LS, Bahia AC, Das S, Souza-Neto JA, Shiao J, Dong Y, et al. Anopheles IMD pathway factors and effectors in infection intensity-dependent anti-Plasmodium action. PLoS Pathog. 2012;8:e1002737. doi: 10.1371/journal.ppat.1002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warr E, Lambrechts L, Koella JC, Bourgouin C, Dimopoulos G. Anopheles gambiae immune responses to sephadex beads: involvement of anti-Plasmodium factors in regulating melanization. Insect Biochem Mol Biol. 2006;36:769–778. doi: 10.1016/j.ibmb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Jaramillo-Gutierrez G, Rodrigues J, Ndikuyeze G, Povelones M, Molina-Cruz A, Barillas-Mury C. Mosquito immune responses and compatibility between Plasmodium parasites and anopheline mosquitoes. BMC Microbiol. 2009;9:154. doi: 10.1186/1471-2180-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Habtewold T, Povelones M, Blagborough AM, Christophides GK. Transmission blocking immunity in the malaria non-vector mosquito Anopheles quadriannulatus species A. PLoS Pathog. 2008;4:e1000070. doi: 10.1371/journal.ppat.1000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Povelones M, Waterhouse RM, Kafatos FC, Christophides GK. Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science. 2009;324:258–261. doi: 10.1126/science.1171400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Povelones M, Upton LM, Sala KA, Christophides GK. Structure-function analysis of the Anopheles gambiae LRIM1/APL1C complex and its interaction with complement C3-like protein TEP1. PLoS Pathog. 2011;7:e1002023. doi: 10.1371/journal.ppat.1002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baxter RH, Steinert S, Chelliah Y, Volohonsky G, Levashina EA, Deisenhofer J. A heterodimeric complex of the LRR proteins LRIM1 and APL1C regulates complement-like immunity in Anopheles gambiae. Proc Natl Acad Sci USA. 2010;107:16817–16822. doi: 10.1073/pnas.1010575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han YS, Thompson J, Kafatos FC, Barillas-Mury C. Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. EMBO J. 2000;19:6030–6040. doi: 10.1093/emboj/19.22.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osta MA, Christophides GK, Kafatos FC. Effects of mosquito genes on Plasmodium development. Science. 2004;303:2030–2032. doi: 10.1126/science.1091789. [DOI] [PubMed] [Google Scholar]
- 40.Marinotti O, Nguyen QK, Calvo E, James AA, Ribeiro JM. Microarray analysis of genes showing variable expression following a blood meal in Anopheles gambiae. Insect Mol Biol. 2005;14:365–373. doi: 10.1111/j.1365-2583.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 41.Feldmann AM, Ponnudurai T. Selection of Anopheles stephensi for refractoriness and susceptibility to Plasmodium falciparum. Med Vet Entomol. 1989;3:41–52. doi: 10.1111/j.1365-2915.1989.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 42.Feldmann AM, Billingsley PF, Savelkoul E. Bloodmeal digestion by strains of Anopheles stephensi Liston (Diptera: Culicidae) of differing susceptibility to Plasmodium falciparum. Parasitology. 1990;101:193–200. doi: 10.1017/S003118200006323X. [DOI] [PubMed] [Google Scholar]
- 43.Galun R. Feeding stimuli and artificial feeding. Bull WHO. 1967;36:590–593. [PMC free article] [PubMed] [Google Scholar]
- 44.Billingsley PF, Rudin W. The role of the mosquito peritrophic membrane in bloodmeal digestion and infectivity of Plasmodium species. J Parasitol. 1992;78:430–440. doi: 10.2307/3283640. [DOI] [PubMed] [Google Scholar]
- 45.Li T, Eappen AG, Richman AM, Billingsley PF, Abebe Y, Li M, et al. Robust, reproducible, industrialized, standard membrane feeding assay for assessing the transmission blocking activity of vaccines and drugs against Plasmodium falciparum. Malar J. 2015;14:150. doi: 10.1186/s12936-015-0665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Handler AM, Harrell RA., 2nd Germline transformation of Drosophila melanogaster with the piggybac transposon vector. Insect Mol Biol. 1999;8:449–457. doi: 10.1046/j.1365-2583.1999.00139.x. [DOI] [PubMed] [Google Scholar]
- 47.Devon RS, Porteous DJ, Brookes AJ. Splinkerettes-improved vectorettes for greater efficiency in PCR walking. Nucleic Acids Res. 1995;23:1644–1645. doi: 10.1093/nar/23.9.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potter CJ, Luo L. Splinkerette PCR for mapping transposable elements in Drosophila. PLoS ONE. 2010;5:e10168. doi: 10.1371/journal.pone.0010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang X, Peery A, Hall AB, Sharma A, Chen X-G, Waterhouse RM, et al. Genome analysis of a major urban malaria vector mosquito Anopheles stephensi. Genome Biology. 2014;15:459. doi: 10.1186/s13059-014-0459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’brochta DA, Alford RT, Pilitt KL, Aluvihare CU, Harrell RA., II Piggybac transposon remobilization and enhancer detection in Anopheles mosquitoes. Proc Natl Acad Sci USA. 2011;108:16339–44. doi: 10.1073/pnas.1110628108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osta MA, Christophides GK, Vlachou D, Kafatos FC. Innate immunity in the malaria vector Anopheles gambiae: comparative and functional genomics. J Exp Biol. 2004;207:2551–2563. doi: 10.1242/jeb.01066. [DOI] [PubMed] [Google Scholar]
- 52.Mendes AM, Awono-Ambene PH, Nsango SE, Cohuet A, Fontenille D, Kafatos FC, et al. Infection intensity-dependent responses of Anopheles gambiae to the African malaria parasite Plasmodium falciparum. Infect Immun. 2011;79:4708–4715. doi: 10.1128/IAI.05647-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blandin S, Moita LF, Kocher T, Wilm M, Kafatos FC, Levashina EA. Reverse genetics in the mosquito Anopheles gambiae: targeted disruption of the defensin gene. EMBO Rep. 2002;3:852–856. doi: 10.1093/embo-reports/kvf180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Catteruccia F, Levashina EA. RNAi in the malaria vector, Anopheles gambiae. Methods Mol Biol. 2009;555:63–75. doi: 10.1007/978-1-60327-295-7_5. [DOI] [PubMed] [Google Scholar]
- 55.Kennerdell JR, Carthew RW. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell. 1998;95:1017–1026. doi: 10.1016/S0092-8674(00)81725-0. [DOI] [PubMed] [Google Scholar]
- 56.Misquitta L, Paterson BM. Targeted disruption of gene function in Drosophila by RNA interference (RNA-i): a role for nautilus in embryonic somatic muscle formation. Proc Natl Acad Sci USA. 1999;96:1451–1456. doi: 10.1073/pnas.96.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown AE, Bugeon L, Crisanti A, Catteruccia F. Stable and heritable gene silencing in the malaria vector Anopheles stephensi. Nucleic Acids Res. 2003;31:e85. doi: 10.1093/nar/gng085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dimopoulos G, Richman A, Muller HM, Kafatos FC. Molecular immune responses of the mosquito Anopheles gambiae to bacteria and malaria parasites. Proc Natl Acad Sci USA. 1997;94:11508–11513. doi: 10.1073/pnas.94.21.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dimopoulos G, Christophides GK, Meister S, Schultz J, White KP, Barillas-Mury C, et al. Genome expression analysis of Anopheles gambiae: responses to injury, bacterial challenge, and malaria infection. Proc Nat Acad Sci USA. 2002;99:8814–8819. doi: 10.1073/pnas.092274999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yassine H, Kamareddine L, Chamat S, Christophides GK, Osta MA. A serine protease homolog negatively regulates TEP1 consumption in systemic infections of the malaria vector Anopheles gambiae. J Innate Immun. 2014;6:806–818. doi: 10.1159/000363296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dekmak AS, Yang X, Dohna HZ, Buchon N, Osta MA. The route of infection influences the contribution of key immunity genes to antibacterial defense in Anopheles gambiae. J Inn Immun. 2020;13:107–126. doi: 10.1159/000511401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohuet A, Osta MA, Morlais I, Awono-Ambene PH, Michel K, Simard F, et al. Anopheles and Plasmodium: from laboratory models to natural systems in the field. EMBO Rep. 2006;7:1285–1289. doi: 10.1038/sj.embor.7400831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. The supplementary materials file contains methods for and results of mosquito infections with P. berghei, summary statistics and data for both P. falciparum and P. berghei infections of mosquitoes, additional molecular methods, and all primers used in the study.
Data Availability Statement
The data generated and/or analysed during the current study are available from the corresponding author on reasonable request.