Abstract
BACKGROUND/OBJECTIVES:
To examine the prevalence of potentially inappropriate medication (PIM) prescribing and its association with healthcare utilization and related expenditures utilizing nationally representative data from the United States.
DESIGN:
Retrospective cohort study.
SETTING:
The 2011–2015 Medical Expenditure Panel Survey (MEPS).
PARTICIPANTS:
Community-dwelling sample of U.S. adults aged 65 and older during the first round of each MEPS cycle.
MEASUREMENTS:
A qualified definition operationalized from the 2019 American Geriatrics Society Beers Criteria® was used to estimate the prevalence of PIM prescribing over the study period. Negative binomial models were assembled to examine associations between PIM exposure and healthcare utilization including hospitalizations, emergency department (ED) visits, and outpatient provider visits. Generalized linear models with the log link function and gamma distribution were used to analyze associations between PIM exposure and healthcare expenditures. Sensitivity analyses were conducted utilizing inverse probability treatment weighting using propensity scores for being prescribed a PIM.
RESULTS:
The period prevalence of PIM prescribing over the 5-year sample was 34.4%. PIM prescribing was positively associated with hospitalizations (adjusted incidence rate ratio [aIRR] = 1.17; 95 confidence interval [CI] = 1.08–1.26; P < .001), ED visits (aIRR = 1.26; 95% CI = 1.17–1.35; P < .001), and outpatient provider visits (aIRR = 1.18; 95% CI = 1.14–1.21; P < .001). PIM exposure was associated with higher marginal costs within outpatient visits ($116; 95% CI = $105–$243; P < .001), prescription medications ($128; 95% CI = $72–$199; P < .001), and total healthcare expenditures ($458; 95% CI = $295–$664; P < .001). Similar results were found in our propensity score analyses.
CONCLUSION:
PIMs continue to be prescribed at a high rate among older adults in the United States. Our results suggest that receipt of PIMs is associated with higher rates of healthcare utilization and increased costs across the healthcare continuum. Further work is needed to implement evidence-based deprescribing interventions that may in turn reduce unnecessary healthcare utilization.
Keywords: potentially inappropriate medications, older adults, healthcare utilization, costs
The average age of the U.S. population is rising, and an estimated 83.7 million Americans will be aged 65 and older by 2050.1 Older adults account for a disproportionate amount of prescription medication and healthcare resource utilization.2 Harm to older frail adults caused by potentially inappropriate medications (PIMS) is a major public health challenge. PIMS are those for which the risks of use may outweigh the potential benefits for most older adults.3 Multiple explicit criteria have been developed to help clinicians identify PIMs,3–5 with the American Geriatrics Society (AGS) Beers Criteria® one of the most commonly used in the United States.3 The Beers Criteria®, originally published in 1991 for application to patients in the nursing home setting, were updated in 2019. The current version is applicable to older adults in all clinical settings and is used as a clinical, educational, and research tool. Nevertheless, PIMs continue to be prescribed at high rates to older adults despite evidence of poor outcomes.6–8
The reported estimated prevalence of PIM exposure varies between 13.8% and 40.7% among community-based populations.6,9–11 Davidoff et al. evaluated a community-dwelling sample of older U.S. adults and found an overall PIM prevalence of 30.7% using their qualified definition.6 Although PIM prevalence is an important key quality measure, their impact on patient and clinical outcomes must also be evaluated. The uptake of value-based systems in health care makes it important to evaluate the impact of inappropriate medications on resource utilization and costs. Although previous studies showed an association between PIMs and resource utilization, most of this work was conducted outside the United States and was confined to specific healthcare systems.8,12–14
The development and implementation of interventions for inappropriate medication prescribing mandate estimates of current PIM prescribing and their associated impact on the healthcare system. Therefore, the objectives of this study were to (1) examine the prevalence of U.S. PIM prescribing using the updated 2019 AGS Beers Criteria® within the Medical Expenditure Panel Survey (MEPS), and (2) evaluate associations between PIM prescribing and healthcare utilization and expenditures across the continuum of care including hospitalizations, emergency department (ED) visits, and outpatient provider visits.
METHODS
Primary Data Source
We conducted this study using MEPS data from 2011 to 2015. MEPS is a nationally representative survey designed to capture information related to the healthcare services people use, the charges for those services, and how those services are paid for. The survey is conducted annually by the U.S. Public Health Service through the Agency for Healthcare Research and Quality (AHRQ) and the Centers for Disease Control and Prevention.15 Each year, a new panel of households is sampled and interviewed in five survey rounds over a 2.5-year period. In each round, MEPS collects information on new medication fills. Additional details about respondents’ medications, such as quantity and days supplied, are collected from dispensing pharmacies. This study used the MEPS Full-Year Consolidated files, Medical Conditions files, and the Prescribed Medicines files from the included study years. The study sample was limited to those aged 65 and older during the first round of data collection for that cycle. All analyses were conducted with deidentified publicly available data, and as such no ethical approval was necessary.
Primary Exposure
The primary exposure was defined as any PIM per the 2019 AGS Beers Criteria.® Specifically, we examined 33 medications or classes of medications considered generally inappropriate for older adults. We adapted the approach described by Davidoff et al. to develop a qualified definition of exposure that accounted for exceptions as mentioned in the criteria.6,7 Similar to these studies, we excluded mineral oil because it is an over-the-counter medication and rapid-acting insulin administered by sliding scale, as we were unable to distinguish it from scheduled dosing.
We identified PIM fills in the Prescribed Medicines files utilizing the drug name and National Drug Code (NDC) variables for each medication. Next, we applied specific qualifications related to dose and dosage form as defined by the 2019 AGS Beers Criteria® recommendations. Finally, we further applied exceptions and qualifications related to patient demographics and comorbidities mentioned in the 2019 AGS Beers Criteria® to create a dichotomous variable to denote exposure to each category. A detailed summary of our operational definitions of PIM exposure is available in Supplementary Table S1.
Outcomes
Period prevalence of PIM prescribing was defined as the number of respondents with any PIM fill as the numerator and the total study population as the denominator. In the same way, we calculated prevalence for individual inappropriate medications. Overall PIM prevalence and that of the individual inappropriate medications was calculated for each year cycle.
Healthcare utilization and expenditure outcomes were identified in the Full-Year Consolidated file and analyzed in a per person, per year fashion for each 2-year panel. Utilization outcomes included the hospital discharge count (as a surrogate of hospitalizations), ED visits, and outpatient and office-based provider visits. MEPS includes outpatient provider and hospital office-based provider visits as two separate count variables; therefore, a new variable was constructed as the sum of outpatient and office-based visits.
Expenditures in MEPS are defined as the sum of direct payments for care provided during the year including out-of-pocket payments and payments by private insurance, Medicaid, Medicare, and other sources. Total expenditures related to inpatient care, ED visits, outpatient care, and prescribed medicines were identified from the Full-Year Consolidated file. Similar to the utilization variable, outpatient and office-based expenditures were summed into one variable. A total expenditure variable was constructed for composite healthcare utilization costs across all four expenditure categories.
Covariates
Demographic and socioeconomic covariates were selected through literature review and identified in the Full-Year Consolidated file.6,7 These included age, sex, race, marital status, education, income, insurance coverage, self-reported general and mental health status, activities of daily living limitations, instrumental activities of daily living limitations, Physical Component Summary (PCS) and Mental Component Summary (MCS) scores of the Medical Outcomes Study 12-Item Short Form Health Survey (SF-12), and geographic region. Age was collected as a continuous variable and stratified into aged 65 to 74, 74 to 85, and 85 and older groups for analysis. SF-12 is a 12-question measure that captures patient self-reported physical and mental health status.16 Because composite PCS and MCS have little intuitive meaning due to inherent variation over the life span, age-specific mean differences were used to assess SF-12 scores.17 Scores were assessed as below average, average, or above average for each respondent’s age group (as previously defined). A variable was constructed to quantify the number of prescribed medicines a patient received over the course of the year by identifying unique drug names and NDCs. Based on this, a dichotomous variable was constructed to categorize those with polypharmacy as prescribed five or more medications and those without polypharmacy (0–4 medications).
Comorbidities collected were part of the Priority Conditions section of the MEPS household component and included coronary heart disease, angina, previous myocardial infarction, cancer, arthritis, hypertension, hyperlipidemia, asthma, stroke, emphysema, chronic bronchitis, and diabetes mellitus, type II. Other diagnoses of interest were identified through truncated International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes or Clinical Classification Software (CCS) codes included in the Medical Conditions file such as congestive heart failure, chronic renal failure, and dementia.
Statistical Analysis
The prevalence of PIM prescribing was calculated for each year studied together with the period prevalence for the 5-year sample. Trends in prevalence of prescribing any PIM were assessed over the study period using the Cochrane-Armitage test for trend. Trends in prescribing individual classes of inappropriate medications between 2011 and 2015 were assessed with a chi-square statistic.
The crude rate of healthcare utilization was compared between PIM users and nonusers using a two-rate chi-square test. The number of events and rate per 100 persons and 95% confidence intervals (CIs) were reported for each utilization outcome. Logistic regression models were constructed to estimate odds ratios (ORs) with 95% CIs for any hospitalization or ED visit within the study period. Negative binomial regression models were used to estimate incidence rate ratios (IRRs) with 95% CIs for healthcare utilization. Poisson and negative binomial models are standard count models used for event occurrence. Negative binomial models are often preferred over Poisson models because they do not need to meet the condition of equality of mean and variance.18 Adjusted models were controlled for clinical and demographic variables associated with PIM prescribing.
Total expenditures for inpatient stays, ED visits, composite outpatient and hospital office-based visits, and prescription medications were standardized to U.S. 2017 dollars using AHRQ price indices.19 Expenditures were modeled using generalized linear regression with a log link function and gamma distribution. We calculated the marginal difference and 95% CI while adjusting for sociodemographics and comorbidities. This approach was selected based on the modified Park test because the expenditure data best fit this distribution.20
Survey weighting procedures were used to produce national estimates of PIM prevalence and in the analysis of healthcare utilization and related expenditures. All statistical tests were 2-tailed with a P < .05 level of significance. All analyses were conducted using SAS v.9.4 (SAS Institute, Cary, NC).
Sensitivity Analysis
Propensity score analyses were conducted to explore the possibility of residual confounding. We calculated the inverse probability of treatment weighting (IPTW) using a propensity score for being prescribed a PIM. Propensity scores were estimated using a logistic regression model with PIM prescribing as the dependent variable and potential confounders listed in Table 1. Following IPTW, covariate balance was assessed in the weighted sample both statistically with a chi-square test and by standardized mean differences. A standardized mean difference less than .25 after weighting is typically considered to indicate adequate covariate balance.21 For analyses, we combined the propensity and survey weights to provide unbiased treatment effect estimates that are generalizable to the survey target population.22
Table 1.
Study Population Characteristics between PIM and Non-PIM Prescribing Groups within the Medical Expenditure Panel Survey, 2011–2015a,b
| Characteristic | Total n = 218,383,123 | SE | PIM exposure n = 75,135,061 | SE | Non-PIM exposure n = 143,248,062 | SE | P |
|---|---|---|---|---|---|---|---|
| Age, y | .026 | ||||||
| 65–74 | 56.9 | .8 | 55.3 | 1.1 | 57.8 | .9 | |
| 75–84 | 30.9 | .7 | 32.7 | .9 | 29.9 | .8 | |
| ≥85 | 12.2 | .6 | 12.0 | .8 | 12.3 | .6 | |
| Sex | .004 | ||||||
| Male | 44.1 | .5 | 41.9 | .9 | 45.2 | .6 | |
| Female | 55.9 | .5 | 58.1 | .9 | 54.8 | .9 | |
| Race/Ethnicity | .001 | ||||||
| White | 77.8 | 1.0 | 79.9 | 1.0 | 76.8 | 1.2 | |
| Black | 8.7 | .5 | 7.8 | .6 | 9.1 | .6 | |
| Hispanic | 7.7 | .5 | 7.3 | .6 | 7.9 | .6 | |
| Other | 5.9 | .7 | 5.1 | .6 | 6.3 | .9 | |
| Marital status | .04 | ||||||
| Married | 55.4 | .9 | 53.6 | 1.3 | 56.3 | 1.0 | |
| Other | 44.7 | .9 | 46.4 | 1.3 | 43.7 | 1.0 | |
| Education | <.001 | ||||||
| High school or less | 49.9 | 1.0 | 51.5 | 1.3 | 49.1 | 1.1 | |
| Some college | 23.2 | .6 | 24.7 | .9 | 22.5 | .7 | |
| College or postgraduate | 26.8 | .9 | 23.8 | 1.2 | 28.5 | .9 | |
| Income, % poverty line | <.001 | ||||||
| High income | 38.1 | 1.0 | 35.0 | 1.2 | 39.7 | 1.0 | |
| Middle income | 28.4 | .6 | 29.4 | .8 | 27.9 | .6 | |
| Low income | 17.6 | .5 | 18.1 | .7 | 17.3 | .6 | |
| Near poor | 6.3 | .3 | 7.5 | .4 | 5.6 | .3 | |
| Poor/negative | 9.7 | .4 | 10.1 | .5 | 9.4 | .4 | |
| Insurance | <.001 | ||||||
| Medicare only | 36.0 | .8 | 35.2 | 1.0 | 36.4 | .9 | |
| Medicare and private | 52.1 | .9 | 52.0 | 1.1 | 52.2 | 1.0 | |
| Medicare and public | 10.8 | .5 | 12.3 | .8 | 10.0 | .5 | |
| No Medicare/uninsured | 1.1 | .1 | .6 | .1 | 1.4 | .2 | |
| General health status | <.001 | ||||||
| Excellent/Very good | 48.2 | .7 | 39.6 | 1.0 | 52.7 | .7 | |
| Good | 31.1 | .5 | 32.9 | .9 | 30.1 | .6 | |
| Fair/poor | 20.8 | .5 | 27.5 | .9 | 17.2 | .5 | |
| Mental health status | <.001 | ||||||
| Excellent/Very good | 61.9 | .7 | 56.3 | 1.0 | 64.9 | .7 | |
| Good | 28.0 | .6 | 30.5 | .8 | 26.7 | .6 | |
| Fair/poor | 10.1 | .4 | 13.2 | .7 | 8.4 | .4 | |
| ADL limitations | <.001 | ||||||
| Yes | 6.6 | .3 | 8.1 | .5 | 5.7 | .3 | |
| IADL limitations | <.001 | ||||||
| Yes | 10.9 | .4 | 13.9 | .8 | 9.4 | .4 | |
| PCS | <.001 | ||||||
| Below average | 47.0 | .7 | 56.7 | 1.0 | 42.0 | .7 | |
| Average | 4.6 | .2 | 4.8 | .4 | 4.5 | .2 | |
| Above average | 48.3 | .7 | 38.5 | 1.0 | 53.5 | .8 | |
| MCS | <.001 | ||||||
| Below average | 40.9 | .6 | 47.6 | 1.0 | 37.5 | .7 | |
| Average | 6.1 | .2 | 6.4 | .4 | 6.0 | .3 | |
| Above average | 52.9 | .6 | 46.1 | .9 | 56.5 | .7 | |
| Census region | <.0001 | ||||||
| Northeast | 19.2 | 1.0 | 16.5 | 1.0 | 20.6 | 1.1 | |
| Midwest | 22.3 | .9 | 23.4 | 1.2 | 21.7 | 1.0 | |
| South | 37.0 | 1.3 | 39.7 | 1.6 | 35.6 | 1.3 | |
| West | 21.4 | .9 | 20.3 | .9 | 22.0 | 1.1 | |
| Usual care source | <.001 | ||||||
| Yes | 93.5 | .3 | 96.6 | .3 | 91.9 | .4 | |
| Comorbidities | |||||||
| Coronary heart disease | 19.5 | .5 | 23.9 | .9 | 17.1 | .6 | <.001 |
| Angina | 7.5 | .3 | 10.2 | .6 | 6.0 | .3 | <.001 |
| Myocardial infarction | 12.3 | .4 | 15.0 | .7 | 10.8 | .4 | <.001 |
| Heart failure | 2.7 | .2 | 3.7 | .4 | 2.2 | .2 | <.001 |
| Chronic renal failure | .5 | .1 | .7 | .1 | .5 | .1 | .097 |
| Cancer | 30.9 | .7 | 34.1 | 1.0 | 29.2 | .8 | <.001 |
| Arthritis | 59.1 | .6 | 70.1 | .9 | 53.3 | .7 | <.001 |
| Hypertension | 68.8 | .6 | 74.6 | .9 | 65.7 | .7 | <.001 |
| Dyslipidemia | 62.5 | .6 | 68.1 | 1.0 | 59.6 | .8 | <.001 |
| Asthma | 8.7 | .3 | 10.6 | .6 | 7.7 | .3 | <.001 |
| Stroke | 12.3 | .4 | 15.4 | .6 | 10.7 | .4 | <.001 |
| Emphysema | 6.4 | .3 | 8.3 | .5 | 5.5 | .3 | <.001 |
| Chronic bronchitis | 4.4 | .2 | 5.7 | .4 | 3.8 | .2 | <.001 |
| Diabetes | 22.4 | .5 | 30.1 | .9 | 18.3 | .6 | <.001 |
| Dementia | 5.0 | .3 | 6.4 | .5 | 4.2 | .3 | <.001 |
| No. of medications | |||||||
| 0–4 | 43.3 | .7 | 18.5 | .7 | 56.3 | .8 | <.001 |
| ≥5 | 56.7 | .7 | 81.5 | .7 | 43.7 | .8 | |
| No. of PIMs | — | ||||||
| 1 | 23.0 | .6 | 66.9 | 1.0 | — | — | |
| 2 | 8.6 | .3 | 25.0 | .8 | — | — | |
| ≥3 | 2.8 | .2 | 8.1 | .5 | — | — |
Abbreviations: ADL, activities of daily living; IADL, instrumental activities of daily living; MCS, Mental Component Summary score; PCS, Physical Component Summary score; PIM, potentially inappropriate medication; SE, standard error.
All percentage are weighted to provide estimates for approximately 218 million older adults in the United States between 2011 and 2015.
Differences were compared between PIM users and nonusers with the chi-square test.
RESULTS
Sample Characteristics
There were 218,383,123 persons aged 65 and older during the study period (2011–2015), of which 75,135,061 (34.4%) were prescribed at least one PIM and 143,248,062 (65.6%) were not prescribed any PIMs (Table 1). The median age of the cohort was 72 years (interquartile range = 68–79 years). Most of the sample was aged 65 to 74 years (56.9%), female (55.9%), and White (77.8%). Most had Medicare and private insurance coverage (52.1%) or Medicare only (36.0%). A greater percentage of those prescribed PIMs reported below-average scores on the PCS (57.6% vs 42.0%) and MCS (47.6% vs 37.5%) for their age group, indicating that those exposed believed they were not in good health relative to others their age. Those in the PIM-exposed group had a higher mean number of medications prescribed (8.7 vs 4.5; P < .001) relative to those not exposed. All comorbidities included were more frequent in those prescribed PIMs (P < .001).
Prevalence of Potentially Inappropriate Medications
The period prevalence of PIM prescribing was 34.4% over the 5-year study sample. There was a statistically significant −2.8% net decrease in prevalence over the study period from 35.3% in 2011 to 32.5% in 2015 (P trend = .045) (Figure 1). On a drug/drug class level, several PIMs showed small but statistically significant changes in prevalence between 2011 and 2015 (Supplementary Table S2). These included antispasmodics (−1.1%; P = .005), digoxin (−.81%; P < .001), antidepressants (−1.4%; P = .003), barbiturates (−.36%; P = .048), non-benzodiazepine hypnotics (−1.6%; P = .01), androgens (−.33%; P = .001), estrogens (−1.2%; P = .005), and metoclopramide (−.37%; P = .03).
Figure 1.
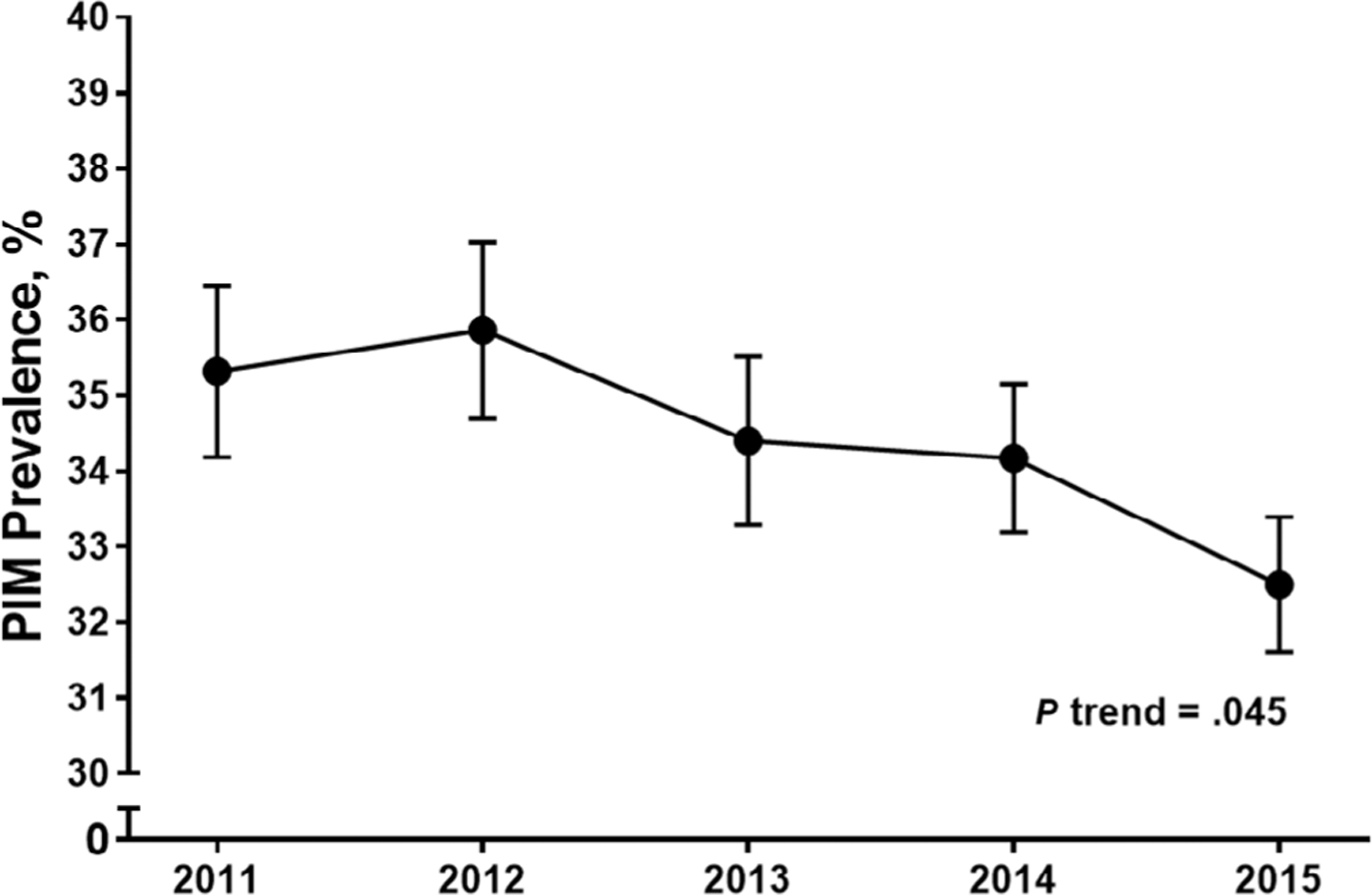
Trend in the Prevalence of Potentially Inappropriate Medication (PIM) Prescribing among Older Adults in the United States, 2011–2015. Note: Prevalence estimates are presented as percentage (standard error) of patients per year with fills for PIM. Cochrane-Armitage test for trend over the study period.
Healthcare Utilization
There were 25,217,427 hospitalizations in those prescribed PIMs and 27,624,024 in those without PIM use (Supplementary Table S3). The crude rate of hospitalizations was higher in those prescribed PIMs at 35.6 per 100 persons (95% CI = 33.55–33.58) versus 19.3 per 100 persons in the non-PIM group (95% CI = 19.28–19.29; P < .001). There was a higher odds of having any hospitalization in adjusted models for those exposed to PIMs (OR = 1.20; 95% CI = 1.06–1.36; P < .004; Supplementary Table S4). In adjusted count models, PIM exposure was associated with an increased rate of hospitalizations (IRR = 1.17; 95% CI = 1.08–1.26; P < .001; Table 2).
Table 2.
Associations between Potentially Inappropriate Medication Prescribing and Healthcare Utilizationa
| Survey weighted, unadjusted analysisb |
Survey weighted, adjusted analysisb,c |
|||
|---|---|---|---|---|
| IRR (95% CI) | P | aIRR (95% CI) | P | |
| Hospitalization | 1.80 (1.67–1.94) | <.001 | 1.17 (1.08–1.26) | <.001 |
| ED visit | 1.81 (1.70–1.94) | <.001 | 1.26 (1.17–1.35) | <.001 |
| Outpatient visit | 1.57 (1.52–1.61) | <.001 | 1.18 (1.14–1.21) | <.001 |
Abbreviations: aIRR, adjusted incidence rate ratio; CI, confidence interval; ED, emergency department; IRR, incidence rate ratio; PIM, potentially inappropriate medication.
Reference for IRR is those with no exposure to PIMS.
Survey weights applied to give a national estimate.
Adjusted models controlled for age, sex, race, marital status, education, income, insurance coverage, self-reported general and mental health status, activities of daily living limitations, instrumental activities of daily living limitations, Mental Component Summary score, Physical Component Summary score, geographic region, coronary heart disease, angina, myocardial infarction, heart failure, chronic renal failure, cancer, arthritis, hypertension, dyslipidemia, asthma, stroke, emphysema, chronic bronchitis, diabetes, dementia, and polypharmacy.
There were 31,103,344 and 33,817,247 ED visits in those prescribed and not prescribed PIMs, respectively. The rate of ED visits was 41.1 per 100 persons (95% CI = 41.38–41.41) in the PIM group compared with 23.6 per 100 persons (95% CI = 23.60–23.63; P < .001) in the non-PIM group. There was a higher odds of having any ED visit in adjusted models for those exposed to PIMs (OR = 1.17; 95% CI = 1.06–1.30); P < .003; Supplementary Table S4). In the adjusted count analysis, PIM prescribing was associated with an increased rate of ED visits (IRR = 1.26; 95% CI = 1.17–1.35; P < .001; Table 2).
The PIM user group had 1,204,981,890 combined outpatient and office-based visits; the non-PIM group had 1,479,524,192 visits. The rate of outpatient provider visits was 160.4 per 100 persons (95% CI = 60.4–160.4) and 103.3 per 100 persons (95% CI = 103.3–103.3; P < .001) in those with and without PIM exposure, respectively. In adjusted models, PIM prescribing was associated with an increased rate of outpatient provider visits (IRR = 1.18; 95% CI = 1.14–1.21; P < .001; Table 2).
Healthcare Expenditures
Mean healthcare expenditures were higher for each type of utilization in those prescribed PIMs (Figure 2). The difference in average total expenditures was higher for those on PIMs within the inpatient ($1,767), ED ($141), and outpatient ($1,568) settings (all P < .001). Prescription drug expenditures were also higher in those prescribed PIMs ($2,263; P < .001). Totaling expenditures across all types resulted in a difference of $5,168 for those exposed to PIMs as compared with the non-PIM group (P < .001).
Figure 2.
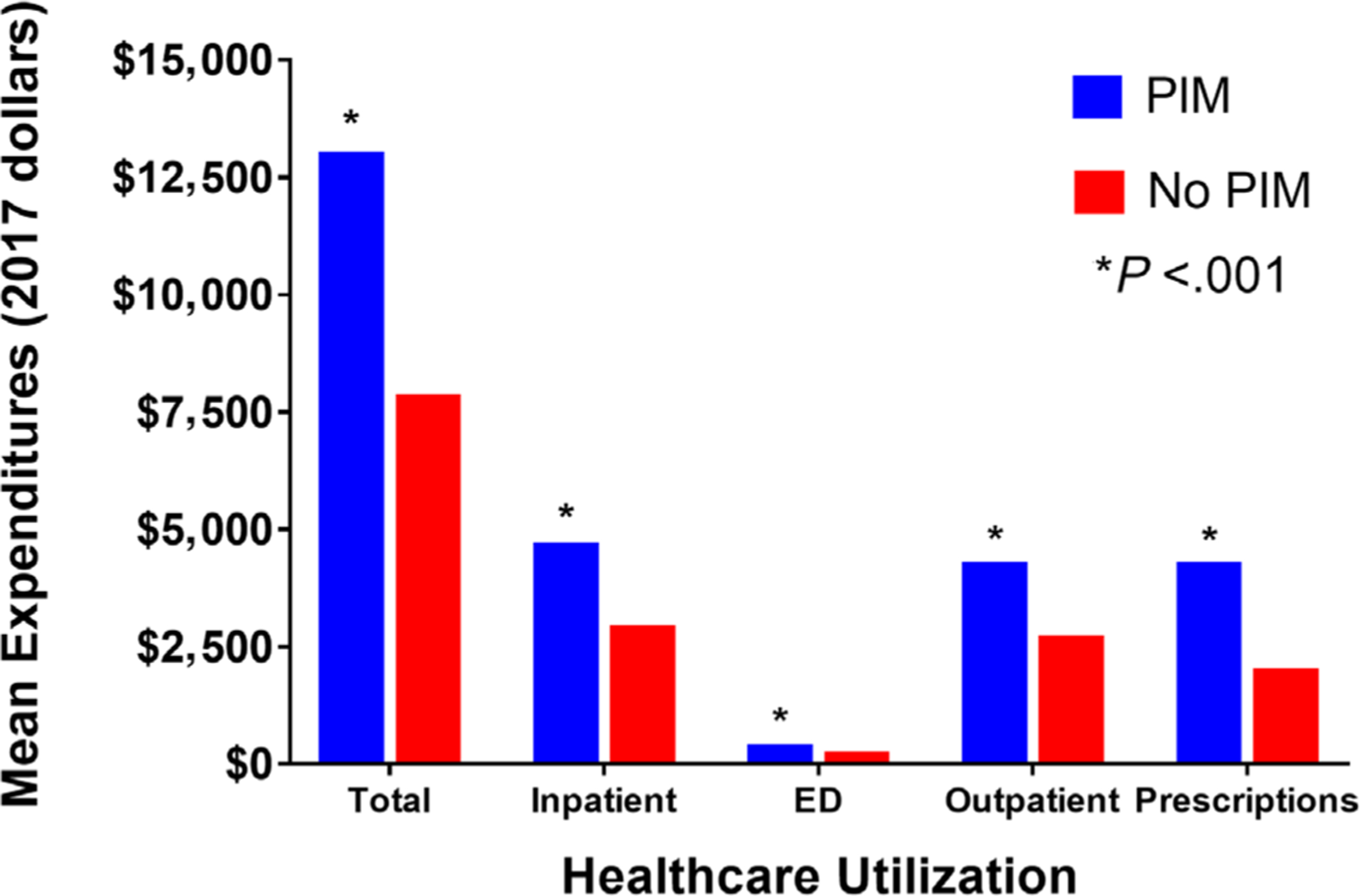
Average Healthcare Expenditures between Potentially Inappropriate Medication (PIM) and Non-PIM Prescribing Groups. * Indicates within-group difference between the PIM and non-PIM group. ED, emergency department.
In the adjusted analysis with total expenditures (composite of inpatient, ED, outpatient, and prescription costs) as the outcome, the mean annual cost difference between PIM users and nonusers was $458 (95% CI = $295–$664; P < .001) (Table 3). Evaluating expenditures related to each type of service and adjusting for the same variables, we found significantly higher costs for those prescribed PIMs for outpatient visits ($116; 95% CI = $105–$243; P < .001) and prescription drug expenditures ($128; 95% CI = $72– $199; P < .001). No significant differences were found for inpatient or ED expenditures.
Table 3.
Marginal Healthcare Expenditures between PIM and Non-PIM Prescribing Groups
| Unadjusted marginal expenditurea (95% CI) | P | Adjusted marginal expenditurea,b (95% CI) | P | |
|---|---|---|---|---|
| Total | $4,617 ($4,072 to $5,199) | <.001 | $458 ($295 to $664) | <.001 |
| Hospitalizations | −$746 (−$2,032 to $774) | .317 | −$461 (−$1,094 to $775) | .381 |
| ED visits | $59 (−$36 to $170) | .241 | $38 (−$21 to $142) | .273 |
| Outpatient visits | $1,357 ($1,177 to $1,551) | <.001 | $116 ($105 to $243) | <.001 |
| Prescription medications | $1,373 ($1,205 to $1,554) | <.001 | $128 ($72 to $199) | <.001 |
Abbreviations: CI, confidence interval; ED, emergency department; PIM, potentially inappropriate medication.
Survey weights applied to give a national estimate.
Marginal expenditures were adjusted for age, sex, race, marital status, education, income, insurance coverage, region, coronary heart disease, angina, myocardial infarction, arthritis, hypertension, hyperlipidemia, asthma, stroke, emphysema, chronic bronchitis, diabetes, and polypharmacy.
Sensitivity Analysis
The balance of covariates was assessed following application of the IPTW to the study sample, and all became balanced with a standardized mean difference of less than .25 (Supplementary Table S5). Adjusted models applying IPTW yielded largely similar results to our initial findings. PIM exposure was associated with an increased odds of having any hospitalization (adjusted OR = 1.26; 95% CI = 1.11–1.43; P < .001) or ED visit (adjusted OR = 1.20; 95% CI = 1.08–1.34; P < .001); Supplementary Table S6). In adjusted models, PIM exposure was associated with an increased rate of hospitalizations (IRR = 1.18; 95% CI = 1.09–1.27; P < .001), ED visits (IRR = 1.26; 95% CI = 1.18–1.36; P < .001), and outpatient provider visits (IRR = 1.18; 95% CI = 1.15–1.21; P < .001; Supplementary Table S7). The marginal expenditures between groups were also similar, although the effect was slightly attenuated in our IPTW analysis. One exception was that cost associated with inpatient stays was found to be lower in the PIM-exposed group (−$1,106; 95% CI −$1,481 to −$290; P = .015; Supplementary Table S8).
DISCUSSION
In this retrospective population-based study, we found that PIM prescribing was highly prevalent in older adults in the United States and associated with increased healthcare utilization and expenditures. PIMs were prescribed in more than one-third of older adults over the 5-year study period. Relative to those without PIM exposure, PIM use was consistently associated with increased rates of healthcare access across the continuum of care. Total healthcare utilization-related expenditures were higher for patients prescribed PIMs, which seemed to be related primarily to prescription medication costs.
The prevalence of PIM prescribing utilizing a qualified version of the 2019 AGS Beers Criteria® is similar to previous estimates applying older versions to MEPS data.6 Davidoff et al. applied a qualified version of the 2012 AGS Beers Criteria® to 2006–2010 MEPS data and reported a prevalence of 30.7% over the study period. Our estimate was slightly higher, most likely due to the inclusion of additional drugs such as proton pump inhibitors (PPIs) that were first included in the 2015 update.23 These authors saw a statistically significant decrease in PIM prevalence over the study period. Our study period included the subsequent 5 years of MEPS data, and we also saw a significant decline in overall PIM prescribing. Although this is encouraging, prescribing of PIMs among older adults still remains high among the general U.S. population. It is worth noting that the guidance recommended by the applied criteria had not yet been published, and previous AGS Beers Criteria® had several differences in medications included. However, as noted by Davidoff et al., the evidence for the new criteria evolved over the years of data included in this study and would have been available to clinicians during the study period.
Although the overall rate of PIM prescribing among older adults appears to be declining, this was not the case for all medications. We saw a small but significant decrease in the use of specific inappropriate medications including antispasmodics, antidepressants, digoxin, non-benzodiazepine hypnotics, androgens, estrogens, and metoclopramide. Of note, some of the agents most commonly targeted for deprescribing in older adults (eg, first-generation antihistamines, antipsychotics, benzodiazepines, nonsteroidal anti-inflammatory drugs, PPIs, and long-acting sulfonylureas) did not change over this time period.24,25 Efforts to deprescribe, where clinicians actively stop medicines that are inappropriate or no longer needed, have increased significantly over the last decade, and evidence-based deprescribing guidelines have been developed to identify inappropriate medications and to help engage patients in conversations about discontinuation.26–28 Continued monitoring of the use of these agents at the population level is warranted as efforts are made to implement these guidelines into clinical practice.
Our results suggest that PIM use is associated with an increased rate of healthcare utilization across the continuum of care. Previous studies showed a positive relationship between PIM exposure and hospitalization. Fillenbaum et al. applied the 1997 Beers Criteria® to a 3-year sample of older adults and found that PIM use was associated with a reduced time to hospitalization but not outpatient visits or nursing home entry.29,30 Klarin et al. applied the same criteria to 785 patients aged 75 and older from a longitudinal population-based cohort of Swedish patients participating in a study on aging and dementia.31 This study found that PIM use was associated with a higher odds of at least one hospitalization but not mortality. A subsequent study applied the 2003 Beers Criteria® to a 3-year sample of claims data for retirees older than 65 years from a single employer.12,32 PIM use was again associated with a higher odds of hospital admissions during the 3 years of available data. There is only limited evidence on the relationship between PIM exposure and subsequent healthcare utilization such as ED and outpatient visits; our study shows a significant increase in utilization of both for those exposed to PIMs. As health care moves toward a value-based system, it will be important to evaluate utilization across the entire healthcare continuum.
PIM prescribing has also been associated with increased healthcare costs in a number of patient populations including ambulatory older adults, those in residential care facilities, cancer patients, and hospitalized patients.8,33–35 However, there are inconsistencies regarding which costs are influenced by these medications. Our study suggests that PIM exposure is associated with higher total expenditures influenced predominantly by prescription medication costs. Fick conducted a retrospective study utilizing claims data for more than 2,000 patients enrolled in a Medicare-managed care plan and found that PIM exposure was associated with higher total, provider, and facility-related costs.8,30 Although mean prescription medication costs were higher in PIM-exposed patients, the difference was not statistically significant. Conversely, and similar to the present study, an Australian study found that PIMs were positively associated with higher total medication costs among residents of aged care facilities.23,33 However, patients in the latter study were prescribed more medications, and a higher percentage were prescribed PIMs. Evidence is limited on the impact of deprescribing PIMs on healthcare utilization in the outpatient setting, but there is evidence that such interventions can reduce prescription medication expenditures.36,37
Our study has several limitations. Due to the observational study design, we cannot exclude the possibility of unmeasured confounding, although supportively there is consistency between the primary and propensity score analyses. A cause-and-effect relationship cannot be inferred because other factors may have influenced healthcare utilization. Although the operationalization of the 2019 AGS Beers Criteria® captures most intended PIM patients, data limitations preclude absolute precision. For example, several of our qualifications used to determine if a medication was considered a PIM relied on truncated ICD-9-CM or CCS codes, several of which may over- or under-include the disease states we intended to consider, therefore altering our prevalence estimate. Additionally, we determined PIM exposure if any fill meeting the necessary qualifications was present in the Prescribed Medicines file.
Of note, the AGS Beers Criteria® has specific recommendations related to duration of therapy for several medications, but we were unable to operationalize these due to a high proportion of missing responses in the quantity and days supplied fields. It is possible that some patients were on multiple medications within the same class (eg, antihistamines), and we were unable to capture that information using our methodology. This may limit the interpretation of a dose-response effect with an increasing number of PIMs prescribed. Additionally, ED visits that lead to an admission would both be counted toward that type of healthcare utilization; however, expenditures were only assessed as part of inpatient costs. Our results are likely not generalizable outside of the United States, where different medications are available and healthcare payment structures differ.
In conclusion, PIMs continue to be prescribed at high rates among older adults in the United States. Our results suggest that PIM use is associated with increased healthcare utilization and costs across the healthcare continuum. Interventions are needed to target unnecessary and inappropriate medications in older adults. Deprescribing is currently in its infancy in the United States, and further work is needed to implement these interventions to reduce unnecessary healthcare utilization.
Supplementary Material
Supplementary Table S1: Qualified definitions for PIM exposure.
Supplementary Table S2: Differences in Potentially Inappropriate Medication Prevalence within the Medical Expenditure Panel Survey, 2011 and 2015.
Supplementary Table S3: Healthcare Utilization Rates between PIM and Non-PIM Prescribing Groups.
Supplementary Table S4: Associations between Potentially Inappropriate Medication Prescribing and any Hospitalizations and Emergency Department Visits.
Supplementary Table S5: Study Population Characteristics between PIM and Non -PIM Prescribing Groups using Inverse Probability of Treatment Weighting.
Supplementary Table S6: Associations between Potentially Inappropriate Medication Prescribing and any Hospitalizations and Emergency Department Visits using Inverse Probability of Treatment Weighting.
Supplementary Table S7: Associations between Potentially Inappropriate Medication Prescribing and Healthcare Utilization using Inverse Probability of Treatment Weighting.
Supplementary Table S8: Marginal Healthcare Expenditures between PIM and Non-PIM Prescribing Groups using Inverse Probability of Treatment Weighting.
ACKNOWLEDGMENTS
Financial Disclosure: Collin M. Clark is supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under Award No. T32HP30035 to the University at Buffalo. Amy L. Shaver is supported in part by Interdisciplinary Training in Cancer Epidemiology (T32CA113951). David M. Jacobs is supported by the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute Loan Repayment Program (2 L30 HL138791-02) and Award No. K12HL138052 to the University at Buffalo. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the NIH under Award No. ULTR001412 to the University at Buffalo. This content is those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by NIH, HRSA, HHS, or the U.S. government.
Previous presentation: These results were previously presented at the American College of Clinical Pharmacy Annual Meeting, October 26, 2019, New York, NY.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
Conflict of Interest: The authors have declared no conflicts of interest for this article.
REFERENCES
- 1.Ortman JM, Velkoff VA, Hogan H. An Aging Nation: The Older Populationin the United States, Current Population Reports, P25–1140. Washington, DC: U.S. Census Bureau; 2014. [Google Scholar]
- 2.Institute of Medicine (US) Committee on the Future Health Care Workforce for Older Americans. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC: National Academies Press (US); 2008. https://www.ncbi.nlm.nih.gov/books/NBK215400/. Accessed December 11, 2019. [PubMed] [Google Scholar]
- 3.By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–694. [DOI] [PubMed] [Google Scholar]
- 4.OʼMahony D, OʼSullivan D, Byrne S, OʼConnor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holt S, Schmiedl S, Thürmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int. 2010;107(31–32):543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidoff AJ, Miller GE, Sarpong EM, Yang E, Brandt N, Fick DM. Prevalence of potentially inappropriate medication use in older adults using the 2012 Beers Criteria. J Am Geriatr Soc. 2015;63(3):486–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller GE, Sarpong EM, Davidoff AJ, Yang EY, Brandt NJ, Fick DM. Determinants of potentially inappropriate medication use among community-dwelling older adults. Health Serv Res. 2017;52(4):1534–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fick D. Potentially inappropriate medication use in a Medicare managed care population: association with higher costs and utilization. J Manag Care Pharm. 2001;7(5):407–413. [Google Scholar]
- 9.Fick DM, Mion LC, Beers MH, Waller JL. Health outcomes associated with potentially inappropriate medication use in older adults. Res Nurs Health. 2008;31(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang YJ, Liu WW, Wang JB, Guo JJ. Potentially inappropriate medication use among older adults in the USA in 2007. Age Ageing. 2011;40(3): 398–401. [DOI] [PubMed] [Google Scholar]
- 11.Bao Y, Shao H, Bishop TF, Schackman BR, Bruce ML. Inappropriate medication in a national sample of US elderly patients receiving home health care. J Gen Intern Med. 2012;27(3):304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albert SM, Colombi A, Hanlon J. Potentially inappropriate medications and risk of hospitalization in retirees: analysis of a US retiree health claims database. Drugs Aging. 2010;27(5):407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heider D, Matschinger H, Meid AD, et al. Health service use, costs, and adverse events associated with potentially inappropriate medication in old age in Germany: retrospective matched cohort study. Drugs Aging. 2017;34 (4):289–301. [DOI] [PubMed] [Google Scholar]
- 14.Endres HG, Kaufmann-Kolle P, Steeb V, Bauer E, Bottner C, Thurmann P. Association between potentially inappropriate medication (PIM) use and risk of hospitalization in older adults: an observational study based on routine data comparing PIM use with use of PIM alternatives. PLoS One. 2016;11 (2):e0146811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey. Rockville, MD: Agency for Healthcare Research and Quality; 2016; https://meps.ahrq.gov/mepsweb/. Accessed December 11, 2019. [Google Scholar]
- 16.Burdine JN, Felix MR, Abel AL, Wiltraut CJ, Musselman YJ. The SF-12 as a population health measure: an exploratory examination of potential for application. Health Serv Res. 2000;35(4):885–904. [PMC free article] [PubMed] [Google Scholar]
- 17.Utah Department of Health. Interpreting the SF-12. Salt Lake City, UT: Utah Department of Health; 2001; http://health.utah.gov/opha/publications/2001hss/sf12/SF12_Interpreting.pdf. Accessed April 5, 2020. [Google Scholar]
- 18.Specification Mullahy J. and testing of some modified count data models. J Econom. 1986;33(3):341–365. [Google Scholar]
- 19.Agency for Healthcare Research and Quality. Using Appropriate Price Indices for Analyses of Health Care Expenditures or Income Across Multiple Years. Washington, DC: Agency for Healthcare Research and Quality; 2016. https://meps.ahrq.gov/about_meps/Price_Index.shtml. Accessed December 11, 2019. [Google Scholar]
- 20.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461–494. [DOI] [PubMed] [Google Scholar]
- 21.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dugoff EH, Schuler M, Stuart EA. Generalizing observational study results: applying propensity score methods to complex surveys. Health Serv Res. 2014;49(1):284–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11): 2227–2246. [DOI] [PubMed] [Google Scholar]
- 24.Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890–898. [DOI] [PubMed] [Google Scholar]
- 26.Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: evidence-based clinical practice guideline. Can Fam Physician. 2017;63 (5):354–364. [PMC free article] [PubMed] [Google Scholar]
- 27.Bjerre LM, Farrell B, Hogel M, et al. Deprescribing antipsychotics for behavioural and psychological symptoms of dementia and insomnia: evidence-based clinical practice guideline. Can Fam Physician. 2018;64(1):17–27. [PMC free article] [PubMed] [Google Scholar]
- 28.Pottie K, Thompson W, Davies S, et al. Deprescribing benzodiazepine receptor agonists: evidence-based clinical practice guideline. Can Fam Physician. 2018;64(5):339–351. [PMC free article] [PubMed] [Google Scholar]
- 29.Fillenbaum GG, Hanlon JT, Landerman LR, et al. Impact of inappropriate drug use on health services utilization among representative older community-dwelling residents. Am J Geriatr Pharmacother. 2004;2(2):92–101. [DOI] [PubMed] [Google Scholar]
- 30.Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Arch Intern Med. 1997;157(14):1531–1536. [PubMed] [Google Scholar]
- 31.Klarin I, Wimo A, Fastbom J. The association of inappropriate drug use with hospitalisation and mortality: a population-based study of the very old. Drugs Aging. 2005;22(1):69–82. [DOI] [PubMed] [Google Scholar]
- 32.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers Criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716–2724. [DOI] [PubMed] [Google Scholar]
- 33.Harrison SL, Kouladjian OʼDonnell L, Milte R, et al. Costs of potentially inappropriate medication use in residential aged care facilities. BMC Geriatr. 2018;18(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagstrom K, Nailor M, Lindberg M, Hobbs L, Sobieraj DM. Associationbetween potentially inappropriate medication use in elderly adults and hospital-related outcomes. J Am Geriatr Soc. 2015;63(1):185–186. [DOI] [PubMed] [Google Scholar]
- 35.Feng X, Higa GM, Safarudin F, Sambamoorthi U, Tan X. Potentially inappropriate medication use and associated healthcare utilization and costs among older adults with colorectal, breast, and prostate cancers. J Geriatr Oncol. 2019;10(5):698–704. [DOI] [PubMed] [Google Scholar]
- 36.Dills H, Shah K, Messinger-Rapport B, Bradford K, Syed Q. Deprescribing medications for chronic diseases management in primary care settings: a systematic review of randomized controlled trials. J Am Med Dir Assoc. 2018; 19(11):923–935.e2. [DOI] [PubMed] [Google Scholar]
- 37.Campins L, Serra-Prat M, Palomera E, Bolibar I, Martinez MA, Gallo P. Reduction of pharmaceutical expenditure by a drug appropriateness intervention in polymedicated elderly subjects in Catalonia (Spain). Gac Sanit. 2019;33(2):106–111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Qualified definitions for PIM exposure.
Supplementary Table S2: Differences in Potentially Inappropriate Medication Prevalence within the Medical Expenditure Panel Survey, 2011 and 2015.
Supplementary Table S3: Healthcare Utilization Rates between PIM and Non-PIM Prescribing Groups.
Supplementary Table S4: Associations between Potentially Inappropriate Medication Prescribing and any Hospitalizations and Emergency Department Visits.
Supplementary Table S5: Study Population Characteristics between PIM and Non -PIM Prescribing Groups using Inverse Probability of Treatment Weighting.
Supplementary Table S6: Associations between Potentially Inappropriate Medication Prescribing and any Hospitalizations and Emergency Department Visits using Inverse Probability of Treatment Weighting.
Supplementary Table S7: Associations between Potentially Inappropriate Medication Prescribing and Healthcare Utilization using Inverse Probability of Treatment Weighting.
Supplementary Table S8: Marginal Healthcare Expenditures between PIM and Non-PIM Prescribing Groups using Inverse Probability of Treatment Weighting.


