Abstract
Background
The relapse and distant metastasis in colon adenocarcinoma (COAD) patients with a poor prognosis. Autophagy has gained increasing attention recently.
Methods
This study utilized univariate Cox analysis from the TCGA database to obtain 10 prognostic autophagy-related genes (ARGs). GO and KEGG functional annotation analysis suggested that the ARGs were significantly enriched in tumor metabolic processes. We verified the autophagy-related genes screened by TCGA clinical data. Then, we compared the expression of SERPINA1 in primary and metastatic tumor cells in the GEO database, and finally verified the relationship between SERPINA1 protein expression and prognosis with the CPTAC database.
Results
The ROC curves showed SERPINA1 had robust prediction capability in judging the prognosis and disease process compared with the other 4 ARGs and risk score in COAD. Clinical relationship analysis further indicated SERPINA1 was related to TMN stage, clinical-stage, OS, RFS, and DMFS in COAD. Besides, survival analysis presented that higher expression of SERPINA1 was significantly associated with the longer OS, RFS, or DMFS. Moreover, SERPINA1 protein was validated to be associated with OS, RFS, and DMFS through our own IHC and CPTAC database. Finally, we exploratoryly combined the SERPINA1 mRNA and SERPINA1 protein as a new index for prognostics.
Conclusion
This new combined index showed the highest prognostic value for OS, RFS, and DMFS, and had the potential to become a practical biomarker for prognosis.
Keywords: SERPINA1, autophagy, COAD, prognostic, relapse, distant metastasis
Introduction
Colorectal cancer (CRC) is one of the most common and lethal malignancies in western countries. Its development is a multi-step process that spans many years, thereby providing an opportunity for prevention and early detection. Colon adenocarcinoma (COAD) is the main type of CRC, approximately accounting for 85% or more.1 In the past thirty years, the survival rate has been improved due to early detection. However, most patients with COAD experience recurrence and metastasis usually exhibit 5-year survival rates <10%,2–4 and the treatments available for these patients are limited. Carcinoembryonic antigen (CEA) has been widely diagnosed with colon cancer, but there are no efficient molecular biomarkers for relapse and metastasis diagnosis, which can improve prognosis and treatment outcomes in colon cancer patients.5,6
Autophagy is a highly conservative “self-phagocytic” process, which ensures the orderly degradation of cytoplasmic contents and the circulation of macromolecular components to maintaining cell homeostasis.7 It plays an important role in a wide range of physiological conditions and diseases, especially in the cancer process.8,9 Besides, exploring the underlying mechanism of autophagy can not only uncover the mystery of tumorigenesis but may also help to provide a new biomarker for cancer.10,11 The Human Autophagy Database provides a detailed and up-to-date list of autophagy-related genes (ARGs).12 There are few systematic comprehensive analyses on the clinical significance and potential biological function of ARGs in colon cancer.13,14 For instance, Wang et al reported the relationships between ARGs and colon cancer15 and made a multi-ARGs-based prognosis model for colon cancer. However, the combined application of multiple ARGs is not easy to popularize and implement.
In the current study, we identified ARGs through univariate Cox regression analysis. GO and KEGG enrichment analysis was carried out based on the ARGs. After combining with the results of clinical, we constructed an autophagy gene model closely related to the prognosis of colon adenocarcinoma patients. We figured out SERPINA1 as a prognostic ARG which was verified by clinical data and immunohistochemistry. Moreover, we found SERPINA1 mRNA and SERPINA1 protein with great clinical value to evaluate the relapse-free survival (RFS) and distant metastasis-free survival (DMFS) in colon cancer.
Materials and Methods
Autophagy Related Genes (ARGs)
The Human Autophagy Database provides a detailed and up-to-date list of autophagy-related genes (ARGs).12 In this study, we obtained the expression profiles of 232 ARGs from HADb.
TCGA Data Acquisition
We downloaded the COAD genes expression dataset from the TCGA database and extracted the expression level of 232 ARGs. TCGA provided gene expression profiles for 398 COAD tissue samples and 39 non-tumor samples. The clinicopathological data of COAD patients is also downloaded from the Genomic Data Commons (https://portal.gdc.cancer.gov). ARGs associated with patient survival were identified using univariate Cox regression for subsequent model construction.
Functional Analysis
The calculation of prognostic ARGs and the functional analysis of their enrichment pathways. In this study, the edgeR software package was used to screen and normalize the expression profiles aiming to analyze the prognostic ARGs in COAD tumors and adjacent normal tissues. The corrected standard value logFC > 2, p<0.01. Besides, to understand the biological functions of the prognostic ARGs in COAD, enrichment analysis including gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis. The enrichment analysis was performed using the R-cluster profile package,16 and the results were displayed using the GOplot package.17
Statistical Analysis
R version 3.5.2 (https://www.R-project.org/) and SPSS program version 25 for statistical analysis and drawing. After normalization, all expression data are converted to log2 (value +1). We utilized a univariate Cox regression analysis to estimate the prognostic value of ARGs, and multivariate Cox regression analysis to obtain the potential prognostic ARGs to construct an autophagy prognostic index (API) model. Independent t-test to compare the prognostic ARGs in COAD and adjacent tissues and to determine the relationship between these genes and the clinicopathological characteristics of COAD patients. The correlation between mRNA expression level and clinical parameter with Pearson correlation analysis, p<0.05 indicates a statistically significant difference. Box plots to show gene expression, and test the ability of prognostic ARGs with a K-M curve analysis to recognize RFS and DMFS related ARGs, then SPSS software to draw and calculate the receiver operating characteristic (ROC) curve of each ARGs, and determine the area under the curve (AUC). Association of gene and protein expression on the overall survival (OS), RFS, and DMFS curves with the TCGA and the CPTAC data.
Patients and Tissue Specimens
Collected tissue samples from patients with COAD in the Gastrointestinal Surgery Ward of the Fourth Hospital of China Medical University from 2008 to 2012, including 55 cases of COAD tissue, 52 cases of normal tissue, and 24 cases of adjacent COAD tissue.
Tissue Microarray (TMA) and Immunohistochemistry (IHC)
The tissue microarray chip is deparaffinized by xylene, and debenzene and hydrated by decreasing gradient ethanol. Immerse the chip in a citrate buffer (pH 6.0), and repair it under 80 kpa high pressure for 10 minutes. After the chip was treated with 3% hydrogen peroxide/methanol and 10% non-immune normal goat serum, mouse anti-human SERPINA1 protein monoclonal antibody (SERPINA1, sc-59438, 1:300 dilution; SANTA CRUZ BIOTECHNOLOGY, INC.) was added dropwise and incubated overnight at 4°C. The next day, the chip was incubated with a biotin-labeled secondary antibody (UltraSensitive SP Mouse/Rabbit IHC Kit, China), and then added dropwise freshly prepared DAB (3, 3-diaminobenzidine). Finally, the sections were counterstained with hematoxylin; dehydrated, transparent with xylene, and mounted with neutral gum for observation.
Evaluation of Immunohistochemistry
Two experienced clinicians evaluated the staining level of the tissue microarray chip. The SERPINA1 protein is expressed in the cytoplasm. The product of the percentage of stained cells (0 ~ 100%) and the staining intensity (no staining is 0, weak positive is 1, medium positive is 2, and strong positive is 3). The total score range was 0 ~ 300%. ROC curve analysis evaluated the survival status of the total score as the boundary value for defining negative and positive protein expression.
CPTAC Data Acquisition
We downloaded the SERPINA1 expression dataset in COAD from the Clinical Proteomic Tumor Analysis Consortium and extracted the expression of 95 COAD tumor-tissue samples. The clinicopathological data of COAD patients were also downloaded from the CPTAC.
Result
Identification of Prognostic ARGs and Functional Enrichment of Prognostic ARGs
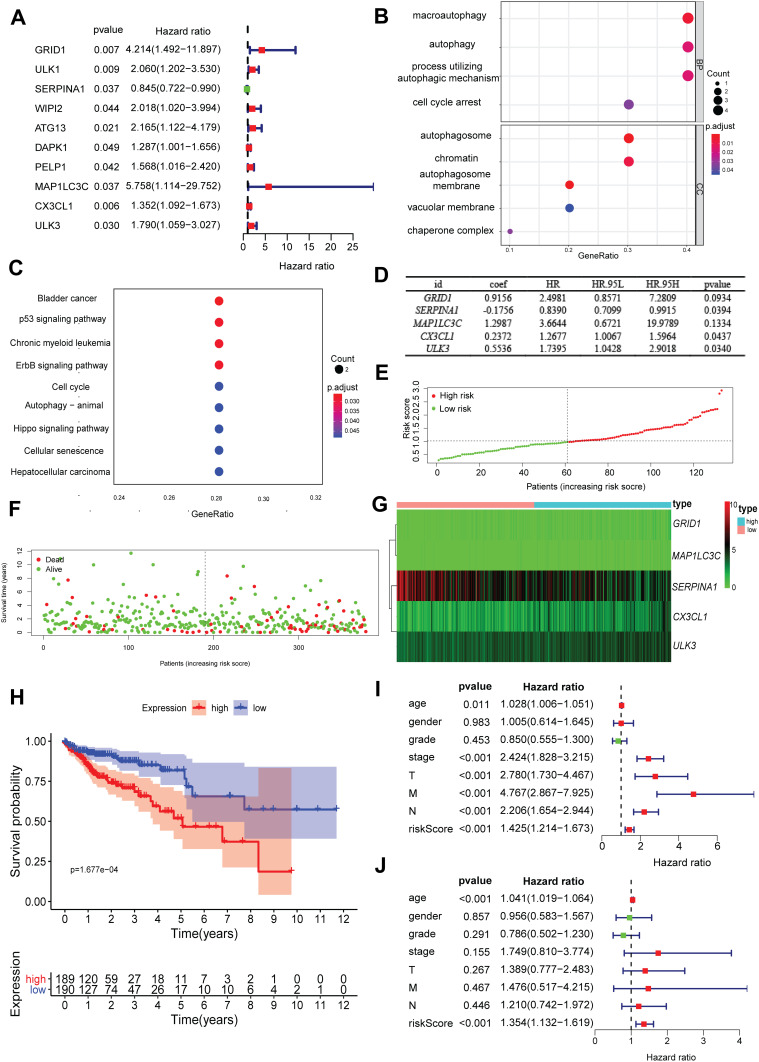
We analyzed the relationship between ARGs and the prognosis of COAD patients. According to the above criteria, we finally obtained 10 prognostic ARGs: GRID1, ULK1, SERPINA1, WIPI2, ATG13, DAPK1, PELP1, MAP1LC3C, CX3CL1, ULK3. They were significantly related to prognosis. The forest chart showed that most of these genes are risk factors except SERPINA1 (Figure 1A). GOplot analysis shows that in biological processes, these genes were related to autophagosome assembly, autophagosome organization, etc. In terms of cellular components, these genes were involved in the functions of the outer mitochondrial membrane, autophagosome, and outer membrane of organelles. In terms of molecular functions, these genes played an indispensable role in certain key functions, such as phagophore assembly site, phagophore assembly site membrane, etc. Besides, KEGG pathway enrichment analysis showed that these genes were mainly enriched in pathways related to the autophagy–animal, spinocerebellar ataxia, autophagy-other, etc. (Figure 1B and C). Multivariate Cox regression was used to achieve 5 ARGs that were significantly related to prognosis independently, and build a risk score for COAD patients (Figure 1D). Based on the results of multivariate Cox regression analysis, we constructed an autophagy prognostic index (API, which is the risk score) to divide COAD patients into high-risk and low-risk groups with discrete clinical outcomes for OS. It showed the distribution of the prognostic index in the TCGA dataset (Figure 1E), the survival time of patients in different groups (Figure 1F). Patients with higher risk scores were more likely to be deceased, and the heatmap was used to show differences in the expression for 5 prognosis-related ARGs between cancer and non-cancer (Figure 1G). A Kaplan-Meier survival curve was drawn to analyze the different survival times between high-risk and low-risk groups (Figure 1H). Univariate and multivariate Cox regression analyses were performed to identify prognosis-related factors in COAD patients. Forest maps showed that clinicopathological characteristics such as age and the risk score were significant after univariate Cox regression analysis (Figure 1I). The risk score was also independently associated with the prognosis of COAD patients for multivariate Cox regression analysis (HR = 1.353, 95% CI = 1.131–1.619; p<0.001; Figure 1J).
Figure 1.
Development of a prognostic index based on ARGs and expression profile and prognostic value of ARGs. (A) Risk ratio forest plot showed the prognostic value of the gene; (B) GO analysis revealed the biological processes and cellular components involved in 10 prognostic-related ARGs; (C) KEGG shows the signaling pathways involved in 10 prognostic-related ARGs; (D) The result of multivariate Cox regression analysis: 5 ARGs were significantly related to prognosis independently; (E) Distribution of prognostic index; (F) Survival status of patients in different groups; (G) Heat map of the expression profile of the included ARGs; (H) Patients in the high-risk group have a shorter OS. A forest plot of univariate (I) and multivariate (J) Cox regression analysis in COAD.
Clinical Utility of Prognostic Markers Diagnostic Value of ARGs in COAD
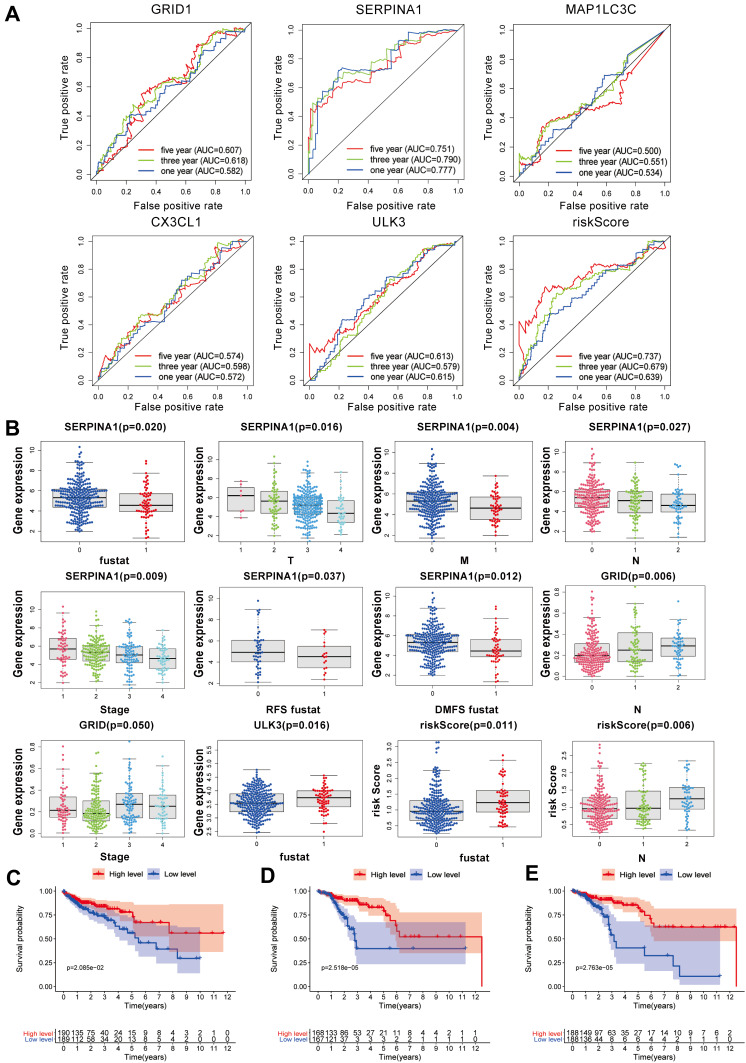
We assessed the diagnostic values of the ARGs for OS status (GRID1, SERPINA1, MAPILC3C, CX3CL1, ULK3, and risk score). The AUC values correlation with the five-year survival rate for GRID1, SERPINA1, MAPILC3C, CX3CL1, ULK3 and risk score were 0.507, 0.751, 0.500, 0.574, 0.613 and 0.737, respectively (Figure 2A).
Figure 2.
ROC curve analysis for ARGs and clinical prognostic diagnostic value of ARGs in COAD and the relationship between SERPINA1 and OS, RFS, and DMFS. (A) ROC curve analysis to determine the potential diagnostic value of the risk ARGs in COAD. The ROC curve plots for 5-years survival rate: GRID1 (AUC = 0.507), SERPINA1 (AUC = 0.751), MAPILC3C (AUC = 0.500), CX3CL1 (AUC = 0.574), ULK3 (AUC = 0.613), risk Score (AUC = 0.737). (B) Clinicopathological significance of the prognostic index of COAD (p<0.05). (C) Kaplan–Meier curve estimated the relationship between SERPINA1 and overall survival (OS). (D) Kaplan–Meier curve estimated the relationship between SERPINA1 and RFS. (E) Kaplan–Meier curve estimated the relationship between SERPINA1 and distant metastasis-free survival DMFS. The Log rank test was performed to test the statistical significance.
This study analyzed the expression of these genes in OS with COX regression analysis, as well as analyzed the relationship between these ARGs and clinical characteristics. According to statistical significance (p<0.05), the level of SERPINA1 gene decreased, the shorter of OS, RFS, DMFS, the more severe with tumor stage and the more serious with TMN stage, the risk Score and survival time was negative-related, with N stage was positive-related. Although GRID1 was related to the tumor stage and N stage, the trend is not obvious. The level of the ULK3 gene was decreased, and the survival status was worse (Figure 2B).
The Relationship Between SERPINA1 and OS, RFS, and DMFS Time
A Kaplan–Meier survival curve was drawn to analyze the overall survival (OS) between high-level and low-level groups. A Kaplan–Meier curve analysis showed that the OS of patients in the low-level group was significantly shorter than that in the high-level group (Figure 2C), which proved SERPINA1 as a protective factor for OS.
RFS refers to the time from the first operation to the earliest evidence of recurrence. DMFS refers to the time when metastasis occurs earliest. In a GEO database, we analyzed from GPL96 (SERPINA1-Colon cancer progression) that the level of SERPINA1 is closely related to metastasis in COAD (Figure S1). GEO data compared SERPINA1 in SW480, a primary colon cancer cell line, to that in SW620, an isogenic metastatic colon cancer cell line. Cell lines are derived from one individual. The results provide the occurrence of metastasis as the level of SERPINA1 decreases. Based on this phenomenon, we determined to analyze the expression of SERPINA1 and RFS (p<0.01) (Figure 2D) and DMFS (p<0.01) (Figure 2E). The results illustrated that SERPINA1 was also a protective factor for RFS and DMFS.
Clinicopathological Characteristics of Colorectal Cancer Patients
In this study, the survival data of 72 COAD patients were collected, and the follow-up time was ranged from 1 to 120 months. Among them, 29 cases died. The 5-year survival rate was 59.7%, and the average survival time was 82.1±14.8 months.
Association of the Expression of SERPINA1 with the Survival of Colorectal Cancer Patients
The results of SERPINA1 protein expression, 72 of 133 colorectal cancer patients completed the scanning of tumor tissue points, were followed up. The median OS of the low expression group was 30 months, and the median OS of the high expression group was 67 months. In the low expression group, 17 cases survived for 3 years, 21 cases died, and the 3-year overall survival rate was 44.74%; in the high expression group, 7 cases survived for 3 years, 28 cases died, and the 3-year survival rate was 20.00%.
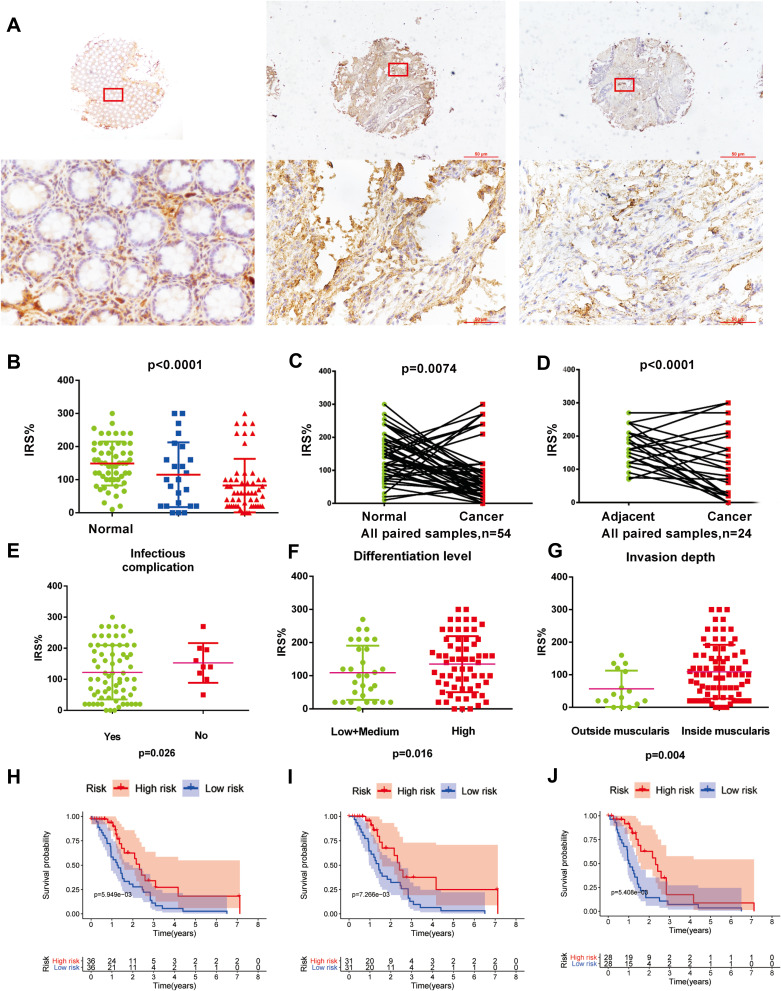
The immunohistochemical method was used to detect the protein expression of SERPINA1 in COAD (Figure 3A), and the comparison found that the expression of SERPINA1 in tumor tissues was significantly lower than that of normal tissues and adjacent tissues (p<0.01, Figure 3B). The immunohistochemical staining results of paired normal and cancerous tissues (Figure 3C) and paired adjacent tissues and cancerous tissues (Figure 3D) from the same patient also showed that the expression of SERPINA1 in tumor tissues was significantly decreased (p<0.01). ROC curve analysis uses the boundary value of the total score to judge the OS status as the cut-off value for the negative expression and positive expression of the protein. ROC curve analysis was related to indicators shown in Figure S2. To investigate the clinical significance of the expression of SERPINA1 in COAD, we analyzed the correlation between OS status and clinical-pathological stratification parameters in COAD patients (Table 1). We found that the number of patients with infectious complications decreased (p=0.026) (Figure 3E). The expression level of SERPINA1 protein increased in those with lower differentiation level (p=0.016) (Figure 3F). And the expression level of SERPINA1 protein decreased significantly in those with deeper intestinal infiltration (p=0.004) (Figure 3G).
Figure 3.
SERPINA1 expression in colon cancer and K-M curve with OS, RFS, DMFS for IHC. (A) Representative micrographs showing immunohistochemical staining of SERPINA1 in normal tissue, tumor-adjacent tissue, and colorectal cancer tissue. Magnification: ×40. Arrows indicate magnified regions in the insert (×400). Scale bar: 50 μm. (B) IRS of normal tissues and adjacent tissues SERPINA1 expression was significantly higher than cancer tissues. (C) IRS of paired cancer samples SERPINA1 expression was significantly lower than normal tissues. (D) IRS of paired samples SERPINA1 expression was significantly lower than tumor-adjacent tissues. the p-value of (B-D) obtained from the Wilcoxon rank-sum test. (E) IRS of SERPINA1 expression with infectious complication. (F) IRS of SERPINA1 expression of differentiation level. (G) IRS of SERPINA1 expression of invasion depth. (H) Kaplan–Meier curve estimates the relationship between SERPINA1 and OS. (I) Kaplan–Meier curve estimates the relationship between SERPINA1 and RFS. (J) Kaplan–Meier curve estimates the relationship between SERPINA1 and DMFS. The Log rank test was performed to test the statistical significance.
Table 1.
Clinicopathological Characteristics of Colon Cancer Patients
| Features | Categories | Frequency | Percent |
|---|---|---|---|
| Sex | Male | 64 | 48.1 |
| Female | 69 | 51.9 | |
| Age(years) | ≤60 | 53 | 39.8 |
| >60 | 80 | 60.2 | |
| Family history | No | 64 | 82.1 |
| Yes | 14 | 17.9 | |
| Infectious complication | No | 69 | 88.5 |
| Yes | 9 | 11.5 | |
| Differentiation level | High | 30 | 32.6 |
| Low+Medium | 62 | 67.4 | |
| Invasion depth | Inside muscular | 16 | 17.0 |
| Outside muscular | 78 | 83.0 | |
| Occupied intestine | ≤0.75 | 28 | 35.4 |
| >0.75 | 51 | 64.6 | |
| CKL | Negative | 26 | 43.3 |
| Positive | 34 | 56.7 | |
| CEA | Negative | 53 | 57.6 |
| Positive | 39 | 42.4 | |
| CA-125 | Negative | 54 | 84.4 |
| Positive | 10 | 15.6 | |
| CA19-9 | Negative | 83 | 83.8 |
| Positive | 16 | 16.2 |
To further investigate the clinical significance of SERPINA1 expression in COAD, the results found that the lower expression of SERPINA1 was significantly correlated with worse patients’ OS, RFS, DMFS status (Figure 3H-J).
The Combined Utilization of SERPINA1 Protein and SERPINA1
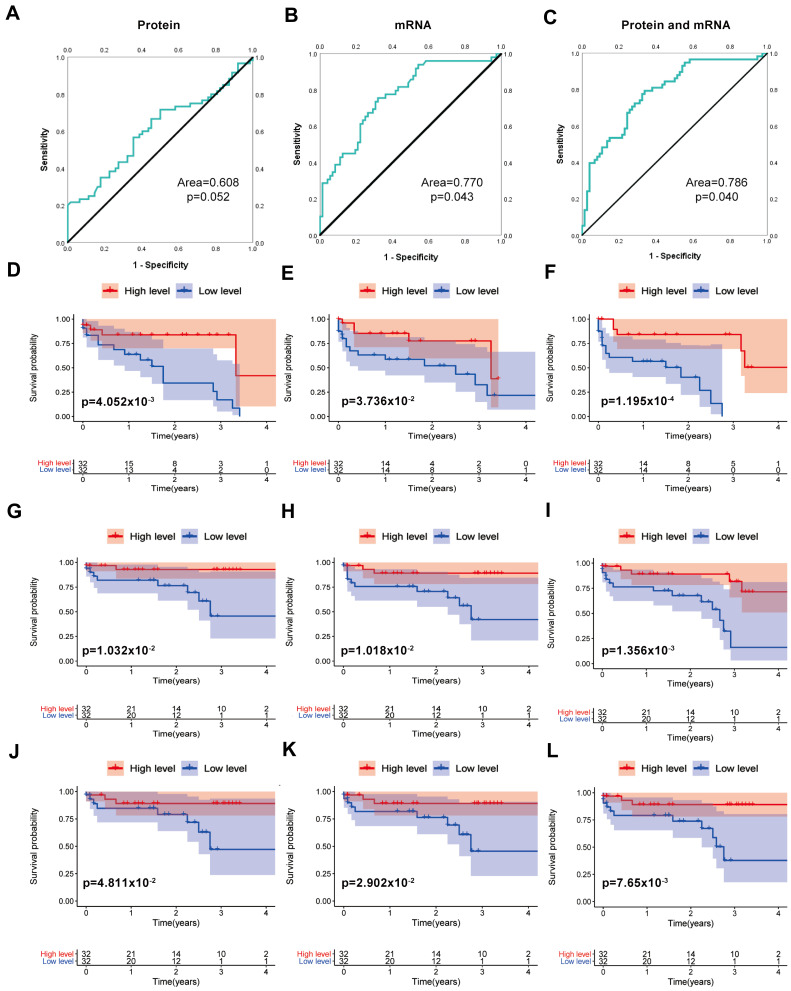
From the above analysis, it is known that SERPINA1 is a protective factor in COAD, and it presents a protected factor in protein expression. We obtained 95 cases of protein expression from the CPTAC Database (https://proteomics.cancer.gov/programs/cptac). We obtained the corresponding mRNA expression from the TCGA database and analyzed the relationship between mRNA and protein expression with OS status. The CPTAC database has 64 clinical cases. We used the median of SERPINA1 mRNA expression and protein expression divided into two groups. ROC curve analysis with survival overall status showed that protein expression (Area=0.608, p=0.052) in Figure 4A, and mRNA (Area=0.770, p=0.043) in Figure 4B. Then, we combined the mRNA and protein as an index for prognosis. We dealt with the data of mRNA expression and protein expression in a quartile. Then, we multiplied the two quartiles processed with the data to get the combined index (CI) of the SERPINA1 mRNA and SERPINA1 protein for each patient when combining protein and mRNA to predict the state of prognosis. It is proved that the accuracy of clinical diagnosis with the combination of SERPINA1 protein expression and mRNA expression is improved (Area=0.786, p =0.04) (Figure 4C).
Figure 4.
The ROC and K-M curve analysis of the influence of SERPINA1 on COAD. (A) SERPINA1 protein, Area=0.773, p=0.004. (B) SERPINA1 mRNA, Area=0.770, p=0.043. (C) SERPINA1 mRNA and protein combine. Area=0.786, p=0.04. (D) Kaplan–Meier curve estimates the relationship between SERPINA1 mRNA and overall survival. (E) Kaplan–Meier curve estimates the relationship between SERPINA1 protein and overall survival. (F) Kaplan–Meier curve estimates the relationship between CI and overall survival. (G) Kaplan–Meier curve estimates the relationship between SERPINA1 mRNA and RFS. (H) Kaplan–Meier curve estimates the relationship between SERPINA1 protein and RFS. (I) Kaplan–Meier curve estimates the relationship between CI with RFS. (J) Kaplan–Meier curve estimates the relationship of SERPINA1 mRNA with DMFS. (K) Kaplan–Meier curve estimates the relationship of SERPINA1 protein with DMFS. (L) Kaplan–Meier curve estimates the relationship between CI with DMFS.
We also analyzed the SERPINA1 mRNA, SERPINA1 protein, and CI for the K-M curve with OS (Figure 4D-F), the SERPINA1 mRNA, SERPINA1 protein, and CI for the K-M curve with RFS (Figure 4G-I), the SERPINA1 mRNA, SERPINA1 protein, and CI for the K-M curve with DMFS (Figure 4J-L).
Discussion
The autophagy process is strictly controlled by a series of ARGs.18 Previous studies have shown that the level of dysregulated autophagy is closely related to tumor growth, survival, and proliferation.19 Therefore, the stable expression of ARGs is essential for suppressing the occurrence of tumors.20 However, the relationship between autophagy-related genes and prognosis in colon cancer patients was reported merely in a few studies. In this study, to examine the prognosis significance of human ARG expression in colon cancer, 10 prognosis-related ARGs were identified. We first found that, among the 232 ARGs, SERPINA1 was the most significant independent protective factor for the occurrence and development of COAD. In COAD, SERPINA1 is more valuable in judging the prognosis and disease process compared with the other 4 independent prognoses ARGs or the risk score which was calculated from the combined 5 independent prognoses ARGs. Therefore, SERPINA1 can be used as an index for clinical application, which may play an important role in causing unregulated autophagy in COAD. A previous study based on ARG analysis had mined prognostic indexes of colon cancer which were abundantly enriched in tumor-related pathways, such as the transcripts of the TGFb/EMT pathway.21 Differentially expressed ARGs: SERPINA1, DAPK1, MAP1LC3C, MAPK9, TSC1, ULK3, CASP3, WIPI1, etc., as risk indexes are used to predict patient prognosis and provide information for individualized treatment. These ARGs are very likely to promote the development of COAD.15,22 Compared with the multi-indexes identified in the studies mentioned above, SERPINA1, a single index, has the advantage of a more convenient application.
was significantly increased in a variety of cancers, such as oral cancer, thyroid papillary cancer, lung cancer, and so on.23–28 AggarwalN5 believed that a large number of cell proliferation and tissue cell necrosis stimulated the release of lysosomal proteases during tumor development, resulting in a compensatory increase in SERPINA1. Kwon CH’s study provided evidence of the key role of SERPINA1 as a regulator of invasion and migration in CRC cells for the first time.28 Some people regarded trypsin as a lymphocyte stimulator, whose inhibitor SERPINA1 had an immunosuppressive effect.29 As SERPINA1 increased, the body lost its immune surveillance effect on mutant cells and induced tumors.30 The high expression of SERPINA1 could be up-regulated fibronectin, and fibronectin promoted tumor progression by activating a variety of oncogenic pathways (such as Akt, extracellular signal-regulated kinase, signal transducer, and activator of transcription 3).31 The above reported research concluded that SERPINA1 is a tumor-promoting factor. However, on the contrary, some studies6,29 have shown that SERPINA1 could have an anti-tumor effect. SERPINA1 was significantly decreased in a variety of cancers (Figure S3), such as pancreatic cancer, kidney cancer, thyroid cancer, and so on. In the current study, for COAD patients, SERPINA1 was shown as a protective factor, which was more valuable in assessing clinical parameters such as survival status, TMN stage, clinical-stage, OS, RFS, and DMFS, based on the TCGA database. Then, SERPINA1 protein expression was further detected with ICH, finding that SERPINA1 was negatively related to an infectious complication, lower differentiation level, and deeper intestinal infiltration, and also correlated with longer OS, RFS, and DMFS. Additionally, similar results were found in the CPTAC database. These results correspond to the results of the TCGA database analysis in the present study. Moreover, recurrence and metastasis are key prognostic factors for colon cancer. We find the prognostic autophagy-related key gene SERPINA1 to be a biomarker for recurrence and metastasis in colon adenocarcinoma. And we used clinical data and IHC for validation, which improved the accuracy of the prognosis model prediction results to make it more clinically significant. Finally, we exploratoryly combined the SERPINA1 mRNA and SERPINA1 protein as a new combined index(CI) for prognostics. It was finally found that CI in COAD was probably the most significant prognostic, recurrence, and distant metastasis biomarker.
It was reported that SERPINA1 protein favored tumor cell growth and inhibited autophagy,32 and was a dangerous ARG that was not conducive to prognosis.22 Meanwhile, autophagy in CRC inhibited the tumor.33 However, Zhang has found that autophagy plays a stimulative role in the development of CRC.34 Meanwhile, autophagy can occur in tumors with the opposite function: protective autophagy and lethal autophagy. In this study, SERPINA1 promotes autophagy, and then autophagy plays an anti-tumor effect in COAD. The SERPINA1 gene-related transcription factors, miRNA, lncRNA, and upstream and downstream-related genes were predicted with gene radar (Figure S4), indicating that SP3, HEB, and PROC were more likely to be involved in the function of SERPINA1 in COAD. GOplot analysis showed that SERPINA1 was related to biological processes, such as acute-phase response, HIF-1 signaling pathway, and so on. Overall, the underlying molecular mechanisms of the key ARGs in the pathogenesis of COAD are not yet clear, and further experimental studies are needed to reveal these mechanisms.
This study highlights the important prognostic significance of SERPINA1 in COAD. These findings indicate that SERPINA1 targeted therapy may have a unique effect on COAD, especially as a diagnostic marker for OS, RFS, and DMFS of COAD patients, and provide clues for an in-depth understanding of the complex biological functions of SERPINA1 in COAD.
Funding Statement
National Natural Science Foundation of China (NSFC, No. 82073884), NSFC-Liaoning joint fund key program (No. U20A20413), Major Special S&T Projects in Liaoning Province (2019JH1/10300005).
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the Medical Ethics Committee of China Medical University. Patients consented to have tissue collected at the time of admission for surgery using protocols approved by the Regional Human Ethics Committee of China Medical University. The patients did not receive radiotherapy or chemotherapy before surgery, and the histological determination operated according to WHO (World Health Organization) standards. All procedures performed in studies involving human participants followed the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent for Publication
Not applicable.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted, and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Devenport SN, Shah YM. Functions and implications of autophagy in colon cancer. Cells. 2019;8(11):1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. [DOI] [PubMed] [Google Scholar]
- 3.Jung K-W, Won Y-J, Oh C-M, Kong H-J, Lee DH, Lee KH. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treatment. 2017;49(2):292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal N, Korenbaum E, Mahadeva R, et al. Α-linoleic acid enhances the capacity of α1-antitrypsin to inhibit lipopolysaccharide-induced IL-1β in human blood neutrophils. Mol Med. 2016;22(1):680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang B, Xu X, Lu Y, et al. Prediction of new targets and mechanisms for quercetin in the treatment of pancreatic cancer, colon cancer, and rectal cancer. Food Funct. 2019;10(9):5339–5349. [DOI] [PubMed] [Google Scholar]
- 7.Jiang P, Mizushima N. Autophagy and human diseases. Cell Res. 2014;24(1):69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockel JS, Kapoor M. Autophagy: controlling cell fate in rheumatic diseases. Nat Rev Rheumatol. 2016;12(9):517. [DOI] [PubMed] [Google Scholar]
- 9.Bravo-San Pedro JM, Kroemer G, Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circ Res. 2017;120(11):1812–1824. [DOI] [PubMed] [Google Scholar]
- 10.Vlodavsky I, Singh P, Boyango I, et al. Heparanase: from basic research to therapeutic applications in cancer and inflammation. Drug Resistance Updates. 2016;29:54–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine B, Packer M, Codogno P. Development of autophagy inducers in clinical medicine. J Clin Invest. 2015;125(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moussay E, Kaoma T, Baginska J, et al. The acquisition of resistance to TNFα in breast cancer cells is associated with constitutive activation of autophagy as revealed by a transcriptome analysis using a custom microarray. Autophagy. 2011;7(7):760–770. [DOI] [PubMed] [Google Scholar]
- 13.Yang M, Bai L, Yu W, et al. Expression of autophagy‑associated proteins in papillary thyroid carcinoma. Oncol Lett. 2017;14(1):411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HM, Kim E-S, Koo JS. Expression of autophagy-related proteins in different types of thyroid cancer. Int J Mol Sci. 2017;18(3):540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Xu Y, Li T, Chen B, Yang W. Development of prognosis model for colon cancer based on autophagy-related genes. World J Surg Oncol. 2020;18(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16(5):284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter W, Sánchez-Cabo F, Ricote M. GOplot: an R package for visually combining expression data with functional analysis. Bioinformatics. 2015;31(17):2912–2914. [DOI] [PubMed] [Google Scholar]
- 18.Hara M, Yonei A, Ayabe T, Tomita M, Nakamura K, Onitsuka T. Postoperative serum C-reactive protein levels in non-small cell lung cancer patients. Ann Thorac Cardiovasc Surg. 2010;16(2):85–90. [PubMed] [Google Scholar]
- 19.Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Lu Q, Xie W, Wang Y, Wang G. Anti-tumor effects of triptolide on angiogenesis and cell apoptosis in osteosarcoma cells by inducing autophagy via repressing Wnt/β-Catenin signaling. Biochem Biophys Res Commun. 2018;496(2):443–449. [DOI] [PubMed] [Google Scholar]
- 21.Zhu S, Wu Q, Zhang B, et al. Autophagy-related gene expression classification defines three molecular subtypes with distinct clinical and microenvironment cell infiltration characteristics in colon cancer. Int Immunopharmacol. 2020;87:106757. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Dai S, Yuan Y, Xiao Q, Ding K. A prognostic model for colon cancer patients based on eight signature autophagy genes. Front Cell Dev Biol. 2020;8:602174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aldonyte R, Tunaitis V, Surovas A, et al. Effects of major human antiprotease α-1-antitrypsin on the motility and proliferation of stromal cells from human exfoliated deciduous teeth. Regen Med. 2010;5(4):633–643. [DOI] [PubMed] [Google Scholar]
- 24.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male: female differences diminishing and adenocarcinoma rates rising. Int j Cancer. 2005;117(2):294–299. [DOI] [PubMed] [Google Scholar]
- 25.Ercetin E, Richtmann S, Delgado BM, et al. Clinical significance of SERPINA1 gene and its encoded alpha1-antitrypsin protein in NSCLC. Cancers. 2019;11(9):1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu H-W, Chang K-P, Hsu C-W, et al. Identification of salivary biomarkers for oral cancer detection with untargeted and targeted quantitative proteomics approaches. Mol Cell Proteomics. 2019;18(9):1796–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarquis M, Moraes DC, Bastos-Rodrigues L, et al. Germline mutations in familial papillary thyroid cancer. Endocr Pathol. 2020;31(1):14–20. [DOI] [PubMed] [Google Scholar]
- 28.Kwon CH, Park HJ, Choi JH, et al. Snail and serpinA1 promote tumor progression and predict prognosis in colorectal cancer. Oncotarget. 2015;6(24):20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashkenazi E, Baranovski BM, Shahaf G, Lewis EC. Pancreatic islet xenograft survival in mice is extended by a combination of alpha-1-antitrypsin and single-dose anti-CD4/CD8 therapy. PLoS One. 2013;8(5):e63625–e63625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erez A, DeBerardinis RJ. Metabolic dysregulation in monogenic disorders and cancer—finding method in madness. Nat Rev Cancer. 2015;15(7):440–448. [DOI] [PubMed] [Google Scholar]
- 31.Shirvaikar NC. Role of membrane-type 1 matrix metalloproteinase in hematopoietic stem/progenitor cell trafficking. 2010.
- 32.Schwarz N, Tumpara S, Wrenger S, et al. Alpha1-antitrypsin protects lung cancer cells from staurosporine-induced apoptosis: the role of bacterial lipopolysaccharide. Sci Rep. 2020;10(1):9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Q, Liu Y, Li X. The interaction mechanism between autophagy and apoptosis in colon cancer. Transl Oncol. 2020;13(12):100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang N, Peng F, Wang Y, et al. Shikonin induces colorectal carcinoma cells apoptosis and autophagy by targeting galectin-1/JNK signaling axis. Int J Biol Sci. 2020;16(1):147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]