Abstract
Background
The absence of specific antivirals to treat COVID-19 leads to the repositioning of candidates’ drugs. Nitazoxanide (NTZ) has a broad antiviral effect.
Methods
This was a randomized, double-blind pilot clinical trial comparing NTZ 600 mg BID versus Placebo for seven days among 50 individuals (25 each arm) with SARS-COV-2 RT-PCR+ (PCR) that were hospitalized with mild respiratory insufficiency from May 20th, 2020, to September 21st, 2020 (ClinicalTrials.gov NCT04348409). Clinical and virologic endpoints and inflammatory biomarkers were evaluated. A five-point scale for disease severity (SSD) was used.
Findings
Two patients died in the NTZ arm compared to 6 in the placebo arm (p = 0.564). NTZ was superior to placebo when considering SSD (p < 0001), the mean time for hospital discharge (6.6 vs. 14 days, p = 0.021), and negative PCR at day 21 (p = 0.035), whereas the placebo group presented more adverse events (p = 0.04). Among adverse events likely related to the study drug, 14 were detected in the NTZ group and 22 in placebo (p = 0.24). Among the 30 adverse events unlikely related, 21 occurred in the placebo group (p = 0.04). A decrease from baseline was higher in the NTZ group for d-Dimer (p = 0.001), US-RCP (p < 0.002), TNF (p < 0.038), IL-6 (p < 0.001), IL-8 (p = 0.014), HLA DR. on CD4+T lymphocytes (p < 0.05), CD38 in CD4+ and CD8+T (both p < 0.05), and CD38 and HLA-DR. on CD4+ (p < 0.01)
Interpretation
Compared to placebo in clinical and virologic outcomes and improvement of inflammatory outcomes, the superiority of NTZ warrants further investigation of this drug for moderate COVID-19 in larger clinical trials. A higher incidence of adverse events in the placebo arm might be attributed to COVID-19 related symptoms.
Keywords: COVID-19, Nitazoxanide, Randomized controlled clinical trial, Lymphocytes cell activation markers, Interleukins
Research in context.
Evidence before this study
We searched the literature for studies in this context, using the terms "SARS-CoV-2 or coronavirus or COVID-19, or virus" and Nitazoxanide". We found some in vitro and some clinical trials showing evidence of the antiviral effect of Nitazoxanide (NTZ) against many distinct viruses, including many influenza virus, respiratory syncytial virus, ebola, norovirus, rotavirus, adenovirus, coronavirus, chikungunya virus, hepatitis B virus, Hepatitis C virus, Japanese encephalitis virus. We found a published larger randomized placebo-controlled clinical trial for mild COVID-19, which documented a significant reduction in the NTZ arm's viral load, although no clinical benefits were detected.
Added value of this study
In this small randomized proof of concept pilot trial, we observed a decrease in the time for hospital discharge of patients with moderate COVID-19 using NTZ. We also documented a faster evolution to SARS-COV-2 RT-PCR negativity, a higher reduction of laboratory inflammatory markers, and a reduction of lymphocyte T cells activation markers among patients treated with NTZ a This results enable us to further explore the potential clinic benefits and the antiviral effect of NTZ for CIVID-19
Implications of all the available evidence
Even in the case of a small clinical study, the positive results suggest that this drug may have future use in treating a disease that still lacks adequate antiviral drug regimens.
Alt-text: Unlabelled box
1. Introduction
Cases of severe COVID-19 can be observed in up to about 14% of patients. Even more critical disease with clear respiratory failure, shock, or multiple organ dysfunction appears in approximately 3.6% to 5% of patients. These compose different clinical phenotypic subgroups [1,2]. In severe cases, the average start time to death has been close to 18.8 days in China and 24.7 days outside China [3,4]. Cases of death usually occur from the tenth day of the onset of symptoms [5,6]. Although most COVID-19 patients recover from the mild and moderate disease within one week, some develop severe pneumonia in the second week, followed by a cytokine storm, adult respiratory distress syndrome (ARDS), multiorgan failure, and disseminated intravascular coagulation in the third week of the disease [7], [8], [9]. A correlation was established between viral load in different sample types and disease severity. In respiratory samples, patients with severe disease presented significantly higher viral loads than patients with mild disease [10,11].
ACE2 is the main receptor of the SARS-Cov-2, allowing the virus to enter the cell. Virus-infected epithelial cells produce interferons via interferon-responsive genes, leading to a robust innate immune response [12]. Dendritic cells, macrophages, and neutrophils subsequently trigger the adaptative immune response. Autopsies in patients who died of COVID-19 revealed a high infiltration of macrophages in the bronchopneumonia area [13]. Besides, ACE2 expressed macrophages containing SARS-CoV-2 nucleoprotein antigen were found infiltrating the spleen and lymph nodes in COVID-19 patients. These macrophages showed significant production of IL-6, suggesting that they may contribute to excessive inflammation in COVID-19 disease [14]. These findings suggest that the inflammatory response may be even more destructive than the virus's direct activity [15]. T cells were more affected by SARS-CoV-2 as the T-cell count was almost half the lower reference limit and tended to be lower in severe cases.
Since pro-inflammatory cytokines play a crucial role in the prognosis of the disease, macrophages’ greater lung damage involvement has been studied [16,17]. More importantly, Macrophage Activation Syndrome (MAS) has been described as a severe risk factor contributing to lung inflammation. Therefore, the intense inflammatory response of IL-6 observed in severe cases of COVID-19 has already been discussed [6,14]. This response, which is usually responsible for health recovery after a viral infection, could impair the recovery of patients who experience the MAS [15,16,18]. The high production of IL-6 along with macrophage activation may explain the high levels of serum reactive C protein (RCP), which are generally not achieved in other viral infections. Similarly, in Severe Acute Respiratory Syndrome (SARS) disease, which represents the closest analog to COVID-19 in humans, the high production of IL-6 has also been described. The IL-6 production intensity in SARS was even higher than in common viral respiratory diseases, such as influenza and parainfluenza [6,19,20]. Conversely, IFN-I (IFN-α and IFN-β) have exhibited a protective effect on SARS-COV and Middle East Respiratory Syndrome (MERS)-COV infection, but the IFN-I pathway have been inhibited in infected mice with SARS CoV-2 [21,22].
Although initially developed for protozoan disease treatment, the antiviral properties of Nitazoxanide (NTZ) and its metabolite, tizoxanide, have generated growing interest in recent years due to its demonstrated ability to act as a broad-spectrum antiviral agent through multiple mechanisms. These seem to vary according to the type of virus but generally interfere with host-regulated pathways, including interferon signaling pathways or mTORC1, which is involved in viral replication [23,24]. As well, it affects antiviral mechanisms that include actions that trigger viral RNA inhibition and DNA replication, direct inhibition of viral protein expression, interference in host cell metabolism, as well as inhibition of viral immune evasion mechanisms [23], [24], [25].
NTZ has been clinically evaluated in treating influenza viruses with selective blockage of viral hemagglutinin's maturation, which impairs its intracellular trafficking and the insertion of viral protein in the host membrane [26,27]. A study comparing the use of 5-day 600 mg and 300 mg dosage regimens with placebo in treating acute uncomplicated influenza infection reported a reduction in symptom duration in the 600 mg treatment group with only mild adverse events [28].
Many clinical trials aiming to use NTZ as the only drug or in combination with other antivirals are proposed (clinicaltrials.gov). Among these, NCT04486313 in the USA seeks to evaluate mild to moderate patients’ efficacy and safety using two 300 mg tablets BID versus Placebo for 800 patients. Phase II/III was also planned in Argentina (NCT04463264) to investigate the efficacy and safety of mild disease using NTZ 500 mg every six hours for 14 days versus placebo in 135 patients. A clinical trial was also planned in Mexico as a prophylactic treatment using 500 mg every 6 h for two days and then every 12 h for four days (NCT04406246, 150 volunteers). Again, in México, a study for safety and efficacy is recruiting to use Hydroxychloroquine 200 mg twice daily for ten days versus NTZ 500 mg BID with Hydroxychloroquine 200 mg BID for ten days 86 patients, NCT04341493). The safety and efficacy of the drug are also planned in a study in Egypt using 500 mg, four times per day for 14 days, versus a regimen combination of Sofosbuvir/ Ledipasvir (400 mg and 90 mg, orally) once daily for 14 days (240 patients, NCT04498936). Also, in Egypt, one study is planning to compare NTZ, ivermectin, and ribavirin 200 mg or 400 mg for seven days versus Placebo (NCT04392427), and some others are planning to use NTZ with Ivermectin, Chloroquine, and Azithromycin (Tanta University Hospital, NCT04382846, NCT04360356, NCT04361318, NCT04351347, and NCT04345419). In Nigeria, a study for efficacy and safety in 98 patients is planning to use NTZ 1000 mg BID and 300/100 mg atazanavir/ritonavir tablets QD for 28 days versus Standard of care treatment (NCT04459286).
One study identified 73 combinations of 32 drugs with potential activity against SARS-CoV-2 using in silico modeling with subsequent validation using in vitro studies. The study reported a high NTZ. synergism with remdesivir, amodiaquine, and umifenovir [29].
NTZ is projected to have average/median Cmax concentrations above its EC50 of viral pathogens in blood plasma and lung during the conventional dosing interval [30]. Thus, the study described here aims to evaluate NTZ's safety and efficacy compared to placebo in a small group of patients with moderate COVID-19. For this, we selected patients with a compatible clinical picture and positive RT-PCR for SARS-COV-2 in the nasal and oropharyngeal swab.
2. Methods
For the in vitro effect evaluation of NTZ, VERO E6 cells were cultured until they achieved a 90% confluent cell monolayer and then treated for 1 h with DMSO and NTZ at the final concentrations of 0.1 µM, 0.5 µM, 1.0 µM, and 10 µM. Mock infected and untreated cells were used as controls. One hour after the incubation, cells were infected with the clinical isolate SARS-CoV-2 EPI_ISL_413,016 at a multiplicity of infection of 0.05. After a 2 h virus adsorption period, each well was washed twice with PBS and completed with 500 µL of fresh medium containing NTZ as described above and cultured for additional 48 h. The supernatants were collected at 48 h post-infection to determine viral load and cellular viability. Cellular viability and cytotoxic effect were determined after 48 h post-infection using a lactate dehydrogenase-based cytotoxicity detection kit (Roche, Basel, Switzerland).
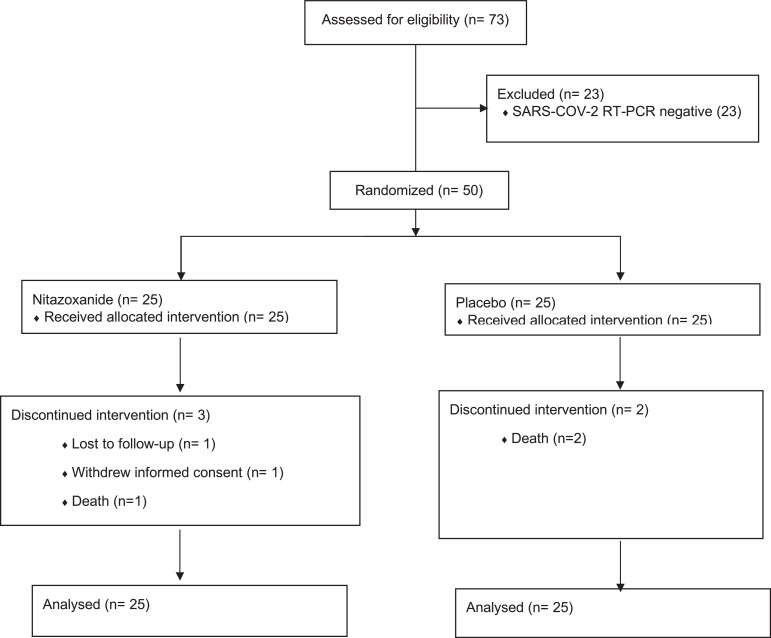
This study was a Pilot Prove of Concept Randomized Double-Blind Clinical Trial. The study's primary objectives were to assess the safety and the effect on the detection of SARS-COV-2 RNA among patients treated with NTZ compared to placebo in preparation for a study with the inclusion of a more significant number of patients. One area of uncertainty to be investigated here was the safety of NTZ among patients with COVID-19. We also want to address if inflammatory markers would be of value as endpoints for COVID-19 treatment clinical trials, especially T cells activation markers. The study population consisted of 50 participants diagnosed with COVID-19 with positive qPCR and compatible symptoms, aged 18 years or older, both genders, hospitalized in a non-critical condition with mild respiratory insufficiency (PO2 saturation inferior to 96%), and with a maximum of 36 h of symptoms (Fig. 1). The sample size of 50 patients was determined during the study design based on the feasibility of conducting this clinical trial. The statistical power calculation for the 50-person sample was calculated after the study had begun. We used a power calculation based on the difference between the proportion of cases with a more significant PCR-RT reduction over the study period (from day 1 to day 21). We considered effect size (the ability to discriminate between the two possible results) of 0.5, which is deemed medium strength. We also used an alpha error of 0.05 and a sample size of 25 per arm. The software used for the calculation was the pwr.2p.test of the pwr package of the R statistical programming system (v.4.0.3), which produced a power of 42.4% [34,35]. Although this is not considered adequate for a full, Phase 3 trial study, we deemed it sufficient for this pilot study. This was a multicenter study with a participation of a total of 6 Hospitals in the State of São Paulo, Brazil, including Instituto do Coração - Incor (city of São Paulo), Beneficiencia Portuguesa (city of São Paulo), Hospital Vera Cruz (Campinas), SPDM / Hospital Geral de Guarulhos (Guarulhos) e Hospital Municipal Dr. Francisco Moran (Barueri) and Hospital Prevent Senior (city of São Paulo). The study ran from May 20th, 2020, to September 21st, 2020. Samples and laboratory testing were processed at Laboratório de Retrovirologia of the Federal University of Sao Paulo, Sao Paulo, Brazil, and interleukine analyses were performed at Laboratório Interdisciplinar de Pesquisa Médicas (Instituto Oswaldo Cruz/FIOCRUZ).
Fig. 1.
Screening and Randomization Patients admitted for hospitalization with mild/moderate hypothesis of COVID-19 were screened (73 patients), and 50 patients were randomized once RT-PCR for SARS-COV-2 was positive in the nasal and throat swabs (RT-PCR results available in the first 12 h of hospital admissions). Patients were allocated to receive Nitazoxanide (25) or placebo (25). Five patients did not receive 7-day treatment with allocated study interventions. From the Nitazoxanide group, one patient dyed on day 2 of the study, one patient received four doses of study drug and withdrew informed consent. Another patient was released from the hospital in good health conditions on day 5 of the study and was lost to follow-up. From the placebo group, one patient dyed on day two and another on day 4. Available data for all patients have been analyzed by the end of the study period.
Patients infected with HIV, HTLV I or II, participants in antineoplastic treatment, patients with severe autoimmune diseases in immunosuppression, transplant recipients, pregnant or lactating women, or any other clinical condition that the Investigator deemed to be an imminent risk to health and participant's life were excluded. Patients who previously received any COVID-19 therapies and medications against specific manifestations of COVID-19, such as monoclonal antibodies or anti-interleukins, were excluded. Due to the small sample size, we used randomization by blocks of size four, using RStudio software, Version 1.2.1335 (Integrated Development for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/). A Contract Research Organization (Azidus, Brazil) generated the random allocation sequence and assigned participants to intervention groups. After randomization, patients received double-blind NTZ 600 mg BID, administered with food, for seven consecutive days (25 patients), or the placebo comparator BID, administered with food, for seven straight days (25 patients). The investigational product and placebo were produced and donated by Farmoaquimica (FQM), Brazil, a Pharmaceutical Company that holds the NTZ production's license issued by the Romark Laboratory (NTZ patent holder, Fl, USA). We selected 600 mg BID doses of NTZ in this study based on experience with pharmacokinetics and tolerability [23,26,28,31]. The clinical evaluations were performed daily during the hospitalization period and, in discharge cases before 21 days of hospitalization, by follow-up at outpatient clinics or telemedicine. This manuscript adheres strictly to CONSORT guidelines (https://www.equator-network.org/wp-content/uploads/2013/09/CONSORT-2010-Checklist-MS-Word.doc).
The viral evaluation was performed by qualitative RT-PCR, and qPCR viral load for SARS-COV-2 collected in nasal and throat swabs on day 1 (baseline, inclusion criteria) and days 4, 7, 14, 21. Evaluations on clinical status, length of hospitalization, in-hospital mortality, need for mechanical ventilation, and need for rehospitalization have been performed. Laboratory parameters accessed at baseline, day 10, and day 21, included ultra-sensitive RCP (US-RCP), d-dimer, CPK, CK-MB, serum interleukins, and lymphocyte activation markers.
According to the manufacturer's instructions, the SARS-COV-2 RT-PCR qualitative analyses were performed by the AllplexTM 2019-nCoV Assay, version 2.1, October 30th 30, 2020. For the SARS-COV-2 RT-PCR viral load, RNA was extracted by using QIAamp RNA Viral kit (Qiagen, German), and RT-qPCR was performed with 5 µL of extracted RNA and 15 µL of reaction buffer containing SARS-CoV-2 primers and probes as previously described [32], and Hot Start go Taq reagent mix (Promega, Madison, Wi). Thermal cycling involved 55 °C for 30 min, followed by 95 °C for 15 min and then 40 cycles of 95 °C for 15 s, 58 °C for 45 s. This protocol detected SARS-CoV-2 RdRp target nCoV_IP2. A real-time PCR instrument (7500 Applied Biosystems) was used with 96 wells plate for qRT-PCR. A standard curve was generated by using synthetic RNA, and then RNA viral load of samples was obtained comparing CT of standard curve and CT of each sample.
Plasma cytokine levels of IL-6, TNF, IL-8, IL-10, IFN-ɣ, IL-1β, IL-4, IL-5, IL-17A, IL-12p70, and IL-13 were assessed, and cytokine contents were calculated by Luminex technology (USA). For this, a multiplex assay was performed to quantify the serum levels of the cytokines. Data analysis was performed using the software provided by the manufacturer (R&D Systems, USA). Recombinant cytokines were used to establish standard curves and the sensitivity of the assay. Results were expressed as mean fluorescent intensity (MFI). The MFI of the last point of each standard curve was used to determine the detection limit of each cytokine.
Lymphocyte activation markers were accessed by flow cytometry and included HLA-DR. and CD38 on CD4+ and CD8+ T cells, as described elsewhere [36]. Briefly, Peripheral blood mononuclear cells (PBMC) were separated and cryopreserved. After thawing, PBMCs were labeled with anti-CD3 APC and anti-CD4 PercP (for the lymphocytic subpopulation) and anti-CD38 FITC and anti-HLA-DR. PE (for cellular activation). Fixed cells were acquired on a FACSCalibur apparatus and analyzed with CellQuest software (BD Biosciences, San Diego CA, USA). The results were expressed as the percentage of CD38+HLA-DR.+ cells among CD4+ and CD8+ T lymphocytes.
All analyses of patients were conducted within the randomized group they were assigned to. Statistical analyses included one-way analyses of variances using the Kruskal-Wallis non-parametric ranks test. Additional statistical questions were addressed with the Wilcoxon Rank Sum Test for numerical variables and Chi-Squared tests for categorical variables. All analyses were conducted using the R Statistical System and language and its appropriate packages [35]. Twelve participants stopped their participation earlier in the study, five on the NTZ arm and seven on the Placebo arm. Participants who discontinued the study prematurely had all their laboratory and clinical data recorded and analyzed up to the time of their withdrawal or death. In the NTZ arm, one patient had the data collected until day two (death), one until day three (withdrew informed consent), one until day five (lost to follow-up), and two until day seven (death and lost to follow-up). On the placebo arm, one patient had data collected until day two (death), one until day four (death), one until day seven (death), one until day ten (death), one until day 14 (death), and two until day 15 (death and informed consent withdrawal). These are the only gaps in the data collection. This study was registered at ClinicalTrials.gov (NCT04348409), and the Brazilian National Institutional Review Board approved it (CONEP; approval # CAAE-30,628,420.0.0000.5412) and each local Institutional Review Boards. Informed consent has been obtained.
2.1. Role of the funding source
FMQ reviewed the data from the study before manuscript submission and wrote a report to the Brazilian National Sanitary Agency (ANVISA). Academic authors retained control in the study design, data collection, data analysis, data interpretation, and manuscript writing.
3. Results
This study was initiated after we confirmed the in vitro activity of NTZ, once viral loads decreased in Vero E6 cells treated with NTZ in a dose-response manner. There were no effects of DMSO on the infection course, and no cytotoxicity effect of NTZ treatment after 48 h was observed (Supplementary Figure 1).
Patients’ characteristics are depicted in Table 1. Groups were well balanced, with higher body weight and BMI in the NTZ group. The prevalence of other comorbidities was equal between groups (data on file). According to the WHO recommendation, the participant's clinical evolution was evaluated through an ordinal scale, which measures the severity of the disease over time [33]. The scale was performed daily, and the worst score was recorded. We adapted the ordinal scale used according to the WHO Special Committee, using fewer anchors (five) to facilitate the evaluation of the reduced sample size of this study as follows: 1= Outpatient; 2 = Hospitalized without oxygen use; 3 = Hospitalized with noninvasive use of oxygen, 4 = Intensive therapy with invasive oxygen; 5 = Death. The longitudinal analysis of clinical evolution scores showed a significant difference between treatments (Table 1, p = 0.001). Both arms showed a decrease in the scale score over time. However, there is a significant difference in the reduction in the NTZ treatment arm (p < 0.001). On day 4, 31.8% of the participants in the NTZ group were undergoing outpatient treatment, and 9% were hospitalized with oxygen or in the Intensive Care Unit (ICU), while only 8.3% of the participants in the placebo group were undergoing outpatient treatment, 29.2% remained hospitalized with oxygen or in the ICU. On day 7, 68.4% of the participants in the NTZ group were undergoing outpatient treatment, compared to 31.8% of the placebo participants, whereas on day 14, 84.2% of participants in the NTZ group were on outpatient treatment, compared to 55% of placebo participants
Table 1.
Patient Characteristics and clinical, inflammatory, and virological Results.
| Arm |
||||||||
|---|---|---|---|---|---|---|---|---|
| Day 21 Difference |
||||||||
| Level | Nitazoxanide | Placebo | Rate Ratio | p-Value | Rate Ratio | p-Value | ||
| Number | 25 | 25 | ||||||
| Gender (%) | Masculine | 7 (28) | 8 (32) | 1143 | ||||
| Feminine | 18 (72) | 17 (68) | 0,944 | |||||
| Race (%) | White | 21 (42) | 16 (32) | 0,762 | ||||
| Mixed | 3 (6) | 7 (14) | 2333 | |||||
| Black | 1 (2) | 2 (4) | 2000 | |||||
| Median Age (median (IQR)) | 64 (17) | 64 (21) | 1000 | |||||
| Age Group (%) | less than 65 | 14 (28) | 13 (26) | 0,929 | ||||
| 65 and older | 11 (22) | 12 (24) | 1091 | |||||
| Height (mean (sd)) | 1.71 (0.08) | 1.68 (0.1) | 0,982 | |||||
| Weight (mean (sd)) | 89.2 (17) | 76.18 (13.04) | 0,854 | |||||
| BMI (mean (sd)) | 30.41 (4.12) | 27.06 (3.7) | 0,890 | |||||
| Day of Release (mean (sd)) | 6.16 (4.07) | 9.12 (4.33) | 1481 | 0,021 | ||||
| Severity of the disease scale score (mean (sd)) | 1.7 (0.8) | 2.13 (1.03) | 1253 | 0,001 | ||||
| Viral Load at Day 1 (mean(sd)) | 29.03 (4.84) | 28.88 (4.89) | 0,995 | 0,984 | ||||
| RT-PCR Difference Day 1 - 21 (mean (sd)) | −30.15 (4.50) | −22.17 (12.67) | 0,735 | 0,035 | ||||
| Removed from Supplemental O2 (mean (sd)) | 3.72 (3.29) | 5.88 (4.14) | 1580 | 0,082 | ||||
| O2 Saturation at Day 1 (Oximeter) (mean(sd)) | 93.00 (1.68) | 93.80 (1.94) | 1009 | 0,984 | ||||
| Patients Hospitalized (#) | Day 4 | 12 | 16 | 1481 | 0,300 | |||
| Day 7 | 5 | 10 | 2222 | 0,134 | ||||
| Day 14 | 2 | 5 | 2778 | 0,202 | ||||
| Day 21 | 1 | 2 | 2222 | 0,503 | ||||
| Oxygen supplementation (#) | Day 4 | 8 | 10 | 1389 | 0,487 | |||
| Day 7 | 5 | 6 | 1333 | 0,634 | ||||
| Day 14 | 2 | 3 | 1667 | 0,572 | ||||
| Day 21 | 1 | 2 | 2222 | 0,503 | ||||
| Invasive mechanical ventilation | Day 4 | 1 | 3 | 3333 | 0,268 | |||
| Day 7 | 1 | 4 | 4444 | 0,144 | ||||
| Day 14 | 1 | 1 | 1111 | 0,941 | ||||
| Day 21 | 0 | 0 | ||||||
| Death | Day 7 | 2 | 3 | 2000 | 0,564 | |||
| Day 14 | 0 | 2 | NA | 0,157 | ||||
| Day 21 | 0 | 1 | 2000 | 0,564 | ||||
| Lymphocytes (mean(sd)) | Day 1 | 1029.36 (526.66) | 944.88 (619.43) | |||||
| Day 7 | 1162.36 (689.14) | 1188.57 (615.77 | ||||||
| D-Dimer (mean (sd)) | Day 1 | 1.15 (1.66) | 1.71 (1.83) | |||||
| Day 10 | 1.16 (1.67) | 1.65 (1.79) | ||||||
| Day 21 | 1.18 (1.71) | 1.82 (1.96) | 0.026€ | 1943 | 0,001 | |||
| US- C Reactive Protein (mean (sd)) | Day 1 | 47.11 (70.51) | 96.23 (110.43) | |||||
| Day 10 | 46.33 (70.56) | 87.85 (106.53) | ||||||
| Day 21 | 45.35 (71.26) | 88.13 (101.72) | <0.001€ | 1544 | 0,002 | |||
| TNF-α (MFI (sd)) | Day 1 | 155.54 (53.46) | 196.17 (62.97) | |||||
| Day 10 | 126.06 (33.76) | 167.69 (132.92) | ||||||
| Day 21 | 136.17 (38.38) | 149.12 (45.88) | <0.001€ | 1095 | 0,038 | |||
| IL-6 (MFI (sd)) | Day 1 | 571.19 (557.70) | 1024.55 (1242.96) | |||||
| Day 10 | 323.14 (685.86) | 943.60 (1395.63) | ||||||
| Day 21 | 114.19 (61.02) | 412.86 (407.52) | <0.001€ | 3616 | <0.001 | |||
| IL-8 (MFI (sd)) | Day 1 | 291.72 (145.17) | 205.88 (167.34) | |||||
| Day 10 | 196.65 (85.82) | 164.25 (56.53) | ||||||
| Day 21 | 180.06 (72.12) | 250.57 (133.17) | <0.001€ | 1392 | 0,014 | |||
Ratio: numbers above 1.0 wt toward the Placebo arm. p-value: non-parametric, Wilcoxon test. Sd = standard deviation.
= Evolution p-value. MFI = Media Intensity Fluorescence.
Considering the number/proportion/frequency of deaths, we observed a total of 8 deaths, 2 for the NTZ group and 6 for the placebo group; all deaths due to ARDS (p = 0.564). When we evaluated the number of participants who required invasive mechanical ventilation, we had a similar difference, with eight patients in total, 2 with NTZ, and 6 with placebo (p = 0.08). In evaluating the time to withdraw from oxygen supplementation, we observed that the median weaning time in the NTZ group was three days. In contrast, the placebo group was eight days (p = 0.08). The mean time to hospital discharge in the NTZ group was 6.2 days compared to 14 days in the placebo group (p = 0.021).
Two patients died in the NTZ arm of the study. One of these 2, patient P3 was a 67-year-old male with systemic arterial hypertension (SAH) and a BMI (body mass index) of 25. Baseline laboratory evaluation revealed a total lymphocyte count of 702 (9% from 7800 total white blood cells), d-dimer of 0.94 (normal up to 0,5 mcg/mL), US-RCP of 193.45 mg/L (normal up to 3 mg/L), and IL-6 of 557.5 MFI. He died of ARDS eight days after entering the study. Another patient in the NTZ group, P6, was a 63-year-old male with type 2 diabetes mellitus and dyslipidemia, with a BMI of 31. Baseline laboratory evaluation revealed a total leukocyte count of 14,250 (1100 lymphocytes), d-dimer of 0.78 mcg/ml, US-RCP 107.07 mcg/L, and IL-6 of 163.0 MFI. He also died of ARDS on day 8 of the study. Among patients who died in the placebo group, P8 was an 88-year-old female with SAH, BMI of 24. Baseline laboratory evaluation revealed a total lymphocyte count of 680 (7560 total leucocytes), d-dimer of 1.56 mcg/ml, US-RCP 345.67 mcg/L, and IL-6 of 4872 MFI. She died on D21 of ARDS and abdominal sepsis due to perforated diverticulitis. Patient P14 was a 65-year-old female with previously not well-defined cardiomyopathy, BMI of 25. Baseline laboratory evaluation revealed a total lymphocyte count of 2320 (total leucocytes 10,340), d-dimer of 0.8 mcg/mL, US-RCP 319.83 mcg/L, and IL-6 of 4658 MFI. She died on D4 of ARDS, refractory cardiogenic shock, and acute renal failure. Patient P24 was a 55-year-old male with type 2 diabetes mellitus, BMI of 26. Baseline laboratory evaluation revealed a total lymphocyte count of 970 (10,500 total leucocytes), d-dimer of 0.58 mcg/mL, US-RCP of 19.36 mcg/L, and IL-6 of 239.5 MFI. He died on D5 of ARDS. Patient P27 was a 71-year-old male with no previous comorbidities, BMI of 24. Baseline laboratory evaluation revealed a total lymphocyte count of 470 (12,950 total leucocytes), d-dimer of 0.94 mcg/mL, US-RCP 166.77 mcg/L, and IL-6 of 849.0 MFI. He died on D14 of ARDS, bacterial sepsis, general bleeding, acute renal failure, and refractory shock. Patient P17 was a 78-year-old male, heavy smoker, BMI of 32. Baseline laboratory evaluation revealed a total lymphocyte count of 460 (total leucocytes 11,710), d-dimer of 0.8 mcg/mL, US-RCP of 94.53 mcg/L, and IL-6 of 585 MFI. He died on D3 of ARDS. The last patient in the placebo group to die was P43, a 73-year-old female with obesity (BMI of 32). Baseline laboratory evaluation revealed a total lymphocyte count of 930 (total leucocytes13,480), d-dimer of 0.82 mcg/mL, US-RCP of 187.59mcg/L, and IL-6 of 417 MFI. She died on D12 of ARDS and acute renal failure.
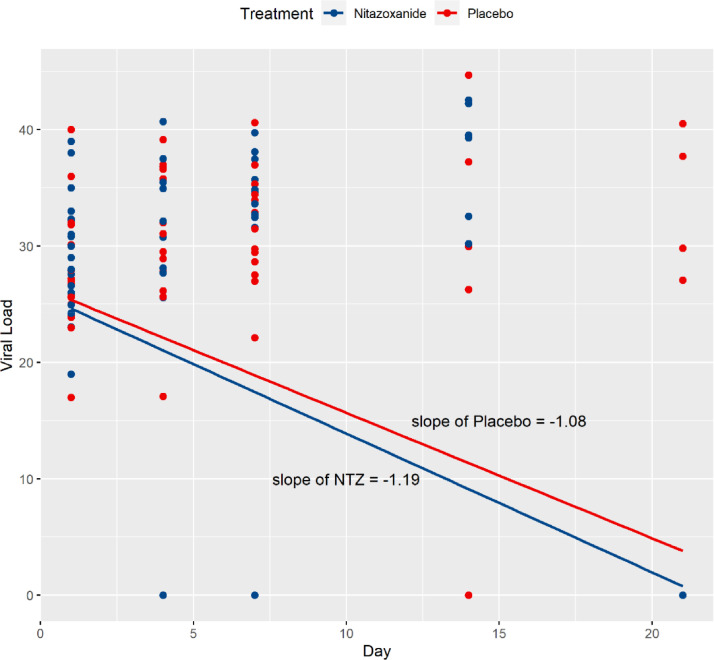
The SARS-COV-2 qualitative RT-PCR results were significantly different between groups over time, favoring the NTZ arm (p = 0.045). There was a significant difference between treatments at day 21 (Table 1, p = 0.035), in which the percentage of negative RT-PCR was 100% in the NTZ compared to 78.9% in the placebo arm (15 out of 19 patients, since six patients had died by day 21). The SARS-COV-2 viral loads were not different between treatment groups, with a significant reduction in both groups’ viral loads over time (p < 0.0001). Fig. 2 shows that the viral load for the NTZ arm of the study dropped slightly faster than the Placebo arm over the 21 days of the study (slope of −1.19 for NTZ against −1.08 for the placebo).
Fig. 2.
Relative slopes for the two treatment groups' viral loads, Nitazoxanide (NTZ) and Placebo.
As seen in Table 1, the unspecific inflammatory markers such as d-Dimer and US-RCP decreased more significantly in the NTZ group than in the placebo group. Similarly, the decrease of plasma levels of interleukins such as TNF, IL-6, and IL-8 was more profound in the NTZ arm, although no significant differences were seen for IL-10, IFN-ɣ, IL-1β, IL-4, IL-5, IL-17A, IL-12p70, and IL-13 (data on file).
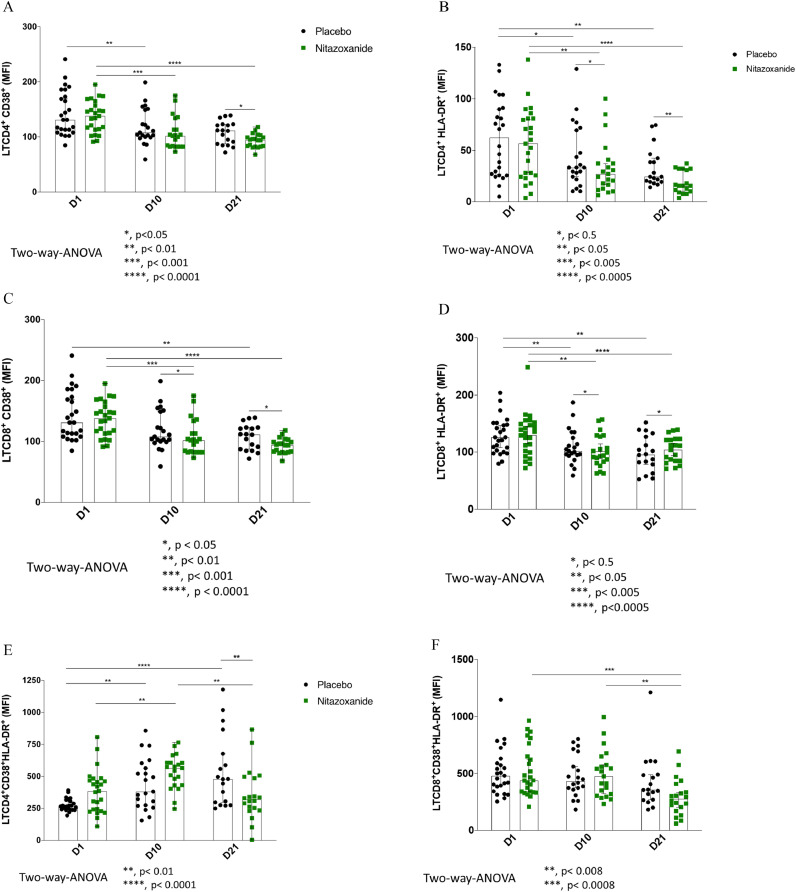
As seen by the CD38 and HLA-DR. in the CD4+ T cells and CD8+ T cells, the lymphocyte T cell activation marker always presented a trend to decrease over time in both NTZ and placebo arm (Fig. 3). The HLA DR. on CD4+ T cells was significantly lower in the NTZ group than placebo on day 21 (p < 0.05; Fig. 3B). Similarly, levels of CD38 in CD4+ and CD8+ T cell lymphocytes are lower in the NTZ arm on day 21 (both p < 0.05; Fig. 3A and 3C). Furthermore, when both markers’ coexpression is evaluated (CD38 and HLA-DR.), there is also a significant difference at day 21 between the two study arms in the CD4+ T cells, favoring NTZ (p < 0.01, Fig. 3E).
Fig. 3.
T cell activation status over time among the NTZ and Placebo groups. Peripheral blood mononuclear cells (PBMC) from patients of all groups were obtained by Ficoll-Hypaque gradient centrifugation. PBMC was thawed and used for immunophenotyping on Day 1, Day 10, and Day 21. The MFI of CD38+CD4+T cells (3A), HLA-DR.+CD4+T cells (3B), CD38+CD8+T cells (3C), HLA-DR.+CD8+T cells (3D), CD38+HLA-DR.+CD4+T cells (3E), and CD38+HLA-DR.+CD8+T cells (3F) are expressed as the median ± standard deviation. Placebo is represented in black dots, whereas Nitazoxanide is represented in green dots.
A total of 36 adverse events were observed, 14 in the NTZ group and 22 with placebo (p = 0.24). Of the six possibly related to the drug, four occurred in the NTZ group and 2 in the Placebo group (p = 0.99). Among the 30 adverse events unlikely to be related to the treatment drug, 21 occurred in the placebo group (p = 0.04).
Among the adverse events with a possible causal relationship with the drug in the NTZ group, one event was mild, and three were moderate, and in the placebo group, only mild adverse events were detected. Two of the adverse events in the NTZ group were severe (death). Considering those that were assumed as adverse events, as traditionally accepted, such as elevation of liver enzymes, renal failure, digestive manifestations, headache, among others, the higher incidence in the placebo group could be possibly attributed to the virus damage on various organs and tissues of the human hosts. A complete list of adverse events is in Supplementary Tables 1 and 2.
4. Discussion
In the absence of a specific antiviral drug that could interrupt the replication cycle of SARS-COV-2, we designed a pilot, placebo-controlled trial to investigate the safety and efficacy of NTZ among patients with moderate COVID-19. First, we demonstrated in our laboratory the existence of an unequivocal antiviral activity against SARS-COV-2, also demonstrating the absence of cytotoxicity in vitro by this drug using a common SARS-COV-2 Brazilian strain (Supplementary Figure 1). However, we recognize that it is not yet clear its effect in other emerging viral variants. We have not examined which variants were present in the patients included in the pilot study and the antiviral activity of NTZ against these specific strains. Although we understand that NTZ is a safe drug, the dosages investigated here were relatively modest and were based on previous studies that explored the antiviral effect for influenza viruses using 600 mg BID [28]. Of note, NTZ has been previously employed at 1000 mg BID without significant safety concerns [29,31,32,37]. Also, previous dose estimates have indicated that higher doses will be required to achieve SARS-COV-2 suppressive concentrations at Concentration trough levels [38]. It has been reported that 1500 mg BID dose of NTZ is currently under investigation in phase I safety trials in the United Kingdom [38]. Although the duration of treatment was short in the current clinical trial, we recognize that sub-optimally dosed monotherapy may enable the virus to replicate under the selective pressure of the drug, with potential risk for the selection of drug-resistant strains, thus jeopardizing this drug activity, in a time antiviral interventions for COVID-19 are urgently required. However, we understand that even drugs considered yet safe should be initially tested without substantial increases in dosages and in patients who do not have high disease severity in the case of a new disease. As a learning experience, one study with hydroxychloroquine led to an unacceptable increase in mortality when higher doses were used for COVID-19 in patients with greater severity [39]. Moreover, the estimated IC50 and IC90 of NTZ against SARS-CoV-2 are 0.68 μg/mL and 1.84 μg/mL [37], and NTZ 300 mg twice-daily dose exhibited peak plasma concentrations of 4.6 μg/mL which is well above its in vitro antiviral concentration [23,28].
Although pilot clinical trials are usually underpowered to test for efficacy or effectiveness, some clinical and laboratory endpoints were evaluated. Even with no statistically significant difference between treatments in the number of death, it is observed that the expected difference between the proportions of deaths between treatments (16%) is considered clinically relevant and a tendency to be considered in studies with a larger sample size. Similarly, the number of participants who required invasive mechanical ventilation, although not statistically significant, was higher in the placebo group (p = 0.08), and again, larger sample sizes may reveal some differences. Also, the time to withdraw from oxygen supplementation (three versus eight days) was not statistically significant (p = 0.08) but may reveal a consistency in the clinical endpoints, added to the mean time to hospital discharge, that was lower in the NTZ group (p = 0.021).
The study's primary outcome was the virological response to treatment with NTZ, and a statistically significant difference was noted in the number of patients who completed the study with negative PCR for SARS-COV-2. Similarly, the acute inflammatory process decreased more significantly in the NTZ arm, as seen by the US-RCP decrease. It is recognized that US-RCP is related to a worse prognosis and worse evolution in COVID-19 [40,41]. We can speculate here that the reduction of this nonspecific marker of inflammation corresponds to better control of viral infection. Not surprisingly, IL-6 was elevated among patients, evolving to a higher decay in the NTZ group. IL-6 is released into the circulation by a variety of different cell types, acting as a significant pro-inflammatory mediator for the induction of the acute phase response, leading to a wide range of local and systemic changes, including fever, leucocytes recruitment, and activation and hemodynamic effects, is also a marker of higher risk of disease deterioration [42].
Besides, we found a statistically significant decrease in TNF levels lower on day 21 in the NTZ arm than placebo. TNF is one of the cytokines that most changes in COVID-19 [43,44], and proposals exist to control the disease's hyperinflammatory phase using anti-TNF drugs. It is conceivable that anti-TNF therapy could mitigate inflammation-driven capillary leak in COVID-19, thus reducing respiratory insufficiency and mortality [45]. It is also plausible that the significant decrease in TNF associated with the use of NTZ corresponds to another advantage in the process of COVID-19 recovery. Furthermore, IL-8 decreased significantly in the NTZ arm compared to the baseline period to day 21 of the disease, which did not occur in the study placebo arm. It has been demonstrated that high serum IL-6, IL-8, and TNF-α levels at the time of hospitalization were strong and independent predictors of patient survival [44]. Also, IL-8 is a potent pro-inflammatory cytokine playing a pivotal role in the recruitment and activation of neutrophils during inflammation, and IL-8 may contribute to the frequent neutrophilia observed in patients with COVID-19 [46]. We have not measured other IFN that might exhibit a protective effect, such as IFN-I (IFN-α and IFN-β) [21,22], and this information is still lacking vis a vis the treatment we proposed.
We explored whether moderate SARS-CoV-2 infection led to a Lymphocyte T cell activation as inferred by the levels of CD38 and HLA-DR................ in CD4+ and CD8+ T cells lymphocytes and if the study drug was able to mitigate this cell activation. Lymphopenia is associated with a worse disease course, and the normalization of lymphocyte count indicated recovery of COVID-19 [47]. Thus, when analyzing the cell activation parameters, we decided to evaluate MFI rather than percentages of cells not to overestimate the lymphocyte T cell activation as disease resolves. Interestingly, we were able to document that all these markers were high in the baseline in both groups of patients and gradually decreased over time.
Moreover, the decrease of the CD4+ HLA-DR.+ T cell lymphocytes on day 21 was significantly higher in the NTZ treated group than the placebo group; one more piece of evidence related to the drug-associated decrease of COVID-19 inflammation among treated patients. Monocytes, which are a source of IL-6, have not been evaluated in this trial. It has been described that a significant expansion of CD14+ CD16+ monocytes producing IL-6 was observed among patients with COVID-19 in ICU [48].
One of the study's main objectives was to evaluate the safety of NTZ among patients with COVID-19, and interestingly, the incidence of adverse events was significantly higher among placebo-treated individuals. Although this difference could be due to chance only, one could say that the adverse events were COVID-19 related symptoms that could have been mitigated in the NTZ-treated arm. According to the study's small sample size nature, it was not expected superiority of treatment arm than placebo when clinical endpoints were analyzed. Nonetheless, the NTZ arm was superior when outcomes such as disease symptoms, oxygen weaning, and hospital discharge were evaluated.
It is conceivable that for the effective treatment of a viral infection such as COVID-19, it is necessary to associate antiviral drugs that act in various virus replication cycle stages, as usually done for some other viral infections. Even with a mechanism for correcting the polymerization of viral nucleic acid carried out by an exonuclease with action 3´−5′ proofreading activity [49], which would decrease the genetic diversity of this virus and mitigate the chance of rapid selection of resistant mutants, it is conceivable that the combination of more than one drug would be necessary at least to increase the potency of the antiviral treatment. In this context and as seen for other viruses, NTZ may have antiviral activity in more than one step of the SARS-COV-2 replication cycle. In different viruses, this drug simultaneously inhibits viral RNA and DNA replication and direct inhibition of viral protein expression [23,25]. Additionally, as a mechanism associated with interference in the host's cellular metabolism, there is a modulation with interferon increment provided by NTZ, which per se would be related to an additional antiviral mechanism [23,24]. We recognize, however, that the mechanism of action responsible for the putative benefits of NTZ in SARS-CoV-2 infection needs to be better elucidated if benefits are confirmed in larger clinical trials. Also, virus evasion to NTZ treatment due to viral resistance needs to be evaluated, especially among high viral loads. As SARS-COV-2 evolves, higher transmissibility and higher viral loads among new emerging strains such as the Brazilian P.1 strain from Amazona have been suggested [50].
We are aware that the results presented here contrast with a larger randomized placebo-controlled clinical trial for mild COVID-19, which despite documenting a significant reduction in the viral load in the NTZ arm, no clinical benefits were detected [51]. This study used 500 mg of NTZ TID for five days, whereas our study gave 600 mg BID for seven days, and in fact, one can speculate that mild COVID-19 will not benefit from an antiviral treatment (if any) since the disease will resolve itself. Among 475 randomized patients with mild COVID-19, no death was reported, and only two patients were admitted to ICU, both from the NTZ group [51].
Some caution is required to interpret this trial's immunological and inflammatory findings, as it is not possible to disentangle a direct immunomodulatory role of NTZ from an indirect immunological impact resulting from its antiviral activity and an associated reduction in viral replication. Also, we recognize that the double-blind nature of a placebo-controlled study is compromised in the case of NTZ, which produces effects visible to the naked eye in some people's urine color using this drug. Also, the inclusion of such a small number of patients could obscure the incidence of less frequent adverse events, which could be severe in the association of this previously unused drug in a disease with such prominent inflammatory characteristics. However, this study's clinical and laboratory outcomes motivate us to proceed with a phase 3 study for moderate/severe COVID-19. We recognize as a limitation of the current study the absence of pharmacokinetic data. Given the lack of current understanding of this drug's most appropriate dose relative to its different action mechanisms, incorporating a sub-study of intensive pharmacokinetic analysis in the phase 3 study will be fundamental.
In conclusion, we were able to observe in that small proof of concept pilot trial an evident decrease in the time for hospital discharge, faster evolution to SARS-COV-2 RT-PCR negativity, and a higher reduction of inflammatory markers such as d-Dimer, US-RCP, TNF, IL-6, IL-8, associated to a higher reduction of lymphocyte T cells activation markers such as HLA DR. on CD4+, CD38 in CD4+ and CD8+ T, and CD38 and HLA-DR. on CD4 among patients treated with NTZ as compared to placebo.
Data sharing statement
The study protocol and all data files from the study results and programs to process the files are deposited in a publicly available repository on the GitLab system for this clinical trial: https://gitlab.com/jameshunterbr/ntz-clinical-trial. Data files are in the form of comma-separated files ("csv"), Excel worksheet files ("xls" or "xlsx"), text files ("txt") or binary R language files (suffix: "rds"). Although these may have suffixes appropriate to the language the program is written in, programs will be stored in text files. All files deposited in this repository will be open to review and downloading publicly.
Contributors
VB lead the project, participated on the study design, oversaw Clinical trial, commented on and edited early versions of the manuscript, and read and approved the final manuscript; SC participated on the study design, read and approved the final manuscript; JH performed statistical analysis, commented on and edited early versions of the manuscript, read and approved the final manuscript; PT, AL, AS and FC attended the patients, read and approved the final manuscript; NB oversaw the implementation of lab experiments, read and approved the final manuscript; JM and LJ oversaw the implementation of lab experiments, conducted the laboratory experiments, performed analysis, read and approved the final manuscript; NM, MV, DD and JG conducted the laboratory experiments, read and approved the final manuscript; JS-O, AMC designed the laboratory study strategies, conducted the laboratory experiments, performed analysis, read and approved the final manuscript; RD designed the study, designed the laboratory study strategies; oversaw the implementation of lab experiments; wrote the manuscript.
Funding
This study was supported by Farmoquimica (FQM), Brazil. Laboratory testing was partially supported by a grant from CNPq, Brazil (RD).
Declaration of Competing Interest
VB exerts activities of clinical research at FQM Farmoquímica, sponsor of the study. AL reports grants from FQM, during the conduct of the study; personal fees from Daiicho Sankyo Brasil, Pfizer, Mantecorp Indústria Química e Farmacêutica, Libs Farmacêutica, Sanofi-Aventis; grants, personal fees and non-financial support from Janssen Pharmaceutical; personal fees and non-financial support from Cristalia Produtos Químicos e Farmacêuticos; grants and personal fees from Eli Lilly; grants from H. Lundbeck A/S, Servier Laboratories, Hoffman-La Roche, Forum Pharmaceuticals, Biophytis, Ganentech, Cellavita, Celltrion (outside the submitted work). JG, DD, JH, and NM report personal fees from FMQ during the conduct of the study. SC reports grants from FQM during the conduct of the study; grants from MERCK SHARP & DOME, NOVARTIS, ROCHE, ABBVIE, GILEAD, and PFIZER (outside the submitted work). Other authors do not have any conflict of interest to declare.
Acknowledgments
We thanks Isaac Barbosa, Daniel Karcher, Fatima Morgana Pio Fonseca, Renan Novaes Pinto for technical laboratory assistance, Isabella Nassar for clerical assistance with the manuscript, and Leila Bertoni Giron for data organization.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100981.
Appendix. Supplementary materials
References
- 1.Valencia D.N. Brief Review on COVID-19: the 2020 pandemic caused by SARS-CoV-2. Cureus. 2020; 12. doi:10.7759/cureus.7386. [DOI] [PMC free article] [PubMed]
- 2.Rello J., Storti E., Belliato M., Serrano R. Clinical phenotypes of SARS-CoV-2: implications for clinicians and researchers. Eur Respir J. 2020;55 doi: 10.1183/13993003.01028-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W., Ni Z., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J., Dong X., Cao Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 5.Romagnoli S., Peris A., De Gaudio A.R., Geppetti P. SARS-CoV-2 and COVID-19: from the bench to the bedside. Physiological Reviews. 2020;100:1455–1466. doi: 10.1152/physrev.00020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang Y., Liu J., Zhang D., Xu Z., Ji J., Wen C. Cytokine Storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm” in COVID-19. Journal of Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng S., Fan J., Yu F. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:1–8. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y., Chen S., Yang Z. SARS-CoV-2 viral load in clinical samples from critically ill patients. Am J Respir Crit Care Med. 2020;201:1435–1438. doi: 10.1164/rccm.202003-0572LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribero M.S., Jouvenet N., Dreux M., Nisole S. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020;16:1–22. doi: 10.1371/journal.ppat.1008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labro G., Jandeaux L.M., Rusu A. Macrophage activation in COVID-19 patients in intensive care unit. J Med Cases. 2020;11:211–214. doi: 10.14740/jmc3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park M.D. Macrophages: a Trojan horse in COVID-19? Nat Rev Immunol. 2020;20 doi: 10.1038/s41577-020-0317-2. 351–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jafarzadeh A., Chauhan P., Saha B., Jafarzadeh S., Nemati M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 17.Gubernatorova E.O., Gorshkova E.A., Polinova A.I., Drutskaya M.S. IL-6: relevance for immunopathology of SARS-CoV-2. Cytokine Growth Fact Rev. 2020;53:13–24. doi: 10.1016/j.cytogfr.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cevik M., Kuppalli K., Kindrachuk J., Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020;371:1–6. doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- 19.Okabayashi T., Kariwa H., Yokota S. Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J Med Virol. 2006;78:417–424. doi: 10.1002/jmv.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García L.F. Immune Response, Inflammation, and the Clinical Spectrum of COVID-19. Front Immunol. 2020;11:4–8. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clinical Infectious Diseases. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossignol J.F. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res. 2014;110:94–103. doi: 10.1016/j.antiviral.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossignol J.F. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J Infect Public Health. 2016;9:227–230. doi: 10.1016/j.jiph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickson S.E., Margineantu D., Hockenbery D.M., Simon J.A., Geballe A.P. Inhibition of vaccinia virus replication by Nitazoxanide. Virology. 2018;518:398–405. doi: 10.1016/j.virol.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossignol J.F., La Frazia S., Chiappa L., Ciucci A., Santoro M.G. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. J Biol Chem. 2009;284:29798–29808. doi: 10.1074/jbc.M109.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pizzorno A., Padey B., Terrier O., Rosa-Calatrava M. Drug repurposing approaches for the treatment of influenza viral infection: reviving old drugs to fight against a long-lived enemy. Front Immunol. 2019;10:531. doi: 10.3389/fimmu.2019.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haffizulla J., Hartman A., Hoppers M. Effect of Nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2014;14:609–618. doi: 10.1016/S1473-3099(14)70717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bobrowski T., Chen L., Eastman R.T. Synergistic and antagonistic drug combinations against SARS-CoV-2. Molecular Therapy. 2021;29:873–885. doi: 10.1016/j.ymthe.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arshad U., Pertinez H., Box H. Prioritization of Anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human Pharmacokinetics. Clin Pharmacol Ther. 2020;108:775–790. doi: 10.1002/cpt.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stockis A., Bruyn S.D., Gengler C., Rosillon D. Nitazoxanide pharmacokinetics and tolerability in man during 7 days dosing with 0.5g and 1g b.i.d. CP. 2002;40:221–227. doi: 10.5414/cpp40221. [DOI] [PubMed] [Google Scholar]
- 32.Pepperrell T., Pilkington V., Owen A., Wang J., Hill A.M. Review of safety and minimum pricing of nitazoxanide for potential treatment of COVID-19. Journal of Virus Eradication. 2020;6:52–60. doi: 10.1016/S2055-6640(20)30017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. 2020 COVID-19 therapeutic trial synopsis. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf
- 34.Helios M., Rosario D. Package PWR title basic functions for power analysis. 2020.
- 35.R: a language and environment for statistical computing. 2020 Available from: https://www.gbif.org/pt/tool/81287/r-a-language-and-environment-for-statistical-computing
- 36.Vergara T.R.C., Samer S., Santos-Oliveira J.R. Thalidomide is associated with increased T cell activation and inflammation in Antiretroviral-naive HIV-infected individuals in a randomised clinical trial of efficacy and safety. E Bio Med. 2017;23:59–67. doi: 10.1016/j.ebiom.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lokhande A.S., Devarajan P.V. A review on possible mechanistic insights of Nitazoxanide for repurposing in COVID-19. Eur J Pharmacol. 2021;891 doi: 10.1016/j.ejphar.2020.173748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajoli R.K.R., Pertinez H., Arshad U. Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis. Br J Clin Pharmacol. 2020;15 doi: 10.1111/bcp.14619. 8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borba M.G.S., Val F.F.A., Sampaio V.S. Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 infection: a randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W., Zheng K., Liu S. Plasma CRP level is positively associated with the severity of COVID-19. Ann Clin Microbiol Antimicrob. 2020;15 doi: 10.1186/s12941-020-00362-2. 19-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intens Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akbari H., Tabrizi R., Lankarani K.B. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Life Sci. 2020;258 doi: 10.1016/j.lfs.2020.118167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Valle D.M., Kim-Schulze S., Huang H.H. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feldmann M., Maini R.N., Woody J.N. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baggiolini M., Walz A., Kunkel S.L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J., Li H., Luo M. Lymphopenia predicted illness severity and recovery in patients with COVID-19: a single-center, retrospective study. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0241659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang D., Guo R., Lei L. Frontline Science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J Leukoc Biol. 2021;109:13–22. doi: 10.1002/JLB.4HI0720-470R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robson F., Khan K.S., Le T.K. Coronavirus RNA proofreading: molecular basis and therapeutic targeting. Molecular Cell. 2020;79:710–727. doi: 10.1016/j.molcel.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naveca F., Nascimento V., Souza V. COVID-19 epidemic in the Brazilian state of Amazonas was driven by long-term persistence of endemic SARS-CoV-2 lineages and the recent emergence of the new Variant of Concern P.1. Review. 2021 doi: 10.21203/rs.3.rs-275494/v1. [DOI] [Google Scholar]
- 51.Rocco P.R.M., Silva P.L., Cruz F.F. Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial. Eur Respir J. 2020 doi: 10.1183/13993003.03725-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.