Abstract
Objectives
Superinfections in patients hospitalized in intensive care unit (ICU) are an important and challenging complication, also in COVID-19. However, no definitive data are available about the role of multidrug-resistant Acinetobacter baumannii (MDR-AB) in COVID-19.
Methods
This was a single-center, cross-sectional study including patients with MDR-AB infections admitted to ICU with or without COVID-19, between January 2019 and January 2021. The primary objective of the study was to evaluate risk factor for MDR-AB infections in ICU patients hospitalized for COVID-19 or other etiology. The secondary endpoints were 30-days mortality in all study population and risk factors associated with development of bloodstream infection (BSI).
Results
During the study period 32 adults with COVID-19 were enrolled and compared with 115 patients admitted in the same ICU for other reasons. We observed a total of 114 deaths, with a survival rate of 29.3%: 18.8% in COVID-19 and 32.2% in control group. Relative risk for MDR-AB infection in COVID-19 showed that serum lactate levels mmol/l > 2, Acinetobacter baumannii colonization, BSI and steroid therapy were observed more frequently in COVID-19 patients. Cox regression analysis showed that serum lactate levels > 2 mmol/l, Acinetobacter baumannii colonization, BSI, and steroid therapy were associated with 30-days mortality. Finally, patients with COVID-19, white blood cells count > 11,000 mm3, serum lactate levels > 2 mmol/l, infections at time of ICU admission, Acinetobacter baumannii colonization, and steroid therapy were independently associated with development of BSI.
Conclusions
Our data highlight the impact of BSI on outcome, the role of Acinetobacter baumannii colonization and the use of steroids on the risk to develop MDR-AB infections also during COVID-19.
Keywords: Acinetobacter baumannii, COVID-19, Bacteraemia, Colonization, Steroids
Introduction
Since the end of 2019 the Coronavirus Disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread globally affecting people worldwide [1, 2]. Patients with severe COVID-19 require intensive care unit (ICU) admission for acute respiratory failure and over 10% need noninvasive and invasive mechanical ventilation [3, 4]. Acute respiratory distress syndrome (ARDS) severity and ventilation management determine a negative outcome and a 90-days mortality of 31% [2].
The data about superinfections complicating COVID-19 are scant, and a significant proportion of these patients are treated with empiric broad spectrum antibiotic therapy that increase the risk to develop infections caused by multidrug-resistant (MDR) pathogens [5, 6]. Finally, the use of drugs targeting cytokines, such as IL-1 and IL-6, might also increase the risk of superinfections in patients with COVID-19 [7].
Infections caused by MDR Acinetobacter baumannii (MDR-AB) represent a major problem in patients admitted to the intensive care unit (ICU) [8, 9]. Inappropriate therapy and limited therapeutic options are responsible for negative impact on outcome and this infection is associated with high mortality rates, especially in ICU patients [10, 11].
The aim of our study was to evaluate the impact of MDR-AB infections on outcome of patients with COVID-19 requiring ICU admission, comparing with non-COVID-19 patients with MDR-AB infections hospitalized in the same ward. We evaluated risk factor for acquisition of MDR-AB infections in ICU patients hospitalized for COVID-19 or other etiology, 30-days mortality in all study population, and risk factors associated with development of bloodstream infection (BSI).
Methods
Study design and patient selection
This was a single-center, cross-sectional study including patients with MDR-AB infections consecutively admitted to the tertiary care Policlinico Umberto I, Sapienza University of Rome, Italy, between January 2019 and January 2021. We compare patients divided in two groups: patients with and without COVID-19 admitted to ICU. Inclusion criteria for all patients were: (1) age ≥ 18 years; (2) clinical signs and symptoms consistent with infection; (3) documented MDR-AB etiology. All patients were managed by the same team of physicians and all antimicrobial therapies were selected according to clinical judgment by infectious disease specialists. The prospective nature of the study was based on the consecutive enrollment of patients. However, all complete data were afterwards retrospectively extracted, and the local Ethics Committee waived the need for informed consent. The study was conducted according to the principles stated in the Declaration of Helsinki.
Patients’ data were collected from medical charts and from hospital computerized databases. The following information were reviewed: demographics; clinical, and laboratory findings; comorbid conditions and the age-adjusted Charlson Comorbidity Index; microbiological data; duration of ICU and hospital stay; any infection during hospitalization; duration of antibiotic therapy, and use of steroid therapy; procedures (e.g., mechanical ventilation, continuous renal replacement therapy [CRRT]), extracorporeal membrane oxygenation [ECMO], carried out during hospitalization; the simplified acute physiology score (SAPS II); sequential organ failure assessment (SOFA) and quick (q)-SOFA, serum lactate levels mmol > 2 at time of infection; anamnestic MDR-AB colonization or infection during hospitalization; source of infection and its adequate control; development of bacteremias and septic shock; 30-days mortality.
Definitions
Septic shock was defined according to international definitions [12].
The severity of clinical conditions was determined using SAPS II, SOFA, and qSOFA scores calculated at the time of infection onset.
The length of hospital and ICU stay were calculated as the number of days from the date of admission to the date of discharge or death. Adequate control of source of infection was defined as the removal of any preexisting contaminated CVC as well as the drainage of intra-abdominal abscesses or other fluid collections have been performed within 24 h after the onset of infection. The timing of CVC removal was based on the medical record review, and was confirmed by review of patient radiographs.
MDR-AB infections were classified in the following categories: ventilator-associated pneumonia (VAP), hospital-acquired pneumonia (HAP), urinary tract infection (UTI), and BSI. Acinetobacter baumannii colonization was weekly evaluated with an active surveillance in hospitalized patients.
Inclusion criteria for patients with COVID-19 were: (1) laboratory confirmed SARS-CoV-2 infection with an RT–PCR test on a nasopharyngeal swab; (2) uni- or bi-lateral interstitial infiltrates confirmed by CT scan or chest X ray; (3) presence of acute hypoxemic respiratory failure requiring mechanical ventilation.
All of these clinically indicated infections were categorized as co-infections or superinfections. If diagnosis was at the time of or within the first 24 h of COVID-19 hospital admission, these infections were defined as community-acquired co-infections. If diagnosis occurred ≥ 48 h after admission for COVID-19, these infections were defined as hospital-acquired superinfections.
MDR-AB definition
Identification of MDR-AB strains was based accordingly with local laboratory techniques. The Vitek 2 automated system (bioMérieux, Marcy l’Etoile, France) was used for isolate identification and antimicrobial susceptibility testing. Minimum inhibitory concentrations (MICs) were established according to the European Committee on Antimicrobial Susceptibility Testing (EU- CAST) breakpoints [13]. Isolated strains were classified as multidrug resistant (MDR), extensively drug resistant (XDR), and pandrug resistant (PDR) [14].
Primary endpoint and statistical analysis
The primary objective of the study was to evaluate risk factor for MDR-AB infections in ICU patients hospitalized for COVID-19 or other etiology. The secondary endpoints were 30-days mortality in all study population and risk factors associated with development of BSI.
All data were analyzed using Statistical Package for Social Science (SPSS) version 20 or Microsoft Excel (Office 2018). Description of mean ± standard deviation (SD), simple frequencies (n), proportions (or percentages), and rates of the given data on each variable has been calculated. The univariate analysis was used to compare patients divided in two groups: MDR-AB infection in patient with COVID-19 vs No COVID-19. T test was conducted for continuous variables and chi-square for categorical variables. The odds ratio (OR) and 95% confidence intervals (CI) were used to quantify the strength of the association between covariates and dependent variable. We have done a standard survival analysis, tracing participants affected, or not by COVID-19 from entry into the clinic to the discharge or death at 30 days. The event‐free survival in follow‐up was depicted graphically by Kaplan–Meier’s survival curve, including the confounding factors with fixed baseline covariates. A p value of less than 0.05 was considered statistically significant.
Results
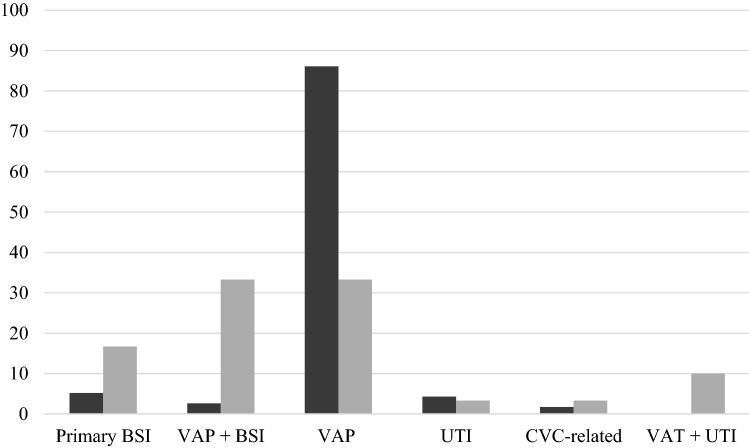
During the study period, 32 adults with COVID-19 and superinfection caused by MDR-AB were prospectively enrolled. This cohort of patients was compared with 115 patients with MDR-AB infection admitted in the same ICU for other etiologies: respiratory failure (29%), septic shock (26%), trauma (20%), stroke (15%), cardiac/hemorrhagic shock/postsurgery (10%). Overall, 147 patients were evaluated in the final analysis. Sites of MDR-AB infection in study population are reported in Fig. 1.
Fig. 1.
Sites of MDR-AB infection in COVID-19 (gray line) or non-COVID-19 (black line). MDR-AB multidrug-resistant Acinetobacter baumannii, BSI bloodstream infection, VAP ventilator-associated pneumonia, UTI urinary tract infection, CVC central venous catheter, VAT ventilator-associated tracheobronchitis
Table 1 shows univariate analysis comparing clinical characteristics of patients affected or not by COVID-19 with documented MDR-AB infection. Differences between COVID-19 and other patients were reported for previous hospitalization (16% vs. 39%, p < 0.015), chronic kidney disease (3% vs. 19%, p < 0.001), COPD (9% vs. 32%, p < 0.009), chronic corticosteroid therapy (0% vs. 27%, p < 0.001), and previous Acinetobacter baumannii colonization or infection (0% vs. 15%, p < 0.020). No differences were observed between COVID-19 and other patients related to the median age, Charlson Comorbidity Index, length of hospitalization, and ICU stay, SAPS II at time of admission, procedures (e.g., mechanical ventilation), clinical and laboratory findings at time of infection, duration of antibiotic therapy, development of septic shock, 30-days mortality.
Table 1.
Univariate analysis about clinical characteristics and outcome of patients with MDR-AB infections affected or not by COVID-19
| Variables | COVID-19 n = 32 (%) |
Non-COVID-19 n = 115 (%) |
p value |
|---|---|---|---|
| Anamnestic factors and comorbidities | |||
| Male sex | 21 (66%) | 73 (63%) | 0.543 |
| Age, mean ± SD (years) | 62.50 ± 10.99 | 62.59 ± 11.31 | 0.969 |
| Previous hospitalization (90 days) | 5 (16%) | 45 (39%) | 0.015 |
| Previous ICU admission (90 days) | 3 (9%) | 22 (19%) | 0.132 |
| > 2 comorbidities | 11 (34%) | 41 (36%) | 0.895 |
| Cardiovascular disease | 17 (53%) | 62 (54%) | 0.938 |
| Heart failure | 3 (9%) | 9 (8%) | 0.791 |
| Charlson Comorbidity Index, mean ± SD | 2.59 ± 1.81 | 2.79 ± 1.78 | 0.605 |
| Diabetes | 4 (13%) | 12 (10%) | 0.756 |
| Chronic kidney disease | 1 (3%) | 22 (19%) | 0.001 |
| Chronic liver disease | 1 (3%) | 8 (7%) | 0.333 |
| Neurologic disease | 3 (9%) | 19 (17%) | 0.267 |
| Vasculitis | 1 (3%) | 9 (8%) | 0.245 |
| COPD | 3 (9%) | 37 (32%) | 0.009 |
| Solid tumor | 2 (6%) | 9 (8%) | 0.755 |
| Hematological malignancies | 1 (3%) | 8 (7%) | 0.333 |
| Chronic corticosteroid therapy | 0 | 31 (27%) | 0.001 |
| Previous Acinetobacter baumannii infection (30 days) | 0 | 17 (15%) | 0.020 |
| Previous endoscopy procedure (30 days) | 3 (9%) | 16 (14%) | 0.464 |
| Intravascular devices | 5 (16%) | 17 (15%) | 0.909 |
| Previous antibiotic therapy (30 days) | 9 (28%) | 45 (39%) | 0.241 |
| Clinical features | |||
| SAPS II at time of admission, mean ± SD | 33.75 ± 15.57 | 37.10 ± 17.75 | 0.296 |
| GCS at the time of admission, mean ± SD | 14.65 ± 0.00 | 15.00 ± 0.00 | 0.330 |
| PaO2/FiO2 < 250 | 26 (81%) | 88 (77%) | 0.560 |
| WBC, mean ± SD | 10,937.81 ± 8794.10 | 13,411.83 ± 12,375.35 | 0.209 |
| Surgery | 2 (6%) | 9 (8%) | 0.755 |
| PLTs, mean ± SD | 254,656.25 ± 101,504.71 | 246,200.00 ± 88,537.26 | 0.679 |
| SOFA at the time of admission, mean ± SD | 5.31 ± 2.95 | 4.92 ± 2.55 | 0.487 |
| Quick SOFA at the time of admission, mean ± SD | 0.72 ± 0.70 | 0.83 ± 0.81 | 0.454 |
| CRRT | 13 (41%) | 46 (40%) | 0.949 |
| ECMO | 4 (13%) | 15 (13%) | 1.000 |
| Surgery source control | 3 (9%) | 20 (17%) | 0.267 |
| Septic shock | 8 (25%) | 43 (37%) | 0.208 |
| SOFA at time of infection onset, mean ± SD | 8.31 ± 4.29 | 7.48 ± 3.76 | 0.312 |
| Quick SOFA at time of infection onset, mean ± SD | 1.25 ± 0.90 | 1.28 ± 0.93 | 0.875 |
| PCT at time of infection onset, mean ± SD | 4.01 ± 6.33 | 3.73 ± 5.23 | 0.825 |
| Serum lactate levels > 2 mmol/l | 21 (66%) | 42 (37%) | 0.003 |
| MDR colonization at the time of ICU admission | 1 (3%) | 16 (14%) | 0.068 |
| Infections at time of ICU admission | 2 (6%) | 70 (61%) | < 0.001 |
| Acinetobacter baumannii colonization | 20 (63%) | 9 (8%) | < 0.001 |
| Time from colonization to MDR-AB infection, mean ± SD (days) | 10.21 ± 9.85 | 11.82 ± 9.2 | 0.89 |
| Bloodstream infection | 18 (56%) | 9 (8%) | < 0.001 |
| Outcomes and therapy | |||
| Steroid therapy | 28 (88%) | 5 (4%) | < 0.001 |
| Total duration of antibiotic therapy, mean ± SD | 25.56 ± 12.66 | 25.35 ± 14.87 | 0.936 |
| Transfer in ICU | 31 (97%) | 115 (100%) | 0.325 |
| Length of ICU stay, mean ± SD (days) | 22.22 ± 9.65 | 22.23 ± 9.53 | 0.997 |
| Length of hospitalization, mean ± SD (days) | 30.41 ± 13.56 | 29.10 ± 10.59 | 0.610 |
| Mortality at 30 days | 26 (81%) | 78 (68%) | 0.154 |
Statistically significant p-values are in bold
MDR-AB multidrug-resistant Acinetobacter baumannii, SD standard deviation, ICU intensive care unit, COPD chronic obstructive pulmonary disease, SAPS simplified acute physiology score, GCS Glasgow coma score, WBC white blood cell, PLT platelets, SOFA sequential organ failure assessment, PICC peripherally-inserted central catheter, CVC central venous catheter, CRRT continuous renal replacement therapy, ECMO extracorporeal membrane oxygenation, PCT procalcitonin, CRP C-reactive protein
Relative risk associated with COVID-19 vs. non-COVID-19 etiology was reported in Table 2: previous hospitalization (RR 0.4; CI 95% 0.2–0.9, p = 0.031), COPD (RR 0.3, CI 95% 0.1–0.9, p = 0.029), chronic corticosteroid therapy (RR 0.1, CI 95% 0.0–0.9, p = 0.041) and infection at time of ICU admission (RR 0.1, CI 95% 0.0–0.9, p = 0.001) were factors associated with non-COVID-19 etiology. Conversely, serum lactate levels > 2 mmol/l at time of infection (RR 1.8, CI 95% 1.3–2.5, p = 0.001), Acinetobacter baumannii colonization (RR 7.9, CI 95% 4.0–15.7, p < 0.001), BSI (RR 6.5, CI 95% 3.2–13.3, p < 0.001), and steroid therapy (RR 18.4, CI 95% 7.6–44.1, p < 0.001) were observed more frequently in COVID-19 patients.
Table 2.
Relative risk* associated or not with MDR-AB infection in patients affected or not by COVID-19
| Variables | RR | CI 95% | p value |
|---|---|---|---|
| Previous hospitalization (90 days) | 0.4 | 0.2–0.9 | 0.031 |
| COPD | 0.3 | 0.1–0.9 | 0.029 |
| Chronic steroid therapy | 0.1 | 0.0–0.9 | 0.041 |
| Infection at time of ICU admission | 0.1 | 0.0–0.4 | 0.001 |
| Serum lactate levels > 2 mmol/l | 1.8 | 1.3–2.5 | 0.001 |
| Acinetobacter baumannii colonization | 7.9 | 4.0–15.7 | < 0.001 |
| Bloodstream infection | 6.5 | 3.2–13.3 | < 0.001 |
| Steroid therapy | 18.4 | 7.6–44.1 | < 0.001 |
RR relative risk, CI confidence interval, COPD chronic obstructive pulmonary disease, ICU intensive care unit
*RR < 1 is associated with non-COVID-19 etiology; > 1 with COVID-19
Cox regression analysis of factors associated with 30-days mortality (see Table 3) showed that serum lactate levels > 2 mmol/l at time of infection (OR 4.9; CI 95% 2.1–11.3, p < 0.001), Acinetobacter baumannii colonization (OR 17.1, CI 95% 5.5–53.3, p < 0.001), BSI (OR 13.6; CI 95% 4.8–38.2, p < 0.001) and steroid therapy (OR 46.9; CI 95% 13.9–157.3, p < 0.001) were associated with 30-days mortality.
Table 3.
Logistic regression analysis about risk factors associated with 30-days mortality
| Variables | OR | CI 95% | p value |
|---|---|---|---|
| Serum lactate levels > 2 mmol/l | 4.9 | 2.1–11.3 | < 0.001 |
| Acinetobacter baumannii colonization | 17.1 | 5.5–53.3 | < 0.001 |
| Bloodstream infection | 13.6 | 4.8–38.2 | < 0.001 |
| Steroid therapy | 46.9 | 13.9–157.5 | < 0.001 |
OR odds ratio, CI confidence interval
The Kaplan–Meier curves for 30-days survival of overall patients with MDR-AB infections (COVID-19 or non-COVID-19 etiology) is reported in Fig. 2. We observed a total of 114 deaths, with a survival rate of 29.3%. Comparing the two groups, we observed a different survival rate: 18.8% (COVID-19) and 32.2% (other patients).
Fig. 2.
Kaplan–Meier curves for 30-days survival in patients affected (green line) or not (blue line) by COVID-19
Table 4 shows univariate analysis comparing patients developing or not BSI caused by MDR-AB. COVID-19 etiology (67% vs. 12%, p < 0.001), serum lactate levels > 2 mmol/l at time of infection (63% vs. 38%, p = 0.018), Acinetobacter baumannii colonization (44% vs. 14%, p = 0.006) and a steroid therapy during the hospitalization (63% vs. 13%, p < 0.001) were more frequently reported in patients developing MDR-AB BSI.
Table 4.
Univariate analysis comparing patients developing or not bloodstream infection
| Variables | No bloodstream infection n = 120 (%) |
Bloodstream infection n = 27 (%) |
p value |
|---|---|---|---|
| Anamnestic factors and comorbidities | |||
| COVID-19 | 14 (12%) | 18 (67%) | < 0.001 |
| Male sex | 77 (64%), | 17 (63%) | 0.922 |
| Age, mean ± SD (years) | 61.84 ± 11.55 | 65.81 ± 9.86 | 0.074 |
| Previous hospitalization (90 days) | 42 (35%) | 8 (30%) | 0.593 |
| Previous ICU admission (90 days) | 22 (18%) | 3 (11%) | 0.315 |
| > 2 comorbidities | 43 (36%) | 9 (33%) | 0.808 |
| Cardiovascular disease | 67 (56%) | 12 (44%) | 0.260 |
| Heart failure | 10 (8%) | 2 (7%) | 0.872 |
| Charlson Comorbidity Index mean ± SD | 2.73 ± 1.78 | 2.85 ± 1.97 | 0.761 |
| Diabetes | 13 (11%) | 3 (11%) | 1.000 |
| Chronic kidney disease | 18 (15%) | 5 (19%) | 0.674 |
| Chronic liver disease | 9 (8%) | 0 (0%) | 0.129 |
| Neurologic disease | 17 (14%) | 5 (19%) | 0.602 |
| Vasculitis | 8 (7%) | 2 (7%) | 1.000 |
| COPD | 34 (28%) | 6 (22%) | 0.508 |
| Solid tumor | 10 (8%) | 1 (4%) | 0.307 |
| Hematological malignancies | 9 (8%) | 0 | 0.129 |
| Chronic corticosteroid therapy | 27 (23%) | 4 (15%) | 0.339 |
| Previous Acinetobacter baumannii infection (30 days) | 16 (13%) | 1 (4%) | 0.051 |
| Previous endoscopy procedure (30 days) | 18 (15%) | 1 (4%) | 0.157 |
| Intravascular devices | 16 (13%) | 6 (22%) | 0.316 |
| Previous antibiotic therapy (30 days) | 47 (39%) | 7 (26%) | 0.179 |
| Clinical features | |||
| SAPS II at time of admission, mean ± SD | 37.01 ± 17.69 | 33.56 ± 15.24 | 0.308 |
| GCS at the time of admission, mean ± SD | 15.00 ± 0.00 | 14.63 ± 1.61 | 0.331 |
| PaO2/FiO2 < 250 | 95 (79%) | 19 (70%) | 0.207 |
| WBC mean ± SD | 13,707.75 ± 12,658.93 | 9164.44 ± 4563.52 | 0.002 |
| Surgery | 10 (8%) | 1 (4%) | 0.307 |
| PTL mean ± SD | 248,166.67 ± 88,895.02 | 247,481.48 ± 106,697.40 | 0.975 |
| SOFA at the time of admission, mean ± SD | 5.02 ± 2.59 | 4.96 ± 2.79 | 0.928 |
| Quick SOFA at the time of admission, mean ± SD | 0.83 ± 0.81 | 0.67 ± 0.62 | 0.241 |
| CRRT | 45 (37.5) | 14 (51.8) | 0.06 |
| ECMO | 18 (15%) | 1 (4%) | 0.157 |
| Surgery source control | 22 (18%) | 1 (4%) | 0.069 |
| Septic shock | 40 (33.3) | 11 (40.7) | 0.384 |
| SOFA at time of infection onset, mean ± SD | 7.44 ± 3.72 | 8.63 ± 4.33 | 0.196 |
| qSOFA at time of infection onset, mean ± SD | 1.24 ± 0.92 | 1.41 ± 0.93 | 0.407 |
| PCT at time of infection onset, mean ± SD | 3.75 ± 5.52 | 3.97 ± 5.18 | 0.847 |
| Serum lactate levels > 2 mmol/l | 46 (38%) | 17 (63%) | 0.018 |
| MDR colonization at the time of ICU admission | 15 (13%) | 2 (7%) | 0.386 |
| Infection at time of ICU admission | 64 (53%) | 8 (30%) | 0.031 |
| Acinetobacter baumannii colonization | 17 (14%) | 12 (44%) | 0.006 |
| Time from colonization to MDR-AB infection, mean ± SD (days) | 13.34 ± 11.81 | 10.91 ± 8.2 | 0.03 |
| Outcomes and therapy | |||
| Steroid therapy | 16 (13%) | 17 (63%) | < 0.001 |
| Total duration of antibiotic therapy, mean ± SD | 25.83 ± 15.19 | 23.44 ± 10.48 | 0.333 |
| Transfer in ICU | 120 (100%) | 26 (96%) | 0.327 |
| Length of ICU stay, mean ± SD (days) | 21.75 ± 9.17 | 24.33 ± 11.51 | 0.283 |
| Length of hospitalization, mean ± SD (days) | 29.46 ± 11.20 | 29.07 ± 11.25 | 0.873 |
| Mortality at 30 days | 84 (70%) | 20 (74%) | 0.673 |
Statistically significant p-values are in bold
MDR-AB multidrug-resistant Acinetobacter baumannii, SD standard deviation, ICU intensive care unit, COPD chronic obstructive pulmonary disease, SAPS simplified acute physiology score, GCS Glasgow coma score, WBC white blood cell, PLT platelets, SOFA sequential organ failure assessment, PICC peripherally-inserted central catheter, CVC central venous catheter, CRRT continuous renal replacement therapy, ECMO extracorporeal membrane oxygenation, PCT procalcitonin, CRP C-reactive protein
Finally, multivariate analysis about risk factors associated with development of BSI (see Table 5) showed that patients with COVID-19 (OR 15.1, CI 95% 3.7–40.1; p < 0.001), white blood cells count > 11,000 mm3 (OR 5.2, CI 95% 2.1–11.5; p < 0.001), serum lactate levels > 2 mmol/l (OR 2.7; CI 95% 1.2–6.4, p = 0.022), infections at time of ICU admission (OR 0.4, CI 95% 0.2–1.0, p = 0.030), Acinetobacter baumannii colonization (OR 4.8, CI 95% 1.9–12.1, p < 0.001), and steroid therapy during hospitalization (OR 8.8, CI 95% 3.5–22.1, p < 0.001) were factors independently associated with development of BSI.
Table 5.
Multivariate analysis about risk factors associated with development of bloodstream infection
| Variables | OR | CI 95% | p value |
|---|---|---|---|
| Severe COVID-19 | 15.1 | 3.7–40.1 | < 0.001 |
| WBC > 11,000 mm3 | 5.2 | 2.1–11.5 | < 0.001 |
| Serum lactate levels > 2 mmol/l | 2.7 | 1.2–6.4 | 0.022 |
| Infections at time of ICU admission | 0.4 | 0.2–1 | 0.030 |
| Acinetobacter baumannii colonization | 4.8 | 1.9–12.1 | < 0.001 |
| Steroid therapy | 8.8 | 3.5–22.1 | < 0.001 |
OR odds ratio, CI confidence interval, WBC white blood cell, ICU intensive care unit
Discussion
To our knowledge, this is the largest experience about risk factors and outcomes of MDR-AB infections in patients affected or not by COVID-19 in ICU. Our study confirms that bacterial superinfections may complicate the hospital course of patients with COVID-19, and we identified peculiar characteristics of COVID-19 patients developing these difficult-to-treat infections. Our data showed that serum lactate levels > 2 mmol/l at time of infection, Acinetobacter baumannii colonization, development of a BSI, and steroid therapy were the most important factors associated with MDR-AB infection in COVID-19 patients and resulted as important determinants of 30-day mortality also in all study population.
Moreover, COVID-19 etiology, white blood cells count > 11,000 mm3, serum lactate levels > 2 mmol/l, infection at time of ICU admission, Acinetobacter baumannii colonization, and steroid therapy during hospitalization were associated with higher risk of BSI development. In this study, our data showed as MDR-AB BSI remain an important ICU-acquired infection [8, 9, 11–15].
Of interest, our experience highlighted the importance of superinfections caused by Gram-negative strains in ICU, including COVID-19 patients. Of importance, a rapid spread of MDR gram-negative bacteria among patients in dedicated coronavirus disease care units was recently observed [16]. In a recent meta-analysis, 19% of patients with COVID-19 showed co-infections and 24% superinfections; the presence of either co-infection or superinfection was associated with poor outcomes, including increased mortality [17].
First of all, this data reflect local epidemiology characterized by a high prevalence of MDR Gram-negatives. The data from literature suggest that COVID-19 was associated with a less effective implementation of infection control procedures for several reasons [18]. As a matter of fact, health-care workers (HCWs) experimented important difficulties to apply standard precautions, and to wear the same equipment for a long time; moreover, HCWs mainly focused on self-protection rather than on cross-transmission of bacteria in the wards. Finally, overcrowded wards, shortages of professionals with appropriate training in infection control procedures, and possible decreased laboratory ability to detect MDR carriage are potentially considered risk factors of MDR spread after the COVID-19 outbreak [19, 20]. Then, it will be crucial to continue monitoring rates of MDR infections and implementing measures of infection control and antimicrobial stewardship [21–23].
Of note, Acinetobacter baumannii intestinal colonization resulted as an independent predictor of infection in COVID-19 patients. As a matter of fact, the association between rectal carriage by carbapenem-resistant pathogens and development of infection is reported as an important predictor of infection, especially in ICU patients [24]. There are many important observations about the role of the gut microbiota during SARS-CoV-2 infection. The gut microbiota of COVID-19 patients is characterized by enrichment of opportunistic pathogens and alterations in gut cells also in the absence of gastrointestinal manifestations [19, 25, 26]. Of interest, these alterations were observed after hospitalization; however, the administration of broad-spectrum antibiotics is the major determinant for intestinal colonization by MDR pathogens.
Our data showed that MDR-AB BSI remains an important ICU-acquired infection, associated with higher mortality. In our interpretation, sepsis and septic shock determine a lethal cascade of events that is unlikely to be interrupted even by an appropriate initial antimicrobial treatment. In addition, most of our patients were severely ill and would probably have been unable to survive their infections independently of the administration of an adequate initial antimicrobial treatment. The data about the high rate of unfavorable outcome in patient with MDR-AB BSI were previously reported and discussed [8, 9, 11]. Finally, MDR-AB can be considered as a marker of the severity for the underlying diseases.
A peculiar aspect of COVID-19 patients, especially during the “second wave”, was the widely use of steroids at high dosages. The use of dexamethasone resulted in lower 28-day mortality, especially in patients receiving invasive mechanical ventilation [27, 28]. However, the prolonged use of high doses of steroids could be associated with the well-known immunomodulant effects of these drugs [29], but the association between steroid treatment and MDR infections deserves further comments. We can hypothesize that patients who received steroids survived longer and were, therefore, more likely to develop an MDR infection during ICU stay. However, in COVID-19 patients with a prolonged ICU stay the use of steroids and immunomodulant drugs may increase the risk of superinfections and should be used with caution [30, 31].
Our study has some limitations. First, it is a single-center study conducted in a setting with a high prevalence of MDR pathogens. Second, the sample size is relatively small, and the CIs of some significant predictors are quite broad. Third, we did not perform a multilocus sequence typing of the strains to understand if we observed an outbreak of infection. Finally, the impact of some therapies, including immunomodulant drugs, and of empiric and definitive antibiotic regimens for treatment of MDR-AB infections were not definitively assessed in the final analysis.
In conclusion, we reported a single-center experience about MDR-AB infection in COVID-19 patients, comparing those with ICU patients hospitalized for other etiologies. Our data highlight the impact of BSI on outcome, the role of Acinetobacter baumannii colonization and the use of steroids on the risk to develop MDR-AB infections also during COVID-19. Antimicrobial stewardship programs are mandatory in this population [32, 33].
Funding
Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement. None.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Transparency declarations
None to declare.
References
- 1.Serafim RB, Póvoa P, Souza-Dantas V, Kalil AC, Salluh JIF. Clinical course and outcomes of critically ill patients with COVID-19 infection: a systematic review. Clin Microbiol Infect. 2021;27:47–54. doi: 10.1016/j.cmi.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Soleimani J, Herasevich S, Pinevich Y, Pennington KM, Dong Y, Pickering BW, Barwise AK. Clinical characteristics, treatment, and outcomes of critically Ill patients with COVID-19: a scoping review. Mayo Clin Proc. 2021;96:183–202. doi: 10.1016/j.mayocp.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rouzé A, Martin-Loeches I, Povoa P, Makris D, Artigas A, Bouchereau M, Lambiotte F, Metzelard M, Cuchet P, Boulle Geronimi C, Labruyere M, Tamion F, Nyunga M, Luyt CE, Labreuche J, Pouly O, Bardin J, Saade A, Asfar P, Baudel JL, Beurton A, Garot D, Ioannidou I, Kreitmann L, Llitjos JF, Magira E, Mégarbane B, Meguerditchian D, Moglia E, Mekontso-Dessap A, Reignier J, Turpin M, Pierre A, Plantefeve G, Vinsonneau C, Floch PE, Weiss N, Ceccato A, Torres A, Duhamel A, Nseir S, coVAPid study Group Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med. 2021;47:188–198. doi: 10.1007/s00134-020-06323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lima WG, Brito JCM, da Cruz Nizer WS. Ventilator-associated pneumonia (VAP) caused by carbapenem-resistant Acinetobacter baumannii in patients with COVID-19: two problems, one solution? Med Hypotheses. 2020;144:110139. doi: 10.1016/j.mehy.2020.110139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contou D, Claudinon A, Pajot O, Micaëlo M, Longuet Flandre P, Dubert M, Cally R, Logre E, Fraissé M, Mentec H, Plantefève G. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care. 2020;10:119. doi: 10.1186/s13613-020-00736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pettit NN, Nguyen CT, Mutlu GM, Wu D, Kimmig L, Pitrak D, Pursell K. Late onset infectious complications and safety of tocilizumab in the management of COVID-19. J Med Virol. 2021;93:1459–1464. doi: 10.1002/jmv.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo A, Bassetti M, Ceccarelli G, Carannante N, Losito AR, Bartoletti M, Corcione S, Granata G, Santoro A, Giacobbe DR, Peghin M, Vena A, Amadori F, Segala FV, Giannella M, Di Caprio G, Menichetti F, Del Bono V, Mussini C, Petrosillo N, De Rosa FG, Viale P, Tumbarello M, Tascini C, Viscoli C, Venditti M, ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva) Bloodstream infections caused by carbapenem-resistant Acinetobacter baumannii: Clinical features, therapy and outcome from a multicenter study. J Infect. 2019;79:130–138. doi: 10.1016/j.jinf.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Russo A, Bassetti M, Bellelli V, Bianchi L, Marincola Cattaneo F, Mazzocchetti S, Paciacconi E, Cottini F, Schiattarella A, Tufaro G, Sabetta F, D'Avino A. Efficacy of a fosfomycin-containing regimen for treatment of severe pneumonia caused by multidrug-resistant Acinetobacter baumannii: a prospective, observational study. Infect Dis Ther. 2021;10:187–200. doi: 10.1007/s40121-020-00357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassetti M, Righi E, Vena A, Graziano E, Russo A, Peghin M. Risk stratification and treatment of ICU-acquired pneumonia caused by multidrug- resistant/extensively drug-resistant/pandrug-resistant bacteria. Curr Opin Crit Care. 2018;24:385–393. doi: 10.1097/MCC.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 11.Russo A, Giuliano S, Ceccarelli G, Alessandri F, Giordano A, Brunetti G, Venditti M. Comparison of septic shock due to multidrug-resistant Acinetobacter baumannii or Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in intensive care unit patients. Antimicrob Agents Chemother. 2018;62:e02562–e2617. doi: 10.1128/AAC.02562-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer M, Deutschman CS, Seymour CW, Shankar- Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Committee on Antimicrobial Susceptibility Testing (EUCAST). Break- point tables for interpretation of MICs and zone diameters, Växjö, Sweden: European Committee on Antimicrobial Susceptibility Testing; 2021, https://eucast.org/clinical_breakpoints/. Accessed 31 Mar 2021.
- 14.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 15.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. Clinical and pathophysiological overview of acinetobacter infections: a century of challenges. Clin Microbiol Rev. 2017;30:409–447. doi: 10.1128/CMR.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel A, Emerick M, Cabunoc MK, Williams MH, Preas MA, Schrank G, Rabinowitz R, Luethy P, Johnson JK, Leekha S. Rapid spread and control of multidrug-resistant gram-negative bacteria in COVID-19 patient care units. Emerg Infect Dis. 2021;27:1234–1237. doi: 10.3201/eid2704.204036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PLoS ONE. 2021;16:e0251170. doi: 10.1371/journal.pone.0251170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.d'Ettorre G, Ceccarelli G, Santinelli L, Vassalini P, Innocenti GP, Alessandri F, Koukopoulos AE, Russo A, d'Ettorre G, Tarsitani L. Post-traumatic stress symptoms in healthcare workers dealing with the COVID-19 Pandemic: a systematic review. Int J Environ Res Public Health. 2021;18:601. doi: 10.3390/ijerph18020601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falcone M, Tiseo G, Giordano C, Leonildi A, Menichini M, Vecchione A, Pistello M, Guarracino F, Ghiadoni L, Forfori F, Barnini S, Menichetti F, Pisa COVID-19 Study Group Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: a prospective observational study. J Antimicrob Chemother. 2021;76:1078–1084. doi: 10.1093/jac/dkaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monnet DL, Harbarth S. Will coronavirus disease (COVID-19) have an impact on antimicrobial resistance? Euro Surveill. 2020;25:2001886. doi: 10.2807/1560-7917.ES.2020.25.45.2001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isler B, Doi Y, Bonomo RA, Paterson DL. New treatment options against carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother. 2018;63:e01110–e1118. doi: 10.1128/AAC.01110-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bengoechea JA, Bamford CG. SARS-CoV-2, bacterial co-infections, and AMR: the deadly trio in COVID-19? EMBO Mol Med. 2020;12:e12560. doi: 10.15252/emmm.202012560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo A. Spotlight on new antibiotics for the treatment of pneumonia. Clin Med Insights Circ Respir Pulm Med. 2020;14:1179548420982786. doi: 10.1177/1179548420982786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cano A, Gutiérrez-Gutiérrez B, Machuca I, et al. Risks of infection and mortality among patients colonized with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: validation of scores and proposal for management. Clin Infect Dis. 2018;66:1204–1210. doi: 10.1093/cid/cix991. [DOI] [PubMed] [Google Scholar]
- 25.Spagnolello O, Pinacchio C, Santinelli L, Vassalini P, Innocenti GP, De Girolamo G, Fabris S, Giovanetti M, Angeletti S, Russo A, Mastroianni CM, Ciccozzi M, Ceccarelli G, d’Ettorre G. Targeting microbiome: an alternative strategy for fighting SARS-CoV-2 infection. Chemotherapy. 2021 doi: 10.1159/000515344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo T, Liu Q, Zhang F, Lui GC, Tso EY, Yeoh YK, Chen Z, Boon SS, Chan FK, Chan PK, Ng SC. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70:276–284. doi: 10.1136/gutjnl-2020-322294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welte T, Ambrose LJ, Sibbring GC, Sheikh S, Müllerová H, Sabir I. Current evidence for COVID-19 therapies: a systematic literature review. Eur Respir Rev. 2021;30:200384. doi: 10.1183/16000617.0384-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko JJ, Wu C, Mehta N, Wald-Dickler N, Yang W, Qiao R. A comparison of methylprednisolone and dexamethasone in intensive care patients with COVID-19. J Intensive Care Med. 2021 doi: 10.1177/0885066621994057. [DOI] [PubMed] [Google Scholar]
- 29.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva MVAO, Baldassare FP, Costa ELV, Moura RAB, Honorato MO, Costa AN, Damiani LP, Lisboa T, Kawano-Dourado L, Zampieri FG, Olivato GB, Righy C, Amendola CP, Roepke RML, Freitas DHM, Forte DN, Freitas FGR, Fernandes CCF, Melro LMG, Junior GFS, Morais DC, Zung S, Machado FR, Azevedo LCP, COALITION COVID-19 Brazil III Investigators Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, Fernandez-Pittol M, Pitart C, Inciarte A, Bodro M, Morata L, Ambrosioni J, Grafia I, Meira F, Macaya I, Cardozo C, Casals C, Tellez A, Castro P, Marco F, García F, Mensa J, Martínez JA, Soriano A, COVID-19 Researchers Group Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Somers EC, Eschenauer GA, Troost JP, Golob JL, Gandhi TN, Wang L, Zhou N, Petty LA, Baang JH, Dillman NO, Frame D, Gregg KS, Kaul DR, Nagel J, Patel TS, Zhou S, Lauring AS, Hanauer DA, Martin E, Sharma P, Fung CM, Pogue JM. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russo A, Venditti M, Ceccarelli G, Mastroianni CM, d'Ettorre G. Procalcitonin in daily clinical practice: an evergreen tool also during a pandemic. Intern Emerg Med. 2021;16:541–543. doi: 10.1007/s11739-021-02659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russo A, Bellelli V, Ceccarelli G, Marincola Cattaneo F, Bianchi L, Pierro R, Russo R, Steffanina A, Pugliese F, Mastroianni CM, d’Ettorre G, Sabetta F. Comparison between hospitalized patients affected or not by COVID-19 (RESILIENCY study) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1745. [DOI] [PMC free article] [PubMed] [Google Scholar]